Abstract
The identification of ferroptosis represents a pivotal advancement in the field of cell death research, revealing an entirely novel mechanism of cellular demise and offering new insights into the initiation, progression, and therapeutic management of various diseases. Ferroptosis is predominantly induced by intracellular iron accumulation, lipid peroxidation, or impairments in the antioxidant defense system, culminating in membrane rupture and consequent cell death. Studies have associated ferroptosis with a wide range of diseases, and by enhancing our comprehension of its underlying mechanisms, we can formulate innovative therapeutic strategies, thereby providing renewed hope for patients.
Keywords: Ferroptosis, Cardiovascular diseases, Neurodegenerative diseases, Cancer, Immunotherapy
Introduction
In 2012, the concept of ferroptosis—a unique kind of programmed cell death—was put forth. It is characterized by aberrant iron metabolism, excessive accumulation of reactive oxygen species (ROS), and lipid peroxides that are dependent on iron [1]. Ferroptosis has recently gained attention in biomedical research, and emergent research elucidates the pivotal role ferroptosis assumes in myriad physiological and pathological episodes, including cardiovascular diseases (CVDs), neurodegenerative diseases (NDs) and cancer. In these three disease types, oxidative stress and inflammation are critical factors that can lead to ferroptosis, which may interact with and exacerbate disease progression. Particularly during cancer treatment, chemotherapy and radiation can contribute to CVDs and NDs. Thus, ferroptosis could be a shared pathological mechanism among these conditions, and targeting it may present a new therapeutic approach [2, 3]. Inhibiting iron ion aggregation, using antioxidants, and ferritin autophagy are the main goals of current treatment approaches to ferroptosis [4, 5]. For clinical use, further research and inquiry are still required, despite certain studies demonstrating the promise of various treatment approaches.
Although research on ferroptosis in CVDs is still in its infancy, there is mounting evidence that it plays a part in their development and progression. At present, the intricate relationship between ferroptosis and CVDs is mainly focused on cardiac ischemia–reperfusion, atherosclerosis, and heart failure [6, 7]. Myocardial cells are harmed by hypoxia and ischemia during myocardial infarction, which causes an imbalance in iron metabolism that ultimately results in ferroptosis [8]. Aggregation of iron ions and oxidative stress may have aided in the death and destruction of endothelial cells in atherosclerotic lesions [9]. Cardiomyocyte damage and death accompany the beginning and progression of heart failure, a complex clinical condition [10]. Thus, targeted ferroptosis becomes a hotspot in the treatment of cardiovascular disease.
Intense research focus also surrounds the role of ferroptosis in NDs. These conditions involve the progressive deterioration of neurons and their myelin sheath, exemplified by Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), among others [11]. Ferroptosis is believed to significantly contribute to their advancement. For instance, AD patients often exhibit increased iron accumulation and reduced ferritin levels in brain regions like the hippocampus and cortex. Treatment with the iron chelator deferriamine has shown potential in mitigating cognitive decline [12]. Similarly, animal models and HD patients have shown signs of lipid peroxidation, iron accumulation, and decreased glutathione (GSH) levels [13]. The regulatory mechanisms of ferroptosis are closely intertwined with treatment and intervention strategies for NDs. Thus, modulating ferroptosis may offer novel approaches for treating these diseases.
Ferroptosis has emerged as a new area of focus for cancer research, and it is anticipated that it will offer fresh approaches and methods for cancer treatment. The metabolism of tumor cells has a direct relationship to ferroptosis. To support their growth and proliferation, tumor cells need a lot of energy and material, so they must use minerals like iron efficiently. Due to the accumulation of iron ions and oxidative stress brought on by excessive iron use, this need may result in ferroptosis [14]. A potential method of treating tumors that are drug-resistant and recurrent may involve ferroptosis. Inducing ferroptosis can successfully destroy tumor cells that are resistant to traditional therapies because they may be extremely sensitive to the condition [15, 16]. At the same time, the exploration of ferroptosis in tumor immunotherapy is also increasing.
In conclusion, ferroptosis has a significant impact on both physiological and pathological processes of organisms and is strongly linked to the onset and progression of numerous diseases, such as CVDs, NDs and cancer. To give a more informed and useful tool for the prevention and treatment of linked diseases, this article delves into an in-depth examination of the regulatory processes underlying ferroptosis and its intricate links to various pathological conditions.
Ferroptosis
The view of ferroptosis
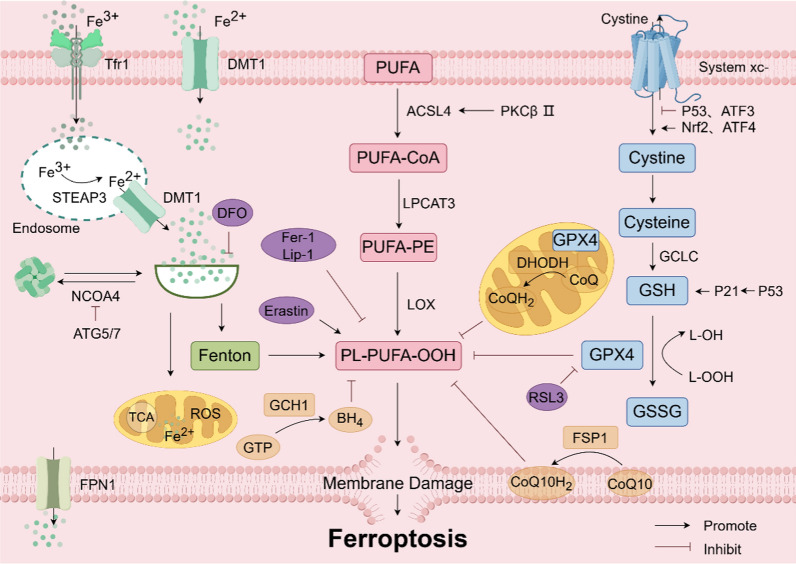
In the panorama of programmed cell death modalities, ferroptosis emerges as a unique entity, differentiated from apoptosis, necrosis, and pyroptosis [17, 18]. Its cardinal mechanism rests upon the catalytic role of ferrous ions or lipoxygenase, instigating a cascade of lipid peroxidation on the cellular membrane, which teems with unsaturated fatty acids, resulting in membrane rupture and subsequent cell death. In addition, the reduction of glutathione peroxidase 4 (GPX4), a key enzyme in the antioxidant system (glutathione system) is also linked to ferroptosis [19] (Fig. 1). Morphologically, ferroptosis results in smaller mitochondria, heightened membrane density, decreased cristae, and minimal changes in nuclear structure. In cellular components, ferroptosis is manifested by increased lipid peroxidation and elevated ROS [20]. Ferroptosis is significantly different from necrosis, pyroptosis and autophagy in cell morphology and function (Table 1).
Fig. 1.
Molecular mechanism of ferroptosis. Ferroptosis primarily occurs due to iron overload within cells, lipid peroxidation, or disruptions in the antioxidant system, leading to membrane rupture and subsequent cell death. ACSL4 acyl-CoA synthetase long chain family member 4, ATF3/4 activating transcription factor ¾, ATG5/7 autophagy related 5/7, BH4 tetrahydrobiopterin, CoQ coenzyme Q, CoQ10 coenzyme Q10, DFO Deferoxamine, DHODH dihydroorotate dehydrogenase, DMT1 divalent metal transporter-1, Fer-1 ferrostatin-1, FPN1 ferroportin1, FSP1 ferroptosis suppressor protein 1, FTH ferritin heavy chain, GCH1 GTP cyclohydrolase 1, GCLC glutamate cysteine ligase, GPX4 glutathione peroxidase 4, GSH glutathione, GSSG glutathione (Oxidized), GTP guanosine triphosphate, Lip-1 liproxstatin-1, LOX lipoxygenase, LPCAT3 lysophosphatidylcholine acyltransferase 3, NCOA4 nuclear receptor coactivator 4, Nrf2 nuclearrespiratoty factor 2, PKCβII protein kinase CβII, PUFA polyunsaturated fatty acid, ROS reactive oxygen species, STEAP3 six-transmembrane epithelial antigen of the prostate 3, Tfr1 transferrin receptor 1
Table 1.
The difference between ferroptosis and other cell death patterns
| Ferroptosis | Autophagy | Necroptosis | Pyroptosis | |
|---|---|---|---|---|
| Biochemical Features | lron accumulation and lipid peroxidation | DNA fagmentation | Increased lysosomal actity | Drop in ATP levels |
| Key genes | GPX4, SLC7A11, | Caspase, Bcl-2, Bax | ATG5, ATG7, LC3 | Caspase-1, IL-1β, IL-18 |
| Morphological features | Mitochondrial membrane density was concentrated, mitochondrial ridge was reduced or disappeared, and mitochondrial outer membrane was ruptured | Formation of double membraned autolysosomes | Plasma membrane rupture; organelle swelling; moderate chromatin condensation | Karyopyknosis, cell edema and membrane rupture |
Molecular mechanisms of ferroptosis
Iron metabolism
Iron metabolism is the process by which organisms absorb, transport, distribute, store, utilize, transform, and excrete iron after absorption. Iron ions typically enter cells bound to transferrin as trivalent iron, are reduced to divalent iron by metal reductases, and then form various iron-containing complexes, exerting various physiological functions [21]. When iron-binding complexes become saturated, excess divalent iron accumulates in cells, forming unstable iron pools, and excess iron ions are stored in the heavy and light chains of ferritin [22]. Fe2+ can enter the cytoplasm via bivalent metal ion transporter-1 (DMT1) or ZRT-IRT-like proteins. After that, part of the cytoplasm Fe2+ binds to ferritin heavy chain 1 (FTH1) and is oxidized to Fe3+, which binds to ferritin light chain (FTL) to form ferritin complex and is stored in the cell. At the same time, the remaining Fe2+ forms free iron pools in the cytoplasm. On the one hand, Fe2+ in the free iron pool binds to poly-binding proteins [23, 24]. On the other hand, Fe2+ can bind to L-Cys residues of GSH to ensure the stability of Fe2+. Additional Fe2+ can be transported out of the cell via the ferroportin 1 (FPN1) and continues to participate in blood transport. Iron overload due to abnormal iron metabolism is a hallmark of ferroptosis. Iron overload is characterized by increased TF saturation and the formation of non-TF-bound iron (NTBI). NTBI is a potential iron that is a direct result of oxidative stress and tissue iron loading. The most common reason for the presence of NTBI is high TF saturation. However, the existence of NTBI cannot be considered a simple TF supersaturation phenomenon; Rather, it is the expression of a kinetic balance between iron excretion in serum, binding to TF, removal from circulation, and utilization in circulation [25]. In addition, some calcium channels may also be involved in intracellular Fe2+ transport. For example, L-type calcium channel blockers significantly reduce the uptake of NTBI by cardiomyocytes. In contrast, the mechanism of iron uptake by thalassemia cardiomyocytes is mainly mediated by T-type calcium channels. The application of corresponding inhibitor channel inhibitors can significantly improve cardiac iron deposition and improve cardiac function [26, 27]. Fang et al. discovered that a high-iron diet can cause cardiac damage and hypertrophic cardiomyopathy in mice, displaying typical molecular features of ferroptosis [28]. Excessive ferrous ions can generate a large amount of ROS through the Fenton reaction, disrupting the balance of redox reactions within cells, causing damage to lipids and proteins in cells, and triggering ferroptosis. FPN1 is the only protein capable of transporting iron ions out of cells, which can inhibit the occurrence of the Fenton reaction within cells, reduce cellular oxidative stress levels, and ultimately inhibit ferroptosis. Knockdown or inhibition of ferritin can promote ferroptosis [29]. Nuclear receptor coactivator 4 (NCOA4) facilitates the transport of ferritin to lysosomes for degradation, raising cellular Fe2+ levels and triggering ferroptosis. On the other hand, autophagy-related proteins 5 and 7 (ATG5/7) can inhibit NCOA4, thereby preventing this process. Additionally, iron chelation has been demonstrated to block erastin-induced cell death [30]. Therefore, the iron ion homeostasis within cells is crucial for regulating ferroptosis.
Lipid peroxidation
Lipid peroxidation refers to the reaction where the side chains of phospholipids, membrane receptors, enzyme-associated polyunsaturated fatty acids (PUFAs), and nucleic acids, among other macromolecules, undergo peroxidation reactions with ROS [31]. PUFA-PEs are synthesized from PUFAs on cell membranes and are mainly composed of arachidonic acid and adrenal acid [32]. The C-H bonds of PUFAs are more susceptible to erosion by a large number of oxygen free radicals, leading to the appearance of numerous hydroxyl groups on their surface, thereby forming a peroxidation state. Free PUFAs do not initiate ferroptosis, only peroxidized PUFAs incorporated into lipids like phospholipids can activate ferroptosis [28]. Research has demonstrated that acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) are vital for the cell membrane, facilitating the production of PUFA-PE. PUFA-PE is highly susceptible to oxidation induced by lipoxygenase (LOX), thereby inducing ferroptosis. ACSL4 and LOX are key to regulating lipid peroxidation in this process. For example, one study found that the ACSL4 gene was expressed at very high levels in breast cancer cells, and knocking out the ACSL4 gene reduced the production of PUFA-PEs (ferroptosis inhibitors), thereby inhibiting RSL3-induced iron fall, and other studies have shown that Ablation and chemical inhibition of the ACSL4 gene increased resistance to RSL3-induced iron sag, while inhibition of 5-LOX and 15-LOX further inhibited RSL3-induced cell death [33, 34]. Furthermore, protein kinase CβII (PKCβII) can directly enhance the phosphorylation of ACSL4 at the Thr32 site, activating ACSL4, increasing lipid peroxidation, and thus inducing ferroptosis [35]. Therefore, suppressing the expression of ACSL4 and LPCAT3 can effectively prevent the excessive buildup of lipid peroxides in cells, thereby inhibiting ferroptosis.
Antioxidant system
GPX4/GSH/ solute carrier family 7 member 11 (SLC7A11) constitutes a crucial antioxidant system in the human body. Disruption of this system accelerates lipid peroxide accumulation and increases ROS levels within cells, thereby inducing ferroptosis [36]. GPX4, a selenium protein, not only effectively reduces peroxides but also inhibits the activation of phospholipid peroxidation enzymes during the process of arachidonic acid metabolism, thereby suppressing ferroptosis [37]. GSH, acting as an essential cofactor for GPX4, facilitates the conversion of lipid peroxides into alcohols, efficiently preventing lipid peroxide buildup and ultimately inhibiting ferroptosis [38]. SLC7A11 is the main subunit of system Xc-, which is a cystine transporter protein facilitating the exchange of cystine and glutamate within cells, thereby promoting GSH synthesis and inhibiting ferroptosis. Nuclear respiratory factor 2 (Nrf2), as one of the transcription factors regulating ferroptosis, under oxidative stress, binds to the antioxidant reaction element in the target gene promoter region, promoting SLC7A11 transcription, accelerating GSH synthesis, and inhibiting ferroptosis [39]. Additionally, P53 increases intracellular levels of GSH and GPX4 by directly targeting GSH through regulating P21. P53 also decreases the expression of system Xc- by reducing the transcription level of SLC7A1 [30]. Additionally, some transcription factors also regulate ferroptosis through the GPX4 / GSH / SLC7A11 pathway. Activating transcription factor 3 (ATF3) represses the transcription of SLC7A11, leading to decreased GSH synthesis and ultimately facilitating ferroptosis. Conversely, activating transcription factor 4 (ATF4) stimulates the expression of SLC7A11, enhancing GSH synthesis and thus inhibiting ferroptosis [40]. The above studies affirm the pivotal role of the GPX4/GSH/SLC7A11 antioxidant system in suppressing ferroptosis through the regulation of pertinent proteins and transcription factors.
Other
Besides the impact of iron metabolism, lipid peroxidation, and the GPX4 / GSH / SLC7A11 system on ferroptosis, other factors like ferroptosis suppressor protein 1 (FSP1)/coenzyme Q10 (CoQ10), dihydroorotate dehydrogenase (DHODH), GTP cyclohydrolase 1 (GCH1)/tetrahydrobiopterin (BH4), among others, also demonstrate antioxidant effects independently of GPX4 regulation [41]. CoQ10 is an endogenous lipid-soluble antioxidant that effectively combats the generation of lipid peroxides. When FSP1 is post-translationally modified by geranylgeranylation, it can promote the re-expression of reducible CoQ10, thereby inhibiting ferroptosis [42]. DHODH, situated in the inner mitochondrial membrane, can convert coenzyme Q (CoQ) into its reduced state, thus exhibiting antioxidative properties that culminate in the suppression of ferroptosis. Furthermore, BH4, functioning akin to CoQ10 as a scavenger of free radicals, and GCH1, a pivotal enzyme controlling the synthesis of BH4, not only modulate BH4 production but also enhance CoQ10 synthesis by regulating tyrosine production. This dual action impedes the buildup of lipid peroxides, thereby ultimately thwarting ferroptosis [43].
Ferroptosis is a meticulously controlled mechanism that encompasses multiple organelles and intricate signaling pathways, such as mitochondria, lysosomes, endoplasmic reticulum, and the Golgi apparatus. Among them, mitochondria and lysosomes are closely associated with ferroptosis. In most mammalian cells, mitochondria are significant sources of ROS, and several mitochondrial antioxidants play crucial roles in inhibiting ferroptosis. GPX4 can localize between the cytoplasm and mitochondrial intermembrane space, playing a role in alleviating lipid peroxidation during ferroptosis [44]. Lysosomes participate in selective autophagy pathways (including ferritinophagy, chaperone-mediated autophagy, clock-mediated phagocytosis, and lipophagy), where lysosomes fuse with autophagosomes to promote ferroptosis by degrading various substrates (including ferritin, SLC40A1, GPX4, ARNTL, and lipid droplets) [45]. When subjected to specific stimuli, endoplasmic reticulum stress initiates the unfolded protein response, aiming to rectify protein equilibrium. However, if the cell cannot restore this equilibrium, it may also instigate ferroptosis. Endoplasmic reticulum stress inducers like AMF-26 and M-COPA can induce ferroptosis, but compared to other organelles, research on the association between the Golgi apparatus and ferroptosis remains limited [46].
CVDs
Ferroptosis in heart
Disturbances in iron metabolism stand as a significant contributor to cardiac ferroptosis. Elevated iron levels within cardiac cells precipitate lipid peroxide buildup, culminating in cell demise. Furthermore, pivotal antioxidant systems like GPX4 and GSH within cardiac cells regulate ferroptosis. Reduced or exhausted antioxidant defenses make heart cells more vulnerable to ferroptosis [47]. Recent studies highlight that ferroptosis occurs in several heart conditions, including myocardial ischemia–reperfusion injury and doxorubicin(DOX)-induced cardiomyopathy [48] (Fig. 2). Moreover, specific drugs or gene modifications have demonstrated efficacy in inhibiting ferroptosis in the heart, thereby ameliorating cardiac damage and enhancing function [49]. Investigations into the subcellular localization of ferroptosis in the heart reveal that in DOX-induced cardiac injury models, iron accumulation and lipid peroxidation predominantly occur within cardiomyocyte mitochondria rather than in the cytoplasm [50]. This suggests mitochondrial impairment is a crucial trigger for cardiac ferroptosis. Given the pivotal role of mitochondria in cardiac function, mitochondrial oxidative phosphorylation defects disrupt cellular oxidation–reduction reaction balance, exacerbating ROS production and activating numerous pro-inflammatory genes and transcription factors like NF-κB, p53, HIF-1α, PPAR-γ, β-catenin/Wnt, and Nrf2. This cascade of events leads to inflammation and contributes to various cardiovascular disease subtypes [50–52]. Studies suggest that mitigating iron ion accumulation or employing antioxidants can reduce ferroptosis incidence, thereby shielding the heart from damage [53], and more and more cardiovascular disease-related drugs targeting ferroptosis have been clinically applied (Table 2).
Fig. 2.
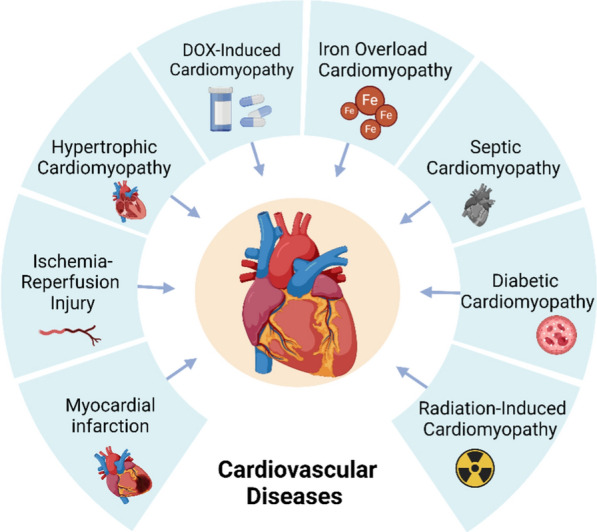
Cardiovascular disease due to ferroptosis
Table 2.
Potential drugs and mechanisms of ferroptosis-targeted for CVDs
| Reagents | Key mechanisms | References |
|---|---|---|
| DFO | Reduce iron overload, inhibit fenton reaction | [118] |
| DXZ | Reduce iron overload、prevents lipid peroxidation | [119] |
| Ferrostatin-1 | Inhibit the iron accumulation, lipid peroxidation and increase the expressions of SLC7A11 and GPX4 | [120] |
| Liproxstatin-1 | Reduce lipid ROS, activate Nrf2 pathway and increase GPX4 levels | [121] |
| Mito TEMPO | Suppress lipid peroxidation | [122] |
| N-acetyl-l-cysteine | Scavenges cellular ROS | [123] |
| Puerarin | Block iron overload and lipid peroxidation | [124] |
| Rapamycin | Target mTOR, activated autophagy | [125] |
| Vitamin E | Reduce lipid ROS, maintain cellular redox homeostasis | [126] |
| XJB-5–131, JP4-039 | Targeted mitochondrial clearance of ROS | [127] |
| Zileuton | Inhibit LOX and maintain cellular redox homeostasis | [128] |
Ferroptosis and CVDs
Myocardial infarction (MI)
MI is myocardial ischemia caused by coronary artery narrowing or obstruction, leading to myocardial cell death. The injured heart tissue is substituted with fibrotic scars. When the fibrotic scar tissue cannot compensate for contractile function, it leads to heart failure [54, 55]. Research indicates that during myocardial infarction, mitochondria in myocardial cells are damaged and dysfunctional, with abnormal accumulation of iron ions and imbalance in redox reactions, further exacerbating myocardial cell death [56]. Quantitative proteomic analysis indicates a significant downregulation of the glutathione metabolism and ROS pathways in the early and middle stages of myocardial infarction, along with a reduction in GPX4, confirming the presence of ferroptosis during this condition [57, 58]. Another study suggests that a high-iron diet induces ferroptosis, leading to severe cardiac damage, hypertrophic cardiomyopathy, and eventual heart failure [59]. Thus, preventing ferroptosis could emerge as a novel approach to the prevention and treatment of myocardial infarction. The mechanism target of rapamycin (mTOR) inhibits ferroptosis and improves left ventricular remodeling by reducing ROS production, indicating that mTOR may be an effective therapeutic target for myocardial infarction by specifically managing iron homeostasis [60]. After heart ischemia–reperfusion(I/R) in adult mice, iron accumulates in cardiomyocytes surrounding myocardium scars. Excess iron leads to cardiac cell death, which can be inhibited by inhibiting the production of lipid-derived ROS. mTOR plays an important role in protecting cardiomyocytes from ferroptosis. mTOR targets a variety of iron transporters, regulates transferrin receptor1, and increases the expression of transferrin [61]. Thus, mTOR can influence ferroptosis by controlling iron metabolism in cardiomyocytes. Research has found that exosomes derived from pericardial adipose tissue can efficiently deliver lipids to myocardial tissue. Additionally, these exosomes interact with iron regulatory protein 2, leading to an increase in ferritin levels in the infarct border zone and a decrease in transferrin receptor levels. This regulation helps maintain iron balance and protects myocardial cells from ferroptosis [62]. Furthermore, miR-23a-3p delivered by umbilical cord blood-derived mesenchymal stem cell exosomes has been shown to inhibit myocardial cell ferroptosis and can be used to mediate myocardial repair in acute myocardial infarction [63]. Some antioxidants or free radical scavengers can also inhibit ferroptosis by regulating intracellular iron ion balance, inhibiting oxidative stress responses, or intervening in ferroptosis-related signaling pathways. With further research into new types of cell death such as ferroptosis, more effective treatment methods may be developed in the future to improve the prognosis of myocardial infarction patients.
I/R
I/R is the most severe complication following acute myocardial infarction, particularly during the reperfusion phase. During this phase, a significant amount of ROS and free radicals are produced, leading to myocardial cell damage, necrosis, and ferroptosis [64]. During rat myocardial I/R injury, there's an elevation in oxidized phosphatidylcholine (OxPCs) production. Fragmented OxPCs have the potential to trigger ferroptosis [65]. In a mouse myocardial ischemia–reperfusion injury model, non-heme iron content in the myocardium increases, and markers of ferroptosis such as prostaglandin-endoperoxide synthase 2 (Ptgs2) mRNA expression are upregulated. Inhibition of ferroptosis with Ferrostatin-1 and RSL3 can alleviate ventricular remodeling and damage [66]. In myocardial ischemia–reperfusion injury, there are no notable changes in the levels of ACSL4, GPX4, iron, and malondialdehyde. However, following myocardial reperfusion, there's an increase in ACSL4, iron, and malondialdehyde levels, alongside a decrease in GPX4 levels. This suggests that ferroptosis predominantly takes place during the reperfusion phase [67]. I/R injury in the myocardium results in excessive iron accumulation caused by the engulfment of iron proteins, leading to iron leakage. Targeting iron protein engulfment with baicalin or the DNA (cytosine-5)-methyltransferase 1 inhibitor 5-aza-CdR notably mitigates myocardial damage in rats [68, 69]. Liproxstatin-1 (Lip-1) shields the myocardium from I/R injury by diminishing voltage-dependent anion channel 1 activity on mitochondria, thereby reducing ROS levels and elevating GPX4 levels. Addressing iron overload using iron chelators represents a promising approach to preventing myocardial I/R injury. Clinical evidence supports the efficacy of the iron chelator deferoxamine (DFO) in this regard [70, 71]. Pre-reperfusion infusion of DFO in primary percutaneous coronary intervention can significantly reduce oxidative stress [72]. Ferroptosis causes I/R damage by inducing ERS. ERS has an ATF4-CHOP pathway. The resulting CHOP can bind to the pro-apoptotic protein PUMA to induce the expression of PUMA and promote apoptosis [73]. An in-depth study of the mechanism revealed that ferroptosis inducers can induce an unfolded protein response, which then activates the PERK/EIF2α/ATF4/CHOP pathway, thereby triggering ERS. The specific process involves the separation of PERK from immunoglobulin-binding proteins and then phosphorylation; PERK is then activated by dimers in the cytoplasm. The α subunit activated by eIF2α can promote the translation of ATF4, and then induce the expression of downstream CHOP molecules, inducing apoptosis and resulting in cell damage [74]. Ferroptosis can activate ERS by promoting the system xc (-). ERS, as a cellular response to ER dysfunction, can be triggered by ROS [75]. Thus, ferroptosis-induced ERS can act as a bridge between ferroptosis and I/R damage. One study found that during reperfusion injury caused by heart transplantation or coronary artery occlusion, cardiomyocytes undergo ferroptosis and then release inflammatory mediators that activate Toll-like receptor 4 (TLR4) /TRI domain adapters to induce interferon (TRIF)/type I interferon (IFN) inflammatory signaling pathways. Promote the adhesion and recruitment of neutrophils and coronary endothelial cells, and aggravate heart injury. However, Fer-1, a ferroptosis inhibitor, reduces the PE level of cardiomyocytes, reduces the infarct size caused by coronary artery ligation, improves left ventricular systolic function, and reduces left ventricular remodeling [76]. Taken together, the above studies provide evidence that ferroptosis plays an important role in I/R injury.
Hypertrophic cardiomyopathy (HCM)
HCM stands as the prevalent primary myocardial condition, distinguished by left ventricular hypertrophy. It represents the leading cause of sudden cardiac death among young adults and athletes [77]. Research has revealed that blocking ferroptosis in a mouse model of hypertrophic cardiomyopathy can shield mice from left ventricular hypertrophy, cardiac damage, and myocardial cell demise. This indicates the potential critical involvement of ferroptosis in the development of HCM [47, 78]. Ferroptosis mediated by Slc7a11 directly promotes the development of HCM in mice with FTH1 knockout, while promoting the expression of x-CT can prevent angiotensin II-induced HCM in a mouse model by inhibiting ferroptosis, providing a new avenue for treating HCM [59, 79]. It's noteworthy that Friedreich's Ataxia (FRDA), a form of HCM, stems from mutations in the FXN gene and is linked to ferroptosis. Botticelli et al. discovered that the ferroptosis inhibitor SRS11-92 can diminish cell mortality in primary fibroblasts from FRDA patients and in mouse fibroblasts carrying FRDA-related mutations [80, 81]. Ferroptosis assumes a pivotal role in DCM via modulation of the Nrf2/HO-1 pathway. Stimulating and enhancing Nrf2 activity leads to elevated levels of GPX4 and HO-1 expression, thereby mitigating DCM symptoms [82].
DOX-induced cardiomyopathy
DOX is renowned as one of the most efficacious chemotherapy agents for combatting a range of cancer types. Nevertheless, its profound cardiotoxic effects, including DICM and congestive heart failure, substantially curtail its clinical usage [83]. Studies suggest that DICM might be linked to apoptosis and autophagy of myocardial cells, mitochondrial impairment, oxidative stress, and excessive calcium accumulation [84]. Recent research has discovered that in a mouse model of DICM, DOX decreases GPX4 levels through the DOX-Fe2+ complex in mitochondria, triggering excessive lipid peroxidation and resulting in mitochondrial-dependent ferroptosis. This finding confirms that mitochondrial-dependent ferroptosis is the main driver of DOX-induced cardiac toxicity [83]. Moreover, in DOX-induced mouse models, the origin of myocardial cell death involves a significant upregulation of heme oxygenase-1 (HO-1) under Nrf2 regulation, promoting the release of free iron and resulting in cardiac ferroptosis, while iron suppressor-1 significantly reduces DICM [53]. It is reported that DOX increases oxidative phospholipids in myocardial cells. Ox-PL reduces cysteine intake and NADPH production, depleting GSH, and leading to GPX4 inactivation, ultimately causing ferroptosis [85]. Liu et al. explored the function of acyl-CoA thioesterase 1 (Acot1) in ferroptosis and discovered that Acot1 regulates the biosynthesis of PUFAs in a mouse model of DICM. Knocking out Acot1 makes myocardial cells sensitive to ferroptosis, while overexpression significantly protects against ferroptosis [86]. Fibroblast growth factor 2-mediated protection of the myocardium against DOX requires activation of the mTOR/Nrf-2/HO-1 pathway. Fer-1 or DXZ can also reverse DOX-induced ferroptosis and cardiac toxicity [85, 87].
Iron overload cardiomyopathy (IOC)
Iron overload in myocardial cells results in myocardial dysfunction, referred to as IOC. Normally, iron is absorbed and transported via the TfR1-DMT1-FPN1 pathway to regulate iron levels and prevent tissue damage [88, 89]. When a significant amount of free iron enters the mitochondria in myocardial cells, the ensuing ROS induces mitochondrial oxidative stress and lipid peroxidation, ultimately causing ferroptosis. It has been shown that mice fed a high-iron diet are susceptible to ferroptosis which can be rescued by the iron prolapse inhibitors Fer-1 and DXZ, as well as by antiferrochelators and TTCC blockers, desferrioxamine, and efronedipine, which can also reduce cardiac Ca2+ and iron levels [90, 91]. Iron chelator and antioxidant combination therapy, compared to monotherapy, has a more significant protective effect on the hearts of iron-overloaded rats [92]. This is demonstrated by the normalization of cardiac iron levels, reduction of oxidative stress, and improvement in mitochondrial function. Furthermore, in the same model, this combination therapy reestablishes cardiac Ca2+ balance and enhances myocardial contractility [93]. Chelator combination therapy also reduces cardiac unstable iron in patients with severe Mediterranean anemia, lowering cardiac toxicity and improving heart function [94].
Septic cardiomyopathy (SCM)
Cardiac dysfunction caused by sepsis, known as SCM, not only results in heart failure but also commonly triggers dysfunction or failure in other organs [95]. Previous research suggests that ferroptosis is a component of the pathogenic mechanism of SCM [96]. Li et al. discovered that lipopolysaccharide (LPS) can increase intracellular Fe2+ levels by upregulating the expression of mitochondrial iron transporters. This leads to the transport of more cytoplasmic Fe2+ into the mitochondria, resulting in the production of mitochondrial ROS and ferroptosis [97]. Cytokines like TNF-α, IL-1β, IL-6, and HMGB1, along with activators of TLRs and NF-κB, have been reported to contribute to the development of SCM and ferroptosis [98]. Jiang et al. identified ZJ01, an inhibitor of the Keap1-Nrf2 protein interaction, characterized by a core structure of sub-amino coumarin benzothiazole. In vitro and in vivo, it can activate Nrf2, inhibiting LPS-induced pro-inflammatory cytokines and ROS production, thereby suppressing ferroptosis and slowing down SCM progression [99].
Diabetic cardiomyopathy (DCM)
DCM stands out as a primary factor contributing to heart failure and mortality in individuals with diabetes. Its characteristics encompass early dysfunction in ventricular diastole, delayed dysfunction in ventricular systole, cardiac hypertrophy, and fibrosis [100]. The onset mechanism is closely linked to the excessive production of ROS and compromised antioxidant capabilities in diabetes [56]. Research has affirmed that ROS and oxidative stress can trigger myocardial necrosis, apoptosis, autophagic inflammation, and fibrosis. Recent evidence suggests a potential involvement of ferroptosis in diabetes and its associated complications [101–104]. In a transgenic mouse model, Baseler et al. identified that GPX4 can ameliorate cardiac injury in diabetes [105]. Behring et al. observed that a diet rich in sugar and fat leads to mitochondrial lipid peroxidation and cardiac hypertrophy in mice [106]. Shu et al. found a correlation between high glucose levels and iron overload, indicating a likelihood of ferroptosis in diabetic patients [107]. Additionally, Bruni et al. noted that iron apoptosis inducers like erastin or RSL3 can impact beta cell function in vitro, heightening sensitivity to ferroptosis by regulating GPX4 expression [108]. Recent studies emphasize that activating Nrf2 can alleviate oxidative damage caused by high glucose levels in cultured myocardial cells, thus preventing the development of DCM [109]. Furthermore, overexpression of mouse HIF-1α has shown potential in averting cardiac damage in diabetic mice [110]. Hence, further exploration is warranted to understand and regulate ferroptosis for the prevention and treatment of DCM.
Radiation-induced cardiomyopathy (RICM)
RICM refers to a persistent impairment of heart muscle function resulting from damage to the inner lining of blood vessels and the development of fibrous tissue in the myocardium [111]. Radiation exposure can result in diverse forms of cell demise, including apoptosis, necrosis, and autophagic cell death. Recently, researchers have also linked iron-dependent cell death, known as ferroptosis, to radiation-induced cell death [112–114]. ROS trigger lipid peroxidation and serve as crucial regulatory factors in ferroptosis. In mouse models, the STING pathway, which detects cytoplasmic DNA, triggers interferon-gamma production and controls the expression of the cyclooxygenase-2 (COX2) gene. Both STING and COX2 are linked to ferroptosis: STING activation preserves GPX4 levels and cellular equilibrium, while COX2 overexpression amplifies the neuroprotective properties of SH-SY5Y human neuroblastoma cells against iron-induced apoptosis by activating miR-137 [115, 116]. In a mouse model of radiation-induced lung fibrosis (RILF), ferroptosis assumes a crucial role, and the ferroptosis inhibitor liproxstatin-1 alleviates RILF by inhibiting TGF-β1. Boerma et al. investigated the antioxidant vitamin E in a rat model and found that it reduces radiation-induced fibrosis by inhibiting ferroptosis [117].
There are many types of cardiovascular disease, such as myocardial infarction, ischemia–reperfusion Injury, hypertrophic cardiomyopathy, DOX-induced cardiomyopathy, iron overload cardiomyopathy, septic cardiomyopathy, diabetic cardiomyopathy and radiation-induced cardiomyopathy, which are associated with ferroptosis.
NDs
Ferroptosis in brain
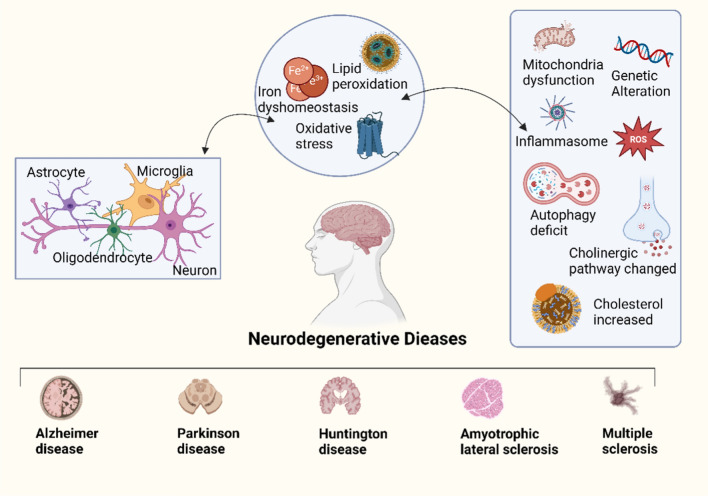
Iron, an essential intracellular element, participates in vital biological functions like oxygen transportation, storage, and utilization. Excessive iron levels can trigger oxidative stress and lipid peroxidation within cells, potentially leading to ferroptosis. Ferroptosis is implicated in various brain-related conditions such as cerebral I/R injury, traumatic brain injury, and neurodegenerative diseases [129]. For instance, in cerebral I/R injury, iron overload can induce brain damage, while mitochondrial ferritin, a crucial protein for storing iron in mitochondria, can mitigate this damage by suppressing ferroptosis [130]. Likewise, the TrkB agonist N-acetyl serotonin facilitates functional recuperation following traumatic brain injury by inhibiting ferroptosis through the PI3K/Akt/Nrf2/H-Ferritin pathway [131]. Neurodegenerative diseases, characterized by progressive neuronal degeneration leading to cognitive and behavioral decline, may benefit from targeting ferroptosis [132] (Fig. 3). Strategies such as using iron chelators to diminish intracellular iron levels or antioxidants to neutralize reactive oxygen species could effectively thwart ferroptosis and safeguard neurons [133]. Furthermore, nuclear transcription factors like Nrf2 have emerged as pivotal players in both neurodegenerative disease treatment and ferroptosis regulation [134].
Fig. 3.
Neurodegenerative diseases caused by ferrioptosis and mechanisms
Ferroptosis and NDs
AD
AD is a neurological condition characterized by progressive memory and behavioral decline, primarily attributed to the accumulation of β-amyloid β-protein (Aβ) and Tau protein [135]. Research has revealed elevated iron levels and reduced ferritin levels in brain regions such as the hippocampus and cortex of AD patients, with iron concentrations positively correlated with disease progression. Excessive Fe2+ levels trigger lipid peroxide production via the Fenton reaction, promoting hippocampal neuron degeneration and exacerbating AD advancement [136]. Aβ precipitation is also associated with iron buildup, with Aβ inducing pericellular mitochondria autophagy, leading to ferroptosis [137]. Furthermore, research has detected reduced expression of FPN in the brains of APP/PS1 mice and individuals with AD, resulting in hippocampal atrophy and memory decline resembling AD symptoms. This underscores the strong link between AD and ferroptosis. Specific inhibitors targeting ferroptosis effectively alleviate neuronal loss and memory deficits induced by Aβ aggregation [12]. Intramuscular desferriamine administration benefits AD patients, while nasal desferriamine administration reverses memory deficits in AD mice. Tetrahydroxy stilbene glycoside administration in AD model mice activates GPX4 and Nrf2, upregulating superoxide dismutase expression, ultimately alleviating AD symptoms [138]. Furthermore, hydroxylated chalcone compounds synthesized by Cong et al. inhibit Aβ accumulation and neuronal ferroptosis, enhancing AD patient behavior [139]. Study has shown that inhibiting ferroptosis by maintaining Ca2+ homeostasis is also an innovative target for the treatment of AD [140]. Hence, targeting neuronal ferroptosis holds promise as a potential AD treatment.
PD
The characteristic traits of PD involve the degeneration of dopamine neurons in the substantia nigra pars compacta and the development of Lewy bodies within these neurons. Iron overload in the body triggers dopamine oxidation and free radical generation, disrupting oxidative balance and accelerating dopaminergic neuron loss, thus contributing to PD pathophysiology [141, 142]. Neuroimaging and postmortem examinations show that there is a buildup of iron in the substantia nigra, and the remaining dopaminergic neurons have higher levels of iron. This emphasizes the significant involvement of iron in the degeneration of dopaminergic neurons in PD [20, 143]. Studies suggest that in both animal models and patients with PD, there is an increase in lipid peroxide levels and impaired mitochondrial function in the substantia nigra pars compacta. This is accompanied by decreased levels of GSH, which ultimately triggers the initiation of ferroptosis [144]. A fascinating aspect is that α-synuclein, a crucial regulator in PD, has been linked to iron and lipid metabolism, contributing to neurodegeneration. Administering the iron chelator deferrone to PD mice diminishes α-synuclein's harmful effects [145]. Pretreatment with ferroptosis inhibitors attenuates cell death in 6-hydroxy-dopamine-induced PD cell models [146]. Deferiprone significantly delays motor disorders and enhances glutathione peroxidase activity in PD patients' cerebrospinal fluid, showing promise in phase II clinical trials and animal models [129]. Research has demonstrated that rapamycin can counteract ferroptosis in PD models induced by MPTP/MPP + by enhancing autophagy [147]. Thus, targeting the iron metabolism-mediated ferroptosis pathway holds promise as a therapeutic approach for PD.
HD
HD is a genetic neurodegenerative condition inherited in an autosomal dominant pattern. It is distinguished by the expansion of CAG repeats within the Huntingtin (HTT) gene. It is distinguished by neurodegeneration in the striatum, cortex, and cerebral cortex, manifesting clinically as dystonia, motor impairment, and cognitive decline [129]. Excessive iron levels are pivotal in ferroptosis onset, as evidenced by iron supplementation exacerbating oxidative stress and hastening disease progression in newborn R6/2 HD mice [148]. Research indicates that individuals with HD exhibit elevated levels of lipid peroxidation in their plasma and reduced levels of GSH, rendering them more vulnerable to ferroptosis. It has been observed that elevated lipid peroxidation occurs in corticostriatal brain slices of HD patients, where it coincides with mHTT inclusions in striatal neurons. This lipid peroxidation impairs axonal signal transmission, ultimately resulting in neuronal degeneration [149, 150]. Skouta et al. administered specific ferroptosis inhibitors to HTT striatal neurons, finding that Fer-1 and SRS11-92 significantly enhanced neuronal survival in a dose-dependent manner [151]. Thus, comprehending ferroptosis's role in HD development offers a promising avenue for HD treatment.
Amyotrophic lateral sclerosis (ALS)
ALS is a fatal and prevalent neurodegenerative disorder affecting the nervous system. Clinical manifestations primarily involve limb weakness and bulbar dysfunction, and death often within two years of diagnosis [152]. Research indicates elevated serum ferritin levels in the cerebrospinal fluid of ALS patients, with early neuronal iron accumulation observed in the corticospinal motor pathway preceding neuropathological changes and microglial activation. Treatment of SodG86R mice and ALS patients with iron chelators improves ALS symptoms, significantly reducing cerebrospinal fluid oxidative stress levels without inducing iron deficiency anemia [153, 154]. ALS progression is characterized not only by disturbances in iron metabolism but also by mitochondrial impairment. Dynamin-related protein 1 (Drp1), a GTP enzyme involved in mitochondrial fission, disrupts intracellular fission–fusion equilibrium, induces oxidative stress, and increases peroxide synthesis. Iron chelators alleviate oxidative stress damage by inhibiting Drp1 dephosphorylation activity [119, 120]. GPX4 deletion is evident in the spinal cords of ALS patients post-mortem and is a common feature in early mouse models of transgenic ALS mutations like SOD1G93A, TDP-43, and C9orf72. GPX4 overexpression in SOD1G93A mice significantly delays ALS onset [155]. Edaravone exhibits antioxidant and lipid-stabilizing properties, inhibits ferroptosis, and thus mitigates ALS progression [156]. Treatments utilizing multifunctional small molecules that target various aspects of mitochondrial dysfunction, oxidative stress, as well as HIF and NF-κB activity, could potentially offer more effective and innovative therapy options compared to drugs that solely focus on a single target [157].
Multiple sclerosis (MS)
The characteristic pathological features of MS encompass the development of plaques within the central nervous system, destruction of neuronal myelin sheaths, and hyperplasia of astrocytes [158]. In the initial stages of MS, there is frequently an association with inflammation. Prolonged inflammation leads to the accumulation of iron and subsequent lipid peroxide increase, ultimately resulting in the initiation of ferroptosis. Ferroptosis, in turn, facilitates T-cell activation-induced neurodegeneration in MS [159, 160]. Active and long-standing MS lesions, along with the cerebrospinal fluid of MS patients, displayed various indications of ferroptosis. This was indicated by increased levels of labile iron, peroxidized phospholipids, and lipid degradation byproducts [161]. Iron is predominantly stored in oligodendrocytes in the healthy brain and plays a role in myelin formation, suggesting that iron release may contribute to demyelinating lesions. Research has demonstrated elevated iron levels in the brains of MS patients, particularly in gray matter and areas adjacent to MS lesions, which could contribute to myelin loss in MS [162]. Schwann cells, peripheral nerve glial cells involved in myelin formation, may also play a role. Knocking out DMT1 and FTH in mouse Schwann cells resulted in decreased axon proliferation, maturation, and myelination, suggesting that ferroptosis induced by abnormal iron metabolism is closely associated with MS [163].
Many neurodegenerative diseases are characterized by the accumulation of local iron in specific regions of the central nervous system and peripheral nervous system, and abnormalities in iron homeostasis in brain tissue can induce large production of ROS in brain cells [164]. This results in catastrophic oxidative damage to sensitive subcellular structures. In mice, GPX4 knockdown leads to age-dependent neurodegenerative changes and neuron loss, exacerbated by dietary vitamin E deficiency [165]. In recent years, with the clear mechanism of ferroptosis, ferroptosis-based ferroptosis inhibitors have developed rapidly. The main function of common ferroptosis inhibitors is to inhibit lipid peroxidation, reduce the concentration of free iron, or inhibit the formation of oxygen free radicals (Table 3).
Table 3.
Drugs and compounds associated with ferroptosis for neurodegenerative diseases treatment
| Drugs | Inhibitor | Target | References |
|---|---|---|---|
| Lipid peroxidation inhibitors | Zileuton | Inhibit 5-LOX | [166, 167] |
| Antioxidants | Ferrostatin-1 | ROS scavenger, increase GSH level, | [168] |
| Antioxidants | SRS11–92 | ROS scavenger, reduce lipid peroxides | [169, 170] |
| Antioxidants | SRS-16–86 | ROS scavenger, reduce lipid peroxide | [171, 172] |
| Lipid soluble antioxidant | Vitamin E | GPX4 | [165] |
Neurodegenerative diseases like Alzheimer's, Parkinson's, Huntington's, Amyotrophic Lateral Sclerosis, and Multiple Sclerosis are connected to ferroptosis. Ferroptosis mainly arises from imbalances in iron, lipid peroxidation, or oxidative stress. It can impact the interactions between nerve cells, and is associated with mitochondrial dysfunction, genetic mutations, inflammasome activity, autophagy deficits, alterations in the cholinergic pathway, increased cholesterol levels, and other physiological changes.
Cancer
Ferroptosis in cancer
Cancer, a malignant growth arising from epithelial tissue, is marked by irregular cell differentiation and proliferation, unrestricted expansion, invasion, and metastasis. It stands as the second most common cause of death worldwide, overtaking CVDs in certain countries with high human development indexes, and emerging as the primary cause of premature mortality. The complex and multi-faceted nature of cancer development involves factors such as smoking, infection, occupational exposure, environmental pollution, unhealthy diet, and genetic predisposition [173]. According to the World Health Organization's 2020 statistics, 9.95 million people worldwide succumbed to cancer, with lung cancer being the most prevalent, accounting for 18.0% of all cancer deaths. Other notable contributors include colorectal cancer (9.4%), liver cancer (8.3%), stomach cancer (7.7%), and female breast cancer (6.9%) [174, 175]. The high mortality rates underscore the formidable challenge in the field of anticancer therapy.
Iron is an essential micronutrient in the human body, pivotal for physiological functions such as hemoglobin formation, DNA synthesis, and energy metabolism. Disruptions in iron metabolism can contribute to the proliferation, infiltration, spread, and reappearance of cancer [176]. Iron deficiency leads to a decrease in red blood cell count, affecting the transport of oxygen and nutrients, resulting in hypoxia and ischemia in various organs and a weakened immune system. For cancer patients, anemia can diminish the effectiveness of chemotherapy and radiotherapy, foster tumor development, and increase tumor aggressiveness [177]. Conversely, cancer cells rely on iron as a raw material for reproduction. Excessive iron in the body becomes fuel for cancer cells, accelerating tumor growth and worsening the disease. This suggests a potential recommendation against iron supplementation or an excess intake of iron-rich foods for cancer patients [178]. Simultaneously, iron deficiency may impact immune cell activity and alter the tumor microenvironment, affecting the immune system's ability to defend against cancer [179].
Ferroptosis, a form of cell demise reliant on iron., is intricately linked to the onset, progression, metastasis, and treatment resistance of cancer [180]. Firstly, tumor cells exhibit a heightened demand for iron, termed "iron addiction," making them more vulnerable to ferroptosis when iron levels rise [181]. Secondly, during ferroptosis, iron ions can initiate the generation of ROS through the Fenton reaction, triggering lipid peroxidation and ultimately causing cell death. This phenomenon is particularly pronounced in tumor cells, characterized by elevated ROS levels and diminished antioxidant capacity [182]. Nevertheless, the occurrence of ferroptosis releases pro-inflammatory factors, inducing inflammation and altering the tumor microenvironment. This, in turn, reduces the immune susceptibility of tumor cells, suggesting that ferroptosis may, to some extent, promote tumor growth and spread [183]. Ferroptosis participates in various crucial molecules and signaling pathways within cancer. Apart from iron and ROS, it is governed by additional pathways like the p53 pathway in cancer. It can be inhibited by several pathways including the FSP1-CoQ10 pathway, GCH1-BH4 pathway, and the DHODH-CoQH2 system [184]. Increasing evidence indicates the pivotal involvement of ferroptosis in cancer therapy, with some anti-cancer medications designed to target ferroptosis already being utilized in clinical settings (Table 4). The merging of classic chemotherapy agents such as cisplatin with substances that trigger ferroptosis has proven to be a potent method for halting the growth of head and neck tumors by working together synergistically [185]. Inducing ferroptosis has the potential to hinder the emergence of resistance in cancer cells to diverse cancer treatments, such as lapatinib, erlotinib, and vemurafenib [186–188]. Significantly, radiation can initiate ferroptosis, which holds comparable importance to apoptosis in thwarting radiation-induced tumors. This indicates that stimulating ferroptosis could potentially heighten the vulnerability of radioresistant cancer cells to radiation therapy [186, 189]. Employing combination therapy centered around ferroptosis presents a highly promising approach that can augment the efficacy of standard treatments, address drug-resistant tumors, and deter tumor recurrence [190]. In summary, the exploration of ferroptosis holds significant research potential in the cancer field. A thorough understanding of its mechanisms and its role in tumor development is anticipated to offer novel insights and approaches for cancer treatment.
Table 4.
Clinical trial drugs inducing ferroptosis for antitumor treatment
| Drugs | Target | Cancer type | References |
|---|---|---|---|
| Sorafenib | SLC7A11 | Hepatocellular carcinoma, gastric cancer, clear cell renal cell carcinoma | [216, 245, 246] |
| Sulfasalazine | SLC7A11 | Prostate cancer, lymphoma, lung cancer, colorectal cancer, head and neck cancer, pancreatic ductal adenocarcinoma, ovarian clear cell carcinoma, breast cancer | [129, 247–250] |
| Lapatinib | Iron | Breast cancer, pancreatic cancer | [251, 252] |
| Neratinib | Iron | Breast cancer | [253] |
| Salinomycin | Iron | Various solid tumour types | [254] |
| Artesunate | Iron | Prostate cancer, pancreatic cancer, hepatocellular carcinoma, head and neck cancer | [251, 253–255] |
| Cisplatin | GSH | Breast cancer, gastric cancer, head and neck cancer, ovarian cancer | [256–259] |
| Gemcitabine | GPX4 | Pancreatic cancer, lung adenocarcinoma | [260, 261] |
| Everolimus | GPX4 | Renal cell carcinoma | [262] |
| Gefitinib | GPX4 | Triple negative breast cancer, lung cancer | [263, 264] |
| Withaferin A | GPX4 | Neuroblastoma, hepatocellular carcinoma | [265, 266] |
| Fluvastatin | HMGCR | Lung adenocarcinoma | [267] |
| Pravastatin | HMGCR | Hepatocellular carcinoma | [268] |
| Simvastatin | HMGCR | Triple-negative breast cancer | [269] |
| Haloperidol | DRD2 | Hepatocellular carcinoma, glioblastoma | [270, 271] |
| Zalcitabine | DNA stress | Pancreatic cancer | [272] |
| β-Elemene | TFEB | Colorectal cancer, non-small cell lung cancer | [272, 273] |
| Buthionine | GCL | Melanoma, neuroblastoma | [274] |
| sulfoximine | GCL | Triple negative breast cancer | [275] |
| Zileuton | 5-LOX | Head cancer | [276] |
| Brequinar | DHODH | Cervical cancer, colon cancer, fibrosarcoma, lung cancer | [277–280] |
| Cetuximab | KRAS | KRAS mutant colorectal cancer | [263] |
| Curcumenol | FTH1 | Lung cancer | [256] |
Ferroptosis and antitumor treatment
Lung cancer
Lung cancer stands as one of the most widespread cancers worldwide, and despite continuous progress in treatment, the overall five-year survival rate for lung cancer patients remains around 16% [191]. Therefore, there is a pressing demand for innovative therapeutic approaches in the treatment of lung cancer. Bioinformatics increasingly indicates a significant correlation between the occurrence of ferroptosis and lung cancer development [192]. The onset of ferroptosis depends on the buildup of iron ions and ROS. In the context of lung cancer cells, disrupted iron and mitochondrial metabolism may elevate intracellular levels of iron ions and ROS, creating favorable conditions for ferroptosis. Moreover, the GSH-dependent GPX4 reduction system stands out as a pivotal pathway. p53 is an oncogene that inhibits cell uptake of cystine by directly inhibiting the transcription of SLC7A11, a key component of system Xc—in the p53-SAT1-ALOX15 pathway. Stimulation by erastin, for example, inhibits System Xc-, leading to the suppression of cysteine or selenocysteine, thereby reducing GPX4 expression and inducing ferroptosis [193]. Adjusting the expression of genes associated with ferroptosis can impact the proliferation and spread of lung cancer cells. For instance, inhibiting GPX4 expression can prompt ferroptosis in lung cancer cells, effectively restraining tumor growth and metastasis [194]. RNA Binding Motif Single Stranded Interacting Protein 1, functioning as a translational enhancer of SLC7A11 in lung cancer, triggers ferroptosis upon its loss, inhibiting lung cancer cell growth and heightening sensitivity to radiotherapy [195]. CoQ-FSP1, an essential element downstream of the KEAP1-Nrf2 pathway, presents itself as a promising target for therapy aimed at addressing KEAP1-mutant lung cancer [196]. As a tumor suppressor, TRIM3 can inhibit the occurrence of non-small-cell carcinoma (NSCLC) by degrading SLC7A11, suggesting a novel strategy for treating NSCLC [62]. Additionally, certain ferroptosis inducers like RSL3 and Erastin have been identified to impede lung cancer cell growth and induce ferroptosis. The manipulation of ferroptosis-related gene expression or the utilization of ferroptosis inducers may pave the way for innovative approaches in lung cancer treatment [197]. As a result, ferroptosis might become a compelling focus for treating lung cancer, and delving deeply into the mechanisms and clinical uses of ferroptosis in lung cancer could provide innovative approaches and strategies for managing this condition.
Colorectal cancer (CRC)
CRC is a malignant growth that impacts the digestive system and ranks as the second most common cause of cancer-related deaths worldwide. The highly migratory and invasive nature of colorectal cancer cells, attributed to epigenetic and metabolic alterations, leads to a mere 12% 5-year survival rate for metastatic colorectal cancer [198]. Recent studies increasingly highlight the clinical significance of inducing ferroptosis in CRC by elevating Fe2+ and ROS levels within CRC cells, diminishing the antioxidant GSH levels, or deactivating GPX4. Conversely, inhibiting ferroptosis may contribute to tumor progression and treatment resistance in CRC [199]. By modulating relevant genes, such as the overexpression of serine and arginine-rich splicing factor 9, the induction of cell lipid peroxidation by erastin and sorafenib can be inhibited, leading to a reduction in GPX4 expression and the suppression of ferroptosis [200]. Heat shock protein family a member 5 also slows down the degradation of GPX4, providing colorectal cancer cells with more time to adapt to the toxicity of erastin [201]. Similarly, genes like IMCA and ACADSB, which down-regulate the expression of SLC7A11, result in decreased levels of cysteine and glutathione. This significantly induces ferroptosis in colorectal cancer cells, inhibiting their migration, invasion, and proliferation [202, 203]. Beyond coding genes, non-coding RNAs, including miRNAs, lncRNAs, and circRNAs, have been reported to mediate ferroptosis in CRC [204]. Intestinal microbiota participates in tumor progression by producing carcinogenic metabolites and can also promote the development of colorectal cancer by inhibiting ferroptosis [205]. Whether administered alone or in conjunction with other chemotherapy agents, ferroptosis inducers can effectively trigger ferroptosis in cancer cells, particularly those resistant to treatment [206]. Furthermore, ferroptosis can selectively target aggressive cancer stem cells, offering potential improvements in immunotherapy efficacy and overcoming resistance to immunotherapy [207].
Hepatocellular carcinoma (HCC)
HCC stands as the most prevalent type of primary liver cancer, with its origins linked to genetic, environmental, and behavioral factors [208]. A potential strategy to eradicate malignant liver cells involves safeguarding healthy cells while selectively inducing the death of tumor cells. Research has demonstrated that ferroptosis inhibits the growth and proliferation of HCC cells in both in vitro and in vivo xenotransplantation models [209, 210]. The mutation or abnormal expression of genes associated with ferroptosis, such as ACSL4, GPX4, Nrf2, SLCA711, heat shock protein family B (small) member 1, etc., may be implicated in the development of hepatocellular carcinoma [211, 212]. Radiotherapy is a primary method for treating hepatocellular carcinoma. In addition to triggering ferroptosis, it also results in the increased expression of genes that inhibit ferroptosis, such as SLC7A11 and GPX4. Tumor cells that overexpress SLC7A11 and GPX4 demonstrate substantial resistance to radiotherapy. Therefore, the combination of radiotherapy with ferroptosis inducers is anticipated as a research direction [213]. Furthermore, ferroptosis plays a role in the drug resistance process of hepatocellular carcinoma, where certain chemotherapy drugs or targeted medications can impede the growth and spread of HCC cells by inducing ferroptosis. Sorafenib, a widely used multikinase inhibitor for HCC treatment, has been found to have enhanced efficacy when combined with ferroptosis induction [214, 215]. Studies suggest that inducing ferroptosis can augment the sensitivity of hepatocellular carcinoma to sorafenib, thereby overcoming drug resistance. Glutathione S-transferase zeta 1 has been recognized as a factor that boosts sorafenib-triggered ferroptosis by suppressing the Nrf2/GPX4 pathway in HCC cells, suggesting a potentially effective treatment approach for HCC that involves combining sorafenib with a ferroptosis inducer [216, 217]. Additionally, ferroptosis is linked to the prognosis of hepatocellular carcinoma, with some research indicating a negative correlation between the expression level of iron-death-related genes and the prognosis of HCC patients. In other words, higher expression of iron-death-related genes is associated with a worse prognosis for patients [218–220]. Traditional Chinese medicine research indicates that Polyphyllin I intervention can enhance mitochondrial damage and induce ferroptosis through the Nrf2/HO-1/GPX4 axis, thereby inhibiting the proliferation, invasion, and metastasis of HCC cells [5]. In conclusion, there exists a significant association between ferroptosis and hepatocellular carcinoma. A thorough exploration of the mechanism of ferroptosis in HCC holds great importance in understanding the onset and progression of hepatocellular carcinoma, unraveling drug resistance mechanisms, and developing novel therapeutic strategies.
Gastric cancer (GC)
GC is among the prevalent malignant tumors, holding the fifth position in terms of occurrence and fourth in mortality rates. Annually, worldwide, more than a million individuals receive a diagnosis of stomach cancer. Risk elements encompass H. pylori infection, tobacco use, alcohol intake, a diet rich in salt, and insufficient physical activity [221, 222]. A study indicates that higher iron content increases the risk of cancer [223]. Ferroptosis-associated RNA contributes to the expression patterns of stomach cancer cells to different extents. For instance, miR103a-3p influences ferroptosis in GC cells by modulating intracellular GSH levels [224]. Additionally, lncLASTR and Circ0000190 regulate the proliferation and migration of GC cells by controlling ferroptosis [225]. Manipulating functional proteins associated with ferroptosis, like GSH and GPX4 in GC cells, can influence the onset and progression of GC [226]. As an example, da2, a novel derivative of Jiyuan oridonin A, has been discovered to selectively suppress the proliferation of GC cells by triggering ferroptosis. This is achieved by reducing GPX4 levels and leading to the accumulation of iron within subcellular compartments [227]. Studies indicate that ferroptosis contributes to the establishment of the tumor microenvironment. Assessing the levels of macrophages and iron within the GC microenvironment may offer valuable insights into predicting the progression of tumors [228, 229].
Pancreatic cancer
Pancreatic cancer is one of the most lethal cancers in the world, characterized by late diagnosis, rapid metastasis, chemotherapy resistance, and poor prognosis [230]. Acute pancreatitis ranks among the most prevalent acute abdominal conditions. In acute pancreatitis, the regulation of ferroptosis can control the excessive activation and release of pancreatic enzymes at the cellular level. By regulating ferritin, ferroptosis in AP can be reduced, and inflammatory factors can be modulated [231, 232]. Pancreatic ductal adenocarcinoma (PDAC) is the most common pathological type of pancreatic cancer, accounting for approximately 90% of cases. Mutations in the KRAS signal lead to increased production of ROS, causing ferroptosis [233]. To avoid excessive ferroptosis in PDAC, substances like miR-125a, erastin, are used to reduce cysteine-induced ferroptosis in PDAC cells. The results confirm that systemic inhibition of x-CT can suppress tumor growth and metastasis [234]. Alternatively, a combination of cisplatin and dihydroartemisinin is used to synergistically inhibit the proliferation of PDAC cells and induce DNA damage. This outcome is mainly accomplished by heightening cellular susceptibility to ferroptosis and elevating intracellular free iron levels, thereby impeding the proliferation of PDAC cells [235]. Lipid peroxidation regulation is also explored, and research indicates that microsomal glutathione transferase 1 can bind with arachidonate 5-lipoxygenase to inhibit cancer cell ferroptosis by reducing lipid peroxidation [236]. Additionally, LncRNA associated with ferroptosis can be used in PDAC to evaluate patient prognosis, molecular characteristics, and treatment modalities. Studies showed that high expression of SLCO4A1-AS1 in PDAC patients is associated with lower sensitivity to ferroptosis, directly leading to a poorer prognosis for patients [237, 238].
Ovarian cancer (OVCA)
OVCA is the leading cause of female reproductive cancer deaths worldwide, with a high recurrence rate [239]. A study has found that the occurrence of ovarian clear cell carcinoma depends on the acquisition of cysteine, and the absence of cysteine leads to the disruption of the main protective pathway for ferroptosis, the GPX4-GSH pathway, triggering oxidative stress-induced ferroptosis [240]. Ferroptosis has a significant connection with the clinical management of OVCA. Current studies suggest that ferroptosis plays a pivotal role in the chemotherapy of ovarian cancer, augmenting the anticancer efficacy of cisplatin in the treatment of this disease [241]. In platinum-resistant OVCA cells, the addition of inhibitors of ferroptosis-related protein GPX4 can increase cancer cell sensitivity to ferroptosis, opening up new avenues for platinum-resistant OVCA treatment [242]. The synergistic effect of erastin and docetaxel also makes drug-resistant cancer cells more prone to ferroptosis [243]. Fortunately, researchers have analyzed and identified mRNA and genes associated with ferroptosis as both treatment targets and prognostic indicators, revealing new therapeutic vulnerabilities in OVCA patients and providing promising prognostic indicators [239, 244].
Ferroptosis and tumor immunotherapy
The immune system's vital function involves identifying and eradicating cancerous cells, thereby actively monitoring and managing tumor expansion. Tumor immunotherapy involves utilizing the immune system's capabilities to target and combat cancer cells, garnering significant attention and research efforts in recent years. An illustrative example is CAR T-cell therapy, a technique that modifies T cells to identify and eliminate tumor cells, demonstrating success, particularly in treating leukemia and lymphoma [281]. Nevertheless, challenges exist within the realm of tumor immunotherapy. Firstly, not all patients exhibit a positive response to this treatment, and some show no response whatsoever. Secondly, immunotherapy may induce adverse effects such as inflammation and autoimmune reactions. Furthermore, the field must address challenges like overcoming tumor immune escape mechanisms and the diverse nature of tumor cells [282, 283]. In conclusion, while tumor immunotherapy holds promise and has achieved notable successes in treating various cancers, ongoing research and enhancements are imperative to refine its effectiveness and safety.
The identification of ferroptosis has opened up new avenues for tumor immunotherapy. On one hand, ferroptosis can eliminate tumor cells, disrupt the tumor's blood supply, incite the immune system's attack, and enhance the responsiveness of tumor cells to other treatments. On the other hand, ferroptosis can also release pro-inflammatory factors, triggering an inflammatory response, altering the tumor's microenvironment, and diminishing the immune resistance of tumor cells [284, 285]. By integrating immunotherapy with strategies that bolster ferroptosis, such as radiotherapy and targeted therapy, it's possible to collaboratively trigger ferroptosis. This approach can either boost anti-tumor immune reactions or inhibit detrimental immune responses [286] (Fig. 4).
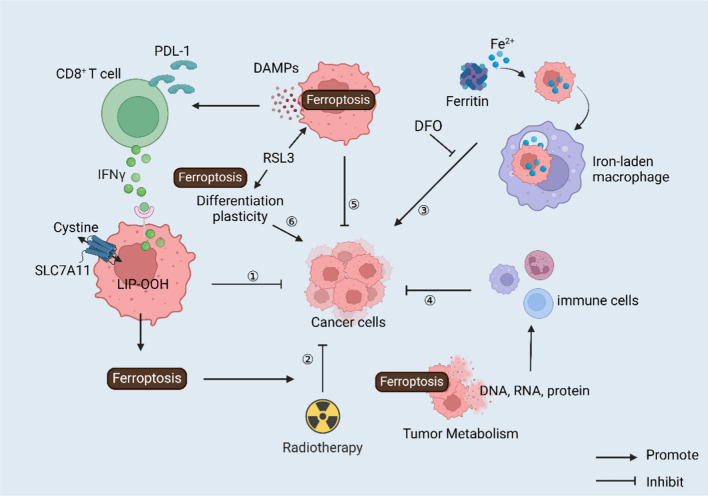
Fig. 4.
Ferroptosis in combination with immunotherapy. Immunotherapy combined with the regulation of ferroptosis: ①tumor immune checkpoint inhibitors; ②revers radiotherapy resistance; ③iron-laden macrophages; ④tumor metabolism; ⑤damage-related molecular patterns; ⑥cancer cell differentiation plasticity
Tumor immune checkpoint inhibitors (ICI)
It has been reported that ICI can significantly improve the treatment efficiency of advanced cancer, including PD-1 inhibitors and cytotoxic T lymphocyte-associated antigen-4 inhibitors. These drugs work by blocking checkpoint molecules on the surface of tumor cells, thereby activating immune cells and enabling them to better attack tumours [287, 288]. Several immune checkpoint inhibitors have been approved for use in the treatment of a wide range of tumors, for example, PD-1 inhibitors are widely used in tumours such as lung cancer, melanoma, renal cell carcinoma and Hodgkin's lymphoma [289]. The effectiveness of immune checkpoint blockade therapy in generating a widespread anti-tumor response for numerous cancers remains limited, primarily due to the complexity of the tumor microenvironment and inadequate stimulation of the host immune system [290]. Ferroptosis has recently been discovered to play a role in the effectiveness of CD8+ T cells against tumors and also affects how well anti-PD-1/PD-L1 immunotherapy works. Studies have shown that when CD8+ T cells are activated by anti-PD-L1 immunotherapy, they trigger ferroptosis in tumor cells by releasing interferon-γ (IFN-γ) following PD-L1 blockade. The released IFN-γ notably decreases the expression of SLC7A11 in tumor cells, which reduces their ability to take up cystine, increases lipid peroxidation, and ultimately leads to ferroptosis [291, 292]. The combined action of cystathionine/cysteine enzymes alongside anti-PD-L1 treatment can generate potent anti-tumor immune responses by triggering ferroptosis [293, 294].
Revers radiotherapy resistance
Radiotherapy is a commonly used cancer treatment that destroys cancer cells through high-energy radiation. Nonetheless, certain tumor cells develop resistance to radiation therapy, often leading to tumor recurrence and metastasis, posing significant challenges in treatment [295]. Previous research has demonstrated that radio resistance in cancer cells was facilitated by increased DNA damage repair, reduced apoptosis, and accelerated autophagy [296–298]. Recent years have seen the development of a novel and significant method for increasing the sensitivity of tumor radiation and immunotherapy: triggering ferroptosis in cancer cells [299]. Effector T cells and radiotherapy engage in a collaborative interaction via ferroptosis to enhance tumor elimination. The use of IFN and CD8+ T lymphocytes encourages tumor cell ferroptosis and causes radio sensitization. By generating IFN in conjunction with radiotherapy-activated ataxia telangiectasia mutated protein targeting SLC7A11 to prevent cystine uptake, immune therapy-activated CD8+T cells cause tumor cells to undergo ferroptosis [189, 197]. In certain mouse models receiving combined treatment of radiation alongside PD-L1/PD-1 or cytotoxic T lymphocyte-associated antigen-4 inhibition, this results in robust tumor immunity, induction of tumor ferroptosis, and subsequent tumor regression [300]. This indicates that tumor ferroptosis represents a new convergence point between radiotherapy and adaptive immunity.
Iron-laden macrophages
Macrophages are a type of leukocyte that are significant components of the tumor microenvironment and have a significant impact on the tumor immune system and immune response. It is involved in the development, proliferation, metastasis, and resistance to drugs of tumors, as well as interactions with ferroptosis and other pathways [301–303]. Normally, the immune system identifies cancer cells and macrophages eliminate them, however, in acidic environments, ferritin phagocytosis facilitates the maximal release of iron, which is eliminated by cancer cells and subsequently assimilated by macrophages to generate iron-laden macrophages [304, 305]. Not only does the presence of iron-laden macrophages in the tumor microenvironment have prognostic significance, but it also represents a significant functional deficit in macrophages' capacity to combat cancer cells. The ability of iron-loaded macrophages to combat cancer cells decreases with increasing iron loading. Reduced serum iron concentration from increased iron uptake by macrophages causes an iron deficit in nearby normal cells, which in turn lowers the activity of iron-dependent enzymes in numerous iron metabolic pathways and generally impairs normal cell function [306, 307]. Accordingly, researchers have demonstrated that using iron chelating agents to reduce the iron load in macrophages can restore the decreased anti-cancer action of iron-laden macrophages and the corresponding iron deficit in normal cells. The most effective iron mobilizer of ferritin and hemosiderin is 1,2-Dimethyl-3-hydroxypyrid-4-one, which has also been demonstrated to remove iron from macrophages and many other cell types during concentration-dependent iron mobilization mediated by chelating agents in inflammatory and cancerous disorders [308–311]. Thus, early after cancer diagnosis, we can introduce iron chelation therapy, which can reduce the iron effect of cancer cells and improve the anti-cancer activity of macrophages and other related therapeutic interventions, in the anti-cancer targeting related to the regulation of iron-laden macrophages. In a similar vein, chelating medications working in tandem with immune agents can enhance cancer treatment.
Tumor metabolism
Tumor cells require a large amount of energy to support their rapid growth and proliferation, which comes mainly from the process of glucose fermentation, however, this metabolism produces large amounts of ROS and iron ions, which, when at a certain concentration within the cell, can trigger tumor cell ferroptosis [14]. In this process, on the one hand, ferroptosis can induce tumor cells to release intra-cellular substances, such as proteins, DNA, and RNA, which can activate the NF-κB signal pathway in immune cells, thereby promoting immune cell activation and anti-tumor immune response. On the other hand, ferroptosis can also cause tumor cell membrane breakage, making it easier for immune cells to identify and attack tumor cells. Research has demonstrated that small-cell lung cancer cells and triple-negative breast cancer cells are susceptible to ferroptosis [312, 313]. Therefore, some drugs and gene therapies can induce an immune response by inducing ferroptosis, which kills tumor cells.
Clever use of damage-related molecular patterns (DAMPs)
DAMPs are endogenous molecules that interact with immune cell receptors, triggering dendritic cell maturation and CD8+ T cell activation, leading to IFN-γ production. Targeting DAMPs holds promise for novel therapies in conditions like sepsis and cancer. Various forms of cell demise, including apoptosis, ferroptosis, and necroptosis, facilitate the liberation of DAMPs [10, 284, 314]. These molecules can also be actively ejected from viable cells through processes such as lysosomal or exosomal secretion, exocytosis of exosomes, and the activation of cell membrane channel pores. In the context of renal fibrosis, ferroptosis is more immunogenic than apoptosis, given the signaling release and activation of DAMPs [315, 316]. Ferroptosis entails the release of cellular components, including DAMPs, which, when outside the cell, serve as immune stimulants, triggering activation of both the innate and adaptive immune systems through binding to pattern recognition receptors. In heart injury, DAMPs release occurs when toll-like receptor 4 (TLR4) signaling is triggered by ferroptosis. This phenomenon is observed in situations such as kidney and brain ferroptosis, wherein cells undergoing ferroptosis release factors that robustly activate the innate immune system [76, 317]. Furthermore, the high mobility group protein B1 (HMGB1) plays a crucial role in DNA regulation, encompassing repair, transcription, and replication. Agents that induce ferroptosis, such as erastin, sorafenib, RSL3, and FIN56, can trigger the release of HMGB1. Subsequently, HMGB1 interacts with the immune system, thereby impacting cancer treatment [318, 319]. As a result, the buildup of DAMPs can trigger tissue inflammation and regulate ferroptosis through an automatic cascade [320]. Strategically utilizing DAMPs can trigger favorable immune responses, hindering tumor proliferation.
Targeting cancer cell differentiation plasticity
Cancer cell plasticity refers to the flexible nature of cancer cells as they change states, affecting tumor growth, spread, and response to treatment. The increasing diversity of cancer cells helps them adapt to the environment and evade anticancer therapies, presenting a major challenge in cancer treatment [321, 322]. Tsoi et al. demonstrate that melanoma can be classified into four subtypes based on a differentiation trajectory. Each subtype exhibits distinct sensitivity to ferroptosis induction, suggesting a therapeutic strategy to target differentiation plasticity. This approach aims to enhance the effectiveness of targeted and immune therapies for melanoma treatment [186]. Changes in cancer metabolism facilitate invasion and metastasis but render cells vulnerable to lipid peroxidation during oxidative stress. Cancers exhibiting mesenchymal characteristics, such as sarcomas found in bone, soft tissue, ovary, and kidney, heavily depend on the lipid peroxidase pathway. They display heightened sensitivity to ferroptosis-inducing agents like RSL3, ML-162, ML210, ML239, and statins compared to cancers originating from epithelial tissues like the esophagus and urinary tract. This implies that ferroptosis inducers may be more efficacious in treating mesenchymal-derived cancers than those derived from epithelial tissues [188]. Under certain metabolic and oxidative stress conditions, cancer cells can evade destruction, and by targeting this distinctive defense mechanism, it becomes possible to selectively eliminate cancer cells [323]. In the treatment of metastatic cancers that exhibit resistance and immune evasion, it is crucial to focus on regulating the GPX4, NF2-YAP signaling pathway, reversible epithelial-to-mesenchymal transition, and the pathways associated with blebbishield metastatic switch. Targeting these processes is essential for inducing ferroptosis and other forms of cell death, addressing the challenges posed by metastatic cancers [324–327].
Conclusion and future direction
Ferroptosis has emerged as a significant research focus in life sciences in recent years. Its discovery not only broadens our understanding of cell death diversity but also introduces new strategies and ideas for treating various diseases. Current research indicates that ferroptosis plays a crucial regulatory role in the onset and progression of cardiovascular diseases, neurodegenerative diseases, cancer, and other conditions. Modulating the ferroptosis pathway could potentially slow disease progression, offering innovative treatment approaches. However, there are challenges associated with ferroptosis, such as its lack of high specificity and difficulty in distinguishing it from other cell death modes. Treatments targeting ferroptosis can have side effects, including the unintended death of immune cells. Additionally, clinical applications face obstacles like targeted drug delivery and off-target effects.
Looking ahead, ferroptosis research can be further explored in several areas: Understanding the molecular mechanisms, including gene regulation, iron metabolism, and lipid metabolism interactions, will enhance our comprehension of ferroptosis in biology and medicine. Clinically, developing ferroptosis-targeting drugs and exploring drug combinations, along with identifying molecular markers for disease risk prediction and treatment evaluation, are crucial. Interdisciplinary research, integrating fields such as chemistry, materials science, and computer science, will advance ferroptosis research using new technologies and methods. In summary, ferroptosis, as a novel form of programmed cell death, holds significant research and clinical potential. With ongoing research and technological advancements, ferroptosis is expected to make substantial contributions to human health.
Acknowledgements
Bio Render
Abbreviations
- Acot1
Acyl-CoA thioesterase 1
- ACSL4
Acyl-CoA synthetase long chain family member 4
- AD
Alzheimer disease
- ALS
Amyotrophic lateral sclerosis
- ATF3/4
Activating transcription factor ¾
- ATG5/7
Autophagy related 5/7
- BH4
Tetrahydrobiopterin
- CRC
Colorectal cancer
- CoQ
Coenzyme Q
- CoQ10
Coenzyme Q10
- COX2
Cyclooxygenase-2
- CVDs
Cardiovascular diseases
- DAMPs
Damage-related molecular patterns
- DCM
Diabetic cardiomyopathy
- DFO
Deferoxamine
- DHODH
Dihydroorotate dehydrogenase
- DMT1
Divalent metal transporter-1
- DOX
Doxorubicin
- Drp1
Dynamin-related protein 1
- FPN1
Ferroportin 1
- FRDA
Friedreich's Ataxia
- FSP1
Ferroptosis suppressor protein 1
- FTH1
Ferritin heavy chain 1
- FTL
Ferritin light chain
- GC
Gastric cancer
- GCH1
GTP cyclohydrolase 1
- GPX4
Glutathione peroxidase 4
- GSH
Glutathione
- HCC
Hepatocellular carcinoma
- HCM
Hypertrophic cardiomyopathy
- HD
Huntington disease
- HMGB1
High mobility group protein B1
- HO-1
Heme oxygenase-1
- HTT
Huntingtin
- Lip-1
Liproxstatin-1
- ICI
Immune checkpoint inhibitors
- IFN-γ
Interferon-γ
- IOC
Iron overload cardiomyopathy
- I/R
Ischemia–reperfusion injury
- LOX
Lipoxygenase
- LPCAT3
Lysophosphatidylcholine acyltransferase 3
- LPS
Lipopolysaccharide
- MI
Myocardial infarction
- MS
Multiple sclerosis
- mTOR
Mechanism target of rapamycin
- NCOA4
Nuclear receptor coactivator 4
- NDs
Neurodegenerative diseases
- Nrf2
Nuclearrespiratoty factor 2
- NSCLC
Non-small-cell carcinoma
- NTBI
Non-TF-bound iron
- OVCA
Ovarian cancer
- OxPCs
Oxidized phosphatidylcholine
- PD
Parkinson disease
- PDAC
Pancreatic ductal adenocarcinoma
- PKCβII
Protein kinase CβII
- Ptgs2
Prostaglandin-endoperoxide synthase 2
- PUFA
Polyunsaturated fatty acid
- RICM
Radiation-induced cardiomyopathy
- RILF
Radiation-induced lung fibrosis
- ROS
Reactive oxygen species
- SCM
Septic cardiomyopathy
- SLC7A11
Solute carrier family 7 member 11
- TLR4
Toll-like receptors 4
Author contributions
Yinghui Li wrote the manuscript and drew diagrams; Cuiyun Liu and Bo Fang revised the manuscript and completed tables; Xinzhe Chen and Kai Wang checked the grammar and polished the manuscript; Hui Xin, Kun Wang and Su-Min Yang responded comments and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (82070313, 82370291).
Availability of data and materials
From Pubmed Data base.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yinghui Li, Cuiyun Liu, and Bo Fang are Co-author.
Contributor Information
Hui Xin, Email: huixin202411@163.com.
Kun Wang, Email: wangk696@qdu.edu.cn.
Su-Min Yang, Email: ysumin2024@126.com.
References
- 1.Jiang X, et al. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan H, et al. Ferroptosis and its potential as a therapeutic target. Biochem Pharmacol. 2021;186:114486. [DOI] [PubMed] [Google Scholar]
- 3.Xu S, et al. The emerging role of ferroptosis in intestinal disease. Cell Death Dis. 2021;12(4):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan H-F, et al. Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther. 2021;6(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang R, et al. Polyphyllin I induced ferroptosis to suppress the progression of hepatocellular carcinoma through activation of the mitochondrial dysfunction via Nrf2/HO-1/GPX4 axis. Phytomedicine Int J Phytother Phytopharm. 2024;122:155135. [DOI] [PubMed] [Google Scholar]
- 6.Li N, et al. Ferroptosis and its emerging roles in cardiovascular diseases. Pharmacol Res. 2021;166:105466. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, et al. The molecular mechanisms of ferroptosis and its role in cardiovascular disease. Biomed Pharmacother Biomedecine Pharmacother. 2022;145:112423. [DOI] [PubMed] [Google Scholar]
- 8.Han X, et al. Targeting ferroptosis: a novel insight against myocardial infarction and ischemia-reperfusion injuries. Apoptosis Int J Program Cell Death. 2023;28(1-2):108-123. [DOI] [PubMed] [Google Scholar]
- 9.Hu G, et al. Autophagy inhibition and ferroptosis activation during atherosclerosis: hypoxia-inducible factor 1α inhibitor PX-478 alleviates atherosclerosis by inducing autophagy and suppressing ferroptosis in macrophages. Biomed Pharmacother Biomedecine Pharmacother. 2023;161:114333. [DOI] [PubMed] [Google Scholar]
- 10.Yang X, et al. Ferroptosis in heart failure. J Mol Cell Cardiol. 2022;173:141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, et al. Pharmacological inhibition of ferroptosis as a therapeutic target for neurodegenerative diseases and strokes. Adv Sci. 2023;10(24):e2300325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao W-D, et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021;28:1548–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reichert CO, et al. Ferroptosis mechanisms involved in neurodegenerative diseases. Int J Mol Sci. 2020;21(22):8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, et al. Ferroptosis and its multifaceted role in cancer: mechanisms and therapeutic approach. Antioxid Basel Switz. 2022;11(8):1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, et al. Overcoming cancer chemotherapy resistance by the induction of ferroptosis. Drug Resist Updat Rev Comment Antimicrob Anticancer Chemother. 2023;66:100916. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C, et al. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer. 2022;21(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, et al. Pyroptosis: role and mechanisms in cardiovascular disease. Front Cardiovasc Med. 2022;9:897815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang R, et al. Molecular therapy of cardiac ischemia-reperfusion injury based on mitochondria and ferroptosis. J Mol Med Berl Ger. 2023;101(9):1059-1071. [DOI] [PubMed] [Google Scholar]
- 19.Ju J, et al. Mechanism of ferroptosis: a potential target for cardiovascular diseases treatment. Aging Dis. 2021;12(1):261-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, et al. Iron overload causes ferroptosis but not apoptosis in MO3.13 oligodendrocytes. Neurochem Res. 2023;48(3):830-838. [DOI] [PubMed] [Google Scholar]
- 21.Sun S, et al. Targeting ferroptosis opens new avenues for the development of novel therapeutics. Signal Transduct Target Ther. 2023;8(1):372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou R-P, et al. Novel insights into ferroptosis: implications for age-related diseases. Theranostics. 2020;10(26):11976-11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoyanovsky DA, et al. Iron catalysis of lipid peroxidation in ferroptosis: regulated enzymatic or random free radical reaction? Free Radic Biol Med. 2019;133:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy JE, et al. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet. 1999;21(4):396-399. [DOI] [PubMed] [Google Scholar]
- 25.Porter JB, et al. Mechanisms of plasma non-transferrin bound iron generation: insights from comparing transfused diamond blackfan anaemia with sickle cell and thalassaemia patients. Br J Haematol. 2014;167(5):692-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumfu S, et al. T-type calcium channel as a portal of iron uptake into cardiomyocytes of beta-thalassemic mice. Eur J Haematol. 2011;86(2):156-166. [DOI] [PubMed] [Google Scholar]
- 27.Jiang L-R, e al. Verapamil downregulates iron uptake and upregulates divalent metal transporter 1 expression in H9C2 cardiomyocytes. Fundam Clin Pharmacol. 2022;36(6):985-991. [DOI] [PubMed] [Google Scholar]
- 28.Dixon SJ, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu Y, et al. The application of ferroptosis in diseases. Pharmacol Res. 2020;159:104919. [DOI] [PubMed] [Google Scholar]
- 30.Zhou D, et al. Ferroptosis and metabolic syndrome and complications: association, mechanism, and translational applications. Front Endocrinol. 2024;14:1248934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin Y, et al. Ferritinophagy and ferroptosis in cardiovascular disease: mechanisms and potential applications. Biomed Pharmacother Biomedecine Pharmacother. 2021;141:111872. [DOI] [PubMed] [Google Scholar]
- 32.Stockwell BR, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang WS, et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113(34):E4966-E4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doll S, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H-L, et al. PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat Cell Biol. 2022;24(1):88-98. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, et al. Ferroptosis: machinery and regulation. Autophagy. 2021;17(9):2054-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedmann Angeli JP, et al. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer. 2019;19(7):405-414. [DOI] [PubMed] [Google Scholar]
- 38.Yan B, et al. Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Mol Cell. 2021;81(2):355-369.e10. [DOI] [PubMed] [Google Scholar]
- 39.Liu M, et al. The critical role and molecular mechanisms of ferroptosis in antioxidant systems: a narrative review. Ann Transl Med. 2022;10(6):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc. Cell Death Differ. 2020;27(2):662-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stockwell BR. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185(14):2401-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bersuker K, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He W, et al. The role of p53 in regulating chronic inflammation and PANoptosis in diabetic wounds. Aging Dis. 2024. 10.14336/AD.2024.0212. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, et al. Organelle-specific regulation of ferroptosis. Cell Death Differ. 2021;28(10):2843-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen F, et al. Autophagy-dependent ferroptosis in cancer. Antioxid Redox Signal. 2023;39(1-3):79-101. [DOI] [PubMed] [Google Scholar]
- 46.Hetz C, et al. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020;21(8):421-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang X, et al. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol. 2023;20(1):7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu X, et al. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 2021;11(7):3052-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu M-L, et al. Klf6 aggravates myocardial ischemia/reperfusion injury by activating Acsl4-mediated ferroptosis. Kaohsiung J Med Sci. 2023;39(10):989-1001. [DOI] [PubMed] [Google Scholar]
- 50.Zhuang S, et al. METTL14 promotes doxorubicin-induced cardiomyocyte ferroptosis by regulating the KCNQ1OT1-miR-7-5p-TFRC axis. Cell Biol Toxicol. 2023;39(3):1015-1035. [DOI] [PubMed] [Google Scholar]
- 51.Jang S, et al. Elucidating the contribution of mitochondrial glutathione to ferroptosis in cardiomyocytes. Redox Biol. 2021;45:102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang W, et al. Canagliflozin attenuates lipotoxicity in cardiomyocytes by inhibiting inflammation and ferroptosis through activating AMPK pathway. Int J Mol Sci. 2023;24(1):858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tadokoro T, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020;5(9):e132747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saito Y, et al. Treatment strategies of acute myocardial infarction: updates on revascularization, pharmacological therapy, and beyond. J Cardiol. 2023;81(2):168-178. [DOI] [PubMed] [Google Scholar]
- 55.Jenča D, et al. Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail. 2021;8(1):222-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ritchie RH, et al. Basic mechanisms of diabetic heart disease. Circ Res. 2020;126(11):1501-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu H, et al. The link between ferroptosis and cardiovascular diseases: a novel target for treatment. Front Cardiovasc Med. 2021;8:710963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park T-J, et al. Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis. 2019;10(11):835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fang X, et al. Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ Res. 2020;127(4):486-501. [DOI] [PubMed] [Google Scholar]
- 60.Baba Y, et al. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2018;314(3):H659-H668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guan P, et al. Mammalian target of rapamycin coordinates iron metabolism with iron-sulfur cluster assembly enzyme and tristetraprolin. Nutr Burbank Los Angel Cty Calif. 2014;30(9):968-974. [DOI] [PubMed] [Google Scholar]
- 62.Wang Z, et al. TRIM3 facilitates ferroptosis in non-small cell lung cancer through promoting SLC7A11/xCT K11-linked ubiquitination and degradation. Cell Death Differ. 2024;31(1):53-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song Y, et al. Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol Toxicol. 2021;37(1):51-64. [DOI] [PubMed] [Google Scholar]
- 64.Ferdinandy P, et al. Interaction of cardiovascular nonmodifiable risk factors, comorbidities and comedications with ischemia/reperfusion injury and cardioprotection by pharmacological treatments and ischemic conditioning. Pharmacol Rev. 2023;75(1):159-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stamenkovic A, et al. Oxidized phosphatidylcholines trigger ferroptosis in cardiomyocytes during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2021;320(3):H1170-H1184. [DOI] [PubMed] [Google Scholar]
- 66.Zhu L, et al. The cyclooxygenase-1/mPGES-1/endothelial prostaglandin EP4 receptor pathway constrains myocardial ischemia-reperfusion injury. Nat Commun. 2019;10:1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiang Q, et al. Regulated cell death in myocardial ischemia-reperfusion injury. Trends Endocrinol Metab. 2024;35(3):219-234. [DOI] [PubMed] [Google Scholar]
- 68.Fan Z, et al. Baicalin prevents myocardial ischemia/reperfusion injury through inhibiting ACSL4 mediated ferroptosis. Front Pharmacol. 2021;12:628988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li W, et al. Inhibition of DNMT-1 alleviates ferroptosis through NCOA4 mediated ferritinophagy during diabetes myocardial ischemia/reperfusion injury. Cell Death Discov. 2021;7(1):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fang X, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116(7):2672-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan W, et al. Effect of iron chelation on myocardial infarct size and oxidative stress in ST-elevation-myocardial infarction. Circ Cardiovasc Interv. 2012;5(2):270-278. [DOI] [PubMed] [Google Scholar]
- 72.Feng Y, et al. Liproxstatin-1 protects the mouse myocardium against ischemia/reperfusion injury by decreasing VDAC1 levels and restoring GPX4 levels. Biochem Biophys Res Commun. 2019;520(3):606-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghosh AP, et al. CHOP potentially co-operates with FOXO3a in neuronal cells to regulate PUMA and BIM expression in response to ER stress. PLoS ONE. 2012;7(6):e39586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee Y-S, et al. Ferroptosis-induced endoplasmic reticulum stress: cross-talk between ferroptosis and apoptosis. Mol Cancer Res MCR. 2018;16(7):1073-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cao SS, et al. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21(3):396-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li W, et al. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J Clin Invest.2019;129(6):2293-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moore B, et al. Sudden cardiac death and ventricular arrhythmias in hypertrophic cardiomyopathy. Heart Lung Circ. 2019;28(1):146-154. [DOI] [PubMed] [Google Scholar]
- 78.Ito J, et al. Iron derived from autophagy-mediated ferritin degradation induces cardiomyocyte death and heart failure in mice. Elife. 2021;10:e62174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang X, et al. SLC7A11/xCT prevents cardiac hypertrophy by inhibiting ferroptosis. Cardiovasc Drugs Ther. 2022;36(3):437-447. [DOI] [PubMed] [Google Scholar]
- 80.Cotticelli MG, et al. Ferroptosis as a novel therapeutic target for Friedreich’s ataxia. J Pharmacol Exp Ther. 2019;369(1):47-54. [DOI] [PubMed] [Google Scholar]
- 81.Turchi R, et al. An overview of the ferroptosis hallmarks in Friedreich’s ataxia. Biomolecules. 2020;10(11):1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu P, et al. Ferroptosis: mechanisms and role in diabetes mellitus and its complications. Ageing Res Rev. 2024;94:102201. [DOI] [PubMed] [Google Scholar]
- 83.DeSantis CE, et al. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66(4):290-308. [DOI] [PubMed] [Google Scholar]
- 84.Li D, et al. Ferroptosis and its role in cardiomyopathy. Biomed Pharmacother. 2022;153:113279. [DOI] [PubMed] [Google Scholar]
- 85.Imai H, et al. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol. 2017;403:143–70. [DOI] [PubMed] [Google Scholar]
- 86.Liuzzi JP, et al. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci U S A. 2006;103(37):13612-13617. [DOI] [PMC free article] [PubMed]
- 87.Zhang H, et al. Protective effects of dexazoxane on rat ferroptosis in doxorubicin-induced cardiomyopathy through regulating HMGB1. Front Cardiovasc Med. 2021;8:685434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Robb A, et al. Regulation of transferrin receptor 2 protein levels by transferrin. Blood. 2004;104(13):4294-4299. [DOI] [PubMed] [Google Scholar]
- 89.Gujja P, et al. Iron overload cardiomyopathy: better understanding of an increasing disorder. J Am Coll Cardiol. 2010;56(13):1001-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fang X, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116(7):2672-2680. [DOI] [PMC free article] [PubMed]
- 91.Khamseekaew J, et al. Effects of the iron chelator deferiprone and the T-type calcium channel blocker efonidipine on cardiac function and Ca2+ regulation in iron-overloaded thalassemic mice. Cell Calcium. 2018;72:18–25. [DOI] [PubMed] [Google Scholar]
- 92.Wongjaikam S, et al. Combined iron chelator and antioxidant exerted greater efficacy on cardioprotection than monotherapy in iron-overloaded rats. PLoS ONE. 2016;11(7):e0159414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wongjaikam S, et al. Restoring the impaired cardiac calcium homeostasis and cardiac function in iron overload rats by the combined deferiprone and N-acetyl cysteine. Sci Rep. 2017;7:44460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berdoukas V, et al. Iron and oxidative stress in cardiomyopathy in thalassemia. Free Radic Biol Med. 2015;88(Pt A):3-9. [DOI] [PubMed] [Google Scholar]
- 95.Hollenberg SM, Singer M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol. 2021;18(6):424-434. [DOI] [PubMed] [Google Scholar]
- 96.Zhai Z, et al. Ferroptosis is a potential novel diagnostic and therapeutic target for patients with cardiomyopathy. Front Cell Dev Biol. 2021;9:649045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li N, et al. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic Biol Med. 2020;160:303-318. [DOI] [PubMed] [Google Scholar]
- 98.Yang M, et al. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci Adv. 2019;5(7):eaaw2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang C-S, et al. Identification of a novel small-molecule Keap1-Nrf2 PPI inhibitor with cytoprotective effects on LPS-induced cardiomyopathy. J Enzyme Inhib Med Chem. 2018;33(1):833-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jia G, et al. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen Y, et al. Distinct types of cell death and the implication in diabetic cardiomyopathy. Front Pharmacol. 2020;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cuomo F, et al. Autophagy function and dysfunction: potential drugs as anti-cancer therapy. Cancers. 2019;11(10):1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang L, et al. Cardioprotective effect of icariin against myocardial fibrosis and its molecular mechanism in diabetic cardiomyopathy based on network pharmacology: Role of ICA in DCM. Phytomedicine Int J Phytother Phytopharm. 2021;91:153607. [DOI] [PubMed] [Google Scholar]
- 104.Huang F, et al. Targeting ferroptosis to treat cardiovascular diseases: a new continent to be explored. Front Cell Dev Biol. 2021;9:737971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baseler WA, et al. Reversal of mitochondrial proteomic loss in type 1 diabetic heart with overexpression of phospholipid hydroperoxide glutathione peroxidase. Am J Physiol Regul Integr Comp Physiol. 2013;304(7):R553-R565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Behring JB, et al. Does reversible cysteine oxidation link the Western diet to cardiac dysfunction? FASEB J Off Publ Fed Am Soc Exp Biol. 2014;28(5):1975-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shu T, et al. Hepcidin as a key iron regulator mediates glucotoxicity-induced pancreatic β-cell dysfunction. Endocr Connect. 2019;8(3):150-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bruni A, et al. Regulated cell death seen through the lens of islet transplantation. Cell Transplant. 2018;27(6):890-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zang H, et al. Autophagy inhibition enables Nrf2 to exaggerate the progression of diabetic cardiomyopathy in mice. Diabetes. 2020;69(12):2720-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xue W, et al. Cardiac-specific overexpression of HIF-1{alpha} prevents deterioration of glycolytic pathway and cardiac remodeling in streptozotocin-induced diabetic mice. Am J Pathol. 2010;177(1):97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang B, et al. Radiation-induced myocardial fibrosis: mechanisms underlying its pathogenesis and therapeutic strategies. J Cell Mol Med. 2020;24(14):7717-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jelonek K, et al. Cardiac endothelial cells isolated from mouse heart - a novel model for radiobiology. Acta Biochim Pol. 2011;58(3):397-404. [PubMed] [Google Scholar]
- 113.Kuwahara Y, et al. Enhancement of autophagy is a potential modality for tumors refractory to radiotherapy. Cell Death Dis. 2011;2(6):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lei G, et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Y, et al. miR-137 boosts the neuroprotective effect of endothelial progenitor cell-derived exosomes in oxyhemoglobin-treated SH-SY5Y cells partially via COX2/PGE2 pathway. Stem Cell Res Ther. 2020;11:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Storozynsky Q, et al. The impact of radiation-induced DNA damage on cGAS-STING-mediated immune responses to cancer. Int J Mol Sci. 2020;21(22):8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li X, et al. Ferroptosis inhibitor alleviates Radiation-induced lung fibrosis (RILF) via down-regulation of TGF-β1. J Inflamm Lond Engl. 2019;16:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ye T, et al. Trastuzumab-induced cardiomyopathy via ferroptosis-mediated mitochondrial dysfunction. Free Radic Biol Med. 2023;206:143–161. [DOI] [PubMed] [Google Scholar]
- 119.Huang C, et al. Pharmacological activation of GPX4 ameliorates doxorubicin-induced cardiomyopathy. Redox Biol. 2024;70:103024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen Y, et al. Targeting iron metabolism and ferroptosis as novel therapeutic approaches in cardiovascular diseases. Nutrients. 2023;15(3):591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Y, et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26(11):2284-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu X, et al. Mitochondrial calpain-1 activates NLRP3 inflammasome by cleaving ATP5A1 and inducing mitochondrial ROS in CVB3-induced myocarditis. Basic Res Cardiol. 2022;117(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zeng X, et al. Activated Drp1 regulates p62-mediated autophagic flux and aggravates inflammation in cerebral ischemia-reperfusion via the ROS-RIP1/RIP3-exosome axis. Mil Med Res. 2022;9(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou Y-X, et al. Effects of puerarin on the prevention and treatment of cardiovascular diseases. Front Pharmacol. 2021;12:771793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mangione MC, et al. Mechanistic target of rapamycin in regulating macrophage function in inflammatory cardiovascular diseases. J Mol Cell Cardiol. 2024;186:111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jomova K, et al. Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch Toxicol. 2024;98(5):1323-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao Z, et al. XJB-5-131 inhibited ferroptosis in tubular epithelial cells after ischemia-reperfusion injury. Cell Death Dis. 2020;11(8):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xie Y, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Costa I, et al. Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacol Ther. 2023;244:108373. [DOI] [PubMed] [Google Scholar]
- 130.Wang P, et al. Mitochondrial ferritin attenuates cerebral ischaemia/reperfusion injury by inhibiting ferroptosis. Cell Death Dis. 2021;12(5):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cheng Y, et al. TrkB agonist N-acetyl serotonin promotes functional recovery after traumatic brain injury by suppressing ferroptosis via the PI3K/Akt/Nrf2/Ferritin H pathway. Free Radic Biol Med. 2023;194:184–98. [DOI] [PubMed] [Google Scholar]
- 132.Weiland A, et al. Ferroptosis and its role in diverse brain diseases. Mol Neurobiol. 2019;56(7):4880-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Long H, et al. Iron homeostasis imbalance and ferroptosis in brain diseases. MedComm. 2023;4(4):e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Song S, et al. Targeting NRF2 to suppress ferroptosis in brain injury. Histol Histopathol. 2021;36(4):383-397. [DOI] [PubMed] [Google Scholar]
- 135.Yang L, et al. Ferroptosis: a potential therapeutic target for Alzheimer’s disease. Rev Neurosci. 2022;34(5):573-598. [DOI] [PubMed] [Google Scholar]
- 136.Lee J, et al. The interplay between intracellular iron homeostasis and neuroinflammation in neurodegenerative diseases. Antioxid Basel Switz. 2023;12(4):918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li J, et al. β-amyloid protein induces mitophagy-dependent ferroptosis through the CD36/PINK/PARKIN pathway leading to blood-brain barrier destruction in Alzheimer’s disease. Cell Biosci. 2022;12(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gao D, et al. Tetrahydroxy stilbene glucoside ameliorates cognitive impairments and pathology in APP/PS1 transgenic mice. Curr Med Sci. 2021;41(2):279-286. [DOI] [PubMed] [Google Scholar]
- 139.Cong L, et al. On the role of synthesized hydroxylated chalcones as dual functional amyloid-β aggregation and ferroptosis inhibitors for potential treatment of Alzheimer’s disease. Eur J Med Chem. 2019;166:11–21. [DOI] [PubMed] [Google Scholar]
- 140.Sun Y, et al. Inhibition of ferroptosis through regulating neuronal calcium homeostasis: an emerging therapeutic target for Alzheimer’s disease. Ageing Res Rev. 2023;87:101899. [DOI] [PubMed] [Google Scholar]
- 141.Wang Z-L, et al. Ferroptosis in Parkinson’s disease: glia-neuron crosstalk. Trends Mol Med. 2022;28(4):258-269. [DOI] [PubMed] [Google Scholar]
- 142.Ding X-S, et al. Ferroptosis in Parkinson’s disease: molecular mechanisms and therapeutic potential. Ageing Res Rev. 2023;91: 102077. [DOI] [PubMed] [Google Scholar]
- 143.Takahashi H, et al. Comprehensive MRI quantification of the substantia nigra pars compacta in Parkinson’s disease. Eur J Radiol. 2018;109:48–56. [DOI] [PubMed] [Google Scholar]
- 144.Lin K-J, et al. Iron brain menace: the involvement of ferroptosis in Parkinson disease. Cells. 2022;11(23):3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.K, Thapa., et al. Therapeutic insights on ferroptosis in Parkinson’s disease. Eur J Pharmacol. 2022;930:175133. [DOI] [PubMed] [Google Scholar]
- 146.Sun Y, et al. Activation of p62-Keap1-Nrf2 pathway protects 6-hydroxydopamine-induced ferroptosis in dopaminergic cells. Mol Neurobiol. 2020;57(11):4628-4641. [DOI] [PubMed] [Google Scholar]
- 147.Liu T, et al. Rapamycin reverses ferroptosis by increasing autophagy in MPTP/MPP+-induced models of Parkinson’s disease. Neural Regen Res. 2023;18(11):2514-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Berggren KL, et al. Neonatal iron supplementation potentiates oxidative stress, energetic dysfunction and neurodegeneration in the R6/2 mouse model of Huntington’s disease. Redox Biol. 2015;4:363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Song S, et al. ALOX5-mediated ferroptosis acts as a distinct cell death pathway upon oxidative stress in Huntington’s disease. Genes Dev. 2023;37(5-6):204-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee J, et al. Modulation of lipid peroxidation and mitochondrial function improves neuropathology in Huntington’s disease mice. Acta Neuropathol (Berl). 2011;121(4):487-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Skouta R, et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136(12):4551-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Allison RL, et al. ALS iPSC-derived microglia and motor neurons respond to astrocyte-targeted IL-10 and CCL2 modulation. Hum Mol Genet. 2024;33(6):530-542. [DOI] [PubMed] [Google Scholar]
- 153.Moreau C, et al. Could conservative iron chelation lead to neuroprotection in amyotrophic lateral sclerosis? Antioxid Redox Signal. 2018;29(8):742-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Feldman EL, et al. Amyotrophic lateral sclerosis. Lancet. 2022;400(10360):1363-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang T, et al. Ferroptosis mediates selective motor neuron death in amyotrophic lateral sclerosis. Cell Death Differ. 2022;29(6):1187-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Singh P, et al. Edaravone oral suspension: a neuroprotective agent to treat amyotrophic lateral sclerosis. Am J Ther. 2024;31(3):e258-e267. [DOI] [PubMed] [Google Scholar]
- 157.Soares P, et al. Drug discovery and amyotrophic lateral sclerosis: emerging challenges and therapeutic opportunities. Ageing Res Rev. 2023;83:101790. [DOI] [PubMed] [Google Scholar]
- 158.Gu S-C, et al. Identification of ferroptosis-related gene signatures associated with multiple sclerosis using weighted gene co-expression network analysis. Medicine. 2022;101(51):e31802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Luoqian J, et al. Ferroptosis promotes T-cell activation-induced neurodegeneration in multiple sclerosis. Cell Mol Immunol. 2022;19(8):913-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Duarte-Silva E, et al. The role of iron metabolism in the pathogenesis and treatment of multiple sclerosis. Front Immunol. 2023;14:1137635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Van San E, et al. Ferroptosis contributes to multiple sclerosis and its pharmacological targeting suppresses experimental disease progression. Cell Death Differ. 2023;30(9):2092-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zierfuss B, et al. Iron in multiple sclerosis - neuropathology, immunology, and real-world considerations. Mult Scler Relat Disord. 2023;78:104934. [DOI] [PubMed] [Google Scholar]
- 163.Santiago González DA, et al. Iron metabolism in the peripheral nervous system: the role of DMT1, ferritin, and transferrin receptor in schwann cell maturation and myelination. J Neurosci Off J Soc Neurosci. 2019;39(50):9940-9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lane DJR, et al. Iron and Alzheimer’s disease: an update on emerging mechanisms. J Alzheimers Dis JAD. 2018;64(s1):S379-S395. [DOI] [PubMed] [Google Scholar]
- 165.Hambright WS, et al. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017;12:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu H, et al. New progress on the role of glia in iron metabolism and iron-induced degeneration of dopamine neurons in Parkinson’s disease. Front Mol Neurosci. 2017;10:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee H-G, et al. Function and therapeutic value of astrocytes in neurological diseases. Nat Rev Drug Discov. 2022;21(5):339-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li S, et al. Ferrostatin-1 alleviates angiotensin II (Ang II)- induced inflammation and ferroptosis in astrocytes. Int Immunopharmacol. 2021;90:107179. [DOI] [PubMed] [Google Scholar]
- 169.Chen L, et al. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. J Biol Chem. 2015;290(47):28097-28106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hoshino T, et al. Susceptibility to erastin-induced ferroptosis decreases during maturation in a human oligodendrocyte cell line. FEBS Open Bio. 2020;10(9):1758-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Apetri MM, et al. Direct observation of α-synuclein amyloid aggregates in endocytic vesicles of neuroblastoma cells. PLoS ONE. 2016;11(4):e0153020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Abounit S, et al. Tunneling nanotubes spread fibrillar α-synuclein by intercellular trafficking of lysosomes. EMBO J. 2016;35(19):2120-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.de Visser KE, et al. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41(3):374-403. [DOI] [PubMed] [Google Scholar]
- 174.Cao M, et al. Natural compounds modulating mitophagy: implications for cancer therapy. Cancer Lett. 2024;582:216590. [DOI] [PubMed] [Google Scholar]
- 175.Cesur-Ergün B, et al. Gene therapy in cancer. J Gene Med. 2023;25(11):e3550. [DOI] [PubMed] [Google Scholar]
- 176.Tomat E. Targeting iron to contrast cancer progression. Curr Opin Chem Biol. 2023;74:102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tisserand J, et al. Association between iron deficiency and survival in older patients with cancer. Cancers. 2023;15(5):1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Morales M, et al. Targeting iron metabolism in cancer therapy. Theranostics. 2021;11(17):8412-8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Phipps O, et al. Iron deficiency, immunology, and colorectal cancer. Nutr Rev. 2021;79(1):88-97. [DOI] [PubMed] [Google Scholar]
- 180.Zhang R, et al. Ferroptosis in cancer progression. Cells. 2023;12(14):1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rodriguez R, et al. Persister cancer cells: iron addiction and vulnerability to ferroptosis. Mol Cell. 2022;82(4):728-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu M-Y, et al. TIGAR drives colorectal cancer ferroptosis resistance through ROS/AMPK/SCD1 pathway. Free Radic Biol Med. 2022;182:219–231. [DOI] [PubMed] [Google Scholar]
- 183.Tang B, et al. Pharmacological inhibition of MELK restricts ferroptosis and the inflammatory response in colitis and colitis-propelled carcinogenesis. Free Radic Biol Med. 2021;172:312–329. [DOI] [PubMed] [Google Scholar]
- 184.Liu Y, et al. The diversified role of mitochondria in ferroptosis in cancer. Cell Death Dis. 2023;14(8):519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Roh J-L, et al. Induction of ferroptotic cell death for overcoming cisplatin resistance of head and neck cancer. Cancer Lett. 2016;381(1):96-103 [DOI] [PubMed] [Google Scholar]
- 186.Tsoi J, et al. Multi-stage differentiation defines melanoma subtypes with differential vulnerability to drug-induced iron-dependent oxidative stress. Cancer Cell. 2018;33(5):890-904.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Viswanathan VS, et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547(7664):453-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hangauer MJ, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551(7679):247-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lang X, et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9(12):1673-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhou Q, et al. Ferroptosis in cancer: from molecular mechanisms to therapeutic strategies. Signal Transduct Target Ther. 2024;9(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Malik PS, et al. Lung cancer: prevalent trends & emerging concepts. Indian J Med Res. 2015;141(1):5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wu S, et al. The role of ferroptosis in lung cancer. Biomark Res. 2021;9(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zou J, et al. Ferroptosis in non-small cell lung cancer: progression and therapeutic potential on It. Int J Mol Sci. 2021;22(24):13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang W, et al. Bufotalin induces ferroptosis in non-small cell lung cancer cells by facilitating the ubiquitination and degradation of GPX4. Free Radic Biol Med. 2022;180:75–84. [DOI] [PubMed] [Google Scholar]
- 195.Zhang W, et al. RBMS1 regulates lung cancer ferroptosis through translational control of SLC7A11. J Clin Invest. 2021;131(22):e152067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Koppula P, et al. A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat Commun. 2022;13(1):2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ye LF, et al. Radiation-Induced Lipid Peroxidation Triggers Ferroptosis and Synergizes with Ferroptosis Inducers. ACS Chem Biol. 2020;15(2):469-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Du S, et al. Prognostic and therapeutic significance of a novel ferroptosis related signature in colorectal cancer patients. Bioengineered. 2022;13(2):2498-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang Y, et al. Ferroptosis in colorectal cancer: potential mechanisms and effective therapeutic targets. Biomed Pharmacother Biomedecine Pharmacother. 2022;153:113524. [DOI] [PubMed] [Google Scholar]
- 200.Wang R, et al. Inhibition of SRSF9 enhances the sensitivity of colorectal cancer to erastin-induced ferroptosis by reducing glutathione peroxidase 4 expression. Int J Biochem Cell Biol. 2021;134:105948. [DOI] [PubMed] [Google Scholar]
- 201.Wang R, et al. HSPA5 repressed ferroptosis to promote colorectal cancer development by maintaining GPX4 stability. Neoplasma. 2022;69(5):1054-1069. [DOI] [PubMed] [Google Scholar]
- 202.Zhang L, et al. IMCA induces ferroptosis mediated by SLC7A11 through the AMPK/mTOR pathway in colorectal cancer. Oxid Med Cell Longev. 2020;2020:1675613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lu D, et al. ACADSB regulates ferroptosis and affects the migration, invasion, and proliferation of colorectal cancer cells. Cell Biol Int. 2020;44(11):2334-2343. [DOI] [PubMed] [Google Scholar]
- 204.Hu B, et al. Insights on Ferroptosis and Colorectal Cancer: Progress and Updates. Molecules. 2022;28(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cui W, et al. Gut microbial metabolite facilitates colorectal cancer development via ferroptosis inhibition. Nat Cell Biol. 2024;26(1):124-137. [DOI] [PubMed] [Google Scholar]
- 206.Wei R, et al. Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. Int J Biol Sci. 2021;17(11):2703-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yang L, et al. Mechanism and application of ferroptosis in colorectal cancer. Biomed Pharmacother. 2023;158:114102. [DOI] [PubMed] [Google Scholar]
- 208.Aizawa S, et al. Cell death and liver disease. Gut Liver. 2020;14(1):20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nie J, et al. Role of ferroptosis in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2018;144(12):2329-2337. [DOI] [PubMed] [Google Scholar]
- 210.Ajoolabady A, et al. Ferroptosis in hepatocellular carcinoma: mechanisms and targeted therapy. Br J Cancer. 2023;128(2):190-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liao H, et al. Molecular targets of ferroptosis in hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:985–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xu F-L, et al. SLC27A5 promotes sorafenib-induced ferroptosis in hepatocellular carcinoma by downregulating glutathione reductase. Cell Death Dis. 2023;14(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mo Y, et al. Targeting ferroptosis in hepatocellular carcinoma. Hepatol Int. 2024;18(1):32-49. [DOI] [PubMed] [Google Scholar]
- 214.Lu Y, et al. Epigenetic regulation of ferroptosis via ETS1/miR-23a-3p/ACSL4 axis mediates sorafenib resistance in human hepatocellular carcinoma. J Exp Clin Cancer Res CR. 2022;41(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li Y, et al. Targeting fatty acid synthase modulates sensitivity of hepatocellular carcinoma to sorafenib via ferroptosis. J Exp Clin Cancer Res CR. 2023;42(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gao R, et al. YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol Med. 2021;13(12):e14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang Q, et al. GSTZ1 sensitizes hepatocellular carcinoma cells to sorafenib-induced ferroptosis via inhibition of NRF2/GPX4 axis. Cell Death Dis. 2021;12(5):426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li G, et al. A novel ferroptosis-related long non-coding RNA prognostic signature correlates with genomic heterogeneity, immunosuppressive phenotype, and drug sensitivity in hepatocellular carcinoma. Front Immunol. 2022;13:929089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tang F, et al. Identification and validation of ferroptosis-related prognostic risk model and immune landscape in hepatocellular carcinoma. Immunobiology. 2023;228(5):152723. [DOI] [PubMed] [Google Scholar]
- 220.Xu Z, et al. Construction of a ferroptosis-related nine-lncRNA signature for predicting prognosis and immune response in hepatocellular carcinoma. Front Immunol. 2021;12:719175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li Y, et al. Ferroptosis: opening up potential targets for gastric cancer treatment. Mol Cell Biochem. 2024;479(11):2863-2874. [DOI] [PubMed] [Google Scholar]
- 222.Gu R, et al. Ferroptosis and its role in gastric cancer. Front Cell Dev Biol. 2022;10:860344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Malfa GA, et al. Betula etnensis Raf. (Betulaceae) extract induced HO-1 expression and ferroptosis cell death in human colon cancer cells. Int J Mol Sci. 2019;20(11):2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hu X, et al. miRNA-103a-3p promotes human gastric cancer cell proliferation by targeting and suppressing ATF7 in vitro. Mol Cells. 2018;41(5):390-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang G, et al. Ferroptosis‑related long non‑coding RNAs and the roles of LASTR in stomach adenocarcinoma. Mol Med Rep. 2022;25(4):118. [DOI] [PMC free article] [PubMed]
- 226.Hao S, et al. Cysteine dioxygenase 1 mediates erastin-induced ferroptosis in human gastric cancer cells. Neoplasia N Y N. 2017;19(12):1022-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Liu Y, et al. Identification of ferroptosis as a novel mechanism for antitumor activity of natural product derivative a2 in gastric cancer. Acta Pharm Sin B. 2021;11(6):1513-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhang Z, et al. The regulatory mechanism and research progress of ferroptosis in gastric cancer. Technol Cancer Res Treat. 2023;22:15330338231168498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chen X, et al. The ferroptosis-related noncoding RNA signature as a novel prognostic biomarker in the tumor microenvironment, immunotherapy, and drug screening of gastric adenocarcinoma. Front Oncol. 2021;11:778557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bao Z-H, et al. The mechanism and progress of ferroptosis in pancreatic cancer. Acta Histochem. 2022;124(6):151919. [DOI] [PubMed] [Google Scholar]
- 231.Liu K, et al. Trypsin-Mediated Sensitization to Ferroptosis Increases the Severity of Pancreatitis in Mice. Cell Mol Gastroenterol Hepatol. 2022;13(2):483-500. [DOI] [PMC free article] [PubMed]
- 232.Fan R, et al. Wedelolactone alleviates acute pancreatitis and associated lung injury via GPX4 mediated suppression of pyroptosis and ferroptosis. Free Radic Biol Med. 2021;173:29–40. [DOI] [PubMed] [Google Scholar]
- 233.Zhang W, et al. Thiostrepton induces ferroptosis in pancreatic cancer cells through STAT3/GPX4 signalling. Cell Death Dis. 2022;13(7):630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Badgley MA, et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368(6486):85-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Du J, et al. DHA exhibits synergistic therapeutic efficacy with cisplatin to induce ferroptosis in pancreatic ductal adenocarcinoma via modulation of iron metabolism. Cell Death Dis. 2021;12(7):705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kuang F, et al. MGST1 is a redox-sensitive repressor of ferroptosis in pancreatic cancer cells. Cell Chem Biol. 2021;28(6):765-775.e5. [DOI] [PubMed] [Google Scholar]
- 237.Chen D, et al. Ferroptosis-related IncRNAs are prognostic biomarker of overall survival in pancreatic cancer patients. Front Cell Dev Biol. 2022;10:819724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhirong Z, et al. Ferroptosis in pancreatic diseases: potential opportunities and challenges that require attention. Hum Cell. 2023;36(4):1233-1243. [DOI] [PubMed] [Google Scholar]
- 239.Li L, et al. Ferroptosis in ovarian cancer: a novel therapeutic strategy. Front Oncol. 2021;11:665945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tan Z, et al. Current progress of ferroptosis study in ovarian cancer. Front Mol Biosci. 2022;9:966007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhao H, et al. Ferroptosis: a new promising target for ovarian cancer therapy. Int J Med Sci. 2022;19(13):1847-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wang Y, et al. Frizzled-7 identifies platinum-tolerant ovarian cancer cells susceptible to ferroptosis. Cancer Res. 2021;81(2):384-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhou H-H, et al. Erastin reverses ABCB1-mediated docetaxel resistance in ovarian cancer. Front Oncol. 2019;9:1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhang J, et al. Comprehensive analysis identifies potential ferroptosis-associated mRNA therapeutic targets in ovarian cancer. Front Med. 2021;8:644053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Xu X, et al. Increased ATF2 expression predicts poor prognosis and inhibits sorafenib-induced ferroptosis in gastric cancer. Redox Biol. 2023;59:102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chang K, et al. DPP9 stabilizes NRF2 to suppress ferroptosis and induce sorafenib resistance in clear cell renal cell carcinoma. Cancer Res. 2023;83(23):3940-3955. [DOI] [PubMed] [Google Scholar]
- 247.Su Y, et al. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020;483:127-136. [DOI] [PubMed] [Google Scholar]
- 248.Zheng Z, et al. The Xc- inhibitor sulfasalazine improves the anti-cancer effect of pharmacological vitamin C in prostate cancer cells via a glutathione-dependent mechanism. Cell Oncol Dordr. 2020;43(1):95-106. [DOI] [PubMed] [Google Scholar]
- 249.Kerkhove L, et al. Repurposing sulfasalazine as a radiosensitizer in hypoxic human colorectal cancer. Cancers. 2023;15(8):2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Idei U, et al. Mechanism of cell death by combined treatment with an xCT inhibitor and paclitaxel: an alternative therapeutic strategy for patients with ovarian clear cell carcinoma. Int J Mol Sci. 2023;24:11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ma S, et al. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016;7(7):e2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Che B, et al. SLC35F2-SYVN1-TRIM59 axis critically regulates ferroptosis of pancreatic cancer cells by inhibiting endogenous p53. Oncogene. 2023;42(44):3260-3273. [DOI] [PubMed] [Google Scholar]
- 253.Nagpal A, et al. Neoadjuvant neratinib promotes ferroptosis and inhibits brain metastasis in a novel syngeneic model of spontaneous HER2+ve breast cancer metastasis. Breast Cancer Res BCR. 2019;21(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhao Y, et al. Salinomycin-loaded gold nanoparticles for treating cancer stem cells by ferroptosis-induced cell death. Mol Pharm. 2019;16(6):2532-2539. [DOI] [PubMed] [Google Scholar]
- 255.Mai TT, et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat Chem. 2017;9(10):1025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhang R, et al. Curcumenol triggered ferroptosis in lung cancer cells via lncRNA H19/miR-19b-3p/FTH1 axis. Bioact Mater. 2021;13:23-36. [DOI] [PMC free article] [PubMed]
- 257.Sha R, et al. Predictive and prognostic impact of ferroptosis-related genes ACSL4 and GPX4 on breast cancer treated with neoadjuvant chemotherapy. EBioMedicine. 2021;71:103560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lou J-S, et al. Ginkgetin derived from Ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer. Phytomed Int J Phytother Phytopharm. 2021;80:153370. [DOI] [PubMed] [Google Scholar]
- 259.Roh J-L, et al. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017;11:254-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Qi R, et al. Cancer-associated fibroblasts suppress ferroptosis and induce gemcitabine resistance in pancreatic cancer cells by secreting exosome-derived ACSL4-targeting miRNAs. Drug Resist Updat Rev Comment Antimicrob Anticancer Chemother. 2023;68:100960. [DOI] [PubMed] [Google Scholar]
- 261.He H, et al. KIF20A is associated with clinical prognosis and synergistic effect of gemcitabine combined with ferroptosis inducer in lung adenocarcinoma. Front Pharmacol. 2022;13:1007429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yangyun W, et al. Everolimus accelerates Erastin and RSL3-induced ferroptosis in renal cell carcinoma. Gene. 2022;809:145992. [DOI] [PubMed] [Google Scholar]
- 263.Chen P, et al. Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics. 2020;10(11):5107-5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Yan W-Y, Cai J, Wang J-N, Gong Y-S, Ding X-B. Co-treatment of betulin and gefitinib is effective against EGFR wild-type/KRAS-mutant non-small cell lung cancer by inducing ferroptosis. Neoplasma. 2022;69(3):648-656. [DOI] [PubMed] [Google Scholar]
- 265.Zhang Y, et al. Implications of Withaferin A for the metastatic potential and drug resistance in hepatocellular carcinoma cells via Nrf2-mediated EMT and ferroptosis. Toxicol Mech Methods. 2023;33(1):47-55. [DOI] [PubMed] [Google Scholar]
- 266.Hassannia B, et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J Clin Invest. 2018;128(8):3341-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wang W, et al. Inhalable biomimetic protein corona-mediated nanoreactor for self-amplified lung adenocarcinoma ferroptosis therapy. ACS Nano. 2022;16(5):8370-8387. [DOI] [PubMed] [Google Scholar]
- 268.Graf H, et al. Chemoembolization combined with pravastatin improves survival in patients with hepatocellular carcinoma. Digestion. 2008;78(1):34-38. [DOI] [PubMed] [Google Scholar]
- 269.Yao X, et al. Simvastatin induced ferroptosis for triple-negative breast cancer therapy. J Nanobiotechnology.2021;19(1):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Shi L, et al. The DRD2 antagonist haloperidol mediates autophagy-induced ferroptosis to increase temozolomide sensitivity by promoting endoplasmic reticulum stress in glioblastoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2023;29(16):3172-3188. [DOI] [PubMed] [Google Scholar]
- 271.Bai T, et al. Haloperidol, a sigma receptor 1 antagonist, promotes ferroptosis in hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2017;491(4):919-925. [DOI] [PubMed] [Google Scholar]
- 272.Li C, et al. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy. 2021;17(4):948-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zhao L-P, et al. β-Elemene induced ferroptosis via TFEB-mediated GPX4 degradation in EGFR wide-type non-small cell lung cancer. J Adv Res. 2024;62:257-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.O’Dwyer PJ, et al. Phase I trial of buthionine sulfoximine in combination with melphalan in patients with cancer. J Clin Oncol Off J Am Soc Clin Oncol. 1996;14(1):249-256. [DOI] [PubMed] [Google Scholar]
- 275.Zeng L, et al. A MOF-based potent ferroptosis inducer for enhanced radiotherapy of triple negative breast cancer. ACS Nano. 2023;17(14):13195-13210. [DOI] [PubMed] [Google Scholar]
- 276.Liu Y, et al. The 5-lipoxygenase inhibitor zileuton confers neuroprotection against glutamate oxidative damage by inhibiting ferroptosis. Biol Pharm Bull. 2015;38(8):1234-1239. [DOI] [PubMed] [Google Scholar]
- 277.Mao C, et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593(7860):586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Amos A, et al. Depletion of SOD2 enhances nasopharyngeal carcinoma cell radiosensitivity via ferroptosis induction modulated by DHODH inhibition. BMC Cancer. 2023;23(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ding Q, et al. Mitochondrial-targeted brequinar liposome boosted mitochondrial-related ferroptosis for promoting checkpoint blockade immunotherapy in bladder cancer. J Control Release Off J Control Release Soc. 2023;363:221–34. [DOI] [PubMed] [Google Scholar]
- 280.Jiang M, et al. DHODH inhibition exerts synergistic therapeutic effect with cisplatin to induce ferroptosis in cervical cancer through regulating mTOR pathway. Cancers. 2023;15(2):546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Baker DJ, et al. CAR T therapy beyond cancer: the evolution of a living drug. Nature. 2023;619(7971):707-715. [DOI] [PubMed] [Google Scholar]
- 282.Lin MJ, et al. Cancer vaccines: the next immunotherapy frontier. Nat Cancer. 2022;3(8):911-926 [DOI] [PubMed] [Google Scholar]
- 283.Zhang Y, et al. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Tang R, et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Chen X, et al. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18(5):280-296. [DOI] [PubMed] [Google Scholar]
- 286.Tang D, et al. Ferroptosis in immunostimulation and immunosuppression. Immunol Rev. 2024;321(1):199-210. [DOI] [PubMed] [Google Scholar]
- 287.Naimi A, et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun Signal CCS. 2022;20(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Tison A, et al. Immune-checkpoint inhibitor use in patients with cancer and pre-existing autoimmune diseases. Nat Rev Rheumatol. 2022;18(11):641-656. [DOI] [PubMed] [Google Scholar]
- 289.Tang Q, et al. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol. 2022;13:964442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Jiang Q, et al. Platelet membrane-camouflaged magnetic nanoparticles for ferroptosis-enhanced cancer immunotherapy. Small Weinh Bergstr Ger. 2020;16(22):e2001704. [DOI] [PubMed] [Google Scholar]
- 291.Jiang Z, et al. TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J Clin Invest. 2021;131(8):e139434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ma X, et al. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33(5):1001-1012.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Cramer SL, et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med. 2017;23(1):120-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wang W, et al. CD8+T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Beretta GL, et al. Radiotherapy-induced ferroptosis for cancer treatment. Front Mol Biosci. 2023;10:1216733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ouellette MM, et al. Cell signaling pathways that promote radioresistance of cancer cells. Diagn Basel Switz. 2022;12(3):656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Zheng W, et al. SDC1-dependent TGM2 determines radiosensitivity in glioblastoma by coordinating EPG5-mediated fusion of autophagosomes with lysosomes. Autophagy. 2023;19(3):839-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Li J, et al. RBBP4 regulates the expression of the Mre11-Rad50-NBS1 (MRN) complex and promotes DNA double-strand break repair to mediate glioblastoma chemoradiotherapy resistance. Cancer Lett. 2023;557:216078. [DOI] [PubMed] [Google Scholar]
- 299.Liu S, et al. Tubastatin A potently inhibits GPX4 activity to potentiate cancer radiotherapy through boosting ferroptosis. Redox Biol. 2023;62:102677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zou W, et al. PD-L1 (B7–H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Furgiuele S, et al. Dealing with macrophage plasticity to address therapeutic challenges in head and neck cancers. Int J Mol Sci. 2022;23(12):6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Zhou Z, et al. Targeting the macrophage-ferroptosis crosstalk: a novel insight into tumor immunotherapy. Front Biosci. 2022;27(7):203. [DOI] [PubMed] [Google Scholar]
- 303.Yang Y, et al. Interaction between macrophages and ferroptosis. Cell Death Dis. 2022;13(4):355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yuan XM, et al. Secretion of ferritin by iron-laden macrophages and influence of lipoproteins. Free Radic Res. 2004;38(10):1133-1142. [DOI] [PubMed] [Google Scholar]
- 305.Wang W, et al. Serum ferritin: past, present and future. Biochim Biophys Acta. 2010;1800(8):760-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kontoghiorghes GJ. Iron mobilization from ferritin using alpha-oxohydroxy heteroaromatic chelators. Biochem J. 1986;233(1):299-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Denz H, et al. Association between the activation of macrophages, changes of iron metabolism and the degree of anaemia in patients with malignant disorders. Eur J Haematol. 1992;48(5):244-248. [DOI] [PubMed] [Google Scholar]
- 308.Kolnagou A, et al. Reduction of body iron stores to normal range levels in thalassaemia by using a deferiprone/deferoxamine combination and their maintenance thereafter by deferiprone monotherapy. Eur J Haematol. 2010;85(5):430-438. [DOI] [PubMed] [Google Scholar]
- 309.Kolnagou A, et al. Prevention of iron overload and long term maintenance of normal iron stores in thalassaemia major patients using deferiprone or deferiprone deferoxamine combination. Drug Res. 2017;67(7):404-411. [DOI] [PubMed] [Google Scholar]
- 310.Vreugdenhil G, et al. Efficacy and safety of oral iron chelator L1 in anaemic rheumatoid arthritis patients. Lancet. 1989;2(8676):1398-1399. [DOI] [PubMed] [Google Scholar]
- 311.Boddaert N, et al. Selective iron chelation in Friedreich ataxia: biologic and clinical implications. Blood. 2007;110(1):401-408. [DOI] [PubMed] [Google Scholar]
- 312.Lei G, et al. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;22(7):381-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Li D, et al. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct Target Ther. 2020;5(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Zhao L, et al. Ferroptosis in cancer and cancer immunotherapy. Cancer Commun Lond Engl. 2022;42(2):88-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Aaes TL, et al. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep. 2016;15(2):274-287. [DOI] [PubMed] [Google Scholar]
- 316.Zhang Y, et al. Therapeutic implications of ferroptosis in renal fibrosis. Front Mol Biosci. 2022;9:890766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Turubanova VD, et al. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J Immunother Cancer. 2019;7(1):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Garg AD, et al. Author correction: hypericin-based photodynamic therapy induces surface exposure of damage-associated molecular patterns like HSP70 and calreticulin. Cancer Immunol Immunother CII. 2018;67(7):1179-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Wen Q, et al. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510(2):278-283. [DOI] [PubMed] [Google Scholar]
- 320.Linkermann A, et al. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol. 2014;14(11):759-767. [DOI] [PubMed] [Google Scholar]
- 321.Bar-Hai N, et al. Engaging plasticity: differentiation therapy in solid tumors. Front Pharmacol. 2022;13:944773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Baghban R, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal CCS. 2020;18(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lee J, Roh JL. Epithelial-mesenchymal plasticity: implications for ferroptosis vulnerability and cancer therapy. Crit Rev Oncol Hematol. 2023;185: 103964. [DOI] [PubMed] [Google Scholar]
- 324.Fukuda M, et al. Down-regulation of glutathione peroxidase 4 in oral cancer inhibits tumor growth through SREBP1 signaling. Anticancer Res. 2021;41(4):1785-1792. [DOI] [PubMed] [Google Scholar]
- 325.Sato T, et al. NF2/Merlin inactivation and potential therapeutic targets in mesothelioma. Int J Mol Sci. 2018;19(4):988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Liu L, et al. SIRT3 inhibits gallbladder cancer by induction of AKT-dependent ferroptosis and blockade of epithelial-mesenchymal transition. Cancer Lett. 2021;510:93–104. [DOI] [PubMed] [Google Scholar]
- 327.Jinesh GG, et al. Classical epithelial-mesenchymal transition (EMT) and alternative cell death process-driven blebbishield metastatic-witch (BMW) pathways to cancer metastasis. Signal Transduct Target Ther. 2022;7(1):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
From Pubmed Data base.