Abstract
Aortic root reconstruction operations have undergone substantial evolution with technical modifications, expanding indications, and the need for increasingly complex decision-making. The purpose of this state-of-the-art review is to detail our approach to contemporary aortic root reconstruction operations. First, we review the evolution of root reconstruction procedures over the years and discuss the approach to the aortic root patient for lifetime management of aneurysm and valvular disease in the modern context of management options. We also discuss state-of-the art technical considerations of valve-sparing root replacement, variations of the Ross operation, aortic valve repair principles and challenges in special populations, and considerations for complication-free coronary button reconstruction. We also discuss root reconstruction in high-risk subpopulations including acute type A aortic dissection, congenital, and reoperative patients. We briefly highlight future directions in transcatheter root replacement as well as the outlook for the next generation of aortic root surgeons.
Keywords: aortic root reconstruction, aneurysm, valvular disease, valve-sparing root replacement
Visual abstract.
Central Message.
There has been an evolution in aortic root reconstruction operations, and future considerations will include emerging technologies and training the next generation of aortic root surgeons.
Introduction
Aortic root reconstruction operations have evolved substantially over the past decades to increasingly emphasize valve repair rather than replacement and aim to optimize long-term outcomes for patients. The contemporary era aortic root patient is assessed and managed while accounting for lifetime considerations and planning for potential reinterventions, through both open surgical and transcatheter approaches. As a result, decision-making for aortic valve and root reconstruction is increasingly multifactorial and complex. Factors include timing of intervention, durability versus preservation of native tissue, and mitigating a patient’s lifelong exposure to thromboembolic/hemorrhagic and reintervention risks. In this state-of-the art review, we describe the evolution of aortic root operations and approach to the modern aortic root patient and detail nuanced technical considerations for aortic root and valve reconstructive operations. We also highlight future considerations with respect to emerging technologies and training the next generation of aortic root surgeons.
Evolution From Bentall to Reconstructive
In individuals requiring an aortic root replacement with an aortic valve unsuitable for repair, a mechanical or biological valved conduit is a class I recommendation. 1 The original mechanical Bentall DeBono procedure in 1968 has evolved from an inclusion technique to a modified button Bentall, to address issues surrounding pseudoaneurysm development at the coronary buttons. Although a mechanical or biological valved conduit remains a preferred choice, there are surgical scenarios in which other prostheses such as xenografts and cryopreserved aortic homografts play a role.
Xenografts such as the porcine Freestyle aortic root graft (Medtronic, Dublin, Ireland) have been in use for more than 30 years, with reported freedom from explant for structural valve deterioration of 83.3% at 15 years. 2 Although the primary indication for a root replacement is for an aortic aneurysm, a xenograft can also be used in patients with a small aortic annulus. A recent study demonstrated favorable results comparing an aortic root replacement with a Freestyle aortic root and an aortic root enlargement. Although results regarding aortic valve reintervention and death were comparable at 5 years, the patients in the Freestyle group had larger valves implanted and a lower incidence of patient-prosthesis mismatch. 3
Previous data from El-Hamamsy et al. demonstrated that compared with the Freestyle aortic root replacement, the homograft has a higher rate of reoperations and aortic valve dysfunction. 4 However, a homograft remains a valuable option in endocarditis cases in which the patient has a periannular abscess, annular destruction, or ventricular-aortic discontinuity because of the pliable nature of the homograft and attached anterior mitral leaflet (Supplemental Video 1). Some guidelines consider homograft as a class IIa recommendation for patients with prosthetic valve endocarditis and periannular abscess. 5
Although historical focus has been on root replacement, the advent of reconstructive techniques including aortic valve-sparing operations and valve repair techniques have aimed to minimize patient exposure to the lifelong risks associated with prosthetic valves. Valve-sparing root replacements (VSRRs) are now in their fourth decade of outcomes with excellent long-term results and have become the standard of care in appropriately selected patients. Another operative approach that has been shown to restore normal life expectancy by means of a living valve substitute is the Ross procedure. Initially sidelined due to several poor outcomes in the era of its initial introduction, pulmonary autograft replacement has now become a mainstay in aortic root centers with excellent and highly reproducible outcomes for experienced surgeons. Patient selection with respect to aortic root and valve pathology, clinical characteristics, and annular/root stabilization remains an area of discussion in order to optimize long-term outcomes several decades postoperatively.
In the current transcatheter era, an endovascular approach to the aortic root, the Endo-Bentall, is gaining interest. To date, only case reports have been published in patients considered at high risk or prohibitive risk for a conventional surgical repair.6,7 Limitations that will prevent widespread adoption of this technique include the need for customized devices given the variability in patient anatomy and limited long-term follow-up.
Lifetime Management of the Aortic Root Patient
It is increasingly recognized that the approach to the aortic root patient should include a plan for lifetime management that accounts for needs and risks of intervention, exposure to long-term risks of thrombotic/hemorrhagic complications, and structural valve deterioration of prosthetic valves. Furthermore, a new variable for consideration is the possibility of transcatheter reinterventions—an option that appears very attractive to patients seeking to avoid long-term anticoagulation but ultimately lacks evidence supported by the test of time. The exact proportion of patients who will ultimately prove to be acceptable candidates for valve-in-valve transcatheter aortic valve implantation (VIV-TAVI) may be fewer than expected, and it is unclear whether the patient-prosthesis mismatch incurred will affect life expectancy. These are all challenging issues and questions to consider when counseling patients in the clinic about options for management of their aortic root.
For patients with greater than a 20-year life expectancy, the implications of these questions are most critical, and the timing of the intervention must be accounted for as well. In a young patient with a likely repairable aortic valve, earlier timing of reintervention may increase the chance of successful repair but exposes them to risk of early aortic replacement if repair proves unfeasible. Furthermore, if the back-up strategy is a Ross, then the earlier it is performed, the more likely it is for cusp adaptation and avoidance of the increased reoperative risks associated with annular dilatation. Conversely, early Ross exposes the patient to more years of risk of failure and reintervention. This is where surgeon experience becomes critical to have a high probability of certainty that the preoperative diagnosis will match intraoperative findings and could be considered as analogous to current practice in mitral repair.
The patient’s native valve, if repairable, remains the best option. As a result, VSRR in well-selected cohorts has demonstrated outstanding long-term freedom from reoperation. However, some patients will inevitably develop aortic sclerosis and stenosis over the long term and thus still require reintervention. When replacement is needed, mechanical aortic valve replacement (AVR) remains the most durable option with a reintervention rate of 2.3% at 12 years. 8 Valve reimplantation in a Dacron graft is highly durable but does expose leaflets to increased stress; therefore, the introduction of VSRR purely for a valve repair indication with relatively normal sinuses requires careful consideration and long-term follow-up. Again, the timing of the intervention is an important consideration to minimize the patient’s exposure to long-term risks of reintervention while maximizing their chance of successful repair before valve destruction is unsalvageable.
Young patients who refuse warfarin and are not candidates for Ross constitute a particular challenge. Although surgical redo AVR can be performed with a similar operative risk as the index procedure, these risks increase by the time of multiple reinterventions. If VIV-TAVI is planned for at least one of the lifelong reinterventions, an adequate-size surgical valve is paramount and likely requires more liberal adoption of root enlargement, even if valve fracture or modification can be performed. Root enlargement likely does increase the technical complexity of redo surgical AVR. Data regarding the technical challenges of redo surgery after VIV-TAVI in tissue AVR with root enlargement are limited. It is important to note that only a proportion of current bioprosthetic Bentall procedures is suitable for VIV-TAVI. Coronary occlusion during VIV-TAVI is increased in the setting of stentless valves or bioprosthetic with externally mounted leaflets, and these should be avoided. 9 A valve-to-coronary distance of less than 4 mm on cardiac computed tomography also predicts coronary occlusion, and the use of a Valsalva graft is helpful to avoid this complication. Patient-prosthesis mismatch with VIV-TAVI is associated with increased rates of mortality and structural valve degeneration. 10 To avoid patient-prosthesis mismatch, the internal orifice area of the bioprosthesis must be considered at the index operation. Liberal use of standard annular/root enlargement techniques has been shown not to increase operative risk. Particular to root surgery, patients with aortoannular ecstasia usually accommodate large valves. In the setting of a smaller annulus, coronary buttons require mobilization, making space for supra-annular anchoring of the valve that could then be upsized.
In general, the clinic discussion with the modern aortic root patient is not always simple. Timing of intervention, choice of operation, and lifelong consequences must be carefully outlined, including the current limitations in long-term evidence. Choosing the correct strategy depends heavily on patient values, and the role of the clinician is to help the patient navigate through these complex issues in the context of valve and root pathology. In addition, the current era aortic root surgeon can anticipate a growing population of patients for whom 1 to 2 additional valve interventions are required over the course of their lives.
Valve-Sparing Aortic Root Reconstruction
VSRR techniques were initially developed to replace aortic root aneurysms while preserving largely normal aortic valve cusps. However, these techniques have expanded to become fundamental to the treatment of patients with significant aortic insufficiency (AI), both in tricuspid aortic valve (TAV) and bicuspid aortic valve (BAV) phenotypes. Most importantly, VSRR techniques preserve native aortic valve cusp function, offer optimal hemodynamic performance, avoid the deleterious effects of prosthetic valve–related complications, and are associated with improved late survival. 11 Thus, patients with isolated aortic root aneurysms and normal cusp function should preferentially undergo VSRR over prosthetic valved conduits. Volume-outcome relationships are well established in aortic surgery, particularly in VSRR operations in which the best clinical outcomes are achieved in high-volume centers with expertise in aortic valve reconstruction. In patients with ascending aortic aneurysms and modest aortic root dilatation, careful decision-making should help guide borderline cases, particularly if the aortic valve is not repairable, as valve replacement will certainly change the natural history of the patient, putting them at risk for future prosthetic valve–related complications and impaired long-term survival.
Both the reimplantation (David) and remodeling (Yacoub) techniques have demonstrated excellent outcomes in expert hands,11–13 and despite manufactured controversies over the years, both operations adhere largely to similar principles and are associated with similarly excellent outcomes. Purists argue that the remodeling technique offers better dynamic, expansile function than the reimplantation technique; however, the commonly required extra-aortic annuloplasty ring likely abolishes the expansibility advantage of the remodeling technique. Although both techniques are most certainly valuable within the aortic root surgeon’s toolbox, the reality is that the reimplantation technique is far more commonly performed, likely due to a perceived shorter learning curve, simplified graft selection and preparation, and some general attraction and admiration of the David procedure. The remodeling technique requires further nuances in graft tailoring and preparation for the proximal suture line, increased concerns of hemostasis in fragile root tissue, and less certainty when addressing asymmetric commissural orientation (CO), particularly in BAV. The key principles of both techniques include restoring normal cusp geometry, optimizing cusp coaptation while maximizing the aortic valve area through cusp preservation, effective height, and aortic annular stabilization. 14 Cusp preservation requires pliable cusp tissue with adequate geometric height and healthy commissural attachments without major fenestrations, significant cusp restriction, or calcification. Effective height after root reconstruction is a key concept that reflects adequate commissural height, correction of any cusp prolapse, and commonly symmetric commissural angle reconstruction. Aortic annular stabilization is a key step to enhance cusp coaptation, ensure long-term repair durability, and restore normal valve physiologic function. Although, intuitively, there should be optimal graft sizing that would maximize the aortic valve area with optimal cusp coaptation and competence, there remain many methods described for graft sizing without an accepted standard. Severe cusp abnormalities and very asymmetric CO constitute the biggest challenges to successful VSRR (Supplemental Video 2).
VSRR has shown strong long-term results, with studies reporting high survival rates and freedom from reoperation at 20 years, especially when performed in expert centers. 12 Comparative studies indicate that VSRR offers better outcomes in terms of survival and reduced complications compared with the composite valved conduits. 11
Ross Procedure
First described in 1967, the Ross procedure reignited interest considering favorable long-term outcomes.15–17 Knowledge regarding patient selection, technical subtleties, mechanism of autograft and homograft failure, as well as the postoperative management of patients undergoing a Ross procedure have helped in making this intervention safe, durable, and reproducible.
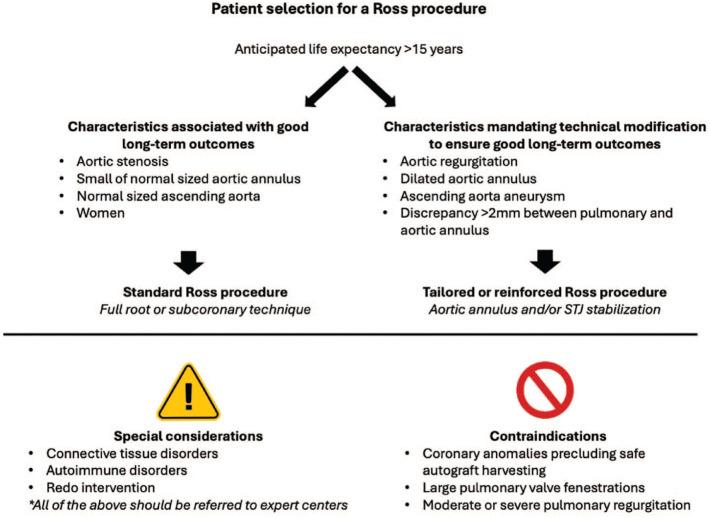
Patients with a life expectancy >15 years are more likely to fully benefit from the advantages of a Ross procedure. Because life expectancy can often be difficult to ascertain, age has often been used as a surrogate. Although most surgeons and clinicians would agree that the Ross procedure is the best option in children and young adults, debate remains regarding the role of the Ross procedure in older adults. Recent studies have shown that older adults enjoy similar benefits when compared with their younger counterparts. 18 Therefore, age alone may not necessarily be used to determine candidacy for a Ross procedure. Conversely, the more advanced the patient’s age, the more risk for failure of adaptation of the autograft and need for reintervention. Careful patient selection is paramount to maintain excellent long-term outcomes of the Ross procedure, although questions remain regarding this. Figure 1 illustrates various patient characteristics and how they may influence long-term outcomes. There are specific conditions that require tailored adaptations and few that may still represent absolute contraindications. In the modern area, these are mainly limited to anatomical factors (large pulmonary valve fenestrations, some coronary anomalies precluding autograft harvesting, and use of bicuspid pulmonary autografts is controversial with limited evidence).
Fig. 1.
Patient selection for a Ross procedure. STJ, sinotubular junction.
Recognizing that one of the main failure mechanisms of the autograft was late dilatation, various approaches to reinforcement have been considered. External reinforcement provides a durable solution to mitigate the risk of late dilatation. Conversely, reinforcement risks restricting or distorting the autograft, thereby limiting the benefits of implanting a living structure in the aortic position. Subcoronary implantation is associated with good long-term outcomes 19 but lacks widespread adoption, likely because of increased technical challenge to avoid autograft distortion during implantation. The best results regarding freedom from reoperation are attributable to the inclusion root technique. Only 5% long-term reintervention was described by Skillington et al., who applied this technique in 100% of cases. 20 Although direct comparison of inclusion root versus full root has not shown a significant difference in long-term freedom from reintervention, in the series by David et al., no late autograft failures were observed in the inclusion root group. 21 The inclusion root technique is technically challenging and intuitively would have some exclusion criteria such as a small aortic root, grossly asymmetric bicuspid roots, coronary orifice malposition, and previous aortic root resection. Nevertheless, Skillington et al. reported the application of this approach in 100% of cases, likely due to 2 technical modifications: (1) the left coronary button is always reimplanted separately, and (2) the root symmetry is restored by downsizing the noncoronary cusp as necessary. 22 Generalizability of these outcomes, which have largely been performed by a single expert surgeon per center only,20,21 remain unclear.
Table 1 summarizes Ross variations, strengths, and limitations. Regardless of the implantation technique used, the following anatomic considerations apply (Supplemental Video 3), namely, valve pathology (aortic regurgitation, aortic stenosis, or mixed disease), as well as the measurements of the aortic annulus, pulmonary valve annulus, aortic root, sinotubular junction, and ascending aorta, all of which need to be obtained to determine the need for aortic annulus and/or sinotubular stabilization. Patients with unicuspid and BAV usually have an asymmetric annulus and possible coronary malposition or anomalies. Autograft position must facilitate tension-free coronary reimplantation. Unicuspid and BAV commissural height is often asymmetric; therefore, autograft implantation should not be at the level of the native commissures or distortion will result.
Table 1.
Strengths and Limitations of the Techniques Used to Prevent Pulmonary Autograft Dilatation.
| Technique | Strengths | Limitations |
|---|---|---|
| External support within a Dacron tube | • Good midterm stability of the neo-sinus diameters | • Might lead to autograft distortion • Risk of early aortic regurgitation • Coronaries can be distorted by the Dacron graft • Limits the natural movements of the autograft • Dacron can cause an inflammatory reaction with the pulmonary autograft • Use of foreign material • Excess stress on aortic valve cusps from rigid external support |
| PEARS | • Does not seem to cause an inflammatory response with the autograft • Prevents dilatation of aneurysms in patients with Marfan syndrome • Prevents dilatation in an animal model of the Ross procedure |
• Might limit the natural movements of the autograft • Sizing of the PEARS might be troublesome knowing that the ideal geometry of the pulmonary autograft in the aortic position is not clearly defined • Excess stress on aortic valve cusps from rigid external support |
| Autologous support (inclusion technique) | • Excellent long-term follow-up • Effectively prevents pulmonary autograft dilatation • Low risk of endocarditis • Reinforcement with compliant structure minimizes stress on valve cusps |
• Might limit the natural movements of the autograft • Challenging to apply to patients with unicuspid and type 0 bicuspid valves or coronary malposition • Might induce distortion of the autograft in patients with large size discrepancies between the aortic root and pulmonary autograft |
| Resorbable scaffold | • Has the potential to combine the advantages of external support and excellent hemodynamics • Prevents dilatation in an animal model |
• Has not been tested in humans yet • Coronary anastomosis might be challenging when implanted in the aortic root position • Potential inflammatory reaction during resorption with unpredictable consequences |
| Tailored approach to prevent pulmonary autograft dilatation | • Maintains the natural elastic recoil of native tissue • Good midterm stability of the neo-sinus diameter |
• Adequate blood pressure control might be difficult to achieve with some patients |
| Subcoronary implantation | • Keeps the native aortic wall • Lower risk of dilatation for most patients • No coronary anastomoses |
• Can be technically challenging, especially in smaller annulus • Risk of early failure due to autograft distortion • Potential for early failure in cases of mismatch between the size of the pulmonary and aortic annuli • Coronary malposition limits generalizability to all anatomic variants |
Abbreviation: PEARS, personalized external aortic root support.
Ross perioperative care has also evolved, although evidence remains scarce. Several centers routinely prescribe anti-inflammatory agents postoperatively to mitigate the risk of homograft dysfunction, which is thought to be caused by an acute postoperative inflammatory reaction. Tight blood pressure control during the first postoperative year, to avoid autograft dilatation, is also part of the postoperative management strategy, and remote blood pressure monitoring is a practice in some institutions.
Aortic Valve Repair and BAV
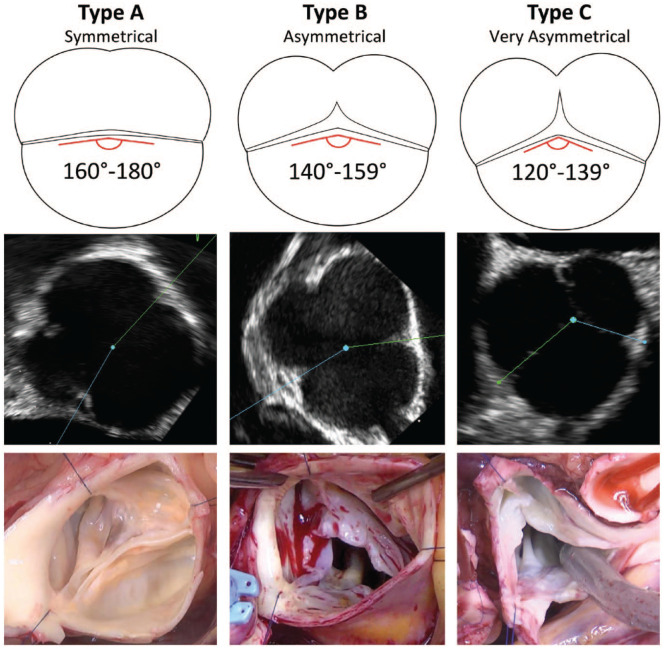
Concomitant aortic valve repair is necessary in many root operations and can generally be approached using the repair-oriented classification of AI, frequently in the context of BAV. Virtually all regurgitant BAVs present with both cusp and annular pathology. In particular, ventriculo-aortic junction (VAJ) dilation is a hallmark of BAV insufficiency. More recently, a BAV-focused, repair-oriented classification system has provided a unifying parameter, CO, that describes the spectrum of morphologies of BAVs, including raphe length and the pseudo-commissure height and spanning from a symmetrical phenotype at one extreme to a “tricuspid-like” phenotype (Fig. 2). 23
Fig. 2.
Schematic, echocardiographic, and intraoperative illustrations of the 3 groups of phenotypes of the repair-oriented BAV classification. Reprinted with permission under STM Signatory agreement from de Kerchove et al., “Variability of repairable bicuspid aortic valve phenotypes: towards an anatomical and repair-oriented classification,” Eur J Cardiothorac Surg, Oxford University Press, © 2019. 23 BAV, bicuspid aortic valve.
For patients with symmetric phenotype (CO: 160° to 180°), a postrepair CO of 180°/180° is optimal. In a very asymmetric valve (CO: 120° to 140°), tricuspidization can be performed by creating a functional commissure at the raphe, often requiring patch material. BAVs with asymmetric phenotype (CO: 140° to 160°) are typically made symmetric to enable greater conjoint leaflet mobility, facilitating leaflet repair. These valves may sometimes be left asymmetric if there is minimal preoperative AI and good leaflet coaptation. Symmetric versus asymmetric reconstruction of the BAV refers to all levels of the valve including VAJ, sinotubular junction, and the leaflet insertion line.
For patients with severe AI and aortic root <45 mm, aortic root replacement may be performed not just to eliminate the risk of aortic complications but rather to stabilize the sinotubular junction and VAJ, to allow symmetric or asymmetric remodeling of the aortic annulus, and to facilitate cusp repair.
In general, repaired valves should have less than mild residual AI and no eccentric jets, coaptation length ≥4 mm, effective height ≥9 mm, and VAJ ≤25 mm to provide a durable result. 24
Outcomes of BAV repair have improved significantly with greater understanding of valve anatomy and predictors of failure. Inadequate annular stabilization and reduction as well as use of a pericardial patch for leaflet augmentation are risk factors for late failure.25,26 Outcomes up to 15 years of follow-up are promising, with freedom from reoperation of ~85%. However, outcomes beyond the second decade are lacking. It remains unclear whether BAV root operations follow the same natural history of TAV counterparts both due to potential intrinsic differences in tissue quality and because of the durability of the repair itself.
Unicuspid aortic valve repair is uncommon and typically involves bicuspidization requiring the use of patch material for cusp augmentation. Although early results are acceptable, there is a significant rate of reoperation at midterm to long-term follow-up, often due to late patch calcification with resulting valve dysfunction.
As aortic valve repair techniques become increasingly common, questions regarding timing of intervention emerge and controversies remain. In general, it is important to separate the indication of valve and (often by extension root) interventions on the basis of primarily root-ascending aneurysm versus primarily valve dysfunction. Timing of intervention for a primarily valve issue may necessitate surgery at subsurgical or borderline aneurysm indications, and therefore, consideration must be given as to whether the added risk of concomitant root/ascending intervention is justified when the valve is repairable. In other words, if the choice of valve operation is Ross or valve sparing in a young patient, this must be counterbalanced against the added risk of root intervention in a relatively normal root. Furthermore, the sooner the root or valve intervention is performed, the more years of life expectancy the patient must be exposed to risk of repair failure and need for reintervention. These complex decisions demonstrate the interplay of many interdependent factors and the paucity of data to guide decision-making. There is a large variability in practice patterns in the modern era, and only time will tell which approaches optimize long-term freedom from reintervention, survival, and quality of life, particularly for young patients.
In the context of this discussion, BAV patients represent a special subgroup. The natural history of BAV aortopathy remains controversial with variability of guidelines regarding the recommended timing of intervention and risk of dissection. The long-term durability of valve repair in BAV patients compared with TAV multiple decades postintervention also remains to be seen. Ultimately, the current era aortic root surgeon must consider potential technical challenges in repairing BAV valve/roots, which may be worsened by delaying intervention, but also understand that delaying intervention may render the patient’s anatomy unrepairable.
Coronary Button Reconstruction
A common feature among all root reconstructive procedures is the need to reimplant coronary buttons and can be a source of devastating complications if not performed with scrupulous attention. Coronary anomalies are common, especially in the BAV population. 27 In both unicuspid and BAV, the coronary ostia are often displaced closer to the commissures than in TAV. This is of particular importance when performing a VSRR, in which great care should be taken when creating the button to ensure annular integrity is preserved (Supplemental Video 4). Further coronary anomalies can range from simply an ostium that is displaced higher than normal in the sinus of Valsalva to complex with single coronary ostium or other patterns of anomalous origin. 28 In the case of a higher-than-normal coronary ostium, care must be taken when creating the coronary button and performing root dissection to avoid injury to the coronary. Intramural coronary arteries may preclude safe coronary button creation and require abandoning or modifying root replacement techniques. When anomalous origin of coronaries is present, a myocardial protection strategy must be adapted to ensure adequate delivery of cardioplegia, including the use of direct ostial and retrograde techniques. Kinking after coronary button reconstruction is associated with younger age, excessive mobilization of coronaries, and indirect coronary reimplantation. 29 Additional risk factors for late complication and reintervention are related to inflammation secondary to implantation into Dacron, compared with native aorta or occult coronary button injury at the initial operation. 30
These technical challenges are further pronounced in situations in which tissue quality is poor such as acute aortic dissection and reoperative root surgery. 31 In acute dissection, it is critical to preserve all layers of the vessel wall when creating the coronary button. This can be particularly challenging in the presence of extensive hematoma, where identifying the adventitia can be difficult. Management of coronary buttons in redo root surgery is a critical step that deserves careful attention. It is advisable to begin with a large button that can be trimmed down after initial excision. Starting with a large button helps to reduce inadvertently damaging the coronary due to significant adhesions, use of prior foreign graft material, and the presence of extensive calcification. If a small button injury occurs, repair with interrupted fine monofilament suture is preferred over coronary artery bypass. If the injury is large and cannot be safely repaired, then coronary artery bypass of the affected vessel should be performed. Bypass should be considered only after exhausting all measures of reconstruction, especially for the left coronary arteries. In some cases of redo root surgery, the aortic root can be “frozen” with adhesions, making mobilization of the coronary buttons extremely challenging. In these cases, indirect coronary reimplantation can be used with either saphenous vein graft or as a Cabrol using an interposition Dacron graft. Once coronary button reconstruction is complete, the anastomosis can be tested by administering cardioplegia down the root, and any repairs can be made before closing the aortotomy.
Aortic Root Reconstruction in Acute Type A Aortic Dissection
Aortic root reconstruction in acute type A aortic dissection (ATAAD) involves complex decision-making, driven primarily by the extent of dissection and the condition of the aortic valve and coronary ostia. When the aortic valve is irreparably diseased, valve replacement is indicated, and root replacement makes logical sense. When the dissection extends into a single sinus without breaching the commissures, the aortic root can often be preserved with single sinus reconstruction with or without felt and Dacron. When the dissection extends into 2 or more sinuses, aortic root replacement is often indicated and far more hemostatic than attempting to salvage the circumferentially dissected native aortic root. If the aortic valve is intact and functional, valve-sparing techniques such as the reimplantation technique can be performed with excellent results 32 but require healthy and intact pericommissural aortic tissue for hemostatic reconstruction and commissural integrity. One unhinged commissure can be successfully reconstructed for VSRR in experienced hands; however, 2 or more unhinged commissures are likely best treated with composite root graft reconstruction. As previously mentioned, the integrity of the coronary buttons remains paramount during aortic root replacement in ATAAD. Overall, the addition of an aortic root replacement to ATAAD repair may provide long-term benefits but is associated with increased early mortality and complication risks. 33 Ultimately, the decision between preserving or replacing the aortic root in ATAAD should be tailored to individual patient anatomy and surgical risk, emphasizing the need for expert surgical judgment and experience (Supplemental Video 5).
Reoperative Root Surgery
Another group of aortic root patients who are associated with increased technical complexity and operative risk are those undergoing reoperative root surgery, an intervention that requires careful planning, thorough review of previous operative reports, and imaging. Ensuring a safe reentry allows for a heparin-free mediastinal dissection. Sternal contact of the aorta may necessitate alternate cannulation sites, rarely requiring hypothermic circulatory arrest upon entry. Mediastinal dissection is often tedious and should be carried out meticulously because final hemostasis will be affected. A short period of circulatory arrest before aortic cross-clamping may facilitate dissection of the aorta from the pulmonary artery and ensure adequate tissue quality and length for the distal anastomosis. An optimal myocardial protection strategy is essential in these long operations. Although dissecting out the previous root conduit may vary according to the type of disease and conduit, principles of coronary button mobilization in redo root surgery may be applied to most situations. To accomplish a safe left main button anastomosis without tension, full mobilization of the left main should be performed. Inserting a metal tip dilatator within the left main as guidance for dissection may ease mobilization and prevent inadvertent left main trauma. In the event of a “frozen” left main, we prefer to perform a “hemi-Cabrol” with an 8 mm Dacron graft or a high thigh vein in severe endocarditis cases. Anastomosis is performed early following root debridement, and the graft is used for cardioplegia infusion, which also allows for verification of hemostasis. The right coronary artery is dealt with in a similar fashion, although we do not hesitate, especially in older patients, to perform a short bypass to the proximal right coronary artery and ligate the frozen/fragile right ostium. Furthermore, the bypass is used to optimize right ventricle protection and facilitates hemostasis in case of low-lying bleeding following reperfusion.
Previous Bentall With Valve Dysfunction
Dissecting out the old conduit is performed by opening the fibrous shell around the Dacron graft. In case of a degenerated bio-Bentall, excision of the bioprosthetic valve may be performed with an 11 blade “hugging” the stent frame. Stitches of the new bioprosthesis incorporate a portion of the left ventricular outflow tract (LVOT) and Dacron graft, hence minimizing the risk of ventriculo-arterial dysfunction.
Homograft Degeneration
Homograft explant requires excising all the calcified root using either a 15 blade or electrocautery dissection. The calcium usually extends deep in the LVOT and aorto-mitral continuity. Meticulous dissection at the LVOT level allows for excision of the calcium and preservation of the thickened fibrotic tissue for strong suture placement. To reinforce the proximal anastomosis of the new root conduit, consideration should be given to performing a second running layer suture. Coronary ostia are usually free of the calcific degeneration process and most of the time may be tailored according to the previous outlined recommendations.
Previous Bentall With Endocarditis or Abscess
Once the aorta is cross-clamped, finding the plane of the graft is usually facilitated by the infectious process surrounding the graft. Eradication of the infectious process depends on extensive debridement of the devitalized/infected tissue up to the presence of healthy tissue. Reconstruction of the LVOT may require pericardial patch reinforcement. In the presence of severe infection, especially with resistant bacteria, reconstruction using a homograft may be advantageous to minimize the risk of reinfection. In case of an aorto-mitral continuity abscess, a “Commando operation” requiring concomitant mitral valve replacement by extending the aortotomy through the dome of the left atrium must be performed. 34 In a reoperative setting, transection of the superior vena cava may increase exposure.
Failed Ross With Annular Dilatation
Root preservation should be considered in young patients with normal autograft leaflets. Excision of the 3 autograft sinuses with deep dissection of the basal aortic ring and annular stabilization is a prerequisite to ensuring successful root preservation. Reconstruction may be performed thereafter using a reimplantation technique with a hemashield graft or a remodeling technique with annular stabilization.
Management of Adult Congenital Patients
Among patients requiring reoperative aortic root intervention, the indication is frequently in the context of previous congenital disease. Specifically, long-term management of congenital patients who undergo surgical repair for truncus arteriosus, arterial switch operation, and the Ross procedure during childhood often carry a significant burden of lifelong reintervention.
Truncus arteriosus is a rare but complex congenital heart defect known for significant early postoperative mortality and lifetime serial reinterventions. Twenty-year survival and freedom from reintervention have been reported to be 70% to 80% and 97% to 98%, respectively. 35 The most common reoperation is for right ventricular outflow tract (RVOT) reconstruction in the setting of right ventricle-pulmonary artery conduit, followed by branch pulmonary artery reconstruction, and third, truncal valve (TV) reintervention. Freedom from TV reoperation is 70% to 80% at 20 years. 36 Quadricuspid TV, baseline TV insufficiency, and TV intervention at index repair were associated with TV reintervention. 35
Arterial switch operation for dexto-transposition of the great arteries has favorable long-term survival of ~95% at 20 years; 37 however, RVOT obstruction, neo-aortic root dilation, AI, and coronary complications call for diligent surveillance. Reoperation for neo-aortic root dilatation and neo-AV insufficiency is ~5% beyond 10 years. 38 Unlike aortic root complications, coronary revision is much rarer but can be associated with significant early and late mortality. 39
In pediatric patients undergoing the Ross procedure, lifelong reintervention on the pulmonary homograft replacement is expected as well as potential autograft reintervention for neo-aortic root dilation and neo-AV insufficiency. Survival at 15 years is ~90%, and freedom from autograft and RVOT reintervention is 80% and 60%, respectively. 40 There is generally no association between age of surgery and autograft reintervention, with recent freedom from LVOT reintervention at 15 years reported to be ~70%. 41
Transcatheter Root Replacement
The evolution of alternative, less invasive options for root replacement has been driven by the need to expand the ability to address aortic root pathology in nonoperative patients. Transcatheter root replacement, or Endo-Bentall, combines TAVI, thoracic endovascular aortic repair, and coronary stenting technology into a single-stage procedure that stabilizes the aortic root. There are no commercially available purpose-built devices for this approach, requiring physician-constructed devices to be preassembled. To date, only a handful of patients have undergone successful transcatheter root replacement with these custom-made devices, with encouraging early results supporting feasibility.7,42 The most common indication has been ATAAD in nonoperative candidates but also in endocarditis. Endo-Bentall remains exclusively investigational and is being done only in highly selected, nonoperative patients by a few expert operators. Endo-Bentall is fraught with potential pitfalls, and there are no long-term data currently; however, these early studies have demonstrated the feasibility of physician-constructed composite graft devices. Challenges include positioning the coronary artery appropriately in the thoracic stent graft and that coronary landing zones are acceptable. Device deployment with appropriate commissural alignment facilitates coronary ostial alignment. The path to wider adoption lies in evaluating long-term durability and the development of off-the-shelf purpose-built devices. Like the evolution of TAVI technology with initial use in nonoperative patients, expanded use in high-risk patients is likely on the horizon.
Learning Curve and the Next Generation of Aortic Root Surgeons
With expanding indications and populations of patients requiring aortic root reconstruction, careful attention must be given to the next generation of aortic root surgeons. Early career aortic root surgeons should ideally advance to more complex cases in a stepwise manner. This is more easily accomplished in an established aortic program, with oversight from senior colleagues. Surgeons should already have reasonable exposure to AVRs, root enlargements, and ascending aorta replacements. Discussing plans with senior aortic surgeons for attempting these cases should be done. More predictable cases, with prescribed surgical steps (i.e., Bentall), are a better starting point than attempting cases with more variability and intraoperative decisions that differ for each case (i.e., Ross, VSRR, root abscess).
Redo root operations will be a part of every aortic root surgeon’s practice in the future. Some factors affecting this are older and sicker patients being offered surgery more frequently, the accelerated degeneration of externally wrapped bioprosthetic valves (Trifecta/Mitroflow), and the prevalence of intravenous drug use and endocarditis. 1
Technical Aspects That Lead to Failure
Inappropriate patient selection is an easily correctable cause of failures. When possible, more inexperienced surgeons should attempt more complex cases with patients who are otherwise healthy, avoid longer cardiopulmonary bypass times and bailout, and perform fewer complex procedures (Bentall vs VSRR). The more features of an ideal aortic valve are present, the greater likelihood of a successful repair (TAV, symmetric, no AI/stenosis or calcification, coaptation length >8 mm, no cusp prolapse, no ventricularization of cusps). Surgeons may attempt to salvage a less-than-perfect valve, as they gain experience and become more facile with aortic valve repair. Mentorship and intraoperative consultation from more senior colleagues are paramount.
Conclusions
In conclusion, aortic root reconstruction operations have undergone significant evolution with respect to technical modifications, indications, and increasingly complex decision-making. In the current era, aortic root patients must be managed in the context of lifetime aortic valve and root function, often planning for potential reinterventions and mitigating lifetime risk of thromboembolic/hemorrhagic complications. Future research regarding the natural history of particular subgroups of patients such as those with BAV, increasingly complex valve repair, acute type A dissection, and the congenital population is required. Furthermore, the role of transcatheter interventions and the effect on long-term survival, freedom from patient-prosthesis mismatch, and freedom from reintervention remain to be seen. Training for the future generation of aortic root surgeons includes being facile with aortic valve repair techniques, increasingly complex reoperative interventions, and scrupulous decision-making regarding the timing of the intervention and ability to accurately predict pathoanatomy from preoperative investigations. As the population of patients requiring root interventions expands, we anticipate increasing adaptation and modification of surgical techniques as well as multiple avenues for ongoing innovation in this field.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.W.C. is supported as the Ray and Elliott Chair in Surgical Innovation and has received speakers honoraria from Medtronic, Edwards Lifesciences, Terumo Aortic, and Artivion.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs: Sabin Bozso  https://orcid.org/0000-0001-7067-3102
https://orcid.org/0000-0001-7067-3102
Michael W. A. Chu  https://orcid.org/0000-0002-9872-399X
https://orcid.org/0000-0002-9872-399X
References
- 1. Isselbacher EM, Preventza O, Black JH, III, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022; 146: e334–e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bach DS, Kon ND. Long-term clinical outcomes 15 years after aortic valve replacement with the Freestyle stentless aortic bioprosthesis. Ann Thorac Surg 2014; 97: 544–551. [DOI] [PubMed] [Google Scholar]
- 3. Yousef S, Serna-Gallegos D, Brown JA, et al. Outcomes of root enlargement vs root replacement for aortic stenosis. Ann Thorac Surg 2023; 115: 1180–1187. [DOI] [PubMed] [Google Scholar]
- 4. El-Hamamsy I, Clark L, Stevens LM, et al. Late outcomes following Freestyle versus homograft aortic root replacement. Results from a prospective randomized trial. J Am Coll Cardiol 2010; 55: 368–376. [DOI] [PubMed] [Google Scholar]
- 5. Pettersson GB, Coselli JS, Coselli JS, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153: 1241–1258. [DOI] [PubMed] [Google Scholar]
- 6. Felipe Gaia D, Bernal O, Castilho E, et al. First-in-human Endo-Bentall procedure for simultaneous treatment of the ascending aorta and aortic valve. JACC Case Rep 2020; 2: 480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghoreishi M, Chahal D, Shah A, et al. First-in-human endovascular aortic root repair (Endo-Bentall) for acute type A dissection. Circ Cardiovasc Interv 2023; 16: E013348. [DOI] [PubMed] [Google Scholar]
- 8. Brennan JM, Edwards FH, Zhao Y, et al. Long-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. Circulation 2013; 127: 1647–1655. [DOI] [PubMed] [Google Scholar]
- 9. Kitamura M, Wilde J, Dumpies O, et al. Risk assessment of coronary obstruction during transcatheter aortic valve replacement: insights from post-BASILICA computed tomography. JACC Cardiovasc Interv 2022; 15: 496–507. [DOI] [PubMed] [Google Scholar]
- 10. Sá MP, Jacquemyn X, Van den Eynde J, et al. Impact of prosthesis-patient mismatch after transcatheter aortic valve replacement: meta-analysis of Kaplan-Meier–derived individual patient data. JACC Cardiovasc Imaging 2023; 16: 298–310. [DOI] [PubMed] [Google Scholar]
- 11. Elbatarny M, Tam DY, Edelman JJ, et al. Valve-sparing root replacement versus composite valve grafting in aortic root dilation: a meta-analysis. Ann Thorac Surg 2020; 110: 296–306. [DOI] [PubMed] [Google Scholar]
- 12. David TE, David CM, Ouzounian M, et al. A progress report on reimplantation of the aortic valve. J Thorac Cardiovasc Surg 2021; 161: 890–899. [DOI] [PubMed] [Google Scholar]
- 13. Mazine A, Chu MWA, El-Hamamsy I, et al. Valve-sparing aortic root replacement: a primer for cardiologists. Curr Opin Cardiol 2022; 37: 156–164. [DOI] [PubMed] [Google Scholar]
- 14. Schneider U, Hofmann C, Schöpe J, et al. Long-term results of differentiated anatomic reconstruction of bicuspid aortic valves. JAMA Cardiol 2020; 5: 1366–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aboud A, Charitos EI, Fujita B, et al. Long-term outcomes of patients undergoing the Ross procedure. J Am Coll Cardiol 2021; 77: 1412–1422. [DOI] [PubMed] [Google Scholar]
- 16. Notenboom ML, Melina G, Veen KM, et al. Long-term clinical and echocardiographic outcomes following the Ross procedure: a post hoc analysis of a randomized clinical trial. JAMA Cardiol 2024; 9: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El-Hamamsy I, Toyoda N, Itagaki S, et al. Propensity-matched comparison of the Ross procedure and prosthetic aortic valve replacement in adults. J Am Coll Cardiol 2022; 79: 805–815. [DOI] [PubMed] [Google Scholar]
- 18. Chauvette V, Bouhout I, Tarabzoni M, et al. The Ross procedure in patients older than 50: a sensible proposition? J Thorac Cardiovasc Surg 2022; 164: 835–844. [DOI] [PubMed] [Google Scholar]
- 19. Sievers HH, Stierle U, Charitos EI, et al. Fourteen years’ experience with 501 subcoronary Ross procedures: surgical details and results. J Thorac Cardiovasc Surg 2010; 140: 816–822. [DOI] [PubMed] [Google Scholar]
- 20. Caldaroni F, Skillington P, Wynne R, et al. 25 years of Ross operation: the inclusion technique keeps up the expectations. In: American Association of Thoracic Surgery (AATS) 104th Annual Meeting, Toronto, Canada, 27–30 April 2024, paper no. 57. Beverly, MA: AATS. [Google Scholar]
- 21. David TE Ouzounian M and David CM. Late results of the Ross procedure. J Thorac Cardiovasc Surg 2019; 157: 201–208. [DOI] [PubMed] [Google Scholar]
- 22. Skillington PD, Flynn CD, Larobina M, et al. The Ross inclusion technique. Ann Cardiothorac Surg 2021; 10: 541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Kerchove L, Mastrobuoni S, Froede L, et al. Variability of repairable bicuspid aortic valve phenotypes: towards an anatomical and repair-oriented classification. Eur J Cardiothorac Surg 2019; 56: 351–359. [DOI] [PubMed] [Google Scholar]
- 24. Guo MH, Boodhwani M. Aortic valve repair: from concept to future targets. Semin Thorac Cardiovasc Surg 2019; 31: 650–655. [DOI] [PubMed] [Google Scholar]
- 25. Boodhwani M, De Kerchove L, Glineur D, et al. Repair of regurgitant bicuspid aortic valves: a systematic approach. J Thorac Cardiovasc Surg 2010; 140: 276–284. [DOI] [PubMed] [Google Scholar]
- 26. Bavaria JE, Desai N, Szeto WY, et al. Valve-sparing root reimplantation and leaflet repair in a bicuspid aortic valve: comparison with the 3-cusp David procedure. J Thorac Cardiovasc Surg 2015; 149: S22–S28. [DOI] [PubMed] [Google Scholar]
- 27. Ouzounian M, Feindel CM, Manlhiot C, et al. Valve-sparing root replacement in patients with bicuspid versus tricuspid aortic valves. J Thorac Cardiovasc Surg 2019; 158: 1–9. [DOI] [PubMed] [Google Scholar]
- 28. O'Blenes SB, Feindel CM. Aortic root replacement with anomalous origin of the coronary arteries. Ann Thorac Surg 2002; 73: 647–649. [DOI] [PubMed] [Google Scholar]
- 29. Nardi P, Pisano C, Bassano C, et al. Bentall operation: early surgical results, seven-year outcomes, and risk factors analysis. Int J Environ Res Public Health 2023; 20: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anastasius M Hillis G and Yiannikas J.. The left main complication of the Bentall’s procedure. Cardiol Res 2013; 4: 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mazine A, David TE, Lafreniere-Roula M, et al. Early outcomes of the Bentall procedure after previous cardiac surgery. J Thorac Cardiovasc Surg 2021; 162: 1063–1071. [DOI] [PubMed] [Google Scholar]
- 32. Leshnower BG, Farrington WJ, Huckaby LV, et al. Long-term results of valve-sparing aortic root replacement in acute type A aortic dissection. Ann Thorac Surg. Epub ahead of print 16 September 2024. DOI: 10.1016/j.athoracsur.2024.09.007. [DOI] [PubMed] [Google Scholar]
- 33. Hage F, Hage A, Dagenais F, et al. Does adding an aortic root replacement or sinus repair during arch repair increase postoperative mortality? Evidence from the Canadian Thoracic Aortic Collaborative. Eur J Cardiothorac Surg 2021; 60: 623–630. [DOI] [PubMed] [Google Scholar]
- 34. David TE, Lafreniere-Roula M, David CM, et al. Outcomes of combined aortic and mitral valve replacement with reconstruction of the fibrous skeleton of the heart. J Thorac Cardiovasc Surg 2022; 164: 1474–1484. [DOI] [PubMed] [Google Scholar]
- 35. Guariento A, Doulamis IP, Staffa SJ, et al. Long-term outcomes of truncus arteriosus repair: a modulated renewal competing risks analysis. J Thorac Cardiovasc Surg 2022; 163: 224–236. [DOI] [PubMed] [Google Scholar]
- 36. Naimo PS, Bell D, Fricke TA, et al. Truncus arteriosus repair: a 40-year multicenter perspective. J Thorac Cardiovasc Surg 2021; 161: 230–240. [DOI] [PubMed] [Google Scholar]
- 37. van der Palen RLF, Blom NA, Kuipers IM, et al. Long-term outcome after the arterial switch operation: 43 years of experience. Eur J Cardiothorac Surg 2021; 59: 968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu MZL, Fricke TA, Buratto E, et al. Outcomes of neo-aortic valve and root surgery late after arterial switch operation. J Thorac Cardiovasc Surg 2024; 167: 1391–1401. [DOI] [PubMed] [Google Scholar]
- 39. Koubský K, Gebauer R, Tláskal T, et al. Long-term survival and freedom from coronary artery reintervention after arterial switch operation for transposition of the great arteries: a population-based nationwide study. J Am Heart Assoc 2021; 10: e020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dib N, Ben Ali W, Ducruet T, et al. The Ross procedure in children and infants: a systematic review with pooled analyses. CJC Pediatr Congenit Heart Dis 2024; 3: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bojko MM, Wiggins L, Cleveland JD, et al. The landscape of congenital heart disease treated with the Ross procedure. J Thorac Cardiovasc Surg. Epub ahead of print 12 September 2024. DOI: 10.1016/j.jtcvs.2024.09.006. [DOI] [PubMed] [Google Scholar]
- 42. Leshnower BG, Duwayri YM, Nicholson WJ, et al. Endo-Bentall procedure using off-the-shelf catheter devices to repair an aorto-atrial fistula. Circ Cardiovasc Interv 2023; 16: E012848. [DOI] [PubMed] [Google Scholar]