Graphical Abstract
Graphical Abstract.
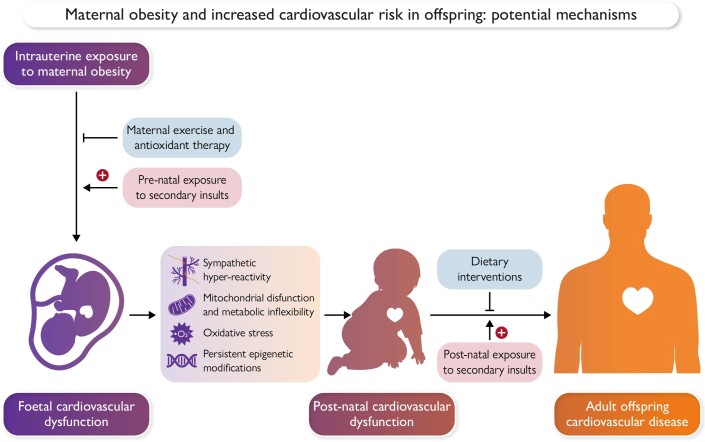
Intrauterine exposure to maternal obesity leads to offspring cardiovascular dysfunction through mechanisms including sympathetic hyper-reactivity, metabolic inflexibility, and mitochondrial dysfunction, as well as oxidative stress. Cardiovascular dysfunction originates in foetal life and persists into postnatal life through persistent epigenetic modifications. Interventions and secondary challenges may occur pre- and post-natally, which may impact progression to overt cardiovascular disease in adulthood. Created with BioRender.com.
Keywords: Foetus, Cardiovascular, Sympathetic, Oxidative stress, Mitochondria, Metabolism, Epigenetics, Pregnancy, Exercise, Antioxidants
Abstract
Pregnancy complicated by maternal obesity contributes to an increased cardiovascular risk in offspring, which is increasingly concerning as the rates of obesity and cardiovascular disease are higher than ever before and still growing. There has been much research in humans and preclinical animal models to understand the impact of maternal obesity on offspring health. This review summarizes what is known about the offspring cardiovascular phenotype, describing a mechanistic role for oxidative stress, metabolic inflexibility, and mitochondrial dysfunction in mediating these impairments. It also discusses the impact of secondary postnatal insults, which may reveal latent cardiovascular deficits that originated in utero. Finally, current interventional efforts and gaps of knowledge to limit the developmental origins of cardiovascular dysfunction in offspring of obese pregnancy are highlighted.
Key points.
Exposure to maternal obesity during pregnancy results in increased risk of cardiovascular disease in offspring.
Maternal obesity-induced cardiovascular dysfunction in offspring has origins in utero.
Cardiovascular dysfunction in offspring of obese pregnancy arises and persists through sympathetic hyper-reactivity, mitochondrial dysfunction, metabolic inflexibility, and epigenetic modification via miRNAs.
Exposure to secondary insults during adulthood, such as dietary modifications, stress, and ageing, can reveal latent cardiovascular impairments in offspring of obese pregnancy.
Interventions established during gestation, such as maternal exercise or antioxidant supplementation, may be key in preventing cardiovascular dysfunction at its origin in offspring of obese pregnancy.
Introduction
The World Health Organization describes obesity as a condition of epidemic proportions, with one in eight individuals and over 1.9 billion adults worldwide living with obesity.1 This rising prevalence of obesity matches a rising incidence of cardiovascular disease, which is now responsible for nearly 30% of all deaths in the UK.2–6 Increased adiposity leads to insulin resistance and hypertension, promoting a greater risk of cardio-metabolic disorders in obese individuals.7,8
The health implications of obesity rise exponentially to a much greater level of importance when considering maternal obesity.9 Over half of women in the UK are now overweight or obese during pregnancy.10 This is of the gravest concern as obesity during pregnancy not only has immediate detrimental effects on the mother but also on her children, thereby propagating adverse health conditions onto the next generation.11 Accumulating evidence derived from human studies and experimental animal models shows that maternal obesity can markedly increase the risk of cardiovascular disease in offspring,6–30 even when the progeny is fed a healthy diet and in the absence of them becoming obese.23 This highlights that it is something about the exposure of the embryo or foetus to an obesogenic environment during gestation itself that either triggers a foetal origin of cardiovascular dysfunction and/or increases susceptibility to heart disease in the adult offspring, consistent with the Developmental Origins of Health and Disease hypothesis.31
In humans, the best evidence to support developmental origins of cardiovascular health and disease in offspring of obese pregnancy comes from studies in women who were obese during a first pregnancy, lost weight through bariatric surgery, and were leaner during a second pregnancy.13,32,33 These studies show that siblings born before bariatric surgery have signs of an increased cardiovascular risk compared with those born after surgery.13,32,33 Therefore, such studies highlight that a different environment in the same womb can programme a differential risk of heart disease in offspring of the same family. This provides compelling evidence in humans that the environment experienced during critical periods of development directly influences long-term cardiovascular health. Therefore, when considering strategies to reduce the burden of heart disease on every nation’s health and wealth, there needs to be a greater focus on prevention rather than treatment (Figure 1).
Figure 1.
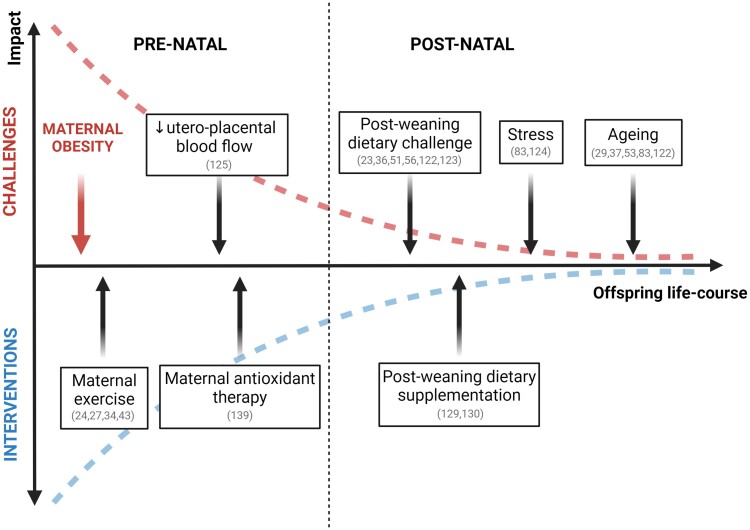
Timeline for intervention and secondary challenges over the life-course in offspring of obese pregnancy. The diagram shows that the younger we are the greater the impact that maternal obesity has upon us. Similarly, the opportunity for correction is greatest in younger life and diminishes progressively as we grow older. Therefore, candidate interventions should start as early as possible during the developmental trajectory, rather than waiting until disease is established. The diagram also shows that exposure to additional challenges in pre-natal and post-natal life, secondary to obese pregnancy, exacerbates the offspring cardiovascular dysfunction. The degree of impact of maternal obesity during pregnancy, superimposed challenges, and interventions is greatest in early pre-natal life, where the environmental sensitivity of progeny is highest, and falls exponentially across the offspring life-course. Key publications supporting statements are cross-referenced. Created with BioRender.com
This review summarizes the evidence derived from human clinical studies and experimental animal models that reflects the impact of maternal obesity on the cardiac and vascular health of the adult offspring. Mechanistically, the work describes how alterations in the intrauterine environment during maternal obesity, such as foetal hypoxia and hyperinsulinaemia34,35 can lead to oxidative stress,20,36 mitochondrial dysfunction and metabolic inflexibility,26,37–41 contributing to sympathetic hyper-reactivity21,22,36,42 and cardiovascular dysfunction21,37,38 in offspring. How postnatal diet, stress, or ageing may reveal or exacerbate an underlying cardiovascular susceptibility originating in utero is also highlighted. Finally, the review focuses on current interventions, such as maternal exercise or dietary supplementation during pregnancy, against the developmental programming of cardiovascular dysfunction in offspring of obese pregnancy.
Maternal obesity impacts offspring cardiovascular function during the postnatal period
Evidence from human studies
An association between maternal obesity and offspring cardiovascular dysfunction postnatally is evident across many studies in humans (Table 1 and Figure 2). Increased maternal body mass index (BMI) during pregnancy is associated with higher rates of hospital admissions due to cardiovascular events in adult offspring aged between 31 and 6412,78 and in a larger cohort aged between 27 and 76,15 with cardiovascular disease risk also higher in young human offspring aged between 1 and 25.14 Studies in children born to obese mothers reveal structural and functional cardiovascular alterations which likely drive this increased disease risk. In young children, increased maternal BMI during pregnancy is associated with left ventricular hypertrophy16 and greater epicardial adiposity.17 This is associated with diastolic dysfunction at 12 months of ages.71
Table 1.
Cardiovascular outcomes in offspring of obese pregnancy
| Species | Control diet | Experimental diet | Diet exposure period | Foetal/neonatal cardiovascular outcomes | Juvenile/adult offspring cardiovascular outcomes | References |
|---|---|---|---|---|---|---|
| Rodents | ||||||
| Mouse C57BL/6J | 3% fat, 7% sugar | 16% fat, 33% sugar | 6 weeks pre-pregnancy, throughout pregnancy and lactation | Altered cardiac lipidome (↑sphingomyelins and acyl-carnitines, ↓cholesteryl esters at d18.5) ↑ primary cardiomyocyte oleate oxidation and expression of genes associated with sterol, fatty acid and carnitine metabolism (at d18.5) ↑ cardiac HIF-1α and Ppara targets (d18.5) |
↑ heart weight and cardiac hypertrophy (at 3 and 8 weeks) Re-expression of foetal genes (↑ NPPB, ACTA1, MYH7:MYH6 ratio at 3 weeks) Cardiac systolic and diastolic dysfunction (at 12 weeks) ↓ LV ejection fraction and cardiac output (at 8 weeks) ↑ cardiac insulin and proliferative signalling (at 8 weeks) Cardiac oxidative stress (at 8 weeks) Cardiac and resistance artery sympathetic dominance (at 12 weeks) ↓ cardiac SERCA2a, total and phosphorylated troponin-I (at 12 weeks) ↑ active SBP and MAP but ↓ active heart rate and locomotion (at 12 weeks) Endothelial dysfunction in resistance arteries (at 12 weeks) |
20,21,23,24,39,42 |
| Mouse C57BL/6J |
10% fat | 40% fat, 20% sucrose soln. | 4–6 weeks pre-pregnancy, throughout pregnancy and lactation | ↑ heart weight (at d18.5) Altered cardiac expression of genes involved in metabolism (↑Pparg, Cd36 and Prkaa1) with predicted ↓ neoplasia and DNA repair/synthesis and ↑ lipid synthesis and metabolism in males, ↓ immune cells and ↑ uptake of mono- and polysaccharides in females |
Cardiac diastolic dysfunction in males (at 3, 6 and 24 months) and females (at 6, 9 and 24 months) ↑ heart weight in females (at 6 months) and ↑ cardiomyocyte cross-sectional area in males (at 3 months) ↑ cardiac foetal gene expression (at 3 months) ↑ cardiac Akt and mTOR signalling in males and ↓ERK1/2 signalling (at 3 months) Dysregulation of metabolism-related genes with ↑ expression of Pparg and targets related to lipid synthesis, storage and oxidation (at 6 months) ↑ myocardial mitochondrial fatty acid oxidation in males (at 6 months) ↓ myocardial glucose uptake in females (at 6 months) |
25,37 |
| Mouse C57BL/6J |
10% fat | 60% fat/45% fat | 8 weeks pre-pregnancy, 45% fat throughout pregnancy | ↓ placental vascular density | 43 | |
| Mouse C57BL/6J |
10% fat | 60% fat | 12 weeks pre-pregnancy, to necroscopy | Systolic dysfunction (↓ ejection fraction and fractional shortening) and diastolic dysfunction (↑ Tei index) at d16.5 ↑ cardiac ROS content (at d16.5) |
44 | |
| Mouse C57BL/6J |
25% fat, 3% sugar | 60% fat, 13% sugar | 6 weeks pre-pregnancy, throughout pregnancy and lactation | ↑ absolute and relative left ventricular mass and internal diameter (at 8 weeks) ↓ fractional shortening in females (at 8 weeks) Circular cardiac mitochondrial morphology and disorganized sarcomere alignment ↓ cardiac mitochondrial oxygen consumption, CI and CII activity (at 8 weeks) |
26 | |
| Mouse C57BL/6N |
10% fat | 45% fat | 13 weeks pre-pregnancy, throughout pregnancy and lactation | Sympathetic dominance (↑ aortic adrenergic vasoconstriction at 14 weeks) | 27 | |
| Mouse C57BL/6 |
10% fat | 45% fat | 6 weeks pre-pregnancy, throughout pregnancy and lactation | ↑ systolic blood pressure (at 10 months) | 28 | |
| Mouse C57BL/6 |
5% fat | 60% fat | 4 weeks pre-pregnancy, throughout pregnancy and lactation | ↑ systolic and diastolic blood pressure (at 12 months) | 29 | |
| Mouse C57BL/6J |
21% | 45% | 4 weeks pre-pregnancy, throughout pregnancy and lactation | ↑ systolic blood pressure (at 30 weeks) ↓ Acetylcholine-mediated vasorelaxation in femoral arteries (at 15 and 30 weeks) ↓ Basal NO production (at 30 weeks) ↑ oxidative stress in femoral arteries (at 15 weeks) |
36 | |
| Rat Sprague-Dawley |
4.3% fat | 24% fat | From weaning, throughout pregnancy | ↑ neonatal heart weight, cardiac fat deposition and apoptosis | ↑ heart weight, cardiac fat deposition and apoptosis (at 1 and 3 months) | 30 |
| Rat Sprague-Dawley |
18% fat | 40% fat | 4 weeks pre-pregnancy, throughout pregnancy and lactation | ↑ heart:body weight ratio and myocardial lipid deposits (in neonates) ↓ heart rate and E:A ventricular filling ratio with ↑ isovolumetric contraction time (in neonates) ↓ cardiomyocyte basal oxygen consumption ↑ smaller, wider and fragmented cardiac mitochondria with ↓ mitochondrial fusion and fission and sex-specific alterations in expression of dynamism-related proteins (in neonates) Cardiac lipid peroxidation (in neonates) ↓ Cardiac FGF-activated PI3K/AKT signalling and ↑ PGC1α mitochondrial biogenesis signalling (in neonates) |
38,40,45 | |
| Rat Sprague-Dawley |
3% fat, 7% sugar | 16% fat, 33% sugar | 6 weeks pre-pregnancy, throughout pregnancy and lactation | ↑ night-time MAP and MAP response to stress (at 4 and 12 weeks) ↓ HR in females (at 4 weeks) and males (at 12 weeks) Sympathetic dominance with ↑ renal tissue noradrenaline (at 4 and 12 weeks) ↑ changes in MAP to NO donor, α−adrenergic agonist and leptin, with ↓ baroreflex sensitivity of HR (at 12 weeks and 6 months) |
22 | |
| Rat Sprague-Dawley |
10% fat | 60% fat | 8 weeks pre-pregnancy, throughout pregnancy and lactation | ↑ systolic and diastolic blood pressure (at 6 months) Altered renin-angiotensin pathway in adipose (at Day 1 and 6 months) |
46 | |
| Rat Sprague-Dawley |
4.5% fat | 12.1% fat | 12 weeks pre-pregnancy, throughout pregnancy and lactation | Mesenteric artery hypertrophy (at 4 months) Sex-specific alterations in vasodilator pathway dependence, with DNA methylation changes in vascular function genes (potassium channels and NO synthase in males, guanylate cyclase and angiotensin receptor I in females) (at 4 months) |
47 | |
| Sheep | ||||||
| Sheep Rambouillet/Columbia cross |
100% NRC | 150% NRC | 60 days pre-pregnancy, to necroscopy at d75 or d135 (.5 or .9 gestation) or term | ↑ left ventricular weight and left ventricular wall thickness (at d135) ↑ hypertrophic signalling (Foxo3a, mTOR and calcineurin pathways) and cardiac hyperplasia (at d75) ↑ cardiomyocyte cross-sectional area Irregular myofibre orientation, and perivascular fibrosis (at d75) ↓ cardiac contractile function in response to a high workload stress challenge (at d135) ↓ cardiomyocyte contractility, disrupted Ca2+ handling and ↑ myosin heavy chain β:α (slow twitch) expression (at d135) ↔ heart rate (at d135) ↓ cardiac insulin signalling Activation of fibrogenic genes (at d75 and d135), associated with increased collagen concentration (at d135) ↓ cotyledonary vascularity (at d75) ↑ aortic wall thickness and collagen:elastin ratio (at d135) |
↑ Left and right ventricular wall thickness, ↑ myocardial collagen content and crosslinking and ↑ expression of pro-inflammatory cytokines (at 22 months, after a 3 month ad libitum feeding challenge) ↑ systolic blood pressure and heart rate at 2.5 months but ↓ systolic and diastolic blood pressure at 9 years Systolic dysfunction with ↓ fractional shortening, cardiac output and ejection fraction (at 9 years) |
48–53 |
| Non-human primates | ||||||
| Baboon | 12% fat, .61% sugar | 45% fat, 12.58% sugar | 4 months pre-pregnancy and throughout pregnancy to d165 (.9 gestation) | ↑ myocardial fibrosis (d165) Dysregulated expression of cardiac microRNAs associated with cardiovascular disease ↑ cardiomyocyte proliferation rates |
54 | |
| Japanese macaque | 14% fat | 32% fat | 4+ years pre-pregnancy to necroscopy at d130 (.75 gestation) | ↓ foeto-placental volume blood flow (at d120) | 55 | |
| Japanese macaque | 14% fat | 36% fat | 4+ years pre-pregnancy, throughout pregnancy and lactation | Altered aortic endothelial function depending on post-weaning diet: ↑ sensitivity to acetylcholine in control diet fed offspring, ↓ sensitivity and max relaxation to acetylcholine and in high fat diet fed offspring associated with hyperinsulinaemia (at 13 months, juvenile) ↑ aortic intima thickness and expression of pro-inflammatory markers and ↓ fibrinolytic signalling, regardless of post-weaning diet (at 13 months) |
56 | |
| Humans | ||||
|---|---|---|---|---|
| Maternal phenotype | Offspring age | Foetal/neonatal offspring | Child/adult offspring | References |
| Obese: mean pre-pregnancy BMI 35 kg/m2 Control: mean pre-pregnancy BMI 21 kg/m2 |
Foetal: 14, 20 and 32 weeks of gestation | ↑ interventricular septum thickness ↓ left and right ventricular global strain rate and strain (at 14, 20 and 32 weeks) ↓ tissue Doppler systolic and late diastolic velocities (at 20 and 32 weeks) ↔ heart rate |
57 | |
| Obese: BMI >30 kg/m2 Control: BMI <30 kg/m2 |
Foetal: 25 weeks of gestation | ↓ left ventricular ejection fraction and strain ↔ intra-ventricular septal thickness, myocardial performance index and mitral E/A ratio |
58 | |
| Obese: pre-pregnancy BMI >30 kg/m2 Control: pre-pregnancy BMI 18.5–24.9 kg/m2 |
Foetal: 26–38 weeks of gestation | ↑ heart rate and heart rate variability ↓ sympathetic dominance (LF:HF ratio) |
59 | |
| Obese: pre-pregnancy BMI of ≥30 kg/m2 Control: pre-pregnancy BMI <25 kg/m2 |
Foetal: 26–38 weeks of gestation | Diastolic dysfunction with ↑isovolumetric relaxation time | 60 | |
| Pre-pregnancy BMI range: 18.2–34.9 kg/m2 | Foetal: during parturition | ↑ sympathetic dominance (LF:HF ratio) with ↑ maternal BMI | 61 | |
| Obese: pre-pregnancy BMI ≥30 kg/m2 Control: pre-pregnancy BMI 18.5–24.9 kg/m2 |
Foetal | ER stress and reduced NO synthesis in HUVECs ↓ NO-dependent dilation of umbilical vein segments in response to insulin ↓ Insulin signalling (↑ inhibitor phosphorylation of IRS-1 and ↓ activator phosphorylation of Akt) |
62,63 | |
| Obese: BMI ≥25 kg/m2 Control: BMI <25 kg/m2 |
Foetal | Alterations in expression of genes related to lipid and mitochondrial metabolism in HUVECs | 64 | |
| Obese: 32 weeks BMI ≥30 kg/m2 Control: 32 weeks BMI 18.5–24.9 kg/m2/37 weeks BMI <30 kg/m2 |
Foetal: 32 and 37 weeks of gestation | ↑ umbilical artery pulsatility at 32 weeks ↔ umbilical artery pulsatility and foetal middle cerebral artery pulsatility at 37 weeks |
65,66 | |
| Obese: parturition BMI ≥30 kg/m2 Control: parturition BMI 19.8–25.9 kg/m2 |
Foetal | ↑ umbilical artery contractility | 67 | |
| Obese: BMI ≥30 kg/m2 Control: BMI 18.5–24.9 kg/m2 |
Foetal | ↓ chorionic plate artery endothelium-independent vasodilatation to nitric oxide donor | 68 | |
| Obese (LGA infant): BMI 31.5 ± 2.5 kg/m2 Control (AGA infant): BMI 22.0 ± .6 kg/m2 |
Foetal | ↓ chorionic plate artery endothelium-dependent vasodilatation to adiponectin in LGA pregnancies | 69 | |
| Obese: Pre-pregnancy BMI ≥30 kg/m2 Control: pre-pregnancy BMI 10–15 kg/m2 |
Neonatal | ↑ heart rate and ↓ heart rate variability ↓ left ventricular end diastolic volume and stroke volume |
70 | |
| Overweight/obese: BMI ≥25 kg/m2 Control: < 25 kg/m2 |
Neonatal and 12 months. | ↑ left ventricular posterior wall ↑end-diastolic and stroke volume |
71 | |
| Obese: BMI >30 kg/m2 Control: BMI 18.5–24.9 kg/m2 |
1–25 years | ↑ rates of cardiovascular disease | 14 | |
| Obese: pre-pregnancy BMI ≥30 kg/m2 Control: pre-pregnancy BMI 18.5–24.9 kg/m2 |
4–6 years | ↑ systolic and diastolic blood pressure ↔ autonomic balance |
18,72 | |
| Obese: BMI >30 kg/m2 Control: BMI 18.5–24.9 kg/m2 |
6 years | ↑ systolic blood pressure ↑ left ventricular eccentric hypertrophy and aortic root diameter with ↑ maternal BMI ↔ fractional shortening, E/A ratio, systolic and diastolic strain |
16,19,73,74 | |
| Obese: gestational BMI of ≥30 kg/m2, body weight >91 kg or above normal by 110%–120% Control: mean gestational BMI 18 kg/m2 |
7–9 years | ↑ epicardial adipose tissue thickness Arterial hypertrophy (↑ carotid intima-media thickness Reduced arterial compliance with ↓ arterial distensibility and strain and ↑ arterial stiffness |
17 | |
| Obese: BMI >30 kg/m2 Control: BMI 18.5–24.9kg/m2 |
6–10 years | ↑ systolic and diastolic blood pressure | 75,76 | |
| Pre-bariatric surgery (obese): mean BMI 45 kg/m2 Post-bariatric surgery: mean BMI 27.6 kg/m2 |
9–15 years | ↑ blood pressure in offspring born pre-bariatric surgery | 13 | |
| Overweight/obese: BMI >25 kg/m2 Control: BMI 18.5–24.9 kg/m2 |
9–15 years | Maternal BMI positively associated with offspring waist circumference, and negatively associated with offspring cardiorespiratory fitness No association between maternal BMI and offspring blood pressure |
77 | |
| Obese: BMI >30 kg/m2 Control: BMI 18.5–24.9 kg/m2 |
Adult: 27–76 years | ↑ risk of hospital admission for cardiovascular event ↑ systolic and diastolic blood pressure |
12,15,78–80 | |
There is extensive evidence of cardiovascular dysfunction in offspring of obese pregnancy, in both pre-natal and post-natal life, across pre-clinical rodent, sheep and non-human primate models, and studies in humans. Diet % in kcal.
Abbreviations: AGA, average for gestational age (foetal weight); Akt, protein kinase B; ACTA1, actin alpha 1; BMI, body mass index; Cd36, a plasma membrane fatty acid translocase; E/A ratio, early/atrial ratio; FGF, fibroblast growth factor; HIF-1α, hypoxia-inducible factor 1 alpha; HUVEC, human umbilical vein endothelial cell; IRS-1, insulin receptor substrate 1; LF:HF ratio, low frequency: high frequency ratio; LGA, large for gestational age (foetal weight); MAP, mean arterial pressure; mTOR, mammalian target of rapamycin; MYH6/7, myosin heavy chain beta 6/7; NO, nitric oxide; NPPB, natriuretic peptide B; Pparg/a, nuclear peroxisome proliferator activated receptor.
Figure 2.
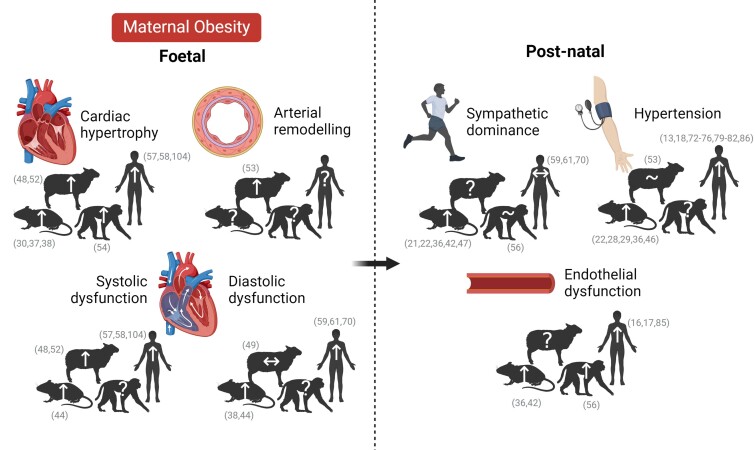
Cardiovascular phenotype of foetal and adult offspring of obese pregnancy in human and pre-clinical animal models. Maternal obesity induces cardiac hypertrophy,30,37,38,48,52,54,57,58,104 arterial remodelling,53 and systolic and diastolic dysfunction38,104,44,48,49,52,57–59,61,70 in the foetal offspring, leading to sympathetic dominance,21,22,36,42,47,56,59,61,70 endothelial dysfunction16,17,36,42,56,85, and hypertension79–82,13,18,22,28,29,36,46,53,72–76,86 in post-natal life. Key publications supporting statements are cross-referenced. Created with BioRender.com
Vascular alterations are also present, with increased aortic root diameter, arterial hypertrophy, and reduced arterial compliance in children of obese pregnancy.16,17,85 Together, these changes likely contribute to the increased prevalence of hypertension in children and adolescents born to mothers with obesity,13,18,72–76,81,86 showing a positive association between maternal pre-pregnancy BMI and offspring blood pressure even in the first year of life,82 that persists into adulthood.79,80 Evidence from human studies also indicates that cardiovascular disease risk in adult offspring is related to the degree of maternal obesity, with increased rates of cardiovascular disease only seen in offspring of mothers with obesity Grade II or higher (BMI over 35 kg/m2), which may be partly due to increased risk of additional complications such as neonatal asphyxia.14
Evidence from animal studies
To further understand the cardiovascular phenotype of offspring of obese pregnancy, rodent, ovine, and non-human primate models of maternal obesity have been generated, each showing different technical and translational advantages and limitations, summarized in Table 2. Across mammalian preclinical models, exposure to maternal obesity during pregnancy leads to alterations in the heart structure and function in the progeny (Table 1 and Figure 2). Rodent offspring of obese pregnancy show increased heart weight,20,21,25,30,83,84 with increased cardiomyocyte size.20,21,25,26,83,84 These alterations occur with the activation of hypertrophic signalling pathways including the re-expression of foetal genes20,25 and increased insulin signalling through AKT, ERK, and the mammalian target rapamycin (mTOR).20,25 Cardiac hypertrophy with greater myocardial collagen content has also been reported in adult offspring in an ovine model of maternal overnutrition.51
Table 2.
Advantages and limitations of rodent, sheep and non-human primate models
| Rodents | Sheep | Non-human primates | |
|---|---|---|---|
| Advantages | |||
| Translational | Invasive, haemochorial placentation with trophoblast-mediated spiral artery remodelling, comparable to humans87 Feeding of highly translational ‘cafeteria’ and ‘Western-style’ diets to rodents leads to obesity and metabolic profiles comparable to humans88 |
Sheep are a precocial species with comparable cardiovascular developmental milestones to human pregnancy89,90 Certain breeds (e.g. welsh Mountain and Merino) are primarily uniparous with neonatal lamb weights comparable to full-term human infants89 Human and sheep placentas have a villous tree structure, concurrent exchange, and similar glucose and amino acid transport systems91,92 Treatments developed in sheep have been highly successful in humans e.g. antenatal maternal corticosteroid therapy for pre-term infants93 |
Relevant for highly species-dependent processes such as the mechanisms promoting parturition94 Similar hormonal changes and duration of menstrual cycle95 |
| Technical | Short gestational length and reproductive cycle facilitates adult offspring and multi-generational studies Multiple pregnancy allows for study of sex differences within litter and using siblings for different outcomes (e.g. freezing vs. fixing) Cross-fostering and embryo transfer possible to isolate the critical periods of peri-conception, gestation and lactation |
Chronic surgical instrumentation of the foetus is possible89,96–99 Longitudinal maternal and foetal blood sampling possible across gestation89,96–99 Ex vivo studies of foetal resistance arteries is possible100 |
Longitudinal maternal blood sampling possible across gestation Ex vivo studies on foetal cardiovascular system possible Life span and time to maturity are shorter than in humans |
| Limitations | |||
| Translational | Rodents are an altricial species with cardiovascular developmental milestones that differ from humans89,91 Differences in basal metabolic rate and glucose disposal between rodents and humans may influence metabolic adaptations occurring with obesity and pregnancy Multiple pregnancy leads to differences from humans in foetal nutrient allocation |
Lack of translational relevance for highly species-dependent processes such as the mechanisms promoting parturition101 Placentation is cotyledonary and synepitheliochorial102 Ruminant metabolism in sheep may result in different metabolic profile occurring with diet-induced obesity |
Non-human primates show differences in placentation, with superficial blastocyst implantation, fewer interstitial trophoblasts and earlier onset of placental circulation103 |
| Technical | Foetal long-term surgical instrumentation not feasible | Length of gestation and life span make adult offspring and multi-generational studies costly and time-consuming | Foetal instrumentation very limited Foetal blood sampling across gestation limited Length of gestation and life span make adult offspring studies costly and time-consuming |
The primary pre-clinical models of maternal obesity in pregnancy are rodent, ovine and non-human primate models. Each of these models presents its own translational and technical advantages and limitations, which relate to the use of this animal model as a study of obesity and as a model of reproductive biology.
Maternal obesity also leads to systolic dysfunction in the adult offspring, with impaired cardiac output seen in mice23,26,83 and sheep.53 Reductions in left ventricular developed pressure,21 fractional shortening,24,26,83 ejection fraction,23,24,26,83 and heart rate22,42 all contribute to a lower cardiac output in rodent offspring. However, there are several discrepancies in heart rate and blood pressure changes in offspring of obese pregnancy; juvenile sheep show a trend towards tachycardia at 2.5 months associated with hypertension, both of which are absent at 9 years.28 In contrast, mouse offspring showing bradycardia at 1 and 3 months that reverses to tachycardia at 6 months, while showing a hypertensive phenotype at all time points.16,35 These differences may be age-dependent and influenced by a range of factors including species differences. Systolic dysfunction is associated with impairments in cardiomyocyte Ca2+ handling and activation of contractile proteins in mouse offspring.21 Diastolic dysfunction in mouse offspring of obese pregnancy results from an increase in left ventricular end-diastolic pressure,21,37 a reduced ratio of early-to-late left ventricular wall displacement and mitral inflow,25,37,83 together with longer isovolumetric relaxation time.83
Vascular alterations have also been reported, with mesenteric artery hypertrophy in adult rat offspring47 and thickening of the aortic intima in non-human primate offspring56 of obese pregnancy. Vascular dysfunction is evident, with a reduction in endothelium-dependent relaxation in resistance arteries of adult mice offspring of obese pregnancy.36,42 The effect on conduit arteries is less clear, with Macaque offspring of obese pregnancy showing increased aortic endothelial sensitivity to acetylcholine.56 In contrast, thoracic aorta endothelium-dependent and independent vasodilatation remained unaltered in adult mice offspring of obese pregnancy.27 This conflicting evidence may arise from species differences, study of resistance versus conduit vessels, and/or due to investigation of outcomes at different stages of maturity of the adult offspring. For instance, Macaque offspring were studied during the juvenile period56 while mouse offspring were studied as mature adults.27 Despite species and vascular bed differences, it is clear that offspring exposed to maternal obesity during gestation show alterations in vascular structure and function, which may contribute to the development of cardiovascular disease later in adulthood.
Maternal obesity impacts offspring cardiovascular dysfunction during the prenatal period
While impacts on adult offspring cardiovascular risk are well established, there is now accumulating evidence suggesting that cardiovascular dysfunction in human offspring of obese pregnancy may originate before birth (Figure 2).
Evidence from human studies
A reduction in bi-ventricular global strain is present in human foetuses of obese mothers at 14 weeks of gestation.57,58,104 Tissue Doppler imaging of foetal cardiac systolic and diastolic velocities and left ventricular ejection fraction reveals a reduction in all variables in human foetuses of obese mothers by 20–25 weeks, while an increased interventricular septum thickness becomes evident by 32 weeks of gestation.57,58,104 Basal foetal heart rate and heart rate variability are increased from mid-gestation in obese compared with healthy human pregnancies, associated with a reduction in the low frequency: high frequency (LF:HF) ratio.59 However, echocardiography studies during stimulated conditions, such as during parturition, revealed an increase in the foetal heart LF:HF ratio in obese pregnancy61 and neonatal recordings show decreased heart rate variability.70
Vascular dysfunction is also apparent in the human foetus of obese pregnancy, with increased umbilical artery constriction to serotonin,67 and impaired endothelium-dependent dilatation of the umbilical vein to insulin, an effect associated with vascular insulin resistance and oxidative and endoplasmic reticulum stress.62,63 These changes occur with an increase in the umbilical artery pulsatility index in offspring of obese women, measured at 32,65 but not at 3766 weeks of gestation. Impairments in endothelium-dependent and independent vasodilatation have also been reported in chorionic plate arteries of obese human pregnancy.68,69 However, no difference was found in the foetal middle cerebral artery pulsatility index with obese pregnancy.66
Evidence from animal studies
Evidence derived from preclinical animal models, including rodent, sheep, and non-human primates, show cardiac structural alterations in offspring of obese pregnancy in prenatal life, which match the hypertrophy seen in adulthood (Table 1 and Figure 2). Maternal obesity leads to increased heart weight in foetal mice37 and neonatal rats.30,38 The late-gestation foetal baboon shows increased cardiomyocyte proliferation and myocardial fibrosis in obese pregnancy, indicative of pathological hypertrophy.54 Similarly, foetal sheep exposed to maternal obesity show increased left ventricular weight and wall thickness with higher cardiomyocyte cross-sectional area, activation of hypertrophic signalling, and evidence of cardiac fibrosis.48,52
Evidence derived from preclinical animal models also supports impairments in cardiac function in foetal life during obese pregnancy. Foetal mice of obese pregnancy show systolic and diastolic dysfunction, with lower values for ejection fraction and fractional shortening, increased time spent in isovolumetric contraction and relaxation, and a reduction in the early atrial to ventricular (E:A) filling ratio.44 Foetal sheep cardiomyocyte contractility is also reduced in obese pregnancy, associated with impaired Ca2+ handling and an increased proportion of slow-twitch myosin heavy chains, resulting in impaired systolic function.49,52
Relatively few animal studies have explored the prenatal origin of vascular dysfunction in offspring of obese pregnancies, in part due to the practical size limitations of evaluating vascular reactivity of resistance circulations in foetal rodents. Placental vascular density is reduced during pregnancy in obese mice and sheep mothers,43,105 and foeto-placental blood flow is impaired in the obese Japanese macaques.55 Arterial hypertrophy is also evident, with an increased aortic wall thickness and in the aortic collagen:elastin ratio in the foetus of over-nourished ewes.53 Therefore, the available literature supports that obese pregnancy leads to alterations in the vascularization of tissues and in vascular structure in foetal life. However, the impact of maternal obesity on foetal vascular function appears entirely unknown, warranting further investigation.
Mechanisms of cardiovascular dysfunction in offspring of obese pregnancy
Animal models have been indispensable to identify causal mechanisms of cardiovascular disease programming by maternal obesity. Although several mechanisms have been proposed, the most prevalent leading to a persistent offspring phenotype can be summarized in four broad areas: sympathetic hyper-reactivity, mitochondrial dysfunction and metabolic inflexibility, oxidative stress, and epigenetic dysregulation including via miRNAs (Figure 3).
Figure 3.
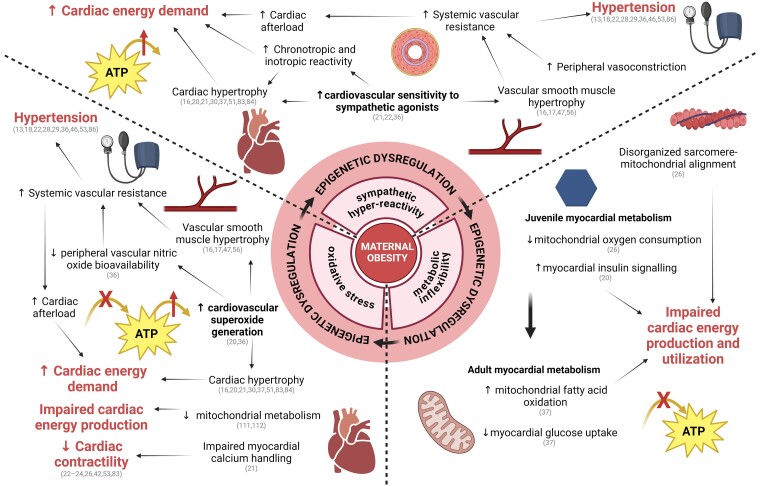
Mechanisms mediating cardiovascular dysfunction in offspring of obese pregnancy. Exposure to maternal obesity in utero leads to sympathetic hyper-reactivity,21,22,36 metabolic inflexibility20,26,37, and oxidative stress20,36 in the offspring, maintained through persistent epigenetic regulation,52,120,122 and eventually leading to overt cardiovascular dysfunction. Key publications supporting statements are cross-referenced. Created with BioRender.com
Sympathetic hyper-reactivity
Sympathetic dominance in the cardiovascular system of adult offspring of obese pregnancy can be seen in many forms, including increased cardiac and vascular sensitivity to sympathetic agonists.21,36,42 There is also greater dose-dependent arterial pressure response to alpha-adrenergic agonists in adult offspring of obese rat pregnancy.22 Sympathetic hyper-reactivity of the peripheral vasculature can precipitate cardiovascular dysfunction as enhanced basal sympathetic tone and arterial hypertrophy independently promote an increase in peripheral vascular resistance, thereby increasing arterial blood pressure.107 Therefore, sympathetic hyper-reactivity contributes to the offspring hypertensive phenotype observed across several animal models of obese pregnancy.22,28,29,36,46,53,86 Increased arterial blood pressure also leads to a greater cardiac afterload, resulting in increased cardiac work. While an enhanced sympathetic drive helps to maintain cardiac output, it is known to be unsustainable, eventually becoming a hallmark of early-stage heart failure.108,109
An increased LF:HF ratio of foetal heart rate variability during parturition has been reported in pregnancies with increased maternal BMI in humans, consistent with a foetal origin of cardiac sympathetic dominance.61 However, a reduction in LF:HF has been measured during mid-to-late gestation,59 and no difference was found in the cardiac autonomic regulation of 5–6-year-old children born to obese compared with healthy weight mothers.18 This suggests that underlying sympathetic hyper-reactivity in the offspring heart resulting from maternal obesity may only be revealed in the presence of a superimposed challenge, such as during labour and delivery. However, detailed studies of the impacts of maternal obesity on foetal cardiovascular function during acute stressful conditions, such as during acute hypoxia, acute asphyxia, or acute hypotension, which trigger foetal sympathetic compensatory responses, await investigation.
Mitochondrial dysfunction and metabolic inflexibility
Mouse offspring exposed to maternal obesity during gestation show increased cardiac insulin signalling20 and a reduction in mitochondrial oxygen consumption26 at 2 months of age. These data suggest that there may be increased dependence on glycolytic pathways for ATP generation. Cardiac mitochondria show circular morphology and a disorganized alignment relative to sarcomeres, which may result in poorer coupling of ATP production with consumption.26 However, -month-old mouse offspring of obese pregnancy show a reversed cardiac metabolic phenotype with increased mitochondrial fatty acid oxidation and a reduction in glucose uptake.37 This cardiac phenotype in adulthood may be an indication of metabolic inflexibility arising due to hyperinsulinaemia resulting from peripheral insulin resistance.20 Interestingly, a metabolic shift with increased dependence on fatty acid metabolism is characteristic of the cardiac phenotype in animal models of diabetic cardiomyopathy.110
The literature also supports that alterations in cardiac metabolism in offspring of obese pregnancy may originate in foetal life. Oleate oxidation is increased in foetal primary cardiomyocytes along with higher cardiac expression of lipid metabolism-related genes in foetal mice of obese pregnancy.37,39 Maternal obesity results in increased cardiac lipid deposition in neonatal rats, likely secondary to changes in myocardial lipid metabolism and hyperlipidaemia.30,38 Metabolic inflexibility is evident at this early stage, presenting a contrasting phenotype to adult offspring, with reductions in cardiac insulin signalling in the foetal sheep49 and fibroblast growth factor (FGF)-activated PI3K/Akt signalling in the neonatal rat45 during exposure to obese gestation. Mitochondrial fragmentation and reduced cardiomyocyte oxygen consumption in the neonatal rat also support that metabolic capacity is impaired and that this may be contributing to the reduced cardiac contractile function in offspring of obese pregnancy.38,40
Oxidative stress
Mouse offspring of obese pregnancy show increased lipid peroxidation consistent with excess superoxide production, which limits basal and acetylcholine-induced nitric oxide production in the femoral artery.36 This shift in vascular oxidant tone results in endothelial dysfunction that becomes exacerbated over time, consistent with an increase in vascular oxidative stress in offspring of obese pregnancy.36 Cardiac oxidative stress is also evident in mouse offspring exposed to maternal obesity. Increased cardiac lipid peroxidation correlates with a reduction in the mitochondrial superoxide dismutase (MnSOD), and an upregulation of catalase levels.20 These alterations in expression of antioxidant enzymes are similar to those described in heart failure, and they are linked with impaired myocardial mitochondrial metabolism.111,112
Excess generation of reactive oxygen species (ROS) and increased HIF-1α target gene expression levels have both been reported in the hearts of foetal mice exposed to maternal obesity.39,44 As ROS generation is reported to stabilize HIF-1α, this is consistent with elevated oxidative stress.39,44 Neonatal rats of obese pregnancy also show higher levels of cardiac lipid peroxidation.38 Damage to mitochondrial metabolism due to oxidative stress further exacerbates existing perturbation of cardiac energy balance, with reduced flexibility of ATP production pathways and poorer coupling of myocardial ATP production and use. Therefore, oxidative stress creates and exacerbates impairments in systolic and diastolic dysfunction in offspring of obese pregnancy.
Epigenetic regulation by miRNAs
Alterations in epigenetic signals, including DNA methylation, histone modifications, and miRNA expression, may provide a mechanism for persistent offspring cardiovascular dysfunction from foetal life into adulthood.113,114 Of particular interest are miRNAs, as their epigenetic dysregulation can modulate networks of genes in a coordinated fashion.115 MiRNAs are small non-coding RNAs that base-pair to specific sequences within the 3′ untranslated region of mRNA-target transcripts and act to decrease mRNA stability and/or block translation.116 Foetal cardiac miRNA expression is dysregulated by maternal high-fat feeding in non-human primates54,106 and in genetically obese-prone mice.117 Predicted targets of miRNAs dysregulated in the foetal baboon heart are p53, PPAR-γ, and HIF-1α, which are known to play key roles in cell cycle regulation, metabolism, and oxidative stress signalling, thus providing a mechanistic framework by which dysregulated miRNA expression could lead to increased risk of cardiovascular disease.54,106 MiRNA dysregulation has been shown to persist into adulthood, with miRNA-15b increased in the myocardium of adult mouse offspring and in the serum of human offspring exposed to obesity during pregnancy.118 MiRNA-15b is released in response to ischaemia-reperfusion of mouse hearts ex vivo, with increased release in hearts of offspring from obese pregnancy.118 MiRNA-15b overexpression reduces cardiomyocyte mitochondrial outer membrane stability and fatty acid oxidation in vitro, demonstrating a role of miRNA-15b in cardiac metabolism.118 Programmed changes in cardiac miRNAs are consistent with a growing body of evidence suggesting that miRNAs play an important role in the pathogenesis of cardiovascular disease (see119). The mechanisms by which an in utero obesogenic environment leads to permanent changes in miRNA expression are unknown but could involve programmed changes in DNA methylation and histone modifications of DNA regions regulating miRNA transcription. In addition to contributing to programming mechanisms, miRNAs could also be exploited as disease biomarkers120 and therapeutic targets.121
Secondary insults reveal latent cardiovascular susceptibility in offspring of obese pregnancy
Offspring of obese pregnancy can show evidence of sympathetic hyper-reactivity, mitochondrial dysfunction, oxidative stress, and epigenetic dysregulation as the most prevalent or persistent phenotype, even decades after birth. However, overt cardiovascular dysfunction is not always seen. It is possible that such aspects of the cardiovascular phenotype in these offspring may enhance sensitivity to secondary insults that unveil latent susceptibility to future cardiovascular risk. Increasing evidence supports this concept, and focuses on alterations in diet, stress and ageing as likely secondary stressors (Figure 1).
Post-weaning diet
Cardiac hypertrophy and inflammation are present in lambs exposed to maternal obesity during gestation only after a 12-week feeding challenge, compared with lambs of control pregnancy exposed to the same feeding challenge.51 While exposure to maternal obesity during gestation leads to cardiac hypertrophy and reduced ejection fraction in 8-week-old mouse offspring, the development of myocardial fibrosis and hypertension is only present at this stage in offspring exposed to an obesogenic post-weaning diet.23 Vascular dysfunction is also evident in macaque offspring of obese pregnancy dependent on post-weaning diet, with offspring on a control diet showing enhanced endothelium-dependent vasodilatation in the aorta, an effect which is reversed in offspring fed a high-fat diet.56 Importantly, in these pre-clinical studies, cardiovascular dysfunction is identified as compared with offspring of control pregnancy also exposed to the same altered post-weaning diet, demonstrating that maternal obesity leads to a heightened susceptibility to cardiovascular dysfunction induced by a dietary challenge.23,51,56 15 week-old mouse offspring of obese pregnancy are hypertensive with impaired basal vascular nitric oxide production only when exposed to a high fat post-weaning diet.36 However, these changes were comparable to offspring of control pregnancy with high-fat post-weaning diet.36 An obesogenic post-weaning diet has also been shown to suppress the compensatory upregulation of myocardial fatty acid oxidation in offspring of obese pregnancy, and to increase expression of uncoupling proteins.122 Similarly, platelet hyperactivation is only observed in male mouse offspring of obese pregnancy which were also exposed to a high fat post-weaning diet.123 Therefore, a superimposed dietary challenge exacerbates cardiac dysfunction in adult offspring of obese pregnancy through structural, inflammatory, and metabolic pathways.
Possible mechanisms for increased sensitivity to a post-weaning dietary challenge in offspring of obese pregnancy include dysregulation of appetite control, poor nutrient handling, and metabolic inflexibility. Mouse offspring of obese pregnancy are hyperphagic,42 increasing susceptibility to diet-induced obesity. Mouse offspring of obese pregnancy also show increased serum insulin levels in the absence of hyperglycaemia, indicative of insulin resistance, resulting in greater metabolic vulnerability.42 For instance, mouse offspring of obese pregnancy show exacerbated hyperinsulinaemia following exposure to a high-fat/high-sugar post-weaning diet.23 Mouse offspring of obese pregnancy also show myocardial metabolic inflexibility, with increased dependence on fatty acid oxidation over glucose metabolism.37
Combined, hyperphagia and dysregulated glucose handling exacerbate disruption to the metabolic and endocrine milieu with a dietary challenge, imposing additional challenges to a heart that already has reduced flexibility in the metabolic pathways available for myocardial ATP production.
Stress
Maternal obesity may also prime offspring to show dysregulated cardiovascular responses to stress, revealing a heightened vulnerability to cardiac injury. Mouse offspring of obese pregnancy show enhanced myocardial fibrosis, systolic and diastolic dysfunction compared with offspring of healthy pregnancy in response to a 2-week stress challenge.83 Similarly, mouse offspring from Ay-mutant obese dams showed significantly higher infarct size than control offspring following an ischaemia-reperfusion challenge,124 indicating reduced coronary reserve to maintain cardiac function with the superimposed challenge. A plausible mechanism for enhanced sensitivity to stress is sympathetic hyper-reactivity. Rodent offspring of obese pregnancy show elevated cardiac and vascular sensitivity to adrenergic agonists, which may result in a greater increase in peripheral and coronary vascular resistance in the presence of stress, leading to increased cardiac afterload and poorer myocardial perfusion, alongside enhanced stimulation of cardiac hypertrophy.21,36,42
Ageing
The onset of maternal obesity-induced hypertension is known to be age-dependent across a range of animal models (Table 1). Mouse offspring of obese pregnancy show no difference in systolic blood pressure at 4–6 months, but by 7–12 months show a significant elevation compared with age-matched controls.29,122 In contrast, juvenile sheep offspring exposed to maternal obesity during gestation show hypertension, which appears to resolve during adulthood.53 However, echocardiography reveals the progression of significant impairments in systolic function in ageing sheep offspring of obese pregnancy compared with ageing offspring of control pregnancy.53 Ageing in mouse offspring of obese pregnancy has also been associated with the development of both systolic and diastolic dysfunction.37,83 These preclinical studies highlight that it is the interaction between developmental exposure to maternal obesity and ageing which mediates cardiovascular dysfunction due to heightened susceptibility, not seen in aged offspring of control pregnancy.
While several studies highlight the impact of exposure to secondary insults postnatally, superimposed challenges in the prenatal environment may also play a significant role in exacerbating maternal obesity-induced cardiovascular dysfunction in offspring. For instance, a study in rats showed that uterine artery ligation in obese pregnancy results in increased relative heart weight and exacerbated alterations in arterial wall structure in 60-day-old offspring.125 Therefore, the interaction between maternal obesity and other intrauterine challenges may also be important in determining offspring cardiovascular health. However, our analysis highlights a gap in the literature of studies investigating the interaction between an in utero obesogenic environment and common stressors in foetal life, such as foetal hypoxia or excess foetal glucocorticoid exposure.
Interventions against the developmental programming of cardiovascular dysfunction in offspring of obese pregnancy
Independent of whether secondary insults occur pre- or post-natally, their occurrence can reveal latent susceptibilities, leading to the expression of overt cardiovascular dysfunction in adult offspring of obese pregnancy later in life. This highlights the need for intervention, while also providing potential windows of opportunity for preventative therapy (Figure 1). To date, interventional strategies have focussed primarily on either maternal exercise or dietary supplementation during obese pregnancy.
Maternal exercise
In mice, maternal exercise ameliorated maternal hyperinsulinaemia, prevented foetal hyperinsulinaemia, and normalized placental HIF-1α expression.34 These changes occurred with attenuation of cardiac hypertrophy and systolic dysfunction in 8-week-old adult offspring of obese pregnancy subjected to a maternal exercise intervention.24 Maternal exercise also alters the vasculature, improving placental vascularization in obese mouse pregnancy,43 and reversing vascular endothelial dysfunction in 23 week-old mouse offspring exposed to maternal obesity and a western diet post-weaning.27 Evidence from mouse models indicates that maternal exercise is an effective intervention to prevent cardiovascular disease programming, with protective effects observed in offspring even when using mild maternal exercise regimes that do not result in the normalization of maternal weight. Exercise interventions that improve the maternal metabolic phenotype, despite no effect on maternal BMI, may prevent the development of oxidative stress and metabolic inflexibility in the offspring cardiovascular system, leading to a reduced cardiovascular risk in offspring. This is an important message to convey to overweight women, that despite having no effect on their body weight, exercise during pregnancy still benefits the cardiometabolic health of their offspring.
Lifestyle interventions, such as maternal exercise, have been trialled in human subjects with no significant improvement in neonatal cardiac structure or function.126 However, a recent systematic review of randomized controlled trials highlighted that maternal lifestyle interventions, such as diet and physical activity, reduced cardiac remodelling and improved systolic and diastolic function in children exposed to maternal obesity in pregnancy.127 Interestingly, maternal lifestyle interventions did not have any effects on offspring blood pressure across trials,127 consistent with the persistence of offspring hypertension in a mouse model of maternal obesity with exercise intervention during pregnancy.24 However, with poor adherence to physical activity guidelines in pregnant women,128 alternative intervention strategies will likely need to be considered.
Offspring and maternal dietary supplementation
Intervention through offspring dietary supplementation with glucose-lowering berberine has been shown to improve cardiac function, together with improved cardiac mitochondrial function in mouse offspring exposed to gestational diabetes.129,130 However, evidence points towards a foetal origin of cardiac dysfunction in obese pregnancy, and so prevention by maternal treatment during pregnancy compared with postnatal intervention may increase the effectiveness of the approach, providing optimal protection against offspring cardiovascular dysfunction (Figure 1). Several studies have reported that maternal antioxidant treatment is effective in protecting against cardiovascular dysfunction in offspring exposed to hypoxic pregnancy by attenuating oxidative stress in the placenta and the foetal cardiovascular system.89,100,131–136 Therefore, as offspring exposed to maternal obesity also show oxidative stress, maternal antioxidant therapy may provide an effective intervention against the programming of cardiovascular dysfunction in offspring of obese pregnancy. For example, antioxidant treatment of obese mice rescues oocyte mitochondrial dysfunction137 and oxidative stress.138 Treatment of obese mice with the antioxidant pyrroloquinoline quinone from conception and throughout lactation increased adult offspring oxidative defences and metabolic flexibility.139 Whether the beneficial effects of maternal antioxidant treatment during obese pregnancy extend to protection against the programming of cardiovascular dysfunction in offspring remains to be tested.
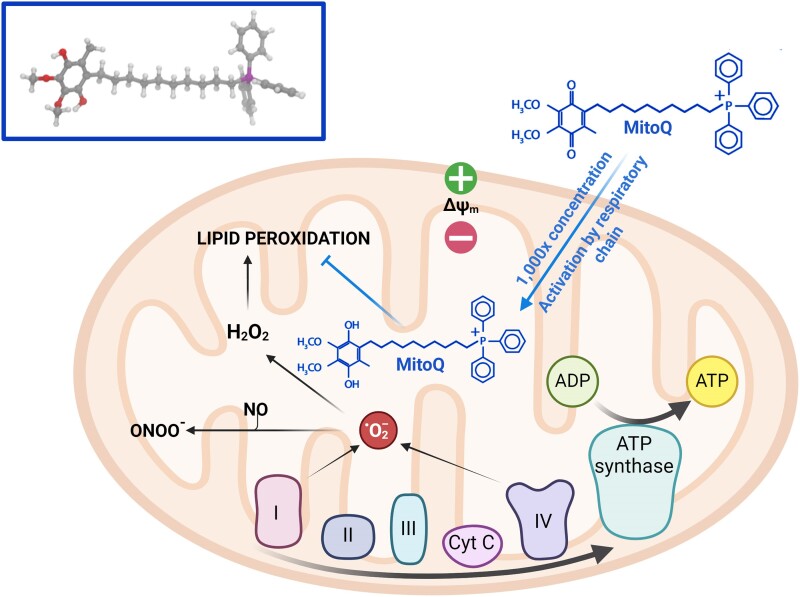
A key limitation of translating antioxidant therapies to human populations lies in identifying a safe, but effective dose. For example, the maternal supplementation with the antioxidant vitamin C during rat pregnancy has been shown to be protective against cardiovascular dysfunction of adult rat offspring exposed to chronic hypoxia in utero, however, the dose used was over 50 times the dose given to pregnant women in clinical trials.133 Therefore, there is an urgent need to identify alternative antioxidant therapies with increased human translational potential. Mitochondria are a major site of ROS production, therefore targeting these organelles should be one of the most effective antioxidant strategies. However, conventional antioxidants are ineffective because they cannot penetrate the mitochondria. A mitochondria-targeted ubiquinone that overcomes the problem of direct delivery to the mitochondria has now been developed (Figure 4). MitoQ is composed of a lipophilic triphenylphosphonium cation covalently attached to a ubiquinol antioxidant.140–142 Lipophilic cations can easily move through phospholipid bilayers without requiring a specific uptake mechanism. Therefore, the triphenylphosphonium cation concentrates MitoQ several hundred-fold within the mitochondria, driven by the large mitochondrial membrane potential.140–142 Only within the mitochondria, MitoQ is reduced by the respiratory chain to its active ubiquinol form, which is a particularly effective antioxidant that prevents lipid peroxidation and mitochondrial damage.140–142 The benefits of MitoQ have been revealed in a range of in vivo studies in rats and mice and have also been assessed in two Phase II human trials.143–147 In contrast to vitamin C and other conventional antioxidants, MitoQ demonstrates no pro-oxidant activity at high doses143 and long-term administration to mice145 and to human patients in Phase II trials, including one that lasted 12 months and revealed no toxicity.146,147 However, the antioxidant benefits of MitoQ in protecting the foetal and adult cardiovascular system in offspring of obese pregnancy remain to be investigated.
Figure 4.
MitoQ: a mitochondria-targeted antioxidant. MitoQ is composed of a lipophilic triphenylphosphonium cation covalently attached to a ubiquinol antioxidant.140–142 The lipophilic cations facilitate free movement of MitoQ through phospholipid bilayers, while the triphenylphosphonium cation concentrates MitoQ ∼1000 fold within the mitochondria, driven by the large mitochondrial membrane potential.140–142 MitoQ is reduced by the respiratory chain to its active ubiquinol form once inside the mitochondrial matrix.140–142 This activated ubiquinol form of MitoQ inhibits lipid peroxidation, ameliorating mitochondrial damage.140–142 Created with BioRender.com
Concluding remarks
There is extensive evidence derived from human studies and preclinical animal models for the programming of an increased risk of cardiovascular disease in offspring exposed to maternal obesity in utero (Table 1 and Figure 2). Cardiovascular susceptibility in offspring has an early origin, with many aspects of the cardiac dysfunctional phenotype emerging in foetal life across mammalian species. This suggests that candidate interventions should start as early as possible during the developmental trajectory, rather than waiting until disease is established and has become irreversible. The effects of novel treatments like mitochondria-targeted antioxidant therapy during obese pregnancy in preclinical animal models should be explored. The literature also highlights a limited understanding of how vascular structure and function is altered in offspring of obese pregnancy before birth. Large mammalian animal models permitting functional assessment of foetal vascular reactivity in resistance circulations must be employed to address this gap in our knowledge. This review also addressed key programming mechanisms linking maternal obesity with offspring cardiovascular dysfunction, including sympathetic hyper-reactivity, the development of oxidative stress, mitochondrial dysfunction, metabolic inflexibility, and epigenetic dysregulation via miRNAs. Data also support that exposure to a secondary insult in adult life, or even the process of ageing, often reveals latent impairments in the cardiovascular system in offspring of obese pregnancy. It is likely that secondary insults occurring prenatally in offspring of obese pregnancy may also exacerbate latent susceptibility to cardiovascular dysfunction. Therefore, further research is required to understand how maternal obesity may impact the foetal cardiovascular defence to common acute stresses in utero, such as acute foetal hypoxia, acute foetal asphyxia, or acute foetal hypotension. In turn, further research is also required to understand how longer-term intrauterine complications in adverse pregnancy, such as chronic foetal hypoxia or excess foetal glucocorticoid exposure, may interact with maternal obesity to affect cardiovascular function in offspring.
Supplementary data
Supplementary data are not available at European Heart Journal online.
Contributor Information
Anna L K Cochrane, Department of Physiology, Development and Neuroscience, University of Cambridge, Downing Street, Cambridge CB2 3EG, UK; Department of Medicine, University of Cambridge, Hills Road, Cambridge CB2 0QQ, UK.
Michael P Murphy, Department of Medicine, University of Cambridge, Hills Road, Cambridge CB2 0QQ, UK; MRC Mitochondrial Biology Unit, University of Cambridge, Hills Road, Cambridge CB2 0XY, UK.
Susan E Ozanne, Metabolic Research Laboratories and MRC Metabolic Diseases Unit, Institute of Metabolic Science, University of Cambridge, Cambridge, UK; Loke Centre for Trophoblast Research, University of Cambridge, Downing Street, Cambridge CB2 3EG, UK; Cambridge Strategic Research Initiative in Reproduction, University of Cambridge, Cambridge, UK; British Heart Foundation, Cambridge Cardiovascular Centre for Research Excellence, University of Cambridge, Cambridge, UK.
Dino A Giussani, Department of Physiology, Development and Neuroscience, University of Cambridge, Downing Street, Cambridge CB2 3EG, UK; Metabolic Research Laboratories and MRC Metabolic Diseases Unit, Institute of Metabolic Science, University of Cambridge, Cambridge, UK; Loke Centre for Trophoblast Research, University of Cambridge, Downing Street, Cambridge CB2 3EG, UK; Cambridge Strategic Research Initiative in Reproduction, University of Cambridge, Cambridge, UK; British Heart Foundation, Cambridge Cardiovascular Centre for Research Excellence, University of Cambridge, Cambridge, UK.
Declarations
Disclosure of Interest
Nothing to declare.
Data Availability
No data were generated or analysed for or in support of this paper.
Funding
This work was supported by the British Heart Foundation (RG/17/8/32924 and PG/14/5/30547; D.A.G.; PG/14/20/3076 and RG/17/12/33167, S.E.O.), the Medical Research Council UK (MC_UU_00028/4; M.P.M.; MRC_MC_UU_00014/4, S.E.O.; MR/V03362X/1, D.A.G.) and by a Wellcome Trust Investigator award (220257/Z/20/Z; M.P.M.).
References
- 1. World Health Organisation . Obesity and overweight. 2018. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (19 January 2023, date last accessed).
- 2. Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res 2016;118:1752–70. 10.1161/CIRCRESAHA.115.306883 [DOI] [PubMed] [Google Scholar]
- 3. British Heart Foundation . Heart and Circulatory Disease Statistics. London, UK: British Heart Foundation, 2020. [Google Scholar]
- 4. Cosentino F, Verma S, Ambery P, Treppendahl MB, van Eickels M, Anker SD, et al. Cardiometabolic risk management: insights from a European Society of Cardiology Cardiovascular Round Table. Eur Heart J 2023;44:4141–56. 10.1093/eurheartj/ehad445 [DOI] [PubMed] [Google Scholar]
- 5. Garcia-Moll X. Obesity and prognosis: time to forget about metabolically healthy obesity. Eur Heart J 2018;39:407–9. 10.1093/eurheartj/ehx535 [DOI] [PubMed] [Google Scholar]
- 6. Lassale C, Tzoulaki I, Moons KGM, Sweeting M, Boer J, Johnson L, et al. Separate and combined associations of obesity and metabolic health with coronary heart disease: a pan-European case-cohort analysis. Eur Heart J 2018;39:397–406. 10.1093/eurheartj/ehx448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lavie CJ, Arena R, Alpert MA, Milani RV, Ventura HO. Management of cardiovascular diseases in patients with obesity. Nat Rev Cardiol 2018;15:45–56. 10.1038/nrcardio.2017.108 [DOI] [PubMed] [Google Scholar]
- 8. Roeters van Lennep JE, Tokgözoğlu LS, Badimon L, Dumanski SM, Gulati M, Hess CN, et al. Women, lipids, and atherosclerotic cardiovascular disease: a call to action from the European Atherosclerosis Society. Eur Heart J 2023;44:4157–73. 10.1093/eurheartj/ehad472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39:3165–241. 10.1093/eurheartj/ehy340 [DOI] [PubMed] [Google Scholar]
- 10. NHS . NHS England Digital 2020. Statistics on Obesity, Physical Activity and Diet, England, 2020. https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-obesity-physical-activity-and-diet/england-2020 (10 June 2024, date last accessed).
- 11. Kaskinen A, Helle E. Unravelling associations between maternal health and congenital heart defect risk in the offspring-the FINNPEDHEART study. Eur Heart J 2023;44:1293–5. 10.1093/eurheartj/ehad033 [DOI] [PubMed] [Google Scholar]
- 12. Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ 2013;347:f4539. 10.1136/bmj.f4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guenard F, Deshaies Y, Cianflone K, Kral JG, Marceau P, Vohl MC. Differential methylation in glucoregulatory genes of offspring born before vs. after maternal gastrointestinal bypass surgery. Proc Natl Acad Sci U S A 2013;110:11439–44. 10.1073/pnas.1216959110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Razaz N, Villamor E, Muraca GM, Bonamy AKE, Cnattingius S. Maternal obesity and risk of cardiovascular diseases in offspring: a population-based cohort and sibling-controlled study. Lancet Diabetes Endocrinol 2020;8:572–81. 10.1016/S2213-8587(20)30151-0 [DOI] [PubMed] [Google Scholar]
- 15. Eriksson JG, Sandboge S, Salonen MK, Kajantie E, Osmond C. Long-term consequences of maternal overweight in pregnancy on offspring later health: findings from the Helsinki Birth Cohort Study. Ann Med 2014;46:434–8. 10.3109/07853890.2014.919728 [DOI] [PubMed] [Google Scholar]
- 16. Toemen L, Gishti O, van Osch-Gevers L, Steegers EAP, Helbing WA, Felix JF, et al. Maternal obesity, gestational weight gain and childhood cardiac outcomes: role of childhood body mass index. Int J Obes 2016;40:1070–8. 10.1038/ijo.2016.86 [DOI] [PubMed] [Google Scholar]
- 17. Avci ME, Tosun Ö. Evaluation of subclinical atherosclerosis and cardiac functions in children of mothers with gestational diabetes and maternal obesity. Cardiol Young 2023;33:1157–64. 10.1017/S1047951122002402 [DOI] [PubMed] [Google Scholar]
- 18. Gademan MGJ, van Eijsden M, Roseboom TJ, van der Post JAM, Stronks K, Vrijkotte TGM. Maternal prepregnancy body mass index and their children’s blood pressure and resting cardiac autonomic balance at age 5 to 6 years. Hypertension 2013;62:641–7. 10.1161/HYPERTENSIONAHA.113.01511 [DOI] [PubMed] [Google Scholar]
- 19. Litwin L, Sundholm JKM, Rönö K, Koivusalo SB, Eriksson JG, Sarkola T. No effect of gestational diabetes or pre-gestational obesity on 6-year offspring left ventricular function—RADIEL study follow-up. Acta Diabetol 2020;57:1463–72. 10.1007/s00592-020-01571-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fernandez-Twinn DS, Blackmore HL, Siggens L, Giussani DA, Cross CM, Foo R, et al. The programming of cardiac hypertrophy in the offspring by maternal obesity is associated with hyperinsulinemia, AKT, ERK, and mTOR activation. Endocrinology 2012;153:5961–71. 10.1210/en.2012-1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blackmore HL, Niu Y, Fernandez-Twinn DS, Tarry-Adkins JL, Giussani DA, Ozanne SE. Maternal diet-induced obesity programs cardiovascular dysfunction in adult male mouse offspring independent of current body weight. Endocrinology 2014;155:3970–80. 10.1210/en.2014-1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Samuelsson AM, Morris A, Igosheva N, Kirk SL, Pombo JMC, Coen CW, et al. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension 2010;55:76–82. 10.1161/HYPERTENSIONAHA.109.139402 [DOI] [PubMed] [Google Scholar]
- 23. Loche E, Blackmore HL, Carpenter AA, Beeson JH, Pinnock A, Ashmore TJ, et al. Maternal diet-induced obesity programmes cardiac dysfunction in male mice independently of post-weaning diet. Cardiovasc Res 2018;114:1372–84. 10.1093/cvr/cvy082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beeson JH, Blackmore HL, Carr SK, Dearden L, Duque-Guimarães DE, Kusinski LC, et al. Maternal exercise intervention in obese pregnancy improves the cardiovascular health of the adult male offspring. Mol Metab 2018;16:35–44. 10.1016/j.molmet.2018.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaughan OR, Rosario FJ, Powell TL, Jansson T. Normalisation of circulating adiponectin levels in obese pregnant mice prevents cardiac dysfunction in adult offspring. Int J Obes (Lond) 2020;44:488–99. 10.1038/s41366-019-0374-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferey JLA, Boudoures AL, Reid M, Drury A, Scheaffer S, Modi Z, et al. A maternal high-fat, high-sucrose diet induces transgenerational cardiac mitochondrial dysfunction independently of maternal mitochondrial inheritance. Am J Physiol Heart Circ Physiol 2019;316:H1202–10. 10.1152/ajpheart.00013.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boonpattrawong NP, Golbidi S, Tai DC, Aleliunas RE, Bernatchez P, Miller JW, et al. Exercise during pregnancy mitigates the adverse effects of maternal obesity on adult male offspring vascular function and alters one-carbon metabolism. Physiol Rep 2020;8:e14582. 10.14814/phy2.14582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elahi MM, Cagampang FR, Mukhtar D, Anthony FW, Ohri SK, Hanson MA. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br J Nutr 2009;102:514–9. 10.1017/S000711450820749X [DOI] [PubMed] [Google Scholar]
- 29. Liang C, Oest ME, Prater MR. Intrauterine exposure to high saturated fat diet elevates risk of adult-onset chronic diseases in C57BL/6 mice. Birth Defects Res B Dev Reprod Toxicol 2009;86:377–84. 10.1002/bdrb.20206 [DOI] [PubMed] [Google Scholar]
- 30. Ma XM, Shi QY, Zhao YX. Maternal exposure to a high-fat diet showed unfavorable effects on the body weight, apoptosis and morphology of cardiac myocytes in offspring. Arch Gynecol Obstet 2020;301:837–44. 10.1007/s00404-020-05470-0 [DOI] [PubMed] [Google Scholar]
- 31. Barker DJP, Bagby SP. Developmental antecedents of cardiovascular disease: a historical perspective. J Am Soc Nephrol 2005;16:2537–44. 10.1681/ASN.2005020160 [DOI] [PubMed] [Google Scholar]
- 32. Van De Maele K, Devlieger R, De Schepper J, Gies I. Endothelial function and its determinants in children born after maternal bariatric surgery. Pediatr Res 2022;91:699–704. 10.1038/s41390-021-01500-y [DOI] [PubMed] [Google Scholar]
- 33. Smith J, Cianflone K, Biron S, Hould FS, Lebel S, Marceau S, et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab 2009;94:4275–83. 10.1210/jc.2009-0709 [DOI] [PubMed] [Google Scholar]
- 34. Fernandez-Twinn DS, Gascoin G, Musial B, Carr S, Duque-Guimaraes D, Blackmore HL, et al. Exercise rescues obese mothers’ insulin sensitivity, placental hypoxia and male offspring insulin sensitivity. Sci Rep 2017;7:44650. 10.1038/srep44650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Evans L, Myatt L. Sexual dimorphism in the effect of maternal obesity on antioxidant defense mechanisms in the human placenta. Placenta 2017;51:64–9. 10.1016/j.placenta.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Torrens C, Ethirajan P, Bruce KD, Cagampang FRA, Siow RCM, Hanson MA, et al. Interaction between maternal and offspring diet to impair vascular function and oxidative balance in high fat fed male mice. PLoS One 2012;7:e50671. 10.1371/journal.pone.0050671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vaughan OR, Rosario FJ, Chan J, Cox LA, Ferchaud-Roucher V, Zemski-Berry KA, et al. Maternal obesity causes fetal cardiac hypertrophy and alters adult offspring myocardial metabolism in mice. J Physiol 2022;600:3169–91. 10.1113/JP282462 [DOI] [PubMed] [Google Scholar]
- 38. Mdaki KS, Larsen TD, Wachal AL, Schimelpfenig MD, Weaver LJ, Dooyema SDR, et al. Maternal high-fat diet impairs cardiac function in offspring of diabetic pregnancy through metabolic stress and mitochondrial dysfunction. Am J Physiol Heart Circ Physiol 2016;310:H681–92. 10.1152/ajpheart.00795.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pantaleão LC, Inzani I, Furse S, Loche E, Hufnagel A, Ashmore T, et al. Maternal diet-induced obesity during pregnancy alters lipid supply to mouse E18.5 fetuses and changes the cardiac tissue lipidome in a sex-dependent manner. Elife 2022;11:e69078. 10.7554/eLife.69078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Larsen TD, Sabey KH, Knutson AJ, Gandy TCT, Louwagie EJ, Lauterboeck L, et al. Diabetic pregnancy and maternal high-fat diet impair mitochondrial dynamism in the developing fetal rat heart by sex-specific mechanisms. Int J Mol Sci 2019;20:E3090. 10.3390/ijms20123090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Diniz MS, Grilo LF, Tocantins C, Falcão-Pires I, Pereira SP. Made in the womb: maternal programming of offspring cardiovascular function by an obesogenic womb. Metabolites 2023;13:845. 10.3390/metabo13070845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EHJM, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 2008;51:383–92. 10.1161/HYPERTENSIONAHA.107.101477 [DOI] [PubMed] [Google Scholar]
- 43. Son JS, Liu X, Tian Q, Zhao L, Chen Y, Hu Y, et al. Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth. J Physiol 2019;597:3333–47. 10.1113/JP277698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu Y, Wang Y, Wang C, Shi R, Zhou X, Li Z, et al. Maternal obesity increases the risk of fetal cardiac dysfunction via visceral adipose tissue derived exosomes. Placenta 2021;105:85–93. 10.1016/j.placenta.2021.01.020 [DOI] [PubMed] [Google Scholar]
- 45. Preston CC, Larsen TD, Eclov JA, Louwagie EJ, Gandy TCT, Faustino RS, et al. Maternal high fat diet and diabetes disrupts transcriptomic pathways that regulate cardiac metabolism and cell fate in newborn rat hearts. Front Endocrinol (Lausanne) 2020;11:570846. 10.3389/fendo.2020.570846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guberman C, Jellyman JK, Han G, Ross MG, Desai M. Maternal high-fat diet programs rat offspring hypertension and activates the adipose renin-angiotensin system. Am J Obstet Gynecol 2013;209:262.e1–8. 10.1016/j.ajog.2013.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Payen C, Guillot A, Paillat L, Fothi A, Dib A, Bourreau J, et al. Pathophysiological adaptations of resistance arteries in rat offspring exposed in utero to maternal obesity is associated with sex-specific epigenetic alterations. Int J Obes 2021;45:1074–85. 10.1038/s41366-021-00777-7 [DOI] [PubMed] [Google Scholar]
- 48. Huang Y, Yan X, Zhao JX, Zhu MJ, McCormick RJ, Ford SP, et al. Maternal obesity induces fibrosis in fetal myocardium of sheep. Am J Physiol Endocrinol Metab 2010;299:E968–75. 10.1152/ajpendo.00434.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang J, Ma H, Tong C, Zhang H, Lawlis GB, Li Y, et al. Overnutrition and maternal obesity in sheep pregnancy alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart. FASEB J 2010;24:2066–76. 10.1096/fj.09-142315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fan X, Turdi S, Ford SP, Hua Y, Nijland MJ, Zhu M, et al. Influence of gestational overfeeding on cardiac morphometry and hypertrophic protein markers in fetal sheep. J Nutr Biochem 2011;22:30–7. 10.1016/j.jnutbio.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ghnenis AB, Odhiambo JF, McCormick RJ, Nathanielsz PW, Ford SP. Maternal obesity in the ewe increases cardiac ventricular expression of glucocorticoid receptors, proinflammatory cytokines and fibrosis in adult male offspring. PLoS One 2017;12:e0189977. 10.1371/journal.pone.0189977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Q, Zhu C, Sun M, Maimaiti R, Ford SP, Nathanielsz PW, et al. Maternal obesity impairs fetal cardiomyocyte contractile function in sheep. FASEB J 2019;33:2587–98. 10.1096/fj.201800988R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pankey CL, Wang Q, King J, Ford SP. Cardiovascular consequences of maternal obesity throughout the lifespan in first generation sheep. PLoS One 2022;17:e0274214. 10.1371/journal.pone.0274214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maloyan A, Muralimanoharan S, Huffman S, Cox LA, Nathanielsz PW, Myatt L, et al. Identification and comparative analyses of myocardial miRNAs involved in the fetal response to maternal obesity. Physiol Genomics 2013;45:889–900. 10.1152/physiolgenomics.00050.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 2011;152:2456–64. 10.1210/en.2010-1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fan L, Lindsley SR, Comstock SM, Takahashi DL, Evans AE, He GW, et al. Maternal high-fat diet impacts endothelial function in nonhuman primate offspring. Int J Obes (Lond) 2013;37:254–62. 10.1038/ijo.2012.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ingul CB, Lorås L, Tegnander E, Eik-Nes SH, Brantberg A. Maternal obesity affects fetal myocardial function as early as in the first trimester. Ultrasound Obstet Gynecol 2016;47:433–42. 10.1002/uog.14841 [DOI] [PubMed] [Google Scholar]
- 58. Kulkarni A, Li L, Craft M, Nanda M, Lorenzo JMM, Danford D, et al. Fetal myocardial deformation in maternal diabetes mellitus and obesity. Ultrasound Obstet Gynecol 2017;49:630–6. 10.1002/uog.15971 [DOI] [PubMed] [Google Scholar]
- 59. Mat Husin H, Schleger F, Bauer I, Fehlert E, Kiefer-Schmidt I, Weiss M, et al. Maternal weight, weight gain, and metabolism are associated with changes in fetal heart rate and variability. Obesity 2020;28:114–21. 10.1002/oby.22664 [DOI] [PubMed] [Google Scholar]
- 60. Ece İ, Uner A, Balli S, Kibar AE, Oflaz MB, Kurdoglu M. The effects of pre-pregnancy obesity on fetal cardiac functions. Pediatr Cardiol 2014;35:838–43. 10.1007/s00246-014-0863-0 [DOI] [PubMed] [Google Scholar]
- 61. Ojala T, Aaltonen J, Siira S, Jalonen J, Ekholm E, Ekblad U, et al. Fetal cardiac sympathetic activation is linked with maternal body mass index. Early Hum Dev 2009;85:557–60. 10.1016/j.earlhumdev.2009.05.009 [DOI] [PubMed] [Google Scholar]
- 62. Villalobos-Labra R, Sáez PJ, Subiabre M, Silva L, Toledo F, Westermeier F, et al. Pre-pregnancy maternal obesity associates with endoplasmic reticulum stress in human umbilical vein endothelium. Biochim Biophys Acta Mol Basis Dis 2018;1864:3195–210. 10.1016/j.bbadis.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 63. Villalobos-Labra R, Westermeier F, Pizarro C, Sáez PJ, Toledo F, Pardo F, et al. Neonates from women with pregestational maternal obesity show reduced umbilical vein endothelial response to insulin. Placenta 2019;86:35–44. 10.1016/j.placenta.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 64. Costa SMR, Isganaitis E, Matthews TJ, Hughes K, Daher G, Dreyfuss JM, et al. Maternal obesity programs mitochondrial and lipid metabolism gene expression in infant umbilical vein endothelial cells. Int J Obes (Lond) 2016;40:1627–34. 10.1038/ijo.2016.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sarno L, Maruotti GM, Saccone G, Morlando M, Sirico A, Martinelli P. Maternal body mass index influences umbilical artery Doppler velocimetry in physiologic pregnancies. Prenat Diagn 2015;35:125–8. 10.1002/pd.4499 [DOI] [PubMed] [Google Scholar]
- 66. Janbakhishov T, Çağlayan E, Acet F, Altunyurt S, Özer E. Effect of vascular endothelial growth factor on fetal vessels among obese pregnant women. Int J Gynaecol Obstet 2020;151:231–6. 10.1002/ijgo.13346 [DOI] [PubMed] [Google Scholar]
- 67. Hehir MP, Moynihan AT, Glavey SV, Morrison JJ. Umbilical artery tone in maternal obesity. Reprod Biol Endocrinol 2009;7:6. 10.1186/1477-7827-7-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hayward CE, Higgins L, Cowley EJ, Greenwood SL, Mills TA, Sibley CP, et al. Chorionic plate arterial function is altered in maternal obesity. Placenta 2013;34:281–7. 10.1016/j.placenta.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Muñoz-Muñoz E, Krause BJ, Uauy R, Casanello P. LGA-newborn from patients with pregestational obesity present reduced adiponectin-mediated vascular relaxation and endothelial dysfunction in fetoplacental arteries. J Cell Physiol 2018;233:6723–33. 10.1002/jcp.26499 [DOI] [PubMed] [Google Scholar]
- 70. Groves AM, Price AN, Russell-Webster T, Jhaveri S, Yang Y, Battersby EE, et al. Impact of maternal obesity on neonatal heart rate and cardiac size. Arch Dis Child Fetal Neonatal Ed 2022;107:481–7. 10.1136/archdischild-2021-322860 [DOI] [PubMed] [Google Scholar]
- 71. Guzzardi MA, Liistro T, Gargani L, Ait Ali L, D’Angelo G, Rocchiccioli S, et al. Maternal obesity and cardiac development in the offspring: study in human neonates and minipigs. JACC Cardiovasc Imaging 2018;11:1750–5. 10.1016/j.jcmg.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 72. Cox B, Luyten LJ, Dockx Y, Provost E, Madhloum N, De Boever P, et al. Association between maternal prepregnancy body mass index and anthropometric parameters, blood pressure, and retinal microvasculature in children age 4 to 6 years. JAMA Netw Open 2020;3:e204662. 10.1001/jamanetworkopen.2020.4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gaillard R, Steegers EAP, Duijts L, Felix JF, Hofman A, Franco OH, et al. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension 2014;63:683–91. 10.1161/HYPERTENSIONAHA.113.02671 [DOI] [PubMed] [Google Scholar]
- 74. Oostvogels AJJM, Stronks K, Roseboom TJ, van der Post JAM, van Eijsden M, Vrijkotte TGM. Maternal prepregnancy BMI, offspring’s early postnatal growth, and metabolic profile at age 5–6 years: the ABCD Study. J Clin Endocrinol Metab 2014;99:3845–54. 10.1210/jc.2014-1561 [DOI] [PubMed] [Google Scholar]
- 75. Perng W, Gillman MW, Mantzoros CS, Oken E. A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Ann Epidemiol 2014;24:793–800.e1. 10.1016/j.annepidem.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fraser A, Tilling K, Macdonald-Wallis C, Sattar N, Brion MJ, Benfield L, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation 2010;121:2557–64. 10.1161/CIRCULATIONAHA.109.906081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Labayen I, Ruiz JR, Ortega FB, Loit HM, Harro J, Veidebaum T, et al. Intergenerational cardiovascular disease risk factors involve both maternal and paternal BMI. Diabetes Care 2010;33:894–900. 10.2337/dc09-1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Forsén T, Eriksson JG, Tuomilehto J, Teramo K, Osmond C, Barker DJ. Mother’s weight in pregnancy and coronary heart disease in a cohort of Finnish men: follow up study. BMJ 1997;315:837–40. 10.1136/bmj.315.7112.837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hochner H, Friedlander Y, Calderon-Margalit R, Meiner V, Sagy Y, Avgil-Tsadok M, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation 2012;125:1381–9. 10.1161/CIRCULATIONAHA.111.070060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cooper R, Pinto Pereira SM, Power C, Hyppönen E. Parental obesity and risk factors for cardiovascular disease among their offspring in mid-life: findings from the 1958 British Birth Cohort Study. Int J Obes (Lond) 2013;37:1590–6. 10.1038/ijo.2013.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Filler G, Yasin A, Kesarwani P, Garg AX, Lindsay R, Sharma AP. Big mother or small baby: which predicts hypertension? J Clin Hypertens (Greenwich) 2011;13:35–41. 10.1111/j.1751-7176.2010.00366.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jansen MAC, Dalmeijer GW, Saldi SR, Grobbee DE, Baharuddin M, Uiterwaal CS, et al. Pre-pregnancy parental BMI and offspring blood pressure in infancy. Eur J Prev Cardiol 2019;26:1581–90. 10.1177/2047487319858157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ahmed A, Liang M, Chi L, Zhou YQ, Sled JG, Wilson MD, et al. Maternal obesity persistently alters cardiac progenitor gene expression and programs adult-onset heart disease susceptibility. Mol Metab 2021;43:101116. 10.1016/j.molmet.2020.101116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Espírito-Santo DA, Cordeiro GS, Oliveira TWS, Santos LS, Silva RT, Costa CAS, et al. Exposure to a high-fat diet during intrauterine life and post-birth causes cardiac histomorphometric changes in rats: a systematic review. Life Sci 2022;303:120658. 10.1016/j.lfs.2022.120658 [DOI] [PubMed] [Google Scholar]
- 85. Sundholm JKM, Litwin L, Rönö K, Koivusalo SB, Eriksson JG, Sarkola T. Maternal obesity and gestational diabetes: impact on arterial wall layer thickness and stiffness in early childhood—RADIEL study six-year follow-up. Atherosclerosis 2019;284:237–44. 10.1016/j.atherosclerosis.2019.01.037 [DOI] [PubMed] [Google Scholar]
- 86. Henry SL, Barzel B, Wood-Bradley RJ, Burke SL, Head GA, Armitage JA. Developmental origins of obesity-related hypertension. Clin Exp Pharmacol Physiol 2012;39:799–806. 10.1111/j.1440-1681.2011.05579.x [DOI] [PubMed] [Google Scholar]
- 87. Hemberger M, Hanna CW, Dean W. Mechanisms of early placental development in mouse and humans. Nat Rev Genet 2020;21:27–43. 10.1038/s41576-019-0169-4 [DOI] [PubMed] [Google Scholar]
- 88. Reynolds CM, Vickers MH. Utility of small animal models of developmental programming. In: Guest PC (ed.), Investigations of Early Nutrition Effects on Long-Term Health: Methods and Applications. New York: Springer, 2018, 145–63. [DOI] [PubMed] [Google Scholar]
- 89. Giussani DA. Breath of life: heart disease link to developmental hypoxia. Circulation 2021;144:1429–43. 10.1161/CIRCULATIONAHA.121.054689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rueda-Clausen CF, Morton JS, Davidge ST. The early origins of cardiovascular health and disease: who, when, and how. Semin Reprod Med 2011;29:197–210. 10.1055/s-0031-1275520 [DOI] [PubMed] [Google Scholar]
- 91. Morrison JL, Berry MJ, Botting KJ, Darby JRT, Frasch MG, Gatford KL, et al. Improving pregnancy outcomes in humans through studies in sheep. Am J Physiol Regul Integr Comp Physiol 2018;315:R1123–53. 10.1152/ajpregu.00391.2017 [DOI] [PubMed] [Google Scholar]
- 92. Barry JS, Anthony RV. The pregnant sheep as a model for human pregnancy. Theriogenology 2008;69:55–67. 10.1016/j.theriogenology.2007.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50:515–25. 10.1542/peds.50.4.515 [DOI] [PubMed] [Google Scholar]
- 94. Nathanielsz PW, Jenkins SL, Tame JD, Winter JA, Guller S, Giussani DA. Local paracrine effects of estradiol are central to parturition in the rhesus monkey. Nat Med 1998;4:456–9. 10.1038/nm0498-456 [DOI] [PubMed] [Google Scholar]
- 95. Wolf DP. The non-human primate oocyte and embryo as a model for women, or is it vice versa? Theriogenology 2008;69:31–6. 10.1016/j.theriogenology.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 96. Burns P, Liu HL, Kuthiala S, Fecteau G, Desrochers A, Durosier LD, et al. Instrumentation of near-term fetal sheep for multivariate chronic non-anesthetized recordings. J Vis Exp 2015;(104):52581. 10.3791/52581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Allison BJ, Brain KL, Niu Y, Kane AD, Herrera EA, Thakor AS, et al. Fetal in vivo continuous cardiovascular function during chronic hypoxia. J Physiol 2016;594:1247–64. 10.1113/JP271091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Brain KL, Allison BJ, Niu Y, Cross CM, Itani N, Kane AD, et al. Induction of controlled hypoxic pregnancy in large mammalian species. Physiol Rep 2015;3:e12614. 10.14814/phy2.12614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Shaw CJ, Botting K, Niu Y, Lees C, Giussani D. Maternal and fetal cardiovascular and metabolic effects of intra-operative uterine handling under general anesthesia during pregnancy in sheep. Sci Rep 2020;10:10867. 10.1038/s41598-020-67714-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Brain KL, Allison BJ, Niu Y, Cross CM, Itani N, Kane AD, et al. Intervention against hypertension in the next generation programmed by developmental hypoxia. PLoS Biol 2019;17:e2006552. 10.1371/journal.pbio.2006552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nathanielsz PW, Giussani DA, Wu WX. Stimulation of the switch in myometrial activity from contractures to contractions in the pregnant sheep and nonhuman primate. Equine Vet J 1997;29:83–8. 10.1111/j.2042-3306.1997.tb05083.x [DOI] [PubMed] [Google Scholar]
- 102. Wooding FBP. The synepitheliochorial placenta of ruminants: binucleate cell fusions and hormone production. Placenta 1992;13:101–13. 10.1016/0143-4004(92)90025-O [DOI] [PubMed] [Google Scholar]
- 103. Carter AM. Animal models of human placentation–a review. Placenta 2007;28:S41–7. 10.1016/j.placenta.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 104. den Harink T, Roelofs MJM, Limpens J, Painter RC, Roseboom TJ, van Deutekom A. Maternal obesity in pregnancy and children’s cardiac function and structure: a systematic review and meta-analysis of evidence from human studies. PLoS One 2022;17:e0275236. 10.1371/journal.pone.0275236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhu MJ, Du M, Nijland MJ, Nathanielsz PW, Hess BW, Moss GE, et al. Down-regulation of growth signaling pathways linked to a reduced cotyledonary vascularity in placentomes of over-nourished, obese pregnant ewes. Placenta 2009;30:405–10. 10.1016/j.placenta.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Schlabritz-Loutsevitch N, Apostolakis-Kyrus K, Krutilina R, Hubbard G, Kocak M, Janjetovic Z, et al. Pregnancy-driven cardiovascular maternal miR-29 plasticity in obesity. J Med Primatol 2016;45:297–303. 10.1111/jmp.12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Brody MJ, Zimmerman BG. Peripheral circulation in arterial hypertension. Prog Cardiovasc Dis 1976;18:323–40. 10.1016/0033-0620(76)90001-3 [DOI] [PubMed] [Google Scholar]
- 108. Danson EJ, Li D, Wang L, Dawson TA, Paterson DJ. Targeting cardiac sympatho-vagal imbalance using gene transfer of nitric oxide synthase. J Mol Cell Cardiol 2009;46:482–9. 10.1016/j.yjmcc.2008.12.013 [DOI] [PubMed] [Google Scholar]
- 109. Bristow MR. beta-adrenergic receptor blockade in chronic heart failure. Circulation 2000;101:558–69. 10.1161/01.CIR.101.5.558 [DOI] [PubMed] [Google Scholar]
- 110. Makrecka-Kuka M, Liepinsh E, Murray AJ, Lemieux H, Dambrova M, Tepp K, et al. Altered mitochondrial metabolism in the insulin-resistant heart. Acta Physiol 2020;228:e13430. 10.1111/apha.13430 [DOI] [PubMed] [Google Scholar]
- 111. Lustgarten MS, Jang YC, Liu Y, Qi W, Qin Y, Dahia PL, et al. MnSOD deficiency results in elevated oxidative stress and decreased mitochondrial function but does not lead to muscle atrophy during aging. Aging Cell 2011;10:493–505. 10.1111/j.1474-9726.2011.00695.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dieterich S, Bieligk U, Beulich K, Hasenfuss G, Prestle J. Gene expression of antioxidative enzymes in the human heart. Circulation 2000;101:33–9. 10.1161/01.CIR.101.1.33 [DOI] [PubMed] [Google Scholar]
- 113. Nicholas LM, Ozanne SE. Early life programming in mice by maternal overnutrition: mechanistic insights and interventional approaches. Philos Trans R Soc Lond B Biol Sci 2019;374:20180116. 10.1098/rstb.2018.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol 2009;5:401–8. 10.1038/nrendo.2009.102 [DOI] [PubMed] [Google Scholar]
- 115. Lock MC, McGillick EV, Orgeig S, McMillen IC, Mühlhäusler BS, Zhang S, et al. Differential effects of late gestation maternal overnutrition on the regulation of surfactant maturation in fetal and postnatal life. J Physiol 2017;595:6635–52. 10.1113/JP274528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wilczynska A, Bushell M. The complexity of miRNA-mediated repression. Cell Death Differ 2015;22:22–33. 10.1038/cdd.2014.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wing-Lun E, Eaton SA, Hur SSJ, Aiken A, Young PE, Buckland ME, et al. Nutrition has a pervasive impact on cardiac microRNA expression in isogenic mice. Epigenetics 2016;11:475–81. 10.1080/15592294.2016.1190895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pantaleão LC, Loche E, Fernandez-Twinn DS, Dearden L, Córdova-Casanova A, Osmond C, et al. Programming of cardiac metabolism by miR-15b-5p, a miRNA released in cardiac extracellular vesicles following ischemia-reperfusion injury. Mol Metab 2024;80:101875. 10.1016/j.molmet.2024.101875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Casas-Agustench P, Iglesias-Gutiérrez E, Dávalos A. Mother’s nutritional miRNA legacy: nutrition during pregnancy and its possible implications to develop cardiometabolic disease in later life. Pharmacol Res 2015;100:322–34. 10.1016/j.phrs.2015.08.017 [DOI] [PubMed] [Google Scholar]
- 120. Wang J, Chen J, Sen S. MicroRNA as biomarkers and diagnostics. J Cell Physiol 2016;231:25–30. 10.1002/jcp.25056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Christopher AF, Kaur RP, Kaur G, Kaur A, Gupta V, Bansal P. MicroRNA therapeutics: discovering novel targets and developing specific therapy. Perspect Clin Res 2016;7:68–74. 10.4103/2229-3485.179431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chiñas Merlin A, Gonzalez K, Mockler S, Perez Y, Jia UTA, Chicco AJ, et al. Switching to a standard chow diet at weaning improves the effects of maternal and postnatal high-fat and high-sucrose diet on cardiometabolic health in adult male mouse offspring. Metabolites 2022;12:563. 10.3390/metabo12060563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gaspar RS, Unsworth AJ, Al-Dibouni A, Bye AP, Sage T, Stewart M, et al. Maternal and offspring high-fat diet leads to platelet hyperactivation in male mice offspring. Sci Rep 2021;11:1473. 10.1038/s41598-020-80373-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Calvert JW, Lefer DJ, Gundewar S, Poston L, Coetzee WA. Developmental programming resulting from maternal obesity: effects on myocardial ischemia/reperfusion injury. Exp Physiol 2009;94:805–14. 10.1113/expphysiol.2009.047183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Miller TA, Dodson RB, Mankouski A, Powers KN, Yang Y, Yu B, et al. Impact of diet on the persistence of early vascular remodeling and stiffening induced by intrauterine growth restriction and a maternal high-fat diet. Am J Physiol Heart Circ Physiol 2019;317:H424–33. 10.1152/ajpheart.00127.2019 [DOI] [PubMed] [Google Scholar]
- 126. Nyrnes SA, Garnæs KK, Salvesen Ø, Timilsina AS, Moholdt T, Ingul CB. Cardiac function in newborns of obese women and the effect of exercise during pregnancy. A randomized controlled trial. PLoS One 2018;13:e0197334. 10.1371/journal.pone.0197334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Burden SJ, Alshehri R, Lamata P, Poston L, Taylor PD. Maternal obesity and offspring cardiovascular remodelling—the effect of preconception and antenatal lifestyle interventions: a systematic review. Int J Obes (Lond) 2024;48:1045–64. 10.1038/s41366-024-01536-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Melzer K, Schutz Y, Boulvain M, Kayser B. Physical activity and pregnancy. Sports Med 2010;40:493–507. 10.2165/11532290-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 129. Cole LK, Zhang M, Chen L, Sparagna GC, Vandel M, Xiang B, et al. Supplemental berberine in a high-fat diet reduces adiposity and cardiac dysfunction in offspring of mouse dams with gestational diabetes mellitus. J Nutr 2021;151:892–901. 10.1093/jn/nxaa408 [DOI] [PubMed] [Google Scholar]
- 130. Cole LK, Sparagna GC, Vandel M, Xiang B, Dolinsky VW, Hatch GM. Berberine elevates cardiolipin in heart of offspring from mouse dams with high fat diet-induced gestational diabetes mellitus. Sci Rep 2021;11:15770. 10.1038/s41598-021-95353-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Giussani DA, Camm EJ, Niu Y, Richter HG, Blanco CE, Gottschalk R, et al. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS One 2012;7:e31017. 10.1371/journal.pone.0031017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Itani N, Skeffington KL, Beck C, Niu Y, Giussani DA. Melatonin rescues cardiovascular dysfunction during hypoxic development in the chick embryo. J Pineal Res 2016;60:16–26. 10.1111/jpi.12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kane AD, Herrera EA, Camm EJ, Giussani DA. Vitamin C prevents intrauterine programming of in vivo cardiovascular dysfunction in the rat. Circ J 2013;77:2604–11. 10.1253/circj.CJ-13-0311 [DOI] [PubMed] [Google Scholar]
- 134. Kane AD, Hansell JA, Herrera EA, Allison BJ, Niu Y, Brain KL, et al. Xanthine oxidase and the fetal cardiovascular defence to hypoxia in late gestation ovine pregnancy. J Physiol 2014;592:475–89. 10.1113/jphysiol.2013.264275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Thompson LP, Al-Hasan Y. Impact of oxidative stress in fetal programming. J Pregnancy 2012;2012:582748. 10.1155/2012/582748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chatterjee P, Holody CD, Kirschenman R, Graton ME, Spaans F, Phillips TJ, et al. Sex-specific effects of prenatal hypoxia and a placental antioxidant treatment on cardiac mitochondrial function in the young adult offspring. Int J Mol Sci 2023;24:13624. 10.3390/ijms241713624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Boots CE, Boudoures A, Zhang W, Drury A, Moley KH. Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Hum Reprod 2016;31:2090–7. 10.1093/humrep/dew181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Han L, Wang H, Li L, Li X, Ge J, Reiter RJ, et al. Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT3-SOD2-dependent pathway. J Pineal Res 2017;63:432–442. 10.1111/jpi.12431 [DOI] [PubMed] [Google Scholar]
- 139. Jonscher KR, Stewart MS, Alfonso-Garcia A, DeFelice BC, Wang XX, Luo Y, et al. Early PQQ supplementation has persistent long-term protective effects on developmental programming of hepatic lipotoxicity and inflammation in obese mice. FASEB J 2017;31:1434–48. 10.1096/fj.201600906R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Murphy MP, Smith RA. Drug delivery to mitochondria: the key to mitochondrial medicine. Adv Drug Deliv Rev 2000;41:235–50. 10.1016/S0169-409X(99)00069-1 [DOI] [PubMed] [Google Scholar]
- 141. Murphy MP. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta 2008;1777:1028–31. 10.1016/j.bbabio.2008.03.029 [DOI] [PubMed] [Google Scholar]
- 142. James AM, Cochemé HM, Smith RAJ, Murphy MP. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem 2005;280:21295–312. 10.1074/jbc.M501527200 [DOI] [PubMed] [Google Scholar]
- 143. Smith RAJ, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci 2010;1201:96–103. 10.1111/j.1749-6632.2010.05627.x [DOI] [PubMed] [Google Scholar]
- 144. Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RAJ, Cochemé HM, et al. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 2009;54:322–8. 10.1161/HYPERTENSIONAHA.109.130351 [DOI] [PubMed] [Google Scholar]
- 145. Rodriguez-Cuenca S, Cochemé HM, Logan A, Abakumova I, Prime TA, Rose C, et al. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic Biol Med 2010;48:161–72. 10.1016/j.freeradbiomed.2009.10.039 [DOI] [PubMed] [Google Scholar]
- 146. Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, et al. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord 2010;25:1670–4. 10.1002/mds.23148 [DOI] [PubMed] [Google Scholar]
- 147. Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM, et al. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int 2010;30:1019–26. 10.1111/j.1478-3231.2010.02250.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were generated or analysed for or in support of this paper.