Abstract
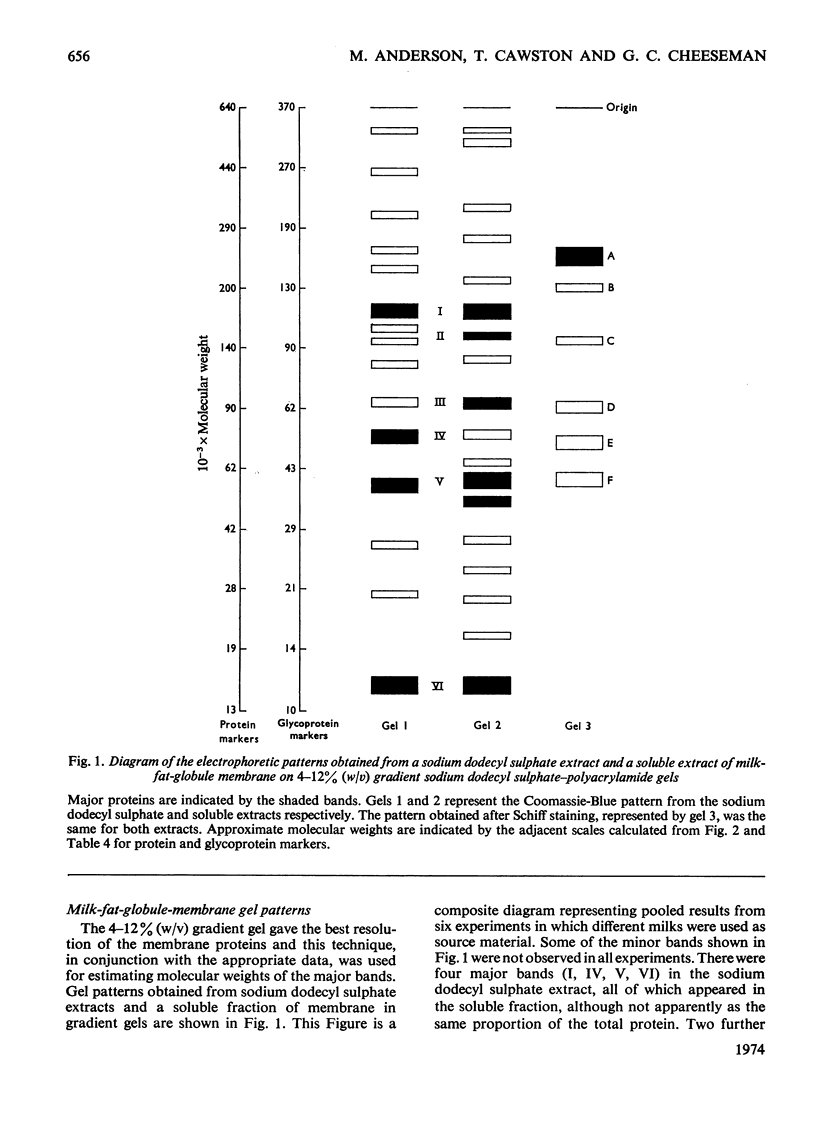
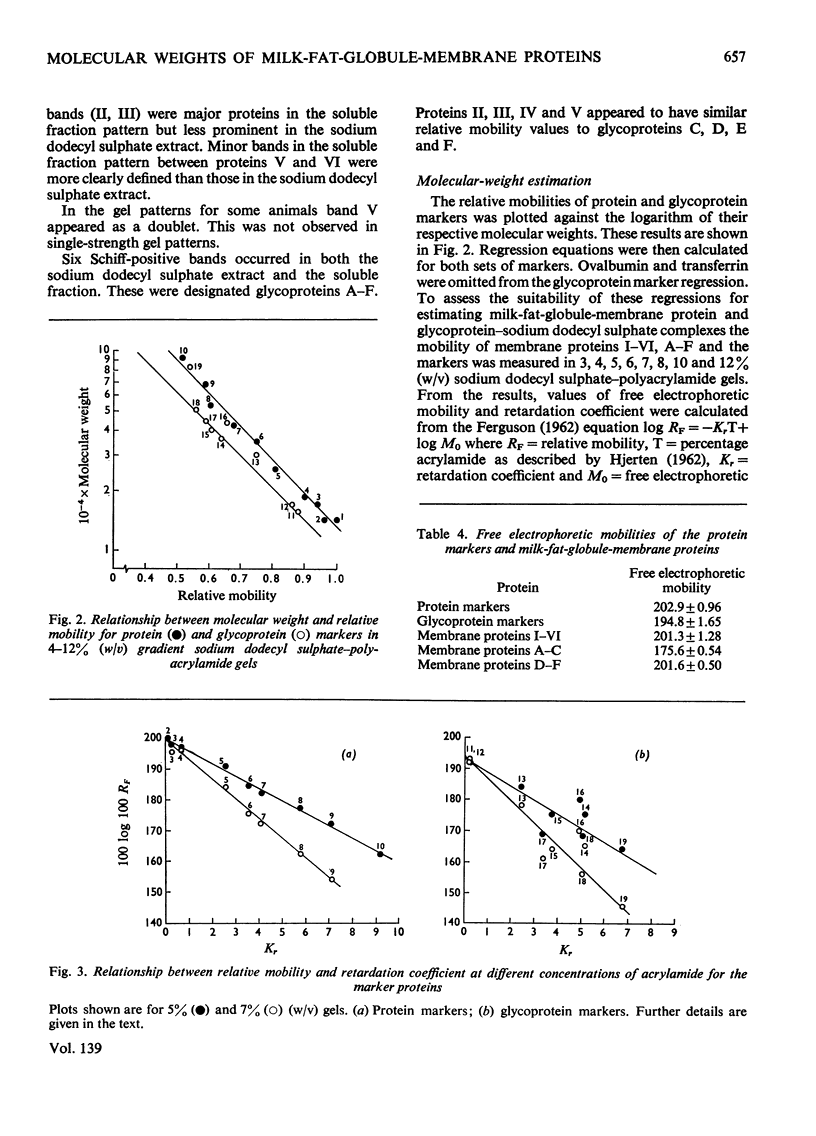
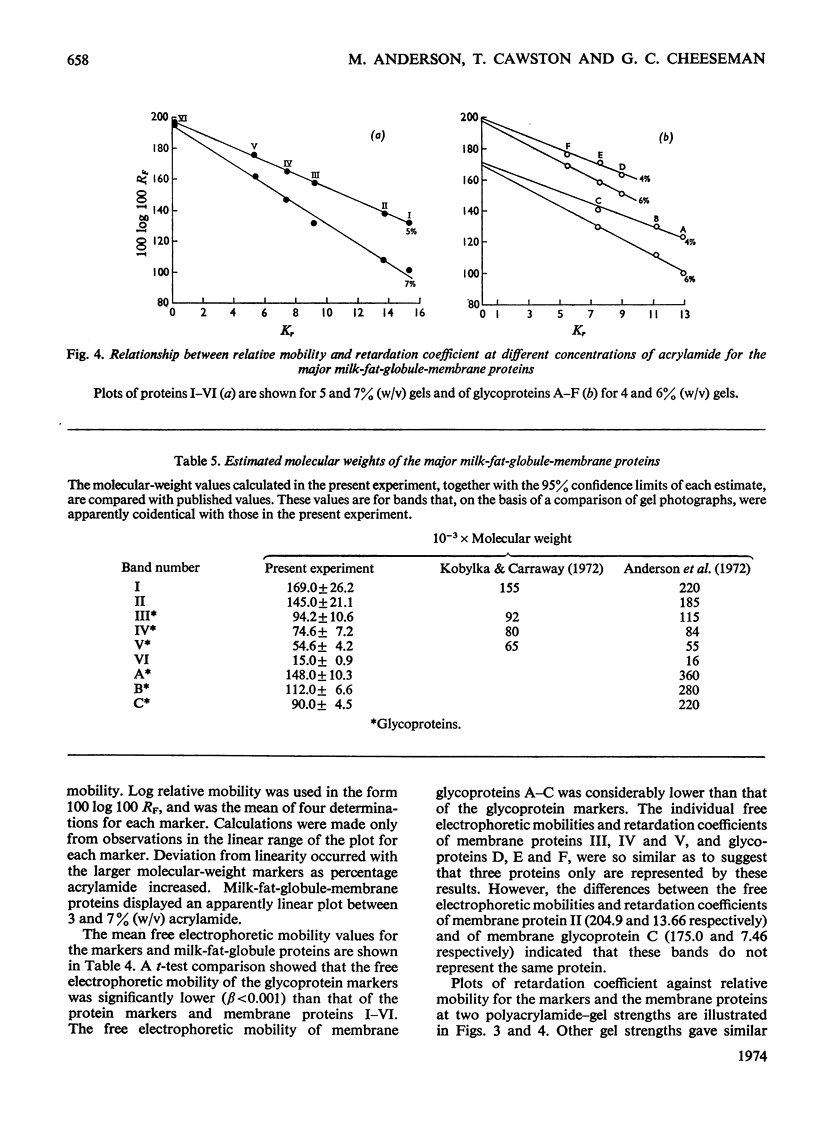
The molecular weights of milk-fat-globule-membrane proteins solubilized in sodium dodecyl sulphate were estimated by gradient gel electrophoresis. Standard curves were calibrated from both protein and glycoprotein markers of known molecular weight. Six major proteins were observed with Coomassie Blue staining and six with periodic acid–Schiff staining. The behaviour of the membrane proteins and the marker proteins was compared on several different single strength sodium dodecyl sulphate–polyacrylamide gels between 3 and 12% (w/v). The results were used to calculate the free electrophoretic mobility and retardation coefficient of each protein. Glycoprotein markers had a significantly lower mean free electrophoretic-mobility value than the protein markers. Three of the milk-fat-globule-membrane glycoproteins were shown to be independent of any of the Coomassie Blue-stained bands. On the basis of a comparison of the free electrophoretic-mobility and retardation- coefficient values of markers and unknown proteins the most appropriate standard curve for molecular-weight estimation was chosen.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banker G. A., Cotman C. W. Measurement of free electrophoretic mobility and retardation coefficient of protein-sodium dodecyl sulfate complexes by gel electrophoresis. A method to validate molecular weight estimates. J Biol Chem. 1972 Sep 25;247(18):5856–5861. [PubMed] [Google Scholar]
- Barman T. E. Purification and properties of bovine milk glyco-alpha-lactalbumin. Biochim Biophys Acta. 1970 Jul 27;214(1):242–244. doi: 10.1016/0005-2795(70)90094-2. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Major human erythrocyte glycoprotein spans the cell membrane. Nat New Biol. 1971 Jun 23;231(25):229–232. doi: 10.1038/newbio231229a0. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM L. W., CLOUSE R. W., FORD J. D. HETEROGENEITY OF THE CARBOHYDRATE MOIETY OF CRYSTALLINE OVALBUMIN. Biochim Biophys Acta. 1963 Oct 29;78:379–381. doi: 10.1016/0006-3002(63)91652-4. [DOI] [PubMed] [Google Scholar]
- Diezel W., Kopperschläger G., Hofmann E. An improved procedure for protein staining in polyacrylamide gels with a new type of Coomassie Brilliant Blue. Anal Biochem. 1972 Aug;48(2):617–620. doi: 10.1016/0003-2697(72)90117-0. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Evans W. H., Gurd J. W. Properties of a 5'-nucleotidase purified from mouse liver plasma membranes. Biochem J. 1973 May;133(1):189–199. doi: 10.1042/bj1330189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- HJERTEN S. "Molecular sieve" chromatography on polyacrylamide gels, prepared according to a simplified method. Arch Biochem Biophys. 1962 Sep;Suppl 1:147–151. [PubMed] [Google Scholar]
- Keenan T. W., Huang C. M. Membranes of mammary gland. VI. Lipid and protein composition of Golgi apparatus and rough endoplasmic reticulum from bovine mammary gland. J Dairy Sci. 1972 Nov;55(11):1586–1596. doi: 10.3168/jds.S0022-0302(72)85725-4. [DOI] [PubMed] [Google Scholar]
- Kobylka D., Carraway K. L. Proteins and glycoproteins of the milk fat globule membrane. Biochim Biophys Acta. 1972 Nov 2;288(2):282–295. doi: 10.1016/0005-2736(72)90249-0. [DOI] [PubMed] [Google Scholar]
- Lyster R. L. Reviews of the progress of dairy science. Section C. Chemistry of milk proteins. J Dairy Res. 1972 Jun;39(2):279–318. doi: 10.1017/s0022029900014114. [DOI] [PubMed] [Google Scholar]
- MORRISON M., HULTQUIST D. E. LACTOPEROXIDASE. II. ISOLATION. J Biol Chem. 1963 Aug;238:2843–2849. [PubMed] [Google Scholar]
- Margolis J., Kenrick K. G. Polyacrylamide gel electrophoresis in a continuous molecular sieve gradient. Anal Biochem. 1968 Oct 24;25(1):347–362. doi: 10.1016/0003-2697(68)90109-7. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Rodbard D., Kapadia G., Chrambach A. Pore gradient electrophoresis. Anal Biochem. 1971 Mar;40(1):135–157. doi: 10.1016/0003-2697(71)90087-x. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Andrews E. P., Marchesi V. T. Human erythrocyte membrane glycoprotein: a re-evaluation of the molecular weight as determined by SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1971 Jul 16;44(2):390–395. doi: 10.1016/0006-291x(71)90612-7. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]


