Abstract
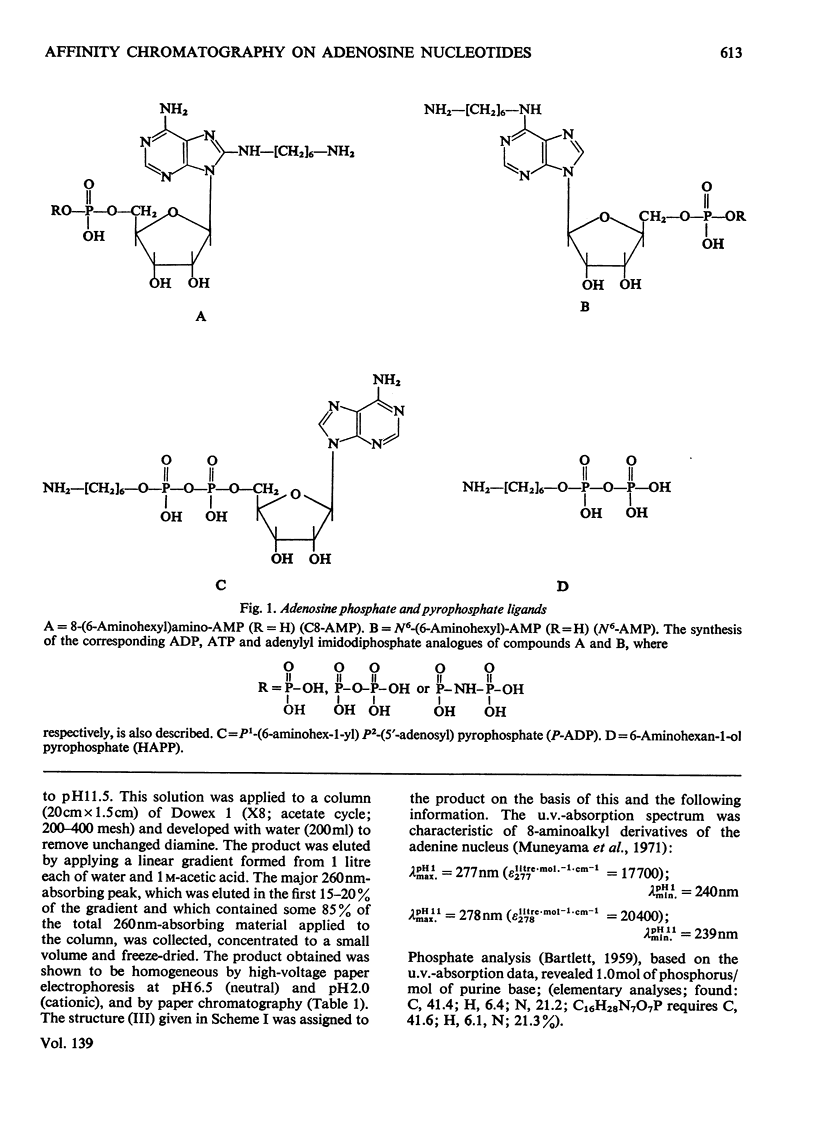
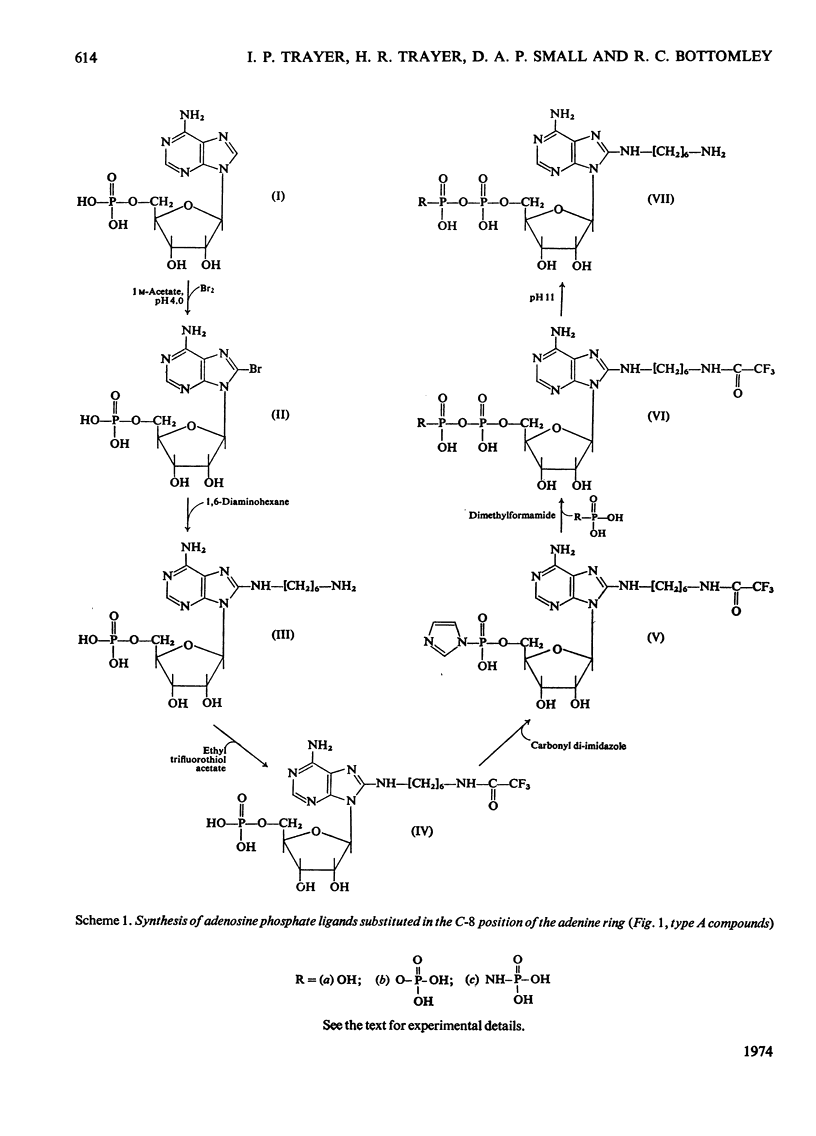
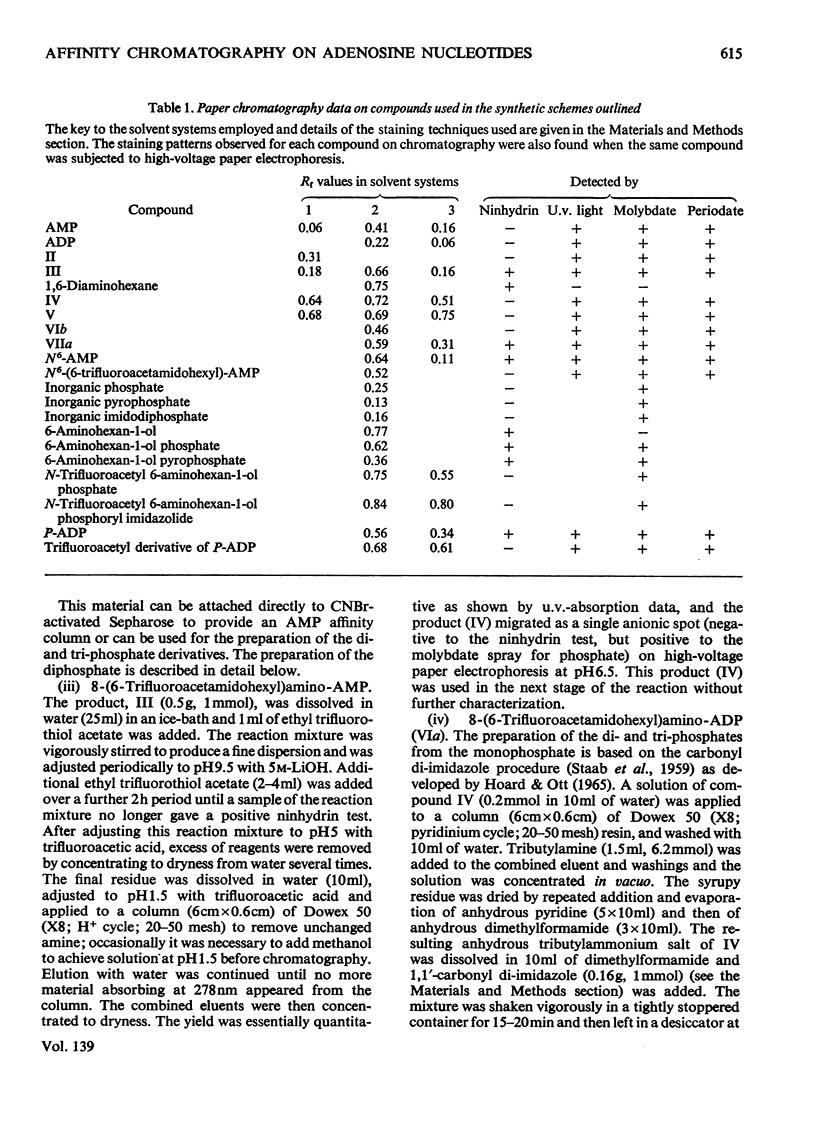
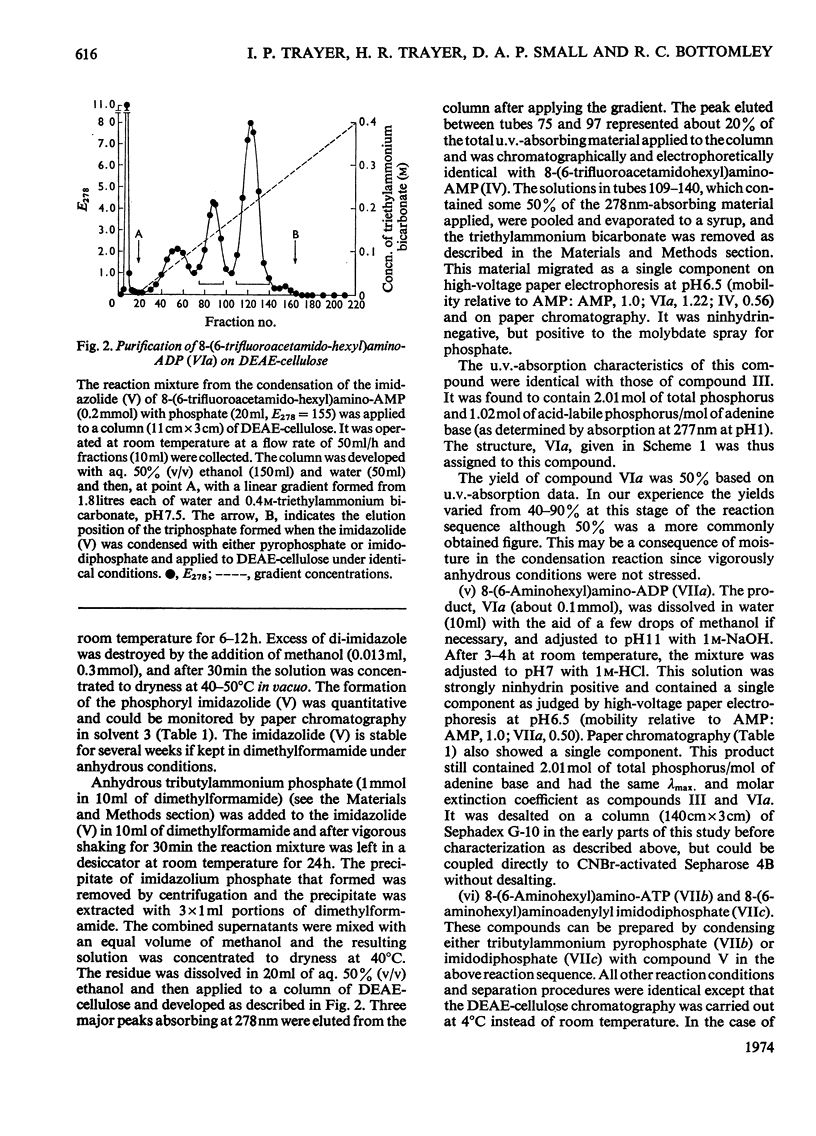
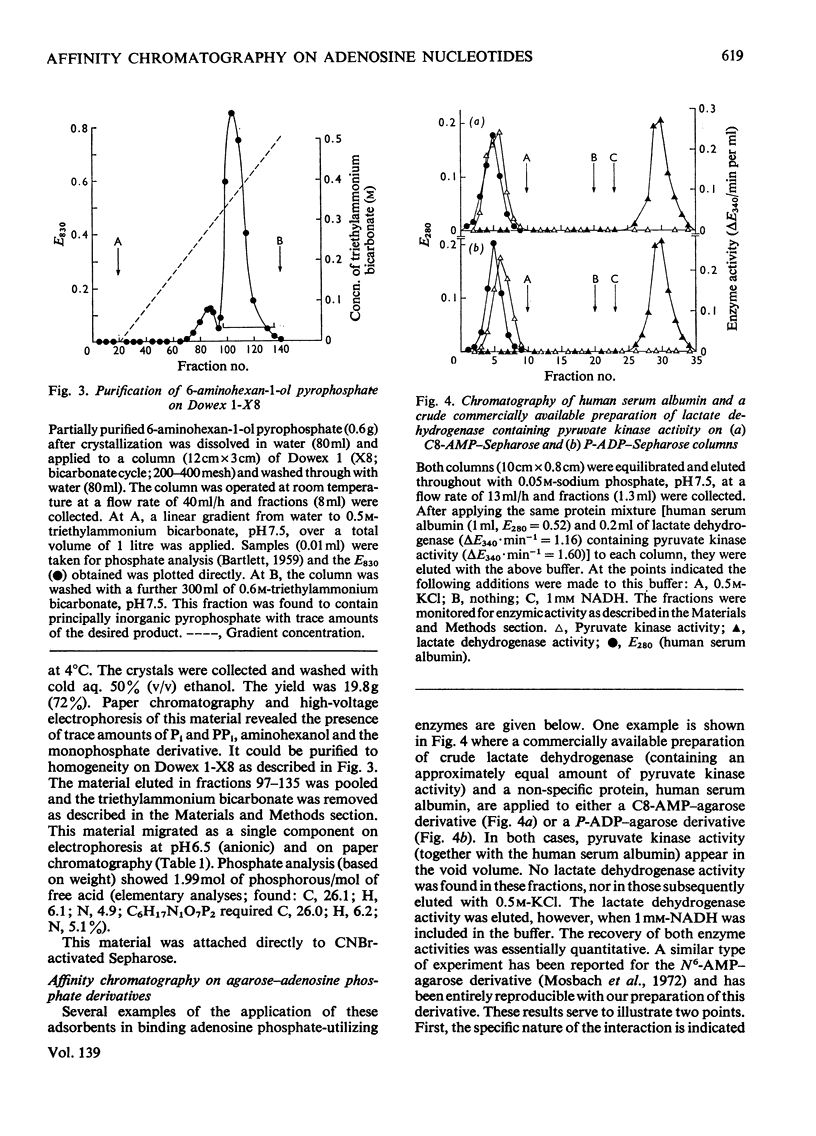
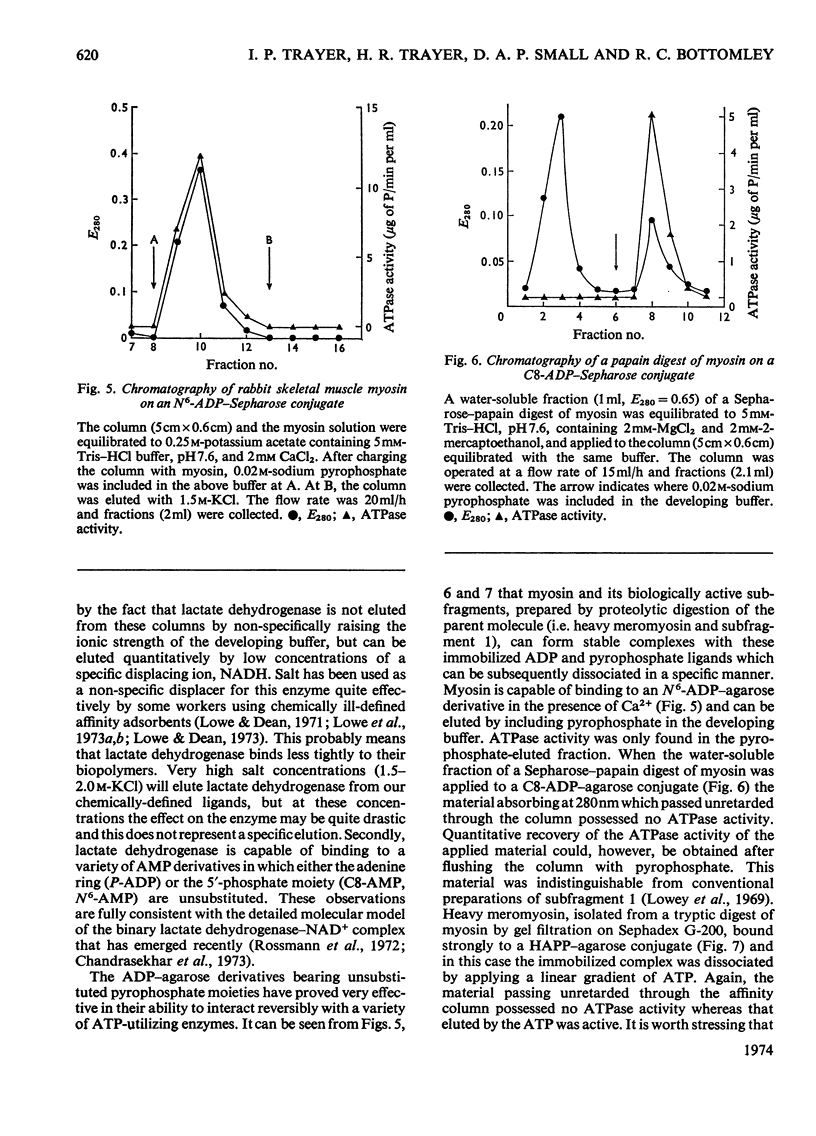
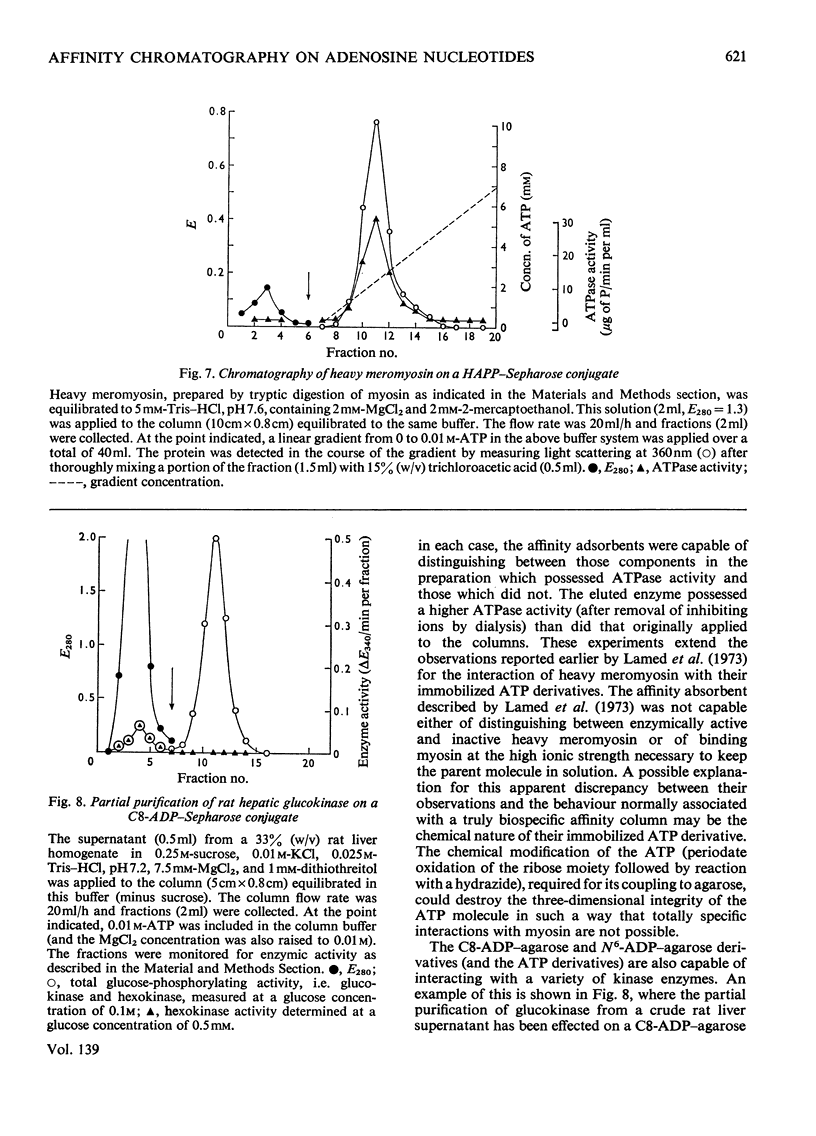
Methods of synthesizing a series of chemically-defined AMP, ADP, ATP, adenylyl imidodiphosphate and pyrophosphate derivatives suitable for affinity chromatography are extensively described. Each derivative has a single primary amino group at the end of a hexamethylene `spacer' chain for attachment to CNBr-activated agarose. The synthesis of the derivative where the `spacer' arm is attached directly to the 8 position of the adenine ring to produce 8-(6-aminohexyl)amino-AMP involves the direct bromination of AMP in the 8 position followed by displacement of the halogen by 1,6-diaminohexane. This monophosphate derivative can then be converted into the corresponding di- or triphosphate forms by direct phosphate condensation with carbonyl di-imidazole. A second series of adenosine phosphate derivatives with the phosphate moieties unsubstituted has been similarly prepared from N6-(6-aminohexyl)-AMP (Guilford et al., 1972). A third type of ligand has been synthesized by condensing the phosphoryl imidazolide of AMP with 6-aminohex-1-yl phosphate. This compound, P1-(6-aminohex-1-yl) P2-(5′-adenosyl) pyrophosphate, has an unsubstituted adenine ring. The synthesis of a fourth type of ligand, 6-aminohex-1-yl pyrophosphate, was done by heating 6-aminohexan-1-ol with crystalline pyrophosphoric acid under reduced pressure. The structures of the synthesized compounds were confirmed by chemical, electrophoretic and chromatographic methods and by u.v. spectrometry. The general applicability of the synthetic methods used is discussed in relation to the preparation of other affinity adsorbents. Examples are given where these derivatives have been successful in reversibly binding dehydrogenases, kinases and myosin and its proteolytic subfragments. The partial purification of rat liver glucokinase on an ADP derivative is shown.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akanuma H., Kasuga A., Akanuma T., Yamasaki M. The effective use of affinity chromatogrphy for the study of complex formation of bovine carboxypeptidase B with basic and aromatic amino acid analogues. Biochem Biophys Res Commun. 1971 Oct 1;45(1):27–33. doi: 10.1016/0006-291x(71)90045-3. [DOI] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BROWN G. B., WELIKY V. S. The synthesis of 9-beta-D-ribofuranosylpurine and the identity of nebularine. J Biol Chem. 1953 Oct;204(2):1019–1024. [PubMed] [Google Scholar]
- Barker R., Olsen K. W., Shaper J. H., Hill R. L. Agarose derivatives of uridine diphosphate and N-acetylglucosamine for the purification of a galactosyltransferase. J Biol Chem. 1972 Nov 25;247(22):7135–7147. [PubMed] [Google Scholar]
- Berglund O., Eckstein F. Synthesis of ATP- and dATP-substituted sepharoses and their application in the purification of phage-T4-induced ribonucleotide reductase. Eur J Biochem. 1972 Aug 4;28(4):492–496. doi: 10.1111/j.1432-1033.1972.tb01936.x. [DOI] [PubMed] [Google Scholar]
- Brodelius P., Mosbach K. Separation of the isoenzymes of lactate dehydrogenase by affinity chromatography using an immobilized AMP-analogue. FEBS Lett. 1973 Sep 15;35(2):223–226. doi: 10.1016/0014-5793(73)80290-x. [DOI] [PubMed] [Google Scholar]
- Campbell D. H., Luescher E., Lerman L. S. Immunologic Adsorbents: I. Isolation of Antibody by Means of a Cellulose-Protein Antigen. Proc Natl Acad Sci U S A. 1951 Sep;37(9):575–578. doi: 10.1073/pnas.37.9.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar K., McPherson A., Jr, Adams M. J., Rossmann M. G. Conformation of coenzyme fragments when bound to lactate dehydrogenase. J Mol Biol. 1973 Jun 5;76(4):503–518. doi: 10.1016/0022-2836(73)90488-9. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography. Annu Rev Biochem. 1971;40:259–278. doi: 10.1146/annurev.bi.40.070171.001355. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new color reaction for the determination of aldopentose in presence of other saccharides. Biochim Biophys Acta. 1957 Mar;23(3):639–642. doi: 10.1016/0006-3002(57)90387-6. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Moos C. The interaction of actin with myosin and heavy meromyosin in solution at low ionic strength. J Biol Chem. 1967 Jun 25;242(12):2945–2951. [PubMed] [Google Scholar]
- Er-el Z., Zaidenzaig Y., Shaltiel S. Hydrocarbon-coated sepharoses. Use in the purification of glycogen phosphorylase. Biochem Biophys Res Commun. 1972 Oct 17;49(2):383–390. doi: 10.1016/0006-291x(72)90422-6. [DOI] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- HOARD D. E., OTT D. G. CONVERSION OF MONO- AND OLIGODEOXYRIBONUCLEOTIDES TO 5-TRIPHOSPHATES. J Am Chem Soc. 1965 Apr 20;87:1785–1788. doi: 10.1021/ja01086a031. [DOI] [PubMed] [Google Scholar]
- Ikehara M., Uesugi S. Studies on nucleosides and nucleotides. 38. Synthesis of 8-bromoadenosine nucleotides. Chem Pharm Bull (Tokyo) 1969 Feb;17(2):348–354. doi: 10.1248/cpb.17.348. [DOI] [PubMed] [Google Scholar]
- Lamed R., Levin Y., Oplatka A. Enzymatic mechanochemistry. I. The interaction of heavy meromyosin with "immobilized adenosine triphosphate". Biochim Biophys Acta. 1973 Apr 27;305(1):163–171. doi: 10.1016/0005-2728(73)90241-7. [DOI] [PubMed] [Google Scholar]
- Lowe C. R., Dean P. D. Affinity chromatography of lactate dehydrogenase on immobilized nucleotides. Biochem J. 1973 Jul;133(3):515–520. doi: 10.1042/bj1330515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C. R., Dean P. D.G. Affinity chromatography of enzymes on insolubilized cofactors. FEBS Lett. 1971 May 20;14(5):313–316. doi: 10.1016/0014-5793(71)80288-0. [DOI] [PubMed] [Google Scholar]
- Lowe C. R., Harvey M. J., Craven D. B., Dean P. D. Some parameters relevant to affinity chromatography on immobilized nucleotides. Biochem J. 1973 Jul;133(3):499–506. doi: 10.1042/bj1330499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C. R., Harvey M. J., Craven D. B., Kerfoot M. A., Hollows M. E., Dean P. D. The purification of nicotinamide nucleotide-dependent dehydrogenases on immobilized cofactors. Biochem J. 1973 Jul;133(3):507–513. doi: 10.1042/bj1330507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- MICHELSON A. M., DONDON J., GRUNBERG-MANAGO M. The action of polynucleotide phosphorylase on 5-halogenouridine-5' pyrophosphates. Biochim Biophys Acta. 1962 Apr 2;55:529–540. doi: 10.1016/0006-3002(62)90986-1. [DOI] [PubMed] [Google Scholar]
- Mosbach K., Guilford H., Ohlsson R., Scott M. General ligands in affinity chromatography. Cofactor-substrate elution of enzymes bound to the immobilized nucleotides adenosine 5'-monophosphate and nicotinamide-adenine dinucleotide. Biochem J. 1972 May;127(4):625–631. doi: 10.1042/bj1270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneyama K., Bauer R. J., Shuman D. A., Robins R. K., Simon L. N. Chemical synthesis and biological activity of 8-substituted adenosine 3',5'-cyclic monophosphate derivatives. Biochemistry. 1971 Jun 8;10(12):2390–2395. doi: 10.1021/bi00788a033. [DOI] [PubMed] [Google Scholar]
- Ohlsson R., Brodelius P., Mosbach K. Affinity chromatography of enzymes on an AMP-analogue: Specific elution of dehydrogenases from a general ligand. FEBS Lett. 1972 Sep 15;25(2):234–238. doi: 10.1016/0014-5793(72)80492-7. [DOI] [PubMed] [Google Scholar]
- Parry M. J., Walker D. G. Purification and properties of adenosine 5'-triphospae-D-glucose 6-phosphotransferase from rat liver. Biochem J. 1966 May;99(2):266–274. doi: 10.1042/bj0990266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Adams M. J., Buehner M., Ford G. C., Hackert M. L., Lentz P. J., Jr, McPherson A., Jr, Schevitz R. W., Smiley I. E. Structural constraints of possible mechanisms of lactate dehydrogenase as shown by high resolution studies of the apoenzyme and a variety of enzyme complexes. Cold Spring Harb Symp Quant Biol. 1972;36:179–191. doi: 10.1101/sqb.1972.036.01.025. [DOI] [PubMed] [Google Scholar]
- SZENT-GYORGYI A. G. Meromyosins, the subunits of myosin. Arch Biochem Biophys. 1953 Feb;42(2):305–320. doi: 10.1016/0003-9861(53)90360-9. [DOI] [PubMed] [Google Scholar]
- Shaltiel S., Er-El Z. Hydrophobic chromatography: use for purification of glycogen synthetase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):778–781. doi: 10.1073/pnas.70.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaper J. H., Barker R., Hill R. L. Purification of wheat germ agglutinin by affinity chromatography. Anal Biochem. 1973 Jun;53(2):564–570. doi: 10.1016/0003-2697(73)90107-3. [DOI] [PubMed] [Google Scholar]
- WINDMUELLER H. G., KAPLAN N. O. The preparation and properties of N-hydroxyethyl derivatives of adenosine, adenosine triphosphate, and nicotinamide adenine dinucleotide. J Biol Chem. 1961 Oct;236:2716–2726. [PubMed] [Google Scholar]
- Wataya Y., Negishi K., Hayatsu H. Debromination of 5-bromo-2'-deoxyuridine by cysteine. Formation of deoxyuridine and S-(5-(2'-deoxyuridyl))cysteine. Biochemistry. 1973 Sep 25;12(20):3992–3998. doi: 10.1021/bi00744a032. [DOI] [PubMed] [Google Scholar]
- Wilchek M., Salomon Y., Lowe M., Selinger Z. Conversion of protein kinase to a cyclic AMP independent form by affinity chromatography on N 6 -caproyl 3',5'-cyclic adenosine monophosphate-sepharose. Biochem Biophys Res Commun. 1971 Dec 3;45(5):1177–1184. doi: 10.1016/0006-291x(71)90142-2. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]


