Abstract
Background
Achieving informed consent is a core clinical procedure and is required before any surgical or invasive procedure is undertaken. However, it is a complex process which requires patients be provided with information which they can understand and retain, opportunity to consider their options, and to be able to express their opinions and ask questions. There is evidence that at present some patients undergo procedures without informed consent being achieved.
Objectives
To assess the effects on patients, clinicians and the healthcare system of interventions to promote informed consent for patients undergoing surgical and other invasive healthcare treatments and procedures.
Search methods
We searched the following databases using keywords and medical subject headings: Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 5, 2012), MEDLINE (OvidSP) (1950 to July 2011), EMBASE (OvidSP) (1980 to July 2011) and PsycINFO (OvidSP) (1806 to July 2011). We applied no language or date restrictions within the search. We also searched reference lists of included studies.
Selection criteria
Randomised controlled trials and cluster randomised trials of interventions to promote informed consent for patients undergoing surgical and other invasive healthcare procedures. We considered an intervention to be intended to promote informed consent when information delivery about the procedure was enhanced (either by providing more information or through, for example, using new written materials), or if more opportunity to consider or deliberate on the information was provided.
Data collection and analysis
Two authors assessed the search output independently to identify potentially‐relevant studies, selected studies for inclusion, and extracted data. We conducted a narrative synthesis of the included trials, and meta‐analyses of outcomes where there were sufficient data.
Main results
We included 65 randomised controlled trials from 12 countries involving patients undergoing a variety of procedures in hospitals. Nine thousand and twenty one patients were randomised and entered into these studies. Interventions used various designs and formats but the main data for results were from studies using written materials, audio‐visual materials and decision aids. Some interventions were delivered before admission to hospital for the procedure while others were delivered on admission.
Only one study attempted to measure the primary outcome, which was informed consent as a unified concept, but this study was at high risk of bias. More commonly, studies measured secondary outcomes which were individual components of informed consent such as knowledge, anxiety, and satisfaction with the consent process. Important but less commonly‐measured outcomes were deliberation, decisional conflict, uptake of procedures and length of consultation.
Meta‐analyses showed statistically‐significant improvements in knowledge when measured immediately after interventions (SMD 0.53 (95% CI 0.37 to 0.69) I2 73%), shortly afterwards (between 24 hours and 14 days) (SMD 0.68 (95% CI 0.42 to 0.93) I2 85%) and at a later date (15 days or more) (SMD 0.78 (95% CI 0.50 to 1.06) I2 82%). Satisfaction with decision making was also increased (SMD 2.25 (95% CI 1.36 to 3.15) I2 99%) and decisional conflict was reduced (SMD ‐1.80 (95% CI ‐3.46 to ‐0.14) I2 99%). No statistically‐significant differences were found for generalised anxiety (SMD ‐0.11 (95% CI ‐0.35 to 0.13) I2 82%), anxiety with the consent process (SMD 0.01 (95% CI ‐0.21 to 0.23) I2 70%) and satisfaction with the consent process (SMD 0.12 (95% CI ‐0.09 to 0.32) I2 76%). Consultation length was increased in those studies with continuous data (mean increase 1.66 minutes (95% CI 0.82 to 2.50) I2 0%) and in the one study with non‐parametric data (control 8.0 minutes versus intervention 11.9 minutes, interquartile range (IQR) of 4 to 11.9 and 7.2 to 15.0 respectively). There were limited data for other outcomes.
In general, sensitivity analyses removing studies at high risk of bias made little difference to the overall results.
Authors' conclusions
Informed consent is an important ethical and practical part of patient care. We have identified efforts by researchers to investigate interventions which seek to improve information delivery and consideration of information to enhance informed consent. The interventions used consistently improve patient knowledge, an important prerequisite for informed consent. This is encouraging and these measures could be widely employed although we are not able to say with confidence which types of interventions are preferable. Our results should be interpreted with caution due to the high levels of heterogeneity associated with many of the main analyses although we believe there is broad evidence of beneficial outcomes for patients with the pragmatic application of interventions. Only one study attempted to measure informed consent as a unified concept.
Keywords: Humans; Surgical Procedures, Operative; Decision Support Techniques; Endoscopy; Informed Consent; Informed Consent/statistics & numerical data; Pamphlets; Patient Education as Topic; Patient Education as Topic/methods; Randomized Controlled Trials as Topic; Teaching Materials
Plain language summary
Interventions to promote informed consent for patients undergoing surgical and other invasive healthcare procedures
Before patients have an operation or other invasive procedure (e.g. endoscopy) it is crucial for the healthcare professional to explain what the treatment involves, what alternatives exist and the risks and benefits of the different treatment options. This process is known as ‘informed consent’ and aims to provide sufficient information to allow patients to understand their treatment options and to choose between them.
Research suggests that when informed consent is obtained, the information provided by healthcare professionals is often unclear or insufficient, leading to misunderstanding, a worse treatment response and even litigation. A number of interventions have been developed to improve the quality of information provided to patients, including written pamphlets, videos and websites. It is unclear whether these interventions work in clinical practice.
In this review we summarise studies of interventions designed to improve information delivery or to improve consideration of information for informed consent.
We searched the scientific literature to identify randomised controlled trials (RCTs) of interventions designed to improve informed consent in clinical practice. We wanted to determine primarily whether these interventions improved all components of ‘informed consent’ (understanding, deliberation and communication of decision). Other individual outcomes of direct relevance to patients (e.g. recall/knowledge, understanding, satisfaction and anxiety), those related to healthcare professionals (e.g. ease of use of intervention, satisfaction) and system outcomes (e.g. cost, rates of procedural uptake) were also assessed.
We included 65 studies involving a total of 9021 patients. The studies varied according to the type of intervention, the procedure for which consent was sought, the clinical setting and the outcomes measured. Most interventions were written or audio‐visual. Only one study assessed all the elements of informed consent, but the design was not robust; all other studies assessed only components of informed consent. When the results of multiple studies were combined, we found that interventions improved knowledge of the planned procedure, immediately (up to 24 hours), in the short term (1 to 14 days) and the long term (more than 14 days). Satisfaction with decision making was increased; decisional conflict was reduced; and consultation length may be increased. There were no differences between the intervention and control for the outcomes of generalised anxiety, and either anxiety or satisfaction associated with the consent process.
Limitations of the review include difficulties combining the results of studies due to variation in the procedures undergone by patients, the interventions used and outcomes measured. This means that we are uncertain as to which specific interventions are most effective but pragmatic steps to improve information delivery and consideration of the information are likely to benefit patients.
Background
Description of the condition
Before many healthcare procedures can be undertaken, there is an accepted legal and ethical principle that a suitably‐trained clinician must obtain informed consent from the patient (or consumer). Obtaining informed consent usually requires a discussion between clinician and patient about a surgical or invasive healthcare intervention which results in the patient understanding what the procedure will involve, the risks and benefits of the procedure and their likelihood, and alternative management options, and then agreeing (or declining) to undergo the procedure. The process of achieving informed consent may occur as a single event or over a series of encounters and discussions in outpatient clinics or, for inpatients, on hospital wards. The consent discussion may be supported to a greater or lesser degree by the provision of comprehensive written, video or web‐based information. However it is achieved, it is most clearly shown as concluding when the patient signs a consent form.
Patients are usually required to give consent for a procedure because, while the intention of the procedure is to diagnose or improve their health, there is a risk of injury or other negative outcomes. The most common use of consent relates to surgical procedures but it is also important for a range of other interventions to investigate or treat diseases. Examples include: diagnostic interventions, for example endoscopy, bronchoscopy and angiography; procedures associated with childbirth and pregnancy, for example delivery by caesarean section, amniocentesis or chorionic villous sampling; and curative procedures, for example chemotherapy and radiotherapy. Consent is usually required for all procedures where the patient is under general anaesthetic, although there may not be a requirement to seek consent for the anaesthetic itself. In emergencies, or when the patient is too ill to provide written consent, clinicians are still expected to seek the oral consent of the patient and document this. In such circumstances they may also seek the consent of a relative or other delegate. Clinicians are also required to seek the consent of a relative or delegate if the patient is under 16 years old, or if the patient is not capable of giving informed consent due, for example, to intellectual disability. If it is not possible to seek consent, for example because the patient is unconscious and no relatives or other delegates are available, clinicians are expected to exercise their judgement and act in the patient's best interests. Clinical trials or research procedures require additional consent, by which the patient confirms their agreement to take part in clinical research. This form of consent (consent for research studies) is excluded from this review and is discussed in other reviews (Ryan 2009;Hon 2012).
The signed consent form is frequently used as evidence of informed consent. However, this is often an oversimplification since there is a risk of acquiescence by a patient who may not be fully informed. For consent to be valid it must be given voluntarily by a patient who has the capacity to consent to the intervention in question and who has done each of the following:
Understood the information provided;
Retained that information long enough to be able to make the decision;
Weighed up the information as part of the decision‐making process; and
Communicated their decision (DoH 2009).
These requirements for informed consent are supported by Marteau who described a model to measure informed choice with regard to antenatal screening (Marteau 2001). This involves assessing the patient's knowledge, attitudes and behaviour. This model aims to measure the patient's knowledge (for example of antenatal screening), their attitude towards the screening (either positive or negative), and whether their behaviour (uptake or refusal of the test) is consistent with their attitudes.
Regulatory bodies such as the General Medical Council, British Medical Association and Department of Health in the United Kingdom (UK), the American Medical Association and the Australian Medical Council provide guidance for clinicians about the information they should discuss with the patient during the informed consent process (AMA 2009; AMC 2009; BMA 2009; DoH 2001DoH 2009; GMC 2008). Information that should be discussed includes the intended benefits and risks and their likelihood, and the alternative options including doing nothing.
There are three standards of disclosure (or information provision) which may be applied when discussing the risks associated with surgical or invasive healthcare procedures:
The ‘professional standard’: a physician is to provide information that a physician of good standing in the physician’s community of peers would provide to his or her patient.
The ‘reasonable person standard’: a physician is to provide that information that a hypothetical reasonable person in the position of the patient would want to know.
The ‘subjective person standard’: a physician is to provide that information that the particular individual patient in question would want to know (Mazur 2009).
Failure to achieve fully‐informed consent has led to a number of legal judgements which have clarified the interpretation of consent with particular emphasis on the provision of information. In Chester v Afshar, a UK case concerning the risks associated with spinal surgery, the House of Lords held that a failure to warn a patient of the risk of injury inherent in surgery, however small the probability, denies the patient the chance to make fully‐informed decisions (Chester v Afshar 2004; DoH 2009). In the United States, in Canterbury v Spence the appellate court held that ‘every human being of adult years and sound mind has a right to determine what shall be done to their own body' and stated that ‘the nature of the doctor‐patient relationship demands that the physician volunteer that information even if the patient does not ask’ (Canterbury v Spence 1972). In Australia, the High Court inRogers v Whitaker found unanimously against a surgeon who was considered to have provided inadequate information. The court stated that it is part of the doctor's duty to disclose 'material' risks. A risk is held to be material if "in the circumstances of the particular case, a reasonable person in the patient's position, if warned of the risk, would be likely to attach significance to it or if the medical practitioner is, or should be aware that the particular patient, if warned of the risk, would be likely to attach significance to it" (Rogers v Whitaker 1992).
These cases indicate that there is a minimum amount of information that the patient needs in order to make an informed choice, although the depth and breadth of this information will vary from procedure to procedure, and some patients will request further details whilst others prefer to have less information. This may require clinicians to give information to patients who have said that they do not want to know more about the planned intervention.
The UK Department of Health emphasises that if the patient has not been given adequate information, or if they do not understand the information, or have not had sufficient opportunity to ask questions, the consent may not be valid even if the patient has signed a consent form. Conversely, properly informed verbal consent, without a signed consent form, is not a bar to treatment (DoH 2001; DoH 2009).
Problems with consent may occur because clinicians sometimes underestimate or undervalue the information needs of patients (Beisecker 1990). Alternatively they may overestimate the amount of information they give (Makoul 1995), lack the skills to give information (Jenkins 1999) or use technical language or jargon. Patients may feel pressured into consenting to a procedure that they have concerns about, or that they have not had adequate opportunity to discuss (Dixon‐Woods 2006). This can be through over‐emphasis of the benefits of a particular treatment, shortage of time, the clinician's manner or lack of empowerment on the part of the patient. Patients’ ability to seek further relevant information may also be influenced by how empowered they feel to ask questions, their knowledge of medical care and their physical condition (Akkad 2004).
A further challenge can involve the clinician translating population‐based estimates to an individual risk for that particular patient (Edwards 2002). Also patients may attach differing significance to different risks and benefits, and their perceptions of them may vary.
Clinicians may focus upon communicating specific technical risks of negative outcomes when talking to patients about the procedure, for example the risk of wound infection, bowel perforation or death (Barkin 2009;Ergina 2009). Some of these are of concern to patients, but sometimes this approach overrides consideration of other concerns of greater importance to the particular patient. These may include the consequences of the procedure, for example pain or length of time off work. The provision of complex information can be even more difficult when caring for a sick patient in an emergency, particularly if the clinician believes the information may add to the patient’s stress (Jefford 2002).
There are also organisational barriers to achieving informed consent. Ideally clinicians will talk to patients some time (at least two or three days) before the intervention or procedure. This allows the patient time to reflect on the discussion and deliberate on their options. However, often the signing of the consent form (and thus the formal consent discussion) is delayed until the patient is admitted (or attends as a day case) for the procedure. Then consenting can become a hurried ritual that does not allow the patient enough time to fully consider their decision (Elwyn 2008).
The use of a standardised consent form may add to the ritualised nature of consent discussion by making the process seem repetitive and ritualistic to the clinician. This can lead to the clinician becoming desensitised to the patient’s fears and concerns, as the clinician may view the treatment as being routine and commonplace (Picano 2004). Notably, only 41% of patients believe that the use of consent forms made their wishes known, and 46% believe that the primary function of the consent form was to protect the hospital (Akkad 2006). Any standardisation of the process runs the risk of failing to promote patient autonomy (Habiba 2004).
Description of the intervention
Ensuring informed consent presents challenges for both clinicians and healthcare organisations. Interventions to promote informed consent usually target patients, clinicians, or both. Interventions for patients generally provide information (ideally evidence based) about the treatment options, associated benefits, harms, probabilities and scientific uncertainties. Where they also encourage the patients to clarify personal values, ask questions and weigh up the pros and cons of choosing surgery (or a procedure), these interventions can be seen to fulfil the definition of 'decision aids' (Stacey 2011). The interventions may involve face to face contact, or online, video, telephone or leaflet‐based information. Interventions for clinicians generally address skills to improve how they share information, or direct them to concise sources of information. Interventions may also be organisational, for example the provision of more time for the patient to consider the procedure and ask questions.
Interventions may be categorised by whom they target (patients or clinicians); the purpose (e.g. general educational, encouraging shared decision making, etc); the format (media used: e.g. electronic, paper) and the timing and method of delivery (e.g. remote patient access at home, access supervised by a clinician).
How the intervention might work
Often patients do not fully understand the information provided during the consent process (Brezis 2008). However a clear set of skills for clinicians can be identified which, if used, increases the likelihood that patients will understand and be able to recall complex clinical information. Furthermore these include specific skills for shared decision making, risk communication and the use of decision aids. Clinicians can be trained in these skills (Silverman 2005).
Decision aids have been shown to improve patients’ knowledge regarding options, clarify perceptions of risk, reduce decisional conflict, reduce the number of people feeling passive about decision making and decrease the proportion of people remaining undecided (Stacey 2011). Where decision aids address conditions for which procedures requiring consent are among the options, they may promote informed consent through greater knowledge and consistency of personal values or attitudes with an enacted choice (for or against the treatment or procedure).
Why it is important to do this review
Over 4 million surgical procedures are undertaken in England each year (Royal College of Surgeons of England 2011), which all require patient consent. Similar volumes of procedures will occur in other similar countries. In addition there will be considerable numbers of other procedures requiring consent. However, the consent consultation frequently does not meet the needs of the patient (Akkad 2004). Reports indicate that patients do not receive sufficient information, that the information is not fully understandable or that the information patients receive is not tailored to their particular needs (Schattner 2006). Failure to achieve informed consent is the basis of many formal complaints, and costly litigation. Adequate information provision has additional wider benefits for patients, including increased satisfaction, more rapid symptom resolution, reduced emotional distress, reduced use of analgesia and possibly shorter hospital admissions (Egbert 1964; Hall 1988;Roter 2006). Informed patients are more likely to make conservative treatment options, such as declining surgical procedures, thus possibly reducing overall health costs (Kennedy 2002). Synthesis of the evidence aimed to establish the most robust evidence for the effectiveness of interventions in this field and thus promote implementation and identify the need for further research.
There may be some overlap with other reviews and protocols. Interventions to improve shared decision making (Duncan 2010;Légaré 2010) and informed consent in research have been reviewed (Ryan 2009), but there are no other Cochrane protocols or reviews that examine informed consent in relation to surgical or other invasive procedures alone. Gøtzsche and Jørgensen (Gøtzsche 2013) examined screening for breast cancer with mammography, noting the need for better information to promote informed consent for screening, but their review was not of interventions to promote informed consent. Other interventions, for example the provision of more information on a surgeon's performance, might also be of benefit; however Henderson and Henderson in their systematic review of this intervention identified no relevant studies (Henderson 2010).
Doust and colleagues (Doust 2007) are conducting a review of interventions to minimise harm from screening, including interventions which enhance knowledge about the benefits and harms of screening, but this does not consider any procedures that may follow the screening. Our own earlier review on interventions before healthcare consultations for helping patients get the information they require (Kinnersley 2007) also does not focus on surgical procedures but covers a range of settings, including primary care and outpatient medical settings. The Cochrane review ‘Decision aids to help people who are facing health treatment or screening decisions’, by Stacey et al (Stacey 2011) includes trials in which decision aids have been used to help patients make a decision about treatment options, but the trials do not analyse the informed consent process itself, and many of them do not address conditions directly related to invasive procedures.
Objectives
To assess the effects of interventions to promote informed consent for patients undergoing surgical or other invasive healthcare treatments and procedures.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) including cluster randomised trials.
Types of participants
Patients aged 16 years and over being asked to give consent for a surgical or other invasive healthcare treatment or procedure, either for themselves, or on behalf of a minor or someone else for whom they have responsibility.
We excluded trials in which:·
the patients were aged 16 years and over but were unable to consent to the procedure themselves (because they lacked capacity);·
the patients were detained in hospital (for example under the Mental Health Act in the UK);
the consent being obtained was to take part in a research trial (even if the trial itself involved an invasive procedure).
Types of interventions
Adhering to our protocol (Kinnersley 2011) we considered studies with interventions that:
targeted healthcare professionals, or patients, or both, who were participating in the consent process for a surgical or other invasive healthcare procedure, or
targeted organisational change of the consenting of these patients.
Interventions, even if targeted at clinicians, were required to have the intention of improving patients' understanding of their treatment options (including declining any intervention) and the procedure under consideration, evaluating their options, or helping them retain and recall the information provided, and thus their ability to provide informed consent.
Where the interventions targeted patients, we included studies in which the participants were undergoing procedures, as well as studies in which patients were considering more generally the possible treatment options for their condition, as long as this included at least one surgical or invasive option.
We excluded interventions that focused on the condition alone, or on conditions for which there was no surgical or invasive option. For example, interventions for patients undergoing cholecystectomy or considering treatment options for gallstones, which included cholecystectomy, were eligible to be included in the review. However, interventions that simply provided more information about gallstones without consideration of treatment options were excluded, as were interventions providing information about treatment options for conditions such as eczema (for which there are, as far as we are aware, none requiring consent).
We included interventions in any format/medium.
Comparisons were made between interventions and usual care (controls). Where there were multiple intervention arms we divided the control groups accordingly.
Types of outcome measures
We took a comprehensive approach to outcomes, as the obtaining of consent is a complex process with effects on the patient, the clinician and the healthcare system (CCCRG 2008).
Primary outcomes
Informed consent
The primary outcome was ‘informed consent’. For us to support an investigator’s view that he or she was measuring informed consent we sought evidence that the outcome measure took account of the patient going through the process of being provided with information about a procedure, which they had understood, retained and weighed up sufficiently to make a decision, which they had then communicated to the clinicians caring for them.
In doing this, we also considered Marteau’s model of informed choice (Marteau 2001) which places emphasis on patients’ decision making being supported by evidence of knowledge and consistent values (or satisfaction with the decision).
Ideally, informed consent or informed choice would be a single measure (dichotomised ‐ achieved or not achieved) of the effects of the intervention for a patient. Although we took the view that it would be more likely that trials reported understanding/knowledge, retention/weighing up, attitudinal and uptake measures at group levels (e.g. Evans 2007) it proved difficult to interpret data on different outcomes within individual studies. Instead we present the data for particular outcomes across studies and consider the meaning of these results in the Discussion.
Secondary outcomes
The initial secondary outcomes were the component elements of informed consent as described above.
Patient understanding
Since understanding has various facets and interpretations, we considered there was overlap between understanding, knowledge and recall. However we used Mazur's model of understanding to develop a framework to assess the patient's level of understanding in more depth or to report single facets (e.g. knowledge) (Mazur 2009). Indeed in some studies authors stated that they were assessing understanding, but in fact used instruments measuring only recall or knowledge. In these cases, we re‐classified the outcomes measured. For example, if a researcher described the measurement of understanding when in fact we believed it to represent the measurement of knowledge, we classified this as knowledge; the distinction being that understanding implied a deeper level of comprehension. For instance, a patient may know that they need surgery because they have appendicitis, without necessarily understanding that they need surgery with an awareness to some extent of the pathophysiological process and the consequences of not having surgery.
We sought data on the following aspects of understanding:
Understanding in terms of asking the patient directly if the information had been understood (patient‐reported understanding);
Understanding in terms of evidence of comprehension of the information provided, and the patient's situation beyond simple factual recall;
Understanding in terms of the way the patient used the information provided. If the information has been understood, subsequent decisions by the patient should be consistent with their personal values. Evidence of this process was considered to be present for the outcomes of deliberation and decisional conflict.
Knowledge/retention/recall
Knowledge/retention/recall were most commonly measured by assessing the extent to which the patient ‘knew’ the information with which they had been provided about the procedure; for example, what the patient knew about appendicectomy. Most often it would be the case that patients were recalling information told to them during the consent consultation. However, in some cases this knowledge may have reflected information that the patient had gathered before the consent process for this particular episode of health care. So, before an episode of appendicitis, many patients will know that the appendix is a part of the bowel (although they may not understand what part of the bowel it is, and the role it plays in sickness and in health). For simplicity we equated ‘recall’ of information provided in the consent consultation with wider knowledge and have reported this as 'knowledge'.The timing of measurement of knowledge also provided information as to the retention of information. To make comparisons we made a pragmatic decision to categorise the outcomes depending upon the time of measurement after the intervention into immediate (24 hours or less), short‐term (between 24 hours and 14 days) and long‐term (15 days or more).
Deliberation (weighing up)
Making an informed decision or choice requires someone to consider carefully (deliberate) or weigh up the information, and how it fits with their personal circumstances and values (Elwyn 2010). We examined studies in which researchers claimed to have measured deliberation, and report on both the methods used and the intervention effects.
Communication of decision
Communication of decision is generally demonstrated (in cases of assent to the procedure) by the patient signing a consent form as discussed above. However, researchers may attempt to measure closely‐related concepts, such as confidence in the decision, consistency of decision making or decisional conflict (Stacey 2011).
Other patient outcomes
We also collected data on:
Satisfaction and anxiety with decision making;
Satisfaction and anxiety with the consent process;
Desire for further information;
Sense of control ‐ locus of control or perception of who made the decision:
Pain experienced or analgesia use.
Clinician outcomes
We collected data on the following clinician outcomes:
Satisfaction with the 'consent consultation' (or process);
Ease of use of intervention(s) to improve gaining of informed consent;
Confidence in patient's decision and whether an informed choice was made.
System outcomes
To further judge the effects of interventions, it was necessary to assess changes to the overall healthcare system, and further evidence of patient implementation of choices. We collected data on the following outcomes:
Rates of uptake (or refusal) of clinical interventions/procedures;
Postponement of clinical interventions/procedures;
Delay in decision making or request for more information/further consultations;
Complaints and litigation;
Adverse procedural outcomes;
Economic/resource use data (e.g. length of consultations, cost of surgery/procedure choices, number of consultations, and length of hospital stay).
Search methods for identification of studies
Electronic searches
We performed an electronic search from database inception to July 2011 in MEDLINE (OvidSP), EMBASE (OvidSP) and PsycINFO (OvidSP) using Medical Subject Headings and text words, applying a randomised controlled trial filter to capture the study types included in the review. We also searched the Cochrane Central Register of Controlled Trials (CENTRAL), The Cochrane Library, issue 5, 2012. We applied no language or date restrictions within the search. The detailed search strategies are in Appendices (1 to 4).
Searching other resources
We searched the reference lists of included trials and relevant published reviews to identify further potentially‐relevant studies. We had planned to search a number of additional sources as specified in the review protocol (Kinnersley 2011), but decided that complete coverage of the area was already ensured by the our search of the above databases.
Data collection and analysis
Selection of studies
Stage 1
We conducted the searches for relevant trials, combining the results into a single database and eliminating duplicates.
Stage 2
We screened titles and abstracts to eliminate obviously irrelevant studies. To ensure consistent application of inclusion criteria, we screened the titles in batches of 20, with 3 authors discussing their results. Disagreements were discussed with the wider author team. Once a high level of consistency was achieved two authors worked independently on larger batches of abstracts, again with disagreements being discussed with the wider team.
Stage 3
We retrieved full text copies of all potentially‐relevant papers, including those for which the description was insufficient to make a decision about inclusion. Disagreement was resolved by discussion between the two assessing authors and an arbiter from the review team (PK or AE). Studies excluded at this stage are listed in the Characteristics of excluded studies table.
Stage 4
Two review authors then reviewed relevant studies to ascertain whether there were multiple reports from single studies and linked these together if applicable to produce a final set of studies for inclusion in the review. These were entered into RevMan 5 software.
Stage 5
We scanned the reference lists of included studies to check for further possibly‐relevant studies which had not been identified. These were re‐entered at Stage 3.
Data extraction and management
Two authors independently extracted data from each included study using a modified Cochrane Consumers and Communication Group data collection checklist that had been predetermined and piloted by the review authors (Appendix 5). The authors each entered the data onto a separate electronic form. Discrepancies in data extraction were resolved by discussion between all review authors or, where this was not possible, between the two review authors extracting data and an arbiter (PK or AE). The data included the study methods, setting and participants, interventions, outcomes and results.
Extracted data were entered into RevMan 5 by one author and checked for accuracy against the original data by a different author (variable combinations of authors for individual studies). In cases of missing data we tried to contact the authors of the studies by email to obtain the relevant information.
Assessment of risk of bias in included studies
When assessing the risk of bias we used criteria in the 'Risk of bias' assessment tool (Higgins 2011). We assessed and reported on the risk of bias of included studies in accordance with the guidelines of the Cochrane Consumers and Communication Review Group (Ryan 2011), which recommends the explicit reporting of the following individual quality elements for RCTs: randomisation; allocation concealment; blinding (participants, personnel); blinding (outcome assessment); completeness of outcome data, selective outcome reporting; other sources of bias. In all cases, two authors independently assessed the risk of bias of included studies, with any disagreements resolved by discussion and consensus. Table 1 shows an overview of the rules that we applied when assessing the risk of bias.
1. Risk of bias: Rules applied when assessing the risk of bias.
| Risk of bias domain | Low risk | Unclear risk | High risk |
| Random sequence generation | Clearly described and appropriate method of randomisation (e.g. computerised randomisation). | No description of random sequence generation. | Alternation or allocation by date or hospital number |
| Allocation concealment | Clearly described and appropriate method of allocation concealment (e.g. central or pharmacy allocation). | No description of allocation concealment. | Inappropriate method of allocation concealment or evidence that allocation procedure was not adhered to. |
| Blinding of participants and personnel | Clearly described and appropriate method of blinding of BOTH participants and personnel. | No description of blinding of participants and personnel. | Inappropriate method of blinding, evidence that blinding procedure was not adhered to or study was ‘unblinded’ for EITHER participants or personnel. |
| Blinding of outcome assessors | Clearly described and appropriate method of blinding of outcome assessors. | No description of blinding of outcome assessors. | Inappropriate method of blinding, evidence that blinding procedure was not adhered to or study was ‘unblinded’ for outcome assessors. |
| Incomplete outcome data | Attrition of participants of < 40%. | No description of attrition. | Attrition of participants of ≥ 40%. |
| Selective outcome reporting | Protocol available and all outcomes pre‐specified were reported in the final publication. | No protocol available. | Protocol available and one or more outcomes pre‐specified were not reported in the final publication. |
| Other sources of bias | No evidence of elements of high risk of bias. | Not applied. | Evidence of any of:
|
When assessing randomisation we checked that the investigators had described an adequate random component in the sequence generation. These were judged to have met this criterion were considered low risk of bias. Those that had used a non‐random component were judged not to have met the criterion and marked as high risk.
We assessed whether allocation concealment was adequate and whether blinding was adequate, (blinding of the participants and personnel, performance bias and blinding of outcome assessment, detection bias) with those that were adequate being categorised as low risk and those that were judged to be inadequate being high risk. Where there was insufficient evidence, studies were classed as unclear.
We also examined outcome data to ensure that the effect of incomplete data (attrition bias) had been adequately addressed. Studies with no loss to follow up or an attrition rate of less than 40% were considered low risk and those that had a greater than 40% attrition were considered high risk. Where there was insufficient evidence, studies were classed as unclear.
We assessed selective outcome reporting as well. If a study had a protocol and this was followed then it was considered low risk, studies were considered high risk if one or more pre‐specified outcomes were not reported. Studies with no available protocol were considered to be unclear.
We then looked at other potential risks of bias; such as threats to validity as detailed in the 'Risk of bias' assessment tool, potential contamination of the intervention or sources of funding leading to competing interests.
We contacted study authors for additional information about the included studies, or for clarification of the study methods as required. If we received no response, the study was marked as unclear for the relevant domain. We incorporated the results of the 'Risk of bias' assessment into the review through systematic narrative description and commentary about each of the items, leading to an overall assessment of the risk of bias of the included studies and a judgement about the internal validity of the review results.
Measures of treatment effect
The two main types of outcomes were continuous and dichotomous. Continuous outcomes were summarised using standardised estimated mean differences. Dichotomous outcomes were summarised using relative risks. For the measures of variance we calculated 95% confidence intervals for the effect estimates. If only non‐parametric data were reported in studies, this was included as narrative description for each relevant outcome to summarise findings in the literature as fully as possible.
For the primary outcome (informed consent/choice), we aimed to identify reports of individual informed consent (achieved or not achieved) for meta‐analysis. If there were studies which reported changes in understanding, values or choices made at group level only, we reported these separately and considered if it was appropriate to include them in a meta‐analysis.
Where studies had multiple outcomes in the same outcome category we identified the main outcome for the study by:
Selecting the primary outcome as identified by the publication authors;
If no primary outcome was specified, selecting the one specified in the sample size calculation;
If there was no sample size calculation, we ranked the effect estimates and selected the median effect estimate.
Unit of analysis issues
Studies in which clusters of individuals were randomised or allocated to intervention groups, but intervention was intended at the level of the individual, are problematic because they may lead to artificially small P values if standard statistical methods are used. We intended to account for this by re‐calculating the statistical significance accounting for intra‐cluster correlations. However, in the event only two studies were found that employed cluster randomisation (Paci 1999; Solberg 2010). Paci 1999 randomised by day of visit which would not be expected to produce appreciable clustering. Solberg 2010 randomised by centre but the impact of clustering possible from this one study was not deemed sufficient to re‐analyse the data. We note the unit‐of‐analysis error.
Dealing with missing data
We tried to contact all authors to obtain additional data. In addition we attempted to clarify the method of randomisation and whether allocation was concealed . We tried to carry out an intention‐to‐treat analysis where this was not reported by the authors and the authors did not respond to our enquiries. If sufficient data were not available, we carried out an available case analysis and consider the implications of the missing data in the Discussion section.
Assessment of heterogeneity
We assessed the statistical heterogeneity in meta‐analyses by visually inspecting the scatter of effect estimates on forest plots and by the I2 statistic (Higgins 2003). We categorised I2 values of 0% to 30% as indicating little evidence of heterogeneity, 31% to 60% as moderate heterogeneity, 61% to 80% as substantial heterogeneity and over 80% as considerable heterogeneity.
Assessment of reporting biases
Where possible, we accessed the Clinical Trials Registry to determine whether studies were reporting their pre‐specified primary outcomes. Where there was evidence of selective outcome reporting, this is reflected in the 'Risk of bias' assessment (see Assessment of risk of bias in included studies). Where sufficient RCTs and data for a given outcome were available, we conducted a visual inspection of the funnel plots to investigate any asymmetry as an indication of publication bias.
Data synthesis
For all studies, we have produced tables of summary statistics and graphs of the data. Although the procedures vary in complexity, we consider it likely that it is the type of intervention which is more important than the type of procedure that was being undertaken, and therefore our main focus was on types of intervention and assessing whether there were consistent benefits (or harms) across similar interventions.
We conducted a meta‐analysis of those studies and outcomes which appear homogenous (minimum of three studies) (Treadwell 2006) using a random‐effects model. Although our original intention was only to conduct meta‐analyses where heterogeneity was low (I2 statistic < 50%) we concluded it was more useful to conduct meta‐analyses regardless of the I2 statistic and leave it to the reader to take note of the heterogeneity of the studies. We performed data synthesis using RevMan 5.
Subgroup analysis and investigation of heterogeneity
Several features of the interaction between patient and clinician may affect communication, the interaction, and thus the opportunity to achieve informed consent for procedures. We planned and attempted to undertake the following comparisons:
face‐to‐face interventions versus distant interventions (for example web‐based);
interventions targeted at clinicians versus those targeted at patients or at organisational change;
the age of patients (young 16 to 35 years; middle‐aged 36 to 60 years; older 61 to 80 years, elderly over 80 years);
interventions targeted at a specific procedure (i.e. whether to undergo, for example, an operation such as knee replacement for osteoarthritis) or at a condition more generally (but for which at least one option may be surgical (e.g. a decision aid addressing menorrhagia)).
For the subgroup analyses we divided the studies into the subgroups of interest and conducted meta‐analyses where possible. We present for each subgroup analysis the standardised mean difference, confidence intervals and measure of heterogeneity for continuous data and, in the case of dichotomous data, relative risks, confidence intervals and measure of heterogeneity.
Sensitivity analysis
We conducted a sensitivity analysis based on the risk of bias identified in the studies. Studies identified as being of greatest risk of bias, specifically regarding randomisation, attrition and blinded outcome assessment were systematically removed from the analysis and we report the effects on the effect estimate.
Consumer participation
We engaged consumer input and representation from Cynnws Pobl (involving people), the consumer network of Clinical Research Collaboration Cymru, for advice on outcome measures, searching for types of interventions and assistance in analysis and interpretation of the effects of interventions across consumer/participant groups.
We also recruited clinician representatives to form a clinician reference group. A range of clinicians (surgical, medical and nursing) who are involved in the consent process were recruited from hospitals in South Wales and South West England, including surgical, radiological and other disciplines. They provided feedback on the results of the review and assisted with the writing of the plain language summary.
Results
Description of studies
Results of the search
The electronic searches conducted yielded 12,283 references and other sources retrieved 1 reference. The number of records after removal of duplicates was 12,067. From these, we identified 271 papers for further examination of the full text. Ninety‐five studies were identified for data extraction and preliminary inclusion but 30 of these studies were subsequently excluded (see Characteristics of excluded studies). Thus Sixty‐five studies met our inclusion criteria and were included in the synthesis. A flow diagram of the study selection process is presented in Figure 1.
1.
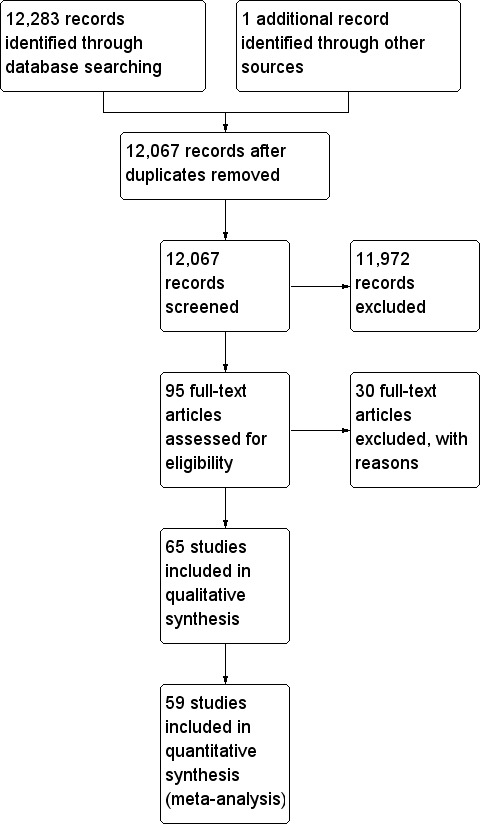
Study flow diagram.
Included studies
Size of review
We included 65 studies with a total of 9021 participants in the review (see Characteristics of included studies). Individual studies ranged from 20 participants (Wadey 1997) to 596 participants (Raynes‐Greenow 2010).
Five studies (six intervention arms) contributed only non‐parametric data so were included in qualitative synthesis only (Astley 2008a; Astley 2008b; Gerancher 2000; Lavelle‐Jones 1993; Mason 2003; Wadey 1997).
In seven studies, two separate interventions were compared with one control (Agre 1994a; Agre 1994b; Astley 2008a; Astley 2008b; Bennett 2009a; Bennett 2009b; Cornoiu 2010a; Cornoiu 2010b;Kang 2009a; Kang 2009b; Mishra 2010a; Mishra 2010b; O'Neill 1996a; O'Neill 1996b). For these studies we split the control group to make independent comparisons with each intervention group. Each of these studies is listed twice in the Included studies list, to enable appropriate reporting and analysis. Overall, there are 72 treatment arms for analysis from the 65 studies.
Settings
Sixty three studies were set in hospital/secondary care and two studies were conducted in dental practice (Johnson 2006; Kang 2009a; Kang 2009b). Twenty five studies were from the USA, 14 from the UK, eight from Australia, seven from Canada, three from Germany, two from Ireland, and one from each of Austria, France, Italy, New Zealand, Switzerland and Turkey. Sixty three studies were written in English; one was translated from French (Danino 2006) and another from German (Hermann 2002).
Participants
Studies focused on adults who were providing informed consent for themselves (60 studies) or were doing this on behalf of a minor (5 studies). Studies had similar exclusion criteria, which referred to a lack of competence to consent or language barriers for the interventions.
Interventions
Intervention types were broadly grouped into audio‐recorded aids (audio‐cassette recording of the consultation or containing information about consent), non‐interactive audio‐visual aids (onscreen DVD, video or software package which the participant watched from beginning to end), interactive multimedia (onscreen DVD, video or software package for which the participant was able to select material to review out of order), written interventions (intervention delivered on paper), decision aids (including multi‐component decision aids), structured consent processes (processes the clinician used to structure the consultation e.g. flip‐sheets, question prompt sheets), question prompt sheets (given to the patient to use) or altering the timing of consent (relative to the time of the procedure). The interventions used in studies are summarised in the following table:
Procedures
Participants were generally those attending for elective procedures but included some emergency procedures. The procedures are summarised in the following table:
| Procedure type | Number of studies (intervention arms) | References |
| Surgical | 32 (35 intervention arms) | Armstrong 1997; Armstrong 2010; Ashraff 2006; Bollschweiler 2008; Chan 2002; Chantry 2010; Cornoiu 2010b; Cornoiu 2010a; Danino 2006; Deyo 2000; Fink 2010; Garrud 2001; Heller 2008; Henry 2008; Hong 2009; Langdon 2002; Lavelle‐Jones 1993; Makdessian 2004; Mason 2003; Masood 2007; Mauffrey 2008; Mishra 2010b; Mishra 2010a; Nadeau 2010; Neary 2010; O'Neill 1996a; O'Neill 1996b; Pesudovs 2006; Rossi 2004; Rossi 2005; Rymeski 2010; Wadey 1997; Walker 2007; Wilhelm 2009; Zite 2011 |
| Invasive medical procedure e.g. endoscopy, colonoscopy, angiogram, bronchoscopy | 10 (12 intervention arms) | Agre 1994a; Agre 1994b; Astley 2008a; Astley 2008b; Elfant 1995; Enzenhofer 2004; Felley 2008; Friedlander 2011; Luck 1999; Phatouros 1995; Tait 2009; Uzbeck 2009 |
| Anaesthetics e.g. general anaesthesia, epidural analgesia, regional anaesthesia | 4 (4 intervention arms) | Garden 1996; Gerancher 2000; Kain 1997; Paci 1999 |
| Electroconvulsive therapy | 1 (1 intervention arm) | Greening 1999 |
| Dental procedures | 2 (3 intervention arms) | Johnson 2006; Kang 2009a; Kang 2009b |
| Paediatrics e.g. neonatal circumcision, inguinal hernia repair, ENT surgery, endoscopy or orthodontics | 5 (6 intervention arms) | Chantry 2010; Friedlander 2011; Kang 2009a; Kang 2009b; Nadeau 2010; Rymeski 2010 |
| Chemotherapy | 2 (2 intervention arms) | Olver 2009; Thomas 2000 |
| Antenatal screening procedures for Down Syndrome (including invasive options for screening) | 1 (1 intervention arm) | Bekker 2004 |
| Invasive radiology (spinal and facet joint injections, urography, intra‐venous contrast CTs) | 5 (6 intervention arms) | Bennett 2009a; Bennett 2009b; Cowan 2007; Hopper 1994; Neptune 1996; Yucel 2005 |
Description of interventions
Development
We analysed included studies for data on how the interventions were developed (Table 2). For 42 of 72 intervention arms (58.3%) it appeared that the interventions were designed for the trial with no validation or piloting (Armstrong 2010; Astley 2008a; Astley 2008b; Bekker 2004; Bennett 2009a; Bennett 2009b; Chan 2002; Chantry 2010; Cowan 2007; Danino 2006; Elfant 1995; Enzenhofer 2004; Fink 2010; Garden 1996; Garrud 2001; Greening 1999; Heller 2008; Henry 2008; Hermann 2002; Hong 2009; Kain 1997; Langdon 2002; Lavelle‐Jones 1993; Makdessian 2004; Mason 2003; Mauffrey 2008; Mishra 2010a; Mishra 2010b; Neary 2010; O'Neill 1996a ;O'Neill 1996b; Olver 2009; Pesudovs 2006; Phatouros 1995; Raynes‐Greenow 2010; Rossi 2004; Tait 2009; Wadey 1997; Walker 2007; Wilhelm 2009; Yucel 2005; Zite 2011). For 16 of 72 intervention arms (22.2%) there appeared to be reasonable efforts to pilot and validate the interventions (Agre 1994a; Agre 1994b; Bollschweiler 2008; Cornoiu 2010a; Cornoiu 2010b; Deyo 2000; Goel 2001; Hopper 1994; Johnson 2006; Morgan 2000; Neptune 1996; Shorten 2005; Solberg 2010; Thomas 2000; Whelan 2003; Wong 2006). For 4 of 72 intervention arms (5.5%) the intervention was modified from standard procedures (Kang 2009a; Kang 2009b; Masood 2007; Uzbeck 2009) and for another 4 intervention arms (5.5%) the intervention was the introduction of a standard procedure not currently in use (Friedlander 2011; Luck 1999; Rossi 2004; Rymeski 2010). For 6 intervention arms (8.3%) no details were provided (Armstrong 1997; Ashraff 2006; Felley 2008; Gerancher 2000; Neary 2010; Paci 1999).
2. Details of the development of the intervention, the exposure to the intervention, the training for delivery of the intervention and evaluation of the intervention delivery; 72 treatment arms reported here.
| Development of the intervention | Number of intervention arms |
| no details | 6 |
| designed for trial ‐ no validation | 42 |
| designed for trial ‐ reasonable effort for validation/piloting | 16 |
| modified from standard information | 4 |
| standard information ‐ no modifications | 4 |
| Total | 72 |
| Exposure to the intervention | |
| once | 65 |
| twice | 1 |
| multiple exposures to the same intervention | 4 |
| two different interventions at different times | 2 |
| Total | 72 |
| Training for delivery of intervention | |
| none needed | 22 |
| no details | 35 |
| no training | 0 |
| brief training | 9 |
| structured/extensive training | 2 |
| all delivered by key researcher | 4 |
| Total | 72 |
| Evaluation of delivery of the intervention | |
| no details | 59 |
| evidence of fidelity/reliability of delivery | 13 |
| Total | 72 |
Exposure to the intervention
Studies were analysed for data on the number of exposures participants had to the interventions (Table 2). In 65 intervention arms (90.2%) participants were exposed to the intervention once (Agre 1994a; Agre 1994b; Armstrong 1997; Ashraff 2006; Astley 2008a; Astley 2008b; Bekker 2004; Bennett 2009a; Bennett 2009b; Bollschweiler 2008; Chan 2002; Chantry 2010; Cornoiu 2010a; Cornoiu 2010b; Cowan 2007; Danino 2006; Deyo 2000; Elfant 1995; Enzenhofer 2004; Felley 2008; Fink 2010; Friedlander 2011; Garden 1996; Garrud 2001; Gerancher 2000; Goel 2001; Greening 1999; Heller 2008; Henry 2008; Hermann 2002; Hong 2009; Hopper 1994; Johnson 2006; Kain 1997; Kang 2009a; Kang 2009b; Langdon 2002; Lavelle‐Jones 1993; Luck 1999; Makdessian 2004; Mason 2003; Masood 2007; Mauffrey 2008; Morgan 2000; Nadeau 2010; Neary 2010; Neptune 1996; O'Neill 1996a; O'Neill 1996b; Olver 2009; Paci 1999; Pesudovs 2006; Phatouros 1995; Raynes‐Greenow 2010; Rossi 2004; Rossi 2005; Rymeski 2010; Tait 2009; Uzbeck 2009; Wadey 1997; Walker 2007; Wilhelm 2009; Wong 2006; Yucel 2005; Zite 2011). One arm (1.4%) gave two exposures to the same intervention (Armstrong 2010). Four arms (5.5%) gave participants multiple exposures to the same Intervention (Mishra 2010a; Mishra 2010b; Thomas 2000; Whelan 2003). Two arms (2.7%) gave the consumers two exposures to two different interventions (Shorten 2005; Solberg 2010).
Training for delivery of intervention
For 35 intervention arms (48.6%) no details were provided of the training given to staff delivering the intervention (Table 2) (Agre 1994b; Astley 2008a; Astley 2008b; Bollschweiler 2008; Chan 2002; Danino 2006; Elfant 1995; Fink 2010; Garden 1996; Garrud 2001; Gerancher 2000; Heller 2008; Henry 2008; Hong 2009; Hopper 1994; Kain 1997; Langdon 2002; Lavelle‐Jones 1993; Makdessian 2004; Mason 2003; Mauffrey 2008; Morgan 2000; Nadeau 2010; Neptune 1996; O'Neill 1996a; O'Neill 1996b; Paci 1999; Pesudovs 2006; Phatouros 1995; Raynes‐Greenow 2010; Rossi 2004; Shorten 2005; Solberg 2010; Walker 2007; Yucel 2005). For 22 intervention arms (30.5%), little or no training appeared necessary, for example if the intervention was delivered by the participants viewing a video (Agre 1994a; Armstrong 1997; Ashraff 2006; Chantry 2010; Felley 2008; Friedlander 2011; Hermann 2002; Kang 2009a; Kang 2009b; Luck 1999; Masood 2007; Mishra 2010a; Mishra 2010b; Neary 2010; Olver 2009; Rossi 2005; Rymeski 2010; Tait 2009; Thomas 2000; Uzbeck 2009; Wilhelm 2009; Wong 2006). For nine intervention arms (12.5%) brief training was given to staff involved (Armstrong 2010; Bennett 2009a; Bennett 2009b; Cornoiu 2010a; Cornoiu 2010b; Cowan 2007; Deyo 2000; Enzenhofer 2004; Goel 2001); for two intervention arms (2.7%) structured and extensive training was given (Johnson 2006; Whelan 2003); and for four intervention arms (5.5%) all interventions were delivered by the key researcher (Bekker 2004; Greening 1999; Wadey 1997; Zite 2011).
Evaluation of the delivery of the intervention
In 59 intervention arms (81.9%) there was no evidence of evaluation of the delivery of the intervention (Agre 1994a; Agre 1994b; Armstrong 1997; Armstrong 2010; Ashraff 2006; Bekker 2004; Bennett 2009a; Bennett 2009b; Chan 2002; Chantry 2010; Cornoiu 2010a; Cornoiu 2010b; Cowan 2007; Danino 2006; Elfant 1995; Fink 2010; Friedlander 2011; Garden 1996; Garrud 2001; Gerancher 2000; Goel 2001; Greening 1999; Heller 2008; Henry 2008; Hermann 2002; Hong 2009; Hopper 1994; Johnson 2006; Kain 1997; Kang 2009a; Kang 2009b; Langdon 2002; Lavelle‐Jones 1993; Luck 1999; Makdessian 2004; Mason 2003; Masood 2007; Mauffrey 2008; Mishra 2010a; Mishra 2010b; Nadeau 2010; Neary 2010; O'Neill 1996a; O'Neill 1996b; Paci 1999; Pesudovs 2006; Phatouros 1995; Raynes‐Greenow 2010; Rossi 2004; Rossi 2005; Solberg 2010; Tait 2009; Uzbeck 2009; Wadey 1997; Walker 2007; Whelan 2003; Wilhelm 2009; Yucel 2005; Zite 2011) and in 13 intervention arms (18.1%) there was evidence of checks on the fidelity and reliability of the delivery of the interventions (Table 2) (Astley 2008a; Astley 2008b; Bollschweiler 2008; Deyo 2000; Enzenhofer 2004; Felley 2008; Morgan 2000; Neptune 1996; Olver 2009; Rymeski 2010; Shorten 2005; Thomas 2000; Wong 2006).
Details of the consent process in the control group
In 33 intervention arms (45.8%) the control groups received verbal information only in their consent consultation (Table 2) (Armstrong 1997; Armstrong 2010; Ashraff 2006; Astley 2008a; Astley 2008b; Bekker 2004; Chantry 2010; Cowan 2007; Elfant 1995; Enzenhofer 2004; Felley 2008; Friedlander 2011; Hermann 2002; Hopper 1994; Johnson 2006; Langdon 2002; Lavelle‐Jones 1993; Makdessian 2004; Mason 2003; Mauffrey 2008; Mishra 2010a; Mishra 2010b; Morgan 2000; Nadeau 2010; Neary 2010; Neptune 1996; Paci 1999; Rossi 2004; Rossi 2005; Rymeski 2010; Wadey 1997; Walker 2007; Wilhelm 2009). In 23 intervention arms (30.9%) participants in the control group received standardised or particular written information as well as verbal information (Bollschweiler 2008; Danino 2006; Deyo 2000; Garden 1996; Garrud 2001; Goel 2001; Greening 1999; Heller 2008; Henry 2008; Kang 2009a; Kang 2009b; Luck 1999; O'Neill 1996a; O'Neill 1996b; Olver 2009; Phatouros 1995; Raynes‐Greenow 2010; Solberg 2010; Thomas 2000; Uzbeck 2009; Whelan 2003; Yucel 2005; Zite 2011). In 12 intervention arms (16.7%) the consenting clinician used a checklist of points to cover when talking to the patients (Agre 1994a; Agre 1994b; Bennett 2009a; Bennett 2009b; Chan 2002; Cornoiu 2010a; Cornoiu 2010b; Gerancher 2000; Hong 2009; Mauffrey 2008; Pesudovs 2006; Tait 2009). In one intervention arm (1.4%) a dummy intervention was used (Wong 2006); in another one (1.4%) an audiovisual intervention was used (Fink 2010); and in two (2.8%) no details were provided (Kain 1997; Shorten 2005). See Table 3 for an overview of the consent process in the control group.
3. Details of the consent process in the control group.
| Details of the consent process in the control group | Number of intervention arms |
| No details | 2 |
| verbal only | 33 |
| verbal + standardised leaflet | 20 |
| verbal + special leaflet | 3 |
| verbal + checklist | 12 |
| verbal + dummy intervention | 1 |
| audiovisual | 1 |
| Total | 72 |
Missing data/contact with authors
For 39 studies we sought clarification from the authors, usually about sources of bias, and we received responses from 28 authors (Armstrong 2010; Ashraff 2006; Bekker 2004; Bollschweiler 2008; Cornoiu 2010a; Cornoiu 2010b; Cowan 2007; Deyo 2000; Fink 2010; Garden 1996; Gerancher 2000; Greening 1999; Heller 2008; Henry 2008; Kain 1997; Kang 2009a; Kang 2009b; Luck 1999; Masood 2007; Mauffrey 2008; Mishra 2010a; Mishra 2010b; Morgan 2000; Nadeau 2010; Olver 2009; Pesudovs 2006; Phatouros 1995;Rossi 2004; Rossi 2005; Tait 2009; Wilhelm 2009) (contacted but no reply – Armstrong 1997; Bennett 2009a; Bennett 2009b; Chan 2002; Elfant 1995; Garrud 2001; Goel 2001; Johnson 2006; Lavelle‐Jones 1993; Neptune 1996; Uzbeck 2009; Whelan 2003). We used unpublished data from 16 studies (Armstrong 2010; Bekker 2004; Cornoiu 2010a; Cornoiu 2010b; Cowan 2007; Fink 2010; Garden 1996; Kain 1997; Kang 2009a; Kang 2009b; Luck 1999; Mauffrey 2008; Mishra 2010a; Mishra 2010b; Morgan 2000; Nadeau 2010; Olver 2009; Tait 2009; Wilhelm 2009).
Excluded studies
We excluded 30 studies (see Characteristics of excluded studies). Seven of these studies (Altaie 2011; Clark 2011; Finch 2009; Gyomber 2010; Migden 2008; Scanlan 2003; Stanley 1998) met the inclusion criteria but had no usable data; details are shown in Table 4.
4. Studies with unusable or insufficient data for analysis.
| Study | Details of study | Details of data |
| Altaie 2011 | A three armed RCT looking at patients recall of knowledge. 36 patients undergoing strabismus surgery were randomised to either standardised consent, standardised plus written information or standardised plus written that included a quiz in the leaflet. A questionnaire was administered on admission which asked the same questions as the quiz. |
No numbers presented in the results. |
| Clark 2011 | RCT looking at patient recall of knowledge. 50 patients admitted for elective laparoscopic cholecystectomy in the USA were randomised to either a power point presentation or to usual care. A questionnaire was completed after the power point (before surgery) to test knowledge. |
Results show mean scores for the two groups but there is not enough detail to extract a SD. Unable to contact authors |
| Finch 2009 | RCT looking at patient recall of knowledge. 100 patients admitted for a transurethral resection of prostate in the UK were randomised to either standard consent or a more detailed written form. These forms were given the night before surgery and recall was tested with a questionnaire three hours later. |
Data not presented in usable form for this review. Unable to make contact with author |
| Gyomber 2010 | RCT looking at patient recall of knowledge. 40 patients admitted for a radical prostatectomy in Australia were randomised to either standard consent or consent in an interactive multimedia form. Recall was tested immediately after the intervention was given. |
Medians and N values are the only data shown |
| Migden 2008 | RCT looking at patient recall of knowledge, satisfaction with the consent process and time of consultation. 11 patients under going Mohs surgery in the USA were randomised to either standard consent or consent with a video. Satisfaction collected on a Likert scale and no details of how knowledge was tested. |
Data not presented in usable form for this review. Time given as a mean with no SD. No data given on knowledge and satisfaction. Contact with author ‐ he is unable to give raw data |
| Scanlan 2003 | RCT looking at patients recall of knowledge . 28 patients undergoing cataract surgery in Canada were randomised to either receive or not to receive further written in formation after the consent consultation. Knowledge was assessed by a questionnaire at time of consultation and one week after surgery. |
Data not presented in usable form for this review. Unable to obtain raw data from authors |
| Stanley 1998 | A four armed RCT that looked at knowledge and anxiety with the consent process. 32 patients undergoing femoral popliteal bypass or carotid surgery in Australia were randomised into groups of normal consent, more detailed written consent, more detailed verbal consent and more detailed written and verbal consent. HADS scores for anxiety and a questionnaire for knowledge were administered after the consent process. |
No extractable data available. |
Risk of bias in included studies
Overall 11 studies were considered to be at high risk of bias regarding random sequence generation, 9 at high risk for attrition bias and 20 studies were considered at high risk for blinding of outcomes (Figure 2). Please refer to Characteristics of included studies for further information on individual studies.
2.
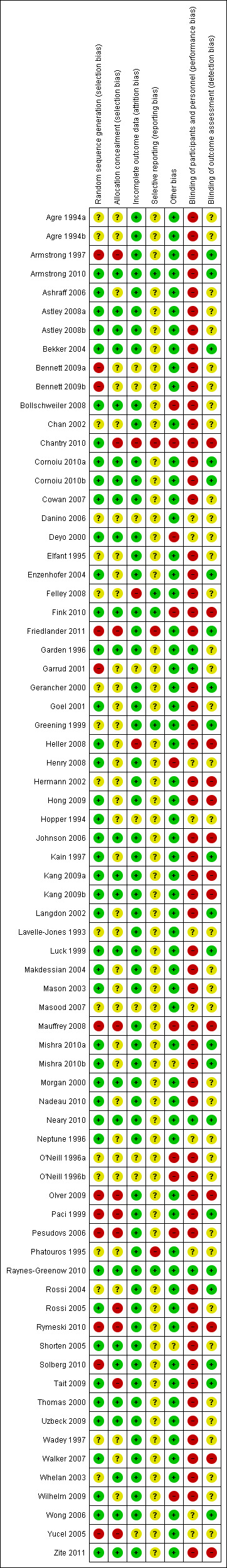
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eleven studies (12 arms) were at high risk of selection bias with poor methods for random sequence generation (Armstrong 1997; Bennett 2009a; Bennett 2009b; Friedlander 2011; Garrud 2001; Mauffrey 2008; Olver 2009; Paci 1999; Pesudovs 2006; Rymeski 2010; Solberg 2010; Yucel 2005).
Eleven studies were at high risk of allocation/selection bias with no rigorous methodology to remove the chance of predicting allocation (Armstrong 2010; Chantry 2010; Friedlander 2011; Mauffrey 2008; Olver 2009; Paci 1999; Pesudovs 2006; Rossi 2005; Rymeski 2010; Tait 2009; Yucel 2005).
Blinding
Due to the nature of the research, informed consent was sought for these studies and some level of understanding of the group allocation by patients or clinicians was likely in most cases. Therefore blinding of participants or personnel was difficult to achieve. Fifty‐one studies (58 arms) were at high risk of performance bias (Agre 1994a; Agre 1994b; Armstrong 1997; Armstrong 2010; Ashraff 2006; Astley 2008a; Astley 2008b; Bekker 2004; Bennett 2009a; Bennett 2009b; Bollschweiler 2008; Chan 2002; Chantry 2010; Cornoiu 2010a; Cornoiu 2010b; Cowan 2007; Elfant 1995; Enzenhofer 2004; Felley 2008; Fink 2010; Friedlander 2011; Gerancher 2000; Goel 2001; Greening 1999; Heller 2008; Hermann 2002; Hong 2009; Johnson 2006; Kain 1997; Kang 2009a; Kang 2009b; Langdon 2002; Luck 1999; Makdessian 2004; Mason 2003; Mauffrey 2008; Mishra 2010a; Mishra 2010b; Morgan 2000; Nadeau 2010; O'Neill 1996a; O'Neill 1996b; Olver 2009; Paci 1999; Pesudovs 2006; Rossi 2004; Rossi 2005; Rymeski 2010; Shorten 2005; Solberg 2010; Tait 2009; Thomas 2000; Uzbeck 2009; Wadey 1997; Walker 2007; Whelan 2003; Wilhelm 2009; Zite 2011).
Twelve studies (13 arms) were at high risk of detection bias (Chantry 2010; Fink 2010; Heller 2008; Hermann 2002; Hong 2009; Johnson 2006; Kang 2009a; Kang 2009b; Mauffrey 2008; Olver 2009; Rymeski 2010; Walker 2007; Zite 2011).
Incomplete outcome data
Three studies were at high risk of attrition bias due to attrition rates above the 40% threshold. In Chantry 2010 43% of the control group and 47% of the intervention group were lost to follow up; in Felley 2008 the drop out rate was 46%; and in Heller 2008 the drop out rate was 51%.
Selective reporting
Three studies were at high risk of reporting bias. In Chantry 2010 data on satisfaction were not included at one month follow up and only 10 of 14 questions were used in a composite knowledge score; in Friedlander 2011we judged there were differences between the registered protocol and the published report; and in Phatouros 1995 again there were missed data on outcomes which were reported in the methods as being measured.
Other potential sources of bias
Nine studies (10 arms) were at high risk of other bias with fidelity or contamination concerns (Bollschweiler 2008; Chantry 2010; Deyo 2000; Fink 2010; Henry 2008; Mauffrey 2008; O'Neill 1996a; O'Neill 1996b; Pesudovs 2006; Wilhelm 2009).
Effects of interventions
Studies using all types of intervention were included in the main analysis and contribute to the results. Subgroup analyses were then performed to evaluate the effects of face‐to‐face versus distant implementation of interventions, effects on different age groups, effects of different types of interventions, and effects of timing of the intervention prior to the procedure being undertaken.
Our main results are summarised in Table 5.
5. Overview of findings.
| Intervention | Number of studies (arms) | Immediate knowledge | Short‐term knowledge | Long‐term knowledge | Generalised anxiety | Anxiety with the consent process | Decisional conflict | Satisfaction with the consent process | Satisfaction with the decision making |
| All interventions | 65 (72 arms) |
22 studies (26 arms) SMD 0.53 (0.37 to 0.69) I2 = 73% |
14 studies (16 arms) SMD 0.68 (0.42 to 0.93) I2 = 85% |
15 studies (17 arms) SMD 0.78 (0.50 to 1.06) I2 = 82% |
12 studies (14 arms ) SMD ‐0.11 (‐0.35 to 0.13) I2 = 82% |
11 studies (13 arms ) SMD 0.01 (‐0.21 to 0.23) I2 = 70% |
3 studies (3 arms) SMD ‐1.80 (‐3.46 to ‐0.14) I2 = 99% |
13 studies (15 arms) SMD 0.12 (‐0.09 to 0.32) I2 = 76% |
8 studies (8 arms) SMD 2.25 (1.36 to 3.15) I2 = 99% |
|
All interventions Dichotomous data |
3 studies (3 arms) RR1.17 (0.85 to 1.60) I2 = 84% |
No data | No data | No data | No data | No data | 10 studies (10arms) RR 1.04 (0.97 to 1.12) I2 = 75% |
No data | |
| Face‐to‐face interventions | 16 (16 arms) |
10 studies (10 arms) SDM 0.52 (0.28 to 0.76) I2 = 80% |
3 studies (3 arms) SMD 0.35 (0.12 to 0.59) I2 = 55% |
No data | No data | 3 arms (3 studies) SMD ‐0.08 (‐0.41 to 0.25) I2 = 73% |
No data | No data | As above in ‘all interventions’ |
| Distant interventions | 51 (56 arms) |
14 studies (16 arms) SDM 0.53 (0.32 to 0.75) I2 = 67% |
11 studies (13 arms) SMD 0.79 (0.44 to 1.14) I2 = 87% |
Not analysed | Not analysed | 8 studies (10 arms) SMD 0.05 (‐0.22 to 0.32) I2 = 58% |
Not analysed | Not analysed | |
| Consent on behalf of a minor | 5 (6 arms) |
2 studies (3 arms) SMD 0.55 (0.15 to 0.96) I2 = 13% |
No data | No data | No data | 2 studies (3 arms) SMD 0.14 (‐0.03 to 0.57) I2 = 51% |
No data | No data | No data |
| Self consent | 60 (66 arms) |
20 studies (23 arms) SMD 0.52 (0.36 to 0.69) I2= 75% |
Not analysed | Not analysed | Not analysed | 9 studies (10 arms) SMD ‐0.02 (0.28 to 0.23) I2=72% |
Not analysed | Not analysed | Not analysed |
| Written | 26 (27 arms) |
6 studies (6 arms) SMD 0.29 (‐0.17 to 0.75) I2 = 65%) |
5 studies (6 arms) SMD 0.99 (0.33 to 1.64) I2 = 80% |
8 studies (8 arms) SMD 0.47 (0.21 to 0.73) I2 = 58% |
3 studies (3 arms) SMD 0.36 (‐0.17 to 0.89) I2 = 83% |
6 studies (6 arms) SMD 0.02 (‐0.38 to 0.43) I2 = 67% |
No data | 5 studies (6 arms) SMD 0.19 (‐0.29 to 0.67) I2 = 82% |
No data |
| Audio‐visual | 19 (19 arms) |
8 studies (8 arms) SMD 0.72 (0.40 to 1.04) I2 = 71% |
4 studies (4 arms) SMD 0.73 (0.14 to 1.32) I2 = 91% |
No data | 5 studies (5 arms) SMD ‐0.48 (‐1.07 to 0.12) I2 = 86% |
4 studies (4 arms) SMD 0.08 (‐0.32 to 0.47) I2 = 53% |
No data | 4 studies (4 arms) SMD 0.05 (‐0.11 to 0.21) I2= 0% Dichotomous data 4 studies RR 1.10 (0.91 to 1.34) I2 = 93% |
No data |
| Interactive multimedia | 6 (6 arms) |
No data | 3 studies (3 arms) SMD 0.47 (0.16 to 0.77) I2 = 43% |
No data | No data | No data | No data | 3 studies (3 arms) SMD 0.23 (‐0.46 to 0.92) I2 = 89% |
No data |
| Structured consent | 6 (6 arms) |
5 studies (5 arms) SMD 0.43 (0.16 to 0.70) I2 = 57% |
No data | No data | No data | No data | No data | No data | No data |
| Decision aids and mixed | 9 (9 arms) |
4 studies (4 arms) SMD 0.64 (0.26 to 1.02) I2 = 81% |
3 studies (3 arms) SMD 0.35 (0.12 to 0.59) I2 = 55% |
No data | No data | No data | As above in ‘all interventions’ | No data | 7 studies (7 arms) SMD 2.64 (1.50 to 3.77) I2 = 99% |
| Before admission for procedure | 38 (42 arms) |
8 studies (10 arms ) SDM 0.50 (0.16 to 0.85) I2 = 86% |
Not analysed | Not analysed | Not analysed | 8 studies (10 arms) SMD ‐0.12 (‐0.33 to 0.09) I2= 50% |
3 studies as above in ‘all interventions’ | 7 studies (8 arms) SMD 0.14 (‐0.12 to 0.41) I2= 74% Dichotomous data 4 studies RR 1.12 (0.94 to 1.33) I2 = 91% |
As above in ‘all interventions’ 8 studies |
| During admission for procedure | 24 (27 arms) |
13 studies (15 arms ) SDM 0.55 (0.40 to 0.70) I2 = 40% |
No data | No data | No data | 3 studies (3 arms) SMD 0.41 (0.19 to 0.62) I2= 0% |
No data | 6 studies (6 arms) SMD 0.10 (‐0.26 to 0.46) I2= 81% Dichotomous data 6 studies RR 1.00 (0.96 to 1.04) I2 = 4% |
No data |
Primary outcome: informed consent
One study with 47 participants in the intervention group and 50 participants in the control group measured informed consent (Friedlander 2011). This study examined the impact of a web‐based learning module for parents of children undergoing endoscopy. The measure of consent was based on a modified questionnaire first published by Woodrow (Woodrow 2006), and included questions to examine the parent’s knowledge of risks, benefits and alternative treatments along with questions which explored if the parent understood what they had been told about risks, benefits, alternatives, their ability to explain what they had been told to another person and if they knew their right to refuse the procedure. A maximum score of 40 was considered to indicate that the participants had given informed consent. The intervention group (47 participants) had a statistically significantly higher mean score than the control group (50 participants) (Intervention group mean 37.4; control group mean 33.2; mean difference 4.16 (95% CI 2.52 to 5.80)) (Analysis 1.1).
1.1. Analysis.
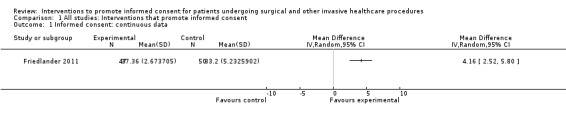
Comparison 1 All studies: Interventions that promote informed consent, Outcome 1 Informed consent: continuous data.
Meta‐analysis for this primary outcome was not possible and the risk of bias was high for most domains, including two of the three deemed most important for this review (randomisation bias and attrition bias), but it was considered at low risk of bias for blinding of outcome assessment.
Secondary outcomes
Patient understanding
One study (two arms) measured patient understanding (Kang 2009a; Kang 2009b). The participants in this study were asked to apply the facts they had learnt to different scenarios, thus giving a measure of understanding rather than recall of simple facts. The participant responses to different scenarios were collected in a qualitative manner and then transcribed and assigned numerical values by the researchers. The trial had two intervention arms; the first intervention arm had a modified informed consent form (29 participants); the second intervention arm had the modified informed consent form and watched a slide‐show (30 participants). The control group had usual care consisting of a standard consent form and standard written information (30 participants ‐ divided into 15 for comparison with the two intervention arms). Those who had the modified informed consent form (the first intervention arm; Kang 2009a) showed no difference in their understanding from the control group (mean difference ‐0.7 (95% CI ‐10.32 to 8.92)). Those who had the modified written information and slide‐show arm (second intervention arm, Kang 2009b) showed a statistically significantly improved understanding compared to the control group (mean difference 11.6 (95% CI 1.15 to 22.05)) (Analysis 1.2).
1.2. Analysis.
Comparison 1 All studies: Interventions that promote informed consent, Outcome 2 Patient understanding: continuous data.
Patient‐reported understanding
Three studies had data that measured how much patients felt they had understood during the informed consent process; two of these studies (Hermann 2002; Walker 2007) reported continuous data and one study (Bollschweiler 2008) reported dichotomous data. The intervention group in Hermann 2002 showed greater self‐reported understanding than those in the ‘usual care’ control group (mean difference 0.79 (95% CI 0.33 to 1.25)). Walker 2007 showed no statistically significant differences for self‐reported understanding (mean difference 0.24 (95% CI ‐0.18 to 0.60)) (Analysis 1.3). Bollschweiler 2008 showed the intervention group had a greater self‐reported understanding than the control group (RR 1.84 (95% CI 1.35 to 2.51)) (Analysis 1.4).
1.3. Analysis.
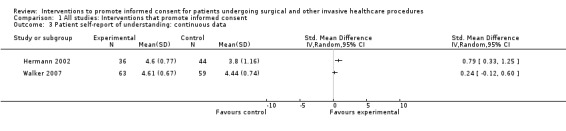
Comparison 1 All studies: Interventions that promote informed consent, Outcome 3 Patient self‐report of understanding: continuous data.
1.4. Analysis.
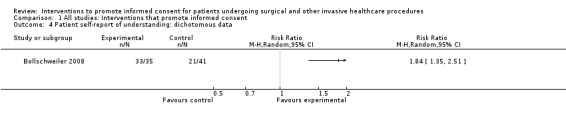
Comparison 1 All studies: Interventions that promote informed consent, Outcome 4 Patient self‐report of understanding: dichotomous data.
Knowledge (recall)
Knowledge was the most frequently‐reported outcome in this review and was generally measured by whether participants could recall information that they had been given while being consented. We categorised this outcome into immediate (i.e. tested within 24 hours of intervention), short‐term (more than 24 hours after intervention but less than 15 days later) and long‐term knowledge (15 days or more after intervention). Many studies reported knowledge at two or more time periods so the studies have contributed data to different meta‐analyses where appropriate. Continuous, dichotomous and non‐parametric data were reported.
Immediate knowledge (tested less than 24 hours after the intervention)
Continuous data
Twenty two studies (26 intervention arms) with 1479 participants in the intervention and 1373 participants in the control groups reported this outcome (Agre 1994a; Agre 1994b; Armstrong 2010; Bekker 2004; Bennett 2009a; Bennett 2009b; Cornoiu 2010a; Cornoiu 2010b; Cowan 2007; Fink 2010; Garden 1996; Greening 1999; Hermann 2002; Hopper 1994; Johnson 2006; Kang 2009a; Kang 2009b; Morgan 2000; Nadeau 2010; Neptune 1996; Pesudovs 2006; Rossi 2004; Rossi 2005; Tait 2009; Walker 2007; Wong 2006). The meta‐analysis showed a statistically significant increase in knowledge for the intervention compared to the control groups, with substantial heterogeneity (SMD 0.53 (95% CI 0.37 to 0.69) I2 73%) (Analysis 1.5).
1.5. Analysis.
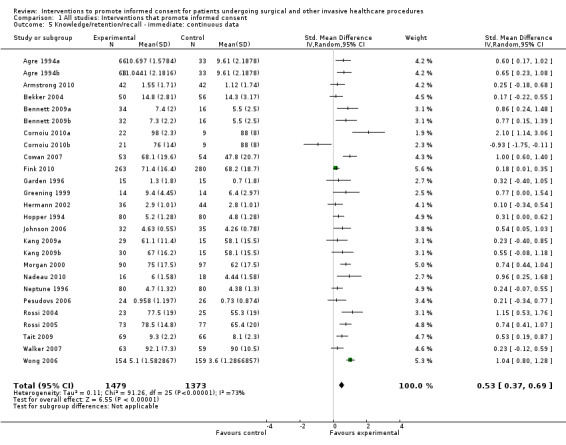
Comparison 1 All studies: Interventions that promote informed consent, Outcome 5 Knowledge/retention/recall ‐ immediate: continuous data.
Sensitivity analysis
The sensitivity analyses did not substantially alter the magnitude or significance of the summary effect size.
1. Random sequence generation
Two studies (three intervention arms) were judged to have a high risk of bias (Bennett 2009a; Bennett 2009b; Pesudovs 2006). Removing these from the meta‐analysis gave a new SMD of 0.52 with substantial heterogeneity ((95% CI 0.35 to 0.69) I2 75%).
2. Attrition bias
None of the studies were at high risk of attrition bias.
3. Blinding of outcome assessment
Five studies (six intervention arms) were judged to have a high risk of bias for blinded outcome assessment (Fink 2010; Hermann 2002; Johnson 2006; Kang 2009a; Kang 2009b; Walker 2007). Removing these from the meta‐analysis gave an SMD of 0.61 with substantial heterogeneity ((95% CI 0.42 to 0.79) I2 71%).
Figure 3 shows a funnel plot of data for immediate knowledge. There is some evidence of publication bias as the bottom left hand corner of the funnel plot shows fewer studies than might be expected.
3.
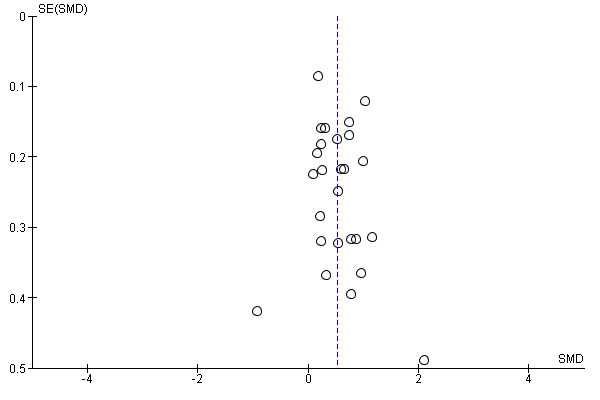
Funnel plot of comparison: 2 All studies: interventions that promote informed consent, outcome: 2.7 Knowledge/retention/recall ‐ Immediate.
Dichotomous data
Three studies (3 intervention arms) with 161 participants in the intervention groups and 170 participants in the control groups reported dichotomous data for immediate knowledge after an intervention (Mason 2003; Pesudovs 2006; Zite 2011). The combined risk ratio showed no difference in knowledge between the two groups, with considerable heterogeneity (RR 1.17 (95% CI 0.85 to 1.60) I2 84%) (Analysis 1.8).
1.8. Analysis.
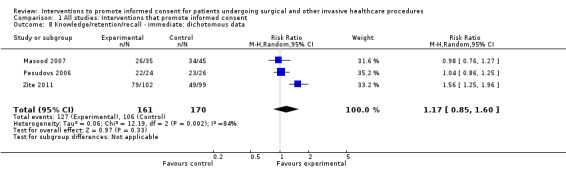
Comparison 1 All studies: Interventions that promote informed consent, Outcome 8 Knowledge/retention/recall ‐ immediate: dichotomous data.
Non‐parametric data
Three studies (4 intervention arms) with 208 participants in intervention groups and 175 in the control groups, report non‐parametric data for immediate knowledge (Astley 2008a; Astley 2008b; Lavelle‐Jones 1993; Mason 2003). Mason 2003 had a knowledge score out of 20: intervention arm median score 18 (Interquartile range (IQR) 16 to 18); control group median score 11.5 (IQR 10 to 15). Astley 2008a had two intervention arms (written information; animated audio‐visual information; control verbal information). A knowledge score out of 12 was recorded for each arm: written information arm median score 4 (IQR 3 to 5); audio‐visual information median score 4 (IQR 3 to 6); control group median score of 3.5 (IQR 2 to 5). Lavelle‐Jones 1993 had a recall score out of a total of 6; both the intervention and control groups scored 4 (both IQR were 2 to 6) (Analysis 1.11).
1.11. Analysis.
Comparison 1 All studies: Interventions that promote informed consent, Outcome 11 Knowledge/retention/recall: non‐parametric data.
| Knowledge/retention/recall: non‐parametric data | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Outcome | Timing of outcome | Intervention group median | Intervention group IQR | Intervention N | Control group median | Control group IQR | Control N | P value |
| Immediate knowledge | |||||||||
| Astley 2008a | Recall ‐ score out of 12 | Immediate | 4 | 3‐5 | 34 | 3.5 | 2‐5 | 16 | |
| Astley 2008b | Recall ‐ score out of 12 | Immediate | 4 | 3‐6 | 33 | 3.5 | 2‐5 | 16 | |
| Lavelle‐Jones 1993 | Recall ‐ score out of 6 | Immediately after consent | 4 | 2‐6 | 126 | 4 | 2‐6 | 127 | P = 0.68 |
| Mason 2003 | Recall ‐ score out of 20 | Immediately after | 18 | 16‐18 | 15 | 11.50 | 10‐15 | 16 | P < 0.001 |
| Short‐term knowledge | |||||||||
| Lavelle‐Jones 1993 | Recall ‐ score out of 6 | On day of discharge (median = day 5, range = 1‐92 | 4 | 2‐6 | 121 | 3 | 1‐6 | 121 | 0.015 |
| Long‐term knowledge | |||||||||
| Astley 2008a | Recall ‐ score out of 12 | 30 days | 2 | 1‐3 | 34 | 3 | 1‐4 | 16 | |
| Astley 2008b | Recall ‐ score out of 12 | 30 days | 3 | 2‐4 | 33 | 3 | 1‐4 | 16 | |
| Gerancher 2000 | recall ‐ score out of 100 | 5‐7 months after | 90 | 80‐100 | 44 | 80 | 70‐90 | 38 | p<0.001 |
| Greening 1999 | Recall | 1‐7 days after full course of ECT. Large range in ECT treatment course length | 8 | 1‐12 | 10 | 4 | 0‐12 | 14 | |
| Lavelle‐Jones 1993 | Recall ‐ score out of 6 | Outpatients clinic at 4‐6 weeks following discharge | 3 | 2‐6 | 112 | 3 | 1‐6 | 111 | P = 0.55 |
| Wadey 1997 | recall‐score out of 3 | 1 month | 3 | 3‐3 | 8 | 2 | 2‐3 | 12 | |
Short‐term knowledge (more than 24 hours, and up to and including 14 days after intervention)
Continuous data
Fourteen studies (16 intervention arms) with 1191 participants in the intervention and 915 participants in the control groups reported this outcome (Ashraff 2006; Chantry 2010; Cornoiu 2010a; Cornoiu 2010b; Enzenhofer 2004; Garrud 2001; Goel 2001; Heller 2008; Luck 1999; Nadeau 2010; O'Neill 1996a; O'Neill 1996b; Raynes‐Greenow 2010; Tait 2009; Whelan 2003; Wilhelm 2009). The meta‐analysis showed a statistically significant increase in knowledge for the intervention compared to the control groups, with considerable heterogeneity (SMD 0.68 (95% CI 0.42 to 0.93) I2 85%) (Analysis 1.6).
1.6. Analysis.
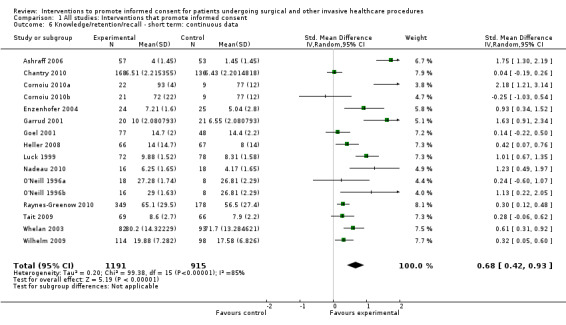
Comparison 1 All studies: Interventions that promote informed consent, Outcome 6 Knowledge/retention/recall ‐ short term: continuous data.
Sensitivity analysis
The sensitivity analyses supported these findings when studies at high risk of attrition bias or poor blinding of outcome assessment were removed from meta‐analysis.
1. Random sequence generation
One study was judged to have a high risk of bias for this domain (Garrud 2001). Removing its data from the meta‐analysis gave an SMD of 0.62 with considerable heterogeneity ((95% CI 0.37 to 0.87) I2 84%).
2. Attrition bias
Three studies were judged to have a high risk of attrition bias (Chantry 2010; Heller 2008; Tait 2009). Removing these from the meta‐analysis gave an SMD of 0.81 with considerable heterogeneity ((95% CI 0.50 to 1.13) I2 85%).
3. Blinding of outcome assessment
Two studies were judged to have a high risk of bias for this domain (Chantry 2010; Heller 2008). Removing these from the meta‐analysis gave an SMD of 0.76 with considerable heterogeneity ((95% CI 0.47 to 1.05) I2 84%).
Figure 4 shows a funnel plot of data for short‐term knowledge. There is spread of effect and study size suggesting low risk of publication bias.
4.
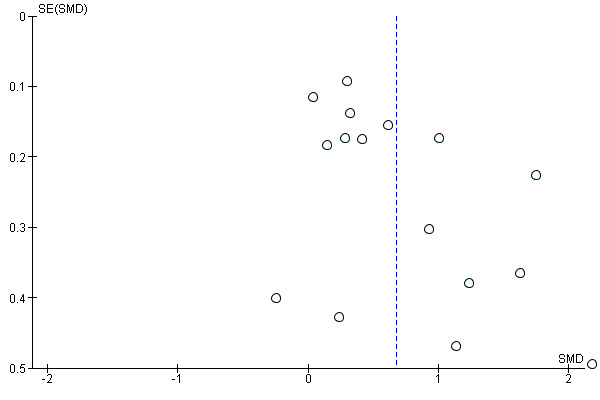
Funnel plot of comparison: 2 All studies: interventions that promote informed consent, outcome: 2.8 Knowledge/Retention/Recall ‐ Short term.
Dichotomous data
Two studies (2 intervention arms) with 167 participants in the intervention group and 162 participants in the control group measured short‐term knowledge recall (Armstrong 1997; Elfant 1995). The results from Armstrong 1997 showed increased knowledge in the intervention group (RR 1.47 (95% CI 1.11 to 1.93)). Elfant’s results showed greater knowledge in the control group compared with the intervention group (RR 0.94 (95% CI 0.57 to 1.53)) (Analysis 1.9).
1.9. Analysis.
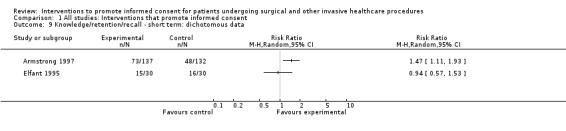
Comparison 1 All studies: Interventions that promote informed consent, Outcome 9 Knowledge/retention/recall ‐ short term: dichotomous data.
Non‐parametric data
One study (one intervention arm) with 121 participants in each group reported a knowledge test with a total score of 6 and showed the intervention increased knowledge (Lavelle‐Jones 1993) (intervention arm median 4, IQR 2 to 6; control arm median 3, IQR 1 to 6 (Analysis 1.11)).
Long‐term knowledge (15 days and longer)
Continuous data
Fifteen studies (17 intervention arms) with 689 participants in the intervention and 664 in the control groups reported this outcome (Bekker 2004; Chan 2002; Cornoiu 2010a; Cornoiu 2010b; Danino 2006; Henry 2008; Hong 2009; Langdon 2002; Makdessian 2004; Mauffrey 2008; Mishra 2010a; Mishra 2010b; Pesudovs 2006; Rossi 2004; Rymeski 2010; Shorten 2005; Solberg 2010). The meta‐analysis showed a statistically significant increase in knowledge for the interventions compared to the control groups with considerable heterogeneity (SMD 0.78 (95% CI 0.50 to 1.06) I2 82%) Analysis 1.7.
1.7. Analysis.
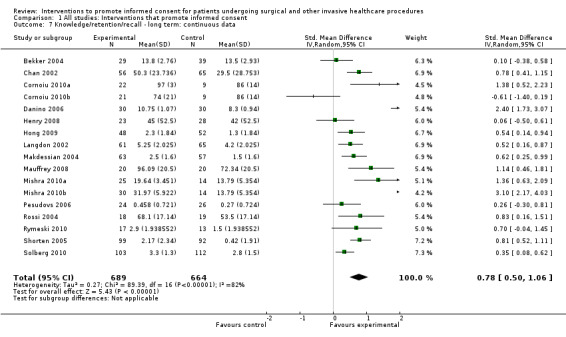
Comparison 1 All studies: Interventions that promote informed consent, Outcome 7 Knowledge/retention/recall ‐ long term: continuous data.
Sensitivity analysis
The sensitivity analyses did not substantially alter the magnitude or significance of the summary effect size:
1. Random sequence generation
Four studies (four intervention arms) were judged to have a high risk of bias (Mauffrey 2008; Pesudovs 2006; Rymeski 2010; Solberg 2010). Removing these from the meta‐analysis gave an SMD of 0.85 with considerable heterogeneity ((95% CI 0.50 to 1.21) I2 85%).
2. Attrition bias
None of the studies were at high risk of attrition bias.
3. Blinding of outcome assessment
Three studies (three intervention arms) were judged to have a high risk of bias for blinded outcome assessment (Hong 2009; Mauffrey 2008; Rymeski 2010). Removing these from the meta‐analysis gave an SMD of 0.79 with considerable heterogeneity ((95% CI 0.46 to 1.12) I2 85%).
Figure 5 shows a funnel plot of data for long‐term knowledge. There is spread of effect and study size suggesting low risk of publication bias.
5.
Funnel plot of comparison: 2 All studies: interventions that promote informed consent, outcome: 2.9 Knowledge/Retention/Recall ‐ Long term.
Dichotomous data
Two studies (2 intervention arms) with 71 participants in the intervention groups and 80 participants in the control groups reported this outcome (Olver 2009; Pesudovs 2006). In each study there was no statistical significance between the groups. Olver showed no statistical improvement in relative risk for the intervention group, RR 1.19 (95% CI 0.81 to 1.76). Pesudovs demonstrates no statistical improvement in relative risk for the control group, RR 0.94 (95% CI 0.75 to 1.18), again without statistical significance (Analysis 1.10).
1.10. Analysis.
Comparison 1 All studies: Interventions that promote informed consent, Outcome 10 Knowledge/retention/recall ‐ long term: dichotomous data.
Non‐parametric data
Five studies (six intervention arms) with 241 participants in the intervention and 207 participants in the control groups reported non‐parametric data for long‐term knowledge (Astley 2008a; Astley 2008b; Gerancher 2000; Greening 1999; Lavelle‐Jones 1993; Wadey 1997). For Astley, the intervention group with written information had a median knowledge of 2 (out of total 12), IQR 1 to 3, the intervention group with audio‐visual information had a median knowledge of 3, IQR 2 to 4, and the control group had median 3, IQR 1 to 4, suggesting that those with written information did more poorly on this test than the other two groups, which were similar. Gerancher reported improved knowledge in the intervention group (median 90 out of 100 versus 80 in the control group). Greening scored knowledge out of 12; the intervention arm showed improved knowledge with a median of 8 (IQR 1 to 12) and the control group had a median of 4 (IQR 0 to 12). Lavelle‐Jones reported the same median of 3 in the intervention and the control groups (IQR for the intervention group was 2 to 6, and 1 to 6 for the control group). Wadey reported a knowledge score out of 6, the median for the intervention group was 3 (IQR 2 to 6) and a median for the control group of 3 (IQR 1 to 6) (Analysis 1.11).
Deliberation
This outcome was not measured directly but one study (Bekker 2004) reported on informed decision making about the prenatal diagnosis of Down's syndrome. This study had 50 participants in the intervention and 56 in the control group. The authors measured informed decision making by coding themes discussed during consultations with the patient. The amount of information sought was given a numerical value for each theme discussed, with a total score of 7 possible (0 to 7; 7 = more informed decision making). The study found no statistically significant differences between the groups, MD 0.17 (95% CI ‐0.35 to 0.69) (Analysis 1.12). However women who received the intervention were found to have evaluated more information during their consultations both positively and negatively, and were more likely to perceive the screening tests to be medium rather than high risk.
1.12. Analysis.
Comparison 1 All studies: Interventions that promote informed consent, Outcome 12 Deliberation: continuous data.
Communication of decision (decision conflict)
There were no studies that directly measured communication of decisions (i.e. choosing surgery or another option). However, it was proposed in the protocol (Kinnersley 2011) that studies may attempt to measure closely‐related concepts such as decision conflict (if someone is in ‘conflict’ or uncertainty, they may be less likely to choose whether or not to undergo the procedure).
Three studies (3 intervention arms) with 526 participants in the intervention and 311 participants in the control groups reported decisional conflict using a common scale (the Decision Conflict Scale) (Goel 2001; Raynes‐Greenow 2010; Shorten 2005). Two studies (Goel 2001; Raynes‐Greenow 2010) reported scores after the intervention was used. Shorten reported a change in scores pre‐ and post‐intervention. The meta‐analysis showed a statistically significant decrease in the decisional conflict score in the intervention groups, with considerable heterogeneity (SMD ‐1.80 (95% CI ‐3.46 to ‐0.14) I2 99%) (Analysis 1.13).
1.13. Analysis.
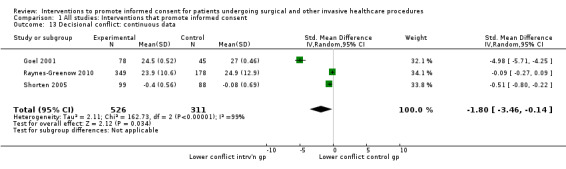
Comparison 1 All studies: Interventions that promote informed consent, Outcome 13 Decisional conflict: continuous data.
No exclusions for high risk of bias were required in the sensitivity analysis.
Other patient outcomes
Anxiety
Anxiety was divided into: general anxiety relating to the hospital stay or the procedure; anxiety concerning the consent process; and anxiety about the decision making process.
General anxiety
Continuous data
Twelve studies (14 intervention arms) with 1134 participants in the intervention and 935 participants in the control groups reported general anxiety (Bollschweiler 2008; Cornoiu 2010a; Cornoiu 2010b; Danino 2006; Felley 2008; Luck 1999; Mishra 2010a; Mishra 2010b; Neary 2010; Olver 2009; Raynes‐Greenow 2010; Thomas 2000; Uzbeck 2009; Whelan 2003). The meta‐analysis showed no statistically significant difference in the intervention groups compared to the control groups, with considerable heterogeneity (SMD ‐0.11 (95% CI ‐0.35 to 0.13) I2 82%) (Analysis 1.14).
1.14. Analysis.
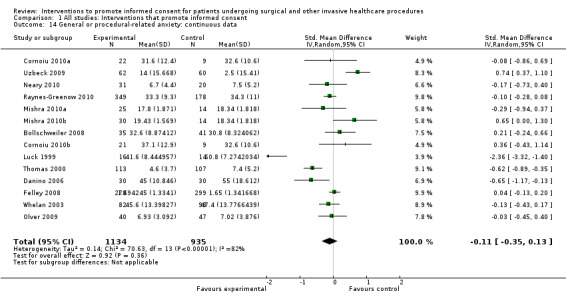
Comparison 1 All studies: Interventions that promote informed consent, Outcome 14 General or procedural‐related anxiety: continuous data.
Sensitivity analysis
The sensitivity analyses did not substantially alter the magnitude or significance of the summary effect size:
1. Random sequence generation
One study was judged to have a high risk of bias for this domain (Olver 2009). Removing this from the meta‐analysis gave an SMD of ‐0.12 with considerable heterogeneity ((95% CI ‐0.38 to 0.13) I2 83%).
2. Attrition bias
One study was judged to have a high risk of attrition bias (Felley 2008). Removing this study from the meta‐analysis gave an SMD of ‐0.14 with considerable heterogeneity ((95% CI ‐0.42 to 0.15) I2 82%).
3. Blinding of outcome assessment
One study was judged to have a high risk of bias for blinded outcome assessment (Olver 2009). Removing this from the meta‐analysis gave an SMD of ‐0.12 with considerable heterogeneity ((95% CI ‐0.38 to 0.13) I2 83%).
Figure 6 shows a funnel plot of data for generalised anxiety. The pattern of effect and study size is symmetrical, suggesting low risk of publication bias.
6.
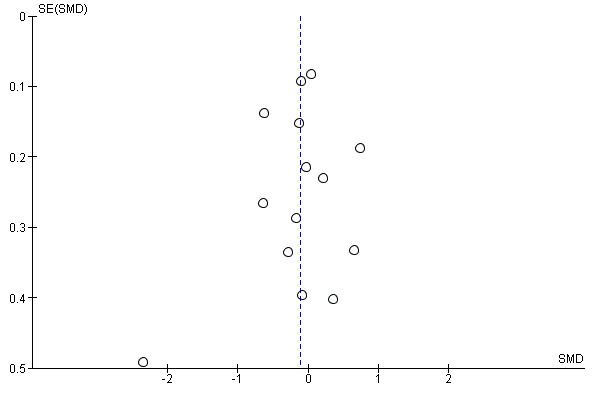
Funnel plot of comparison: 2 All studies: interventions that promote informed consent, outcome: 2.16 General or procedural‐related anxiety.
Dichotomous data
Two studies (2 intervention arms) with 145 participants in the intervention groups and 142 participants in the control groups reported the outcome of general anxiety (Johnson 2006; Thomas 2000). Johnson reported no statistically significant difference between groups (RR 0.67 (95% CI 0.32 to 1.41)). Thomas reported reduced anxiety in the intervention group compared to the control group (RR 0.47 (95% CI 0.31 to 0.72)) (Analysis 1.15).
1.15. Analysis.
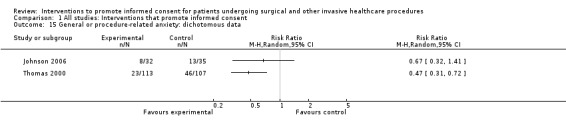
Comparison 1 All studies: Interventions that promote informed consent, Outcome 15 General or procedure‐related anxiety: dichotomous data.
Anxiety with consent process
Continuous data
Eleven studies (13 intervention arms) with 727 participants in the intervention groups and 680 participants in the control groups reported data on anxiety with the consent process (Bekker 2004; Cornoiu 2010a; Cornoiu 2010b; Danino 2006; Fink 2010; Friedlander 2011; Garden 1996; Garrud 2001; Kain 1997; Kang 2009a; Kang 2009b; Walker 2007; Yucel 2005). The meta‐analysis showed no overall differences between the groups with substantial heterogeneity (SMD 0.01 (95% CI ‐0.21 to 0.23) I2 70%) (Analysis 1.16).
1.16. Analysis.
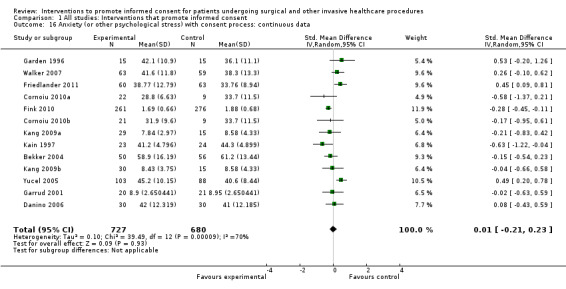
Comparison 1 All studies: Interventions that promote informed consent, Outcome 16 Anxiety (or other psychological stress) with consent process: continuous data.
Sensitivity analysis
None of the sensitivity analyses substantially altered the magnitude or significance of the summary effect size:
1. Random sequence generation
Three studies (three intervention arms) were judged to have a high risk of bias for this domain (Friedlander 2011; Garrud 2001; Yucel 2005). Removing these from the meta‐analysis gave an SMD of ‐0.12 with moderate heterogeneity ((95% CI ‐0.31 to 0.08) I2 41%).
2. Attrition bias
None of the reporting anxiety with the consent process studies at high risk of attrition bias.
3. Blinding of outcome assessment
Three studies (four intervention arms) were judged to have a high risk of bias for this domain (Fink 2010; Kang 2009a; Kang 2009b; Walker 2007). Removing these from the meta‐analysis gave an SMD of ‐0.05 with moderate heterogeneity ((95% CI ‐0.23 to 0.33) I2 64%).
Dichotomous data
One study (one intervention arm) reported data on anxiety with the consent process with 29 participants in the intervention group and 36 in the control group (Phatouros 1995). This study found no statistically significant difference between the groups (RR 2.90 (95% CI 0.82 to 10.22)) (Analysis 1.17).
1.17. Analysis.
Comparison 1 All studies: Interventions that promote informed consent, Outcome 17 Anxiety (or other psychological stress) with consent process: dichotomous data.
Non‐parametric data
Two studies (3 intervention arms) with 82 participants in the intervention groups and 48 in the control groups reported data on anxiety with the consent process (Astley 2008a; Astley 2008b; Mason 2003). In each study no statistically significant differences were found between the groups. Astley reports a median of 3 out of 5 on a Likert scale for both intervention and control groups (IQR 2 to 4). Mason reports on a State Trait Anxiety Inventory scale with median of 9 for the intervention group versus 10 for the control group (IQR 6 to 15 for both arms) (Analysis 1.18).
1.18. Analysis.
Comparison 1 All studies: Interventions that promote informed consent, Outcome 18 Anxiety (or other psychological stress) with consent process: non‐parametric data.
| Anxiety (or other psychological stress) with consent process: non‐parametric data | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Outcome | Timing of Assessment | Intervention Group Median | Intervention Group IQR | Intervention Group N | Control Group Median | Control Group IQR | Control Group N | Notes |
| Astley 2008a | 5 point anxiety scale with 5=anxious | Immediate | 3 | 2‐4 | 34 | 3 | 2‐4 | 16 | |
| Astley 2008b | 5 point anxiety scale with 5=anxious | Immediate | 3 | 2‐4 | 33 | 3 | 2‐4 | 16 | |
| Mason 2003 | 6 Item version of Speilberger State Anxiety Inventory | Immediately after intervention | 9.0 | 6‐15 | 15 | 10 | 6‐15 | 16 | P = N.S. |
Decision‐related anxiety
Continuous data
One study reported data on anxiety with the decision process with 154 participants in the intervention group and 159 in the control group (Wong 2006). No differences were found between the two groups (MD 0.00 (95% CI ‐3.54 to 3.54)) (Analysis 1.19).
1.19. Analysis.

Comparison 1 All studies: Interventions that promote informed consent, Outcome 19 Anxiety (or other psychological stress) with decision‐making: continuous data.
Satisfaction
This outcome was divided into satisfaction with the consent process and satisfaction with decision making.
Satisfaction with the consent process
Continuous data
Thirteen studies (15 intervention arms) with 1046 participants in the intervention groups and 978 in the control groups reported satisfaction with the consent process (Armstrong 2010; Bekker 2004; Chantry 2010; Cornoiu 2010b; Cornoiu 2010a; Enzenhofer 2004; Felley 2008; Garrud 2001; Hopper 1994; O'Neill 1996b; O'Neill 1996a; Tait 2009; Uzbeck 2009; Walker 2007; Wilhelm 2009). The meta‐analysis showed no statistically significant difference between groups with substantial heterogeneity (SMD 0.12 (95% CI ‐0.09 to 0.32) I2 76%) (Analysis 1.20).
1.20. Analysis.
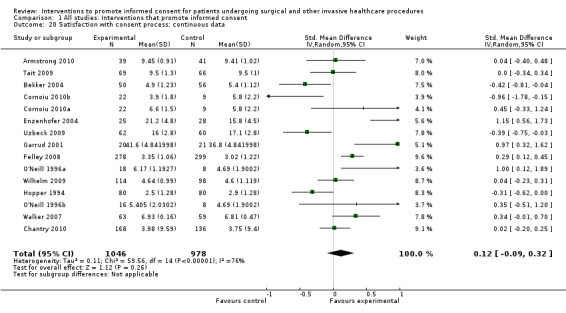
Comparison 1 All studies: Interventions that promote informed consent, Outcome 20 Satisfaction with consent process: continuous data.
Sensitivity analysis
None of the sensitivity analyses substantially altered the summary effect size:
1. Random sequence generation
One study was judged to have a high risk of bias for this domain (Garrud 2001). Removing this study from the meta‐analysis gave an SMD of 0.07 with substantial heterogeneity ((95% CI ‐0.13 to 0.27) I2 75%).
2. Attrition bias
Two studies were judged to have a high risk of attrition bias (Chantry 2010; Felley 2008). Removing these from the meta‐analysis gave an SMD of 0.12 with substantial heterogeneity ((95% CI ‐0.14 to 0.39) I2 77%).
3. Blinding of outcome assessment
Three studies were judged to have a high risk of bias (Chantry 2010; Walker 2007; Wilhelm 2009). Removing these from the meta‐analysis gave an SMD of 0.12 with substantial heterogeneity (95% CI ‐0.13 to 0.37) I2 79%).
Figure 7 shows a funnel plot of the data from the 'satisfaction with the consent process' outcome, There may be a small bias as a result of small studies with negative effects not being published.
7.
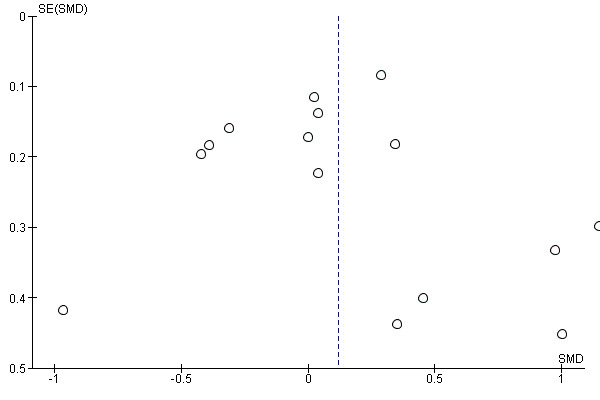
Funnel plot of comparison: 2 All studies: interventions that promote informed consent, outcome: 2.22 Satisfaction with consent process.
Dichotomous data
Ten studies (10 intervention arms) with 515 participants in the intervention groups and 530 in the control groups reported data on satisfaction with the consent process (Bollschweiler 2008; Cowan 2007; Heller 2008; Johnson 2006; Olver 2009; Paci 1999; Pesudovs 2006; Phatouros 1995; Rossi 2005; Thomas 2000). The meta‐analysis showed no difference in satisfaction with the consent process between the two groups, with substantial heterogeneity (RR 1.04 (95% CI 0.97 to 1.12) I2 75%) (Analysis 1.21).
1.21. Analysis.
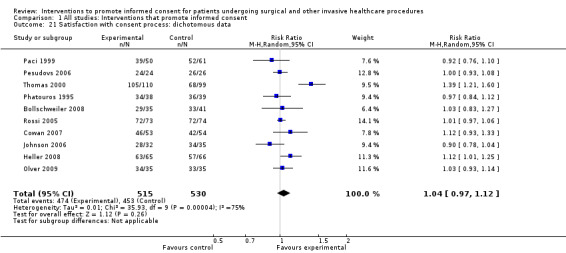
Comparison 1 All studies: Interventions that promote informed consent, Outcome 21 Satisfaction with consent process: dichotomous data.
Sensitivity analysis:
None of the sensitivity analyses substantially altered the magnitude or significance of the summary effect size:
1. Random sequence generation
Three studies were judged to have a high risk of bias (Olver 2009; Paci 1999; Pesudovs 2006). Removing these from the meta‐analysis gave an RR of 1.07 with considerable heterogeneity ((95% CI 0.95 to 1.19) I2 84%).
2. Attrition bias
One study were judged to have a high risk of attrition bias (Heller 2008). Removing this study from the meta‐analysis gave an RR of 1.03 with substantial heterogeneity ((95% CI 0.96 to 1.11) I2 76%).
3. Blinding of outcome assessment
Three studies were judged to have a high risk of bias for this domain (Heller 2008; Johnson 2006; Olver 2009). Removing these from the meta‐analysis gave an RR of 1.05 with considerable heterogeneity ((95% CI 0.95 to 1.16) I2 82%).
Non‐parametric data
Two studies (3 intervention arms) with 98 participants in the intervention groups and 52 participants in the control groups report data on this outcome (Astley 2008a; Astley 2008b; Neary 2010). In both intervention groups for Astley, the intervention group had lower median satisfaction than in the control group (medians 4, 4, 5 respectively; IQR 4 to 5 for all 3 groups). Neary 2010 reported the same median satisfaction for the intervention arm and control arm (median 28 out of possible score of 30; IQR 26 to 30 for intervention; 25.3 to 30 for control) (Analysis 1.22).
1.22. Analysis.
Comparison 1 All studies: Interventions that promote informed consent, Outcome 22 Satisfaction with consent process: non‐parametric data.
| Satisfaction with consent process: non‐parametric data | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Outcome | Timing of Assessment | Intervention group median | Intervention Group IQR | Intervention Group N | Control Group median | Control Group IQR | Control group N | Notes |
| Astley 2008a | Satisfaction with the consent process | Immediate | 4 | 4‐5 | 34 | 5 | 4‐5 | 16 | |
| Astley 2008b | Satisfaction with the consent process | Immediate | 4 | 4‐5 | 33 | 5 | 4‐5 | 16 | |
| Neary 2010 | Satisfaction | Post‐op | 28 | 26‐30 | 31 | 28 | 25.3‐30 | 20 | P = 0.976 |
Satisfaction with decision making
Continuous data
Eight studies (8 intervention arms) with 1147 participants in the intervention groups and 997 participants in the control groups report continuous data for this outcome (Bekker 2004; Fink 2010; Goel 2001; Morgan 2000; Raynes‐Greenow 2010; Solberg 2010; Whelan 2003; Wong 2006). The meta‐analysis showed a statistically‐significant increase in satisfaction in the intervention groups with considerable heterogeneity (SMD 2.25 (95% CI 1.36 to 3.15) I2 99%) (Analysis 1.23).
1.23. Analysis.
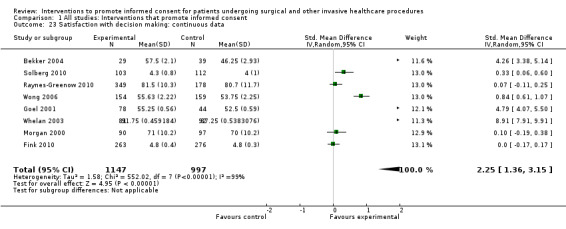
Comparison 1 All studies: Interventions that promote informed consent, Outcome 23 Satisfaction with decision making: continuous data.
Sensitivity analysis:
None of the sensitivity analyses substantially altered the magnitude or significance of the summary effect size:
1. Random sequence generation
One study was judged to have a high risk of bias for this domain (Solberg 2010). Removing this study from the meta‐analysis gave an SMD of 2.57 with considerable heterogeneity ((95% CI 1.52 to 3.61) I2 99%).
2. Attrition bias
There were no studies at high risk for attrition bias.
3. Blinding of outcome assessment
One study was judged to have a high risk of bias (Fink 2010). Removing this from the meta‐analysis gave an SMD of 3.07 with considerable heterogeneity ((95% CI 1.65 to 4.48) I2 99%).
Dichotomous data
One study (one intervention arm) with 171 participants in the intervention group and 172 participants in the control group reported data for this outcome (Deyo 2000). Deyo reported no statistically‐significant difference between the groups (RR 0.94, 95% CI 0.79 to 1.11) (Analysis 1.24).
1.24. Analysis.
Comparison 1 All studies: Interventions that promote informed consent, Outcome 24 Satisfaction with decision making: dichotomous data.
Pain
Five studies reported data on pain. Two studies (Felley 2008; Neary 2010) reported continuous data on pain levels; two studies (Deyo 2000; Phatouros 1995) reported dichotomous data on pain levels; and one study (Neary 2010) reported non‐parametric data on analgesia use as per the WHO analgesic ladder.
Continuous data
Two studies (2 intervention arms) reported continuous data on pain with 309 participants in the intervention groups and 319 participants in the control groups (Felley 2008; Neary 2010). There were no statistically‐significant differences between the groups in either study; Felley MD 0.11 (95% CI ‐0.06 to 0.27), Neary MD 0.03 (95% CI ‐0.54 to 0.59) (Analysis 1.25).
1.25. Analysis.
Comparison 1 All studies: Interventions that promote informed consent, Outcome 25 Pain levels: continuous data.
Dichotomous data
Two studies (2 intervention arms) with 210 participants in the intervention groups and 213 participants in the control groups reported data for this outcome (Deyo 2000; Phatouros 1995). There were no statistically‐significant differences between the groups in either study; Deyo RR 0.77 (95% CI 0.54 to 1.09) and Phatouros RR 1.41 (95% CI 0.64 to 3.13) (Analysis 1.26).
1.26. Analysis.
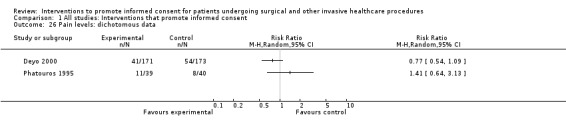
Comparison 1 All studies: Interventions that promote informed consent, Outcome 26 Pain levels: dichotomous data.
Non‐parametric data
One study reported data on analgesia use 24 hours after radio‐guided parathyroidectomy, with 31 participants in the intervention group and 20 participants in the control group (Neary 2010). Results were similar between the two trial arms with a median in both groups of 1 (IQR 1 to 2; Analysis 1.27).
1.27. Analysis.
Comparison 1 All studies: Interventions that promote informed consent, Outcome 27 Analgesia use: non‐parametric data.
| Analgesia use: non‐parametric data | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Outcome | Timing | Intervention Group: Median | Intervention Group: IQR | Intervention Group: N | Control Group: Median | Control Group: IQR | Control Group: N | Notes |
| Neary 2010 | Analgesia Requirement (WHO) | 24 hours | 1 | 1‐2 | 31 | 1 | 1‐2 | 20 | P = 0.769 |
Desire for further information
Dichotomous data
Four studies (4 intervention arms) with 503 participants in the intervention arms and 346 participants in the control arms report the outcome of desire for further information (Heller 2008; Paci 1999; Phatouros 1995; Raynes‐Greenow 2010). The combined RR showed no statistical difference between participants' desire for further information, with moderate heterogeneity (RR 0.65 (95% CI 0.35 to 1.22) I2 57%) (Analysis 1.28).
1.28. Analysis.
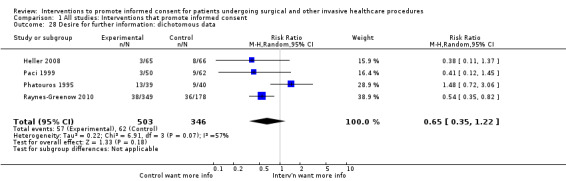
Comparison 1 All studies: Interventions that promote informed consent, Outcome 28 Desire for further information: dichotomous data.
Sense of control – locus of control or perception of who made the decision
Continuous data
One study (one intervention arm) with 103 participants in the intervention group and 112 in the control group reported continuous data on the patients’ locus of control (Solberg 2010). Participants in the intervention group felt more strongly that it was their decision to make (regarding treatment options for uterine fibroids) than those in the control group, with statistical significance between groups (MD 0.30 (95% CI 0.07 to 0.53)) (Analysis 1.29).
1.29. Analysis.

Comparison 1 All studies: Interventions that promote informed consent, Outcome 29 Sense of control ‐ locus of control or perception of who made the decision: continuous data.
Dichotomous data
Three studies (3 intervention arms) with 561 participants in the intervention groups and 410 participants in the control groups reported data on the patients’ locus of control (Deyo 2000; Raynes‐Greenow 2010; Whelan 2003). Meta‐analysis showed no significant difference between the groups, with substantial heterogeneity (RR 1.03 (95% CI 0.98 to 1.09) I2 62%) (Analysis 1.30).
1.30. Analysis.
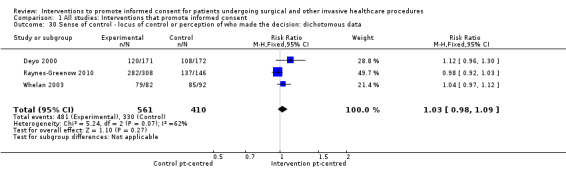
Comparison 1 All studies: Interventions that promote informed consent, Outcome 30 Sense of control ‐ locus of control or perception of who made the decision: dichotomous data.
Clinician outcomes
Satisfaction with the consent consultation
Continuous data
One study (one intervention arm) with 22 participants in the intervention group and 22 participants in the control group reported data from clinicians on their satisfaction with the consent consultation (Whelan 2003). Whelan et al report no statistically‐significant differences between groups (MD 0.02 (95% CI ‐0.23 to 0.27)) (Analysis 1.31).
1.31. Analysis.

Comparison 1 All studies: Interventions that promote informed consent, Outcome 31 Clinician outcome: satisfaction with the consent consultation: continuous data.
Dichotomous data
One study (one intervention arm) with 13 participants in the intervention group and 13 participants in the control group reported data from clinicians on their satisfaction with the consent consultation (Solberg 2010). No statistically‐significant differences were found between the groups (RR 0.82 (95% CI 0.53 to 1.26)) (Analysis 1.32).
1.32. Analysis.

Comparison 1 All studies: Interventions that promote informed consent, Outcome 32 Clinician outcome: satisfaction with the consent consultation: dichotomous data.
Ease of use of intervention(s) to improve gaining of informed consent
No studies reported this outcome.
Confidence in patients’ decision and whether an informed choice was made
No studies reported this outcome.
Systems/organisational outcomes
Rates of uptake (or refusal) of clinical interventions/procedures
For the purposes of analysis, uptake of the invasive procedure is presented as the outcome of interest.
Dichotomous data
Ten studies (10 intervention arms) with 1613 participants in the intervention groups and 1462 in the control groups reported data on this outcome (Bekker 2004; Cowan 2007; Deyo 2000; Felley 2008; Morgan 2000; Paci 1999; Raynes‐Greenow 2010; Shorten 2005; Whelan 2003; Wong 2006). The meta‐analysis showed no statistically‐significant differences between the groups, with little heterogeneity (RR 0.98 (95% CI 0.95 to 1.02) I2 25%) (Analysis 1.33).
1.33. Analysis.
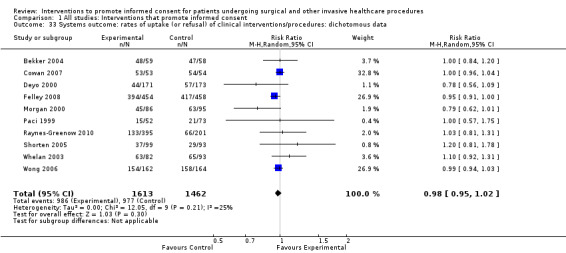
Comparison 1 All studies: Interventions that promote informed consent, Outcome 33 Systems outcome: rates of uptake (or refusal) of clinical interventions/procedures: dichotomous data.
Postponement of clinical interventions/procedures
No studies reported this outcome.
Delay in decision making or request for more information. Further consultations
No studies reported this outcome.
Complaints and litigation
No studies reported this outcome.
Adverse procedural outcomes
No studies reported this outcome.
Economic/resource use data
Length of consultation
Continuous data
Five studies (6 intervention arms) with 271 participants in the intervention groups and 246 participants in the control groups reported continuous data on the length of the consultation (Bekker 2004; Bennett 2009a; Bennett 2009b; Enzenhofer 2004; Hopper 1994; Whelan 2003). The meta‐analysis showed that the control consultations were statistically‐significantly shorter than the intervention consultations by a mean of 1.66 minutes, with little heterogeneity ((95% CI 0.82 to 2.50) I2 0%) (Analysis 1.34).
1.34. Analysis.
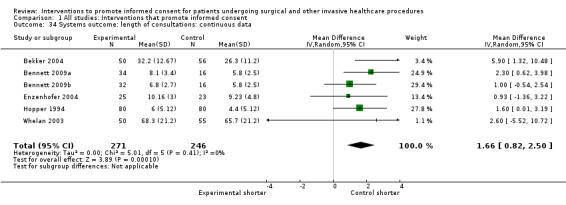
Comparison 1 All studies: Interventions that promote informed consent, Outcome 34 Systems outcome: length of consultations: continuous data.
Non‐parametric data
One study (one intervention arm) with 251 participants in the intervention group and 258 participants in the control group reported non‐parametric data on the consultation length (Fink 2010). This showed a lower median consultation time in the control group than the intervention group (8.0 minutes versus 11.9 minutes, IQR of 4 to 11.9 and 7.2 to 15.0 respectively) (Analysis 1.35).
1.35. Analysis.
Comparison 1 All studies: Interventions that promote informed consent, Outcome 35 Systems outcome: economic‐time taken to consent: non‐parametric data.
| Systems outcome: economic‐time taken to consent: non‐parametric data | ||||||
|---|---|---|---|---|---|---|
| Study |
Intervention: median (mins) |
IQR (mins) |
N |
Control: median (mins) |
IQR (mins) |
N |
| Fink 2010 | 11.92 | 7.2‐15 | 251 | 8.00 | 4‐11.89 | 258 |
Subgroup analyses
Face‐to‐face interventions versus distant interventions (e.g. web‐based)
Interventions were categorised as either being delivered face‐to‐face with a clinician, or delivered without immediate contact with the clinician.
Face‐to‐face interventions: Sixteen studies (16 intervention arms) with 1549 participants in the intervention groups and 1321 participants in the control groups used interventions that the participant completed face‐to‐face with the clinician as part of the informed consent process (Agre 1994b; Bekker 2004; Bennett 2009a; Elfant 1995; Fink 2010; Goel 2001; Greening 1999; Johnson 2006; Morgan 2000; Neptune 1996; Raynes‐Greenow 2010; Solberg 2010; Wadey 1997; Walker 2007; Whelan 2003; Wong 2006).
Interventions not requiring direct clinician contact to complete the intervention: 51 studies (56 intervention arms) with 3169 participants in the intervention groups and 2982 participants in the control groups were classified as not requiring clinician presence to complete the intervention part of the informed consent process (Agre 1994a; Armstrong 1997; Armstrong 2010; Ashraff 2006; Astley 2008a; Astley 2008b; Bennett 2009b; Bollschweiler 2008; Chan 2002; Chantry 2010; Cornoiu 2010b; Cornoiu 2010a; Cowan 2007; Danino 2006; Deyo 2000; Enzenhofer 2004; Felley 2008; Friedlander 2011; Garden 1996; Garrud 2001; Gerancher 2000; Heller 2008; Henry 2008; Hermann 2002; Hong 2009; Hopper 1994; Kain 1997; Kang 2009a; Kang 2009b; Langdon 2002; Lavelle‐Jones 1993; Luck 1999; Makdessian 2004; Mason 2003; Masood 2007; Mauffrey 2008; Mishra 2010b; Mishra 2010a; Nadeau 2010; Neary 2010; O'Neill 1996b; O'Neill 1996a; Olver 2009; Paci 1999; Pesudovs 2006; Phatouros 1995; Rossi 2004; Rossi 2005; Rymeski 2010; Shorten 2005; Tait 2009; Thomas 2000; Uzbeck 2009; Wilhelm 2009; Yucel 2005; Zite 2011).
We performed subgroup analyses for these studies for the outcomes of immediate and short‐term knowledge, and anxiety with the consent process. Other meta‐analyses were not possible, with less than three studies contributing data to other outcomes of direct comparable interest to those analysed in the main 'Effects of interventions' section. Meta‐analysis of ‘satisfaction with the decision making’ was possible only for the face‐to‐face interventions, so does not differ from results already reported for this outcome.
Immediate knowledge
Face‐to‐face interventions: Ten studies (10 intervention arms) with 848 participants in the intervention groups and 829 participants in the control groups reported results for immediate knowledge (Agre 1994b; Bekker 2004; Bennett 2009a; Fink 2010; Greening 1999; Johnson 2006; Morgan 2000; Neptune 1996; Walker 2007; Wong 2006). Meta‐analysis showed statistically‐significantly improved knowledge in the face‐to‐face intervention groups compared with the control groups with substantial heterogeneity (SMD 0.52 (95% CI 0.28 to 0.76) I2 80%) (Analysis 2.5).
2.5. Analysis.
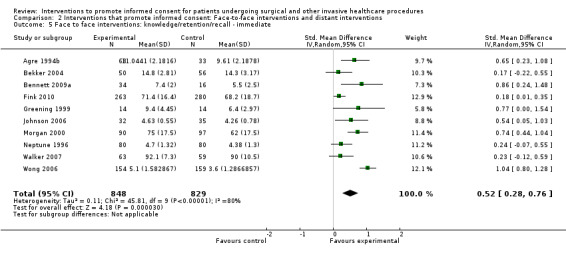
Comparison 2 Interventions that promote informed consent: Face‐to‐face interventions and distant interventions, Outcome 5 Face to face interventions: knowledge/retention/recall ‐ immediate.
Interventions not requiring direct clinician contact to complete the intervention: Fourteen studies (16 intervention arms) with 631 participants in the intervention groups and 544 participants in the control groups reported results for immediate knowledge (Agre 1994a; Armstrong 2010; Bennett 2009b; Cornoiu 2010b; Cornoiu 2010a; Cowan 2007; Garden 1996; Hermann 2002; Hopper 1994; Kang 2009a; Kang 2009b; Nadeau 2010; Pesudovs 2006; Rossi 2004; Rossi 2005; Tait 2009). Meta‐analysis showed statistically‐significantly improved knowledge in the intervention groups compared with the control groups with substantial heterogeneity (SMD 0.53 (95% CI 0.32 to 0.75) I2 67%) (Analysis 2.1).
2.1. Analysis.
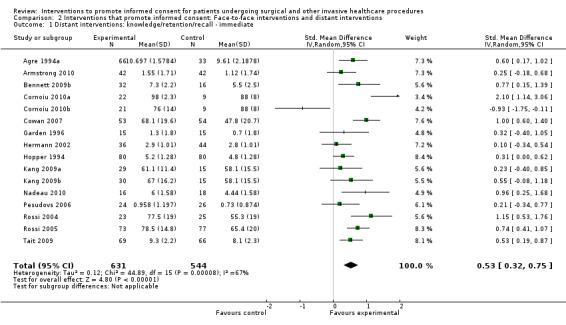
Comparison 2 Interventions that promote informed consent: Face‐to‐face interventions and distant interventions, Outcome 1 Distant interventions: knowledge/retention/recall ‐ immediate.
Short‐term knowledge
Face‐to‐face interventions: Three studies (3 intervention arms) with 508 participants in the intervention groups and 319 participants in the control groups reported results for short‐term knowledge (Goel 2001; Raynes‐Greenow 2010; Whelan 2003). Meta‐analysis showed a statistically‐significantly improvement in knowledge in the intervention groups compared to the control groups with moderate heterogeneity (SMD 0.35 (95% CI 0.12 to 0.59) I2 55%) (Analysis 2.6).
2.6. Analysis.
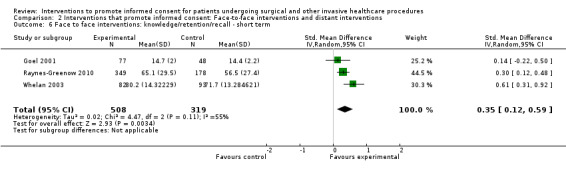
Comparison 2 Interventions that promote informed consent: Face‐to‐face interventions and distant interventions, Outcome 6 Face to face interventions: knowledge/retention/recall ‐ short term.
Interventions not requiring direct clinician contact to complete the intervention: Eleven studies (13 intervention arms) with 683 participants in the intervention groups and 596 participants in the control groups reported results for short‐term knowledge (Ashraff 2006; Chantry 2010; Cornoiu 2010b; Cornoiu 2010a; Enzenhofer 2004; Garrud 2001; Heller 2008; Luck 1999; Nadeau 2010; O'Neill 1996b; O'Neill 1996a; Tait 2009; Wilhelm 2009). Meta‐analysis also showed a statistically‐significant improvement in knowledge with considerable heterogeneity (SMD 0.79 (95% CI 0.44 to 1.14), I2 87%) (Analysis 2.2).
2.2. Analysis.
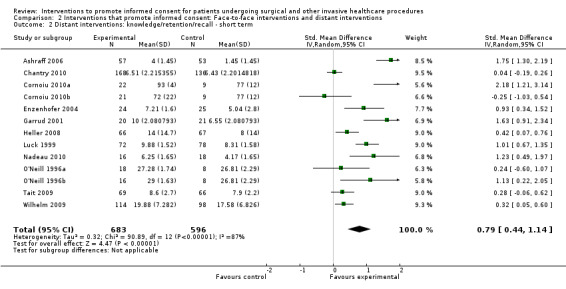
Comparison 2 Interventions that promote informed consent: Face‐to‐face interventions and distant interventions, Outcome 2 Distant interventions: knowledge/retention/recall ‐ short term.
Anxiety with the consent process
Face‐to‐face interventions: Three studies (3 intervention arms) with 374 participants in the intervention groups and 391 in the control groups reported results for anxiety with the consent process (Bekker 2004; Fink 2010; Walker 2007). There was no statistical difference in anxiety in the intervention groups compared with the control groups with substantial heterogeneity (SMD ‐0.08 (95% CI ‐0.41 to 0.25), I2 73%) (Analysis 2.7).
2.7. Analysis.
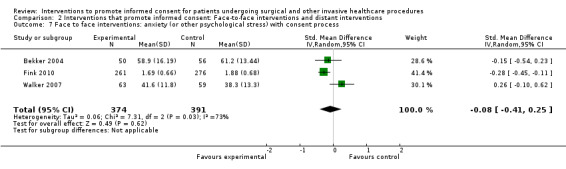
Comparison 2 Interventions that promote informed consent: Face‐to‐face interventions and distant interventions, Outcome 7 Face to face interventions: anxiety (or other psychological stress) with consent process.
Interventions not requiring direct clinician contact to complete the intervention: Eight studies (10 intervention arms) with 353 participants in the intervention groups and 289 in the control groups reported results for anxiety with the consent process (Cornoiu 2010b; Cornoiu 2010a; Danino 2006; Friedlander 2011; Garden 1996; Garrud 2001; Kain 1997; Kang 2009a; Kang 2009b; Yucel 2005). There was no statistical difference in anxiety in the intervention groups compared with the control groups with moderate heterogeneity (SMD 0.05 (95% CI ‐0.22 to 0.32) I2 58%) (Analysis 2.3).
2.3. Analysis.
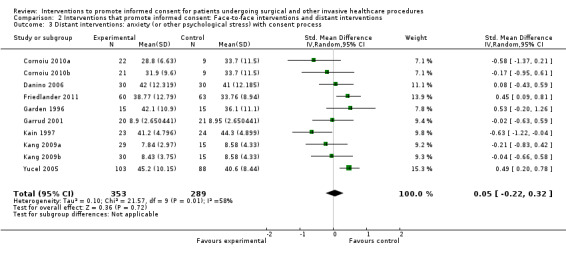
Comparison 2 Interventions that promote informed consent: Face‐to‐face interventions and distant interventions, Outcome 3 Distant interventions: anxiety (or other psychological stress) with consent process.
Length of consent consultation
Continuous data
Face‐to‐face interventions: Three studies (3 intervention arms) with 134 participants in the intervention groups and 127 participants in the control groups reported results for length of consultation (Bekker 2004, Bennett 2009a, Whelan 2003). The consultation was statistically‐significantly longer in the intervention groups compared to the control groups with little heterogeneity (MD 2.81 minutes (95% CI 1.07 to 4.55) I2 5%) (Analysis 2.8).
2.8. Analysis.
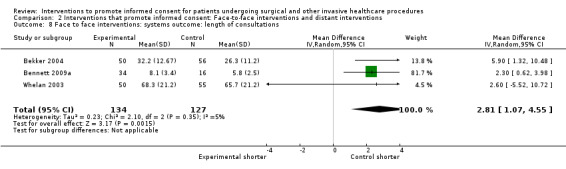
Comparison 2 Interventions that promote informed consent: Face‐to‐face interventions and distant interventions, Outcome 8 Face to face interventions: systems outcome: length of consultations.
Interventions not requiring direct clinician contact to complete the intervention: Three studies (3 intervention arms) with 137 participants in the intervention arms and 119 participants in the control arms reported results (Bennett 2009b; Enzenhofer 2004; Hopper 1994). The consultation was statistically‐significantly longer in the intervention groups compared to the control groups with little heterogeneity (MD 1.22 minutes (CI 95% 0.23 to 2.22) I2 0%) (Analysis 2.4).
2.4. Analysis.
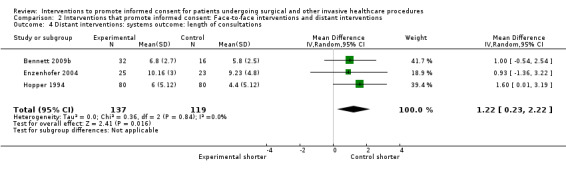
Comparison 2 Interventions that promote informed consent: Face‐to‐face interventions and distant interventions, Outcome 4 Distant interventions: systems outcome: length of consultations.
Interventions targeted at clinicians versus those targeted at patients or at organisational change
There were no studies with interventions that targeted clinicians, therefore this planned subgroup analysis was not possible.
Age of patients (young, middle‐aged, older, elderly)
Subgroup analyses were only possible under this heading by considering studies in which parents or guardians provided consent on behalf of a minor, in contrast to other studies where mixed age‐ranges of adult participants were used who were consenting for procedures for themselves (self‐consent).
Five studies (6 intervention arms) with a total of 307 participants in the intervention groups and 247 participants in the control groups reported data for consent on behalf of a minor (Chantry 2010; Friedlander 2011; Kang 2009a; Kang 2009b; Nadeau 2010; Rymeski 2010). Meta‐analysis was only possible on two outcomes: immediate knowledge and anxiety with the consent process.
Immediate knowledge
Consent on behalf of a minor: Two studies (3 arms) with 75 participants in the intervention groups and 48 in the control groups reported results in studies with parents or guardians providing consent (Kang 2009a; Kang 2009b; Nadeau 2010). There was a statistically‐significant increase in knowledge in the intervention groups compared with the control groups with little heterogeneity (SMD 0.55 (95% CI 0.15 to 0.96) I2 13%) (Analysis 3.1).
3.1. Analysis.
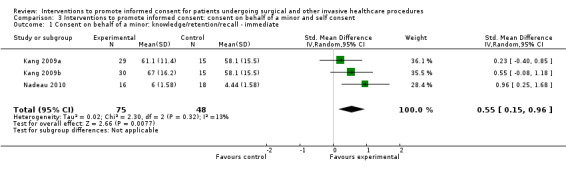
Comparison 3 Interventions to promote informed consent: consent on behalf of a minor and self consent, Outcome 1 Consent on behalf of a minor: knowledge/retention/recall ‐ immediate.
Self‐consent: Twenty studies (23 intervention arms) with 1404 participants in the intervention groups and 1325 participants in the control groups report results for comparison on this outcome (Agre 1994a; Agre 1994b; Armstrong 2010; Bekker 2004; Bennett 2009a; Bennett 2009b; Cornoiu 2010b; Cornoiu 2010a; Cowan 2007; Fink 2010; Garden 1996; Greening 1999; Hermann 2002; Hopper 1994; Johnson 2006; Morgan 2000; Neptune 1996; Pesudovs 2006; Rossi 2004; Rossi 2005; Tait 2009; Walker 2007; Wong 2006). Meta‐analysis shows a significant increase in immediate knowledge with substantial heterogeneity (SMD 0.52 (CI 95% 0.36 to 0.69) I2 75%) (Analysis 3.3).
3.3. Analysis.
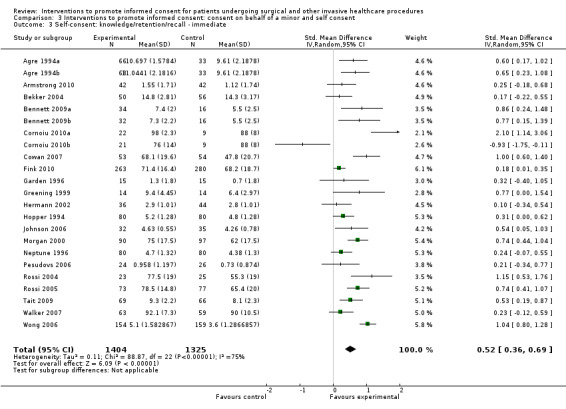
Comparison 3 Interventions to promote informed consent: consent on behalf of a minor and self consent, Outcome 3 Self‐consent: knowledge/retention/recall ‐ immediate.
Anxiety with the consent process
Consent on behalf of a minor: Two studies (3 intervention arms) with 119 participants in the intervention groups and 93 in the control groups reported results in studies with parents or guardians providing consent (Friedlander 2011; Kang 2009a; Kang 2009b). There was no statistically‐significant difference between the intervention groups compared with the control groups with moderate heterogeneity (SMD 0.14 (95% CI ‐0.30 to 0.57), I2 51%) (Analysis 3.2).
3.2. Analysis.
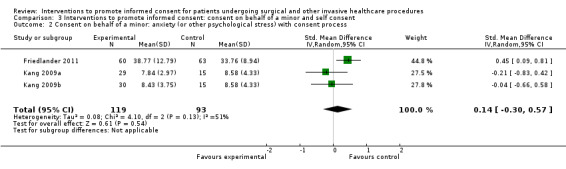
Comparison 3 Interventions to promote informed consent: consent on behalf of a minor and self consent, Outcome 2 Consent on behalf of a minor: anxiety (or other psychological stress) with consent process.
Self‐consent: Nine studies (10 intervention arms) with 608 participants in the intervention groups and 587 participants in the control groups reported results for comparison on this outcome (Bekker 2004; Cornoiu 2010a; Cornoiu 2010b; Danino 2006; Fink 2010; Garden 1996; Garrud 2001; Kain 1997; Walker 2007; Yucel 2005). Meta‐analysis shows no difference statistically between groups with substantial heterogeneity (SMD ‐0.02 (CI 95% ‐0.28 to 0.23) I2 72%) (Analysis 3.4).
3.4. Analysis.
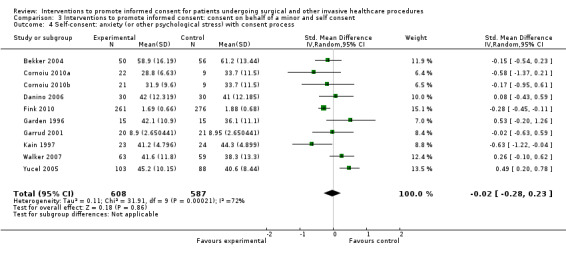
Comparison 3 Interventions to promote informed consent: consent on behalf of a minor and self consent, Outcome 4 Self‐consent: anxiety (or other psychological stress) with consent process.
Interventions targeted at a specific procedure (e.g. knee replacement) or condition more generally (e.g. decision‐aid addressing menorrhagia including a surgical option)
There were insufficient data to report on this subgroup analysis for specific procedures. Specific types of interventions e.g. decision‐aids, are addressed in the following post‐hoc analyses.
Post‐hoc analyses
We performed two post‐hoc analyses. The first was based on classification of the main component of the interventions using the following categories: written, audio‐visual; interactive multimedia; structured consent; and decision aids. The second additional subgroup analysis reports data split between interventions happening before admission to hospital and those happening at the time of admission.
Classification of the intervention
Written interventions
Written interventions were classified as interventions that use additional written materials. Twenty six studies (27 intervention arms) used written interventions (Armstrong 1997; Ashraff 2006; Astley 2008a; Bennett 2009b; Chan 2002; Cornoiu 2010b; Felley 2008; Garden 1996; Garrud 2001; Gerancher 2000; Henry 2008; Hong 2009; Kain 1997; Kang 2009a; Langdon 2002; Lavelle‐Jones 1993; Makdessian 2004; Masood 2007; Mauffrey 2008; Nadeau 2010; O'Neill 1996b; O'Neill 1996a; Pesudovs 2006; Phatouros 1995; Uzbeck 2009; Yucel 2005; Zite 2011). Meta‐analysis was possible for knowledge (immediate/short‐term/long‐term), generalised anxiety, anxiety with the consent process, and satisfaction with the consent process.
Immediate knowledge
Six studies (6 intervention arms) with 137 participants in the intervention and 99 in the control groups reported this outcome using written interventions (Bennett 2009b; Cornoiu 2010b; Garden 1996; Kang 2009a; Nadeau 2010; Pesudovs 2006). The meta‐analysis showed no difference between the groups with substantial heterogeneity (SMD 0.29 (95% CI ‐0.17 to 0.75) I2 65%) (Analysis 4.1).
4.1. Analysis.
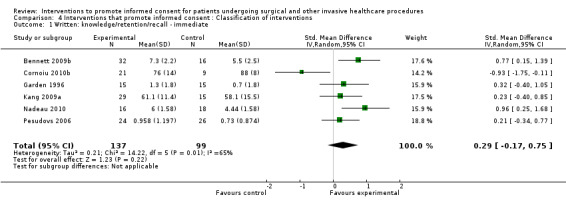
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 1 Written: knowledge/retention/recall ‐ immediate.
Short‐term knowledge
Five studies (6 intervention arms) with 148 participants in the intervention and 117 in the control groups reported this outcome using written interventions (Ashraff 2006; Cornoiu 2010b; Garrud 2001; Nadeau 2010; O'Neill 1996b; O'Neill 1996a). The meta‐analysis showed statistically‐significant improved knowledge in the intervention group with substantial heterogeneity (SMD 0.99 (95% CI 0.33 to 1.64) I2 80%) (Analysis 4.2).
4.2. Analysis.
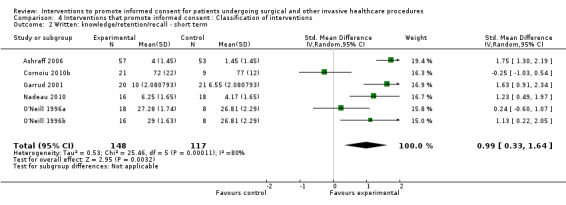
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 2 Written: knowledge/retention/recall ‐ short term.
Long‐term knowledge
Eight studies (8 intervention arms) with 296 participants in the intervention and 302 in the control groups reported this outcome using written interventions (Chan 2002; Cornoiu 2010b; Henry 2008; Hong 2009; Langdon 2002; Makdessian 2004; Mauffrey 2008; Pesudovs 2006). The meta‐analysis showed statistically significant improved knowledge in the intervention group with moderate heterogeneity (SMD 0.47 (95% CI 0.21 to 0.73) I2 58%) (Analysis 4.3).
4.3. Analysis.
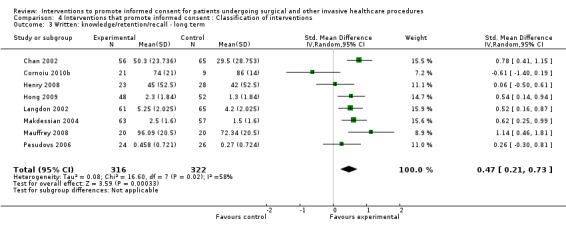
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 3 Written: knowledge/retention/recall ‐ long term.
General anxiety
Three studies (3 intervention arms) with 361 participants in the intervention and 368 in the control groups reported this outcome using written interventions (Cornoiu 2010b; Felley 2008; Uzbeck 2009). The meta‐analysis showed no differences between the groups with considerable heterogeneity (SMD 0.36 (95% CI ‐0.17 to 0.89) I2 83%) (Analysis 4.4).
4.4. Analysis.
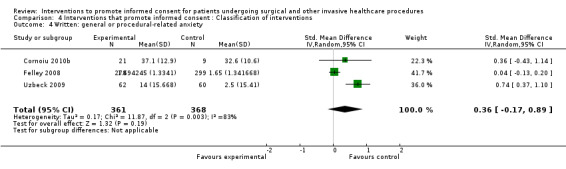
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 4 Written: general or procedural‐related anxiety.
Anxiety with the consent process
Six studies (6 intervention arms) with 211 participants in the intervention and 172 in the control groups reported this outcome using written interventions (Cornoiu 2010b; Garden 1996; Garrud 2001; Kain 1997; Kang 2009a; Yucel 2005). The meta‐analysis showed no differences between the groups with substantial heterogeneity (SMD 0.02 (95% CI ‐0.38 to 0.43) I2 67%) (Analysis 4.5).
4.5. Analysis.
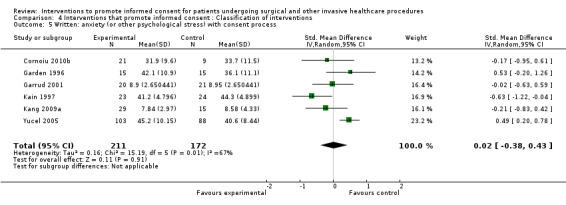
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 5 Written: anxiety (or other psychological stress) with consent process.
Satisfaction with the consent process
Five studies (6 intervention arms) with 416 participants in the intervention and 405 participants in the control groups reported this outcome using written interventions (Cornoiu 2010b; Felley 2008; Garrud 2001; O'Neill 1996b; O'Neill 1996a; Uzbeck 2009). The meta‐analysis showed no difference between the groups with considerable heterogeneity (SMD 0.19 (95% CI ‐0.29 to 0.67) I2 82%) (Analysis 4.6).
4.6. Analysis.
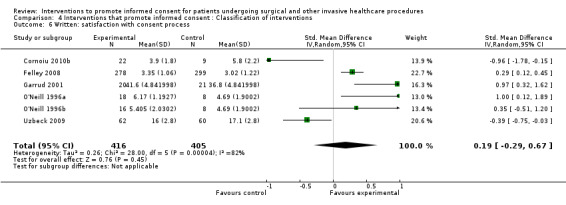
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 6 Written: satisfaction with consent process.
Audio‐visual Interventions
Audio‐visual interventions were classified as interventions that used voice and pictures, for example a video. There were 19 studies (19 intervention arms) that used audio‐visual interventions (Agre 1994a; Armstrong 2010; Astley 2008b; Bollschweiler 2008; Chantry 2010; Cornoiu 2010a; Cowan 2007; Danino 2006; Friedlander 2011; Hermann 2002; Kang 2009b; Luck 1999; Mason 2003; Olver 2009; Rossi 2004; Rossi 2005; Rymeski 2010; Thomas 2000; Wilhelm 2009). Meta‐analysis was possible for knowledge (immediate/short‐term), generalised anxiety, anxiety with the consent process and satisfaction with the consent process.
Immediate knowledge
Eight studies (8 intervention arms) with 345 participants in the intervention and 299 in the control groups reported this outcome using audio‐visual interventions (Agre 1994a; Armstrong 2010; Cornoiu 2010a; Cowan 2007; Hermann 2002; Kang 2009b; Rossi 2004; Rossi 2005). The meta‐analysis showed statistically‐significantly improved knowledge in the intervention group with substantial heterogeneity (SMD 0.72 (95% CI 0.40 to 1.04) I2 71%) (Analysis 4.7).
4.7. Analysis.
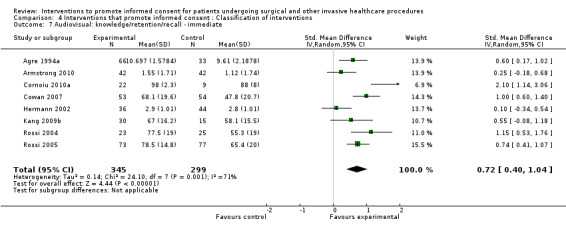
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 7 Audiovisual: knowledge/retention/recall ‐ immediate.
Short‐term knowledge
Four studies (4 intervention arms) with 376 participants in the intervention and 321 in the control groups reported this outcome using audio‐visual interventions (Chantry 2010; Cornoiu 2010a; Luck 1999; Wilhelm 2009). The meta‐analysis showed statistically‐significantly improved knowledge in the intervention groups with considerable heterogeneity (SMD 0.73, 95% CI 0.14 to 1.32) I2 91%) (Analysis 4.8).
4.8. Analysis.
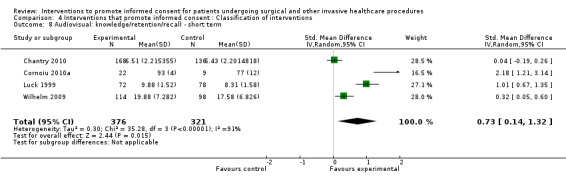
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 8 Audiovisual: knowledge/retention/recall ‐ short term.
General anxiety
Five studies (5 intervention arms) with 226 participants in the intervention and 218 in the control groups reported this outcome using audio‐visual interventions (Bollschweiler 2008; Cornoiu 2010a; Luck 1999; Olver 2009; Thomas 2000). The meta‐analysis showed no difference between groups with considerable heterogeneity (SMD ‐0.48 (95% CI ‐1.07 to 0.12), I2 86%) (Analysis 4.9).
4.9. Analysis.

Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 9 Audiovisual: general or procedural‐related anxiety.
Anxiety with the consent process
Four studies (4 intervention arms) with 142 participants in the intervention and 117 in the control groups reported this outcome using audio‐visual interventions (Cornoiu 2010a; Danino 2006; Friedlander 2011; Kang 2009b). The meta‐analysis showed no statistically‐significant difference between the groups with moderate heterogeneity (SMD 0.08 (95% CI ‐0.32 to 0.47) I2 53%) (Analysis 4.10).
4.10. Analysis.
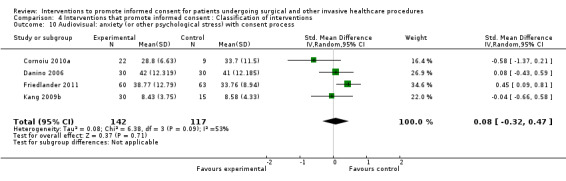
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 10 Audiovisual: anxiety (or other psychological stress) with consent process.
Satisfaction with the consent process
Continuous data: Four studies (4 intervention arms) with 343 participants in the intervention and 284 in the control groups reported this outcome using audio‐visual interventions (Armstrong 2010; Chantry 2010; Cornoiu 2010a; Wilhelm 2009). The meta‐analysis showed no statistically significant difference between the groups with little heterogeneity (SMD 0.05 (95% CI ‐0.11 to 0.21) I2 0%) (Analysis 4.11).
4.11. Analysis.
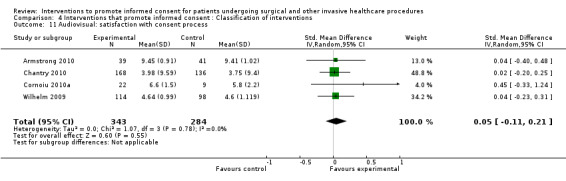
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 11 Audiovisual: satisfaction with consent process.
Dichotomous data: Four studies (4 intervention arms) with 252 participants in the intervention groups and 249 participants in the control groups reported dichotomous data for this outcome (Bollschweiler 2008; Olver 2009; Rossi 2005; Thomas 2000). The meta‐analysis showed no difference between the groups with considerable heterogeneity (RR 1.10 (95% CI 0.91 to 1.34) I2 93%) (Analysis 4.12).
4.12. Analysis.
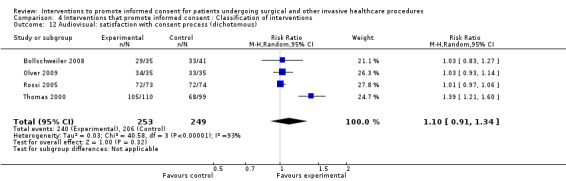
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 12 Audiovisual: satisfaction with consent process (dichotomous).
Interactive multimedia
Interactive multimedia interventions were classified as any intervention that used pictures and voice but also required the user to actively participate in the process. There were six studies (six intervention arms) that used these interventions (Deyo 2000; Enzenhofer 2004; Heller 2008; Hopper 1994; Neary 2010; Tait 2009). Meta‐analysis was possible for short‐term knowledge and satisfaction with the consent process.
Short‐term knowledge
Three studies (3 intervention arms) with 159 participants in the intervention and 158 participants in the control groups reported this outcome using interactive multimedia interventions (Enzenhofer 2004; Heller 2008; Tait 2009). The meta‐analysis showed statistically‐significantly improved knowledge in the intervention group with moderate heterogeneity (SMD 0.47 (95% CI 0.16 to 0.77) I2 43%) (Analysis 4.13).
4.13. Analysis.
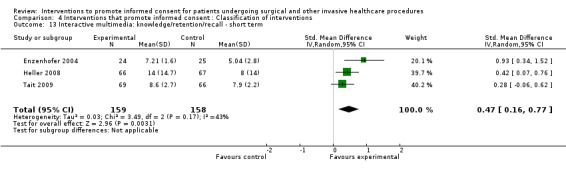
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 13 Interactive multimedia: knowledge/retention/recall ‐ short term.
Satisfaction with the consent process
Three studies (three intervention arms) with 174 participants in the intervention and 174 participants in the control groups reported this outcome using interactive multimedia interventions (Enzenhofer 2004; Hopper 1994; Tait 2009). The meta‐analysis showed no statistically‐significant difference between the groups with considerable heterogeneity (SMD 0.23 (95% CI ‐0.46 to 0.92) I2 89%) (Analysis 4.14).
4.14. Analysis.
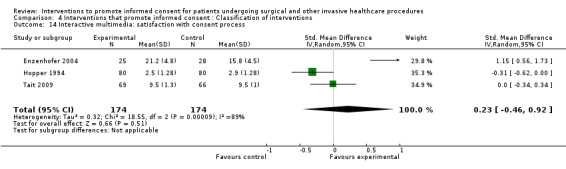
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 14 Interactive multimedia: satisfaction with consent process.
Structured consent
Structured consent interventions were classified as interventions that involved providing additional structuring of the consent process, such as the clinician being trained to ask the patient to repeat back what they had been told. There were six studies (six intervention arms) that used these interventions (Agre 1994b; Bennett 2009a; Fink 2010; Greening 1999; Wadey 1997; Walker 2007). Meta‐analysis was possible for immediate knowledge.
Immediate knowledge
Five studies (5 intervention arms) with 442 participants in the intervention and 402 in the control groups reported this outcome using interventions focusing on structuring the consent consultation (Agre 1994b; Bennett 2009a; Fink 2010; Greening 1999; Walker 2007). The meta‐analysis showed statistically‐significantly improved knowledge in the intervention group with moderate heterogeneity (SMD 0.43 (95% CI 0.16 to 0.70) I2 57%) (Analysis 4.15).
4.15. Analysis.
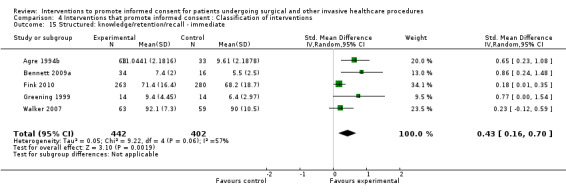
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 15 Structured: knowledge/retention/recall ‐ immediate.
Decision aids
Decision aid interventions were classified as interventions that used decision aids, either alone or in combination with other components. There were nine studies (nine intervention arms) that used these interventions (Bekker 2004; Goel 2001; Johnson 2006; Morgan 2000; Raynes‐Greenow 2010; Shorten 2005; Solberg 2010; Whelan 2003; Wong 2006). Meta‐analysis was possible for knowledge (immediate/short‐term) and satisfaction with the consent process.
Immediate knowledge
Four studies (4 intervention arms) with 326 participants in the intervention and 347 in the control groups reported this intervention using interventions with decision aids (Bekker 2004; Johnson 2006; Morgan 2000; Wong 2006). The meta‐analysis showed statistically‐significantly improved knowledge in the intervention group with considerable heterogeneity (SMD 0.64 (95% CI 0.26 to 1.02) I2 81%) (Analysis 4.16).
4.16. Analysis.
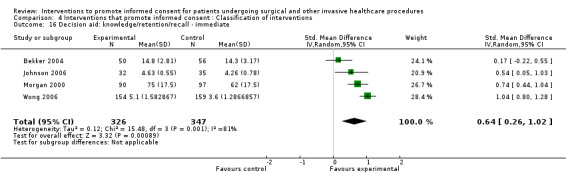
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 16 Decision aid: knowledge/retention/recall ‐ immediate.
Short‐term knowledge
Three studies (3 intervention arms) with 508 participants in the intervention and 319 in the control groups reported this intervention using interventions with decision aids (Goel 2001; Raynes‐Greenow 2010; Whelan 2003). The meta‐analysis showed statistically‐significantly improved knowledge in the intervention group with moderate heterogeneity (SMD 0.35 (95% CI 0.12 to 0.59) I2 55%) (Analysis 4.17).
4.17. Analysis.
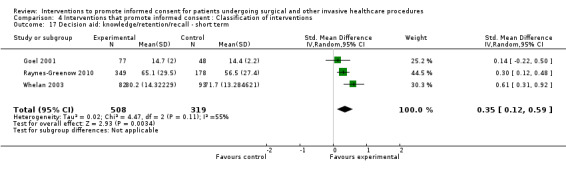
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 17 Decision aid: knowledge/retention/recall ‐ short term.
Satisfaction with the decision‐making process
Seven studies (7 intervention arms) with 884 participants in the intervention and 721 in the control groups reported this outcome using interventions with decision aids (Bekker 2004; Goel 2001; Morgan 2000; Raynes‐Greenow 2010; Solberg 2010; Whelan 2003; Wong 2006). The meta‐analysis showed statistically‐significantly improved satisfaction in the intervention group with considerable heterogeneity (SMD 2.64 (95% CI 1.50 to 3.77), I2 99%) (Analysis 4.18).
4.18. Analysis.
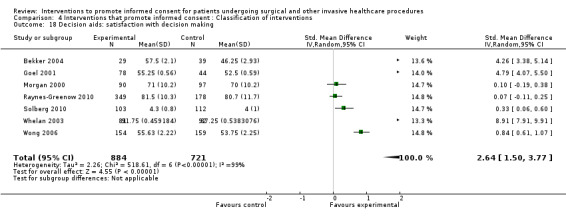
Comparison 4 Interventions that promote informed consent : Classification of interventions, Outcome 18 Decision aids: satisfaction with decision making.
Timing of intervention
Interventions were divided into two groups, the first being interventions that happened before admission to hospital/place of procedure and the second being interventions that happened at the time of admission for a procedure. Classification was split according to when the intervention group had an intervention in studies where the control group had a component which was more than usual care. Two studies were excluded from this subgroup analysis because they used timing of consent as the intervention (Elfant 1995; Neptune 1996).
Subgroup meta‐analysis was possible for the following outcomes: Immediate knowledge, short‐term knowledge, long‐term knowledge, generalised anxiety, anxiety with the consent process, and satisfaction with the consent process.
Intervention before admission for a procedure
Thirty nine studies (43 intervention arms) with 3211 participants in the intervention groups and 2898 participants in the control arms report data when the intervention happened before admission for a procedure (Ashraff 2006; Bekker 2004; Chan 2002; Chantry 2010; Cornoiu 2010a; Cornoiu 2010b; Danino 2006; Deyo 2000; Felley 2008; Fink 2010; Friedlander 2011; Garrud 2001; Goel 2001; Heller 2008; Henry 2008; Hong 2009; Kain 1997; Kang 2009a; Kang 2009b; Langdon 2002; Luck 1999; Makdessian 2004; Mason 2003; Mauffrey 2008; Mishra 2010a; Mishra 2010b; Morgan 2000; Nadeau 2010; Neary 2010; O'Neill 1996a; O'Neill 1996b; Olver 2009; Pesudovs 2006; Raynes‐Greenow 2010; Rymeski 2010; Shorten 2005; Solberg 2010; Thomas 2000; Wadey 1997; Whelan 2003; Wilhelm 2009; Wong 2006; Zite 2011).
Immediate knowledge
Continuous data: Eight studies (10 intervention arms) with 699 participants in the intervention groups and 684 participants in the control groups reported results for interventions before admission for a procedure (Bekker 2004; Cornoiu 2010a; Cornoiu 2010b; Fink 2010; Kang 2009a; Kang 2009b; Morgan 2000; Nadeau 2010; Pesudovs 2006; Wong 2006). Meta‐analysis showed a statistically‐significant difference between the intervention and control groups with greater knowledge reported in the intervention group, however with considerable heterogeneity (SMD 0.50 (95% CI 0.16 to 0.85) I2 86%) (Analysis 5.1).
5.1. Analysis.
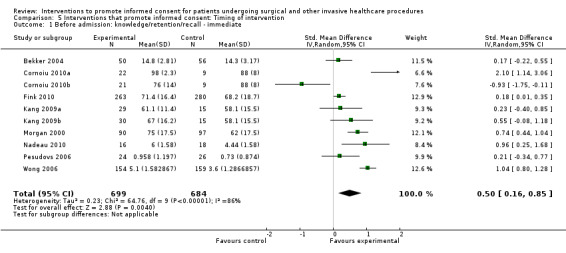
Comparison 5 Interventions that promote informed consent: Timing of intervention, Outcome 1 Before admission: knowledge/retention/recall ‐ immediate.
Anxiety with the consent process
Continuous data: Eight studies (10 intervention arms) with 546 participants in the intervention groups and 518 participants in the control groups reported results for interventions before admission for a procedure (Bekker 2004; Cornoiu 2010a;Cornoiu 2010b; Danino 2006; Fink 2010; Friedlander 2011; Garrud 2001; Kain 1997; Kang 2009a; Kang 2009b). Meta‐analysis showed no significant difference in anxiety between the two groups with substantial heterogeneity (SMD ‐0.12 (95% CI ‐0.33 to 0.09) I2 50%) (Analysis 5.2).
5.2. Analysis.
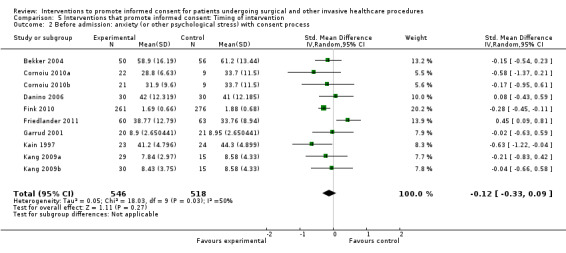
Comparison 5 Interventions that promote informed consent: Timing of intervention, Outcome 2 Before admission: anxiety (or other psychological stress) with consent process.
Satisfaction with the consent process
Continuous data: Seven studies (9 intervention arms) with 708 participants in the intervention groups and 644 participants in the control groups reported results for interventions before admission for a procedure (Bekker 2004; Chantry 2010; Cornoiu 2010b; Cornoiu 2010a; Felley 2008; Garrud 2001; O'Neill 1996b; O'Neill 1996a; Wilhelm 2009). There was no significant difference in satisfaction between groups with substantial heterogeneity (SMD 0.14 (95% CI ‐0.12 to 0.41) I2 74%) (Analysis 5.3).
5.3. Analysis.
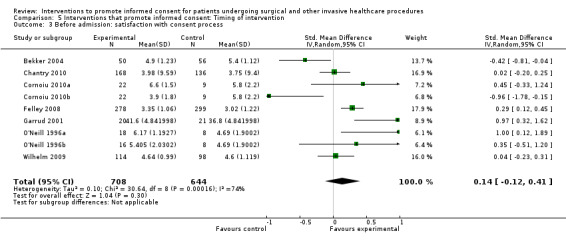
Comparison 5 Interventions that promote informed consent: Timing of intervention, Outcome 3 Before admission: satisfaction with consent process.
Dichotomous data: Four studies (4 intervention arms) with 234 participants in the intervention groups and 226 participants in the control groups reported dichotomous results for interventions before admission for a procedure (Heller 2008; Olver 2009; Pesudovs 2006 ;Thomas 2000). There was no significant difference in satisfaction between groups with considerable heterogeneity (RR1.12 (95% CI 0.94 to 1.33) I2 91%) (Analysis 5.4).
5.4. Analysis.
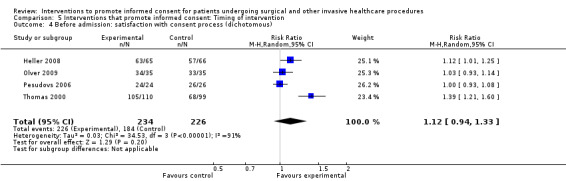
Comparison 5 Interventions that promote informed consent: Timing of intervention, Outcome 4 Before admission: satisfaction with consent process (dichotomous).
Intervention after admission for a procedure
Twenty four studies (27 intervention arms) with 1412 participants in the intervention groups and 1281 participants in the control groups report data where the interventions happened after admission for a procedure (Agre 1994a; Agre 1994b; Armstrong 1997; Armstrong 2010; Astley 2008a; Astley 2008b; Bennett 2009a; Bennett 2009b; Bollschweiler 2008; Cowan 2007; Enzenhofer 2004; Garden 1996; Gerancher 2000; Greening 1999; Hermann 2002; Hopper 1994; Johnson 2006; Lavelle‐Jones 1993; Masood 2007; Paci 1999; Phatouros 1995; Rossi 2004; Rossi 2005; Tait 2009; Uzbeck 2009; Walker 2007; Yucel 2005).
Immediate knowledge
Continuous data: Thirteen studies (15 intervention arms) with 700 participants in the intervention groups and 609 in the control groups reported results for this outcome for interventions used after admission for a procedure (Agre 1994a; Agre 1994b; Armstrong 2010; Bennett 2009a; Bennett 2009b; Cowan 2007; Garden 1996; Greening 1999; Hermann 2002; Hopper 1994; Johnson 2006; Rossi 2004; Rossi 2005; Tait 2009; Walker 2007). Meta‐analysis showed a statistically‐significant difference between the intervention and the control groups with greater knowledge reported in the intervention groups, with moderate heterogeneity (SDM 0.55 (95% CI 0.40 to 0.70) I2 40%) (Analysis 5.5).
5.5. Analysis.
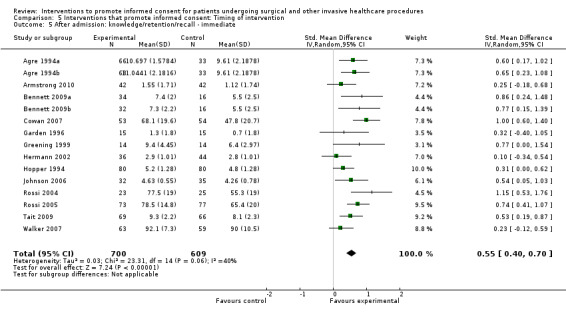
Comparison 5 Interventions that promote informed consent: Timing of intervention, Outcome 5 After admission: knowledge/retention/recall ‐ immediate.
Anxiety with the consent process
Continuous data: Three studies (3 intervention arms) with 181 participants in the intervention groups and 162 in the control groups reported results for interventions used after admission for a procedure (Garden 1996; Walker 2007; Yucel 2005). In contrast to the before‐admission interventions, meta‐analysis showed statistically‐significantly increased anxiety for the intervention groups with little or no heterogeneity (SDM 0.41 (95% CI 0.19 to 0.62) I2 0%) (Analysis 5.6).
5.6. Analysis.
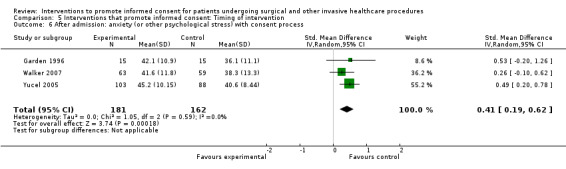
Comparison 5 Interventions that promote informed consent: Timing of intervention, Outcome 6 After admission: anxiety (or other psychological stress) with consent process.
Satisfaction with the consent process
Continuous data: Six studies (6 intervention arms) with 338 participants in the intervention groups and 334 participants in the control groups reported results for interventions used after admission for a procedure (Armstrong 2010; Enzenhofer 2004; Hopper 1994; Tait 2009; Uzbeck 2009; Walker 2007). Meta‐analysis showed no significant difference between the two groups with considerable heterogeneity (SDM 0.10 (95% CI ‐0.26 to 0.46) I2 81%) (Analysis 5.7).
5.7. Analysis.
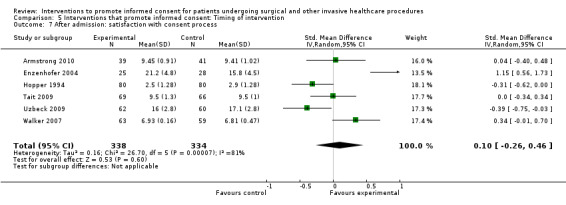
Comparison 5 Interventions that promote informed consent: Timing of intervention, Outcome 7 After admission: satisfaction with consent process.
Dichotomous data: Six studies (6 intervention arms) with 281 participants in the intervention groups and 304 participants in the control arms reported dichotomous results for interventions used after admission for a procedure (Bollschweiler 2008; Cowan 2007; Johnson 2006; Paci 1999; Phatouros 1995; Rossi 2005). There was no significant difference in satisfaction with the consent process between the two groups with little heterogeneity (RR 1.00 (95% CI 0.96 to 1.04) I2 4%) (Analysis 5.8).
5.8. Analysis.
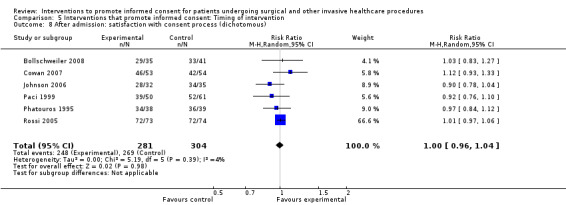
Comparison 5 Interventions that promote informed consent: Timing of intervention, Outcome 8 After admission: satisfaction with consent process (dichotomous).
Discussion
Summary of main results
In this review we found that interventions to promote informed consent for patients undergoing surgical and other invasive healthcare procedures generally increased patients’ perceived knowledge and understanding. More specifically we found that immediate, short‐term and long‐term patient knowledge and satisfaction with decision making were increased, and decisional conflict was reduced. Satisfaction with the consent process, generalised anxiety and anxiety with the consent process were unchanged. We also found that clinician and organisational outcomes were measured in a very limited number of studies. Where there were data, we found that for participants receiving the intervention, clinician satisfaction was unchanged, consultation length was slightly increased and uptake of procedures was unchanged.
This review also shows that further research is required; in particular, further consideration of how to measure informed consent as a unified concept. In addition, more information is needed about the impact of interventions on clinicians of providing informed consent, the impact on healthcare organisations and particularly the uptake of procedures. More recent studies appeared to be informed by the development of decision aids and an appreciation that consent should be a process rather than an event.
Overall completeness and applicability of evidence
We identified a large number of relevant studies from a wide variety of settings. Given the importance of informed consent to clinical care, it is encouraging to see the efforts being made to improve this process and the evidence that patients can benefit. While it is disappointing to identify that a unified measure of informed consent was only attempted in one study, using interventions that improve components of informed consent should lead to improvements in consent itself. It is also encouraging that potentially negative impacts of providing patients with more information, such as increased patient anxiety or increased consultation length, may be limited.
Increases in patient perceived understanding and knowledge seem beneficial since patient perception of care is important and this is consistent with the finding of an increase in satisfaction with decision making. However more convincing is the evidence of increases in actual knowledge. Since the interventions were generally based around providing the patient with more information, it is probably not surprising that the interventions increased immediate knowledge. However, the evidence of growth in long‐term knowledge (at longer than two weeks) suggests that the interventions had a real impact on patients’ understanding of the procedures they were undergoing.
Several of the meta‐analyses are difficult to interpret because the levels of heterogeneity are high. The studies we were considering took place in a variety of settings and included patients undergoing a range of procedures. In addition, varied measures were used for measuring outcomes. In these circumstances high levels of heterogeneity might be anticipated.
The timing of interventions to improve consent may be important. In the past, consent was seen as an event, usually occurring shortly before the procedure itself, at the time when the patient arrived at the hospital. This is clearly problematic since opportunities for the patient to deliberate on the information provided and consider their options or even simply ask questions is restricted by lack of time and the patient’s need to prepare him or herself for the procedure. Making consent a process, whereby clinician and patient identify and plan the most appropriate treatment options, sharing information as they do so, and the patient is provided with time to consider the information before a final decision is made on which treatment is appropriate (including the option of doing nothing), may be more likely to result in informed consent. Support for this approach may come from this review. Our subgroup analyses looking at the timing of interventions identified that interventions both before and at the time of admission increased immediate knowledge, but suggest that those at the time of admission to hospital may increase anxiety with the consent process. It is a logical supposition that getting consent at the time of admission adds to the burden/work for the patient, but we have very low confidence in this result, given the analysis came from three small trials with variable risk of bias (Oxman 2004). This approach (ie seeking informed consent before the procedure) is also supported by the use of decision aids which, by their nature, have to be presented to the patient in advance of the procedure and provide more opportunity to deliberate. Our subgroup finding that these interventions increased knowledge and reduced decisional conflict is encouraging and consistent with the review conducted by Stacey (Stacey 2011).
It is notable that limitation attention was paid in the studies we identified to both clinician and organisational outcomes. For interventions to be taken up and used widely they need support from clinicians. At the least they should not have negative impacts. Some effects such as complaints and litigation which are of considerable significance around informed consent will occur too infrequently to be used as outcomes in relatively short‐term trials, but when significant change occurs in a healthcare system it would be important to gather data on these outcomes.
Rate of uptake of procedures is another complex outcome. It was measured in 10 studies with over 3000 participants. Interpretation is difficult because patients who were more fully informed might choose to have the procedure if this process indicated the procedure would produce benefits, alternatively they might be more likely to decline the procedure if little benefit was likely. In addition, the timing of the intervention would again be important. This is illustrated in the nature of the procedures being considered. Some studies were designed to promote the non‐uptake of invasive procedures (for example caesarean section (Shorten 2005)) while others were more neutral in their approach to treatments such as chemotherapy after breast cancer (Whelan 2003).
With regard to the particular format of the interventions used, for example written or audio‐visual, it is difficult to draw conclusions. Generally all formats improved knowledge but the findings across other outcomes are less consistent. A number of factors will influence the impact of interventions employing different formats. For example, if written materials are used in advance of admission to hospital they may act as ‘prompt sheets’ which are known to increase the question‐asking by patients (Kinnersley 2007).
Quality of the evidence
The particular risks of bias that were judged as most likely to affect the results of studies in this review included random sequence generation flaws, attrition bias and poor blinding of outcome assessment. Attrition bias was generally low since studies mainly collected outcome data at the time of consent or at the time of the procedure.
Blinding of outcomes was at high risk in about a quarter of included studies. This was most commonly because research assistants were not employed and clinical staff appeared to be conducting the studies and collecting data. However, in general when studies at high risk of bias were removed from the meta‐analyses no major differences were apparent.
Potential biases in the review process
These results of this review need interpreting with caution. Strengths of our review are that we undertook comprehensive search and review strategies and identified a large number of trials. In addition we attempted to contact authors to clarify or obtain further data and we have considered and reported on a comprehensive group of outcomes. However we only found one study reporting data on our primary outcome (Friedlander 2011) and, as has been discussed, the level of heterogeneity is high in many of the meta‐analyses. However, the heterogeneity of this review can also be seen as a strength. There appear to be enough data and significant findings on multiple outcomes to form broad conclusions for pragmatic application. The review does not answer questions for specific procedures, or identify how best to consent patients for one particular operation in one part of the world. We believe, however, there is useful evidence here regarding the impact of a number of essentially similar interventions on elements that can enhance informed consent.
Fidelity
Few studies in this review reported directly on adherence to the intervention. It is important to know to what extent participants actually used the intervention, and lack of adherence is more likely if the intervention is complex as was the case in many of the studies reported here. If there is significant lack of adherence to the intervention, clearly the outcomes cannot then be attributed to the intended intervention. Notably, when internet‐based interventions were used (e.g. Rymeski 2010) the inclusion of a tracking system to monitor participant progress through modules allowed for adherence to be reported relatively straight‐forwardly.
Agreements and disagreements with other studies or reviews
To date, other systematic reviews have either looked specifically at interventions promoting informed consent for clinical research trials (e.g. Ryan 2009), or have included randomised and non‐randomised studies (Schenker 2011). Schenker et al concluded that interventions improve comprehension, and that research is still needed to clarify and evaluate the effects of interventions on ‘informed consent’. Our review supports these findings but adds more robust data. Like Schenker et al, we are unable to comment on whether interventions promote a wider concept of informed consent.
Ryan 2009 systematically reviewed the effects of audio‐visual aids on informed consent for clinical trials. The findings in our review focusing on consent for procedures are more positive, demonstrating that audio‐visual aids improve immediate and short‐term knowledge and reduce generalised anxiety, without increasing anxiety with the consent process. Our review further looks at different types of interventions and compares the efficacy of these over within a range of outcomes. However, as has been found elsewhere, we are unable to clearly identify whether one particular type of intervention is better than another (Cohn 2007).
Authors' conclusions
Implications for practice.
For patients
This review identifies benefits for patients of using interventions to enhance the process of informed consent for invasive clinical procedures. Emergency procedures present particular challenges, but for routine, planned procedures it would appear that efforts should be made to provide patients with additional materials which provide information about the procedure, and patients should also be provided with time to consider the information. We were unable to make comparisons between different groups of patients but the variety of patients included in our review would suggest that the results are reasonably generalisable. In the case of vulnerable groups it would appear even more important that clinicians make efforts to ensure informed consent.
For clinicians
For clinicians, the studies reported have concluded no significant change in satisfaction with the consent consultation, as a result of interventions to promote informed consent. There were no data available on interventions targeted at clinicians and this may be an area of research in the future. Rates of uptake of procedures were also unchanged, although this may be confounded by preferred treatment choices influencing patient choice in some areas.
We present here evidence that indicates that for a wide range of procedures and settings, elements of the consent process can be improved. Since clinicians take responsibility for the consenting of patients they should also take responsibility for improving this process if possible. The previously routine process of simply providing patients with verbal information at the time of their admission to hospital may not benefit patients, and a more considered approach with enhanced information provision and time for consideration may be more beneficial.
Clinicians, those responsible for training clinicians, and researchers considering the design of interventions need to consider both ‘what’ information is provided to patients and ‘how’ this information is delivered. Standardised consent forms in which clinicians are expected to confirm that they have provided particular information to patients may be helpful, but they may also promote a ‘tick‐box’ approach to giving information, and risk the patient being overloaded with factual information which they may struggle to comprehend. Silverman and colleagues (Silverman 2005) have summarized many of the deficiencies in information provision to patients and have identified key skills for enhanced information provision and discussion of plans. These skills need to be combined with the skills for shared decision making (Elwyn 2012). Informed consent is most likely to be achieved when the patient has had a discussion with a clinician who is both well informed and skilled at providing information, and who uses interventions as described in this review to at least enhance patient knowledge. Patients then need an opportunity to deliberate on the choices available to them, and then be able to express their decision without feeling pressurized.
Implications for research.
Achieving informed consent is a complex process. Further research should appreciate this complexity and consider the overall pattern of outcomes achieved. It would also be helpful if there could be greater consensus on appropriate validated, reliable tools which measure outcomes such as knowledge and satisfaction, allowing improved comparison between studies.
If outcome measurements are more robust, research could benefit from looking in several directions. Further evaluation of different types of interventions is required so that conclusions can be drawn regarding which form of intervention (such as audio‐visual, interactive multimedia, written information) is most beneficial in different settings, and whether there is any benefit in re‐enforcing information with more than one intervention or if interventions are repeated. The timing of interventions during the process from diagnosis to procedure appears to be of importance and deserves further study. There is little evidence at present to suggest the impact of interventions on clinicians. Further research in this area may be very informative and define the way that informed consent is addressed.
Acknowledgements
We thank the translators of three papers, Katy Wilkinson (French paper, Danino 2006), Michaela Gal (excluded German paper, Lembcke 1998), and Jana Witt (German paper, Hermann 2002). James Ellis Jones, a patient with recent experience of health care in the UK; Mr Hunaid Vohra, cardiothoracic surgeon, Bristol; and Professor Steve Thomas, maxillo‐facial surgeon, Bristol provided feedback and suggestions to improve the plain language summary. We also thank Sophie Hill, Megan Prictor and Jessica Thomas of the Cochrane Consumers and Communication Review Group for their help and support.
Appendices
Appendix 1. MEDLINE (OvidSP) (1947 to July 2011)
1. exp informed consent/
2. (informed adj2 (consent or decision* or choice*)).tw.
3. informed decision making.tw.
4. consent comprehension.tw.
5. informed choice.tw.
6. informed consent recall.tw.
7. (consent* adj (process or form* or document*)).tw.
8. improving informed consent.tw.
9. (improv* adj2 consent).tw.
10. (understanding adj2 consent).tw.
11. consent process.tw.
12. *"Parental Consent"/
13. Parental Education as topic/
14. or/1‐13
15. exp health education/
16. health knowledge attitudes practice/
17. ((health or patient or client) adj (education or knowledge or information or communication)).tw.
18. education/
19. ((education* or teaching or learning or training or skills or online or on‐line or web* or internet or video* or multimedia or multi‐media) adj (intervention* or session* or course* or program* or material* or package*)).tw.
20. ((improv* or increas* or enhanc*) adj3 (understanding or comprehension)).tw.
21. ((educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn*) and (Patient* or client* or consumer* or user* or carer* or caregiver* or care giver*)).tw.
22. exp professional patient relations/
23. hospital patient relations/
24. "referral and consultation"/
25. interviews as topic/
26. (consult* or interview).tw.
27. information services/
28. information dissemination/
29. access to information/
30. (information* adj (service* or system* or dissemination or seeking or provision or aid* or material* or sheet* or package*)).tw.
31. ((patient or client or written or print* or visual* or providing) adj information).tw.
32. (inform* adj2 (patient* or client*)).tw.
33. communication/
34. exp communications media/
35. ((mass or communication* or electronic or multi or print* or social or new) adj media).tw.
36. video recording/
37. (radio or televisions or audio* or video* or tape or recording* or cassette* or cd‐rom* or dvd* or film* or mulimedia or hypermedia or telephon* or phone or sms or short message* or text message* or internet or web* or email* or electronic mail* or online or on‐line or blog* or telemedicine or telehealth or virtual reality).tw.
38. ((print* adj (media or material* or based)) or paper based or publication* or brochure* or pamphlet* or leaflet* or flyer* or handout* or poster* or illustrat* or picture* or image* or pictorial* or pictogram*).tw.
39. exp computer systems/
40. online systems/
41. medical informatics/
42. exp informatics/
43. information systems/
44. software/
45. computer assisted instruction/
46. (computer* adj1 (system* or network* or program* or terminal* or interface* or interact* or intervention* or graphic* or game* or simulation* or searching or mediated or based or tailored or communication or assisted)).tw.
47. (interactive adj3 (program* or software or online or on‐line or media or technolog* or communication or health*)).tw.
48. user computer interface/
49. computer graphics/
50. video games/
51. decision making/
52. decision support techniques/
53. (decision adj (aid* or support or tool*)).tw.
54. exp counseling/
55. translating/
56. multilingualism/
57. cultural* competen*.mp.
58. ((cultural* adj3 communication) or interpreter* or interpreting or translator* or translating).tw.
59. exp education, medical/
60. (((continuing or residency or distance) adj2 education) or internship or interns or inservice or in‐service or staff‐development).mp.
61. or/15‐60
62. 14 and 61
63. randomized controlled trial.pt.
64. controlled clinical trial.pt.
65. randomized.ab.
66. placebo.ab.
67. randomly.ab.
68. trial.ab.
69. groups.ab.
70. or/63‐69
71. exp animals/not humans.sh.
72. 70 not 71
73. 62 and 72
Appendix 2. EMBASE (OvidSP) (1980 to July 2011)
1. exp informed consent/
2. (informed adj2 (consent or decision* or choice*)).tw.
3. informed decision making.tw.
4. consent comprehension.tw.
5. informed choice.tw.
6. informed consent recall.tw.
7. (consent* adj (process or form* or document*)).tw.
8. improving informed consent.tw.
9. (improv* adj2 consent).tw.
10. (understanding adj2 consent).tw.
11. consent process.tw.
12. "Parental Consent"/
13. Parental Education as topic/
14. or/1‐13
15. exp health education/
16. health knowledge attitudes practice/
17. ((health or patient or client) adj (education or knowledge or information or communication)).tw.
18. education/
19. ((education* or teaching or learning or training or skills or online or on‐line or web* or internet or video* or multimedia or multi‐media) adj (intervention* or session* or course* or program* or material* or package*)).tw.
20. ((improv* or increas* or enhanc*) adj3 (understanding or comprehension)).tw.
21. ((educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn*) and (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*)).tw.
22. exp professional patient relations/
23. hospital patient relations/
24. "referral and consultation"/
25. interviews as topic/
26. (consult* or interview).tw.
27. information services/
28. information dissemination/
29. access to information/
30. (information* adj (service* or system* or dissemination or seeking or provision or aid* or material* or sheet* or package*)).tw.
31. ((patient or client or written or print* or visual* or providing) adj information).tw.
32. (inform* adj2 (patient* or client*)).tw.
33. communication/
34. exp communications media/
35. ((mass or communication* or electronic or multi or print* or social or new) adj media).tw.
36. video recording/
37. (radio or television or audio* or video* or tape or recording* or casette* or cd‐rom* or dvd* or film* or multimedia or hypermedia or telephon* or phone or sms or short message* or text message* or internet or web* or email* or elctronic mail* or online or on‐line or blog* or telemedicine or telehealth or virtual reality).tw.
38. ((print* adj (media or material* or based)) or paper based or publication* or brochure* or pamphlet* or leaflet* or flyer* or handout* or poster* or illustrat* or picture* or image* or pictorial* or pictogram*).tw.
39. exp computer systems/
40. online systems/
41. medical informatics/
42. exp informatics/
43. information systems/
44. software/
45. computer assisted instruction/
46. (computer* adj1 (system* or network* or program* or terminal* or interface* or interact* or intervention* or graphic* or game* or simulation* or searching or mediated or based or tailored or communication or assisted)).tw.
47. (interactive adj3 (program* or software or online or on‐line or media or technolog* or communication or health*)).tw.
48. user computer interface/
49. computer graphics/
50. video games/
51. decision making/
52. decision support techniques/
53. (decision adj (aid* or support or tool*)).tw.
54. exp counseling/
55. translating/
56. multilingualism/
57. cultural* competen*.mp.
58. ((cultural* adj3 communication) or interpreter* or interpreting or translator* or translating).tw.
59. exp education, medical/
60. (((continuing or residency or distance) adj2 education) or internship or interns or inservice or in‐service or staff‐development).mp.
61. or/15‐60
62. 14 and 61
63. randomized controlled trial/
64. random$.tw.
65. exp controlled study/
66. double blind procedure/
67. single blind procedure/
68. crossover procedure/
69. latin square design/
70. multicenter study/
71. ((clinical or controlled or comparative or placebo or prospective or random$) adj3 (trial or study)).tw.
72. ((single$ or doubl$ or trebl$ or tripl$) adj7 (blind$ or mask$)).tw.
73. (crossover$ or cross‐over$ or (cross adj1 over$)).tw.
74. ((allocat$ or allot$ or assign$ or divid$) adj3 (condition$ or experiment$ or intervention$ or treatment$ or therap$ or control$ or group$)).tw.
75. or/63‐74
76. 62 and 75
Appendix 3. PsycINFO (OvidSP) (1801 to July 2011)
1. exp informed consent/
2. (informed adj2 (consent or decision* or choice*)).tw.
3. informed decision making.tw.
4. consent comprehension.tw.
5. informed choice.tw.
6. informed consent recall.tw.
7. (consent* adj (process or form* or document*)).tw.
8. improving informed consent.tw.
9. (improv* adj2 consent).tw.
10. (understanding adj2 consent).tw.
11. consent process.tw.
12. parent* consent.tw.
13. exp PARENT TRAINING/
14. or/1‐13
15. exp health education/
16. exp Health Behavior/or exp Knowledge Level/or exp Client Education/or exp Health Education/or exp Health Knowledge/or exp Health Attitudes/
17. ((health or patient or client) adj (education or knowledge or information or communication)).tw.
18. exp Health Education/or exp Education/
19. ((education* or teaching or learning or training or skills or online or on‐line or web* or internet or video* or multimedia or multi‐media) adj (intervention* or session* or course* or program* or material* or package*)).tw.
20. ((improv* or increas* or enhanc*) adj3 (understanding or comprehension)).tw.
21. ((educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn*) and (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*)).tw.
22. exp Information Systems/or exp Health Care Delivery/or exp Technology/
23. exp THERAPEUTIC PROCESSES/
24. exp Professional Referral/
25. exp Interviews/
26. (consult* or interview).tw.
27. information services/
28. information dissemination/
29. exp Information Seeking/
30. (information* adj (service* or system* or dissemination or seeking or provision or aid* or material* or sheet* or package*)).tw.
31. ((patient or client or written or print* or visual* or providing) adj information).tw.
32. (inform* adj2 (patient* or client*)).tw.
33. exp Communication Barriers/or exp Computer Mediated Communication/or exp Communication/or exp Oral Communication/or exp Verbal Communication/or exp Written Communication/or exp Nonverbal Communication/or exp Interpersonal Communication/
34. exp communications media/
35. ((mass or communication* or electronic or multi or print* or social or new) adj media).tw.
36. exp Videotapes/or exp Videotape Recorders/
37. (radio or television or audio* or video* or tape or recording* or casette* or cd‐rom* or dvd* or film* or multimedia or hypermedia or telephon* or phone or sms or short message* or text message* or internet or web* or email* or elctronic mail* or online or on‐line or blog* or telemedicine or telehealth or virtual reality).tw.
38. ((print* adj (media or material* or based)) or paper based or publication* or brochure* or pamphlet* or leaflet* or flyer* or handout* or poster* or illustrat* or picture* or image* or pictorial* or pictogram*).tw.
39. exp Computer Applications/
40. exp Internet/
41. exp information technology/
42. exp computer applications/
43. information systems/
44. exp Computer Software/
45. computer assisted instruction/
46. (computer* adj1 (system* or network* or program* or terminal* or interface* or interact* or intervention* or graphic* or game* or simulation* or searching or mediated or based or tailored or communication or assisted)).tw.
47. (interactive adj3 (program* or software or online or on‐line or media or technolog* or communication or health*)).tw.
48. exp Human Computer Interaction/
49. exp computer simulation/
50. exp graphical displays/
51. exp Computer Games/
52. video game*.tw.
53. decision making/
54. exp Decision Support Systems/
55. (decision adj (aid* or support or tool*)).tw.
56. exp counseling/
57. exp Foreign Language Translation/
58. multilingualism/
59. cultural* competen*.mp.
60. ((cultural* adj3 communication) or interpreter* or interpreting or translator* or translating).tw.
61. exp Medical Education/
62. (((continuing or residency or distance) adj2 education) or internship or interns or inservice or in‐service or staff‐development).mp.
63. or/15‐62
64. 14 and 63
65. random*.ti,ab,hw,id.
66. trial*.ti,ab,hw,id.
67. control*.ti,ab,hw,id.
68. placebo*.ti,ab,hw,id.
69. ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id.
70. (cross over or crossover or factorial* or latin square).ti,ab,hw,id.
71. (assign* or allocat* or volunteer*).ti,ab,hw,id.
72. or/65‐71
73. 64 and 72
Appendix 4. CENTRAL (Cochrane Central Register of Controlled Trials), The Cochrane Library
| #1 | MeSH descriptor Informed Consent explode all trees |
| #2 | (informed NEAR decision*) |
| #3 | (informed NEAR Choice*) |
| #4 | "informed decision making" |
| #5 | (improv* NEAR consent) |
| #6 | (#1 OR ( #2 AND OR #3 ) OR #4 OR #5) |
| #7 | (health or patient or client NEAR education or knowledge or information or communication) |
| #8 | (healthcare treatment):ti,ab,kw |
| #9 | (Surgery):ti,ab,kw |
| #10 | (health knowledge):ti,ab,kw |
| #11 | (education* or teaching or learning or training or skills or online or on‐line or web* or internet or video* or multimedia or multi‐media NEAR intervention* or session* or course* or program* or material* or package*) |
| #12 | (#7 OR #8 OR #9 OR #10 OR #11) |
| #13 | MeSH descriptor Decision Making explode all trees |
| #14 | MeSH descriptor Decision Support Techniques explode tree 2 |
| #15 | (decision NEAR aid* or support or tool*) |
| #16 | (#13 OR #14 OR #15) |
| #17 | (#6 AND #12 AND #16), from 1950 to 2011 |
Appendix 5. Data extraction sheet
| IDENTIFICATION AND SUMMARY | |
| Form version/date | |
| Review title | INTERVENTIONS THAT PROMOTE INFORMED CONSENT FOR PATIENTS UNDERGOING SURGICAL AND INVASIVE PROCEDURES |
| Study ID | Surname Year as it appears in RevMan |
| Eligibility | Yes = 1, No = 2 |
| Reason for exclusion | |
| Notes | |
| Source of information (esp if multiple reports of same trial, or unpublished data/personal communication included) |
|
| Aim of study | |
| Aim of intervention | e.g. deliberation/recall/satisfaction/choice etc. |
| Study design | |
| Informed consent for study | 1 = Yes, 2 = No, 3 = Unclear. Details if no. |
| Ethical approval | 1 = Yes, 2 = No, 3 = Unclear |
| Funding | 1 = Yes, 2 = No, 3 = Unclear. If yes, who? (not stated = no) |
| Outcomes measured | Primary = x; Secondary = y. a) Selecting the primary outcome as identified by the publication authors b) If no primary outcome specified, select the one specified in the sample size calculation c) If there are no sample size calculations, rank the effect estimates and select the median effect estimate. |
| Outcomes relevant to our review |
Primary outcomes 1.Informed consent – all elements Secondary outcomes 2.1 Patient Outcomes 2.1.1 Patient understanding 2.1.2 Knowledge/Retention/Recall 2.1.3 Deliberation (Weighing up) 2.1.4 Communication of decision 2.1.5 Other patient outcomes 2.1.5.1 Satisfaction with decision making 2.1.5.2 Anxiety (or other psychological stress) with decision making 2.1.5.3 Satisfaction with consent process 2.1.5.4 Anxiety (or other psychological stress) with consent process 2.1.5.5 Desire for further information 2.1.5.6 Sense of control – locus of control or perception of who made the decision. 2.2 Clinician Outcomes 2.2.1 Satisfaction with the 'consent consultation' 2.2.2 Ease of use of intervention(s) to improve gaining of informed consent 2.2.3 Confidence in patient's decision and whether an informed choice was made 2.3 Systems outcome 2.3.1 Rates of uptake (or refusal) of clinical interventions/procedures 2.3.2 Postponement of clinical interventions/procedures 2.3.3 Delay in decision making or request for more information/further consultations 2.3.4 Complaints and litigation 2.3.5 Adverse outcomes 2.3.6 Economic/resource use data (e.g. length of consultations, cost of surgery/procedure choices, number of consultations, length of hospital stay) |
| Consumer involvement | 1 = Yes, 2 = No, 3 = Unclear (not mentioned = no) |
| BIAS – please refer to Cochrane Handbook chapter on bias which has definitions of low and high risk, see alsoTable 1 | ||
| Domain | Review authors' judgement | Description |
| Was the allocation sequence adequately generated? | Yes/low risk of bias = 1 No/high risk of bias = 2 Unclear = 3 No information = 4 |
Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. |
| Was allocation adequately concealed? | Yes/low risk of bias = 1 No/high risk of bias = 2 Unclear = 3 No information = 4 |
Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. |
|
Blinding: Was knowledge of the allocation intervention adequately prevented during the study? 1) Blinding of participants and personnel?; 2) Blinding of outcome measurement |
Yes/low risk of bias = 1 No/high risk of bias = 2 Unclear = 3 No information = 4 |
Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Also describe measures use to ensure measurement of outcomes was also blinded. Provide any information relating to whether the intended blinding was effective Overall opinion on whether blinding was sufficient/suitable. |
| Were incomplete outcome data adequately addressed? | Yes/low risk of bias = 1 No/high risk of bias = 2 Unclear = 3 No information = 4 |
Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors. Rough estimate of 40% lost to follow up = high risk of bias (excludes legitimate reasons for drop out) |
| Are reports of the study free from suggestion of selective outcome reporting? | Yes/low risk of bias = 1 No/high risk of bias = 2 Unclear = 3 No information = 4 |
State how the possibility of selective outcome reporting was examined by the review authors, and what was found. 5 min check for published protocol |
| Other sources of bias | State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were pre‐specified in the review’s protocol, responses should be provided for each question/entry. | Design of trial, contamination, fidelity/integrity, reliability of outcome, etc |
| Were the intervention and control groups comparable at baseline? | Yes/low risk of bias = 1 No/high risk of bias = 2 Unclear = 3 |
In what way? |
| Have measures been taken within the study to protect against contamination? | Yes/low risk of bias = 1 No/high risk of bias = 2 Unclear = 3 No information = 4 |
|
| Other quality indicators | Note: A potential source of bias must be able to change the magnitude of the effect estimate, whereas sources of imprecision affect only the uncertainty in the estimate (i.e. its confidence interval). Potential factors affecting precision of an estimate include technological variability (e.g. measurement error), and observer variability. | |
| PARTICIPANTS | |
| Description | (eg. Patients/consumers; carers; parents of patients/consumers; health professionals; well people in the community) |
| Geographical location and country | (eg. City/State/Country) If unclear try to check, if still unclear assume location of first author |
| Setting | (eg. Community, home, primary health centre, acute care hospital, extended care facility) |
| Inclusion criteria | |
| Exclusion criteria | |
| Invasive procedure undergone | |
| Age | + range |
| Gender | + % male |
| Ethnicity | |
| Social/demographic details | (eg. Literacy or reading level) |
| Methods of recruitment of participants | |
| Total eligible participants: | |
| Excluded | |
| Randomised | Intervention: Control: |
| Withdrew | |
| Died | |
| Lost to follow‐up | |
| Included in analysis | |
| Total | |
| Control | |
| Intervention | |
| INTERVENTIONS | |
| Number of intervention groups | |
| Number of control groups | |
| Type of Intervention in study | e.g. CD ROM, given lists of questions to ask etc. |
| Details of intervention (Capture this information for each arm of the study, eg. Intervention A, Intervention B) |
Theoretical basis (with key references); Content; Format(s) (media); Source; Setting |
| Details of control/usual or routine care | |
| Details of co‐interventions in all groups | (co‐interventions may be separate to the intervention of interest for this review, or they may be other similar elements in a suite of interventions having a common purpose. Record all relevant information). i.e. above and beyond usual care |
| Delivery of intervention | (eg. stages, timing, frequency, duration) (for each intervention included in the study, eg. Intervention A; Intervention B?) |
| Details of providers | Who delivers the intervention? Number of providers Training of providers in delivery of intervention |
| Intervention quality (if relevant): | (record any information on the quality of the intervention ‐ assessed by study authors, others, or by you ‐ such as the evidence base of the intervention, or the quality of staff training for intervention delivery) |
| Fidelity/integrity | Adherence ‐ Was the intervention delivered as intended? Programme differentiation ‐ received only the planned interventions Exposure ‐ number, length and frequency of implementation Quality of delivery ‐ implanter enthusiasm, attitude to intervention Participant responsiveness ‐ levels of participation and enthusiasm Record any assessment of this |
| OUTCOME 1 | |||
| Power calculation performed? | 1 = Yes, 2 = No, 3 = Unclear + who did it | ||
| Sample size calculated achieved? | 1 = Yes, 2 = No, 3 = Unclear, 4 = NA | ||
| Confounding? | State potential confounders and if this controlled for in analysis? | ||
| Outcome Measure 1: | |||
| Methods of assessing outcome measure | (eg. phone survey, questionnaire, physical measurements (for each outcome)) | ||
| Method of follow‐up for non‐respondents | |||
| Timing of outcome assessment | (including frequency, length of follow up (for each outcome)) | ||
| Interpretation of scale scores | |||
| Adverse events | (eg. complaints, levels of dissatisfaction, adverse incidents, side effects) | ||
| Statistical analysis performed | e.g. t‐test, ANOVA, ANCOVA, linear regression (for continuous data); Chi2, logistic regression (for dichotomous outcomes); Mann‐Whitney (ordinal data). | ||
| Were outcome measurement tools validated? | Yes = 1 No = 2 Unclear = 3 |
Methods of validation/supporting literature | |
| Are the outcome measures reliable? | Yes = 1 No = 2 Unclear = 3 No information = 4 |
Test re‐test – proven with supporting literature? | |
| Dichotomous outcomes | ||||||||||||||
| Outcome | Timing of outcome assessment | Intervention group | Control group | P values, CIs, Notes | ||||||||||
| Observed (n) | Total (N) | Observed (n) | Total (N) | |||||||||||
| |
||||||||||||||
| Continuous outcomes | ||||||||||||||
| Outcome | Timing of outcome assessment | Intervention group | Control group | P values, CIs, Notes | ||||||||||
| Mean/mean change | Standard deviation | N | Mean/mean change | Standard deviation | N | |||||||||
| Non‐parametric outcomes | ||||||||
| Outcome | Timing of outcome assessment | Intervention group | Control group | Notes | ||||
| Median | Inter‐quartile range | N | Median | Inter‐quartile range | N | |||
| OUTCOME 2 | |||||
| Power calculation performed? | 1 = Yes, 2 = No, 3 = Unclear + who did it | ||||
| Sample size calculated achieved? | 1 = Yes, 2 = No, 3 = Unclear, 4 = NA | ||||
| Confounding? | State potential confounders and if this controlled for in analysis? | ||||
| Outcome Measure 2: | |||||
| Methods of assessing outcome measure | (eg. Phone survey, questionnaire, physical measurements (for each outcome)) | ||||
| Method of follow‐up for non‐respondents | |||||
| Timing of outcome assessment | (including frequency, length of follow up (for each outcome)) | ||||
| Interpretation of scale scores | |||||
| Adverse events | (eg. Complaints, levels of dissatisfaction, adverse incidents, side effects) | ||||
| Statistical analysis performed | e.g. t‐test, ANOVA, ANCOVA, linear regression (for continuous data); Chi2, logistic regression (for dichotomous outcomes); Mann‐Whitney (ordinal data). | ||||
| Were outcome measurement tools validated? | Yes = 1 No = 2 Unclear = 3 |
Methods of validation/supporting literature | |||
| Are the outcome measures reliable? | Yes = 1 No = 2 Unclear = 3 No information = 4 |
Test re‐test – proven with supporting literature? | |||
| Dichotomous outcomes | ||||||||||||||
| Outcome | Timing of outcome assessment | Intervention group | Control group | P values, CIs, Notes | ||||||||||
| Observed (n) | Total (N) | Observed (n) | Total (N) | |||||||||||
| |
||||||||||||||
| |
||||||||||||||
| Continuous outcomes | ||||||||||||||
| Outcome | Timing of outcome assessment | Intervention group | Control group | P values, CIs, Notes | ||||||||||
| Mean/mean change | Standard deviation | N | Mean/mean change | Standard deviation | N | |||||||||
| Non‐parametric outcomes | ||||||||
| Outcome | Timing of outcome assessment | Intervention group | Control group | Notes | ||||
| Median | Inter‐quartile range | N | Median | Inter‐quartile range | N | |||
| OUTCOME 3 | |||||
| Power calculation performed? | 1 = Yes, 2 = No, 3 = Unclear + who did it | ||||
| Sample size calculated achieved? | 1 = Yes, 2 = No, 3 = Unclear, 4 = NA | ||||
| Confounding? | State potential confounders and if this controlled for in analysis? | ||||
| Outcome Measure 3: | |||||
| Methods of assessing outcome measure | (eg. Phone survey, questionnaire, physical measurements (for each outcome)) | ||||
| Method of follow‐up for non‐respondents | |||||
| Timing of outcome assessment | (including frequency, length of follow up (for each outcome)) | ||||
| Interpretation of scale scores | |||||
| Adverse events | (eg. Complaints, levels of dissatisfaction, adverse incidents, side effects) | ||||
| Statistical analysis performed | e.g. t‐test, ANOVA, ANCOVA, linear regression (for continuous data); Chi2, logistic regression (for dichotomous outcomes); Mann‐Whitney (ordinal data). | ||||
| Were outcome measurement tools validated? | Yes = 1 No = 2 Unclear = 3 |
Methods of validation/supporting literature | |||
| Are the outcome measures reliable? | Yes = 1 No = 2 Unclear = 3 No information = 4 |
Test re‐test – proven with supporting literature? | |||
| Dichotomous outcomes | ||||||||||||||
| Outcome | Timing of outcome assessment | Intervention group | Control group | P values, CIs, Notes | ||||||||||
| Observed (n) | Total (N) | Observed (n) | Total (N) | |||||||||||
| |
||||||||||||||
| Continuous outcomes | ||||||||||||||
| Outcome | Timing of outcome assessment | Intervention group | Control group | P values, CIs, Notes | ||||||||||
| Mean/mean change | Standard deviation | N | Mean/mean change | Standard deviation | N | |||||||||
| Non‐parametric outcomes | ||||||||
| Outcome | Timing of outcome assessment | Intervention group | Control group | Notes | ||||
| Median | Inter‐quartile range | N | Median | Inter‐quartile range | N | |||
| The studies’ key conclusions | |
| References to other studies | |
| Eg Contact with author, if study translated, if study duplicate publication |
Data and analyses
Comparison 1. All studies: Interventions that promote informed consent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Informed consent: continuous data | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Patient understanding: continuous data | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Patient self‐report of understanding: continuous data | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Patient self‐report of understanding: dichotomous data | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Knowledge/retention/recall ‐ immediate: continuous data | 26 | 2852 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [0.37, 0.69] |
| 6 Knowledge/retention/recall ‐ short term: continuous data | 16 | 2106 | Std. Mean Difference (IV, Random, 95% CI) | 0.68 [0.42, 0.93] |
| 7 Knowledge/retention/recall ‐ long term: continuous data | 17 | 1353 | Std. Mean Difference (IV, Random, 95% CI) | 0.78 [0.50, 1.06] |
| 8 Knowledge/retention/recall ‐ immediate: dichotomous data | 3 | 331 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.85, 1.60] |
| 9 Knowledge/retention/recall ‐ short term: dichotomous data | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 Knowledge/retention/recall ‐ long term: dichotomous data | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11 Knowledge/retention/recall: non‐parametric data | Other data | No numeric data | ||
| 11.1 Immediate knowledge | Other data | No numeric data | ||
| 11.2 Short‐term knowledge | Other data | No numeric data | ||
| 11.3 Long‐term knowledge | Other data | No numeric data | ||
| 12 Deliberation: continuous data | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13 Decisional conflict: continuous data | 3 | 837 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.46, ‐0.14] |
| 14 General or procedural‐related anxiety: continuous data | 14 | 2069 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.35, 0.13] |
| 15 General or procedure‐related anxiety: dichotomous data | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 16 Anxiety (or other psychological stress) with consent process: continuous data | 13 | 1407 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.21, 0.23] |
| 17 Anxiety (or other psychological stress) with consent process: dichotomous data | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 18 Anxiety (or other psychological stress) with consent process: non‐parametric data | Other data | No numeric data | ||
| 19 Anxiety (or other psychological stress) with decision‐making: continuous data | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20 Satisfaction with consent process: continuous data | 15 | 2024 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.09, 0.32] |
| 21 Satisfaction with consent process: dichotomous data | 10 | 1045 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.97, 1.12] |
| 22 Satisfaction with consent process: non‐parametric data | Other data | No numeric data | ||
| 23 Satisfaction with decision making: continuous data | 8 | 2144 | Std. Mean Difference (IV, Random, 95% CI) | 2.25 [1.36, 3.15] |
| 24 Satisfaction with decision making: dichotomous data | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 25 Pain levels: continuous data | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 26 Pain levels: dichotomous data | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 27 Analgesia use: non‐parametric data | Other data | No numeric data | ||
| 28 Desire for further information: dichotomous data | 4 | 849 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.35, 1.22] |
| 29 Sense of control ‐ locus of control or perception of who made the decision: continuous data | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 30 Sense of control ‐ locus of control or perception of who made the decision: dichotomous data | 3 | 971 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.98, 1.09] |
| 31 Clinician outcome: satisfaction with the consent consultation: continuous data | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 32 Clinician outcome: satisfaction with the consent consultation: dichotomous data | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 33 Systems outcome: rates of uptake (or refusal) of clinical interventions/procedures: dichotomous data | 10 | 3075 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.02] |
| 34 Systems outcome: length of consultations: continuous data | 6 | 517 | Mean Difference (IV, Random, 95% CI) | 1.66 [0.82, 2.50] |
| 35 Systems outcome: economic‐time taken to consent: non‐parametric data | Other data | No numeric data |
Comparison 2. Interventions that promote informed consent: Face‐to‐face interventions and distant interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Distant interventions: knowledge/retention/recall ‐ immediate | 16 | 1175 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [0.32, 0.75] |
| 2 Distant interventions: knowledge/retention/recall ‐ short term | 13 | 1279 | Std. Mean Difference (IV, Random, 95% CI) | 0.79 [0.44, 1.14] |
| 3 Distant interventions: anxiety (or other psychological stress) with consent process | 10 | 642 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.22, 0.32] |
| 4 Distant interventions: systems outcome: length of consultations | 3 | 256 | Mean Difference (IV, Random, 95% CI) | 1.22 [0.23, 2.22] |
| 5 Face to face interventions: knowledge/retention/recall ‐ immediate | 10 | 1677 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [0.28, 0.76] |
| 6 Face to face interventions: knowledge/retention/recall ‐ short term | 3 | 827 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [0.12, 0.59] |
| 7 Face to face interventions: anxiety (or other psychological stress) with consent process | 3 | 765 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.41, 0.25] |
| 8 Face to face interventions: systems outcome: length of consultations | 3 | 261 | Mean Difference (IV, Random, 95% CI) | 2.81 [1.07, 4.55] |
Comparison 3. Interventions to promote informed consent: consent on behalf of a minor and self consent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Consent on behalf of a minor: knowledge/retention/recall ‐ immediate | 3 | 123 | Std. Mean Difference (IV, Random, 95% CI) | 0.55 [0.15, 0.96] |
| 2 Consent on behalf of a minor: anxiety (or other psychological stress) with consent process | 3 | 212 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.30, 0.57] |
| 3 Self‐consent: knowledge/retention/recall ‐ immediate | 23 | 2729 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [0.36, 0.69] |
| 4 Self‐consent: anxiety (or other psychological stress) with consent process | 10 | 1195 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.28, 0.23] |
Comparison 4. Interventions that promote informed consent : Classification of interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Written: knowledge/retention/recall ‐ immediate | 6 | 236 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.17, 0.75] |
| 2 Written: knowledge/retention/recall ‐ short term | 6 | 265 | Std. Mean Difference (IV, Random, 95% CI) | 0.99 [0.33, 1.64] |
| 3 Written: knowledge/retention/recall ‐ long term | 8 | 638 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.21, 0.73] |
| 4 Written: general or procedural‐related anxiety | 3 | 729 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.17, 0.89] |
| 5 Written: anxiety (or other psychological stress) with consent process | 6 | 383 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.38, 0.43] |
| 6 Written: satisfaction with consent process | 6 | 821 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.29, 0.67] |
| 7 Audiovisual: knowledge/retention/recall ‐ immediate | 8 | 644 | Std. Mean Difference (IV, Random, 95% CI) | 0.72 [0.40, 1.04] |
| 8 Audiovisual: knowledge/retention/recall ‐ short term | 4 | 697 | Std. Mean Difference (IV, Random, 95% CI) | 0.73 [0.14, 1.32] |
| 9 Audiovisual: general or procedural‐related anxiety | 5 | 444 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.07, 0.12] |
| 10 Audiovisual: anxiety (or other psychological stress) with consent process | 4 | 259 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.32, 0.47] |
| 11 Audiovisual: satisfaction with consent process | 4 | 627 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.11, 0.21] |
| 12 Audiovisual: satisfaction with consent process (dichotomous) | 4 | 502 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.91, 1.34] |
| 13 Interactive multimedia: knowledge/retention/recall ‐ short term | 3 | 317 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.16, 0.77] |
| 14 Interactive multimedia: satisfaction with consent process | 3 | 348 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.46, 0.92] |
| 15 Structured: knowledge/retention/recall ‐ immediate | 5 | 844 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.16, 0.70] |
| 16 Decision aid: knowledge/retention/recall ‐ immediate | 4 | 673 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [0.26, 1.02] |
| 17 Decision aid: knowledge/retention/recall ‐ short term | 3 | 827 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [0.12, 0.59] |
| 18 Decision aids: satisfaction with decision making | 7 | 1605 | Std. Mean Difference (IV, Random, 95% CI) | 2.64 [1.50, 3.77] |
Comparison 5. Interventions that promote informed consent: Timing of intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Before admission: knowledge/retention/recall ‐ immediate | 10 | 1383 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [0.16, 0.85] |
| 2 Before admission: anxiety (or other psychological stress) with consent process | 10 | 1064 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.33, 0.09] |
| 3 Before admission: satisfaction with consent process | 9 | 1352 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.12, 0.41] |
| 4 Before admission: satisfaction with consent process (dichotomous) | 4 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.94, 1.33] |
| 5 After admission: knowledge/retention/recall ‐ immediate | 15 | 1309 | Std. Mean Difference (IV, Random, 95% CI) | 0.55 [0.40, 0.70] |
| 6 After admission: anxiety (or other psychological stress) with consent process | 3 | 343 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [0.19, 0.62] |
| 7 After admission: satisfaction with consent process | 6 | 672 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.26, 0.46] |
| 8 After admission: satisfaction with consent process (dichotomous) | 6 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.96, 1.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agre 1994a.
| Methods | Single‐centre RCT Three armed trial Data analysed for each intervention separately Presented in this table is information for Intervention A |
|
| Participants | 201 patients attending for endoscopy Recruited between 18 March and 21 October 1992 New York, USA Numbers of participants in analysis: 99 Intervention: 66 Control: 33 (the control group was split between the two arms of this study) |
|
| Interventions |
Intervention A: Audio‐visual, non‐interactive 5 minute video designed for the trial (approved by 5 endoscopy/GI specialists), same content as standardised oral discussion but with graphics to aid explanation Intervention development: designed for the trial with reasonable effort for validation/piloting Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control group characteristics:verbal consent with a check list to ensure all points covered Done with clinician?: distant without clinician Intervention type: audiovisual Time of delivery: on admission |
|
| Outcomes |
Immediate knowledge: continuous data reported 13‐item MCQ (validated in the department through edits by the clinicians working there and then asking 50 patients to pilot who were having colonoscopy) completed 10 minutes after consent signed for procedure Anxiety with consent process: from STAI questionnaire measured 10 minutes after consent ‐ insufficient data reported to permit analysis |
|
| Notes | Aim: to examine the role of videotape in increasing patients' knowledge in preparation for informed consent and to examine whether an increase in knowledge resulted in increased anxiety Conclusion: knowledge improved for intervention A with no significant increase in anxiety |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details: random envelopes prepared by 'Department of Biostats' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details: no details on concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded, unclear whether clinicians blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient details: no details of blinding |
Agre 1994b.
| Methods | Single‐centre RCT Three armed trial Data analysed for each intervention separately Presented in this table is information for Intervention B |
|
| Participants | 201 patients attending for endoscopy Recruited between 18 March and 21 October 1992 New York, USA Numbers of participants in analysis:101 Intervention: 68 Control: 33 (the control group was split between the two arms of this study) |
|
| Interventions |
Intervention B: Interactive multimedia: 5 minute video designed for the trial (approved by 5 endoscopy/GI specialists), same content as standardised oral discussion but with graphics to aid explanation on the video and time given with the endoscopist for further explanation Intervention development: designed for the trial with reasonable effort for validation/piloting Exposure: once Training for delivery of intervention:no details Evaluation of the delivery of intervention: no details Control group characteristics:verbal consent with a check list to ensure all points covered Done with clinician?: face to face Intervention type: interactive multimedia Time of delivery: on admission |
|
| Outcomes |
Immediate knowledge: continuous data reported 13‐item MCQ (validated in the department through edits by the clinicians working there and then asking 50 patients to pilot who were having colonoscopy) completed 10 minutes after consent signed for procedure Anxiety with consent process: from STAI questionnaire measured 10 minutes after consent ‐ insufficient data reported to permit analysis |
|
| Notes | Aim: to examine the role of videotape in increasing patients' knowledge in preparation for informed consent and to examine whether an increase in knowledge resulted in increased anxiety Conclusion: knowledge improved for intervention B with no significant increase in anxiety |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details: random envelopes prepared by 'Department of Biostats' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details: no details on concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded, unclear whether clinicians blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient details: no details of blinding |
Armstrong 1997.
| Methods | RCT comparing usual (verbal) consent to showing patient a list of potential side‐effects for cosmetic, hand and minor skin tumour surgery | |
| Participants | Patients undergoing cosmetic surgery/elective hand surgery/excision of minor skin tumours Sheffield, United Kingdom Numbers of participants in analysis: 269 Intervention: 137 Control: 132 |
|
| Interventions | Written information sheet with lists of 7 risks, signed by patient to confirm understanding and retained in patient's notes Intervention development: no details Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: written Time of delivery: on admission |
|
| Outcomes | Short‐term knowledge: Recall of risks recorded in post‐op interview by nurse experienced in plastic surgery, at mean of 9 days post surgery (data extracted on recall of “Wound” taken from table 5, this was the median effect size), dichotomous data | |
| Notes | Aim: To determine if written pre‐operative warnings about risks of surgery improve patients recall of risks post‐operatively compared with a group given verbal warning alone Conclusion: Post‐op recall of patients given written warning sheets was improved, although the relevance of the difference in recall between the two groups of fewer than 1 warning is debatable |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Odd/even hospital numbers ‐ quasi‐randomisation |
| Allocation concealment (selection bias) | High risk | Allocation predictable because of method of random sequence generation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measured by nurse who was blinded to allocation |
Armstrong 2010.
| Methods | RCT to evaluate the effectiveness of video information on patients' immediate knowledge for either punch or shave biopsy | |
| Participants | Patients attending a dermatology clinic from July 2009 to February 2010 USA Numbers of participants in analysis: 84 Intervention: 42 Control: 42 |
|
| Interventions | Dermatologists used an MP3 video (3.5 screen‐ipod touch or iphone 3) to show an education video detailing the 3 aspects of a skin biopsy Intervention development: no detail Exposure: once Training for delivery of intervention: brief training Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: audio‐visual Time of delivery: on admission |
|
| Outcomes | Immediate knowledge | |
| Notes | Aim: to determine if a video‐based education delivered through mobile, video devices improves patient knowledge and satisfaction in the informed consent and post op educational processes compared with conventional verbal instruction Conclusion: study demonstrated a significant increase in knowledge score following video education, but not following oral education. Although group comparisons did not achieve statistical significant, portable video media for presenting informed consent and wound care instructions for skin biopsies appear to be more effective and result in higher satisfaction than traditional oral education |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Performed using the Graphpad Prism 2009 statistical software |
| Allocation concealment (selection bias) | Low risk | Group allocation sequence was kept in sealed envelopes until each participant was ready to be randomised |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Protocol published and checked for consistency, all outcomes reported |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unclear if participants knew that they were in a study or not Dermatologists were aware of group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Author email reply states outcome measurement blinded, stated outcome measurement researcher blinded to randomisation |
Ashraff 2006.
| Methods | RTC ‐ 2 groups, leaflet posted 2 weeks before admission, recall of information tested at admission | |
| Participants | Elective orthopaedic patients under going THR, TKR, knee arthroscopy, shoulder surgery inc shoulder decompression, shoulder replacement and Bankarts Repair Numbers of participants in analysis: 110 Intervention: 57 Control: 53 |
|
| Interventions | A4 information leaflet post 2 weeks prior to admission, describing operation and included a list of complications and information re post op care such as length of stay and return to work Intervention development: no details Exposure: once Training for delivery of intervention: non needed Evaluation of the delivery of intervention: no details Control group characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes | Short‐term knowledge: score out of 10, assessed on admission to the ward, 2 weeks after receiving the leaflet | |
| Notes | Aim: to determine whether patient information leaflets help to improve patient recall during the process of informed consent Conclusion: patient information leaflets are a useful tool for the surgeon to improve the recall of the information given to the patient, in order to facilitate informed consent Author contacted: yes, no further data available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Questionnaire, but unclear if anonymously assessed Insufficient detail |
Astley 2008a.
| Methods | 3 armed randomised controlled trial Data analysed for each intervention separately Presented in this table is information for Intervention A (written information) |
|
| Participants | Patients were undergoing coronary angiography. All completed primary end point, immediately after procedure (> 4hrs and < 24hrs), but 10 were lost to follow‐up at 30 days and 2 died before 30 day follow‐up (from the three arms) Australia Numbers of participants in analysis: 50 Intervention: 34 Control: 16 |
|
| Interventions | Content of all interventions standardised by a coronary angiogram risk pro forma, developed at Flinders medical centre with input from the cardiology and cardiac surgery departments. This formed checklist for verbal, text for written and script for audio visual technique After receiving information, followed up by doctor who asked for and fielded questions from the patient, prior to consent Prior to the patient signing the consent form the same doctor verbally explained the process of coronary angioplasty and stenting in case this intervention became part of the same procedure Intervention development: designed for the trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control group characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: written Time of delivery: on admission |
|
| Outcomes |
Immediate, short‐term, long‐term knowledge: assessed at < 4 hrs, 4 to 24 hours and 30 days (median score out of 12) non‐parametric data Satisfaction with the consent process: assessed at < 4 hrs, 4 to 24 hours and 30 days (5 point Likert scale, 5 = satisfied) Anxiety with the consent process: assessed at < 4 hrs, 4 to 24 hours and 30 days (5 point Likert scale 5 = anxious) |
|
| Notes | Aim: to compare verbal, written and animated audiovisual information prior to coronary angiography by assessing risk recall, satisfaction and anxiety Conclusion: interventions had no effect on recall, anxiety or satisfaction |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomised in 1:1:1 ratio Balanced randomisation generated via computer program |
| Allocation concealment (selection bias) | Low risk | Computer print‐out enabled study staff to implement next allocation in sequence, research subjects and staff were unable to view print‐out |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All completed primary outcome of recall post procedure 10 lost to follow up at 30 days and 2 died; attrition less than 40% |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and clinicians were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail Outcomes data collected by 2 trained study staff from the Flinders Medical Centre, cardiovascular outcomes research unit, unclear if blinded |
Astley 2008b.
| Methods | 3 armed randomised controlled trial Data analysed for each intervention separately Presented in this table is information for Intervention B (audiovisual information) |
|
| Participants | Patients were undergoing coronary angiography. All completed primary end point, immediately after procedure (> 4hrs and < 24hrs), but 10 were lost to follow‐up at 30 days and 2 died before 30 day follow‐up (from the three arms) Australia Numbers of participants in analysis: 49 Intervention: 33 Control: 16 |
|
| Interventions | Content of all interventions standardised by a coronary angiogram risk pro forma, developed at Flinders medical centre with input from the cardiology and cardiac surgery departments. This formed checklist for verbal, text for written and script for audio visual technique Audiovisual video was produced at Flinders medical centre and designed to include four types of memory cues: photographic explanation, demonstration, animation, and bullet point text After receiving information, followed up by doctor who asked for and fielded questions from the patient, prior to consent Prior to the patient signing the consent form the same doctor verbally explained the process of coronary angioplasty and stenting in case this intervention became part of the same procedure Intervention development: designed for the trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: audiovisual Time of delivery: on admission |
|
| Outcomes |
Immediate, short‐term, long‐term knowledge: assessed at < 4 hrs, 4 to 24 hours and 30 days (median score out of 12) non‐parametric data Satisfaction with the consent process: assessed at < 4 hrs, 4 to 24 hours and 30 days (5 point Likert scale , 5 = satisfied) Anxiety with the consent process: assessed at < 4 hrs, 4 to 24 hours and 30 days (5 point Likert scale 5 = anxious) |
|
| Notes | Aim: to compare verbal, written and animated audiovisual information prior to coronary angiography by assessing risk recall, satisfaction and anxiety Conclusion: interventions had no effect on recall, anxiety or satisfaction |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomised in 1:1:1 ratio Balanced randomisation generated via computer program |
| Allocation concealment (selection bias) | Low risk | Computer print‐out enabled study staff to implement next allocation in sequence, research subjects and staff were unable to view print‐out |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All completed primary outcome of recall post procedure 0 lost to follow up at 30 days and 2 died; attrition less than 40% |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and clinicians were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail Outcomes data collected by 2 trained study staff from the Flinders Medical Centre, Cardiovascular outcomes research unit, unclear if blinded |
Bekker 2004.
| Methods | RCT evaluating decision analysis for Down Syndrome screening, measuring outcomes immediately after intervention and one month later | |
| Participants | Pregnant mothers having received a positive (> 1 in 250) maternal serum screening test (MSS) over a 15 month period Leeds, United Kingdom 178 assessed for eligibility, of whom 46 not eligible & 15 declined; 117 randomised, intervention 59, control 58 (See incomplete outcome section of risk of bias table for further details regarding attrition rates) Numbers of participants in analysis: 106 Intervention: 50 Control: 56 |
|
| Interventions | Routine information, plus decisional analysis consisting of 3 components
Intervention development: designed for the trial with no validation Exposure: once Training for delivery of intervention: all delivered by key researcher Evaluation of the delivery of intervention: no details Control group characteristics: verbal consent Done with clinician?: face to face Intervention type: decision aid Time of delivery: before admission |
|
| Outcomes |
Deliberation: 'seeking information' score from coding of themes from consultation transcripts. 'Seek info' outcome used which is on scale 0 to 7, higher score is ‘good’ Immediate knowledge: MCQ questionnaire immediately after initial consultation (after intervention) Long‐term knowledge: MCQ questionnaire at one month follow‐up (after results of further investigations have been given to patient) Satisfaction with decision making: 'effective' arm of decisional conflict score, at 1 month follow‐up Length of consultation: initial consultation Rates of uptake: patients' notes checked for diagnostic test results Generalised anxiety: short form STAI (20‐80, low‐high anxiety), measured at initial appointment Satisfaction with consent process: "overall, how useful was the information given during this consultation?", measured at initial consultation and at follow‐up at 1 month following receipt of results of diagnostic tests. Initial consultation assessed to be appropriate measure for our review as closer in time to intervention, and less effected by results of diagnostic tests. |
|
| Notes | Aim: to evaluate decisional analysis as a technique to facilitate women's decision making about prenatal diagnosis for Down Syndrome using measures of effective decision making Conclusion: decision analysis consultations enabled women to make more informed prenatal diagnosis decisions. Informed decision making was higher, perceived risk more realistic, and decisional conflict lower in the intervention group. Intervention had no impact on knowledge or subjective expected utility scores, and was no more or less directive, useful or anxiety provoking than control. Consultations were six minutes longer in intervention group Author contacted: yes, data used for Risk of Bias table |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated.The method of randomisation was a random number generator programme (SPSS) carried out in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Previously numbered, sealed opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full details on this in data ‐ exclusion between randomisation and time 1 (T1) was 15% in intervention, 3% in control. Attrition rate at 1 month (T2) was 42% in intervention, 30% in control. Fully documented and analysed with no statistical difference between return rates in groups Overall attrition not > 40% |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not told which consultation was routine. Personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Questionnaires were completed by patients without the researcher present. The questionnaires were entered onto the computer with their study number identified. At the final stage of analysis, the SN and arm allocation were matched to reveal the trial group |
Bennett 2009a.
| Methods | RCT with two intervention arms (teach‐the‐teacher/diagrams) and one control Data analysed for each intervention separately versus half of the control group Presented in this table is information for Intervention A versus Control group This entry is for Intervention A: TEACH THE TEACHER group |
|
| Participants | 109 eligible participants, 10 declined, therefore 99 patients undergoing a spinal injection (epidural steroid, nerve root or facet joint injection) in musculoskeletal radiology. Iowa, USA Numbers of participants in analysis: 50 Intervention: 34 Control: 16 (half the number of total control group to allow for analysis of each intervention arm) |
|
| Interventions |
Intervention A: Teach the teacher group, had to verbally repeat the twelve key points without error to the physician before the patient could sign the informed consent form Intervention development:designed for trial with no validation Exposure: once Training for delivery of intervention: brief training Evaluation of the delivery of intervention: no details Control group characteristics: verbal consent and used a checklist Done with clinician?: face‐to‐face Intervention type: decision aid Time of delivery: on admission |
|
| Outcomes |
Immediate knowledge: MCQ scored out of 10, with negative marking Length of consultation: time in minutes Generalised anxiety: after procedure, patients asked to rank calmness from 1 to 10 (1 = completely calm). Lacking data to derive SDs from data, therefore information cannot be used in the meta‐analysis. TTT 3/10, control 2/10. Pain: after procedure, patients asked to rank during the procedure from 1 to 10 (1 = no pain). Cannot include in the meta‐analysis with not enough raw data available. Control 2/10, TTT 2/10. On a scale of 1 to 10 with 1 being completely painless. |
|
| Notes | Aim: a method using diagrams will improve patient‐physician communication without increasing the time required to obtain informed consent over the teach the teacher method as well as over current informed consent protocol. Conclusion: diagram method was most successful requiring less time than the teach the teacher method and had no negative correlation with age, and had improved survey scores over the control group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Research assistant randomly assigned patient to groups in a rotatory basis |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient detail |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
Bennett 2009b.
| Methods | RCT with two intervention arms (teach‐the‐teacher/diagrams) and one control. Data analysed for each intervention separately versus half of the control group. Presented in this table is information for Intervention B versus Control group. This entry is for Intervention B: Diagram group. |
|
| Participants | 109 eligible participants, 10 declined, therefore 99 patients undergoing a spinal injection (epidural steroid, nerve root or facet joint injection) in musculoskeletal radiology. Iowa, USA Numbers of participants in analysis: 48 Intervention: 32 Control: 16 (half of the total control group) |
|
| Interventions |
Intervention B: Diagram group viewed a set of diagrams illustrating the twelve key points before signing the informed consent form. Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: brief training Evaluation of the delivery of intervention: no details Control group characteristics: verbal consent and used a checklist Done with clinician?: distant without clinician Intervention type: written Time of delivery: on admission |
|
| Outcomes |
Immediate knowledge: MCQ scored out of 10, with negative marking Length of consultation: time in minutes Generalised anxiety: after procedure, patients asked to rank calmness from 1 to 10 (1 = completely calm). Lacking data to derive SDs, therefore information cannot be used in the meta‐analysis. Diagram and control group both scored 2/10. Pain: after procedure, patients asked to rank during the procedure from 1 to 10 (1 = no pain). Cannot include in the meta‐analysis with insufficient raw data. Control 2/10, diagram 3/10. |
|
| Notes | Aim: a method using diagrams will improve patient‐physician communication without increasing the time required to obtain informed consent over the teach the teacher method as well as over current informed consent protocol. Conclusion: diagram method was most successful requiring less time than the teach the teacher method and had no negative correlation with age, and had improved survey scores over the control group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Research assistant randomly assigned patient to groups in a rotatory basis |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient detail |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
Bollschweiler 2008.
| Methods | 4‐centre RCT comparing standard methods of consent to the addition of a multimedia program to aid consent for laparoscopic cholecystectomy. | |
| Participants | 80 patients were recruited and 76 randomised to intervention or control. Patients attending 1 of 4 hospitals were randomised usually day before surgery to either group. Different levels of education between 2 groups were noted and therefore were not comparable at baseline Germany Numbers of participants in analysis: 76 Intervention: 35 Control: 41 |
|
| Interventions | Computer program (MM‐IP) which was developed over 3 years by a team of medics, linguist and psychologists and evaluated by patients and staff. High quality. Based on powerpoint, with progressive levels of information for patients from easy to harder. Intervention development: designed for trial with reasonable effort for validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: evidence of fidelity/reliability of delivery Control characteristics: verbal consent and used a special leaflet Done with clinician?: distant without clinician Intervention type: non‐interactive audio‐visual Time of delivery: on admission |
|
| Outcomes | After informed consent process completed, questionnaires were given to both groups. No details of timing Self‐report of understanding: of general information and risks of surgery, using a previously validated tool (VAS validated version). The report asked 8 questions of self‐perceived knowledge and it is difficult to take an average score. The paper summed the total and used this in secondary analysis against education scores ‐ mean and SD of each group's total score not available in report Satisfaction with consent process: evaluated using VAS (also validated) scores Statistics note for satisfaction with consent process ‐ took question 1 as representative and most relevant General anxiety: with the process, measured after consent process using KASA scores (also validated) Statistics note for general anxiety ‐ SDs obtained from SEM |
|
| Notes | Aim: patients' perception of their understanding of important aspects of their illness (disease, therapeutic alternatives, operation, risks) and satisfaction with the consenting process Conclusion: before an elective procedure, patients using an MM‐IP as a supplement to the traditional informed consent process feel better informed about their disease and its treatment than patients going through the traditional consent process alone. Improved patient understanding of illness is the basis of the informed consent process. Personal consultation with the physician and written informed consent remains indispensable to this process |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central allocation, random list |
| Allocation concealment (selection bias) | Low risk | Centrally allocated, random process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rate (lost 4/80) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | High risk | Groups not comparable for education level, not accounted for in analysis |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
Chan 2002.
| Methods | RCT comparing written information with illustrations plus usual consent to usual consent alone. | |
| Participants | 125 consecutive patients of greater than 16 years of age, who were undergoing thyroidectomy or parotidectomy. Four patients excluded from analysis as lost to follow‐up Toronto, Canada Numbers of participants in analysis: 121 Intervention: 56 Control: 65 |
|
| Interventions | Intervention: an information leaflet with written information and illustrations plus usual consent. Control: Usual consent whereby surgeons were given a specific checklist of risks to outline to the patient according to the planned surgical procedure with an equal emphasis on each risk. During the consent process in both groups any additional questions from patients were answered and patients were asked to sign a standard surgical consent document. Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent and used a checklist Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes | Long‐term knowledge: Average rate of risk recall ‐ performed 3 to 7 weeks after consultation by telephone interview where patients were asked to recall specific risks of their operation. Thyroidectomy scored out of 3, parotidectomy scored out of 4 | |
| Notes | Aim: to examine the effects of an educational intervention on patient knowledge and recall of possible risks from parotidectomy or thyroidectomy. Conclusion: study found that a patient's ability to recall potential complications of surgery was significantly increased by the intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail. Patients were 'randomised'. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40% |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail: Unclear who outcomes assessors were |
Chantry 2010.
| Methods | Single‐centre RCT assessing videotape intervention to improve informed consent for neonatal circumcision, control group watched placebo video followed by traditional 1:1 informed consent discussion in both groups with physician about risks and benefits | |
| Participants | 579 Mothers on a labour ward who were thinking about neonatal circumcision of whom 306 were randomised. 2 were excluded from analysis as trial group not recorded. 304 included in analysis. English speaking. Convenience sample USA Numbers of participants in analysis: 304 Intervention: 168 Control: 136 |
|
| Interventions | 11‐minute videotape giving explanation of risks and benefits as listed in the 1999 AAP Circumcision Policy Statement. Also included interviews with parents of older infants who had opted for and against newborn circumcision. Used still photographs depicting the procedure as part of this accompanied by narration. Produced for the trial, moderate quality. Watched through without navigation menu, approximately 1 to 2 hours after enrolment into trial Placebo video was regarding breast feeding produced by the Supplemental Nutrition Program for Women, Infants and Children, watched at similar time Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: non‐interactive audio‐visual Time of delivery: before admission |
|
| Outcomes |
Satisfaction with consent process: verbal interview with 4 yes/no questions and a 5‐point Likert scale. Gave composite mean satisfaction score where yes = 1, no = 0 for questions, and on Likert scale satisfied/very satisfied = 1 point (all other responses = 0). Not validated. Assessed after intervention, approximately 1 day. Secondary analysis of satisfaction was done 1 month post‐discharge but not included since no data available Short‐term knowledge: assessed by 10‐point composite knowledge score administered verbally (post‐intervention, approx 1 day later, before discharge). Not validated. t‐test analysis on composite score out of total 10 Perception of risk‐provider bias ‐ not used in this review |
|
| Notes | Aim: to determine if videotapes about newborn circumcision would be superior to traditional physician 'informed consent' discussion for maternal knowledge, satisfaction and perception of provider bias Conclusion: videotaped information and an opportunity to ask questions was equivalent to provision of standard informed consent by a physician for maternal knowledge and satisfaction for the group of mothers in the study. Similar perception of provider bias for both groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was achieved by ordering envelopes which contained a descriptor of the video to which the mother was assigned, chosen by roll of the dice |
| Allocation concealment (selection bias) | High risk | Uneven group size resulted from inadvertent use of a series of 20 envelopes not previously randomised, this was picked up quickly indicating concealment concerns. Unclear whether used opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In immediate knowledge scores each question is missing 1 or 2 answers (minimal). At follow‐up, 43% control and 47% intervention lost to follow‐up, so greater than 40% attrition |
| Selective reporting (reporting bias) | High risk | Data collected in a telephone follow‐up that was not reported. Asked 14 knowledge questions verbally but included results on 10 |
| Other bias | High risk | Convenience sampling, high contamination rates (all on same labour ward), fidelity issues with 18 different clinicians used with differing training and skills |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Verbally administered survey, outcome assessors not blinded |
Cornoiu 2010a.
| Methods | Single centre RCT comparing the use of multimedia presentations, pamphlets and standard verbal consent when consenting patients for knee arthroscopy surgery. For the purposes of this review, the study has been split in two: Pamphlet versus Control and Interactive Multimedia versus Control. The N value for the control was divided by 2 |
|
| Participants | 100 eligible participants undergoing knee arthroscopy, of whom 39 were excluded with the main reason being due to poor English skills. 61 patients were randomised with 22 participants in the Multimedia group, 21 in the pamphlet group and 18 in the control group. Melbourne, Australia Numbers of participants in analysis: 31 Intervention: 22 Control: 9 |
|
| Interventions |
This study entry compares the standard verbal consent to the interactive multimedia group. Prior to study a literature review of the published complications of knee arthroscopy was performed. Based on this review, consensus was reached between the authors for the average risk of each complication, to be presented in the pre‐operative information. A focus group of patients who had previously undergone knee arthroscopy was also undertaken to determine what information they would like to have been told prior to surgery. From this a list was derived that would form the core content for the verbal consent, the pamphlet and the multimedia intervention. Standard Verbal Consent consisted of a verbal consent script developed using the core information derived from the literature review and focus group. This ensured that all patients received the same quality and amount of information. The multimedia presentation consisted of an educational module including a mixture of voice, text, photographs and 3D computer animation, which was revised after piloting. The text included was identical to the verbal consent script, with the speech reflecting this and no extra information. The animation and audio tracks were integrated with appropriate text into an interactive linear program that allowed participants to progress and review information as desired. Intervention development:designed for trial with reasonable effort for validation/piloting Exposure: once Training for delivery of intervention: brief training Evaluation of the delivery of intervention: no details Control characteristics: verbal consent and used a checklist Done with clinician?: distant from clinician Intervention type: non‐interactive audio‐visual Time of delivery: before admission |
|
| Outcomes |
Immediate, short‐term and long‐term knowledge: assessed 3 to 6 weeks prior to surgery, after receiving intervention, on the day of surgery and 6 weeks after surgery. Assessed using a 10‐question knowledge quiz. In the paper only the data for immediate knowledge was presented in a numerically extractable form Satisfaction with consent process: assessed using a 4 question survey with Likert scales relating to satisfaction with the amount, method and content of the information provided during the consent process. There were 5 possible responses to the questionnaire: Strongly agree (2 points), Agree (1 point), Undecided (0 points), Disagree (‐1 point), Strongly disagree (‐2 points). For the four questions the maximum response could be 8 and lowest –8 Anxiety: assessed 3 to 6 weeks prior to surgery, after receiving intervention, on the day of surgery and 6 weeks after surgery. assessed using an abbreviated mental state score and the Stait Trait Anxiety Index. For purposes of our review, assessment immediately after receiving intervention deemed to be "Anxiety with the consent process" and assessment on day of surgery deemed to be "general anxiety" |
|
| Notes | Aim: to compare the efficacy of a computer based multimedia presentation against standard verbal consent and information pamphlets for patients considering knee arthroscopy surgery Conclusion: delivery of information using a combination of high quality computer animation, voice and text in this study appeared to provide improved patient understanding of surgery and complications. Higher satisfaction in the multimedia and verbal groups compared to those in the pamphlet arm. Anxiety levels were not significantly different between any groups Author contacted: yes, contact was successful and original data were obtained for knowledge outcomes ‐ original values and standard deviations obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by numbered ball allocation |
| Allocation concealment (selection bias) | Low risk | Not stated in paper, but author contact: "Randomisation was carried out by a numbered ball method placed in a box which the patient drew from. The number on the ball corresponded to the group which the patient would be allocated to (pamphlet, verbal or multimedia PC). There were only 3 balls and the patient was enrolled to the study prior to them choosing the ball. The research team did not have any direct influence on the allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | Potential risk of bias due to amount of information provided by treating surgeon at first consultation before entry into study. However, retrospective review revealed even spread of patients through all three consent groups with no correlation between surgeon and recall responses. Unlikely to have biased results |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No data in paper, author contact: the resident in the clinic administered the initial questionnaire. The preoperative questionnaire and post‐operative questionnaire were administered by the research staff. They were not formally blinded to which group the patient belonged however the patient filled in the questionnaire without any direct intervention from the staff (except for being given instructions that all questions must be answered) The main analyst was not blinded, however the statistician was. |
Cornoiu 2010b.
| Methods | Single centre RCT comparing the use of interactive multimedia presentations, pamphlets and standard verbal consent when consenting patients for knee arthroscopy surgery. For the purposes of this review, the study has been split in two: Pamphlet versus Control and Interactive Multimedia versus Control. The N value for the control was divided by 2. |
|
| Participants | 100 eligible participants undergoing knee arthroscopy, of whom 39 were excluded with the main reason being due to poor English skills. 61 patients were randomised with 22 participants in the Multimedia group, 21 in the pamphlet group and 18 in the control group. Melbourne, Australia Numbers of participants in analysis: 30 Intervention: 21 Control: 9 (half of the control group) |
|
| Interventions |
This study entry compares the standard verbal consent to the pamphlet group. Prior to study a literature review of the published complications of knee arthroscopy was performed. Based on this review, consensus was reached between the authors for the average risk of each complication, to be presented in the pre‐operative information. A focus group of patients who had previously undergone knee arthroscopy was also undertaken to determine what information they would like to have been told prior to surgery. From this a list was derived that would form the core content for the verbal consent, the pamphlet and the multimedia intervention. Standard Verbal Consent consisted of a verbal consent script developed using the core information derived from the literature review and focus group. This ensured that all patients received the same quality and amount of information. The pamphlet consisted of a single A4 page of 12 point Arial font with no pictures. Plain English at a grade 8 reading level was used, outlining the procedure and post‐operative course, with most detail clarifying the possible risks and complications of the procedure. Intervention development: designed for trial with reasonable effort for validation /piloting Exposure: once Training for delivery of intervention: brief training Evaluation of the delivery of intervention: no details Control characteristics: verbal consent and used a checklist Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes |
Immediate, short‐termand long‐termknowledge: assessed 3 to 6 weeks prior to surgery, after receiving intervention, on the day of surgery and 6 weeks after surgery. Assessed using a 10 question knowledge quiz. In the paper only the data for immediate knowledge was presented in a numerically extractable form Satisfaction with consent process: assessed using a 4 question survey with Likert scales relating to satisfaction with the amount, method and content of the information provided during the consent process. There were 5 possible responses to the questionnaire: Strongly agree (2 points), Agree (1 point), Undecided (0 points), Disagree (‐1 point), Strongly Disagree (‐2 points). For the four questions the maximum response could be 8 and lowest –8. Anxiety: assessed 3 to 6 weeks prior to surgery, after receiving intervention, on the day of surgery and 6 weeks after surgery. assessed using an abbreviated mental state score and the Stait Trait Anxiety Index. For purposes of our review, assessment immediately after receiving intervention deemed to be "Anxiety with the consent process" and assessment on day of surgery deemed to be "General Anxiety" |
|
| Notes | Aim: to compare the efficacy of a computer‐based multimedia presentation against standard verbal consent and information pamphlets for patients considering knee arthroscopy surgery Conclusion: delivery of information using a combination of high quality computer animation, voice and text in this study appeared to provide improved patient understanding of surgery and complications. Higher satisfaction in the multimedia and verbal groups compared to those in the pamphlet arm. Anxiety levels were not significantly different between any groups Author contacted: yes, contact was successful and original data were obtained for knowledge outcomes ‐ original values and standard deviations obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by numbered ball allocation |
| Allocation concealment (selection bias) | Low risk | Not stated in paper, but author contact: "Randomisation was carried out by a numbered ball method placed in a box which the patient drew from. The number on the ball corresponded to the group which the patient would be allocated to (pamphlet, verbal or multimedia PC). There were only 3 balls and the patient was enrolled to the study prior to them choosing the ball. The research team did not have any direct influence on the allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | Potential risk of bias due to amount of information provided by treating surgeon at first consultation before entry into study. However, retrospective review revealed even spread of patients through all three consent groups with no correlation between surgeon and recall responses. Unlikely to have biased results |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No data in paper, author contact: The resident in the clinic administered the initial questionnaire. The preoperative questionnaire and post‐operative questionnaire were administered by the research staff. They were not formally blinded to which group the patient belonged however the patient filled in the questionnaire without any direct intervention from the staff (except for being given instructions that all questions must be answered). The main analyst was not blinded, however the statistician was. |
Cowan 2007.
| Methods | RCT comparing a video intervention in either Spanish or English language as appropriate, versus routine discussion for intravenous contrast media with their Emergency Physician. Patients watched DVD at their bedside prior to CT scan in the Emergency Department | |
| Participants | 202 enrolled participants of whom 49 were excluded and 41 refused participation. Total of 112 randomised, of which 107 completed the study. Intervention 53, control 54 USA Numbers of participants in analysis: 107 Intervention: 53 Control: 54 |
|
| Interventions | IV contrast media video developed by panel of 4 Emergency Physicians, based on expert opinion, pharmaceutical package inserts and current radiology literature. Videos were 5 minutes long, contained information on risks, benefits and alternatives of IVC. Language level was 8th grade level. Following watching DVD, patients were given the opportunity to speak to their Physician with any questions Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: brief training Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant from clinician Intervention type: non‐interactive audio‐visual Time of delivery: on admission |
|
| Outcomes |
Immediate knowledge: assessed by 10 question MCQ questionnaire ‐ questions structured by the same physicians who made the video, and was administered immediately after the consultation Satisfaction with the consent process: assessed by 4‐point ordinal satisfaction scale. Excellent and good dichotomised to satisfied, poor and fair dichotomised to not satisfied. Satisfaction rated immediately after consultation Rates of uptake: assessed by refusal to sign consent form |
|
| Notes | Aim: to determine whether Spanish & English educational videos are superior to routine discussion for informing emergency department patients about risks, benefits and alternatives to receiving IV contrast for CT Conclusion: use of Spanish and English educational videos was superior to routine informed consent. Video assisted informed consent may also increase patient satisfaction with the informed consent process Author contacted: yes, data available for risk of bias table and SDs for immediate knowledge scores |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated using a standard computer generated block randomisation routine available on‐line at www.randomization.com |
| Allocation concealment (selection bias) | Low risk | Group assignments placed in sealed opaque envelopes, sequentially opened after patients signed informed consent for the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data accounted for, only 5 patients withdrew post‐randomisation (clarified by author email) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Specifically states not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Pilot of this study was blinded in this respect. Unable to assume study itself was also blinded |
Danino 2006.
| Methods | Patients were given standard information on the surgical procedure and at a second consultation were randomised into the two groups during which they received a surgical consultation and examination. Both consultations were carried out by the surgeon. The intervention group then used the CD‐ROM delivered by a psychologist | |
| Participants | All patients attending for aesthetic abdominoplasty between September 2002 and April 2004 France Numbers of participants in analysis: 60 Intervention: 30 Control: 30 |
|
| Interventions | CDROM with images explaining the procedure, risks and usual results. Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal and used standardised leaflet Done with clinician?: distant from clinician Intervention type: non‐interactive audio‐visual Time of delivery: before admission |
|
| Outcomes |
Long‐term knowledge Anxiety with consent process: STAI General anxiety: STAI day of the operation Economic: time taken to consent No data reported in paper, only: “the consultation time between the patient and surgeon was not statistically significant between the two groups (+/‐ 3mm)” |
|
| Notes | Published in French, translated by Elinor Farrell and checked by Katy Wilkinson Aim: the aim was to explore the effect of the introduction of images on CD‐ROM on the knowledge and anxiety of the patient before abdominal plastic surgery Conclusion: the introduction of images on CD‐ROM has a beneficial effect on preoperative anxiety on patients undergoing abdominoplasty for aesthetic reasons |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail. States that they were randomly assigned, no further detail |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient detail |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
Deyo 2000.
| Methods | Multicentre RCT, comparing video disc and written booklet versus booklet alone | |
| Participants | 393 elective candidates for lower back surgery were randomised. Patients were identified from referrals to neurosurgery and primary care clinics. Patients from Iowa were selected by study surgeons if they believed that lumbar surgery was a treatment option and if they had received non surgical therapy for greater than 4 weeks. Participants were comparable at baselines, age, gender, ethnicity, education level, married/living as married, employment and smoker/non‐smoker demographics measured Seattle, USA Numbers of participants in analysis: 343 Intervention: 171 Control: 172 |
|
| Interventions | Video disc plus written booklet versus written booklet alone. Patients in the intervention groups used an interactive programme on a videodisk player, modified micro‐computer, monitor with touch screen and printer. They entered their ages and diagnoses (herniated disc, spinal stenosis or non‐specific back pain) into the session and then viewed material specific to their condition. The video programme included animated graphics of spinal anatomy, discussion of causes of back pain, ambiguities in diagnosis, interviews with patients including good and bad outcomes. Subjects were able to control the order of the presentation, repeat segments and obtain further information on other topics. They then were given a printed copy of outcome probabilities and the surgeon was informed of segments watched Control group received a written booklet containing illustrations of the lumbar spine, discussion of surgical and non‐surgical treatments for herniated disks and spinal stenosis, general description of expected outcome and a short self test Intervention development: designed for trial with reasonable effort for validation/piloting Exposure: once Training for delivery of intervention: brief training Evaluation of the delivery of intervention: evidence of fidelity/reliability of delivery Control characteristics: verbal with special leaflet Done with clinician?: distant from clinician Intervention type: interactive audio‐visual Time of delivery: before admission |
|
| Outcomes |
Satisfaction with decision making: information taken from satisfaction with decision making process, table 3. Five questions were taken to include satisfaction with consent therefore the median effect size was taken as per protocol Locus of control: information was taken from table 3. Three questions were felt to reflect locus of control, therefore the question with the median effect size was selected as per protocol Pain: both back and leg pain results were combined as these were both felt to be clinically important. An average pain answer was calculated from given percentages, and used to work out the N of people who would have pain for this hypothetical average question Rate of uptake of surgery: telephoned patients at 3 months and 1 year Economic resource use: information was only available for one of the study sites (Group Health Coop‐Seattle)stated that there were no significant differences in the number of physician visits or physical therapy visits for low back pain, spine imaging studies, pharmacy or laboratory use between the two groups. The only significant difference in utilization was for outpatient back surgery amongst patients with herniated discs; the proportions of subjects undergoing surgery was significantly lower in the video group |
|
| Notes | Aim: to determine the impact of shared decision making on patient satisfaction outcomes and choices of surgical or non‐surgical treatment Conclusion: the programme appears to facilitate decision making and may help ensure informed consent. For patients with herniated discs it reduced the surgery rate without diminishing patient outcomes. its impact on cost of care depends on the proportion of patients with various diagnoses and on local surgery rates Author contacted: yes, no further data available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation |
| Allocation concealment (selection bias) | Low risk | Numbered opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40% |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | High risk | Surgeons in the trial had already viewed both sources of information. Possible contamination. Utlisation of services data used to check service use, however comprehensive utilisation data were unavailable for one of the study sites |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
Elfant 1995.
| Methods | RCT‐ single centre, looking at the effect of time on recall of elements of informed consent | |
| Participants | 60 patients scheduled to undergo elective colonoscopy or endoscopy. Unclear if groups were comparable at baseline although age and gender were recorded New Jersey, USA Numbers of participants in analysis: 60 Intervention: 30 Control: 30 |
|
| Interventions | Time‐standard care was informed consent on the day of the procedure, intervention was informed consent 24 to 72 hours prior to the procedure. One clinical investigator consented both groups, no standardised sheet was used. The same content was used, timing of the consent information was the only difference between the groups Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: face‐to‐face Intervention type: alteration of timing Time of delivery: before (intervention group) and on admission (control group) |
|
| Outcomes | Short‐term knowledge: total recall of consent information over the telephone‐short term (1 to 3 days after the procedure) | |
| Notes | Aim: to determine if recall of informed consent is affected by the timing of obtaining informed consent before endoscopic procedures Conclusion: despite similar rates of recall at follow‐up, we recommend pre‐procedure discussion and obtaining informed consent a few days before the procedure whenever possible |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly assigned to one of two groups. No other information given |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Used one investigator, no standardised script used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail. Post‐op telephone recall was done by another investigator who may have been blinded but no information was given |
Enzenhofer 2004.
| Methods | Patients admitted to the ward had a conversation with the physician, +/‐ intervention and then filled out a questionnaire on satisfaction and knowledge. | |
| Participants | 56 patients undergoing either cardiology procedures or endoscopy procedures. 28 in control and intervention. For patient satisfaction 3 patients were lost to follow up in the intervention group and none in the control. For the patient knowledge test 4 patients were lost to follow up in the intervention group and 3 in the control group Germany Numbers of participants in analysis: 49 Intervention: 24 Control: 25 |
|
| Interventions | A 5 minute computer based visualisation programme. Containing conversation between a physician and a patient, pictures which patients could point on and receive a short explanation. Patients were also given a information brochure to both the intervention and control groups Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: brief training Evaluation of the delivery of intervention: evidence of fidelity/reliability of delivery Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: interactive audio‐visual Time of delivery: on admission |
|
| Outcomes |
Satisfaction with the consent process: asked shortly after intervention, 5 questions with a five point ranking scale used, max score was 25 which indicates good satisfaction. Average scores were reported Short‐term knowledge: within 3 days of the intervention, 10 questions asked right or wrong scoring, score out of 10, 10 = good knowledge, average scores re‐reported, recorded as Intermediate recall Economic‐time for consultation: was recorded by the physician, average time for consultation was reported |
|
| Notes | Aim: does using computer based visualisation of a procedure improve patient satisfaction with the consent process and knowledge of the procedure Conclusion: computer based intervention improves patient satisfaction and knowledge |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffled envelopes, doctors unaware of frequency of allocation within their envelopes |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail. No details on whether the envelopes were opaque |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40% |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent supervisors were blinded and they collected the questionnaires. Statisticians were blinded |
Felley 2008.
| Methods | 2‐centre RCT comparing the combination of written and oral or oral information alone before endoscopy on the patient's assessment of quality of information and level of anxiety | |
| Participants | 912 randomised patients of whom 577 were included in final analysis. Patients in 2 hospitals undergoing elective upper or lower GI endoscopy. Enrolled over a 3‐month period Switzerland Numbers of participants in analysis: 577 Intervention: 278 Control: 299 |
|
| Interventions | Written information booklet all the standard information regularly given to patients and detailing risks and benefits of procedure, treatment of complications and possibility of receiving hypnotic drug during the procedure (with related risks). This was posted to randomised intervention participants along with appointment details approximately 1 week before procedure and before informed consent for the study discussed Intervention development: no details Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: evidence of fidelity/reliability of delivery Control characteristics: verbal consent Done with clinician?: distant from clinician Intervention type: written Time of delivery: before for intervention |
|
| Outcomes |
Satisfaction with consent process: assessed with mean score of 8‐questions posted back day after endoscopy General anxiety: post‐event, posted back. Rating scale none‐strong ‐ dichotomous outcome Rates of uptake: (or refusal) of clinical procedures (numbers cancelling procedure out of original 912 patients) ‐ given cancellation rates but converted to uptake rates for comparison with other studies Pain: during procedure (rating scale none‐strong) ‐ dichotomous outcome All outcomes assessed by questionnaire that was taken home and posted back after endoscopy |
|
| Notes | Aim: to assess the effects of combined written and oral information compared to oral information alone on the quality of information before endoscopy and level of anxiety Conclusion: study found that structured and comprehensive written information is perceived as beneficial by patients, without a negative impact on anxiety. There was also a statistically significant increase in cancellation rates in the intervention group over the control. This was not followed up and its unclear whether there were any adverse events from the cancellations |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 46% attrition |
| Selective reporting (reporting bias) | Low risk | Has trial protocol ISRCTN 34382782. Details all outcomes included |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail; no information on whether questionnaires were anonymised |
Fink 2010.
| Methods | Multicentre RCT at 7 Veterans Health Administration Medical Centers where informed consent is obtained using iMedConsent, the VA’s computer based platform. iMedConsent is a computer generated informed consent document with the patient when reviewing the indications, risks, benefits and alternatives specific to the operation. Comprehension and satisfaction was measured directly after completion of informed consent discussion | |
| Participants | 502 patients scheduled for 1 of 4 elective surgical procedures (carotid endarterectomy, laparoscopic cholecystectomy, radical prostatectomy and total hip arthroplasty) scheduled between August 2006 and June 2008. Patients were comparable at baseline, measured on age, gender, ethnicity, marital status, education, reading ability, employment, health status and health literacy USA Numbers of participants in analysis: 539 Intervention: 263 Control: 276 |
|
| Interventions | Patient proceeded with the informed consent process as in the standard iMedConsent process however, when the provider and the patient were ready to sign the consent a RB dialogue was initiated. RB dialogue consisted of the subject being able to describe the diagnosis, procedure, anatomic location, risks, benefits and alternatives to the proposed procedure Patients in the control group received standard consent using the iMedConsent process where consent is sort by following the computer‐generated informed consent document with the patient when reviewing the indications, risks, benefits and alternatives Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: audio‐visual Done with clinician?: face‐to‐face Intervention type: structured consent Time of delivery: before admission |
|
| Outcomes |
Immediate knowledge: after consent discussion‐used customized VHA questionnaire Satisfaction with decision making: independent questionnaire for this study found at http://links.lww.com/SLA/A52. Measured satisfaction with decision making as a sub scale with 6 questions each on a 1 to 5 scale, therefore out of total 30 points Anxiety with the consent process: STAI Economic‐time taken to consent: iMedConsent programme was reconfigured with internal time stamps which recorded the length of consultation time Time was reported using median, mean and SD. Authors used Ilcoxon test indicating that results were not evenly distributed. Therefore contact with author was established and the IQR obtained. Non‐parametric data Comprehension and satisfaction were measured directly after completion of the informed consent process. Patient anxiety was measured before and after the consent discussion. Provider attitudes were measured at the end of study enrolment/after the residents surgical rotation was finished |
|
| Notes | Aim: use of repeat back will: 1) improve the surgical patient's comprehension, 2) lead to better patient satisfaction with the consent process and the healthcare received, 3) lessen patient anxiety about the surgical procedure, 3) lessen patient anxiety about the surgical procedure, 4) be acceptable to surgical providers Conclusion: repeat back implemented within an electronic informed consent system improved patient comprehension. The additional time required was acceptable to providers. Repeat back should be considered as an enhancement to surgical informed consent Author contacted: yes, data available for outcome 'time taken to consent' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using an internet based programme which used a concealed, computer‐generated simple randomisation scheme without stratification |
| Allocation concealment (selection bias) | Low risk | The randomisation sequence was concealed from each centre's personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Clinical trial was registered (NCT 00288899), outcomes reported |
| Other bias | High risk | Concern with contamination as some providers were assigned to both groups. No information about controlling information between the two groups There were small differences in baseline characteristics between the 2 groups, with a higher percentage of males and 'white' ethnic origin in the RB group The RB group were also older, likely to be retired and had a better SF12 mental score and less anxiety at study entry (P < 0.05) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial was not blinded |
Friedlander 2011.
| Methods | Single‐centre RCT looking at improving parental informed consent for paediatric upper GI endoscopy with an interactive on‐line video module the night before endoscopy versus usual care consent consultations | |
| Participants | 220 consecutive parents of patients were eligible; 190 were randomised and then 42 withdrew leaving a total of 148 (74 in each group). Intention to treat analysis not performed. Included patients from a pilot study due to under‐powered numbers in secondary analysis after drop‐out Comparable at baseline, similar education levels, 60 to 70% Caucasian. USA Numbers of participants in analysis: 97 Intervention: 47 Control: 50 |
|
| Interventions |
Intervention group: Interactive internet‐based video module covering the information required to be delivered by a physician obtaining informed consent. 6th‐grade reading level. Commercially available. Viewed from home the night before endoscopy, took a minimum of 20 minutes to complete, families able to pause and repeat sections as needed. Tracked via a monitored website, total time spent on the video recorded but not presented in paper Control group: also used internet the night before endoscopy but only to fill out baseline questionnaires. On day of endoscopy, both groups received same treatment (form‐based consent consultation with clinician) Intervention development: standard information with no modifications Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: non‐interactive audio‐visual Time of delivery: before admission |
|
| Outcomes |
Informed consent: this was measured on a questionnaire published by Woodrow et al. 2006 with some modifications (modified Consent‐20 form). Not validated in current form and no reliability testing. Maximum score of 40 possible, some free‐text answers included, 2 points scored for correct answer versus 0 for incorrect answer. Completed immediately after informed consent consultation Anxiety with consent process: state scale of STAI, measured at baseline the night before endoscopy and immediately after informed consent consultation. Satisfaction: measure this with questions that assess the whole hospital stay rather than the consent process or decision‐making, therefore not included as an outcome in this study Desire for further information: number of questions that parents in each group wanted to ask were written by the parents the night before endoscopy and counted when attending for the procedure. This information is not available in a form that could be used in the meta‐analyses. We have medians and ranges for each group (intervention median 1 question, range 0 to 14, n = 60; control median 3 questions, range 0 to 9, n = 63; P = 0.0053 with Mann‐Whitney test) |
|
| Notes | Aim: to evaluate the adequacy of paediatric informed consent and its augmentation by a supplemental computer‐based module in paediatric endoscopy Conclusion: this study demonstrates the limitations of form‐based informed consent methods for paediatric endoscopy. It also shows that even when necessary information was repeated electronically in a comprehensive and standardised video, informed consent as measured by our instrument was incompletely achieved. The supplemental information did, however, significantly improve understanding in a manner that did not negatively impact workflow, subject anxiety or satisfaction. Additional study of informed consent is required |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation by alternating numbers |
| Allocation concealment (selection bias) | High risk | Alternating numbers therefore high risk of predicting next participant's allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates for primary analysis 51/148 = 34% (i.e. < 40%). Used pilot data to increase numbers for secondary analysis as a post‐hoc strategy |
| Selective reporting (reporting bias) | High risk | Registered trial at http://clinicaltrials.gov with identifier NCT00899392. State that they are looking at flow through the endoscopy suite but do not report this in the paper |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinicians blinded but participants gave informed consent for the study and were not. Outcomes included subjective measure e.g. anxiety, so possibility of Hawthorne effect after consent given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All questions were read and answered in private. Answers were entered by the subject on a laptop computer and recorded electronically into a secure database |
Garden 1996.
| Methods | 3 armed RCT, assessing outcomes at base line and after delivery of intervention on the day before cardiac surgery. One arm excluded as they were given less information | |
| Participants | Pre‐op patients waiting for cardiac surgery (consent for the anaesthetic for the cardiac surgery). 57 eligible patients of whom 9 declined and 3 were excluded and 1 withdrew due to anxiety. The remaining 44 were randomised into 3 groups. 2 groups of interest to our study consisted of 15 participants of whom all were included in analysis New Zealand Numbers of participants in analysis: 30 Intervention: 15 Control: 15 |
|
| Interventions | Information booklet with different amount of details. The routine care group had a booklet with details of anaesthetic risks already widely used in New Zealand and enforced by the New Zealand Society of Anaesthetists content is based on what a reasonable doctor thought a patients needs to be told. The detailed booklet gave even more detailed information about anaesthetics and the risks involved Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent and standardised leaflet Done with clinician?: distant without clinician Intervention type: written Time of delivery: on admission |
|
| Outcomes |
Anxiety with consent process: STAI measured after intervention Immediate knowledge: score out of 10, difference between scores measured at baseline and then after the intervention (both before surgery) |
|
| Notes | Aim: to evaluate anxiety and knowledge using different information disclosure levels in patient information booklets Conclusion: anxiety not affected by level of information disclosed but knowledge and recall of facts did improve with full information Author contacted: yes, data available for risk of bias table |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised" no further details. Response from author – a table of random numbers was used to undertake block randomisation |
| Allocation concealment (selection bias) | Low risk | Response from author – no chance that the intervention allocation could have been foreseen in advance or during patient enrolment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40%. Full breakdown given, loss to follow up due to deaths and strokes. 32% attrition |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Insufficient detail in report Response from authors: the patients were blinded to the group allocation. They received leaflets that were numbered 1‐n, and had been prearranged in that order that reflected the randomisation. The leaflets were handed out by a senior registrar and that was his sole role. He didn't participate in the randomisation or the analysis |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | All data collection questionnaires delivered by study investigator Response from author: the assessment was a questionnaire. All participants filled out the same questionnaire Insufficient detail to assess if personnel analysing the questionnaire were blinded or if questionnaire anonymised |
Garrud 2001.
| Methods | RCT in Nottingham UK, comparing detailed leaflet to standard leaflet in patients undergoing elective gynaecological laparoscopy. Assessed using knowledge, satisfaction and anxiety via telephone interview 2 to 3 days after the intervention was delivered | |
| Participants | 41 female patients undergoing gynaecological laparoscopy, of whom all completed the study United Kingdom Numbers of participants in analysis: 41 Intervention: 20 Control: 21 |
|
| Interventions | Revised detailed leaflet including more information on the risks of laparoscopy ‐ bullet pointing text and lower reading age (16 years) Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent and standardised leaflet Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes |
Short‐term knowledge: assessed via telephone interview, 2 to 3 days following appointment. 5 open ended questions about what a laparoscopy is, why it is carried out, what the risks and complications are, where you can get further information, what happens afterwards and what pain or discomfort may be experienced. Scored for presence of different factual elements, to a maximum score of 23 Satisfaction with consent process: telephone survey, 2 to 3 days following appointment using a modification of medical interview satisfaction scale cognitive sub‐scale, where referent for each of the nine items was changed from “the doctor” to “the leaflet” Possible scores range from minimum of 9 ‐ maximum of 45, with higher scores being more satisfied Anxiety with consent process: telephone survey using 6 item version of Spielberger State‐Trait Anxiety Inventory. Possible scores ranged from 6 to 24, with higher scores representing greater anxiety |
|
| Notes | Aim: to compare detailed risk leaflet to a standard one in terms of knowledge, satisfaction and anxiety. Conclusion: detailed leaflet resulted in higher knowledge and satisfaction without increasing anxiety. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Block randomisation based on the week they attended clinic |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient detail |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Two leaflets and patients unaware of what the other contained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
Gerancher 2000.
| Methods | RCT‐Women admitted in labour to delivery suite but before the initiation of labour analgesia or administration of parenteral medications. Interview with anaesthetist lasted 10 mins for both control and intervention groups | |
| Participants | 113 labouring women, considering epidural or GA as part of labour USA Numbers of participants in analysis: 82 Intervention: 44 Control: 38 |
|
| Interventions | Interview between the patient and the anaesthetist with a review of the patient's medical condition and presentation of anaesthetic options. Investigator used a 10 point checklist and if in intervention group, patient and doctor reviewed and then signed a consent document Intervention development: no details Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent and used a checklist Done with clinician?: distant without clinician Intervention type: written Time of delivery: on admission |
|
| Outcomes | Long‐term knowledge: phone survey 5 to 7 months after delivery, a score was given out of 100, 10 questions asked, 10 point for correct answer, 0 for incorrect and 5 for I don't know, median scores and IQR reported | |
| Notes | Aim: to determine the ability of a woman in labour to recall preanaesthesia discussion with her anaesthesiologist and to determine if written consent added to this discussion improves recall Conclusion: regardless of the provision of a written document being used as part of the consent process, women in labour were able to recall the risks of epidural labour analgesia and the process of consent with a high degree of reliability.the practice of adding written to verbal consent during labour analgesia may increase recall of medical info slightly Author contacted: yes, no further data available to check excluded numbers and complete risk of bias table |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail. Random assignment ‐ no further description |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40% (27%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes measured by blinded research assistant who read out questions over the telephone |
Goel 2001.
| Methods | Multi‐centre RCT looking at the effect of a decision aid for the surgical treatment in early breast cancer. Randomisation was of surgeons rather than patients since intervention directed at surgeon‐patient interaction. Consent for the study was obtained from patients the surgeons saw, then the patients given baseline questionnaires before accessing intervention/control pamphlet. A second (post‐intervention) patient questionnaire was completed at home with telephone prompting 48 to 72 hours later to check completed. Looked at satisfaction with decision making, anxiety with decision making and short‐term knowledge recall. A further questionnaire was mailed at 6 months. No raw data for 6 months' results | |
| Participants | 232 surgeons were eligible, and 69 were randomised after showing some interest. Study nurses visited surgeons after randomisation with more information, and 57 surgeons were used for study results; 29 recruiting patients into the control group and a separate 28 recruiting patients into the intervention group. 164 patients with newly‐diagnosed stage I or II breast cancer with no prior history of cancer and who were suitable to have either mastectomy or breast conservation therapy were recruited, who could consent and complete the study questionnaires. No difference in baseline demographics ‐ looked at education, employment and language. In total, 136 patients were included in analysis; 50 in the control group and 86 in the intervention group Canada Numbers of participants in analysis: 123 Intervention: 78 Control: 45 |
|
| Interventions |
Development: the decision aid was developed previously (Sawka et al. 1998. Development of a patient decision aid for choice of surgical treatment for breast cancer. Health Expect 1:22‐36). High quality intervention ‐ development included literature review, focus groups of women with breast cancer, consultation with experts and sequential pilot studies. Information was updated where required in line with current evidence. Content: grade 8 reading‐level. Contains a 3‐step process of 1) women asked to review advantages/disadvantages of each procedure, 2) consideration of value of each of the advantages/disadvantages, 3) examination of worksheet to identify which procedure she is leaning towards. Format: audiotape and workbook to be used in the consultation and at home. Booklet uses colour photographs and describes the likelihood of events in graphical form using 100 figures to provide quantitative information. Tape supplements workbook. Setting: given to take home after initial decision on type of surgery made with the surgeon Control group: had similar consultation with the surgeon and given all the same information to take home, but in a non‐interactive form. This was in the form of a tri‐fold pamphlet with no numbers, photographs, graphics or values ‐ clarification exercise Intervention development: designed for trial with reasonable effort for validation/piloting Exposure: once Training for delivery of intervention: brief training Evaluation of the delivery of intervention: no details Control characteristics: verbal consent and special leaflet Done with clinician?: face‐to‐face Intervention type: multiple including decision aid Time of delivery: before admission |
|
| Outcomes |
Satisfaction with decision making: measured on the Decisional Conflict Scale (we are using the perceived effectiveness sub‐scale to estimate satisfaction, in line with other similar study data). Validated and reliable tool. Questionnaire completed within 72 hours of consultation with surgeon and choice of treatment, then mailed back to study organisers. Score converted from total 5 to total 100 in keeping with reporting convention from other systematic reviews Anxiety with decision process: measured on STAI scale. Done after initial consultation with choice of treatment, then again within 72 hours after consultation. Also completed at 6 months'. No raw data for this outcome ‐ "There were no differences in anxiety across the 2 study groups. Both groups showed high levels of anxiety at enrolment and preoperatively, above 50 points. At the 6 month follow‐up, these dropped equally to about 35 points,within the normal population range" Short‐term knowledge: measured on BCIT‐R scores (Breast Cancer Information Test‐Revised), a validated and reliable tool. Consists of 18 true/false questions (Ward and Griffin, 1990. Developing a test of knowledge of surgical options for breast cancer. Cancer Nurse 13:191‐6).Completed the questionnaire at home and mailed back approximately 72 hours after consultation where made treatment choice and intervention/control pamphlet were given Decisional Conflict: measured on the Decisional Conflict Scale using total score for all sub‐scales. Validated and reliable tool |
|
| Notes | Aim: to evaluate the effect of a decision aid for the surgical treatment in early breast cancer Conclusion: "Although the decision aid had minimal impact on the main study outcomes, a subgroup may have benefited. Such subgroups should be identified, and appropriate decision support interventions should be developed and evaluated" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Surgeons were randomised prior to the nurse visit to either the decision aid or pamphlet intervention in blocks of 8 based on a random number generator |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail. The allocation was not revealed to the surgeon until after agreement to participate in the study was obtained, but unclear how this was done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate of participating surgeons of 18% from those who engaged in study after recruitment (57/69) Attrition rate of participating patients 7% for pre‐op follow‐up (mailing the questionnaire back after 72 hours to complete it from initial consultation) and 20% for follow‐up at 6‐months |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
Greening 1999.
| Methods | RCT, inpatients approached and structured interviews given before ECT, outcomes measured before ECT and 1 to 7 days following completion of ECT (number of treatment medians 9 range 4 to 19) | |
| Participants | Informal patients with depression in a psychiatric hospital awaiting ECT Birmingham, United Kingdom Numbers of participants in analysis: 28 Originally the groups were 16 in control and intervention. 3 were lost to follow‐up. We have assumed 14 per group at follow‐up. Unable to gain correct details from author Intervention: 14 Control: 14 |
|
| Interventions | Structured consent process/interview, 10 basic points were covered and short specific verbal and written statements were given simultaneously with pictures. Subjects were asked to recall these points and the process was repeated three times or until all ten items were remembered Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: all delivered by key researcher Evaluation of the delivery of intervention: no details Control characteristics: verbal consent and standardised leaflet Done with clinician?: face‐to‐face Intervention type: structured consent Time of delivery: on admission |
|
| Outcomes |
Short‐term and long‐term knowledge: questionnaire marked out of 20 measured before ECT. Total number measured was 29, details of N values for each group not available (continuous data) Recall measured after ECT finished ‐ this varied from one course to 9 months (long‐term knowledge ‐ non‐parametric data) |
|
| Notes | Aim: to see if structured consent improved knowledge of ECT Conclusion: knowledge on ECT was improved with the structured interview warranting further study (this was a pilot ‐ no further study has been done to date by these authors) Author contacted: yes, no further data accessible |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Sealed envelope technique" ‐ not stated how random sequence generated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail; sealed envelopes, unclear if opaque |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40%. Small loss to follow up for pre‐ECT data, details of drop‐outs given, 2 withdrew consent one absconded, one had treatment terminated due to PE |
| Selective reporting (reporting bias) | Low risk | Protocol pilot study, outcomes all reported |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded and intervention delivered by trial investigator/author |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome rated by blinded investigator |
Heller 2008.
| Methods | Prospective RCT, evaluating the impact of an interactive digital education aid on knowledge and satisfaction in patients undergoing breast reconstruction for breast cancer. Assessed at clinic visit before receiving information, immediately before surgery after having received intervention and one month after surgery | |
| Participants | 274 breast cancer patients undergoing breast reconstruction were randomised, of whom 133 completed the study, 66 in the intervention group, 67 in the control group. Texas, USA Numbers of participants in analysis: 133 Intervention: 66 Control: 67 |
|
| Interventions | The interactive digital education aid is a menu driven, interactive software program that includes high‐quality, three‐dimensional animated graphics, patient testimonials, before‐and‐after photographs, and video explanations from plastic surgeons and clinical specialists in surgical, medical, and radiation oncology. It required 3 years to produce Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal and standardised leaflet Done with clinician?: distant without clinician Intervention type: interactive audio‐visual Time of delivery: before admission |
|
| Outcomes |
Short‐term knowledge: assessed using 12 answer questionnaire, completed at three time points: 1) Before intervention, 2) Immediately before surgery after having received information and 3) 1 month after surgery. Data presented in paper was "mean change between time 1 and time 2" Satisfaction with consent process: assessed using a 1 to 5 Likert scale. Data presented in paper is dichotomous. Clarification from author sought who was unable to recall how dichotomised. Entered data as dichotomous data from Table 5 in paper Generalised anxiety: assessed using Spielberger State Trait Anxiety Index at 3 time‐points listed above. Data presented in graphical format in paper (Fig. 3). No extractable data in paper. Authors contacted but no longer has access to the data. Agreed with statistician that unable to include this outcome in our review Desire for further information: participants asked "Did you receive all the necessary information?" Data presented as dichotomous, "Yes/No." For purpose of this review, No = desire for further information present |
|
| Notes | Aim: to assess the effectiveness of an interactive digital education aid Conclusion: this study found that an interactive digital education aid that explains the various methods of breast reconstruction can contribute significantly to the patient’s education. Having the opportunity to obtain information from both the interactive educational aid and the medical team appeared to be beneficial, particularly in terms of increasing the patient’s knowledge about the procedures themselves and increasing the patient’s satisfaction with how the information was delivered Author contact: yes, for raw data for outcomes 'short‐term knowledge', 'satisfaction with consent process' and 'generalised anxiety'. No further data available from the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail; author response insufficient to make judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of initial randomised patients, 51% dropped out once randomised to leaflet (n = 73) or because did not complete all questionnaires (n = 68) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded; large dropout rate when found were not in intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No form of outcome assessment blinding |
Henry 2008.
| Methods | RCT comparing a leaflet with pictures of common otological surgery risks with a leaflet (usual care) without pictures in one tertiary‐referral centre | |
| Participants | 51 consecutive patients on the list for 4 otologic procedures under 2 consultants conducted over a 14 month period. 51 completed initial outcome measures, but then sub‐sample of 31 looked at (with 26 responding) for secondary outcomes at 1 year Canada Numbers of participants in analysis: 51 Intervention: 23 Control: 28 |
|
| Interventions | Leaflet detailing common surgical risks with pictures versus non‐pictorial usual care standard leaflet. Handed out after informed consent consultation for the procedure to take home. Rated by BM and KP as poor quality ‐ pictures differ in style and clarity, no background preparation described and no previous validation. List of complications included facial nerve injury, worsened hearing, vertigo, operation failure, wound infection, tympanic membrane perforation, alteration in taste and complete sensorineural hearing loss Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal with standardised leaflet Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes |
Long‐term knowledge: recall of as many complications as possible via a telephone communication with a separate investigator a mean duration of 19 days (range 14 to 49 days) after the intervention given out (+consent given). Standardised questions, no prompting or suggestion Measured as percentage of those risks discussed with the surgeon |
|
| Notes | Aim: to test whether pictures in a handout improved patients' recall of otological surgical risks Conclusion: pictorial cues do not improve patients' recall of surgical risks but education level does Author contacted: yes, no further data accessible |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Performed using a computer‐generated binomial randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up at initial outcome measures. Although only 26 (50%) followed‐up at one year we have not used this data for the outcomes of interest in this review |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | High risk | Contamination risks ‐ not addressed in study design Secondly, post‐hoc alteration in study design to collect more data on last 31 participants ‐ unanonymised data on these to enable follow‐up, of which 26 replied when contacted |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail; outcome assessed by a separate investigator but unclear whether aware of allocations |
Hermann 2002.
| Methods | Single‐centre RCT evaluating the effects of using a non‐interactive animated video lasting 7 minutes on knowledge prior to elective thyroidectomy | |
| Participants | 80 participants (22.5% male) were randomised to intervention (n = 36) or control (n = 44). All were undergoing elective thyroidectomy. No other demographic baseline details were given. Inclusion criteria included those undergoing thyroidectomy, there were no exclusion criteria given Vienna, Austria Numbers of participants in analysis: 80 Intervention: 36 Control: 44 |
|
| Interventions | Intervention: participants in this arm were first asked to watch a 7 minute video detailing the steps of thyroidectomy surgery, then the outcome assessments were made. Participants in this arm then had an informed consent discussion with clinicians Video: designed by the researchers for the trial, no formal assessment of quality made. 7 minutes in total, used 3D models (although appears in study that presented to patients in 2D only). Setting was in hospital on the morning of scheduled operation Control: participants in this arm were given written information similar in content to the information included in the video, and more detailed than information usually used in standard care. They had 10 minutes to read this, and then the outcome assessments were done. Participants then got a chance to watch the video before participating in an informed consent discussion with clinicians. Note: all outcome data were collected before they watched the video, so do qualify as a control group Intervention development: designed for the trial with no validation Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: non‐interactive audio‐visual Time of delivery: on admission |
|
| Outcomes |
Patient' self‐report of understanding: measured with 2 written questions measured on 1 to 5 point Likert scales with high scores = better knowledge. These were not validated previously. The questions were: Qu1: 'After this explanation, can you image what happens during the operation?' Qu2: 'Have you understood the steps in the operation?' We have taken Qu2 as relevant to this review and used the data from this question in our outcome analysis (continuous data) Immediate knowledge: measured with 2 free‐text written questions: Qu1: 'Describe the important steps of the operation in your own words?' Qu2: 'Which risks are part of this operation?' We have taken Qu2 to represent knowledge required for informed consent (continuous data) Fear, "inner yes" and perceived competency of doctor and the service outcomes not included in this review |
|
| Notes | Aim: to analyse the merits of computerized animation to illustrate a difficult treatment process i.e. the progressive steps of a thyroid operation, in comparison to the use of conventional flyers Conclusion: “Preoperative surgical information can be optimised by presenting the operative procedure via computer animation. Nowadays, several types of new media such as the Internet, CD, DVD and digital TV are readily available and –as shown here – suitable for effective visual explanation. Most patients are familiar with acquiring new information by one of these means. An appropriately designed 3D representation is met with a high level of acceptance, as the present study clearly shows. Modern patient‐based information systems are necessary. They can no longer be the sole responsibility of the medical profession, but must be on the agenda of hospital managements and of medical care systems as well.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. Analysed with intention‐to‐treat |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded No information on researchers being blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Additional questions were included on control questionnaire, therefore easy to guess grouping. Researchers scored free‐text objective questions and converted them to a 1 to 5 point scale so potential for detection bias |
Hong 2009.
| Methods | Multicentre‐RCT assessing the effectiveness of written information versus. traditional oral dialogue, in rhinoplasty. No information was given in the paper on when the pamphlet was given to the intervention group | |
| Participants | 100 consecutive patients for rhinoplasty, multi centre trial. None of the patients were aware that they were in a study until they were phoned two weeks later after surgery and when authors completed knowledge questionnaire Canada Numbers of participants in analysis: 100 Intervention: 48 Control: 52 |
|
| Interventions | Both groups received a standard initial consultation including a detailed discussion of the potential risks and complication of the operation. Prior to the commencement of the study, a list of the most common and significant risks of rhinoplasty was generated. The participating surgeons used a set form during the consultation, which included a checklist of the 5 potential complications, to strive for a consistent discussion with the patients. In addition the intervention group received a written pamphlet outlining the risks of rhinoplasty Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent and used a checklist Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes | Long‐term knowledge: 14 to 18 days after initial consultation | |
| Notes | Aim: to determine the effectiveness of providing written information in enhancing patient understanding and retention Conclusion: risk recall in rhinoplasty is improved with the addition of written information during the informed consent process. More specifically female patients and those with a higher level of education seemed to benefit from receiving supplementary information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated roll of die then allocated according to odd/even numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unaware of being in a trial, however were then informed when the outcome was measured 14 to 18 days later. Impossible to blind participants as received different interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. Telephone interviewers were aware of allocation |
Hopper 1994.
| Methods | RCT patients either receive standard written consent form or use video information program. Knowledge assessed by questionnaire after consent, prior to scan. Satisfaction with consent process measured at same time. Time taken was timed during the process | |
| Participants | 160 consecutive patients, referred for imaging examination (venography, excretory urography and CT requiring IV contrast) during week day hours Pennsylvania USA Numbers of participants in analysis: 160 Intervention: 80 Control: 80 |
|
| Interventions | Interactive video providing information on risks of IV contrast material. Patients were able to select as much or as little information as they chose Intervention development: designed for trial with reasonable effort for validation/piloting Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: interactive audio‐visual Time of delivery: on admission |
|
| Outcomes |
Immediate knowledge: MCQ ‐ 7 questions relating to knowledge Satisfaction with the consent process: 5‐point scale 1 = much more than satisfied, 5 = much less than satisfied (scores inverted when entered into RevMan) Economic ‐ length of consultation: time reported in minutes Standard deviations for all outcome measurements calculated using usablestats.com |
|
| Notes | Aim: to evaluate interactive computer‐based informed consent (interactive video) for use of contrast material versus the same information in a written format Conclusion: the video informed consent for use of IVCM is a possible alternative to the written consent form |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised into one of 8 groups, then computer generated randomisation to then allocate to intervention or control |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient details |
Johnson 2006.
| Methods | Single‐centre RCT using normal consent process versus consent process with aid of a face:face decision aid to clarify treatment options, benefits, risks, prognosis and costs when root canal therapy or extraction of a tooth is indicated | |
| Participants | 80 eligible participants were approached and 70 recruited. 3 were lost to follow‐up and analysis was based on the 67 that completed outcome questionnaires. All were consecutive patients in a postgraduate endodontics clinic who had been through preliminary dental screening and whom had all been considered eligible for root canal treatment Chicago, USA Numbers of participants in analysis: 67 Intervention: 32 Control: 35 |
|
| Interventions | 4 second‐year endodontic residents underwent training with a pilot of 40 patients, learning how to present information with the decision board. The principal investigator oversaw delivery discretely to ensure consistent use of the decision aid The decision aid was a face:face paper aid on one page addressing 5 treatment options and including information for each option on; time required/appointment numbers necessary, costs, risks of treatment and infection, chance of keeping the tooth/replacement for 5 years or more, a diagram to illustrate each option. The decision aid was developed at the University of Illinois with involvement from experienced staff and dental experts, using evidence‐based data. The control group had 'usual care' informed consent process ? delivered in clinic on day that treatment was initiated ‐ emailed authors to check but no response. Consensus of 2 review authors that occurred on day treatment initiated Intervention development: designed for trial with reasonable effort for validation/piloting Exposure: once Training for delivery of intervention: structured/extensive training Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: face‐to‐face Intervention type: decision aid Time of delivery: on admission |
|
| Outcomes |
Immediate knowledge: measured on 5 questions (no validity or reliability testing). Given a point for each correct answer. Results presented as mean score/5 and SD for each trial arm Satisfaction with consent process: measured with one question (no validity or reliability testing). "How satisfied were you with the explanation of your treatment options?". 7‐point Likert scale indicating satisfaction. Reported as number per group giving each possible answer on Likert scale. For this Review, taken all 'satisfied' options (somewhat satisfied/satisfied/very satisfied) and used as dichotomous data ‐ those satisfied per trial arm General anxiety: measured with one question (no validity or reliability testing). "Did the explanation of treatment options make you more or less anxious about the treatment?". 7‐point Likert scale indicating anxiety. Reported as number per group giving each possible answer on Likert scale. For this Review, taken all 'anxious' options (slightly more anxious/more anxious/much more anxious) and used as dichotomous data ‐ those anxious per trial arm |
|
| Notes | Aim: to develop and test an Endodontic Decision Board for chairside use to help clarify treatment alternatives, benefits, risks, prognosis, and costs when root canal therapy or extraction of a tooth was indicated. The hypothesis was that the use of the EndoDB would lead to improved patient knowledge, greater satisfaction with the decision‐making process, and no difference in anxiety when compared to the standard discussion and informed consent process (usual care) Conclusion: patients in the EndoDB group demonstrated a small, but statistically significant increase in knowledge compared to the usual care group. There was no difference between groups in the measures of satisfaction or anxiety. Decision aids may emerge as a useful tool to facilitate SDM and evidence‐based clinical practice |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation lists |
| Allocation concealment (selection bias) | Low risk | Allocation concealed from patients |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40% (3/70) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinicians were not blinded and delivered consent for both arms of the trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome responses labelled with participant information |
Kain 1997.
| Methods | RCT‐Anxiety was measured at 4 different time points‐pre and post intervention, on day of surgery and after the child was taken into the operating room using STAI | |
| Participants | 47 parents of 4 to 12 year olds undergoing elective surgery and a general anaesthetic. 23 in the intervention group and 24 in the control group USA Numbers of participants in analysis: 47 Intervention: 23 Control: 24 |
|
| Interventions | Intervention was detailed anaesthetic information as opposed to control which was a leaflet containing standard anaesthetic information.The intervention contained statistics about adverse outcomes associated with anaesthesia Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: no details Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes |
Anxiety with the consent process: measured at 4 different points, used STAI‐T and STAI‐S at base line, and then STAI‐S at T1 pre‐anaesthetic interview, T2 after the intervention and T3 prior to surgery in the pre‐op area and T4 after the child was taken into surgery We have usedT2 for analysis. Data extracted from graph in the paper |
|
| Notes | Aim: hypothesized that the provision of detailed information about anaesthesia‐related risk, including incidence of adverse outcome, is associated with increased parental anxiety Conclusion: when provided with highly detailed anaesthetic risk data parental anxiety does not increase Author contacted: yes, response for risk of bias table |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Response from author: doctor reading risks not blinded but all medical staff blinded. Patients not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Response from author: all personnel involved in outcomes were blinded |
Kang 2009a.
| Methods | Assigned to 1 of 3 groups for patient‐parent groups for patients undergoing orthodontic work. 45 minutes later after intervention delivery they were interviewed to check for understanding and recall Data analysed for each intervention separately versus half of the control group Presented in this table is information for Intervention A (MIC ‐ Modified informed consent) versus Control group (AAO ‐ existing consent form) |
|
| Participants | Parents of patients aged between 12 to 18 years USA Numbers of participants in analysis: 44 Intervention: 29 Control: 15 (half of the control group) |
|
| Interventions | Standard consent was an American Association of Orthodontists informed consent form (AAO) Intervention group A were given a modified informed consent form which was created from the AAO document and an existing informed consent document used already Intervention development: modified from standardised information Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal and standardised leaflet Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes |
Understanding: assessed by asking the parents to apply their knowledge to different scenarios to prove their understanding ‐ qualitative analysis of transcribed interviews. Interviews were classified on a 1 to 4 scale and the outcome tools were previously validated and are reliable Immediate knowledge: (45 minutes) as above Anxiety with the consent process: STAI |
|
| Notes | Aim: to improve recall and comprehension Conclusion: improving the readability of consent material made little difference, but combining improved readability and processability improved parents and patients comprehension and recall Author contacted: yes, further information for risk of bias assessment obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Author states "Randomised using Random.org. The allocation was then followed by stratification for age by year from 12‐18. So, if allocation was to group A and the age group was filled, it was allocated to the next sequential group, B. The code book rater was blind to group allocation" |
| Allocation concealment (selection bias) | Low risk | Response from author stating that allocation was concealed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Research assistants collecting the data would have been aware of which group the patients were in and what the outcomes measured were (qualitative results) |
Kang 2009b.
| Methods |
As follow on fromKang 2009aabove, intervention group B versus control: Assigned to 1 of 3 groups for patient‐parent groups for patients undergoing orthodontic work. 45 minutes later after intervention delivery they were interviewed to check for understanding and recall Data analysed for each intervention separately versus half of the control group Presented in this table is information for Intervention B (MIC + SS ‐ Modified informed consent + slide show) versus control group (AAO ‐ existing informed consent form) |
|
| Participants | Parents of patients aged between 12 to 18 years USA Numbers of participants in analysis: 45 Intervention: 30 Control: 15 |
|
| Interventions | Standard consent (control group) used the AAO (see above for details) Intervention B used the modified consent form (details as above) and additionally had a narrated slide show presentation from power point with audio and visual cues representing the 18 elements of orthodontic informed consent Intervention development: modified from standardised information Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal and standardised leaflet Done with clinician?: distant without clinician Intervention type: non‐interactive audio‐visual Time of delivery: before admission |
|
| Outcomes |
Understanding: assessed by asking the parents to apply their knowledge to different scenarios to prove their understanding ‐ qualitative analysis of transcribed interviews. Interviews were classified on a 1 to 4 scale and the outcome tools were previously validated and are reliable Immediate knowledge: (45 minutes) as above Anxiety with the consent process: STAI |
|
| Notes | Aim: to improve recall and comprehension Conclusion: improving the readability of consent material made little difference, but combining improved readability and processability improved parents and patients comprehension and recall Author contacted: yes, further information for risk of bias assessment obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Author states "Randomised using Random.org. The allocation was then followed by stratification for age by year from 12‐18. So, if allocation was to group A and the age group was filled, it was allocated to the next sequential group, B. The code book rater was blind to group allocation" |
| Allocation concealment (selection bias) | Low risk | Response from author stating that allocation was concealed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Research assistants collecting the data would have been aware of which group the patients were in and what the outcomes measured were (qualitative results) |
Langdon 2002.
| Methods | RCT, patients randomly allocated to standard verbal consent or standard verbal consent with written information | |
| Participants | 126 patients undergoing hip arthroplasty were randomised. Were comparable at baseline based on age and previous hip arthroplasty UK Numbers of participants in analysis: 126 Intervention: 61 Control: 65 |
|
| Interventions | Written informed consent sheet vs standard verbal consent only. Information sheet contained a picture of total hip replacement and informations about the operation, hospital stay, types of implants and anaesthetic and risks Control group was given structured verbal information during the consent interview (same information as that on the written document Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes |
Long‐term knowledge: 18.5 days Measured using MCQs |
|
| Notes | Aim: to ascertain whether written information sheets are acceptable to patients and improves recall of the consent interview Conclusion: written information sheets contribute to the process of informed consent. As patients' recall of information is generally poor the sheets may also be useful medicolegally as a permanent record of what was discussed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was undertaken by blinded author |
Lavelle‐Jones 1993.
| Methods | RCT of patients undergoing surgery. All patients received standard consent, but half randomised to receive an operation information card | |
| Participants | 265 Randomised patients undergoing intra‐thoracic, intraperitoneal and arterial procedures. 130 randomised to intervention group, 135 randomised to control group. 192 completed study. Dundee, United Kingdom Numbers of participants in analysis: 253 Intervention: 126 Control: 127 |
|
| Interventions | Operational Information card consisting of 3 items of information relating to the nature of the operation and 3 items on recovery, side effects and after treatment Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: written Time of delivery: on admission |
|
| Outcomes |
Immediate, short‐term and long‐term knowledge: assessed at 5 time points:
Assessed on scale of 0 ‐ 6 according to the 6 items listed on the intervention cards, non‐parametric data reported |
|
| Notes | Aim: to examine factor influencing quality of informed consent, using operation information cards Conclusion: patients who received an operation information card were significantly better informed on the day of discharge only, but seemed to have no advantage immediately after the consent form was signed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Half were provided with operation information cards on a random basis. The randomisation was performed after entry into the study” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full break down of patients analysed in each category at each time point. Control – 6 months = loss to follow up of 27%, intervention = 22.8% |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
Luck 1999.
| Methods | RCT looking at knowledge and general anxiety pre‐colonoscopy | |
| Participants | 150 patients scheduled for colonoscopy in a Day Surgery Unit (Jan to Aug 1998). Comparable at baseline‐measured age, gender, education, previous colonoscopy. Australia Numbers of participants in analysis: 150 Intervention: 72 Control: 78 |
|
| Interventions | 10‐minute videotape discussing procedure then watching the procedure. Rated as medium quality. Sourced from Australian Gastroenterology Institute. All patients were given the standard surgical and anaesthetic information process before enrolment. (control + intervention) then the intervention group watched video Intervention development: standardised information with no modifications Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal and standardised leaflet Done with clinician?: distant without clinician Intervention type: non‐interactive audio‐visual Time of delivery: before admission |
|
| Outcomes |
General anxiety: about procedure (measured 1 week before colonoscopy and then on morning pre‐op) using STAI, however results were only reported for patients that were severely anxious at baseline (STAI > 50). n = 16 intervention and 14 for control Statistics note for general anxiety: the difference between the two groups pre‐procedure rather than the change from baseline based on the information that the paper provided. We have used the excel spreadsheet to calculate the SD for both groups from the confidence intervals. To put into Revman we have subtracted the amounts from the total of 80 (max possible score on the STAI) to reflect that the intervention group were better with a lower score than the control group Short‐term knowledge: 1 week later. Tested with a questionnaire developed for the study asking about purpose, procedure details and potential complications on morning of colonoscopy (pre‐op). SDs from the confidence intervals were calculated manually by the Review team |
|
| Notes | Aim: to assess the value of an information video in the provision of information before colonoscopy to improve knowledge and anxiety Conclusion: an information video increases knowledge and decreases anxiety in patients preparing for colonoscopy Author contacted: yes, confirmed severe anxiety measured with STAI scores > 50; no further raw data usable |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random shuffled cards |
| Allocation concealment (selection bias) | Low risk | Thoroughly shuffled marked cards placed into sequentially numbered, sealed, opaque envelopes by clerical staff not involved in the rest of the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients who reached the randomisation stage completed the trial |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Different researcher marked the outcome and was blinded to the groups allocation |
Makdessian 2004.
| Methods | RCT of patients attending an ambulatory facial plastic surgery centre comparing standard pre‐surgery consultation with a standard pre‐surgery consultation plus written pamphlet | |
| Participants | 120 patients undergoing either rhinoplasty, face‐lift surgery or laser resurfacing. paper fails to state if the groups were comparable at baseline, although age, gender and education level were measured Canada Numbers of participants in analysis: 120 Intervention: 63 Control: 57 |
|
| Interventions | Control group had the standard initial consultation discussing nature, purpose, complications of the operation only. The intervention group had the same but in addition received a written pamphlet, however no clear information was actually given about its contents. Assumed that the information was to do with the risks that were orally discussed with the patients during the surgical consultation Intervention development:designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes |
Long‐term recall: median 15 days Answers were obtained by telephone consultation with the patient and patients were asked to repeat the risks and complications of the particular procedure that was discussed during the intimal consultation with the surgeon. The answers were recorded on a standard checklist outlining the risks of that particular procedure |
|
| Notes | Aim: to evaluate the effectiveness of oral communication about the risks of facial cosmetic procedures compared with oral and written communication Conclusion: written disclosure of the risks of cosmetic procedures enables patients to retain and understand more clearly those potential risks. They are, therefore, able to give informed consent to the proposed procedure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was via a computer generated die, even numbers placed into the intervention group and odd into control |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although patients were initially unaware they were participating in a trial, they were then informed before providing answers to the questionnaire |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail; unclear whether the researchers performing the test were aware of the allocation of study groups |
Mason 2003.
| Methods | 2 centre RCT comparing standard consent plus a video disk to standard consent alone | |
| Participants | 38 women attending for planned sterilisation. 31 completed study United Kingdom Numbers of participants in analysis: 31 Intervention: 15 Control: 16 |
|
| Interventions | Video consisting of diagrams, text, shots of the QMC Day Theatre and laparoscopic equipment, as well as the presenter (VM) talking directly to camera. The programme lasted approximately 5 minutes Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: non‐interactive audio‐visual Time of delivery: before admission |
|
| Outcomes |
Immediate knowledge: non‐parametric data Anxiety with the consent process: non‐parametric data |
|
| Notes | Aim: to test whether a video intervention in addition to standard consultation in women requesting sterilisation improves patient’s knowledge without increasing anxiety Conclusion: women receiving video information as well as the standard consultation had significantly higher knowledge scores compared with women only receiving the conventional consultation. There were no differences in anxiety levels between the groups. Information giving by video was acceptable to the majority of women |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation programme |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded, personnel aware of content of video and that patient was participating in study, but not aware of if had seen video or not |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail; no details in paper, response from author stated that they were blinded to intervention groups |
Masood 2007.
| Methods | Single centre RCT, comparing verbal consent with consent using the written standardised consent form (modified from BAUS) | |
| Participants | 80 patients were randomised with no attrition. Patients were undergoing either TURP or TURBT for the first time. Unclear if patients were comparable at baseline, although "no significant difference in the mean ages." Age and sex comparable with no significant difference in social class stratification Essex, United Kingdom Numbers of participants in analysis: 80 Intervention: 35 Control: 45 |
|
| Interventions | Modified version of standardised consent form produced by BAUS‐no information given on the modifications carried out. Given in addition to verbal consultation. Control group received verbal consent as per standard practice. Intervention quality‐moderate Intervention development: modified from standardised information Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: written Time of delivery: on admission |
|
| Outcomes |
Immediate knowledge: before discharge but after procedure Stats analysis: effect sizes for outcome 1 = knowledge were obtained by averaging percentages for the first 2 questions. These were rounded to integer values but note the percentages quoted in paper are not possible ‐ ? attrition which not reported |
|
| Notes | Aim: to determine the degree to which patients understood the nature and risks associated with routine urological surgical procedures and whether providing additional detailed written information improved their understanding Conclusion: verbal and written information supplied to a patient might be understood, but is easily and quickly forgotten. In an increasingly medicolegal environment, it is essential to gain informed consent from a patient before and intervention. The provision of an information booklet might provide nothing more than proof for the surgeon of information provided to the patient. Verbal and written information seems inadequate for obtaining informed consent, and the whole informed consent issue needs revisiting Author contacted: yes, no further information or data accessible |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient detail; outcome measure completed before discharge so unlikely to have a high attrition rate |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
Mauffrey 2008.
| Methods | RCT of patients scheduled for spinal surgery with the control group receiving a standard verbal discussion of the risks during and after the operation and the intervention group in addition to the verbal discussion were given a written A4 sheet reiterating the information given | |
| Participants | 43 consecutive patients from a pre‐op assessment clinic scheduled to undergo elective spinal surgery between February‐November 2006. Participants were comparable at baseline with age, social class and education levels recorded United Kingdom Numbers of participants in analysis: 40 Intervention: 20 Control: 20 |
|
| Interventions | Informative spreadsheet (A4 size sheet) containing a written explanation (identical to one provided verbally to both groups) of the risks during or after the operation Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent and used a checklist Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes |
Long‐term knowledge: 15 days An A4 size questionnaire was given to the patients the day prior to their operation, about 2 to 3 weeks after the consent process. This questionnaire assessed whether patients remembered having been told about the various risks of surgery. For each of these risks the answer was yes, no, cannot remember or not applicable. This form was filled in by the specialist registrar himself to avoid having patients using their A4 explanation sheet to fill in the questionnaire |
|
| Notes | Aim: to assess the influence of written information provided to the patients during the consenting process on their recall of operative risks Conclusion: the addition of a written sheet given to patients during the consenting process makes a significant difference in terms of their recall of the surgical risks in elective lumbar spine surgery Author contacted: yes, information available to aid assessment of risk of bias |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generation was done using the last digit of the hospital number: odd/even (information was obtained from contact with author) |
| Allocation concealment (selection bias) | High risk | Risk of allocation prediction due to use of odd or even hospital record numbers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up Typing error within the published paper was reported following contact with author, therefore no‐one was excluded. 43 is the correct number enrolled in the study |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | High risk | Possibility that patients could have obtained information sheets from the other patients, contamination |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome measurements were done by the registrars who delivered the intervention |
Mishra 2010a.
| Methods |
As follow on fromMishra 2010babove, intervention group B versus control: RCT with two intervention groups and one control. Patients' outpatient consultation with the surgeon was recorded, then patients either received one of the intervention audio‐cassettes or received standard care |
|
| Participants | 84 elective first time CABG at a tertiary health centre under the care of one surgeon. Participants were comparable at baseline, according to age, gender, ability to speak English, NART score and Area of Deprivation Index United Kingdom Numbers of participants in analysis: 38 Intervention: 24 Control: 14 (half of the control group) |
|
| Interventions | All participants were audio‐recorded at their outpatient appointment with the surgeon. Following this: the second intervention group (intervention generic) received a generic tape containing information about CABG, scripted to include information covering each of the domains described by the GMC. Both groups received a letter encouraging them to listen to the tapes as many times as they wished and with others The control group did not receive any tape Intervention development: designed for trial with no validation Exposure: multiple exposures to the same intervention Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: audio‐recorded consultation and audio‐tape with standardised information Time of delivery: before admission |
|
| Outcomes |
Long‐term knowledge: length of time not stated, following discussion in meeting 30/3/12 decided that it was long term as likely to be > 2 weeks between outpatient appointment and CABG General anxiety: measured using the HAD questionnaire (scale 0 to 21) at presumed to be measured on admission for CABG |
|
| Notes | Aim: to evaluate the effect of audio‐taping outpatient consultations on informed consent for cardiac surgery Conclusion: providing an audio‐taped recording of the consultation before cardiac surgery appears to improve patients knowledge and perceptions of control of their health status and to reduce anxiety and depression Author contacted: yes, further information used to assess risk of bias |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised by minimization using age and sex as stratification factors‐computer programme used |
| Allocation concealment (selection bias) | Unclear risk | Contact with author "allocation was by the computer generated software" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All consultations were recorded so that the patients and surgeons were blinded to which arm of the trial that they were in. However the patients were then sent a tape therefore blinding not robust |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Personnel administering and assessing the outcome measurements were blinded |
Mishra 2010b.
| Methods | RCT with two intervention groups and one control. Patients' outpatient consultation with the surgeon was recorded, then patients either received one of the intervention audio‐cassettes or not at all Data analysed for each intervention separately versus half of the control group Presented in this table is information for Intervention A versus control group |
|
| Participants | 84 Elective first time CABG at a tertiary health centre under the care of one surgeon. Participants were comparable at baseline, according to age, gender, ability to speak English, NART score and Area of Deprivation Index United Kingdom Numbers of participants in analysis: 38 Intervention: 24 Control: 14 (half of the control group) |
|
| Interventions | All participants were audio‐recorded at their outpatient appointment with the surgeon. Following this: one group (intervention consultation) received an audio tape of their consultation with the surgeon The control group did not receive any tape Intervention development: designed for the trial with no validation Exposure: multiple exposures of the same intervention Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: audio‐recorded Time of delivery: before admission |
|
| Outcomes |
Long‐term knowledge: length of time not stated, following discussion in meeting 30/3/12 decided that it was long term as likely to be > 2 weeks between outpatient appointment and CABG General anxiety: measured using the HAD questionnaire (scale 0 to 21) at presumed to be measured on admission for CABG |
|
| Notes | Aim: to evaluate the effect of audio‐taping outpatient consultations on informed consent for cardiac surgery Conclusion: providing an audio‐taped recording of the consultation before cardiac surgery appears to improve patients knowledge and perceptions of control of their health status and to reduce anxiety and depression Author contacted: yes, further information used to assess risk of bias |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised by minimization using age and sex as stratification factors‐computer programme used |
| Allocation concealment (selection bias) | Unclear risk | Contact with author "allocation was by the computer generated software" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Unclear risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All consultations were recorded so that the patients and surgeons were blinded to which arm of the trial that they were in. However the patients were then sent a tape therefore blinding not robust |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Personnel administering and assessing the outcome measurements were blinded |
Morgan 2000.
| Methods | RCT using questionnaire at time of treatment decision to measure knowledge and satisfaction, and follow‐up data at 6 months to assess rate of uptake | |
| Participants | Patients with ischaemic heart disease who could be treated by either elective revascularization or medical therapy. 279 eligible participants, of whom 39 were excluded. 120 were randomised to intervention and 120 to control. 53 patients either withdrew or were lost to follow up Toronto, Canada Numbers of participants in analysis: 181 Intervention: 86 Control: 95 |
|
| Interventions | Interactive videodisc presenting information about the risks and benefits associated with the three treatment alternatives for IHD; medical therapy, angioplasty or bypass surgery Intervention development: designed for trial with reasonable effort for validation/piloting Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: evidence of fidelity/reliability of delivery Control characteristics: verbal consent Done with clinician?: face‐to‐face Intervention type: multiple interventions including decision aid Time of delivery: before admission |
|
| Outcomes |
Patient satisfaction with decision making process: 12 point MCQ, reported as percentage score. Confidence Interval for difference is reported and is non‐symmetrical, indicating unequal variance between groups. We've matched P value as opposed to Confidence Interval (usablestat.com) Immediate knowledge: 20 true/false questions, reported as percentage of correct answers. Confidence Interval is symmetrical for this outcome. We have matched P value and Confidence Interval (usablestats.com) Rate of uptake: using measurement of patients who had revascularisation by 6 months 'Bodily pain' was also measured at 6 months ‐ but not used for our review ‐ as is a measure of generalised pain, rather than pain from the procedure being considered |
|
| Notes | Aim: to determine the effect of the IHD shared decision making program, an interactive video disc, designed to assist patients in the decision making process involving treatment choices for IHD on patient decision making Conclusion: no significant difference in satisfaction with the decision making process scores between the IHD shared decision program and usual practice groups. the IHDSDP groups were more knowledgeable, underwent less revascularisation and demonstrated increased patient decision making autonomy without apparent impact on quality of life See notes on standard deviation calculations in outcome section above Author contacted: yes, further information available for risk of bias assessment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Two randomisation schedules and a blocking factor |
| Allocation concealment (selection bias) | Low risk | Done by statistician over the phone who was the only person privy to the information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40% |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Following email contact with author: neither the investigators nor the patients were blinded to the intervention, the angiographers may have known about the allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
Nadeau 2010.
| Methods | Single centre RCT with one control and one intervention group (handout) | |
| Participants | 34 Military parents of children undergoing ENT surgery (tonsillectomy and adenoidectomy or bilateral myringotomy with tympanic tubes. All completed study. Comparable at baseline USA Numbers of participants in analysis: 34 Intervention: 16 Control: 18 |
|
| Interventions | 2‐stage intervention: Stage 1 = consented with a surgical risk sheet prompt in the intervention group (procedure specific for either tonsillectomy or ear tubes). Control group had consultation without prompt Stage 2 = general surgical sheet (operative instructive sheet) given to intervention group and control group to take home Source and content of sheets not stated Unclear quality assessment ‐ no information available to make judgement Intervention development: no details Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes |
Immediate and short‐term knowledge: recall of 9 specific risks of surgery (T1 = post‐consent at time of intervention, and T2 = post‐op on day of surgery ‐ mean 6.3 days, range 1 to 22 days after intervention). Note, this was measured immediately after surgery Secondary: general knowledge on a test ‐ scored as percentage correct answers, no information on what questions asked or style of test. This outcome measure would be included under same ‘Secondary Outcome Measure’ for our review as the previous recall of 9 risks was. Since the Risk recall was the primary outcome of Nadeau et al, we are using the data from that outcome rather than this ‘General Knowledge Test’ outcome |
|
| Notes | Aim: investigate parent understanding of risks of paediatric surgery after counselling with/without use of an information leaflet Conclusions: parents of children undergoing ENT surgery recall far less than 100% risks. The use of detailed surgical risk counselling aids can improve measured parental risk recall Author contacted: yes, further information available to assess risk of bias |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by numbered card technique (from email correspondence: "Groups stratified by type of procedure consenting for before randomisation") |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
Neary 2010.
| Methods | RCT comparing an interactive website to a control website | |
| Participants | 81 eligible Patients undergoing minimally invasive radio guided parathyroidectomy, of whom 51 completed the trial. Ireland Numbers of participants in analysis: 51 Intervention: 31 Control: 20 |
|
| Interventions | The use of an interactive individualised online patient pathway. This presented the patient with a clear stepwise description of their expected clinical course, from initial diagnosis at their general practitioner’s surgery to eventual discharge from the hospital. There was the option of requesting more information if required, and if dissatisfied with results, the option of email a consultant for further information Control consisted of a web site with only limited information such as patient name, date of birth, address, and background information about the surgeon and hospital as is available via the standard hospital web site Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: interactive multimedia Time of delivery: before admission |
|
| Outcomes |
Generalised anxiety: using HAD scale , evening before surgery Pain levels: post‐op pain measured on VAS Analgesia use: analgesia requirements as defined by intervals on WHO pain ladder Satisfaction with the consent process: with capacity to consent measured by total score to 6 questions using a Likert scale ranging from 1 to 5, 5 = Strongly Agree. Gives non‐parametric data Perception of website utility, measured by 9 questions on 5‐point Likert scales |
|
| Notes | Aim: to determine the usefulness of an interactive, individualized online patient pathway to patients undergoing elective operation for nonmalignant disease Conclusion: this study demonstrates the proof of concept of the subjective usefulness of web‐based information for patients in the preoperative period. Although it did not influence patient anxiety or analgesic requirements, the novel online, interactive patient pathway makes a positive impression on our patients’ journey through the healthcare system and so would seem to provide added value to the overall experience |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assigned by permuted block randomisation, randomisation was performed by a person not involved in recruitment or data collection and the recruiter and interviewer were not aware that the study was block randomised prior to its completion |
| Allocation concealment (selection bias) | Low risk | The recruiter and interviewer were not aware that the study was block randomised prior to its completion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40%. 64 eligible, with 13 excluded due to not accessing website = 51 included in analysis = 80% |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and all study personnel were blinded as to which group patients were allotted. Patients were informed only that the website they accessed would give them basic details about the surgery and were provided with a username and password that allowed access to their allotted website |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only after study was completed were the group composition and assessments revealed to the study authors |
Neptune 1996.
| Methods | RCT comparing the same consent form for IV pyelogram or IV contrast CT, delivered either 24 to 72 hours prior to procedure in intervention group, or 15 to 60 minutes prior to procedure in control group | |
| Participants | Patients undergoing IV pyelogram or IV contrast CT. 80 patients in both groups ‐ from 160 consecutive out‐patients awaiting the relevant diagnostic test Pennsylvania, USA, Numbers of participants in analysis: 160 Intervention: 80 Control: 80 |
|
| Interventions | Intervention group received their contrast information sheet by post 24 to 72 hours prior to radiology appointment. The same information was provided 15 to 60 minutes prior to the diagnostic procedure in the control group. Intervention group were telephoned to check they had received the information sheet, and were willing to read it. The form had been published previously by the same authors ‐ and was designed for 8th grade reading level Intervention development: designed for trial with reasonable effort for validation/piloting Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: evidence of fidelity/reliability of delivery Control characteristics: verbal consent Done with clinician?: face‐to‐face Intervention type: alteration of timing Time of delivery: intervention group consented before admission and control group consented on admission |
|
| Outcomes |
Immediate knowledge: assessed by 7 question questionnaire administered in the radiography department, after receiving intervention or control, and signing consent form, but before scan Satisfaction assessed via a questionnaire ‐ no details given, data not extractable for this outcome |
|
| Notes | Aim: to improve knowledge & satisfaction by providing informed consent form 24 to 72 hours prior to appointment for IV pyelogram or IV contrast CT Conclusion: providing informed consent 24 to 72 hours in advance of diagnostic procedures did not improve knowledge and satisfaction |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40%; paper states that approximately 10% of intervention group patients were disqualified from study as could not be reached, denied receipt of information sheet or were reluctant to read information. No n values given for analyses, so unclear whether 72 included in analysis, or 89 initially recruited (i.e. < 40%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
O'Neill 1996a.
| Methods | Single‐centre RCT of patients scheduled to undergo wisdom tooth removal. Comprised of four groups, wisdom tooth leaflet (WTL) prompt by dentist to read the leaflet versus WTL only. Two control groups, one group were given a leaflet on dental health education and the other control group were given nothing. It was decided that the control group who were given the leaflet were to be excluded because this did not represent normal treatment. Control group two was therefore split between the two intervention groups Data analysed for each intervention separately versus half of the control group Presented in this table is information for Intervention WTL versus half of the control group |
|
| Participants | 66 patients were randomised into 4 groups. Patients were comparable according to age, sex and exodontia. All patients referred to the university hospital for surgical removal of wisdom teeth under local anaesthesia were invited to take part in the study. No exclusion criteria given Liverpool, United Kingdom Numbers of participants in analysis: 26 Intervention: 18 Control: 8 |
|
| Interventions |
Intervention 2 was a wisdom tooth leaflet only (WTL) Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal and standardised leaflet Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes |
Immediate knowledge: approximately 2 weeks prior to treatment Satisfaction with the consent process: questionnaire 1 to 7 (1 extremely satisfied, 7 extremely dissatisfied) |
|
| Notes | Aim: to determine the effect of an information leaflet on patients' knowledge of wisdom tooth removal and to assess satisfaction with the information supplied to patients attending the Oral Surgery Department Conclusion: the WTL and prompt group showed increased knowledge on retest. The WTL group showed a trend greater knowledge, however the control groups showed no improvement. Patient satisfaction, although greatest in the group given a leaflet without prompting, was not simply related to leaflet provision |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail; states 'randomised' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient detail; unclear how many initially randomised and then lost |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | High risk | Possibility of contamination |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail; unclear who the outcome assessors were |
O'Neill 1996b.
| Methods | Single‐centre RCT of patients scheduled to undergo wisdom tooth removal. Comprised of four groups, wisdom tooth leaflet (WTL) prompt by dentist to read the leaflet versus WTL only. Two control groups, one group were given a leaflet on dental health education and the other control group were given nothing. It was decided that the control group who were given the leaflet were to be excluded because this did not represent normal treatment. Control group two was therefore split between the two intervention groups Data analysed for each intervention separately versus half of the control group Presented in this table is information for Intervention WTL and prompt versus half of control group |
|
| Participants | 66 patients were randomised into four groups. Patients were comparable at baseline according to age, sex and exodontia. All patients referred to the university hospital for surgical removal of wisdom teeth under local anaesthesia were invited to take part in the study. No exclusion criteria Liverpool, United Kingdom Numbers of participants in analysis: 24 Intervention: 16 Control: 8 |
|
| Interventions |
Intervention 1 was a wisdom tooth leaflet plus prompting by the dentist to read the leaflet (WTL and prompt) Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal and standardised leaflet Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes |
Immediate knowledge: approximately 2 weeks prior to treatment satisfaction with the consent process: questionnaire 1 to 7 (1 extremely satisfied, 7 extremely dissatisfied) |
|
| Notes | Aim: to determine the effect of an information leaflet on patients' knowledge of wisdom tooth removal and to assess satisfaction with the information supplied to patients attending the Oral Surgery Department Conclusion: the WTL and prompt group showed increased knowledge on retest. The WTL group showed a trend greater knowledge, however the control groups showed no improvement. Patient satisfaction, although greatest in the group given a leaflet without prompting, was not simply related to leaflet provision |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail; states 'randomised' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient detail |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | High risk | Possibility of contamination |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail; unclear who outcome assessors were |
Olver 2009.
| Methods | RCT | |
| Participants | 101 participants were randomised, all of whom were to undergo chemotherapy. Patients who were chemotherapy naive who were not involved in clinical trials. Over 18 yrs, life expectancy of at least 12 weeks, English speaking, ability to provide consent. Participants were comparable at baseline, with age, gender, nationality, level of education and occupation measured Adelaide, Australia Numbers of participants in analysis: 101 Intervention: 47 Control: 54 |
|
| Interventions | CD ROM for intervention group versus written information for controls. The CD‐ROM was locally produced using the clinical researchers and local media. Content included the same information as the standard information sheet and consent forms about the treatment, however included additional information about cancer and its treatment. Not validated and only 28% of those using the CD‐ROM felt that they understood all the information. The control group had written information before signing the consent form about which no details were provided Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal and standardised leaflet Done with clinician?: distant without clinical Intervention type: non‐interactive audio‐visual Time of delivery: before admission |
|
| Outcomes |
Long‐term knowledge: 3 to 4 weeks after intervention/consent consultation Statistics note on long‐term recall data: took the median value for 3 different recall assessments (correct recall of number of drugs instead of recall of treatment length or recall of treatment goal) Generalised anxiety: HADs score at 3 to 4 weeks but not presented for individual trial arms in report. Raw data available for each trial arm from authors on request Satisfaction with the consent process: Dichotomous data but not presented for individual trial arms in report. Questionnaire at 3 ‐ 4 weeks asking how helpful was the CD ROM? Answers were Very helpful, somewhat helpful, unhelpful, unnecessary or can't remember. Helpful and very helpful were grouped together for a positive response. Raw data available for each trial arm from authors on request |
|
| Notes | Aim: to determine whether an interactive CD‐ROM improved cancer patients’ recall of chemotherapy treatment information over standard written information, and whether demographic, cognitive, and psychological factors better predicted recall than this format of delivery Conclusion: an interactive CD‐ROM did not improve cancer patients' recall of treatment information enough to warrant changes in consent procedures Author contacted: yes, raw data available for outcomes 'generalised anxiety' and 'satisfaction with the consent process' ‐ see meta‐analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Data managers performed randomisation using odd/even hospital identification numbers |
| Allocation concealment (selection bias) | High risk | Able to predict allocation due to method of quasi‐randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. Flow‐diagram page 199 (fig 1) clearly shows intention‐to‐treat and what happened to other patients after recruitment. However, no comment on how authors undertook ITT |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention not concealed for nursing staff who gave the patients the CD‐ROM or written information. Nurses were responsible for giving the majority of information to over 30% patients, therefore not blinding may introduce bias. Patients not concealed since consented to study, but interviewed individually by Psychologist administered tests. Clinicians were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Nurses giving out the CD‐ROM or written information are 'the principle nurses involved in treating the patient' that record the patient's recall for the primary outcome measure. Since they are recording a binary 'correct' or 'incorrect' recall outcome, this leads to high risk of bias of outcome assessment |
Paci 1999.
| Methods | Day before surgery patient was seen as outpatient. Patients unaware they were enrolled in a study and the nurse gave them a list of questions to ask in the consultation. The doctors recorded how many of the questions the patients asked and the type of anaesthetic was recorded. Patients were called 2 weeks later asked about satisfaction | |
| Participants | Patients attending for minor surgical procedures, ASA I and II; varicose vein stripping, inguinal herniorrhaphy, haemorrhoidectomy, trans‐urethral resection of the prostate, or hydrocelectomy Numbers of participants in analysis: 112 Intervention: 50 Control: 62 |
|
| Interventions | List of seven questions designed to facilitate the patients active engagement in the medical encounter was given to the patient prior to seeing the doctor. Patients were asked to think about these and ask any questions they thought relevant Intervention development: no details Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: prompt questions Time of delivery: on admission |
|
| Outcomes |
Rates of uptake: percentage of patients that choose general anaesthetic as opposed to regional anaesthetic Desire for further information: assessed by patient responses to 'did you think of any other questions you would have liked to have asked?' Satisfaction with the consent process: (yes/no answer ‐ dichotomous data) telephone interview 2 weeks after surgery |
|
| Notes | Aims: to assess an intervention aimed to facilitate the patient’s active engagement and satisfaction in the medical encounter Conclusion: method did not change proportion who chose specific anaesthesia, but does seem to improve patient participation in decision making process. Satisfaction unchanged |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Cluster randomisation by day of visit was performed |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed to the anaesthetists as the patients brought along their lists of questions. P164 column 2 last paragraph. Patients however were unaware they were in a trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40%; 73 patients in control group, 52 in survey group. 11 patients in control group (15%) and 2 (4%) in the survey group were unable to be reached for telephone interviews. No evidence of exclusion from analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants were unaware they were in a trial – completely blind Personnel were not blind as patients in study group brought along their question list The anaesthetists all said they would prefer regional anaesthetic so may have influenced consultation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The post operation telephone interview was blind with regard to the patient group |
Pesudovs 2006.
| Methods | RCT with patients being assigned to either standard verbal consent, where the clinician used a standardised information sheet which explained the risks and benefits of cataract surgery or the same verbal consent with the addition of a take home copy of the information sheet | |
| Participants | 50 patients undergoing elective cataract surgery who completed the study, unclear how many were initially eligible Australia Numbers of participants in analysis: 50 Intervention: 24 Control: 26 |
|
| Interventions | For the control group, they were consented verbally using an information sheet explaining the risks and benefits of cataract surgery. This was written in as simple terms as possible, minimising jargon, and including all important content areas for cataract surgery informed consent The reading age of the information was independently assessed to be 8 years of age The intervention group received the same consent, but were issued a copy of the information sheet to take home Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: evidence of fidelity/reliability of delivery Control characteristics: verbal consent and used a checklist Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes |
Immediate and long‐term knowledge: assessed using eight multiple choice questions. Administered immediately and at average of 79 days, standard deviation of 53 days after pre‐op completion. Seven questions were dichotomous, correct versus Incorrect (Q 2, 6, 7, 8, 9, 10a & 10b). To enter data for this review, the median effect size was calculated by ranking the difference between written information and no written information from the greatest positive change to the greatest negative change and the Median effect size calculated. This was Q9 for Immediate knowledge and Q6 for long‐term knowledge. Q11 was a continuous measure of number of risks recalled of a possible 5, expressed as percentage of group. To enter this data that original N values were calculated for "Number of risks recalled" and from this the "average number of risks recalled" was calculated Satisfaction with consent process: measured using three multiple choice questions, (Q 3,4 & 5). These were dichotomised (Q3: enough = satisfied, too much/too little = not satisfied. Q4&5: yes = satisfied, no = not satisfied) and a median effect size was calculated. Satisfaction was measured immediately and at long term. For review have entered immediate data as fewer confounding factors |
|
| Notes | Aim: to investigate the effect of giving written material on information recall from informed consent counselling for cataract surgery Conclusion: patient’s ability to recall information provided during the informed consent process is poor. Recall deteriorates with time after surgery and is not improved by the provision of written material Author contacted: yes, no further data available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation by hospital number (even numbers given written consent) ‐ quasi‐randomisation |
| Allocation concealment (selection bias) | High risk | No concealment as by hospital number |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | High risk | Large range of time for follow‐up questionnaire completion (average of 79 days, SD of 53 days) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded ‐ they were aware of the interventions delivered |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail; no comment on blinding of outcome assessment, but questionnaires completed by unblinded patients |
Phatouros 1995.
| Methods | Pilot RCT comparing two information leaflets for angiography and angioplasty, one detailed and one basic. Outcomes assessed by nurse post‐procedure | |
| Participants | 100 patients in an in‐ or out‐patient setting at an acute care hospital, due to undergo angiography or angioplasty. 81 completed the study Numbers of participants in analysis: 65 Intervention: 29 Control: 36 |
|
| Interventions | Control group received basic information sheet, providing no information regarding specific complications ‐ but advised this could be discussed if the patient required. Intervention group received a longer information sheet providing identical information, but included information detailing possible complications or the procedure, and those of IV contrast administration ‐ including mortality rate Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal and standardised leaflet Done with clinician?: distant without clinician Intervention type: written Time of delivery: on admission |
|
| Outcomes |
Anxiety with consent process: patients response to question regarding whether information provided made them more anxious ‐ measured on single Likert Scale. Results dichotomised by authors into agree/disagree that more anxious, data for 'don't know' excluded by us (but reported by authors) Rates of uptake: treatment uptake rate – paper reported that overall 3 participants did not have test, however, breakdown between groups not provided, so data not usable for review Satisfaction with consent process: measured by three Likert scale questions Desire for further information: single Likert Scale question N.B. For all dichotomous outcomes have excluded 'don't knows' ‐ although they were reported in paper Paper also measured patients self reported increased likelihood of cancelling procedure ‐ decided not to include as hypothetical treatment uptake rate not realistic. Whether extra time taken to answer patients questions (as reported by "proceduralist"). Paper states “Two patients from each group required specific information of the proceduralist. This took less than 5 min in each case". No assessment of how long the consent process was in general and impact 5 min made, so not extractable for this review |
|
| Notes | Aim: to test levels of risk disclosure with respect to anxiety, usefulness of information and treatment uptake rates Conclusion: information sheets were well accepted and provide a practical way of disseminating advice on procedure protocol, and adverse outcomes. Furthermore, the provision of extra information on adverse outcomes did not lead to a statistically significant increased level of subjective reported anxiety or risk of procedural cancellation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail; patients were randomised ‐ but no further details given |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40%; 81% response rate |
| Selective reporting (reporting bias) | High risk | Not all stated outcomes reported ‐ did not include all data e.g. extra time taken; whether information was useful |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
Raynes‐Greenow 2010.
| Methods | RCT, outcomes assessed at baseline, 1 week following intervention and 12 to 16 weeks post‐partum | |
| Participants | Pregnant women 37 weeks gestation, prior to entering labour. 627 were approached, 31 refused, 596 randomised. 395 intervention, 201 control at baseline ‐ 349 intervention, 178 control at 1 week ‐ 308 intervention & 146 control 3 months post‐partum. Participants were divided between 2 obstetric hospitals, enrolled Sept 2004 ‐ April 2006 Australia Numbers of participants in analysis: 596 Intervention: 395 Control: 201 |
|
| Interventions | 55 page decision aid, with or without audio guide, and four A3 page workbook, which women were given to take home and use. Content was information on analgesia options in labour Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent with special leaflet Done with clinician?: face‐to ‐face Intervention type: multiple interventions and decision aids Time of delivery: before admission |
|
| Outcomes |
Decisional conflict: self‐administered decisional conflict scale (DCS) validated & reliable questionnaire at baseline, 1 week after intervention (38 weeks gestation) and 3 months post‐partum. We used 1 week data Short‐term knowledge: 16 true/false questions at baseline, and 1 week after intervention. Questions related to general knowledge about labor analgesia risks & benefits. Questionnaire not validated Generalised anxiety: state component of short Spielberger anxiety scale (20 to 80, 20 = low anxiety), at baseline, 1 week & 3 months post‐partum. We used 1 week data as generalised anxiety outcome Satisfaction with decision making: using validated satisfaction with decision scale at 1 week and 3 months. (SWD validated scale used) Expressed as percent satisfied in a continuous manner Desire for further information: yes/no question "Enough information to make the decision?" at 1 week Rates of uptake: extracted from routinely collected hospital database. Percentage who had an epidural used for this review NB: that study design gave info to all women who were 37 weeks gestation, and had outcome data for all of them ‐ not everyone in the study did, or would have been expected to, go on an have an invasive intervention e.g. epidural. This makes interpretation of scores difficult ‐ was 'having an epidural' a favourable outcome or not? Sense of control: at 3 months, a 'participation in decision making' scale used ‐ patients chose one of 5 statements ‐ either 'I chose by myself' 'I chose after seriously considering my care‐providers opinion' ' shared decision with care‐providers' 'care provider made decisions' 'other'. We summed first 3 options as patient locus, and last 2 as physician locus of control (following D/W statistician) |
|
| Notes | Aim: to test the effectiveness of a decision aid for labour analgesia for primiparous women Conclusion: decision aid improved women's labour analgesia knowledge, without increasing anxiety. Significantly the decision aid group were more informed of labour analgesia options and considered the options of their care‐providers more often when making their decisions, thus improving informed decision making |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Remote telephone randomisation generated by computer using random variable block sizes |
| Allocation concealment (selection bias) | Low risk | Allocated at remote location |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40%. 1st follow‐up overall response rate was 88%, with no significant difference between the two groups. At 2nd follow‐up overall response was 78%, with no significant difference between groups |
| Selective reporting (reporting bias) | Low risk | Protocol published and checked, all outcomes reported |
| Other bias | Low risk | Authors combined two arms of intervention groups, but had pre‐specified this in protocol, so deemed not to introduce a high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants consented but most women who received control pamphlet didn't know it wasn't the intervention. Usual antenatal care providers trained on study protocol and risks of contamination, and blinded to content & format of the decision aid |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers were kept blinded to intervention allocation as much as possible. Research assistant followed an interview protocol at each follow‐up, and had been trained in the implementation of keeping the follow‐up standardised regardless of intervention allocation |
Rossi 2004.
| Methods | RCT comparing video information to standard verbal consent for patients undergoing surgical management of closed ankle fracture, at 2 acute care centres | |
| Participants | 48 consecutive patients from the 2 centres, enrolled over 9 months, or whom 100% completed initial follow‐up, and 77% completed 10‐week follow‐up. USA Numbers of participants in analysis: 48 Intervention: 23 Control: 25 |
|
| Interventions | 9‐minute video including information on risks, benefits and alternatives to treatment, as well as a description of the procedure, pertinent anatomy of the ankle, post‐operative care and follow‐up, possible complications and supplemental visual aids. Assessed using the Fry formula for readability, which detected a 7th grade reading level Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: non‐interactive audiovisual Time of delivery: on admission |
|
| Outcomes | Immediate and long‐term knowledge: knowledge assessed by a 12 MCQ questionnaire immediately after consent intervention, and at a mean of 9.8 weeks post‐surgery (range 3.7 to 20.0 weeks) | |
| Notes | Aim: to evaluate the effectiveness of a video tape information over standard verbal consultation in terms of knowledge for informed consent of ankle fracture surgery. Conclusion: patients who received information about their surgery on a video tape before giving their consent demonstrated a significant increase in knowledge recall compared to those consented verbally alone. It is unclear if the difference noted in this study is based on a presentation that is easier to understand or exposure to more accurate information given during the consent process SD for knowledge scores calculated using "usablestats.com" (assumed SD for intervention/control were the same) Author contact: no further data available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail; block randomisation by educational level, no details |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40%; 100% initial follow‐up, 23% lost to follow‐up at 10 weeks |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel who provided consent information were aware that a study was being conducted, but blinded to content of video Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data analysed by an independent statistician blinded to the study and control groups |
Rossi 2005.
| Methods | RCT‐single centre. Comparing patient comprehension and satisfaction with informed consent when using a video vs verbal discussion. | |
| Participants | 152 eligible of which 150 were randomised, undergoing knee arthroscopy under a single surgeon. Patients were comparable at baseline, age, gender, ethnicity and social characteristics recorded. Baseline characteristics: stratified for educational level below and above 12th grade in analysis (prior to randomisation) Taos, USA Numbers of participants in analysis: 180 Intervention: 73 Control: 77 |
|
| Interventions | Videotape prepared by the American Academy of Orthopaedic surgeons and National Association Orthopaedic nurses. Title: Arthroscopic knee surgery: return to action. Intervention was watched in a "patient education room" and a orthopaedic technologist or a physicians assistant provided unlimited time for patients to ask questions and sign a consent form Control group received conventional verbal consent (included unlimited question time) before signing a consent form. Intervention ranked as moderate to high quality Intervention development: standardised information with no modifications Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: non‐interactive audio‐visual Time of delivery: on admission |
|
| Outcomes |
Immediate knowledge: Statistics note for immediate knowledge: using percentage scores in continuous data for immediate knowledge, but note that not normally distributed (with 2 SDs being over 100%) Satisfaction with consent process |
|
| Notes | Aim: to test the hypothesis that video informed consent improves knee arthroscopy patient comprehension and satisfaction compared with traditional verbal informed consent Conclusion: video informed consent improves knee arthroscopy patient comprehension compared with traditional verbal informed consent Author contacted: yes, but no further data available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patient data collection packages (containing demographic forms and an outcome questionnaire) were labelled as video or verbal in equal numbers for each subgroup, shuffled face down, and picked from the top of a stack for each patient who was entered into the study |
| Allocation concealment (selection bias) | High risk | High risk of bias if researcher wanted to pick which one to give to the participant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40%; 2 participants dropped out |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
Rymeski 2010.
| Methods | RCT using an Internet based aid to look at the rate of recall of nine surgical complications | |
| Participants | 30 parents of children having elective inguinal hernia repair or hydroceles in an outpatient clinic. 30 included in analysis. Similar for age USA Numbers of participants in analysis: 30 Intervention: 17 Control: 13 |
|
| Interventions | an Internet programme called EMMI which parents were given a code to access over the Internet after a preoperative clinic before surgery around one month later Intervention development: standard information with no modifications Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: evidence of fidelity/reliability of delivery Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: non‐interactive audio‐visual Time of delivery: before admission |
|
| Outcomes |
Long‐term knowledge: recall of 9 complications of surgery measured approximately one month after the intervention, results collected on a blank sheet Statistics note: taken comparison at time point 2 instead of change over time in score. Using the P value of 0.06 and the excel spreadsheet SD calculated as 1.93 |
|
| Notes | Aim: to examine the effect of an Internet based aid to informed consent on parent recall of potential surgical complications Conclusion: although overall recall of potential surgical complication was poor in both groups, there was a trend towards a significant improvement in recall in the study group after viewing the Internet based programme |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Odds/even system based on the last digit of the patients medical record number ‐ quasi‐randomisation |
| Allocation concealment (selection bias) | High risk | Predictable randomisation due to the sequence generation method |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded Staff were aware of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Did not blind the marker of the recall sheets |
Shorten 2005.
| Methods | RCT, intervention delivered at 28 weeks. Women were surveyed on knowledge and decisional conflict at 28 weeks and again at 36 Rate of uptake‐ preferred preference for birth was recorded at 36 weeks and then compared to actual method of delivery |
|
| Participants | Pregnant women who had previously had a caesarean section and considering method of delivery of subsequent pregnancies (either for a trial of labour or an elective caesarean section) 252 eligible participants, 115 randomised to the intervention and 112 to the control. At follow up at 36 weeks ‐ 92 in the control group replied and 99 in their intervention group. For follow up for the decisional conflict score 99 in the intervention group and 88 in the control group had details recorded Australia Numbers of participants in analysis: 191 Intervention: 99 Control: 92 |
|
| Interventions | Decisional aid booklet using the Ottawa decision framework as a format and incorporating evidence based information, explicit probability illustrations and values clarification exercises Intervention development: designed for trial with reasonable efforts for validation/piloting Exposure: two exposures and two different interventions Training for delivery of intervention: no details Evaluation of the delivery of intervention: evidence of fidelity/reliability of delivery Control characteristics: no details Done with clinician?: distant without clinician Intervention type: decision aid Time of delivery: before admission |
|
| Outcomes |
Long‐term knowledge: change in score between 28 (before intervention given)and 36 weeks therefore long term (8 weeks) difference in knowledge reported as mean difference Decisional conflict ‐ decisional conflict scale was used, a score out of 5, change in score between 28 to 36 weeks was reported Rates of uptake: caesarean section or trial of labour were reported for each group. Results of a preferred elective caesarean section were reported as the invasive option |
|
| Notes | Aim: to determining if a decision aid about mode of birth after a caesarean section facilitates informed decision‐making about birth in subsequent pregnancies Conclusion: a decisional aid for women facing choices about birth after caesarean section is effective in improving knowledge and reducing decisional conflict. However, little evidence suggested that this process led to an informed choice |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomised numbers |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes containing a random allocation for each participant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40% |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Unclear risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail; measured by questionnaire, unclear if anonymised |
Solberg 2010.
| Methods | Cluster randomised controlled trial comparing an extensive intervention to standard care. Block randomised between eight centres | |
| Participants | Women considering treatment options for uterine fibroids, 526 assessed for eligibility, 226 were excluded as asymptomatic/incidental fibroids or as the participant had already made their treatment choice. 300 were randomised to 136 for the intervention and 164 for the control group. 24 from the intervention group and 32 from the control group were either lost to follow up or excluded because of language barriers. 112 were analysed in the intervention group and 132 in the control group. These values differ from the N values stated in the tables so the author was contacted. Difference due to skip patterns within the administered questionnaires (Intervention 103 and control 112) USA Numbers of participants in analysis: 215 Intervention: 103 Control: 112 |
|
| Interventions | Control group had information sheets and normal care, no extra decisional support The ICA intervention included the following components:
Intervention development: designed for trial with reasonable effort for validation/piloting Exposure: two exposures and two different interventions Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent and standardised leaflet Done with clinician?: face‐to‐face Intervention type: multiple interventions including decision aids Time of delivery: before admission |
|
| Outcomes |
Long‐term knowledge: knowledge score out of 5 measured 4 to 5 weeks following consultation Satisfaction with decision making: measured 4 to 5 weeks following the consultation (5 point Likert scale: 5 = satisfied) Sense of control: measured at 4 to 5 weeks following the consultation (5 point Likert scale: 5 = strongly agree that my decision was consistent with my personal values) Clinician satisfaction with the consent consultation: staff emailed |
|
| Notes | Aim: to test a decision support intervention for uterine fibroid interventions Conclusion: intervention participants were more aware of treatment options felt more informed and had more knowledge about fibroids and were more satisfied Clinicians were less satisfied with the intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Block randomised of population size of clinics, first 2 large centres sorted into opposite arms Nurses decided who should be enrolled based on whether they had made up their mind on treatment or not |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40% |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Insufficient detail in report, emailed authors Response from authors: surveys were completed by patients and returned by mail to a data collection centre which optically scanned surveys. Since there was no manual entry of surveys, there was no opportunity for the scanning operator to adjust data based on study arm. The completed data file was provided to the study statistician, who collated and summarized the data. The statistician was aware of group assignment for the purposes of data analysis |
Tait 2009.
| Methods | RCT. Subjects interviewed at baseline, prior to intervention to ascertain baseline knowledge. They were then interviewed after catheterization and again around two weeks later, to assess understanding and satisfaction with the information received. Responses to questions were transcribed verbatim and scored at a later time independently by two assessors who were blinded to the groups assignments. Guidelines for scoring were determined a priori to give a score out of 12. The scoring system was based on the Deaconess Informed Consent Comprehension Test and has been described previously | |
| Participants | 155 eligible consecutive adult patients. Aged over 18 yrs, scheduled for elective diagnostic cardiac catheterization. 13 were excluded and 7 were lost to follow up or withdrew, 71 were randomised to intervention, 71 to control. 135 were included in the analysis, with 66 in the control group and 69 in the intervention group Patients that had undergone a catheterization within the past 3 years and those undergoing emergency catheterization were excluded 13 participants declined participation and 7 were lost due to withdrawal or incomplete data Michigan, USA Numbers of participants in analysis: 135 Intervention: 69 Control: 66 |
|
| Interventions | Interactive computer programme using 2D & 3D graphics. 5th author is president and chief medical officer of the company who designed the intervention Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal consent and used a checklist Done with clinician?: distant without clinician Intervention type: interactive multimedia Time of delivery: on admission |
|
| Outcomes |
Immediate knowledge: 24 hours post procedure Short‐term knowledge: around 2 weeks Satisfaction with the consent process: overall satisfaction with perceptions of the message delivery were recorded using a ten point scale, 24 hours post procedure |
|
| Notes | Aim: evaluation of whether an interactive computer based information program for cardiac catheterization would result in improved patient understanding compared with standard verbal and written information Conclusion: subjects who received information about cardiac catheterization using the intervention demonstrated significantly greater improvement in overall early understanding from baseline than those receiving standard information Author contacted: yes, further information available for assessment of risk of bias |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | High risk | Insufficient detail in report, emailed authors. Author replied and stated that they "did not use any specific concealment technique such as sealed envelopes" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40% |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded Response from email regarding personnel: "On any given day the authors had no knowledge of which patient was randomised to which group, and had no knowledge of data collection" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Personnel who transcribed interviews and scored understanding were blinded to patient allocation. Response from email: "All data was collected by research assistants with no vested interest in the study. At the end of the study, measures of understanding were scored independently by two of the authors who were blinded to the allocation of subjects to the control or intervention groups" |
Thomas 2000.
| Methods | Well‐designed RCT of videotape information versus usual discussion for chemo/radiotherapy after initial consultation recommending the procedure | |
| Participants | 235 of whom 220 patients were randomised and analysed (113 to intervention and 107 to control). Inclusion = recommended by the clinician for either chemotherapy or radiotherapy with a diagnosis of cancer (breast, bowel, lymphoma or other) UK Numbers of participants in analysis: 135 Intervention: 69 Control: 66 |
|
| Interventions | Post‐consultation provided with a high‐quality professionally‐made 20 minute video consisting of comprehensive description of therapy, associated risks and patients describing their own experiences. Presented by Sue Lawley and Anton Rodgers. Participants took video home to watch after the initial consultation with clinician Control group: involved same initial consultation and everyone given routine written information booklets (BACUP ‐ British Association of Cancer United Patients) Intervention development: designed for trial with reasonable effort for validation/piloting Exposure: multiple exposures of same intervention Training for delivery of intervention: none needed Evaluation of the delivery of intervention: evidence of fidelity/reliability of delivery Control characteristics: verbal and standardised leaflet Done with clinician?: distant without clinician Intervention type: non‐interactive audiovisual Time of delivery: before admission |
|
| Outcomes |
Satisfaction with consent process: assessed on 5‐point scale, not validated but used in a previous audit and published before Statistics note for 'satisfaction with consent process' ‐ report gives frequency of responses for each point on a 5‐point Likert scale. Data dichotomised by review team into (very satisfied + satisfied) = satisfied and (equivocal, unsatisfied and very unsatisfied) = dissatisfied for each group. The 'Unknown category' was excluded. Data entered for proportion in each group that had 'satisfied' responses Generalised anxiety: HAD scores for depression and anxiety ‐ using scores for anxiety only at present, reversed scores for entry into data tables since lower anxiety score is an improvement in outcome (total score = 21) Outcomes measured 3 weeks into treatment (3 weeks after intervention) |
|
| Notes | Aim: to assess the benefits of receiving a cassette to take home following patients' first consultation Conclusion: improved satisfaction and reduced treatment‐related anxiety and depression |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated independently at trial centre: 240 randomisation cards written, 120 for control and intervention each. Cards placed in sealed opaque envelopes which were shuffled and placed in tight‐fitting trial boxes. Order of envelopes remained the same and batches of 20 were sent to lead trial nurses at each Unit for opening at a time after written consent |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. 100% follow‐up except for 'minor details' omitted on follow‐up from 12 patients |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail. Data analysed centrally, but unclear whether outcome assessors blinded to allocation |
Uzbeck 2009.
| Methods | RCT looking at more detailed written risk information versus briefer written risk information anxiety and satisfaction for bronchoscopy | |
| Participants | 142 eligible patients for elective day‐case bronchoscopy, of which 122 randomised and used in analysis in one centre Galway, Ireland Numbers of participants in analysis: 120 Intervention: 60 Control: 60 |
|
| Interventions | Written pages of risk information from 'Queensland Health consent form' providing basis for more detailed form before consent versus 'control group' of risk information from 'Addenbrooke's Hospital consent form'. Given in the day case suite and patients could read for 30‐40 minutes before post‐intervention evaluation completed. Then all patients discussed with doctor and consent taken Intervention development: modified from standardised information Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal and special leaflet Done with clinician?: distant without clinician Intervention type: written Time of delivery: on admission |
|
| Outcomes |
Generalised anxiety: measured using VAS scale and modified APAIS (Amsterdam pre‐op anxiety and information scale) pre‐ and post‐intervention. Immediate assessment after intervention Satisfaction with consent process: measured by four questions on 5‐point scale made for the study |
|
| Notes | Aim: to determine the effect of more detailed risk disclosure on anxiety and satisfaction Conclusion: provision of more detailed risk information before bronchoscopy may come at the cost of a small but significant increase in anxiety. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by random placement of thoroughly shuffled marked cards into sequentially numbered, sealed, opaque envelopes by staff not involved in the rest of the trial |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40%; only 3 participants lost to follow‐up (total 122) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail; if aware of which arm of trial randomised to, would impact on outcome assessment. However, since both groups given an information leaflet this may not have occurred |
Wadey 1997.
| Methods | RCT comparing effectiveness of standard consent process versus standard with repeat‐back for patients undergoing ACL reconstruction. Outcome‐knowledge was tested by questionnaire one month later, 3 question questionnaire | |
| Participants | 20 patients from referral‐based, outpatient, sport medicine clinic, referred for reconstruction of ACL‐deficient knee Calgary, Canada Numbers of participants in analysis: 20 Intervention: 8 Control: 12 |
|
| Interventions | Standard surgical consultation with 3 steps: 1. surgeon drawing diagram of knee. 2. surgeon explaining deficiency of ACL using 3D model. 3. Patient had the opportunity to hold and manipulate the model. Following this, intervention group were required to accurately verbalize the associated benefits and risks of the procedure back to the surgeon in their own words. Any errors were corrected until verbalization was accurate Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: all delivered by key research Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: face‐to‐face Intervention type: structured consent Time of delivery: before admission |
|
| Outcomes | Long‐term knowledge: one month later | |
| Notes | Aim: to determine if preoperative patient verbalization of the risks and benefits of anterior cruciate ligament reconstruction enhances understanding of the risks and benefits of that procedure. Conclusion: patients who verbalized the risks and benefits during their surgical consultation demonstrated a significantly greater understanding of the risks and benefits of an ACL reconstruction repair |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail. States 'randomly assigned' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail. Unclear whether author who measured outcome was blinded or not to the groups' allocation |
Walker 2007.
| Methods | RCT‐single centre. Patients were randomised to receive a flip chart intervention as part of the informed consent consultation or not. All patients received a standard informed consent discussion delivered by the radiologists as part of routine care. Outcome measurements were a mixture of objective and subjective topics and therefore were selected according to protocol guidelines | |
| Participants | 122 patients (inc in analysis) aged over 18 years who were referred for an image‐guided core biopsy (stereotactic or US‐guided) between February and October 2003. Patients were comparable at baseline, according to age, ethnicity and education level USA Numbers of participants in analysis: 122 Intervention: 63 Control: 59 |
|
| Interventions | 47 page flip chart. Containing artwork with photos and line drawings. Key topics covered: breast anatomy, common breast abnormalities, diagnostic procedures, treatment, reconstruction and clinical trials. Addressed voluntarism, disclosure and understanding elements of the informed consent process. The chart presented information at an introductory level, including colourful graphics, designed to be culturally sensitive and suitable for high and low grade literacy patients and their families Participants in the control group received a standard informed consent discussion delivered by the radiologists in practice at the breast health centre Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: face‐to‐face Intervention type: structured consent Time of delivery: on admission |
|
| Outcomes |
Immediate knowledge: post informed consent discussion, no information on questionnaire Satisfaction with consent process: Likert scale‐not validated Anxiety with consent process: STAI Self‐reported understanding: decision was taken to report the information post consultation as this was nearer to the consent procedure as opposed to the information gained from follow up Desire for further information: no raw data for this outcome. |
|
| Notes | Aim: to examine the effect of an educational intervention used during the informed consent discussion for women referred for breast biopsy Conclusion: the usual care consent process is effective for many but not all patients. Informed consent that employs visual aids may help overcome characteristics of the consent process that are ineffective for some patients |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generates sequence, random in equal proportions |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail; email from author stated that they used sealed, consecutive envelopes, no details as to whether these were opaque or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40% |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Email from author stated that the researchers were not blinded to the patient allocations |
Whelan 2003.
| Methods | Multicentre RCT in USA comparing decision boards with usual care in making decisions to have chemotherapy as adjuvant to breast cancer treatment | |
| Participants | 176 participants were randomised, 1 was then excluded. Total 175 in analysis: 82 in control and 93 in intervention. Patients were women with breast cancer who had been identified by clinician after primary surgery to be suitable for adjuvant chemotherapy. Similar demographics and baseline characteristics. Recruitment occurred between October 1995 and March 2000 Numbers of participants in analysis: 176 Intervention: 83 Control: 93 |
|
| Interventions | Both groups had initial meeting with clinician to discuss treatment options in 'usual fashion' Intervention group then met with a nurse who introduced and explained the intervention (decision board). Patients encouraged to ask question during and after the presentation. Given a copy to take home Control group: met with nurse after discussion as well (which was usual care) and encouraged to ask any questions. After meeting with the primary care nurse patients in both groups received the same lymph‐node negative breast cancer pamphlet. Patients were asked to return one week later to see the medical oncologist who would then answer any further questions regarding the consultation or materials received and to make a decision regarding treatment. The Decision Board "contains detailed information tailored to the individual on a patient’s treatment choices (chemotherapy or no chemotherapy); outcomes (recurrence or not); probability of outcomes and their meaning; and quality of life associated with treatment choice and outcome. … The treatment choices and outcomes are described by detailed information cards, and the probabilities of recurrence are described by colour‐coded probability wheels. Probabilities for recurrence with or without chemotherapy are tailored to the patient’s risk on the basis of tumour size and histologic tumour grade.” Format: "Empty initially, the patient and nurse read each information card and then attach it to the board." This aid has been developed to a high quality and used in previous publications by this research group (see Levine et al., 1992 ‐ ref in paper) Intervention development: designed for trial with reasonable effort for validation/piloting Exposure: multiple exposures of same intervention Training for delivery of intervention: structured/extensive training Evaluation of the delivery of intervention: no details Control characteristics: verbal and standardised leaflet Done with clinician?: face‐to‐face Intervention type: multiple interventions including decision aid Time of delivery: before admission |
|
| Outcomes |
Short‐term knowledge: 25 item questionnaire, validated, covers natural history of breast cancer, risk of recurrence, what chemo is and how given, benefits and risks of chemotherapy. Assessed at one week post‐intervention. Each question had true/false/unsure responses. Scored on percentage correct (total 100%) Satisfaction with decision making: 4‐item questionnaire (effective decision‐making subscale of Decisional Conflict Scale (DCS)), validated. Assessed at 1 week, 3, 6 and 12 months after intervention. Mean score obtained per person, they then reversed scores so 5 = strongly agree. Data extracted from graphical presentation in paper Generalised anxiety: assessed by STAI at 1 week, then at 3, 6, 12 months' Sense of control: asked if were offered a choice to have chemotherapy. Not validated Uptake rates: numbers deciding to have chemotherapy as expressed by patient Clinician satisfaction with decision making: modified form of questionnaire given to patients for satisfaction (from DCS). Mean scores given. Completed immediately after initial consultation Length of consultation: total taken for duration of first consultation and follow‐up after the intervention |
|
| Notes | Aim: to determine whether adding the Decision Board to the medical consultation improved patient knowledge and satisfaction compared with the medical consultation alone Conclusion: when making decisions regarding adjuvant chemotherapy, patients with early breast cancer who had been exposed to the Decision Board had better knowledge of the disease and treatment options and greater satisfaction with their decision making than those who received the standard consultation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail: patients were stratified by medical oncologist/primary care nurse team before randomisation. No details on methods of randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40%. Low attrition rates: only 1 patient lost to follow‐up in short‐term analysis. For long term follow‐up: 78/92 control group followed‐up at 12 months’ and 72/81 = 13% attrition rate over 1 year |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail. No information on who the outcome assessors were or whether blinded |
Wilhelm 2009.
| Methods | RCT, patients shown a DVD one week prior to surgery, data collected in outpatients one week after discharge | |
| Participants | Patients undergoing laparoscopic cholecystectomy between May 2005 and May 2007 (259 recruited and 212 completed) USA Numbers of participants in analysis: 212 Intervention: 114 Control: 98 |
|
| Interventions | DVD with 5 parts, 26 minutes, based on discussion with surgeon and actor patient with the use of written text and picture information and complex 3D computer animation Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: no details Control characteristics: verbal consent Done with clinician?: distant without clinician Intervention type: non‐interactive audio‐visual Time of delivery: before admission |
|
| Outcomes |
Short‐term knowledge: knowledge at 1 week post op ‐ knowledge test score out of 30 Satisfaction with the consent process: 1 week post op ‐ satisfaction score out of 5 |
|
| Notes | Aims: to evaluate the impact of an extended education in the form of a DVD on patients understanding of cholecystectomy Conclusions: extended education using additional tools such as multimedia DVD has a significant impact on post‐operative patient knowledge and improves the quality of informed consent Author contacted: details on risk of bias and standard deviations gained for short‐term knowledge and satisfaction with the consent process |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Report quotes 'using a specifically designed randomisation list'. Author confirms this in email, using a die |
| Allocation concealment (selection bias) | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40%; 81.8% completed questionnaires |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | High risk | Patient groups not comparable at baseline with respect to education level, intervention group had higher education status |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
Wong 2006.
| Methods | RCT looking at effect of a decisional‐aid booklet on choice of termination method, measures of effective decision making (including risk perception, attitudes and knowledge of both the medical and surgical methods), decisional conflict, anxiety and usefulness of the leaflet | |
| Participants | 1177 patients were approached, of which 326 were randomised to receive either intervention or control. These were patients visiting the Unit for 'Fertility Control' for pregnancy termination (under 9 weeks of gestation) presenting for a decision‐discussion with a clinician and then booking to return in a fortnight for the procedure. Participants were comparable at baseline, age, marital status, education level, ethnicity, prior obstetric history were recorded Leeds, United Kingdom Numbers of participants in analysis: 191 Intervention: 103 Control: 88 |
|
| Interventions | Decisional‐aid leaflet with previous validation and piloting ‐ of good quality. The booklet was produced by the research team which had been previously piloted and based on evidence on EBM. (references given) Given out in the waiting room before discussion with the clinician. Control group given a leaflet on contraception so everyone seen to be reading Intervention development: designed for trial with reasonable efforts for validation/piloting Exposure: once Training for delivery of intervention: none needed Evaluation of the delivery of intervention: evidence of fidelity/reliability of delivery Control characteristics: verbal consent and dummy intervention Done with clinician?: face‐to‐face Intervention type: decision aid Time of delivery: before admission |
|
| Outcomes |
Rates of uptake: postponement of clinical interventions ‐ from clinic data Statistics note on rates of uptake: taking medical or surgical termination to both be invasive procedures in this review, and contrasting to rates of refusal (continuing with pregnancy, not going through with termination). Focus on rate of uptake of invasive procedures requiring consent rather than refusal Satisfaction with decision making: measured by effective sub‐scale of 'decisional conflict' tool in DCS questionnaire ‐ previously validated and reliability‐checked Immediate knowledge: scale score, no information on what tested, questionnaire assessment Anxiety with decision making: from modified STAI, previously validated in modified form |
|
| Notes | Aim: to evaluate the effectiveness of a decision aid to help women choose between surgical and medical methods of pregnancy termination. “Specifically, the study investigated the impact of the decision aid to:
Increase the perceived usefulness of written information provided by the termination services” Conclusion: “A simple decision‐aid leaflet read before a routine consultation enables women to make better and more informed choices about which method of pregnancy termination to have. In addition, as the intervention had a sustained effect on women’s views, it is feasible that a longer term impact may be observed on women’s subsequent use of termination services" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling cards or envelopes, drawing of lots |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40%. Note ‐ not used intention to treat analysis, but equal loss to follow‐up in each group and unlikely to bias outcome |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient detail. Dummy intervention used in control group, but unclear if clinicians blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from report: “to minimise bias in this real‐world setting, study identifiers were only referred to at the end of the recruitment phase during data entry by SSMW” |
Yucel 2005.
| Methods | Multicentre‐RCT. Patients received either an informed consent form or a more detailed informed consent form STAI measured by questionnaire prior to intervention. Trait anxiety was repeated after the intervention |
|
| Participants | 265 eligible patients, 74 of whom were excluded. Remaining 191 were randomised: 103 to intervention, 88 to control. Patients referred between Nov 2003 and march 2004 for CT or excretory urography requiring IV contrast material. Must have graduated from primary school Turkey Numbers of participants in analysis: 191 Intervention: 103 Control: 88 |
|
| Interventions | Control group had a consent form with brief information including only the most common risks and risk factors associated with IVCM. The intervention group had more comprehensive information Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: no details Evaluation of the delivery of intervention: no details Control characteristics: verbal and standardised leaflet Done with clinician?: distant without clinician Intervention type: written Time of delivery: on admission |
|
| Outcomes |
Anxiety with consent process: authors measured state and trait anxiety prior to the consent process, and again after the intervention. We have taken trait anxiety after intervention as our outcome measure because trait anxiety measures how the patients feel in that particular situation or moment whereas state anxiety evaluates how the patient feels independently of this situation or condition at that moment The authors also asked patients if they desired to be informed about intra venous contrast material prior to the consent procedure. Authors note that informed consent is not usually obtained in Turkey. This outcome was not included in our study because it did not meet the criteria of desire for further information |
|
| Notes | Aim: to compare the effect of two different consent forms on patients; anxiety level prior to intravenous contrast material injection Conclusion: informed consent including general information about the risk factors and potential adverse reactions of IV contrast material reduced anxiety level, but detailed informed consent increased anxiety level before the procedure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Divided according to file number, odd or even number. Uncertain about how patients were selected, states 'cooperative' patients |
| Allocation concealment (selection bias) | High risk | Clear by the file number which group they were allocated to |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient detail |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No identified areas of concern |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
Zite 2011.
| Methods | Two armed RCT comparing a low literacy level information sheet/consent form to the standard, higher reading age form currently used. Patients were randomised and shown form, then knowledge was assessed immediately | |
| Participants | Between May and July 2010, 210 participants who were undergoing tubal sterilisation were eligible, of whom 201 completed and were analysed USA Numbers of participants in analysis: 201 Intervention: 102 Control: 99 |
|
| Interventions | A low‐literacy version of the Medicaid‐Title XIX SCF, written at the 6th grade reading level compared to the standard "Medicaid‐Title XIX SCF" which is a one page document, currently used throughout the United States and written at a high school reading level Intervention development: designed for trial with no validation Exposure: once Training for delivery of intervention: all delivered by key researcher Evaluation of the delivery of intervention: no details Control characteristics: verbal and standardised leaflet Done with clinician?: distant without clinician Intervention type: written Time of delivery: before admission |
|
| Outcomes |
Immediate knowledge: assessed immediately via five closed‐ended questions, addressing content outlined on both the standard and the low‐literacy Medicaid‐Title XIX SCF, to assess sterilization‐related knowledge. An overall sterilization‐related knowledge composite was calculated based on participants’ total number of correct responses to these five close‐ended items. Participants were categorized as having limited (zero to three correct responses) or adequate (four or more correct responses) sterilization‐related knowledge Women’s preferences for either the standard or the low‐literacy Medicaid‐Title XIX SCF |
|
| Notes | Aim: To estimate whether Medicaid‐Title XIX SCF format “standard” compared with “low literacy” was associated with women’s understanding of tubal sterilization Conclusion: The study's results support replacing the standard Medicaid‐Title XIX SCF with the low‐literacy version to foster increased understanding of sterilization |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated sequence with permuted blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Assignment was concealed by placing a photocopy of either the standard or the low‐literacy Medicaid‐Title XIX SCF into a beige manila file folder. The research assistant always opened the next manila file folder in the pre‐randomised stack, which allocated participants to one of the two study groups in accordance with the randomisation sequence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition less than 40%. 1% loss to follow up |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | Participants were paid $10 to compensate their time, but unlikely to affect outcome |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Knowledge assessment not blinded and performed by research assistant. Coding of responses regarding preference of forms was blinded |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ader 1992 | No control group within the randomised arms of trial. Comparing interactive with non‐interactive video of same material, but no usable data for the review |
| Altaie 2011 | No usable data, Table 4 |
| Broyles 1992 | Looking at an intervention for a procedure (neonatal mechanical ventilation) which does not require informed consent |
| Clark 2011 | No usable data, Table 4 |
| Dawes 1992 | Control group not randomised. Randomisation happened between two interventions, therefore no comparable data for this review |
| Dawes 1993 | Study examining the patients' attitudes towards informed consent. No outcomes relevant to our review. Same data set as Dawes 1992 |
| Eggers 2007 | Not an RCT ‐ no randomisation performed |
| Finch 2009 | No usable data, Table 4 |
| Fink 2010a | Same data as Fink 2010 but no outcomes relevant to our review |
| Graham 2000 | Not targeting consent – instead targeting all pregnant women to improve knowledge about prenatal testing |
| Grawe 2010 | Impact of general education (not on consent or decision making) on pain |
| Gyomber 2010 | No usable data, Table 4 |
| Hewison 2001 | Not about consent for invasive procedure, but information for all pregnant women about prenatal testing |
| Hilzenrat 2006 | Anxiety for liver biopsy, not targeting consent or decision making process |
| Jlala 2010 | Anxiety for surgery, not targeting consent or decision making process |
| Johnson 2011 | No control group ‐ comparator group has usual consent and customised written handout |
| Kasper 2008 | Immunotherapy ‐ not invasive healthcare procedure |
| Lembcke 1998 | Three leaflets as three intervention arms in trial, no control group. Unable to differentiate between leaflets as individual leaflets not accessible |
| Lipp 1991 | Not an RCT |
| Migden 2008 | No usable data, Table 4 |
| Nagle 2008 | Information given to all pregnant women to improve their knowledge of prenatal testing – not targeted at women having an intervention that requires consent |
| O'Cathain 2002 | Detailed information around areas of pregnancy and childbirth but no specific healthcare procedure addressed |
| Roth‐Isigkeit 2001a | Not invasive healthcare procedure |
| Scanlan 2003 | No usable data, Table 4 |
| Schenker 2010 | Editorial discussing paper by Tait 2009 |
| Shurnas 2003 | Only difference in the two groups was that one group signed the list of risks that a surgeon used as a prompt during consultation whereas the other group did not sign it. Neither group had any longer exposure to the list or any use of the list. Consensus opinion of the review authors that signing the form not an intervention that directly aims to improve informed consent |
| Stanley 1998 | No usable data, Table 4 |
| Steckelberg 2011 | Interventions to improve screening uptake, but not looking at interventions to help choose between screening methods |
| Taylor 2010 | Randomisation collapsed before analysis of results |
| Wright 2010 | Anxiety for procedure, not about consent or decision making |
Differences between protocol and review
The protocol (Kinnersley 2011) and the review differ in the following ways:
We developed three timeframes for the outcome 'knowledge'. and categorised results into immediate, short‐term and long‐term assessments for this outcome.
The subgroup analyses of face‐to‐face versus distant intervention use, classification of intervention and timing of intervention were developed post‐hoc.
Our approach diverged from that stated in the protocol by presenting meta‐analyses including those with high heterogeneity, and including non‐parametric data in results, in a comprehensive approach.
Finally, Katie Phillips, Katherine Savage, Ben Morgan, Elinor Farrell, Vicky Lewis and Robert Whistance joined the review team after the protocol was completed.
Contributions of authors
Glyn Elwyn, Paul Kinnersley, Bethan Stephens, Adrian Edwards and Jane Blazeby were involved in conceiving the idea for the review. Mala Mann supervised the search strategy. Katie Phillips and Katherine Savage led the searching, data extraction and analyses with Ben Morgan, Elinor Farrell, Vicky Lewis and Robert Whistance. Mark Kelly provided statistical expertise. All the authors were involved in the consideration and interpretation of the results. Katie Phillips, Katherine Savage, Adrian Edwards and Paul Kinnersley produced the final report.
Sources of support
Internal sources
-
School of Medicine, Cardiff University, UK.
Infrastructure and salary support to Kinnersley, Elwyn, Stephens, Savage, Lewis, Edwards and Kelly.
-
School of Social and Community Medicine, Bristol University, UK.
Infrastructure and salary support to Blazeby.
-
Support Unit for Research Evidence, Cardiff University, UK.
Infrastructure and salary support to Mann.
External sources
-
NIHR, UK.
Whistance holds a Doctoral Research Fellowship award
Declarations of interest
Glyn Elwyn, Adrian Edwards, Paul Kinnersley have been involved in the evaluation of decision aids. None of the authors have any financial interest in the results of the review.
New
References
References to studies included in this review
Agre 1994a {published data only}
- Agre P, Kurtz RC, Krauss BJ. A randomized trial using videotape to present consent information for colonoscopy. Gastrointestinal Endoscopy 1994;40(3):271‐6. [DOI] [PubMed] [Google Scholar]
Agre 1994b {published data only}
- Agre P, Kurtz RC, Krauss BJ. A randomized trial using videotape to present consent information for colonoscopy. Gastrointestinal Endoscopy 1994;40(3):271‐6. [DOI] [PubMed] [Google Scholar]
Armstrong 1997 {published data only (unpublished sought but not used)}
- Armstrong AP, Cole AA, Page RE. Informed consent: Are we doing enough?. British Journal of Plastic Surgery 1997;50(8):637‐40. [DOI] [PubMed] [Google Scholar]
Armstrong 2010 {published and unpublished data}
- Armstrong AW, Alikhan A, Cheng LS, Schupp C, Kurlinkus C, Eisen DB. Portable video media for presenting informed consent and wound care instructions for skin biopsies: a randomized controlled trial. British Journal of Dermatology 2010;163(5):1014‐9. [DOI] [PubMed] [Google Scholar]
Ashraff 2006 {published data only (unpublished sought but not used)}
- Ashraff S, Malawa G, Dolan T, Khanduja V. Prospective randomised controlled trial on the role of patient information leaflets in obtaining informed consent. ANZ Journal of Surgery 2006;76(3):139‐41. [DOI] [PubMed] [Google Scholar]
Astley 2008a {published data only}
- Astley CM, Chew DP, Aylward PE, Molloy DA, Pasquale CG. A randomised study of three different Informational aids prior to coronary angiography, measuring patient recall, satisfaction and anxiety. Heart Lung and Circulation 2008;17(1):25‐32. [DOI] [PubMed] [Google Scholar]
Astley 2008b {published data only}
- Astley CM, Chew DP, Aylward PE, Molloy DA, Pasquale CG. A randomised study of three different informational aids prior to coronary angiography, measuring patient recall, satisfaction and anxiety. Heart Lung and Circulation 2008;17(1):25‐32. [DOI] [PubMed] [Google Scholar]
Bekker 2004 {published and unpublished data}
- Bekker HL, Hewison J, Thornton JG. Applying decision analysis to facilitate informed decision making about prenatal diagnosis for Down syndrome: a randomised controlled trial. Prenatal Diagnosis 2004;24(4):265‐75. [DOI] [PubMed] [Google Scholar]
Bennett 2009a {published data only (unpublished sought but not used)}
- Bennett DL, Dharia CV, Ferguson KJ, Okon AE. Patient‐physician communication: informed consent for imaging‐guided spinal injections. Journal of the American College of Radiology 2009;6(1):38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 2009b {published data only (unpublished sought but not used)}
- Bennett DL, Dharia CV, Ferguson KJ, Okon AE. Patient‐physician communication: informed consent for imaging‐guided spinal injections. Journal of the American College of Radiology 2009;6(1):38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bollschweiler 2008 {published data only (unpublished sought but not used)}
- Bollschweiler E, Apitzsch J, Obliers R. Results of a prospective randomized multicenter study of patients before cholecystectomy. Annals of Surgery 2008;248(4):694. [DOI] [PubMed] [Google Scholar]
Chan 2002 {published data only (unpublished sought but not used)}
- Chan Y, Irish JC, Wood SJ, Rotstein LE, Brown DH, Gullane PJ, et al. Patient education and informed consent in head and neck surgery. Archives of Otolaryngology ‐ Head and Neck Surgery 2002;128(11):1269‐74. [DOI] [PubMed] [Google Scholar]
Chantry 2010 {published data only}
- Chantry CJ, Byrd RS, Sage AC, Calvert EE. Video versus traditional informed consent for neonatal circumcision. Acta Paediatrica 2010;99(9):1418‐24. [DOI] [PubMed] [Google Scholar]
Cornoiu 2010a {published and unpublished data}
- Cornoiu A, Beischer AD, Donnan L, Graves S, Steiger R. Multimedia patient education to assist the informed consent process for knee arthroscopy. ANZ Journal of Surgery 2010;81(3):176‐80. [DOI] [PubMed] [Google Scholar]
Cornoiu 2010b {published and unpublished data}
- Cornoiu A, Beischer AD, Donnan L, Graves S, Steiger R. Multimedia patient education to assist the informed consent process for knee arthroscopy. ANZ Journal of Surgery 2010;81(3):176‐80. [DOI] [PubMed] [Google Scholar]
Cowan 2007 {published and unpublished data}
- Cowan EA, Calderon Y, Gennis P, Macklin R, Ortiz C, Wall SP. Spanish and English video‐assisted informed consent for intravenous contrast administration in the emergency department: a randomized controlled trial. Annals of Emergency Medicine 2007;49(2):221‐30. [DOI] [PubMed] [Google Scholar]
Danino 2006 {published data only}
- Danino AM, Sultan SD, Weber ID, Herve C, Malka G. Effect of information by images on patients' anxiety and comprehension before esthetic surgery on the abdominal wall: a prospective randomised trial with 60 patients [Impact de l'introduction d'images sur l'anxiete et la connaissance des patientes avant une abdominoplastie esthetique: Une etude prospective randomisee incluant 60 patientes]. Annales de Chirurgie Plastique et Esthetique 2006;51(6):517‐24. [DOI] [PubMed] [Google Scholar]
Deyo 2000 {published data only (unpublished sought but not used)}
- Deyo RA, Cherkin DC, Weinstein J, Howe J, Ciol M, Mulley Jr AG. Involving patients in clinical decisions: impact of an interactive video program on use of back surgery. Medical Care 2000;38(9):959‐69. [DOI] [PubMed] [Google Scholar]
Elfant 1995 {published data only (unpublished sought but not used)}
- Elfant AB, Korn C, Mendez L, Pello MJ, Peikin SR. Recall of informed consent after endoscopic procedures. Diseases of the Colon and Rectum 1995;38(1):1‐3. [DOI] [PubMed] [Google Scholar]
Enzenhofer 2004 {published data only}
- Enzenhofer M, Bludau HB, Komm N, Wild B, Mueller K, Herzog W, et al. Improvement of the educational process by computer‐based visualization of procedures: randomized controlled trial. Journal of Medical Internet Research 2004;6(2):e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Felley 2008 {published data only}
- Felley C, Perneger TV, Goulet I, Rouillard C, Azar‐Pey N, Dorta G, et al. Combined written and oral information prior to gastrointestinal endoscopy compared with oral information alone: a randomized trial. BMC Gastroenterology 2008;8(22):n.p. [DOI: 10.1186/1471-230X-8-22] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fink 2010 {published and unpublished data}
- Fink AS, Prochazka AV, Henderson WG, Bartenfeld D, Nyirenda C, Webb A, et al. Enhancement of surgical informed consent by addition of repeat back: a multicenter, randomized controlled clinical trial. Annals of Surgery 2010;252(1):27‐36. [DOI] [PubMed] [Google Scholar]
Friedlander 2011 {published data only}
- Friedlander JA, Loeben GS, Finnegan PK, Puma AE, Zhang X, Zoeten EF, et al. A novel method to enhance informed consent: a prospective and randomised trial of form‐based versus electronic assisted informed consent in paediatric endoscopy. Journal of Medical Ethics 2011;37(4):194‐200. [DOI] [PubMed] [Google Scholar]
Garden 1996 {published and unpublished data}
- Garden AL, Merry AF, Holland RL, Petrie KJ. Anaesthesia information ‐ what patients want to know. Anaesthesia and Intensive Care 1996;24(5):594‐8. [DOI] [PubMed] [Google Scholar]
Garrud 2001 {published data only (unpublished sought but not used)}
- Garrud P, Wood M, Stainsby L. Impact of risk information in a patient education leaflet. Patient Education and Counseling 2001;43(3):301‐4. [DOI] [PubMed] [Google Scholar]
Gerancher 2000 {published data only (unpublished sought but not used)}
- Gerancher JC, Grice SC, Dewan DM, Eisenach J. An evaluation of informed consent prior to epidural analgesia for labor and delivery. International Journal of Obstetric Anesthesia 2000;9(3):168‐73. [DOI] [PubMed] [Google Scholar]
Goel 2001 {published data only (unpublished sought but not used)}
- Goel V, Sawka CA, Thiel EC, Gort EH, O'Connor AM. Randomized trial of a patient decision aid for choice of surgical treatment for breast cancer. Medical Decision Making 2001;21:1‐6. [DOI] [PubMed] [Google Scholar]
Greening 1999 {published data only (unpublished sought but not used)}
- Greening J, Bentham P, Stemman J, Staples V, Ambegaokar S, Upthegrove R, et al. The effect of structured consent on recall of information pre‐ and post‐electroconvulsive therapy: a pilot study. Psychiatric Bulletin 1999;23(8):471‐4. [Google Scholar]
Heller 2008 {published data only (unpublished sought but not used)}
- Heller L, Parker PA, Youssef A, Miller MJ. Interactive digital education aid in breast reconstruction?. Plastic and Reconstructive Surgery 2008;122(3):717‐24. [DOI] [PubMed] [Google Scholar]
Henry 2008 {published data only (unpublished sought but not used)}
- Henry E, Brown T, Bartlett C, Massoud E, Bance M. Informed consent in otologic surgery: Prospective randomized study comparing risk recall with an illustrated handout and a nonillustrated handout. Journal of Otolaryngology ‐ Head and Neck Surgery 2008;37(2):273‐8. [PubMed] [Google Scholar]
Hermann 2002 {published data only}
- Hermann M. 3‐dimensional computer animation‐‐a new medium for supporting patient education before surgery Acceptance and assessment of patients based on a prospective randomized study‐‐picture versus text [Dreidimensionale Computeranimation‐‐neues Medium zur Unterstutzung des Aufklarungsgesprachs vor Operationen Akzeptanz und Bewertung der Patienten anhand einer prospektiv randomisierten Studie‐‐Bild versus Text]. Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen 2002;73(5):500‐7. [DOI] [PubMed] [Google Scholar]
Hong 2009 {published data only}
- Hong P, Makdessian AS, Ellis DAF, Taylor SM. Informed consent in rhinoplasty: prospective randomized study of risk recall in patients who are given written disclosure of risks versus traditional oral discussion groups. Journal of Otolaryngology ‐ Head and Neck Surgery 2009;38(3):369‐74. [PubMed] [Google Scholar]
Hopper 1994 {published data only}
- Hopper KD, Zajdel M, Hulse SF, Yoanidis NR, TenHave TR, Labuski MR, et al. Interactive method of informing patients of the risks of intravenous contrast media. Radiology 1994;192(1):67‐71. [DOI] [PubMed] [Google Scholar]
Johnson 2006 {published data only (unpublished sought but not used)}
- Johnson BR, Schartz A, Goldberg J, Koerber A. A chairside aid for shared decision making in dentistry: a randomized controlled trial. Journal of Dental Education 2006 Feb;70(2):133‐41. [PubMed] [Google Scholar]
Kain 1997 {published and unpublished data}
- Kain ZN, Wang SM, Caramico LA, Hofstadter M, Mayes LC. Parental desire for perioperative information and informed consent: a two‐phase study. Anesthesia and Analgesia 1997;84(2):299‐306. [DOI] [PubMed] [Google Scholar]
Kang 2009a {published and unpublished data}
- Kang EY, Fields HW, Kiyak A, Beck FM, Firestone AR. Informed consent recall and comprehension in orthodontics: traditional vs improved readability and processability methods. American Journal of Orthodontics and Dentofacial Orthopedics 2009;136(4):488.e1‐e13. [DOI] [PubMed] [Google Scholar]
Kang 2009b {published and unpublished data}
- Kang EY, Fields HW, Kiyak A, Beck FM, Firestone AR. Informed consent recall and comprehension in orthodontics: traditional vs improved readability and processability methods. American Journal of Orthodontics and Dentofacial Orthopedics 2009;136(4):488.e1‐e13. [DOI] [PubMed] [Google Scholar]
Langdon 2002 {published data only}
- Langdon IJ, Hardin R, Learmonth ID. Informed consent for total hip arthroplasty: does a written information sheet improve recall by patients?. Annals of the Royal College of Surgeons of England 2002;84(6):404‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavelle‐Jones 1993 {published data only (unpublished sought but not used)}
- Lavelle‐Jones C, Byrne DJ, Rice P, Cuschieri A. Factors affecting quality of informed consent. BMJ 1993;306(6882):885‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luck 1999 {published and unpublished data}
- Luck A, Pearson S, Maddern G, Hewett P. Effects of video information on precolonoscopy anxiety and knowledge: a randomised trial. Lancet 1999;354(9195):2032‐5. [DOI] [PubMed] [Google Scholar]
Makdessian 2004 {published data only}
- Makdessian AS, Ellis DA, Irish JC. Informed consent in facial plastic surgery: effectiveness of a simple educational intervention. Archives of Facial Plastic Surgery 2004;6(1):26‐30. [DOI] [PubMed] [Google Scholar]
Mason 2003 {published data only}
- Mason V, McEwan A, Walker D, Barrett S, James D. The use of video information in obtaining consent for female sterilisation: a randomised study. British Journal of Obstetrics and Gynaecology 2003;110(12):1062‐71. [PubMed] [Google Scholar]
Masood 2007 {published data only (unpublished sought but not used)}
- Masood J, Hafeez A, Wiseman O, Hill JT. Informed consent: are we deluding ourselves? A randomized controlled trial. British Journal of Urology International 2007;99(1):4‐5. [DOI] [PubMed] [Google Scholar]
Mauffrey 2008 {published and unpublished data}
- Mauffrey C, Prempeh EM, John J, Vasario G. The influence of written information during the consenting process on patients' recall of operative risks: a prospective randomised study. International Orthopaedics 2008;32(4):425‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishra 2010a {published and unpublished data}
- Mishra PK, Mathias H, Millar K, Nagrajan K, Murday A. A randomized controlled trial to assess the effect of audiotaped consultations on the quality of informed consent in cardiac surgery. Archives of Surgery 2010;145(4):383‐8. [DOI] [PubMed] [Google Scholar]
Mishra 2010b {published and unpublished data}
- Mishra PK, Mathias H, Millar K, Nagrajan K, Murday A. A randomized controlled trial to assess the effect of audiotaped consultations on the quality of informed consent in cardiac surgery. Archives of Surgery 2010;145(4):383‐8. [DOI] [PubMed] [Google Scholar]
Morgan 2000 {published and unpublished data}
- Morgan MW, Deber RB, Llewellyn‐Thomas HA, Gladstone P, Cusimano RJ, O'Rourke K, et al. Randomized, controlled trial of an interactive videodisc decision aid for patients with ischemic heart disease. Journal of General Internal Medicine 2000;15(10):685‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nadeau 2010 {published and unpublished data}
- Nadeau DP, Rich JN, Brietzke SE. Informed consent in pediatric surgery: do parents understand the risks?. Archives of Otolaryngology ‐ Head and Neck Surgery 2010;136(3):265‐9. [DOI] [PubMed] [Google Scholar]
Neary 2010 {published data only}
- Neary PM, Sung R, Corrigan M, O'Donovan M, Cahill RA, Redmond HP. The benefits of an interactive, individualized online patient pathway for patients undergoing minimally invasive radioguided parathyroidectomy: a prospective, double‐blinded, randomized clinical trial. Surgical Innovation 2010;17(3):236‐41. [DOI] [PubMed] [Google Scholar]
Neptune 1996 {published data only (unpublished sought but not used)}
- Neptune SM, Hopper KD, Houts PS, Hartzel JS, Have TR, Loges IRJ. Take‐home informed consent for intravenous contrast media. Investigative Radiology 1996;31(2):109‐13. [DOI] [PubMed] [Google Scholar]
O'Neill 1996a {published data only}
- O'Neill P, Humphris GM, Field EA. The use of an information leaflet for patients undergoing wisdom tooth removal. British Journal of Oral and Maxillofacial Surgery 1996;34(4):331‐4. [DOI] [PubMed] [Google Scholar]
O'Neill 1996b {published data only}
- O'Neill P, Humphris GM, Field EA. The use of an information leaflet for patients undergoing wisdom tooth removal. British Journal of Oral and Maxillofacial Surgery 1996;34(4):331‐4. [DOI] [PubMed] [Google Scholar]
Olver 2009 {published and unpublished data}
- Olver IN, Whitford HS, Denson LA, Peterson MJ, Olver SI. Improving informed consent to chemotherapy: a randomized controlled trial of written information versus an interactive multimedia CD‐ROM. Patient Education and Counseling 2009;74(2):197‐204. [DOI] [PubMed] [Google Scholar]
Paci 1999 {published data only}
- Paci E, Barneschi MG, Miccinesi G, Falchi S, Metrangolo L, Novelli GP. Informed consent and patient participation in the medical encounter: a list of questions for an informed choice about the type of anaesthesia. European Journal of Anaesthesiology 1999;16(3):160‐5. [DOI] [PubMed] [Google Scholar]
Pesudovs 2006 {published data only (unpublished sought but not used)}
- Pesudovs K, Luscombe CK, Coster DJ. Recall from informed consent counselling for cataract surgery. Journal of Law and Medicine 2006;13(4):496‐504. [PubMed] [Google Scholar]
Phatouros 1995 {published data only (unpublished sought but not used)}
- Phatouros CC, Blake MP. Patients' attitudes to an information sheet prior to angiography and angioplasty. Australasian Radiology 1995;39(2):135‐9. [DOI] [PubMed] [Google Scholar]
Raynes‐Greenow 2010 {published data only}
- Raynes‐Greenow CH, Nassar N, Torvaldsen S, Trevena L, Roberts CL. Assisting informed decision making for labour analgesia: a randomised controlled trial of a decision aid for labour analgesia versus a pamphlet. BMC Pregnancy and Childbirth 2010;10(15). [DOI: 10.1186/1471-2393-10-15] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossi 2004 {published data only (unpublished sought but not used)}
- Rossi M, McClellan R, Chou L, Davis K. Informed consent for ankle fracture surgery: patient comprehension of verbal and videotaped information. Foot and Ankle International 2004;25(10):756‐62. [DOI] [PubMed] [Google Scholar]
Rossi 2005 {published data only (unpublished sought but not used)}
- Rossi MJ, Guttmann D, MacLennan MJ, Lubowitz JH. Video informed consent improves knee arthroscopy patient comprehension. Arthroscopy 2005;21(6):739‐43. [DOI] [PubMed] [Google Scholar]
Rymeski 2010 {published data only}
- Rymeski B, Marchildon M, Katz DA, Vinocur CD, Dunn SP, Reichard KW, et al. Pilot study using an Internet‐based program in informed consent. Journal of Pediatric Surgery 2010;45(6):1137‐41. [DOI] [PubMed] [Google Scholar]
Shorten 2005 {published data only}
- Shorten A, Shorten B, Keogh J, West S, Morris J. Making choices for childbirth: a randomized controlled trial of a decision‐aid for informed birth after cesarean. Birth 2005;32(4):252‐61. [DOI] [PubMed] [Google Scholar]
Solberg 2010 {published data only}
- Solberg LI, Asche SE, Sepucha K, Thygeson NM, Madden JE, Morrissey L, et al. Informed choice assistance for women making uterine fibroid treatment decisions: a practical clinical trial. Medical Decision Making 2010;30(4):444‐52. [DOI] [PubMed] [Google Scholar]
Tait 2009 {published and unpublished data}
- Tait AR, Voepel‐Lewis T, Moscucci M, Brennan‐Martinez CM, Levine R. Patient comprehension of an interactive, computer‐based information program for cardiac catheterization: a comparison with standard information. Archives of Internal Medicine 2009;169(20):1907‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2000 {published data only}
- Thomas R, Daly M, Perryman B, Stockton D. Forewarned is forearmed ‐ benefits of preparatory information on video cassette for patients receiving chemotherapy or radiotherapy ‐ a randomised controlled trial. European Journal of Cancer 2000;36(12):1536‐43. [DOI] [PubMed] [Google Scholar]
Uzbeck 2009 {published data only (unpublished sought but not used)}
- Uzbeck M, Quinn C, Saleem I, Cotter P, Gilmartin JJ, O'Keeffe ST. Randomised controlled trial of the effect of standard and detailed risk disclosure prior to bronchoscopy on peri‐procedure anxiety and satisfaction. Thorax 2009;64(3):224‐7. [DOI] [PubMed] [Google Scholar]
Wadey 1997 {published data only}
- Wadey V, Frank C. The effectiveness of patient verbalization on informed consent. Canadian Journal of Surgery 1997;40(2):124‐8. [PMC free article] [PubMed] [Google Scholar]
Walker 2007 {published data only}
- Walker MS, Farria D, Schmidt M, Monsees B, Wiele K, Bokern J, et al. Educational intervention for women undergoing image‐guided breast biopsy: results of a randomized clinical trial. Cancer Control 2007;14(4):380‐7. [DOI] [PubMed] [Google Scholar]
Whelan 2003 {published data only (unpublished sought but not used)}
- Whelan T, Sawka C, Levine M, Gafni A, Reyno L, Willan A, et al. Helping patients make informed choices: a randomized trial of a decision aid for adjuvant chemotherapy in lymph node‐negative breast cancer. Journal of the National Cancer Institute 2003;95(8):581‐7. [DOI] [PubMed] [Google Scholar]
Wilhelm 2009 {published and unpublished data}
- Wilhelm D, Gillen S, Wirnhier H, Kranzfelder M, Schneider A, Schmidt A, et al. Extended preoperative patient education using a multimedia DVD‐impact on patients receiving a laparoscopic cholecystectomy: a randomised controlled trial. Langenbeck's Archives of Surgery 2009;394(2):227‐33. [DOI] [PubMed] [Google Scholar]
Wong 2006 {published data only}
- Wong SSM, Thornton JG, Gbolade B, Bekker HL. A randomised controlled trial of a decision‐aid leaflet to facilitate women's choice between pregnancy termination methods. British Journal of Obstetrics and Gynaecology 2006;113(6):688‐94. [DOI] [PubMed] [Google Scholar]
Yucel 2005 {published data only}
- Yucel A, Gecici O, Emul M, Oyar O, Gulsoy UK, Dayanir YO, et al. Effect of informed consent for intravascular contrast material on the level of anxiety: how much information should be given?. Acta Radiologica 2005;46(7):701‐7. [DOI] [PubMed] [Google Scholar]
Zite 2011 {published data only}
- Zite NB, Wallace LS. Use of a low‐literacy informed consent form to improve women's understanding of tubal sterilization: a randomized controlled trial. Obstetrics and Gynecology 2011;117(5):1160‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ader 1992 {published data only}
- Ader DN, Seibring AR, Bhaskar P, Melamed BG. Information seeking and interactive videodisc preparation for third molar extraction. Journal of Oral & Maxillofacial Surgery 1992;50(1):27‐31; discussion ‐2. [DOI] [PubMed] [Google Scholar]
Altaie 2011 {published data only}
- Altaie RR, Ku JJ, Mora JJ. Informed consent in strabismus surgery. Journal of American Association of Pediatric Ophthalmology and Strabismus 2011;15(1):e12. [DOI] [PubMed] [Google Scholar]
Broyles 1992 {published data only}
- Broyles S, Sharp C, Tyson J, Sadler J. An exploratory trial in the neonatal period. Early Human Development 1992;31(1):67‐75. [DOI] [PubMed] [Google Scholar]
Clark 2011 {published data only}
- Clark S, Mangram A, Ernest D, Lebron R, Peralta L. The informed consent: a study of the efficacy of informed consents and the associated role of language barriers. Journal of Surgical Education 2011;68(2):143‐7. [DOI] [PubMed] [Google Scholar]
Dawes 1992 {published data only}
- Dawes PJ, O'Keefe L, Adcock S. Informed consent: the assessment of two structured interview approaches compared to the current approach. Journal of Laryngology & Otology 1992;106(5):420‐4. [DOI] [PubMed] [Google Scholar]
Dawes 1993 {published data only}
- Dawes PJD, O'Keefe L, Adcock S. Informed consent: using a structured interview changes patients' attitudes towards informed consent. Journal of Laryngology and Otology 1993;107(9):775‐9. [DOI] [PubMed] [Google Scholar]
Eggers 2007 {published data only}
- Eggers C, Obliers R, Koerfer A, Thomas W, Koehle K, Hoelscher AH, et al. A multimedia tool for the informed consent of patients prior to gastric banding. Obesity 2007;15(11):2866‐73. [DOI] [PubMed] [Google Scholar]
Finch 2009 {published data only}
- Finch WJG, Rochester MA, Mills RD. A randomised trial of conventional versus BAUS procedure‐specific consent forms for transurethral resection of prostate. Annals of the Royal College of Surgeons of England 2009;91(3):232‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fink 2010a {published data only}
- Fink AS, Prochazka AV, Henderson WG, Bartenfeld D, Nyirenda C, Webb A, et al. Enhancement of surgical informed consent by addition of repeat back: a multicenter, randomized controlled clinical trial. Annals of Surgery 2010;252(1):27‐36. [DOI] [PubMed] [Google Scholar]
Graham 2000 {published data only}
- Graham W, Smith P, Kamil A, Fitzmaurice A, Smith N, Hamilton N. Randomised controlled trial comparing effectiveness of touchscreen system with leaflet for providing women with information on prenatal tests. BMJ 2000;320:155‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grawe 2010 {published data only}
- Grawe JS, Mirow L, Bouchard R, Lindig M, Huppe M. [Impact of preoperative patient education on postoperative pain in consideration of the individual coping style]. Der Schmerz 2010;24(6):575‐86. [DOI] [PubMed] [Google Scholar]
Gyomber 2010 {published data only}
- Gyomber D, Lawrentschuk N, Wong P, Parker F, Bolton DM. Improving informed consent for patients undergoing radical prostatectomy using multimedia techniques: a prospective randomized crossover study. British Journal of Urology International 2010;106(8):1152‐6. [DOI] [PubMed] [Google Scholar]
Hewison 2001 {published data only}
- Hewison J, Cuckle H, Baillie C, Sehmi I, Lindow S, Jackson F, et al. Use of videotapes for viewing at home to inform choice in Down syndrome screening: a randomised controlled trial. Prenatal Diagnosis 2001;21(2):146‐9. [DOI] [PubMed] [Google Scholar]
Hilzenrat 2006 {published data only}
- Hilzenrat N, Yesovitch R, Shrier I, Stavrakis M, Deschenes M. The effect of information level and coping style on pain and anxiety in needle liver biopsy. Canadian Journal of Gastroenterology 2006;20(9):597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jlala 2010 {published data only}
- Jlala HA, French JL, Foxall GL, Hardman JG, Bedforth NM. Effect of preoperative multimedia information on perioperative anxiety in patients undergoing procedures under regional anaesthesia. British Journal of Anaesthesia 2010;104(3):369‐74. [DOI] [PubMed] [Google Scholar]
Johnson 2011 {published data only}
- Johnson MR, Singh JA, Stewart T, Terance JG. Patient understanding and satisfaction in informed consent for total knee arthroplasty: a randomized study. American College of Rheumatology July 2011;63(7):1048‐54. [DOI] [PubMed] [Google Scholar]
Kasper 2008 {published data only}
- Kasper J, Kopke S, Muhlhauser I, Nubling M, Heesen C. Informed shared decision making about immunotherapy for patients with multiple sclerosis (ISDIMS): a randomized controlled trial. European Journal of Neurology 2008;15(12):1345‐52. [DOI] [PubMed] [Google Scholar]
Lembcke 1998 {published data only}
- Lembcke B, Specht J, Nippel G, Caspary WF. Prospective randomized investigation of three printed information forms for upper GI‐endoscopy [Struktur‐ und ergebnisqualitat deutschsprachiger gastroskopie‐ aufkdarungsbogen aus Patientensicht]. Zeitschrift fur Gastroenterologie 1998;36(9):829‐38. [PubMed] [Google Scholar]
Lipp 1991 {published data only}
- Lipp M, Dick W, Daublander M, Bertram M. Different information patterns and their influence on patient anxiety prior to dental local anesthesia [Beeinflussung der Patientenangst vor der zahnarztlichen Lokalanasthesie mit verschiedenen Aufklarungsformen]. Deutsche Zeitschrift fur Mund‐, Kiefer‐ und Gesichts‐Chirurgie 1991;15(6):449‐57. [PubMed] [Google Scholar]
Migden 2008 {published data only}
- Migden M, Chavez‐Frazier A, Nguyen T. The use of high definition video modules for delivery of informed consent and wound care education in the Mohs Surgery Unit. Seminars in Cutaneous Medicine and Surgery 2008;27(1):89‐93. [DOI] [PubMed] [Google Scholar]
Nagle 2008 {published data only}
- Nagle C, Gunn J, Bell R, Lewis S, Meiser B, Metcalfe S, et al. Use of a decision aid for prenatal testing of fetal abnormalities to improve women’s informed decision making: a cluster randomised controlled trial. BJOG An International Journal of Obstetrics and Gynaecology 2008;115:339‐47. [DOI] [PubMed] [Google Scholar]
O'Cathain 2002 {published data only}
- O'Cathain A, Walters SJ, Nicholl JP, Thomas KJ, Kirkham M. Use of evidence based leaflets to promote informed choice in maternity care: randomised controlled trial in everyday practice. BMJ 2002;324(7338):643‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roth‐Isigkeit 2001a {published data only}
- Roth‐Isigkeit A, Brechmann J, Schwarzenberger J, Bruckner S, Schmucker P. Preoperative preparation of patients undergoing cardiac surgery and satisfaction ratings [Auswirkungen praoperativer vorbereitung auf zufriedenheitseinschatzungen kardiochirurgischer patienten]. Zeitschrift fur Herz‐, Thorax‐ und Gefasschirurgie 2001;15(2):62‐7. [Google Scholar]
Scanlan 2003 {published data only}
- Scanlan D, Siddiqui F, Perry G, Hutnik CML. Informed consent for cataract surgery: what patients do and do not understand. Journal of Cataract and Refractive Surgery 2003;29(10):1904‐12. [DOI] [PubMed] [Google Scholar]
Schenker 2010 {published data only}
- Schenker Y. Computer information program improves patient understanding in informed consent for cardiac catheterization. Journal of Clinical Outcomes Management 2010;17(1):10‐2. [Google Scholar]
Shurnas 2003 {published data only}
- Shurnas PS, Coughlin MJ. Recall of the risks of forefoot surgery after informed consent. Foot and Ankle International 2003;24(12):904‐8. [DOI] [PubMed] [Google Scholar]
Stanley 1998 {published data only}
- Stanley BM, Walters DJ, Maddern GJ. Informed consent: how much information is enough?. Australian & New Zealand Journal of Surgery 1998;68(11):788‐91. [DOI] [PubMed] [Google Scholar]
Steckelberg 2011 {published data only}
- Steckelberg A, Hulfenhaus C, Haastert B, Muhlhauser I. Effect of evidence based risk information on "informed choice" in colorectal cancer screening: randomised controlled trial. BMJ 2011;342(7810):d3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2010 {published data only}
- Taylor KL, Davis KM, Lamond T, Williams RM, Schwartz MD, Lawrence W, et al. Use and evaluation of a CD‐ROM‐based decision aid for prostate cancer treatment decisions. Behavioral Medicine 2010;36(4):130‐40. [DOI] [PubMed] [Google Scholar]
Wright 2010 {published data only}
- Wright NS, Fleming PS, Sharma PK, Battagel J. Influence of supplemental written information on adolescent anxiety, motivation and compliance in early orthodontic treatment. Angle Orthodontist 2010;80(2):329‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Akkad 2004
- Akkad A, Jackson C, Kenyon S, Dixon‐Woods M, Taub N, Habiba M. Informed consent for elective and emergency surgery: questionnaire study. British Journal of Obstetrics and Gynaecology 2004;111(10):1133‐8. [DOI] [PubMed] [Google Scholar]
Akkad 2006
- Akkad A, Jackson C, Kenyon S, Dixon‐Woods M, Taub N, Habiba M. Patients' perceptions of written consent: questionnaire study. BMJ 2006;333:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
AMA 2009
- American Medical Association. Informed Consent. Code of Medical Ethics. Revised. American Medical Association, 2009:438. [Google Scholar]
AMC 2009
- Australian Medical Council Ltd. Good Medical Practice: A Code of Conduct for Doctors in Australia. http://www.amc.org.au/images/Final_Code.pdf 2009 (accessed 9 Sep 2011).
Barkin 2009
- Barkin JS, Aronson JK, Feldman LS, Maddern GJ, Strasberg SM. Evaluation and stages of surgical innovations. Lancet 2009;374:1089‐96. [DOI] [PubMed] [Google Scholar]
Beisecker 1990
- Beisecker AE, Beisecker TD. Patient information‐seeking behaviors when communicating with doctors. Medical Care 1990;28(1):19‐28. [DOI] [PubMed] [Google Scholar]
BMA 2009
- British Medical Association. Consent Tool Kit. http://www.bma.org.uk/images/consenttoolkitdec2009_tcm41‐193139.pdf 2009 (accessed 9 Sep 2011).
Brezis 2008
- Brezis M, Israel S, Weinstein‐Birenshtock A, Pogoda P, Sharon A, Tauber R. Quality of informed consent for invasive procedures. International Journal of Quality in Health Care 2008;20(5):352‐7. [DOI] [PubMed] [Google Scholar]
Canterbury v Spence 1972
- United States Court of Appeals for the District of Columbia Circuit. Canterbury v Spence. Federal Reporter 1972; Vol. 464.
CCCRG 2008
- Cochrane Communication and Consumers Review Group. Outcomes of interest to the Cochrane Communication and Consumers Review Group. (http://www.latrobe.edu.au/chcp/assets/downloads/Outcomes.pdf) 2008, accessed 12 September 2011.
Chester v Afshar 2004
- House of Lords. Chester v Afshar. House of Lords opinions of the Lords of Appeal for Judgement 2004; Vol. UKHL41.
Cohn 2007
- Cohn E, Larson E. Improving participant comprehension in the informed consent process. Journal of Nursing Scholarship 2007;39:273‐80. [DOI] [PubMed] [Google Scholar]
Dixon‐Woods 2006
- Dixon‐Woods M, Williams SJ, Jackson CJ, Akkad A, Kenyon S, Habiba M. Why women consent to surgery, even when they don't want to: a qualitative study. Clinical Ethics 2006;1(3):153. [Google Scholar]
DoH 2001
- Department of Health (UK). Reference Guide to Consent for Examination or Treatment. London: Department of Health (UK), 2001. [Google Scholar]
DoH 2009
- Department of Health (UK). Reference Guide to Consent for Examination or Treatment. 2nd Edition. London: Department of Health (UK), 2009. [Google Scholar]
Doust 2007
- Doust J, Mannes P, Bastian H, Edwards AGK. Interventions for improving understanding and minimising the psychological impact of screening. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD001212.pub2] [DOI] [Google Scholar]
Duncan 2010
- Duncan E, Best C, Hagen S. Shared decision making interventions for people with mental health conditions. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007297.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2002
- Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ 2002;324(7341):827‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egbert 1964
- Egbert LD, Battit GE, Welch CE, Bartlett MK. Reduction of postoperative pain by encouragement and instruction of patients. a study of doctor‐patient rapport. New England Journal of Medicine 1964;270:825‐7. [DOI] [PubMed] [Google Scholar]
Elwyn 2008
- Elwyn G. Patient consent: decision or assumption?. BMJ 2008;336(7656):1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2010
- Elwyn G, Miron‐Shatz T. Deliberation before determination: the definition and evaluation of good decision making. Health Expectations June 2010;13(2):139‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2012
- Elwyn G, Frosch D, Thomson R, Joseph‐Williams N, Lloyd A, Kinnersley P, et al. Shared decision making: a model for clinical practice. Journal of General Internal Medicine 2012;May 23. [DOI: 10.1007/s11606-012-2077-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ergina 2009
- Ergina PL, Cook JA, Blazeby JM, Boutron I, Clavien P, Reeves BC, et al. Challenges in evaluating surgical innovations. Lancet 2009;374:1097‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2007
- Evans R, Elwyn G, Edwards A, Newcombe R, Kinnersley P, Wright P, et al. A randomised controlled trial of the effects of a web‐based PSA decision aid, Prosdex. Protocol. BMC Family Practice 2007;8(1):58. [DOI: 10.1186/1471-2296-8-58] [DOI] [PMC free article] [PubMed] [Google Scholar]
GMC 2008
- General Medical Council. Consent: Patients and Doctors Making Decisions Together. General Medical Council 2008. [ISBN 978‐0‐901458‐31‐5]
Gøtzsche 2013
- Gøtzsche PC, Jørgensen KJ. Screening for breast cancer with mammography. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD001877.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Habiba 2004
- Habiba M, Jackson C, Akkad A, Kenyon S, Dixon‐Woods M. Women's accounts of consenting to surgery: is consent a quality problem?. Quality & Safety in Health Care 2004;13(6):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 1988
- Hall JA, Dornan MC. What patients like about their medical care and how often they are asked: a meta‐analysis of the satisfaction literature. Social Science in Medicine 1988;27(9):935‐9. [DOI] [PubMed] [Google Scholar]
Henderson 2010
- Henderson A, Henderson S. Provision of a surgeon's performance data for people considering elective surgery. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD006327.pub2] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (eds). Chapter 8: Assessing the risk of bias in included studies. In: Higgins JPT, Greene S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration, 2011. [Google Scholar]
Hon 2012
- Hon YK, Narayanan P, Goh PP. Extended discussion of information for informed consent for participation in clinical trials. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009835] [DOI] [Google Scholar]
Jefford 2002
- Jefford M, Tattersall MH. Informing and involving cancer patients in their own care. Lancet Oncology 2002;3(10):629‐37. [DOI] [PubMed] [Google Scholar]
Jenkins 1999
- Jenkins VA, Fallowfield LJ, Souhami A, Sawtell M. How do doctors explain randomised clinical trials to their patients?. European Journal of Cancer 1999;35(8):1187‐93. [DOI] [PubMed] [Google Scholar]
Kennedy 2002
- Kennedy AD, Sculpher MJ, Coulter A, Dwyer N, Rees M, Abrams KR, et al. Effects of decision aids for menorrhagia on treatment choices, health outcomes, and costs: a randomized controlled trial. Journal of the American Medical Association 2002;288(21):2701‐8. [DOI] [PubMed] [Google Scholar]
Kinnersley 2007
- Kinnersley P, Edwards AGK, Hood K, Cadbury N, Ryan R, Prout H, et al. Interventions before consultations for helping patients address their information needs. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004565.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Légaré 2010
- Légaré F, Ratte S, Stacey D, Kryworuchko J, Gravel K, Graham ID, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD006732.pub2] [DOI] [PubMed] [Google Scholar]
Makoul 1995
- Makoul G, Arntson P, Schofield T. Health promotion in primary care: physician‐patient communication and decision making about prescription medications. Social Science and Medicine 1995;41(9):1241‐54. [DOI] [PubMed] [Google Scholar]
Marteau 2001
- Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expectations 2001;4(2):99‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazur 2009
- Mazur DJ. Medical‐legal aspects of evidence‐based choice and shared decision making. In: Edwards A, Elwyn G editor(s). Shared Decision‐Making in Healthcare: Achieving Evidence‐Based Patient Choice. 2nd Edition. Oxford: OUP, 2009:165‐70. [Google Scholar]
Oxman 2004
- Oxman A. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Picano 2004
- Picano E. Informed consent and communication of risk from radiological and nuclear medicine examinations: how to escape from a communication inferno. BMJ 2004;329(7470):849‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rogers v Whitaker 1992
- High Court of Australia. Rogers v Whitaker. High Court of Australia, Canberra 1992; Vol. 58:175 CLR 479.
Roter 2006
- Roter DL, Hall JA. Doctors Talking to Patients/Patients Talking to Doctors: Improving Communication in Medical Visits. 2nd Edition. Westport, CT: Praeger Publishing, 2006. [Google Scholar]
Royal College of Surgeons of England 2011
- Royal College of Surgeons of England. Surgery and the NHS in numbers. http://www.rcseng.ac.uk/media/media‐background‐briefings‐and‐statistics/surgery‐and‐the‐nhs‐in‐numbers 2011.
Ryan 2009
- Ryan R, Prictor M, McLaughlin KJ, Hill S. Audio‐visual presentation of information for informed consent for participation in clinical trials. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD003717.pub2] [DOI] [PubMed] [Google Scholar]
Ryan 2011
- Ryan R, Hill S, Prictor M, McKenzie J. Study Quality Guide. Cochrane Consumers and Communication Review Group. May 2011 (accessed 12 Sep 2011).
Schattner 2006
- Schattner A, Bronstein A, Jellin N. Information and shared decision‐making are top patients' priorities. BMC Health Services Research 2006;6(1):21. [DOI: 10.1186/1472-6963-6-21] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schenker 2011
- Schenker Y, Fernandez A, Sudore R, Schillinger D. Interventions to improve patient comprehension in informed consent for medical and surgical procedures: a systematic review. Medical Decision Making 2011;31(1):151‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silverman 2005
- Silverman J, Kurtz S, Draper J. Skills for Communicating with Patients. Oxford: Radcliffe, 2005. [Google Scholar]
Stacey 2011
- Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes‐Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD001431.pub2] [DOI] [PubMed] [Google Scholar]
Treadwell 2006
- Treadwell JR, Tregear SJ, Reston JT, Turkelson CM. A system for rating the stability and strength of medical evidence. BMC Medical Research Methodologies 2006;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woodrow 2006
- Woodrow SR, Jenkins AP. How thorough is the process of informed consent prior to outpatient gastroscopy? A study of practice in a United Kingdom District Hospital. Digestion 2006;73(2‐3):189‐97. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kinnersley 2011
- Kinnersley P, Stephens BL, Elwyn GJ, Blazeby J, Kelly MJ, Savage K, et al. Interventions to promote informed consent for patients undergoing surgical and other invasive healthcare procedures. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD009445] [DOI] [PMC free article] [PubMed] [Google Scholar]


