Abstract
Ferroptosis is a novel form of programmed cell death characterized by iron accumulation, lipid peroxidation, and a decline in antioxidant capacity, all of which are regulated by gene expression. The onset of numerous diseases is closely associated with ferroptosis. Common diseases affect a large population, reduce the quality of life, and impose an increased burden on the healthcare system. The role of ferroptosis in common diseases, its therapeutic potential, and even its translation into clinical drug treatments are currently significant research topics worldwide. This study preliminarily explores the theoretical basis of ferroptosis, its mechanism and treatment prospect in common diseases including ischaemia-reperfusion injury, inflammatory bowel diseases, liver fibrosis, acute kidney injury, diabetic kidney disease, stroke, Alzheimer’s disease, cardiovascular disease, immune and cancer. This review provides a theoretical foundation for the further study and development of ferroptosis, as well as for the prevention and treatment of common diseases.
Keywords: Ferroptosis, Mechanism, Inducer, Inhibitor, Treatment
Introduction
Death is the ultimate fate of all cells. Scholars originally believed that cell death was unregulated until the concept of programmed cell death was raised in the 1960s (Hirschhorn & Stockwell, 2019). Unlike non-programmed cell death, which is caused by intense stress (such as severe heat shock, the use of detergents, pore-forming agents, or highly active alkylating agents), programmed cell death refers to a pattern of cell death that relies on specific molecular involvement and can be regulated by specific pharmacological and genetic means (Galluzzi et al., 2018). The discovery of programmed cell death patterns has aroused great interest among scholars. Subsequently, researchers found a series of programmed cell death such as autophagy, apoptosis and necroptosis. Unlike previous patterns of cell death, ferroptosis first proposed by Dixon et al. (2012) in 2012 is a novel cell death model. It is characterized by excess iron accumulation in the cytoplasm, lipid peroxidation of the cell membrane, mitochondrial morphology, and changes in genes related to ferroptosis. The reactive oxygen species (ROS) initiated ferroptosis include iron-mediated Fenton reactions, mitochondrial ROS, and the NOX protein family-mediated ROS. Polyunsaturated fatty acid (PUFA)-containing phospholipids are the main substrates of lipid peroxidation in ferroptosis, which is active by enzymes, such as ACSL4, LPCAT3, LOX. The decrease of anti-lipid peroxidation ability of System xc-, glutathione-GPX4 axis, etc., is also an important manifestation of ferroptosis (Liu, Kang & Tang, 2022). In 2018, the Committee on Cell Death Nomenclature (NCCD) added ferroptosis to the family of programmed cell death (Galluzzi et al., 2018). At present, ferroptosis has been found to exist in many common diseases including ischaemia-reperfusion injury, inflammatory bowel diseases, liver fibrosis, acute kidney injury, diabetic kidney disease, stroke, Alzheimer’s disease, cardiovascular disease, immune and cancer. This provides insights into the pathogenesis and treatment of common diseases. By reviewing the mechanism and treatment of ferroptosis in common diseases, we aim to summarize the achievements and shortcomings of current ferroptosis research, so as to better guide the direction of future research..
Survey methodology
We searched related literature by pubmed using the key words (ferroptosis) AND (intestine)/(ferroptosis) AND (liver)/(ferroptosis) AND (kidney)/(ferroptosis) AND (cerebrovascular disease)/(ferroptosis) AND (cardiovascular disease)/(ferroptosis) AND (tumor)/(ferroptosis) AND (neutrophils)/(ferroptosis) AND (B cell)/(ferroptosis) AND (T cell)/(ferroptosis) AND (macrophages). These terms were selected to better capture a view of ferroptosis across different organ systems and immune cell types. However, the articles included only published materials and excludednot research articles or reviews.
Ferroptosis and intestinal disease
Ischaemia-reperfusion (I/R) injury is defined as the paradoxical exacerbation of cellular dysfunction and death, following restoration of blood flow to ischemic tissues. Although, reestablishment of blood flow is essential to salvage ischemic tissues, reperfusion would paradoxically further injury the function and viability of organ (Sorby-Adams et al., 2024). Intestinal I/R injury is common in various clinical diseases, such as trauma, hemorrhagic shock, mesenteric ischemia, small intestine torsion and so on. Necrotizing enterocolitis is a common disease in newborns, and I/R injury is an important pathogenesis of this condition (Klinke et al., 2020; Knowles et al., 2021).
The damage of I/R to the intestinal tract is multifactorial, such as damage to the intestinal mucosal barrier, inflammation, and ectopia of the flora. The damage of intestinal mucosal barrier, inflammation and ectopic flora could start from the death of intestinal epithelial cells. I/R injury can lead to various forms of cell death, including necrosis, apoptosis, and autophagy (Li et al., 2023c; Xi et al., 2024). In recent years, researchers have found that ferroptosis can also be induced by I/R. Reactive oxygen species (ROS) are highly reactive forms of molecular oxygen, including the superoxide anion radical, hydrogen peroxide, singlet oxygen, and hydroxyl radical (Sahoo et al., 2022). During I/R, a large amount of ROS may be produced, and ROS is an important initiation of lipid peroxidation and ferroptosis. Malondialdehyde (MDA) is a product of lipid peroxidation and a marker of ferroptosis (Qiu et al., 2022). I/R leads to the increase of ROS and MDA, the decrease of Glutathione (GSH), which protected reactive molecules in living cells against oxidative damage. In addition, I/R damage can increase the iron content of intestinal tissues or cells, and iron accumulation is also necessary for ferroptosis (Dong et al., 2021). Hu et al. (2018), Ozkan et al. (2009), Stefanutti et al. (2008), Li et al. (2019b) showed that intestinal I/R could induce ferroptosis in the early stage. They also found that the degree of intestinal damage induced by I/R was improved by the use of the ferroptosis inhibitor liproxstatin-1 (Lip-1). Although it has been reported that lipid peroxidation and iron accumulation are important role of ferroptosis in intestinal I/R injury, the mechanism of ferroptosis still need to be further studied (Xu et al., 2021).
Inflammatory bowel diseases (IBDs), which include ulcerative colitis (UC) and Crohn’s disease (CD), are chronic inflammatory disease of the gastrointestinal tract most diagnosed in adolescence and young adulthood, with an increasing incidence in pediatric populations. However, because its pathogenesis is not clear, the current treatment for IBDs is not effective. Chen et al. (2020) found that ferrostain-1 (Fer-1) can alleviate the symptoms of dss induced colitis, and NRF2/HO-1 signaling pathway plays an important role in inhibiting the process of intestinal ferroptosis. In addition, ferroptosis suppressant drugs Lip-1 and deferoxamine (DFO) can also treat dss induced colitis, reduce disease symptoms and increase colon length (Xu et al., 2020, 2021). Kobayashi et al. (2019) have found that high iron intake increases the risk of UC, and the use of DFO can reduce ROS production and improve colonic symptoms of UC (Millar, Rampton & Blake, 2000; Minaiyan, Mostaghel & Mahzouni, 2012). Acyl-CoA synthetase long-chain family member 4 (ACSL4) is an important isozyme for polyunsaturated fatty acids (PUFAs) metabolism that dictates ferroptosis sensitivity (Cui et al., 2021). Glutathione peroxidase 4 (GPX4) is a selenoenzyme that uses GSH as a co-factor to regulate lipid peroxidation of cell membranes by eliminating phospholipid hydroperoxides (Xue et al., 2023). Gao, Sun & Kong (2023) found that vitamin D can improve UC by inhibiting ACSL4 and upregulating GPX4 expression in the ferroptosis pathway. Another exciting discovery is that Curculigoside, naturally occurring compounds can mitigate DSS-induced UC in mice by promoting GPX4 expression in a manner that depends on selenium (Wang et al., 2020).
Ferroptosis and liver disease
The liver is an important organ for iron storage and metabolism. Primary and secondary abnormalities in iron levels can affect the normal function of liver cells and liver diseases. Zhou et al. (2019) showed that in alcoholic liver disease, fat-specific lipin-1 overexpression accelerates iron accumulation, causes lipid peroxidation, reduces GSH, induces ferroptosis and thus promotes liver injury in mice. Concurrently, adipose lipin-1 overexpression induced defective adiponectin signaling pathways in ethanol-fed mice, including impaired adiponectin-SIRT1 signaling and disrupted adiponectin-FGF15 axis after ethanol administration in mice. Unlike the relatively mild liver damage caused by alcohol, acute liver failure is a clinical critical disease characterized by rapid loss of liver function with high mortality. The common inducing factors in clinic are virus, drug and alcohol. It had been reported that the nonsteroidal anti-inflammatory drug acetaminophen can induce ferroptosis and lead to liver damage, and ferroptosis inhibitors can improve liver cell death (Lorincz et al., 2015). Carlson et al. (2016) showed that GPX4 content and vitamin E administration could protect liver function. It is suggested that hepatocyte-specific Gpx4−/− pups were found to die by 48 h due to the lipid peroxidation-mediated cell death. The supplemented vitamin E form gestation period and newborns could protect membranes from oxidative damage, with the decreasing plasma levels of MDA. Ferroptosis inhibitor Lip-1 can improve I/R liver function, suggesting that ferroptosis plays an important role in ischemia-perfusion-related liver diseases (Chen et al., 2022b).
Liver fibrosis is a chronic progressive process, and the end-stage can lead to a series of liver dysfunction and cirrhosis symptoms. The persistent liver damage due to infection of chronic hepatitis B virus (HBV) and/or hepatitis C virus (HCV) is an important cause of liver fibrosis. Kanda et al. (2019) found that both HBV and HCV infections change hepcidin levels and other iron-related parameters, which are closely linked to ferroptosis. Liver fibrosis is characterized by excessive accumulation of extracellular matrix (ECM) proteins. ECM secreted by myofibroblasts proteins are mainly transformed from hepatic stellate cells (HSCs). Sui et al. (2018) showed that the knockout of HO-1 could inhibit the ferroptosis of liver trait cells, thus aggravating the liver fibrosis process. Kong, Liu & Cheng (2019) showed that artesunate alleviated carbon tetrachloride (CCl4)-induced liver fibrosis in mice by promoting the ferritinophagy-mediated ferroptosis pathway of HSCs. Sorafenib, well known for its anti-tumour effect, was also found to exhibit anti-fibrosis effect in liver by inhibiting proliferation and promoting apoptosis of HSCs. Ttransmembrane protein of solute carrier 7A11 (SLC7A11), combined with solute carrier 3A2 (SLC3A2), are the composition unit of system XC- for exchanging extracellular cysteine with intracellular glutamate (Ma et al., 2021). After being transported into the cell by system xc−, cystine is oxidizedto cysteine, which is then used for glutamate-cysteine ligase catalytic subunit (GCLC/GCL)-mediated GSH synthesis. Recently, it was observed that sorafenib attenuated liver injury and ECM accumulation in CCl4-induced fibrotic livers via HIF-1α/SLC7A11 (Yuan et al., 2022). Induction of ferroptosis by RSL3 treatment in HSCs results in decreased glutathione peroxidase 4, glutathione deficiency, reactive oxygen species generation, and lipid peroxidation. Moreover, RSL3 treatment upregulated the expression of plasminogen activator inhibitor-1, a representative fibrogenic marker of HSCs (Cho et al., 2022). Regulatory molecules of ferroptosis in HSCs, including p53, ELAV-like protein 1 (ELAVL1), and zinc finger protein 36 (ZFP36), have been reported as promising targets in preventing liver fibrosis (Chen et al., 2022b). Another exciting finding is that ginsenoside Rb1 (GRb1), a major active component extracted from Panax ginseng, inhibits HSC activation by promoting SLC7A11 expression (Lin et al., 2024). On the contrary, however, hepatic iron accumulation and ferroptosis deteriorate liver injury and fibrosis induced by acetaminophen, which can be regressed by ferrostatin-1 (Fer-1) (Wang et al., 2017). It seems that ferroptosis play a bidirectional role in the development of liver fibrosis. However, the role and effect of ferroptosis in the early stages of liver fibrosis is unclear. In the future, early prevention of liver fibrosis and even reversal of liver fibrosis may be an important research direction. Ferroportin (Fpn), also known as SLC40A1, is a the only known iron exporter and plays an essential role in iron homeostasis by releasing ferrous ion (Fe2+) from cells (Jiang et al., 2021a; Shen et al., 2023). In addition, it’s worth mentioning that the liver can secrete hepcidin to regulate the level of FPN protein in itself and other organs (Collins, Wessling-Resnick & Knutson, 2008). This suggested that the regulation of ferroptosis may be a multi-system or intercellular process, rather than confined to a single organ or cell.
Ferroptosis and kidney disease
Acute kidney injury (AKI) is commonly seen in clinical conditions such as bleeding, dehydration, postoperative hypoperfusion, sepsis and shock. Linkermann et al. (2014) found that the function of kidney injury induced by I/R could be relieved by administration of Fer-1. In renal I/R, the iron accumulation, through the Fenton reaction, may generate a large amount of ROS (also increased by mitochondrial dysfunction and NOX family activity) that can severely enhance intra-cellular oxidative stress and lipid peroxidation (Granata et al., 2022). Fer-1 can prevented AKI in lipid levels of ferroptosis, such as the decrease in PE and lyso-sulfatide species, without changing the gene expression of lipid metabolism enzymes (Martin-Saiz et al., 2022). It is also suggested that Fer-1 reduce iron content and some critical ferroptosis-related proteins in AKI, such as GPX4, SLC7A11, NRF2, and FTH1 (Zhao et al., 2023). Su et al. (2019) further demonstrated that ferroptosis inhibitors could also protect cells from hypoxic damage in isolated tubule cells. Ferroptosis suppressor protein 1 (FSP1) and GPX4 are important molecules in the body to play an antioxidant role in resisting ferroptosis. Ferroptosis suppressor protein 1 (FSP1), operating independently of the canonical system xc-/GPX4 pathway, is an NAD(P)H-ubiquinone oxidoreductase that reduces ubiquinone to ubiquinol (Lee & Roh, 2023). It has been shown that loss of function of GPX4 or FSP1 proteins can increase susceptibility of mice to ferroptosis during acute kidney injury (Tonnus et al., 2021). It is worth mentioning that the damage of renal cell morphology and function is rapid in acute renal injury. In the case of ferroptosis cells, the researchers found that ferroptosis cells could impair the surrounding cells in a lipid peroxide- and iron-dependent manner (Kim et al., 2016; Linkermann et al., 2014). Riegman et al. (2020) showed that the rupture of ferroptostic cells was due to plasma membrane pores, similar to cell lysis in pyroptosis and necroptosis. Ferroptosis inducers can cause transmission of intercellular death, which does not directly depend on GPX4 inhibition. Changes in ferroptosis signaling precede cell rupture, leading to cell swelling and intercellular transmission in a lipid peroxide and iron-dependent manner (Riegman et al., 2020). Excitingly, it has shown that acute kidney injury can be mitigated by targeted delivery of vitamin E using nanoparticles, which is related to its strong ability to inhibit ROS production and ferroptosis (Zhang et al., 2024a).
Diabetic kidney disease (DKD) is a common chronic complication of diabetes and has become the leading cause of end-stage kidney disease (Ma et al., 2023). Diabetic kidney disease has a high global disease burden and the single strongest predictor of mortality in patients with diabetes (Reidy et al., 2014). However, existing treatments are less effective and there is still a risk of worsening (Tuttle et al., 2022). Current therapeutic regimens, such as angiotensin converting enzyme inhibitors (ACEIs) and angiotensin II receptor antagonists (ARBs), can only alleviate diabetic nephropathy to a certain extent. Based on the huge population of diabetes and may result in heavy financial burden, increase the pathogenesis and treatment of diabetic nephropathy the urgent demand (Wang et al., 2023a). Previous studies have shown that iron overload is directly related to proteinuria and tubule injury in DKD patients and STZ-induced type 1 diabetes rat models (Dominguez, Liu & Kelly, 2015; Howard, Buddington & Alfrey, 1991). Matsumoto et al. (2013) found that limiting iron intake in diabetic mice could delay the development of diabetic nephropathy, indicating that iron overload could aggravate kidney damage in DKD. In addition to iron overload, lipid peroxidation also plays an important role in DKD. In China, Wang et al. (2022b) found that GPX4 was mainly expressed in renal tubule interstitium, especially in tubular epithelial cells of patients with diabetic nephropathy. GPX4 decreased significantly in diabetic nephropathy patients, which was strongly associated with worsening renal function in type 2 diabetic nephropathy, including urinary protein, Scr, eGFR, and sclerosed glomerular percentage in renal specimens. In particular, GPX4 levels in the renal tubulointerstitium have been shown to be an independent predictor of renal prognosis (Wang et al., 2022b). In addition to the decrease of antioxidant capacity, ROS levels in the kidney will also be significantly increased in diabetic nephropathy due to various NADPH oxidase and mitochondrial dysfunction (Jha et al., 2016), thus aggravating the process of lipid peroxidation. In addition to ferroptosis inhibitors like Fer-1 and DFO, many traditional Chinese herb components have a beneficial effect in suppressing ferroptosis in DKD (Wang et al., 2023a). Schisandrin A, isolated from the fruits of Schisandra chinensis, protect against kidney injury by reducing ROS production, alleviating mitochondrial damage through NRF2/HO-1/GPX4 pathway in HFD/STZ-induced diabetic mice (Wang et al., 2022a). Germacrone, the main bioactive component of Rhizoma Curcuma, improve ferroptosis induced kidney injury in mice by the ROS/GSH/GPX4 axis (Jin et al., 2022).
Ferroptosis and cerebrovascular disease
Stroke is an acute cerebrovascular syndrome with a high incidence, disability and recurrence rate, especially in elderly patients. Studies have shown that ferroptosis process exists in both ischemic stroke and hemorrhagic stroke (Cui et al., 2021; Karuppagounder et al., 2018). The severity of ischemic stroke and hemorrhagic stroke can be improved by the use of ferroptosis inhibitors DFO and Fer-1, respectively (Hanson et al., 2009; Zhang et al., 2018). Ischemic stroke contains ischemia and reperfusion injury (IRI). In ischemic stroke, iron accumulation has emerged after ischemia through inhibiting the expression of tau (Tuo et al., 2017). The excess iron would also flow into the brain parenchyma through the interrupted blood brain barrier, boosting the generation of ROS via Fenton reaction, ultimately inducing ferroptosis (Zhang et al., 2021). Hemorrhagic stroke was defined as a rupture of cerebral vessels that causes blood to flow into subarachnoid space, brain parenchyma, or ventricular system. The blood accumulates and compresses the surrounding brain tissue, causing tissue damage and neuronal death. Hemoglobin (Hb) released from lysed erythrocytes can be taken into microglia and metabolized into ferrous/ferric iron, which induces lethal reactive oxygen species (ROS) and lipid membranes damage (Li et al., 2017). In addition, the ferroptosis, including increased iron, the increased iron, MDA and shrunken mitochondria can be found in the early stage (3h) of the hemorrhagic stroke mouse (Chen et al., 2019a). Inhibition of ferroptosis exerts a long-term cerebroprotective effects in the hemorrhagic stroke (Chen et al., 2019a). Although hemorrhagic stroke and ischemic stroke have different pathologic processes, there is still crossover in the pathogenesis of ferroptosis. The inactivation of GPX4 plays an important role in both hemorrhagic and ischemic diseases, and supplementation of GSH and selenoprotein plays an important role in the activation and treatment of GPX4 (Wei, 2024). The existence of blood-brain barrier is an important factor restricting the effect of stroke treatment. How to improve the efficiency of drugs entering the blood-brain barrier is the key to improve the therapeutic effect. Excitingly, the researchers found that the assembly of recombinant human heavy chain ferritin (rHF) through nanomaterials can improve the efficiency of drug entry into the blood-brain barrier and reduce infarct size by anti-ROS and ferroptosis effect (Qi et al., 2024). In hemorrhagic stroke, Li et al. (2024) found that Selenium nanoparticles can improve the cognitive deficits and ameliorate the damage of hippocampal by promoting NRF2/GPX4 axis expression neuron.
In addition to Stork, acute cerebrovascular syndrome, cerebrovascular chronic diseases and the resulting damage are also of concern. Alzheimer’s disease, which is also common in the elderly, is a progressive neurodegenerative disease of development, characterized by dementia symptoms, including memory impairment and aphasia. Most patients with AD have Aβ amyloid angiopathy, degenerative capillaries changes and ischaemic parenchymal abnormalities. It is suggested that hypoperfusion exists in the early and late stages of Alzheimer’s disease (Love & Miners, 2016). It is also suggested that ferroptosis exits in Alzheimer’s disease (Lane et al., 2021). Neurons in forebrain regions (cerebral cortex and hippocampus) are severely afflicted in AD patients, which might be vulnerable to ferroptosis. Hambright et al. (2017) generated Gpx4BIKO mouse, a mouse model with conditional deletion in forebrain neurons of Gpx4. It is suggested that Gpx4BIKO mice have significant imperfection in spatial learning and memory function vs. control mice as estimated by the Morris water maze task. Further examinations revealed that Gpx4BIKO mice exhibited hippocampal neurodegeneration (Hambright et al., 2017). Moreover, Hambright et al. (2017) also found Lip-1 can improve Gpx4BIKO mice ameliorated neurodegeneration, while the vitamin E had an expedited rate of hippocampal neurodegeneration and behavior dysfunction. In addition, studies have also found that the hippocampus of Alzheimer’s patients is severely damaged and its iron content is significantly elevated, which increases the risk of Fenton reaction in brain tissue (Lane, Ayton & Bush, 2018; Raven et al., 2013). In recent studies, new therapeutic agents, such as thonningianin A and selenium nanosphere, were also found to improve cognitive function in mice with Alzheimer’s disease by promoting GPX4 expression (Wang et al., 2023b; Yong et al., 2024).
Ferroptosis and cardiovascular disease
Myocardial ischemia is a common disease of the cardiac system. Studies have shown that ferroptosis plays an important role in the onset and progression of myocardial ischemia. Ischemia can lead to the increase of ROS and lipid peroxidation in myocardium (Hausenloy & Yellon, 2013). Fang et al. (2019) showed that ischemia reperfusion resulted in the increase of non-heme iron and Fth in the heart, and intervention in the process of ferroptosis could improve cardiac function. Dabkowski, Williamson & Hollander (2008) showed that decreased expression of anti-lipid peroxidation protein GPX4 could improve the function of cardiac contraction after ischemia/reperfusion injury. Myocardial infarction is a serious complication of myocardial ischemia. Park et al. (2019) showed that ferroptosis process existed in myocardial cells during myocardial infarction. The protein level of GPX4 was significantly down-regulated in the early and middle stages of myocardial infarction by proteomic analysis of the heart tissue in mice that were lapped to simulate myocardial infarction. Toll-like receptor 4(TLR4), a transmembrane receptor, plays a central role in the innate immune response (Kim et al., 2023). Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 (NOX4) is a constitutive enzyme and primarily contributes to the growth in ROS, and exerts an important role in the regulation of oxidative stress and downstream signals (Li et al., 2023b). In the process of heart failure, when ferroptosis occurs, TLR4 and NOX4 are up-regulated, and inhibition of TLR4 or NOX4 can reduce cardiomyocyte death through ferroptosis and autophagy (Chen et al., 2019b). It is worth mentioning that coenzyme Q10(COQ10), as a clinical drug commonly used to improve myocardial ischemia, has also been found to improve the lipid peroxidation process in ferroptosis (Awad, Sayed & Banach, 2022; Li et al., 2023a; Liang, Ping & Ge, 2017). As a clinical drug, the rationale of the use of CoQ10 in cardiovascular diseases is that the loss of contractile function due to an energy depletion status in the mitochondria and reduced levels of NO for vasodilatation. Clinical evidence shows that CoQ10 supplementation for prolonged periods is safe, well-tolerated. Moreover, CoQ10 supplementation reduces mortality in cardiovascular causes and improves clinical outcome in from and patients undergoing coronary artery bypass graft surgery, including decreased vascular stiffness and hypertension (Rabanal-Ruiz, Llanos-Gonzalez & Alcain, 2021). COQ10, existing in oxidized form (ubiquinone) and reduced form (ubiquinol), is a vitamin-like endogenously produced isoprenyl benzoquinone compound. It is an essential part of the mitochondrial respiratory chain and works as a powerful antioxidant by neutralizing free radicals in various membrane structures (Kuang et al., 2020). FSP1-CoQ10-NAD(P)H pathway operates independently from the GPX4 pathway, functioning to scavenge lipid radicals by reducing ubiquinone to ubiquinol (Doll et al., 2019).
Cardiomyopathy is a type of abnormal mechanical and electrical activity of the heart caused by different causes, which is manifested by improper hypertrophy or dilation of the ventricles. The most common cardiomyopathy were hypertrophic cardiomyopathy and dilated cardiomyopathy. Fang et al. (2019) found that the lipid peroxidation pathway in mitochondria mediated ferroptosis plays an important role in cardiomyopathy. The use of mitochondria-targeted antioxidant MitoTEMPO improved doxorubicin (DOX) -induced cardiomyopathy. In addition to heart disease, there is also an ferroptosis process in the process of blood vessel damage. Some cardiovascular risk factors, such as smoking and PM2.5, can damage smooth muscle cells and endothelial cells in blood vessels by inducing ferroptosis (Sampilvanjil et al., 2020).
Ferroptosis and immune disease
Ferroptosis broadly regulates immune system function. On the one hand, ferroptosis can affect the function and number of immune cells. On the other hand, ferroptosis cells and their products can be recognized by immune cells, and thus induce an immune response. Macrophages are an important part of innate immunity. Under normal physiological effects, macrophages are an important mechanism for the clearance of senescent red blood cells in the body. Youssef et al. (2018) showed that ferroptosis inhibitors could reduce the phagocytosis of macrophages on red blood cells in the spleen. Toll-like Receptor 2 (TLR2) is a protein coding gene that belongs to the Toll-like receptor family and plays a role in pathogen recognition and innate immunity. Luo et al. (2021) showed that ferroptostic cells could produce oxidized phosphatidylethanolamine, which was recognized and cleared by TLR2 on macrophages. The polarization function of macrophages is also regulated to some extent by ferroptosis. Neutrophils are another important part of innate immunity. Recruitment of neutrophils in the early stages of inflammation can inhibit infection and clear pathogens. It is shown that ferroptostic cells in heart transplantation can initiate neutrophils recruitment by activating TLR4/Trif signals, thus leading to tissue damage, and further use of ferroptosis inhibitor Fer-1 can inhibit the expansion of inflammation (Li et al., 2019a). T cells and B cells are important parts of adaptive immunity. Muri et al. (2019) showed that B1 cells and marginal B cells but not follicular B2 cells needed GXP4 to inhibit lipid peroxidation and ferroptosis, and knocking out GPX4 in B1 and marginal B cells caused ferroptosis and IgM antibody response. Similarly, loss of GPX4 expression plays an important role in the induction of ferroptosis in T cells. It is shown that the occurrence of ferroptosis can lead to the failure of CD4+ or CD8+ T cells to expand in the case of acute infection, but the ability to expand can be restored by administering the anti-lipid peroxidation drug vitamin E (Matsushita et al., 2015). Infection is an important factor leading to immune cell activation and tissue damage. It is reported that infection can induce ferroptosis. The main ways of infection induced ferroptosis include: (1) transcriptional regulation of the ferroptosis-related proteins, mainly GPX4; (2) control of lipid ROS generations through lipid, iron, and mitochondria metabolism (Gao et al., 2023). Dar et al. (2018) found that Gram-negative Pseudomonas aeruginosa could induce lipid peroxidation and ferroptosis in human lung epithelial cells. Cecal ligation and puncture (CLP) and Lipopolysaccharide (LPS) are methods to make sepsis mouse models and acute tissue injury (Xu et al., 2018). Myeloid cell-specific Gpx4-deficient mice are sensitive to LPS or CLP induced septic death with increasing lipid peroxidation (Kang et al., 2018). Chu et al. (2023) found neutrophils in sepsis-associated acute lung injury accumulation, which is strongly associated with ferroptosis and decrease GPX4. Ferroptosis inhibitors (e.g., Fer-1) prevent CLP or LPS-induced tissue damage (Li et al., 2020c; Wei et al., 2020). It is worth mentioning that in the non-infectious inflammatory response, the increase of TLR4 level caused by heart failure is closely related to ferroptosis, and inhibition of TLR4 or NOX4 can reduce myocardial cell death through ferroptosis and autophagy (Chen et al., 2019b). Although the previously reported results support the important role of ferroptosis in immune cells and infection, there are still many questions to be solved about the process and mechanism of ferroptosis in immune cells caused by infection.
Ferroptosis and tumor
In many diseases, the existence of ferroptosis aggravates the disease process, and it is necessary to intervene in the process of ferroptosis to inhibit its occurrence. In tumor-related diseases, the effect of ferroptosis on tumor cells is complex and even two-sided. Loss of GPX4 in tumor cells leads to an increase in lipid peroxidation products such as 5-HETE, 11-HETE, and 15-HETE (Friedmann Angeli et al., 2014). These lipid peroxidation substances can assist immune cells to detect tumor cells and regulate the activity of immune cells (Li & Li, 2020). On the other hand, immune cells can also regulate the ferroptosis of tumor cells. It is reported that CD8+T cells can release the cytokine interferon gamma (IFNγ), which in turn down-regulates the expression of SLC3A2 and SLC7A11. The absence of SLC3A2 and SLC7A11 led to the impairing the uptake of cystine and decrease of GSH synthesis in tumor cells, which in turn reduced the anti-lipid peroxidation effect of GPX4, thus inducing ferroptosis in tumor cells. In fact, clinical studies have shown that the decreased expression of SLC3A2 and the increased IFN-γ in patients treated with anti-tumor drugs can help improve the clinical therapeutic effect (Wang et al., 2019). At present, many studies have found that classical anti-tumor drugs can affect the target of ferroptosis pathway and played the role of anti-tumor therapy. Sulfasalazine and sorafenib can affect the content of GSH in tumor cells by inhibiting system xc-, but cannot well inhibit lipid peroxidation during ferroptosis (Dixon et al., 2014; Louandre et al., 2015; Sleire et al., 2015). Lapatinib can induce ferroptosis by inhibiting iron transport in breast cancer cells, thus playing a therapeutic role. In addition to the therapeutic effect, ferroptosis is also deeply involved in the occurrence, development, metastasis and invasion of tumors. Knockout of ACSL4 reduced 17β-estradiol induced migration, proliferation, and invasion of cancer cells, while increasing ACSL4 enhanced tumor growth and proliferation (Belkaid, Ouellette & Surette, 2017; Wu et al., 2015, 2013). In mechanism, the silence of ACSL4 decrease the uptake of in arachidonic acid, eicosapentaenoic acid and the activation of Akt/GSK3β/E-cadherin pathway, which is the requisites for migration and invasion capacities. It is exciting to see that nanotechnology and traditional Chinese medicine extract ingredients, such as Formosanin C, can effectively promote iron death and thus improve the treatment of breast cancer (Chen et al., 2022a; Mu et al., 2024; Shi et al., 2024; Zhang et al., 2024b). In addition, studies have shown that GPX4 expression can influence tumor cell invasion and metastasis. Overexpression of GPX4 in L929 cells can reduce the metastatic ability of tumor cells, whereas increasing the level of GXP4 in B16BL6 cells can increase the ability of lung metastasis to colonize (Heirman et al., 2006). The increase of NRF2 inhibits the increase of vascular cell adhesion molecule-1 (VCAM-1), thereby reducing vascular inhibition and tumor migration (Banning & Brigelius-Flohe, 2005). Iron chelating agent and promotion of SLC40A1 expression can reduce the migration of tumor cells (Guo et al., 2015; Wang et al., 2014).
Induction and treatment of ferroptosis
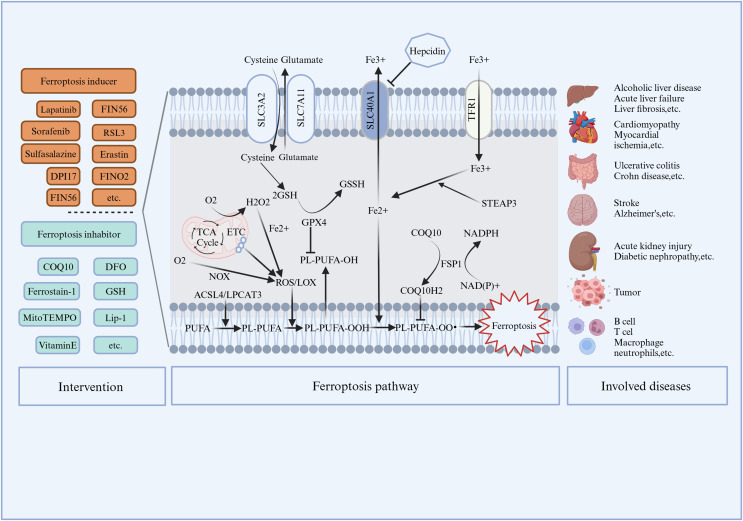
In 2003, Dolma et al. (2003) discovered a new compound, erastin, which has a selective lethal effect on RAS expressing cancer cells. Subsequently, Yang & Stockwell (2008) and Yagoda et al. (2007) showed that this cell death pattern could be inhibited by iron chelating agent (DFO), while RAS-selective lethal 3 (RSL3), could cause this cell death pattern. Various substances that induce ferroptosis mainly include four categories (Li et al., 2020a): (1) Erastin; (2) RSL3 and DPI7; (3) FIN56; (4) FINO2. Erastin is induced mainly by action on GPX4. Erastin can also directly inhibit System Xc-, thus inhibiting cystine import and depriving cells of cysteine, an essential composition of GSH. The lack of GSH inactivate GPX4, leading to phospholipid hydroperoxides (PLOOHs) accumulate, causing damage of plasma membrane, leading to ferroptosis cells (Jiang, Stockwell & Conrad, 2021b). Recent studies have also found that Erastin activation of ferroptosis can increase the level of lysosomal associated membrane protein 2a, thereby promoting chaperone protein-mediated autophagy and further promoting GPX4 degradation (Wu et al., 2019). Erastin can also induce mitochondrial dysfunction through the volt-dependent anion channels pathway, thus producing the ROS, Fenton reaction, and lipid peroxidation, leading to ferroptosis cells (Li et al., 2020b). RSL3 and DPI7 directly inhibit GPX4 activity and induce ferroptosis. FIN56, which has two ways of inducing ferroptosis. On the one hand, FIN56 promotes the degradation of GPX4. Another way of acting, FIN56 binds to squalene synthetase, leading to depletion of endogenous antioxidant COQ10. This process enhances the sensitivity of cells to FIN56-induced iron poisoning (Liang et al., 2019). FINO2, an organic peroxide that shares many characteristics with artemisinin, causes iron poisoning due to the combined action of direct oxidation of unstable iron and inactivation of GPX4 (Liang et al., 2019). For the treatment of ferroptosis, in addition to the use of iron chelating agents to target the accumulation of iron in cells, there are many inhibitors that target the lipid process in the final stage of ferroptosis, such as Fer-1, Lip-1, and vitamin E.
Issues and prospects
The role of ferroptosis causing pathological cell death associated with I/R injury, IBDs, liver fibrosis, AKI, DKD, stroke, degenerative diseases, cardiovascular disease, immune and cancer is increasingly being recognized. However, the specific role of ferroptosis in disease and its mechanisms are still poorly understood. In tumor, ferroptosis can promote tumor growth and may also promote tumor formation, depending on the type, stage and microenvironment of the tumor (Kuang et al., 2020). On the other hand, we noted that during liver fibrosis, the ferroptosis can both promote liver fibrosis and inhibit liver fibrosis, which is related to the specific cell type (Chen et al., 2022b). Therefore, focusing on the effects of ferroptosis at different stages of the disease process and on specific cell functions is a very important to provide us with more precise prevention and treatment strategies. Moreover, while GPX4, FSP1, and SLC7A11 are known to play an important role in ferroptosis, the specific mechanism of regulating the change of these molecular at the gene level is still not clear. In addition to the genetic level, the clinical risk factors that induce ferroptosis still need to be further explored. Although iron overload is thought to be the key factor to trigger ferroptosis, whether other metal ions are involved in ferroptosis remains unclear, as the latest researchhas revealed that copper and calcium may be also associated with the initiation of ferroptosis (Maher et al., 2018; Xue et al., 2023).
Another unresolved important issue is that whether there is a synergistic or antagonistic effect of ferroptosis with other modes of cell death. It has been shown that ferroptosis, autophagy,and apoptosis will occur together in neuronal death after ICH via ultrastructural analysis (Zhang et al., 2021). In the ATG5-ATG7-NCOA4 pathway, the process of ferritin-associated autophagy that is mediated by NCOA4 can increase the content of unstable iron in cells, thus promoting ferroptosis (Li et al., 2020a). These results suggest that ferroptosis can coexist with other forms of cell death, and that other forms of cell death have an effect on ferroptosis.
The discovery and proposal of ferroptosis greatly promoted the understanding and research of programmed cell death. Firstly, description of ferroptosis as a unique cell death modality brings together into a cohesive network previously disparate elements of cell metabolism, involving iron, selenium, amino acids, lipids and redox chemistry (Jiang, Stockwell & Conrad, 2021b). In addition, since the introduction and identification in 2012, there has been a rapid increase in the number and results of studies on ferroptosis. In addition to the classical system xc−(SLC7A11/SLC3A2) and GPX4/GSH pathways, we have successively discovered FSP1/COQ10 and GCH1/BH4 pathways. In terms of drug therapy, unlike Fer-1, Lip-1 used to treat drugs in animals, some metabolites in the new target, such as GSH and COQ10, have been safely applied clinically, which provides a theoretical basis for the new application of old drugs. A successful example is that researchers found that Bitopertin originally developed to treat schizophrenia successfully treat osteoporosis by inhibiting the ferroptosis pathway and with fewer side effects (Dong et al., 2024). We summarized preliminarily summarized ferroptosis and disease (Table 1). Further, for more accurate treatment, the researchers proposed to design developing nanoparticles to target ferroptosis from multiple levels (Sun et al., 2023). Thus, as our increasing understanding of ferroptosis regulatory pathways such as iron metabolism, lipid metabolism and new drug targets, ferroptosis may provide new opportunities for the treatment of many currently incurable diseases.
Table 1. Therapeutic targets of ferroptosis in common diseases.
| Disease | Model | Key mechanism | Drug | Reference |
|---|---|---|---|---|
| Ulcerative colitis | In vivo | Downregulate NRF2/HO-1, GPX4, FTH1; Upregulate ACSL4; |
Inhibitor: Ferrostain-1, Liproxstatin-1, Deferoxamine |
Chen et al. (2020) |
| In vivo/In vitro | Downregulate NF-κBp65 |
Inhibitor: Ferrostain-1 |
Xu et al. (2020) | |
| In vivo/In vitro | Downregulate GPX4 | Inhibitor: Curculigoside Ferrostain-1 |
Wang et al. (2020) | |
| In vivo/In vitro | Upregulate ACSL4; Downregulate GPX4 |
Inhibitor: Vitamin D |
Gao, Sun & Kong (2023) | |
| In vivo/In vitro | Downregulate GPX4 | Inhibitor: Curculigoside |
Wang et al. (2020) | |
| Acute liver failure | In vivo | Downregulate GPX4 | Inhibitor: Vitamin E |
Xue et al. (2023) |
| I/R Liver | In vivo | Upregulate ACSL4 | Inhibitor: Liproxstatin-1 |
Rokop et al. (2024) |
| Liver fibrosis | In vivo | Downregulate HO-1 | — | Sui et al. (2018) |
| In vivo/In vitro | Downregulate HIF-1α/SLC7A11 |
Inducer: Sorafenib |
Yuan et al. (2022) | |
| In vitro | Downregulate GPX4 | Inducer: RSL3, Erastin | Cho et al. (2022) | |
| In vivo | Downregulate SLC7A11 | Inhibitor: Ferrostatin-1; Inducers: iron |
Wang et al. (2017) | |
| In vivo/In vitro | Downregulate SLC7A11 | Inhibitor: Ginsenoside Rb1 |
Lin et al. (2024) | |
| Acute kidney injury | In vivo | Imbalance of lipid composition and gene expression of lipid metabolism enzymes | Inhibitor: Ferrostatin-1 |
Martin-Saiz et al. (2022) |
| In vivo/In vitro | Downregulate GPX4, SLC7A11, NRF2, FTH1 | Inhibitor: Ferrostatin-1 |
Zhao et al. (2023) | |
| In vivo/In vitro | Downregulate GPX4, FSP1 | Inhibitor: Ferrostatin-1 Nec-1f |
Tonnus et al. (2021) | |
| In vivo/In vitro | Upregulate ROS | Inhibitor: Vitamin E nanoparticles |
Zhang et al. (2024a) | |
| Diabetic kidney disease | In vivo | Downregulate NRF2/HO-1/GPX4 | Inhibitor: Schisandrin A |
Wang et al. (2022a) |
| In vivo | Downregulate GPX4 | Inhibitor: Germacrone |
Jin et al. (2022) | |
| In vivo/In vitro | Downregulate NRF2,SLC7A11, GPX4 | Inhibitor: Ferroastatin-1 |
Kim et al. (2021) | |
| In vitro | Downregulate GPX4,FTH1, SLC7A11 | Inhibitor: Deferoxamine |
Huang et al. (2022) | |
| Ischemic stroke | In vivo | Iron accumulation | Inhibitor: Deferoxamine |
Hanson et al. (2009) |
| In vivo | Downregulate Tau, Iron accumulation |
Inhibitor: Liproxstatin-1, Ferrostatin-1 |
Tuo et al. (2017) | |
| In vivo/In vitro | Upregulate ROS | Inhibitor: Recombinant human heavy chain ferritin nanoparticles |
Qi et al. (2024) | |
| Hemorrhagic stroke | In vivo | Downregulate GPX4 | Inhibitor: Ferrostatin-1 |
Zhang et al. (2018) |
| In vivo/In vitro | Downregulate NRF2/GPX4 | Inhibitor: Selenium nanoparticles |
Li et al. (2024) | |
| In vivo | Downregulate cyclooxygenase-2 |
Inhibitor: Ferrostatin-1 |
Li et al. (2017) | |
| Ischemic stroke and Hemorrhagic stroke | In vitro | Downregulate GPX4 | Inhibitor: GSH, Selenoprotein |
Wei (2024) |
| Alzheimer’s disease | In vivo | Downregulate GPX4 | Inhibitor: Liproxstatin-1 Inducer: Vitamin E |
Hambright et al. (2017) |
| In vivo/In vitro | Downregulate GPX4 | Inhibitor: selenium nanosphere |
Wang et al. (2023b) | |
| In vivo/In vitro | Downregulate GPX4 | Inhibitor: Thonningianin A |
Yong et al. (2024) | |
| Myocardial infarction | In vivo/In vitro | Downregulate GPX4 | Inhibitor: Liproxstatin-1 Ferrostatin-1 Inducer: RSL3 |
Park et al. (2019) |
| Myocardial ischemia | In vivo/In vitro | Upregulate ROS/AMPK/mTOR |
Inhibitor: Idebenone (CoQ10 analog) |
Li et al. (2023a) |
| I/R heart | In vitro | Downregulate antioxidative stress | Inhibitor: COQ10 |
Awad, Sayed & Banach (2022), Liang, Ping & Ge (2017) |
| In vivo | Downregulate GPX4 | — | Dabkowski, Williamson & Hollander (2008) | |
| Heart failure | In vivo/In vitro | Downregulate SLC7A11 | Inhibitor: Liproxstatin-1 Inducers: Erastin |
Ma et al. (2020) |
| In vivo | Upregulate TLR4, NOX4; Downregulate GPX4, FTH1 |
— | Chen et al. (2019b) | |
| Cardiomyopathy | In vivo | Upregulate NRF2, HO-1 |
Inhibitors: MitoTEMPO, Zinc protoporphyrin IX, Ferrostatin-1, Deferoxamine |
Fang et al. (2019) |
| Aortic aneurysm and dissection | In vivo/In vitro | Downregulate GPX4; Upregulate Ptgs2 |
Inducer: Cigarette, RSL3 Inhibitor: Liproxstatin-1, Ferrostatin-1, Deferoxamine |
Sampilvanjil et al. (2020) |
| Sepsis | In vitro | Downregulate GPX4 | Inducer: RSL3 Inhibitor: Irisin |
Wei et al. (2020) |
| In vivo/In vitro | Upregulate NCOA4, Ferritinophagy |
Inhibitor: Ferrostatin-1 | Li et al. (2020c) | |
| Fibrosarcoma | In vitro | Downregulate SLC3A2, SLC7A11 |
Inducer: RSL3, Erastin Inhibitor: Nivolumab, Liproxstatin-1 |
Wang et al. (2019) |
| Fibrosarcoma, Prostate cancer | In vitro | Downregulate SLC7A11 |
Inducer: Sorafenib, Erastin |
Dixon et al. (2014) |
| Glioblastomas | In vivo/In vitro | Downregulate SLC7A11 |
Inducer: Sulfasalazine |
Sleire et al. (2015) |
| Breast cancer | In vitro | Upregulate FPN |
Inducer: Lapatinib |
Ma et al. (2016) |
| In vitro | Downregulate ACSL4 |
Inducer: 17β-estradiol |
Belkaid, Ouellette & Surette (2017) | |
| In vitro | Upregulate GSH/GPX4 |
Inducer: GSH-depleted photodynamic nanoadjuvant |
Shi et al. (2024) | |
| In vivo/In vitro | Downregulate ROS |
Inducer: Mitochondria-targeted nanoreactor (Ce3+, EGCG and IR780) | Mu et al. (2024) | |
| In vitro | Upregulate SlC7A11, FPN; Downregulate Ferritinophagy |
Inducer: Formosanin C |
Chen et al. (2022a) |
Conclusion
In summary, ferroptosis, as a new programmed cell death mode, plays an important role in the occurrence and development of a wide range of diseases (e.g., Ischaemia-Reperfusion injury, liver fibrosis, neurodegenerative diseases, cancer, etc.) (Fig. 1). The targets of iron metabolism, lipid peroxidation and key pathways during ferroptosis provide new ideas for disease occurrence and treatment. To explore the role and specific mechanism of ferroptosis pathway in various stages of disease has revolutionize clinical significance to change the treatment strategy and curative effect of existing diseases. Further research on ferroptosis drugs and their clinical transformation may be important research directions in the future.
Figure 1. Intervention, mechanism and related diseases of ferroptosis.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Pengjian Zou performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Qiuming He performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Huimin Xia analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Wei Zhong conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
This is a literature review.
References
- Awad, Sayed & Banach (2022).Awad K, Sayed A, Banach M. Coenzyme Q(10) reduces infarct size in animal models of myocardial ischemia-reperfusion injury: a meta-analysis and summary of underlying mechanisms. Frontiers in Cardiovascular Medicine. 2022;9:857364. doi: 10.3389/fcvm.2022.857364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banning & Brigelius-Flohe (2005).Banning A, Brigelius-Flohe R. NF-kappaB, Nrf2, and HO-1 interplay in redox-regulated VCAM-1 expression. Antioxidants & Redox Signaling. 2005;7(7–8):889–899. doi: 10.1089/ars.2005.7.889. [DOI] [PubMed] [Google Scholar]
- Belkaid, Ouellette & Surette (2017).Belkaid A, Ouellette RJ, Surette ME. 17beta-estradiol-induced ACSL4 protein expression promotes an invasive phenotype in estrogen receptor positive mammary carcinoma cells. Carcinogenesis. 2017;38(4):402–410. doi: 10.1093/carcin/bgx020. [DOI] [PubMed] [Google Scholar]
- Carlson et al. (2016).Carlson BA, Tobe R, Yefremova E, Tsuji PA, Hoffmann VJ, Schweizer U, Gladyshev VN, Hatfield DL, Conrad M. Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biology. 2016;9(5):22–31. doi: 10.1016/j.redox.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2019a).Chen B, Chen Z, Liu M, Gao X, Cheng Y, Wei Y, Wu Z, Cui D, Shang H. Inhibition of neuronal ferroptosis in the acute phase of intracerebral hemorrhage shows long-term cerebroprotective effects. Brain Research Bulletin. 2019a;153:122–132. doi: 10.1016/j.brainresbull.2019.08.013. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2022b).Chen J, Li X, Ge C, Min J, Wang F. The multifaceted role of ferroptosis in liver disease. Cell Death & Differentiation. 2022b;29(3):467–480. doi: 10.1038/s41418-022-00941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2022a).Chen HC, Tang HH, Hsu WH, Wu SY, Cheng WH, Wang BY, Su CL. Vulnerability of triple-negative breast cancer to saponin formosanin C-induced ferroptosis. Antioxidants (Basel) 2022a;11(2):298. doi: 10.3390/antiox11020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2019b).Chen X, Xu S, Zhao C, Liu B. Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochemical and Biophysical Research Communications. 2019b;516(1):37–43. doi: 10.1016/j.bbrc.2019.06.015. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2020).Chen Y, Zhang P, Chen W, Chen G. Ferroptosis mediated DSS-induced ulcerative colitis associated with Nrf2/HO-1 signaling pathway. Immunology Letters. 2020;225(12):9–15. doi: 10.1016/j.imlet.2020.06.005. [DOI] [PubMed] [Google Scholar]
- Cho et al. (2022).Cho SS, Yang JH, Lee JH, Baek JS, Ku SK, Cho IJ, Kim KM, Ki SH. Ferroptosis contribute to hepatic stellate cell activation and liver fibrogenesis. Free Radical Biology and Medicine. 2022;193(9615):620–637. doi: 10.1016/j.freeradbiomed.2022.11.011. [DOI] [PubMed] [Google Scholar]
- Chu et al. (2023).Chu C, Wang X, Yang C, Chen F, Shi L, Xu W, Wang K, Liu B, Wang C, Sun D, Ding W. Neutrophil extracellular traps drive intestinal microvascular endothelial ferroptosis by impairing Fundc1-dependent mitophagy. Redox Biology. 2023;67(10):102906. doi: 10.1016/j.redox.2023.102906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, Wessling-Resnick & Knutson (2008).Collins JF, Wessling-Resnick M, Knutson MD. Hepcidin regulation of iron transport. The Journal of Nutrition. 2008;138(11):2284–2288. doi: 10.3945/jn.108.096347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui et al. (2021).Cui Y, Zhang Y, Zhao X, Shao L, Liu G, Sun C, Xu R, Zhang Z. ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain, Behavior, and Immunity. 2021;93(5):312–321. doi: 10.1016/j.bbi.2021.01.003. [DOI] [PubMed] [Google Scholar]
- Dabkowski, Williamson & Hollander (2008).Dabkowski ER, Williamson CL, Hollander JM. Mitochondria-specific transgenic overexpression of phospholipid hydroperoxide glutathione peroxidase (GPx4) attenuates ischemia/reperfusion-associated cardiac dysfunction. Free Radical Biology and Medicine. 2008;45(6):855–865. doi: 10.1016/j.freeradbiomed.2008.06.021. [DOI] [PubMed] [Google Scholar]
- Dar et al. (2018).Dar HH, Tyurina YY, Mikulska-Ruminska K, Shrivastava I, Ting H-C, Tyurin VA, Krieger J, St. Croix CM, Watkins S, Bayir E, Mao G, Armbruster CR, Kapralov A, Wang H, Parsek MR, Anthonymuthu TS, Ogunsola AF, Flitter BA, Freedman CJ, Gaston JR, Holman TR, Pilewski JM, Greenberger JS, Mallampalli RK, Doi Y, Lee JS, Bahar I, Bomberger JM, Bayır H, Kagan VE. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. Journal of Clinical Investigation. 2018;128(10):4639–4653. doi: 10.1172/JCI99490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon et al. (2012).Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon et al. (2014).Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, Stockwell BR. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll et al. (2019).Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius E, Scheel CH, Mourao A, Buday K, Sato M, Wanninger J, Vignane T, Mohana V, Rehberg M, Flatley A, Schepers A, Kurz A, White D, Sauer M, Sattler M, Tate EW, Schmitz W, Schulze A, O’Donnell V, Proneth B, Popowicz GM, Pratt DA, Angeli JPF, Conrad M. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- Dolma et al. (2003).Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3(3):285–296. doi: 10.1016/S1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- Dominguez, Liu & Kelly (2015).Dominguez JH, Liu Y, Kelly KJ. Renal iron overload in rats with diabetic nephropathy. Physiological Reports. 2015;3(12):e12654. doi: 10.14814/phy2.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong et al. (2024).Dong Y, Kang H, Peng R, Liu Z, Liao F, Hu SA, Ding W, Wang P, Yang P, Zhu M, Wang S, Wu M, Ye D, Gan X, Li F, Song K. A clinical-stage Nrf2 activator suppresses osteoclast differentiation via the iron-ornithine axis. Cell Metabolism. 2024;36(8):1679–1695 e1676. doi: 10.1016/j.cmet.2024.03.005. [DOI] [PubMed] [Google Scholar]
- Dong et al. (2021).Dong H, Xia Y, Jin S, Xue C, Wang Y, Hu R, Jiang H. Nrf2 attenuates ferroptosis-mediated IIR-ALI by modulating TERT and SLC7A11. Cell Death & Disease. 2021;12(11):1027. doi: 10.1038/s41419-021-04307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang et al. (2019).Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, Cheng Q, Zhang P, Dai W, Chen J, Yang F, Yang HT, Linkermann A, Gu W, Min J, Wang F. Ferroptosis as a target for protection against cardiomyopathy. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(7):2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann Angeli et al. (2014).Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Radmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Forster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O’Donnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nature Cell Biology. 2014;16(12):1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi et al. (2018).Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D’Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, Garcia-Saez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jaattela M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, Lopez-Otin C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Munoz-Pinedo C, Nagata S, Nunez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G. Vander, Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Sun & Kong (2023).Gao S, Sun C, Kong J. Vitamin D attenuates ulcerative colitis by inhibiting ACSL4-mediated ferroptosis. Nutrients. 2023;15(22):4845. doi: 10.3390/nu15224845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al. (2023).Gao J, Wang Q, Tang YD, Zhai J, Hu W, Zheng C. When ferroptosis meets pathogenic infections. Trends in Microbiology. 2023;31(5):468–479. doi: 10.1016/j.tim.2022.11.006. [DOI] [PubMed] [Google Scholar]
- Granata et al. (2022).Granata S, Votrico V, Spadaccino F, Catalano V, Netti GS, Ranieri E, Stallone G, Zaza G. Oxidative stress and ischemia/reperfusion injury in kidney transplantation: focus on ferroptosis, mitophagy and new antioxidants. Antioxidants. 2022;11(4):769. doi: 10.3390/antiox11040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo et al. (2015).Guo W, Zhang S, Chen Y, Zhang D, Yuan L, Cong H, Liu S. An important role of the hepcidin-ferroportin signaling in affecting tumor growth and metastasis. Acta Biochimica et Biophysica Sinica. 2015;47(9):703–715. doi: 10.1093/abbs/gmv063. [DOI] [PubMed] [Google Scholar]
- Hambright et al. (2017).Hambright WS, Fonseca RS, Chen L, Na R, Ran Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biology. 2017;12(1):8–17. doi: 10.1016/j.redox.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson et al. (2009).Hanson LR, Roeytenberg A, Martinez PM, Coppes VG, Sweet DC, Rao RJ, Marti DL, Hoekman JD, Matthews RB, Frey WH, 2nd, Panter SS. Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke. Journal of Pharmacology and Experimental Therapeutics. 2009;330(3):679–686. doi: 10.1124/jpet.108.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy & Yellon (2013).Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. Journal of Clinical Investigation. 2013;123(1):92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heirman et al. (2006).Heirman I, Ginneberge D, Brigelius-Flohe R, Hendrickx N, Agostinis P, Brouckaert P, Rottiers P, Grooten J. Blocking tumor cell eicosanoid synthesis by GP x 4 impedes tumor growth and malignancy. Free Radical Biology and Medicine. 2006;40(2):285–294. doi: 10.1016/j.freeradbiomed.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Hirschhorn & Stockwell (2019).Hirschhorn T, Stockwell BR. The development of the concept of ferroptosis. Free Radical Biology and Medicine. 2019;133(1988):130–143. doi: 10.1016/j.freeradbiomed.2018.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, Buddington & Alfrey (1991).Howard RL, Buddington B, Alfrey AC. Urinary albumin, transferrin and iron excretion in diabetic patients. Kidney International. 1991;40(5):923–926. doi: 10.1038/ki.1991.295. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2018).Hu Y, Mao Z, Xu L, Yin L, Tao X, Tang Z, Qi Y, Sun P, Peng J. Protective effect of dioscin against intestinal ischemia/reperfusion injury via adjusting miR-351-5p-mediated oxidative stress. Pharmacological Research. 2018;137:56–63. doi: 10.1016/j.phrs.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2022).Huang J, Chen G, Wang J, Liu S, Su J. Platycodin D regulates high glucose-induced ferroptosis of HK-2 cells through glutathione peroxidase 4 (GPX4) Bioengineered. 2022;13(3):6627–6637. doi: 10.1080/21655979.2022.2045834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha et al. (2016).Jha JC, Banal C, Chow BS, Cooper ME, Jandeleit-Dahm K. Diabetes and kidney disease: role of oxidative stress. Antioxidants & Redox Signaling. 2016;25(12):657–684. doi: 10.1089/ars.2016.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Stockwell & Conrad (2021b).Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nature Reviews Molecular Cell Biology. 2021b;22(4):266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang et al. (2021a).Jiang L, Wang J, Wang K, Wang H, Wu Q, Yang C, Yu Y, Ni P, Zhong Y, Song Z, Xie E, Hu R, Min J, Wang F. RNF217 regulates iron homeostasis through its E3 ubiquitin ligase activity by modulating ferroportin degradation. Blood. 2021a;138(8):689–705. doi: 10.1182/blood.2020008986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin et al. (2022).Jin J, Wang Y, Zheng D, Liang M, He Q. A novel identified circular RNA, mmu_mmu_circRNA_0000309, involves in germacrone-mediated improvement of diabetic nephropathy through regulating ferroptosis by targeting miR-188-3p/GPX4 signaling axis. Antioxidants & Redox Signaling. 2022;36(10–12):740–759. doi: 10.1089/ars.2021.0063. [DOI] [PubMed] [Google Scholar]
- Kanda et al. (2019).Kanda T, Goto T, Hirotsu Y, Moriyama M, Omata M. Molecular mechanisms driving progression of liver cirrhosis towards hepatocellular carcinoma in chronic hepatitis b and c infections: a review. International Journal of Molecular Sciences. 2019;20(6):1358. doi: 10.3390/ijms20061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang et al. (2018).Kang R, Zeng L, Zhu S, Xie Y, Liu J, Wen Q, Cao L, Xie M, Ran Q, Kroemer G, Wang H, Billiar TR, Jiang J, Tang D. Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host & Microbe. 2018;24(1):97–108 e104. doi: 10.1016/j.chom.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppagounder et al. (2018).Karuppagounder SS, Alin L, Chen Y, Brand D, Bourassa MW, Dietrich K, Wilkinson CM, Nadeau CA, Kumar A, Perry S, Pinto JT, Darley-Usmar V, Sanchez S, Milne GL, Pratico D, Holman TR, Carmichael ST, Coppola G, Colbourne F, Ratan RR. N-acetylcysteine targets 5 lipoxygenase-derived, toxic lipids and can synergize with prostaglandin E(2) to inhibit ferroptosis and improve outcomes following hemorrhagic stroke in mice. Annals of Neurology. 2018;84(6):854–872. doi: 10.1002/ana.25356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2021).Kim S, Kang SW, Joo J, Han SH, Shin H, Nam BY, Park J, Yoo TH, Kim G, Lee P, Park JT. Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death & Disease. 2021;12(2):160. doi: 10.1038/s41419-021-03452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2023).Kim HJ, Kim H, Lee JH, Hwangbo C. Toll-like receptor 4 (TLR4): new insight immune and aging. Immunity & Ageing. 2023;20(1):67. doi: 10.1186/s12979-023-00383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2016).Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I, Conrad M, Turker MZ, Gao M, Jiang X, Monette S, Pauliah M, Gonen M, Zanzonico P, Quinn T, Wiesner U, Bradbury MS, Overholtzer M. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nature Nanotechnology. 2016;11(11):977–985. doi: 10.1038/nnano.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinke et al. (2020).Klinke M, Wiskemann H, Bay B, Schafer HJ, Pagerols Raluy L, Reinshagen K, Vincent D, Boettcher M. Cardiac and inflammatory necrotizing enterocolitis in newborns are not the same entity. Frontiers in Pediatrics. 2020;8:593926. doi: 10.3389/fped.2020.593926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles et al. (2021).Knowles TA, Hosfield BD, Pecoraro AR, Li H, Shelley WC, Markel TA. It’s all in the milk: chondroitin sulfate as potential preventative therapy for necrotizing enterocolitis. Pediatric Research. 2021;89(6):1373–1379. doi: 10.1038/s41390-020-01125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi et al. (2019).Kobayashi Y, Ohfuji S, Kondo K, Fukushima W, Sasaki S, Kamata N, Yamagami H, Fujiwara Y, Suzuki Y, Hirota Y, for the Japanese Case–Control Study Group for Ulcerative Colitis Association between dietary iron and zinc intake and development of ulcerative colitis: a case–control study in Japan. Journal of Gastroenterology and Hepatology. 2019;34(10):1703–1710. doi: 10.1111/jgh.14642. [DOI] [PubMed] [Google Scholar]
- Kong, Liu & Cheng (2019).Kong Z, Liu R, Cheng Y. Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway. Biomedicine & Pharmacotherapy. 2019;109:2043–2053. doi: 10.1016/j.biopha.2018.11.030. [DOI] [PubMed] [Google Scholar]
- Kuang et al. (2020).Kuang F, Liu J, Tang D, Kang R. Oxidative damage and antioxidant defense in ferroptosis. Frontiers in Cell and Developmental Biology. 2020;8:586578. doi: 10.3389/fcell.2020.586578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, Ayton & Bush (2018).Lane DJR, Ayton S, Bush AI. Iron and Alzheimer’s disease: an update on emerging mechanisms. Journal of Alzheimer’s Disease. 2018;64(s1):S379–S395. doi: 10.3233/JAD-179944. [DOI] [PubMed] [Google Scholar]
- Lane et al. (2021).Lane DJR, Metselaar B, Greenough M, Bush AI, Ayton SJ. Ferroptosis and NRF2: an emerging battlefield in the neurodegeneration of Alzheimer’s disease. Essays in Biochemistry. 2021;65(7):925–940. doi: 10.1042/EBC20210017. [DOI] [PubMed] [Google Scholar]
- Lee & Roh (2023).Lee J, Roh JL. Unleashing ferroptosis in human cancers: targeting ferroptosis suppressor protein 1 for overcoming therapy resistance. Antioxidants. 2023;12(6):1218. doi: 10.3390/antiox12061218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2020a).Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: past, present and future. Cell Death & Disease. 2020a;11(2):88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2023c).Li Z, Dai R, Chen M, Huang L, Zhu K, Li M, Zhu W, Li Y, Xie N, Li J, Wang L, Lan F, Cao CM. p55gamma degrades RIP3 via MG53 to suppress ischaemia-induced myocardial necroptosis and mediates cardioprotection of preconditioning. Cardiovascular Research. 2023c;119(14):2421–2440. doi: 10.1093/cvr/cvad123. [DOI] [PubMed] [Google Scholar]
- Li et al. (2019a).Li W, Feng G, Gauthier JM, Lokshina I, Higashikubo R, Evans S, Liu X, Hassan A, Tanaka S, Cicka M, Hsiao HM, Ruiz-Perez D, Bredemeyer A, Gross RW, Mann DL, Tyurina YY, Gelman AE, Kagan VE, Linkermann A, Lavine KJ, Kreisel D. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. Journal of Clinical Investigation. 2019a;129(6):2293–2304. doi: 10.1172/JCI126428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2019b).Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, Liu D, Zhang F, Ning S, Yao J, Tian X. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death & Differentiation. 2019b;26(11):2284–2299. doi: 10.1038/s41418-019-0299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2017).Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, Cheng T, Yang J, Wang Z, Jiang C, Ying M, Koehler RC, Stockwell BR, Wang J. Inhibition of neuronal ferroptosis protects hemorrhagic brain. Journal of Clinical Investigation Insight. 2017;2(7):e90777. doi: 10.1172/jci.insight.90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li & Li (2020).Li D, Li Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduction and Targeted Therapy. 2020;5(1):108. doi: 10.1038/s41392-020-00216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2024).Li XN, Lin L, Li XW, Zhu Q, Xie ZY, Hu YZ, Long QS, Wei XB, Wen YQ, Zhang LY, Zhang QK, Jing YC, Wei XH, Li XS. BSA-stabilized selenium nanoparticles ameliorate intracerebral hemorrhage’s-like pathology by inhibiting ferroptosis-mediated neurotoxicology via Nrf2/GPX4 axis activation. Redox Biology. 2024;75(9675):103268. doi: 10.1016/j.redox.2024.103268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2020b).Li M, Wang X, Lu S, He C, Wang C, Wang L, Wang X, Ge P, Song D. Erastin triggers autophagic death of breast cancer cells by increasing intracellular iron levels. Oncology Letters. 2020b;20:57. doi: 10.3892/ol.2020.11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2023b).Li J, Wang L, Wang B, Zhang Z, Jiang L, Qin Z, Zhao Y, Su B. NOX4 is a potential therapeutic target in septic acute kidney injury by inhibiting mitochondrial dysfunction and inflammation. Theranostics. 2023b;13(9):2863–2878. doi: 10.7150/thno.81240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2020c).Li N, Wang W, Zhou H, Wu Q, Duan M, Liu C, Wu H, Deng W, Shen D, Tang Q. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radical Biology and Medicine. 2020c;160:303–318. doi: 10.1016/j.freeradbiomed.2020.08.009. [DOI] [PubMed] [Google Scholar]
- Li et al. (2023a).Li D, Zhang G, Wang Z, Guo J, Liu Y, Lu Y, Qin Z, Xu Y, Cao C, Wang B, Guo Q, Wang Y, Liu G, Cui X, Zhang J, Tang J. Idebenone attenuates ferroptosis by inhibiting excessive autophagy via the ROS-AMPK-mTOR pathway to preserve cardiac function after myocardial infarction. European Journal of Pharmacology. 2023a;943(5):175569. doi: 10.1016/j.ejphar.2023.175569. [DOI] [PubMed] [Google Scholar]
- Liang, Ping & Ge (2017).Liang S, Ping Z, Ge J. Coenzyme Q10 regulates antioxidative stress and autophagy in acute myocardial ischemia-reperfusion injury. Oxidative Medicine and Cellular Longevity. 2017;2017:9863181. doi: 10.1155/2017/9863181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang et al. (2019).Liang C, Zhang X, Yang M, Dong X. Recent progress in ferroptosis inducers for cancer therapy. Advanced Materials. 2019;31(51):e1904197. doi: 10.1002/adma.201904197. [DOI] [PubMed] [Google Scholar]
- Lin et al. (2024).Lin L, Li X, Li Y, Lang Z, Li Y, Zheng J. Ginsenoside Rb1 induces hepatic stellate cell ferroptosis to alleviate liver fibrosis via the BECN1/SLC7A11 axis. Journal of Pharmaceutical Analysis. 2024;14(5):100902. doi: 10.1016/j.jpha.2023.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkermann et al. (2014).Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz PS, Weinlich R, Vanden Berghe T, Vandenabeele P, Pasparakis M, Bleich M, Weinberg JM, Reichel CA, Brasen JH, Kunzendorf U, Anders HJ, Stockwell BR, Green DR, Krautwald S. Synchronized renal tubular cell death involves ferroptosis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(47):16836–16841. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Kang & Tang (2022).Liu J, Kang R, Tang D. Signaling pathways and defense mechanisms of ferroptosis. The FEBS Journal. 2022;289(22):7038–7050. doi: 10.1111/febs.16059. [DOI] [PubMed] [Google Scholar]
- Lorincz et al. (2015).Lorincz T, Jemnitz K, Kardon T, Mandl J, Szarka A. Ferroptosis is involved in acetaminophen induced cell death. Pathology & Oncology Research. 2015;21(4):1115–1121. doi: 10.1007/s12253-015-9946-3. [DOI] [PubMed] [Google Scholar]
- Louandre et al. (2015).Louandre C, Marcq I, Bouhlal H, Lachaier E, Godin C, Saidak Z, Francois C, Chatelain D, Debuysscher V, Barbare JC, Chauffert B, Galmiche A. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Letters. 2015;356(2):971–977. doi: 10.1016/j.canlet.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Love & Miners (2016).Love S, Miners JS. Cerebrovascular disease in ageing and Alzheimer’s disease. Acta Neuropathologica. 2016;131(5):645–658. doi: 10.1007/s00401-015-1522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo et al. (2021).Luo X, Gong HB, Gao HY, Wu YP, Sun WY, Li ZQ, Wang G, Liu B, Liang L, Kurihara H, Duan WJ, Li YF, He RR. Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death & Differentiation. 2021;28(6):1971–1989. doi: 10.1038/s41418-020-00719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al. (2016).Ma S, Henson ES, Chen Y, Gibson SB. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death & Disease. 2016;7(7):e2307. doi: 10.1038/cddis.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher et al. (2018).Maher P, van Leyen K, Dey PN, Honrath B, Dolga A, Methner A. The role of Ca2+ in cell death caused by oxidative glutamate toxicity and ferroptosis. Cell Calcium. 2018;70:47–55. doi: 10.1016/j.ceca.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al. (2023).Ma X, Ma J, Leng T, Yuan Z, Hu T, Liu Q, Shen T. Advances in oxidative stress in pathogenesis of diabetic kidney disease and efficacy of TCM intervention. Renal Failure. 2023;45(1):2146512. doi: 10.1080/0886022X.2022.2146512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al. (2020).Ma S, Sun L, Wu W, Wu J, Sun Z, Ren J. USP22 protects against myocardial ischemia-reperfusion injury via the SIRT1-p53/SLC7A11-dependent inhibition of ferroptosis-induced cardiomyocyte death. Frontiers in Physiology. 2020;11:551318. doi: 10.3389/fphys.2020.551318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al. (2021).Ma L, Zhang X, Yu K, Xu X, Chen T, Shi Y, Wang Y, Qiu S, Guo S, Cui J, Miao Y, Tian X, Du L, Yu Y, Xia J, Wang J. Targeting SLC3A2 subunit of system X(C)(-) is essential for m(6)A reader YTHDC2 to be an endogenous ferroptosis inducer in lung adenocarcinoma. Free Radical Biology and Medicine. 2021;168(6):25–43. doi: 10.1016/j.freeradbiomed.2021.03.023. [DOI] [PubMed] [Google Scholar]
- Martin-Saiz et al. (2022).Martin-Saiz L, Guerrero-Mauvecin J, Martin-Sanchez D, Fresnedo O, Gomez MJ, Carrasco S, Cannata-Ortiz P, Ortiz A, Fernandez JA, Sanz AB. Ferrostatin-1 modulates dysregulated kidney lipids in acute kidney injury. The Journal of Pathology. 2022;257(3):285–299. doi: 10.1002/path.5882. [DOI] [PubMed] [Google Scholar]
- Matsumoto et al. (2013).Matsumoto M, Sasaki N, Tsujino T, Akahori H, Naito Y, Masuyama T. Iron restriction prevents diabetic nephropathy in Otsuka Long-Evans Tokushima fatty rat. Renal Failure. 2013;35(8):1156–1162. doi: 10.3109/0886022X.2013.819729. [DOI] [PubMed] [Google Scholar]
- Matsushita et al. (2015).Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. Journal of Experimental Medicine. 2015;212(4):555–568. doi: 10.1084/jem.20140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar, Rampton & Blake (2000).Millar AD, Rampton DS, Blake DR. Effects of iron and iron chelation in vitro on mucosal oxidant activity in ulcerative colitis. Alimentary Pharmacology & Therapeutics. 2000;14(9):1163–1168. doi: 10.1046/j.1365-2036.2000.00828.x. [DOI] [PubMed] [Google Scholar]
- Minaiyan, Mostaghel & Mahzouni (2012).Minaiyan M, Mostaghel E, Mahzouni P. Preventive therapy of experimental colitis with selected iron chelators and anti-oxidants. International Journal of Preventive Medicine. 2012;3:S162–169. [PMC free article] [PubMed] [Google Scholar]
- Mu et al. (2024).Mu M, Chen B, Li H, Fan R, Yang Y, Zhou L, Han B, Zou B, Chen N, Guo G. Augmented the sensitivity of photothermal-ferroptosis therapy in triple-negative breast cancer through mitochondria-targeted nanoreactor. Journal of Controlled Release. 2024;375:733–744. doi: 10.1016/j.jconrel.2024.09.042. [DOI] [PubMed] [Google Scholar]
- Muri et al. (2019).Muri J, Thut H, Bornkamm GW, Kopf M. B1 and marginal zone b cells but not follicular B2 cells require gpx4 to prevent lipid peroxidation and ferroptosis. Cell Reports. 2019;29(9):2731–2744.e4. doi: 10.1016/j.celrep.2019.10.070. [DOI] [PubMed] [Google Scholar]
- Ozkan et al. (2009).Ozkan OV, Yuzbasioglu MF, Ciralik H, Kurutas EB, Yonden Z, Aydin M, Bulbuloglu E, Semerci E, Goksu M, Atli Y, Bakan V, Duran N. Resveratrol, a natural antioxidant, attenuates intestinal ischemia/reperfusion injury in rats. The Tohoku Journal of Experimental Medicine. 2009;218(3):251–258. doi: 10.1620/tjem.218.251. [DOI] [PubMed] [Google Scholar]
- Park et al. (2019).Park TJ, Park JH, Lee GS, Lee JY, Shin JH, Kim MW, Kim YS, Kim JY, Oh KJ, Han BS, Kim WK, Ahn Y, Moon JH, Song J, Bae KH, Kim DH, Lee EW, Lee SC. Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death & Disease. 2019;10(11):835. doi: 10.1038/s41419-019-2061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi et al. (2024).Qi M, Cheng Y, Liu K, Cai J, Liu T, Wu X, Tang H, Huang H, Chen Q, Zhou X. Recombinant human heavy chain ferritin nanoparticles serve as ROS scavengers for the treatment of ischemic stroke. International Journal of Nanomedicine. 2024;19:2285–2299. doi: 10.2147/IJN.S449606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu et al. (2022).Qiu W, An S, Wang T, Li J, Yu B, Zeng Z, Chen Z, Lin B, Lin X, Gao Y. Melatonin suppresses ferroptosis via activation of the Nrf2/HO-1 signaling pathway in the mouse model of sepsis-induced acute kidney injury. International Immunopharmacology. 2022;112(8):109162. doi: 10.1016/j.intimp.2022.109162. [DOI] [PubMed] [Google Scholar]
- Rabanal-Ruiz, Llanos-Gonzalez & Alcain (2021).Rabanal-Ruiz Y, Llanos-Gonzalez E, Alcain FJ. The use of coenzyme Q10 in cardiovascular diseases. Antioxidants. 2021;10(5):755. doi: 10.3390/antiox10050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven et al. (2013).Raven EP, Lu PH, Tishler TA, Heydari P, Bartzokis G. Increased iron levels and decreased tissue integrity in hippocampus of Alzheimer’s disease detected in vivo with magnetic resonance imaging. Journal of Alzheimer’s Disease. 2013;37(1):127–136. doi: 10.3233/JAD-130209. [DOI] [PubMed] [Google Scholar]
- Reidy et al. (2014).Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. Journal of Clinical Investigation. 2014;124(6):2333–2340. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegman et al. (2020).Riegman M, Sagie L, Galed C, Levin T, Steinberg N, Dixon SJ, Wiesner U, Bradbury MS, Niethammer P, Zaritsky A, Overholtzer M. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nature Cell Biology. 2020;22(9):1042–1048. doi: 10.1038/s41556-020-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokop et al. (2024).Rokop ZP, Zhang W, Ghosh N, Biswas N, Das A, Lin J, Sen CK, Kubal C. Exacerbated ischemia-reperfusion injury in fatty livers is mediated by lipid peroxidation stress and ferroptosis. Surgery. 2024;175(6):1539–1546. doi: 10.1016/j.surg.2024.02.001. [DOI] [PubMed] [Google Scholar]
- Sahoo et al. (2022).Sahoo BM, Banik BK, Borah P, Jain A. Reactive oxygen species (ROS): key components in cancer therapies. Anti-Cancer Agents in Medicinal Chemistry. 2022;22(2):215–222. doi: 10.2174/1871520621666210608095512. [DOI] [PubMed] [Google Scholar]
- Sampilvanjil et al. (2020).Sampilvanjil A, Karasawa T, Yamada N, Komada T, Higashi T, Baatarjav C, Watanabe S, Kamata R, Ohno N, Takahashi M. Cigarette smoke extract induces ferroptosis in vascular smooth muscle cells. American Journal of Physiology-Heart and Circulatory Physiology. 2020;318(3):H508–H518. doi: 10.1152/ajpheart.00559.2019. [DOI] [PubMed] [Google Scholar]
- Shen et al. (2023).Shen J, Wilbon AS, Zhou M, Pan Y. Mechanism of Ca(2+) transport by ferroportin. Elife. 2023;12:e82947. doi: 10.7554/eLife.82947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al. (2024).Shi J, Cui G, Jin Y, Mi B, Liu K, Zhao L, Bao K, Lu Z, Liu J, Wang Y, He H, Guo Z. Glutathione-depleted photodynamic nanoadjuvant for triggering nonferrous ferroptosis to amplify radiotherapy of breast cancer. Advanced Healthcare Materials. 2024 doi: 10.1002/adhm.202402474. Epub ahead of print 13 October 2024. [DOI] [PubMed] [Google Scholar]
- Sleire et al. (2015).Sleire L, Skeie BS, Netland IA, Forde HE, Dodoo E, Selheim F, Leiss L, Heggdal JI, Pedersen PH, Wang J, Enger PO. Drug repurposing: sulfasalazine sensitizes gliomas to gamma knife radiosurgery by blocking cystine uptake through system Xc-, leading to glutathione depletion. Oncogene. 2015;34(49):5951–5959. doi: 10.1038/onc.2015.60. [DOI] [PubMed] [Google Scholar]
- Sorby-Adams et al. (2024).Sorby-Adams A, Prime TA, Miljkovic JL, Prag HA, Krieg T, Murphy MP. A model of mitochondrial superoxide production during ischaemia-reperfusion injury for therapeutic development and mechanistic understanding. Redox Biology. 2024;72(11):103161. doi: 10.1016/j.redox.2024.103161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanutti et al. (2008).Stefanutti G, Pierro A, Parkinson EJ, Smith VV, Eaton S. Moderate hypothermia as a rescue therapy against intestinal ischemia and reperfusion injury in the rat. Critical Care Medicine. 2008;36(5):1564–1572. doi: 10.1097/CCM.0b013e3181709e9f. [DOI] [PubMed] [Google Scholar]
- Su et al. (2019).Su L, Jiang X, Yang C, Zhang J, Chen B, Li Y, Yao S, Xie Q, Gomez H, Murugan R, Peng Z. Pannexin 1 mediates ferroptosis that contributes to renal ischemia/reperfusion injury. Journal of Biological Chemistry. 2019;294(50):19395–19404. doi: 10.1074/jbc.RA119.010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui et al. (2018).Sui M, Jiang X, Chen J, Yang H, Zhu Y. Magnesium isoglycyrrhizinate ameliorates liver fibrosis and hepatic stellate cell activation by regulating ferroptosis signaling pathway. Biomedicine & Pharmacotherapy. 2018;106(4):125–133. doi: 10.1016/j.biopha.2018.06.060. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2023).Sun S, Shen J, Jiang J, Wang F, Min J. Targeting ferroptosis opens new avenues for the development of novel therapeutics. Signal Transduction and Targeted Therapy. 2023;8(1):372. doi: 10.1038/s41392-023-01606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnus et al. (2021).Tonnus W, Meyer C, Steinebach C, Belavgeni A, von Massenhausen A, Gonzalez NZ, Maremonti F, Gembardt F, Himmerkus N, Latk M, Locke S, Marschner J, Li W, Short S, Doll S, Ingold I, Proneth B, Daniel C, Kabgani N, Kramann R, Motika S, Hergenrother PJ, Bornstein SR, Hugo C, Becker JU, Amann K, Anders HJ, Kreisel D, Pratt D, Gutschow M, Conrad M, Linkermann A. Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury. Nature Communications. 2021;12(1):4402. doi: 10.1038/s41467-021-24712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo et al. (2017).Tuo QZ, Lei P, Jackman KA, Li XL, Xiong H, Li XL, Liuyang ZY, Roisman L, Zhang ST, Ayton S, Wang Q, Crouch PJ, Ganio K, Wang XC, Pei L, Adlard PA, Lu YM, Cappai R, Wang JZ, Liu R, Bush AI. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Molecular Psychiatry. 2017;22(11):1520–1530. doi: 10.1038/mp.2017.171. [DOI] [PubMed] [Google Scholar]
- Tuttle et al. (2022).Tuttle KR, Agarwal R, Alpers CE, Bakris GL, Brosius FC, Kolkhof P, Uribarri J. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney International. 2022;102(2):248–260. doi: 10.1016/j.kint.2022.05.012. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2017).Wang H, An P, Xie E, Wu Q, Fang X, Gao H, Zhang Z, Li Y, Wang X, Zhang J, Li G, Yang L, Liu W, Min J, Wang F. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology. 2017;66(2):449–465. doi: 10.1002/hep.29117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2022b).Wang YH, Chang DY, Zhao MH, Chen M. Glutathione peroxidase 4 is a predictor of diabetic kidney disease progression in type 2 diabetes mellitus. Oxidative Medicine and Cellular Longevity. 2022b;2022(4):2948248. doi: 10.1155/2022/2948248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2019).Wang W, Green M, Choi JE, Gijon M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, Xia H, Zhou J, Li G, Li J, Li W, Wei S, Vatan L, Zhang H, Szeliga W, Gu W, Liu R, Lawrence TS, Lamb C, Tanno Y, Cieslik M, Stone E, Georgiou G, Chan TA, Chinnaiyan A, Zou W. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2022a).Wang X, Li Q, Sui B, Xu M, Pu Z, Qiu T. Schisandrin a from schisandra chinensis attenuates ferroptosis and NLRP3 inflammasome-mediated pyroptosis in diabetic nephropathy through mitochondrial damage by AdipoR1 ubiquitination. Oxidative Medicine and Cellular Longevity. 2022a;2022:5411462. doi: 10.1155/2022/5411462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2020).Wang S, Liu W, Wang J, Bai X. Curculigoside inhibits ferroptosis in ulcerative colitis through the induction of GPX4. Life Sciences. 2020;259(1):118356. doi: 10.1016/j.lfs.2020.118356. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2023a).Wang H, Liu D, Zheng B, Yang Y, Qiao Y, Li S, Pan S, Liu Y, Feng Q, Liu Z. Emerging role of ferroptosis in diabetic kidney disease: molecular mechanisms and therapeutic opportunities. International Journal of Biological Sciences. 2023a;19(9):2678–2694. doi: 10.7150/ijbs.81892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2023b).Wang J, Wang Z, Li Y, Hou Y, Yin C, Yang E, Liao Z, Fan C, Martin LL, Sun D. Blood brain barrier-targeted delivery of double selenium nanospheres ameliorates neural ferroptosis in Alzheimer’s disease. Biomaterials. 2023b;302(3):122359. doi: 10.1016/j.biomaterials.2023.122359. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2014).Wang J, Yin D, Xie C, Zheng T, Liang Y, Hong X, Lu Z, Song X, Song R, Yang H, Sun B, Bhatta N, Meng X, Pan S, Jiang H, Liu L. The iron chelator Dp44mT inhibits hepatocellular carcinoma metastasis via N-Myc downstream-regulated gene 2 (NDRG2)/gp130/STAT3 pathway. Oncotarget. 2014;5(18):8478–8491. doi: 10.18632/oncotarget.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei (2024).Wei C. The role of glutathione peroxidase 4 in neuronal ferroptosis and its therapeutic potential in ischemic and hemorrhagic stroke. Brain Research Bulletin. 2024;217(7):111065. doi: 10.1016/j.brainresbull.2024.111065. [DOI] [PubMed] [Google Scholar]
- Wei et al. (2020).Wei S, Bi J, Yang L, Zhang J, Wan Y, Chen X, Wang Y, Wu Z, Lv Y, Wu R. Serum irisin levels are decreased in patients with sepsis, and exogenous irisin suppresses ferroptosis in the liver of septic mice. Clinical and Translational Medicine. 2020;10(5):e173. doi: 10.1002/ctm2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2015).Wu X, Deng F, Li Y, Daniels G, Du X, Ren Q, Wang J, Wang LH, Yang Y, Zhang V, Zhang D, Ye F, Melamed J, Monaco ME, Lee P. ACSL4 promotes prostate cancer growth, invasion and hormonal resistance. Oncotarget. 2015;6:44849–44863. doi: 10.18632/oncotarget.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2019).Wu Z, Geng Y, Lu X, Shi Y, Wu G, Zhang M, Shan B, Pan H, Yuan J. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proceedings of the National Academy of Sciences of the United States of America. 2019;116:2996–3005. doi: 10.1073/pnas.1819728116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2013).Wu X, Li Y, Wang J, Wen X, Marcus MT, Daniels G, Zhang DY, Ye F, Wang LH, Du X, Adams S, Singh B, Zavadil J, Lee P, Monaco ME. Long chain fatty Acyl-CoA synthetase 4 is a biomarker for and mediator of hormone resistance in human breast cancer. PLOS ONE. 2013;8:e77060. doi: 10.1371/journal.pone.0077060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi et al. (2024).Xi ZC, Ren HG, Ai L, Wang Y, Liu MF, Qiu YF, Feng JL, Fu W, Bi QQ, Wang F, Xu HX. Ginsenoside Rg1 mitigates cerebral ischaemia/reperfusion injury in mice by inhibiting autophagy through activation of mTOR signalling. Acta Pharmacologica Sinica. 2024;45:2474–2486. doi: 10.1038/s41401-024-01334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2021).Xu S, He Y, Lin L, Chen P, Chen M, Zhang S. The emerging role of ferroptosis in intestinal disease. Cell Death & Disease. 2021;12(4):289. doi: 10.1038/s41419-021-03559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2018).Xu F, Lin S, Yan X, Wang C, Tu H, Yin Y, Cao J. Interleukin 38 protects against lethal sepsis. The Journal of Infectious Diseases. 2018;218:1175–1184. doi: 10.1093/infdis/jiy289. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2020).Xu M, Tao J, Yang Y, Tan S, Liu H, Jiang J, Zheng F, Wu B. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death & Disease. 2020;11:86. doi: 10.1038/s41419-020-2299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue et al. (2023).Xue Q, Yan D, Chen X, Li X, Kang R, Klionsky DJ, Kroemer G, Chen X, Tang D, Liu J. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy. 2023;19(7):1982–1996. doi: 10.1080/15548627.2023.2165323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagoda et al. (2007).Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM, Boniface JJ, Smith R, Lessnick SL, Sahasrabudhe S, Stockwell BR. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447(7146):864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang & Stockwell (2008).Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chemistry & Biology. 2008;15(3):234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong et al. (2024).Yong Y, Yan L, Wei J, Feng C, Yu L, Wu J, Guo M, Fan D, Yu C, Qin D, Zhou X, Wu A. A novel ferroptosis inhibitor, Thonningianin A, improves Alzheimer’s disease by activating GPX4. Theranostics. 2024;14(16):6161–6184. doi: 10.7150/thno.98172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef et al. (2018).Youssef LA, Rebbaa A, Pampou S, Weisberg SP, Stockwell BR, Hod EA, Spitalnik SL. Increased erythrophagocytosis induces ferroptosis in red pulp macrophages in a mouse model of transfusion. Blood. 2018;131:2581–2593. doi: 10.1182/blood-2017-12-822619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan et al. (2022).Yuan S, Wei C, Liu G, Zhang L, Li J, Li L, Cai S, Fang L. Sorafenib attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis via HIF-1alpha/SLC7A11 pathway. Cell Proliferation. 2022;55(1):e13158. doi: 10.1111/cpr.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2024b).Zhang S, Guo L, Tao R, Liu S. Ferroptosis-targeting drugs in breast cancer. Journal of Drug Targeting. 2024b doi: 10.1080/1061186X.2024.2399181. Epub ahead of print 5 September 2024. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2021).Zhang Y, Lu X, Tai B, Li W, Li T. Ferroptosis and its multifaceted roles in cerebral stroke. Frontiers in Cellular Neuroscience. 2021;15:615372. doi: 10.3389/fncel.2021.615372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2024a).Zhang J, Ren X, Nie Z, You Y, Zhu Y, Chen H, Yu H, Mo GP, Su L, Peng Z, Tang MC. Dual-responsive renal injury cells targeting nanoparticles for vitamin E delivery to treat ischemia reperfusion-induced acute kidney injury. Journal of Nanobiotechnology. 2024a;22(1):626. doi: 10.1186/s12951-024-02894-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2018).Zhang Z, Wu Y, Yuan S, Zhang P, Zhang J, Li H, Li X, Shen H, Wang Z, Chen G. Glutathione peroxidase 4 participates in secondary brain injury through mediating ferroptosis in a rat model of intracerebral hemorrhage. Brain Research. 2018;1701:112–125. doi: 10.1016/j.brainres.2018.09.012. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2023).Zhao Y, Jiang B, Huang D, Lou J, Li G, Liu J, Duan F, Yuan Y, Su X. Ferrostatin-1 post-treatment attenuates acute kidney injury in mice by inhibiting ferritin production and regulating iron uptake-related proteins. PeerJ. 2023;11:e15786. doi: 10.7717/peerj.15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. (2019).Zhou Z, Ye TJ, Bonavita G, Daniels M, Kainrad N, Jogasuria A, You M. Adipose-specific lipin-1 overexpression renders hepatic ferroptosis and exacerbates alcoholic steatohepatitis in mice. Hepatology Communications. 2019;3(5):656–669. doi: 10.1002/hep4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
This is a literature review.