Abstract
Transcranial magnetic stimulation (TMS) is applied both in research settings and clinically, notably in treating depression through the dorsolateral prefrontal cortex (dlPFC). We have recently shown that transcranial alternating current stimulation of the dlPFC partially entrains muscle sympathetic nerve activity (MSNA) to the stimulus. We, therefore, aimed to further explore the sympathetic properties of the dlPFC, hypothesizing that single-pulse TMS could generate de novo MSNA bursts. Microneurography was performed on the right common peroneal nerve in 12 participants. TMS pulses were then delivered to the ipsilateral dlPFC at resting motor threshold (MT) of the first dorsal interosseous muscle and at powers 20 below, 10 below, 10% above, and 20% above MT. The MT and 10% above MT intensities were also used to stimulate the right motor cortex and shoulder. Comparisons between stimulus intensities at the same site and between sites at the same intensities revealed no differences in MSNA burst frequency, burst incidence, or single MSNA spikes. Most stimulus trains, however, showed reduced burst frequency and incidence from baseline, regardless of site. This suggests that the TMS itself was evoking arousal-based sympathoinhibition, independent of dlPFC influences. It seems the dlPFC is capable of modulating MSNA but cannot directly generate bursts.
Keywords: dorsolateral prefrontal cortex, motor cortex, muscle sympathetic nerve activity, transcranial magnetic stimulation
Introduction
Transcranial magnetic stimulation (TMS) is a non-invasive and pain-free means of focally stimulating the human brain via an electrical field capable of eliciting action potentials in cortical neurons (Siebner et al. 2022). First introduced in the modern application by Barker et al. (1985), TMS usage has grown exponentially through the years and is applied in three distinct ways: through single-pulse, paired-pulse, and repetitive TMS (rTMS). The latter is especially common in therapeutic treatments of psychiatric and neurological disorders such as depression and obsessive-compulsive disorder. In treating depression (Fitzgerald 2020), protocols target the left dorsolateral prefrontal cortex (dlPFC), given its well-documented role in mood regulation and altered activity in psychological disease states (Li et al. 2022; Piretti et al. 2022). It is important to note that clinical usage of TMS is not restricted to rTMS, however. For example, single-pulse TMS has been used to diminish the impact of migraine episodes (Lipton et al. 2010).
In addition to clinical applications, TMS is used in neurological research to evaluate the disruption of cortical processing in collaboration with brain imaging techniques (Koponen and Peterchev 2020). Of particular interest regarding the present study, however, TMS has also been used to evaluate altered neuronal function on sympathetic responses measured by muscle sympathetic nerve activity (MSNA). With this being said, the body of literature is small, and the findings are relatively varied and inconsistent. For instance, when targeting the spinal cord with rTMS, Paxton et al. (2011) report no changes in MSNA. Contrary to this, Kimmerly et al. (2018), using theta burst TMS over the motor cortex; report an increase in MSNA when the stimuli are delivered continuously but no change when the stimuli are intermittent. Similarly, while Silber et al. (2000) reported no MSNA change during single-pulse TMS over the motor cortex, Macefield et al. (1998) reported that single pulses at the same area had inhibitory effects on MSNA.
A role for the dlPFC in regulating MSNA has also been reported from functional magnetic resonance imaging (fMRI) studies. That is, concurrent fMRI and MSNA recordings have revealed that the dlPFC is one cortical area in which fMRI signal intensity covaries with fluctuations in spontaneous bursts of MSNA (James et al. 2013; Macefield and Henderson 2019), suggesting that the dlPFC contributes to its generation. Moreover, we have recently shown that transcranial alternating current stimulation (tACS) of the dlPFC is capable of entraining bursts of MSNA and skin sympathetic nerve activity (SSNA) to the stimuli, regardless of which side was stimulated (Sesa-Ashton et al. 2022; Wong et al. 2023). This was also explored alongside the vestibulosympathetic reflexes, where it was concluded that the dlPFC was even capable of inhibiting the MSNA modulation produced by selective stimulation of the vestibular apparatus (McCarthy et al. 2024b).
Given these results and the growing use of rTMS of the dlPFC in treatment of psychiatric and neurological disorders, we reasoned that single-pulse TMS of the dlPFC could evoke increases in MSNA—specifically the generation of bursts unrelated to the normal baroreflex MSNA entrainment. Indeed, we have previously shown sinusoidal galvanic vestibular stimulation to evoke de novo bursts of MSNA unrelated to the cardiac cycle but time-locked to the vestibular stimulus (Bent et al. 2006). Additionally, superentrainment of MSNA was even observed with regard to the cyclic vestibular stimuli (Macefield and James 2016). The use of single-pulse TMS in the present study addresses a limitation of the methods used in our previous studies—namely, the off-target side effects of tACS—by directly activating the cell bodies of the dlPFC. In this way, it could be determined whether or not the predicted generation of MSNA bursts is specific to the stimulus. We hypothesized that single pulses of TMS to the dlPFC would generate de novo bursts of MSNA when delivered at a sufficient intensity, i.e. at 110% of motor threshold (MT)—the intensity at which most treatment protocols are delivered (Bourla et al. 2023).
Methods
Participants and ethics
Studies were performed on seven male and five female participants (age: 24 to 31 yrs, mean ± standard deviation (SD), 27.0 ± 2.4 yrs; height: 154 to 194 cm, 170.5 ± 12.6 cm; weight: 41 to 100 kg, 65.4 ± 16.2 kg). All participants provided informed written consent in line with the Declaration of Helsinki. The study was conducted with approval of the Human Research Ethics Committee (HREC) of the Alfred Hospital (HREC approval 89575) and endorsed by Monash University HREC.
Participants were instructed to refrain from the intake of nicotine and caffeinated food and beverages for at least four hours prior to the beginning of their experiment, as doing so has been shown to have influences on sympathetic nerve activity (Corti et al. 2002; Dimitriadis et al. 2022; Butler et al. 2023) Similarly, exclusion criteria included those taking anti-depressant medications due to the inhibitory properties of some of those drugs on sympathetic activity (Tank et al. 2003; Barton et al. 2007; Scalco et al. 2009). Further exclusion criteria included those with known neurological and cardiovascular diseases, those with metallic implants within their heads, and those with a history of seizures, each to ensure participant safety regarding the experimental protocol.
Recording procedures
Participants were seated in a semi-recumbent position on a procedure chair with their legs extended and arms supported on armrests on their respective sides. The right leg was elevated and supported at the level of the lower thigh by a casting cushion (Germa Protec, Sweden) to allow ease of access to the right common peroneal nerve at the fibular head and to minimize leg movements. After this setup, the nerve was initially located through manual palpation before a 35 mm adhesive hydrogel Ag/AgCl surface electrode (Covidien, Ireland; Cardinal Health, USA) was placed on the opposite side of the leg (i.e. medially) to the location of the nerve. This acted as the anode for a search probe using short (0.2 ms) pulses of electrical current at 1 Hz delivered from an isolated stimulator (Stimulus Isolator, ADInstruments, Australia). The probe was used to determine the area of the nerve showing the lowest threshold for evoking a muscle twitch, and after the skin had been disinfected with an alcohol swab (Briemarpak, Australia; Covidien, USA), the area would then serve as the site for microneurography—percutaneous insertion of a sterile insulated tungsten recording microelectrode (FHC Inc., Bowdoin, USA). This microelectrode was used to record MSNA, while a similar subcutaneous microelectrode (with 1 mm of insulation removed) was placed approximately 1 cm away to act as a reference electrode. Both microelectrodes were connected to a low-noise, electrically isolated headstage (NeuroAmpEX, ADInstruments, Australia) used to amplify the neural activity (gain 2 × 104, bandpass 0.3 to 5.0 kHz; sampling frequency 10 kHz) and grounded with another Ag/AgCl surface electrode placed on the knee. The recording microelectrode was manually guided into a nerve fascicle supplying the muscle fibers of the leg by using progressively weaker electrical stimuli (1.0 to 0.01 mA, 0.2 ms, 1 Hz) as it approached. Twitches of the foot at a current intensity of ≤0.02 mA indicated a muscle fascicle had been penetrated. Percussion of the muscle belly or tendon evoked stretch-related activity of muscle spindle afferents, confirming the identity of the muscle fascicle. Small adjustments of the microelectrode tip were used until spontaneous bursts of MSNA were encountered, with clear evidence of several negative-going spikes with clear cardiac rhythmicity. Real-time data were generated and connected to an audio system to aid in the identification of bursts of MSNA. Once a suitable site had been obtained, a five-minute baseline period was recorded prior to the commencement of the stimulation protocol.
Microneurography and further physiological data were visualized, recorded, and stored in real-time using a computer-based data acquisition system (LabChart 7 for Macintosh, PowerLab 16S, ADInstruments, Australia). This further data included recordings of the electrocardiogram (ECG) through three more 35 mm adhesive hydrogel Ag/AgCl surface electrodes (Covidien, Ireland; Cardinal Health, USA) placed on the chest and sampled at 2 kHz; recordings of the electromyographic (EMG) signal on the left hand through Ag/AgCl surface electrodes placed on the belly of the left first dorsal interosseous (FDI) muscle and laterally on the proximal phalanx of the left index finger (ground on wrist), sampled at 2 kHz; and continuous non-invasive blood pressure recordings taken from two finger cuffs placed on the right hand, sampled at 400 Hz and calibrated to a sphygmomanometer cuff on the contralateral arm (NOVA, Finapres Medical System BV, the Netherlands).
Stimulation procedures
Before commencing microneurography, electroencephalogram (EEG) sites F4 (corresponding to the right dlPFC) and C4 (corresponding to the right motor cortex) were marked on the heads of participants according to the international 10-20 system of electrode placement. Single pulses of TMS were delivered through a figure-eight coil (double 70 mm remote control coil, Magstim 2002, Magstim, UK) to area C4 with increasing pulse intensity (indicated as a percentage value of magnetic flux on the TMS machine) until resting MT was reached. This was indicated by a resting EMG signal of 0.05 mV from the FDI of the contralateral hand and a latency of approximately 22 ms from the artifact generated by the pulse to the peak of the EMG signal. Once determined, the MT was used as a primer for the stimulation delivered after the microneurographic setup.
Single pulses were delivered to the dlPFC at a power intensity 20 lower than MT, 10 lower than MT, at MT, at a power of 110% of MT, and at a power of 120% of MT. For example, a participant with a resting MT value of 60% would have pulses delivered at 40%, 50%, 60%, 66%, and 72%. The MT and 110% values were then also used to sequentially stimulate area C4 and the right shoulder just below the collarbone. The extra stimulus intensities chosen for the dlPFC were used for comparisons to the focal intensities—MT and 110%. Stimulus trains delivered to each participant were conducted in the order described above (i.e. without randomization). This was done for participant safety in order to reduce and monitor for any possible risk of seizures that higher doses of flux could promote (Rossi et al. 2021). Due to deterioration of the recording site over time and other technical difficulties, the 110% stimulation of the shoulder could not be carried out in three experiments, reducing the sample size for that particular stimulation to nine. Five pulses were delivered at each intensity at each site, with each pulse spaced at least 10 s apart to avoid inhibitory properties (Vasegi et al. 2015). Further to this, pulse trains were separated by a period of at least 15 s. TMS pulses were delivered manually by an investigator so as to be asynchronous with bursts of MSNA as viewed in real-time on the computer monitor. In cases in which a pulse was delivered in time with a burst, another compensatory pulse was delivered during that pulse train.
Data analysis
To prevent misrepresentation of the MSNA data by positive-going spikes from myelinated axons or single motor units (Macefield 2021), analysis was performed on the raw, negative-going spikes. These spikes were defined with a half-width of 0.2 to 0.5 ms and isolated using window discriminator software on LabChart 7 (Spike Histogram for Macintosh, v2.5.1, ADInstruments, Australia), as were the R-waves of the ECG and stimulus artifacts generated by TMS pulses.
The primary results were obtained using peristimulus time histogram (PSTH) analysis in two ways: single MSNA spikes referenced to the R-waves of the ECG (spikes/R-wave) and single MSNA spikes referenced to the TMS pulses (spikes/pulse). The average burst referenced to the R-waves of the ECG across the entire data file was first determined in each participant (R-wave triggered average). Data were separated into 10 ms time bins and the bin count from the average burst of the whole file PSTH was used as a primer for subsequent PSTH analysis. This included analysis of a five-minute baseline period at the beginning of each experiment, as well as a period of time beginning two seconds before the onset of the first pulse of a given intensity and ending two seconds after the last pulse of that train. The start and end points of the bin selection from the PSTH referenced to the R-waves during each stimulation period were consistent within each participant; however, the bin selection of the spikes referenced to the TMS pulses was shifted in time relative to the bins encompassing the most spikes in the PSTH (i.e. a burst of activity). In each case, the bin count remained consistent within each participant—only the start and end points were altered.
The standard measures of multi-unit sympathetic nerve recordings—MSNA burst frequency (bursts/min) and burst incidence (bursts/100 heartbeats)—were further analyzed alongside blood pressure data separated into both systolic and diastolic pressure. MSNA burst count was determined by the cyclic measurements feature of LabChart 7 from the root-mean-square (RMS)-processed nerve signal (200 ms moving average). Each metric was gathered during baseline and during the time period, encapsulating each pulse intensity as described above. Technical difficulties prevented the acquisition of blood pressure data during one experiment, thereby reducing that sample by one.
All data were exported to a statistical and graphical analysis program (Prism 10 for Macintosh, v10.2.0, GraphPad Software, USA). Normality was determined using D’Agostino and Pearson normality tests—data that passed normality underwent a repeated measures one-way analysis of variance (RM one-way ANOVA) or a two-tailed paired t-test, while data that were not normally distributed underwent a Friedman or two-tailed Wilcoxon test. Further post hoc analysis was also conducted: normally distributed data underwent Dunnett’s multiple comparisons tests to compare baseline values to stimulus trains and Tukey’s multiple comparisons tests to compare stimulus intensities at each stimulus site and matching stimulus intensities at different sites; data which were not normally distributed underwent Dunn’s multiple comparisons tests in the same manner as above. In all analyses, data are presented as mean ± SD and were considered significantly different if they reached a P value of ≤0.05.
Results
Sympathetic nerve activity was recorded in all 12 participants (Fig. 1), with single pulses of TMS delivered at five intensities (MT -20%, MT -10%, MT ± 0%, 110% of MT and 120% of MT) to the right dlPFC at EEG site F4 and at two intensities (MT ± 0% and 110% of MT) to the right motor cortex at EEG site C4 and to the right shoulder. During three experiments, technical issues prevented the acquisition of shoulder data at 110% of MT, reducing the sample size of that stimulation to nine and, to facilitate paired analysis, each comparison to the shoulder at that intensity had a reduced sample size accordingly.
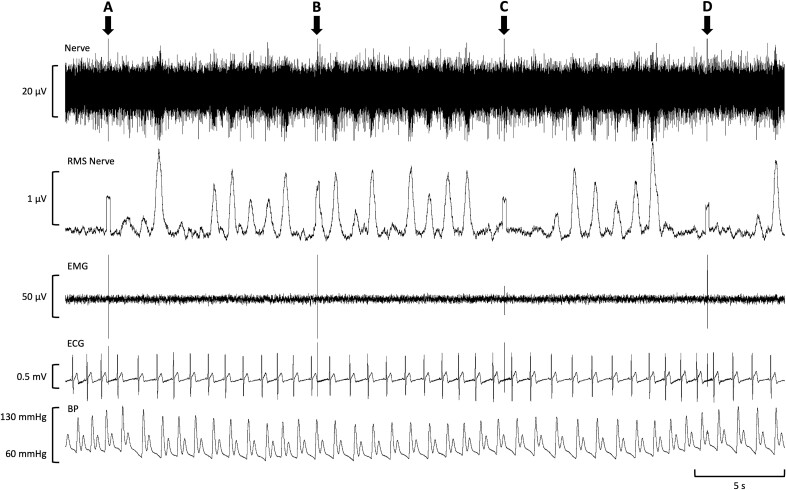
Fig. 1.
Raw physiological data during stimulation of the dorsolateral prefrontal cortex (dlPFC). A single participant’s real-time data during a 40 s time period of right dlPFC stimulation at 110% of motor threshold (MT) is displayed. Muscle sympathetic nerve activity (MSNA) data is shown in the first channel (nerve) and used to calculate the processed signal in the second channel (Root-mean-square (RMS) nerve). Additionally, data from the electromyogram (EMG) and electrocardiogram (ECG), as well as blood pressure (BP), can be seen in the EMG, ECG, and BP channels, respectively. Single transcranial magnetic stimulation (TMS) pulses were delivered at the time points denoted by arrows (A) to (D), each generating a signal artifact that can be seen in all channels except BP. While all pulses were aimed to be delivered out-of-phase with MSNA burst trains, an example of a miss-timed pulse can be seen in (B). The Y-axis on each channel represents signal range.
PSTH analysis of MSNA referenced to R-waves
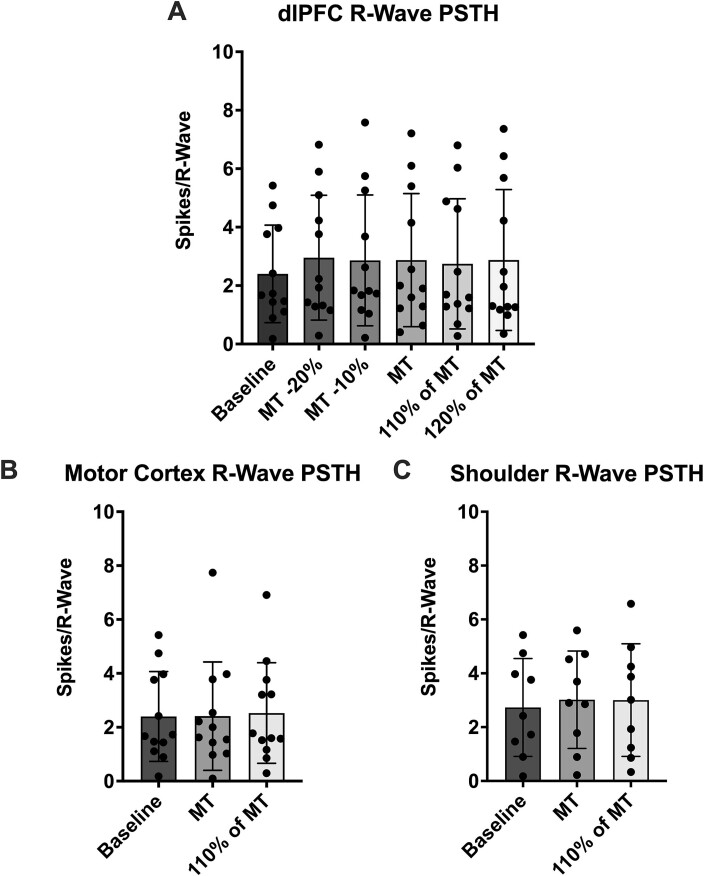
Firstly, each TMS stimulus intensity delivered to the dlPFC was analyzed through PSTH analysis of MSNA spikes referenced to R-waves of the ECG (Table 1; Fig. 2). No statistically significant (P > 0.05) differences were found between the baseline period and any stimulus intensity (RM one-way ANOVA, P = 0.20). Further to this, comparisons between each of the stimulus intensities (RM one-way ANOVA, P = 0.79) likewise revealed no statistically significant (P > 0.05) differences.
Table 1.
PSTH analysis of MSNA spikes during each stimulus train. PSTH analysis of MSNA spikes referenced to the R-waves of the ECG (spikes per R-wave) and referenced to the TMS pulses (spikes/pulse) of any given intensity are displayed during a five-minute baseline period as well as during a period encapsulating each TMS pulse train. Pulses were delivered at five intensities to the dlPFC (n = 12 at each intensity) and at two intensities to the motor cortex (n = 12 at each intensity) and shoulder (n = 12 at MT; n = 9 at 110% of MT). Baseline values for each region are the same, as they derive from one period at the beginning of each experiment. No baseline is given for spikes referenced to the TMS pulses, as no pulses were delivered during the baseline period for the PSTH analysis to be referenced to. No values were found to be significantly different from baseline (P > 0.05). Data are presented as mean ± SD.
| Baseline | MT -20% | MT -10% | MT | 110% of MT | 120% of MT | ||
|---|---|---|---|---|---|---|---|
| MSNA spikes referenced to the R-waves of the ECG (spikes/R-wave) | dlPFC | 2.40 ± 1.67 | 2.95 ± 2.14 | 2.86 ± 2.24 | 2.87 ± 2.28 | 2.74 ± 2.23 | 2.87 ± 2.41 |
| Motor cortex | 2.40 ± 1.67 | 2.41 ± 2.01 | 2.53 ± 1.87 | ||||
| Shoulder | 2.40 ± 1.67 | 2.57 ± 1.87 | 3.00 ± 2.09 | ||||
| MSNA spikes referenced to the TMS pulses (spikes/pulse) | dlPFC | 7.86 ± 5.04 | 7.35 ± 4.02 | 7.61 ± 4.03 | 7.36 ± 3.64 | 8.02 ± 4.74 | |
| Motor cortex | 7.77 ± 4.30 | 7.85 ± 5.19 | |||||
| Shoulder | 9.18 ± 7.03 | 10.18 ± 8.94 | |||||
Fig. 2.
Peristimulus time histogram (PSTH) analysis of MSNA spikes referenced to the R-waves during each stimulus train. (A) Shows spikes of MSNA during stimulation of the dlPFC (n = 12) at five different intensities as well as spikes during baseline. (B) Presents sympathetic spikes during stimulation of the motor cortex (n = 12) and (C) presents spikes during stimulation of the shoulder (n = 9). Baseline values are taken from the same timepoint at the beginning of each experiment and, so, are consistent in (A) and (B). The reduced sample size in (C) has shifted the baseline value slightly. No significant differences (P > 0.05) were found between any stimulation intensities when compared to each other and baseline at each site. Data are presented as mean ± SD.
In comparing the two stimulus intensities delivered to the motor cortex (Table 1; Fig. 2) to the baseline period as above (Friedman test, P = 0.78), again no statistically significant (P > 0.05) differences were found. The same was true for comparing the stimulations to each other (Wilcoxon test, P = 0.62). Likewise, stimulation of the shoulder (Table 1; Fig. 2) resulted in no significant (P > 0.05) differences when compared to baseline (RM one-way ANOVA, P = 0.45) and when the stimuli were compared to each other (Paired t-test, P = 0.96).
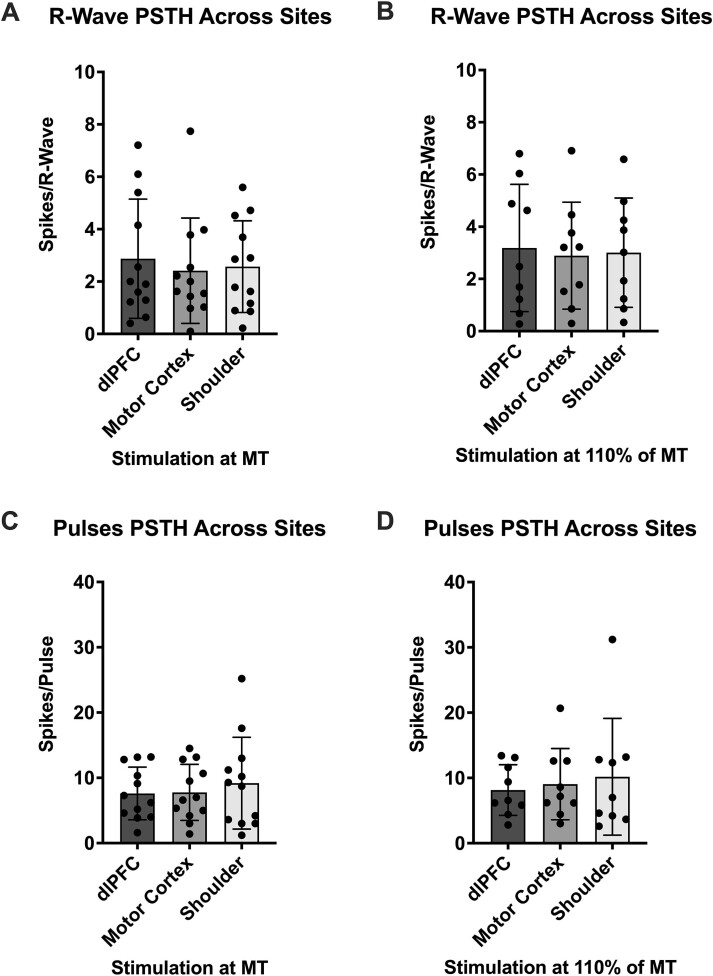
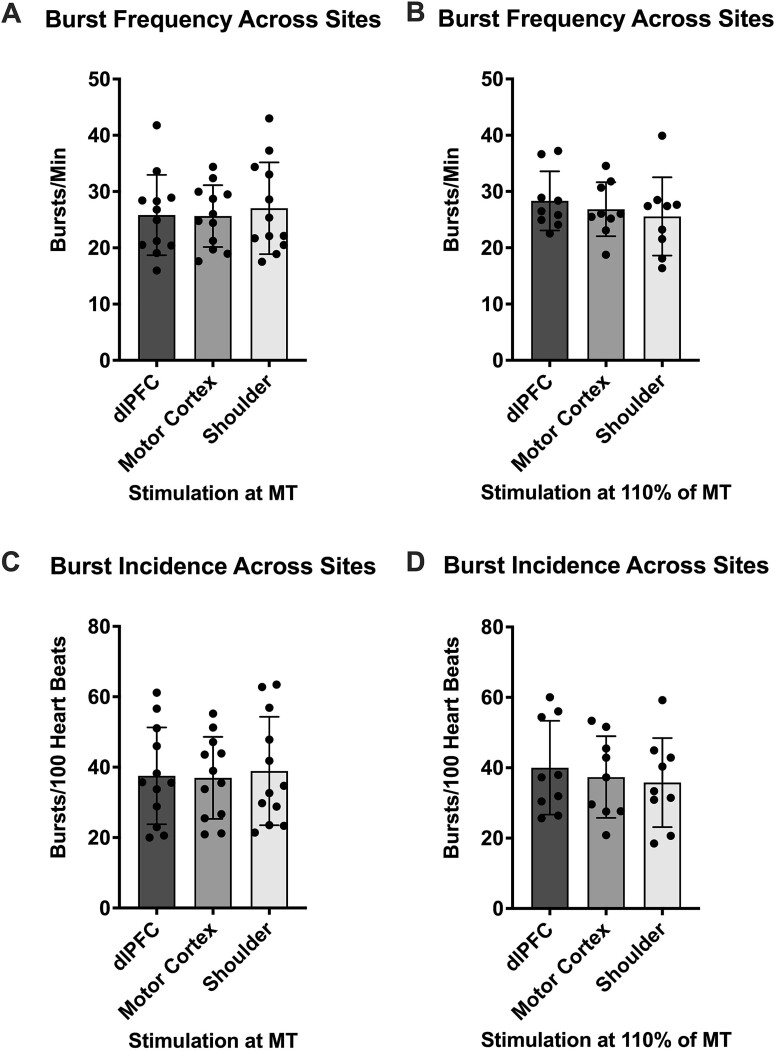
PSTH spike counts were also compared between each site at the same stimulus intensity (Table 1; Fig. 3). Analysis of the three sites when stimulated at MT (Friedman test, P = 0.21) revealed no significant differences (F4 vs C4, P = 0.46; F4 vs shoulder, P = 0.31; C4 vs shoulder, P > 0.99). Similar results were seen in analysis of the sites at 110% of MT (RM one-way ANOVA, P = 0.34)—no statistically significant differences were found (F4 vs C4, P = 0.58; F4 vs shoulder, P = 0.60; C4 vs shoulder, P = 0.63).
Fig. 3.
PSTH comparisons of MSNA spikes across different stimulation sites. (A) and (B) depict MSNA spikes referenced to the R-waves of the ECG when stimuli were delivered to the dlPFC, motor cortex and shoulder at MT (n = 12) and at 110% of MT (n = 9), respectively. (C) and (D) depict MSNA spikes referenced to the TMS pulses when stimuli were delivered to the dlPFC, motor cortex and shoulder at MT (n = 12) and at 110% of MT (n = 9), respectively. No significant differences (P > 0.05) were found between sites at either intensity during both PSTH analyses. Data are presented as mean ± SD.
PSTH analysis of MSNA referenced to TMS pulses
Each of the stimulus intensities was also analyzed with PSTHs of MSNA spikes referenced to the TMS pulses (Table 1; Fig. 3). This analysis did not compare stimuli to the baseline period, as the baseline did not have any pulses to which the MSNA spikes could be referenced.
In comparisons between each of the five dlPFC stimulations (RM one-way ANOVA, P = 0.73), no significant (P > 0.05) differences were found. This was also the case for comparisons of the two motor cortex stimulations (Wilcoxon test, P = 0.98) and the two shoulder stimulations (Wilcoxon test, P = 0.73).
PSTH spike counts were analyzed across areas as well, just as above (Table 1; Fig. 3). At MT, analysis (RM one-way ANOVA, P = 0.33) showed no statistically significant (P > 0.05) differences (F4 vs C4, P = 0.94; F4 vs shoulder, P = 0.52; C4 vs shoulder, P = 0.59). The same results were found in analysis of the sites at 110% of MT (Friedman test, P = 0.81)—no statistically significant (P > 0.05) differences (F4 vs C4, P > 0.99; F4 vs shoulder, P > 0.99; C4 vs shoulder, P > 0.99).
Standard analysis of MSNA
In addition to PSTH analysis, MSNA burst frequency (bursts/min) was analyzed during each stimulus train in the same manner (Table 2). Similar to the PSTH analysis, no statistically significant (P > 0.05) differences were found when comparing stimulus intensities to each other (Fig. 4) at the dlPFC (RM one-way ANOVA, P = 0.35), the motor cortex (paired t-test, P = 0.67) and the shoulder (paired t-test, P = 0.18). This was also true of comparisons (P > 0.05) between the three sites (Fig. 5) at MT (RM one-way ANOVA, P = 0.61; F4 vs C4, P = 0.99; F4 vs shoulder, P = 0.59; C4 vs shoulder, P = 0.73), as well as at 110% of MT (RM one-way ANOVA, P = 0.24; F4 vs C4, P = 0.49; F4 vs shoulder, P = 0.22; C4 vs shoulder, P = 0.78).
Table 2.
RMS-processed standard metrics during each stimulus train. MSNA burst frequency (bursts per minute) and burst incidence (bursts per 100 heartbeats) are displayed during a five-minute baseline period as well as during each TMS pulse train. Pulses were delivered at five intensities to the dlPFC (n = 12 at each intensity) and at two intensities to the motor cortex (n = 12 at each intensity) and shoulder (n = 12 at MT; n = 9 at 110% of MT). Baseline values for each region are the same, as they derive from one period at the beginning of each experiment. Values found to be significantly different from baseline are denoted with an asterisk (*P < 0.05; **P < 0.01; ***P < 0.001). Data are presented as mean ± SD.
| Baseline | MT -20% | MT -10% | MT | 110% of MT | 120% of MT | ||
|---|---|---|---|---|---|---|---|
| Burst frequency (bursts/min) | dlPFC | 34.93 ± 5.13 | 27.05 ± 5.87* | 28.49 ± 7.83 | 25.82 ± 7.14* | 27.90 ± 4.66** | 27.42 ± 4.70** |
| Motor cortex | 34.93 ± 5.13 | 25.64 ± 5.49*** | 26.23 ± 4.27*** | ||||
| Shoulder | 34.93 ± 5.13 | 27.03 ± 8.15 | 25.56 ± 6.96** | ||||
| Burst incidence (bursts/100 heartbeats) | dlPFC | 48.99 ± 12.94 | 39.71 ± 13.89 | 41.61 ± 15.94 | 37.55 ± 13.76* | 40.85 ± 12.05* | 39.15 ± 9.83* |
| Motor cortex | 48.99 ± 12.94 | 36.97 ± 11.65*** | 37.85 ± 10.10** | ||||
| Shoulder | 48.99 ± 12.94 | 38.92 ± 15.40 | 35.78 ± 12.65* | ||||
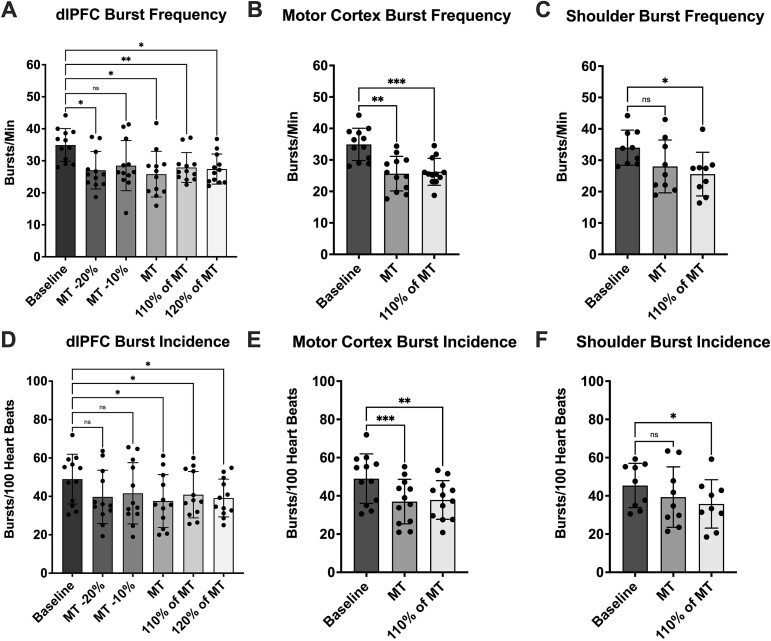
Fig. 4.
RMS-processed MSNA metrics. (A), (B), and (C) present MSNA burst frequency (bursts/min) data during stimulus trains delivered to the dlPFC (n = 12), motor cortex (n = 12) and shoulder (n = 9), respectively. In each graph, stimuli are compared to a baseline value obtained at the beginning of the experiments. (D), (E) and (F) present MSNA burst incidence (bursts/100 heartbeats) data during stimulus trains delivered to the dlPFC (n = 12), motor cortex (n = 12) and shoulder (n = 9), respectively. In each graph, stimuli are compared to a baseline value obtained at the beginning of the experiments. Time points that were found to be significantly different from baseline are denoted with asterisks (*P < 0.05; **P < 0.01; ***P < 0.001). No significant differences (P > 0.05) were found between stimulation intensities at each site. Data are presented as mean ± SD.
Fig. 5.
RMS-processed MSNA metric comparison across different stimulation sites. (A) and (B) show MSNA burst frequency (bursts/min) when stimuli were delivered to the dlPFC, motor cortex and shoulder at MT (n = 12) and at 110% of MT (n = 9), respectively. (C) and (D) show MSNA burst incidence (bursts/100 heartbeats) when stimuli were delivered to the dlPFC, motor cortex and shoulder at MT (n = 12) and at 110% of MT (n = 9), respectively. No significant differences (P > 0.05) were found between sites at either intensity through either metric. Data are presented as mean ± SD.
However, unlike the PSTH analysis, differences were observed when burst frequencies during stimulation were compared to baseline (Fig. 4). Analysis of each stimulus intensity delivered to the dlPFC (RM one-way ANOVA, P = 0.0010) revealed significantly lower MSNA burst frequency when compared to baseline, with the exception of the MT −10% stimulus train (baseline vs MT -20%, P = 0.013; baseline vs MT -10%, P = 0.068; baseline vs MT, P = 0.017; baseline vs 110% of MT, P = 0.0038; baseline vs 120% of MT, P = 0.011). Likewise, stimulation of the motor cortex reduced burst frequency (RM one-way ANOVA, P < 0.0001) compared to baseline (baseline vs MT, P = 0.0013; baseline vs 110% of MT, P = 0.00050), as did stimulation of the shoulder (RM one-way ANOVA, P = 0.016), but only at 110% of MT (baseline vs MT, P = 0.13; baseline vs 110% of MT, P = 0.015).
The MSNA burst incidence (bursts/100 heartbeats) analysis displayed results much like the burst frequency analysis (Table 2). Stimulus intensities were not found to be statistically significantly (P > 0.05) different when compared to each other (Fig. 4) at the dlPFC (RM one-way ANOVA, P = 0.29), the motor cortex (paired t-test, P = 0.68) and the shoulder (paired t-test, P = 0.20). Comparisons between sites (Fig. 5) at MT (RM one-way ANOVA, P = 0.67) saw no significant differences (F4 vs C4, P = 0.98; F4 vs shoulder, P = 0.70; C4 vs shoulder, P = 0.79), akin to comparisons at 110% of MT (RM one-way ANOVA, P = 0.22; F4 vs C4, P = 0.37; F4 vs shoulder, P = 0.21; C4 vs shoulder, P = 0.84).
Just as with burst frequency, burst incidence was decreased during stimulus trains when compared to baseline (Fig. 4). During stimulation of the dlPFC, stimuli below MT did not differ significantly from baseline (P > 0.05), but stimuli at or above MT did (RM one-way ANOVA, P = 0.0045; baseline vs MT -20%, P = 0.066; baseline vs MT -10%, P = 0.14; baseline vs MT, P = 0.035; baseline vs 110% of MT, P = 0.026; baseline vs 120% of MT, P = 0.015). Both stimulus trains applied to the motor cortex reduced burst incidence from baseline (RM one-way ANOVA, P = 0.00010; baseline vs MT, P = 0.00090; baseline vs 110% of MT, P = 0.0028) and, as before, stimulation of the shoulder (RM one-way ANOVA, P = 0.038), reduced burst incidence, but only at 110% of MT (baseline vs MT, P = 0.27; baseline vs 110% of MT, P = 0.021).
Hemodynamic data analysis
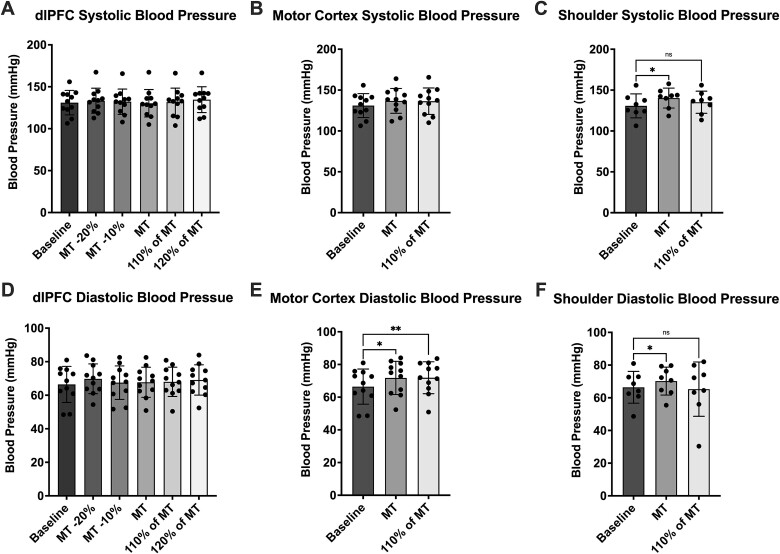
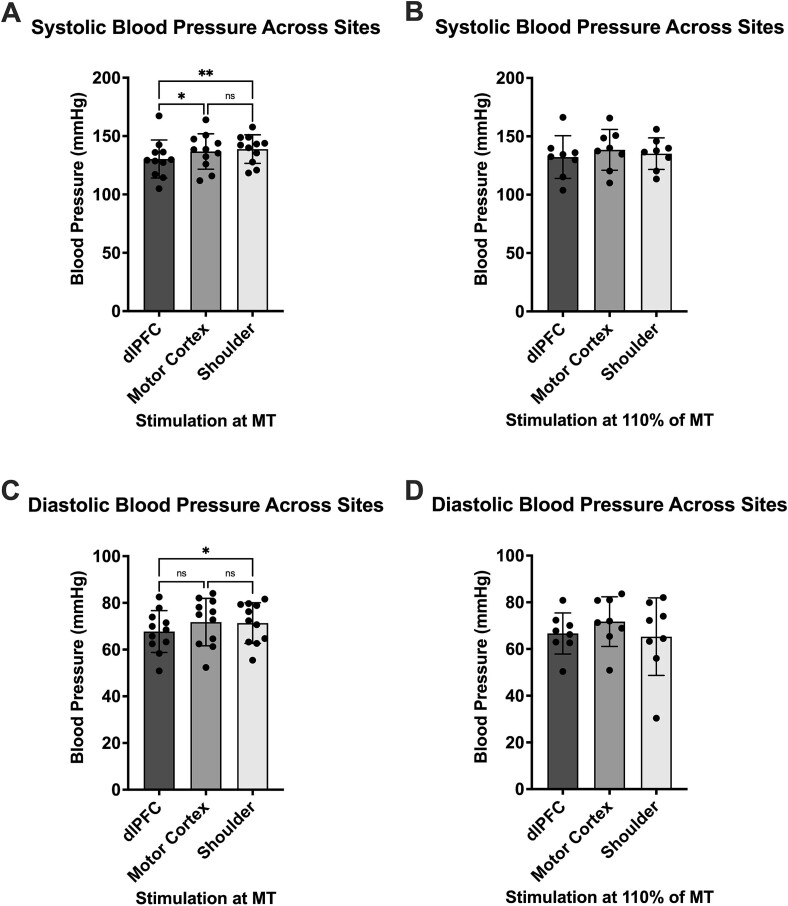
Systolic blood pressure was analyzed as well, in the same way as the previous data (Table 3). No significant (P > 0.05) differences were found between stimulation intensities (Fig. 6) at the dlPFC (RM one-way ANOVA, P = 0.047), the motor cortex (paired t test, P = 0.84) and the shoulder (paired t test, P = 0.43). There was also a lack of significant (P > 0.05) differences in the multiple comparisons post hoc analysis of dlPFC stimulations to baseline (RM one-way ANOVA, P = 0.18) and C4 stimulations to baseline (RM one-way ANOVA, P = 0.035). However, during baseline vs shoulder stimulation (RM one-way ANOVA, P = 0.27), TMS pulses delivered at MT increased blood pressure (P = 0.013). This was not true of stimulation at 110% of MT (P = 0.77). Further statistically significant differences were found in comparisons between areas (RM one-way ANOVA, P = 0.0018) (Fig. 7)—both motor cortex and shoulder stimulation increased systolic blood pressure more than stimulation of the dlPFC at MT (F4 vs C4, P = 0.028; F4 vs shoulder, P = 0.0049; C4 vs shoulder, P = 0.62). Again, this was not reflected in the data from stimulation at 110% of MT (RM one-way ANOVA, P = 0.59; F4 vs C4, P = 0.26; F4 vs shoulder, P = 0.93; C4 vs shoulder, P = 0.90).
Table 3.
Hemodynamic data during each stimulus train. Systolic and diastolic blood pressure (mmHg) are displayed during a five-minute baseline period as well as during each TMS pulse train. Pulses were delivered at five intensities to the dlPFC (n = 11 at each intensity) and at two intensities to the motor cortex (n = 11 at each intensity) and shoulder (n = 11 at MT; n = 8 at 110% of MT). Baseline values for each region are the same, as they derive from one period at the beginning of each experiment. Values found to be significantly different from baseline are denoted with an asterisk (*P < 0.05; **P < 0.01). Data are presented as mean ± SD.
| Baseline | MT -20% | MT -10% | MT | 110% of MT | 120% of MT | ||
|---|---|---|---|---|---|---|---|
| Systolic Blood Pressure (mmHg) | dlPFC | 131.0 ± 14.61 | 133.4 ± 14.91 | 132.2 ± 15.09 | 130.3 ± 16.34 | 131.7 ± 16.72 | 134.6 ± 15.40 |
| Motor Cortex | 131.0 ± 14.61 | 136.8 ± 15.18 | 136.4 ± 16.16 | ||||
| Shoulder | 131.0 ± 14.61 | 138.9 ± 12.22* | 135.2 ± 13.54 | ||||
| Diastolic Blood Pressure (mmHg) | dlPFC | 66.44 ± 10.75 | 69.78 ± 8.88 | 67.49 ± 9.97 | 67.72 ± 8.96 | 67.99 ± 8.72 | 69.09 ± 9.03 |
| Motor Cortex | 66.44 ± 10.75 | 71.78 ± 10.17* | 71.89 ± 9.76** | ||||
| Shoulder | 66.44 ± 10.75 | 71.35 ± 8.71* | 65.29 ± 16.60 | ||||
Fig. 6.
Systolic and diastolic blood pressure during each stimulus train. (A), (B), and (C) present systolic blood pressure data during stimulus trains delivered to the dlPFC (n = 11), motor cortex (n = 11) and shoulder (n = 8), respectively. In each graph, stimuli are compared to a baseline value obtained at the beginning of the experiments. (D), (E), and (F) present diastolic blood pressure data during stimulus trains delivered to the dlPFC (n = 11), motor cortex (n = 11) and shoulder (n = 8), respectively. In each graph, stimuli are compared to a baseline value obtained at the beginning of the experiments. Time points which were found to be significantly different from baseline are denoted with asterisks (*P < 0.05; **P < 0.01). No significant differences (P > 0.05) were found between stimulation intensities at each site. Data are presented as mean ± SD.
Fig. 7.
Blood pressure comparison across different stimulation sites. (A) and (B) show systolic blood pressure when stimuli were delivered to the dlPFC, motor cortex and shoulder at MT (n = 11) and at 110% of MT (n = 8), respectively. (C) and (D) show diastolic blood pressure when stimuli were delivered to the dlPFC, motor cortex and shoulder at MT (n = 11) and at 110% of MT (n = 8), respectively. Significant differences were found between sites only at MT in both metrics and are denoted with asterisks (*P < 0.05; **P < 0.01). Data are presented as mean ± SD.
In a similar fashion, diastolic blood pressure analysis (Table 3) saw no significant (P > 0.05) differences between stimulation intensities (Fig. 6) at the dlPFC (RM one-way ANOVA, P = 0.11), the motor cortex (paired t-test, P = 0.88) and the shoulder (paired t-test, P = 0.46). In comparing each dlPFC stimulation to baseline (RM one-way ANOVA, P = 0.20), no significant (P > 0.05) differences were found either. Unlike the systolic blood pressure, however, analysis of motor cortex stimulation (RM one-way ANOVA, P = 0.0036) showed significantly increased blood pressure at both intensities (baseline vs MT, P = 0.018; baseline vs 110% of MT, P = 0.0044). Shoulder stimulation (RM one-way ANOVA, P = 0.54) saw an increase at MT (P = 0.043) but not at 110% of MT (P = 0.98). When comparing between areas (Fig. 7), only one significant difference was found—shoulder stimulation increased diastolic blood pressure more than dlPFC stimulation at MT (MT analysis: RM one-way ANOVA, P = 0.039; F4 vs C4, P = 0.13; F4 vs shoulder, P = 0.039; C4 vs shoulder, P = 0.91; 110% of MT analysis: RM one-way ANOVA, P = 0.45; F4 vs C4, P = 0.15; F4 vs shoulder, P = 0.98; C4 vs shoulder, P = 0.60).
Discussion
In a continuation of our previous work on the sympathetic influences of the dlPFC (Sesa-Ashton et al. 2022; Wong et al. 2023; McCarthy et al. 2024b), the current study has evaluated the effects of single-pulse TMS of the dlPFC on MSNA. This had the added value of potential clinical relevance, given that the dlPFC is targeted with TMS for treatment of major depressive disorder. By carrying out the present study, the work also reinforces the sparse existing literature regarding the ability of TMS to generate changes in MSNA. To address this, the standard metric analyses of MSNA (burst frequency and burst incidence) were used alongside PSTH analysis. This latter analysis method facilitated direct referencing of spikes of MSNA to the TMS pulses, as well as to the R-waves of the ECG—a method previously used within our laboratory to assess sympathetic nerve activity (Boulton et al. 2018; McCarthy et al. 2024a).
Perhaps the most distinctive results obtained from the present study are the lack of single-pulse TMS effects in all of the MSNA metrics studied—spikes per R-wave, spikes per pulse, bursts per minute, and bursts per 100 heartbeats. No differences were found using PSTH analysis, both with different intensities at the same site and with the same intensities at different sites. The same was observed when comparing across sites using the standard MSNA analysis techniques. However, most stimulations did evoke a significant decrease in MSNA burst frequency and incidence when compared to the baseline value. Given that this response was not dependent upon the site of stimulation (further exemplified in stimulation of the shoulder), it would seem likely that the stimulation itself is affecting the sympathetic outflow rather than the specific cortical area targeted—TMS of the dlPFC would appear to have no effects on MSNA.
Our findings are consistent with the work of Wallin et al. (2003), in which they showed MSNA decreases with short arousal stimuli independent of cortical stimulation. Electrical skin pulses on the finger and visual flash stimuli both had this effect, while a sham consisting of a trigger without sensory stimulation did not influence MSNA. Furthermore, trains of five electrical pulses only had minor additional inhibitory effects, as arousal responses are subject to habituation. Some parallels can be drawn between the previous study by Wallin et al. (2003) and the present study in that each of our participants perceived the TMS pulses being delivered as a minor “tingling” or “tapping” sensation on the targeted site, thereby constituting a sensory stimulation. Thus, this could potentially be responsible for the MSNA inhibition. Such an idea also extends into the auditory component of the TMS pulses, with the click that accompanies each pulse. In their work, Eder et al. (2009) reported MSNA inhibition much the same as Wallin et al. (2003), but with regard to startling auditory stimuli. In this way, any startle component of our TMS protocol is potentially accounted for, too.
The results of the present study also revealed a lack of a distinct dose–response curve. The multiple comparisons analysis of the PSTH data shows all stimulation intensities to be equal in their effects on MSNA, and the same was true for the standard metric analysis when comparing between intensities. Given that the stimuli did not produce an effect in a stepwise fashion (with each increasing stimulation intensity producing a greater decrease in MSNA), the lack of dose–response gives further credit to the idea that the effects on MSNA seen here are arousal-dependent. Of course, it is a possibility that the stimuli were simply not delivered at a sufficient enough intensity to realize this potential. While 110% of MT is used for treatment of depression, this may not be optimal for evaluating sympathetic activity. As Kähkönen et al. (2004) report, TMS-evoked responses from stimulation of the prefrontal cortex were significantly smaller than those produced using the same intensity at the motor cortex. Therefore, it could be worthwhile using stimulus intensities even higher than 120% of MT to evaluate MSNA responses.
The apparent absence of any sympathetic responses to single-pulse TMS might be beneficial with respect to treatment options. For example, if these single-pulse results are indicative of MSNA responses in rTMS, then they have clinical relevance in the field of depression treatment. Lambert et al. (2010) have suggested that patients with depression have elevated resting MSNA levels, so providing evidence showing that TMS does nothing to expound this factor and possibly even reduces MSNA may prove insightful to our understanding of the field. It would, of course, not be ideal to treat depression with rTMS only to increase the risk of adverse cardiac events by perhaps increasing an already elevated MSNA baseline (Charkoudian and Rabbitts 2009; Malpas 2010). Whether or not the sympathoinhibitory effects are short or possibly longer-term, especially over repeated TMS sessions, is worth investigating further. On the other hand, Darling et al. (2024) have reported that MSNA reactivity, as well as resting MSNA, remains unchanged in the depressed state. If such is the case, the sympathoinhibition seen in our study may not have actively beneficial effects but is important nonetheless for the information it provides at the foundational level. This extends to the context of anti-depressant use, with several medications being known to have sympathoinhibitory effects (Tank et al. 2003; Barton et al. 2007; Scalco et al. 2009). Granted, TMS for depression treatment is typically only used when other treatment options have proven ineffective; however, TMS and anti-depressants can be used simultaneously, and there is even evidence to show that doing so may have additive effects in the treatment response (Rakesh et al. 2024). Similar to the statements above, should inhibitory TMS couple with inhibitory anti-depressants, this could potentially prove detrimental to the cardiovascular health of the patients. With this being said, if the stimulation of the dlPFC is, indeed, having no discernible effects on MSNA other than short-term arousal, then the safety of the combined depression treatment can be further justified.
The lack of significant differences extends into the blood pressure data, with dlPFC stimulation showing no difference between timepoints or even in comparison to the baseline, both for systolic and diastolic blood pressure. These results are consistent with the study by Campana et al. (2021), who showed no changes in blood pressure with rTMS over the left dlPFC. It is worth discussing, though, that stimulation of the motor cortex and shoulder at MT increased systolic blood pressure significantly more than stimulation of the dlPFC at the same intensity. Pulses delivered to the shoulder also increased diastolic blood pressure significantly more than those delivered to the dlPFC, but this was not reflected in stimulation of the motor cortex. Interestingly, increasing stimulation to 110% of MT removed any significant differences between stimulation sites. The shoulder stimulation may be accounted for here by the fact that pulses were delivered first at MT, then at the increased intensity. Arm twitches evoked by the stimulation likely contributed to a startle response, which increased blood pressure during the initial pulses and which participants habituated to by the time the increased intensity pulses were delivered (Rettig et al. 1986; Eder et al. 2009). However, this does not account for the motor cortex response, which had the additional effect of increasing diastolic (but not systolic) blood pressure from baseline at both intensities. This is somewhat of a surprising result, as studies exploring the cardiovascular effects of single-pulse TMS over the motor cortex state that mean arterial pressure and heart rate remain stable during stimulation (Keller-Ross et al. 2019), as does blood pressure (Macefield et al. 1998), so the sympathoinhibition cannot be explained by baroreflex-mediated inhibition. Chantigian et al. (2021), however, report blood pressure results from single-pulse TMS more similar to those seen in our current study. Still, other studies, have even shown motor cortex stimulation to decrease blood pressure, albeit with TMS protocols other than single-pulse TMS (Foerster et al. 1997). Therefore, the influences of TMS on blood pressure may well be a matter worth investigating further.
Moreover, in direct relation to our initial hypothesis, all of these results suggest that the dlPFC is not capable of generating de novo bursts of MSNA. Given our previous work on dlPFC-mediated MSNA responses (Sesa-Ashton et al. 2022; McCarthy et al. 2024b), it would seem that the dlPFC can play a modulatory role on MSNA, but not a deterministic one. The properties of the tACS used in the previous studies meant that membrane excitability within the dlPFC was being altered, but action potentials were not being directly generated (Elyamany et al. 2021), hence the use of TMS for the present study. Therefore, it is possible that the dlPFC is exerting its actions on MSNA through a network that the single-pulse TMS does not have the capacity to engage in the same manner that continuous tACS does. This network, as first introduced by James et al. (2013), has been further described and speculated upon in our previous work (Sesa-Ashton et al. 2022; McCarthy et al. 2024b), with emphasis placed on the involvement of the insula, hypothalamus, and periaqueductal gray. How each of these regions may or may not be taking part in the TMS response may prove interesting to examine in the context of real-time brain imaging.
Limitations
A primary limitation of the present study involved the positioning of the TMS coil. While the dlPFC and motor cortex were marked on each participant at the beginning of each experiment, the TMS coil was held over the stimulation site by an investigator and each pulse was delivered manually. As such, in the absence of a stereotactic neuronavigation system, slight changes in position may have influenced the strength and area of stimulation (de Geode et al. 2018). This was not such an issue with shoulder stimulation, as the coil could be held lower and rested on a flatter surface.
Additionally, the experimental protocol aimed to deliver five TMS pulses out-of-phase with MSNA bursts. However, due to the manual delivery of the pulses, occasionally, a pulse would be delivered in phase with a burst of sympathetic activity. In such cases, extra pulses were delivered during the train to compensate for the miss-timed pulses. During analysis, however, the window discriminator software often made it impossible to remove the in-phase pulses, and so, PSTH analysis was conducted using some of the miss-timed pulses. The nature of PSTH analysis using an averaged burst profile reduced the consequence of this factor, but it is, nevertheless, worth noting.
Finally, it is also important to note that no recovery period was obtained after stimulus trains. Given the amount of different stimulus intensities used in the protocol, a recovery period of significant length after each train was not viable. Nevertheless, this means that whether or not the sympathoinhibition seen during stimulation periods is preserved remains to be seen. Supposing that the effects are, indeed, arousal-dependent, it would be assumed that MSNA levels would shortly return to baseline values; however, this is worth investigating further.
Conclusion
The current project has displayed evidence to suggest that the dlPFC is not capable of generating de novo bursts of MSNA through single-pulse TMS, contrary to our initial hypothesis. This comes from the basis of our previous work in which the dlPFC was evidenced to have a modulatory effect on MSNA, entraining the activity to the sinusoidal stimulus with tACS (Sesa-Ashton et al. 2022; McCarthy et al. 2024b). The single pulses of TMS were here shown to reduce MSNA (in burst frequency and incidence) from baseline. However, stimulation delivered to the motor cortex and to the shoulder was seen to have the same effect. Comparisons between stimulation sites and stimulation intensities further displayed no differences, as did all comparisons evaluated through PSTH analysis of single spikes of MSNA. This led to the conclusion that any observed differences in sympathetic activity are attributable to arousal rather than the magnetic stimulus directly activating a cortical area or network. With this being said, it may very well be that the stimuli were simply not sufficient enough to evoke activity within the dlPFC and it may prove interesting to further explore this concept with the use of rTMS and/or higher stimulation intensities.
Acknowledgments
Brendan McCarthy was supported by an Australian Government Research Training Program (RTP) scholarship.
Contributor Information
Brendan McCarthy, Baker Heart and Diabetes Institute, 75 Commercial Road, Melbourne, VIC 3004, Australia; Baker Department of Cardiometabolic Health, The University of Melbourne, Grattan Street, Parkville, VIC 3010, Australia.
Gianni Sesa-Ashton, Baker Heart and Diabetes Institute, 75 Commercial Road, Melbourne, VIC 3004, Australia.
Donggyu Rim, Department of Neuroscience, School of Translational Medicine, Monash University, The Alfred Centre, 99 Commercial Road, Melbourne, VIC 3004, Australia.
Luke A Henderson, School of Medical Sciences (Neuroscience), Brain and Mind Centre, The University of Sydney, Paramatta Road, Camperdown, NSW 2050, Australia.
Vaughan G Macefield, Baker Department of Cardiometabolic Health, The University of Melbourne, Grattan Street, Parkville, VIC 3010, Australia; Department of Neuroscience, School of Translational Medicine, Monash University, The Alfred Centre, 99 Commercial Road, Melbourne, VIC 3004, Australia.
Author contributions
Brendan McCarthy (Data curation, Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review & editing), Gianni Sesa-Ashton (Data curation, Investigation, Methodology, Writing—review & editing), Donggyu Rim (Data curation, Investigation, Writing—review & editing), Luke A. Henderson (Conceptualization, Supervision, Writing—review & editing), and Vaughan G. Macefield (Conceptualization, Data curation, Methodology, Project administration, Resources, Supervision, Writing—review & editing).
Funding
This work did not receive funding.
Conflict of interest statement: The authors declare no conflicts of interest.
Data availability
The data are available upon reasonable request.
References
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985:325:1106–1107. [DOI] [PubMed] [Google Scholar]
- Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, Socratous F, Kaye DM, Schlaich MP, Hickie Iet al. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens. 2007:25:2117–2124. 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- Bent LR, Boulton PS, Macefield VG. Modulation of muscle sympathetic bursts by sinusoidal galvanic vestibular stimulation in human subjects. Exp Brain Res. 2006:174:701–711. 10.1007/s00221-006-0515-6. [DOI] [PubMed] [Google Scholar]
- Boulton D, Taylor CE, Green S, Macefield VG. The metaboreflex does not contribute to the increase in muscle sympathetic nerve activity to contracting muscle during static exercise in humans. J Physiol. 2018:596:1091–1102. 10.1113/JP275526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourla A, Mouchabac S, Lorimy L, Crette B, Millet B, Ferreri F. Variability in motor threshold during transcranial magnetic stimulation treatment for depression: neurophysiological implications. Brain Sci. 2023:13:1246. 10.3390/brainsci13091246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM, Frampton CM, Moore G, Barclay ML, Jardine DL. Immediate effects of caffeine on sympathetic nerve activity: why coffee is safe? A single-centre crossover study. Clin Auton Res. 2023:33:623–633. 10.1007/s10286-023-00967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana M, Wagner E, Wobrock T, Langguth B, Landgrebe M, Eichhammer P, Frank E, Cordes J, Wölwer W, Winterer Get al. Effects of high-frequency prefrontal rTMS on heart frequency rates and blood pressure in schizophrenia. J Psychiatr Res. 2021:140:243–249. 10.1016/j.jpsychires.2021.06.010. [DOI] [PubMed] [Google Scholar]
- Chantigian DP, Larson M, Chen M, Gillick BT, Cross TJ, Keller-Ross ML. Heart rate and blood pressure responses to corticomotor stimulation in young, healthy adults. Clin Neurophsiol Prac. 2021:6:206–208. 10.1016/j.cnp.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N, Rabbitts JA. Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin Proc. 2009:84:822–830. 10.4065/84.9.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti R, Binggeli C, Sudano I, Spieker L, Hänseler E, Ruschitzka F, Chaplin WF, Lüscher TF, Noll G. Coffee acutely increases sympathetic nerve activity and blood pressure independently of caffeine content: role of habitual vs nonhabitual drinking. Circ. 2002:106:2935–2940. 10.1161/01.CIR.0000046228.97025.3A. [DOI] [PubMed] [Google Scholar]
- Darling AM, Young BE, Skow RJ, Dominguez CM, Saunders EF, Fadel PJ, Greaney JL. Sympathetic and blood pressure reactivity in young adults with major depressive disorder. J Affect Disord. 2024:361:322–332. 10.1016/j.jad.2024.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis K, Narkiewicz K, Leontsinis I, Konstantinidis D, Mihas C, Andrikou I, Thomopoulos C, Tousoulis D, Tsioufis K. Acute effects of electronic and tobacco cigarette smoking on sympathetic nerve activity and blood pressure in humans. Int J Environ Res Public Health. 2022:19:3237. 10.3390/ijerph19063237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder DN, Elam M, Wallin BG. Sympathetic nerve and cardiovascular responses to auditory startle and prepulse inhibition. Int J Psychophysiol. 2009:71:149–155. 10.1016/j.ijpsycho.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Elyamany O, Leicht G, Hermann CS, Mulert C. Transcranial alternating current stimulation (tACS): from basic mechanisms towards first applications in psychiatry. Eur Arch Psychiatry Clin Neurosci. 2021:271:135–156. 10.1007/s00406-020-01209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB. An update on the clinical use of repetitive transcranial magnetic stimulation in the treatment of depression. J Affect Disord. 2020:276:90–103. 10.1016/j.jad.2020.06.067. [DOI] [PubMed] [Google Scholar]
- Foerster A, Schmitz JM, Nouri S, Claus D. Safety of rapid-rate transcranial magnetic stimulation: heart rate and blood pressure changes. Electroencephalogr Clin Neurophysiol. 1997:104:207–212. 10.1016/S0168-5597(97)00016-6. [DOI] [PubMed] [Google Scholar]
- Geode AA, Ter Braack EM, Putten MJ. Accurate coil positioning is important for single and paired pulse TMS on the subject level. Brain Topogr. 2018:31:917–930. 10.1007/s10548-018-0655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C, Macefield VG, Henderson LA. Real-time imaging of cortical and subcortical control of muscle sympathetic nerve activity in awake human subjects. NeuroImage. 2013:70:59–65. 10.1016/j.neuroimage.2012.12.047. [DOI] [PubMed] [Google Scholar]
- Kähkönen S, Wilwnius J, Komssi S, Ilmoniemi RJ. Distinct differences in cortical reactivity of motor and prefrontal cortices to magnetic stimulation. Clin Neurophysiol. 2004:115:583–588. 10.1016/j.clinph.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Keller-Ross ML, Chantigian DP, Chen M, Rich TL, Chen CY, Gillick BT. Stability of the cardiovascular response during single-pulse TMS in perinatal stroke. Brain Stimul. 2019:12:371–373. 10.1016/j.brs.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerly DS, Boe S, Ladouceur M, Weir JA. Does temporary inhibition of the human primary motor cortex alter resting and isometric handgrip exercise-mediated sympathetic neural responses? FASEB J. 2018:31:865.6. 10.1096/fasebj.31.1_supplement.865.6. [DOI] [Google Scholar]
- Koponen LM, Peterchev AV. Transcranial magnetic stimulation: principles and applications. In: He B, editors. Neural Engineering. Cham: Springer; 2020, p. 245–270. 10.1007/978-3-030-43395-6_7. [DOI] [Google Scholar]
- Lambert E, Dawood T, Straznicky N, Sari C, Schlaich M, Esler M, Lambert G. Association between the sympathetic firing pattern and anxiety level in patients with the metabolic syndrome and elevated blood pressure. J Hypertens. 2010:28:543–550. 10.1097/HJH.0b013e3283350ea4. [DOI] [PubMed] [Google Scholar]
- Li Q, Fu Y, Liu C, Meng Z. Transcranial direct current stimulation of the dorsolateral prefrontal cortex for treatment of neuropsychiatric disorders. Front Behav Neurosci. 2022:16:893955. 10.3389/fnbeh.2022.893955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Pearlman SH, Fischell RE, Ruppel PL, Goadsby PJ. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010:9:373–380. 10.1016/S1474-4422(10)70054-5. [DOI] [PubMed] [Google Scholar]
- Macefield VG. Recording and quantifying sympathetic outflow to muscle and skin in humans: methods, caveats and challenges. Clin Auton Res. 2021:31:59–75. 10.1007/s10286-020-00700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Henderson LA. Identification of the human sympathetic connectome involved in blood pressure regulation. NeuroImage. 2019:202:116119. 10.1016/j.neuroimage.2019.116119. [DOI] [PubMed] [Google Scholar]
- Macefield VG, James C. Superentrainment of muscle sympathetic nerve activity during sinusoidal galvanic vestibular stimulation. J Neurophysiol. 2016:116:2689–2694. 10.1152/jn.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Taylor JL, Wallin BG. Inhibition of muscle sympathetic outflow following transcranial cortical stimulation. J Autonom Nerv Syst. 1998:68:49–57. 10.1016/S0165-1838(97)00117-3. [DOI] [PubMed] [Google Scholar]
- Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010:90:513–557. 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- McCarthy B, Datta S, Sesa-Ashton G, Wong R, Henderson LA, Dawood T, Macefield VG. Differential control of sympathetic outflow to muscle and skin during physical and cognitive stressors. Clin Auton Res. 2024a:34:177–189. 10.1007/s10286-024-01015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy B, Datta S, Sesa-Ashton G, Wong R, Henderson LA, Dawood T, Macefield VG. Non-additive effects of electrical stimulation of the dorsolateral prefrontal cortex and the vestibular system on muscle sympathetic nerve activity in humans. Exp Brain Res. 2024b:174:701–711. 10.1007/s00221-024-06852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton RJ, Malcolm MP, Newsom SA, Richards JC, Rynn GM, Bell C. Sympathetic responses to repetitive trans-spinal magnetic stimulation. Clin Auton Res. 2011:21:81–87. 10.1007/s10286-010-0092-4. [DOI] [PubMed] [Google Scholar]
- Piretti L, Pappaianni E, Gobbo S, Rumiati RI, Job R, Grecucci A. Dissociating the role of dACC and dlPFC for emotion appraisal and mood regulation using cathodal tDCS. Cogn Affect Behav Neurosci. 2022:22:304–315. 10.3758/s13415-021-00952-3. [DOI] [PubMed] [Google Scholar]
- Rakesh G, Cordero P, Khanal R, Himelhoch SS, Rush CR. Optimally combined transcranial magnetic stimulation with antidepressants in major depressive disorder: a systematic review and meta-analysis. J Affect Disord. 2024:358:432–439. 10.1016/j.jad.2024.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig R, Geyer MA, Printz MP. Cardiovascular concomitants of tactile and acoustic startle responses in spontaneously hypertensive and normotensive rats. Physiol Behav. 1986:36:1123–1126. 10.1016/0031-9384(86)90489-0. [DOI] [PubMed] [Google Scholar]
- Rossi S, Antal A, Bestmann S, Bikson M, Brewer C, Brockmöller J, Carpenter LL, Cincotta M, Chen R, Daskalakis JDet al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin Neurophysiol. 2021:132:269–306. 10.1016/j.clinph.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalco AZ, Rondon MU, Trombetta IC, Laterza MC, Azul JB, Pullenayegum EM, Scalco MZ, Kuniyoshi FH, Wajngarten M, Negrāo CEet al. Muscle sympathetic nervous activity in depressed patients before and after treatment with sertraline. J Hypertens. 2009:27:2429–2436. 10.1097/HJH.0b013e3283310ece. [DOI] [PubMed] [Google Scholar]
- Sesa-Ashton G, Wong R, McCarthy B, Datta S, Henderson LA, Dawood T, Macefield VG. Stimulation of the dorsolateral prefrontal cortex modulates muscle sympathetic nerve activity and blood pressure in humans. Cerebral Cortex Commun. 2022:3:tgac017. 10.1093/texcom/tgac017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Funke K, Aberra AS, Bestmann S, Chen R, Classen J, Davare M, Di Lazzaro V, Fox PT, Hallett M. Transcranial magnetic stimulation of the brain: what is stimulated? – a consensus and critical position paper. Clin Neurophysiol. 2022:140:59–97. 10.1016/j.clinph.2022.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber DH, Sinoway LI, Leuenberger UA, Amassian VE. Magnetic stimulation of the human motor cortex evokes skin sympathetic nerve activity. J Appl Physiol. 2000:88:126–134. 10.1152/jappl.2000.88.1.126. [DOI] [PubMed] [Google Scholar]
- Tank J, Schroeder C, Diedrich A, Szczech E, Haertter S, Sharma AM, Luft FC, Jordan J. Selective impairment in sympathetic vasomotor control with norepinephrine transporter inhibition. Circ. 2003:107:2949–2954. 10.1161/01.CIR.0000072786.99163.FE. [DOI] [PubMed] [Google Scholar]
- Vasegi B, Zoghi M, Jaberzadeh S. Inter-pulse interval affects the size of single-pulse TMS-induced motor evoked potentials: a reliability study. Basic Clin Neurosci. 2015:6:44–51. [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Donadio V, Karlsson T, Kallio M, Nordin M, Elam M. Arousal increases baroreflex inhibition of muscle sympathetic activity. Acta Physiol Scand. 2003:177:291–298. 10.1046/j.1365-201X.2003.01071.x. [DOI] [PubMed] [Google Scholar]
- Wong R, Sesa-Ashton G, Datta S, McCarthy B, Henderson LA, Dawood T, Macefield VG. The role of the dorsolateral prefrontal cortex in control of skin sympathetic nerve activity in humans. Cereb Cortex. 2023:33:8265–8272. 10.1093/cercor/bhad112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available upon reasonable request.