Abstract
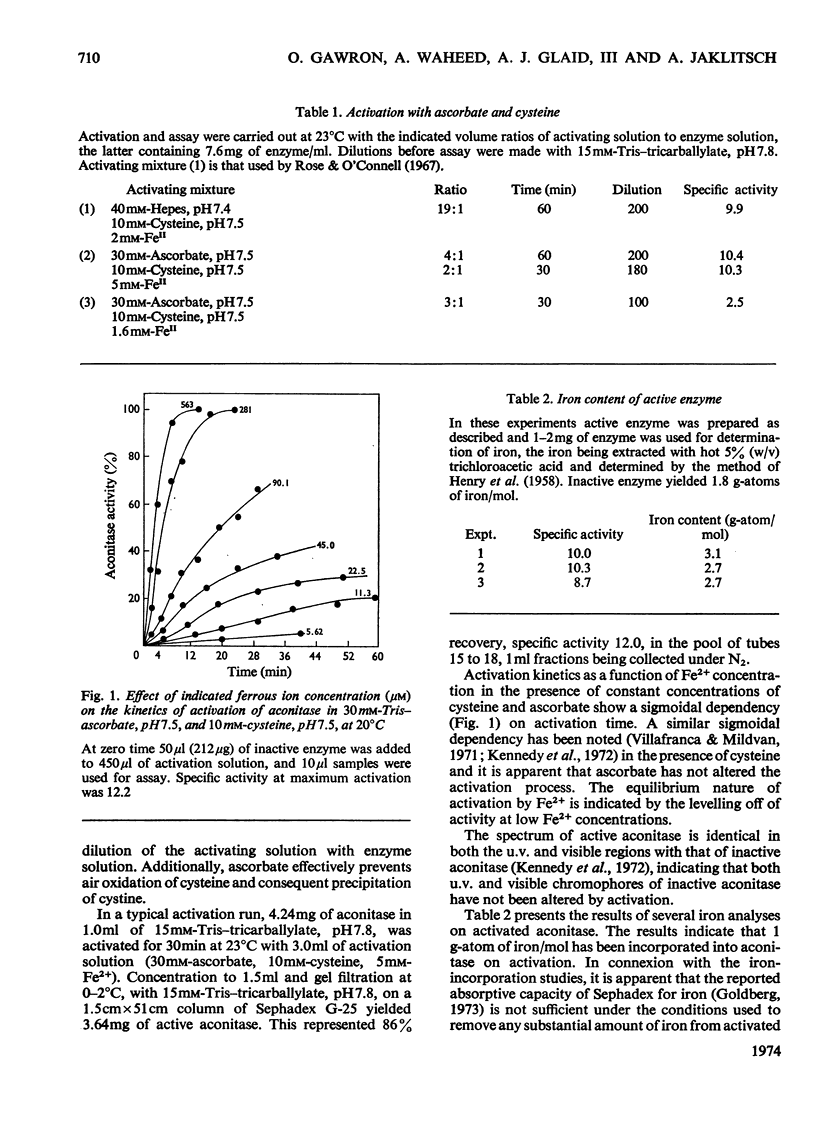
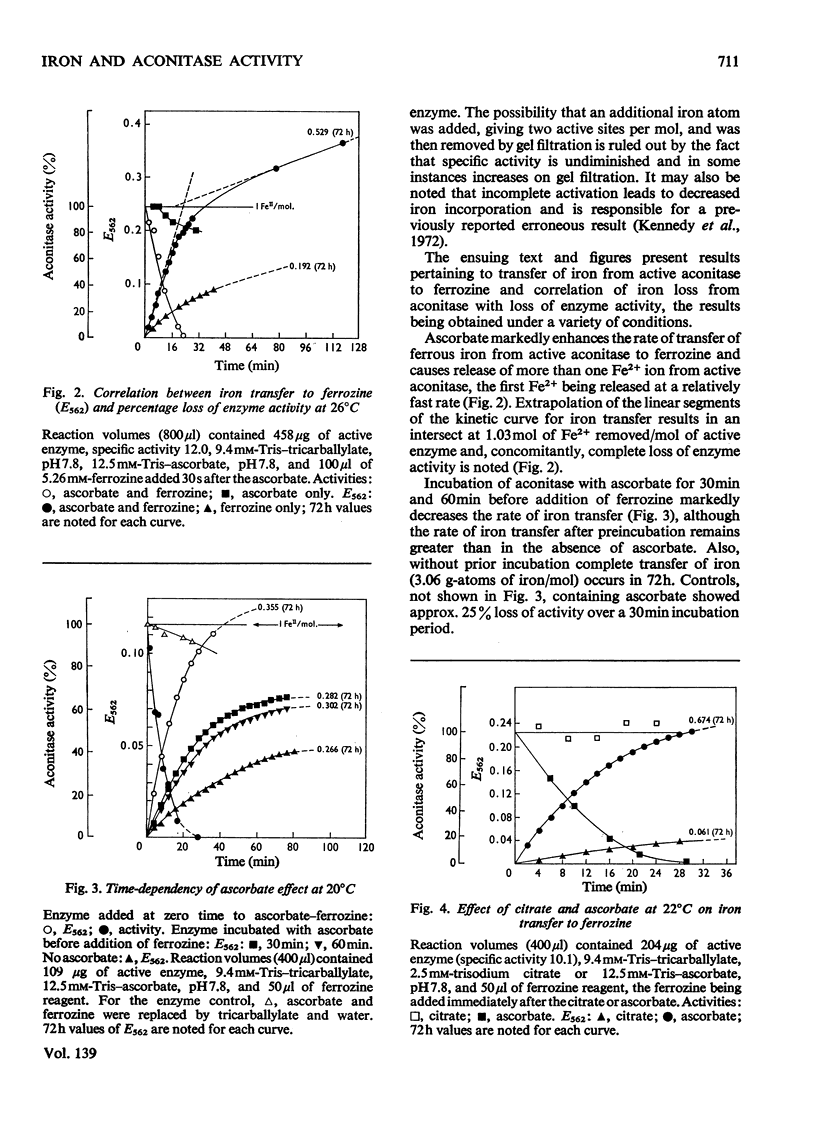
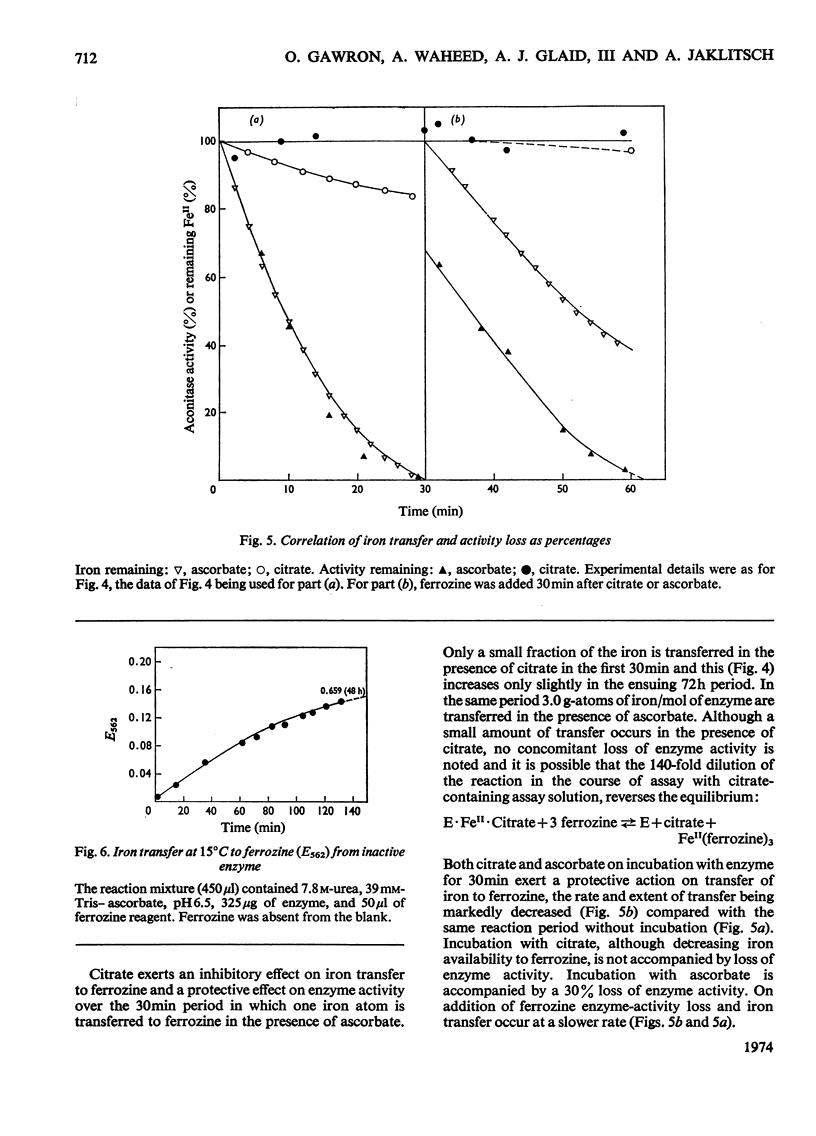
Aconitase activated with Fe2+, cysteine and ascorbate incorporates 1 g-atom of Fe2+/mol. Loss of this Fe2+ by transfer to ferrozine, a Fe2+ chelator, results in loss of activity. Ascorbate increases the rate of transfer of the essential Fe2+ whereas citrate retards the rate of transfer. Transfer of Fe2+ from inactive aconitase, 2 g-atoms of Fe/mol, can be accomplished in the presence of urea and ascorbate. The correlation of activity with the presence of an added g-atom of Fe2+/mol leads to the conclusion that active aconitase has only one active site per mol.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal Biochem. 1971 Apr;40(2):450–458. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- GAWRON O., CHESLOCK K. E. The activation of papain by arylamides of mercaptoacetic acid. Arch Biochem Biophys. 1951 Nov;34(1):38–49. doi: 10.1016/s0003-9861(51)80006-7. [DOI] [PubMed] [Google Scholar]
- Glusker J. P. Mechanism of aconitase action deduced from crystallographic studies of its substrates. J Mol Biol. 1968 Dec 14;38(2):149–162. doi: 10.1016/0022-2836(68)90403-8. [DOI] [PubMed] [Google Scholar]
- Goldberg M. L. Quantitative assay for submicrogram amounts of protein. Anal Biochem. 1973 Jan;51(1):240–246. doi: 10.1016/0003-2697(73)90471-5. [DOI] [PubMed] [Google Scholar]
- HENRY R. J., SOBEL C., CHIAMORI N. On the determination of serum iron and iron-binding capacity. Clin Chim Acta. 1958 Nov;3(6):523–530. doi: 10.1016/0009-8981(58)90004-4. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. S., Srere P. A. Molecular models of metal chelates to illustrate enzymatic reactions. J Chem Educ. 1968 Aug;45(8):539–540. doi: 10.1021/ed045p539. [DOI] [PubMed] [Google Scholar]
- Kennedy S. C., Rauner R., Gawron O. On pig heart aconitase. Biochem Biophys Res Commun. 1972 May 26;47(4):740–745. doi: 10.1016/0006-291x(72)90554-2. [DOI] [PubMed] [Google Scholar]
- MORRISON J. F. The activation of aconitase by ferrous ions and reducing agents. Biochem J. 1954 Dec;58(4):685–692. doi: 10.1042/bj0580685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H., Shinitzky M., Smith R. A. The activation of papain and ficin by phosphorothioate. Biochemistry. 1967 May;6(5):1421–1428. doi: 10.1021/bi00857a026. [DOI] [PubMed] [Google Scholar]
- Rose I. A., O'Connell E. L. Mechanism of aconitase action. I. The hydrogen transfer reaction. J Biol Chem. 1967 Apr 25;242(8):1870–1879. [PubMed] [Google Scholar]
- Villafranca J. J., Mildvan A. S. The mechanism of aconitase action. 3. Detection and properties of enzyme-metal-substrate and enzyme-metal-inhibitor bridge complexes with manganese(II) and iron(II). J Biol Chem. 1972 Jun 10;247(11):3454–3463. [PubMed] [Google Scholar]
- Villafranca J. J., Mildvan A. S. The mechanism of aconitase action. I. Preparation, physical properties of the enzyme, and activation by iron (II). J Biol Chem. 1971 Feb 10;246(3):772–779. [PubMed] [Google Scholar]


