Abstract
Background:
Intimate partner violence affects about a third of women in their lifetimes and can result in short- and long-term health consequences, including less favorable performance on measures of cognitive function.
Objectives:
We assess whether experiencing physical intimate partner violence in midlife was associated with steeper declines in subsequent tests of cognitive performance.
Design:
This study used data from 1713 women in the longitudinal cohort Study of Women’s Health Across the Nation to relate baseline information on physical intimate partner violence to declines in scores from the Symbol Digit Modalities Test, the East Boston Memory Test and the Digit Span Backwards spanning follow-up visits 7 through 15.
Methods:
Separate linear mixed models were constructed for each cognitive test outcome. Analyses were adjusted for race-ethnicity, education, financial strain, depressive symptoms, trouble sleeping, and bodily pain.
Results:
At Study of Women’s Health Across the Nation baseline, 3.1% of participants reported experiencing physical intimate partner violence in the prior year. In adjusted models, women who reported violence evidenced a statistically significant greater annualized decline (−0.17 points, 95% CI: −0.28, −0.06) in working memory (Digit Span Backwards test), compared to women who had not reported prior-year violence at baseline.
Conclusion:
Midlife women with a history of physical intimate partner violence exhibited a persistent decrease in the trajectory of working memory. These longitudinal findings extend previous cross-sectional reports which found that physical intimate partner violence had detrimental effects on working memory. These findings provide additional evidence that intimate partner violence is associated with decreases in working memory performance. They underscore the importance of further research into intimate partner violence and cognition during middle age, a particularly understudied life stage.
Keywords: abuse, violence, physical abuse, intimate partner violence, cognition, working memory
Plain language summary
Physical intimate partner violence is associated with declines in test of working memory
Introduction:
Approximately a third of women in the United States experience intimate partner violence (IPV) during their lifetimes. Physical IPV is one form of partner violence characterized by physical harm perpetrated by a current or former intimate partner. Physical IPV has previously been linked to poorer cognitive performance, indicated by lower scores on tests of working memory and concentration. Few studies have examined the cognitive performance of women in midlife who have experienced physical IPV.
Methods:
This study included 1,713 women in the Study of Women’s Health Across the Nation. Participants are from seven sites in the United States and were in midlife and premenopausal at study enrollment. This study includes data spanning the baseline study visit (1996-1997) through Study Visit 15 (2015-2016). Surveys captured experience of physical IPV, health conditions, and sociodemographic factors at the initial study visit. Participants completed several cognitive tests at multiple subsequent study visits. Statistical analysis examined the relationship between reported physical IPV at the initial visit and later changes in cognitive performance.
Results:
About 3% of participants had experienced physical IPV in the year before their first SWAN study visit. Women who experienced physical IPV showed greater decreases on a test of working memory than women who had not experienced IPV.
Conclusion:
Over time, physical IPV may be associated with greater declines in working memory in women moving from midlife into older adulthood. Future studies should investigate potential mechanisms for this greater decline. This study offers additional evidence of the harms of physical IPV.
Introduction
Upwards of 30% of women experience intimate partner violence (IPV) at some point in their lives. 1 IPV comes in many forms, including physical violence, emotional violence, and sexual violence, and many women experience overlapping forms of violence. IPV may be an isolated event but is more often repeated and experienced over many years. 2 While it is critical to address structural drivers of IPV that may leave women vulnerable, identifying survivors of IPV and addressing potential health consequences is an essential path toward supporting women’s health and well-being. 3 Although women ages 18–24 are at greatest risk of violence from an intimate partner, women of all ages experience IPV.1,2 Women in midlife and older adulthood are often excluded in research related to IPV, even though approximately 4.1% of U.S. women ages 45–54 and 1.4% of U.S. women ages 55 or older have experienced physical violence, sexual violence, or stalking from an intimate partner in the past year.1,4 Further research is needed to illuminate how violence and aging interact to influence health, particularly in midlife. 5
Although few studies have examined whether physical IPV influences cognitive function, existing work points to associations between physical IPV and cognitive function, including working memory and concentration.6,7 However, most previous studies are cross-sectional, and the few extant longitudinal studies have small sample sizes. 8
When considering the cognitive effects of physical IPV, traumatic brain injury (TBI) may be one mechanism of cognitive change. Of women who have experienced IPV, a significant proportion report experiencing trauma to the head, neck, and face, or attempted strangulation. 9 Direct trauma to the head, neck, or face can result in a TBI, and strangulation can result in an acquired brain injury (ABI). Both forms of brain injury can have marked effects on physical, emotional, and cognitive function.7,9 –12
Estimates of the prevalence of TBI in female IPV survivors range from 19% to 100%.9,11,13 –16 As TBI can adversely influence cognitive functioning, with recurrent TBI increasing risk, physical violence at the hands of an intimate partner may be associated with cognitive decline as women age. 17 Only about a quarter of IPV survivors seek clinical care for a potential TBI, and these women are vulnerable to experiencing adverse cognitive health consequences, especially in the case of repetitive TBI.7,17,18
Using data from the prospective Study of Women’s Health Across the Nation (SWAN), this article examined whether reports of physical IPV at the cohort baseline visit were associated with subsequent declines in cognitive performance among midlife women. We hypothesized that women who reported having experienced physical IPV, compared to those who have not, would have greater declines in measured cognitive performance over time.
Methods
This study is a longitudinal analysis of the association between exposure to physical IPV assessed once at baseline and trajectories of cognitive performance measured longitudinally starting from 7 years after baseline. The SWAN is a longitudinal cohort of 3302 midlife women who were enrolled in 1996–1997 when they were aged 42–52 years old and pre- or early-perimenopausal. 19 To be eligible for the cohort, women had to have had a menstrual period in the previous 3 months, could not be pregnant, lactating, or using hormones, and had to have a uterus and at least one ovary. The seven clinical sites enrolled Black (Boston, Chicago, Pittsburgh, and southeast Michigan), Chinese (northern California), Japanese (Los Angeles), or Hispanic (New Jersey) women with all sites also enrolling white women. After the baseline clinical visit, participants were followed approximately annually for up to 15 follow-up clinic visits, at which time SWAN remained in contact with 75% of surviving participants. The study protocol was approved by the institutional review board at each clinical site and women provided informed consent prior to each clinic visit. The reporting of this study conforms to the STROBE reporting guidelines. 20
Sociodemographic information was ascertained at baseline. At all study visits, women completed questionnaires about their medical history, menopausal stage, mental health, sleep, lifestyle, and stressful life events. Cognitive measures were introduced at follow-up visit 4 (2000–2001).
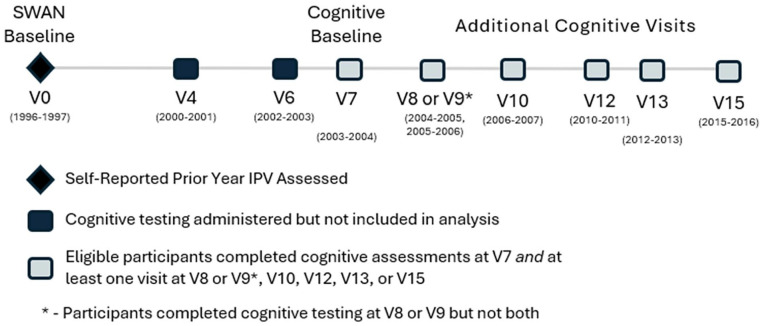
To be eligible for this analysis, women had to participate in cognitive testing at visits 4, 6, 7, and have at least one additional visit with cognitive testing. Study participants who reported a stroke prior to visit 7 (2003–2004) were ineligible for analysis, leaving 1718 (52.0% of the original cohort) eligible women. 21 As cognitive measures were not assessed at all of the three qualifying visits for both Hispanic and White women at the New Jersey site, participants from this site are not included in this analysis. Additionally, five women were excluded from the analysis as they were missing information on their experience of physical IPV at baseline. In sum, women were excluded from analyses: (a) if they were missing data on physical IPV at baseline; or (b) did not have cognitive measures from all three initial cognitive testing visits (4, 6, and 7); or (c) did not have at least one additional visit with cognitive testing subsequent to visit 7. Women were also excluded if they reported a stroke prior to Visit 7 and were censored if a stroke occurred thereafter. Thus, the final analytic sample included 1713 (51.9% of the original cohort) women, who participated at study baseline (1996–1997) and in cognitive assessments beginning in 2000 and continuing through 2016. Study timeline is shown in Figure 1.
Figure 1.
Timeline of SWAN study visits.
Cognitive measures
Cognitive performance was assessed at follow-up visits 4, 6 through 10, 12, 13, and 15. Visit 4 was administered between 2000 and 2001 and Visit 15 was administered in 2015–2016. Measures included the Symbol Digit Modalities Test (SDMT), for which scores range from 0 to 110, the East Boston Memory Test (EBMT) and the Digit Span Backwards (DSBs) for which scores range from 0 to 12. In each test, higher scores indicate better performance. The SDMT measures cognitive processing speed and complex attention by asking participants to match as many digits to given symbols as possible within 90 s. 22 The EBMT measures verbal episodic memory by prompting women to repeat a story they hear from the interviewer. The EBMT has two portions – immediate (EBMT-I), which is assessed directly after hearing the story, and delayed (EBMT-D), which is assessed later in the cognitive assessment. 23 The EBMT-I did not decline with age, in this or in prior SWAN analyses; therefore, we include only the EBMT-D. DSB measures working memory by having the interviewer read a series of numbers to participants and asking participants to repeat the numbers in reverse order. 24 Average SDMT, EBMT-D, and DSB scores decline in midlife, indicating sensitivity to aging-related change.21,25 –27 Tests were administered in English, Chinese, and Japanese, with testing language accounted for in all analyses. To minimize practice effects, we consider Visit 7 (2003–2004), the third application of cognitive measures, to be the baseline cognitive measure in these analyses.21,28
Experience of physical IPV was assessed as one item in an assessment of prior-year experience of stressful life events. This assessment was part of a self-administered questionnaire women completed during the SWAN baseline visit. In response to the question, “During the last 12 months, have you experienced. . . ‘Slapped, kicked, or otherwise hurt by husband/partner or someone else important to you’,” women could respond “No,” “Yes Not at all upsetting,” “Yes Somewhat upsetting,” or “Yes Very upsetting.” We dichotomized responses into “No,” indicating a participant did not report any prior-year physical IPV, or “Yes,” a participant reported prior-year physical IPV. Small cell sizes prevented analysis of level of distress; however, 92.5% of those who reported violence at baseline indicated the violence was somewhat or very upsetting.
Covariates
Sociodemographic covariates included self-reported race and ethnicity (Black, Chinese, Japanese, and White), level of education (high school or less versus some college or more), and financial strain, assessed by a woman’s self-reported difficulty paying for basic necessities (not at all versus somewhat or very difficult). These covariates were ascertained contemporaneously at cognitive testing visits. Alcohol use was categorized as none, infrequent (<2 servings per week), light to moderate (2–7 servings per week), or heavy (>7 servings per week). Depressive symptoms (yes/no based on the top quartile (⩾13) of scores) were assessed by the Center for Epidemiological Studies Depression Scale.29,30 Anxiety symptoms were assessed via four questions regarding anxiety symptoms in the past 4 weeks, including irritability or grouchiness, feeling tense or nervous, heart pounding or racing, and feeling fearful for no reason. Response options included not at all (0), 1–5 days (1), 6–8 days (2), 9–13 days (3), and every day (4), which were then summed across the four questions; women were classified as having anxiety symptoms based on scoring in the top quintile (⩾4). 31 Trouble sleeping (yes/no) was defined as having trouble falling asleep one or more times per week in the past 2 weeks. Pain was assessed based on the SF-36 scale of bodily pain, which combines responses to two questions about how much bodily pain a woman had in the past 4 weeks (none to very severe) and how much pain interfered with normal work (not at all to extremely); scores are then scaled from 1 to 100, and greater values indicate less pain. 32 Covariates were selected based on a priori knowledge regarding factors known to be associated with IPV and/or known to influence cognitive performance.
Statistical analyses
To minimize practice effects, follow-up visit 7, which was the third time cognitive performance was tested in SWAN, is the cognitive baseline in this analysis. 21 Practice effects may occur when a participant encounters the same assessment multiple times. Scores may show an improvement due to “practice” rather than a true improvement in cognitive function. Practice effects may be especially strong in the visits closest to first administration; thus, we define the third visit with cognitive testing as the cognitive baseline to diminish the impact of expected practice effects.28,30 We calculated means and standard deviations of continuous variables and frequencies of categorical variables. We ran separate linear mixed models for each cognitive test outcome with the primary exposure of physical IPV reported at the SWAN baseline (Table 2). Linear mixed models allow for the analysis of repeated measures, and repeated observations from women in this analysis contribute to the model at different ages. The cognitive baseline (Visit 7) and all subsequent cognitive test scores were included in the model. Women who reported a stroke after cognitive baseline were censored at time of stroke. Based on prior SWAN work, we modeled the SDMT and DSB slope over time with a knot at age 61 and the EBMT-D with a knot at age 58. 33 Initial models were adjusted for number of missed cognitive testing visits and, in SDMT models, residual practice effects. An indicator variable for fourth and later cognition testing visits was included to capture practice-related improvement in test scores from third to subsequent visits. 21 Only SDMT exhibited a significant residual practice effect. No practice effect was observed for DSB or EBMT-D outcomes. 33 We subsequently adjusted for race/ethnicity, testing language (English, Cantonese, or Japanese), education (some college or more versus high school degree or less), and difficulty paying for basics (very/somewhat versus not at all; time-varying). To this model, we added each of the following covariates individually: depressive symptoms, pain, trouble sleeping, anxiety, and alcohol use. We retained covariates that were significantly associated with cognition for our final adjusted model. Final models were further adjusted for depressive symptoms, pain, and trouble sleeping. We included random intercepts for each woman and random slopes for each woman before and after age 61 (SDMT/DSB) or 58 (EBMT-D). All covariates were assessed at SWAN baseline (V0), except for difficulty paying for basics, which was measured at cognitive baseline (V7) and allowed to vary over time. Time-invariant covariates (including physical IPV) were modeled as affecting both cognitive test level and annualized cognitive test slope; time-varying difficulty paying for basics was modeled as affecting cognitive test level, and values at cognitive baseline were allowed to affect annualized cognitive test slope. In models of the SDMT, all covariates were modeled as having the same effect on annualized cognitive test slope before and after age 61; for the DSB and EBMT-D, covariates were modeled as affecting only the slope after the knots at ages 61 and 58, respectively. We also modeled potential interactions between IPV and depressive symptoms and IPV and anxiety symptoms. All statistical analyses were run using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA). Statistical significance was defined as α < 0.05.
Table 2.
Unadjusted and adjusted linear mixed models.
| Model | DSB | SDMT | EBMT-D | |||
|---|---|---|---|---|---|---|
| DSB level | DSB annualized slope | SDMT level | SDMT annualized slope | EBMT-D level | EBMT-D annualized slope | |
| β (95% CI) p-value | β (95% CI) p-value | β (95% CI) p-value | β (95% CI) p-value | β (95% CI) p-value | β (95% CI) p-value | |
| Model 1: Base model | ||||||
| Physical IPV | ||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | −0.40 (−0.95, 0.15) p = 0.158 | −0.18 (−0.29, −0.07) p = 0.001 | −1.23 (−4.00, 1.54) p = 0.385 | −0.04 (−0.21, 0.13) p = 0.683 | −0.06 (−0.40, 0.28) p = 0.722 | −0.001 (−0.069, 0.066) p = 0.976 |
| Model 2: Adjusted for sociodemographics | ||||||
| Physical IPV | ||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | −0.17 (−0.69, 0.35) p = 0.527 | −0.17 (−0.28, −0.07) p = 0.002 | 0.64 (−1.87, 3.16) p = 0.616 | −0.02 (−0.19, 0.15) p = 0.821 | 0.06 (−0.27, 0.38) p = 0.729 | 0.003 (−0.064, 0.069) p = 0.934 |
| Model 3: Fully adjusted | ||||||
| Physical IPV | ||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | −0.12 (−0.64, 0.40) p = 0.655 | −0.17 (−0.28, −0.06) p = 0.002 | 0.83 (−1.67, 3.33) p = 0.515 | −0.01 (−0.19, 0.16) p = 0.865 | 0.09 (−0.24, 0.41) p = 0.601 | 0.00008 (−0.066, 0.066) p = 0.998 |
Model 1: Adjusted for number of missed cognitive test visits, practice effect indicator (SDMT only), and slopes before and after age 61 (SDMT/DSB) or 58 (EBMT-D).
Model 2: Adjusted for variables in model 1 and race/ethnicity, education, financial strain, and testing language.
Model 3: Adjusted for variables in model 2 and depressive symptoms, bodily pain, and trouble sleeping (see section “Methods” and Table 1 for definitions of these variables).
Results
This study included 454 (26.5%) Black, 868 (50.7%) White, 181 (10.6%) Chinese, and 210 (12.3%) Japanese women. At baseline, study participants had a mean age of 46.5 years (range 42–52), 51% had a college degree or more education, and 25% reported that it was very or somewhat difficult to pay for basic necessities (Table 1). Depressive symptoms, anxiety symptoms, and trouble sleeping were reported by 26.5%, 19.1%, and 21.2% of women, respectively. The mean SF-36 score for bodily pain was 72.1 (standard deviation = 21.1). The average follow-up time between baseline IPV and the final cognitive observation was 17.6 years (range 8.8–20.9).
Table 1.
Descriptive characteristics of study population (N = 1713).
| Characteristic | N (%) |
|---|---|
| Mean (SD) | |
| Race/ethnicity | |
| Black | 454 (26.50) |
| White | 868 (50.67) |
| Chinese | 181 (10.57) |
| Japanese | 210 (12.26) |
| Missing | 0 |
| Education | |
| Less than high school | 49 (2.86) |
| High school degree | 246 (14.36) |
| Some college | 547 (31.93) |
| College degree | 394 (23.00) |
| Post-college | 477 (27.85) |
| Missing | 0 |
| Difficulty paying for basic necessities | |
| Very | 72 (4.20) |
| Somewhat | 347 (20.26) |
| Not at all | 1270 (75.14) |
| Missing | 24 (1.40) |
| Testing language | |
| English | 1568 (91.54) |
| Cantonese | 69 (4.03) |
| Japanese | 76 (4.44) |
| Missing | 0 |
| Depressive symptoms | |
| No | 1259 (73.50) |
| Yes | 454 (26.50) |
| Missing | 0 |
| Anxiety | |
| No | 1369 (79.92) |
| Yes | 324 (18.91) |
| Missing | 20 (1.17) |
| Trouble sleeping | |
| No | 1345 (78.52) |
| Yes | 362 (21.13) |
| Missing | 6 (0.35) |
| Alcohol use | |
| None | 807 (47.11) |
| Infrequent (<2 servings/week) | 158 (9.22) |
| Light to moderate (2–7 servings/week) | 418 (25.40) |
| Heavy (>7 servings/week) | 255 (14.89) |
| Missing | 75 (4.38) |
| Menopausal stage | |
| Premenopausal | 32 (1.87) |
| Early perimenopausal | 427 (24.93) |
| Late perimenopausal | 170 (9.92) |
| Natural postmenopausal | 870 (50.79) |
| Surgical postmenopausal | 111 (6.48) |
| Unknown due to hormone therapy use | 103 (6.01) |
| Missing | 0 |
| SF-36 bodily pain | 72.07 (21.06) |
Difficulty paying for basic necessities (not at all versus somewhat or very difficult) is a measure of financial strain. Depressive symptoms (yes/no based on the top quartile (⩾13) of scores) were assessed by the Center for Epidemiological Studies Depression Scale. 30 Anxiety symptoms were assessed via four questions regarding anxiety symptoms in the past 4 weeks, including irritability or grouchiness, feeling tense or nervous, heart pounding or racing, and feeling fearful for no reason. Response options included not at all (0), 1–5 days (1), 6–8 days (2), 9–13 days (3), and every day (4), which were then summed across the four questions; women were classified as having anxiety symptoms based on scoring in the top quintile (⩾ 4). 31 Trouble sleeping (yes/no) was defined as having trouble falling asleep one or more times per week in the past 2 weeks. Alcohol use was categorized as none, infrequent (<2 servings per week), light to moderate (2–7 servings per week), or heavy (>7 servings per week). Menopausal stage was assessed by asking about bleeding history in previous 12 months. Premenopausal indicates a menstrual period in previous 3 months with no change in regularity in previous 12 months, early perimenopausal indicates a menstrual period in previous 3 months with a change in regularity in previous 12 months, late perimenopausal indicates a menstrual period more than 3 months but less than 12 months ago, natural menopause indicates no menstrual period within past 12 months, and surgical menopause indicates those who had a bilateral oophorectomy with or without hysterectomy. Pain was assessed based on the SF-36 scale of bodily pain, which combines responses to two questions about how much bodily pain a woman had in the past 4 weeks (none to very severe) and how much pain interfered with normal work (not at all to extremely); scores are then scaled from 1 to 100, and greater values indicate less pain.32,34
At baseline, 53 (3.1%) reported experiencing physical IPV by a husband/partner or someone else important to her in the previous year. Women who reported physical IPV at baseline were slightly less likely to be included in the analytic sample than those who had not reported physical IPV, though this difference was not statistically significant (p = 0.118). Of those women included in the analytic sample, 3.1% had reported physical IPV. Of those women not included in the analytic sample, 4.2% had reported physical IPV.
In the base model, experience of physical IPV was associated with a statistically significant −0.18 (95% confidence interval (CI) = −0.29, 0.07) greater decline per year in DSB scores after age 61 compared to women who did not report experience of physical IPV (Table 2). Adjustment for race and ethnicity, level of education, level of financial strain, and testing language reduced this decline only slightly to −0.17 (95% CI = −0.28, −0.07) per year. Additional adjustment for depressive symptoms, trouble sleeping, and pain had no further influence on the estimate for decline in DSB score associated with experience of physical IPV (−0.17 (95% CI = −0.28, −0.06)). There was no interaction between IPV and depressive symptoms or IPV and anxiety symptoms. Physical IPV was not associated with baseline levels of any cognitive test or longitudinal declines in the SDMT or EBMT scores.
Discussion
This is one of the few prospective studies to examine the longitudinal association between women’s experience of physical IPV and subsequent decline in cognitive performance in a community-based sample of midlife women. We found that women who had experienced physical IPV in the year prior to the baseline SWAN interview demonstrated greater declines on an assessment of working memory after age 61 than women who had not reported prior-year physical IPV. These longitudinal findings support and extend the results of prior cross-sectional studies, lending further credibility to the idea that physical IPV can have long-lasting repercussions for cognitive performance.
Physical IPV was associated with a 10-year decline in baseline DSB scores by 0.73 standard deviations (SDs), equating to a decrease of 24.5% of the average DSB score 10 years after age 61 (Figure 2). Physical IPV was not associated with cognitive baseline levels at Visit 7 of DSB, SDMT, or EBMT or with declines in the SDMT or EBMT scores.
Figure 2.
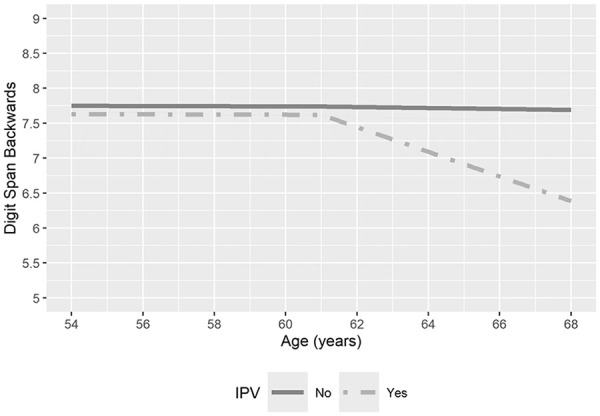
Age and digit span backwards scores, by self-report of physical IPV in the year prior to SWAN baseline interview. These trends are for a referent woman (White, age 54 at cognitive baseline, no missed visits, no difficulty paying for basics, at least some college education, testing language English, no depressive symptoms, no trouble sleeping, and average bodily pain score).
Results presented here suggest that physical IPV has a long-lasting effect on working memory. Several mechanisms exist through which the experience of physical IPV may affect working memory. First, physical IPV can lead to brain injury directly through a blow to the head, neck, or face or indirectly through a hit to another part of the body or through whiplash. Direct and indirect brain injury can damage neurons and set off an inflammatory response, disrupting connectivity within regions of the brain like the prefrontal cortex, the parietal cortex, the cingulate gyrus, and the basal ganglia that are involved in working memory.10,35 –43 We acknowledge that these brain regions are not exclusive to working memory and may be involved in other dimensions of cognitive function; however, we observed an association with working memory only. Since other measures of cognitive performance did not show similar associations in this study, further work should examine whether other cognitive processes may be affected by IPV as women age. Second, experiencing partner violence can result in adverse mental health conditions such as posttraumatic stress disorder (PTSD) that can have long-term negative impacts on cognition. Longitudinal analyses with the Nurses’ Health Study II cohort indicated that women with elevated PTSD symptoms demonstrated worse performance on tests of memory and attention, even after adjusting for comorbid depression. 44 Previous cross-sectional studies have reported associations between IPV-related PTSD and processing speed. 45 Thus, evaluation of mental health conditions as potential mediators of the association between IPV and cognitive performance is also needed.
This study has some limitations. First, a major limitation is that physical IPV was assessed only once through a single item that inquired about physical violence in the prior year measured at SWAN cohort baseline, which took place an average of 7 years prior to the cognitive analysis baseline at follow-up Visit 7. This question also did not capture specific forms of violence that could lead to brain injury, like non-fatal strangulation. The participant may have experienced physical IPV prior to study baseline. By the time of the cognitive assessments, a participant may have experienced continual violence or may have stopped experiencing violence; these two experiences may lead to distinct profiles of cognitive performance which cannot be differentiated in this analysis. Moreover, IPV is often experienced repeatedly, so it is possible that women who reported IPV at baseline continued to experience IPV in the years thereafter.46 –48 Because of the stigma associated with experiencing violence, women may have under-reported this experience. Also, women who reported physical IPV were marginally less likely to be included in the analytic sample. Women may have been excluded from analysis due to death, loss to follow-up, or missing data, reasons which could be linked to experiencing IPV. However, these limitations in ascertainment and possible differential loss to follow-up of women who experienced violence would likely lead to an underestimation of the association between physical IPV and cognitive performance.
Cognitive measures were objective and assessed at multiple study visits, but only a few domains of cognitive performance were evaluated. A plausible hypothesis is that the link between IPV and a greater rate of decline in cognitive performance is brain injury; however, we have no direct data (e.g., imaging studies or medical records) as part of this study to support this. Additionally, only working memory was associated with IPV, and if the mechanism were TBI, we would expect a more global effect. 49 It is plausible that SWAN’s small test battery, which assessed only a few domains, was not sufficient to capture other outcomes of IPV. Other aspects of cognitive performance, including those influenced by TBI such as visual memory, are not captured in these measures. 50 Mental health conditions, which can co-occur with a history of IPV, were assessed in these visits through self-report of symptoms. Further work should explore the association between physical IPV and mental health conditions. There is the possibility of unmeasured confounders which may account for all of part of the decline in working memory scores. Finally, we did not do a power analysis; this is a secondary analysis of cohort data; thus, the sample size was fixed.
This study also has several notable strengths. The data come from a large, prospective, multiracial, and multiethnic cohort of women who have been followed since 1996 through and beyond the menopausal transition. The cognitive assessments were objective and validated measures of cognitive performance that were repeated at multiple study visits, allowing for the tracking of performance over time while also accounting for learning effects. Only a small number of factors have been linked to decline in cognitive performance during midlife.30,33,51 This study explored one factor, IPV, that may influence the rate of cognitive aging. Cognitive aging may or may not lead to a tangible functional deficit; one theory of cognitive aging is that it eventually crosses a threshold after which functional decline is manifest. 52 Thus, minimizing the rate of cognitive decline could be a means of maintaining optimal cognitive function. This study suggests that physical IPV may also lead to decline in cognitive performance over time, offering a novel area of further exploration.
Current clinical guidelines for IPV screening recommend screening for women of reproductive age, based on evidence of harm from IPV for this age group and the availability of evidence-based interventions to improve their health.53 –55 Evidence for similar guidelines and interventions in older adults, including postmenopausal women, is currently deemed insufficient by the U.S. Preventive Services Task Force. The current study underscores the importance of expanding our knowledge about the possible harms of IPV in peri- and postmenopausal women, to whom the present screening guidelines do not apply.
Conclusion
In conclusion, this study indicates that midlife women who experienced physical IPV may have greater declines in working memory performance during the menopause transition and early postmenopause compared to women without this experience, thereby broadening available information about the deleterious health effects of IPV to include older, non-reproductive age women.
Acknowledgments
Clinical Centers: University of Michigan, Ann Arbor – Carrie Karvonen-Gutierrez, PI 2021–present, Siobán Harlow, PI 2011–2021, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Sherri-Ann Burnett-Bowie, PI 2020–Present; Joel Finkelstein, PI 1999–2020; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL – Imke Janssen, PI 2020–Present; Howard Kravitz, PI 2009–2020; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser – Elaine Waetjen and Monique Hedderson, PIs 2020–Present; Ellen Gold, PI 1994–2020; University of California, Los Angeles – Arun Karlamangla, PI 2020–Present; Gail Greendale, PI 1994–2020; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA – Rebecca Thurston, PI 2020–Present; Karen Matthews, PI 1994–2020. NIH Program Office: National Institute on Aging, Bethesda, MD – Rosaly Correa-de-Araujo 2020–present; Chhanda Dutta 2016–present; Winifred Rossi 2012–2016; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD – Program Officers. Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell and Wade Sanders (Central Ligand Assay Satellite Services). Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA – Sonja McKinlay, PI 1995–2001. Steering Committee: Susan Johnson, Current Chair. Chris Gallagher, Former Chair. We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
ORCID iDs: Jillian S Baker  https://orcid.org/0000-0001-5472-6315
https://orcid.org/0000-0001-5472-6315
Michelle M Hood  https://orcid.org/0000-0002-1049-8426
https://orcid.org/0000-0002-1049-8426
Siobán D Harlow  https://orcid.org/0000-0002-9899-356X
https://orcid.org/0000-0002-9899-356X
Declarations
Ethics approval and consent to participate: Study activities were approved by the Institutional Review Board at each SWAN Study Site: University of Michigan IRB #00000245; Partners Healthcare IRB#: 1999P006353/MGH; Rush University Medical Center IRB#: 13021201-IRB01-AM04; University of California, Davis IRB# 260339-17; UCLA Office of the Human Research Protection Program IRB#11-002274-AM-00009; Albert Einstein American College of Medicine of Yeshiva University IRB#: 2005-012; University of Pittsburgh IRB #: REN16020248/IRB0402168; SWAN Coordinating Center IRB at University of Pittsburgh IRB#: REN15070236/IRB0709006. Informed consent was collected at each study visit, written in the case of in-person visits and verbal in the case of phone visits.
Consent for publication: Not applicable as all data are presented as aggregated.
Author contribution(s): Jillian S Baker: Writing – original draft; Writing – review & editing; Methodology.
Gail A Greendale: Conceptualization; Methodology; Writing – original draft; Writing – review & editing; Funding acquisition.
Michelle M Hood: Writing – original draft; Formal analysis; Writing – review & editing; Methodology; Software; Data curation.
Arun S Karlamangla: Conceptualization; Funding acquisition; Writing – original draft; Writing – review & editing; Methodology.
Siobán D Harlow: Writing – review & editing; Conceptualization; Methodology; Writing – original draft; Funding acquisition; Supervision.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). JB was supported by the National Institute on Aging (Grant T32AG027708, PI: Kobayashi, Mezuk). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: SWAN provides access to public use datasets that include data from SWAN screening, the baseline visit, and follow-up visits (https://agingresearchbiobank.nia.nih.gov/). To preserve participant confidentiality, some, but not all, of the data used for this article are contained in the public use datasets. A link to the public-use datasets is also located on the SWAN website: http://www.swanstudy.org/swan-research/data-access/. Investigators who require assistance accessing the public use dataset may contact the SWAN Coordinating Center at the following email address: swanaccess@edc.pitt.edu.
References
- 1. Breiding MJ, Chen J, Black MC. Intimate partner violence in the United States—2010. Atlanta: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, 2014. [Google Scholar]
- 2. Thompson RS, Bonomi AE, Anderson M, et al. Intimate partner violence: prevalence, types, and chronicity in adult women. Am J Prev Med 2006; 30: 447–457. [DOI] [PubMed] [Google Scholar]
- 3. Bonomi AE, Anderson ML, Rivara FP, et al. Health outcomes in women with physical and sexual intimate partner violence exposure. J Womens Health 2007; 16: 987–997. [DOI] [PubMed] [Google Scholar]
- 4. Chang JC, Miller E, Thurston RC. Addressing intimate partner violence with midlife women: awareness, support, empowerment. Menopause 2021; 28: 1313. [DOI] [PubMed] [Google Scholar]
- 5. Pathak N, Dhairyawan R, Tariq S. The experience of intimate partner violence among older women: a narrative review. Maturitas 2019; 121: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stein MB, Kennedy CM, Twamley EW. Neuropsychological function in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry 2002; 52: 1079–1088. [DOI] [PubMed] [Google Scholar]
- 7. Campbell JC, Anderson JC, McFadgion A, et al. The effects of intimate partner violence and probable traumatic brain injury on central nervous system symptoms. J Womens Health 2018; 27: 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams MR, Murphy CM, Dore GA, et al. Intimate partner violence victimization and cognitive function in a mixed-sex epidemiological sample of urban adults. Violence Vict 2017; 32: 1133–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kwako LE, Glass N, Campbell J, et al. Traumatic brain injury in intimate partner violence: a critical review of outcomes and mechanisms. Trauma Violence Abuse 2011; 12: 115–126. [DOI] [PubMed] [Google Scholar]
- 10. Valera EM, Daugherty JC, Scott OC, et al. Strangulation as an acquired brain injury in intimate–partner violence and its relationship to cognitive and psychological functioning: a preliminary study. J Head Trauma Rehabil 2022; 37: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Esopenko C, Jain D, Adhikari SP, et al. Intimate partner violence-related brain injury: unmasking and addressing the gaps. J Neurotrauma 2024; 41(19–20): 2219–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rowe RK, Murphy SM, Handmaker H, et al. Population-level epidemiology of concussion concurrent with domestic violence in Arizona, USA. J Neurotrauma 2021; 38: 2301–2310. [DOI] [PubMed] [Google Scholar]
- 13. Haag H, Jones D, Joseph T, et al. Battered and brain injured: traumatic brain injury among women survivors of intimate partner violence—a scoping review. Trauma Violence Abuse 2022; 23: 1270–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iverson KM, Pogoda TK. Traumatic brain injury among women veterans: an invisible wound of intimate partner violence. Med Care 2015; 53: S112–S119. [DOI] [PubMed] [Google Scholar]
- 15. Valera E, Kucyi A. Brain injury in women experiencing intimate partner-violence: neural mechanistic evidence of an “invisible” trauma. Brain Imaging Behav 2017; 11: 1664–1677. [DOI] [PubMed] [Google Scholar]
- 16. Campbell JK, Joseph A-LC, Rothman EF, et al. The prevalence of brain injury among survivors and perpetrators of intimate partner violence and the prevalence of violence victimization and perpetration among people with brain injury: a scoping review. Curr Epidemiol Rep 2022; 9: 290–315. [Google Scholar]
- 17. Zieman G, Bridwell A, Cárdenas JF. Traumatic brain injury in domestic violence victims: a retrospective study at the Barrow Neurological Institute. J Neurotrauma 2017; 34: 876–880. [DOI] [PubMed] [Google Scholar]
- 18. McAllister T, McCrea M. Long-term cognitive and neuropsychiatric consequences of repetitive concussion and head-impact exposure. J Athl Train 2017; 52: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sowers M, Crawford S, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Kelsey J, Marcus R. (eds.) Menopause: biology and pathobiology. San Diego: Academic Press, 2000, pp. 175–188. [Google Scholar]
- 20. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiol Camb Mass 2007; 18: 800–804. [DOI] [PubMed] [Google Scholar]
- 21. Karlamangla AS, Lachman ME, Han W, et al. Evidence for cognitive aging in midlife women: study of women’s health across the nation. PLoS One 2017; 12: e0169008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith A. Symbol digit modality test manual. Los Angeles, CA: Western Psychological Services, 1982. [Google Scholar]
- 23. Albert M, Smith LA, Scherr PA, et al. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci 1991; 57: 167–178. [DOI] [PubMed] [Google Scholar]
- 24. Wechsler D. WMS-R: Wechsler Memory Scale-Revised: manual. Agra, India: Psychological Corporation, 1987. [Google Scholar]
- 25. Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev 1996; 103: 403–428. [DOI] [PubMed] [Google Scholar]
- 26. Hughes ML, Agrigoroaei S, Jeon M, et al. Change in cognitive performance from midlife into old age: findings from the Midlife in the United States (MIDUS) study. J Int Neuropsychol Soc 2018; 24: 805–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salthouse TA. Aging and measures of processing speed. Biol Psychol 2000; 54: 35–54. [DOI] [PubMed] [Google Scholar]
- 28. Bartels C, Wegrzyn M, Wiedl A, et al. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci 2010; 11: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1: 385–401. [Google Scholar]
- 30. Greendale GA, Wight RG, Huang M-H, et al. Menopause-associated symptoms and cognitive performance: results from the study of women’s health across the nation. Am J Epidemiol 2010; 171: 1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bromberger JT, Kravitz HM, Chang Y, et al. Does risk for anxiety increase during the menopausal transition? Study of women’s health across the nation. Menopause 2013; 20: 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ware JE, Snow KK, Kosinski M, et al. SF-36® Health Survey Manual and Interpretation Guide. Boston, MA: New England Medical Center, The Health Institute, 1993. [Google Scholar]
- 33. Greendale GA, Han W, Huang M, et al. Longitudinal assessment of physical activity and cognitive outcomes among women at midlife. JAMA Netw Open 2021; 4: e213227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–483. [PubMed] [Google Scholar]
- 35. McDowell S, Whyte J, D’Esposito M. Working memory impairments in traumatic brain injury: evidence from a dual-task paradigm. Neuropsychologia 1997; 35: 1341–1353. [DOI] [PubMed] [Google Scholar]
- 36. McAllister TW, Flashman LA, Sparling MB, et al. Working memory deficits after traumatic brain injury: catecholaminergic mechanisms and prospects for treatment—a review. Brain Inj 2004; 18: 331–350. [DOI] [PubMed] [Google Scholar]
- 37. Graham DI, Gennarelli TA. Pathology of brain damage after head injury. In: Cooper PR, Golfinos JE. (eds.) Head injury. New York, NY: McGraw-Hill, 2000, pp. 133–153. [Google Scholar]
- 38. McKee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol 2015; 127: 45–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adams JH, Doyle D, Ford I, et al. Diffuse axonal injury in head injury: definition, diagnosis, and grading. Histopathology 1989; 15: 49–59. [DOI] [PubMed] [Google Scholar]
- 40. Adams J, Doyle D, Graham DI, et al. Deep intracerebral (basal ganglia) haematomas in fatal non-missile head injury in man. J Neurol Neurosurg Psychiatry 1986; 49: 1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Graham DI, Gennarelli TA, McIntosh TK. Trauma. In: Graham DI, Lantos PL. (eds.) Greenfield’s neuropathology. London: Arnold, 2002, pp. 823–898. [Google Scholar]
- 42. St Ivany A, Schminkey D. Intimate partner violence and traumatic brain injury: state of the science and next steps. Fam Community Health 2016; 39: 129–137. [DOI] [PubMed] [Google Scholar]
- 43. Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol 2015; 72: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sumner JA, Hagan K, Grodstein F, et al. Posttraumatic stress disorder symptoms and cognitive function in a large cohort of middle-aged women. Depress Anxiety 2017; 34: 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Twamley EW, Allard CB, Thorp SR, et al. Cognitive impairment and functioning in PTSD related to intimate partner violence. J Int Neuropsychol Soc 2009; 15: 879–887. [DOI] [PubMed] [Google Scholar]
- 46. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl 1983; 30: 325–339. [Google Scholar]
- 47. Smith PH, Tessaro I, Earp JAL. Women’s experiences with battering: a conceptualization from qualitative research. Womens Health Issues 1995; 5: 173–182. [DOI] [PubMed] [Google Scholar]
- 48. Gomez-Casillas A, Lozano M, Rentería E. Expected years lived with intimate partner violence: a new approach for public health. Glob Health Action 2021; 14: 1976442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rabinowitz AR, Levin HS. Cognitive sequelae of traumatic brain injury. Psychiatr Clin North Am 2014; 37: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Levin HS, Temkin NR, Barber J, et al. Association of sex and age with mild traumatic brain injury–related symptoms: a TRACK-TBI study. JAMA Netw Open 2021; 4: e213046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Greendale GA, Huang M-H, Wight RG, et al. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology 2009; 72: 1850–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lindenberger U. Human cognitive aging: Corriger la fortune? Science 2014; 346: 572–578. [DOI] [PubMed] [Google Scholar]
- 53. US Preventive Services Task Force; Curry SJ, Krist AH, et al. Screening for intimate partner violence, elder abuse, and abuse of vulnerable adults: US Preventive Services Task Force final recommendation statement. JAMA 2018; 320: 1678–1687. [DOI] [PubMed] [Google Scholar]
- 54. Sharps PW, Bullock LF, Campbell JC, et al. Domestic violence enhanced perinatal home visits: the DOVE randomized clinical trial. J Womens Health 2016; 25: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bair-Merritt MH, Jennings JM, Chen R, et al. Reducing maternal intimate partner violence after the birth of a child: a randomized controlled trial of the Hawaii Healthy Start Home Visitation Program. Arch Pediatr Adolesc Med 2010; 164: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]