Abstract
Background
Allo-HSCT is a curative therapy for patients with transfusion-dependent thalassemia (TDT). The high incidence of transplant-related complications is becoming an obstacle to safe and effective unrelated donor (URD) transplantation.
Methods
In this retrospective study, we reported the survival outcomes and complications of transplantation in thalassemia patients using a novel regimen consisting of pre-transplantation immunosuppression (PTIS) and modified myeloablative conditioning based on intravenous busulfan, cyclophosphamide, fludarabine, and rabbit anti-human thymocyte immunoglobulin.
Results
A total of 88 thalassemia patients received the novel conditioning regimen (NCR group), while 118 patients received the conventional conditioning regimen (CCR group). The median age at HSCT in the NCR group was older (7 years vs. 4 years, p < 0.05). No patient in the NCR group experienced primary graft failure, while the 3-year probabilities of OS and TFS were 96.6% and 93.2%, respectively. Even when the intensity of conditioning was reduced, OS (94.8% vs. 94.3%, p = 0.848) and TFS (89.8% vs. 92.5%, p = 0.663) in URD transplants in the NCR group were comparable to those in the CCR group, while the risk of autoimmune hemolytic anemia (AIHA) (0% vs. 15.1%) was lower. In addition, the NCR group had lower rates of mixed chimerism (7.1%).
Conclusions
URD transplantation can achieve a comparable prognosis to matched sibling donor (MSD) transplantation with a lower incidence of AIHA due to PTIS and modified myeloablative conditioning regimen.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-024-04103-6.
Keywords: transfusion-dependent β-thalassemia, hematopoietic stem cell transplantation, unrelated donor transplantation, pre-transplantation immunosuppression, children
Background
Thalassemia is a major health problem for millions of people worldwide and is the most common single-gene disorder. Although transfusion and iron chelation could improve the life expectancy of patients with transfusion-dependent thalassemia (TDT), a significant proportion of the population does not have the adequate access to the regular transfusion and iron chelation in the real world. Although gene therapy strategies have shown encouraging results in recent trials [1, 2], most of them are only available in clinical trials and their long-term safety is unclear. Allogeneic hematopoietic stem cell transplantation (HSCT) remains the most available curative therapy for patients with TDT.
Human leukocyte antigen (HLA)-matched siblings are recognized as the ideal donors for allogeneic HSCT in TDT, but their availability is limited. Alternative donor transplantation strategies have been investigated to overcome the higher rates of graft-versus-host disease (GVHD) and the delayed immune reconstitution. Given the unique clinical and biological aspects of TDT, investigators have developed various protocols to enhance immunosuppression, including azathioprine and hydroxyurea, courses of dexamethasone (Dex) and fludarabine (Flu) before conditioning regimen, Flu or anti-human thymocyte immunoglobulin (ATG) containing conditioning regimen, post-transplant cyclophosphamide (PTCY) or T-cell depletion strategies [3–8].
In previous studies, the myeloablative conditioning regimen consisting of a combination of busulfan (Bu), Flu, cyclophosphamide (Cy) and rabbit anti-human thymocyte immunoglobulin (ATG) has shown promising results in unrelated donor peripheral blood stem cell transplantation (UD-PBSCT) with a 3-year overall survival (OS) of 94% and thalassemia-free survival (TFS) of 92%. In particularly, an increasing proportion of patients with an unfavorable high-risk subgroup are now undergoing HSCT nowadays. The complications of bacterial and fungal infections [9], mixed chimerism [10], and immune-mediated cytopenia [11, 12] have been the major challenges following HSCT in the high-risk subgroup. Anurathapan and colleagues performed an intensive pre-conditioning immunoablation followed by a myeloablative conditioning regimen in patients, classified as a very high-risk subgroup or undergoing haploidentical related donor transplantation, with encouraging results [3–5]. The same approach was also applied to patients with thalassemia and sickle cell disease (SCD), demonstrating that pre-transplantation immunosuppression (PTIS) effectively eliminated the antibodies against donor-specific HLA (DSA) and facilitated engraftment with sustained donor chimerism [13]. However, this strategy has not yet been widely adopted in unrelated donor transplantation.
Therefore, we introduced this concept into the regimen of TDT patients receiving unrelated or sibling donor transplantation. Multidrug PTIS therapy followed by a modified myeloablative conditioning regimen was introduced to prolong the duration of immunosuppression treatment and reduce the transplant conditioning intensity (TCI) score. In this retrospective study, we report the effects of the novel conditioning regimen (NCR) in reducing chemotherapy side effects of chemotherapy and transplant-related mortality in pediatric patients with TDT.
Methods
Patients
This retrospective study was approved by the Ethics Committee of Sun Yat-sen Memorial Hospital of Sun Yat-sen University and registered in the Chinese medical research Registry (MR-44-23-018929). Consent had been sought from patients and their parents for research. From July 2007 to December 2022, 206 consecutive patients with TDT who received their first HSCT from either well-matched unrelated donors (n = 113) or matched sibling donors (n = 93) at Sun Yat-Sen Memorial Hospital. 88 patients with TDT accepted PTIS systemic therapy in the NCR protocol between August 2018 and December 2022. To evaluate the safety and efficacy of the novel conditioning regimen, 118 patients with TDT from the same center who received conventional conditioning regimen (CCR) without PTIS between July 2007 and September 2017 were included as a control group. The follow-up continues until 5 years after transplant. All patients were diagnosed with TDT based on their genotype and transfusion-dependent phenotype.
Conditioning regimens
Conventional conditioning regimen
The conventional conditioning regimen (CCR) consisted of intravenous Bu (12.8 mg/kg, divided day − 9 to day − 6), Flu (150 mg/m2, divided day − 7 to day − 3), Cy (200 mg/kg for class I and II patients, while 180 mg/kg for class III according to the Nanfang (NF) risk classification [14], divided day − 5 to day − 2) and ATG (10-11 mg/kg for URD while 7.5 mg/kg for MSD, divided day − 5 to day − 2). All patients received eight weeks of oral azathioprine (1.5 mg/kg, daily) and hydroxyurea (15 mg/kg, daily) prior to Bu conditioning or until intolerable side effects of azathioprine and hydroxyurea occurred.
Novel conditioning regimen (NCR)
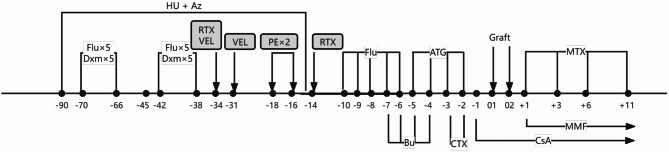
A novel conditioning regimen (NCR) is consisted by multidrug PTIS including courses of Dex and Flu and a modified version of CCR. The NCR was administered in two phases. The multidrug PTIS included oral azathioprine (1.5 mg/kg, daily) and hydroxyurea (15 mg/kg, daily) from day − 90 to day − 14 to suppress bone marrow expansion until leukocytes < 1.5 × 109/L or platelets < 50 × 109/L. Patients received one or two courses of intravenous Flu (40 mg/m2, daily) and Dex (25 mg/m2, daily) for 5 days, administered from day − 70 to day − 38. One course of Flu and Dex was given to patients who received an MSD transplant if they were older than 7 years or classified as a high-risk subgroup. One course of PTIS was given in 10/10 and 9/10 HLA-matched URD transplants. Two courses of PTIS were given in 8/10 HLA-matched URD transplants or high-risk subgroup without MSD. Panel reactive antibody (PRA) titers were monitored before and during PTIS, and intravenous immunoglobulin (IVIG) at 0.5 g/kg was given on day 1 and day 2 if PRA was positive. Rituximab, bortezomib or plasmapheresis were used in patients with high PRA positive (> 30%). Subsequently, all patients received a myeloablative conditioning regimen, including Flu (total dose of 150 mg/m2, divided day − 10 to day − 6), Bu (total dose of 12.8 mg/kg, divided day − 7 to day − 4), Cy (total dose of 120 mg/kg, divided day − 3 to day − 2), and ATG (total dose of 10 mg/kg for URD, 6-7.5 mg/kg for MSD, divided day − 5 to day − 2) (Fig. 1).
Fig. 1.
Schematic diagram of the program consisting of multidrug pretransplant immune hematopoietic suppression therapy including courses of dexamethasone and fludarabine followed by a modified myeloablative conditioning regimen. The post-transplant GVHD prophylaxis consisted of CsA, MMF and short-term MTX. See Methods section for details
Graft-versus-host disease prophylaxis
Post-transplant GVHD prophylaxis consisted of cyclosporin A (CsA) given at a dose of 2.5-3 mg/kg daily adjusted according to plasma concentration (200 ± 50 ng/mL), and mycophenolate mofetil (MMF) 20 mg/kg daily until day + 28. Methotrexate (MTX) was administered on days + 1, +3, + 6 and + 11 post-transplant at 15, 10, 10 and 10 mg/m2, respectively.
End-points and definitions
The risk classification of HSCT was based on the Nanfang (NF) classification [14], according to age at transplantation, serum ferritin level, and liver size. The primary endpoints were overall survival (OS, death from any cause) and thalassemia-free survival (TFS, graft failure or death from any cause). Engraftment was defined as the first of three consecutive days with an ANC greater than 0.5 × 109 /L and a platelet count greater than 20 × 109 /L for seven consecutive days without transfusion support. Hemoglobin engraftment was defined as the first of seven consecutive days on which the hemoglobin count exceeded 80 g/L without transfusion support. Quantitative chimerism monitoring was performed using short tandem repeat (STR)-based PCR techniques [15]. Complete donor chimerism was defined as more than 95% donor cells in peripheral blood after HSCT, whereas stable mixed chimerism (MC) was defined as having less than 95% donor cells without transfusion-dependency. Primary graft failure was defined as zero of donor chimerism and lack of hematological recovery within the first month after transplantation, while secondary graft rejection was defined as decline in donor chimerism to less than 5% and recurrence of transfusion dependence after engraftment. Acute and chronic GVHD were diagnosed and graded according to consensus criteria [16, 17].
Invasive fungal diseases are diagnosed clinically through pathogen culture and high-resolution chest CT scan. Patient with positive blood culture was diagnosed with bacteremia. AIHA was diagnosed when patients had a positive direct antiglobulin test and clinical hemolysis. Posterior reversible encephalopathy syndrome (PRES) was diagnosed by magnetic resonance imaging and clinical symptoms, including headache, seizures, impaired consciousness and visual disturbances. Post-transplant lymphoproliferative disorders (PTLD) were diagnosed according to the 2017 WHO classification [18]. Before transplantation, patients’ serum samples were collected for measurement of panel reactive antibody (PRA) titer using the enzyme-labelled immunosorbent assay method. PRA > 10% was considered positive, while PRA ≥ 30% was considered strong positive. Pre- and post-transplant lymphocyte subsets, including CD3-CD16 + CD56+ (natural killer [NK] cells), CD3+ (total T-cells), CD3 + CD4+ (helper T-cells), CD3 + CD8+ (cytolytic T-cells) and CD19+ (B-cells) subsets, were measured using the flow cytometry method described in previous literature [19]. Fluorescence-conjugated monoclonal antibodies (CD3, CD16, CD56, CD4, CD8, CD19, BD multitest 6-color TBNK, San Jose, CA, USA) were added to the mononuclear cells. Samples were analyzed on a Beckman navios cytometer (Beckman Coulter Life Science) and then analyzed using Navios tetra software (Beckman Coulter Life Science).
Statistical analysis
Specific database and follow-up Center made sure the data integrity in the certain degree. There was no absence of data on survival outcomes in this study. A portion of the patients had their PRA titers and lymphocyte subsets monitored during PTIS, the missing data were excluded from the analysis. Statistical analysis was performed using SPSS version 28.0 (IBM Corp.). All p values were two-sided, and statistical significance was set at p < 0.05. Quantitative variables were presented as median and interquartile range, and qualitative variables as frequencies and percentages. The Wilcoxon Mann-Whitney U test or Kruskal-Wallis H test was used for comparisons of quantitative variables, while Fisher’s exact test was used for qualitative variables. Repeated measures pre- and post-transplant data were evaluated using the Wilcoxon signed rank test. The primary endpoints were calculated using the Kaplan-Meier estimates and the log-rank test. Confounding was controlled by propensity score and Cochran-Mantel-Haenszel test. Comparing the pre-transplantation characteristics, it was found that the transplant ages was significantly older in NCR group compared to CCR group (p < 0.05), which was considered as confounding factor. Propensity score was calculated based on Logistic regression and transformed to quadripartite variation by quartile and the median as the dividing values. Subsequently, Cochran-Mantel-Haenszel test was conducted to explore the association between different conditioning regimens and transplant complications.
Results
Patient characteristics
206 patients with TDT were included in this study. The comparison of the characteristics of the NCR and CCR groups were list in Table 1. Of all transplants, 88 patients received NCR with a median age of 7 years (range, 4–10), while 118 patients received CCR with a median age of 4 years (range, 3–7) and the median follow-up of 46 and 46.3 months, respectively. No patient was lost to follow-up. The majority of the patients in two groups were classified as intermediate and high risk before transplantation according to the NF risk stratification criteria. Prior to transplantation, 27.1% and 13.5% of the patients in the NCR and CCR groups, respectively, had a strong positive PRA titer.
Table 1.
Characteristics of the NCR and CCR groups
| Characteristics | CCR group (N = 118) | NCR group (N = 88) | p |
|---|---|---|---|
| Age, median (range) [years] | 4(3,7) | 7(4,10) | < 0.001 |
| Sex | |||
| Male, n (%) | 84(71.2) | 54(61.4) | 0.138 |
| Female, n (%) | 34(28.8) | 34(38.6) | |
|
Serum ferritin, median (range) [µg/L] |
1802.0(1254.0,2689.5) | 2113.5 (1199.5, 2700.8) | 0.552 |
| Risk group, n (%) | (N = 116) | (N = 88) | |
| Low-risk | 30(25.9) | 13((14.8) | 0.019 |
| Medium-risk | 86(74.1) | 72 (81.8) | |
| High-risk | 0(0) | 3(3.4) | |
| Donor/patient ABO-match, n (%) | |||
| Match | 75(63.6) | 41(46.6) | 0.015 |
| Mismatch | 43(36.4) | 47 (53.4) | |
| PRA before, n (%) | [N = 89] | [N = 70] | |
| Negative (≤ 10%) | 52(58.4) | 42 (60.0) | 0.018 |
| Positive (11–29%) | 25(28.1) | 9(12.9) | |
| Strongly positive (≥ 30%) | 12(13.5) | 19(27.1) | |
| Donor source, n (%) | |||
| Matched sibling donor | 65(55.1) | 28(31.8) | |
| Unrelated donor | 53(44.9) | 60(68.2) | |
| HLA matching status, n (%) | |||
| 10/10 matched | 99(83.9) | 60(68.2) | |
| 9/10 matched | 16(13.6) | 26(29.5) | |
| 8/10 matched | 3(2.5) | 2(2.3) | |
| Stem cell source, n (%) | |||
| BM | 2(1.7) | 0 | |
| PBSC | 51(43.2) | 60(68.2) | |
| UCB | 1(0.8) | 0 | |
| BM + PBSC | 50(42.4) | 17(19.3) | |
| BM + UCB | 8(6.8) | 5(5.7) | |
| PBSC + UCB | 2(1.7) | 0 | |
| BM + PBSC + UCB | 4(3.4) | 6(6.8) | |
| Follow-up, median (range) [months] | 46.3(24.7,68.6) | 46.0(25.0,52.6) |
* Abbreviations: CCR, conventional conditioning regimen; NCR, novel conditioning regimen; PRA, panel reactive antibody; BM, bone marrow; PBSC, peripheral blood stem cells; UCB, umbilical cord blood. Median (range) refers to the median value and the interquartile range
Engraftment and transplant-related complications
No patients in the NCR group experienced primary graft failure. The median neutrophil engraftment time was 13 days (range, 12 to14 days). The median platelet and hemoglobin recovery times were 17 days (range, 13 to 26.5 days) and 15 days (range, 12 to 19 days), respectively. In the NCR group, thirteen patients (14.8%) had late phase grade III-IV hemorrhagic cystitis. Eight patients (9.1%) had VOD. Fungal infection and bacteremia occurred in 4.5% (4/88) and 8.0% (7/88) of cases respectively. Twenty-two patients (25.0%) had CMV DNAemia and three had CMV disease without associated mortality. Forty-nine patients (55.7%) experienced Epstein-Barr virus reactivation while two patients (2.3%) developed PTLD. None of the patients in the NCR group developed AIHA. Two patients (2.3%) were diagnosed with PRES (Supplementary Information).
The complications after unrelated and sibling donor transplantation undergoing different conditioning regimens were compared in Table 2. It was observed that the incidence of fungal infection was significantly lower in the NCR group (5.0%) compared to the CCR group (26.4%) among URD grafts (p = 0.004). Meanwhile, the novel conditioning regimen showed a positive effect on immune-related complications. The incidence of AIHA was significantly lower in the NCR group compared to the CCR group in both unrelated (p = 0.006) and related (p = 0.014) transplants. In addition, the NCR group had significantly lower rates of mixed chimerism in MSD transplants (p = 0.007).
Table 2.
Complications after unrelated and sibling donor transplantation undergoing different conditioning regimen
| Variables, n (%) | Unrelated donor transplantation | Sibling donor transplantation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CCR group (n = 53) |
NCR group (n = 60) |
OR/ difference value |
95% CI | p | CCR group (n = 65) |
NCR group (n = 28) |
OR/ difference value |
95% CI | p | |
| Mixed chimerism | 5(9.4) | 1(1.7) | 0.247 | 0.029–2.074 | 0.198 | 27(41.5) | 2(7.1) | 0.112 | 0.023–0.554 | 0.007* |
| GVHD | ||||||||||
| Acute, grade II-IV | 23(43.4) | 28(46.7) | 1.355 | 0.611–3.007 | 0.455 | 15(23.1) | 3(10.7) | 0.460 | 0.118–1.798 | 0.264 |
| Chronic | 10(18.9) | 15(25.0) | 1.380 | 0.538–3.540 | 0.503 | 8(12.3) | 2(7.1) | 0.388 | 0.071–2.125 | 0.275 |
| Fungal infection | 14(26.4) | 3(5.0) | 0.164 | 0.048–0.560 | 0.004 | 14(21.5) | 1(3.6) | 0.123 | 0.013–1.198 | 0.071 |
| Bacteremia | 6(11.3) | 4(6.7) | 0.716 | 0.185–2.774 | 0.629 | 8(12.3) | 3(10.7) | 0.634 | 0.137–2.932 | 0.560 |
| PRES | 5(9.4) | 2(3.3) | 0.356 | 0.069–1.836 | 0.217 | 5(7.7) | 0(0) | 0.077 | 0.012–0.142 | 0.314▲ |
| PTLD | 2(3.8) | 2(3.3) | 1.263 | 0.155–10.314 | 0.828 | 0(0) | 0(0) | |||
| AIHA | 8(15.1) | 0(0) | 6.763 | 0.055–0.247 | 0.006▲ | 15(23.1) | 0(0) | 0.231 | 0.129–0.333 | 0.014▲ |
Abbreviations: CCR, conventional conditioning regimen; NCR, novel conditioning regimen; ANC, absolute neutrophil count; PLT, blood platelet; GVHD, graft versus host disease; AIHA, autoimmune hemolytic anemia; PRES, posterior reversible encephalopathy syndrome; PTLD, posttransplant lymphoproliferative disorders
*The differences are statistically significant. The difference analysis for complications except AIHA and PRES between two groups was performed using the propensity score and Cochran-Mantel-Haenszel test
▲The difference analysis for AIHA and PRES between two groups was performed using the chi-square test
Thirty-one patients (35.2%) experienced grade II-IV acute GVHD while seventeen patients (19.3%) experienced grade III-IV acute GVHD. None of the patients died from severe aGVHD. Twelve patients (13.6%) developed limited chronic GVHD and five (5.7%) developed extensive chronic GVHD. There was no difference in the incidence of GVHD between the NCR and CCR groups in URD grafts.
Survival
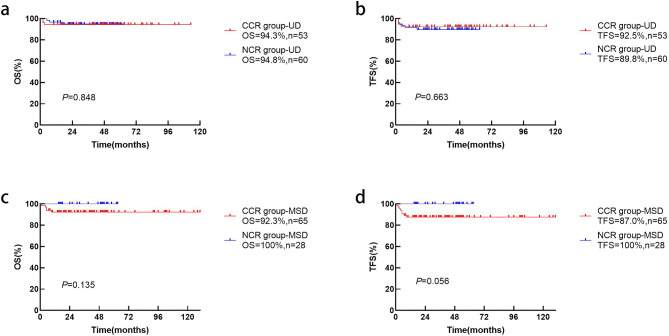
The OS (p = 0.848) and TFS (p = 0.663) in different conditioning regimens for unrelated donor transplantation were not significantly different (Fig. 2a and b). In MSD transplantation, the OS (p = 0.135) and TFS (p = 0.056) are slightly higher in the NCR group than in the CCR group without statistical significance (Fig. 2c and d).
Fig. 2.
Estimated OS (a) and TFS (b) for thalassemia patients receiving UD-HSCT and the OS (c) and TFS (d) for patients receiving MSD-HSCT in the NCR and CCR groups
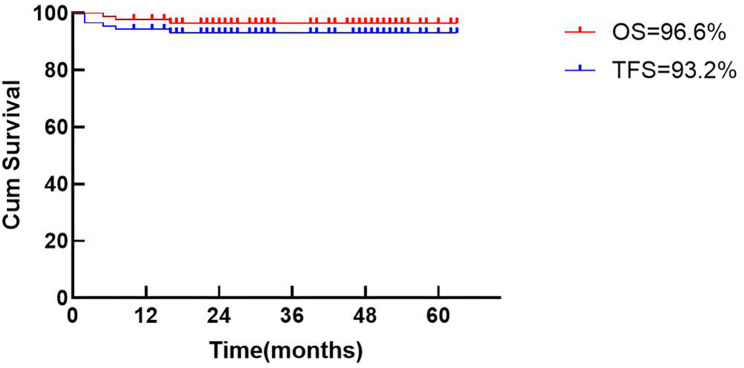
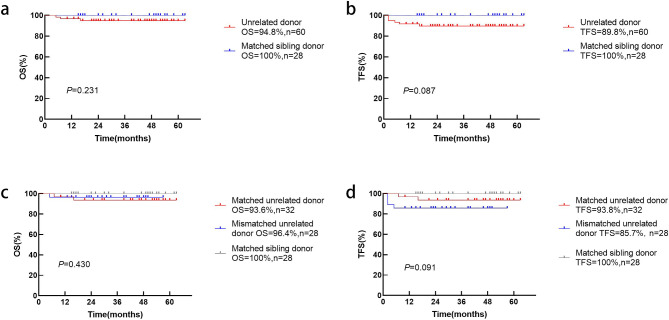
In the NCR group, the 3-year probabilities of OS and TFS were 96.6% (confidence interval (CI), 90.4–99.3%) and 93.2% (CI, 85.7–97.5%), respectively (Fig. 3). There were three cases of transplant-related mortality. One patient died of pneumonia and acute cerebral infarction. One patient developed intestinal cGVHD and died of multi-organ failure. One patient developed severe cGVHD and died of respiratory failure. Three patients developed secondary graft failure. One had AIHA pre-transplantation and platelet antibody positive with an 8/10 HLA-matched unrelated donor, while the other two patients had strong positive PRA titer prior to transplantation. When sibling (MSD) and unrelated (MUD) donors were compared, the OS (100% vs. 94.8%, p = 0.231) and TFS (100% vs. 89.8%, p = 0.087) were not statistically different (Fig. 4a and b). Taking into account the degree of allelic HLA matching, outcomes were compared between matched sibling donor (MSD), matched unrelated donor (MUD) and mismatched unrelated donor (MMUD). The 3-year OS in the MMUD group was 96.4% compared to 93.6% in the MUD group and 100% in the MSD group (p = 0.091, Fig. 4c). The corresponding probabilities for TFS were 85.7%, 93.8% and 100% (p = 0.430, Fig. 4d).
Fig. 3.
Estimated OS and TFS of 88 thalassemia patients undergoing HSCT based on the novel conditioning regimen
Fig. 4.
Estimated OS (a) and TFS (b) for thalassemia patients receiving MSD and UD-HSCT based on the novel conditioning regimen (MSD: UD = 28:60). Taking into account the degree of allelic HLA matching, the OS (c) and TFS (d) were further compared between MSD, MUD and MMUD (MSD: MUD: MMUD = 28:32:28)
Immune reconstitution
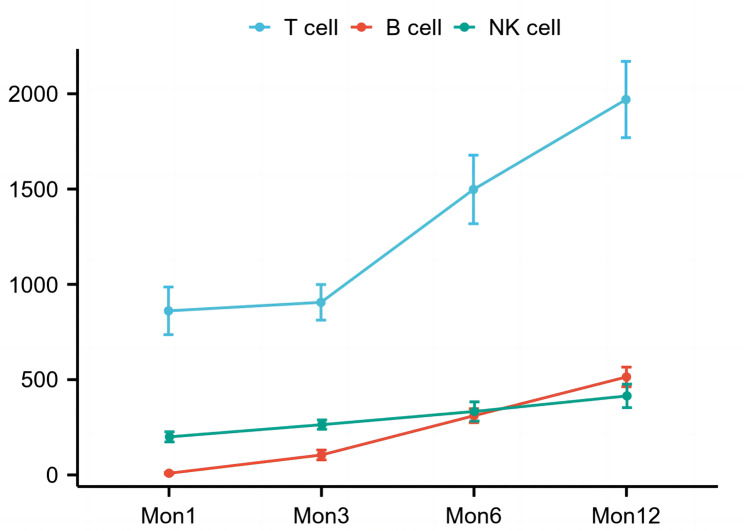
Twenty-eight patients had their PRA titers monitored during PTIS. PRA titers decreased significantly after PTIS (p = 0.002, Table 3). Meanwhile, lymphocyte subsets were measured in forty-six patients. The number of all lymphocyte types decreased significantly after PTIS (p < 0.001, Table 3). The kinetics of immune reconstitution were studied in forty-one patients (Fig. 5). At month 6 post-SCT, all patients with sustained engraftment had regained the mean absolute counts of 1498 ± 1093 cells/uL for T cells, 310 ± 221 cells/uL for B cells, and 333 ± 306 cells/uL for NK cells.
Table 3.
The change of immune status under immunosuppressive therapy before transplantation
| Variables, median(range) | before FD | after FD | p |
|---|---|---|---|
| PRA titers (%)(N = 28) | 22.5(3.3–54.5) | 11.5(0-37.3) | 0.002 |
| Class I HLA | 19.5(2.5–41.0) | 7.5(0–31.0) | 0.003 |
| Class II HLA | 0(0-15.5) | 0(0-1.8) | 0.043 |
| Lymphocyte subsets (N = 46) | |||
| WBC (×109/L) | 6.4(4.4, 8.6) | 3.3(2.3, 5.0) | < 0.001 |
| LYM (×109/L) | 2.5(1.9, 3.6) | 0.6(0.5, 1.0) | < 0.001 |
| TCL (cells/uL) | 1746.7(1370.7, 2147.3) | 521.0(337.9, 782.4) | < 0.001 |
| BCL (cells/uL) | 501.4(289.9, 900.2) | 35.8(8.3, 112.2) | < 0.001 |
| NK (cells/uL) | 218.8(163.6, 358.8) | 84.4(54.9, 112.5) | < 0.001 |
| CD3 + CD4+ (cells/uL) | 827.7(650.2, 1068.1) | 231.6(158.1, 370.6) | < 0.001 |
| CD3 + CD8+ (cells/uL) | 662.2(489.9, 899.1) | 217.8(142.8, 308.2) | < 0.001 |
| CD4+/CD8+ | 1.14(0.95, 1.50) | 1.20(0.88, 1.62) | 0.701 |
Abbreviations: FD, courses of dexamethasone (Dex) and fludarabine (Flu); PRA, panel reactive antibody; HLA, Human leukocyte antigen; WBC, white blood cell; LYM, lymphocyte; TCL, T-cell; BCL, B-cell; NK, natural kill cell; CD, cluster of differentiation. Median (range) refers to the median value and the interquartile range
Fig. 5.
Immune reconstitution in 41 patients with thalassemia undergoing hematopoietic stem cell transplantation based on the novel conditioning regimen
Discussion
This is the first study on the use of PTIS in both related and unrelated donor transplantation for TDT. In this study, we introduced a novel program consisting of multidrug PTIS therapy and a modified myeloablative conditioning regimen. The safety and efficacy of this novel conditioning regimen in children with TDT was presented by the retrospective analysis. A notable finding of this study is that the novel conditioning regimen reduced the risk of fungal infection, mixed chimerism, and immune-related complications, while the outcome of unrelated donor transplantation is equivalent to matched sibling donor transplantation, despite the older age and higher risk of transplantation and higher probability of positive PRA titers.
Older transplant age and hepatomegaly have been shown to be associated with inferior outcomes. It has been suggested that patients older than 7 years and with hepatomegaly should be defined as a very high-risk subgroup [20–22]. A multicenter study [23] reported that, after adjusting for donor type and conditioning regimen, the 5-year OS were 84% for patients aged 7 to 15 years and 63% for those aged 16 to 25 years. In another Indian study [24], the estimated OS for recipients aged > 7 years was only 90%. It is recommended that HSCT should be performed before the age of 7 years or before the onset of organ damage caused by severe iron overload and other thalassemia-related complications [22, 25]. In this study, despite the older age of transplantation of median age of over 7 years in NCR group, the NCR has overcome the disadvantages of age and unrelated donor, achieving comparable prognosis as in MSD.
Furthermore, PRA is another risk factor affecting engraftment in patients with thalassemia, which has been associated with multiple transfusions before transplantation [26, 27]. Our previous study found that PRA had a negative effect on DNA synthesis and the colony formation of CD34+ cord blood cells in vitro [28]. Transplants in PRA-positive recipients had a higher transplant-related mortality associated with poor graft function and vascular complications [27, 29]. Taking up the challenge of the high-risk subgroup with older transplant age and strong positive PRA, our center is trying to optimize this preparative backbone by adding PTIS and reducing the intensity of the conditioning regimen. It was showed that exposure to fludarabine led to a sustained loss of STAT1, which was essential for cell-mediated immunity, contributing to the prolonged period of immunosuppression [30], while dexamethasone-mediated T cell suppression diminished immature T cells [31]. Rituximab or bortezomib were used to eliminate plasma cells and memory B cells responsible for the production of anti-HLA antibodies, while plasmapheresis contributed to the elimination of PRA. Prior HSCT, low-dose chemotherapy with fludarabine and dexamethasone, or plasmapheresis alone or in combination with rituximab, bortezomib, sirolimus, high-dose IVIG, splenectomy [32], or T-cell depletion [11, 12] can prevent AIHA and pure red cell aplasia in PRA-positive recipients. According to the results, PTIS suppressed the immunity and depleted the lymphocytes from the recipients, resulting in stable implantation, less AIHA, and less graft failure, while the immune reconstitution post-transplantation was not impaired.
TDT is characterized by hypercellular bone marrow, which may contribute to higher risk of graft failure [11, 12]. Therefore, the ideal conditioning regimen must enhance the suppression of marrow hyperplasia, as well as the heighten immune system, to achieve stable engraftment. Bu-Cy has been the cornerstone conditioning regimen in most studies, although it is associated with hepatic and vascular complications. Various centers have endeavored to optimize protocols to minimize organ damage and enhance graft success. In a recent international study, the TCI score was developed as a new tool to define and measure the intensity of the conditioning regimen [33]. The NF-08-TM protocol reduced the dose of both Cy and Bu, but added Thiotepa (TT), with a TCI score of 4.5. The reported OS and TFS for matched and mismatched unrelated donor HSCT were 93.6% vs. 84.6% (p = 0.058), 90.4% vs. 82.1% (p = 0.089) [34]. In a study conducted at the Guangzhou Women and Children’s Medical Centre, 257 patients underwent a modified NF-08-TM conditioning regimen for both fully-matched and mismatched donor grafts, with the high incidence of immune-related complications, including AIHA ranging from 19.17 to 25.37%, PRES from 6.74 to 11.94%, and PTLD from 0.52 to 1.49%. Notably, patients receiving grafts with two or more HLA-allele mismatches were more susceptible to develop AIHA (40.91% vs. 17.78%, p = 0.041) [7]. A study from India also reported a higher incidence of PRES and immune-mediated cytopenia in patients undergoing unrelated donor transplantation [24]. In this study, all transplants received a myeloablative conditioning regimen consisting of Bu, Cy, Flu, and ATG. Our previous study based on CCR had observed a high incidence of serious bacterial infections and fungal pneumonia, which could be related to the immunosuppression of high-dose ATG. In addition, Cy had shown dose-related cardiotoxicity [35]. Therefore, in the NCR group, the lower dose of Cy (120 mg/kg instead of 180–200 mg/kg) has the same TCI score as the NF-08-TM protocol. Considering that a lower dose of Cy could have a negative impact on the maintenance of donor engraftment [11, 12], PTIS plays the role of providing additional immunosuppression prior to conditioning regimen without compromising immune reconstitution. It’s worth noting that the incidence of immune-related complications decreased significantly. Interestingly, the proportion of mixed chimerism was significantly lower in the NCR group compared to the CCR group for MSD transplants, which might be attributed to the prolonged and intensive suppression of recipient immune cells before transplantation. Furthermore, URD transplantation was comparable to MSD transplantation for TDT under the novel conditioning regimen. In the setting of UD-HSCT, reducing the intensity of the conditioning regimen did not affect OS and TFS. The novel conditioning regimen is able to be applied cost-effectively and accessibly in developing countries.
In addition to addressing the early transplantation complications, we place significant emphasis on the long-term outcomes for pediatric transplant recipients, which includes monitoring and managing issues such as iron overload, endocrine function and growth development, as well as quality of life and psychological well-being post-transplantation. Each pediatric patients performed growth development and endocrinology assessments prior to transplantation, and it is recommended that they attend regular outpatient follow-ups to monitor their growth and development post-transplantation. Our group had revealed that thalassemia patients with iron overload were at an increased risk of developing abnormal glucose metabolism before transplantation [36]. Additionally, premature ovarian failure was observed in some patients. Numerous studies have documented a significant depletion of the primordial follicle pool following CTX treatment [37, 38], which partly underpins our decision to reduce the single dose of CTX in novel conditioning regimen. Therefore, we will continue to pay attention to the effect of preconditioning regimen on growth and development post transplantation in future follow-up.
In conclusion, URD transplantation in patients with TDT can achieve a comparable prognosis to MSD transplantation without increasing the treatment-related mortality or graft failure rate by PTIS and modified myeloablative conditioning regimen. Based on the favorable outcome in the high-risk subpopulation with mismatched unrelated donors in this study, mismatched unrelated donors PBSC transplantation may be an optimized alternative for patients with TDT in the absence of MSD or MUD.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors declare that they have not use AI-generated work in this manuscript. The authors would like to thank all the patients and their guardians who participated in this study. The authors would like to thank the hardworking nurses and the outpatient assistants at the follow-up center for providing data collection and important information.
Author contributions
Huaqing Yang, Xin-Yu Li, Liping Que, and Han Chen contributed equally to the manuscript. Ke Huang, Honggui Xu, and Jian-Pei Fang designed the study. Huaqing Yang, Xin-Yu Li, Liping Que, and Han Chen analyzed the data and draft the manuscript. Huaqing Yang, Xin-Yu Li, Liping Que, Han Chen, and Danping Zhong contributed to the acquisition and analysis of important data. Liping Zhan, Dunhua Zhou, Yang Li, Shaofen Lin, Yin Wang, Xiaojun Wu, Xiawei Han, and Zhengzhou Wu participated in part in the clinical study. All authors contributed to the drafting, critical revision and final approval of the manuscript.
Funding
The work has been supported by the Science and Technology Project of Guangzhou (No.202201010962), Science and Technology Project of Sun Yat-sen Memorial Hospital (No. YXQH201913) and grants for a Clinical Key Discipline (The Subtropical Disease Center for Thalassemia) from the Chinese Ministry of Health (No. 1311200006107).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. All additional files are included in the manuscript.
Declarations
Ethics approval and connect to participate
The research protocol ‘The Impact of Modified Pretreatment Scheme on the Efficacy of Hematopoietic Stem Cell Transplantation for Treatment of Thalassemia’ was approved by the Ethics Committee of Sun Yat-sen Memorial Hospital of Sun Yat-sen University (SYSKY-2023-425-01) on 11 May 2023. Informed consent was obtained from all participants and the parents/guardians of the children under 18 years of age. This study was conducted in accordance with the Declaration of Helsinki. The protocol, amendments, and informed consent forms were approved by the appropriate institutional review board and independent ethics committee before the study began. Written informed consent was obtained from all patients prior to enrolment.
Conflict of interest
The authors have declared that no conflicts of interest. No financial or non-financial benefits have been received or will be received from any party directly or indirectly related to the subject of this article.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huaqing Yang, Xinyu Li, Liping Que and Han Chen contributed equally to this work.
Contributor Information
Ke Huang, Email: hke@mail.sysu.edu.cn.
Honggui Xu, Email: xuhgui@mail.sysu.edu.cn.
Jianpei Fang, Email: fangjpei@mail.sysu.edu.cn.
References
- 1.Frangoul H, et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N Engl J Med. 2021;384(3):252–60. [DOI] [PubMed] [Google Scholar]
- 2.Fu B, et al. CRISPR-Cas9-mediated gene editing of the BCL11A enhancer for pediatric β(0)/β(0) transfusion-dependent β-thalassemia. Nat Med. 2022;28(8):1573–80. [DOI] [PubMed] [Google Scholar]
- 3.Anurathapan U, et al. Hematopoietic Stem Cell Transplantation for Severe Thalassemia Patients from Haploidentical Donors Using a Novel Conditioning Regimen. Biol Blood Marrow Transpl. 2020;26(6):1106–12. [DOI] [PubMed] [Google Scholar]
- 4.Anurathapan U, et al. Outcomes of thalassemia patients undergoing hematopoietic stem cell transplantation by using a standard myeloablative versus a novel reduced-toxicity conditioning regimen according to a new risk stratification. Biol Blood Marrow Transpl. 2014;20(12):2066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anurathapan U, et al. Hematopoietic stem cell transplantation for homozygous β-thalassemia and β-thalassemia/hemoglobin E patients from haploidentical donors. Bone Marrow Transpl. 2016;51(6):813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Koning C, Nierkens S, Boelens JJ. Strategies before, during, and after hematopoietic cell transplantation to improve T-cell immune reconstitution. Blood. 2016;128(23):2607–15. [DOI] [PubMed] [Google Scholar]
- 7.Huang C, et al. Hematopoietic stem cell transplantation for thalassemia major using HLA fully-matched and mismatched donor grafts. Transl Pediatr. 2021;10(6):1552–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oevermann L, et al. HLA-haploidentical hematopoietic stem cell transplantation in pediatric patients with hemoglobinopathies: current practice and new approaches. Bone Marrow Transpl. 2019;54(Suppl 2):743–8. [DOI] [PubMed] [Google Scholar]
- 9.Huang K, et al. Modified conditioning regimen improves outcomes of unrelated donor peripheral blood stem cell transplantation for β-thalassaemia major patients. Pediatr Blood Cancer. 2018;65(7):e27026. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, et al. Prediction, management, and prognosis of mixed chimerism after hematopoietic stem cell transplantation in transfusion-dependent pediatric thalassemia patients. Pediatr Transpl. 2020;24(8):e13876. [DOI] [PubMed] [Google Scholar]
- 11.Lucarelli G, et al. Marrow transplantation for patients with thalassemia: results in class 3 patients. Blood. 1996;87(5):2082–8. [PubMed] [Google Scholar]
- 12.Hu J, et al. Haploidentical transplant for paediatric patients with severe thalassaemia using post-transplant cyclophosphamide and methotrexate: A prospectively registered multicentre trial from the Bone Marrow Failure Working Group of Hunan Province, China. Br J Haematol. 2023;200(3):329–37. [DOI] [PubMed] [Google Scholar]
- 13.Furst S, et al. Durable engraftment after pharmacological pre-transplant immune suppression followed by reduced-toxicity myeloablative haploidentical stem cell transplantation in highly HLA-immunized adults with sickle cell disease. Bone Marrow Transpl. 2024;59(7):918–27. [DOI] [PubMed] [Google Scholar]
- 14.Li C, et al. A novel conditioning regimen improves outcomes in β-thalassemia major patients using unrelated donor peripheral blood stem cell transplantation. Blood. 2012;120(19):3875–81. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y, et al. Chimerism status is correlated to acute graft-versus-host disease after allogeneic stem cell transplantation. Int J Hematol. 2014;99(3):323–8. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant, 1995;15(6):825-8. [PubMed]
- 17.Atkinson K, et al. Consensus among bone marrow transplanters for diagnosis, grading and treatment of chronic graft-versus-host disease. Committee of the International Bone Marrow Transplant Registry. Bone Marrow Transpl. 1989;4(3):247–54. [PubMed] [Google Scholar]
- 18.Swerdlow SH, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayello J, et al. Characterization of cord blood natural killer and lymphokine activated killer lymphocytes following ex vivo cellular engineering. Biol Blood Marrow Transpl. 2006;12(6):608–22. [DOI] [PubMed] [Google Scholar]
- 20.Mathews V, et al. A new stratification strategy that identifies a subset of class III patients with an adverse prognosis among children with beta thalassemia major undergoing a matched related allogeneic stem cell transplantation. Biol Blood Marrow Transpl. 2007;13(8):889–94. [DOI] [PubMed] [Google Scholar]
- 21.Sabloff M, et al. HLA-matched sibling bone marrow transplantation for β-thalassemia major. Blood. 2011;117(5):1745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yesilipek MA, et al. Thalassemia-free and graft-versus-host-free survival: outcomes of hematopoietic stem cell transplantation for thalassemia major, Turkish experience. Bone Marrow Transpl. 2022;57(5):760–7. [DOI] [PubMed] [Google Scholar]
- 23.Li C, et al. Related and unrelated donor transplantation for beta-thalassemia major: results of an international survey. Blood Adv. 2019;3(17):2562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swaminathan VV, et al. Matched Family versus Alternative Donor Hematopoietic Stem Cell Transplantation for Patients with Thalassemia Major: Experience from a Tertiary Referral Center in South India. Biol Blood Marrow Transpl. 2020;26(7):1326–31. [DOI] [PubMed] [Google Scholar]
- 25.Algeri M, Lodi M, Locatelli F. Hematopoietic Stem Cell Transplantation in Thalassemia. Hematol Oncol Clin North Am. 2023;37(2):413–32. [DOI] [PubMed] [Google Scholar]
- 26.Lv M, et al. Class I and II human leukocyte antibodies in pediatric haploidentical allograft candidates: prevalence and risk factors. Bone Marrow Transpl. 2019;54(8):1287–94. [DOI] [PubMed] [Google Scholar]
- 27.Malbora B, et al. Effect of serum panel reactive antibodies on allogeneic hematopoietic stem cell transplantation in pediatric thalassemia patients: A single-center experience. Pediatr Transpl. 2024;28(1):e14648. [DOI] [PubMed] [Google Scholar]
- 28.Fang JP, et al. Panel reactive antibody in thalassemic serum inhibits proliferation and differentiation of cord blood CD34 + cells in vitro. Pediatr Hematol Oncol. 2009;26(5):338–44. [DOI] [PubMed] [Google Scholar]
- 29.Detrait M, et al. Impact of anti-HLA antibodies on allogeneic hematopoietic stem cell transplantation outcomes after reduced-intensity conditioning regimens. Exp Hematol. 2012;40(10):792–9. [DOI] [PubMed] [Google Scholar]
- 30.Frank DA, Mahajan S, Ritz J. Fludarabine-induced immunosuppression is associated with inhibition of STAT1 signaling. Nat Med. 1999;5(4):444–7. [DOI] [PubMed] [Google Scholar]
- 31.Giles AJ, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang YJ, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol. 2015;8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spyridonidis A, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transpl. 2020;55(6):1114–25. [DOI] [PubMed] [Google Scholar]
- 34.He Y, et al. Long-term results of the NF-08-TM protocol in stem cell transplant for patients with thalassemia major: A multi-center large-sample study. Am J Hematol. 2020;95(11):E297–9. [DOI] [PubMed] [Google Scholar]
- 35.Iqubal A, et al. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: Old drug with a new vision. Life Sci. 2019;218:112–31. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, et al. Indicators of glucose dysregulation and the relationship with iron overload in Chinese children with beta thalassemia major. Pediatr Diabetes. 2022;23(5):562–8. [DOI] [PubMed] [Google Scholar]
- 37.Shai D, et al. Ovaries of patients recently treated with alkylating agent chemotherapy indicate the presence of acute follicle activation, elucidating its role among other proposed mechanisms of follicle loss. Fertil Steril. 2021;115(5):1239–49. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen QN, et al. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Mol Hum Reprod. 2019;25(8):433–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. All additional files are included in the manuscript.