Abstract
Lenvatinib is a multi-target tyrosine kinase inhibitor widely used in the treatment of hepatocellular carcinoma (HCC). Its primary mechanism of action involves inhibiting signal pathways such as vascular endothelial growth factor receptors (VEGFR) and fibroblast growth factor receptors (FGFR), thereby reducing tumor cell proliferation and angiogenesis and affecting the tumor’s immune microenvironment. In the treatment of liver cancer, although lenvatinib monotherapy has shown good clinical effect, the problem of drug resistance is becoming more and more serious. This resistance may be caused by a variety of factors, including genetic mutations, signaling pathway remodeling, and changes in the tumor microenvironment. In order to overcome drug resistance, the combination of lenvatinib and other therapeutic strategies has gradually become a research hotspot, and it is worth noting that the combination of lenvatinib and immune checkpoint inhibitors (ICIs) has shown a good application prospect. This combination not only enhances the anti-tumor immune response but also helps improve therapeutic efficacy. However, combination therapy also faces challenges regarding safety and tolerability. Therefore, studying the mechanisms of resistance and identifying relevant biomarkers is particularly important, as it aids in early diagnosis and personalized treatment. This article reviews the mechanisms of lenvatinib in treating liver cancer, the mechanisms and efficacy of its combination with immune checkpoint inhibitors, the causes of resistance, the exploration of biomarkers, and other novel combination therapy strategies for lenvatinib. We hope to provide insights into the use and research of lenvatinib in clinical and scientific settings, offering new strategies for the treatment of liver cancer.
Keywords: Lenvatinib, Tyrosine kinase inhibitor (TKI), Immune-checkpoint inhibitors (ICIs), Hepatocellular carcinoma
Introduction
Overview of hepatocellular carcinoma (HCC)
Hepatocellular carcinoma (HCC) stands as a prevalent primary hepatic malignancy with profound global health implications. It exhibits an aggressive phenotype, high recurrence rates, and limited therapeutic avenues, culminating in a bleak prognosis characterized by a five-year overall survival rate below 20%. Chronic liver conditions such as viral hepatitis, alcohol-related liver disease, non-alcoholic fatty liver disease, and cirrhosis are closely linked to HCC, contributing to its escalating global incidence. Despite strides in early detection and treatment modalities, managing HCC remains a formidable challenge. Current therapeutic strategies encompass surgical resection, liver transplantation, locoregional interventions, and systemic treatments like sorafenib and lenvatinib. However, these options are encumbered by efficacy constraints, drug resistance issues, and substantial toxicities. Hence, there is an urgent call for innovative treatment paradigms to enhance outcomes for individuals afflicted with HCC.
Historical context of sorafenib as a first-line therapy
Sorafenib, a multikinase inhibitor, plays a key role in the treatment of HCC as a first-line therapy. The historical context of sorafenib as a breakthrough therapeutic option for HCC traces back to its landmark clinical trial, which demonstrated its efficacy and established it as the first systemic therapy to show a survival benefit in advanced HCC. The phase III trial, known as the SHARP trial [1], compared sorafenib to placebo in patients with unresectable HCC and demonstrated a significant improvement in median overall survival. Following the positive results of the SHARP trial, sorafenib received regulatory approval in several countries, transforming the treatment landscape for advanced HCC. Despite the subsequent emergence of other systemic therapies, sorafenib’s historical significance as the first-line therapy for HCC sets the stage for subsequent developments in the field and highlights the importance of targeted therapies in improving patient outcomes. However, despite its success, sorafenib has its limitations, including modest response rates and the development of drug resistance over time.
Emergence of lenvatinib as a multi-target TKI
The development of lenvatinib as a multipotent tyrosine kinase inhibitor (TKI) is a promising alternative to these problems. Over the last few years, lenvatinib has emerged as an additional prominent multipotent tyrosine kinase (TKI) inhibitor in the first-line treatment of liver cancer (HCC). Lenvatinib’s unique mechanism of action, targeting multiple kinases involved in tumor angiogenesis, proliferation, and immune regulation, has shown efficacy and safety in clinical trials. Lenvatinib acts by targeting multiple kinases involved in tumor angiogenesis, tumor proliferation, and immune regulation. Lenvatinib has been shown to be effective in REFLECT [2], a phase III trial comparing lenvatinib with sorafenib as the first-line therapy for unresectable HCC. The trial showcased non-inferiority in overall survival and demonstrated favorable outcomes in terms of progression-free survival and objective response rate, solidifying lenvatinib as a viable alternative to sorafenib. The emergence of lenvatinib as a multi-target TKI has expanded the treatment options available to HCC patients and sparked further investigations into combination therapies, such as lenvatinib in conjunction with immune-checkpoint inhibitors, to enhance treatment responses and improve patient outcomes.
Introduction to immune-checkpoint inhibitors (ICIs) in HCC
The advent of immune-checkpoint inhibitors (ICIs) has brought significant advancements to the field of cancer immunotherapy, including their application in hepatocellular carcinoma (HCC). ICIs work by targeting key immune checkpoint molecules, such as PD-1 and PD-L1, to release the ability of the immune system to identify and eradicate cancer cells. In HCC, ICIs have shown promising clinical efficacy, and studies have demonstrated durable responses and improved survival in a subset of patients. However, the majority of HCC patients do not respond to ICIs as monotherapy, prompting the exploration of combination strategies. One such approach involves the combination of ICIs with lenvatinib, a multi-target tyrosine kinase inhibitor. Lenvatinib’s anti-angiogenic properties, combined with the immunomodulatory effects of ICIs, offer a rationale for the synergistic potential of this combination therapy in HCC. Preclinical and early clinical data suggest enhanced antitumor activity and improved response rates when lenvatinib is administered in conjunction with ICIs. Understanding the mechanistic insights, evaluating the clinical efficacy, and exploring the future perspectives of the lenvatinib and ICIs combination therapy in HCC are essential for optimizing treatment outcomes and shaping the future landscape of HCC management.
This review aims to delve into the therapeutic mechanisms, clinical efficacy, and prospective directions concerning lenvatinib, particularly in combination with immune checkpoint inhibitors (ICIs), with the goal of elucidating strategies to enhance treatment efficacy and influence the evolving landscape of hepatocellular carcinoma (HCC) management.
Mechanisms of action of lenvatinib in HCC
Lenvatinib is a multi-target tyrosine kinase inhibitor (TKI) that has emerged as a promising therapeutic option in the treatment of various cancers, including hepatocellular carcinoma (HCC). It exerts its pharmacological effects by targeting multiple receptors involved in tumor angiogenesis, proliferation, and immune regulation. As a multi-target TKI, lenvatinib inhibits the activity of several key kinases involved in the development and progression of cancer. These encompass vascular endothelial growth factor receptors (VEGFR) 1–3, fibroblast growth factor receptors (FGFR) 1–4, platelet-derived growth factor receptor alpha (PDGFRα), rearranged during transfection (RET), and KIT proto-oncogene receptor tyrosine kinase (KIT). By simultaneously targeting these receptors, lenvatinib disrupts multiple signaling pathways that are critical for tumor growth and angiogenesis. The mechanisms underlying lenvatinib’s anti-tumor effects involve its ability to inhibit tumor angiogenesis. By targeting VEGFRs, lenvatinib suppresses the formation of new blood vessels that supply nutrients to the tumor, thereby limiting its growth. This is especially useful for HCC, which is a highly vascularized cancer that not only receives nutrients from blood vessels but also distributes thousands of cancer cells to initiate metastasis. In addition, VEGFRs contribute to the delivery of oxygen to cancer cells. Thus, hypoxia and cell hunger are induced by lenvatinib, resulting in cell death [3]. Additionally, lenvatinib’s inhibition of other receptors, such as FGFRs and PDGFRα, may further contribute to its anti-tumor activity by interfering with tumor cell proliferation and survival.
Molecular targets and their roles in tumor proliferation and angiogenesis:
Lenvatinib has a broad spectrum of target receptors, which allows it to disrupt multiple pathways involved in tumor proliferation and angiogenesis. This multi-pronged attack is key to its effectiveness in treating advanced hepatocellular carcinoma, where traditional treatments might fail or be insufficient.
VEGFR1-3 (Vascular endothelial growth factor receptors)
Lenvatinib primarily inhibits tumor angiogenesis by suppressing VEGFR1-3. Tumor vascularization is intimately linked to vascular endothelial growth factor (VEGF) and its receptors (VEGFR1-3). VEGF acts directly on endothelial cells or induces angiogenesis through paracrine pathways, and it can bind to VEGFR-1 and VEGFR-2 expressed on vascular endothelial cells, triggering specific downstream signaling pathways that promote angiogenesis. Notably, VEGFR is also highly expressed in hepatocellular carcinoma [4]. Compared to normal tissue vessels, tumor blood vessels exhibit high permeability and tortuous dilation. VEGF acts on the interaction between tumor stromal cells through its specific structure, which influences the microenvironment of the tumor and plays an important role in the development of cancer.
The multiple regulatory mechanisms of VEGF give hepatocellular carcinoma a growth advantage, with VEGFR2 mediating angiogenesis and playing a central role. This makes VEGFR2 an important target for lenvatinib, which is particularly sensitive to VEGFR2-related kinases [5]. Therefore, lenvatinib can effectively inhibit tumor angiogenesis through its high affinity for VEGFR2. VEGFR2, a type I transmembrane kinase receptor, comprises distinct domains: the extracellular domain, the transmembrane domain, and the cytoplasmic tyrosine kinase domain. Lenvatinib, akin to numerous pharmaceutical agents, exerts its effects specifically on the tyrosine kinase domain. Based on the conformation when complexed with VEGFR2, tyrosine kinase inhibitors (TKIs) are categorized into various inhibitor types (I, II, III, IV). Structural elucidation of lenvatinib demonstrates its interaction with the ATP binding site and neighboring allosteric domains in a distinct conformation (Asp-Phe-Gly, known as the “DFG-in” conformation), thereby yielding notable specificity towards kinases. Okamoto et al. suggested categorizing lenvatinib as a type V novel inhibitor [6]. In contrast, sorafenib (SOR) binds to the DFG-out state of VEGFR2, making it a type II inhibitor [7]. The differences in molecular characteristics between lenvatinib and sorafenib may account for the variations in their pharmacological activities and mechanisms of action [8]. The specific mechanisms through which lenvatinib targets VEGFR to inhibit tumor angiogenesis and affect the tumor microenvironment still require further clarification.
FGFR1-4 (Fibroblast growth factor receptors)
Lenvatinib exhibits antitumor effects not only by inhibiting angiogenesis but also by suppressing the growth of tumor cells through the inhibition of FGFR1-4. Unlike sorafenib, lenvatinib also targets FGFR1-4, which plays a crucial role in tumor growth and serves as an important targets against HCC [3]. Analysis of the co-crystal structure of the lenvatinib-FGFR1-4 complex reveals that lenvatinib also binds in a type V manner to FGFR1-4. This indicates that lenvatinib has high affinity and selectivity for FGFR in addition to VEGFR. Lenvatinib is significantly related to fibroblast growth factor (FGF) and fibroblast growth factor receptors (FGFR1-4); the FGF family promotes the proliferation and invasion of liver cancer cell lines through the FGF signaling pathway, particularly the FGF19/FGFR4 pathway [9]. Overexpression of FGF19 in liver cancer cells regulates lipid, glucose, bile acid, and energy metabolism, aiding in the survival of liver cancer cells in nutrient-limited tumor microenvironments [10]. Abnormal expression of FGF19 interacts with the β-Klotho co-receptor, activating the FGF19/FGFR4 signaling pathway, which promotes the proliferation, migration, and invasion of liver cancer cell lines while inhibiting apoptosis [11]. In FGF19-overexpressing HCC xenograft models, lenvatinib can inhibit the FGF signaling pathway and suppress tumor growth [8]. Clinical results also show that lenvatinib is more effective in treating HCC cases with FGF19-FGFR4 expression compared to those without this expression [12].
Research on the downstream pathways affected by lenvatinib’s impact on FGFR4 indicates that lenvatinib can inhibit the phosphorylation of the downstream signaling molecule FRS2, thereby suppressing the proliferation and growth of liver cancer cells [8, 13]. Additionally, lenvatinib can reduce xCT expression by inhibiting FGFR4, ultimately inducing lipid ROS accumulation and leading to ferroptosis in liver cancer cells [14]. In the context of the immune microenvironment, empirical investigations conducted both in vitro and in vivo have revealed that lenvatinib diminishes tumor PD-L1 expression and inhibits Treg cell differentiation through FGFR4 blockade, consequently augmenting the effectiveness of anti-PD-1 immunotherapy. These findings furnish critical theoretical underpinnings for the synergistic application of lenvatinib in conjunction with anti-PD-1 therapy [15].
In addition to FGFR4, a preclinical study using an HCC PDX model indicated that models with high FGFR1 expression respond better to lenvatinib than to sorafenib. Prolonged exposure to sorafenib can lead to upregulation of FGFR1, causing HCC cells to develop resistance to sorafenib. Therefore, lenvatinib may potentially overcome this sorafenib resistance by blocking FGFR1 [8]. Lenvatinib can also reduce cancer stem-like cells (CSCs) in HCC by inhibiting FGFR1-3 signaling pathways.
As two important target receptors of lenvatinib, VEGFR and FGFR are closely interconnected after lenvatinib treatment. Suppression of FGFR activity triggers a compensatory activation of the EGFR–PAK2–ERK5 signaling cascade, which can be effectively attenuated by targeting EGFR, thereby interrupting this signaling pathway. Therefore, combined inhibition of FGFR and EGFR makes liver cancer more sensitive to lenvatinib [16]. Additionally, the FGF-FGFR-MAPK axis mediates the survival of HCC cells under nutrient-deprived conditions due to vascular suppression. Consequently, using drugs like lenvatinib to dual-target both VEGFR and FGFR provides enhanced antitumor activity against HCC with activated FGF signaling pathways compared to therapies that only target VEGF signaling [17]. These findings suggest that lenvatinib significantly enhances the antitumor response in liver cancer compared to sorafenib, due to its simultaneous action on both VEGFR and FGFR.
Other target receptor: PDGFRα (Platelet-derived growth factor receptor), RET (Rearranged during transfection), and KIT (KIT proto-oncogene receptor tyrosine kinase)
Compared to the extensively studied target receptors VEGFR and FGFR, research on the remaining receptors, PDGFRα, RET, and KIT, is relatively limited. PDGFR is a receptor tyrosine kinase widely distributed on the surface of various cells, existing in two structurally similar subtypes: PDGFRα and PDGFRβ. Dysregulated activation of the PDGF/PDGFR pathway has the potential to stimulate angiogenesis, facilitating the development of neovascularization within tumors, while concurrently influencing tumor cell proliferation and migration through direct or indirect mechanisms. The RET protein, encoded by the RET gene, belongs to the receptor tyrosine kinase family. Pathogenic mutations—either mutations or rearrangements—of the RET gene can lead to its abnormal activation, resulting in tumor cell proliferation, differentiation, and migration. However, research on lenvatinib targeting RET has largely been limited to thyroid cancer, likely due to the high mutation rate of RET in that context, drawing researchers’ attention [18]. Both in vitro and in vivo studies have shown that lenvatinib can inhibit RET phosphorylation and subsequently suppress RET-ERK signaling in cases with genetic alterations in RET (such as activating mutations or gene rearrangements) [19, 20], ultimately affecting the antitumor process. Kato et al. reported that among 4871 cases of other cancer types analyzed for RET mutations and amplifications, only 88 cases (1.8%) were observed, with mutations found in just one of 44 HCC cases[21]. Therefore, the frequency of RET mutations in HCC is low, and the inhibitory effect of lenvatinib on RET may be limited to certain cases. A comprehensive understanding of the mechanisms by which lenvatinib affects the PDGFR, RET, and KIT pathways is crucial for revealing its therapeutic mechanisms. Future research is anticipated to delve deeper into the specific roles of these pathways and provide stronger support for personalized treatment strategies.
Impact on the tumor immune microenvironment:
Lenvatinib not only inhibits tumor cell growth in the treatment of hepatocellular carcinoma (HCC) but also significantly impacts the tumor immune microenvironment. It alters the immune status of the tumor microenvironment by regulating the activity and distribution of immune cells, thereby enhancing the body’s immune response against the tumor. This immunomodulatory effect may contribute to improved treatment outcomes and better prognosis for HCC patients. Additionally, understanding lenvatinib’s influence on the immune microenvironment will provide new insights for future combination immunotherapy strategies.
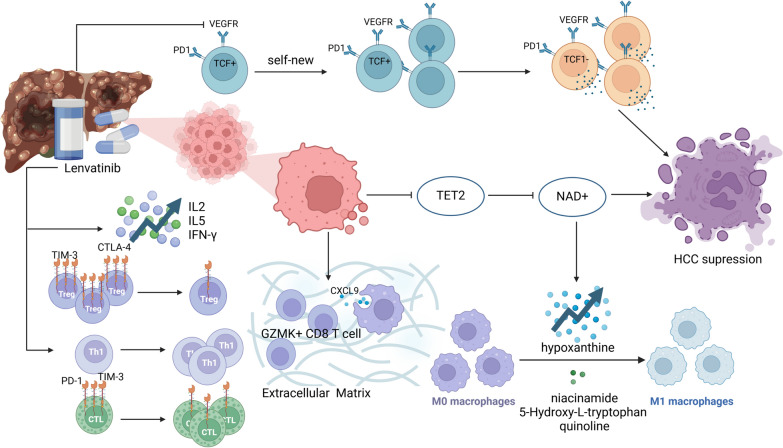
Lenvatinib regulates the tumor immune microenvironment in hepatocellular carcinoma (HCC) through multiple mechanisms, promoting immune cell infiltration and function, thus enhancing treatment efficacy. It plays a significant role in the HCC immune microenvironment by targeting the VEGFR pathway. Research shows that lenvatinib downregulates the VEGFR pathway and promotes the infiltration of GZMK + CD8 T cells in the tumor microenvironment, mediated by macrophage-derived CXCL9. However, the recruited T cells may lose their original antitumor activity due to the physical barriers of the extracellular matrix (ECM). Therefore, compounds targeting the ECM could facilitate the penetration of cytotoxic GZMK + CD8 T cells into HCC cell areas, ultimately improving drug efficacy [22]. Additionally, lenvatinib can impact the PI3K/Akt/mTOR signaling pathway in PD1 + CD8 + T cells by targeting VEGFR2 on these cells, influencing their differentiation, promoting the self-proliferation of TCF1 + PD1 + Ki-67 + TIM3 − T progenitor cells, and enhancing the tumor-killing function of intermediate TCF1 − PD1 + Ki-67 − TIM3 − T cells[23]. Beyond targeting VEGFR, lenvatinib also participates in the HCC tumor immune process by targeting FGFR [8]. Moreover, lenvatinib affects metabolic pathways that influence the immune microenvironment. Research by Sun et al. indicates that lenvatinib targets TET2 synthesis to increase the levels of nicotinamide adenine dinucleotide (NAD +), enhances the secretion of inosine, and inhibits the secretion of nicotinamide, 5-hydroxy-L-tryptophan, and quinolinic acid, thereby promoting macrophage proliferation and migration and facilitating M1 polarization [24]. Under the influence of lenvatinib, the properties of immune cells also change. Besides inducing macrophage polarization, lenvatinib significantly reduces the expression of PD-1 and TIM-3 on CTLs, as well as TIM-3 and CTLA-4 on Treg cells [25]. This provides a theoretical basis for the combined application of lenvatinib with immune checkpoint inhibitors, potentially further enhancing antitumor immune responses and improving treatment outcomes.
There is extensive research on the components of immune cells and immune factors in the tumor microenvironment after lenvatinib treatment. Following treatment, the frequency of T helper (Th) cells and regulatory T (Treg) cells decreases, while cytotoxic T lymphocytes (CTL) significantly increase. The CTL/Treg ratio has been identified as a new prognostic indicator for HCC patients [25]. Additionally, the ratios of monocytes and macrophages, as well as neutrophils and lymphocytes, are significantly reduced, while the numbers of CD4 + T cells, CD8 + T cells, and Th1 cells increase [26–28]. The cytokine profile shows an increase in IL-2, IL-5, and IFN-γ, while IL-6, IL-10, TNF-α, and TNF-β levels decrease [25]. These changes reflect a re-regulation of the immune system and are closely associated with patient prognosis. Future research will further explore the roles of these immune cells and factors, providing more evidence for personalized treatment strategies.
Overall, lenvatinib exhibits dual efficacy by suppressing tumor cell proliferation in hepatocellular carcinoma (HCC) and substantially altering the tumor immune microenvironment, thereby augmenting the host immune response. This immunomodulatory effect may improve treatment efficacy and patient prognosis. Further research on lenvatinib’s impact on the immune microenvironment will provide an important theoretical foundation for future combination immunotherapy strategies (Fig. 1).
Fig. 1.
Effect of lenvatinib on the tumour immune microenvironment
Antitumor proliferation and immunomodulatory activity in preclinical models
Researchers have investigated the cellular-level mechanism of action of lenvatinib, validating its antitumor proliferation and immunomodulatory activity through cell experiments. Subsequently, the research progressed to preclinical models, assessing the drug's efficacy and safety in more complex biological systems. This process not only enhances the credibility of the research but also lays a solid foundation for future clinical applications, allowing the findings to better inform the development of actual treatment plans. Encouragingly, preclinical models also demonstrate that lenvatinib has significant antitumor proliferation and immunomodulatory activity in the treatment of liver cancer.
Lenvatinib has demonstrated impressive performance in preclinical models, particularly in anti-angiogenesis. The strong activity of lenvatinib against VEGF signaling in xenograft models of HCC with overexpressed VEGF provides the basis for its antitumor and antiangiogenic effects in vascularized HCC models [29]. Additionally, evidence shows that lenvatinib’s ability to inhibit tumor growth in mice is dose-dependent, meaning that as the dose of lenvatinib increases, tumor vascularity and microvascular density significantly decrease, while necrosis rates markedly increase [30]. Interestingly, some studies have approached this from an imaging perspective. Results indicate that early in lenvatinib treatment, CT scans show no significant decrease in tumor blood vessel volume density, with a notable decline in TBV (tumor blood volume) density occurring by day fourteen. This suggests that the normalization of TBV, which begins early in treatment, is a unique feature of lenvatinib therapy. It appears that lenvatinib does not affect existing vascular structures but rather induces a reduction in vessel diameter in terms of TBV normalization [31]. This research helps to better understand how lenvatinib influences vascular changes.
In addition to affecting tumor angiogenesis, preclinical models treated with lenvatinib have also shown changes in the immune microenvironment. In an orthotopic rat model carrying liver cancer cells, administering 5 mg/kg of lenvatinib demonstrated that lenvatinib had no direct effect on macrophages, but instead led to their polarization towards the M1 phenotype. This polarization improves the tumor immune microenvironment and contributes to antitumor effects [32].
Many preclinical studies have compared lenvatinib and sorafenib. In HCC patient-derived xenograft (PDX) models, research has confirmed that lenvatinib exhibits superior anti-angiogenic capabilities compared to sorafenib. FGF, a pro-angiogenic factor, can induce escape from anti-VEGF therapy, while lenvatinib targets FGFR, a capacity that sorafenib lacks [8]. In the context of FGF-induced angiogenesis, examination through histological analysis of xenograft tumors overexpressing FGF19 in mice treated with lenvatinib demonstrated that FGF facilitates the survival of hepatocellular carcinoma (HCC) cells characterized by activated FGF signaling pathways within tumor microenvironments deprived of adequate nutrients, operating through the MAPK cascade [17]. Moreover, in murine models harboring subcutaneously implanted hepatocellular carcinoma (HCC), the administration of 3 mg/kg of lenvatinib demonstrated a significant reduction in microvascular density and the normalization of tumor vasculature compared to treatment with 50 mg/kg of sorafenib. These findings indicate that lenvatinib expedites tumor vascular normalization and improves the intra-tumoral microenvironment more rapidly and effectively than sorafenib [33].
These studies indicate that lenvatinib not only suppresses tumor cell growth but also effectively regulates immune cells in the tumor microenvironment, boosting the host's immune response. By thoroughly analyzing the results from these models, researchers can attain a more comprehensive comprehension of lenvatinib’s mechanisms of action, providing crucial evidence for its efficacy in clinical applications.
Immune-checkpoint inhibitors in HCC
Immune checkpoint inhibitors in oncology are a class of innovative drugs used for cancer treatment. They activate the patient's immune system to attack and suppress tumor growth by blocking the inhibitory signals between tumor cells and the immune system. These inhibitors primarily target immune checkpoints, which are key molecules that regulate immune responses. Immune checkpoint inhibitors, such as PD-1 and PD-L1 inhibitors, have demonstrated significant efficacy across various cancer types. For example, in melanoma, non-small cell lung cancer, and renal cell carcinoma, immune checkpoint inhibitors have become part of standard treatment, significantly improving patient survival rates and quality of life. Additionally, ICIs have broadened treatment options, maintained treatment durability, and provided new approaches for personalized therapy.
Typical immune checkpoint inhibitors (ICIs) encompass anti-programmed cell death-1 (PD-1) and programmed cell death ligand 1 (PD-L1) therapeutics, exemplified by nivolumab, pembrolizumab, durvalumab, and atezolizumab, along with anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) agents such as tremelimumab and ipilimumab [34]. Lymphocyte activation gene 3 (LAG-3) is the most mature immune checkpoint studied after CTLA-4 and PD-1/PD-L1, with LAG-3 antibodies like Opdualag and Relatlimab already approved for clinical anti-tumor therapy. Additionally, T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), CD47, T cell immunoglobulin and ITIM domain protein (TIGIT), and V-domain Ig suppressor of T-cell activation (VISTA) are also under extensive research and clinical trials, which is significant for overcoming acquired resistance to immune checkpoint inhibitors and preventing the emergence of intrinsic resistance mechanisms.
Mechanisms of action of ICIs
PD-1/PD-L1
Programmed cell death protein 1 (PD-1) is a negative co-stimulatory receptor expressed on activated T cells, natural killer (NK) cells, and monocytes. Its ligand, programmed death-ligand 1 (PD-L1), engages PD-1 on T cells, triggering tyrosine phosphorylation within the immune receptor tyrosine motif of PD-1. This event recruits Src homology region 2-containing protein tyrosine phosphatase 2 (SHP-2), leading to the dephosphorylation of downstream protein kinases like spleen tyrosine kinase (Syk) and phosphatidylinositol 3-kinase (PI3K). Consequently, this process disrupts signaling pathways involving protein kinase B (PKB or AKT) and extracellular regulated protein kinase (ERK), impeding gene transcription and cytokine production necessary for T cell activation, ultimately imposing negative regulation on T cell function [35]. Tumor cells exhibiting elevated PD-L1 levels can interact with PD-1 on tumor-specific T cells, impeding T cell activation, proliferation, and cytokine release, thereby facilitating immune evasion by the tumor. Consequently, interruption of the PD-1/PD-L1 signaling axis can revive T cell cytotoxicity and instigate inherent anti-tumor immune responses, thereby manifesting anti-tumor properties.
Since 2012, following the favorable safety profile and anti-tumor efficacy demonstrated by PD-1 monoclonal antibodies in phase I/II clinical trials, multiple phase III clinical trials have been initiated targeting various malignancies. Presently, a total of 10 PD-1 monoclonal antibodies and 3 PD-L1 monoclonal antibodies have received approval for treating 11 cancer types [35], including liver cancer.
Although PD-1/PD-L1 antibodies exhibit significant anti-tumor activity across various malignancies, their response rates are relatively low in most tumors. Developing combination strategies with PD-1/PD-L1 antibodies is one approach to enhance the response rates of PD-1/PD-L1 monotherapy. Clinical trials combining PD-1/PD-L1 therapies with other anti-tumor agents are underway, including other immunotherapies (such as other ICIs, oncolytic viruses, stimulator of interferon genes (STING) agonists, and chimeric antigen receptor T-cell (CAR-T) therapy), radiotherapy, chemotherapy, and molecularly targeted therapies. However, currently, FDA-approved PD-1/PD-L1 combination strategies are limited to combinations with chemotherapy, vascular endothelial growth factor (VEGF) targeted agents, and CTLA-4 monoclonal antibodies.
CTLA-4
CTLA-4 is widely expressed in activated T cells. The binding of the T cell surface receptor CD28 to CD80/CD86 on antigen-presenting cells (APCs) generates a co-stimulatory signal, which, along with the signal from the antigen-specific T cell receptor (TCR) recognizing major histocompatibility complex (MHC), constitutes the dual signal necessary for T cell activation. CTLA-4 shares a high degree of homology with the extracellular domain of CD28, and compared to CD28, CTLA-4 has a higher affinity for CD80/CD86. This high affinity allows CTLA-4 to antagonize the interaction between CD28 and its ligands, thereby reducing the generation of co-stimulatory signals and inhibiting T-cell activation. Additionally, CTLA-4 is also expressed on regulatory T cells (Tregs), where it promotes the internalization of CD80/CD86 on APCs through trans-endocytosis, reducing their expression levels and further enhancing immunosuppressive functions. Therefore, targeting and blocking CTLA-4 can relieve T cell immune suppression and allow for T cell activation, thereby facilitating tumor cytotoxicity.
Recent investigations indicate that antibody-dependent cell-mediated cytotoxicity (ADCC) may represent a fundamental mechanism through which ipilimumab mediates its anti-neoplastic actions. Research indicates that ADCC, mediated by the Fc region, can target and eliminate Tregs that express high levels of CTLA-4, thereby relieving the immune suppression caused by these Tregs. Consequently, enhancing Fc-mediated ADCC has become an important direction in the development of CTLA-4 monoclonal antibodies.
One phase III clinical trial of tremelimumab [36] (Clinical Trial Number: NCT03298451) showed significant improvement in overall survival (OS) when combined with durvalumab in patients with liver cancer. Based on this study, tremelimumab has received FDA priority review and is expected to become the second approved CTLA-4 monoclonal antibody. Besides these drugs, numerous CTLA-4 antibody candidates have entered clinical and preclinical research stages, focusing primarily on the efficacy and safety of the monoclonal antibodies.
In terms of combination therapy, CTLA-4 monoclonal antibodies target the early stages of anti-tumor immunity and have a long-lasting effect. When combined with PD-1/PD-L1 monoclonal antibodies, which act during the T cell effector phase, this approach can significantly improve response rates and enhance anti-tumor efficacy. The amalgamation of the anti-PD-1 antibody nivolumab with the anti-CTLA-4 antibody ipilimumab was initially evaluated in the phase I/II clinical trial CheckMate-040 [37] (NCT01658878) in liver cancer. Compared to monotherapy, the nivolumab and ipilimumab combination appears to be safe and effective.
LAG-3
LAG-3 is another important immune checkpoint following CTLA-4 and PD-1/PD-L1. It is expressed on the surface of various lymphocytes and is structurally similar to CD4, with a stronger affinity for MHC class II. LAG-3 can stably bind to peptide-MHC class II complexes, inhibiting T-cell activation. Additionally, fibrinogen-like protein 1 (FGL1) has been identified as a ligand for LAG-3. Blocking the FGL1-LAG-3 interaction can also enhance tumor immunity and inhibit tumor growth in tumor-bearing mice.
Relatlimab is the first LAG-3 monoclonal antibody developed for clinical use. Although LAG-3 antibody monotherapy has good safety, its diverse ligands and mechanisms of action limit its anti-tumor efficacy. Current clinical studies indicate that the combination of LAG-3 with PD-1/PD-L1 antibodies holds significant promise in terms of efficacy and safety. Several other LAG-3 antibodies, such as GSK2831781, HLX26, and Sym022, are currently in clinical trials.
Other ICIs
TIM-3 is also a negative co-stimulatory molecule, widely expressed on various immune cells. Currently, four ligands have been identified for TIM-3, including galectin-9 (Gal-9), carcinoembryonic antigen-related cell adhesion molecule 1, high mobility group protein B1, and phosphatidylserine, with Gal-9 being the primary ligand. TIM-3 mediates dysfunction and exhaustion of TIM-3 + T cells through its interaction with Gal-9. Currently, antibody drugs targeting TIM-3, such as Sym023, INCAGN2390, and LY3321367, are undergoing clinical trials as monotherapies for advanced solid tumors and lymphomas (NCT03489343, NCT03652077, NCT04443751). LY3321367 has shown good safety in phase I trials, but its anti-tumor activity is limited. Additionally, studies indicate that the development of resistance to PD-1 antibodies is associated with the upregulation of TIM-3. A clinical trial of TIM-3 and PD-1 antibody combination therapy for advanced solid tumors showed that the combination group not only had good tolerability but also demonstrated improved anti-tumor activity. Research on the combination of TIM-3 and PD-1 antibodies is currently being widely conducted.
CD47 is widely expressed in normal cells and is overexpressed in various types of solid tumors and hematological malignancies. The regulation of tumor immunity by CD47 primarily occurs through its interaction with signal regulatory protein α (SIRPα) on macrophages, inhibiting the clearance of tumor cells by these immune cells. To enhance the efficacy of CD47 antibodies, numerous clinical trials combining CD47 antibodies with other immune checkpoint inhibitors (ICIs) are currently being conducted, pending further research for confirmation.
TIGIT is primarily expressed in T cells and NK cells, with three ligands: CD155, CD112, and CD113 [38]. Among these, TIGIT has a higher binding affinity for CD155 compared to CD112 and CD113. The interaction between TIGIT and CD155 inhibits the activity of T cells and NK cells, leading to reduced antigen presentation by dendritic cells and decreased secretion of anti-inflammatory cytokines, which ultimately impairs T cell activation. Research has shown that TIGIT antibody monotherapy is insufficient to suppress tumor growth in mice, but administering TIGIT monoclonal antibodies before tumor formation can delay tumor development and metastasis. Additionally, studies indicate that combining TIGIT antibodies with PD-1/PD-L1 antibodies can enhance the anti-tumor effects of TIGIT antibodies. Currently, several TIGIT inhibitors are in clinical studies but none have been approved for marketing. Among them, only four TIGIT antibodies have reached phase III clinical trials: tiragolumab, vibostolimab, ociperlimab, and domvanalimab.
VISTA exhibits sequence similarity with PD-L1 and PD-L2 and demonstrates notable expression in myeloid-derived suppressor cells and various immune cell populations. Currently, two immunosuppressive ligands have been confirmed for VISTA: V-set and immunoglobulin domain containing 3 (VSIG3) and P-selectin glycoprotein ligand 1 (PSGL-1). At physiological pH, VISTA interacts with VSIG3, while at acidic pH, VISTA-expressing cells can bind to PSGL-1 on T cells. Both interactions suppress T cell function. Although antibodies targeting VISTA have shown significant therapeutic effects in various preclinical tumor models, there are currently no approved antibody drugs on the market. Most effective VISTA treatment models also rely on combination approaches.
Specific ICIs used in HCC
In the era of immunotherapy, the use of immune checkpoint inhibitors (ICIs) in liver cancer is of significant importance and drives the development of personalized and precision medicine. ICIs have also been tested in HCC patients, with nivolumab and pembrolizumab (both anti-PD-1 antibodies) approved for second-line treatment.
In 2012, a phase I/II clinical trial, CheckMate 040, was initiated to evaluate the efficacy and safety of nivolumab, either as a monotherapy or in combination, for second-line treatment of advanced hepatocellular carcinoma (HCC) [39]. Following the release of preliminary results from CheckMate 040 in 2017, the FDA approved nivolumab for second-line treatment of HCC, making it the first immunotherapy approved for this indication. A study comparing nivolumab monotherapy to sorafenib monotherapy for first-line treatment of advanced HCC showed that nivolumab did not significantly improve overall survival compared to sorafenib, but clinical activity and good safety were observed in advanced HCC patients [40]. Therefore, nivolumab may be considered a treatment option for patients contraindicated or at significant risk with tyrosine kinase inhibitors and anti-angiogenic agents. Regarding the long-term effects of nivolumab monotherapy, a five-year follow-up demonstrated that it provided durable clinical benefits and manageable safety for both treatment-naive and sorafenib-treated advanced HCC patients, with the five-year survival rate for advanced HCC increasing from 3 to 12%−14% [41].
KEYNOTE-224 was a pioneering study of pembrolizumab monotherapy for second-line treatment of advanced HCC [42], and due to its outstanding results, pembrolizumab was approved by the FDA. Second-line treatment is the primary focus for pembrolizumab. Following the KEYNOTE-224 study, researchers initiated two large multicenter phase III trials: KEYNOTE-240 and KEYNOTE-394. In the global KEYNOTE-240 study, the primary endpoint did not achieve statistical significance [43]. However, the KEYNOTE-394 trial in the Asian population met its primary endpoint [44], making it the first phase III clinical trial of an immune monotherapy to achieve positive results in liver cancer. The trial demonstrated positive outcomes for overall survival (OS), progression-free survival (PFS), and overall response rate (ORR). The OS in the pembrolizumab group reached 14.6 months, representing the longest OS data for immune monotherapy in liver cancer to date, while the duration of response (DOR) was 23.9 months, offering hope for prolonged survival in liver cancer patients.
In numerous instances, sole targeting of immune checkpoints has demonstrated inadequacy, given that these checkpoints represent only a fraction of the complexities inherent in the immune system and tumor microenvironment. As the application of ICIs expands, resistance phenomena have gradually emerged, with many patients experiencing tumor recurrence or progression after initial treatment. This resistance mechanism may be associated with alterations in the tumor microenvironment, activation of immune evasion pathways, and the genomic diversity of tumor cells. As a result, a comprehensive investigation into resistance mechanisms and the establishment of strategies to overcome them are crucial for enhancing the therapeutic efficacy of immune checkpoint inhibitors. The realm of combination therapies based on immune checkpoint inhibitors is progressively gaining traction, supplanting tyrosine kinase inhibitor monotherapy in advanced hepatocellular carcinoma (HCC). Nevertheless, challenges such as resistance, the identification of predictive biomarkers for treatment response, and potential adverse effects in combined therapies persist. The development of safer and more effective combination treatment protocols for advanced HCC is paramount, underscoring the need for further research endeavors.
Synergy between lenvatinib and ICIs:
Not all HCC patients, especially those awaiting liver transplantation, respond to ICI immunotherapy. More importantly, monotherapy with immunotherapy does not significantly improve overall survival (OS). Given this data, researchers are exploring more effective combination therapies for HCC, including the combined use of ICIs and TKIs [34].
Role of lenvatinib in modulating the tumor microenvironment to enhance ICI efficacy
The mechanism by which lenvatinib enhances ICI efficacy is closely related to its target pathways. Lenvatinib’s modulation of the VEGF pathway can diminish the activity of cytotoxic T cells and dendritic cells, while facilitating the infiltration of immunosuppressive cell populations such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and M2 macrophages, thereby exerting a direct immunosuppressive impact within the tumor microenvironment [45]. Additionally, lenvatinib can induce the formation of the NRP-1-PDGFRβ complex and activate the Crkl-C3G-Rap1 signaling pathway in endothelial cells, further optimizing the immature and dysfunctional tumor vasculature. This enhances the infiltration of immune cells, primarily CD8 + T cells, reshaping the tumor microenvironment (TME) and increasing sensitivity to immune checkpoint blockade (ICB) therapies [46]. Moreover, the inhibition of VEGFR-2 in endothelial cells triggers the expression of PD-L1 in hepatocellular carcinoma (HCC) cells in a paracrine fashion, partially mediated by interferon-γ expression. Concurrently, VEGFR-2 blockade enhances PD-1 expression in CD4 + cells infiltrating the tumor. In the presence of anti-PD-1 therapy, CD4 + cells facilitate the normalization of vascular structures when exposed to anti-VEGFR-2 antibodies during anti-angiogenic treatment, laying the groundwork for potential combined therapeutic approaches [47].
Similarly, the FGFR-FGF pathway is actively involved in the mechanism by which lenvatinib enhances ICI efficacy. In vitro and in vivo experiments have demonstrated that lenvatinib improves anti-PD-1 efficacy by reducing tumor PD-L1 levels and Treg differentiation through FGFR4 blockade. The expression levels of FGFR4 and Treg infiltration in tumors can serve as biomarkers for screening HCC patients for combination therapy with lenvatinib and anti-PD-1[15]. Activation of FGFR signaling inhibits the JAK/STAT signaling pathway stimulated by IFN-γ, reducing the expression of its target genes, including B2M, CXCL10, and PD-L1 [48]. Inhibiting FGFR with lenvatinib can enhance anti-tumor immunity and the activity of anti-PD-1 antibodies. Additionally, lenvatinib’s suppression of FGFR signaling can increase the number of PD-L1-positive cells by downregulating lipid metabolism-related genes, transforming the tumor immune microenvironment (TIME) into a hot immune state [49].
Lenvatinib also has potential effects on the inhibition of other targets such as rearranged during transfection (RET) and platelet-derived growth factor (PDGF), carrying additional immunological and molecular significance [45]. Furthermore, lenvatinib can restore sensitivity to ICIs by blocking the PKCα/ZFP64/CSF1 axis, thereby reshaping the tumor microenvironment [50].
By enhancing the efficacy of ICIs within the tumor microenvironment of HCC, the combination of lenvatinib and immune checkpoint inhibitors has become an important treatment strategy. This combination can improve the tumor microenvironment, promote the infiltration and activity of immune cells, and enhance the body’s immune response to tumors. This combined treatment approach offers new hope for HCC patients, particularly those who do not respond well to traditional therapies, further advancing the development of personalized medicine.
Preclinical data showing enhanced anti-tumor immune response
Lenvatinib and ICI combination therapy have shown significant superiority in preclinical liver cancer animal models. In murine models, the combined treatment exhibited tumor regression and quicker response durations in contrast to monotherapy (P < 0.001). Monotherapy involving anti-PD-1 triggered infiltration of dendritic cells and T cells, whereas lenvatinib decreased the percentage of regulatory T cells (Tregs). Nevertheless, solely the combined therapy notably suppressed immunosuppressive signaling associated with the TGFβ pathway and fostered an immune-active microenvironment (P < 0.05) [45]. In a study conducted by Chen et al., it was observed that the co-administration of lenvatinib with siRNA targeting PD-L1 effectively impeded tumor progression in H22 tumor-bearing mice by impeding tumor cell proliferation and fostering apoptotic processes. This combined therapeutic regimen synergistically downregulated the expression of vascular endothelial growth factor (VEGF) and PD-L1, facilitating heightened infiltration of T cells within tumor tissues and an increased T cell population in the spleen. Furthermore, the combined treatment amplified the presence of granzyme B-positive T cells, suggesting a notable reinforcement of the mice's anti-tumor immune response [51].
A study comparing anti-PD-1 monoclonal antibodies combined with lenvatinib or axitinib (a VEGFR-selective inhibitor) showed that both combination therapies exhibited higher anti-tumor activity and longer survival in mouse models compared to monotherapy. Furthermore, the anti-tumor activity of the anti-PD-1 monoclonal antibody combined with lenvatinib was enhanced compared to the combination with axitinib. Mechanistically, lenvatinib targeting FGFR was shown to reduce the number of tumor-associated macrophages and increase CD8 T cell counts, further highlighting its specificity and superior efficacy [48].
These preclinical data demonstrate the promising efficacy of lenvatinib combined with ICIs, which will facilitate the clinical translation of combination therapy. Further clinical trials are needed to validate the effectiveness and safety of this combination therapy in different liver cancer patient populations. With a deeper understanding of the mechanisms behind lenvatinib and ICI combination therapy, there is hope for optimizing treatment regimens to improve patient survival and quality of life. Ultimately, the successful implementation of this combination therapy could change the treatment standards for liver cancer and bring breakthroughs to clinical practice.
Clinical efficacy of lenvatinib and ICIs in HCC
Comparative efficacy with sorafenib
REFLECT trial: lenvatinib vs. sorafenib in advanced HCC
Sorafenib is the standard first-line treatment for advanced liver cancer. Since its approval in 2007, multiple drugs have attempted to challenge Sorafenib's position but have failed. The REFLECT Trial [52] aimed to explore whether Lenvatinib could be the first drug to match or even surpass Sorafenib. The study collected data from 954 treatment-naive, unresectable advanced HCC patients across 154 centers in 20 countries from March 1, 2013, to July 30, 2015. Patients were randomly assigned in a 1:1 ratio to receive Lenvatinib (N = 478) or Sorafenib (N = 476). Participants took Lenvatinib (12 mg/d for those weighing ≥ 60 kg; 8 mg/d for those < 60 kg) or Sorafenib (400 mg bid), with each cycle lasting 28 days. The median follow-up was 27.7 months for the Lenvatinib group and 27.2 months for the Sorafenib group. Results showed that for the primary endpoint, the median overall survival (OS) was 13.6 months for the Lenvatinib group and 12.3 months for the Sorafenib group, achieving non-inferiority (HR 0.92) but not superiority. However, all secondary endpoints (progression-free survival (PFS), time to progression (TTP), objective response rate (ORR), quality of life, and plasma pharmacokinetic exposure parameters) were significantly better in the Lenvatinib group. Additionally, Lenvatinib outperformed Sorafenib in all PFS subgroups. Both treatments had manageable safety profiles. This large, multicenter study demonstrated that Lenvatinib is not inferior to Sorafenib in overall survival for untreated advanced hepatocellular carcinoma, positioning Lenvatinib as a potential first-line standard treatment for advanced liver cancer.
Other clinical trials: Lenvatinib vs. sorafenib in HCC
Based on the REFLECT Trial, many subsequent studies have explored health-related quality of life (HRQOL) during treatment [53], subset analyses [54, 55], side effect studies [56, 57], and drug monitoring [58]. These clinical trials have laid a theoretical foundation for the broader application of Lenvatinib.
Results from two meta-analyses showed that the overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR) in the Lenvatinib monotherapy group were significantly higher than those in the Sorafenib group. However, the incidence of adverse events was higher in patients receiving Lenvatinib compared to those on Sorafenib [59], particularly regarding hypertension [60]. Additionally, studies indicated increased rates of hepatic encephalopathy [61] and renal impairment [62] in the Lenvatinib group. The incidence of hand-foot skin reactions was lower in the Lenvatinib group (P < 0.05), while the rates of nausea, fatigue, urinary frequency, and dizziness showed no statistically significant difference between the two groups (P > 0.05) [63]. For drug selection after HCC progression, one study evaluated the safety and efficacy of Lenvatinib versus Sorafenib in treating uHCC patients who progressed to Child–Pugh B (CP-B). The results indicated that Lenvatinib was more effective, with manageable side effects, allowing patients with deteriorating liver function to continue Lenvatinib treatment [64]. Compared to Sorafenib, Lenvatinib may yield more favorable outcomes in advanced HCC with Vp3/4 [65]. Another study suggested that Lenvatinib could overcome Sorafenib resistance in HCC by intervening in the FGFR4-ERK signaling pathway, making it a suitable second-line treatment for unresectable HCC patients with Sorafenib resistance and FGFR4 expression [66].
Multiple studies have explored the benefits of Lenvatinib versus Sorafenib treatment. A cost-effectiveness analysis in China for patients with unresectable hepatocellular carcinoma showed that for patients weighing ≥ 60 kg, the incremental cost-effectiveness ratio (ICER) was close to an acceptable threshold, with Lenvatinib having a slightly higher probability of being cost-effective compared to Sorafenib. For patients weighing < 60 kg, Lenvatinib demonstrated a comparative cost-effectiveness advantage [67]. The results of this study were more favorable than those from Japan [68]. In China, the direct medical cost and quality-adjusted life years (QALY) was 2.916, significantly higher than Japan’s 1.88. A probabilistic sensitivity analysis from Canada indicated that in 64.87% of simulations, Lenvatinib remained a cost-saving option. Nevertheless, in the event of a 57% reduction in the cost of sorafenib, lenvatinib would cease to uphold its dominance as the primary strategy [69]. Similarly, a study from Australia also demonstrated that Lenvatinib is a cost-effective treatment choice compared to Sorafenib [70]. Through continuous assessments of treatment expenses and clinical results, Lenvatinib could emerge as the favored therapeutic choice for individuals afflicted with unresectable hepatocellular carcinoma. Future research should further investigate its long-term effects and adaptability in different patient populations to advance personalized treatment and provide better options for patients.
Comparing the mechanism of action of lenvatinib with sorafenib
Lenvatinib and Sorafenib are standard first-line treatments for HCC, both being TKIs, but they differ significantly in their mechanisms of action, primarily due to differences in targeted signaling pathways. Sorafenib targets the Raf/MEK/ERK pathway, as well as RTK, VEGFR1, VEGFR2, VEGFR3, and PDGFR-β, while Lenvatinib targets VEGFR1–3, FGFR1–4, PDGFR-α, c-Kit, and RET, showing a notable difference in their kinase affinity profiles [71]. Both drugs share VEGFR as a common target; however, when VEGF and VEGFR are inhibited, FGF is activated to enhance FGFR signaling, promoting tumor angiogenesis. The activation of the FGF-FGFR signaling pathway serves as a compensatory mechanism for tumor angiogenesis when VEGF-VEGFR pathways are suppressed. Compared to Sorafenib, Lenvatinib uniquely inhibits FGFR signaling and has a higher affinity for quickly binding to factors involved in tumor angiogenesis (such as VEGFR and FGFR), resulting in a strong inhibitory effect on tumor growth in vivo [30]. FGF21 may be a candidate biomarker for predicting Lenvatinib’s potential to extend OS [72]. Additionally, Lenvatinib can reduce cancer stem-like cells (CSCs) in HCC by inhibiting FGFR1-3 signaling, a capability not possessed by Sorafenib [71].
Moreover, as a type V inhibitor, Lenvatinib binds to VEGFR2 more rapidly and with higher affinity, resulting in stronger kinase inhibitory activity compared to type II inhibitors like Sorafenib [72]. In cellular comparisons, while Lenvatinib is less effective than Sorafenib in inhibiting cell growth in vitro, it outperforms Sorafenib in inhibiting tumor growth in vivo [30]. Sorafenib is particularly active in highly differentiated cells with wild-type p53 and increased mitochondrial respiration. In contrast, Lenvatinib appears more effective in moderately to poorly differentiated cells with p53 mutations and lower mitochondrial respiration [73].
Understanding the different mechanisms and effects of these two drugs is crucial for developing personalized treatment plans. Continued research into the biological characteristics of HCC, combined with patient-specific factors, will aid in selecting the appropriate medication, thereby improving treatment outcomes and patient survival rates. Future clinical studies should focus on exploring the application potential of Lenvatinib in various types of liver cancer patients to support broader treatment strategies.
Synergistic effects of lenvatinib and ICIs:
KEYNOTE-524: lenvatinib plus pembrolizumab
The principle behind the combination of Lenvatinib and Pembrolizumab is that Lenvatinib can simultaneously inhibit tumor microenvironment-induced angiogenesis and immune suppression. This inhibition may enhance the clinical efficacy of PD-1 antibodies by boosting anti-tumor immune responses [74, 75]. KEYNOTE-524 is an open-label, single-arm Phase Ib clinical trial designed to explore the use of Lenvatinib and Pembrolizumab in unresectable HCC. The study included 104 patients with unresectable HCC, who received Lenvatinib (12 mg/day for patients weighing ≥ 60 kg; 8 mg/day for those < 60 kg) in combination with Pembrolizumab (200 mg every 3 weeks). According to mRECIST and RECIST v1.1, the objective response rates (ORR) were 46.0% and 36.0%, respectively, with median durations of response of 8.6 and 12.6 months. The median progression-free survival (mPFS) was 9.3 and 8.6 months, while the median overall survival (mOS) was 22 months. The results indicate that the combination therapy of Lenvatinib and Pembrolizumab yields a better response rate in unresectable HCC compared to Lenvatinib alone, with adverse events occurring within an acceptable range, demonstrating good anti-tumor activity and safety [76].
LEAP-002: lenvatinib plus pembrolizumab vs. lenvatinib alone
LEAP-002 [77] is a global, randomized, double-blind Phase III study aimed at assessing the efficacy and safety of Pembrolizumab in combination with Lenvatinib compared to Lenvatinib alone as a first-line treatment for advanced HCC. The results indicated that at the data cutoff, there were 252 deaths (64%) in the combination group and 282 deaths (71%) in the monotherapy group. The median overall survival (OS) was 21.2 months (95% CI: 19.0–23.6 months) for the combination group and 19.0 months (95% CI: 17.2–21.7 months) for the monotherapy group (HR: 0.84, p = 0.023, not reaching the one-sided p = 0.019 superiority threshold), suggesting that the addition of Pembrolizumab did not significantly improve OS. In terms of progression-free survival (PFS), the median PFS was 8.2 months (95% CI: 6.4–8.4 months) for the combination group and 8.0 months (95% CI: 6.3–8.2 months) for the monotherapy group, indicating no significant difference in PFS between the two treatments. The combination of Pembrolizumab and Lenvatinib did not demonstrate the expected outcomes, with neither primary endpoint being achieved. Despite not reaching the primary endpoints, the combination therapy resulted in the longest OS observed to date for first-line treatment (21.2 months), while the OS for Lenvatinib monotherapy also exceeded expectations at 19 months. This clinical trial further supports the use of Lenvatinib monotherapy as a first-line treatment option for HCC patients.
Other clinical trials combining lenvatinib and ICIs
A study recruited adult patients with unresectable Barcelona Clinic Liver Cancer stage B or C HCC who were newly treated with systemic therapy. They received oral Lenvatinib in combination with intravenous anti-PD-1 agents (sintilimab/pembrolizumab/toripalimab/tislelizumab). The results showed a conversion success rate of 55.4% (31/56) for the primary endpoint. The objective response rate (ORR) according to mRECIST was 53.6% and 44.6% per RECIST 1.1. The median progression-free survival (PFS) was 8.9 months, and the median overall survival (OS) was 23.9 months. Among the 31 successfully converted patients, 21 underwent surgery, with an R0 resection rate of 85.7% and a pathological complete response rate of 38.1%. Grade ≥ 3 treatment-related adverse events were observed in 42.9% of patients [78]. The results of the study on the efficacy and adverse reactions of Nivolumab combined with Lenvatinib for advanced HCC indicate that this combination can improve tumor control, reduce tumor burden, and enhance liver and immune function. Compared to Lenvatinib alone, the increase in adverse effects from the combination therapy was manageable [79, 80]. Additionally, clinical trials involving other ICIs [76, 81–84], including Tislelizumab combined with Lenvatinib, have shown good anti-tumor activity and tolerability, confirming the superiority of combination therapy.
In investigating treatment regimens, Wang et al. explored whether synchronous or asynchronous treatment with Lenvatinib and PD-1 inhibitors affected efficacy. The results indicated that the synchronous treatment group exhibited significantly improved OS and PFS compared to the asynchronous group, suggesting that administering Lenvatinib and PD-1 inhibitors together can significantly enhance survival in HCC patients [85]. Another study examined the impact of dosing and frequency on the efficacy of combination treatment, dividing patients into two groups: one receiving 6 mg/kg of Cadonilimab plus Lenvatinib every two weeks, and the other receiving 15 mg/kg every three weeks. Both groups achieved the expected treatment effects, but there were no significant differences in tumor control and safety between the two [86]. For patients excluded from the KEYNOTE-524 trial with tumor volume ≥ 50% of liver volume (TO ≥ 50%) or Vp4 infiltration, Lenvatinib combined with Pembrolizumab may provide survival benefits for those with Vp4-infiltrating HCC, potentially expanding the indications for this combination therapy [87].
Compared to monotherapy, combination therapy has not shown significant statistical differences in safety across most clinical trials, but this does not mean that the side effects of combination therapy can be overlooked in clinical practice. Common adverse reactions include fatigue, loss of appetite, increased blood pressure, hand-foot skin reactions, diarrhea, rashes, acute asthma attacks, and muscle wasting, which should be managed during treatment [88, 89].
The broad application prospects of combination therapy have also led to the development of various predictive efficacy methods. Peripheral naive CD8 + T cells, peripheral Th cells, and NK cells can predict responses to first-line combination therapy of Lenvatinib and anti-PD-1 antibodies in patients with advanced or unresectable HBV-related HCC [90, 91]. Using multivariate Firth logistic regression, Xu et al. confirmed that their proposed criteria are independent predictors for surgical conversion after Lenvatinib combined with anti-PD-1 antibody therapy [92]. Additionally, liver tumor stiffness measured by shear wave elastography [93], ALBI grading [94], and MRI-based nomograms [95] have all been shown to predict the efficacy of Lenvatinib combined with PD-1 antibodies.
Lenvatinib in combination with immune checkpoint inhibitors (ICIs) urgently requires larger-scale clinical trials to validate its effectiveness and safety. These studies should encompass various types of hepatocellular carcinoma patients, considering factors such as age, sex, and disease duration to comprehensively assess treatment outcomes. Furthermore, exploring optimal dosing regimens and combination strategies will help improve patient survival and quality of life. Through systematic clinical research, we can better understand the mechanisms of this combination therapy, providing a solid theoretical foundation for clinical practice (Table 1).
Table 1.
Clinical trials combining lenvatinib and ICIs
| Clinical trial registration number | Nature of the test | Basis for clustering | Treatment target | ORR | PFS(months) | OS(months) | Safety and adverse reactions | Number of persons included | References |
|---|---|---|---|---|---|---|---|---|---|
| NCT03006926 | Phase Ib multicenter, single-arm,open-label study | Lenvatinib + Pembrolizumab | Unresectable hepatocellular carcinoma | 46% | 9.3 | 22 | TRAE Grade ≥ 3: 67% | 104 | [76] |
| ChiCTR1900023914 | A single-arm, phase II trial | Lenvatinib + sintilimab/pembrolizumab/toripalimab/tislelizumab | Unresectable liver Cancer stage B or C HCC | 53.60% | 8.9 | 23.9 | TRAE Grade ≥ 3: 42.9% | 56 | [78] |
| Prospective clinical trial | Lenvatinib vs. lenvatinib + nivolumab | HCC | 45.65% vs. 23.91% | NA | 92 | [79] | |||
| A retrospective study | Lenvatinib + nivolumab vs. lenvatinib | Advanced hepatocellular carcinoma (aHCC) | 45.0% vs. 23.4% | 7.5 vs. 4.8 | 22.9 vs. 10.3 | HR 0.2,95% C.I. 0.1–0.7 | 87 | [80] | |
| NCT04401800 | A multicenter, single-arm, phase 2 trial | Tislelizumab + lenvatinib | Unresectable hepatocellular carcinoma | 38.70% | 8.2 | 12: 88.6% | TRAE Grade ≥ 3: 28.1% | 64 | [81] |
| A retrospective, unpaired, single-center study | Lenvatinib + anti‐PD‐1 antibodies | Advanced or unresectable hepatocellular carcinoma (HCC) | 28.10% | 8.4 | 17.2 | AE 79.5% | 210 | [82] | |
| NCT03892577 | A retrospective study | Lenvatinib + anti‐PD‐1 antibodies | Unresectable hepatocellular carcinoma | 19.60% | 6.9 | 17.8 | AE Grade ≥ 3: 57.9 | 378 | [83] |
| A retrospective study | Lenvatinib + anti‐PD‐1 antibodies vs. lenvatinib | Unresectable hepatocellular carcinoma | 41.5% vs. 20.0% | 8.0 vs. 3.0 | not reached vs. 13.0 | hypertension: 20% vs. 17.8% | 127 | [84] | |
| A prospective trial | Synchronous + asynchronous of lenvatinib + PD‐1 inhibitor | HCC | 35.9% vs.19.8% | 10.5 vs. 3.6 | 24.6 vs. 14.8 | 40.2% vs. 41.3% | 213 | [85] | |
| NCT04444167 | A phase Ib/II single-arm clinical trial | Time and dose of cardonizumab combined with lenvatinib | Advanced hepatocellular carcinoma (aHCC) | 6 mg/kg/2 weeks: 35.5% 15 mg/kg/3 weeks: 35.7% | 6 mg/kg/2 weeks: 8.6 months15 mg/kg/3 weeks: 9.8 months | 6 mg/kg/2 weeks: 27.1 months15 mg/kg/3 weeks: NA | NA | 59 | [86] |
| A retrospective study | Lenvatinib + pembrolizumab/toripalimab/sintilimab/nivolumab/camrelizumab | Tumor occupation ≥ 50% volume of liver (TO ≥ 50%) or invasion in Vp4 | 20.20% | 6.6 | 11.4 | NA | 84 | [87] | |
| NCT03892577 | A multicenter observational retrospective real-world study | ICI + lenvatinib vs. ICI + others (apatinib/regorafenib/bevacizumab/sorafenib/donafenib) | 13.8% vs. 3.7% | 6.1 vs. 3.4 | 22.1 vs. 21.3 | TRAE: 46.6% vs. 70.4% | 85 | [96] |
Multiple combination therapies based on ICI + lenvatinib
In current research on hepatocellular carcinoma treatment, various combination therapies based on immune checkpoint inhibitors (ICIs) and Lenvatinib have garnered significant attention. This combinatorial approach aims to leverage the strengths of each agent, enhancing treatment efficacy and improving patient outcomes through synergistic effects. As exploration in this field deepens, an increasing number of studies are evaluating the potential applications of these combinations in diverse clinical contexts.
ICI + lenvatinib + molecular targeted agent (MTA):
In the treatment of hepatocellular carcinoma, exploring combination therapies for patients who experience progression after Lenvatinib monotherapy is particularly important. For these patients, the combination of immune checkpoint inhibitors (ICIs) with molecular targeted agents (MTAs) has demonstrated high anti-tumor activity and favorable safety profiles. Patients can receive ICIs and localized regional treatments while continuing Lenvatinib therapy, aiming for improved overall survival (OS) [96]. Research has also indicated that the failure to combine MTA adjunct therapy is considered an independent risk factor for tumor recurrence [97], further emphasizing the importance of comprehensive treatment strategies. Future studies should continue to focus on this area to optimize treatment regimens and enhance patient outcomes.
ICI + lenvatinib + Hepatic artery infusion chemotherapy (HAIC):
Compared to Lenvatinib alone, the combination of Lenvatinib, hepatic artery infusion chemotherapy (HAIC), and PD-1 inhibitors demonstrates a safe and promising anti-tumor activity in hepatocellular carcinoma (HCC) patients with high-risk features. The combination of hepatic artery infusion of FOLFOX with Lenvatinib and PD-1 inhibitors has proven effective as a first-line treatment, with manageable adverse effects [98–102]. It is noteworthy that HCC patients receiving HAIC combined with Lenvatinib and PD-1 inhibitors may experience HBV reactivation. Individuals experiencing HBV reactivation exhibit reduced survival durations in contrast to those without such reactivation. Consequently, it is advisable for patients with HBV-related hepatocellular carcinoma to undergo antiviral treatment and regular monitoring of HBV-DNA levels before and during combined therapeutic interventions [103]. Monitoring and managing HBV reactivation should be incorporated into clinical practice to ensure optimal treatment efficacy and patient outcomes. In the context of the Lenvatinib, HAIC, and PD-1 triplet therapy, levels of BTC (Betacellulin) and CCL28 (C–C motif chemokine ligand 28) may serve as predictive biomarkers for this combination treatment [104]. Additionally, AKR1C2 + and CFHR4 + hepatocyte subtypes may be predictive biomarkers for resistance to the combined therapy [105]. Future research should continue to focus on the exploration of biomarkers to optimize individualized treatment strategies.
ICI + lenvatinib + transarterial chemoembolization (TACE):
The combination of transarterial chemoembolization (TACE), Lenvatinib, and PD-1 inhibitors appears to significantly improve overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR) in patients with advanced hepatocellular carcinoma (HCC) without significantly increasing the risk of all-grade adverse events [106–119]. Nomograms can aid in stratifying treatment decisions and selecting appropriate populations for patients with unresectable HCC [120, 121]. Independent prognostic factors for survival include Child–Pugh classification, portal vein tumor thrombus (PVTT) classification, levels of IL-6, IL-17, IFN-α, and VEGF [122], as well as TAE scores [123]. The neutrophil-to-lymphocyte ratio and early tumor regression may serve as predictive biomarkers for patients with unresectable HCC [124]. These findings provide new insights for clinical treatment, suggesting that combinations of various therapeutic modalities may yield improved efficacy.
Other combination therapies
Furthermore, findings from clinical trials have indicated the tolerability and efficacy of Lenvatinib as a viable second-line systemic treatment after the administration of Atezolizumab and Bevacizumab in individuals diagnosed with advanced hepatocellular carcinoma (HCC) [125]. Furthermore, more complex combination therapies have shown promising clinical results, such as HAIC combined with Lenvatinib and PD-1 antibodies. Concurrently, transarterial chemoembolization (TACE) and portal vein embolization (PVE) have been validated as safe and effective treatment options that can promote tumor necrosis in advanced HCC patients and enhance future liver volume [126]. The combination of TACE, Lenvatinib, PD-1 inhibitors, and I125 seed brachytherapy significantly improves overall survival (OS), progression-free survival (PFS), and disease control rate (DCR) in HCC patients with portal vein tumor thrombus (PVTT), demonstrating better prognosis [127].
In the treatment of liver cancer, the combined application of various therapeutic approaches is of great significance. This integrative treatment strategy maximizes the advantages of different modalities, addressing the complexity and heterogeneity of liver cancer to enhance treatment efficacy. By combining surgical interventions, pharmacotherapy (such as targeted drugs and immunotherapy), and localized treatments (like hepatic artery infusion chemotherapy and radiation therapy), more effective tumor control can be achieved while reducing the risk of recurrence and improving patient survival rates. Moreover, combination therapies can optimize individualized treatment plans, allowing for flexible adjustments based on disease progression, thus enhancing overall efficacy and quality of life. Therefore, exploring and implementing the combined use of multiple therapeutic strategies will be an important development direction in the field of liver cancer treatment (Table 2).
Table 2.
Multiple combination therapies based on ICI and Lenvatinib
| Other treatments | Research type | Treatment type | ORR | PFS | OS | TRAE | Number of people | References |
|---|---|---|---|---|---|---|---|---|
| TACE + MWA + lenvatinib + anti-PD-1 antibodies vs. TACE + MWA | A retrospective study | HCC | 21.257 months vs. 11.795 months | 65.52% | 67 | [97] | ||
| HAIC-FOLFOX-lenvatinib-anti-PD-1 antibodies | A retrospective study | HCC with macrovascular invasion(MaVI) | 31.20% | 179 days | no reach | 68.75% | 32 | [98] |
| HAIC-LEN-PD1 vs. LEN-PD1 | A retrospective study | HCC patients exhibiting high-risk characteristics (Vp4, and/or bile duct invasion, and/or tumor occupancy ≥ 50%) | 76.7% vs. 23.0% | 9.6 months vs. 4.9 months | 19.3 months vs. 9.8 months | higher | 164 | [99] |
| HAIC-FOLFOX-lenvatinib-anti-PD-1 antibodies | A retrospective study | uHCC(major portal vein tumor thrombosis (Vp3 and Vp4)) or tumor occupancy exceeding 50% of the liver | 52.7% (RECIST 1.1) 72.5% (mRECIST) | 8.8 months | 14.3 months | 94.50% | 91 | [100] |
| HAIC-Len-ICI vs. Len-ICI | A retrospective study | Intermediate or advanced TACE-refractory HCC | 48.30% vs. 23.80% | 13 months vs. 7.2 months | 24 months vs. 13 months | NA | 121 | [101] |
| HAIC(FOLFOX)-Len-PD1 vs. Len-PD1 | A retrospective study | HCC patients with PVTT | 61.8% vs. 20.8% | 11.5 months vs. 5.5 months | 26.3 months vs. 13.8 months | higher | 142 | [102] |
| HAIC-lenvatinib-anti-PD-1 antibodies | A retrospective study | HCC without HBV reactivation vs. CC with HBV reactivation | 56.1% vs. 32.5% | 6 months vs. 11.3 months | 12.6 months vs. 25 months | lower | 213 | [103] |
| HAIC-lenvatinib-PD-1-inhibitors vs. HAIC-lenvatinib | a retrospective study | Advanced hepatocellular carcinoma refractory to hepatic arterial infusion chemotherapy | 43.6 months vs. 18.9 months | NA | 145 | [117] | ||
| Lenvatinib, toripalimab plus FOLFOX-HAIC | phase II, single-centre, single-arm trial | 63.90% | 10.4 months | no reach | 36 | [104] | ||
| Lenvatinib, Toripalimab + FOLFOX | HCC with Extrahepatic Metastasis | 43.30% | 8.3 months | 13.7 months | 30 | [105] | ||
| Pembrolizumab-lenvatinib-TACE vs. lenvatinib-TACE | A retrospective study | UHCC | 47.1% vs. 27.8% | 9.2 months vs. 5.5 months | 18.1 months vs. 14.1 months | higher | 142 | [106] |
| PTL + TACE + Lenvatinib vs. TACE + Lenvatinib | A retrospective study | HCC patients with PVTT (portal vein tumor thrombus) types I-IV | NA | 5.4 months vs. 2.7 months | NA | lower | 41 | [107] |
| TACE-lenvatinib-PD-1 inhibitor (TACE-L-P) vs. TACE-TACE-L | A retrospective cohort study | AHCC | 7.3 months vs. 4.0 months | 16.9 months vs. 12.1 months | 81 | [108] | ||
| Lenvatinib-PD-1 inhibitor-TACE | A retrospective study | BCLC stage C HCC | mRECIST: 60% RECIST 1.1: 30% | 8 months | 18.4 months | 93.30% | 30 | [112] |
| TACE-Lenvatinib and Camrelizumab vs. TACE + Lenvatinib | A retrospective study | Unresectable Multiple Nodular and Large Hepatocellular Carcinoma (> 5 cm) | 51.5% vs. 46.9% | 9.4 months vs. 5.9 months | 16.4 months vs. 11.0 months | 33% vs49% | 82 | [113] |
| TACE-lenvatinib -camrelizumab | A single-arm, open-label, multicenter, and prospective study | HCC with portal vein tumor thrombus | 26.10% | 9.3 months | 18.2 months | TRAE ≥ 3: 34.8% | 69 | [114] |
| TACE-PD-1 inhibitors-lenvatinib vs.PD-1 inhibitors-lenvatinib | An observational, retrospective, cohort study | UHCC | 54.3% vs. 25.4% | 10.2 months vs. 7.4 months | 20.5 months vs. 12.6 months | NA | 105 | [115] |
| TACE + LEN + PD-1 vs. LEN + PD-1 | A retrospective case–control study | UHCC | 76.7 vs. 44.9% | 16.2 months vs. 10.2 months | 29.0 months vs. 17.8 months | NA | 118 | [116] |
| TACE- lenvatinib-PD-1 inhibitors vs. TACE + len vs. TACE | A retrospective study | Unresectable recurrent HCC | 70.4% vs. 48.9% vs. 42.5% | 24.1 months vs. 17.3 months vs. 13.7 months | no reach vs. 25.6 months vs. 15.7 months | NA | 204 | [118] |
| PD-1 inhibitors, lenvatinib, TACE (PD-1-Lenv-T) vs. lenvatinib, TACE (Lenv-T) | A retrospective study | unresectable recurrent HCC | 44.4% vs. 20% | 11.7 months vs. 8.5 months | 26.8 months vs. 14.0 months | NA | 65 | [128] |
| TACE-L-P vs. TACE-S (Sorafenib) -P | A retrospective study | HCC with Portal Vein Tumor Thrombus | 41.25% vs. 30.59% | 6.3 months vs. 3.2 months | 21.7 months vs. 15.6 months | NA | 165 | [129] |
| TACE-lenvatinib (TACE-L)-PD-1 inhibitor vs (TACE-L) | A retrospective study | uHCC | 54.0% vs. 32.8% | 14.0 months vs. 9.0 months | 24.0 months vs. 15.0 months | NA | 241 | [120] |
| TACE + LEN + PD-1 inhibitor vs. TACE + LEN | A retrospective study | HCC with portal vein tumor thrombus | 38.57% vs. 24.45% | 7.5 months vs. 4.3 months | 23.5 months vs. 18.3 months | NA | 160 | [122] |
| lenvatinib- atezolizumab -bevacizumab | A retrospective study | Advanced Hepatocellular Carcinoma | 21.40% | 4.2 months | 8.3 months | 100% | 14 | [125] |
| TACE-lenvatinib-PD-1 inhibitor, and iodine-125 seed brachytherapy vs. TACE + len vs. TACE + len + PD-1 | A retrospective study | HCC with portal vein tumor thrombosis | 13 months vs. 5 months vs. 9 months | 21 months vs. 10 months vs. 14 months | NA | 150 | [127] |
Future Perspectives of Lenvatinib in HCC Management
Biomarker-guided precision medicine:
Biomarkers play a crucial role in the treatment of liver cancer with lenvatinib, as they can help predict patient responses and guide personalized treatment plans. Specific biomarkers are associated with treatment outcomes and can identify patients who are more likely to benefit from lenvatinib. Additionally, biomarkers can be used to monitor treatment responses and detect potential resistance mechanisms early, allowing for timely adjustments to the treatment regimen. Overall, integrating biomarkers into clinical practice enhances the precision of lenvatinib therapy, ultimately improving treatment outcomes and quality of life for liver cancer patients.
Potential biomarkers for predicting response
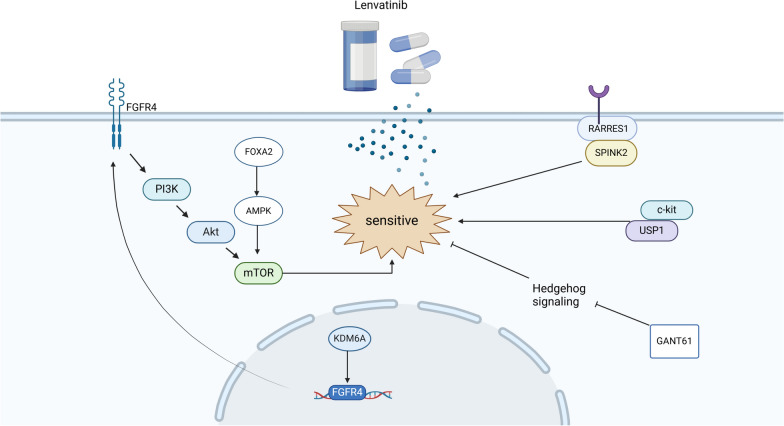
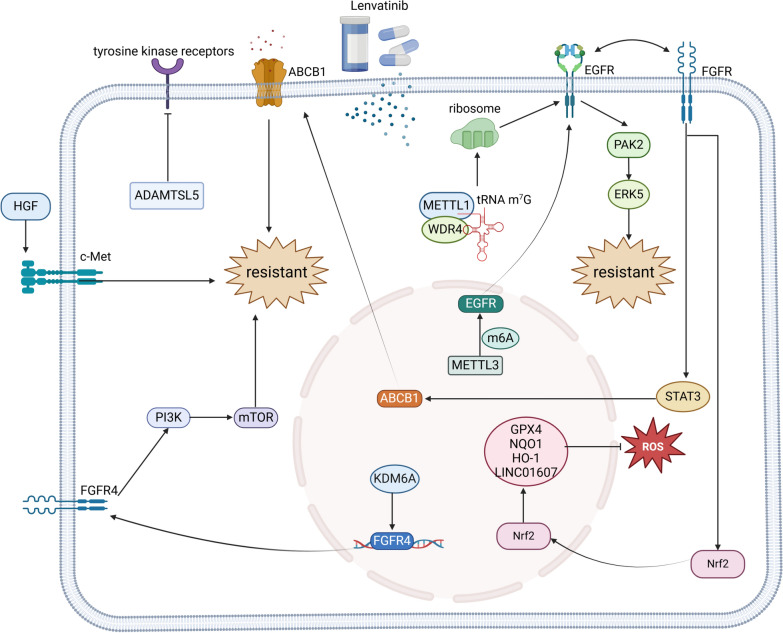
Specific gene expression in tumor tissues is significantly associated with the efficacy and survival benefits of lenvatinib. Research suggests that FGFR4 may predict the clinical benefits of lenvatinib for HCC patients[130]. KDM6A promotes HCC progression by activating FGFR4 expression, making it an important molecule influencing the efficacy of lenvatinib treatment in HCC; patients with high KDM6A levels are more likely to benefit from lenvatinib therapy[131]. Additionally, the expression of miR-3154 in tumor tissues may also predict the clinical benefits of lenvatinib for HCC patients[132].
At the cellular level, circulating tumor DNA (ctDNA) analysis stands out as a promising methodology offering significant clinical insights. Monitoring the dynamics of ctDNA within somatic cells of hepatocellular carcinoma (HCC) patients undergoing lenvatinib therapy emerges as a valuable biomarker for gauging disease progression. Frequently altered genes encompass TP53 (54%), CTNNB1 (42%), TERT (42%), ATM (25%), and ARID1A (13%). A reduction in the average variant allele frequency (VAF) following four weeks of lenvatinib treatment correlates with extended progression-free survival. While the mean mutation frequency during treatment bears prognostic relevance, it is important to note the absence of a discernible link between initial mutational status and the therapeutic efficacy of lenvatinib [133].
The physical function of patients is a crucial prognostic factor for the treatment of any disease, including hepatocellular carcinoma (HCC) treated with lenvatinib. In HCC patients receiving lenvatinib, skeletal muscle volume (SMV)[134], skeletal muscle index (SMI) [135, 136], geriatric nutritional risk index (GNRI) [137], fatigue status [138], and body composition assessments [139] are all significantly correlated with patient prognosis following treatment.
Imaging monitoring also plays a vital role in predicting the efficacy of lenvatinib treatment for HCC. The baseline volume index from 68 Ga-FAPI PET/CT is a potential independent prognostic factor for predicting durable clinical benefits, progression-free survival (PFS), and overall survival (OS) in uHCC patients receiving combined PD-1 and lenvatinib therapy [140]. Early modifications in the time-intensity curve (TIC) detected during the arterial phase of contrast-enhanced ultrasound (CEUS) represent promising early imaging markers for assessing the efficacy of lenvatinib [141]. Additionally, measuring changes in tumor blood flow via CEUS is one of the indicators for evaluating the effectiveness of lenvatinib in advanced HCC [142]. A reduction rate of CT attenuation values ≥ 40% is indicative of a favorable response to lenvatinib in highly malignant tumors [143]. Furthermore, CT values of necrosis post-lenvatinib treatment (N-CTav) and pre-treatment 18F-fluorodeoxyglucose PET scans can predict overall outcomes following lenvatinib therapy [144, 145]. Additionally, Bo et al. developed a valuable machine-learning radiomics model that demonstrates good performance in predicting the response of unresectable HCC to monotherapy with lenvatinib [146].
In addition to the biomarkers mentioned above, numerous studies have developed new grading systems or models to monitor the efficacy of lenvatinib. Examples include the PIMET score [147], albumin-bilirubin (ALBI) grade [148], modified ALBI (mALBI) grade [149], the corresponding albumin simplified (ALBS) grade [150], the glasgow prognostic score (GPS) is determined based on serum levels of C-reactive protein and albumin [151], the AFP-GGT-AGH scoring system [152], Child–Pugh classification, prognostic nutritional index (PNI)[153], lenvatinib prognostic index (LEP)[154], onodera’s prognostic nutritional index (O-PNI) [155], CONUT score [156], and mG8 score [157]. Predictive models such as the “lenvatinib multivariable prognostic model” (MPML) [158] and the risk classifier of South China cohort (RCOSC) [159] demonstrate good discriminative ability, calibration, and applicability.
These biomarkers derived from tissue and cellular sources, along with imaging methods and the establishment of new evaluation grades or models, show strong performance in predicting lenvatinib efficacy. They can also identify patients who would benefit most from lenvatinib treatment, providing reliable tools for precision clinical decision-making in HCC patients. These biomarkers not only reflect the biological characteristics of tumors but also assist physicians in assessing patients’ treatment tolerability and potential risks of adverse effects. Through personalized evaluations for different patients, medical teams can develop treatment plans that better align with individual circumstances, thereby enhancing treatment success rates and patients' quality of life. Moreover, regular monitoring of changes in these biomarkers allows for timely adjustments to treatment strategies, optimizing therapeutic outcomes and further advancing the development of personalized treatments for liver cancer.
Liquid biopsy approaches
Liquid biopsy is a technique that detects circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), and circulating tumor RNA (ctRNA) through the collection of body fluid samples such as blood, urine, and saliva. Its non-invasive and highly repeatable nature provides comprehensive and personalized information in the diagnosis, treatment, and monitoring of tumors, thereby helping to improve patient survival rates and quality of life.
In the context of lenvatinib treatment for liver cancer, numerous studies have identified various circulating tumor biomarkers that can predict the efficacy of lenvatinib. Lenvatinib improves the immune microenvironment in HCC patients, preventing the exhaustion of effector cells and inhibiting the quantity and function of immunosuppressive cells. Consequently, many immune markers can validate the efficacy of lenvatinib, including the CTL/Treg ratio [25], Th1 cells, and serum TNF-α levels [28]. The neutrophil-to-lymphocyte ratio (NLR) [160] also plays a significant role in efficacy prediction, while the proportion of eosinophils in the blood before and after treatment is not a viable marker [161]. Additionally, inflammatory indicators and their combination with other types of markers can effectively predict lenvatinib efficacy. For instance, C-reactive protein (CRP) levels [162], the C-reactive protein-to-albumin ratio (CAR) [163], and the platelet-to-lymphocyte ratio (PLR) [164] are all predictive of overall survival in unresectable HCC patients receiving lenvatinib.
Lenvatinib exerts its anti-cancer effects by targeting receptors, and high levels of VEGF, FGF21, and FGF19 in the blood often indicate poor patient outcomes. Other predictive indicators for HCC patients undergoing lenvatinib treatment include serum angiopoietin 2 (ANG2) [72, 165], M2BPGi [166], ST6GAL [167], changes in nitric oxide levels [168], alpha-fetoprotein, and the ratio of branched-chain amino acids to tyrosine (BTR) [169].
Genotypically, the GALNT14-rs9679162 “GG” genotype has been associated with longer overall survival in HBsAg-negative aHCC patients treated with lenvatinib [170]. The SNP combination patterns of NOS3 rs2070744 and FGFR4 rs351855 may serve as predictive factors for treatment response and prognosis in HCC patients receiving lenvatinib [171].
The importance of liquid biomarkers in lenvatinib treatment for liver cancer is increasingly recognized. These biomarkers can non-invasively reflect the dynamic changes in tumors, aiding early assessment of patient responses to lenvatinib and monitoring potential resistance mechanisms during treatment. Liquid biomarkers hold significant clinical application value in the treatment of liver cancer with lenvatinib, advancing the progress of personalized therapy for this disease (Table 3).
Table 3.
Biomarkers and other predictive tools
| Predictor molecule | Location | Relationship with prognosis | References |
|---|---|---|---|
| FGFR4 | Tissue | Positive correlation | [130] |
| miR-3154 | Tissue | Negative correlation | [132] |
| Skeletal muscle volume (SMV) | Muscle | Positive correlation | [134–136] |
| KDM6A | Tissue | Positive correlation | [131] |
| Neutrophil-to-lymphocyte ratio | Blood | Negative correlation | [124] |
| ctDNA | Blood | [133] | |
| CD8 + T cell | Blood | Negative correlation | [90] |
| C-reactive protein to albumin ratio (CAR) | Blood | Negative correlation | [163] |
| Th cell, NK cell | Blood | Positive correlation | [91] |
| VEGF、FGF21 和 ANG2 | Serum | Negative correlation | [72] |
| Mac-2-binding protein glycosylation isomer (M2BPGi) | Serum | Negative correlation | [166] |
| Platelet-lymphocyte ratio(PLR) | Blood | Negative correlation | [164] |
| CTL/Treg 比率 | Serum | Positive correlation | [25] |
| GALNT14-rs9679162 genotype | Blood | Positive correlation | [170] |
| Eosinophil count | Blood | NA | [161] |
| ANG2 and VEGF | Serum |
VEGF: Positive correlation ANG2: Negative correlation |
[165] |
| TH1 cell, TNF-α | Blood | Positive correlation | [28] |
| CRP | Serum | Negative correlation | [162] |
| ST6GAL | Serum | Positive correlation | [167] |
| NOS3 rs2070744 genotypes | Blood | [171] | |
| 2 M-DBR (DBR for the first 60 days), α-fetoprotein and branched-chain Amino acid to tyrosine ratio (BTR) | Blood | Positive correlation | [138, 169] |
| Reduction in tumor stain at 2 weeks | Blood, CT | Negative correlation | [172] |
| Predictive model/grading | Relationship with prognosis | References |
|---|---|---|
| A tumor-to-normal liver ratio (TLR) | Negative correlation | [145] |
| Cirrhotic PDX model | [173] | |
| Modified albumin-bilirubin grade(mALBI) | Negative correlation | [149] |
| Onodera's prognostic nutritional index (O-PNI) | Negative correlation | [155] |
| The time-intensity curve (TIC) of arterial phase on contrast-enhanced ultrasound (CEUS) | [141] | |
| The albumin-bilirubin (ALBI) grade | Negative correlation | [148] |
| Modified Geriatric 8 (mG8) score | Positive correlation | [157] |
| The Geriatric Nutritional Risk Index (GNRI) | Positive correlation | [137] |
| PIMET score | Positive correlation | [147] |
| Blood flow reduction | Positive correlation | [142] |
| MPML model | Negative correlation | [158] |
| CONUT score | Negative correlation | [156] |
| The albumin simplified (ALBS) grade | Negative correlation | [150] |
| Glasgow prognostic score (GPS) | Negative correlation | [151] |
| AFP-GGT-Hangzhou (AGH) scoring system | Negative correlation | [152] |
| Body composition parameters | [139] | |
| Prognostic nutrition index (PNI) | Positive correlation | [153] |
| Machine learning (ML) radiomics models | [146] | |
| Reductions in the rate of mean computed tomography (CT) attenuation values | Negative correlation | [143] |
| The lenvatinib prognostic index (LEP) index | Negative correlation | [154] |
| A Risk Classifier of South China Cohort (RCOSC) | [159] | |
| The computed tomography (CT) attenuation value (CTav) | Positive correlation | [144] |
Positive correlation: the higher the content or value, the better the prognosis
Negative correlation: the lower the content or value, the better the prognosis
Combination strategies:
Lenvatinib plus other TKIs
Combining lenvatinib with other tyrosine kinase inhibitors (TKIs) can integrate multiple target inhibitions, thereby addressing the biological characteristics of tumors more comprehensively, optimizing treatment strategies, and improving patient outcomes. Regorafenib, an oral broad-spectrum tyrosine kinase inhibitor, received FDA approval in April 2017 for use as a second-line therapy in patients displaying resistance to sorafenib. However, resistance to regorafenib also occurs. Research by Zhang et al. demonstrated that lenvatinib can reverse regorafenib resistance in hepatocellular carcinoma (HCC) by downregulating the IGF1R/Mek/Erk signaling pathway, highlighting the potential of combining regorafenib with lenvatinib in HCC treatment [174]. Furthermore, prolonged exposure to sorafenib can lead to upregulation of FGFR1 in HCC cells, resulting in resistance to sorafenib, while lenvatinib may overcome this resistance by blocking FGFR1 [8]. Therefore, clinical studies on the combination of lenvatinib after sorafenib resistance are also anticipated.
Lenvatinib plus anti-angiogenic agents
One of the primary mechanisms by which lenvatinib exerts its anti-cancer effects is through the inhibition of tumor angiogenesis. When combined with other anti-angiogenic agents, it can enhance the overall anti-cancer efficacy. Concurrently, hepatocellular carcinoma (HCC) cells acquire resistance to lenvatinib through the activation of the epidermal growth factor receptor (EGFR) pathway, thereby inducing the EGFR-STAT3-ABCB1 axis. Targeting EGFR can help counteract the resistance induced by lenvatinib, as EGFR inhibition increases the sensitivity of liver cancer to lenvatinib. The combination of lenvatinib with epidermal growth factor receptor (EGFR) inhibitors represents a promising strategy that may improve clinical outcomes for certain patients [175]. Erlotinib, an inhibitor of the epidermal growth factor receptor (EGFR), has exhibited the capability to suppress ABCB1 activity, consequently diminishing the efflux of lenvatinib. Co-administration of lenvatinib with erlotinib has demonstrated substantial synergistic effects on hepatocellular carcinoma, as evidenced in both in vitro and in vivo studies [176]. Ramucirumab, a targeted agent that binds selectively to vascular endothelial growth factor receptor 2 (VEGFR-2) to hinder its activation, demonstrates promise in halting disease advancement among individuals with unresectable hepatocellular carcinoma (HCC) who have prior exposure to lenvatinib, all the while preserving hepatic function throughout treatment [177, 178]. Additionally, clinical trials have confirmed the efficacy and safety of the triple combination of lenvatinib with anti-angiogenic agents (such as bevacizumab) and PD-1 inhibitors (like atezolizumab) [179, 180].
The synergistic utilization of lenvatinib in conjunction with other tyrosine kinase inhibitors (TKIs) and anti-angiogenic agents displays auspicious clinical prospects. This strategy not only addresses numerous biological pathways implicated in tumor advancement but also proficiently retards the emergence of resistance mechanisms.
In addition, combinations of lenvatinib with proton beam therapy [181], hepatic arterial infusion chemotherapy [182–185], transarterial chemoembolization (TACE) [186], and lenvatinib-eluting microspheres [187, 188] have demonstrated promising clinical application potential. By integrating drugs with different mechanisms, these treatment regimens can more comprehensively address the complexities of liver cancer, thereby improving patient survival and quality of life. Future research will further validate the safety and efficacy of these combination therapies, providing more effective treatment options for liver cancer patients.
Overcoming resistance to lenvatinib
Mechanisms of resistance
In the treatment of liver cancer, the emergence of resistance to lenvatinib limits its long-term efficacy. Data indicate that once patients develop resistance, the average duration of treatment is only 7.4 months [3]. Research indicates that resistance mechanisms are closely associated with factors such as genetic mutations in tumor cells, reprogramming of signaling pathways, and alterations in the tumor microenvironment. A deeper understanding of these resistance mechanisms is crucial for optimizing treatment strategies and improving patient outcomes.
Mechanisms for increasing sensitivity to lenvatinib
In the treatment of liver cancer, enhancing patient sensitivity to lenvatinib is crucial for improving efficacy. This can be explored from three aspects: coding genes, non-coding RNAs, and drug strategies. First, the regulation of certain coding genes can influence tumor cell responses to lenvatinib by optimizing target expression and signaling pathway activity. Second, non-coding RNAs, particularly specific miRNAs and long non-coding RNAs (lncRNAs) can promote drug sensitivity by modulating the expression of resistance-related genes. Finally, appropriate drug combination strategies and administration methods can significantly enhance the efficacy of lenvatinib through synergistic effects. By comprehensively studying these factors, new approaches for personalized treatment of liver cancer may be developed.
Coding genes in the context of coding genes, FOXA2 is a transcription factor essential for normal liver development and metabolism. Its overexpression in hepatocellular carcinoma (HCC) is often associated with better prognosis, as it enhances the inhibitory effect of lenvatinib on HCC cells by upregulating the AMPK-mTOR pathway [189]. Ubiquitin-specific protease 1 (USP1) is a key deubiquitinating enzyme that is overexpressed in HCC patients and plays a critical role in tumor progression. Mechanistic studies indicate that USP1 enhances the stability of the c-kit through interaction, making the c-kit an important target for lenvatinib treatment. Thus, the upregulation of USP1 sensitizes HCC cells to lenvatinib therapy [190]. GANT61, a selective inhibitor of GLI that suppresses the hedgehog signaling pathway, has demonstrated potential anti-cancer effects. In HCC cells, particularly those with high CD133 expression, the combination of lenvatinib and GANT61 can reverse lenvatinib resistance and improve treatment efficacy [191]. RARRES1, a gene encoding a membrane protein, has been shown to interact with SPINK2 in HCC, promoting SPINK2 expression and inhibiting tumor cell proliferation and migration, thus increasing sensitivity to lenvatinib [192]. In HCC, KDM6A is significantly upregulated and associated with poor prognosis. KDM6A promotes FGFR4 expression, further activating the PI3K-AKT-mTOR signaling pathway, leading to metabolic reprogramming of glucose and lipids. Patients with high KDM6A expression in HCC exhibit greater sensitivity to lenvatinib-targeted therapy [131]. DUSP4 inhibits the lenvatinib resistance process by suppressing MAPK/ERK signaling. These mechanisms highlight the importance of specific coding genes in enhancing lenvatinib sensitivity in HCC treatment [193] (Fig. 2).
Fig. 2.
Coding gene promotes lenvastinib sensitivity
Noncoding RNA in the realm of non-coding RNA, miR-23b, as one of the most extensively studied miRNAs, plays a significant role in the development of many diseases, and hence is commonly targeted as a therapeutic intervention point. In the context of lenvatinib resistance in liver cancer research, it has been confirmed that miR-23b can inhibit the proliferation, migration, invasion, and angiogenesis of lenvatinib in human hepatocellular carcinoma cells, ultimately enhancing the sensitivity of lenvatinib [194]. In lenvatinib-resistant cell lines, elevated expression of Lnc-ZEB2-19, by specifically binding with TRA2A, leads to destabilization and subsequent degradation of RSPH14 mRNA, further resulting in the transcriptional downregulation of Rela (p65) and p-Rela (p-p65), inhibiting the stemness and migration of liver cancer, ultimately suppressing the progression and drug resistance of HCC [195] (Fig. 3).
Fig. 3.
Noncoding RNA promotes lenvastinib sensitivity
Drugs in terms of pharmaceuticals, cisplatin, a common chemotherapy drug, induces phosphorylation of Chk1 and Chk2 by promoting ATM/ATR, further inhibiting cyclin B1 and Cdc2, preventing cell transition from G2 phase to M phase, thus inhibiting LR cell proliferation. Simultaneously, cisplatin is involved in the regulation of drug-resistant miRNAs (including 17 upregulated miRNAs and 6 downregulated miRNAs) [196]. Losartan, an angiotensin II receptor blocker, reduces lenvatinib-mediated VEGF-A and IL-8 production, directly inhibiting endothelial cell growth, thereby increasing sensitivity to lenvatinib-mediated vascular inhibition [197]. Metformin, besides its application in diabetes, also plays a role in anticancer activities. The combination of metformin and lenvatinib inhibits the AKT signaling pathway, thereby suppressing FOXO3 phosphorylation, leading to its nuclear accumulation, and synergistically inhibiting HCC growth and migration [198]. Secukinumab, the first approved IL-17A inhibitor globally, commonly used to treat psoriasis, when combined with lenvatinib, enhances tumor autophagy, better-suppressing tumor development compared to using lenvatinib alone [199]. Flubendazole, a common parasite treatment drug, has recently been reported for its role in cancer treatment, such as in breast cancer [200]. Its advantages in combination with lenvatinib have also been discovered in liver cancer [201]. Fasudil, a vasodilator, overcomes lenvatinib resistance in HCC by inhibiting the upstream switch IHH of the Hedgehog pathway, further inhibiting the GLI2-ABCC1 pathway activated by lenvatinib, ultimately overcoming lenvatinib resistance in HCC [202]. Traditional Chinese medicine also demonstrates great potential in enhancing sensitivity to lenvatinib Betulinic acid, a natural pentacyclic triterpenoid compound widespread in birch trees and various plants, has shown promising prospects in anticancer applications and has recently emerged in enhancing the efficacy of lenvatinib. Betulinic acid inhibits SREBP2, further inhibiting the mTOR/IL-1β signaling pathway, demonstrating good sensitization effects both in vitro and in vivo. The combination therapy of lenvatinib and betulinic acid synergistically inhibits HCC cell proliferation [203]. Amentoflavone enhances sensitization effects by further enhancing lenvatinib’s inhibition on the AKT/ERK signaling pathways and inducing apoptosis in liver cancer cells [204]. Sophoridine effectively inhibits tumor cell proliferation, particularly in LR cells, by reducing VEGFR2 expression and further suppressing the RAS/MEK/ERK axis to inhibit lenvatinib-resistant liver cancer cell growth [205]. Oxysophocarpine suppresses FGFR1 expression, subsequently inhibiting AKT and ERK signal transduction, making FGFR1-overexpressing HCC more sensitive to lenvatinib [206]. Compound Phyllanthus urinaria L. (CP), known for its potent anticancer effects, is extensively used in clinical treatment of liver cancer. The combination therapy of CP and lenvatinib effectively controls HCC progression by promoting exosome-mediated autophagy inhibition [207]. Curcumol effectively induces apoptosis in many cancer cells by targeting key signaling pathways such as MAPK/ERK, PI3K/Akt, and NF-κB. In LR cells, EGFR activation is significantly inhibited, and Curcumol enhances the efficacy of lenvatinib by inhibiting the EGFR-PI3K-AKT pathway [208]. Additionally, Curcumin has been reported to increase the expression of Bax, E-cadherin, ULK, and LC3B II/I while decreasing Bcl-2, N-cadherin, and JAK2/STAT3 expression, thereby enhancing lenvatinib’s anti-tumor effects on liver cancer cells [209].
In conclusion, exploring strategies to enhance sensitivity to lenvatinib in liver cancer from the perspectives of coding genes, non-coding RNA, and pharmaceuticals holds significant clinical importance. Regulating the expression of key genes, targeting specific miRNAs, and utilizing pharmaceutical agents in combination therapies are promising approaches to overcome lenvatinib resistance and improve treatment outcomes in hepatocellular carcinoma (Fig. 4).
Fig. 4.
Drugs promotes lenvastinib sensitivity
Mechanisms of increasing resistance to lenvatinib
Tyrosine kinase receptors lenvatinib exerts its anti-tumor effects by targeting tyrosine kinase receptors, including FGFR, EGFR, and MET receptors. Numerous studies have indicated that the activation or increased expression of these targeted receptors can promote the development of resistance to Lenvatinib. Lenvatinib inhibits FGFR4, further affecting GPX4 to induce ROS elevation, thereby mediating ferroptosis in tumor cells. Research has shown that Nrf2, a key regulator of antioxidant responses, can counteract Lenvatinib-induced ferroptosis by inhibiting ROS generation, and the inhibition of activated Nrf2 can resensitize HCC cells to Lenvatinib [14]. The upregulation of EGFR may lead to resistance development by promoting ROS accumulation [16, 210]. Additionally, studies have reported that the activation of the EGFR-STAT3-ABCB1 axis significantly enhances Lenvatinib-mediated efflux-induced resistance, and the combination of Erlotinib, an EGFR inhibitor, with Lenvatinib has been shown to improve Lenvatinib resistance issues [176]. Moreover, it has been found that the two targets of Lenvatinib, EGFR, and FGFR, are not entirely independent, and their inhibition may lead to feedback activation of the EGFR-PAK2-ERK5 signaling axis, which is also a contributing factor to Lenvatinib resistance [16]. Activation of the hepatocyte growth factor (HGF)/c-MET signaling pathway is implicated in inducing resistance to Lenvatinib in hepatocellular carcinoma (HCC) cells exhibiting heightened c-MET expression levels. Combining Lenvatinib treatment with c-MET inhibitors can enhance systemic treatment efficacy in HCC patients [211]. Overexpression of ADAMTSL5 is associated with increased expression or phosphorylation of TKI receptors such as MET, EGFR, PDGFRβ, IGF1Rβ, or FGFR4, thereby contributing to reduced sensitivity of HCC cells to TKIs including Lenvatinib[212]. METTL1 activates the EGFR pathway through m7G tRNA modification and participates in the development of Lenvatinib resistance in HCC patients [213]. Significantly increased METTL3 in Lenvatinib-resistant cells affects EGFR activation through m6A modification, further promoting Lenvatinib resistance [214]. The close relationship between the target receptors of Lenvatinib and resistance highlights the intricate nature of resistance mechanisms. In-depth exploration of these target receptors not only enhances our understanding of resistance mechanisms but also offers novel therapeutic avenues to overcome resistance. By optimizing targeted treatment strategies and combining them with other therapies, there is potential to further enhance the efficacy of Lenvatinib, thereby improving treatment outcomes for liver cancer patients (Fig. 5).
Fig. 5.
Targeting tyrosine kinase receptors promotes lenvastinib resistance
Signal pathways in the treatment of liver cancer with lenvatinib, the role of signaling pathways is particularly crucial. These pathways not only regulate tumor cell growth and survival but also directly influence drug sensitivity and resistance. Investigating key signaling pathways can provide insights into how tumor cells develop resistance to lenvatinib. ITGB8, a member of the integrin beta chain family that encodes a type I membrane protein, promotes lenvatinib resistance by regulating AKT stability and enhancing AKT signaling through HSP90 [215]. The IGFBP-1-integrin α5β1 pathway facilitates tumor angiogenesis, contributing to HCC resistance [216]. In hypoxic environments, the PPARGC1A/BAMBI/ACSL5 axis is activated, regulating ROS production to inhibit ferroptosis in HCC cells [217]. Additionally, pathways such as TM4SF1-MYH9-NOTCH [218], FAK-WNK1 [219], USP22-JMJD8 [220], and METTL3-FZD10/β-catenin/c-Jun/MEK/ERK are all implicated in the development of lenvatinib resistance [221]. A deeper exploration of the significance of these signaling pathways in lenvatinib resistance will provide new insights and strategies for the personalized treatment of liver cancer. The abnormal activation or reprogramming of these pathways is often a primary cause of resistance, highlighting their potential therapeutic value as targets for intervention (Fig. 6).
Fig. 6.
Promoting lenvatinib resistance through signaling pathways
Metabolic factor in the treatment of liver cancer with lenvatinib, metabolic factors play a significant role and have a notable impact on the development of resistance. The metabolic reprogramming of tumor cells can alter their response to drugs, affecting drug uptake, metabolism, and excretion. Specific metabolic pathways, such as glycolysis, lipid metabolism, and amino acid metabolism, may play critical roles in resistance mechanisms. By studying these metabolic factors, we can gain a comprehensive understanding of how liver cancer cells adapt to lenvatinib treatment, providing new targets and strategies to overcome resistance.
During lenvatinib treatment, the drug can increase the recruitment of neutrophils by promoting the secretion of CXCL2 and CXCL5. Once in the tumor microenvironment (TME), neutrophils polarize to the N2 phenotype. Tumor-derived lactate can induce the expression of PD-L1 on neutrophils through the MCT1/NF-κB/COX-2 pathway. The increase in PD-L1-expressing neutrophils leads to the upregulation of Tregs and a decrease in T cell cytotoxicity. COX-2 inhibitors have been shown to reduce PD-L1-expressing neutrophils and restore T-cell cytotoxicity, which may provide a basis for improving lenvatinib resistance [222]. SLP2, a mitochondrial protein, can bind to JNK2, maintaining its stability. The increase in JNK2 enhances SREBP1 activity, promoting its translocation to the nucleus and facilitating de novo lipogenesis, thereby counteracting the effects of lenvatinib. This has also been validated in animal models [223]. ACYP1 can form a trimeric complex with HSP90 and MYC, promoting MYC nuclear entry and mediating the transcription of LDHA, which regulates glycolysis and promotes the progression of HCC. Targeting ACYP1 may synergistically enhance the effects of lenvatinib [224]. High expression of NQO1 reduces ROS generation induced by lenvatinib, thereby inhibiting apoptosis and promoting resistance to lenvatinib in liver cancer cells [225]. The significance of metabolic factors in resistance will provide important insights for the personalization and optimization of liver cancer treatment (Fig. 7).
Fig. 7.
Promoting lenvatinib resistance through metabolic factors
Transcription factor in the treatment of liver cancer with lenvatinib, transcription factors play a crucial role in regulating the expression of related genes, thereby influencing tumor cell responses and adaptations to the drug. Specific transcription factors may enhance the expression of resistance-related genes, leading to decreased sensitivity of tumor cells to lenvatinib. LEF1 has emerged as a new target to overcome lenvatinib resistance; studies have shown that in resistant cells, both LEF1 and its upstream regulator, β-catenin, are significantly elevated. This elevation affects the transcription of EMT-related genes (snail1, snail2, twist) in the nucleus, promoting resistance [226]. Under lenvatinib treatment, HIF-1α levels increase in HCC cells, leading to enhanced transcription of STOML2. Increased STOML2 stabilizes PINK1, further enhancing mitochondrial autophagy, which supports normal physiological activities of tumor cells, promotes metastasis, and modulates HCC responses to lenvatinib [227]. HIF-1α can also regulate autophagy levels by increasing the transcription of NRP1, contributing to the development of cellular resistance [228]. The DAGLA/2-AG axis promotes YAP nuclear translocation by inhibiting the phosphorylation of LATS1 and YAP, ultimately increasing the nuclear expression of PHLDA2, which can promote resistance [229]. WDR4 is highly expressed in liver cancer and contributes to lenvatinib resistance by promoting the expression of TRIM28, thus influencing downstream target gene expression [230]. The upregulation of CDK6 mediated by ERK/YAP signaling binds to and phosphorylates GSK3β, affecting LEF1 transcription and leading to the activation of Wnt/β-catenin, which in turn increases the expression of EMT-related genes, promoting lenvatinib resistance [220]. YTHDF1 drives HCC resistance through the YTHDF1–m6A–NOTCH1 epitranscriptomic axis. Targeting YTHDF1 with lipid nanoparticles has been shown to significantly improve the efficacy of lenvatinib in HCC [231]. Investigating the mechanisms of these transcription factors not only helps unveil the fundamental causes of resistance but also provides potential targets for developing new therapeutic strategies (Fig. 8).
Fig. 8.
Promoting lenvatinib resistance through transcription factor
Noncoding RNA in the realm of non-coding RNAs, lnc MT1JP promotes resistance to lenvatinib treatment by targeting miR-24-3p, which elevates the levels of BCL2L2 and inhibits tumor cell apoptosis [232]. Studies have also shown that lncRNA MKLN1-AS plays a critical role in resisting lenvatinib-induced apoptosis [233]. Additionally, lncRNA AC026401.3 can bind to OCT1, facilitating its accumulation in the promoter region of E2F2, thereby activating the transcription of E2F2 and contributing to lenvatinib resistance in HCC [234]. LINC01607 competes with miRNA-892b, reducing its levels while upregulating P62. The overexpression of P62 can induce protective mitophagy and modulate the transcription of ferroptosis-related genes (such as GPX4 and HO-1) by promoting the nuclear entry of Nrf2, ultimately leading to decreased ROS production and enhanced lenvatinib resistance[235]. Lenvatinib-resistant cells can secrete circPAK1, which is transmitted to sensitive cells via exosomes, inducing resistance in those cells [236]. This underscores the critical role of ceRNAs in promoting lenvatinib resistance in HCC. Addressing the issue of lenvatinib resistance in liver cancer necessitates further exploration of ceRNA regulatory networks for targeted therapies (Fig. 9).
Fig. 9.
Promoting lenvatinib resistance through Noncoding RNA
Tumor immunity in terms of immunity, SPP1 derived from cancer-associated fibroblasts (CAFs) influences the epithelial-mesenchymal transition (EMT) in liver cancer cells through the PKCα-RAF-MAPK-PI3K-AKT-MTOR pathway, leading to the development of resistance [237]. The combination of lenvatinib with immune checkpoint inhibitors has shown promising prospects compared to monotherapy; however, resistance issues still arise with combination treatments, and relevant studies have reported on this resistance. Zhou et al. found that lenvatinib combined with anti-PD1 antibody therapy leads to increased secretion of TNF by mucosal-associated invariant T (MAIT) cells in the immune microenvironment. TNF acts on TNFRSF1B on Treg cells, which subsequently suppresses anti-tumor immunity [238] (Fig. 10).
Fig. 10.
Promoting lenvatinib resistance through tumor immunity
Drugs in terms of pharmacology, targets that promote lenvatinib resistance have the potential as new therapeutic targets. Silencing or knocking out resistance-promoting genes has yielded promising results in cellular and preclinical studies, indicating potential for clinical translation. Notably, treatment strategies targeting HIF-1α have attracted significant attention. In vitro, experiments have demonstrated that the use of attenuated Salmonella delivering siRNA-HIF-1α in combination with lenvatinib effectively reduces the proliferation and angiogenesis of liver cancer cells while promoting apoptosis. Moreover, this combined therapeutic approach has the potential to augment the infiltration of T cells and M1 macrophages within the tumor microenvironment, amplifying the presence of immune cells in the spleen and thereby enhancing the host's anti-tumor immune response [239].
Predicting the occurrence of resistance in liver cancer patients during lenvatinib treatment is critically significant. Early identification of resistance risks allows for timely adjustments to treatment plans, improving therapeutic outcomes. Circulating tumor markers, due to their safety and sensitivity, are increasingly being utilized in cancer diagnosis and treatment. Analysis of circulating tumor DNA (ctDNA) in peripheral blood provides a non-invasive method to identify cancer-specific mutations. Discovering heterogeneous genomic changes associated with lenvatinib resistance during treatment will greatly expand the application of ctDNA.
In summary, studying the mechanisms of lenvatinib resistance in liver cancer carries profound implications. This research not only helps elucidate the complex processes by which tumor cells adapt to drug pressure but also provides new insights and strategies for clinical treatment. By identifying and understanding these resistance mechanisms, a foundation can be established for developing new targeted therapies and combination treatment strategies, thereby improving patient outcomes and survival rates. Future research should continue to focus on the diversity and complexity of resistance mechanisms to advance liver cancer treatment further.
Emerging therapeutic modalities:
Linkage with nanotechnology
The introduction of nanotechnology can enhance drug targeting and bioavailability, reduce side effects, and improve therapeutic efficacy. Lenvatinib, as a targeted therapy, can precisely target cancer cells and inhibit their growth. When combined with nanotechnology, the stability of the drug in the body is further improved, prolonging its circulation time while minimizing toxicity to normal cells, and offering new hope in cancer treatment.
Zhang et al. developed magnetic nanoliposomes (MNL) with dual-targeting capabilities for epithelial cell adhesion molecule (EpCAM) and wave protein, capable of encapsulating lenvatinib. In vitro and in vivo experiments demonstrated low cytotoxicity, effective inhibition of HCC cell proliferation, promotion of HCC cell apoptosis, and specific targeting capabilities with MRI tracing ability [240]. Wu et al. successfully constructed a biomimetic nanodrug delivery platform with lenvatinib at its core, wrapped in a pH-sensitive polymer, poly(β-amino ester)-polyethylene glycol amine (PAE-PEG-NH2), and coated with cancer cell membrane (CCM). In vivo experiments indicated that this nanodrug exhibited superior therapeutic effects in subcutaneous tumor mouse models, effectively eliminating tumors [241]. Liu et al. developed Bi/Se nanoparticles (NPs) loaded with lenvatinib (Bi/Se-Len NPs), which demonstrated excellent performance in reversing hypoxic and immunosuppressive states in HCC. Additionally, these nanoparticles can be used for CT image-guided stereotactic body radiotherapy (SBRT) in mice, providing accurate imaging guidance [242].
Metal-based nano drugs show promising clinical translational potential when combined with lenvatinib. Zeng et al. developed a targeted therapeutic strategy that co-assembled lenvatinib, adriamycin, and Fe3 + , along with metal nanodrugs to enhance tumor vascular normalization and increase the infiltration of T lymphocytes within tumors. This approach improves anti-tumor immunity mediated by calreticulin by alleviating hypoxia, reducing regulatory T cells, and downregulating tumor PD-L1 expression, effectively inhibiting the progression of HCC in both in situ models and patient-derived organoids [243]. Another metal nanodrug, CAL@PG, was successfully constructed by Xu et al. In this technique, ultrasmall gold nanoparticles (AuNPs) and ultrafine copper sulfide nanocrystals (Cu2-xS NCs) were uniformly encapsulated within galactosamine-conjugated poly(lactide-co-glycolide) (PLGA) to form drug delivery nanoparticles. CAL@PG nanoparticles exhibited excellent stability under physiological conditions, while rapidly releasing lenvatinib in the unique tumor microenvironment (TME) and at elevated temperatures. This combination therapy significantly enhanced the efficacy of lenvatinib through near-infrared II (NIR-II) photothermal effects [244, 245]. Luo et al. loaded lenvatinib onto mesoporous Fe3O4 (mFe) nanoparticles, with bovine serum albumin (BSA) attached to the NP surface to create a metal drug (BSA-mFe@Len NPs). This metal drug induces immune modulation in the TME against HCC through multiple functions of mFe NPs and lenvatinib, including triggering tumor apoptosis, vascular normalization, recruitment of cytotoxic T lymphocytes, and elimination of regulatory T cells. In vivo experiments demonstrated that this metal drug significantly inhibited hepatocellular carcinoma growth and evoked long-term anti-tumor immune memory, paving a new pathway for treating advanced HCC patients [246].
The combination with nanotechnology provides a multifunctional delivery system for lenvatinib, enriching the multimodal synergistic treatment approach for HCC. This method effectively enhances survival rates and quality of life for cancer patients while introducing new ideas and methods in cancer treatment. However, despite the potential shown in laboratory and clinical trials, further research and validation are needed to ensure safety and efficacy. As the fields of nanotechnology and biomedicine continue to advance, the application prospects of combining nanotechnology with targeted drugs like lenvatinib will become even broader.
Autologous platelets encapsulated the drugs
Hepatocellular carcinoma activates platelets, which then bind to tumor-associated endothelial cells and release growth factors, promoting tumor progression. Hiroki et al. utilized platelets as drug carriers for tumor therapy. In both in vitro and in vivo experiments, autologous platelets encapsulating various tyrosine kinase inhibitors (lenvatinib or sorafenib) were shown to effectively deliver anti-cancer drugs to tumor tissues. The use of autologous platelets for drug encapsulation may represent a novel therapeutic strategy for HCC [247].
Combination with Histone Deacetylase Inhibitors
Histone deacetylase inhibitors (HDAC inhibitors) alter chromatin structure and gene expression by inhibiting the activity of histone deacetylases, leading to increased histone acetylation and enhanced gene transcription. This process affects cell proliferation, differentiation, and apoptosis. HDAC inhibitors show significant potential in cancer therapy and can enhance the effects of other treatments. In hepatocellular carcinoma, the combination of HDAC inhibitors with lenvatinib has demonstrated synergistic anti-tumor effects. Research by Ryo et al. indicated that class IIa inhibitors combined with lenvatinib induce apoptosis by downregulating FGFR4 and blocking FGFR signaling in FGFR4-positive HCC cell lines[248]. Moreover, Jane et al. discovered that the synergistic action of entinostat, an HDAC inhibitor, in conjunction with lenvatinib induces cell death by triggering ROS-dependent ATM activation and eIF2α inactivation. Consequently, this series of events results in heightened cytotoxic autophagosome generation and reduced levels of protective mitochondrial protein expression [249].
Discussion and conclusion
Lenvatinib is a multi-targeted tyrosine kinase inhibitor that exerts its anti-tumor effects primarily by inhibiting vascular endothelial growth factor receptors (VEGFR), fibroblast growth factor receptors (FGFR), and other related signaling pathways. By blocking these pathways, lenvatinib effectively suppresses tumor cell proliferation and survival, inhibits tumor angiogenesis, and reduces tumor blood supply, thereby achieving therapeutic efficacy. Additionally, lenvatinib has shown improvements in the liver cancer microenvironment, capable of altering the tumor-associated immune status and promoting anti-tumor immune responses.
In multiple clinical trials, lenvatinib has demonstrated good anti-tumor activity and a high objective response rate, particularly among patients with advanced hepatocellular carcinoma (HCC), where its efficacy has been widely recognized. Compared to traditional treatment methods, lenvatinib not only prolongs patient survival but also enhances quality of life. However, its clinical application is not without side effects, such as hypertension, fatigue, and liver function impairment. These adverse reactions may affect patient compliance and overall survival rates. Therefore, in clinical practice, it is essential to strengthen the monitoring and management of these side effects to improve the patient treatment experience.
Although Lenvatinib demonstrates promising efficacy in the treatment of liver cancer, the issue of drug resistance is becoming increasingly severe. Studies indicate that HCC cells may develop resistance through various mechanisms, including genetic mutations, signaling pathway alterations, and changes in the tumor microenvironment. The emergence of these resistance mechanisms not only impacts the effectiveness of individual drugs but may also lead to disease progression. Understanding the complexity of drug resistance mechanisms is crucial for the development of new therapeutic strategies. Research into resistance mechanisms can provide novel biomarkers for clinical use. Biomarkers play a significant role in early diagnosis, prognosis assessment, and treatment monitoring of liver cancer. By identifying resistance-related biomarkers, it becomes possible to better predict patient responses and tailor treatment plans. Additionally, biomarkers can aid in identifying patient populations suitable for combination therapies, thereby enhancing overall treatment outcomes.
Combination therapies not only enhance anti-tumor immune responses but may also overcome resistance issues associated with single treatments. However, combining immune checkpoint inhibitors with Lenvatinib presents challenges in terms of safety and tolerability. Studies suggest that immune checkpoint inhibitors may trigger immune-related adverse events such as rash, hepatitis, and endocrine disorders, while Lenvatinib itself can lead to issues like hypertension and abnormal liver function. When these two drugs are used together, the safety risks for patients may significantly increase. Therefore, close monitoring of patient health status is essential during combination therapy to promptly identify and manage potential side effects, ensuring treatment safety and efficacy. Furthermore, further research should focus on the long-term safety of such combination therapies to provide more reliable treatment options for patients.
In conclusion, Lenvatinib holds significant clinical value in the treatment of liver cancer. Given the advantages of combination therapy, future research is anticipated to explore emerging treatment modalities involving Lenvatinib, such as combinations with novel ICIs (anti-LAG-3), CAR-T cell therapy, oncolytic viruses, ablation techniques, and other drugs or technologies. Understanding Lenvatinib resistance mechanisms, identifying biomarkers, and optimizing combination treatment strategies with immune checkpoint inhibitors offer new possibilities for improving the survival rates and quality of life for liver cancer patients. Future research should continue to focus on these areas to drive further advancements in liver cancer treatment.
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- ALBI
Albumin-bilirubin
- ALBS
Albumin simplified
- ANG2
Angiopoietin 2
- APCs
Antigen-presenting cells
- AuNPs
Ultrasmall gold nanoparticles
- Bi/Se-Len NPs
Bi/Se nanoparticles loaded with lenvatinib
- BSA
Bovine serum albumin
- BTR
Branched-chain amino acids to tyrosine
- CAFs
Cancer-associated fibroblasts
- CAR
C-reactive protein to albumin ratio
- CAR-T
Chimeric antigen receptor T-cell
- CCM
Cancer cell membrane
- CCL28
C–C motif chemokine ligand 28
- CEUS
Contrast-enhanced ultrasound
- CP
Compound Phyllanthus urinaria L.
- CP-B
Child–Pugh B
- CRP
C-reactive protein
- CSCs
Stem-like cells
- CTAv
CT values of necrosis post-lenvatinib treatment
- CTLA-4
Cytotoxic T lymphocyte-associated protein 4
- CTLs
Cytotoxic T lymphocytes
- CTCs
Circulating tumor cells
- CTD
Circulating tumor DNA
- CTIC
Time-intensity curve
- CTIM-3
T cell immunoglobulin and mucin domain-containing protein 3
- CTIGIT
T cell immunoglobulin and ITIM domain protein
- Cu2-xS NCs
Ultrafine copper sulfide nanocrystals
- DCR
Disease control rate
- DOR
Duration of response
- ECM
Extracellular matrix
- EMT
Epithelial-mesenchymal transition
- EpCAM
Epithelial cell adhesion molecule
- ERK
Extracellular regulated protein kinase
- FGL1
Fibrinogen-like protein 1
- FGF
Fibroblast growth factor
- FGFR
Fibroblast growth factor receptors
- FOLFOX
Oxaliplatin, 5-fluorouracil, and leucovorin
- Gal-9
Galectin-9
- GNRI
Geriatric nutritional risk index
- GPS
Glasgow Prognostic Score
- HAIC
Hepatic artery infusion chemotherapy
- HCC
Hepatocellular carcinoma
- HDAC inhibitors
Histone deacetylase inhibitors
- HRQOL
Health-related quality of life
- ICB
Immune checkpoint blockade
- ICER
Incremental cost-effectiveness ratio
- ICIs
Immune checkpoint inhibitors
- KIT
KIT proto-oncogene receptor tyrosine kinase
- LAG-3
Lymphocyte activation gene 3
- LEP
Lenvatinib Prognostic Index
- lncRNAs
Long non-coding RNAs
- MAIT
Mucosal-associated invariant T
- MDSCs
Myeloid-derived suppressor cells
- mALBI
Modified ALBI
- MHC
Major histocompatibility complex
- MNL
Magnetic nanoliposomes
- mOS
Median overall survival
- MPML
Lenvatinib multivariable prognostic model
- MSI-H
Microsatellite instability
- mFe
Fe3O4
- mPFS
Median progression-free survival
- MTA
Molecular targeted agent
- NCTAv
CT values of necrosis post-lenvatinib treatment
- NIR-II
Near-infrared II
- NAD +
Nicotinamide adenine dinucleotide
- NLR
Neutrophil-to-lymphocyte ratio
- OS
Overall survival
- O-PNI
Onodera's Prognostic Nutritional Index
- ORR
Overall response rate
- PAE-PEG-NH2
Poly(β-amino ester)-polyethylene glycol amine
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death ligand 1
- PDX
Patient-derived xenograft
- PDGF
Platelet-derived growth factor
- PDGFRα
Platelet-derived growth factor receptor alpha
- PFS
Progression-free survival
- PLGA
Galactosamine-conjugated poly(lactide-co-glycolide)
- PLR
Platelet-to-lymphocyte ratio
- PKB/AKT
Protein kinase B
- PNI
Prognostic nutritional index
- PSGL-1
P-selectin glycoprotein ligand 1
- PVE
Portal vein embolization
- PVTT
Portal vein tumor thrombus
- QALY
Quality-adjusted life years
- RCOSC
Risk classifier of south China cohort
- RET
Rearranged during transfection
- SBRT
Stereotactic body radiotherapy
- SHP-2
Src homology region 2-containing protein tyrosine phosphatase 2
- SIRPα
Signal regulatory protein α
- SMI
Skeletal muscle index
- SMV
Skeletal muscle volume
- Syk
Spleen tyrosine kinase
- STING
Stimulator of interferon genes
- TACE
Transarterial chemoembolization
- TCR
T cell receptor
- TBV
Tumor blood volume
- TCTDNA
Circulating tumor DNA
- TCTRNA
Circulating tumor RNA
- Th
T helper
- TIGIT
T cell immunoglobulin and ITIM domain protein
- TIME
Tumor immune microenvironment
- TIM-3
T cell immunoglobulin and mucin domain-containing protein 3
- TILs
Tumor-infiltrating lymphocytes
- TME
Tumor microenvironment
- TNPs
Bi/Se nanoparticles loaded with lenvatinib
- Tregs
Regulatory T cells
- TTP
Time to progression
- TRAE
Treatment-related adverse events
- USP1
Ubiquitin-specific protease 1
- VAF
Variant allele frequency
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptors
- VISTA
V-domain Ig suppressor of T-cell activation
- VSIG3
V-set and immunoglobulin domain containing 3
Author contributions
YC and SD retrieved the data and drafted the manuscript. CC retrieved the data and revised the manuscript. LC initiated the idea and drafted the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82374246, 82174169).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuhang Chen and Suoyi Dai have contributed equally to this work.
Contributor Information
Chien-shan Cheng, Email: natcheng@connect.hku.hk.
Lianyu Chen, Email: lianyu-chen@alu.fudan.edu.cn.
References
- 1.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;4:378–90. [DOI] [PubMed] [Google Scholar]
- 2.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Cheng AL, et al. Overall survival and objective response in advanced unresectable hepatocellular carcinoma: a subanalysis of the REFLECT study. J Hepatol. 2023;1:133–41. [DOI] [PubMed] [Google Scholar]
- 3.Buttell A, Qiu W. The action and resistance mechanisms of Lenvatinib in liver cancer. Mol Carcinog. 2023;12:1918–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar C, Chakroborty D, Goswami S, Fan H, Mo X and Basu S. VEGF-A controls the expression of its regulator of angiogenic functions, dopamine D2 receptor, on endothelial cells. J Cell Sci. 2022; 11. [DOI] [PMC free article] [PubMed]
- 5.Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014; 18. [DOI] [PMC free article] [PubMed]
- 6.Zschabitz S and Grullich C. Lenvantinib: A Tyrosine Kinase Inhibitor of VEGFR 1–3, FGFR 1–4, PDGFRalpha, KIT and RET. Recent Results Cancer Res. 2018; 187–98. [DOI] [PubMed]
- 7.Wang Y, Peng C, Wang G, Xu Z, Luo Y, Wang J, et al. Exploring binding mechanisms of VEGFR2 with three drugs lenvatinib, sorafenib, and sunitinib by molecular dynamics simulation and free energy calculation. Chem Biol Drug Des. 2019;5:934–48. [DOI] [PubMed] [Google Scholar]
- 8.Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, et al. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;6:2641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prieto-Dominguez N, Shull AY, Teng Y. Making way for suppressing the FGF19/FGFR4 axis in cancer. Future Med Chem. 2018;20:2457–70. [DOI] [PubMed] [Google Scholar]
- 10.Johansson H, Sondergaard J N, Jorns C, Kutter C and Ellis E C S. Chenodeoxycholic Acid Modulates Bile Acid Synthesis Independent of Fibroblast Growth Factor 19 in Primary Human Hepatocytes. Front Endocrinol (Lausanne). 2020; 554922. [DOI] [PMC free article] [PubMed]
- 11.Phan P, Saikia BB, Sonnaila S, Agrawal S, Alraawi Z, Kumar TKS, et al. The saga of endocrine FGFs. Cells. 2021;9:2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibata O, Kamimura K, Ko M, Sakai N, Abe H, Morita S, et al. Effect of lenvatinib on a hepatocellular carcinoma with fibroblast growth factor receptor 4 expression: a case report and review of the literature. Intern Med. 2021;11:1709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang K, Chu K, Wu X, Gao H, Wang J, Yuan YC, et al. Amplification of FRS2 and activation of FGFR/FRS2 signaling pathway in high-grade liposarcoma. Cancer Res. 2013;4:1298–307. [DOI] [PubMed] [Google Scholar]
- 14.Iseda N, Itoh S, Toshida K, Tomiyama T, Morinaga A, Shimokawa M, et al. Ferroptosis is induced by lenvatinib through fibroblast growth factor receptor-4 inhibition in hepatocellular carcinoma. Cancer Sci. 2022;7:2272–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, et al. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology. 2021;5:2544–60. [DOI] [PubMed] [Google Scholar]
- 16.Jin H, Shi Y, Lv Y, Yuan S, Ramirez CFA, Lieftink C, et al. EGFR activation limits the response of liver cancer to lenvatinib. Nature. 2021;7869:730–4. [DOI] [PubMed] [Google Scholar]
- 17.Hoshi T, Watanabe Miyano S, Watanabe H, Sonobe RMK, Seki Y, Ohta E, et al. Lenvatinib induces death of human hepatocellular carcinoma cells harboring an activated FGF signaling pathway through inhibition of FGFR-MAPK cascades. Biochem Biophys Res Commun. 2019;1:1–7. [DOI] [PubMed] [Google Scholar]
- 18.Moura MM, Cavaco BM, Pinto AE, Domingues R, Santos JR, Cid MO, et al. Correlation of RET somatic mutations with clinicopathological features in sporadic medullary thyroid carcinomas. Br J Cancer. 2009;11:1777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tohyama O, Matsui J, Kodama K, Hata-Sugi N, Kimura T, Okamoto K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014; 638747. [DOI] [PMC free article] [PubMed]
- 20.Okamoto K, Kodama K, Takase K, Sugi NH, Yamamoto Y, Iwata M, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;1:97–103. [DOI] [PubMed] [Google Scholar]
- 21.Kato S, Subbiah V, Marchlik E, Elkin SK, Carter JL, Kurzrock R. RET aberrations in diverse cancers: next-generation sequencing of 4871 patients. Clin Cancer Res. 2017;8:1988–97. [DOI] [PubMed] [Google Scholar]
- 22.Yamada T, Fujiwara N, Kubota N, Matsushita Y, Nakatsuka T, Kurosaki S, et al. Lenvatinib recruits cytotoxic GZMK+CD8 T cells in hepatocellular carcinoma. Hepatol Commun. 2023; 8. [DOI] [PMC free article] [PubMed]
- 23.Mei Z, Gao X, Pan C, Wu Q, Wang S, Qian J, et al. Lenvatinib enhances antitumor immunity by promoting the infiltration of TCF1(+) CD8(+) T cells in HCC via blocking VEGFR2. Cancer Sci. 2023;4:1284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Q, Shen M, Zhu S, Liao Y, Zhang D, Sun J, et al. Targeting NAD(+) metabolism of hepatocellular carcinoma cells by lenvatinib promotes M2 macrophages reverse polarization, suppressing the HCC progression. Hepatol Int. 2023;6:1444–60. [DOI] [PubMed] [Google Scholar]
- 25.Zhu J, Fang P, Wang C, Gu M, Pan B, Guo W, et al. The immunomodulatory activity of lenvatinib prompts the survival of patients with advanced hepatocellular carcinoma. Cancer Med. 2021;22:7977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura T, Kato Y, Ozawa Y, Kodama K, Ito J, Ichikawa K, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;12:3993–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin YY, Tan CT, Chen CW, Ou DL, Cheng AL, Hsu C. Immunomodulatory effects of current targeted therapies on hepatocellular carcinoma: implication for the future of immunotherapy. Semin Liver Dis. 2018;4:379–88. [DOI] [PubMed] [Google Scholar]
- 28.Nagai H, Mukozu T, Kobayashi K, Nogami A, Nagumo H, Mohri K, et al. Lenvatinib might induce activation of host immunity in patients with hepatocellular carcinoma. Oncology. 2023;1:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adachi Y, Matsuki M, Watanabe H, Takase K, Kodama K, Matsui J, et al. Antitumor and antiangiogenic activities of lenvatinib in mouse xenograft models of vascular endothelial growth factor-induced hypervascular human hepatocellular carcinoma. Cancer Invest. 2019;4–5:185–98. [DOI] [PubMed] [Google Scholar]
- 30.Ogasawara S, Mihara Y, Kondo R, Kusano H, Akiba J, Yano H. Antiproliferative effect of lenvatinib on human liver cancer cell lines in vitro and in vivo. Anticancer Res. 2019;11:5973–82. [DOI] [PubMed] [Google Scholar]
- 31.Muraishi N, Kawamura Y, Akuta N, Shindoh J, Matsumura M, Okubo S, et al. The impact of lenvatinib on tumor blood vessel shrinkage of hepatocellular carcinoma during treatment: an imaging-based analysis. Oncology. 2023;2:134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueshima E, Sofue K, Takaki H, Hirata Y, Kodama H, Okada T, et al. Lenvatinib mitigates transarterial embolization-induced polarization of tumor-associated macrophages in a rat hepatocellular carcinoma model. J Vasc Interv Radiol. 2023;11(1977–85): e4. [DOI] [PubMed] [Google Scholar]
- 33.Une N, Takano-Kasuya M, Kitamura N, Ohta M, Inose T, Kato C, et al. The anti-angiogenic agent lenvatinib induces tumor vessel normalization and enhances radiosensitivity in hepatocellular tumors. Med Oncol. 2021;6:60. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Hu H, Yuan X, Fan X, Zhang C. Advances in immune checkpoint inhibitors for advanced hepatocellular carcinoma. Front Immunol. 2022;13:896752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;1:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau G, Abou-Alfa G K, Cheng A L, Sukeepaisarnjaroen W, Dao T V, Kang Y K, et al. Outcomes in the Asian subgroup of the phase III randomised HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. J Hepatol. 2024. [DOI] [PubMed]
- 37.Melero I, Yau T, Kang YK, Kim TY, Santoro A, Sangro B, et al. Nivolumab plus ipilimumab combination therapy in patients with advanced hepatocellular carcinoma previously treated with sorafenib: 5-year results from CheckMate 040. Ann Oncol. 2024;6:537–48. [DOI] [PubMed] [Google Scholar]
- 38.O’Donnell J S, Madore J, Li X Y and Smyth M J. Tumor intrinsic and extrinsic immune functions of CD155. Semin Cancer Biol. 2020; 189–96. [DOI] [PubMed]
- 39.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;10088:2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;1:77–90. [DOI] [PubMed] [Google Scholar]
- 41.El-Khoueiry AB, Trojan J, Meyer T, Yau T, Melero I, Kudo M, et al. Nivolumab in sorafenib-naive and sorafenib-experienced patients with advanced hepatocellular carcinoma: 5-year follow-up from CheckMate 040. Ann Oncol. 2024;4:381–91. [DOI] [PubMed] [Google Scholar]
- 42.Kudo M, Finn R S, Edeline J, Cattan S, Ogasawara S, Palmer D H, et al. Updated efficacy and safety of KEYNOTE-224: a phase II study of pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Eur J Cancer. 2022; 1–12. [DOI] [PubMed]
- 43.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind phase III trial. J Clin Oncol. 2020;3:193–202. [DOI] [PubMed] [Google Scholar]
- 44.Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo BY, et al. Pembrolizumab versus placebo as second-line therapy in patients from Asia with advanced hepatocellular carcinoma: a randomized, double-blind phase III trial. J Clin Oncol. 2023;7:1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torrens L, Montironi C, Puigvehi M, Mesropian A, Leslie J, Haber PK, et al. Immunomodulatory effects of lenvatinib plus anti-programmed cell death protein 1 in mice and rationale for patient enrichment in hepatocellular carcinoma. Hepatology. 2021;5:2652–69. [DOI] [PubMed] [Google Scholar]
- 46.Yang J, Guo Z, Song M, Pan Q, Zhao J, Huang Y, et al. Lenvatinib improves anti-PD-1 therapeutic efficacy by promoting vascular normalization via the NRP-1-PDGFRbeta complex in hepatocellular carcinoma. Front Immunol. 2023;14:1212577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology. 2020;4:1247–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adachi Y, Kamiyama H, Ichikawa K, Fukushima S, Ozawa Y, Yamaguchi S, et al. Inhibition of FGFR reactivates IFNgamma signaling in tumor cells to enhance the combined antitumor activity of lenvatinib with anti-PD-1 antibodies. Cancer Res. 2022;2:292–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki H, Iwamoto H, Tanaka T, Sakaue T, Imamura Y, Masuda A, et al. Fibroblast growth factor inhibition by molecular-targeted agents mitigates immunosuppressive tissue microenvironment in hepatocellular carcinoma. Hepatol Int. 2024;2:610–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei CY, Zhu MX, Zhang PF, Huang XY, Wan JK, Yao XZ, et al. PKCalpha/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. J Hepatol. 2022;1:163–76. [DOI] [PubMed] [Google Scholar]
- 51.Chen P, Li Y, Wei P, Liang L, Li B, Cao Y, et al. siRNA targeting PD-L1 delivered with attenuated Salmonella enhanced the anti-tumor effect of lenvatinib on mice bearing Hepatocellular carcinoma. Int Immunopharmacol. 2022;111:109127. [DOI] [PubMed] [Google Scholar]
- 52.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;10126:1163–73. [DOI] [PubMed] [Google Scholar]
- 53.Vogel A, Qin S, Kudo M, Su Y, Hudgens S, Yamashita T, et al. Lenvatinib versus sorafenib for first-line treatment of unresectable hepatocellular carcinoma: patient-reported outcomes from a randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;8:649–58. [DOI] [PubMed] [Google Scholar]
- 54.Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M, et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol. 2020;1:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ikeda S, Kudo M, Izumi N, Kobayashi M, Azuma M, Meier G, et al. Cost-effectiveness of lenvatinib in the treatment of patients with unresectable hepatocellular carcinomas in Japan: an analysis using data from japanese patients in the REFLECT trial. Value Health Reg Issues. 2021;24:82–9. [DOI] [PubMed] [Google Scholar]
- 56.Rinninella E, Cintoni M, Raoul P, Mele MC, De Gaetano AM, Marini MG, et al. Minimal impact of lenvatinib (Lenvima(R)) on muscle mass in advanced hepatocellular carcinoma and implications for treatment duration. two cases from the REFLECT study. Eur Rev Med Pharmacol Sci. 2019;22:10132–8. [DOI] [PubMed] [Google Scholar]
- 57.Piscaglia F, Ikeda K, Cheng AL, Kudo M, Ikeda M, Breder V, et al. Association between treatment-emergent hypertension and survival with lenvatinib treatment for patients with hepatocellular carcinoma in the REFLECT study. Cancer. 2024;8:1281–91. [DOI] [PubMed] [Google Scholar]
- 58.Evans TRJ, Kudo M, Finn RS, Han KH, Cheng AL, Ikeda M, et al. Urine protein: creatinine ratio vs 24-hour urine protein for proteinuria management: analysis from the phase 3 REFLECT study of lenvatinib vs sorafenib in hepatocellular carcinoma. Br J Cancer. 2019;3:218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu L, Zheng Y, Lin J, Shi X, Wang A. Comparison of the effects of lenvatinib and sorafenib on survival in patients with advanced hepatocellular carcinoma: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2023;1: 102061. [DOI] [PubMed] [Google Scholar]
- 60.Hua X, Yin Z, Liang J, Chen W, Gong H. Efficacy and safety comparison between lenvatinib and sorafenib in hepatocellular carcinoma treatment: a systematic review and meta-analysis of real-world study. Eur J Gastroenterol Hepatol. 2024;1:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen B, Zhang L, Cheng J, Wu T, Lei J, Yang X, et al. Risk factors for hepatic encephalopathy in hepatocellular carcinoma after sorafenib or lenvatinib treatment: a real-world study. Drug Des Devel Ther. 2022; 4429–37. [DOI] [PMC free article] [PubMed]
- 62.Sasaki R, Fukushima M, Haraguchi M, Honda T, Miuma S, Miyaaki H, et al. Impact of lenvatinib on renal function compared to sorafenib for unresectable hepatocellular carcinoma. Medicine (Baltimore). 2022;19: e29289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, Wang L, Xiao B, Cui M and Zhang B. Differences between sorafenib and lenvatinib treatment from genetic and clinical perspectives for patients with hepatocellular carcinoma. Med Sci Monit. 2022; e934936. [DOI] [PMC free article] [PubMed]
- 64.Huynh J, Cho M T, Kim E J, Ren M, Ramji Z and Vogel A. Lenvatinib in patients with unresectable hepatocellular carcinoma who progressed to child-Pugh B liver function. Ther Adv Med Oncol. 2022; 17588359221116608. [DOI] [PMC free article] [PubMed]
- 65.Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, et al. Sorafenib vs. lenvatinib as first-line therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Anticancer Res. 2020;4:2283–90. [DOI] [PubMed] [Google Scholar]
- 66.Shi T, Iwama H, Fujita K, Kobara H, Nishiyama N, Fujihara S, et al. Evaluating the effect of lenvatinib on sorafenib-resistant hepatocellular carcinoma cells. Int J Mol Sci. 2021; 23. [DOI] [PMC free article] [PubMed]
- 67.Cai H, Zhang L, Li N, Zheng B, Liu M. Lenvatinib versus sorafenib for unresectable hepatocellular carcinoma: a cost-effectiveness analysis. J Comp Eff Res. 2020;8:553–62. [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi M, Kudo M, Izumi N, Kaneko S, Azuma M, Copher R, et al. Cost-effectiveness analysis of lenvatinib treatment for patients with unresectable hepatocellular carcinoma (uHCC) compared with sorafenib in Japan. J Gastroenterol. 2019;6:558–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim JJ, McFarlane T, Tully S, Wong WWL. Lenvatinib versus sorafenib as first-line treatment of unresectable hepatocellular carcinoma: a cost-utility analysis. Oncologist. 2020;3:e512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saiyed M, Byrnes J, Srivastava T, Scuffham P, Downes M. Cost-effectiveness of lenvatinib compared with sorafenib for the first-line treatment of advanced hepatocellular carcinoma in Australia. Clin Drug Investig. 2020;12:1167–76. [DOI] [PubMed] [Google Scholar]
- 71.Shigesawa T, Maehara O, Suda G, Natsuizaka M, Kimura M, Shimazaki T, et al. Lenvatinib suppresses cancer stem-like cells in HCC by inhibiting FGFR1-3 signaling, but not FGFR4 signaling. Carcinogenesis. 2021;1:58–69. [DOI] [PubMed] [Google Scholar]
- 72.Finn RS, Kudo M, Cheng AL, Wyrwicz L, Ngan RKC, Blanc JF, et al. Pharmacodynamic biomarkers predictive of survival benefit with lenvatinib in unresectable hepatocellular carcinoma: from the phase III REFLECT study. Clin Cancer Res. 2021;17:4848–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodriguez-Hernandez MA, Chapresto-Garzon R, Cadenas M, Navarro-Villaran E, Negrete M, Gomez-Bravo MA, et al. Differential effectiveness of tyrosine kinase inhibitors in 2D/3D culture according to cell differentiation, p53 status and mitochondrial respiration in liver cancer cells. Cell Death Dis. 2020;5:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gunda V, Gigliotti B, Ashry T, Ndishabandi D, McCarthy M, Zhou Z, et al. Anti-PD-1/PD-L1 therapy augments lenvatinib’s efficacy by favorably altering the immune microenvironment of murine anaplastic thyroid cancer. Int J Cancer. 2019;9:2266–78. [DOI] [PubMed] [Google Scholar]
- 75.Allen E, Jabouille A, Rivera L B, Lodewijckx I, Missiaen R, Steri V, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017; 385. [DOI] [PMC free article] [PubMed]
- 76.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;26:2960–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Llovet JM, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, et al. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;12:1399–410. [DOI] [PubMed] [Google Scholar]
- 78.Zhang W, Tong S, Hu B, Wan T, Tang H, Zhao F, et al. Lenvatinib plus anti-PD-1 antibodies as conversion therapy for patients with unresectable intermediate-advanced hepatocellular carcinoma: a single-arm, phase II trial. J Immunother Cancer. 2023; 9. [DOI] [PMC free article] [PubMed]
- 79.Wen S, Zeng J, Zhong L, Ye J, Lai X. The efficacy and adverse effects of nivolumab and lenvatinib in the treatment of advanced hepatocellular carcinoma. Cell Mol Biol. 2022;11:53–7. [DOI] [PubMed] [Google Scholar]
- 80.Wu WC, Lin TY, Chen MH, Hung YP, Liu CA, Lee RC, et al. Lenvatinib combined with nivolumab in advanced hepatocellular carcinoma-real-world experience. Invest New Drugs. 2022;4:789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu L, Chen J, Liu C, Song X, Zhang Y, Zhao H, et al. Efficacy and safety of tislelizumab plus lenvatinib as first-line treatment in patients with unresectable hepatocellular carcinoma: a multicenter, single-arm, phase 2 trial. BMC Med. 2024;1:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu MH, Huang C, Li ML, Zhu XD, Tan CJ, Zhou J, et al. Effectiveness and safety of lenvatinib plus anti-programmed death-1 antibodies in patients with hepatocellular carcinoma: a real-world cohort study. Cancer Med. 2023;8:9202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang X, Chen B, Wang Y, Wang Y, Long J, Zhang N, et al. Real-world efficacy and prognostic factors of lenvatinib plus PD-1 inhibitors in 378 unresectable hepatocellular carcinoma patients. Hepatol Int. 2023;3:709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen K, Wei W, Liu L, Deng ZJ, Li L, Liang XM, et al. Lenvatinib with or without immune checkpoint inhibitors for patients with unresectable hepatocellular carcinoma in real-world clinical practice. Cancer Immunol Immunother. 2022;5:1063–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang XH, Liu CJ, Wen HQ, Duan XH, Jiao YQ, Liu YJ, et al. Effectiveness of lenvatinib plus immune checkpoint inhibitors in primary advanced hepatocellular carcinoma beyond oligometastasis. Clin Transl Med. 2023;3: e1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qiao Q, Han C, Ye S, Li J, Shao G, Bai Y, et al. The efficacy and safety of cadonilimab combined with lenvatinib for first-line treatment of advanced hepatocellular carcinoma (COMPASSION-08): a phase Ib/II single-arm clinical trial. Front Immunol. 2023; 1238667. [DOI] [PMC free article] [PubMed]
- 87.Sun X, Zhang Q, Mei J, Yang Z, Chen M, Liang T. Real-world efficiency of lenvatinib plus PD-1 blockades in advanced hepatocellular carcinoma: an exploration for expanded indications. BMC Cancer. 2022;1:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun W, Yin X, Liu X, Wei J, Yu M, Li W, et al. The clinical significance of sarcopenia in patients with hepatocellular carcinoma treated with lenvatinib and PD-1 inhibitors. Front Immunol. 2024; 1380477. [DOI] [PMC free article] [PubMed]
- 89.Wang H, Shi J, Wang X, Zhao H and Wang H. Acute exacerbation of asthma induced by combined therapy of programmed death-1 Blocker plus lenvatinib in a patient with advanced hepatocellular carcinoma. Eur J Cancer. 2021; 122–4. [DOI] [PubMed]
- 90.Huang C, Xu B, Zhu XD, Shen YH, Li ML, Zhu JJ, et al. Peripheral naive CD8(+) T cells as a predictive biomarker of response to lenvatinib plus an anti-PD-1 antibody in advanced hepatocellular carcinoma: a biomarker study. Cancer Commun (Lond). 2022;11:1226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zou Y, Chen Z, Chen Z, Zhang Y, Zheng Z, Wu F, et al. Peripheral Th and NK cells are associated with response to lenvatinib plus PD-1 inhibitor in unresectable hepatocellular carcinoma. Clin Immunol. 2023; 109290. [DOI] [PubMed]
- 92.Xu B, Zhu X D, Shen Y H, Zhu J J, Liu J, Li M L, et al. Criteria for identifying potentially resectable patients with initially oncologically unresectable hepatocellular carcinoma before treatment with lenvatinib plus an anti-PD-1 antibody. Front Immunol. 2022; 1016736. [DOI] [PMC free article] [PubMed]
- 93.Yuan G, Xie F, Song Y, Li Q, Li R, Hu X, et al. Hepatic tumor stiffness measured by shear wave elastography is prognostic for HCC progression following treatment with anti-PD-1 antibodies plus lenvatinib: a retrospective analysis of two independent cohorts. Front Immunol. 2022; 868809. [DOI] [PMC free article] [PubMed]
- 94.Lin PT, Teng W, Jeng WJ, Lin CY, Lin SM, Sheen IS. Combining immune checkpoint inhibitor with lenvatinib prolongs survival than lenvatinib alone in sorafenib-experienced hepatocellular carcinoma patients. Eur J Gastroenterol Hepatol. 2022;2:213–9. [DOI] [PubMed] [Google Scholar]
- 95.Sheng R, Zeng M, Jin K, Zhang Y, Wu D, Sun H. MRI-based nomogram predicts the risk of progression of unresectable hepatocellular carcinoma after combined lenvatinib and anti-pd-1 antibody therapy. Acad Radiol. 2022;6:819–29. [DOI] [PubMed] [Google Scholar]
- 96.Xie F, Chen B, Yang X, Wang H, Zhang G, Wang Y, et al. Efficacy of immune checkpoint inhibitors plus molecular targeted agents after the progression of lenvatinib for advanced hepatocellular carcinoma. Front Immunol. 2022; 1052937. [DOI] [PMC free article] [PubMed]
- 97.Men B, Cui H, Han Z, Jin X, Xu Q, Jin Y, et al. Evaluation of the efficacy of transarterial chemoembolization combined with microwave ablation followed by adjuvant therapy in patients with hepatocellular carcinoma. Front Immunol. 2024; 1337396. [DOI] [PMC free article] [PubMed]
- 98.Zhang Y, Zhang H, Xu H, Wang Y, Feng L, Yi F. Efficacy and safety of hepatic arterial infusion chemotherapy combined with lenvatinib and PD-1 inhibitors for advanced hepatocellular carcinoma with macrovascular invasion. World J Surg Oncol. 2024;1:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang X, Li X, Sun P, Li Z, Sun P, Ning S. HAIC Combined with lenvatinib plus PD-1 versus lenvatinib Plus PD-1 in patients with high-risk advanced HCC: a real-world study. BMC Cancer. 2024;1:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fu S, Xu Y, Mao Y, He M, Chen Z, Huang S, et al. Hepatic arterial infusion chemotherapy, lenvatinib plus programmed cell death protein-1 inhibitors: a promising treatment approach for high-burden hepatocellular carcinoma. Cancer Med. 2024;9: e7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diao L, Wang C, You R, Leng B, Yu Z, Xu Q, et al. Hepatic arterial infusion chemotherapy combined with lenvatinib and PD-1 inhibitors versus lenvatinib and PD-1 inhibitors for HCC refractory to TACE. J Gastroenterol Hepatol. 2024;4:746–53. [DOI] [PubMed] [Google Scholar]
- 102.Fu Y, Peng W, Zhang W, Yang Z, Hu Z, Pang Y, et al. Induction therapy with hepatic arterial infusion chemotherapy enhances the efficacy of lenvatinib and pd1 inhibitors in treating hepatocellular carcinoma patients with portal vein tumor thrombosis. J Gastroenterol. 2023;4:413–24. [DOI] [PubMed] [Google Scholar]
- 103.Yang Z, Guan R, Fu Y, Hu D, Zhou Z, Chen M, et al. Risk of hepatitis B virus reactivation and its effect on survival in advanced hepatocellular carcinoma patients treated with hepatic arterial infusion chemotherapy and lenvatinib plus programmed death receptor-1 inhibitors. Front Cell Infect Microbiol. 2024;14:1336619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lai Z, He M, Bu X, Xu Y, Huang Y, Wen D, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: a biomolecular exploratory, phase II trial. Eur J Cancer. 2022;174:68–77. [DOI] [PubMed] [Google Scholar]
- 105.He M, Huang Y, Du Z, Lai Z, Ouyang H, Shen J, et al. Lenvatinib, toripalimab plus FOLFOX chemotherapy in hepatocellular carcinoma patients with extrahepatic metastasis: a biomolecular exploratory, phase II trial (LTSC). Clin Cancer Res. 2023;24:5104–15. [DOI] [PubMed] [Google Scholar]
- 106.Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F, et al. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: a retrospective study. J Cancer Res Clin Oncol. 2022;8:2115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu HX, Ding XY, Xu YW, Yu MH, Li XM, Deng N, et al. Transcatheter arterial chemoembolization combined with PD-1 inhibitors and Lenvatinib for hepatocellular carcinoma with portal vein tumor thrombus. World J Gastroenterol. 2024;8:843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, et al. Transarterial chemoembolization combined with lenvatinib plus pd-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022; 848387. [DOI] [PMC free article] [PubMed]
- 109.Wang L, Lin L, Zhou W. Efficacy and safety of transarterial chemoembolization combined with lenvatinib and PD-1 inhibitor in the treatment of advanced hepatocellular carcinoma: a meta-analysis. Pharmacol Ther. 2024;257:108634. [DOI] [PubMed] [Google Scholar]
- 110.Wu J, Wu J, Li S, Luo M, Zeng Z, Li Y, et al. Effect of transcatheter arterial chemoembolization combined with lenvatinib plus anti-PD-1 antibodies in patients with unresectable hepatocellular carcinoma: a treatment with Chinese characteristics. Biosci Trends. 2024;1:42–8. [DOI] [PubMed] [Google Scholar]
- 111.Peng W, Wu Y, Zhang X, Li C, Shen J, Chen W, et al. Liver transplantation for advanced hepatocellular carcinoma after downstaging with consequential lenvatinib, transcatheter arterial chemoembolization and camrelizumab. Br J Surg. 2024; 3. [DOI] [PMC free article] [PubMed]
- 112.Cai M, Huang W, Liang W, Guo Y, Liang L, Lin L, et al. Lenvatinib, sintilimab plus transarterial chemoembolization for advanced stage hepatocellular carcinoma: a phase II study. Liver Int. 2024;4:920–30. [DOI] [PubMed] [Google Scholar]
- 113.Xiang Z, Li G, Mu L, Wang H, Zhou C, Yan H, et al. TACE combined with lenvatinib and camrelizumab for unresectable multiple nodular and large hepatocellular carcinoma (>5 cm). Technol Cancer Res Treat. 2023; 15330338231200320. [DOI] [PMC free article] [PubMed]
- 114.Li X, Ding X, Liu M, Wang J, Sun W, Teng Y, et al. A multicenter prospective study of TACE combined with lenvatinib and camrelizumab for hepatocellular carcinoma with portal vein tumor thrombus. Cancer Med. 2023;16:16805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang J, Zhao M, Han G, Han X, Shi J, Mi L, et al. Transarterial chemoembolization combined with PD-1 inhibitors plus lenvatinib showed improved efficacy for treatment of unresectable hepatocellular carcinoma compared with PD-1 inhibitors plus lenvatinib. Technol Cancer Res Treat. 2023; 15330338231166765. [DOI] [PMC free article] [PubMed]
- 116.Xin Y, Zhang X, Liu N, Peng G, Huang X, Cao X, et al. Efficacy and safety of lenvatinib plus PD-1 inhibitor with or without transarterial chemoembolization in unresectable hepatocellular carcinoma. Hepatol Int. 2023;3:753–64. [DOI] [PubMed] [Google Scholar]
- 117.An C, Fu Y, Li W, Zuo M, Wu P. Postprogression treatment of lenvatinib plus PD-1 inhibitor in advanced hepatocellular carcinoma refractory to hepatic arterial infusion chemotherapy. Cancer. 2023;14:2235–44. [DOI] [PubMed] [Google Scholar]
- 118.Wang WJ, Liu ZH, Wang K, Yu HM, Cheng YQ, Xiang YJ, et al. Efficacy and safety of TACE combined with lenvatinib and PD-1 inhibitors for unresectable recurrent HCC: a multicenter, retrospective study. Cancer Med. 2023;10:11513–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.xxxx
- 120.Sheng Y, Wang Q, Liu H, Wang Q, Chen W, Xing W. Prognostic nomogram model for selecting between transarterial chemoembolization plus lenvatinib, with and without PD-1 inhibitor in unresectable hepatocellular carcinoma. Br J Radiol. 2024;1155:668–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li S, Wu J, Wu J, Fu Y, Zeng Z, Li Y, et al. Prediction of early treatment response to the combination therapy of TACE plus lenvatinib and anti-PD-1 antibody immunotherapy for unresectable hepatocellular carcinoma: Multicenter retrospective study. Front Immunol. 2023; 1109771. [DOI] [PMC free article] [PubMed]
- 122.Zou X, Xu Q, You R, Yin G. Correlation and efficacy of TACE combined with lenvatinib plus PD-1 inhibitor in the treatment of hepatocellular carcinoma with portal vein tumor thrombus based on immunological features. Cancer Med. 2023;10:11315–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zeng ZX, Wu JY, Wu JY, Li YN, Fu YK, Zhang ZB, et al. The TAE score predicts prognosis of unresectable HCC patients treated with TACE plus lenvatinib with PD-1 inhibitors. Hepatol Int. 2024;2:651–60. [DOI] [PubMed] [Google Scholar]
- 124.Qu S, Wu D and Hu Z. Neutrophil-to-lymphocyte ratio and early tumor shrinkage as predictive biomarkers in unresectable hepatocellular carcinoma patients treated with lenvatinib, PD-1 inhibitors, in combination with TACE. Technol Cancer Res Treat. 2023; 15330338231206704. [DOI] [PMC free article] [PubMed]
- 125.Chen YH, Chen YY, Wang JH, Hung CH. Efficacy and safety of lenvatinib after progression on first-line atezolizumab plus bevacizumab treatment in advanced hepatocellular carcinoma patients. Anticancer Res. 2023;3:1377–84. [DOI] [PubMed] [Google Scholar]
- 126.Luo X, Chang RZ, Kuang D, Yuan M, Li GX, Zhang B, et al. Case report: successful conversion and salvage resection of huge hepatocellular carcinoma with portal vein tumor thrombosis and intrahepatic metastasis via sequential hepatic arterial infusion chemotherapy, lenvatinib plus PD-1 antibody followed by simultaneous transcatheter arterial chemoembolization, and portal vein embolization. Front Immunol. 2023;14:1285296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lin LW, Yan LY, Ke K, Yang WZ, Lin JQ, Huang N. Efficacy and safety of transarterial chemoembolization combined with lenvatinib, programmed death-1 inhibitor, and iodine-125 seed brachytherapy for hepatocellular carcinoma with portal vein tumor thrombosis. Brachytherapy. 2023;6:858–71. [DOI] [PubMed] [Google Scholar]
- 128.Wang YY, Yang X, Wang YC, Long JY, Sun HS, Li YR, et al. Clinical outcomes of lenvatinib plus transarterial chemoembolization with or without programmed death receptor-1 inhibitors in unresectable hepatocellular carcinoma. World J Gastroenterol. 2023;10:1614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zou X, Xu Q, You R, Yin G. Evaluating the benefits of tace combined with lenvatinib plus PD-1 inhibitor for hepatocellular carcinoma with portal vein tumor thrombus. Adv Ther. 2023;4:1686–704. [DOI] [PubMed] [Google Scholar]
- 130.Yamauchi M, Ono A, Ishikawa A, Kodama K, Uchikawa S, Hatooka H, et al. Tumor fibroblast growth factor receptor 4 level predicts the efficacy of lenvatinib in patients with advanced hepatocellular carcinoma. Clin Transl Gastroenterol. 2020;5: e00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guo W, Li S, Qian Y, Li L, Wang F, Tong Y, et al. KDM6A promotes hepatocellular carcinoma progression and dictates lenvatinib efficacy by upregulating FGFR4 expression. Clin Transl Med. 2023;10: e1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wei Y, Wei L, Han T, Ding S. miR-3154 promotes hepatocellular carcinoma progression via suppressing HNF4alpha. Carcinogenesis. 2022;10:1002–14. [DOI] [PubMed] [Google Scholar]
- 133.Fujii Y, Ono A, Hayes CN, Aikata H, Yamauchi M, Uchikawa S, et al. Identification and monitoring of mutations in circulating cell-free tumor DNA in hepatocellular carcinoma treated with lenvatinib. J Exp Clin Cancer Res. 2021;1:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fujita M, Abe K, Kuroda H, Oikawa T, Ninomiya M, Masamune A, et al. Influence of skeletal muscle volume loss during lenvatinib treatment on prognosis in unresectable hepatocellular carcinoma: a multicenter study in Tohoku. Japan Sci Rep. 2022;1:6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chihiro K, Kawaoka T, Uchikawa S, Kinami T, Yamasaki S, Kosaka M, et al. The analysis of muscle volume measured by bioelectrical impedance in patients with hepatocellular carcinoma treated with first-line atezolizumab plus bevacizumab combination therapy or first-line lenvatinib. Oncology. 2023;8:491–501. [DOI] [PubMed] [Google Scholar]
- 136.Yamamoto T, Imai N, Kuzuya T, Yokoyama S, Yamamoto K, Ito T, et al. Changes in body composition predict the time to treatment failure of lenvatinib in patients with advanced hepatocellular carcinoma: a pilot retrospective study. Nutr Cancer. 2022;9:3118–27. [DOI] [PubMed] [Google Scholar]
- 137.Kinoshita A, Hagiwara N, Osawa A, Akasu T, Matsumoto Y, Ueda K, et al. The geriatric nutritional risk index predicts tolerability of lenvatinib in patients with hepatocellular carcinoma. In Vivo. 2022;2:865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shimose S, Koya S, Kawaguchi T, Hirota K, Yoshio S, Niizeki T, et al. Impact of branched-chain amino acids and frailty on the management of lenvatinib-related fatigue in patients with hepatocellular carcinoma. Clin Mol Hepatol. 2021;4:616–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yamaoka K, Kodama K, Kawaoka T, Kosaka M, Johira Y, Shirane Y, et al. The importance of body composition assessment for patients with advanced hepatocellular carcinoma by bioelectrical impedance analysis in lenvatinib treatment. PLoS ONE. 2022;1: e0262675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu M, Wang Y, Yang Q, Wang X, Yang X, Xing H, et al. Comparison of baseline (68)Ga-FAPI and (18)F-FDG PET/CT for prediction of response and clinical outcome in patients with unresectable hepatocellular carcinoma treated with PD-1 inhibitor and lenvatinib. J Nucl Med. 2023;10:1532–9. [DOI] [PubMed] [Google Scholar]
- 141.Kuorda H, Abe T, Fujiwara Y, Okamoto T, Yonezawa M, Sato H, et al. Change in arterial tumor perfusion is an early biomarker of lenvatinib efficacy in patients with unresectable hepatocellular carcinoma. World J Gastroenterol. 2019;19:2365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kamachi N, Nakano M, Okamura S, Niizeki T, Iwamoto H, Shimose S, et al. Evaluating the therapeutic effect of lenvatinib against advanced hepatocellular carcinoma by measuring blood flow changes using contrast-enhanced ultrasound. Cancer Rep (Hoboken). 2022;2: e1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Muraishi N, Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Okubo S, et al. Changes in the mean intrahepatic target computed tomography attenuation value during treatment may be a useful new predictor of the post-progression survival associated with lenvatinib treatment. Intern Med. 2022;7:951–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chuma M, Yokoo H, Hiraoka A, Ueda K, Yokoyama T, Tsuji K, et al. Identification of CT values that could be predictive of necrosis (N-CTav) in hepatocellular carcinoma after lenvatinib treatment. Curr Oncol. 2022;5:3259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Okubo S, Muraishi N, et al. Pretreatment positron emission tomography with 18F-fluorodeoxyglucose may be a useful new predictor of overall prognosis following lenvatinib treatment. Oncology. 2021;10:611–21. [DOI] [PubMed] [Google Scholar]
- 146.Bo Z, Chen B, Zhao Z, He Q, Mao Y, Yang Y, et al. Prediction of response to lenvatinib monotherapy for unresectable hepatocellular carcinoma by machine learning radiomics: a multicenter cohort study. Clin Cancer Res. 2023;9:1730–40. [DOI] [PubMed] [Google Scholar]
- 147.Guo D Z, Zhang S Y, Dong S Y, Yan J Y, Wang Y P, Cao Y, et al. Prognostic model for predicting outcome and guiding treatment decision for unresectable hepatocellular carcinoma treated with lenvatinib monotherapy or lenvatinib plus immunotherapy. Front Immunol. 2023; 1141199. [DOI] [PMC free article] [PubMed]
- 148.Enomoto D, Yamamoto K, Matsumoto Y, Morioka A, Omura T, Komatsu S, et al. ALBI grade is a predictive factor of lenvatinib treatment discontinuation due to adverse events in hepatocellular carcinoma. Anticancer Res. 2023;3:1317–23. [DOI] [PubMed] [Google Scholar]
- 149.Tada T, Kumada T, Hiraoka A, Atsukawa M, Hirooka M, Tsuji K, et al. Impact of modified albumin-bilirubin grade on survival in patients with HCC who received lenvatinib. Sci Rep. 2021;1:14474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kariyama K, Hiraoka A, Kumada T, Yasuda S, Toyoda H, Tsuji K, et al. Chronological change in serum albumin as a prognostic factor in patients with hepatocellular carcinoma treated with lenvatinib: proposal of albumin simplified grading based on the modified albumin-bilirubin score (ALBS grade). J Gastroenterol. 2022;8:581–6. [DOI] [PubMed] [Google Scholar]
- 151.Tada T, Kumada T, Hiraoka A, Hirooka M, Kariyama K, Tani J, et al. Glasgow prognostic score predicts survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib: a multicenter analysis. Eur J Gastroenterol Hepatol. 2022;8:857–64. [DOI] [PubMed] [Google Scholar]
- 152.Li JH, Chen T, Xing H, Li RD, Shen CH, Zhang QB, et al. The AGH score is a predictor of disease-free survival and targeted therapy efficacy after liver transplantation in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2023;3:245–52. [DOI] [PubMed] [Google Scholar]
- 153.Shibano M, Takahashi K, Takahashi M, Uchida-Kobayashi S, Kawada N, Nakamura Y, et al. Prognostic nutrition index as an indicator of therapeutic response to lenvatinib therapy in hepatocellular carcinoma. Anticancer Res. 2022;12:6019–26. [DOI] [PubMed] [Google Scholar]
- 154.Rapposelli IG, Shimose S, Kumada T, Okamura S, Hiraoka A, Di Costanzo GG, et al. Identification of lenvatinib prognostic index via recursive partitioning analysis in advanced hepatocellular carcinoma. ESMO Open. 2021;4: 100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hiraoka A, Kumada T, Tada T, Fukunishi S, Atsukawa M, Hirooka M, et al. Nutritional index as prognostic indicator in patients receiving lenvatinib treatment for unresectable hepatocellular carcinoma. Oncology. 2020;5:295–302. [DOI] [PubMed] [Google Scholar]
- 156.Shimose S, Kawaguchi T, Iwamoto H, Tanaka M, Miyazaki K, Ono M, et al. Controlling nutritional status (CONUT) score is associated with overall survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib: a multicenter cohort study. Nutrients. 2020; 4. [DOI] [PMC free article] [PubMed]
- 157.Sekiguchi S, Tsuchiya K, Yasui Y, Inada K, Kirino S, Yamashita K, et al. Clinical usefulness of geriatric assessment in elderly patients with unresectable hepatocellular carcinoma receiving sorafenib or lenvatinib therapy. Cancer Rep (Hoboken). 2022;11: e1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li X, Ding X, Liu M, Wang J, Li W, Chen J. Development of a multivariate prognostic model for lenvatinib treatment in hepatocellular carcinoma. Oncologist. 2023;10:e942–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Deng Y, Yang J, Chen Y, Wang J, Fu B, Zhang T, et al. Development of a risk classifier to predict tumor recurrence and lenvatinib benefits in hepatocellular carcinoma after liver transplantation. Transplant Proc. 2023;1:153–63. [DOI] [PubMed] [Google Scholar]
- 160.Tada T, Kumada T, Hiraoka A, Michitaka K, Atsukawa M, Hirooka M, et al. Neutrophil-to-lymphocyte ratio is associated with survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Liver Int. 2020;4:968–76. [DOI] [PubMed] [Google Scholar]
- 161.Toshida K, Itoh S, Yoshiya S, Nagao Y, Tomino T, Izumi T, et al. Pretreatment eosinophil count predicts response to atezolizumab plus bevacizumab therapy in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2024;3:576–86. [DOI] [PubMed] [Google Scholar]
- 162.Hayashi T, Shibata M, Oe S, Miyagawa K, Honma Y, Harada M. C-reactive protein can predict dose intensity, time to treatment failure and overall survival in HCC treated with lenvatinib. PLoS ONE. 2020;12: e0244370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tada T, Kumada T, Hiraoka A, Hirooka M, Kariyama K, Tani J, et al. C-reactive protein to albumin ratio predicts survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Sci Rep. 2022;1:8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tada T, Kumada T, Hiraoka A, Michitaka K, Atsukawa M, Hirooka M, et al. Platelet-lymphocyte ratio predicts survival in patients with hepatocellular carcinoma who receive lenvatinib: an inverse probability weighting analysis. Eur J Gastroenterol Hepatol. 2021;2:261–8. [DOI] [PubMed] [Google Scholar]
- 165.Shigesawa T, Suda G, Kimura M, Maehara O, Tokuchi Y, Kubo A, et al. Baseline serum angiopoietin-2 and VEGF levels predict the deterioration of the liver functional reserve during lenvatinib treatment for hepatocellular carcinoma. PLoS ONE. 2021;3: e0247728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Eso Y, Nakano S, Mishima M, Arasawa S, Iguchi E, Takeda H, et al. Mac-2 binding protein glycosylation isomer predicts tolerability and clinical outcome of lenvatinib therapy for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2021;6:498–507. [DOI] [PubMed] [Google Scholar]
- 167.Myojin Y, Kodama T, Maesaka K, Motooka D, Sato Y, Tanaka S, et al. ST6GAL1 is a novel serum biomarker for lenvatinib-susceptible FGF19-driven hepatocellular carcinoma. Clin Cancer Res. 2021;4:1150–61. [DOI] [PubMed] [Google Scholar]
- 168.Kawamura A, Uojima H, Chuma M, Shao X, Hidaka H, Nakazawa T, et al. The change rate in serum nitric oxide may affect lenvatinib therapy in hepatocellular carcinoma. BMC Cancer. 2022;1:912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Eso Y, Nakano S, Mishima M, Arasawa S, Iguchi E, Takeda H, et al. Branched-chain amino acid to tyrosine ratio is an essential pre-treatment factor for maintaining sufficient treatment intensity of lenvatinib in patients with hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2020;12:913–21. [DOI] [PubMed] [Google Scholar]
- 170.Lin CL, Chien RN, Chen LW, Chu YD, Yeh CT. Rs9679162 genotype predicts prognosis of real-world advanced hepatocellular carcinoma treated by sorafenib. Cancer Biomark. 2023;3:251–66. [DOI] [PubMed] [Google Scholar]
- 171.Azuma S, Uojima H, Chuma M, Shao X, Hidaka H, Nakazawa T, et al. Influence of NOS3 rs2070744 genotypes on hepatocellular carcinoma patients treated with lenvatinib. Sci Rep. 2020;1:17054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kunimoto H, Shakado S, Tanaka T, Takata K, Yamauchi R, Fukuda H, et al. Reduction in tumor stain at 2 weeks after treatment initiation is a predictor of the efficacy of lenvatinib in patients with unresectable hepatocellular carcinoma. Oncology. 2020;11:779–86. [DOI] [PubMed] [Google Scholar]
- 173.Huang DQ, Muthiah MD, Zhou L, Jumat H, Tan WX, Lee GH, et al. Predicting HCC response to multikinase inhibitors with in vivo cirrhotic mouse model for personalized therapy. Cell Mol Gastroenterol Hepatol. 2021;5:1313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang J, Kuang SW, Huang N, Zhang JJ. Liu M and Wang L M [lenvatinib down-regulates IGF1R/Mek/Erk signaling pathway in the treatment of regorafenib-resistant hepatocellular carcinoma]. Zhonghua Zhong Liu Za Zhi. 2023;6:490–8. [DOI] [PubMed] [Google Scholar]
- 175.Hindson J. Lenvatinib plus EGFR inhibition for liver cancer. Nat Rev Gastroenterol Hepatol. 2021;10:675. [DOI] [PubMed] [Google Scholar]
- 176.Hu B, Zou T, Qin W, Shen X, Su Y, Li J, et al. Inhibition of EGFR overcomes acquired lenvatinib resistance driven by STAT3-ABCB1 signaling in hepatocellular carcinoma. Cancer Res. 2022;20:3845–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kasuya K, Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Kajiwara A, et al. Efficacy and safety of ramucirumab in patients with unresectable hepatocellular carcinoma with progression after treatment with lenvatinib. Intern Med. 2021;3:345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, et al. Initial experience of ramucirumab treatment after lenvatinib failure for patients with advanced hepatocellular carcinoma. Anticancer Res. 2020;4:2089–93. [DOI] [PubMed] [Google Scholar]
- 179.Muto H, Kuzuya T, Kawabe N, Ohno E, Funasaka K, Nagasaka M, et al. Clinical outcomes with lenvatinib in patients previously treated with atezolizumab/bevacizumab for advanced hepatocellular carcinoma. Anticancer Res. 2023;10:4673–82. [DOI] [PubMed] [Google Scholar]
- 180.Hiraoka A, Kumada T, Tada T, Hirooka M, Kariyama K, Tani J, et al. Lenvatinib as second-line treatment after atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma: clinical results show importance of hepatic reserve function. Oncology. 2023;10:624–33. [DOI] [PubMed] [Google Scholar]
- 181.Cheng JY, Huang BS, Chen YY, Wang CC, Chen YH. Combination of lenvatinib and proton beam therapy in the management of patients with advanced hepatocellular carcinoma. Anticancer Res. 2023;3:1361–71. [DOI] [PubMed] [Google Scholar]
- 182.Yuan W, Yue W, Wen H, Wang X, Wang Q. Analysis on efficacy of hepatic artery infusion chemotherapy with or without lenvatinib for unresectable hepatocellular carcinoma. Eur Surg Res. 2023;2:268–77. [DOI] [PubMed] [Google Scholar]
- 183.Liu Y, Qiao Y, Zhou M, Guo J, Lin Y, Li W, et al. Efficacy and safety of hepatic arterial infusion chemotherapy combined with lenvatinib and sequential ablation in the treatment of advanced hepatocellular carcinoma. Cancer Med. 2023;5:5436–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wu DD, He XF, Tian C, Peng P, Chen CL, Liu XH, et al. Tumor-feeding artery diameter reduction is associated with improved short-term effect of hepatic arterial infusion chemotherapy plus lenvatinib treatment. World J Gastroenterol. 2022;26:3232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ando Y, Kawaoka T, Amioka K, Naruto K, Ogawa Y, Yoshikawa Y, et al. Efficacy and safety of lenvatinib-transcatheter arterial chemoembolization sequential therapy for patients with intermediate-stage hepatocellular carcinoma. Oncology. 2021;8:507–17. [DOI] [PubMed] [Google Scholar]
- 186.Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (UHCC): a retrospective controlled study. Hepatol Int. 2021;3:663–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pe J, Choi B, Choi H, Kwon SW, Kim DH. Preclinical therapeutic evaluation of lenvatinib-eluting microspheres for transcatheter arterial chemoembolization of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2022;12:1834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fan W, Zhu B, Yue S, Zheng X, Zou X, Li F, et al. Idarubicin-loaded DEB-TACE plus lenvatinib versus lenvatinib for patients with advanced hepatocellular carcinoma: a propensity score-matching analysis. Cancer Med. 2023;1:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang Z, Shen J, Chen C, Wen T, Li C. FOXA2 plays a critical role in hepatocellular carcinoma progression and lenvatinib-associated drug resistance. Biosci Trends. 2023;2:136–47. [DOI] [PubMed] [Google Scholar]
- 190.Chen Z, Ma Y, Guo Z, Song D, Chen Z, Sun M. Ubiquitin-specific protease 1 acts as an oncogene and promotes lenvatinib efficacy in hepatocellular carcinoma by stabilizing c-kit. Ann Hepatol. 2022;2: 100669. [DOI] [PubMed] [Google Scholar]
- 191.Hu Q, Hu X, Zhang L, Zhao Y, Li L. Targeting Hedgehog signalling in CD133-positive hepatocellular carcinoma: improving lenvatinib therapeutic efficiency. Med Oncol. 2021;4:41. [DOI] [PubMed] [Google Scholar]
- 192.Guo Y, Chai B, Zhang H, Chai X, Chen Y, Xu J, et al. RARRES1 inhibits hepatocellular carcinoma progression and increases its sensitivity to lenvatinib through interaction with SPINK2. Biol Direct. 2024;1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Huang S, Ma Z, Zhou Q, Wang A, Gong Y, Li Z, et al. Genome-wide CRISPR/Cas9 library screening identified that DUSP4 deficiency induces lenvatinib resistance in hepatocellular carcinoma. Int J Biol Sci. 2022;11:4357–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yan WN, Li SB, Ma HJ, Chen DD, Wang J, Le T, et al. Effect of miR-23b on the malignant phenotype and the sensitivity of lenvatinib in human hepatocellular carcinoma cells. Zhonghua Gan Zang Bing Za Zhi. 2021;5:433–8. [DOI] [PubMed] [Google Scholar]
- 195.Cao M, Ren Y, Li Y, Deng J, Su X, Tang Y, et al. Lnc-ZEB2-19 inhibits the progression and lenvatinib resistance of hepatocellular carcinoma by attenuating the NF-kappaB signaling pathway through the TRA2A/RSPH14 axis. Int J Biol Sci. 2023;12:3678–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hamaya S, Fujihara S, Iwama H, Fujita K, Shi T, Nakabayashi R, et al. Characterization of cisplatin effects in lenvatinib-resistant hepatocellular carcinoma cells. Anticancer Res. 2022;3:1263–75. [DOI] [PubMed] [Google Scholar]
- 197.Takagi H, Kaji K, Nishimura N, Ishida K, Ogawa H, Takaya H, et al. The angiotensin II receptor blocker losartan sensitizes human liver cancer cells to lenvatinib-mediated cytostatic and angiostatic effects. Cells. 2021;3:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cheng Y, Zhan P, Lu J, Lu Y, Luo C, Cen X, et al. Metformin synergistically enhances the antitumour activity of Lenvatinib in hepatocellular carcinoma by altering AKT-FOXO3 signalling pathway. Liver Int. 2023;7:1577–92. [DOI] [PubMed] [Google Scholar]
- 199.Tang R, Zheng L, Zheng J, Wu J, Chen P, Chen J, et al. Secukinumab plays a synergistic role with starvation therapy in promoting autophagic cell death of hepatocellular carcinoma via inhibiting IL-17A-increased BCL2 level. In Vitro Cell Dev Biol Anim. 2023;5:381–93. [DOI] [PubMed] [Google Scholar]
- 200.Zhen Y, Zhao R, Wang M, Jiang X, Gao F, Fu L, et al. Flubendazole elicits anti-cancer effects via targeting EVA1A-modulated autophagy and apoptosis in triple-negative breast cancer. Theranostics. 2020;18:8080–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jin W, Yu J, Su Y, Lin H, Liu T, Chen J, et al. Drug repurposing flubendazole to suppress tumorigenicity via PCSK9-dependent inhibition and potentiate lenvatinib therapy for hepatocellular carcinoma. Int J Biol Sci. 2023;7:2270–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Huang Y, Wang S, Zhang X, Yang C, Wang S, Cheng H, et al. Identification of Fasudil as a collaborator to promote the anti-tumor effect of lenvatinib in hepatocellular carcinoma by inhibiting GLI2-mediated hedgehog signaling pathway. Pharmacol Res. 2024;200:107082. [DOI] [PubMed] [Google Scholar]
- 203.Fan M, Chen Z, Shao W, Chen Y, Lin Z, Yi C, et al. SREBP2 inhibitor betulin sensitizes hepatocellular carcinoma to lenvatinib by inhibiting the mTOR/IL-1beta pathway. Acta Biochim Biophys Sin (Shanghai). 2023;9:1479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yang CJ, Wu MH, Tsai JJ, Hsu FT, Hsia TC, Liu KC, et al. Inactivation of AKT/ERK signaling and induction of apoptosis are associated with amentoflavone sensitization of hepatocellular carcinoma to lenvatinib. Anticancer Res. 2022;5:2495–505. [DOI] [PubMed] [Google Scholar]
- 205.Zhao Z, Zhang D, Wu F, Tu J, Song J, Xu M, et al. Sophoridine suppresses lenvatinib-resistant hepatocellular carcinoma growth by inhibiting RAS/MEK/ERK axis via decreasing VEGFR2 expression. J Cell Mol Med. 2021;1:549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhao Z, Song J, Zhang D, Wu F, Tu J and Ji J. Oxysophocarpine suppresses FGFR1-overexpressed hepatocellular carcinoma growth and sensitizes the therapeutic effect of lenvatinib. Life Sci. 2021; 118642. [DOI] [PubMed]
- 207.Liao M, Qin M, Liu L, Huang H, Chen N, Du H, et al. Exosomal microRNA profiling revealed enhanced autophagy suppression and anti-tumor effects of a combination of compound Phyllanthus urinaria and lenvatinib in hepatocellular carcinoma. Phytomedicine. 2024; 155091. [DOI] [PubMed]
- 208.Miyazaki K, Morine Y, Xu C, Nakasu C, Wada Y, Teraoku H, et al. Curcumin-Mediated Resistance to Lenvatinib via EGFR Signaling Pathway in Hepatocellular Carcinoma. Cells. 2023; 4. [DOI] [PMC free article] [PubMed]
- 209.Wu Z, Wang J. Curcumol enhances the antitumor effect of lenvatinib on hepatocellular carcinoma Cells. Discov Med. 2024;180:199–208. [DOI] [PubMed] [Google Scholar]
- 210.He X, Hikiba Y, Suzuki Y, Nakamori Y, Kanemaru Y, Sugimori M, et al. EGFR inhibition reverses resistance to lenvatinib in hepatocellular carcinoma cells. Sci Rep. 2022;1:8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fu R, Jiang S, Li J, Chen H, Zhang X. Activation of the HGF/c-MET axis promotes lenvatinib resistance in hepatocellular carcinoma cells with high c-MET expression. Med Oncol. 2020;4:24. [DOI] [PubMed] [Google Scholar]
- 212.Arechederra M, Bazai SK, Abdouni A, Sequera C, Mead TJ, Richelme S, et al. ADAMTSL5 is an epigenetically activated gene underlying tumorigenesis and drug resistance in hepatocellular carcinoma. J Hepatol. 2021;4:893–906. [DOI] [PubMed] [Google Scholar]
- 213.Huang M, Long J, Yao Z, Zhao Y, Zhao Y, Liao J, et al. METTL1-mediated m7G tRNA modification promotes lenvatinib resistance in hepatocellular carcinoma. Cancer Res. 2023;1:89–102. [DOI] [PubMed] [Google Scholar]
- 214.Wang L, Yang Q, Zhou Q, Fang F, Lei K, Liu Z, et al. METTL3-m(6)A-EGFR-axis drives lenvatinib resistance in hepatocellular carcinoma. Cancer Lett. 2023; 216122. [DOI] [PubMed]
- 215.Hou W, Bridgeman B, Malnassy G, Ding X, Cotler SJ, Dhanarajan A, et al. Integrin subunit beta 8 contributes to lenvatinib resistance in HCC. Hepatol Commun. 2022;7:1786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Suzuki H, Iwamoto H, Seki T, Nakamura T, Masuda A, Sakaue T, et al. Tumor-derived insulin-like growth factor-binding protein-1 contributes to resistance of hepatocellular carcinoma to tyrosine kinase inhibitors. Cancer Commun (Lond). 2023;4:415–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang Q, Xiong L, Wei T, Liu Q, Yan L, Chen J, et al. Hypoxia-responsive PPARGC1A/BAMBI/ACSL5 axis promotes progression and resistance to lenvatinib in hepatocellular carcinoma. Oncogene. 2023;19:1509–23. [DOI] [PubMed] [Google Scholar]
- 218.Yang SB, Zhou ZH, Lei J, Li XW, Chen Q, Li B, et al. TM4SF1 upregulates MYH9 to activate the NOTCH pathway to promote cancer stemness and lenvatinib resistance in HCC. Biol Direct. 2023;1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hou W, Gad SA, Ding X, Dhanarajan A, Qiu W. Focal adhesion kinase confers lenvatinib resistance in hepatocellular carcinoma via the regulation of lysine-deficient kinase 1. Mol Carcinog. 2024;1:173–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Leung CON, Yang Y, Leung RWH, So KKH, Guo HJ, Lei MML, et al. Broad-spectrum kinome profiling identifies CDK6 upregulation as a driver of lenvatinib resistance in hepatocellular carcinoma. Nat Commun. 2023;1:6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang J, Yu H, Dong W, Zhang C, Hu M, Ma W, et al. N6-methyladenosine-mediated up-regulation of FZD10 regulates liver cancer stem cells’ properties and lenvatinib resistance through WNT/beta-catenin and hippo signaling pathways. Gastroenterology. 2023;6:990–1005. [DOI] [PubMed] [Google Scholar]
- 222.Deng H, Kan A, Lyu N, He M, Huang X, Qiao S, et al. Tumor-derived lactate inhibit the efficacy of lenvatinib through regulating PD-L1 expression on neutrophil in hepatocellular carcinoma. J Immunother Cancer. 2021;6:e002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu Y, Sun L, Guo H, Zhou S, Wang C, Ji C, et al. Targeting SLP2-mediated lipid metabolism reprograming restricts proliferation and metastasis of hepatocellular carcinoma and promotes sensitivity to lenvatinib. Oncogene. 2023;5:374–88. [DOI] [PubMed] [Google Scholar]
- 224.Wang S, Zhou L, Ji N, Sun C, Sun L, Sun J, et al. Targeting ACYP1-mediated glycolysis reverses lenvatinib resistance and restricts hepatocellular carcinoma progression. Drug Resist Updat. 2023;69:100976. [DOI] [PubMed] [Google Scholar]
- 225.Xue W, Wang T, Tian WJ, Pang SQ, Zhang HF, Jia WD. NQO1 mediates lenvatinib resistance by regulating ROS-induced apoptosis in hepatocellular carcinoma. Curr Med Sci. 2024;1:168–79. [DOI] [PubMed] [Google Scholar]
- 226.Li X, Su H, Tang W, Shu S, Zhao L, Sun J, et al. Targeting LEF1-mediated epithelial-mesenchymal transition reverses lenvatinib resistance in hepatocellular carcinoma. Invest New Drugs. 2024;2:185–95. [DOI] [PubMed] [Google Scholar]
- 227.Zheng Y, Huang C, Lu L, Yu K, Zhao J, Chen M, et al. STOML2 potentiates metastasis of hepatocellular carcinoma by promoting PINK1-mediated mitophagy and regulates sensitivity to lenvatinib. J Hematol Oncol. 2021;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fernandez-Palanca P, Payo-Serafin T, San-Miguel B, Mendez-Blanco C, Tunon MJ, Gonzalez-Gallego J, et al. Hepatocellular carcinoma cells loss lenvatinib efficacy in vitro through autophagy and hypoxia response-derived neuropilin-1 degradation. Acta Pharmacol Sin. 2023;5:1066–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yan YC, Meng GX, Yang CC, Yang YF, Tan SY, Yan LJ, et al. Diacylglycerol lipase alpha promotes hepatocellular carcinoma progression and induces lenvatinib resistance by enhancing YAP activity. Cell Death Dis. 2023;7:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Han WY, Wang J, Zhao J, Zheng YM, Chai XQ, Gao C, et al. WDR4/TRIM28 is a novel molecular target linked to lenvatinib resistance that helps retain the stem characteristics in hepatocellular carcinomas. Cancer Lett. 2023;568:216259. [DOI] [PubMed] [Google Scholar]
- 231.Zhang X, Su T, Wu Y, Cai Y, Wang L, Liang C, et al. N6-methyladenosine reader YTHDF1 promotes stemness and therapeutic resistance in hepatocellular carcinoma by enhancing NOTCH1 expression. Cancer Res. 2024;6:827–40. [DOI] [PubMed] [Google Scholar]
- 232.Yu T, Yu J, Lu L, Zhang Y, Zhou Y, Zhou Y, et al. MT1JP-mediated miR-24-3p/BCL2L2 axis promotes lenvatinib resistance in hepatocellular carcinoma cells by inhibiting apoptosis. Cell Oncol (Dordr). 2021;4:821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chen X, Ye Q, Chen Z, Lin Q, Chen W, Xie C, et al. Long non-coding RNA muskelin 1 antisense RNA as a potential therapeutic target in hepatocellular carcinoma treatment. Bioengineered. 2022;5:12237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wang Y, Tan K, Hu W, Hou Y and Yang G. LncRNA AC026401.3 interacts with OCT1 to intensify sorafenib and lenvatinib resistance by activating E2F2 signaling in hepatocellular carcinoma. Exp Cell Res. 2022; 1:113335. [DOI] [PubMed]
- 235.Zhang Y, Zhang Y, Tao H, Zhu J, Lu Y, Cheng F, et al. Targeting LINC01607 sensitizes hepatocellular carcinoma to lenvatinib via suppressing mitophagy. Cancer Lett. 2023; 216405. [DOI] [PubMed]
- 236.Hao X, Zhang Y, Shi X, Liu H, Zheng Z, Han G, et al. CircPAK1 promotes the progression of hepatocellular carcinoma via modulation of YAP nucleus localization by interacting with 14-3-3zeta. J Exp Clin Cancer Res. 2022;1:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Eun JW, Yoon JH, Ahn HR, Kim S, Kim YB, Lim SB, et al. Cancer-associated fibroblast-derived secreted phosphoprotein 1 contributes to resistance of hepatocellular carcinoma to sorafenib and lenvatinib. Cancer Commun (Lond). 2023;4:455–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhou C, Sun B Y, Zhou P Y, Yang Z F, Wang Z T, Liu G, et al. MAIT cells confer resistance to Lenvatinib plus anti-PD1 antibodies in hepatocellular carcinoma through TNF-TNFRSF1B pathway. Clin Immunol. 2023; 109770. [DOI] [PubMed]
- 239.Chen P, Wang Y, Zhu X, Huang Y, Chen J, Sun H, et al. SiRNA-HIF-1alpha delivered by attenuated Salmonella enhances the efficacy of lenvatinib against hepatocellular carcinoma. Int Immunopharmacol. 2024; 111728. [DOI] [PubMed]
- 240.Zhang Y, Wu X, Zhu H, Cong Y. Development and in functional study of a bi-specific sustained release drug-loaded nano-liposomes for hepatocellular carcinoma. J Biomater Appl. 2023;1:97–108. [DOI] [PubMed] [Google Scholar]
- 241.Wu Y, Zhu R, Zhou M, Liu J, Dong K, Zhao S, et al. Homologous cancer cell membrane-camouflaged nanoparticles target drug delivery and enhance the chemotherapy efficacy of hepatocellular carcinoma. Cancer Lett. 2023; 216106. [DOI] [PubMed]
- 242.Liu J, Chen J, Liu H, Zhang K, Zeng Q, Yang S, et al. Bi/Se-based nanotherapeutics sensitize CT image-guided stereotactic body radiotherapy through reprogramming the microenvironment of hepatocellular carcinoma. ACS Appl Mater Interfaces. 2021;36:42473–85. [DOI] [PubMed] [Google Scholar]
- 243.Zhang D, Jiang C, Zheng X, Lin Z, Zhuang Q, Xie H, et al. Normalization of tumor vessels by lenvatinib-based metallo-nanodrugs alleviates hypoxia and enhances calreticulin-mediated immune responses in orthotopic HCC and organoids. Small. 2023;29: e2207786. [DOI] [PubMed] [Google Scholar]
- 244.Xu Q, Hu H, Mo Z, Chen T, He Q and Xu Z. A multifunctional nanotheranostic agent based on Lenvatinib for multimodal synergistic hepatocellular carcinoma therapy with remarkably enhanced efficacy. J Colloid Interface Sci. 2023; 375–91. [DOI] [PubMed]
- 245.Xu Q, Li Q, Yang Z, Huang P, Hu H, Mo Z, et al. Lenvatinib and Cu(2–x)S nanocrystals co-encapsulated in poly(D, L-lactide-co-glycolide) for synergistic chemo-photothermal therapy against advanced hepatocellular carcinoma. J Mater Chem B. 2021;48:9908–22. [DOI] [PubMed] [Google Scholar]
- 246.Luo Y, Wang J, Xu L, Du Q, Fang N, Wu H, et al. A theranostic metallodrug modulates immunovascular crosstalk to combat immunosuppressive liver cancer. Acta Biomater. 2022; 478–96. [DOI] [PubMed]
- 247.Tanaka H, Horioka K, Hasebe T, Sawada K, Nakajima S, Konishi H, et al. Treatment of hepatocellular carcinoma with autologous platelets encapsulating sorafenib or lenvatinib: a novel therapy exploiting tumor-platelet interactions. Int J Cancer. 2022;10:1640–53. [DOI] [PubMed] [Google Scholar]
- 248.Ito R, Miyanishi K, Kubo T, Hamaguchi K, Osuga T, Tanaka S, et al. Synergistic antitumor effect of histone deacetylase class IIa inhibitor with lenvatinib in hepatocellular carcinoma. Hepatol Int. 2023;3:735–44. [DOI] [PubMed] [Google Scholar]
- 249.Roberts J L, Poklepovic A, Booth L and Dent P. The multi-kinase inhibitor lenvatinib interacts with the HDAC inhibitor entinostat to kill liver cancer cells. Cell Signal. 2020; 109573. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.