Abstract
Purpose
To evaluate the efficacy and safety of intranasal dexmedetomidine (Dex), oral lorazepam, and a placebo in managing preoperative anxiety-related insomnia.
Patients and Methods
A total of 90 patients exhibiting symptoms of preoperative anxiety and insomnia were randomly assigned to three groups: Dex (receiving 2.5 µg/kg Dex intranasally and starch tablets orally), lorazepam (receiving saline intranasally and 2 mg lorazepam orally), and placebo (receiving saline intranasally and starch tablets orally). Interventions were conducted the night before surgery. The primary outcome was measured using the Leeds Sleep Evaluation Questionnaire (LSEQ) to evaluate changes in sleep quality pre- and post-intervention. Secondary outcomes included monitoring sleep on the night of the intervention, sleep satisfaction scores, changes in vital signs within 2 hours post-intervention, and adverse reaction rates.
Results
According to sleep assessments using the LSEQ, the Dex group demonstrated significant improvements in ease of getting to sleep (GTS), ease of awakening (AFS), and alertness and behavior after waking (BFW) compared to the lorazepam group (p < 0.05). However, no significant differences were observed in the quality of sleep (QOS) between the two groups (p > 0.05). Sleep monitoring indicated that the Dex group had a median sleep onset latency (SOL) of 19.0 min, significantly shorter than those recorded for the lorazepam group at 33.5 min and the placebo group at 57.0 min (p < 0.001). The total sleep time (TST) and sleep efficiency (SE) were 403.7 min and 84.5% for the Dex group, similar to the lorazepam group (408.6 min, 83.2%)(p >0.999) and superior to the placebo group (278.8 min, 57.4%)(p < 0.001). Sleep satisfaction scores did not significantly differ between the Dex and lorazepam groups (p > 0.999). No serious adverse reactions were reported across the groups.
Conclusion
Both 2.5 μg/kg intranasal Dex and 2 mg oral lorazepam effectively improved sleep quality in patients with preoperative anxiety-related insomnia. While both treatments were comparable in maintaining sleep, intranasal Dex was more effective in initiating sleep and enhancing daytime functionality than lorazepam.
Keywords: intranasal dexmedetomidine, preoperative anxiety insomnia, sleep disorder, therapeutic efficacy safety assessment
Introduction
Insomnia, a common sleep disorder, involves difficulties in initiating or maintaining sleep, causing significant distress and daytime dysfunction.1 Both the prevalence and severity of insomnia are observed to increase during the perioperative period. Recent studies indicate that nearly 60% of patients experience insomnia before surgery, primarily due to anxiety.2,3 This decline in sleep quality not only exacerbates anxiety but also poses negative health implications, including increased risks for cardiovascular diseases, cognitive impairment, heightened pain perception, compromised immune function, and prolonged hospital stays.4–7 Non-pharmacological interventions such as environmental management, sleep hygiene education, and cognitive-behavioral therapy (CBT) are commonly employed but often show limited efficacy and may necessitate pharmacological support.8 Benzodiazepines, particularly lorazepam at dosages of 2 mg, are frequently prescribed to address insomnia linked with preoperative anxiety.9,10 Nevertheless, they can disrupt normal sleep patterns by reducing rapid eye movement (REM) sleep and deep sleep stages, causing side effects such as dizziness and daytime drowsiness.9,11,12 Given these limitations, the exploration of effective and safer alternatives for managing preoperative insomnia is critical.
Dexmedetomidine (Dex), a specific α2-adrenoceptor agonist, exhibits sympatholytic, anxiolytic, sedative, and analgesic properties and is extensively used in clinical anesthesia and intensive care units (ICU).13 Distinctly from other sedatives, Dex promotes a sleep pattern analogous to non-rapid eye movement sleep stages 2 (N2) and 3 (N3) by activating intrinsic sleep-facilitating pathways.14–16 The N3 sleep is closely related to cognitive improvement and synaptic plasticity enhancement.17–20 In the ICU, Dex has proven efficacious in increasing sleep duration and efficiency and in preventing delirium.21–24 These qualities make Dex a potential treatment option for insomnia, as it improves sleep quality while reducing nervous system side effects. Our previous study found that a 2.5 μg/kg dose of intranasal Dex effectively alleviated preoperative anxiety-insomnia.25 However, the comparative efficacy and side effects between Dex and benzodiazepines remain unclear. Therefore, we hypothesized that intranasal Dex at 2.5 μg/kg might have efficacy similar to that of oral 2 mg lorazepam in treating preoperative anxiety-insomnia while reducing adverse effects on the central nervous system.
Clinical Trial Oversight
This randomized, triple-blind, parallel-group clinical trial was carried out at the First Affiliated Hospital of Gannan Medical University, Jiangxi Province, China, from January 1 to September 30, 2023. The protocol was approved by the Clinical Ethics Committee of the First Affiliated Hospital of Gannan Medical University (Approval No. LLSC-2022121901) and registered with the Chinese Clinical Trial Registry (ChiCTR2200067165). This clinical trial adhered to the Declaration of Helsinki, and informed consent was obtained from all participants before the start of the study.
Study Participants
Two investigators screened potential participants among patients scheduled for elective surgery. Eligibility criteria were: ages 18–65 years, insomnia severity index (ISI) > 726 (range: 0–28, scores > 7 indicating insomnia and higher scores indicating greater severity), hospital anxiety and depression scale-anxiety subscale (HADS-A) > 727 (range: 0–21, scores > 7 indicating anxiety, higher scores indicating more severe anxiety), and oxygen saturation (SpO2) > 95% when breathing room air. The exclusion criteria were: (1) Hospital anxiety and depression scale-depression subscale (HADS-D) score27 > 7 (range: 0–21, with scores > 7 indicating depression, higher scores indicating greater depression); (2) numeric rating scale (NRS) for pain > 3; (3) history of alcohol or drug dependence; (4) psychotropic medication use in the past two weeks; (5) confirmed or suspected obstructive sleep apnea syndrome; (6) intranasal pathology or structural nasal abnormalities; (7) heart rate (HR) < 60 bpm or preoperative sick sinus syndrome or second-degree (or higher) atrioventricular block without a pacemaker; (8) Serious liver or kidney dysfunction; (9) known allergies to study medications; (10) pregnancy, breastfeeding, or planned pregnancy; (11) inability to understand study questionnaires or severe visual impairment precluding study participation.
Randomization and Blindness
SPSS software (version 26.0, USA) was used to randomly assign eligible participants in a 1:1:1 ratio to one of three groups: Dex, lorazepam, and placebo. Group allocations were concealed in sequentially numbered opaque envelopes that were opened after participants were enrolled. A nurse who was uninvolved in the study prepared the medications, ensuring that both the nasal drops and oral tablets appeared identical across all group allocations. The Dex group received Dex solution (2 mL: 200 µg, Yangtze River Pharmaceutical Group Co., Ltd., China) and starch tablets; the lorazepam group received saline (10 mL: 0.09 g, Otsuka Pharmaceutical Co., Ltd., China) and lorazepam tablets (1 mg each, Shandong Xinyi Pharmaceutical Co., Ltd., China); the placebo group received saline and starch tablets. Nasal drops were dispensed into 1 mL syringes that appeared identical, while starch tablets were designed to replicate the appearance, size, color, weight, and odor of lorazepam tablets. Once prepared, medications were numbered and handed over to the anesthesiologist for administration. Blinding was maintained throughout the study; both participants and personnel involved in recruitment, administration, data collection, and analysis were unaware of group allocations. An independent research administrator conducted the randomization process. In emergencies, the physician or researcher could unblind the group allocation for the corresponding patient. To ensure patient safety, this patient would be excluded from further study participation.
Study Procedures
On the night before the surgery, patients’ bedtimes were set between 21:00 and 23:00. The study was conducted in a ward setting where patients lay supine on their beds without supplemental oxygen, undergoing monitoring for blood pressure, SpO2, HR, and bispectral index (BIS). After monitoring was established, patients took tablets (2 mg lorazepam or starch tablets) with 10 mL of water. Subsequently, an anesthesiologist administered the drug (Dex solution or saline) intranasally using a needleless 1 mL syringe. The concentration of Dex used in this experiment is 100 μg/mL, with a predefined dosing amount of 2.5 μg/kg. To calculate the liquid volume of the nasal medication, we use the following formula: volume (mL) = required drug amount / drug concentration = (2.5 × body weight) ÷ 100. During administration, 0.1 mL of the solution was instilled into one nostril at a time, with gentle pressure applied to facilitate absorption, then alternated to the other nostril until the full dose was administered. The entire administration process was completed within 5 min. Afterwards, lights and sounds were minimized to create a conducive sleep environment. Over the next two hours, anesthesiologists closely monitored patients’ vital signs. If the mean arterial pressure (MAP) deviated from baseline by more than 30%, adjustments were made using norepinephrine or nitroglycerin; atropine was used if the HR dropped below 45 bpm; metoprolol was administered if the HR exceeded 100 bpm; and if SpO2 fell below 92%, oxygen was provided via nasal cannula. Two hours post-drug administration, all monitoring except BIS was discontinued to minimize nighttime sleep disturbances. The next morning between 07:00 and 08:00, researchers assessed patients’ sleep quality and adverse effects. Patients were also invited to complete sleep satisfaction scores and the Leeds Sleep Evaluation Questionnaire (LSEQ).
Outcome Variables
The primary outcome was assessed using the LSEQ to evaluate changes in sleep quality pre- and post-intervention. The LSEQ is a standardized self-assessment tool with ten visual analog scales for evaluating different aspects of sleep.28 These aspects include ease of getting to sleep (GTS), quality of sleep (QOS), ease of awakening from sleep (AFS), and alertness and behavior following wakefulness (BFW).28 Higher scores indicate better sleep conditions.28 The LSEQ has been validated as an effective tool for assessing pharmacological impacts on sleep quality, showing strong internal consistency and reliability across multiple studies.29,30
Secondary outcomes included monitoring sleep on the night of the intervention, sleep satisfaction scores, changes in vital signs within 2 hours post-intervention, and adverse reaction rates.
Polysomnography (PSG) is regarded as the gold standard for objective sleep monitoring. However, it requires patients to undergo testing in a specialized sleep lab, which involves adapting to a new environment and connecting to multiple monitoring devices. These factors can increase patient anxiety and potentially disrupt sleep quality. To overcome these limitations, this study employs a more portable and simplified monitoring device—the BIS—which enables sleep monitoring in the patient’s familiar hospital room. The BIS is a non-invasive tool commonly used to assess anesthesia depth. Given the neurophysiological similarities between anesthesia and sleep, researchers have proposed using BIS to evaluate sleep depth.31–34 Sandra et al found a significant correlation between BIS scores and PSG.32 A BIS value below 80 typically indicates the onset of sleep, with lower values indicating deeper sleep.33,35 In this study, BIS values were used to quantify several sleep quality metrics, including: (1) Sleep onset latency (SOL)—the time from lights out to the first BIS value drop below 80; (2) Total sleep time (TST)—the total duration during which BIS remains below 80; (3) Sleep efficiency (SE)—the percentage of TST relative to total time in bed; (4) Number of awakenings—the number of instances BIS exceeded 80 and lasted for more than 5 min; and (5) Wake after sleep onset (WASO)—the wakefulness duration post-sleep onset. Additionally, the study divided nighttime sleep into two phases: the first half (the initial four hours) and the second half (the remaining period). The number of awakenings and WASO were recorded and analyzed separately for each phase.
Sleep satisfaction was assessed using a 0 to 10 scale, where higher scores corresponded to greater satisfaction.
Within two hours following the intervention, continuous monitoring of SpO2, HR, and BIS was conducted. Intermittent blood pressure measurements were taken at seven time points: pre-administration (T0), and 10 min (T1), 20 min (T2), 30 min (T3), 60 min (T4), 90 min (T5), and 120 min (T6) post-administration. Concurrently, the anesthesiologist recorded MAP, SpO2, HR, and BIS values at each time point. The study also monitored for potential clinical adverse events, such as hypotension (systolic pressure < 90 mmHg or diastolic pressure < 60 mmHg), hypertension (systolic pressure > 160 mmHg or diastolic pressure > 100 mmHg), bradycardia (HR < 50 bpm), tachycardia (HR > 100 bpm), and respiratory depression (SpO2 < 92%). Any adverse events were to be immediately documented and addressed according to predefined intervention protocols.
Adverse reaction monitoring focused on symptoms like nasal discomfort, bitter taste, dry mouth, dizziness, headache, fatigue, drowsiness, unsteady gait, gastrointestinal discomfort, hypotension, hypertension, bradycardia, tachycardia, and respiratory depression.
Statistical Analysis
The sample size was determined using preliminary findings, which assessed the changes in the LSEQ’s four domains (GTS, QOS, AFS, and BFW). In the Dex group, mean score changes were 6.6 ± 2.0 (GTS), 4.7 ± 0.7 (QOS), 1.2 ± 1.7 (AFS), 2.9 ± 1.7 (BFW), while in the lorazepam group, these were 4.8 ± 2.0 (GTS), 5.5 ± 1.2 (QOS), −0.5 ± 1.7 (AFS), and −0.4 ± 1.2 (BFW). In the placebo group, the scores were 1.0 ± 2.4 (GTS), 1.4 ± 1.4 (QOS), −0.2 ± 1.3 (AFS), and −0.4 ± 1.3 (BFW). PASS software (version 15.0, USA) was used to calculate the required sample size for each domain, targeting a 0.05 two-sided significance level and 90% statistical power. The largest estimated sample size was 24 participants per group. In order to account for a 20% dropout rate, 30 participants were enrolled per group, ie, a total of 90 individuals.
Sample Size Estimation
Outcome Analysis
Statistical analyses were conducted using IBM SPSS (version 26.0, USA) and GraphPad Prism (version 9.2.0, USA). Normally distributed data were presented as means with standard deviation (SD), and group differences were evaluated using one-way analysis of variance (ANOVA) with Bonferroni correction for post-hoc pairwise comparisons. Non-normally distributed data were expressed as medians and interquartile ranges (IQR), with group differences assessed via the Kruskal–Wallis H-test. Categorical data were shown as frequencies and percentages, analyzed using Pearson’s chi-square test or Fisher’s exact test as appropriate, with pairwise comparisons made via chi-squared segmentation or Fisher’s exact probability methods. A P-value < 0.05 was deemed statistically significant.
Results
Patient Disposition and Baseline Demographics
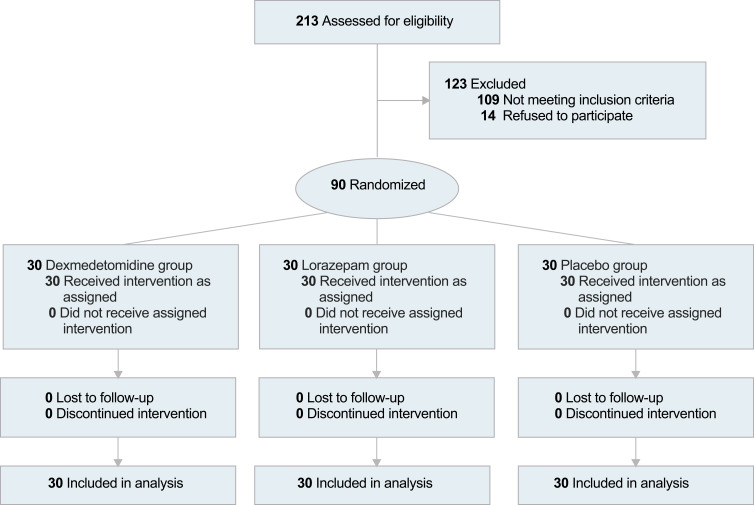
A total of 213 patients were screened between January 1 and September 30, 2023. Among them, 90 eligible patients were enrolled in this study (Figure 1). The cohort comprised 57 females (63.3%) and 33 males (36.7%), with an average age of 50.5 years (SD = 5.4). No patients dropped out during the study period. Demographic and clinical characteristics were comparable across the three groups (Table 1). The severity of insomnia and anxiety was deemed mild to moderate according to baseline ISI and HADS scores in all groups.
Figure 1.
Study population flow diagram.
Table 1.
Patient Demographics and Baseline Characteristics
| Characteristic | Dex Group (n=30) | Lorazepam Group (n=30) | Placebo Group (n=30) |
|---|---|---|---|
| Age, mean (SD), y | 50.1 (5.7) | 50.1 (5.8) | 51.3 (4.7) |
| Female, No. (%) | 17 (57) | 20 (67) | 20 (67) |
| Height, mean (SD), cm | 161.7 (8.7) | 159.7 (7.0) | 159.8 (6.5) |
| Weight, mean (SD), Kg | 61.0 (9.6) | 61.7 (8.8) | 58.3 (8.1) |
| Standard body weight, mean (SD), Kg | 56.3 (6.6) | 54.7 (5.3) | 54.8 (5.0) |
| BMI, mean (SD), Kg/m2 | 23.3 (2.8) | 24.1 (2.3) | 22.8 (2.4) |
| ASA, No. (%) | |||
| I | 15 (50) | 13 (43) | 13 (43) |
| II | 15 (50) | 17 (57) | 17 (57) |
| Education level, median (IQR), y | 9.0 (9.0,12.0) | 9.0 (9.0,12.0) | 9.0 (9.0,12.0) |
| NRS, median (IQR) | 0 (0,2.0) | 0 (0,2.0) | 0 (0,2.3) |
| ISI, median (IQR) | 11.0 (11.0,14.0) | 12.0 (10.0,14.3) | 11.0 (10.0,14.0) |
| HADS, median (IQR) | |||
| HADS-A | 9.0 (8.0,10.0) | 9.0 (8.0,11.0) | 9.0 (8.0,10.3) |
| HADS-D | 2.0 (2.0,3.0) | 2.0 (1.0,3.0) | 2.0 (1.0,3.0) |
| LSEQ, median (IQR) | |||
| GTS | 11.0 (10.0,12.0) | 10.0 (9.5,12.0) | 11.0 (9.0,12.0) |
| QOS | 7.0 (6.0,7.0) | 7.0 (6.0,7.0) | 7.0 (6.0,7.3) |
| AFS | 10.0 (9.0,10.0) | 10.0 (9.0,10.0) | 10.0 (9.0,10.0) |
| BFW | 14.0 (13.0,14.0) | 14.0 (13.0,14.0) | 14.0 (12.8,14.0) |
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiologists; ISI, Insomnia severity index; HADS, Hospital Anxiety and Depression Scale; HADS-A, Hospital Anxiety and Depression Scale-Anxiety subscale; HADS-D, Hospital Anxiety and Depression Scale-Depression subscale; NRS, Numeric Rating Scale for Pain; LSEQ, Leeds Sleep Evaluation Questionnaire; GTS, ease of getting to sleep; QOS, quality of sleep; AFS, ease of awakening from sleep; BFW, alertness and behavior following wakefulness.
Primary Outcomes
Before the intervention, no significant differences in scores were found among the three groups across the four dimensions: GTS, QOS, AFS, and BFW (Table 1, P > 0.05). The post-intervention changes in LSEQ scores are detailed in Table 2. The results demonstrate that the Dex group experienced significant improvements across four dimensions (P < 0.001). The lorazepam group exhibited significant enhancements in GTS and QOS scores (P < 0.001), while AFS scores declined (P > 0.05). The placebo group showed a significant increase only in GTS scores (P < 0.05).
Table 2.
LSEQ Scores Among Study Participants
| Outcome Assessment | Mean Change in LSEQ Score from Baseline (95% CI) | Contrast | Effect Size | 95% CI | P-valuea | ||
|---|---|---|---|---|---|---|---|
| Dex Group (n=30) | Lorazepam Group (n=30) | Placebo Group (n=30) | |||||
| GTS | 6.8 (6.1 to 7.5) *** | 5.4 (4.5 to 6.2)*** | 1.2 (0.3 to 2.1) * | D-L | 1.5 | 0.1 to 2.9 | 0.035 |
| D-P | 5.7 | 4.3 to 7.1 | < 0.001 | ||||
| L-P | 4.2 | 2.8 to 5.6 | < 0.001 | ||||
| QOS | 5.2 (4.7 to 5.7) *** | 5.6 (5.1 to 6.1)*** | 0.5 (−0.1 to 1.3) | D-L | −0.4 | −1.3 to 0.5 | 0.851 |
| D-P | 4.7 | 3.8 to 5.6 | < 0.001 | ||||
| L-P | 5.1 | 4.2 to 6.0 | < 0.001 | ||||
| AFS | 2.2 (1.8 to 2.7) *** | −1.3 (−1.8 to −0.8)*** | −0.4 (−0.9 to 0.1) | D-L | 3.5 | 2.7 to 4.4 | < 0.001 |
| D-P | 2.6 | 1.8 to 3.4 | < 0.001 | ||||
| L-P | −0.9 | −1.8 to −0.1 | 0.024 | ||||
| BFW | 3.3 (2.8 to 3.8) *** | 0.1 (−0.4 to 0.6) | −0.3 (−0.8 to 0.3) | D-L | 3.2 | 2.4 to 4.1 | < 0.001 |
| D-P | 3.6 | 2.7 to 4.5 | < 0.001 | ||||
| L-P | 0.4 | −0.5 to 1.3 | 0.934 | ||||
Notes: aThe P-value has been adjusted for multiple tests using Bonferroni correction; P < 0.05 is statistically significant. *Significant differences between pre-treatment and post-treatment, P < 0.05; *P < 0.05, ***P < 0.001.
Abbreviations: LSEQ, Leeds Sleep Evaluation Questionnaire; GTS, getting to sleep; QOS, quality of sleep; AFS, ease of awakening from sleep; BFW, alertness and behavior following wakefulness.
Comparative analysis between groups indicated that the Dex group exhibited significantly greater improvements than the placebo group across all measured dimensions. Specifically, the GTS showed a mean increase of 5.7 (95% confidence interval (CI): 4.3 to 7.1; P < 0.001), QOS by 4.7 (95% CI: 3.8 to 5.6; P < 0.001), AFS by 2.6 (95% CI: 1.8 to 3.4; P < 0.001), and BFW by 3.6 (95% CI: 2.7 to 4.5; P < 0.001). Compared to the placebo group, the lorazepam group demonstrated notably greater enhancements in both the GTS and QOS, with mean differences of 4.2 (95% CI: 2.8 to 5.6; P < 0.001) and 5.1 (95% CI: 4.2 to 6.0; P < 0.001), respectively. Conversely, there was a slight reduction in the AFS score, with a mean difference of −0.9 (95% CI: −1.8 to −0.1; P < 0.05). Changes in the BFW score were not statistically significant, with a mean difference of 0.4 (95% CI: −0.5 to 1.3; P > 0.05). Notably, Dex was more efficacious than lorazepam in GTS, AFS, and BFW, with mean differences of 1.5 (95% CI: 0.1 to 2.9; P < 0.05), 3.5 (95% CI: 2.7 to 4.4; P < 0.001), and 3.2 (95% CI: 2.4 to 4.1; P < 0.001), respectively. The QOS scores did not show significant differences between the Dex and lorazepam groups, with a mean difference of −0.4 (95% CI: −1.3 to 0.5; P > 0.05).
Secondary Outcomes
Based on objective sleep parameters quantified by BIS values (Table 3), the Dex group had a median SOL of 19.0 min (IQR 16.0–22.3), significantly shorter than that of the lorazepam group, which was 33.5 min (IQR 30.0–50.0), and the placebo group, which was 57.0 min (IQR 36.8–160.5) (both P < 0.001). TST and SE for the Dex group were 403.7 min and 84.5%, respectively, similar to the lorazepam group (408.6 min, 83.2%; P > 0.999) but significantly higher than those in the placebo group (278.8 min, 57.4%; P < 0.001). Both the Dex and lorazepam groups showed reductions in the number of awakenings and WASO compared to the placebo group (both P < 0.05). However, no significant differences were found between the Dex and lorazepam groups in these measures (p > 0.05). To further explore sleep details, we analyzed awakenings during the first and second half of the night (eTable 1 in the Supplementary Materials). Results indicate that during the first half of the night, the Dex group exhibited significantly fewer number of awakenings compared to the placebo group (P < 0.05), with no significant difference between the lorazepam and placebo groups (P > 0.05). During the second half of the night, the Lorazepam group experienced significantly less WASO compared to the placebo group (P < 0.01), while no significant differences were observed between the Dex and placebo group. No significant differences were detected in the number of awakenings and WASO between the Dex and lorazepam groups in either half of the night (P > 0.05).
Table 3.
Secondary Outcomes Between Groups
| Measurement | Dex Group (n=30) | Lorazepam Group (n=30) | Placebo Group (n=30) | Contrast | P-value |
|---|---|---|---|---|---|
| Objective sleep measurementa | |||||
| SOL, median (IQR), min | 19.0 (16.0,22.3) | 33.5 (30.0,50.0) | 57.0 (36.8,160.5) | D-L | < 0.001 |
| D-P | < 0.001 | ||||
| L-P | 0.021 | ||||
| TST, Mean (SD), min | 403.7 (41.1) | 408.6 (55.1) | 278.8 (49.7) | D-L | >0.999 |
| D-P | < 0.001 | ||||
| L-P | < 0.001 | ||||
| SE, Mean (SD),% | 84.5 (4.9) | 83.2 (7.1) | 57.4 (9.5) | D-L | >0.999 |
| D-P | < 0.001 | ||||
| L-P | < 0.001 | ||||
| No. Of awakenings, median (IQR) | 3.0 (1.8,4.0) | 3.0 (2.0,4.0) | 5.5 (3.0,7.0) | D-L | >0.999 |
| D-P | 0.002 | ||||
| L-P | 0.005 | ||||
| WASO, median (IQR), min | 53.5 (33.8,65.5) | 41.5 (21.8,58.8) | 106.0 (62.0,164.0) | D-L | 0.898 |
| D-P | 0.002 | ||||
| L-P | < 0.001 | ||||
| Satisfaction, median (IQR)a | 9.0 (8.0,9.0) | 9.0 (8.0,9.0) | 5.0 (4.0–6.0) | D-L | >0.999 |
| D-P | < 0.001 | ||||
| L-P | < 0.001 | ||||
| Adverse reactionb | |||||
| Tachycardia, No. (%) | 0 (0) | 0 (0) | 0 (0) | D-L-P | 1.000 |
| Hypertension, No. (%) | 0 (0) | 0 (0) | 0 (0) | D-L-P | 1.000 |
| Respiratory depression, No. (%) | 0 (0) | 0 (0) | 0 (0) | D-L-P | 1.000 |
| Nasal discomfort, No. (%) | 0 (0) | 0 (0) | 0 (0) | D-L-P | 1.000 |
| Gastrointestinal discomfort, No. (%) | 0 (0) | 0 (0) | 0 (0) | D-L-P | 1.000 |
| Bitter taste, No. (%) | 1 (3) | 2 (4) | 1 (3) | D-L-P | >0.999 |
| Headache, No. (%) | 0 (0) | 3 (10) | 1 (3) | D-L-P | 0.318 |
| Unsteady gait, No. (%) | 0 (0) | 3 (10) | 1 (3) | D-L-P | 0.318 |
| Dry mouth, No. (%) | 5 (17) | 1 (3) | 2 (7) | D-L-P | 0.263 |
| Bradycardia, No. (%) | 3 (10) | 0 (0) | 0 (0) | D-L-P | 0.104 |
| Hypotension, No. (%) | 6 (20) | 3 (10) | 0 (0) | D-L | 0.472 |
| D-P | 0.024 | ||||
| L-P | 0.237 | ||||
| Dizziness, No. (%) | 1 (3) | 9 (30) | 6 (20) | D-L | 0.012 |
| D-P | 0.103 | ||||
| L-P | 0.552 | ||||
| Fatigue, No. (%) | 0 (0) | 6 (20) | 4 (13) | D-L | 0.024 |
| D-P | 0.112 | ||||
| L-P | 0.731 | ||||
| Drowsiness, No. (%) | 0 (0) | 8 (27) | 12 (40) | D-L | 0.005 |
| D-P | < 0.001 | ||||
| L-P | 0.412 |
Notes: aIn the selected outcome measures, Bonferroni correction was applied to adjust the P-values for multiple testing, with P < 0.05 considered statistically significant. bIn the selected outcome measures, the significance level was initially set at α = 0.05 for the comparisons between the three groups. When conducting pairwise comparisons between the groups, Bonferroni correction was applied with a significance level of α = 0.017, and P-value < 0.017 was considered statistically significant.
Abbreviations: SOL, Sleep Onset Latency; TST, Total Sleep Time; SE, Sleep efficiency; WASO, wake-after sleep onset.
Sleep satisfaction scores were equivalent between the Dex and lorazepam groups, with both groups having a median score of 9.0 (IQR 8.0–9.0; P > 0.999). This score significantly exceeded that of the placebo group, which was 5.0 (IQR 4.0–6.0; p < 0.001) (Table 3).
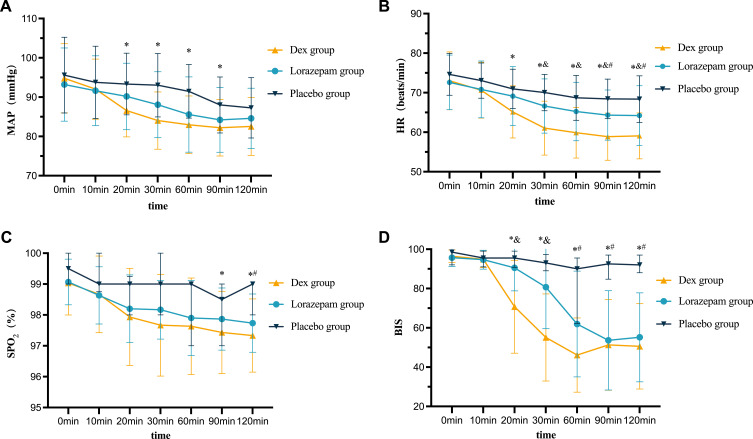
Figure 2 and eTable 2 in the Supplementary Materials present the changes in vital signs after 2 hours of intervention. During the observation period, three Dex group patients developed bradycardia, with a minimum HR of 49 bpm, ie, above the 45 bpm intervention threshold; hence, no pharmacological intervention was required. Additionally, six Dex and three lorazepam groups’ patients experienced hypotension without requiring intervention, as the MAP decrease was below 30% of the baseline. The comparison results of MAP, HR, SpO2, and BIS values among the three groups of patients at seven specific time points are as follows: (1) MAP: No significant differences were observed at T0, T1, and T6 (P > 0.05). From T2 to T5, Dex group’s MAP was lower than the placebo group (P < 0.05) but similar to the lorazepam group (P > 0.05). (2) HR: Similar across groups at T0 and T1 (P > 0.05). HR was lower in the Dex group than the placebo group (P < 0.05) from T2 to T6 and the lorazepam group from T3 to T6 (P < 0.05). (3) SpO2: No significant differences were observed from T0 to T2 and T4 (P > 0.05). However, at T3, T5, and T6, SpO2 was lower in the Dex group than in the placebo group (P < 0.05), and at T6, SpO2 was also lower in the lorazepam than in the placebo group (P < 0.05) but not different from Dex group (P > 0.05). The SpO2 levels in all groups were above 92%, rendering these differences clinically insignificant. (4) BIS value: No significant differences were observed at T0 and T1 (P > 0.05). From T2 to T6, the BIS values were lower in the Dex than in the placebo group (P < 0.05). At T2 and T3, the BIS value was lower in the Dex than in the lorazepam group (P < 0.05).
Figure 2.
The results of repeated measurements of vital sign parameters. Mean arterial pressure (A), heart rate (B), pulse oxygen saturation (C), and bispectral index (D) within 2 hours post-administration in patients. *P < 0.05 between the Dex and placebo groups; and P < 0.05 between the Dex and Lorazepam groups; #P < 0.05 between the Lorazepam and placebo groups.
Abbreviations: MAP, mean arterial pressure; HR, heart rate; SpO2, pulse oxygen saturation; BIS, bispectral index; Dex, dexmedetomidine.
Adverse reaction analysis (Table 3) revealed the following: (1) No instances of tachycardia, hypertension, respiratory depression or gastrointestinal discomfort across all groups (P = 1); (2) Bitter taste, headache, unsteady gait, dry mouth, and bradycardia were observed, but did not differ significantly among the groups (all P > 0.05); (3) Significant differences in hypotension, dizziness, fatigue, and drowsiness rates were observed among the three groups (P < 0.05). However, post-Bonferroni adjustment (α =0.017), only the differences in the incidence of dizziness and fatigue remained statistically significant. Dizziness was higher in the lorazepam (30%) compared to the Dex group (3%, P < 0.017) but did not differ from that in the placebo group (20%, P > 0.017). Fatigue was absent in the Dex group, lower than that in the lorazepam (27%, P < 0.017) and placebo (47%, P < 0.017) groups.
Discussion
This clinical trial primarily investigated the differences in efficacy and safety between intranasal Dex, oral lorazepam, and placebo for treating preoperative anxiety insomnia. Subjective and objective sleep assessments demonstrated that both Dex and lorazepam significantly improved patients’ sleep quality on the night before surgery, compared to placebo. Regarding sleep maintenance, Dex and lorazepam exhibited similar efficacy. However, Dex outperformed lorazepam in initiating sleep and enhancing daytime functionality. Throughout the study, there were no reports of serious adverse reactions associated with the treatment.
Daytime Function
An ideal insomnia treatment should facilitate sleep onset, sustain sleep, enhance daytime functionality, and improve overall health.36,37 In this study, benzodiazepine administration was frequently associated with daytime impairments, including difficulties in awakening, dizziness, fatigue, and somnolence, aligning with prior research.9,11,12,38 In contrast, patients undergoing Dex therapy exhibited marked improvements in daytime functionality, as demonstrated by easier arousal, enhanced alertness, and elevated activity levels. The enhancement in daytime function observed with Dex may be attributed to multiple factors. Firstly, as an α2-adrenergic receptor agonist, Dex inhibits activity from the locus coeruleus (LC) to wakefulness centers such as the basal forebrain (BF), the pre-optic area (POA) of the hypothalamus, the intralaminar nucleus (ILN) of the thalamus, and the cortex, thereby inducing sedation similar to non-rapid eye movement (NREM) sleep.39 Under moderate sedation, Dex-induced electroencephalogram (EEG) patterns resemble those of N2 sleep, which is associated with emotional regulation and physical relaxation.39,40 During deep sedation, EEG patterns from Dex show strong and slow delta waves similar to N3 sleep, enhancing cognitive functions and synaptic plasticity.17–20,39 Consequently, Dex may confer benefits analogous to physiological sleep regarding physical and neurological functions. Second, Dex is mostly eliminated from the system within 8–10 h post-intranasal administration, reducing the residual effect.13 Finally, based on the strong association between insomnia and neuroinflammation, the anti-inflammatory and neuroprotective effects of Dex may also play a significant role.41–44
Sleep Initiation
Difficulty falling asleep is a common challenge faced by individuals with insomnia. In the present study, intranasal administration of 2.5 µg/kg Dex significantly reduced the SOL to 19.0 min (IQR 16.0–22.3), compared to 33.5 min (IQR 30–50) for lorazepam. According to clinical practice guidelines from the American Academy of Sleep Medicine, a reduction in SOL of more than 10 min is clinically significant.45 Therefore, Dex demonstrated superior efficacy over lorazepam in facilitating sleep initiation. Traditional pharmacological treatments for insomnia, particularly oral medications, are often hindered by gastrointestinal transit times and the hepatic first-pass effect, which can delay the onset of therapeutic action and reduce overall efficacy. As a result, oral medications frequently fail to provide satisfactory results in promoting sleep initiation. In contrast, intranasal administration leverages the nasal mucosa’s extensive vascular network for rapid drug absorption, bypassing both gastrointestinal retention and the first-pass effect. This route of delivery enhances the bioavailability and pharmacokinetic profile of the drug. Moreover, intranasal administration allows direct access to the central nervous system by crossing the blood-brain barrier via the olfactory and trigeminal nerve pathways, thereby accelerating the therapeutic effects of psychotropic drugs.46 Previous studies have reported that 2.0 µg/kg intranasal Dex reduced SOL to 27.0 min (IQR 20.0–35.0) in elderly patients with chronic insomnia experiencing postoperative sleep disturbances.47 This result is slightly higher than the SOL observed in our study, which may be attributed to differences in drug dosages and the additional influence of postoperative pain on sleep.
Sleep Maintenance
Continuous intravenous infusion of Dex at night significantly reduces sleep interruptions, extends total sleep time, and enhances sleep efficiency in ICU patients.24,48 However, this method requires establishing an intravenous pathway and using an infusion pump, which not only inconveniences patients but also increases medical costs. Conversely, intranasal administration provides a non-invasive alternative, greatly enhancing treatment comfort and convenience. Our studies indicate that intranasal Dex also has a good effect on sleep maintenance. When assessing objective sleep parameters—TST, SE, number of awake, and WASO—the Dex and lorazepam groups showed similar results, both outperforming the placebo. Furthermore, this study also focused on sleep maintenance across different stages of the night. The analysis revealed that the Dex group experienced significantly fewer awakenings than the placebo group in the first half of the night, while the lorazepam group’s effects were similar to those of the placebo. This phenomenon could be attributed to Dex’s rapid peak concentration (38 min) via intranasal administration, thereby maintaining sleep in the early night half.14 In the second half, the lorazepam group outperformed in WASO reduction compared to the placebo group, and the Dex group showed similar effects to the placebo group. These findings might be attributed to a prolonged half-life of lorazepam (12 h), which enhances its effectiveness during the later sleep stages, while Dex has a shorter half-life of only 2 h.49,50 Despite these findings, the limited sample size precluded our ability to statistically differentiate lorazepam’s and Dex’s sleep maintenance effects during the respective night halves. Consequently, future studies should broaden the sample population for precise evaluation of the efficacy of these drugs throughout the sleep stages.
Safety
Dex exhibits a biphasic effect on blood pressure; it induces vasoconstriction at high concentrations, increasing blood pressure, and promotes vasodilation at lower concentrations, decreasing blood pressure.51,52 In our study, we observed no hypertension events, which could be linked to the slower absorption rate of Dex administered via nasal drops, resulting in lower peak plasma concentrations compared to intravenous delivery. Although the data indicated a rise in hypotension and bradycardia incidents with Dex use, these findings were not statistically significant, and no patients required intervention. During natural sleep, as sleep depth increases, enhanced parasympathetic nerve activity leads to a slowing of heart rate and a decrease in blood pressure. The study by Donghee et al corroborates our findings, suggesting that Dex-induced bradycardia may be a physiological response during sleep and does not necessitate intervention.53 Additionally, we did not observe any respiratory suppression with Dex use, aligning with previous studies.13 Given these findings, intranasal administration of 2.5μg/kg Dex appears to be a relatively safe option for managing anxiety-induced insomnia, although caution is advised in patients with compromised cardiac function, reduced blood volume, or pre-existing hypotension and bradycardia.
In conclusion, both intranasal Dex and oral lorazepam can safely and effectively improve preoperative anxiety and insomnia, with Dex showing better performance in initiating sleep and improving individual daytime functioning. Nevertheless, the present study has some limitations. Firstly, it predominantly encompassed patients exhibiting mild to moderate symptoms of anxiety and insomnia, while those experiencing significant pain (NRS score > 3) or depressive symptoms were excluded, potentially limiting the broad applicability of our results. Secondly, the evaluation of sleep quality did not incorporate polysomnography, which is considered the gold standard for sleep assessment. Finally, the study focused only on short-term efficacy and safety, necessitating in-depth research on long-term adverse effects and tolerance.
Conclusion
Employing both subjective and objective evaluation methods, our research established that 2.5 μg/kg of intranasal Dex and 2 mg of oral lorazepam are safe and efficacious in alleviating preoperative anxiety-induced insomnia. Although both interventions equivalently sustained sleep, intranasal Dex demonstrated superior efficacy in sleep initiation and improved daytime functionality compared to lorazepam.
Acknowledgments
We thank the study staff and participants for their essential contributions to this research, including assistance with data collection. This work was supported by the Natural Science Foundation of Jiangxi Province (grant no. 20232ACB206050).
Data Sharing Statement
Data underpinning this study’s findings are accessible upon reasonable request to the corresponding author, Li Chen, at zgx8778@126.com.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Morin CM, Drake CL, Harvey AG, et al. Insomnia disorder. Nat Rev Dis Primers. 2015;1:15026. doi: 10.1038/nrdp.2015.26 [DOI] [PubMed] [Google Scholar]
- 2.Sun GW, Yang YL, Yang XB, et al. Preoperative insomnia and its association with psychological factors, pain and anxiety in Chinese colorectal cancer patients. Support Care Cancer. 2020;28(6):2911–2919. doi: 10.1007/s00520-019-05151-y [DOI] [PubMed] [Google Scholar]
- 3.Butris N, Tang E, Pivetta B, et al. The prevalence and risk factors of sleep disturbances in surgical patients: a systematic review and meta-analysis. Sleep Med Rev. 2023;69:101786. doi: 10.1016/j.smrv.2023.101786 [DOI] [PubMed] [Google Scholar]
- 4.Huang BH, Duncan MJ, Cistulli PA, Nassar N, Hamer M, Stamatakis E. Sleep and physical activity in relation to all-cause, cardiovascular disease and cancer mortality risk. Br J Sports Med. 2022;56(13):718–724. doi: 10.1136/bjsports-2021-104046 [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Fang Z, Wu YY, et al. Association between perioperative self-reported sleep disturbances and delirium risk in elderly patients following total joint arthroplasty: a cohort study. J Sleep Res. 2024;33(5):e14168. doi: 10.1111/jsr.14168 [DOI] [PubMed] [Google Scholar]
- 6.Nowakowski S, Levy-Meeks ME, Dawson DB, et al. Association of preoperative sleep pattern with posthysterectomy pain: a pilot study. J Clin Sleep Med. 2020;16(11):1901–1908. doi: 10.5664/jcsm.8730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irwin MR, Olmstead R, Carroll JE. Disturbance sleep sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillman DR, Carlucci M, Charchaflieh JG, et al. Society of anesthesia and sleep medicine position paper on patient sleep during hospitalization. Anesth Analg. 2023;136(4):814–824. [DOI] [PubMed] [Google Scholar]
- 9.Krystal AD, Prather AA, Ashbrook LH. The assessment and management of insomnia: an update. World Psychiatry. 2019;18(3):337–352. doi: 10.1002/wps.20674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paymaster NJ. Lorazepam (WY 4036) as a pre-operative medication. Anaesthesia. 1973;28(5):521–526. doi: 10.1111/j.1365-2044.1973.tb00518.x [DOI] [PubMed] [Google Scholar]
- 11.de Mendonca FMR, de Mendonca G, Souza LC, et al. Benzodiazepines and sleep architecture: a systematic review. CNS Neurol Disord Drug Targets. 2023;22(2):172–179. doi: 10.2174/1871527320666210618103344 [DOI] [PubMed] [Google Scholar]
- 12.Marron L, Segurado R, Kenny RA, McNicholas T. The association between benzodiazepine use and falls, and the impact of sleep quality on this association: data from the TILDA study. QJM. 2020;113(1):31–36. doi: 10.1093/qjmed/hcz217 [DOI] [PubMed] [Google Scholar]
- 13.Weerink MAS, Struys M, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. doi: 10.1007/s40262-017-0507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iirola T, Vilo S, Manner T, et al. Bioavailability of dexmedetomidine after intranasal administration. Eur J Clin Pharmacol. 2011;67(8):825–831. doi: 10.1007/s00228-011-1002-y [DOI] [PubMed] [Google Scholar]
- 15.Akeju O, Hobbs LE, Gao L, et al. Dexmedetomidine promotes biomimetic non-rapid eye movement stage 3 sleep in humans: a pilot study. Clin Neurophysiol. 2018;129(1):69–78. doi: 10.1016/j.clinph.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98(2):428–436. doi: 10.1097/00000542-200302000-00024 [DOI] [PubMed] [Google Scholar]
- 17.Molle M, Marshall L, Gais S, Born J. Learning increases human electroencephalographic coherence during subsequent slow sleep oscillations. Proc Natl Acad Sci U S A. 2004;101(38):13963–13968. doi: 10.1073/pnas.0402820101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315(5817):1426–1429. doi: 10.1126/science.1138581 [DOI] [PubMed] [Google Scholar]
- 19.Prehn-Kristensen A, Munz M, Goder R, et al. Transcranial oscillatory direct current stimulation during sleep improves declarative memory consolidation in children with attention-deficit/hyperactivity disorder to a level comparable to healthy controls. Brain Stimul. 2014;7(6):793–799. doi: 10.1016/j.brs.2014.07.036 [DOI] [PubMed] [Google Scholar]
- 20.Wood KH, Memon AA, Memon RA, et al. Slow wave sleep and EEG delta spectral power are associated with cognitive function in Parkinson’s disease. J Parkinsons Dis. 2021;11(2):703–714. doi: 10.3233/JPD-202215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Qin M, Lu W, Shen X. Dexmedetomidine improved sleep quality in the intensive care unit after laryngectomy. Drug Des Devel Ther. 2023;17:1631–1640. doi: 10.2147/DDDT.S413321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moller MH, Alhazzani W, Lewis K, et al. Use of dexmedetomidine for sedation in mechanically ventilated adult ICU patients: a rapid practice guideline. Intensive Care Med. 2022;48(7):801–810. doi: 10.1007/s00134-022-06660-x [DOI] [PubMed] [Google Scholar]
- 23.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499. doi: 10.1001/jama.2009.56 [DOI] [PubMed] [Google Scholar]
- 24.Alexopoulou C, Kondili E, Diamantaki E, et al. Effects of dexmedetomidine on sleep quality in critically ill patients: a pilot study. Anesthesiology. 2014;121(4):801–807. doi: 10.1097/ALN.0000000000000361 [DOI] [PubMed] [Google Scholar]
- 25.Zeng W, Chen L, Liu X, et al. Intranasal dexmedetomidine for the treatment of pre-operative anxiety and insomnia: a prospective, randomized, controlled, and clinical trial. Front Psychiatry. 2022;13:816893. doi: 10.3389/fpsyt.2022.816893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 27.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. an updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/S0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 28.Parrott AC, Hindmarch I. The Leeds sleep evaluation questionnaire in psychopharmacological investigations - A review. Psychopharmacology. 1980;71(2):173–179. doi: 10.1007/BF00434408 [DOI] [PubMed] [Google Scholar]
- 29.Zisapel N, Laudon M. Subjective assessment of the effects of CNS-active drugs on sleep by the Leeds sleep evaluation questionnaire: a review. Hum Psychopharmacol. 2003;18(1):1–20. doi: 10.1002/hup.455 [DOI] [PubMed] [Google Scholar]
- 30.Tarrasch R, Laudon M, Zisapel N. Cross-cultural validation of the Leeds sleep evaluation questionnaire (LSEQ) in insomnia patients. Hum Psychopharmacol. 2003;18(8):603–610. doi: 10.1002/hup.534 [DOI] [PubMed] [Google Scholar]
- 31.Dahaba AA, Xue JX, Xu GX, Liu QH, Metzler H. Bilateral Bispectral Index (BIS)-Vista as a measure of physiologic sleep in sleep-deprived anesthesiologists. Minerva Anestesiol. 2011;77(4):388–393. [PubMed] [Google Scholar]
- 32.Gimenez S, Romero S, Alonso JF, et al. Monitoring sleep depth: analysis of bispectral index (BIS) based on polysomnographic recordings and sleep deprivation. J Clin Monit Comput. 2017;31(1):103–110. doi: 10.1007/s10877-015-9805-5 [DOI] [PubMed] [Google Scholar]
- 33.Tung A, Lynch JP, Roizen MF. Use of the BIS monitor to detect onset of naturally occurring sleep. J Clin Monit Comput. 2002;17(1):37–42. doi: 10.1023/A:1015404803637 [DOI] [PubMed] [Google Scholar]
- 34.Sleigh JW, Andrzejowski J, Steyn-Ross A, Steyn-Ross M. The bispectral index: a measure of depth of sleep? Anesth Analg. 1999;88(3):659–661. doi: 10.1213/00000539-199903000-00035 [DOI] [PubMed] [Google Scholar]
- 35.Benini F, Trapanotto M, Sartori S, et al. Analysis of the bispectral index during natural sleep in children. Anesth Analg. 2005;101(3):644. doi: 10.1213/01.ANE.0000157566.41739.2C [DOI] [PubMed] [Google Scholar]
- 36.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. doi: 10.5664/jcsm.27286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunz D. Rethinking the use of hypnotics for treatment of insomnia in the elderly. Expert Opin Pharmacother. 2021;22(8):953–957. doi: 10.1080/14656566.2021.1900116 [DOI] [PubMed] [Google Scholar]
- 38.Scharf MB, Kales A, Bixler EO, Jacoby JA, Schweitzer PK. Lorazepam-efficacy, side effects, and rebound phenomena. Clin Pharmacol Ther. 1982;31(2):175–179. doi: 10.1038/clpt.1982.27 [DOI] [PubMed] [Google Scholar]
- 39.Purdon PL, Sampson A, Pavone KJ, Brown EN. Clinical electroencephalography for anesthesiologists: part I: background and basic signatures. Anesthesiology. 2015;123(4):937–960. doi: 10.1097/ALN.0000000000000841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou Y, Xia H, He T, Zhang B, Qiu G, Chen A. N2 responses in youths with psychosis risk syndrome and their association with clinical outcomes: a cohort follow-up study based on the three-stimulus visual oddball paradigm. Am J Psychiatry. 2024;181(4):330–341. doi: 10.1176/appi.ajp.20221013 [DOI] [PubMed] [Google Scholar]
- 41.Gao H, Zhang Y, Luo D, et al. Activation of the hippocampal DRD2 alleviates neuroinflammation, synaptic plasticity damage and cognitive impairment after sleep deprivation. Mol Neurobiol. 2023;60(12):7208–7221. doi: 10.1007/s12035-023-03514-5 [DOI] [PubMed] [Google Scholar]
- 42.Pak VM, Onen SH, Bliwise DL, Kutner NG, Russell KL, Onen F. Sleep disturbances in MCI and AD: neuroinflammation as a possible mediating pathway. Front Aging Neurosci. 2020;12:69. doi: 10.3389/fnagi.2020.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang S, Zhang Y, Zheng Y, et al. Dexmedetomidine attenuates sleep deprivation-induced inhibition of hippocampal neurogenesis via VEGF-VEGFR2 signaling and inhibits neuroinflammation. Biomed Pharmacother. 2023;165:115085. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Tan SL, Du J, et al. Dexmedetomidine alleviates neuroinflammation, restores sleep disorders and neurobehavioral abnormalities in rats with minimal hepatic encephalopathy. Int Immunopharmacol. 2021;96:107795. doi: 10.1016/j.intimp.2021.107795 [DOI] [PubMed] [Google Scholar]
- 45.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307–349. doi: 10.5664/jcsm.6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crowe TP, Greenlee MHW, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52. doi: 10.1016/j.lfs.2017.12.025 [DOI] [PubMed] [Google Scholar]
- 47.Wu J, Liu X, Ye C, Hu J, Ma D, Wang E. Intranasal dexmedetomidine improves postoperative sleep quality in older patients with chronic insomnia: a randomized double-blind controlled trial. Front Pharmacol. 2023;14:1223746. doi: 10.3389/fphar.2023.1223746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu XH, Cui F, Zhang C, et al. Low-dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit: a pilot randomized controlled trial. Anesthesiology. 2016;125(5):979–991. doi: 10.1097/ALN.0000000000001325 [DOI] [PubMed] [Google Scholar]
- 49.Li A, Yuen VM, Goulay-Dufay S, et al. Pharmacokinetic and pharmacodynamic study of intranasal and intravenous dexmedetomidine. Br J Anaesth. 2018;120(5):960–968. doi: 10.1016/j.bja.2017.11.100 [DOI] [PubMed] [Google Scholar]
- 50.Kyriakopoulos AA, Greenblatt DJ, Shader RI. Clinical pharmacokinetics of lorazepam: a review. J Clin Psychiatry. 1978;39(10 Pt 2):16–23. [PubMed] [Google Scholar]
- 51.Belleville JP, Ward DS, Bloor BC, Maze M. Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology. 1992;77(6):1125–1133. doi: 10.1097/00000542-199212000-00013 [DOI] [PubMed] [Google Scholar]
- 52.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77(6):1134–1142. [DOI] [PubMed] [Google Scholar]
- 53.Kang D, Lim C, Shim DJ, et al. The correlation of heart rate between natural sleep and dexmedetomidine sedation. Korean J Anesthesiol. 2019;72(2):164–168. doi: 10.4097/kja.d.18.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underpinning this study’s findings are accessible upon reasonable request to the corresponding author, Li Chen, at zgx8778@126.com.