Abstract
Periodontal ligament fibroblasts (PDLFs) play a crucial role in the etiology of periodontitis and periodontal tissue regeneration. In healthy periodontal tissues, PDLFs maintain the homeostasis of periodontal soft and hard tissues as well as the local immune microenvironment. PDLFs also have the potential for multidirectional transdifferentiation and are involved in periodontal tissue regeneration. On the other hand, PDLFs can become dysfunctional and acquire an inflammatory phenotype to secret various inflammatory cytokines when affected by pathological factors. These cytokines further trigger immune and inflammatory events, and lead to destruction of periodontal soft and hard tissues as well as damage to the regenerative potential of PDLFs. This review summarizes the physiological functions of PDLFs. Meanwhile, this review also highlights recent insights into the pathological mechanisms driving the development of periodontitis through dysfunctional PDLFs and the negative impact on periodontal tissue regeneration. Additionally, this paper summarizes strategies for targeting PDLFs to treat periodontitis, involving blocking multiple stages of the inflammatory response induced by PDLFs and promoting the multidirectional transdifferentiation of PDLFs. Future research directions are proposed to address important questions that have not yet been answered in this field. This article provides a reference for understanding the important role of PDLFs in the pathological mechanisms of periodontitis and for developing new strategies for targeting PDLFs in periodontitis treatment.
Graphical Abstract
Keywords: Periodontal Ligament Fibroblasts, Periodontitis, Pathological Mechanisms, Regeneration of periodontal tissue
Introduction
Periodontal disease is an inflammatory disease that is caused by an imbalance between the subgingival flora and the host immune response, and characterized by damage of the tooth-supportive tissues, which can lead to destruction of the alveolar bone, cementum and periodontal ligament [1]. From 2011 to 2020, it was estimated that more than 62% of the adult population worldwide suffered from periodontitis [2]. Moreover, the collective occurrence of moderate to severe periodontitis is estimated to be 53.2% [2]. Periodontitis can not only damage oral function and reduce patient quality of life but also increase the risk of systemic health complications and have adverse economic and social impacts [2]. Therefore, the prevention and treatment of periodontitis have become global public health issues [2]. However, the currently available strategies for preventing and treating periodontitis are not satisfactory [3]. This is primarily due to the complex pathogenesis of periodontitis, which, in addition to factors related to genetics and the subgingival flora, involves the dysfunction of multiple histiocytes and immune cells, including periodontal ligament fibroblasts (PDLFs) [4].
PDLFs, which are periodontal-specific histiocytes, play a pivotal role in maintaining periodontal tissue integrity [5]. Physiologically, PDLFs can maintain the homeostasis of periodontal soft and hard tissues as well as the local immune microenvironment [4, 6, 7]. As mesenchymal cells, PDLFs can undergo multidirectional differentiation, which allows PDLFs to contribute to the restoration and regeneration of periodontal tissues after injury [8]. However, PDLFs can become dysfunctional and acquire an inflammatory phenotype when affected by pathological factors, such as stimulation by subgingival flora [9], paracrine actions of immune cells [10] and abnormal glucose metabolism [11]. PDLF dysfunction subsequently triggers a cascade of immunoinflammatory events, which lead to the destruction of periodontal soft and hard tissues and inhibit periodontal regeneration [12, 13]. Given the importance of PDLFs in the pathogenesis of periodontitis, new therapeutic strategies that combine traditional periodontal treatment with PDLF-targeted treatment have been developed, and these strategies have shown initial, predictable effects in clinical practice [14].
Numerous studies have highlighted the significant role of PDLFs in the etiology of periodontitis and periodontal tissue regeneration. However, these results have not been well summarized. This review provides a comprehensive summary of recent in-depth insights into PDLFs, including their growth and development, physiological function, and identification and characterization. In addition, this paper focuses on the potential mechanisms by which dysfunctional PDLFs promote the development of periodontitis and negatively impact periodontal tissue regeneration. Moreover, this paper summarizes strategies for targeting PDLFs to periodontitis treatment, including strategies that block multiple stages of the inflammatory response associated with PDLFs and promote the multidirectional transdifferentiation of PDLFs to facilitate tissue regeneration after periodontal injury. Finally, future research directions for this field are proposed based on issues that have not yet been elucidated.
Physiology of PDLFs
Sources
PDLFs are spindle-shaped, elongated, connective histiocytes that are located in periodontal tissue, and they account for 50–60% of the total number of periodontal cells [15]. In adult human premolars, PDLFs occupy approximately 25% of the periodontal ligament space, while in rodent molars, they occupy approximately 35% of the space [15]. PDLFs originate from the ectomesenchyme tissue of dental follicles during embryonic development [16, 17]. Subsequently, epithelial–mesenchymal interactions induce the differentiation of ectomesenchyme tissue into dental follicle cells, and then, the enamel organ induces the differentiation of dental follicle cells into PDLFs [18]. In contrast, during the healing of periodontal injuries in adult rodents, PDLFs are derived from a paravascular population of progenitor cells [19], including periodontal ligament stem cells [20]. These progenitor cells migrate from the endosteal spaces of the alveolar bone to reach the periodontal tissue and further differentiate into PDLFs [17, 20].
Growth and development
During the normal development of rodent molar roots, mesenchymal stem cells (MSCs) are enriched in the population of newly formed periodontal ligament cells at the root diaphragm [18]. These MSCs undergo division and form daughter cells that migrate toward the crown, where they subsequently develop into fibroblasts with secretory functions [18, 21]. The fibroblasts then continue to migrate along collagen fibers to the cementum and alveolar bone with tooth eruption, migrating in the direction of the crown to differentiate into PDLFs [21]. Afterward, the PDLFs are aligned in a discrete linear fashion and parallel to collagen fiber bundles, forming an extensive cellular network between the cementum and alveolar bone [22].
In adults, PDLFs can regenerate and remodel periodontal tissue that is destroyed by periodontitis [1] via an alternative developmental mechanism [15]. In mice, following periodontal tissue destruction, a distinct paravascular population of progenitor cells is present within 200 µm of the trauma margin [19]. Within 30 – 72 h after injury, these progenitor cells undergo multiple divisions, and the resulting daughter cells migrate to the damaged site and divide again during the following 70 – 120 h [19]. Interactions between the fully differentiated enamel organ and the overlying connective tissue induce these dividing cells to eventually differentiate and develop into PDLFs [18].
Studies in rodent models have provided morphological evidence for the growth and development of PDLFs. However, the underlying mechanisms that regulate the cell division, migration, and functional arrangement of PDLFs are largely unknown. A better comprehension of these underlying molecular mechanisms will increase our understanding of the functional properties of PDLFs, which is very important for developing strategies to promote tissue-engineered regeneration in adult individuals in the future.
Physiological functions
Physiologically, PDLFs can maintain the homeostasis of periodontal soft and hard tissues, as well as the local immune microenvironment, to maintain periodontal health [4, 6, 7]. First, PDLFs can remodel the extracellular matrix (ECM) and regulate the differentiation and migration of gingival epithelial cells, maintaining soft tissue homeostasis [21]. PDLFs can secrete collagen and organize it into fibers to form the ECM [22, 23]. Moreover, PDLFs can secrete matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) to regulate ECM degradation, thereby dynamically remodeling the ECM [24]. PDLFs can secrete keratinocyte growth factor and hepatocyte growth factor [25], thus regulating the differentiation and migration of gingival epithelial cells and maintaining soft tissue boundaries [21]. The combined effects of these two aspects help to maintain local soft tissue homeostasis in periodontal tissues [21].
In addition, PDLFs can secrete a range of cytokines that regulate the functions of both osteoclasts (OCs) and osteoblasts (OBs), thereby maintaining alveolar bone homeostasis. PDLFs can promote the proliferation and differentiation of OC precursor cells by increasing the nuclear factor receptor activator-κB ligand (RANKL)/osteoprotegerin (OPG) ratio [22]. PDLFs can also secrete high levels of transforming growth factor-β (TGF-β) to promote the activity of OBs, which are involved in physiological alveolar bone remodeling [26]. To control the degree of osteogenesis that occurs during physiological alveolar bone remodeling, thereby preserving the width of the periodontal ligament and preventing ankylosis, PDLFs secrete prostaglandin E2 (PGE2) to control the excessive differentiation of OB precursors in the periodontal ligament [27]. These functions of PDLFs ensure the maintenance of periodontal soft and hard tissue homeostasis in the resting state or during physiological tooth movement [28, 29].
In addition to maintaining soft and hard tissue homeostasis, PDLFs also play a pivotal role in balancing the local periodontal immune microenvironment [7]. PDLFs can promote the migration of immune cells, such as dendritic cells (DCs) and macrophages to periodontal tissues and induce their maturation, increasing phagocytosis by professional antigen-presenting cells (APCs) [7]. This function of PDLFs can maintain the balance between the local pro- and anti-inflammatory responses in periodontal tissues [7].
It is well known that subgingival flora homeostasis is critical for the maintenance of periodontal health [30, 31]. However, whether PDLFs play a role in maintaining this homeostasis has not been reported. Research has shown that gingival fibroblasts can secrete antimicrobial peptides and chemokines [32]. Antimicrobial peptides can directly eliminate subgingival flora [33], and chemokines can attract immune cells to resist bacterial infection, thus maintaining a balance in the subgingival flora [32, 34]. Therefore, we hypothesize that PDLFs can also maintain subgingival flora homeostasis through similar mechanisms. This possibility warrants further investigation.
PDLFs also play a very important role in periodontal tissue regeneration. Cytokines, growth factors, and drugs can induce the transdifferentiation of PDLFs into OBs and cementoblasts [8], which are crucial for periodontal tissue regeneration. Therefore, in the field of tissue engineering, PDLFs are increasingly being recognized as targets for promoting periodontal regeneration [1, 35, 36].
Identification and characterization
There are currently no accepted markers for identifying and characterizing PDLFs [37]. Only a limited number of studies have reported specific gene expressed by PDLFs in particular environments [37]. For instance, one study showed that PDLFs exhibit high expression of the PDLs-5, PDLs-17, PDLs-22, PDLs-25 and PDLs-31 genes under resting conditions, and this gene expression pattern can be used to distinguish PDLFs from other cell types [37]. Among these genes, PDLs-17 is thought to be important for the differentiation of PDLFs [37]. However, the functions of the PDLs-5, PDLs-22, PDLs-25 and PDLs-31 genes are not known. Further research is needed to characterize the functions of these genes and determine their validity as specific markers of PDLFs.
Furthermore, researchers have identified secretory proteins that are specifically produced by PDLFs in different functional states and can be used as markers. For instance, during ECM remodeling, PDLFs exhibit high expression of α-smooth muscle actin and XII-collagen [17]. Moreover, during the osteogenic differentiation and mineralization of bone tissues [37], PDLFs express significant levels of osteoblast-specific factor 2 (OSF2) [38] and calcium-binding protein (S100A4) [39].
Currently, the absence of reliable and specific markers is a major limitation in the study of PDLFs. Detection technologies such as RNA sequencing, tissue single-cell mapping, and spatial transcriptomic technologies are advancing quickly and have been used to identify fibroblasts in rheumatoid arthritis (RA) and inflammatory bowel disease [40]. The identification and characterization of PDLFs are anticipated to be feasible in the future with the help of these technologies.
Underlying mechanisms of dysfunctional PDLFs involved in periodontitis
Factors leading to dysfunction of PDLFs
As previously mentioned, the main functions of PDLFs are the secretion of various cytokines to regulate local periodontal homeostasis and self-differentiation to promote periodontal regeneration. However, PDLFs can acquire an inflammatory phenotype following dysfunction, leading to the excessive secretion of tissue destruction-related cytokines, decreased secretion of tissue repair-related cytokines, as well as impaired self-differentiation and even programmed cell death (PCD), which promote the occurrence and development of periodontitis. It is currently thought that PDLF dysfunction can be caused by various factors, such as stimulation by subgingival flora, the paracrine effects of immune cells and abnormal glucose metabolism.
Stimulation by subgingival flora
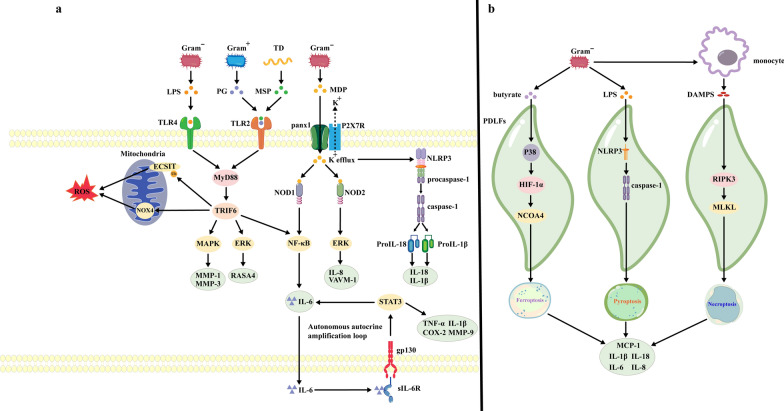
Dysbiosis of the subgingival flora can lead to increased numbers of pathogenic bacteria and an increase in the levels of their toxic products, which in turn, can modulate pattern recognition receptors (PRRs) to promote the generation of inflammatory phenotypes (Fig. 1a) and also induce the PCD of PDLFs (Fig. 1b), ultimately resulting in dysfunction of PDLFs.
Fig. 1.
Stimulation by oral pathogenic bacteria induce dysfunctional PDLFs. a Toxic products, secreted by pathogenic bacteria, lead to the production of inflammatory factors and the activation of multiple signaling pathways through pattern recognition receptors. b Metabolites and virulence factors produced by pathogenic bacteria induce inflammatory death in PDLFs, resulting in the release of inflammatory factors. The black arrows indicate activation. Gram− gram-positive bacteria, Gram + gram-negative bacteria, TD Treponema denticola, LPS lipopolysaccharide, PG peptidoglycan, MSP major surface protein, MDP muramyl dipeptide MurNAc-L-Ala-D-isoGln, TLR toll-like receptor, panx1 Pannexin-1, P2XR7 P2RX7 receptor, MyD88 myeloid differentiation factor 88, TRIF6 Tumor necrosis factor receptor associated factor 6, NLR NOD-like receptor, NLRP3 NLRP3 inflammasome, ROS Reactive oxygen species, NCOA4 cargo receptor nuclear receptor coactivator 4, HIF-1α Hypoxia-inducible factor-1 α, DAMPs death-associated molecular patterns, RIPK3 receptor-interacting protein serine-threonine kinases-3, MLKL mixed lineage kinase domain-like protein, VCAM-1 vascular cellular adhesion molecule-1, RANKL receptor activator of nuclear factor-kappa B ligand, MMP Matrix metalloproteinase, NOD nucleotide-binding oligomerization domain, ECSIT, evolutionarily conserved signaling intermediate in Toll pathways, NOX NADPH Oxidases, ERK Extracellular signal-regulated kinases, COX-2 cyclooxygenase-2, GP130 Glycoprotein 130, sIL-6R soluble interleukin-6 receptor, TNF-α Tumor necrosis factor α, IL-1 interleukin-1, IL-1β interleukin-1β, RASA4 RAS p21 protein activator 4, MCP-1 Monocyte chemoattractant protein-1
PDLFs express a range of PRRs, such as Toll-like receptors (TLRs) on the cell surface and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) in the cytoplasm [41]. Bacteria and their toxic products can interact with these receptors to initiate inflammatory responses [16]. The virulence factor lipopolysaccharide (LPS), which is secreted by gram-negative bacteria, including Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans, is a potent activator of TLR4 on the surface of PDLFs [16]. The virulence factor peptidoglycan, which is secreted by gram-positive bacteria, such as Actinomyces viscosus [9], as well as major surface protein (MSP), which is secreted by Treponema denticola [42], can bind to and activate TLR2 [43]. Activated TLR2/4 trigger an intracellular signaling cascade that is mediated by MyD88 and tumor necrosis factor receptor-associated factor 6 (TRAF-6) [9], which promotes the ubiquitination of downstream ECSIT proteins in the mitochondrial outer membrane [44] and the expression of NOX4 on the mitochondrial inner membrane [45] to increase reactive oxygen species (ROS) production [46]. Activated TLR2/4 upregulate the mitogen-activated protein kinase (MAPK) pathway, which can increase MMP1 and MMP3 expression, leading to ECM degradation [47]. Additionally, the ERK pathway is also activated, which inhibits Ca2+ influx and further upregulates RASA4 expression, leading to fibrin cleavage and promoting the development of periodontitis [24].
Notably, activation of TLR2/4 signaling can also lead to an autonomous autocrine amplification loop in PDLFs, which is a key mechanism that drives the amplification of inflammation [40]. Activation of NF-κB, which is downstream of TLR2/4 [48], results in the secretion of interleukin-6 (IL-6) [49]. IL-6 can bind to extracellular soluble IL-6R (sIL-6R) produced by PDLFs and immune cells; this interaction further activates the gp130 coreceptor/STAT3 pathway in PDLFs [50], ultimately increasing the synthesis and secretion of other inflammatory factors, such as MMP-9, interleukin-1β (IL-1β), tumor necrosis factor (TNF-α), and cyclooxygenase (COX-2) [48], via a positive feedback mechanism [50].
In addition to TLRs, PDLFs also express NLRs in the cytoplasm [41]. Research has shown that pannexin-1 (panx-1) is released from the surface of PDLFs in response to bacterial stimulation [51]. The muramyl dipeptide MurNAc-L-Ala-D-isoGln (MDP) originates from the cell wall of gram-negative bacteria, such as P. gingivalis, P. intermedia, A. actinomycetemcomitans and F. nucleatum [52], and can enter the cytoplasm of PDLFs via panx-1, binding to and activating NOD1 and NOD2 [53, 54]. Activation of NOD1 and NOD2 can upregulate the NF-κB and ERK pathways, promoting the synthesis and secretion of IL-6 and interleukin-8 (IL-8) [53]. The opening of panx-1 can also trigger the exocytosis of cytoplasmic K+ via the P2X7 receptor (P2X7R) [51], thus activating NLRP3, which contains an NLR structural domain [51, 55], and ultimately leading to pro-caspase-1 maturation [41]. Mature caspase-1 can promote the development of periodontitis by inducing the maturation and release of IL-1β and interleukin-18 (IL-18) [9, 41].
In addition to promoting an inflammatory phenotype in PDLFs, the toxic products of subgingival flora can cause PCD of PDLFs, such as ferroptosis, pyroptosis, and necrotic apoptosis [56–58]. For instance, the metabolite butyrate from P. gingivalis can activate the p38/hypoxia-inducible factor-1α (HIF-1α) pathway in PDLFs, thus inducing the upregulation of nuclear receptor coactivator 4 (NCOA4) and subsequently leading to ferritinophagy and ferroptosis of PDLFs [57]. LPS can activate NLRP3 in PDLFs, which recruits pro-caspase-1 and induces its proteolytic cleavage to form active caspase-1, leading to pyroptosis [56]. Furthermore, P. intermedia can trigger the release of death-associated molecular patterns (DAMPs) from monocytes, which then induce the necroptosis of PDLFs through receptor-interacting protein serine-threonine kinase-3 (RIPK3) and mixed lineage kinase domain-like protein (MLKL) [58]. The PCD of PDLFs results in osmotic lysis, which leads to the release of IL-1β, MCP-1, IL-6, IL-8 and IL-18, promoting the destruction of periodontal tissues [56].
The occurrence of PCD of PDLFs is a fascinating subject, and available evidence from in vitro studies provides a solid foundation for an in-depth understanding the roles of these PCD mechanisms in the development of periodontitis. Future studies should further validate the importance of the PCD of PDLFs in periodontitis through in vivo experiments and analysis of clinical samples.
Paracrine actions of immune cells
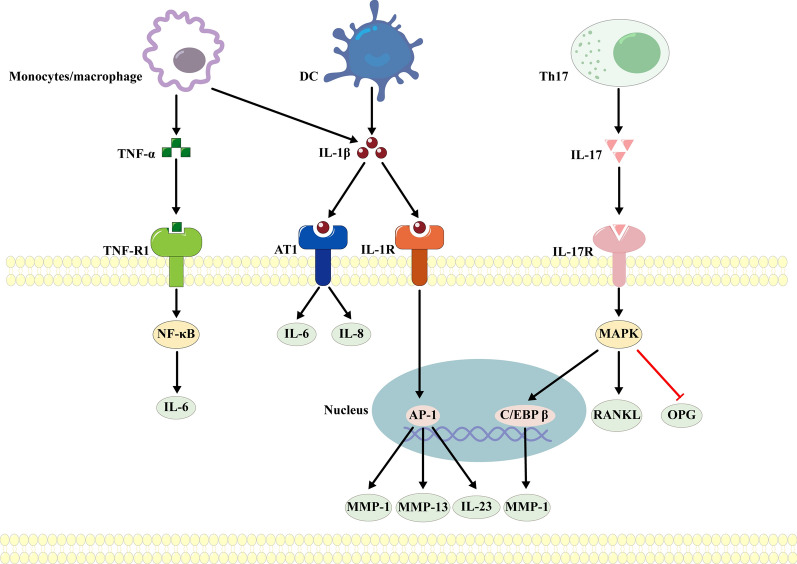
During the development of periodontitis, innate and adaptive immune cells may induce PDLFs to acquire an inflammatory phenotype in a paracrine manner [10] (Fig. 2). Research has demonstrated that circulating monocytes/macrophages and DCs can be recruited to and invade periodontal tissues, where they can secrete IL-1β and TNF-α [10]. IL-1β can bind to the IL-1 type I receptor on the surface of PDLFs and activate activator protein-I, which upregulates MMP-1 and MMP-13 to degrade collagen [59–61] and increases the synthesis and secretion of interleukin-23 (IL-23) to promote maturation of OCs [62, 63]. Furthermore, IL-1β can also bind to the angiotensin II type 1 receptor (AT1) on the cell surface, upregulating IL-6 and IL-8 and leading to the destruction of alveolar bone [64]. In addition, TNF-α can upregulate IL-6 through the NF-κB pathway [65], which is an efficient activator of OC precursors [10].
Fig. 2.
Paracrine actions of immune cells induce dysfunctional PDLFs. Innate immune cells associated with periodontitis, such as macrophages and dendritic cells, and adaptive immune cells, such as Th17 cells, regulate the induction of an inflammatory phenotype in PDLFs in a paracrine manner. The black arrows indicate activation, and the red horizontal lines indicate inhibition. DC Dendritic cell, Th17 T helper cell 17, TNF-α tumor necrosis factor α, IL-8 interleukin-8, IL-1β interleukin-1β, IL-17 interleukin-17, TNF-R1 tumor necrosis factor receptor 1, IL-6 interleukin-6, RANKL receptor activator of nuclear factor-kappa B ligand, AT1 angiotensin II type 1 receptor, IL-1R interleukin-1R, IL-17R interleukin-17R, NF-κB nuclear factor-kappa B, MAPK mitogen-activated protein kinase, PKA protein kinase A AP-1, activating protein-1, C/EBPβ acetylated CCAAT/enhancer binding protein β, MMP matrix metalloproteinase, IL-23 interleukin-23, OPG Osteoprotegerin
In the adaptive immune system, CD4+ T lymphocytes, including helper T lymphocytes (Th17), recognize antigens presented by DCs, and then, these cells leave the lymph nodes, enter circulation and infiltrate infected periodontal tissues [66]. Th17 helper lymphocytes secrete interleukin-17 (IL-17), which can bind to IL-17R on the surface of PDLFs and activate the MAPK pathway, ultimately increasing RANKL expression and decreasing OPG expression [67]. The binding of IL-17 to IL-17R can also upregulate the transcription of NF-κB and CCAAT/enhancer-binding protein β (C/EBP β) [68, 69], thus increasing the secretion of MMP-1 [68]. In addition to Th17 cells, Th22 cells are also important lymphocytes that are associated with periodontitis [70, 71]. However, until now, no study has examined whether Th22 cells can mediate the participation of dysfunctional PDLFs in the progression of periodontitis, and this topic should be further explored in future studies.
Abnormal glucose metabolism
Clinically, diabetic patients often exhibit severe inflammation and periodontal destruction, which is associated with dysfunctional PDLFs induced by the production of advanced glycation end products (AGEs) due to hyperglycemia [72, 73]. AGEs can bind to receptor for advanced glycation end products (RAGE) on the surface of PDLFs [72], which activates the NF-κB pathway and upregulates the expression of CXCL2, RANKL, IL-6, and ROS [11], thereby enhancing neutrophil recruitment [74], increasing OC-mediated bone resorption and leading to oxidative stress [65, 75].
In fact, patients with abnormal glucose metabolism often have abnormal lipid metabolism [11], which may also be an important factor that contributes to dysfunctional PDLFs [72]. Clinically, the levels of oxidized low-density lipoprotein (ox-LDL) are increased in the gingival crevicular fluid of hyperlipidemic patients, and these levels are closely associated with periodontitis [76, 77]. Moreover, PDLFs express the structural domains of oxidized LDL receptor 1 (LOX-1), named LDL receptor-related protein 4 (LPR4) and LPR5 [78]. In studies of dysfunctional synovial fibroblasts, it was reported that ox-LDL can bind to LOX-1 and activate the NF-κB signaling pathway, further leading to the secretion of MMP-1 and MMP-3 [79, 80]. Thus, we hypothesize that elevated levels of ox-LDL in gingival crevicular fluid may cause dysfunctional PDLFs through a similar mechanism. This hypothesis requires further testing in future studies.
Underlying mechanisms involved in periodontitis
Alveolar bone resorption
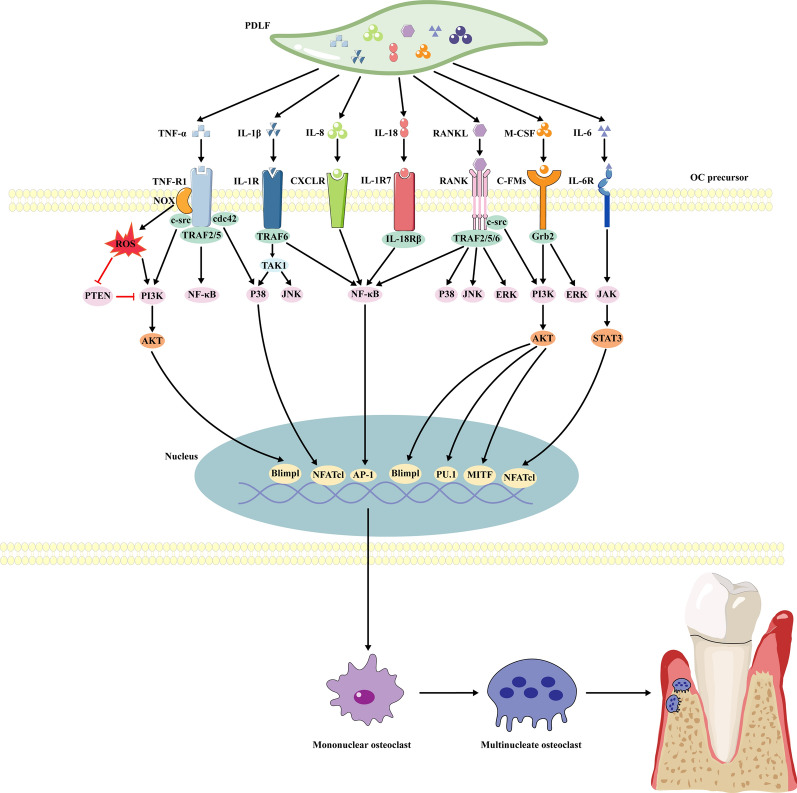
Animal studies have shown that dysfunctional PDLFs can secrete excessive levels of inflammatory cytokines, which promote OC differentiation and lead to alveolar bone destruction by modulating various signaling pathways [81] (Fig. 3).
Fig. 3.
Mechanisms by which inflammatory factors derived from dysfunctional PDLFs modulate alveolar bone destruction. Multiple inflammatory factors secreted by PDLFs promote the maturation and differentiation of osteoclast precursors, leading to the resorption of alveolar bone. PDLFs Periodontal ligament fibroblasts, IL-8 interleukin-8, IL-18 interleukin-18, IL-1β interleukin-1β, TNF-α Tumor necrosis factor α, IL-6 interleukin-6, RANKL receptor activator of nuclear factor-kappa B ligand, M-CSF macrophage colony-stimulating factor, TNF-R1 Tumor necrosis factor receptor 1, NOX NADPH Oxidases, IL-1R interleukin-1R CXCLR, C-X-C motif ligand receptor; IL-1R7 IL-1 receptor 7, IL-6R interleukin-6 receptor, ROS Reactive oxygen species, TRAF TNF receptor associated factor, IL-18Rβ interleukin-18 receptor β, Grb2 growth factor receptor-bound protein-2, TAK1 transforming growth factor-β-activated kinase 1, PTEN Phosphatase and tensin homolog, NF-κB nuclear factor-kappa B, PI3K phosphatidylinositol 3-phosphate kinase, AKT serine/threonine kinas, MAPK mitogen-activated protein kinases, JAK Janus kinase, STAT3 signal transducer and activator of transcription 3, P38 p38 Mitogen-Activated Protein Kinases, JNK The c-Jun N-terminal kinase, ERK Extracellular signal-regulated kinases, Blimpl a transcription factor, AP-1 activating protein-1, NFATcl nuclear factor of activated T cells cl, PU.1 an ETS-family transcription factor, MITF microphthalmia-associated transcription factor, c-Fms colony-stimulating factor-1
NF-κB pathway: The NF-κB pathway is a crucial signaling pathway that induces the formation of mononuclear OCs from OC precursors, resulting in the destruction of alveolar bone. It was reported that dysfunctional PDLFs can secrete excessive amounts of TNF-α, IL-1β, IL-18, IL-8, and RANKL [82–87]. TNF-α can bind to the type I p55 TNF receptor (TNF-R1) on the surface of OC precursors and activate TNF receptor-associated factor 2/5 (TRAF2/5), thus upregulates the NF-κB pathway [82]. In contrast, the binding of IL-1β to the type I receptor (IL-1RI) on the surface of OC precursors can activate Toll and IL-1R-like (TIR) intracellular interface proteins to phosphorylate TRAF6, subsequently leading to upregulation of the NF-κB pathway [83]. IL-18, which is a member of the IL-1 family [84], can bind to IL-1R7 and activate the NF-κB pathway by recruiting the IL-18Rβ chain [85]. Similarly, IL-8 can activate the NF-κB pathway by binding to the CXCR1 precursor on the surface of OCs [86]. Additionally, RANKL can bind to RANK on the surface of OC precursors and recruit TRAF2, TRAF5 and TRAF6, which work together to upregulate the NF-κB pathway, ultimately promoting the formation of mononucleated OCs from OC precursors and inducing alveolar bone resorption [87].
PI3K/Akt pathway: Inflammatory cytokines that are produced by dysfunctional PDLFs, such as M-CSF [88], TNF-α [89] and RANKL [90], can promote the proliferation of OC precursors and their differentiation into mature OCs through activation of the PI3K/Akt pathway, leading to bone resorption [91]. It was found that M-CSF can bind to colony-stimulating factor-1 (c-Fms) on the surface of OC precursors and recruit growth factor receptor-bound protein 2 (Grb2) [91], which activates the PI3K/Akt pathway, upregulating the transcription factor PU.1 and MITF to promote OC proliferation [90]. TNF-α can bind to TNF-R1, and RANKL can bind to RANK on OC precursors [91], and both of these receptors recruit the adaptor c-src to activate the PI3K/Akt pathway, subsequently upregulating B-lymphocyte-induced maturation protein-1 (Blimp1) and promoting maturation of OCs [88]. Furthermore, as previously mentioned, dysfunctional PDLFs can generate excessive levels of ROS [46]. ROS can not only directly activate the PI3K/Akt pathway but also indirectly enhance it by inactivating phosphatase and tensin homolog (PTEN), thus promoting the differentiation and maturation of OCs [89].
MAPK pathway: The MAPK pathway is also regulated by a variety of cytokines derived from dysfunctional PDLFs, such as TNF-α, M-CSF, IL-1β, and RANKL; these cytokines induce the differentiation of OC precursors into mature OCs and lead to alveolar bone destruction [91]. TNF-α can bind to TNF-R1 on the surface of OC precursors and recruit the small G protein cdc42, activating p38 to induce NFATc1-dependent maturation of OCs [89]. The interaction of M-CSF with c-Fms can activate Grb2, which upregulates ERK expression, leading to MAPK pathway activation [91]. IL-1β can bind to type I (IL-1RI) to activate TRAF-6, which recruits TGF-β-activated protein kinase (TAK-1) [83]. TAK-1 upregulates JNK and p38 expression, resulting in maturation of OCs and alveolar bone destruction [83]. RANKL can bind to RANK on the surface of OC precursors to activate the junction molecule TRAF6, which then recruits TAB1, TAB2, and TAK1 and activates the three MAPK signaling cascades, inducing the differentiation of OC precursors into mature OCs [92].
JAK/STAT pathway: The JAK/STAT pathway in OC precursors can modulate NFATc1 to induce the differentiation of these cells into mature OCs, thus playing a crucial role in the alveolar bone resorption that is regulated by dysfunctional PDLFs [93]. IL-6, which is secreted by dysfunctional PDLFs, can bind to the IL-6 receptor (IL-6R) on the surface of OC precursors, upregulates the JAK/STAT3 pathway [94], thereby promoting the differentiation and maturation of OCs [95].
The studies mentioned above reported that inflammatory cytokines that are secreted by dysfunctional PDLFs can induce the formation of mononuclear OCs. However, a key step in OC maturation and bone resorption is the fusion of mononuclear OCs to form multinucleated OCs [96]. The mechanism by which these cytokines promote the multinucleation of mononuclear OCs is currently unknown. Research has shown that the process of OC multinucleation is initiated by two transmembrane proteins, namely DC-STAMP and OC-STAMP, that are localized to the surface of mononuclear OCs [96]. Further studies are necessary to determine whether the inflammatory cytokines secreted by dysfunctional PDLFs induce OC multinucleation through these two receptor-mediated signaling pathways.
Cementum destruction
Physiologically, the cementum is divided into two main components: acellular and cellular cementum [97]. Acellular cementum is mainly composed of hydroxyapatite and collagen, while cellular cementum contains a small number of cementoblasts [97]. Dysfunctional PDLFs secrete multiple cytokines that can destroy hydroxyapatite in the cementum and inhibit the differentiation and mineralization of cementoblasts, thereby preventing restoration of the cementum [98].
It has been demonstrated that dysfunctional PDLFs can secrete MCP-1 to recruit circulating monocytes/macrophages into periodontal tissues, which then migrate to the surface of the cementum [99]. In the inflammatory microenvironment, these monocytes/macrophages further differentiate into mature OCs, which directly destroy hydroxyapatite, leading to resorption of the cementum [98].
Additionally, cytokines that are produced by dysfunctional PDLFs, such as IL-1β and TNF-α, can inhibit the differentiation and mineralization of cementoblasts. Research has shown that excess IL-1β can bind to IL-1R2 on the surface of cementoblasts [100] and increase the transcription of miR-325-3p [101]. The latter inhibits runt-related transcription factor 2 (Runx2), thus suppressing the differentiation of cementoblasts [101]. TNF-α can inhibit both cementoblast differentiation and cementum mineralization. TNF-α can bind to TNF-R1 on the surface of cementoblasts, upregulate miR-155-3p transcription and inhibit the expression of potassium channel tetramerization domain containing 1 [101]. Kctd1 regulates the classic Wnt/β-catenin pathway, inhibiting cementoblast differentiation [101]. Furthermore, TNF-α not only activates the p53 [102] and STAT3 pathways [103] in cementoblasts to induce autophagy but also activates the NF-κB pathway to inhibit the expression of bone sialoprotein (BSP), collagen type I (COL I), and OPG, thus inhibiting cementum mineralization [104]. To date, few studies have been conducted on cementoblasts differentiation and cementum mineralisation inhibited by dysfunctional PDLFs, and the underlying mechanisms have not been fully elucidated; thus, this topic warrants further attention in future studies.
Periodontal ligament breakage
The periodontal ligament is primarily composed of type I, III, and XII collagen fibers as well as ECM [105]. Dysfunctional PDLFs can secrete various MMPs, which degrade collagen fibers and ECM, ultimately leading to periodontal ligament breakage [106].
MMP-14 and MMP-13 that are produced by dysfunctional PDLFs can undergo autoactivation [107, 108]. Activated MMP-14 can continue to activate MMP-8 [105], and activated MMP-8 can combine with activated MMP-13 to cleave the natural triple-helix molecules of type I, type III and type XII fibrillar collagen [109] into multiple denatured collagen fragments [108]. Subsequently, MMP-13 can activate MMP-2 and MMP-9 [110], thus facilitating the further cleavage of denatured collagen fragments into smaller protein fragments [111]. Furthermore, MMP-13 can also upregulate MMP-3, which degrades proteoglycans and matrix glycoproteins in the ECM into small organic molecules, ultimately leading to the destruction of the periodontal ligament [112].
The integrity of periodontal ligaments does not rely on collagen fibers alone, but Malassez epithelial remnant cells also play an important role. Malassez epithelial remnant cells can secrete matrix proteins and collagenases to maintain periodontal ligament integrity [113, 114]. Research has shown that during the initial stages of periodontitis, PDLFs can interact with Malassez epithelial cells, resulting in the loss of attachment and the formation of periodontal pockets [115]. This phenomenon may be related to the negative impact of dysfunctional PDLF-derived inflammatory cytokines on the secretory function of Malassez epithelial cells. However, further studies are needed to confirm this hypothesis.
Mechanisms of inhibiting periodontal regeneration
The multidirectional differentiation of PDLFs is crucial for the regeneration of periodontal tissue. However, the transdifferentiation potential of dysfunctional PDLF is attenuated, and thus, periodontal tissue regeneration is inhibited.
The MAPK pathway is activated in dysfunctional PDLFs, which leads to the downregulation of osteogenic differentiation marker genes, such as ColA1, osteocalcin (OCN), OPN and BSP, and the inhibition of PDLFs transdifferentiation into osteoblasts [13]. On the other hand, LPS derived from Pg suppresses the transdifferentiation of PDLFs by regulating their epigenetic mechanisms, such as DNA methylation, histone modifications, and changes in non-coding RNA expression. Studies have found that LPS can enter the nucleus of PDLFs and induce the hypermethylation of RUNX2 DNA, decreasing RUNX2 expression and inhibiting osteogenic differentiation in PDLFs [116]. Additionally, LPS can induce the posttranslational modification of histone 1 lysine 2 (H1K2) and H5K1 in the promoters of matrix-associated genes, such as COL3A27, COL3A4, and RUNX1, as well as osteogenic genes, such as COL1A1, COL3A1, and RUNX2 [116]. As a result, the transcriptional program that is associated with the transdifferentiation of PDLFs is suppressed [116], and alveolar bone regeneration is inhibited [117]. Furthermore, LPS derived from Pg can upregulate the expression of the long noncoding RNA MIAT, which subsequently modulates miR-204-5p/Dicckopf-1 (DKK1) axis, thereby inhibiting transdifferentiation of PDLFs [118].
Interestingly, a recent study has documented that Pg-LPS can actually facilitate the osteogenic differentiation of PDLFs at concentrations below 0.01 μg/mL and with exposure times shorter than 8 h [119], which seems to contradict the aforementioned research. Pg-LPS, secreted by Gram-negative bacteria as a virulence factor, modulates cellular functions by time and concentration-dependent factors. Research has demonstrated that low concentration and short-term stimulation of Pg-LPS has been shown to initiate autophagy, which enhance the proliferative capacity of cells [120]. This phenomenon may be linked to the activation of the integrated stress response, which helps to sustain the cells’ capacity for proliferation and regeneration [121]. Conversely, under the influence of prolonged and high-concentration stimuli, the cellular stress response becomes imbalanced, ultimately leading to the inhibition of regeneration [121]. Xi Wu et al [47] reported that Pg-LPS, exceeding a concentration of 0.1 μg/mL and an exposure duration beyond 8 h, can suppress the osteogenic differentiation of PDLFs. This finding consists with the phenotype observed in patients with periodontitis in clinical. Uncontrolled dysbiosis of subgingival flora leads to continuous stimulation of PDLFs by Pg-LPS, exceeding the stress compensatory capacity of PDLFs, ultimately inhibiting their osteogenic potential and contributing to the destruction of alveolar bone and the progression of periodontitis.
In summary, many studies have shown that transdifferentiation is inhibited in dysfunctional PDLFs, thereby impeding alveolar bone formation. In fact, cementum regeneration and the subsequent functional periodontal ligament reconstruction are considered the gold standards for periodontal tissue regeneration [101, 122]. However, only in vitro experiments have reported that the transdifferentiation of PDLFs into cementoblasts is blocked after exposure to stimuli such as bacteria [122]. The relationship between the pathological changes and transdifferentiation into cementoblast of PDLFs have not been reported in vivo. Further research is needed to thoroughly investigate the impact of dysfunctional PDLFs on their transdifferentiation into cementoblast and the underlying mechanisms involved.
Periodontal treatment strategies targeting PDLFs
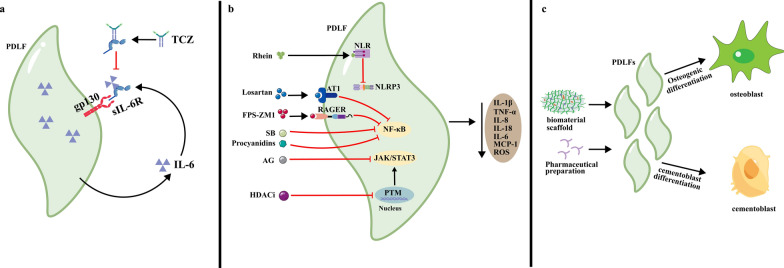
Here, we described insights into the pathological mechanism by which dysfunctional PDLFs are involved in periodontitis, and these insights may provide a novel potential strategy for periodontal treatment. Periodontal treatment strategies that target PDLFs have good clinical results following conventional periodontal treatment [123]. Such treatments include reducing periodontal inflammation by inhibiting the autonomous autocrine amplification loop of PDLFs, preventing the synthesis and secretion of inflammatory cytokines by PDLFs, and inducing the transdifferentiation of PDLFs to promote periodontal tissue regeneration (Fig. 4).
Fig. 4.
Strategies for targeting PDLFs in the treatment of periodontitis. a The synthesis of inflammatory factors can be reduced by blocking the sIL-6R signaling pathway and inhibiting the autonomous autocrine amplification loop in PDLFs. b The synthesis and secretion of inflammatory factors can be reduced by targeting and inhibiting the PDLF surface receptor, the postreceptor signaling pathway and epigenetic inheritance. c Biomaterials and pharmacological agents can promote the regeneration of inflamed periodontal tissues by inducing osteogenic differentiation in PDLFs and cementoblast differentiation. The black arrows indicate activation, and the red horizontal lines indicate inhibition. IL-6, interleukin-6, sIL-6R soluble interleukin-6 receptor, gp130 Glycoprotein 130, TCZ, tocilizumab, NLR NOD-like receptor, AT1 angiotensin II type 1 receptor, RAGER receptor for advanced glycation end products; FPS-ZM1 RAGE inhibitor, SB Silibinin, AG andrographolide, HDACis small molecule inhibitors of histone deacetylases, NF-κB nuclear factor-kappa B, PI3K phosphatidylinositol 3-phosphate kinase, AKT serine/threonine kinase, JAK Janus kinase, STAT3 signal transducer and activator of transcription 3, PTM posttranslational modification, IL-1β interleukin-1β, TNF-α tumor necrosis factor α, IL-8 interleukin-8, IL-18 interleukin-18
Inhibition of the autonomous autocrine amplification loop
Persistent activation of the proinflammatory phenotype in PDLFs can be inhibited by blocking the autonomous autocrine amplification loop, which effectively reduces the synthesis of inflammatory cytokines. Tocilizumab (TCZ)® is a commercial drug that has been shown to block the binding of IL-6 to IL-6R, exerting anti-inflammatory effects in the treatment of rheumatoid arthritis [124]. Research has also shown that TCZ, when used to treat patients with chronic periodontitis, can specifically inhibit the IL-6-mediated autonomous autocrine amplification loop [124, 125], thereby inhibiting the inflammatory phenotype of PDLFs and significantly reducing periodontal inflammation [125].
Despite the predictable anti-inflammatory effects, there are relatively few therapeutic strategies that target the autonomous autocrine amplification loop. A possible reason is that researchers have not fully recognized the autonomous autocrine amplification loop as a key step in the development of periodontitis. Future studies should focus on this topic.
Blockade of inflammatory cytokine synthesis and secretion
As previously stated, the synthesis and secretion of inflammatory cytokines by PDLFs involve receptor and postreceptor signaling pathways as well as epigenetic regulation. Treatments that target these three steps have been reported to effectively control periodontal inflammation.
First, blocking receptors on PDLFs inhibits the synthesis and secretion of inflammatory cytokines, attenuating local periodontal inflammation in vivo [64, 126, 127]. Losartan was shown to block the AT1 receptor on the surface of PDLFs, thereby inhibiting the NF-κB pathway and suppressing IL-8 expression [64]. Under hyperglycemic conditions, the RAGE inhibitor FPS-ZM1 can prevent the binding of AGEs to RAGE on the surface of PDLFs and inhibit the activation of the NF-κB pathway, which downregulates TNF-α [126]. Additionally, rhein can block the NLR in PDLFs, inhibit NLRP3 inflammasome formation and activation, and decrease IL-1β synthesis and secretion [127].
In addition to receptor blockers, a series of drugs can target the postreceptor signaling pathway in PDLFs to inhibit inflammatory cytokine synthesis and secretion. Oral or topical administration of silymarin [128] can inhibit the NF-κB pathway in PDLFs, downregulating TNF-a, IL-1β, MCP-1, and IL-18 [128]. Andrographolide can inhibit the JAK/STAT3 pathway in PDLFs, downregulating IL-1β and TNF-α mRNA expression [129]. Although these drugs can suppress periodontal inflammation, their long-term efficacy remains uncertain. In the future, it will be necessary to focus on how effective therapeutic concentrations of these drugs can be maintained locally in periodontal tissues in the long term. The use of nanocarriers may help to address these issues [130, 131]. Optimizing the conditions of nanocarriers considering their order of drug release, release rate [132], and degradation rate can help to increase the local anti-inflammatory effects of these drugs in periodontal tissue [130].
In recent years, several studies have shown that epigenetic regulation can suppress the production of inflammatory cytokines in PDLFs. For instance, small-molecule inhibitors of histone deacetylases can suppress histone deacetylation in the nucleus of PDLFs, leading to decreases in TNF-α and IL-1β expression and ROS production [116, 133]. However, previous studies have not focused sufficiently on epigenetics, and research has been limited to in vitro experiments. In contrast, epigenetic modulation has been reported to have great potential in the treatment of inflammatory diseases. For instance, azacitidine can inhibit DNA methylation in synovial fibroblasts and thus ameliorate rheumatoid arthritis [134]. Therefore, targeting epigenetic regulation in PDLFs for the treatment of periodontitis is an area that warrants further investigation.
Promotion of periodontal regeneration
Biomaterials
Currently, the use of biomaterials that target PDLFs to promote periodontal tissue regeneration is receiving widespread attention. Some of these biomaterials can directly promote transdifferentiation of PDLFs, while others act as carriers for exogenous signaling molecules that indirectly promote transdifferentiation of PDLFs, ultimately leading to periodontal tissue regeneration.
Nanoscale biomaterials are now known to have beneficial biological effects, which directly induce the transdifferentiation of PDLFs [135, 136]. Studies have reported that nanohydroxyapatite particles are internalized by PDLFs and then stimulate increased alkaline phosphatase (ALP) expression, promoting the proliferation and transdifferentiation of PDLFs [135]. In contrast, metal nanoparticles appear to be more effective at regulating the transdifferentiation of PDLFs [136]. It has been demonstrated that metal nanoparticles, including silver nanoparticles (AgNPs), when internalized by PDLFs, elicit a dose-dependent activation of the RhoA-TAZ signalling pathway, thereby promoting transdifferentiation of PDLFs [136]. In a rat model of periodontitis, MgO nanoparticles promoted the transdifferentiation of PDLFs, which in turn increased neoplastic periodontal tissue formation [137].
Some biomaterials can incorporate exogenous signaling molecules as hybrid scaffolds which can be implanted into periodontal defects to induce the transdifferentiation of PDLFs and the regeneration of alveolar bone [138, 139]. COMP-Ang1 scaffolds loaded with angiopoietin-1 have been demonstrated to stimulate osteogenesis and the transdifferentiation of PDLFs by releasing angiopoietin-1 [138]. Similarly, biocompatible alginate/nano bioactive glass ceramic composite scaffolds were shown to release significant amounts of silicon and calcium, upregulating ALP and OPN expression in PDLFs and inducing their transdifferentiation into OCs [139]. All these materials mentioned above have been suggested to be effective in promoting alveolar bone regeneration in vivo.
Notably, the biomaterials mentioned above are constructed as bilayer or hybrid scaffolds and show excellent potential for inducing the differentiation of PDLFs to promote alveolar bone regeneration [138]. However, ideal periodontal tissue regeneration should mimic physiological developmental processes, which can achieve the multidirectional differentiation and functional regeneration of alveolar bone, cementum and periodontal ligament in a spatiotemporal sequence [139]. From this point of view, periodontal regeneration strategies that target PDLFs have significant potential for research and development [139]. A trilayered nanocomposite hydrogel scaffold structure has been reported to induce the differentiation of dental follicle stem cells into OBs, cementoblasts, or fibroblasts, which ultimately promote the regeneration of periodontal soft and hard tissues [140]. Multiphase nanocomposite scaffolds have the potential to induce the multidirectional transdifferentiation of PDLFs to promote the complete and orderly regeneration of periodontal tissues. Future studies on periodontal tissue regeneration should focus on this area.
Pharmaceutical agents
In addition to biomaterials, certain biologics and herbal preparations can target PDLFs to promote periodontal tissue regeneration.
Among the biologics, calcitonin can activate the BMP-2/4 pathway in PDLFs [141], which upregulates the expression of type I and type III collagen, OCN and ALP and promotes transdifferentiation [141]. Pigment epithelium-derived factor can bind to lipoprotein receptor-related protein 6, inhibit Wnt/β-catenin signaling and promote transdifferentiation and mineralization in PDLFs thus facilitating alveolar bone regeneration [142].
Recently, herbal preparations have been shown to target PDLFs to promote alveolar bone regeneration. For example, icariin [143] and proanthocyanin [144] have been shown to inhibit the TLR-4/NF-κB pathway in PDLFs, enhancing their transdifferentiation and inhibiting their apoptosis in in vivo experiments [143, 144].
In fact, other biological agents, such as etanercept [145], and herbal agents, such as tripterine and methotrexate [146], are used to target synovial fibroblasts to treat rheumatoid arthritis and have shown significant efficacy [145–147]. However, despite their potential efficacy, these agents have not attracted widespread attention in the treatment of periodontitis. In the future, the development or identification of new pharmacological agents that target PDLFs could be very beneficial for the treatment of periodontitis.
Limitations and prospects
At present, fibroblasts are mesenchymal cells that are receiving increasing amounts of attention due to their important roles in the pathological mechanisms of a variety of inflammatory diseases [40]. The identification of fibroblast subtypes has become a popular topic in recent years. Different fibroblast subtypes are closely associated with disease progression and regression [40]. In the oral cavity, subtypes of gingival fibroblasts were identified and characterized by single-cell sequencing, and correlations between these subtypes and periodontitis have been reported [148–150]. However, no studies have been conducted on the subtypes of PDLFs and the associations between these subtypes and periodontitis. It is hypothesized that different subtypes of PDLFs may emerge in response to different pathological stimuli. Identifying subtypes of PDLFs is crucial for understanding the pathological mechanisms involved, developing treatments that target inflammation and promoting periodontal regeneration. Therefore, future studies should carefully consider the potential impact of PDLF subtypes on periodontal health.
The microbiota is always present in the periodontal microenvironment. The state of periodontal health is determined by the interplay between subgingival flora and surrounding histiocytes and immune cells [151]. Numerous studies have focused on the interactions between microbiota dysbiosis and immune cells [16]. However, little is known about the regulatory relationship between the subgingival flora and histiocytes, such as PDLFs. Previous studies have reported only the effect of toxic products of the subgingival flora on PDLF function [116]. However, how cytokines or other products that are secreted by PDLFs affect the homeostasis of the subgingival flora is unclear. Further exploration will help us understand the importance of PDLFs in periodontal tissues from a bacterial microecological perspective.
The oral cavity is an important organ that performs the function of mastication, and periodontal tissue is subjected to various mechanical stresses, including masticatory forces [152, 153]. PDLFs are among the most important cell types for withstanding mechanical forces [154]. Therefore, mechanical factors should not be neglected in periodontitis treatment with targeted PDLFs. However, current therapeutic strategies that target PDLFs have not yet considered the influence of mechanical forces. Controlling mechanical forces in order to allow PDLFs to function optimally during the treatment of periodontitis and promotion of periodontal tissue regeneration will be a new strategy for future periodontal treatments.
Acknowledgements
This work was supported by Guangdong Natural Science Foundation (No.2024A1515012892).
Authors’ contribution
SY and LYT are the co-corresponding authors responsible for the overall conception, framework design, and revision of the review. HYJ and TY are the co-first authors. HYJ and TY contributed to the in-depth analysis of key topics. HYJ was responsible for drafting sections on 'Introduction', 'Physiology of PDLFs' and 'Underlying mechanisms of dysfunctional PDLFs involved in periodontitis'. TY authored the sections 'Periodontal treatment strategies targeting PDLFs' and 'Limitations and prospects'. ZRQ, WU, and YL led the literature collection and preliminary screening, as well as the creation of schematic diagrams, ensuring the accuracy and readability of the information presented. CXL, WQQ, and CYY refined the language of the review draft, enhancing the article's fluency. All authors read and approved the final manuscript.
Funding
Guangdong Natural Science Foundation, 2024A1515012892, Yuan Su.
Data availability
Not applicable to this review as as no datasets were generated or analyzed in this review.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yijie Huang and Ying Tang have contributed equally to this work. Author order was determined by drawing straws.
References
- 1.Chang Y-T, Lai C-C, Lin D-J. Collagen scaffolds laden with human periodontal ligament fibroblasts promote periodontal regeneration in SD rat model. Polymers. 2023;15:2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trindade D, Carvalho R, Machado V, Chambrone L, Mendes JJ, Botelho J. Prevalence of periodontitis in dentate people between 2011 and 2020: a systematic review and meta-analysis of epidemiological studies. J Clin Periodontol. 2023;50:604–26. [DOI] [PubMed] [Google Scholar]
- 3.Graziani F, Karapetsa D, Alonso B, Herrera D. Nonsurgical and surgical treatment of periodontitis: how many options for one disease? Periodontol. 2000;2017(75):152–88. [DOI] [PubMed] [Google Scholar]
- 4.Lekic P, McCulloch CAG. Periodontal ligament cell populations: the central role of fibroblasts in creating a unique tissue. Anat Rec. 1996;245:327–41. [DOI] [PubMed] [Google Scholar]
- 5.Song S, Yan Z, Wu W. MiR-874-3p inhibits osteogenic differentiation of human periodontal ligament fibroblasts through regulating Wnt/β-catenin pathway. J Dent Sci. 2021;16:1146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lekic P, Sodek J, McCulloch CAG. Osteopontin and bone sialoprotein expression in regenerating rat periodontal ligament and alveolar bone. Anat Rec. 1996;244:50–8. [DOI] [PubMed] [Google Scholar]
- 7.Konermann A, Stabenow D, Knolle PA, Held SAE, Deschner J, Jäger A. Regulatory role of periodontal ligament fibroblasts for innate immune cell function and differentiation. Innate Immun. 2012;18:745–52. [DOI] [PubMed] [Google Scholar]
- 8.Heo JS, Lee S-Y, Lee J-C. Wnt/β-catenin signaling enhances osteoblastogenic differentiation from human periodontal ligament fibroblasts. Mol Cells. 2010;30:449–54. [DOI] [PubMed] [Google Scholar]
- 9.Hatakeyama J, Tamai R, Sugiyama A, Akashi S, Sugawara S, Takada H. Contrasting responses of human gingival and periodontal ligament fibroblasts to bacterial cell-surface components through the CD14/Toll-like receptor system. Oral Microbiol Immunol. 2003;18:14–23. [DOI] [PubMed] [Google Scholar]
- 10.Rajeshwari HRS, Kishen A. Periodontal fibroblasts—macrophage crosstalk in external inflammatory root resorption. J Endod. 2023;49:1145-1153.e3. [DOI] [PubMed] [Google Scholar]
- 11.Graves DT, Ding Z, Yang Y. The impact of diabetes on periodontal diseases. Periodontol. 2000;2020(82):214–24. [DOI] [PubMed] [Google Scholar]
- 12.Papadopoulou A, Cantele A, Koletsi D, Eliades T, Kletsas D. Short- and long-term treatment with TNF-α inhibits the induction of osteoblastic differentiation in cyclic tensile-stretched periodontal ligament fibroblasts. Eur J Orthod. 2020;42:396–406. [DOI] [PubMed] [Google Scholar]
- 13.Diercke K, Sen S, Kohl A, Lux CJ, Erber R. Compression-dependent up-regulation of ephrin-A2 in PDL fibroblasts attenuates osteogenesis. J Dent Res. 2011;90:1108–15. [DOI] [PubMed] [Google Scholar]
- 14.Dogan A, Ozdemir A, Kubar A, Oygür T. Assessment of periodontal healing by seeding of fibroblast-like cells derived from regenerated periodontal ligament in artificial furcation defects in a dog: a pilot study. Tissue Eng. 2002;8:273–82. [DOI] [PubMed] [Google Scholar]
- 15.Beertsen W, McCulloch CAG, Sodek J. The periodontal ligament: a unique, multifunctional connective tissue. Periodontol. 2000;1997(13):20–40. [DOI] [PubMed] [Google Scholar]
- 16.Sokos D, Everts V, de Vries TJ. Role of periodontal ligament fibroblasts in osteoclastogenesis: a review. J Periodontal Res. 2015;50:152–9. [DOI] [PubMed] [Google Scholar]
- 17.McCulloch CA. Origins and functions of cells essential for periodontal repair: the role of fibroblasts in tissue homeostasis. Oral Dis. 1995;1:271–8. [DOI] [PubMed] [Google Scholar]
- 18.Pitaru S, McCulloch CA, Narayanan SA. Cellular origins and differentiation control mechanisms during periodontal development and wound healing. J Periodontal Res. 1994;29:81–94. [DOI] [PubMed] [Google Scholar]
- 19.Gould TR, Melcher AH, Brunette DM. Migration and division of progenitor cell populations in periodontal ligament after wounding. J Periodontal Res. 1980;15:20–42. [DOI] [PubMed] [Google Scholar]
- 20.Chen SC, Marino V, Gronthos S, Bartold PM. Location of putative stem cells in human periodontal ligament. J Periodontal Res. 2006;41:547–53. [DOI] [PubMed] [Google Scholar]
- 21.Hirashima S, Kanazawa T, Ohta K, Nakamura K-I. Three-dimensional ultrastructural imaging and quantitative analysis of the periodontal ligament. Anat Sci Int. 2020;95:1–11. [DOI] [PubMed] [Google Scholar]
- 22.Müssig E, Tomakidi P, Steinberg T. Molecules contributing to the maintenance of periodontal tissues. Their possible association with orthodontic tooth movement. J Orofac Orthop. 2005;66:422–33. [DOI] [PubMed] [Google Scholar]
- 23.Meazzini MC, Toma CD, Schaffer JL, Gray ML, Gerstenfeld LC. Osteoblast cytoskeletal modulation in response to mechanical strain in vitro. J Orthop Res. 1998;16:170–80. [DOI] [PubMed] [Google Scholar]
- 24.Malone ET, Ganther S, Mena N, Radaic A, Shariati K, Kindberg A, et al. Treponema denticola-Induced RASA4 upregulation mediates cytoskeletal dysfunction and MMP-2 activity in periodontal fibroblasts. Front Cell Infect Microbiol. 2021;11:671968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locke M, Hyland PL, Irwin CR, Mackenzie IC. Modulation of gingival epithelial phenotypes by interactions with regionally defined populations of fibroblasts. J Periodontal Res. 2008;43:279–89. [DOI] [PubMed] [Google Scholar]
- 26.Morandini AC, Sipert CR, Ramos-Junior ES, Brozoski DT, Santos CF. Periodontal ligament and gingival fibroblasts participate in the production of TGF-β, interleukin (IL)-8 and IL-10. Braz Oral Res. 2011;25:157–62. [DOI] [PubMed] [Google Scholar]
- 27.Ogiso B, Hughes FJ, Melcher AH, McCulloch CA. Fibroblasts inhibit mineralised bone nodule formation by rat bone marrow stromal cells in vitro. J Cell Physiol. 1991;146:442–50. [DOI] [PubMed] [Google Scholar]
- 28.Jiang N, Guo W, Chen M, Zheng Y, Zhou J, Kim SG, et al. Periodontal ligament and alveolar bone in health and adaptation: tooth movement. Front Oral Biol. 2016;18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki T, Shimizu T, Watanabe C, Hiyoshi Y. Cellular roles in physiological root resorption of deciduous teeth in the cat. J Dent Res. 1990;69:67–74. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Qian Y, Jia S, Shi Z, Zhong Q. Comparative analysis of subgingival microbiota in patients with mild, moderate, and severe chronic periodontitis. Oral Dis. 2023;29:2865–77. [DOI] [PubMed] [Google Scholar]
- 31.Lu X, Liu T, Zhou J, Liu J, Yuan Z, Guo L. Subgingival microbiome in periodontitis and type 2 diabetes mellitus: an exploratory study using metagenomic sequencing. J Periodontal Implant Sci. 2022;52:282–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang JY, Song I-S, Baek KJ, Choi Y, Ji S. Immunologic characteristics of human gingival fibroblasts in response to oral bacteria. J Periodontal Res. 2017;52:447–57. [DOI] [PubMed] [Google Scholar]
- 33.Ji S, Hyun J, Park E, Lee B-L, Kim K-K, Choi Y. Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J Periodontal Res. 2007;42:410–9. [DOI] [PubMed] [Google Scholar]
- 34.Jang JY, Choi GH, Ji S. IFN-γ or IL-4 polarization impacts the response of gingival fibroblasts to oral bacteria. J Periodontal Res. 2021;56:462–70. [DOI] [PubMed] [Google Scholar]
- 35.Seubbuk S, Surarit R, Stephens D, Hasturk H, Van Dyke TE, Kantarci A. TLR2 and TLR4 differentially regulate the osteogenic capacity of human periodontal ligament fibroblasts. J Int Acad Periodontol. 2021;23:3–10. [PMC free article] [PubMed] [Google Scholar]
- 36.Elango J, Selvaganapathy PR, Lazzari G, Bao B, Wenhui W. Biomimetic collagen-sodium alginate-titanium oxide (TiO2) 3D matrix supports differentiated periodontal ligament fibroblasts growth for periodontal tissue regeneration. Int J Biol Macromol. 2020;163:9–18. [DOI] [PubMed] [Google Scholar]
- 37.Park JC, Kim YB, Kim HJ, Jang HS, Kim HS, Kim BO, et al. Isolation and characterization of cultured human periodental ligament fibroblast-specific cDNAs. Biochem Biophys Res Commun. 2001;282:1145–53. [DOI] [PubMed] [Google Scholar]
- 38.Pi S-H, Lee S-K, Hwang Y-S, Choi M-G, Lee S-K, Kim E-C. Differential expression of periodontal ligament-specific markers and osteogenic differentiation in human papilloma virus 16-immortalized human gingival fibroblasts and periodontal ligament cells. J Periodontal Res. 2007;42:104–13. [DOI] [PubMed] [Google Scholar]
- 39.Duarte WR, Kasugai S, Iimura T, Oida S, Takenaga K, Ohya K, et al. cDNA cloning of S100 calcium-binding proteins from bovine periodontal ligament and their expression in oral tissues. J Dent Res. 1998;77:1694–9. [DOI] [PubMed] [Google Scholar]
- 40.Wei K, Nguyen HN, Brenner MB. Fibroblast pathology in inflammatory diseases. J Clin Invest. 2021;131: e149538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu WL, Song DZ, Yue JL, Wang TT, Zhou XD, Zhang P, et al. NLRP3 inflammasome may regulate inflammatory response of human periodontal ligament fibroblasts in an apoptosis-associated speck-like protein containing a CARD (ASC)-dependent manner. Int Endod J. 2017;50:967–75. [DOI] [PubMed] [Google Scholar]
- 42.Hinson AN, Hawkes CG, Blake CS, Fitzsimonds ZR, Zhu B, Buck G, et al. Treponema denticola induces interleukin-36γ expression in human oral gingival keratinocytes via the parallel activation of NF-κB and mitogen-activated protein kinase pathways. Infect Immun. 2022;90:e00247-e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Tang X, Li C, Pan C, Li Q, Geng F, et al. Porphyromonas gingivalis promotes the cell cycle and inflammatory cytokine production in periodontal ligament fibroblasts. Arch Oral Biol. 2015;60:1153–61. [DOI] [PubMed] [Google Scholar]
- 44.Fock EM, Parnova RG. Protective effect of mitochondria-targeted antioxidants against inflammatory response to lipopolysaccharide challenge: a review. Pharmaceutics. 2021;13:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gölz L, Memmert S, Rath-Deschner B, Jäger A, Appel T, Baumgarten G, et al. LPS from P. gingivalis and hypoxia increases oxidative stress in periodontal ligament fibroblasts and contributes to periodontitis. Mediators Inflamm. 2014;2014:986264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu X, Zhang G, Feng X, Li P, Tan Y. Transcriptome analysis of human periodontal ligament fibroblasts exposed to Porphyromonas gingivalis LPS. Arch Oral Biol. 2020;110:104632. [DOI] [PubMed] [Google Scholar]
- 48.Ambili R, Janam P, Saneesh Babu PS, Prasad M, Vinod D, Anil Kumar PR, et al. Differential expression of transcription factors NF-κB and STAT3 in periodontal ligament fibroblasts and gingiva of healthy and diseased individuals. Arch Oral Biol. 2017;82:19–26. [DOI] [PubMed] [Google Scholar]
- 49.Greenhill CJ, Rose-John S, Lissilaa R, Ferlin W, Ernst M, Hertzog PJ, et al. IL-6 trans-signaling modulates TLR4-dependent inflammatory responses via STAT3. J Immunol. 2011;186:1199–208. [DOI] [PubMed] [Google Scholar]
- 50.Hernández-Caldera A, Vernal R, Paredes R, Veloso-Matta P, Astorga J, Hernández M. Human periodontal ligament fibroblasts synthesize C-reactive protein and Th-related cytokines in response to interleukin (IL)-6 trans-signalling. Int Endod J. 2018;51:632–40. [DOI] [PubMed] [Google Scholar]
- 51.Paik S, Kim JK, Silwal P, Sasakawa C, Jo E-K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol Immunol. 2021;18:1141–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Y, Ye Q, Feng Y, Chen Y, Tan L, Ouyang Z, et al. Prevotella genus and its related NOD-like receptor signaling pathway in young males with stage III periodontitis. Front Microbiol. 2022;13:1049525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang L, Zhou X, Wang Q, Zhang L, Wang Y, Li X, et al. Expression of TRAF6 and pro-inflammatory cytokines through activation of TLR2, TLR4, NOD1, and NOD2 in human periodontal ligament fibroblasts. Arch Oral Biol. 2011;56:1064–72. [DOI] [PubMed] [Google Scholar]
- 54.Inohara N, Nuñez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3:371–82. [DOI] [PubMed] [Google Scholar]
- 55.Platnich JM, Muruve DA. NOD-like receptors and inflammasomes: a review of their canonical and non-canonical signaling pathways. Arch Biochem Biophys. 2019;670:4–14. [DOI] [PubMed] [Google Scholar]
- 56.Cheng R, Feng Y, Zhang R, Liu W, Lei L, Hu T. The extent of pyroptosis varies in different stages of apical periodontitis. Biochim Biophys Acta Mol Basis Dis. 2018;1864:226–37. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Y, Li J, Guo W, Li H, Lei L. Periodontitis-level butyrate-induced ferroptosis in periodontal ligament fibroblasts by activation of ferritinophagy. Cell Death Discov. 2020;6:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi J, Li J, Su W, Zhao S, Li H, Lei L. Loss of periodontal ligament fibroblasts by RIPK3-MLKL-mediated necroptosis in the progress of chronic periodontitis. Sci Rep. 2019;9:2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Havemose-Poulsen A, Holmstrup P. Factors affecting IL-1-mediated collagen metabolism by fibroblasts and the pathogenesis of periodontal disease: a review of the literature. Crit Rev Oral Biol Med. 1997;8:217–36. [DOI] [PubMed] [Google Scholar]
- 60.Halazonetis TD, Georgopoulos K, Greenberg ME, Leder P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell. 1988;55:917–24. [DOI] [PubMed] [Google Scholar]
- 61.Rossa C, Liu M, Patil C, Kirkwood KL. MKK3/6-p38 MAPK negatively regulates murine MMP-13 gene expression induced by IL-1beta and TNF-alpha in immortalized periodontal ligament fibroblasts. Matrix Biol. 2005;24:478–88. [DOI] [PubMed] [Google Scholar]
- 62.Zhu L, Wu Y, Wei H, Yang S, Zhan N, Xing X, et al. Up-regulation of IL-23 p19 expression in human periodontal ligament fibroblasts by IL-1β via concurrent activation of the NF-κB and MAPKs/AP-1 pathways. Cytokine. 2012;60:171–8. [DOI] [PubMed] [Google Scholar]
- 63.Chen L, Wei X-Q, Evans B, Jiang W, Aeschlimann D. IL-23 promotes osteoclast formation by up-regulation of receptor activator of NF-kappaB (RANK) expression in myeloid precursor cells. Eur J Immunol. 2008;38:2845–54. [DOI] [PubMed] [Google Scholar]
- 64.Gabriele LG, Morandini AC, Dionísio TJ, Santos CF. Angiotensin II type 1 receptor knockdown impairs Interleukin-1β-induced cytokines in human periodontal fibroblasts. J Periodontol. 2017;88:e1-11. [DOI] [PubMed] [Google Scholar]
- 65.Okada N, Kobayashi M, Mugikura K, Okamatsu Y, Hanazawa S, Kitano S, et al. Interleukin-6 production in human fibroblasts derived from periodontal tissues is differentially regulated by cytokines and a glucocorticoid. J Periodontal Res. 1997;32:559–69. [DOI] [PubMed] [Google Scholar]
- 66.Jang H-M, Park J-Y, Lee Y-J, Kang M-J, Jo S-G, Jeong Y-J, et al. TLR2 and the NLRP3 inflammasome mediate IL-1β production in Prevotella nigrescens-infected dendritic cells. Int J Med Sci. 2021;18:432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nong D, Qin Y, Zhou H. Kang N [IL-17 regulates the expression of RANKL and OPG in human periodontal ligament fibroblasts by activating p38MAPK signaling pathway]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2019;35:545–51. [PubMed] [Google Scholar]
- 68.Cortez DM, Feldman MD, Mummidi S, Valente AJ, Steffensen B, Vincenti M, et al. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am J Physiol Heart Circ Physiol. 2007;293:H3356-3365. [DOI] [PubMed] [Google Scholar]
- 69.Bai Y, Wei Y, Wu L, Wei J, Wang X, Bai Y. C/EBP β mediates endoplasmic reticulum stress regulated inflammatory response and extracellular matrix degradation in LPS-stimulated human periodontal ligament cells. Int J Mol Sci. 2016;17:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Becerra-Ruiz JS, Guerrero-Velázquez C, Martínez-Esquivias F, Martínez-Pérez LA, Guzmán-Flores JM. Innate and adaptive immunity of periodontal disease. From etiology to alveolar bone loss. Oral Dis. 2022;28:1441–7. [DOI] [PubMed] [Google Scholar]
- 71.Cavalla F, Hernández M. Polarization profiles of T lymphocytes and macrophages responses in periodontitis. Adv Exp Med Biol. 2022;1373:195–208. [DOI] [PubMed] [Google Scholar]
- 72.Zhou X, Zhang W, Liu X, Zhang W, Li Y. Interrelationship between diabetes and periodontitis: role of hyperlipidemia. Arch Oral Biol. 2015;60:667–74. [DOI] [PubMed] [Google Scholar]
- 73.Iacopino AM. Diabetic periodontitis: possible lipid-induced defect in tissue repair through alteration of macrophage phenotype and function. Oral Dis. 1995;1:214–29. [DOI] [PubMed] [Google Scholar]
- 74.Kondo T, Gleason A, Okawa H, Hokugo A, Nishimura I. Mouse gingival single-cell transcriptomic atlas: an activated fibroblast subpopulation guides oral barrier immunity in periodontitis. bioRxiv. 2023. 10.1101/2023.04.13.536751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng J, Chen S, Albiero ML, Vieira GHA, Wang J, Feng JQ, et al. Diabetes activates periodontal ligament fibroblasts via NF-κB in vivo. J Dent Res. 2018;97:580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fentoğlu Ö, Tözüm Bulut M, Doğan B, Kırzıoğlu FY, Kemer Doğan ES. Is the relationship between periodontitis and hyperlipidemia mediated by lipoprotein-associated inflammatory mediators? J Periodontal Implant Sci. 2020;50:135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hong R, Wang Z, Sui A, Liu X, Fan C, Lipkind S, et al. Gingival mesenchymal stem cells attenuate pro-inflammatory macrophages stimulated with oxidized low-density lipoprotein and modulate lipid metabolism. Arch Oral Biol. 2019;98:92–8. [DOI] [PubMed] [Google Scholar]
- 78.Jeon H, Blacklow SC. Structure and physiologic function of the low-density lipoprotein receptor. Annu Rev Biochem. 2005;74:535–62. [DOI] [PubMed] [Google Scholar]
- 79.Kattoor AJ, Kanuri SH, Mehta JL. Role of Ox-LDL and LOX-1 in atherogenesis. Curr Med Chem. 2019;26:1693–700. [DOI] [PubMed] [Google Scholar]
- 80.Ishikawa M, Ito H, Akiyoshi M, Kume N, Yoshitomi H, Mitsuoka H, et al. Lectin-like oxidized low-density lipoprotein receptor 1 signal is a potent biomarker and therapeutic target for human rheumatoid arthritis. Arthritis Rheum. 2012;64:1024–34. [DOI] [PubMed] [Google Scholar]
- 81.Mo S, Jang JS, Lee SH, Kim H-H. Single-cell transcriptome analysis reveals periodontal ligament fibroblast heterogeneity with distinct IL-1β and RANKL expression in periodontitis. Mol Cells. 2024;47:100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858–64. [DOI] [PubMed] [Google Scholar]
- 83.Ruscitti P, Cipriani P, Carubbi F, Liakouli V, Zazzeroni F, Di Benedetto P, et al. The role of IL-1β in the bone loss during rheumatic diseases. Mediators Inflamm. 2015;2015:782382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang W, Cong X-L, Qin Y-H, He Z-W, He D-Y, Dai S-M. IL-18 upregulates the production of key regulators of osteoclastogenesis from fibroblast-like synoviocytes in rheumatoid arthritis. Inflammation. 2013;36:103–9. [DOI] [PubMed] [Google Scholar]
- 85.Kaplanski G. Interleukin-18: biological properties and role in disease pathogenesis. Immunol Rev. 2018;281:138–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kitaura H, Marahleh A, Ohori F, Noguchi T, Shen W-R, Qi J, et al. Osteocyte-related cytokines regulate osteoclast formation and bone resorption. Int J Mol Sci. 2020;21:5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abu-Amer Y. NF-κB signaling and bone resorption. Osteoporos Int. 2013;24:2377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu L, Guo Q, Yang J, Ni B. Tumor necrosis factor alpha promotes osteoclast formation via PI3K/Akt pathway-mediated Blimp1 expression upregulation. J Cell Biochem. 2017;118:1308–15. [DOI] [PubMed] [Google Scholar]
- 89.Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, et al. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016;2016:4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. [DOI] [PubMed] [Google Scholar]
- 91.Amarasekara DS, Yun H, Kim S, Lee N, Kim H, Rho J. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 2018;18: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Agidigbi TS, Kim C. Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ROS-mediated osteoclast diseases. Int J Mol Sci. 2019;20:3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanpaolo ER, Rotondo C, Cici D, Corrado A, Cantatore FP. JAK/STAT pathway and molecular mechanism in bone remodeling. Mol Biol Rep. 2020;47:9087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018;27:1984–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheon H, Rho YH, Choi SJ, Lee YH, Song GG, Sohn J, et al. Prostaglandin E2 augments IL-10 signaling and function. J Immunol. 2006;177:1092–100. [DOI] [PubMed] [Google Scholar]
- 96.Kodama J, Kaito T. Osteoclast multinucleation: review of current literature. Int J Mol Sci. 2020;21:5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Diekwisch TG. The developmental biology of cementum. Int J Dev Biol. 2001;45:695–706. [PubMed] [Google Scholar]
- 98.Feller L, Khammissa RAG, Thomadakis G, Fourie J, Lemmer J. Apical external root resorption and repair in orthodontic tooth movement: biological events. Biomed Res Int. 2016;2016:4864195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mulholland BS, Forwood MR, Morrison NA. Monocyte chemoattractant protein-1 (MCP-1/CCL2) drives activation of bone remodelling and skeletal metastasis. Curr Osteoporos Rep. 2019;17:538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huynh NC-N, Everts V, Pavasant P, Ampornaramveth RS. Interleukin-1β induces human cementoblasts to support osteoclastogenesis. Int J Oral Sci. 2017;9: e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Y, Li Y, Shao P, Wang L, Bao X, Hu M. IL1β inhibits differentiation of cementoblasts via microRNA-325-3p. J Cell Biochem. 2020;121:2606–17. [DOI] [PubMed] [Google Scholar]
- 102.Wang YL, He H, Liu ZJ, Cao ZG, Wang XY, Yang K, et al. Effects of TNF-α on cementoblast differentiation, mineralization, and apoptosis. J Dent Res. 2015;94:1225–32. [DOI] [PubMed] [Google Scholar]
- 103.Wang L, Wang Y, Du M, Liu Z, Cao Z, Hao Y, et al. Inhibition of Stat3 signaling pathway decreases TNF-α-induced autophagy in cementoblasts. Cell Tissue Res. 2018;374:567–75. [DOI] [PubMed] [Google Scholar]
- 104.Wang Y, He H, Cao Z, Fang Y, Du M, Liu Z. Regulatory effects of bone morphogenetic protein-4 on tumour necrosis factor-α-suppressed Runx2 and osteoprotegerin expression in cementoblasts. Cell Prolif. 2017;50: e12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Surlin P, Rauten A-M, Mateescu G-O, Oprea B, Mariş M, Manolea H. The involvement of metalloproteinases and their tissular inhibitors in the processes of periodontal orthodontic remodeling. Rom J Morphol Embryol. 2009;50:181–4. [PubMed] [Google Scholar]
- 106.Reynolds JJ, Meikle MC. Mechanisms of connective tissue matrix destruction in periodontitis. Periodontol. 2000;1997(14):144–57. [DOI] [PubMed] [Google Scholar]
- 107.Franco C, Patricia H-R, Timo S, Claudia B, Marcela H. Matrix metalloproteinases as regulators of periodontal inflammation. Int J Mol Sci. 2017;18:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, Lee H-M, et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006;38:306–21. [DOI] [PubMed] [Google Scholar]
- 109.Hannas AR, Pereira JC, Granjeiro JM, Tjäderhane L. The role of matrix metalloproteinases in the oral environment. Acta Odontol Scand. 2007;65:1–13. [DOI] [PubMed] [Google Scholar]
- 110.Beklen A, Tüter G, Sorsa T, Hanemaaijer R, Virtanen I, Tervahartiala T, et al. Gingival tissue and crevicular fluid co-operation in adult periodontitis. J Dent Res. 2006;85:59–63. [DOI] [PubMed] [Google Scholar]
- 111.Reynolds JJ. Collagenases and tissue inhibitors of metalloproteinases: a functional balance in tissue degradation. Oral Dis. 1996;2:70–6. [DOI] [PubMed] [Google Scholar]
- 112.Luchian I, Goriuc A, Sandu D, Covasa M. The Role of Matrix metalloproteinases (MMP-8, MMP-9, MMP-13) in periodontal and peri-implant pathological processes. Int J Mol Sci. 2022;23:1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Berkovitz BK. Periodontal ligament: structural and clinical correlates. Dent Update. 2004;31:46–50. [DOI] [PubMed] [Google Scholar]
- 114.Xiong J, Gronthos S, Bartold PM. Role of the epithelial cell rests of Malassez in the development, maintenance and regeneration of periodontal ligament tissues. Periodontol. 2000;2013(63):217–33. [DOI] [PubMed] [Google Scholar]
- 115.Ohshima M, Ogoshi T, Ogawa H, Muto A, Suzuki K, Otsuka K. Effect of dental cyst epithelium-conditioned medium on collagenase production by periodontal ligament fibroblasts. J Nihon Univ Sch Dent. 1997;39:31–3. [DOI] [PubMed] [Google Scholar]
- 116.Jurdziński KT, Potempa J, Grabiec AM. Epigenetic regulation of inflammation in periodontitis: cellular mechanisms and therapeutic potential. Clin Epigenetics. 2020;12:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Francis M, Pandya M, Gopinathan G, Lyu H, Ma W, Foyle D, et al. Histone methylation mechanisms modulate the inflammatory response of periodontal ligament progenitors. Stem Cells Dev. 2019;28:1015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wei Y, Peng Z. Hsa_circ_0099630 knockdown induces the proliferation and osteogenic differentiation and attenuates the apoptosis of porphyromonas gingivalis lipopolysaccharide–induced human periodontal ligament fibroblasts. Ann Transl Med. 2022;10:993–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang Y, Li X. Lipopolysaccharide-regulated production of bone sialoprotein and interleukin-8 in human periodontal ligament fibroblasts: the role of toll-like receptors 2 and 4 and the MAPK pathway. J Periodontal Res. 2015;50:141–51. [DOI] [PubMed] [Google Scholar]
- 120.Xu MX, Sun XX, Li W, et al. LPS at low concentration promotes the fracture healing through regulating the autophagy of osteoblasts via NF-κB signal pathway. Eur Rev Med Pharmacol Sci. 2018;22(6):1569–79. [DOI] [PubMed] [Google Scholar]
- 121.Costa-Mattioli M, Walter P. The integrated stress response: from mechanism to disease. Science. 2020;368:eaat5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu J, Ruan J, Weir MD, Ren K, Schneider A, Wang P, et al. Periodontal bone-ligament-cementum regeneration via scaffolds and stem cells. Cells. 2019;8:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sato A, Suzuki S, Yuan H, Fahreza RR, Wang X, Nemoto E, et al. Pharmacological activation of YAP/TAZ by Targeting LATS1/2 enhances periodontal tissue regeneration in a murine model. Int J Mol Sci. 2023;24:970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kobayashi T, Ito S, Kobayashi D, Kojima A, Shimada A, Narita I, et al. Interleukin-6 receptor inhibitor tocilizumab ameliorates periodontal inflammation in patients with rheumatoid arthritis and periodontitis as well as tumor necrosis factor inhibitors. Clin Exp Dent Res. 2015;1:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kobayashi T, Okada M, Ito S, Kobayashi D, Ishida K, Kojima A, et al. Assessment of interleukin-6 receptor inhibition therapy on periodontal condition in patients with rheumatoid arthritis and chronic periodontitis. J Periodontol. 2014;85:57–67. [DOI] [PubMed] [Google Scholar]
- 126.Zhan D, Guo L, Zheng L. Inhibition of the receptor for advanced glycation promotes proliferation and repair of human periodontal ligament fibroblasts in response to high glucose via the NF-κB signaling pathway. Arch Oral Biol. 2018;87:86–93. [DOI] [PubMed] [Google Scholar]
- 127.Huang X, Zhu L, Gong Y. Rhein induces bone regeneration via alleviating inflammation in murine periodontitis model. Oral Dis. 2024;30(3):1506–15. [DOI] [PubMed] [Google Scholar]
- 128.Li X, Zhou R, Han Y, Zeng J, Shi L, Mao Y, et al. Silibinin attenuates experimental periodontitis by downregulation of inflammation and oxidative stress. Oxid Med Cell Longev. 2023;2023:5617800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ambili R, Janam P, Saneesh Babu PS, Prasad M, Vinod D, Anil Kumar PR, et al. An ex vivo evaluation of the efficacy of and rographolide in modulating differential expression of transcription factors and target genes in periodontal cells and its potential role in treating periodontal diseases. J Ethnopharmacol. 2017;196:160–7. [DOI] [PubMed] [Google Scholar]
- 130.Pouroutzidou GK, Lazaridou M, Papoulia C, Tsamesidis I, Chrissafis K, Vourlias G, et al. Electrospun PLGA membranes with incorporated moxifloxacin-loaded silica-based mesoporous nanocarriers for periodontal regeneration. Nanomaterials. 2022;12:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Madhumathi K, Sampath Kumar TS. Regenerative potential and anti-bacterial activity of tetracycline loaded apatitic nanocarriers for the treatment of periodontitis. Biomed Mater. 2014;9:035002. [DOI] [PubMed] [Google Scholar]
- 132.Cafferata EA, Alvarez C, Diaz KT, Maureira M, Monasterio G, González FE, et al. Multifunctional nanocarriers for the treatment of periodontitis: Immunomodulatory, antimicrobial, and regenerative strategies. Oral Dis. 2019;25:1866–78. [DOI] [PubMed] [Google Scholar]
- 133.Kim T-I, Han J-E, Jung H-M, Oh J-H, Woo KM. Analysis of histone deacetylase inhibitor-induced responses in human periodontal ligament fibroblasts. Biotechnol Lett. 2013;35:129–33. [DOI] [PubMed] [Google Scholar]
- 134.Ciechomska M, Roszkowski L, Maslinski W. DNA methylation as a future therapeutic and diagnostic target in rheumatoid arthritis. Cells. 2019;8:953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Alliot-Licht B, De Lange GL, Gregoire M. Effects of hydroxyapatite particles on periodontal ligament fibroblast-like cell behavior. J Periodontol. 1997;68:158–65. [DOI] [PubMed] [Google Scholar]
- 136.Xu Y, Zheng B, He J, Cui Z, Liu Y. Silver nanoparticles promote osteogenic differentiation of human periodontal ligament fibroblasts by regulating the RhoA-TAZ axis. Cell Biol Int. 2019;43:910–20. [DOI] [PubMed] [Google Scholar]
- 137.Wang W, Song Y, Tian Y, Chen B, Liang Y, Liang Y, et al. TCPP/MgO-loaded PLGA microspheres combining photodynamic antibacterial therapy with PBM-assisted fibroblast activation to treat periodontitis. Biomater Sci. 2023;11:2828–44. [DOI] [PubMed] [Google Scholar]
- 138.Bhattarai G, Kook S-H, Kim J-H, Poudel SB, Lim S-S, Seo Y-K, et al. COMP-Ang1 prevents periodontitic damages and enhances mandible bone growth in an experimental animal model. Bone. 2016;92:168–79. [DOI] [PubMed] [Google Scholar]
- 139.Srinivasan S, Jayasree R, Chennazhi KP, Nair SV, Jayakumar R. Biocompatible alginate/nano bioactive glass ceramic composite scaffolds for periodontal tissue regeneration. Carbohydr Polym. 2012;87:274–83. [DOI] [PubMed] [Google Scholar]
- 140.Sowmya S, Mony U, Jayachandran P, Reshma S, Kumar RA, Arzate H, et al. Tri-layered nanocomposite hydrogel scaffold for the concurrent regeneration of cementum, periodontal ligament, and alveolar bone. Adv Healthc Mater. 2017. 10.1002/adhm.201601251. [DOI] [PubMed] [Google Scholar]
- 141.Wei Y, Ye Q, Tang Z, Tian G, Zhu Q, Gao H, et al. Calcitonin induces collagen synthesis and osteoblastic differentiation in human periodontal ligament fibroblasts. Arch Oral Biol. 2017;74:114–22. [DOI] [PubMed] [Google Scholar]
- 142.Xu C, Du Y, Tian J, Liu C, Huang Y, Zhou T, et al. Pigment epithelium-derived factor modulates periodontal homeostasis in mice and induces osteogenic differentiation of human periodontal ligament fibroblasts. Connect Tissue Res. 2022;63:485–97. [DOI] [PubMed] [Google Scholar]
- 143.Liu H-J, Liu X-Y, Jing D-B. Icariin induces the growth, migration and osteoblastic differentiation of human periodontal ligament fibroblasts by inhibiting Toll-like receptor 4 and NF-κB p65 phosphorylation. Mol Med Rep. 2018;18:3325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang J, Liu L, Jin S, Zhang Y, Zhang L, Li S, et al. Proanthocyanidins promote osteogenic differentiation of human periodontal ligament fibroblasts in inflammatory environment via suppressing NF-κB signal pathway. Inflammation. 2020;43:892–902. [DOI] [PubMed] [Google Scholar]
- 145.Xiao Q, Li X, Li Y, Wu Z, Xu C, Chen Z, et al. Biological drug and drug delivery-mediated immunotherapy. Acta Pharm Sin B. 2021;11:941–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guo B-J, Bian Z-X, Qiu H-C, Wang Y-T, Wang Y. Biological and clinical implications of herbal medicine and natural products for the treatment of inflammatory bowel disease. Ann N Y Acad Sci. 2017;1401:37–48. [DOI] [PubMed] [Google Scholar]
- 147.Wang Y, Chen S, Du K, Liang C, Wang S, Owusu Boadi E, et al. Traditional herbal medicine: therapeutic potential in rheumatoid arthritis. J Ethnopharmacol. 2021;279:114368. [DOI] [PubMed] [Google Scholar]
- 148.Häkkinen L, Larjava H, Fournier BPJ. Distinct phenotype and therapeutic potential of gingival fibroblasts. Cytotherapy. 2014;16:1171–86. [DOI] [PubMed] [Google Scholar]
- 149.Irwin CR, Picardo M, Ellis I, Sloan P, Grey A, McGurk M, et al. Inter- and intra-site heterogeneity in the expression of fetal-like phenotypic characteristics by gingival fibroblasts: potential significance for wound healing. J Cell Sci. 1994;107(Pt 5):1333–46. [DOI] [PubMed] [Google Scholar]
- 150.Fujita T, Iwata T, Shiba H, Igarashi A, Hirata R, Takeda K, et al. Identification of marker genes distinguishing human periodontal ligament cells from human mesenchymal stem cells and human gingival fibroblasts. J Periodontal Res. 2007;42:283–6. [DOI] [PubMed] [Google Scholar]
- 151.Niemiec BA. Periodontal disease. Top Companion Anim Med. 2008;23:72–80. [DOI] [PubMed] [Google Scholar]
- 152.Yuan L, Zhao Y, Zhou W. Expression of IL-6 and it’s mRNA transcription in the process of rat periodontium remodeling influenced by various bite forces. Zhonghua Kou Qiang Yi Xue Za Zhi. 2002;37:54–7. [PubMed] [Google Scholar]
- 153.Yamashiro K, Myokai F, Hiratsuka K, Yamamoto T, Senoo K, Arai H, et al. Oligonucleotide array analysis of cyclic tension-responsive genes in human periodontal ligament fibroblasts. Int J Biochem Cell Biol. 2007;39:910–21. [DOI] [PubMed] [Google Scholar]
- 154.Chukkapalli SS, Lele TP. Periodontal cell mechanotransduction. Open Biol. 2018;8:180053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable to this review as as no datasets were generated or analyzed in this review.