Abstract
Background:
Several studies have indicated that the use of immune checkpoint inhibitors (ICI) can prolong the survival of patients with advanced gastric cancer (AGC). However, it remains unclear whether the presence of liver metastasis leads to systemic immune suppression, resulting in poorer immune therapy outcomes. This study aims to investigate whether liver metastasis affects the efficacy of ICI in first-line treatment for AGC patients.
Methods:
The data of AGC patients undergoing combined immunotherapy and chemotherapy treatment at Harbin Medical University Cancer Hospital and the First Hospital of Shanxi Medical University from January 2018 to January 2023 were collected. The Kaplan–Meier method and Cox proportional hazards regression analysis were employed to analyze the overall survival (OS) and progression-free survival (PFS) of the patients.
Results:
A total of 162 patients with AGC who were human epidermal growth factor receptor 2 (Her 2) negative and treated with immunotherapy in the first line were included in the study. Patients were divided into two groups, the liver metastasis group (LM group, n = 40) and the group without liver metastasis (NLM group, n = 122) according to the presence of liver metastasis. The results of the present study indicate that there was no statistically significant difference in the median OS, with median OS of 17 and 15 months, respectively (p = 0.29). Similarly, no significant difference was observed in the median PFS between the two groups (p = 0.65).
Conclusion:
This study suggests that the presence or absence of liver metastasis does not significantly affect the prognosis of AGC patients receiving first-line treatment with ICI.
Keywords: advanced gastric cancer, first-line treatment, immune checkpoint inhibitors, liver metastasis, prognosis
Introduction
According to the latest data from GLOBOCAN in 2022, gastric cancer (GC) ranks seventh in incidence and third in mortality among all cancers in China. There were approximately 358,700 new cases of GC, accounting for about 7.43% of all cancer cases, with 260,400 deaths attributed to GC, accounting for approximately 10.12%. 1 Early symptoms of GC are often subtle, and there is a lack of specific biomarkers, leading to around 70% of patients being diagnosed only in advanced stages. 2 Despite continuous advancements in first-line treatment strategies for advanced gastric cancer (AGC), the median overall survival (OS) of patients remains difficult to surpass 1 year, indicating poor prognosis.3–5
The liver is the most common target organ for hematogenous metastasis in GC. The incidence of liver metastasis in stage IV GC patients is as high as 41.30%, significantly higher than other organs. 6 Studies have suggested that patients with liver metastasis may experience reduced benefits from immunotherapy, a phenomenon confirmed in melanoma and non-small-cell lung cancer.7,8 By contrast, in patients with mismatch repair deficiency colorectal cancer, those with liver metastasis showed poorer progression-free survival (PFS; p = 0.03) and diminished tumor response (p = 0.01) following first-line treatment with immune checkpoint inhibitors (ICI). 9 This could be attributed to the immunosuppressive characteristics of the liver metastatic microenvironment, with liver metastasis often indicating worse objective response rate (ORR), PFS, and OS.10–14 Furthermore, a recent study from Japan suggested that the occurrence of liver metastasis in GC patients may serve as a predictive factor for systemic progression during immunotherapy, correlating with poorer OS. 15
However, several studies indicate that the presence of liver metastasis in AGC does not affect the efficacy of immunotherapy.16–19 In the international multicenter phase III CheckMate 649 study, subgroup analysis showed that for patients with programmed death-ligand 1 (PD-L1) combined positive score ⩾5, the administration of nivolumab in combination with chemotherapy resulted in an OS extension of 2.4 months in patients without liver metastasis compared to those with liver metastasis. Similarly, in all randomized patients, those without liver metastasis had an OS extension of 1.6 months compared to those with liver metastasis, although the differences were not statistically significant. 17 Subgroup analysis of the 2-year follow-up of CheckMate 649 in China showed that the presence of liver metastasis in AGC patients receiving immunotherapy was not associated with poor prognosis. 20 In the ATTRACTION-4 study, survival benefits from nivolumab were observed in AGC patients regardless of the presence of liver metastasis. 19 Likewise, in the REGONIVO and LENPEM studies, the combination of anti-programmed cell death protein 1 (PD-1) antibodies with regorafenib or lenvatinib for AGC demonstrated promising antitumor activity with a longer follow-up, irrespective of liver metastasis status. This suggests that immunotherapy displays favorable long-term antitumor immune responses for GC, regardless of the presence of liver metastasis.21,22
Currently, there is significant controversy regarding whether the use of immunotherapy results in poorer OS for patients with AGC with liver metastasis compared to those without liver metastasis. Our retrospective study aims to explore the impact of liver metastasis on the efficacy of immunotherapy in patients with AGC, providing further evidence for subsequent clinical practice.
Methods
Study design and participants
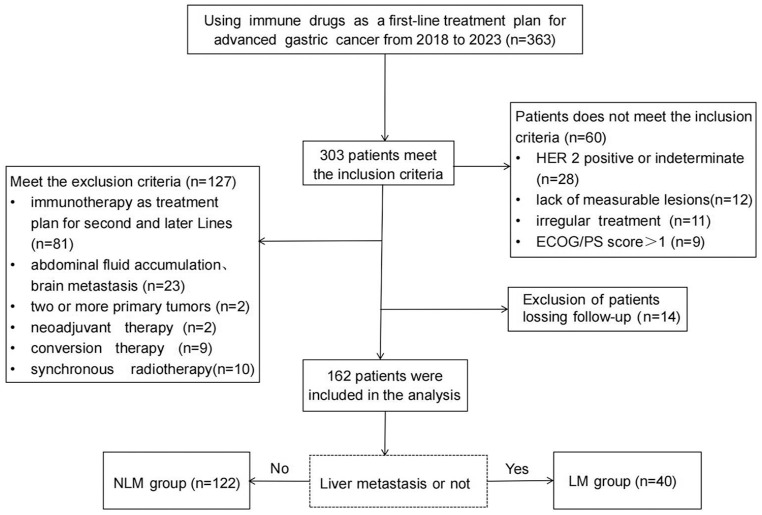
This retrospective study enrolled a total of 162 patients, all of whom were AGC patients treated with immunotherapy combined with chemotherapy at Harbin Medical University Cancer Hospital and the First Hospital of Shanxi Medical University from January 2018 to January 2023, with follow-up until April 28, 2023. Initially, 343 AGC patients with Eastern Cooperative Oncology Group/Performance Status (ECOG/PS) scores of 0 or 1, who were treated with ICI either alone or in combination with other drugs as part of their first-line treatment regimen, were initially enrolled. Further patient selection was conducted based on inclusion and exclusion criteria (Figure 1), resulting in the final inclusion of 162 patients. Patients were divided into two groups based on the presence or absence of liver metastases: the liver metastasis group (LM group, n = 40) and the non-liver metastasis group (NLM group, n = 122). This study was approved by the Ethics Committees of Harbin Medical University Cancer Hospital and the First Hospital of Shanxi Medical University (Ethics No. KY2022-32) and adhered to the principles outlined in the Helsinki Declaration. All patients provided written informed consent for chemotherapy and immunotherapy prior to treatment. Patient data were kept confidential.
Figure 1.
Flowchart of the study population.
ECOG/PS, Eastern Cooperative Oncology Group/Performance Status; HER 2, human epidermal growth factor receptor 2; LM, liver metastasis; NLM, no liver metastasis.
The immunotherapeutic agents utilized by the patients included in this study were predominantly PD-1 inhibitors, such as but not limited to sintilimab, nivolumab, and tislelizumab. A small subset of patients received PD-1 inhibitors before formal approval by the National Medical Products Administration and were enrolled in clinical trials. Their participation was post-unblinding, constituting experimental treatment groups. The remainder of the patients received medication in accordance with clinical standards. The reporting of this study conforms to the ESMO Guidance for Reporting Oncology real-world evidence (GROW) statement 23 (Supplemental Material).
Inclusion criteria:
(1) Pathologically diagnosed with GC/gastroesophageal junction cancer;
(2) Initial treatment for locally advanced, unresectable, or metastatic GC/gastroesophageal junction cancer;
(3) ECOG/PS score of 0 or 1;
(4) Human epidermal growth factor receptor 2 (HER 2) negative;
(5) Received immunotherapy combined with chemotherapy as first-line treatment for advanced disease for a minimum of two cycles.
Exclusion criteria:
(1) Presence of ascites or brain metastases at the time of initial diagnosis;
(2) Presence of two or more primary lesions;
(3) Received radiotherapy, conversion therapy, or neoadjuvant therapy;
(4) The time from completion of postoperative adjuvant chemotherapy to recurrence or metastasis was less than 6 months.
Study endpoints
Patient follow-up information was obtained from hospital follow-up center records or telephone interviews with patients and their families. OS was the primary endpoint of the study, defined as the time from initial standard treatment to death from any cause. PFS, ORR, and disease control rate (DCR) were secondary endpoints, and PFS was defined as the time from initial standard treatment to disease progression. For patients who died without experiencing PFS, the time of death was recorded as PFS. For patients with missing follow-up data for OS or PFS, we recorded the time from the initiation of standard treatment to the last follow-up as OS or PFS, respectively. At baseline, all patients had at least one evaluable lesion, and the patients were imaged after every 2–3 treatment cycles performed and were evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Efficacy was assessed. ORR was defined as the proportion of patients with a tumor volume reduction of at least 30%, sustained for at least 4 weeks, which includes both complete response (CR) and partial response (PR) patients. DCR was defined as the number of cases achieving remission and lesion stabilization after treatment as a percentage of the number of evaluable cases, as the proportions of patients with optimal CR, PR, and stable disease (SD).
Statistical analysis
This study utilized the chi-square test or Fisher’s exact test to analyze the clinical characteristics of the patients included in this study and employed a Cox proportional hazards regression model to analyze the multifactorial effects on the survival prognosis of AGC patients. The confounding factors in the multifactorial analysis included age (⩽65 vs >65), gender (male vs female), tumor site (cardia, whole stomach and gastric body, gastric antrum, gastroesophageal junction), World Health Organization (WHO) classification (adenocarcinoma, signet ring cell carcinoma, low adhesion carcinoma, mixed cancer), histological classification (poorly differentiated, moderately differentiated, well differentiated), primary lesion surgical status (no surgery, radical surgery, palliative surgery), adjuvant chemotherapy (yes vs no), solitary liver metastasis (yes vs no), organs with metastases (⩽1 vs ⩾2), combined chemotherapy regimen (S-1 plus oxaliplatin (SOX), capecitabine plus oxaliplatin (XELOX), albumin-bound paclitaxel plus S-1 (AS), others), PD-L1 expression (positive vs negative), and microsatellite instability (MSI) status (microsatellite stable or microsatellite instability-low vs microsatellite instability-high). The relationship between liver metastasis and prognosis was assessed. Univariate analysis and multivariable analysis were performed using the Log-rank test and Kaplan–Meier method, and PFS and OS survival curves were plotted and compared. p < 0.05 was considered to indicate statistically significant differences. The median period of follow-up and its interquartile range were calculated for the entire study cohort according to the reverse Kaplan–Meier method. The statistical analysis was conducted using SPSS (version 27.0.1) and R software (version 4.3.1).
Results
Patient clinical characteristics
The study initially collected data from 176 eligible GC patients from January 2018 to January 2023, with follow-up until April 28, 2023. Among these 176 patients, 14 patients were lost to follow-up and therefore excluded from this study, resulting in the inclusion of 162 patients. Regarding OS, 11 patients were lost to follow-up, and regarding PFS, 7 patients were lost to follow-up. For these lost follow-up patients, we recorded the last follow-up date as their OS or PFS. With a median follow-up of 15 months (95% CI: 12.440–17.560), 110 patients had experienced disease progression and 79 patients died.
In the final analysis, a total of 162 patients with AGC were included, with a mean age of 57 years (range 20–82 years), among whom 61.7% were male. 12.3% of the patients had a family history of tumors. Adenocarcinoma was the most common pathological type, accounting for 72.2%. Patients were divided into the LM group (n = 40) and NLM group (n = 122) based on the presence or absence of liver metastasis.
A comparison of patients’ general data and clinical characteristics is shown in Table 1. There were significantly more male patients than female patients in the LM (83% vs 17%), whereas the proportion of male and female patients in the NLM group was similar (56% vs 44%), and the difference was statistically significant (p = 0.002). In WHO staging, adenocarcinoma was the most common type, and the predominance of adenocarcinoma was 88% and 67% in the LM and NLM groups, respectively (p = 0.017). The differences in terms of histologic typing and adjuvant chemotherapy between the two cohorts were also statistically significant (p = 0.030; p = 0.032). Furthermore, there were statistically significant differences between the two groups for solitary liver metastasis and organs with metastases (p < 0.001). There were no statistically significant differences between the two groups in terms of age, tumor site, surgery, combination chemotherapy regimen, PD-L1 expression, or MSI status (all p > 0.05).
Table 1.
Clinical characteristics of patients enrolled in this study.
| Characteristic | Liver metastasis (n = 40) | No liver metastasis (n = 122) | χ2 | p-Value |
|---|---|---|---|---|
| Age | ||||
| ⩽65 years | 31 (78%) | 99 (81%) | 0.253 | 0.615 |
| >65 years | 9 (22%) | 23 (19%) | ||
| Gender | ||||
| Male | 33 (83%) | 68 (56%) | 9.190 | 0.002 |
| Female | 7 (17%) | 54 (44%) | ||
| Tumor site | ||||
| Cardia | 4 (10%) | 5 (4%) | 6.814 | 0.235 |
| Gastric body and the whole stomach | 12 (30%) | 53 (43%) | ||
| Gastric antrum | 20 (50%) | 44 (36%) | ||
| Gastroesophageal junction | 2 (5%) | 4 (3%) | ||
| Both a | 1 (3%) | 10 (8%) | ||
| Missing | 1 (3%) | 6 (5%) | ||
| WHO grade | ||||
| Adenocarcinoma | 35 (88%) | 82 (67%) | 12.03 | 0.017 |
| Signet ring cell carcinoma | 0 (0%) | 8 (7%) | ||
| Low adhesion carcinoma | 0 (0%) | 5 (4%) | ||
| Mixed cancer | 3 (8%) | 26 (21%) | ||
| Missing | 2 (5%) | 1 (<1%) | ||
| Histological classification | ||||
| Poorly differentiated | 13 (33%) | 71 (58%) | 8.984 | 0.030 |
| Moderately differentiated | 9 (23%) | 22 (18%) | ||
| Well differentiated | 1 (3%) | 1 (<1%) | ||
| Missing | 17 (43%) | 28 (23%) | ||
| Surgery | ||||
| No | 27 (68%) | 67 (55%) | 3.492 | 0.174 |
| Radical resection | 13 (33%) | 48 (39%) | ||
| Palliative resection | 0 (0%) | 7 (6%) | ||
| Adjuvant chemotherapy | ||||
| Yes | 5 (13%) | 36 (30%) | 4.610 | 0.032 |
| No | 35 (88%) | 86 (70%) | ||
| Solitary liver metastasis | ||||
| Yes | 18 (45%) | 0 (0%) | 162.00 | <0.001 |
| No (multi-organ metastasis) | 22 (55%) | 0 (0%) | ||
| No (Others) | 0 (0%) | 122 (100%) | ||
| Organs with metastases | ||||
| ⩽1 | 18 (45%) | 94 (77%) | 14.500 | <0.001 |
| ⩾2 | 22 (55%) | 28 (23%) | ||
| Combined chemotherapy regimen | ||||
| SOX | 24 (60%) | 66 (54%) | 1.583 | 0.663 |
| XELOX | 3 (8%) | 5 (4%) | ||
| AS | 3 (8%) | 13 (11%) | ||
| Others | 10 (25%) | 38 (31%) | ||
| PD-L1 expression | ||||
| Positive (CPS ⩾1) | 13 (33%) | 25 (20%) | 4.733 | 0.094 |
| Negative | 2 (5%) | 20 (16%) | ||
| Unknown | 25 (63%) | 77 (63%) | ||
| Microsatellite instability status | ||||
| MSS or MSI-L | 18 (45%) | 55 (45%) | 0.044 | 0.978 |
| MSI-H | 3 (8%) | 8 (7%) | ||
| Unknown | 19 (48%) | 59 (48%) | ||
AS, albumin-bound paclitaxel plus S-1; CPS, Combined Positive Score; MSI-H, microsatellite instability-high; MSI-L, microsatellite instability-low; MSS, Microsatellite stable; PD-L1, programmed cell death ligand 1; SOX, S-1 plus oxaliplatin; WHO, World Health Organization; XELOX, capecitabine plus oxaliplatin.
Both: Gastric body and the whole stomach and gastric antrum.
In the Cox regression univariable and multivariable analyses, organs with metastases and combined chemotherapy regimen had statistical significance in their impact on PFS (p = 0.031; p = 0.023), indicating that AGC patients with two or more organ metastases may have a worse prognosis than those with only one organ metastasis (hazard ratios (HR) = 1.560; 95% CI: 1.042–2.337). The results indicated that the AS regimen combined with immunotherapy may have a poorer prognosis than the SOX regimen combined with immunotherapy in the first line of treatment (HR = 2.196; 95% CI: 1.112–4.338; Table 2). Besides, only the WHO classification exhibited statistical significance (p = 0.035), indicating that patients with low adhesion carcinoma may have a worse long-term prognosis than those with adenocarcinoma (HR = 3.093; 95% CI: 1.084–8.829; Table 3).
Table 2.
Univariable and multivariable analyses for progression-free survival.
| Variables | Category | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Age (years) | >65 (vs ⩽65) | 0.842 (0.523–1.356) | 0.479 | ||
| Gender | Female (vs male) | 0.955 (0.641–1.422) | 0.821 | ||
| Tumor site | Gastric antrum (vs cardia) | 1.997 (0.717–5.567) | 0.186 | ||
| WHO grade | Low adhesion carcinoma (vs adenocarcinoma) | 1.131 (0.356–3.590) | 0.835 | ||
| Histological classification | Poorly differentiated (vs well differentiated) | 1.485 (0.205–10.760) | 0.695 | ||
| Surgery | Radical surgery (vs no) | 1.094 (0.739–1.621) | 0.653 | ||
| Adjuvant chemotherapy | No (vs yes) | 0.801 (0.523–1.225) | 0.305 | ||
| Liver metastasis | No (vs yes) | 0.908 (0.590–1.397) | 0.661 | ||
| Solitary liver metastasis | No (multi-organ metastasis) (vs yes) | 2.080 (0.965–4.480) | 0.062 | ||
| Organs with metastases | ⩾2 (vs ⩽1) | 1.529 (1.026–2.279) | 0.037 | 1.560 (1.042–2.337) | 0.031 |
| Combined chemotherapy regimen | AS (vs SOX) | 2.121 (1.076–4.181) | 0.030 | 2.196 (1.112–4.338) | 0.023 |
| PD-L1 expression | Negative (vs positive) | 1.055 (0.544–2.047) | 0.873 | ||
| Microsatellite instability status | MSS or MSI-L (vs MSI-H) | 1.326 (0.597–2.943) | 0.488 | ||
AS, albumin-bound paclitaxel plus S-1; HR, hazard ratios; MSI-H, microsatellite instability-high; MSI-L, microsatellite instability-low; MSS, microsatellite stable; PD-L1, programmed cell death ligand 1; SOX, S-1 plus oxaliplatin; WHO, World Health Organization.
Table 3.
Univariable and multivariable analyses for overall survival.
| Variables | Category | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Age (years) | >65 (vs ⩽65) | 0.978 (0.548–1.744) | 0.940 | ||
| Gender | Female (vs male) | 1.011 (0.638–1.600) | 0.964 | ||
| Tumor site | Gastric antrum (vs cardia) | 1.649 (0.503–5.407) | 0.409 | ||
| WHO grade | Low adhesion carcinoma (vs adenocarcinoma) | 3.517 (1.249–9.901) | 0.017 | 3.093 (1.084–8.829) | 0.035 |
| Histological classification | Poorly differentiated (vs well differentiated) | 1.231 (0.169–8.979) | 0.837 | ||
| Surgery | Radical surgery (vs no) | 1.376 (0.868–2.184) | 0.175 | ||
| Adjuvant chemotherapy | No (vs yes) | 0.572 (0.351–0.933) | 0.025 | 0.665 (0.394–1.124) | 0.128 |
| Liver metastasis | No (vs yes) | 1.357 (0.761–2.420) | 0.301 | ||
| Solitary liver metastasis | No (multi-organ metastasis) (vs yes) | 1.305 (0.450–3.780) | 0.624 | ||
| Organs with metastases | ⩾2 (vs ⩽1) | 1.298 (0.794–2.124) | 0.299 | ||
| Combined chemotherapy regimen | AS (vs SOX) | 1.324 (0.642–2.731) | 0.447 | ||
| PD-L1 expression | Negative (vs positive) | 1.241 (0.564–2.731) | 0.592 | ||
| Microsatellite instability status | MSS or MSI-L (vs MSI-H) | 1.193 (0.420–3.384) | 0.741 | ||
AS, albumin-bound paclitaxel plus S-1; HR, hazard ratios; MSI-H, microsatellite instability-high; MSI-L, microsatellite instability-low; MSS, microsatellite stable; PD-L1, programmed cell death ligand 1; SOX, S-1 plus oxaliplatin; WHO, World Health Organization.
Comparison of short-term and long-term efficacy of immunotherapy between LM group and NLM group
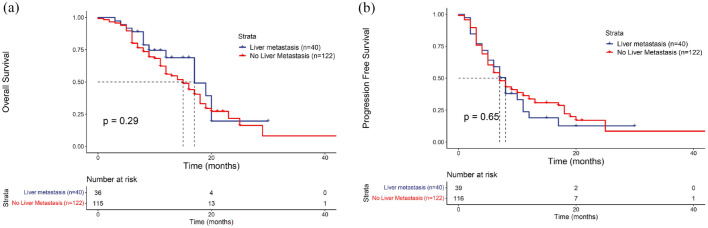
The results of Kaplan–Meier survival analysis showed that patients in the LM and NLM groups had a median OS of 17 and 15 months, respectively, p = 0.29, HR = 1.357 (95% CI: 0.761–2.420; Figure 2(a)), and a median PFS of 8 and 7 months, respectively, p = 0.65, HR = 0.908, (95% CI: 0.590–1.397; Figure 2(b)), and the results were not statistically significant. The 6-month PFS rate of patients in the LM group was 59%, and the 1-year OS rate was 68.8%; the 6-month PFS rate of patients in the NLM group was 54.3%, and the 1-year OS rate was 56%. The results showed that the prognosis of AGC patients after immunotherapy was independent of the presence of liver metastases.
Figure 2.
Kaplan–Meier survival curves are shown for overall survival (a) and progression-free survival (b) in the liver metastasis and no liver metastasis groups.
Evaluating the therapeutic efficacy based on the RECIST 1.1 criteria, 31 patients (31/162, 19%) among all patients applying ICI achieved PR with an ORR of 19% and a DCR of 74%. In the LM group, 11 patients (11/40, 28%) obtained PR and 19 patients (19/40, 48%) achieved SD; in the NLM group, 20 patients (20/122, 16%) obtained PR and 70 patients (70/122, 57%) achieved SD. The ORRs of the LM group and NLM group were 28% and 16%, while the DCRs of 75% and 74%, respectively (Table 4). The comparison of ORR and DCR between the two groups showed no statistically significant differences, with p-values of 0.168 and 0.620.
Table 4.
Response of patients with measurable disease.
| Response | All | Liver metastasis | No liver metastasis |
|---|---|---|---|
| n = 162 | n = 40 | n = 122 | |
| PR | 31 (19%) | 11 (28%) | 20 (16%) |
| SD | 89 (55%) | 19 (48%) | 70 (57%) |
| PD | 27 (17%) | 8 (20%) | 19 (16%) |
| Missing | 15 (9%) | 2 (5%) | 13 (11%) |
| ORR | 19% | 28% | 16% |
| p-Value | – | 0.168 | |
| DCR (%) | 74 | 75 | 74 |
| p-Value | – | 0.620 | |
DCR, disease control rate; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Subgroup analysis of PFS and OS in patients with and without liver metastasis
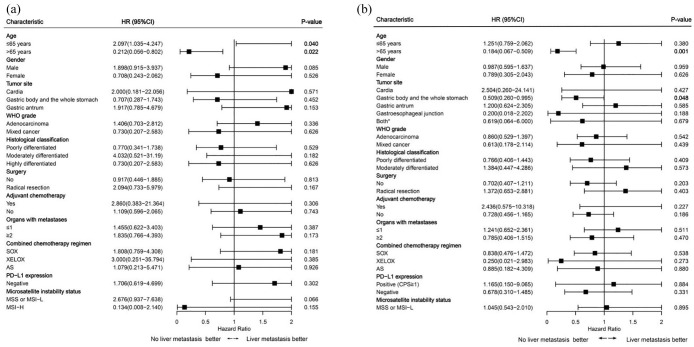
Subgroup analyses revealed that among AGC patients aged ⩽65 years, those with liver metastasis had a reduced risk of death after first-line immune therapy compared to those without liver metastasis (p = 0.040; Figure 3(a)). Conversely, among patients aged >65 years, those with liver metastasis had an increased risk of death and disease progression after first-line immune therapy, with liver metastasis being a risk factor (OS, p = 0.022; PFS, p = 0.001; Figure 3(a) and (b)). Regarding tumor location, among patients with tumors located in the body or throughout the stomach, those with liver metastasis had an increased risk of disease progression after first-line immune therapy (p = 0.048; Figure 3(b)). However, the presence or absence of liver metastasis had little influence on the risk of disease progression and death in terms of gender, WHO grading, histological classification, surgery, adjuvant chemotherapy, solitary liver metastasis, organs with metastases, combined chemotherapy regimen, PD-L1 expression, and MSI status, with all p-values >0.05 (Figure 3).
Figure 3.
Subgroup analysis of overall survival (a) and progression-free survival (b) in the liver metastasis and no liver metastasis groups.
AS, albumin-bound paclitaxel plus S-1; CPS, Combined Positive Score; MSI-H, microsatellite instability-high; MSI-L, microsatellite instability-low; MSS, microsatellite stable; PD-L1, programmed cell death ligand 1; SOX, S-1 plus oxaliplatin; WHO, World Health Organization; XELOX, capecitabine plus oxaliplatin.
*Both: Gastric body and the whole stomach and gastric antrum.
Discussion
Recently, a retrospective study from Japan suggested that the occurrence of liver metastasis in GC patients might serve as a predictive factor for overall progression during immunotherapy, correlating with poorer OS. It was also found that the presence of liver metastasis could be a potential negative baseline factor for ICI monotherapy, with the treatment of liver metastasis possibly enhancing the efficacy of immunotherapy. 15 Another multicenter retrospective study demonstrated a significant increase in the rate of hyperprogressive disease in patients with liver metastasis (p < 0.001), which was identified as an independent prognostic factor for poorer PFS in GC patients. 24 In addition, Liang et al. conducted a retrospective study where patients received immune combined chemotherapy or targeted therapy. The data indicated that in the NLM group, the median PFS and median OS were 8.7 and 13.43 months, respectively (p = 0.584), while in the LM group, the median PFS and median OS were 4.9 and 10.53 months, respectively (p = 0.026). There was a significant difference in median PFS between the two groups, with the LM group showing a higher ORR (47% vs 38.9%). 25 These three studies support the notion that liver metastasis in AGC patients might be an adverse factor for the prognosis of immunotherapy. However, in several prospective studies such as CheckMate 649 and ATTRACTION-4, subgroup analyses have consistently shown survival benefits from ICI regardless of the presence of liver metastasis, with generally consistent degrees of benefit and no statistically significant differences.17,19 In the real world, the impact of liver metastasis on the efficacy of immunotherapy in GC patients remains controversial.
As is well known, the liver serves as an immune-exempt organ. It has been suggested that liver metastasis may decrease the likelihood of a systemic response to ICI therapy through liver-induced immune tolerance. 8 Yu et al. proposed a preclinical model elucidating the potential mechanisms of immune tolerance, suggesting that liver metastasis sequesters and eliminates antigen-specific CD8+ T cells induced by the liver, leading to systemic immune suppression. The study included patients with metastatic melanoma, non-small-cell lung cancer, urothelial carcinoma, and renal cell carcinoma, but did not involve GC patients. 13 Furthermore, the team led by Lee demonstrated that the presence of liver metastasis in melanoma patients is associated with reduced activation of CD8+ tumor-infiltrating lymphocytes and decreased expression of functional markers during biopsy of skin tumors.26,27 Previous research indicates that liver-induced immune tolerance mechanisms are associated with effector T-cell exhaustion mediated by the FAS/FASL pathway. Inspired by this, Lee et al. discovered that the immune response to liver metastatic tumors can activate regulatory T cells (Tregs). Enhanced Tregs, activated through specific pathways, modulate tumor antigen-specific myeloid-derived suppressor cells, which then migrate to distant tumor sites. This migration inhibits the activation of peripheral CD8+ T cells incapable of clonal suppression. Moreover, CD11b+ suppressive macrophages induce CD8+ T-cell apoptosis via FASL-dependent mechanisms, ultimately leading to the suppression of anti-tumor immunity locally and systemically. However, this dysfunctional immune state cannot be reversed by anti-PD-1 monotherapy. 14 Due to tumor heterogeneity, it remains unclear whether liver metastasis in GC patients also leads to systemic “immune desertification,” and further exploration is needed to elucidate the specific mechanisms.
In our study, patients were divided into LM group and NLM group according to the presence or absence of liver metastasis. The LM group comprised only 40 patients, approximately 25% of the total population enrolled. Statistics indicate that up to 41.30% of GC patients may experience liver metastasis. Factors contributing to the difference in proportions of liver metastasis besides varying inclusion criteria may also be associated with ethnicity. Studies have reported that the proportion of liver metastasis occurrence in Asian GC patients is the lowest, approximately 12.32%. 6 Looking at the clinical characteristics of the two cohorts, significant differences were observed in gender, WHO classification, and histological subtypes, with p-values of 0.002, 0.017, and 0.030, respectively. Furthermore, there were significant differences in adjuvant chemotherapy, solitary liver metastasis, and organs with metastases, with p-values of 0.032, <0.001, and <0.001, respectively. These disparities represent inevitable biases that could potentially impact subsequent multifactorial analyses and the estimation of risk ratios in Cox regression models.
Interestingly, significant differences were observed in univariable and multivariable analyses between the two cohorts in the combined chemotherapy regimen (AS vs SOX; p = 0.023), indicating that patients with AGC who used AS combined with immunotherapy experienced a higher HR compared to those who used SOX (HR = 2.196; 95% CI: 1.112–4.338). In daily clinical practice, we employ XELOX and SOX as first-line combination regimens more often. If oxaliplatin is not tolerated by patients, we commonly use albumin-bound paclitaxel instead. However, in this study, the risk of death was increased by 2.196 times compared to the SOX plan. As a result, this serves as a reminder that the survival benefits of an AS regimen paired with immunotherapy may be limited. It is not recommended as the ideal plan.
Subgroup analysis using Cox proportional hazards models illustrated that among patients aged ⩽65 years with AGC, those with liver metastasis experienced a reduced risk of death after first-line immune therapy (p = 0.040). Conversely, among patients aged >65 years with AGC, those with liver metastasis had increased risks of death and disease progression after first-line immune therapy, with liver metastasis serving as a risk factor (OS, p = 0.022; PFS, p = 0.001). These findings suggest that immune therapy following liver metastasis may improve survival rates in younger GC patients, whereas for older patients, immune therapy after liver metastasis may not reduce the risk of disease progression and death. Regarding tumor location, among patients with tumors located in the body or throughout the stomach, those with liver metastasis had an increased risk of disease progression after first-line immune therapy (p = 0.048), although the specific reasons await further exploration.
In terms of survival analysis, our study found that the median OS for patients in the LM group and NLM group were 17 and 15 months, respectively (p = 0.29). The median PFS for the LM group and NLM group were 8 and 7 months, respectively (p = 0.65). The ORR was 28% in the LM group and 16% in the NLM group (p = 0.168), while the disease control rate (DCR) was 75% in the LM group and 74% in the NLM group (p = 0.620). There were no significant differences between the two groups statistically. The study results are generally consistent with the survival data obtained from subgroup analyses of CheckMate 649 and ATTRACTION-4. In the CheckMate 649 study, the median OS for the LM group and NLM group was 12.6 and 14.2 months, respectively, with no statistically significant difference. 17 In the ATTRACTION-4 study, patients treated with nivolumab combined with chemotherapy, the median PFS for those with liver metastases was 10.94 months (7.13–NR), median OS was 18.33 months (14.92–23.82), while for those without liver metastases, median PFS was 10.45 months (8.15–14.75), median OS was 17.35 months (14.62–20.83), with no significant difference. 19 Moreover, in higher-grade evidence meta-analyses, some evidence supporting the results of this study was also found. A meta-analysis showed that the OS and PFS of liver metastatic patients receiving immunotherapy were worse than those without liver metastasis, and varied across different cancers. This difference was more pronounced in non-small-cell lung cancer, melanoma, and genitourinary tumors, while in GC, the impact of liver metastasis on post-immunotherapy survival prognosis was relatively small (HR = 1.17, 95% CI: 0.90–1.52). 10 In another meta-analysis, it was found that in the subset of patients with immunotherapy benefits in AGC, the risk of death in liver metastasis patients was similar to that of non-liver metastasis patients. The HR for liver metastasis patients was 0.72 (0.62–0.84), and for non-liver metastasis patients, it was 0.75 (0.63–0.90). 28
In both the REGONIVO 21 and LENPEM 22 studies, patients with AGC demonstrated favorable outcomes when treated with ICI and multi-targeted tyrosine kinase inhibitors, regardless of their liver metastasis status. Among patients with liver metastasis, the ORR was 40% in the REGONIVO study and 54% in the LENPEM study, with promising survival outcomes. Combining the results of both studies, the median PFS for patients with liver metastasis was 7.8 months (95% CI, 4.3–13.7), compared to 6.9 months (95% CI, 4.6–9.8) for those without liver metastasis, with an HR of 0.817 (95% CI, 0.462–1.444), p = 0.4813. The median OS for patients with liver metastasis was 15.6 months (95% CI, 9.8–not reached), while for those without liver metastasis, it was 15.5 months (95% CI, 7.2–22.2), with an HR of 0.723 (95% CI, 0.371–1.411), p = 0.3398. Interestingly, in the anti-PD-1 monotherapy cohort, patients with liver metastasis had poorer PFS (1.4 months vs 2.3 months, p = 0.0009) compared to those without liver metastasis, consistent with previous reports. 29 Exploring the reasons behind this, it may be related to a synergistic effect between multi-targeted tyrosine kinase inhibitors and ICI, as multi-targeted tyrosine kinase inhibitors can target immune-suppressive cells, alleviating the systemic immune suppression caused by liver metastasis. 22 Thus, single immunotherapy alone cannot reverse the systemic immune suppression caused by liver metastasis. 14
When analyzing the study results, it is important to consider the limitations of this study. First, all cases included in this study were sourced from only two centers, which may introduce some selection bias into the data. Second, the follow-up time for some patients was insufficient. In addition, the collection of PD-L1 and MSI status data was not complete. Because the pathology of some patients was in the outer hospital and was unrequited, nearly half of the patients had unknown data which may have a certain impact on the accuracy of the results. Finally, due to the challenges posed by immune-related adverse events, the data collected in this study are insufficient regarding safety. Therefore, for further validation of the experimental results, it is necessary to conduct large-scale meta-analyses or prospective randomized controlled trials.
Conclusion
The results of this study indicate that first-line AGC patients can benefit from immunotherapy regardless of the presence of liver metastasis, with generally consistent degrees of benefit. This may be due to the heterogeneity of GC leading to reduced immunosuppression in liver metastases, reversing the therapeutic limitations imposed by liver metastasis, and thereby overcoming systemic immune tolerance. However, the specific mechanisms need to be further elucidated through large-scale preclinical studies.
Supplemental Material
Supplemental material, sj-pdf-1-tam-10.1177_17588359241308389 for A multicenter retrospective study of the combination of immune checkpoint inhibitors and chemotherapy regimens with or without liver metastasis for the first-line treatment of advanced gastric cancer by Jing Ren, Ke Wang, Qianhao Meng, Chang Xu, Changqing Liu, Yusheng Wang and Guangyu Wang in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank Spandidos Publications for English language editing.
Footnotes
ORCID iDs: Jing Ren  https://orcid.org/0009-0005-9787-3100
https://orcid.org/0009-0005-9787-3100
Ke Wang  https://orcid.org/0000-0003-4248-8836
https://orcid.org/0000-0003-4248-8836
Qianhao Meng  https://orcid.org/0000-0003-1225-9004
https://orcid.org/0000-0003-1225-9004
Chang Xu  https://orcid.org/0009-0002-7798-6907
https://orcid.org/0009-0002-7798-6907
Changqing Liu  https://orcid.org/0009-0001-5477-2837
https://orcid.org/0009-0001-5477-2837
Yusheng Wang  https://orcid.org/0000-0002-9681-2876
https://orcid.org/0000-0002-9681-2876
Guangyu Wang  https://orcid.org/0000-0002-8171-515X
https://orcid.org/0000-0002-8171-515X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jing Ren, Department of Gastrointestinal Medical Oncology, Harbin Medical University Cancer Hospital, Harbin, China.
Ke Wang, Department of Gastrointestinal Medical Oncology, Harbin Medical University Cancer Hospital, Harbin, China.
Qianhao Meng, Department of Oncology, Shanghai Pudong New Area People’s Hospital, Shanghai, China.
Chang Xu, Department of Gastrointestinal Medical Oncology, Harbin Medical University Cancer Hospital, Harbin, China.
Changqing Liu, Department of Gastrointestinal Medical Oncology, Harbin Medical University Cancer Hospital, Harbin, China.
Yusheng Wang, Department of Oncology Digestive, the First Hospital of Shanxi Medical University, Taiyuan 030001, China.
Guangyu Wang, Department of Gastrointestinal Medical Oncology, Harbin Medical University Cancer Hospital, Harbin 150040, China.
Declarations
Ethics approval and consent to participate: This study was reviewed and approved by the Ethics Committee of Harbin Medical University Cancer Hospital and the First Hospital of Shanxi Medical University (Ethics No. KY2022-32) and was conducted in accordance with the committee’s charter and the Helsinki Declaration of 1975 as revised in 2013. The patients provided written informed consent to participate in this study. All patients included in this study signed the Second Use Informed Consent for Historical Data/Biospecimens at Harbin Medical University affiliated Oncology Hospital.
Consent for publication: Not applicable.
Author contributions: Jing Ren: Data curation; Formal analysis; Investigation; Methodology; Project administration; Software; Writing – original draft; Writing – review & editing.
Ke Wang: Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing – original draft.
Qianhao Meng: Data curation; Investigation; Resources.
Chang Xu: Data curation; Investigation.
Changqing Liu: Data curation; Investigation.
Yusheng Wang: Data curation; Investigation; Resources.
Guangyu Wang: Conceptualization; Funding acquisition; Methodology; Resources; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present study was supported by grants from Beijing Medical Award Foundation (grant no. YXJL-2020-0785-1198; Guangyu Wang), Haiyan Fund’s General Projects (grant no. JJMS2023-01), Wu Jieping Foundation (grant no. 320.6750.2022-18-56), and Program for Young Talents of Basic Research in Universities of Heilongjiang Province (grant no. YQJH2024123; Guangyu Wang).
The authors declare that there is no conflict of interest.
Availability of data and materials: The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
References
- 1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024; 74: 229–263. [DOI] [PubMed] [Google Scholar]
- 2. Digklia A, Wagner AD. Advanced gastric cancer: current treatment landscape and future perspectives. World J Gastroenterol 2016; 22: 2403–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol 2008; 19: 1450–1457. [DOI] [PubMed] [Google Scholar]
- 4. Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 2009; 20: 666–673. [DOI] [PubMed] [Google Scholar]
- 5. Yoon HH, Bendell JC, Braiteh FS, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter Phase II trial. Ann Oncol 2016; 27: 2196–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qiu MZ, Shi SM, Chen ZH, et al. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: a SEER-based study. Cancer Med 2018; 7: 3662–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Topalian SL, Hodi FS, Brahmer JR, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol 2019; 5: 1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tumeh PC, Hellmann MD, Hamid O, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 2017; 5: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saberzadeh-Ardestani B, Jones JC, McWilliams RR, et al. Metastatic site and clinical outcome of patients with deficient mismatch repair metastatic colorectal cancer treated with an immune checkpoint inhibitor in the first-line setting. Eur J Cancer 2024; 196: 113433. [DOI] [PubMed] [Google Scholar]
- 10. Tian BW, Han CL, Wang HC, et al. Effect of liver metastasis on the efficacy of immune checkpoint inhibitors in cancer patients: a systemic review and meta-analysis. Clin Exp Metastasis 2023; 40: 255–287. [DOI] [PubMed] [Google Scholar]
- 11. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018; 19: 521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu J, Green MD, Li S, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 2021; 27: 152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee JC, Mehdizadeh S, Smith J, et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol 2020; 5: eaba0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Motoo I, Ando T, Hamashima T, et al. Liver metastasis affects progression pattern during immune checkpoint inhibitors monotherapy in gastric cancer. Front Oncol 2023; 13: 1193533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu J, Jiang H, Pan Y, et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial. JAMA 2023; 330: 2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021; 398: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rha SY, Oh DY, Yañez P, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2023; 24: 1181–1195. [DOI] [PubMed] [Google Scholar]
- 19. Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022; 23: 234–247. [DOI] [PubMed] [Google Scholar]
- 20. Liu T, Bai Y, Lin X, et al. First-line nivolumab plus chemotherapy vs chemotherapy in patients with advanced gastric, gastroesophageal junction and esophageal adenocarcinoma: CheckMate 649 Chinese subgroup analysis. Int J Canc Prev 2023; 152: 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fukuoka S, Hara H, Takahashi N, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol 2020; 38: 2053–2061. [DOI] [PubMed] [Google Scholar]
- 22. Yukami H, Kawazoe A, Lin YT, et al. Updated efficacy outcomes of anti-PD-1 antibodies plus multikinase inhibitors for patients with advanced gastric cancer with or without liver metastases in clinical trials. Clin Cancer Res 2022; 28: 3480–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Castelo-Branco L, Pellat A, Martins-Branco D, et al. ESMO guidance for reporting oncology real-world evidence (GROW). Ann Oncol 2023; 34: 1097–1112. [DOI] [PubMed] [Google Scholar]
- 24. Hagi T, Kurokawa Y, Kawabata R, et al. Multicentre biomarker cohort study on the efficacy of nivolumab treatment for gastric cancer. Br J Cancer 2020; 123: 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liang H, Li Z, Huang Z, et al. Prognostic characteristics and clinical response to immunotherapy targeting programmed cell death 1 for patients with advanced gastric cancer with liver metastases. Front Immunol 2022; 13: 1015549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Daud AI, Loo K, Pauli ML, et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest 2016; 126: 3447–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loo K, Tsai KK, Mahuron K, et al. Partially exhausted tumor-infiltrating lymphocytes predict response to combination immunotherapy. JCI Insight 2017; 2: e93433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoon HH, Jin Z, Kour O, et al. Association of PD-L1 expression and other variables with benefit from immune checkpoint inhibition in advanced gastroesophageal cancer: systematic review and meta-analysis of 17 phase 3 randomized clinical trials. JAMA Oncol 2022; 8: 1456–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fucà G, Cohen R, Lonardi S, et al. Ascites and resistance to immune checkpoint inhibition in dMMR/MSI-H metastatic colorectal and gastric cancers. J Immunother Cancer 2022; 10: e004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tam-10.1177_17588359241308389 for A multicenter retrospective study of the combination of immune checkpoint inhibitors and chemotherapy regimens with or without liver metastasis for the first-line treatment of advanced gastric cancer by Jing Ren, Ke Wang, Qianhao Meng, Chang Xu, Changqing Liu, Yusheng Wang and Guangyu Wang in Therapeutic Advances in Medical Oncology