Abstract
Background
Postoperative complications are prone to occur in patients after radical pancreaticoduodenectomy (PD). This study aimed to construct and validate a model for predicting postoperative major complications in patients after PD.
Methods
The clinical data of 360 patients who underwent PD were retrospectively collected from two centers between January 2019 and December 2023. Visceral adipose volume (VAV) and subcutaneous adipose volume (SAV) were measured using three-dimensional (3D) computed tomography (CT) reconstruction. According to the Clavien-Dindo classification system, the postoperative complications were graded. Subsequently, a predictive model was constructed based on the results of least absolute shrinkage and selection operator (LASSO) multivariate logistic regression analysis and stepwise (stepAIC) selection. The nomogram was internally validated by the training and test cohort. The discriminatory ability and clinical utility of the nomogram were evaluated by area under the receiver operating characteristic (ROC) curve (AUC), calibration curve, and decision curve analysis (DCA).
Results
The major complications occurred in 13.3% (n = 48) of patients after PD. The nomogram revealed that high VAV/SAV, high system inflammation response index (SIRI), high triglyceride glucose-body mass index (TyG-BMI), low prognostic nutritional index (PNI) and CA199 ≥ 37 were independent risk factors for major complications. The C-index of this model was 0.854 (95%CI [0.800–0.907]), showing excellent discrimination. The calibration curve demonstrated satisfactory concordance between nomogram predictions and actual observations. The DCA curve indicated the substantial clinical utility of the nomogram.
Conclusion
The model based on clinical and CT indices demonstrates good predictive performance and clinical benefit for major complications in patients undergoing PD.
Keywords: Pancreaticoduodenectomy, Postoperative complications, Three-dimensional CT reconstruction, Predictive model
Introduction
Pancreatoduodenectomy (PD), acknowledged as a classic surgical method, is commonly performed for malignant pancreatic tumors, adenocarcinoma of ampulla, duodenal tumor, cholangiocarcinoma and some benign tumors. Although with the improvement of the PD and the standardization of perioperative management, operative mortality, and morbidity rates have decreased dramatically, postoperative complication rates still vary from 20% to 60% (Menahem et al., 2015). Common complications after PD comprise pancreatic fistula, bile leakage, intestinal fistula, abdominal infection, pulmonary infection, postpancreatectomy hemorrhage (PPH), delayed gastric emptying (DGE), and organ dysfunction (Karim et al., 2018). Such complications would ultimately prolong hospital stays, increase hospitalization costs, and even lead to readmission in the post-discharge period. Major complications, typically classified as Clavien-Dindo grade ≥3, significantly impact the short-term outcomes of PD and may also negatively influence long-term survival according to the expert consensus (Study Group of Pancreatic Surgery in China Society of Surgery of Chinese Medical Association, Pancreatic Disease Committee of China Research Hospital Association, and Editorial Board of Chinese Journal of Surgery, 2023). Therefore, correctly evaluating the occurrence of major complications after PD is of great significance for guiding clinical practice. To further decrease the incidence of postoperative complications after PD, there is a clinical need to clarify potential risk factors in the perioperative period. According to results, clinical doctors can intervene early and ultimately improve postoperative clinical outcomes.
Currently, researches showed that advanced age (Faraj et al., 2013), a high subcutaneous adipose (SAT) area in the paralumbar section of the third lumbar spine (L3) (van Dijk et al., 2017), and high body mass index (BMI) (Shen et al., 2021a) are considered as risk factors for postoperative morbidity and mortality following PD (Matsui et al., 2022; Watanabe et al., 2012). Moreover, other clinical indicators including preoperative nutritional condition, hyperglycemia, hyperlipidemia, and inflammatory status can also increase the risk (Chen et al., 2022; Gouillat & Gigot, 2001; Shi et al., 2023). However, BMI and L3 VAT cannot adequately reflect the fat distribution of the body. Visceral adipose volume (VAV) and subcutaneous adipose volume (SAV) at the L3 vertebral level were measured using three-dimensional (3D) computed tomography (CT) reconstruction technology to calculate the VAV/SAV ratio in patients. This approach was applied to provide a more accurate representation of body composition in the patient cohort. The triglyceride glucose-body mass index (TyG-BMI), derived from the combination of the TyG index with obesity indices, reflects the blood glucose, lipid levels, and insulin resistance, which is closely associated with various postoperative complications (Yang et al., 2023). Recent studies showed that the occurrence and development of pancreatic cancer are directly related to high insulin levels (Zhang et al., 2023). Consequently, we explored the value of the TyG-BMI index in predicting the risk of major complications in patients undergoing PD.
Additionally, recent studies have highlighted the significant role of inflammation and nutritional status in influencing the outcomes of PD (Gilliland et al., 2017; Ma et al., 2023). Preoperative inflammation, often driven by systemic immune responses to malignancy, and poor nutritional status have both been linked to increased rates of postoperative complications. The system inflammation response index (SIRI) is a novel, non-invasive inflammatory biomarker that comprehensively reflects the balance between the host’s immune and inflammatory states (Geng et al., 2018). The prognostic nutritional index (PNI), a simple and effective indicator reflecting the nutritional status of the body, has been demonstrated in multiple studies to have predictive value for the prognosis of gastrointestinal malignancies (Ding et al., 2022). However, there have been relatively few studies exploring their impact on the occurrence of major complications after PD. Therefore, we have also included them in our analysis.
The nomogram is a statistical tool for predicting individual-specific outcomes by transforming complex regression equations into visual graphs. Numerous studies have demonstrated the clinical utility of the nomogram in predicting complications and prognosis after PD (Zhang et al., 2022b; Zhu et al., 2022). Therefore, this study aimed to develop a novel nomogram based on clinical and CT indices to predict the risk factors of patients after PD on the basis of preoperative clinical indicators that can be easily obtained in routine clinical practice.
Methods
Study population
The study population were retrospectively enrolled between January 2019 and December 2023 from two centers, Jinling Hospital and The Affiliated People’s Hospital of Jiangsu University. The flowchart of this study is shown in Fig. S1.
All patients admitted would first receive an assessment of surgical resectability by the multidisciplinary team (MDT) through imaging to determine curative resectability of tumors. The inclusion criteria are all patients that underwent upfront PD during the study period at the designated institutions by expert pancreatic surgeons. Finally, a total of 360 patients were included in the study. Subsequently, their medical records, laboratory results, and imaging data of these patients were collected and systematically organized for further analysis. In terms of the Clavien-Dindo system, the cohort was divided into those with or those without major complications. Written informed consent was obtained from patients prior to the research. This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Jinling Hospital (2023DZKY-049-03) and registered at ClinicalTrials.gov (NCT06378853).
Surgical procedures and perioperative management
All patients underwent an MDT discussion before treatment. The open Kausch-Whipple procedure is performed according to the NCCN guidelines (Tempero et al., 2021). The surgical procedure generally includes: opening the Kocher incision, organ resection, anastomotic reconstruction in hand-sewn by continuous suturing techniques, and irrigating the peritoneal cavity.
Drugs inhibiting secretion of digestive fluid such as proton-pump inhibitors and somatostain were administered routinely. Laboratory results, including routine blood and biochemical examinations, were collected on postoperative day (POD) 1. All patients in the study underwent abdominal CT or trans-abdominal ultrasound within the first week after surgery. Additional abdominal imaging was conducted when there was a sign of possible intra-abdominal complications. Oral diet, walking, and early withdrawal of drainage tubes were advocated early postoperatively. Liquid diet was gradually resumed around POD 2–5 and soft diet after defecation. Drainage tubes were removed appropriately on day 3 and day 5 after surgery if there were no signs of leakage and drainage fluid amylase levels were within normal range.
Data collection
The clinical information of all patients included age, sex, and BMI, diabetes, hypertension and ASA. The preoperative data closest to the time of surgery were collected for analysis, including white blood cell (WBC) count, red blood cell (RBC) count, platelet (PLT) count, hemoglobin (Hb) level, albumin (ALB) level, lymphocyte count, monocyte count, neutrophil count, triglyceride (TG) level, fasting plasma glucose (FPG) level, and CA199 level. The prognosis nutrition index (PNI) was calculated using the formula: PNI = serum ALB level (g/L) + 5 × total number of peripheral blood lymphocytes (×109/L) (Onodera, Goseki & Kosaki, 1984), and the systemic inflammation response index (SIRI) was calculated using the formula: SIRI = total number of peripheral blood neutrophils (×109/L) × total number of peripheral blood monocytes (×109/L)/total number of peripheral blood lymphocytes (×109/L) (Qi et al., 2016). The TyG index was calculated using the formula: TyG = Ln [TG (mg/dl) × FPG (mg/dl)/2] (Simental-Mendia, Rodriguez-Moran & Guerrero-Romero, 2008). The TyG-BMI index was defined as the TyG index multiplied by BMI (Kg/m2). The postoperative data such as maximum tumor size, length of hospital stay, and postoperative complications were analyzed. Postoperative complications were mainly evaluated based on the diagnostic criteria established by the International Study Group of Pancreatic Surgery (ISGPS) (Bassi et al., 2017; Koch et al., 2011; Wente et al., 2007a, 2007b).
Collection of CT data
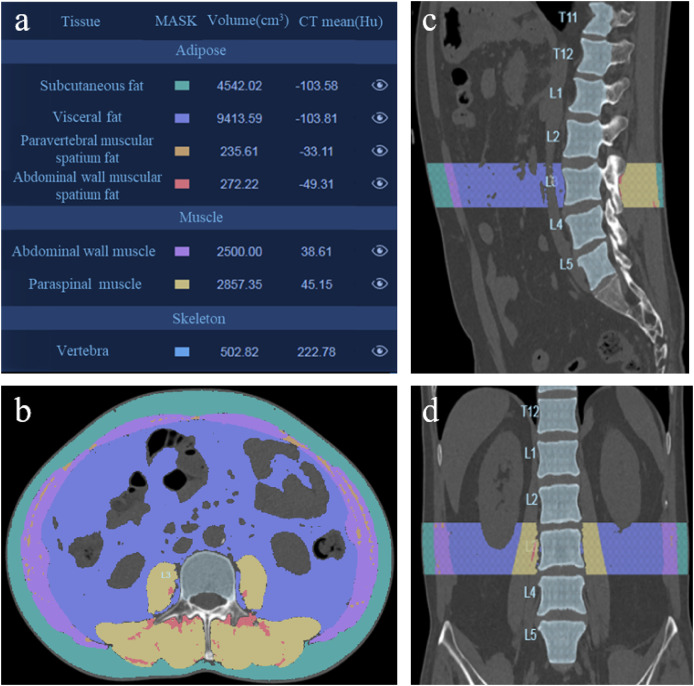
A single trained radiologist analyzed CT images preoperatively using the HY-QCT medical software version V2.5.0. The software is based on high-quality CT imaging data and simulates the human visual nervous system’s method of detecting target objects in images through convolutional neural networks. It trains the computer to become a perceptual system composed of neurons that are extremely sensitive to the features of the spinal region in CT images. By processing, analyzing, and calculating the spinal region in CT images, it achieves segmentation detection of spinal vertebrae, morphological analysis of vertebrae, and tissue analysis. Then, the tissue composition, such as L3 SAV, VAV, abdominal wall muscular spatium fat, paravertebral muscular spatium fat, abdominal wall muscle, and paraspinal muscle were segmented automatically and their volume were calculated (Fig. 1).
Figure 1. 3D CT reconstruction to analyze the L3 adipose and muscle tissue.
(A) Analysis results of patient’s adipose and muscle tissue. (B) Axial slice reformation of a preoperative CT scan at the L3 level. (C) Sagittal reformation at the L3 level of the same patient. (D) Coronal plane reformation at the L3 level of the same patient.
Definition of cut-off value
The BMI ≤ 18.5 kg/m2 indicates that weight is below the healthy range, suggesting the possibility of malnutrition, chronic illness, or other health issues according to clinical management and treatment of obesity in China (Zeng et al., 2021). CA199 ≥ 37 U/ml indicates that it exceeds the normal healthy range, suggesting the possibility of pancreatic cancer or other gastrointestinal tumors based on comprehensive guidelines for the diagnosis and treatment of pancreatic cancer (Pancreatic Cancer Committee of Chinese Anticancer A, 2021). Due to the lack of specific cut-off values in previous studies for VAV/SAV, SIRI, PNI, and TyG-BMI, we determined the optimal cut-off value for the classification performance of these indicators using the area under the receiver operating characteristic (ROC) curves. The optimal cut-off value was determined by maximizing specificity and sensitivity using Youden’s Index. Therefore, SIRI ≥ 0.86 was defined as high SIRI, PNI ≤ 47.65 as low PNI, TyG-BMI ≥ 200.11 as high TyG-BMI, and VAV/SAV ≥ 1.59 as high VAV/SAV.
Feature selection and nomogram development
From all demographic features and preoperative clinical data, to prevent overfitting and handle multicollinearity, these variables which showed differences between the two groups (P < 0.05) and other potential risk factors were evaluated by the LASSO regression. Then it produced 11 significant parameters (age ≥ 65, diabetes, hypertension, CA199 ≥ 37, NWR, SIRI, PNI, TyG-BMI, VAV/SAV, jaundice and Hb that had nonzero coefficients. And we obtained the lambda min value when the MSE is minimized, which indicates that the prediction error of the model is at its lowest.).
Then those significant parameters were inputted into the final multivariate regression analysis. The multivariate regression analysis conducted as described demonstrated that CA199 ≥ 37, high SIRI, low PNI, high TyG-BMI, and high VAV/SAV were independent risk factors for major complications after PD. Next, the stepAIC selection was performed to obtain the best prediction model. Based on the results, a nomogram model was established to predict major complications after PD.
Statistical analysis
Continuous variables with a normal distribution are presented as the mean and standard deviation (SD) and were analyzed using the Student’s t-test. Continuous variables with a non-normal distribution are presented as the median with interquartile range (IQR), and the Mann-Whitney U test was used for analysis between the two groups. Chi-squared or Fisher test was used to compare the distribution of categorical variables between groups. Two-sided p-values less than 0.05 were considered significant. All statistical analyses were conducted using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA) and R version 4.0.5 (R Core Team, 2021).
The model building followed a predefined plan. Firstly, data cleaning and imputation were conducted for datasets. Variables with missing percentages of more than 10% were deleted, and other variables were filled with multiple imputation methods (Mice package in R). Secondly, least absolute shrinkage and selection operator analysis was used to filter valuable diagnostic variables. Next, multivariate logistic regression analyses were used to determine independent risk factors for major complications in patients undergoing PD. StepAIC selection was then performed to obtain the best prediction model. Nomograms were constructed based on the proportional conversion of each regression coefficient from multivariate logistic regression onto a 0- to 100-point scale. Then, the nomogram was internally validated by the training and test database.
The optimal cut-off value of the nomogram score was calculated using the maximum Youden index method. The area under the curve (AUC) was subsequently corrected by bootstrapping validation (1,000 bootstrap resamples) (Harrell, Lee & Mark, 1996; Steyerberg et al., 2001). The Cox-Snell R2, Nagelkerke R2 and Hosmer-Lemeshow R2 (HL R2) were used to fit of the model. The calibration curve, and decision curve analysis (DCA) were used to evaluate this model.
Results
Clinicopathological characteristics of patients and incidence of postoperative complications
Of 415 PDs performed at the participating institutions during the study period, 360 (86.7% in relation to 415) met the criteria for analysis. The clinical pathological characteristics of these patients were shown in Table 1. There were 173 males (48.1%) and 187 females (51.9%). The median age of the patients was 61.0 ± 10.6 years. Among baseline characteristics, significant differences were noted for age, diabetes BMI, lymphocyte, neutrophil, FPG, TG, and CA199 between the two groups. Tumor size and duration of hospital stay differed significantly between the two groups.
Table 1. Patient demographics and clinical characteristics are divided by with or without major complications.
| Variables | Total | Without major complications |
With major complications |
p value |
|---|---|---|---|---|
| Total number | 360 | 312 | 48 | |
| Age, year | 61.0 ± 10.6 | 60.4 ± 10.9 | 64.5 ± 7.6 | 0.013* |
| Sex, female/male | 0.066 | |||
| Female | 187 | 168 | 19 | |
| Male | 173 | 144 | 29 | |
| BMI ≤ 18.5 kg/m2, n | 45 | 34 | 11 | 0.019* |
| Diabetes | 0.041 | |||
| No | 255 | 227 | 28 | |
| Yes | 105 | 85 | 20 | |
| Hypertension | 0.145 | |||
| No | 250 | 221 | 29 | |
| Yes | 110 | 91 | 19 | |
| Jaundice | 0.664 | |||
| No | 235 | 205 | 30 | |
| Yes | 125 | 107 | 18 | |
| ASA | 0.130 | |||
| 1 | 143 | 128 | 15 | |
| 2 | 183 | 158 | 25 | |
| ≥ 3 | 34 | 26 | 8 | |
| WBC, 109/L | 6.54 (5.20–7.30) | 6.52 (5.04–7.27) | 6.90 (5.92–7.35) | 0.059 |
| RBC, 1012/L | 3.96 (3.79–4.15) | 3.96 (3.79–4.15) | 3.95 (3.78–4.14) | 0.639 |
| PLT, 109/L | 202 (186–212) | 202 (186–215) | 200 (187–207) | 0.151 |
| Hb, g/L | 123 (113–135) | 123 (113–137) | 121 (110–131) | 0.156 |
| Lymphocyte, 109/L | 1.59 (1.22–1.95) | 1.63 (1.24–1.98) | 1.37 (1.08–1.68) | 0.002** |
| Monocyte, 109/L | 0.47 (0.38–0.60) | 0.47 (0.37–0.60) | 0.50 (0.41–0.61) | 0.142 |
| Neutrophil, 109/L | 3.95 (2.93–5.20) | 3.90 (2.78–5.29) | 4.12 (3.63–5.10) | 0.037* |
| NLR | 2.59 (1.84–3.32) | 2.51 (1.73–3.21) | 3.07 (2.43–4.42) | <0.001*** |
| NWR | 0.64 (0.56–0.72) | 0.63 (0.55–0.72) | 0.65 (0.57–0.78) | 0.200 |
| MWR | 0.08 (0.06–0.10) | 0.08 (0.06–0.10) | 0.08 (0.06–0.09) | 0.556 |
| High SIRI, n | 252 | 206 | 46 | <0.001*** |
| ALB ≤ 35 g/L, n | 65 | 51 | 11 | 0.262 |
| FPG, mmol/L | 4.50 (3.90–5.40) | 4.42 (3.90–5.30) | 5.10 (4.46–5.62) | 0.011* |
| TG, mmol/L | 1.72 (1.36–2.08) | 1.63 (1.30–1.91) | 2.09 (1.95–2.27) | <0.001*** |
| Low PNI, n | 172 | 131 | 41 | <0.001*** |
| High TyG-BMI, n | 53 | 37 | 16 | <0.001*** |
| CA199 ≥ 37 U/ml, n | 153 | 126 | 27 | 0.038* |
| VAV, cm3 | 3,587.90 (2,413.52–4,739.48) | 3,415.08 (2,310.62–4,593.01) | 4,238.64 (3,100.14–5,360.96) | 0.004** |
| SAV, cm3 | 3,470.36 (2,400.66–4,512.26) | 3,620.71 (2,400.66–4,818.90) | 2,941.49 (2,379.68–3,885.39) | 0.032* |
| Abdominal wall muscle volume, cm3 | 1,608.5 (1,310.1–1,955.9) | 1,610.5 (1,291.8–1,943.5) | 1,607.0 (1,364.1–1,999.7) | 0.631 |
| Paraspinal muscle volume, cm3 | 1,955.8 (1,618.1–2,456.4) | 1,958.5 (1,613.2–2,471.8) | 1,929.9 (1,647.8–2,386.3) | 0.918 |
| Total muscle volume, cm3 | 3,567.0 (2,860.6–4,360.4) | 3,571.0 (2,844.5–4,356.8) | 3,527.0 (2,946.3–4,406.3) | 0.791 |
| High VAV/SAV, n | 74 | 48 | 26 | <0.001*** |
| Tumor size, (cm, n) | <0.001*** | |||
| <3 | 178 | 165 | 13 | |
| ≥3 | 182 | 147 | 35 | |
| Duration of hospital stay, day | 21.3 ± 9.3 | 19.8 ± 8.2 | 31.2 ± 10.5 | <0.001*** |
| Pancreatic texture, n | ||||
| soft | 256 | 224 | 32 | 0.466 |
| hard | 104 | 88 | 16 | |
| Pancreas duct size, (mm, n) | 0.563 | |||
| ≤3 | 179 | 157 | 22 | |
| >3 | 181 | 155 | 26 | |
| Operative time, min | 300.0 (280.0–320.0) | 300.0 (280.0–320.0) | 305.0 (282.5–327.5) | 0.099 |
| Blood loss, ml | 300.0 (250.0–350.0) | 300.0 (250.0–350.0) | 320.0 (262.5–390.0) | 0.058 |
| Pathology | 0.412 | |||
| PDAC | 204 | 182 | 22 | |
| Adenocarcinoma of ampulla | 53 | 43 | 10 | |
| Duodenal tumor | 37 | 32 | 5 | |
| Cholangiocarcinoma | 32 | 25 | 7 | |
| IPMN | 22 | 20 | 2 | |
| Others | 12 | 10 | 2 |
Notes:
P < 0.05.
P < 0.01.
P < 0.001.
BMI, body mass index; ASA, American Society of Anesthesiologists; WBC, white blood cell; RBC, red blood cell; PLT, platelet; Hb: hemoglobin; ALB, albumin; FPG, fasting plasma glucose; TG, triglyceride; NLR, neutrophil to lymphocyte ratio; NWR, neutrophil to white blood cell ratio; MWR, monocyte to white blood cell ratio; SIRI, systemic inflammation response index; PNI, prognosis nutrition index; TyG-BMI, triglyceride glucose-body mass index; VAV, visceral adipose volume; SAV, subcutaneous adipose volume; PDAC, pancreatic ductal adenocarcinoma; IPMN, intraductal papillary mucinous tumor.
All postoperative complications were present in Tables S1 and S2. There were 48 cases (13.3%) of major complications in the total cohort. A total of 53 (14.7%) patients experienced pancreatic fistula, 16 (4.4%) patients had bile leakage and 6 (1.7%) patients had intestinal fistula. The rates of abdominal and pulmonary infection were 14.2% and 6.9% respectively. The PPH occurred in 28 (7.8%) patients. The DGE and organ dysfunction rates were 9.2% and 2.5%, respectively.
Risk-associated factors of major complications after PD
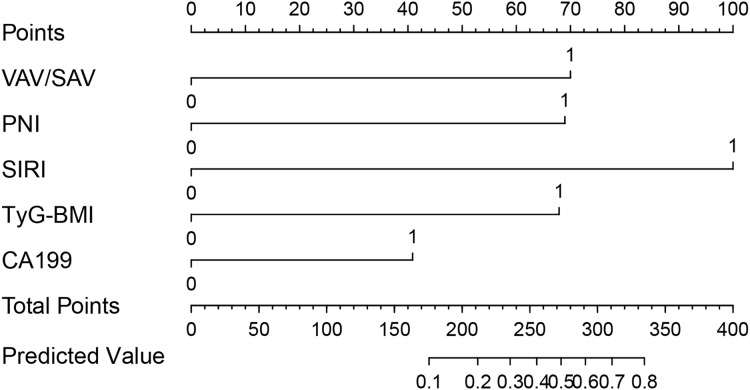
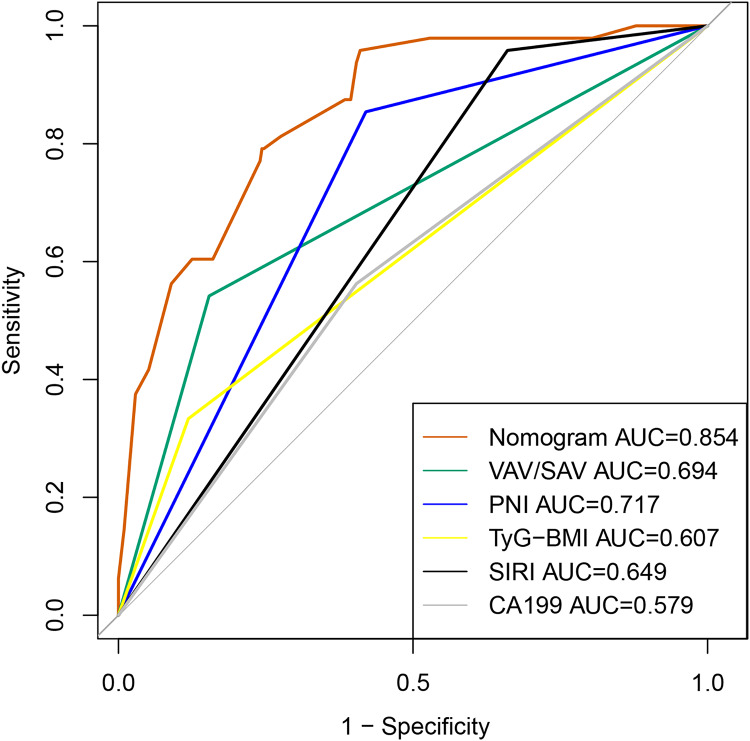
The results of LASSO are shown in Figs. S2 and S3. Then those significant parameters were inputted into the multivariate regression analysis. The results of multivariate regression analysis for risk factor selection are as follows (Table 2). To obtain the best model, we used the stepAIC method to select variables: one variable is added at a time, but at each step, the variables are re-evaluated. Variables that do not contribute to the model will be removed. The AIC value is calculated to assess the quality of each candidate model, and this process continues until the optimal model is obtained. Cox-Snell R2, Nagelkerke R2 and HL R2 are commonly used metrics to assess the goodness of fit of the selected model. As demonstrated in Table 3, the goodness-of-fit statistics for this model outperform those of the alternative models, suggesting that it exhibits superior explanatory power, accuracy, and a better fit to the data. The nomogram model to predict major complications after PD was established according to these results shown in Fig. 2. The total score was calculated using VAV/SAV, PNI, SIRI, TyG-BMI, and CA199. Each of these variables was assigned a score based on a scaled axis. The individual scores were then summed to obtain the total score. By mapping this total score to the corresponding point scale, we were able to estimate the risk of major postoperative complications in patients after PD. Contribution of each risk factor to the prediction model were shown in Table 3. The AUC and C-index of the nomogram was 0.854 (95% CI [0.800–0.907]), the positive and negative predictive value were 0.700 and 0.883 respectively, and accuracy was 0.878, indicating that the model exhibited good discrimination and substantial accuracy in predicting major complications after PD (Fig. 3).
Table 2. Multivariate analyses of risk factors for major complications after PD.
| Factor | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| β coefficient | SE | Wald | OR | 95% CI | p value | |
| High SIRI | 1.952 | 0.777 | 6.310 | 7.045 | [1.536–32.319] | 0.012* |
| NWR | −0.394 | 0.839 | 0.220 | 0.675 | [0.130–3.495] | 0.639 |
| Low PNI | 1.483 | 0.461 | 10.349 | 4.406 | [1.785–10.876] | 0.001** |
| High TyG-BMI | 1.349 | 0.452 | 8.907 | 3.852 | [1.589–9.338] | 0.003** |
| High VAV/SAV | 1.611 | 0.396 | 16.591 | 5.009 | [2.307–10.877] | <0.001*** |
| Age ≥ 65 | 0.317 | 0.381 | 0.692 | 1.373 | [0.650–2.901] | 0.406 |
| CA199 ≥ 37 | 0.839 | 0.388 | 4.667 | 2.314 | [1.081–4.954] | 0.031* |
| Jaundice | −0.376 | 0.399 | 0.888 | 0.687 | [0.314–1.501] | 0.346 |
| Hb | −0.011 | 0.012 | 0.724 | 0.989 | [0.966–1.014] | 0.395 |
| Diabetes | 0.190 | 0.567 | 0.112 | 1.209 | [0.398–3.677] | 0.738 |
| Hypertension | 0.138 | 0.563 | 0.060 | 1.148 | [0.381–3.464] | 0.806 |
Notes:
P < 0.05.
P < 0.01.
P < 0.001.
SE, standard error; OR, odds ratio; CI, confidence interval; SIRI, systemic inflammation response index; NWR, neutrophil to white blood cell ratio; PNI, prognosis nutrition index; TyG-BMI, triglyceride glucose-body mass index; VAV, visceral adipose volume; SAV, subcutaneous adipose volume; Hb, hemoglobin.
Table 3. Contribution of variables to the prediction of major complications in patients.
| Variables | AIC | Cox-Snell R2 | Nagelkerke R2 | HL R2 | AUC |
|---|---|---|---|---|---|
| High VAV/SAV | −813.39 | 0.084 | 0.155 | 0.112 | 0.694 (0.620–0.768) |
| +Low PNI | −834.28 | 0.144 | 0.264 | 0.198 | 0.793 (0.724–0.862) |
| +High TyG-BMI | −844.08 | 0.163 | 0.300 | 0.227 | 0.809 (0.746–0.872) |
| +CA199 ≥ 37 | −848.53 | 0.179 | 0.330 | 0.252 | 0.834 (0.777–0.891) |
| +High SIRI | −851.54 | 0.198 | 0.365 | 0.281 | 0.854 (0.800–0.907) |
Note:
AIC, akaike information criterion; HL, Hosmer-Lemeshow test; AUC, area under the curve; VAV, visceral adipose volume; SAV, subcutaneous adipose volume; PNI, prognosis nutrition index; SIRI, systemic inflammation response index; TyG-BMI, triglyceride glucose-body mass index.
Figure 2. Nomogram to predict the probability of major complications after PD.
Figure 3. The ROC curve of the predictive model for major complications after PD.
Calibration and validation of the nomogram
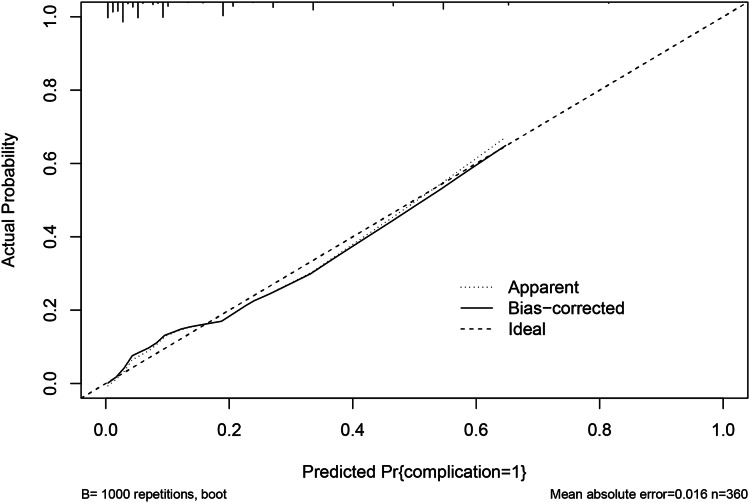
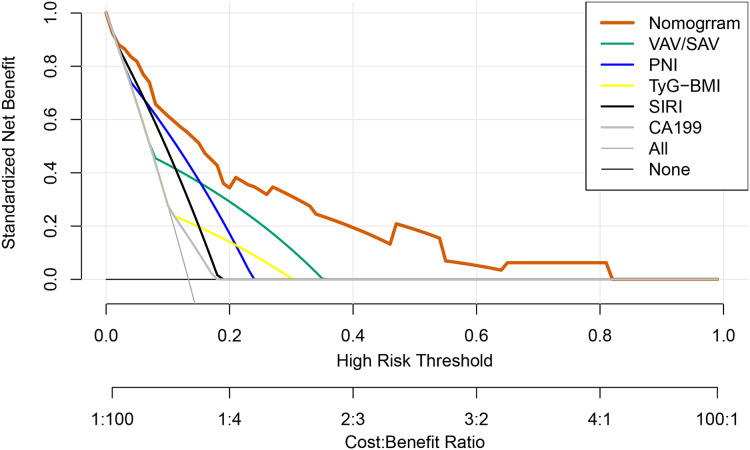
The calibration curve via 1,000 bootstrap resampling was used to assess the predictive accuracy of the nomogram. The calibration curve for the probability showed good concordance between the predicted and actual observations and the absolute error between the simulated curve and the actual curve is 0.016 (Fig. 4), which demonstrated the nomogram had great accurate predictive ability. The DCA converts complex mathematical models into simple and easy-to-understand graphics to intuitively judge the practicability and net benefits of different models. Therefore, in order to further investigate the clinical utility of the prediction model, the DCA (Fig. 5) was drawn with the threshold probability as the abscissa and the net benefit rate of patients as the ordinate. It can be clearly shown that patients whose postoperative major complications were predicted were predicted based on the nomogram have a higher net benefit than any patient predicted by a single indicator, and the nomogram had a better potential for clinical application when the threshold probability was 0.10 to 0.80, indicating that the prediction model has great clinical value.
Figure 4. The calibration curve of the nomogram for predicting the probability of major complications (bootstrap = 1,000 repetitions).
Figure 5. DCA curves of the prediction model to predict the probability of major complications.
To test the applicability of our prediction model, patients were divided into the training database (n = 251) and test database (n = 109) randomly with a ratio of 7:3. Internal validation of this model showed that the AUC was 0.864 and data from the test database was used for cross validation of the model, showing the AUC of 0.824 (Fig. S4). The calibration curves of the training and test database also demonstrated that the prediction model was in great agreement with actual observations (Figs. S5, S6). The DCA curves also revealed that the model provided great clinical benefit in the training and test database (Figs. S7, S8).
Discussion
Due to all cause-morbidity rates after PD are reported to vary from 38% to 77%, whilst procedure specific complications vary between 13% to 50% (Bassi et al., 2017), we should emphasize the importance of recognizing and detecting postoperative complications early. Several studies (El Nakeeb et al., 2013; Mintziras et al., 2020; Nakata et al., 2013; Palumbo et al., 2021) have established some prediction models to predict the occurrence of postoperative complications after PD, but the major complications are one of the most concerning issues to surgeons. Because they influence short-term outcomes and may also impact long-term survival severely.
In this study, clinical data from patients who underwent PD at two centers were retrospectively analyzed to identify risk factors associated with major complications. Additionally, a nomogram predictive model was developed based on 3D CT reconstruction, offering clinicians an intuitive and simple tool to identify risk factors and implement active medical interventions for prevention. The multivariable regression analysis identified the preoperative CA199 ≥ 37, VAV/SAV, PNI, SIRI, and TyG-BMI as independent risk factors for major complications. Consequently, these parameters were incorporated into the nomogram predictive model. Further internal validation, calibration curve, and DCA curve showed this model had good predicting ability and great value in clinical application. Additionally, this represents the first prediction model based on 3D CT reconstruction which combines multiple comprehensive indicators simultaneously.
Previous studies have explored the impact of body composition on postoperative complications in patients undergoing abdominal surgery. The high VAT/SAT ratio was significantly associated with postoperative complications and survival (Matsui, Inaki & Tsuji, 2021; Pacquelet et al., 2022). Visceral obesity has already proven to be associated with complications such as anastomotic leakage and postoperative pneumonia (Ballian et al., 2012). However, most current investigators analyzed CT images through SliceOmatic software to calculate parameters such as L3 skeletal muscle tissue (cm2), VAT (cm2), and SAT area (cm2), which may not accurately reflect the true composition of patients. At the same time, traditional technology uses serial processing methods in CT image feature extraction. It is prone to loss of image data, which results in problems such as ring distortion of the reconstructed image and long reconstruction time. Therefore, we segmented each structure of interest based on convolutional neural networks to calculate parameters including abdominal wall muscle (cm3), paraspinal muscle (cm3), VAV (cm3), and SAV (cm3). This study found that high VAV/SAV ratio was significantly associated with postoperative complications after PD, which is consistent with the literature before. It is considered that excessive fat accumulation may lead to the generation of adipokines and the infiltration of pro-inflammatory macrophages, thereby increasing the risk of postoperative complications (Deng et al., 2016).
Similar to high VAV/SAV, studies have reported associations between FPG and TG levels and postoperative complications in abdominal surgery. The pathological mechanism of glucotoxicity affecting the function of the circulatory system involves hyperglycemia and its secondary oxidative stress. Elevated FPG and TG levels result in increased glycosylated hemoglobin, which exerts pro-inflammatory and thrombotic effects, potentially leading to postoperative complications (Daryabor et al., 2020; Libby, 2007). For the pancreas, insulin resistance can lead to dysfunction of pancreatic islets β cells, resulting in persistent hyperglycemia (Chinese Elderly Type 2 Diabetes P et al., 2022). The TyG-BMI index, which is calculated using TG, FPG, and BMI has become an attractive option for predicting insulin resistance. Several studies have shown that the TyG-BMI index is associated with inflammation, glucolipid metabolism disorders, and microvascular complications (Yang et al., 2023). However, the correlation between the TyG-BMI index and complications after PD remains unclear. Thus, this study aimed to investigate whether the TyG-BMI index can predict major complications in patients undergoing PD.
SIRI is a novel systemic inflammatory biomarker based on the counts of neutrophils, monocytes, and lymphocytes in peripheral blood, which can reflect the immune and inflammatory status in patients. It was initially used to predict the survival of patients undergoing chemotherapy for pancreatic cancer (Qi et al., 2016). It can also be used to predict the risk of postoperative complications, assess postoperative recovery, and guide postoperative interventions. This study found that patient group with major complications had higher SIRI. The findings may be explained by the following mechanisms. Monocytes promote inflammatory response by releasing pro-inflammatory cytokines and interacting with other immune cells, thereby increasing the risk of postoperative complications.
PNI is an important indicator reflecting the nutritional status of patients and is of great significance for perioperative nutritional assessment. PNI, as a combined indicator of serum ALB and lymphocytes, effectively enhances the sensitivity of their predictive power. Its predictive effect on the prognosis of malignant tumors of the digestive tract, such as pancreatic cancer and gastric cancer, has been reported in many studies (Huang et al., 2023; Pan, Ma & Dai, 2023). Especially in pancreatic cancer patients, the preoperative nutritional status is crucial for postoperative complications and prognosis (Tumas et al., 2020). Due to factors such as tumor location and malignancy, most pancreatic cancer patients develop symptoms related to malnutrition, with unintentional weight loss being the primary manifestation. Although various nutritional assessment tools are currently available and widely used in hospital, such as the Nutrition Risk Screening 2002 (NRS 2002) and the Malnutrition Universal Screening Tool (MUST), their results are often influenced by subjective descriptions and subjective judgments from evaluators (Hersberger et al., 2020; Zhang et al., 2021). In contrast, PNI, as an objective calculation formula based on laboratory tests, shows significant potential in the preoperative assessment of patients with cancer. This study indicates that patients with a low preoperative PNI are more likely to occur major complications. Therefore, using PNI to assess preoperative nutritional status and the risk of major complications can be beneficial for the long-term outcomes of patients.
Another risk factor for major complications in our model is CA199 ≥ 37. Serum tumor markers are a commonly used diagnostic and therapeutic tool in clinical practice, known for their convenience, cost-effectiveness, and repeatability. CA199 is the most commonly used serum tumor marker in the diagnosis and treatment of pancreatic tumors and cholangiocarcinoma (Engle et al., 2019). In healthy individuals, serum CA199 levels are typically less than 37 U/ml. Studies have shown that patients with elevated preoperative CA199 levels are more prone to postoperative complications and that it is associated with poor prognosis in various tumors (Cai et al., 2023; Wang et al., 2023; Zhang et al., 2022a). In this study, CA199 was found to be an independent risk factor for major complications after PD. The possible explanation is that in the tumor microenvironment, hypoxia induces tumor cells to proliferate extensively and produce more CA199, which is released into the bloodstream after cell destruction. Additionally, tumor infiltration promotes angiogenesis, increasing vascular permeability, making it easier for CA199 to enter the bloodstream (Galli, Basso & Plebani, 2013).
Therefore, our study developed a nomogram focused on predicting postoperative major complications in patients undergoing PD. In addition, we compared our model with existing new methods for predicting postoperative complications after PD. The AUC of our model is 0.854, which is higher than the AUC1: 0.723 (Zhu et al., 2022), AUC2: 0.701 (Shen et al., 2021b), AUC3: 0.821 (Li et al., 2019), and AUC4: 0.776 (Li et al., 2021). The results showed that our model has good predictive capability for major complications after PD. Additionally, there are also some limitations in this study that need to be considered. Firstly, although this study included two centers, it was a retrospective study and the sample size was small, so inherent bias was inevitable. Larger, prospective, and multi-center studies are required to confirm the efficacy of this research. Secondly, the risk factors in our prediction model were derived from routine laboratory tests, potentially lacking some important parameters from specialist examinations. Thirdly, due to the limited duration of laparoscopic PD in our centers, the method of PD in this research is the open approach.
Conclusion
In conclusion, this two-center study identified the preoperative CA199 ≥ 37, SIRI, PNI, TyG-BMI, and VAV/SAV as significant risk factors in the nomogram for predicting major complications after PD. The nomogram exhibited good performance in predicting risk factors with high degrees of stability and accuracy, enabling clinicians to carry out necessary treatment strategies early to improve patient outcomes in the future.
Supplemental Information
The lasso and multivariate logistic regression analysis results.
Funding Statement
This work was supported by the National Natural Science Foundation of China (82170575, 82370900). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Jiaqi Wang conceived and designed the experiments, performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Kangjing Xu conceived and designed the experiments, performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Changsheng Zhou performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Xinbo Wang performed the experiments, prepared figures and/or tables, and approved the final draft.
Junbo Zuo analyzed the data, prepared figures and/or tables, and approved the final draft.
Chenghao Zeng analyzed the data, prepared figures and/or tables, and approved the final draft.
Pinwen Zhou analyzed the data, prepared figures and/or tables, and approved the final draft.
Xuejin Gao analyzed the data, prepared figures and/or tables, and approved the final draft.
Li Zhang analyzed the data, prepared figures and/or tables, and approved the final draft.
Xinying Wang conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Jinling Hospital (2023DZKY-049-03) and registered at ClinicalTrials.gov (NCT06378853).
Data Availability
The following information was supplied regarding data availability:
The raw data is available in the Supplemental File.
References
- Ballian et al. (2012).Ballian N, Lubner MG, Munoz A, Harms BA, Heise CP, Foley EF, Kennedy GD. Visceral obesity is associated with outcomes of total mesorectal excision for rectal adenocarcinoma. Journal of Surgical Oncology. 2012;105(4):365–370. doi: 10.1002/jso.22031. [DOI] [PubMed] [Google Scholar]
- Bassi et al. (2017).Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer C, Wolfgang CL, Yeo CJ, Salvia R, Buchler M. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584–591. doi: 10.1016/j.surg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Cai et al. (2023).Cai M, Guo T, Chen Z, Li W, Pu T, Zhang Z, Huang X, Guo X, Yu Y. Development and validation of a network calculator model for safety and efficacy after pancreaticoduodenectomy in the elderly patients with pancreatic head cancer. Cancer Medicine. 2023;12(19):19673–19689. doi: 10.1002/cam4.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2022).Chen JY, Nassereldine H, Cook SB, Thornblade LW, Dellinger EP, Flum DR. Paradoxical association of hyperglycemia and surgical complications among patients with and without diabetes. JAMA Surgery. 2022;157(9):765–770. doi: 10.1001/jamasurg.2021.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Elderly Type 2 Diabetes P et al. (2022).Chinese Elderly Type 2 Diabetes P. Treatment of Clinical Guidelines Writing G. Geriatric E, Metabolism Branch of Chinese Geriatric S. Geriatric Professional Committee of Beijing Medical Award F. National Clinical Medical Research Center for Geriatric D Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition) Zhonghua Nei Ke Za Zhi. 2022;61:12–50. doi: 10.3760/cma.j.cn112138-20211027-00751. [DOI] [PubMed] [Google Scholar]
- Daryabor et al. (2020).Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Frontiers in Immunology. 2020;11:1582. doi: 10.3389/fimmu.2020.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng et al. (2016).Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annual Review of Pathology: Mechanisms of Disease. 2016;11(1):421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- Ding et al. (2022).Ding P, Guo H, Sun C, Yang P, Kim NH, Tian Y, Liu Y, Liu P, Li Y, Zhao Q. Combined systemic immune-inflammatory index (SII) and prognostic nutritional index (PNI) predicts chemotherapy response and prognosis in locally advanced gastric cancer patients receiving neoadjuvant chemotherapy with PD-1 antibody sintilimab and XELOX: a prospective study. BMC Gastroenterol. 2022;22:121. doi: 10.1186/s12876-022-02199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Nakeeb et al. (2013).El Nakeeb A, Salah T, Sultan A, El Hemaly M, Askr W, Ezzat H, Hamdy E, Atef E, El Hanafy E, El-Geidie A, Abdel Wahab M, Abdallah T. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors, and management (single center experience) World Journal of Surgery. 2013;37(6):1405–1418. doi: 10.1007/s00268-013-1998-5. [DOI] [PubMed] [Google Scholar]
- Engle et al. (2019).Engle DD, Tiriac H, Rivera KD, Pommier A, Whalen S, Oni TE, Alagesan B, Lee EJ, Yao MA, Lucito MS, Spielman B, Da Silva B, Schoepfer C, Wright K, Creighton B, Afinowicz L, Yu KH, Grutzmann R, Aust D, Gimotty PA, Pollard KS, Hruban RH, Goggins MG, Pilarsky C, Park Y, Pappin DJ, Hollingsworth MA, Tuveson DA. The glycan CA19-9 promotes pancreatitis and pancreatic cancer in mice. Science. 2019;364(6446):1156–1162. doi: 10.1126/science.aaw3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraj et al. (2013).Faraj W, Alameddine R, Mukherji D, Musallam K, Haydar A, Eloubiedi M, Shamseddine A, Halal A, Abou-Alfa GK, O’Reilly EM, Jamali F, Khalife M. Postoperative outcomes following pancreaticoduodenectomy: how should age affect clinical practice? World Journal of Surgical Oncology. 2013;11(1):131. doi: 10.1186/1477-7819-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli, Basso & Plebani (2013).Galli C, Basso D, Plebani M. CA 19-9: handle with care. Clinical Chemistry and Laboratory Medicine. 2013;51(7):1369–1383. doi: 10.1515/cclm-2012-0744. [DOI] [PubMed] [Google Scholar]
- Geng et al. (2018).Geng Y, Zhu D, Wu C, Wu J, Wang Q, Li R, Jiang J, Wu C. A novel systemic inflammation response index (SIRI) for predicting postoperative survival of patients with esophageal squamous cell carcinoma. International Immunopharmacology. 2018;65:503–510. doi: 10.1016/j.intimp.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Gilliland et al. (2017).Gilliland TM, Villafane-Ferriol N, Shah KP, Shah RM, Tran Cao HS, Massarweh NN, Silberfein EJ, Choi EA, Hsu C, McElhany AL, Barakat O, Fisher W, Van Buren G. Nutritional and metabolic derangements in pancreatic cancer and pancreatic resection. Nutrients. 2017;9(3):243. doi: 10.3390/nu9030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouillat & Gigot (2001).Gouillat C, Gigot JF. Pancreatic surgical complications--the case for prophylaxis. Gut. 2001;49(Suppl 4):iv32–iv39. doi: 10.1136/gut.49.suppl_4.iv29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell, Lee & Mark (1996).Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in Medicine. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hersberger et al. (2020).Hersberger L, Bargetzi L, Bargetzi A, Tribolet P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, Kutz A, Kagi-Braun N, Hoess C, Pavlicek V, Schmid S, Bilz S, Sigrist S, Brandle M, Benz C, Henzen C, Nigg M, Thomann R, Brand C, Rutishauser J, Aujesky D, Rodondi N, Donze J, Stanga Z, Mueller B, Schuetz P. Nutritional risk screening (NRS 2002) is a strong and modifiable predictor risk score for short-term and long-term clinical outcomes: secondary analysis of a prospective randomised trial. Clinical Nutrition. 2020;39:2720–2729. doi: 10.1016/j.clnu.2019.11.041. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2023).Huang JC, Pan B, Jiang T, Zhang XX, Lyu SC, Lang R. Effect of the preoperative prognostic nutritional index on the long-term prognosis in patients with borderline resectable pancreatic cancer after pancreaticoduodenectomy. Frontiers in Oncology. 2023;13:1098459. doi: 10.3389/fonc.2023.1098459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim et al. (2018).Karim SAM, Abdulla KS, Abdulkarim QH, Rahim FH. The outcomes and complications of pancreaticoduodenectomy (Whipple procedure): cross sectional study. International Journal of Surgery. 2018;52:383–387. doi: 10.1016/j.ijsu.2018.01.041. [DOI] [PubMed] [Google Scholar]
- Koch et al. (2011).Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C, Banting S, Brooke-Smith M, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Nimura Y, Figueras J, DeMatteo RP, Buchler MW, Weitz J. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Li et al. (2021).Li D, Du C, Zhang J, Xing Z, Liu J. Nomogram and a predictive model for postoperative hemorrhage in preoperative patients of laparoscopic pancreaticoduodectomy. Scientific Reports. 2021;11(1):14822. doi: 10.1038/s41598-021-94387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2019).Li Y, Zhou F, Zhu DM, Zhang ZX, Yang J, Yao J, Wei YJ, Xu YL, Li DC, Zhou J. Novel risk scoring system for prediction of pancreatic fistula after pancreaticoduodenectomy. World Journal of Gastroenterology. 2019;25(21):2650–2664. doi: 10.3748/wjg.v25.i21.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby (2007).Libby P. Fat fuels the flame: triglyceride-rich lipoproteins and arterial inflammation. Circulation Research. 2007;100(3):299–301. doi: 10.1161/01.RES.0000259393.89870.58. [DOI] [PubMed] [Google Scholar]
- Ma et al. (2023).Ma M, Li G, Zhou B, Li K, Wu Z, Kong L, Liu M, Liu M, Zhang C, Yu H, Wang S, Huang Z, Zong K. Comprehensive analysis of the association between inflammation indexes and complications in patients undergoing pancreaticoduodenectomy. Frontiers in Immunology. 2023;14:1303283. doi: 10.3389/fimmu.2023.1303283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, Inaki & Tsuji (2021).Matsui R, Inaki N, Tsuji T. Impact of visceral adipose tissue on compliance of adjuvant chemotherapy and relapse-free survival after gastrectomy for gastric cancer: a propensity score matching analysis. Clinical Nutrition. 2021;40(5):2745–2753. doi: 10.1016/j.clnu.2021.04.019. [DOI] [PubMed] [Google Scholar]
- Matsui et al. (2022).Matsui R, Watanabe J, Banno M, Inaki N, Fukunaga T. Association of visceral adipose tissue with postoperative outcome in upper gastrointestinal cancer: a systematic review and meta-analysis. The American Journal of Clinical Nutrition. 2022;116(6):1540–1552. doi: 10.1093/ajcn/nqac273. [DOI] [PubMed] [Google Scholar]
- Menahem et al. (2015).Menahem B, Guittet L, Mulliri A, Alves A, Lubrano J. Pancreaticogastrostomy is superior to pancreaticojejunostomy for prevention of pancreatic fistula after pancreaticoduodenectomy: an updated meta-analysis of randomized controlled trials. Annals of Surgery. 2015;261(5):882–887. doi: 10.1097/SLA.0000000000000806. [DOI] [PubMed] [Google Scholar]
- Mintziras et al. (2020).Mintziras I, Maurer E, Kanngiesser V, Bartsch DK. C-reactive protein and drain amylase accurately predict clinically relevant pancreatic fistula after partial pancreaticoduodenectomy. International Journal of Surgery. 2020;76(2):53–58. doi: 10.1016/j.ijsu.2020.02.025. [DOI] [PubMed] [Google Scholar]
- Nakata et al. (2013).Nakata B, Ishikawa T, Amano R, Kimura K, Hirakawa K. Impact of preoperative diabetes mellitus on clinical outcome after pancreatectomy. International Journal of Surgery. 2013;11(9):757–761. doi: 10.1016/j.ijsu.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Onodera, Goseki & Kosaki (1984).Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–1005. [PubMed] [Google Scholar]
- Pacquelet et al. (2022).Pacquelet B, Morello R, Pelage JP, Eid Y, Lebreton G, Alves A, Fohlen A. Abdominal adipose tissue quantification and distribution with CT: prognostic value for surgical and oncological outcome in patients with rectal cancer. European Radiology. 2022;32(9):6258–6269. doi: 10.1007/s00330-022-08697-4. [DOI] [PubMed] [Google Scholar]
- Palumbo et al. (2021).Palumbo D, Tamburrino D, Partelli S, Gusmini S, Guazzarotti G, Cao R, Crippa S, Falconi M, De Cobelli F. Before sentinel bleeding: early prediction of postpancreatectomy hemorrhage (PPH) with a CT-based scoring system. European Radiology. 2021;31(9):6879–6888. doi: 10.1007/s00330-021-07788-y. [DOI] [PubMed] [Google Scholar]
- Pan, Ma & Dai (2023).Pan Y, Ma Y, Dai G. The prognostic value of the prognostic nutritional index in patients with advanced or metastatic gastric cancer treated with immunotherapy. Nutrients. 2023;15(19):4290. doi: 10.3390/nu15194290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancreatic Cancer Committee of Chinese Anticancer A (2021).Pancreatic Cancer Committee of Chinese Anticancer A Comprehensive guidelines for the diagnosis and treatment of pancreatic cancer (2020 version) Zhonghua Wai Ke Za Zhi [Chinese Journal of Surgery] 2021;59:81–100. doi: 10.3760/cma.j.cn112139-20201113-00794. [DOI] [PubMed] [Google Scholar]
- Qi et al. (2016).Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, Liu L, Meng Z, Wang P, Chen Z. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122(14):2158–2167. doi: 10.1002/cncr.30057. [DOI] [PubMed] [Google Scholar]
- R Core Team (2021).R Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2021. Version 4.0.5. [Google Scholar]
- Shen et al. (2021a).Shen J, Guo F, Sun Y, Zhao J, Hu J, Ke Z, Zhang Y, Jin X, Wu H. Predictive nomogram for postoperative pancreatic fistula following pancreaticoduodenectomy: a retrospective study. BMC Cancer. 2021a;21(1):550. doi: 10.1186/s12885-021-08201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen et al. (2021b).Shen Z, Xu Z, Wang W, Xu W, Zhou Y, Lu X, Deng X, Weng Y, Shen B. A novel nomogram for predicting the risk of major complications after pancreaticoduodenectomy in patients with obstructive jaundice. Clinica Chimica Acta. 2021b;517(2):162–170. doi: 10.1016/j.cca.2021.02.018. [DOI] [PubMed] [Google Scholar]
- Shi et al. (2023).Shi J, Liu T, Ge Y, Liu C, Zhang Q, Xie H, Ruan G, Lin S, Zheng X, Chen Y, Zhang H, Song M, Zhang X, Hu C, Li X, Yang M, Liu X, Deng L, Shi H. Cholesterol-modified prognostic nutritional index (CPNI) as an effective tool for assessing the nutrition status and predicting survival in patients with breast cancer. BMC Medicine. 2023;21:512. doi: 10.1186/s12916-023-03225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simental-Mendia, Rodriguez-Moran & Guerrero-Romero (2008).Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metabolic Syndrome and Related Disorders. 2008;6(4):299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- Steyerberg et al. (2001).Steyerberg EW, Harrell FE, Jr., Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. Journal of Clinical Epidemiology. 2001;54(8):774–781. doi: 10.1016/S0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- Study Group of Pancreatic Surgery in China Society of Surgery of Chinese Medical Association, Pancreatic Disease Committee of China Research Hospital Association, and Editorial Board of Chinese Journal of Surgery (2023).Study Group of Pancreatic Surgery in China Society of Surgery of Chinese Medical Association, Pancreatic Disease Committee of China Research Hospital Association, and Editorial Board of Chinese Journal of Surgery The guideline for prevention and treatment of common complications after pancreatic surgery (2022) Zhonghua Wai Ke Za Zhi [Chinese Journal of Surgery] 2023;61:1–18. doi: 10.3760/cma.j.cn112139-20230419-00173. [DOI] [PubMed] [Google Scholar]
- Tempero et al. (2021).Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. JNCCN. 2021;19:439–457. doi: 10.6004/jnccn.2021.0017. [DOI] [PubMed] [Google Scholar]
- Tumas et al. (2020).Tumas J, Tumiene B, Jurkeviciene J, Jasiunas E, Sileikis A. Nutritional and immune impairments and their effects on outcomes in early pancreatic cancer patients undergoing pancreatoduodenectomy. Clinical Nutrition. 2020;39(11):3385–3394. doi: 10.1016/j.clnu.2020.02.029. [DOI] [PubMed] [Google Scholar]
- van Dijk et al. (2017).van Dijk DP, Bakens MJ, Coolsen MM, Rensen SS, van Dam RM, Bours MJ, Weijenberg MP, Dejong CH, Olde Damink SW. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. Journal of Cachexia, Sarcopenia and Muscle. 2017;8(2):317–326. doi: 10.1002/jcsm.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2023).Wang R, Xu B, Sun M, Pang X, Wang X, Zhu J, Lian J, Lu H. Dynamic monitoring of serum CEA and CA19-9 predicts the prognosis of postoperative stage II colon cancer. European Journal of Surgical Oncology. 2023;49(12):107138. doi: 10.1016/j.ejso.2023.107138. [DOI] [PubMed] [Google Scholar]
- Watanabe et al. (2012).Watanabe F, Noda H, Kamiyama H, Kato T, Kakizawa N, Ichida K, Toyama N, Konishi F. Risk factors for intra-abdominal infection after pancreaticoduodenectomy-a retrospective analysis to evaluate the significance of preoperative biliary drainage and postoperative pancreatic fistula. Hepato-Gastroenterology. 2012;59:1270–1273. doi: 10.5754/hge12060. [DOI] [PubMed] [Google Scholar]
- Wente et al. (2007a).Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Buchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007a;142(5):761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Wente et al. (2007b).Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Buchler MW. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007b;142(1):20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2023).Yang Q, Xu H, Zhang H, Li Y, Chen S, He D, Yang G, Ban B, Zhang M, Liu F. Serum triglyceride glucose index is a valuable predictor for visceral obesity in patients with type 2 diabetes: a cross-sectional study. Cardiovascular Diabetology. 2023;22(1):98. doi: 10.1186/s12933-023-01834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng et al. (2021).Zeng Q, Li N, Pan XF, Chen L, Pan A. Clinical management and treatment of obesity in China. The Lancet Diabetes & Endocrinology. 2021;9(6):393–405. doi: 10.1016/S2213-8587(21)00047-4. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2022b).Zhang XP, Gao YX, Xu S, Zhao GD, Hu MG, Tan XL, Zhao ZM, Liu R. A novel online calculator to predict early recurrence and long-term survival of patients with resectable pancreatic ductal adenocarcinoma after pancreaticoduodenectomy: a multicenter study. International Journal of Surgery. 2022b;106:106891. doi: 10.1016/j.ijsu.2022.106891. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2022a).Zhang J, Qin SD, Li Y, Lu F, Gong WF, Zhong JH, Ma L, Zhao JF, Zhan GH, Li PZ, Song B, De Xiang B. Prognostic significance of combined alpha-fetoprotein and CA19-9 for hepatocellular carcinoma after hepatectomy. World Journal of Surgical Oncology. 2022a;20(1):346. doi: 10.1186/s12957-022-02806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2021).Zhang Z, Wan Z, Zhu Y, Zhang L, Zhang L, Wan H. Prevalence of malnutrition comparing NRS2002, MUST, and PG-SGA with the GLIM criteria in adults with cancer: a multi-center study. Nutrition. 2021;83:111072. doi: 10.1016/j.nut.2020.111072. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2023).Zhang AMY, Xia YH, Lin JSH, Chu KH, Wang WCK, Ruiter TJJ, Yang JCC, Chen N, Chhuor J, Patil S, Cen HH, Rideout EJ, Richard VR, Schaeffer DF, Zahedi RP, Borchers CH, Johnson JD, Kopp JL. Hyperinsulinemia acts via acinar insulin receptors to initiate pancreatic cancer by increasing digestive enzyme production and inflammation. Cell Metabolism. 2023;35(12):2119–2135 e2115. doi: 10.1016/j.cmet.2023.10.003. [DOI] [PubMed] [Google Scholar]
- Zhu et al. (2022).Zhu L, Li T, Yang Y, Tang N, Fu X, Qiu Y. Development and validation of a nomogram for predicting post-operative abdominal infection in patients undergoing pancreaticoduodenectomy. Clinica Chimica Acta. 2022;534(1):57–64. doi: 10.1016/j.cca.2022.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The lasso and multivariate logistic regression analysis results.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available in the Supplemental File.