Abstract
Background
Poststroke irritability (PSI) is common among stroke survivors and can lead to a poor quality of life, difficulties in social interactions, criticism from caregivers, and caregiver stress. The planned study will evaluate the clinical, neuropsychological, and magnetic resonance imaging (MRI) correlates of PSI in a cohort of stroke survivors. In addition, the study will examine the 15-month progression of PSI.
Methods
This will be a prospective cohort study that will recruit 285 participants. Participants and their caregivers will undergo detailed assessments at a research clinic at 3, 9, and 15 months after stroke onset (T1/T2/T3). The irritability/lability subscale of the Chinese version of the Neuropsychiatric Inventory (CNPI) will be completed by caregivers. Potential covariates will also be measured. Patients will undergo MRI, including diffusion-weighted imaging, within 1 week of stroke onset. A stepwise logistic regression will be performed to evaluate the importance of lesions in the regions of interest (ROIs) along with other significant variables identified in univariate analyses. These analyses will be repeated for patients with and without PSI at T2 and T3. Repeated measures analysis of covariance (ANCOVA) will be used to assess changes in CNPI scores for the entire sample. In ANCOVA analyses, the frequency of infarcts in the ROIs will be treated as the predictor.
Discussion
This will be the first MRI study on PSI in stroke survivors. The findings will provide insights into the association of the orbitofrontal cortex, anterior cingulate cortex, anterior temporal lobe, insula, amygdala, thalamus, and basal ganglia lesions with the risk of PSI.
Keywords: stroke, irritability, MRI, prefrontal cortex, anterior cingulate cortex, anterior temporal lobe, insula, amygdala
Background
Irritability traditionally has been defined as a temporary psychological state characterized by impatience, intolerance, and poorly controlled anger, incorporating elements of aggression and decreased impulse control (1, 2). More recently, irritability has been described as a “mood that predisposes individuals toward certain emotions (e.g., anger), cognitions (e.g., hostile appraisals), and actions (e.g., aggression)” that is “subjectively unpleasant and objectively characterized by expressions of negative emotion in interpersonal relationships” (2, 3). Irritability is subjectively unpleasant and can either be brief or prolonged. Irritability should be distinguished from long-term traits, which are characterized by stable personality styles. Patients with irritability are often difficult to interact with, display emotional lability, and have outbursts in response to minor provocations. This neuropsychiatric symptom is strongly associated with functional disability (4).
In the context of psychiatric illnesses, irritability is a transdiagnostic phenomenon that occurs across all age groups and often leads to significant distress and impairment. Irritability is especially prevalent in mood disorders, where it plays a central role in diagnosis. Despite its ubiquity, irritability remains poorly understood (5). As a multidimensional construct, irritability is strongly associated with other psychological difficulties, such as depression and anxiety (6). There is considerable overlap between irritability and these psychiatric disorders, especially depression and anxiety. Many patients with depression report irritability, with nearly half experiencing a high level of this condition (7). In addition, irritability is listed as a symptom or associated feature of several anxiety disorders in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (8). A study indicated that clinically significant irritability is common in patients with anxiety disorders, particularly generalized anxiety disorder, where it is a diagnostic criterion (9). However, irritability can also occur independently of depression or anxiety (1, 2). Neurobiologically, irritability is associated with activity in the amygdala, orbitofrontal cortices, and hypothalamus. However, the patterns of activity in these areas differ from findings in youth. From a neurochemical perspective, irritability in adults is associated with increased monoamine transmission, whereas in youth, it involves abnormal dopamine (a monoamine transmitter) levels and responses (5).
Irritability is a common phenomenon in various cerebral diseases, such as Huntington’s disease (3, 4), traumatic brain injury (10, 11), dementia (12, 13), Parkinson’s disease (14, 15), multiple sclerosis (16, 17), and stroke (18, 19). For instance, the prevalence of irritability in Huntington’s disease ranges from 38 to 73% (3, 18). Similarly, irritability is frequently observed in patients with head injury, with a prevalence ranging from 15 to 74% (10, 11) and is associated with poor functioning and greater impairment in activities of daily living (20, 21). In patients with dementia or Huntington’s disease, irritability is a major source of distress for caregivers (22), often contributing to social and family dysfunction (3). In addition, irritability is a leading cause of hospitalization and institutionalization (23, 24). Studies have also linked irritability to suicidal ideation (25), violence (3) in patients, and harmful behavior by caregivers (26).
Poststroke irritability (PSI) is highly prevalent among stroke survivors, often manifesting as impatience over minor inconveniences (e.g., waiting or delays), flashes of anger (62%), rapid mood changes, and quarrels (29%) (27). Studies have reported that 29 to 70% of stroke patients experience irritability within 1 month to 7 years after stroke (19, 28–31). The frequency of PSI, as defined by the Neuropsychiatric Inventory (32), ranges from 12 to 53% (18, 27, 33, 34). A local study of 77 Chinese stroke survivors found a PSI prevalence of 9% (35). Possible clinical correlates of PSI include younger age, aphasia, premorbid personality traits (e.g., high neuroticism and low agreeableness) (27, 33), depressive and anxiety symptoms, and cognitive deficits (36). Irritability is particularly common in patients with poststroke aphasia, as impaired functional communication can lead to frustration and decreased tolerance for trivial matters (19). In addition, irritability is a common symptom in dementia, affecting nearly 30% of patients (35), and is found in 13–45% of patients with mild cognitive impairment (37). Furthermore, irritability may reflect difficulties in coping with newly acquired stroke-related disabilities. Even after a mild stroke, survivors often struggle to cope with the uncertainty of their condition, which can contribute to irritability (38). Despite its prevalence, PSI is often undiagnosed and thus untreated in stroke survivors (17), leading to poor recovery (39) and quality of life (36), difficulties in social interactions (40), criticism from caregivers (41), and caregiver stress (42).
The course of PSI remains unclear. In a study including 128 stroke patients, irritability was observed in 40, 40, and 34% of patients at 1, 6, and 12 months after stroke, respectively (29). Another study involving 124 stroke survivors reported that PSI may peak at 1 year poststroke, indicating its potential chronicity (27). A pilot study of 11 stroke survivors with PSI reported a remission rate of 63% at the 1-year follow-up (34). In addition, in a 2-year longitudinal study, recovery rates for poststroke depression (PSD) were 75% for dysthymia, 100% for minor depression, and 75% for major depression. Recovery rates for poststroke anxiety were 83% for panic disorder, 60% for generalized anxiety disorder, and 50% for social phobia (43).
To date, no high-quality trials have been conducted on pharmacological or psychosocial treatments for irritability in stroke patients. Although carbamazepine may be effective in managing PSI (44), antidepressants have not shown efficacy (36). Studies on patients with Huntington’s disease have indicated that serotonin reuptake inhibitors, valproate, atypical antipsychotics, beta-blockers, and synthetic cannabinoids may be effective in treating apathy and aggression (3, 4, 45). Similarly, studies on patients with traumatic head injury have suggested that amantadine (37) and sertraline may be effective for treating irritability (46–48). Regarding nonpharmacological interventions, validation therapy, music therapy, aromatherapy, and cognitive-behavioral interventions may offer benefits (49, 50). A recent review of PSD treatments supported the efficacy and safety of selective serotonin reuptake inhibitors (SSRIs) and recommended nortriptyline for patients who do not respond to SSRIs. Cognitive behavioral therapy (CBT), virtual reality, and repetitive transcranial magnetic stimulation (rTMS) have also shown promise in treating PSD. CBT, a widely recognized psychotherapeutic intervention, helps individuals reshape negative thought patterns and develop effective coping strategies tailored to their unique poststroke challenges. rTMS, a noninvasive brain stimulation technique, demonstrated effectiveness in rebalancing neural activity and reducing depressive symptoms in stroke survivors (51). In addition, some clinical trials have suggested that acupuncture and reminiscence therapy are effective in treating poststroke anxiety (52, 53).
Starkstein and Robinson (54) indicated that PSI may result from damage to the orbito-temporal-limbic feedback loop, where the inhibitory control of the cortex over the amygdala is disrupted, reducing the ability to suppress instinctive emotional reactions. The key components of this brain circuit are the medial orbitofrontal cortex (OFC), amygdala, and connecting tracts (55). In Huntington’s disease, irritability is caused by disruptions in the emotional circuitry involving the medial OFC and amygdala (56). A functional magnetic resonance imaging (fMRI) study of the brain’s response to frustration revealed that exposure to frustrating stimuli leads to increased activation in various regions, including the amygdala and dorsomedial and ventromedial prefrontal cortices (57). Motivational deficits in PSD were associated with lesions in the OFC (58). The relationship between irritability and dysfunctions in the primary components of the fronto-amygdala circuit is discussed in the following paragraphs.
Irritability is commonly observed in patients with frontal lobe pathologies, such as frontotemporal dementia (13, 59). A single case report demonstrated a relationship between irritability and frontal lobe stroke (44). Furthermore, irritability is a common sequela following traumatic frontal lobe injury (60–63). Irritability has been associated with damage to the OFC (64–66). Moreover, in primary progressive aphasia, irritability was related to atrophy of the lateral OFC and anterior cingulate cortex (ACC) (67). An fMRI study on Huntington’s disease reported that irritability results from volume reduction and dysfunction in the medial OFC (56, 68). In addition, fMRI studies have shown that individuals with chronic irritability exhibited dysfunctions in the medial frontal gyrus (69), middle/superior frontal gyrus (70), inferior frontal gyrus (49), and ACC (69, 71). ACC lesions and decreased functional connectivity have also been associated with PSD (72, 73).
In addition to the frontal cortex, temporal lobe structures have been implicated in the pathophysiology of irritability. In patients with traumatic head injury, Gualtieri (65) observed an association of irritability with damage to the anterior temporal lobe (ATL). In individuals with chronic irritability, a reduction in gray matter volume was observed in the insula (49). Irritability in patients with Alzheimer’s disease (AD) has been associated with insula atrophy (74) and abnormal functional connectivity (75). Task-related fMRI studies of facial emotion processing in individuals with chronic irritability have shown dysfunctions in the superior temporal gyrus (76) and posterior insula (77).
The amygdala plays a central role in regulating emotions and is implicated in the pathophysiology of irritability in various clinical populations (78). In patients with dementia, atrophy of the amygdala was associated with the severity of irritability (79). The volume of the amygdala was significantly decreased in patients with dysphoric disorder associated with epilepsy, and this reduction was correlated with the level of irritability (80). An fMRI study in patients with AD found that amygdala dysfunction and hypersensitivity were correlated with the severity of irritability (81). In addition, several fMRI studies using facial emotion processing tasks have shown that individuals with chronic irritability exhibited dysfunction in the amygdala (70, 77, 82, 83). In poststroke patients, somatic symptoms associated with depressive states are related to amygdala lesions (58, 84).
Evidence suggests that decreased white matter integrity is associated with irritability in neuropsychiatric disorders (85). In a diffusion tensor imaging study of 45 individuals with mild cognitive impairment or AD, lower integrity of the anterior cingulum, as measured through fractional anisotropy, was related to a higher risk of irritability (86). In a study on depression, irritability was found to be associated with decreased integrity in the anterior corona radiata, inferior longitudinal fasciculus, and inferior fronto-occipital fasciculus (55). In addition, white matter structural and functional changes are also associated with PSD (87–89).
Irritability is a known feature of basal ganglia (BG) disorders (3, 4, 14, 90, 91). One possible explanation for irritability in BG disorders is the development of “rigidity” in thinking, where patients fixate on a particular desire or idea, leading to outbursts when their perceived needs are unmet (91). Individuals with chronic irritability have a decreased gray matter volume in the globus pallidus (49). A case report indicated an association of irritability with an infarction in the left subthalamic nucleus (92). Task-related fMRI studies have also revealed dysfunction in the BG in individuals with chronic irritability (49, 83, 93, 94). In addition, BG lesions are associated with poststroke depressive symptoms (95–97).
Many neuroimaging studies on poststroke anxiety and depression have been published (58, 89). However, few structural brain imaging studies have focused on irritability following stroke (28, 36). Single case reports have demonstrated an association of irritability with anterior (98), paramedian thalamic (99), and subthalamic (92) infarctions. In a study including 16 patients with malignant middle cerebral artery infarction (100), 53% showed an increase in irritability. Folstein et al. (28) reported that 70% of patients with right hemisphere stroke exhibited irritability, whereas no patients with left hemisphere stroke displayed irritability. Chan et al. (36) found that irritability in 92 stroke patients was related to lesions near the frontal pole. However, these studies have several limitations, including small sample sizes (28, 36, 100), the inclusion of participants with current psychiatric diagnoses (36), and a lack of detailed radiological examinations. Furthermore, the classification of infarct locations is often crude, such as hemispheric versus brainstem (36), left versus right (28), and anterior versus posterior (36).
Aims and hypotheses to be tested
The primary objective of the proposed study is to evaluate the clinical and magnetic resonance imaging (MRI) correlates of PSI in a cohort of stroke survivors, an area that has not been explored in published studies. The secondary objective is to examine the 12-month progression of PSI.
Hypotheses
The first hypothesis is that individuals with PSI have more infarcts in the regions of interest (ROIs) but not in the control region than those without PSI. The ROIs will be the OFC, ACC, ATL, insula, amygdala, thalamus, and BG. The occipital lobe will be included as the control region. The second hypothesis is that the number of infarcts in the ROIs is significantly and positively correlated with the severity of PSI. The third hypothesis is that 37% (34) of individuals with PSI at baseline continue to exhibit PSI 12 months after the initial assessment.
Methods
Participant recruitment
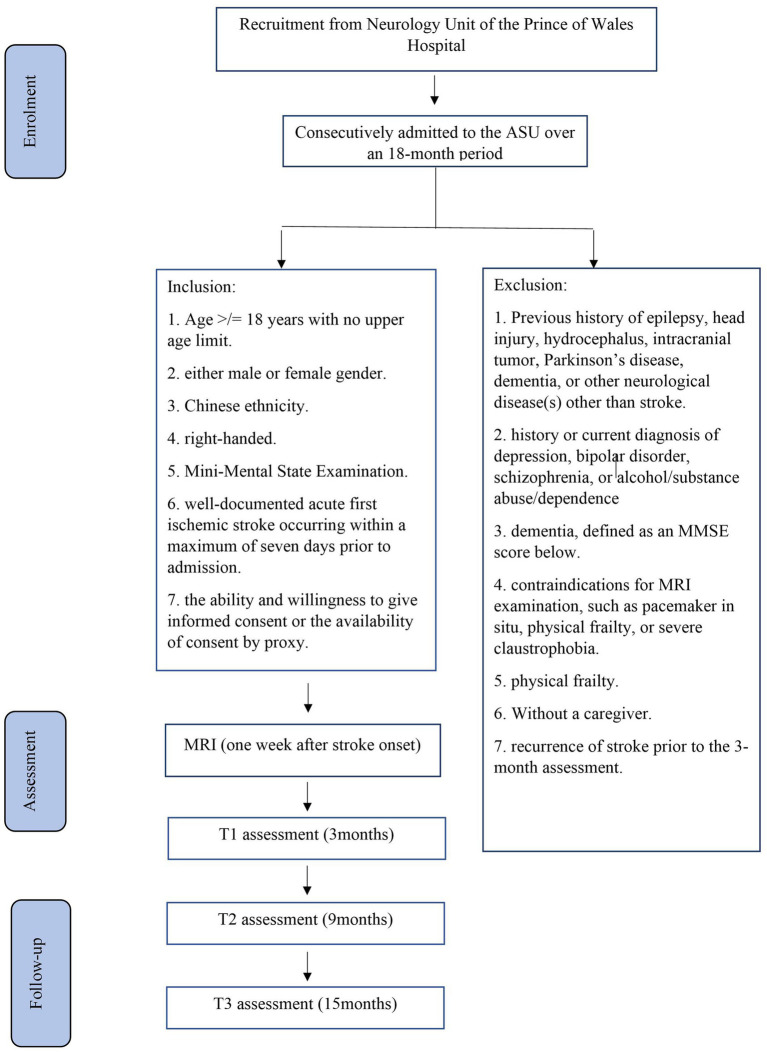
The planned study is a prospective nested case–control study of stroke survivors. Details of the recruitment process are shown in Figure 1. Participants will be recruited from patients consecutively admitted with a first-ever stroke to the Acute Stroke Unit (ASU) at the Prince of Wales Hospital (PWH). The PWH is a general hospital serving a population of 800,000 in Hong Kong. The ASU treats approximately 93% of all patients with acute stroke admitted to the PWH, with the remaining 7% admitted to the neurosurgery unit. All patients with acute stroke (n = 1,000) who are consecutively admitted to the ASU over a 24-month period will be invited to participate. A trained research assistant (RA) will visit the ASU daily to identify eligible patients and obtain written consent. Approximately 80% of these 1,000 patients (n = 1,000 × 80% = 800) are expected to have ischemic stroke. MRI examination will be contraindicated in 10% of these patients, leaving 720 potential participants (800 × 90%). Based on our previous findings (101), the mortality rate at 3 months poststroke is approximately 12%, reducing the number of potential participants to 634 [720 × (100% − 12%)]. Assuming a 25% dropout rate and that approximately 60% of the survivors will meet the inclusion criteria (101), we will recruit approximately 285 participants [634 × (100% − 25%) × 60%]. Follow-up assessments at 6 and 12 months will be conducted for all 285 participants.
Figure 1.
Details of recruitment.
Eligibility criteria
Inclusion and exclusion criteria
The inclusion criteria will be as follows: (1) age ≥ 18 years with no upper limit; (2) either male or female sex; (3) Chinese ethnicity; (4) right-handed; (5) a clinical diagnosis of acute first ischemic stroke occurring within a maximum of 7 days prior to admission, as recorded in the medical notes; and (6) ability and willingness to provide informed consent or availability of consent by proxy.
The exclusion criteria will be as follows: (1) previous history of epilepsy, head injury, hydrocephalus, intracranial tumor, Parkinson’s disease, dementia, or any other neurological diseases other than stroke; (2) history or current diagnosis of depression, bipolar disorder, schizophrenia, or alcohol/substance abuse or dependence; (3) dementia; (4) contraindications for MRI examination, such as the presence of a pacemaker, physical frailty that prevents attendance at the research clinic, or severe claustrophobia; (5) physical frailty; (6) lack of a caregiver; and (7) recurrence of stroke prior to the 3-month assessment.
Data collection
The data collection schedule is presented in Table 1. Written consent will be obtained from all patients. The number of exclusions and reasons for exclusion will be recorded. The following demographic, psychosocial, and medical data will be collected from all participants: age, sex, education level, and date of stroke onset. Clinical data, including information on neurological impairments such as aphasia and dysarthria, will be measured using the National Institute of Health Stroke Scale (NIHSS) (102) and extracted from the Stroke Registry, which is maintained by a full-time trained research nurse.
Table 1.
Data collection schedule.
| Study period | T0 | T1 | T2 | T3 |
|---|---|---|---|---|
| Months | 0 | 3 | 9 | 15 |
| Visits | 0 | 1 | 2 | 3 |
| MRI | X | |||
| Review of inclusion/exclusion criteria | X | |||
| Informed consent | X | |||
| IDA, BI, MMSE, BDI, HADSA | X | X | X |
Assessment of PSI
Assessment of irritability
Three months after the onset of the index stroke (T1), patients and their caregivers will undergo assessments at a research clinic. The timing of the assessments will be consistent with other studies on PSI (31, 33).
A trained RA will conduct a clinical interview at the research clinic. PSI will be evaluated using the irritability/lability subscale of the Chinese version of the Neuropsychiatric Inventory (CNPI). The CNPI is based on a structured interview with the caregiver, during which a screening question is asked to determine the presence or absence of irritability over the past month (32, 103). The behavior must reflect a change from the patient’s pre-stroke condition. If the response is positive, the behavior will be further explored with sub-questions. Frequency is rated from 1 to 4, and severity is scored from 1 to 3. The product of severity and frequency is calculated to quantify the severity of PSI. The Neuropsychiatric Inventory is the most commonly used tool for assessing irritability in stroke (18, 27, 33, 34, 104).
Physical functioning, depressive and anxiety symptoms, aphasia, and cognitive functioning will be assessed using the Barthel Index (BI) (105), the Beck Depression Inventory (BDI) (106), the anxiety subscale of the Hospital Anxiety Depression Scale (HADSA) (107), the Language Screening Test (LAST) (108), and the Montreal Cognitive Assessment (MoCA) (109), respectively. For participants with substantial aphasia, proxy ratings will be obtained from caregivers. Pre-stroke personality will be assessed using the Chinese version of the NEO-Five-Factor Inventory, which will be completed by the caregiver (110, 111).
Follow-up assessments of irritability will be conducted for all participants at 9 months (T2) and 15 months (T3) poststroke, corresponding to 6 and 12 months after the initial assessment. Any new episode of depression or anxiety disorder, as well as subsequent pharmacological treatments, will be recorded. The assessments (BI, MoCA, BDI, and HADSA) will be repeated during follow-up (Table 1).
MRI examination and analysis
Stroke patients will undergo MRI within 1 week of stroke onset. All scans will be performed using a 3 T scanner (Philips Achieve 3.0 T, X Series, Quasar Dual MRI System) with standardized sequences, including diffusion-weighted imaging (DWI); three-dimensional T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR); and susceptibility-weighted imaging (SWI). An experienced neuroradiologist blinded to the participants’ psychiatric diagnoses and PSI status will assess the MRI images. Acute infarcts will be identified as hyperintense lesions on DWI with corresponding hypointensity on the apparent diffusion coefficient map. White matter hyperintensities (WMHs) will be defined as ill-defined hyperintensities 5 mm on FLAIR, which are isointense with a normal brain parenchyma on T1-weighted images. Lesions that exhibit signal characteristics of the cerebrospinal fluid on T1-weighted images and measure more than 3 mm in diameter, as well as wedge-shaped cortico-subcortical lesions, will be classified as old/lacunar infarcts. Microbleeds will be defined as dot-like hypointense regions on SWI, and the total number of microbleeds will be determined. The number of microbleeds in the BG and thalamus will be noted separately. All raw data will be transferred to the PALS system (Carestream Solutions).
MRI preprocessing
Preprocessing steps will include nonuniformity correction (112), spatial standardization, and brain extraction (removal of the skull). To ensure that brain structure volumes are comparable among participants, the MRI data of each participant will be transformed from its original space to a common stereotactic space using multiscale affine registration (113). Brain regions will be automatically segmented from the MRI data by using the brain extraction tool (114).
Brain segmentation
The brain tissue will be classified into gray matter, white matter, and cerebrospinal fluid (115). Whole-brain segmentation will be conducted using an atlas-based approach (116), which automatically adjusts the existing atlas intensity model to new data. The ROIs and other brain regions will be segmented, and their volumes will be quantified using the Taahirah brain atlas (107) and demon registration (117).
Infarct segmentation and quantification
Infarcts will be delineated semi-automatically as high-intensity regions on DWI, whereas WMHs will be identified as high-density regions on FLAIR (and isointense on T1-weighted images) using ITK-SNAP software. The segmented infarct and WMH regions will be combined with the ROIs and other brain-region masks generated in the previous step. The infarct and WMH pixels within the ROIs and other brain regions will then be calculated.
Sample size estimation
A total of 287 patients will be recruited, with the expectation that 22% will present with PSI, resulting in 63 patients with PSI (18, 27, 33, 34, 104). If no significant correlations between irritability and lesion location are found in this sample, it is unlikely that clinically meaningful effects of lesions would be detected in a larger sample. Because no published data exist on the specific locations of infarcts in PSI, our estimates were based on figures reported for other poststroke neurobehavioral disorders. Tang et al. (118) reported that frontal infarcts were present in 15 and 5% of patients with and without poststroke emotional incontinence (PSEI), respectively. In another study on poststroke anxiety (PSAn), 21.4% of patients with PSAn had frontal infarcts, compared with only 8.6% of patients without PSAn (119). Similar to PSI, frontal lobe dysfunction is believed to be involved in PSEI and PSAn. A sample size of 285 will provide 80–86% power to identify frontal infarcts as a predictor of irritability in stroke based on a one-degree-of-freedom chi square test (120).
Statistical analysis
All variables will be tested for normality using the Kolmogorov–Smirnov test with a significance threshold of p < 0.05. Demographic, clinical, and MRI variables (age, sex, NIHSS, BI, HADSA, BDI, MoCA, and NEO-Five-Factor Inventory scores; infarcts in ROIs; microbleeds; and WMH volumes) will be compared between groups using the chi-square test, Student’s t test, or Mann–Whitney U test, as appropriate. A stepwise logistic regression will be performed to evaluate the importance of lesions in the ROIs, along with other significant variables identified in the univariate analyses. These analyses will be repeated for patients with and without PSI at T2 and T3.
Repeated measures analysis of covariance (ANCOVA) will be used to assess changes in the CNPI scores for the entire sample. In the ANCOVA analysis, the frequency of infarcts in the ROIs will be treated as the predictor, and the statistical model will be adjusted for age, aphasia (LAST), personality traits (NEO-Five-Factor Inventory scores), cognitive function (MoCA score), depressive and anxiety symptoms (BDI and HADSA scores), new episodes of anxiety disorder, and subsequent pharmacological treatments. At each time point, the CNPI scores will be analyzed using linear regression while controlling for the same covariates as in the ANCOVA. Multiple testing will be controlled using false discovery rate correction. The level of significance will be set at 0.05.
Discussion
We aim to include a homogenous patient population by narrowing the criteria for age, ethnicity, handedness, and PSI duration. Patients with other causes of PSI, such as psychiatric or neurological disorders, will be excluded. This will be the first longitudinal study to examine the role of the OFC, ACC, ATL, insula, amygdala, thalamus, and BG in a large sample of consecutively admitted stroke survivors with PSI. The results will provide insights into the association between these brain regions and PSI. The findings will be applicable to a broad population of neurological patients at risk of irritability and stimulate further research in this field.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Ethics statement
The studies involving humans were approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee, Hong Kong SAR, China (reference number: 2023.383). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WKT: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. EH: Conceptualization, Methodology, Writing – review & editing. TWHL: Conceptualization, Methodology, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Snaith RP, Constantopoulos AA, Jardine MY, McGuffin P. A clinical scale for the self-assessment of irritability. Br J Psychiatry. (1978) 132:164–71. doi: 10.1192/bjp.132.2.164, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Craig KJ, Hietanen H, Markova IS, Berrios GE. The irritability questionnaire: a new scale for the measurement of irritability. Psychiatry Res. (2008) 159:367–75. doi: 10.1016/j.psychres.2007.03.002, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Karagas NE, Rocha NP, Stimming EF. Irritability in Huntington’s disease. J Huntington’s Dis. (2020) 9:107–13. doi: 10.3233/JHD-200397, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Duijn E. Treatment of irritability in Huntington’s disease. Curr Treat Options Neurol. (2010) 12:424–33. doi: 10.1007/s11940-010-0088-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell E, Boyce P, Porter RJ, Bryant RA, Malhi GS. Irritability in mood disorders: neurobiological underpinnings and implications for pharmacological intervention. CNS Drugs. (2021) 35:619–41. doi: 10.1007/s40263-021-00823-y, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Simpson J, Dale M, Theed R, Gunn S, Zarotti N, Eccles FJ. Validity of irritability in Huntington's disease: a scoping review. Cortex. (2019) 120:353–74. doi: 10.1016/j.cortex.2019.06.012, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Verhoeven FE, Booij L, Van der Wee NJ, Penninx BW, Van der Does AW. Clinical and physiological correlates of irritability in depression: results from the Netherlands study of depression and anxiety. Depress Res Treat. (2011) 2011:126895. doi: 10.1155/2011/126895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Psychiatric Association DS, American Psychiatric Association DS . Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association; (2013). [Google Scholar]
- 9.Stoddard J, Stringaris A, Brotman MA, Montville D, Pine DS, Leibenluft E. Irritability in child and adolescent anxiety disorders. Depress Anxiety. (2014) 31:566–73. doi: 10.1002/da.22151, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang C-C, Huang S-J, Lin W-C, Tsai Y-H, Hua M-S. Divergent manifestations of irritability in patients with mild and moderate-to-severe traumatic brain injury: perspectives of awareness and neurocognitive correlates. Brain Inj. (2013) 27:1008–15. doi: 10.3109/02699052.2013.794975, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Miles SR, Hammond FM, Neumann D, Silva MA, Tang X, Kajankova M, et al. Evolution of irritability, anger, and aggression after traumatic brain injury: identifying and predicting subgroups. J Neurotrauma. (2021) 38:1827–33. doi: 10.1089/neu.2020.7451, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns A, Folstein S, Brandt J, Folstein M. Clinical assessment of irritability, aggression, and apathy in Huntington and Alzheimer disease. J Nerv Ment Dis. (1990) 178:20–6. doi: 10.1097/00005053-199001000-00004, PMID: [DOI] [PubMed] [Google Scholar]
- 13.Schwertner E, Pereira JB, Xu H, Secnik J, Winblad B, Eriksdotter M, et al. Behavioral and psychological symptoms of dementia in different dementia disorders: a large-scale study of 10,000 individuals. J Alzheimers Dis. (2022) 87:1307–18. doi: 10.3233/JAD-215198, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aarsland D, Larsen JP, Lim NG, Janvin C, Karlsen K, Tandberg E, et al. Range of neuropsychiatric disturbances in patients with parkinson’s disease. J Neurol Neurosurgery Psychiatry. (1999) 67:492–6. doi: 10.1136/jnnp.67.4.492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jose A, Bhargavan A, Appireddy R, Raghunath P S, Rajan R, Iype T. Neuropsychiatric symptoms and Caregiver's burden in Parkinson's disease patients in a tertiary care teaching Hospital in South India. Neurol India. (2021) 69:1706–10. doi: 10.4103/0028-3886.333437 [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Olavarrieta C, Cummings JL, Velazquez J, Garcia de al Cadena C. Neuropsychiatric manifestations of multiple sclerosis. The journal of neuropsychiatry and clinical. Neurosciences. (1999) 11:51–7. doi: 10.1176/jnp.11.1.51 [DOI] [PubMed] [Google Scholar]
- 17.Vanotti S, Caceres FJ. Cognitive and neuropsychiatric disorders among MS patients from Latin America. Multiple Sclerosis J Exp Trans Clin. (2017) 3:205521731771750. doi: 10.1177/2055217317717508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Almenkerk S, Depla MFIA, Smalbrugge M, Eefsting JA, Hertogh CMPM. Institutionalized stroke patients: status of functioning of an under researched population. J Am Med Dir Assoc. (2012) 13:634–9. doi: 10.1016/j.jamda.2012.05.008, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Edelkraut L, López-Barroso D, Torres-Prioris MJ, Starkstein SE, Jorge RE, Aloisi J, et al. Spectrum of neuropsychiatric symptoms in chronic post-stroke aphasia. World J Psychiatry. (2022) 12:450–69. doi: 10.5498/wjp.v12.i3.450, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SH, Manes F, Kosier T, Baruah S, Robinson RG. Irritability following traumatic brain injury. J Nervous Mental Dis. (1999) 187:327–35. doi: 10.1097/00005053-199906000-00001 [DOI] [PubMed] [Google Scholar]
- 21.Jacobsson L, Lexell J. Functioning and disability from 10 to 16 years after traumatic brain injury. Acta Neurol Scand. (2019) 141:115–22. doi: 10.1111/ane.13194 [DOI] [PubMed] [Google Scholar]
- 22.Baillon S, Gasper A, Wilson-Morkeh F, Pritchard M, Jesu A, Velayudhan L. Prevalence and severity of neuropsychiatric symptoms in early- versus late-onset Alzheimer’s disease. Am J Alzheimers Dis Other Dement. (2019) 34:433–8. doi: 10.1177/1533317519841191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuoka T, Manabe T, Akatsu H, Hashizume Y, Yamamoto S, Ogawa N, et al. Factors influencing hospital admission among patients with autopsy-confirmed dementia. Psychogeriatrics. (2019) 19:255–63. doi: 10.1111/psyg.12393, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Castillo-García IM, López-Álvarez J, Osorio R, Olazarán J, Ramos García MI, Agüera-Ortiz L. Clinical trajectories of neuropsychiatric symptoms in mild-moderate to advanced dementia. J Alzheimers Dis. (2022) 86:861–75. doi: 10.3233/JAD-215133, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Holmstrand C, Rahm Hallberg I, Saks K, Leino-Kilpi H, Renom Guiteras A, Verbeek H, et al. Associated factors of suicidal ideation among older persons with dementia living at home in eight European countries. Aging Ment Health. (2020) 25:1730–9. doi: 10.1080/13607863.2020.1745143 [DOI] [PubMed] [Google Scholar]
- 26.Toda D, Tsukasaki K, Itatani T, Kyota K, Hino S, Kitamura T. Predictors of potentially harmful behaviour by family caregivers towards patients treated for behavioural and psychological symptoms of dementia in Japan. Psychogeriatrics. (2018) 18:357–64. doi: 10.1111/psyg.12328, PMID: [DOI] [PubMed] [Google Scholar]
- 27.Angelelli P, Paolucci S, Bivona U, Piccardi L, Ciurli P, Cantagallo A, et al. Development of neuropsychiatric symptoms in poststroke patients: a cross-sectional study. Acta Psychiatr Scand. (2004) 110:55–63. doi: 10.1111/j.1600-0447.2004.00297.x, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Maiberger R, McHugh PR. Mood disorder as a specific complication of stroke. J Neurol Neurosurg Psychiatry. (1977) 40:1018–20. doi: 10.1136/jnnp.40.10.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.House A, Dennis M, Mogridge L, Warlow C, Hawton K, Jones L. Mood disorders in the year after first stroke. Br J Psychiatry. (1991) 158:83–92. doi: 10.1192/bjp.158.1.83, PMID: [DOI] [PubMed] [Google Scholar]
- 30.Dam H. Depression in stroke patients 7 years following stroke. Acta Psychiatr Scand. (2001) 103:287–93. doi: 10.1034/j.1600-0447.2001.103004287.x, PMID: [DOI] [PubMed] [Google Scholar]
- 31.Skånér Y, Nilsson GH, Sundquist K, Hassler E, Krakau I. Self-rated health, symptoms of depression and general symptoms at 3 and 12 months after a first-ever stroke: a municipality-based study in Sweden. BMC Fam Pract. (2007) 8:61. doi: 10.1186/1471-2296-8-61, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory. Neurology. (1994) 44:2308–8. doi: 10.1212/WNL.44.12.2308, PMID: [DOI] [PubMed] [Google Scholar]
- 33.Greenop KR, Almeida OP, Hankey GJ, van Bockxmeer F, Lautenschlager NT. Premorbid personality traits are associated with post-stroke behavioral and psychological symptoms: a three-month follow-up study in Perth, Western Australia. Int Psychogeriatr. (2009) 21:1063–71. doi: 10.1017/S1041610209990457, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Buijck BI, Zuidema SU, Spruit-van Eijk M, Geurts AC, Koopmans RT. Neuropsychiatric symptoms in geriatric patients admitted to skilled nursing facilities in nursing homes for rehabilitation after stroke: a longitudinal multicenter study. Int J Geriatr Psychiatry. (2011) 27:734–41. doi: 10.1002/gps.2781 [DOI] [PubMed] [Google Scholar]
- 35.Vaingankar JA, Chong SA, Abdin E, Picco L, Jeyagurunathan A, Seow E, et al. Behavioral and psychological symptoms of dementia: prevalence, symptom groups and their correlates in community-based older adults with dementia in Singapore. Int Psychogeriatr. (2017) 29:1363–76. doi: 10.1017/S1041610217000564, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Chan K-L, Campayo A, Moser DJ, Arndt S, Robinson RG. Aggressive behavior in patients with stroke: association with psychopathology and results of antidepressant treatment on aggression. Arch Phys Med Rehabil. (2006) 87:793–8. doi: 10.1016/j.apmr.2006.02.016, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Martin E, Velayudhan L. Neuropsychiatric symptoms in mild cognitive impairment: a literature review. Dement Geriatr Cogn Disord. (2020) 49:146–55. doi: 10.1159/000507078, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Carlsson GE, Möller A, Blomstrand C. Managing an everyday life of uncertainty–a qualitative study of coping in persons with mild stroke. Disabil Rehabil. (2009) 31:773–82. doi: 10.1080/09638280802638857, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Cao K-G, Fu C-H, Li H-Q, Xin X-Y, Gao Y. A new prognostic scale for the early prediction of ischemic stroke recovery mainly based on traditional Chinese medicine symptoms and NIHSS SCORE: a retrospective cohort study. BMC Complement Altern Med. (2015) 15:407. doi: 10.1186/s12906-015-0903-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlsson G, Möller A, Blomstrand C. A qualitative study of the consequences of “hidden dysfunctions” one year after a mild stroke in persons <?75 years. Disabil Rehabil. (2004) 26:1373–80. doi: 10.1080/09638280400000211 [DOI] [PubMed] [Google Scholar]
- 41.Angeleri F, Angeleri VA, Foschi N, Giaquinto S, Nolfe G. The influence of depression, social activity, and family stress on functional outcome after stroke. Stroke. (1993) 24:1478–83. doi: 10.1161/01.STR.24.10.1478, PMID: [DOI] [PubMed] [Google Scholar]
- 42.Williams A. What bothers caregivers of stroke victims? J Neurosci Nurs. (1994) 26:155–61. doi: 10.1097/01376517-199406000-00009, PMID: [DOI] [PubMed] [Google Scholar]
- 43.Sagen-Vik U, Finset A, Moum T, Vik TG, Dammen T. The longitudinal course of anxiety, depression and apathy through two years after stroke. J Psychosom Res. (2022) 162:111016. doi: 10.1016/j.jpsychores.2022.111016, PMID: [DOI] [PubMed] [Google Scholar]
- 44.Nagata T, Harada D, Aoki K, Kada H, Miyata H, Kasahara H, et al. Effectiveness of carbamazepine for benzodiazepine-resistant impulsive aggression in a patient with frontal infarctions. Psychiatry Clin Neurosci. (2007) 61:695–7. doi: 10.1111/j.1440-1819.2007.01737.x, PMID: [DOI] [PubMed] [Google Scholar]
- 45.Harris KL, Kuan W-L, Mason SL, Barker RA. Antidopaminergic treatment is associated with reduced chorea and irritability but impaired cognition in Huntington’s disease (enroll-HD). J Neurol Neurosurg Psychiatry. (2020) 91:622–30. doi: 10.1136/jnnp-2019-322038, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammond FM, Bickett AK, Norton JH, Pershad R. Effectiveness of amantadine hydrochloride in the reduction of chronic traumatic brain injury irritability and aggression. J Head Trauma Rehabil. (2014) 29:391–9. doi: 10.1097/01.HTR.0000438116.56228.de, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Kant R, Smith-Seemiller L, Zeiler D. Treatment of aggression and irritability after head injury. Brain Inj. (1998) 12:661–6. doi: 10.1080/026990598122223 [DOI] [PubMed] [Google Scholar]
- 48.Hammond FM, Zafonte RD, Tang Q, Jang JH. Carbamazepine for irritability and aggression after traumatic brain injury: a randomized, placebo-controlled study. J Neurotrauma. (2021) 38:2238–46. doi: 10.1089/neu.2020.7530, PMID: [DOI] [PubMed] [Google Scholar]
- 49.Adleman NE, Fromm SJ, Razdan V, Kayser R, Dickstein DP, Brotman MA, et al. Cross-sectional and longitudinal abnormalities in brain structure in children with severe mood dysregulation or bipolar disorder. J Child Psychol Psychiatry. (2012) 53:1149–56. doi: 10.1111/j.1469-7610.2012.02568.x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dimitriou T, Papatriantafyllou J, Konsta A, Kazis D, Athanasiadis L, Ioannidis P, et al. Assess of combinations of non-pharmacological interventions for the reduction of irritability in patients with dementia and their caregivers: a cross-over RCT. Brain Sci. (2022) 12:691. doi: 10.3390/brainsci12060691, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raggi A, Serretti A, Ferri R. A comprehensive overview of post-stroke depression treatment options. Int Clin Psychopharmacol. (2024) 39:127–38. doi: 10.1097/YIC.0000000000000532, PMID: [DOI] [PubMed] [Google Scholar]
- 52.Zhang SH, Wang YL, Zhang CX, Zhang CP, Xiao P, Li QF, et al. Effect of interactive dynamic scalp acupuncture on post-stroke cognitive function, depression, and anxiety: a multicenter, randomized, controlled trial. Chin J Integr Med. (2022) 28:106–15. doi: 10.1007/s11655-021-3338-1 [DOI] [PubMed] [Google Scholar]
- 53.Cheng C, Fan W, Liu C, Liu Y, Liu X. Reminiscence therapy–based care program relieves post-stroke cognitive impairment, anxiety, and depression in acute ischemic stroke patients: a randomized, controlled study. Ir J Med Sci (1971 -). (2021) 190:345–55. doi: 10.1007/s11845-020-02273-9 [DOI] [PubMed] [Google Scholar]
- 54.Starkstein SE, Robinson RG. The role of the human lobes in affective disorder following stroke In: Frontal lobe function and dysfunction. Ed. H. S. Levin, H. M. Eisenberg, and A. L. Benton. Oxford: Oxford University Press; (1991) [Google Scholar]
- 55.Henderson SE, Johnson AR, Vallejo AI, Katz L, Wong E, Gabbay V. A preliminary study of white matter in adolescent depression: relationships with illness severity, anhedonia, and irritability. Front Psych. (2013) 4:152. doi: 10.3389/fpsyt.2013.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klöppel S, Stonnington CM, Petrovic P, Mobbs D, Tüscher O, Craufurd D, et al. Irritability in pre-clinical Huntington’s disease. Neuropsychologia. (2010) 48:549–57. doi: 10.1016/j.neuropsychologia.2009.10.016, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryan ND. Severe irritability in youths: disruptive mood dysregulation disorder and associated brain circuit changes. Am J Psychiatry. (2013) 170:1093–6. doi: 10.1176/appi.ajp.2013.13070934, PMID: [DOI] [PubMed] [Google Scholar]
- 58.Krick S, Koob JL, Latarnik S, Volz LJ, Fink GR, Grefkes C, et al. Neuroanatomy of post-stroke depression: the association between symptom clusters and lesion location. Brain. Communications. (2023) 5:fcad275. doi: 10.1093/braincomms/fcad275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mourik JC, Rosso SM, Niermeijer MF, Duivenvoorden HJ, van Swieten JC, Tibben A. Frontotemporal dementia: behavioral symptoms and caregiver distress. Dement Geriatr Cogn Disord. (2004) 18:299–306. doi: 10.1159/000080123 [DOI] [PubMed] [Google Scholar]
- 60.Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression. Neurology. (1996) 46:1231–1. doi: 10.1212/WNL.46.5.1231, PMID: [DOI] [PubMed] [Google Scholar]
- 61.Tateno A, Jorge RE, Robinson RG. Clinical correlates of aggressive behavior after traumatic brain injury. J Neuropsychiatry Clin Neurosci. (2003) 15:155–60. doi: 10.1176/jnp.15.2.155, PMID: [DOI] [PubMed] [Google Scholar]
- 62.Vasa RA, Suskauer SJ, Thorn JM, Kalb L, Grados MA, Slomine BS, et al. Prevalence and predictors of affective lability after paediatric traumatic brain injury. Brain Inj. (2015) 29:921–8. doi: 10.3109/02699052.2015.1005670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hocaoğlu C, Okumuş B. Psychiatric manifestations and brain tumor: a case report and brief review. Mustafa Kemal Üniversitesi Tıp Dergisi. (2018) 9:42–9. doi: 10.17944/mkutfd.435908 [DOI] [Google Scholar]
- 64.Eames PG. Organic bases of behaviour disorders after traumatic brain injury In: Neurobehavioural sequelae of traumatic brain injury. Ed. R. L. Wood. Hove, UK: Psychology Press; (1990) [Google Scholar]
- 65.Gualtieri CT. Neuropsychiatry and behavioral pharmacology. New York: Springer; (1991). [Google Scholar]
- 66.Metin O, Tufan AE, Cevher Binici N, Saracli O, Atalay A, Yolga TA. Executive functions in frontal lob syndrome: a case report. Turk J Psychiatry. (2016). doi: 10.5080/u14849, PMID: [DOI] [PubMed] [Google Scholar]
- 67.Rohrer JD, Warren JD. Phenomenology and anatomy of abnormal behaviours in primary progressive aphasia. J Neurol Sci. (2010) 293:35–8. doi: 10.1016/j.jns.2010.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez-Horta S, Sampedro F, Horta-Barba A, Perez-Perez J, Pagonabarraga J, Gomez-Anson B, et al. Structural brain correlates of irritability and aggression in early manifest Huntington’s disease. Brain Imaging Behav. (2020) 15:107–13. doi: 10.1007/s11682-019-00237-x [DOI] [PubMed] [Google Scholar]
- 69.Rich BA, Carver FW, Holroyd T, Rosen HR, Mendoza JK, Cornwell BR, et al. Different neural pathways to negative affect in youth with pediatric bipolar disorder and severe mood dysregulation. J Psychiatr Res. (2011) 45:1283–94. doi: 10.1016/j.jpsychires.2011.04.006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomas LA, Brotman MA, Muhrer EJ, Rosen BH, Bones BL, Reynolds RC, et al. Parametric modulation of neural activity by emotion in youth with bipolar disorder, youth with severe mood dysregulation, and healthy volunteers. Arch Gen Psychiatry. (2012) 69:1257. doi: 10.1001/archgenpsychiatry.2012.913, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tseng W-L, Deveney CM, Stoddard J, Kircanski K, Frackman AE, Yi JY, et al. Brain mechanisms of attention orienting following frustration: associations with irritability and age in youths. Am J Psychiatry. (2019) 176:67–76. doi: 10.1176/appi.ajp.2018.18040491, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang JJ, Gu MM, Jiang SY, Yin DW, Wang P, Sun W, et al. Association between lesion location and depressive symptoms in acute ischemic stroke patients using voxel-based lesion-symptom mapping. Zhonghua Nei Ke Za Zhi. (2023) 62:70–5. doi: 10.3760/cma.j.cn112138-20220107-00022, PMID: [DOI] [PubMed] [Google Scholar]
- 73.Shi Y, Zeng Y, Wu L, Liu W, Liu Z, Zhang S, et al. A study of the brain abnormalities of post-stroke depression in frontal lobe lesion. Sci Rep. (2017) 7:13203. doi: 10.1038/s41598-017-13681-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moon Y, Moon W-J, Kim H, Han S-H. Regional atrophy of the insular cortex is associated with neuropsychiatric symptoms in Alzheimer’s disease patients. Eur Neurol. (2014) 71:223–9. doi: 10.1159/000356343 [DOI] [PubMed] [Google Scholar]
- 75.Balthazar ML, Pereira FR, Lopes TM, da Silva EL, Coan AC, Campos BM, et al. Neuropsychiatric symptoms in Alzheimer’s disease are related to functional connectivity alterations in the salience network. Hum Brain Mapp. (2013) 35:1237–46. doi: 10.1002/hbm.22248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas LA, Brotman MA, Bones BL, Chen G, Rosen BH, Pine DS, et al. Neural circuitry of masked emotional face processing in youth with bipolar disorder, severe mood dysregulation, and healthy volunteers. Dev Cogn Neurosci. (2014) 8:110–20. doi: 10.1016/j.dcn.2013.09.007, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thomas LA, Kim P, Bones BL, Hinton KE, Milch HS, Reynolds RC, et al. Elevated amygdala responses to emotional faces in youths with chronic irritability or bipolar disorder. Neuroimage. (2013) 2:637–45. doi: 10.1016/j.nicl.2013.04.007, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leocadi M, Canu E, Cividini C, Russo T, Cecchetti G, Celico C, et al. Brain structural abnormalities and cognitive changes in a patient with 17q21.31 microduplication and early onset dementia: a case report. J Neurol. (2022) 270:1127–34. doi: 10.1007/s00415-022-11423-1 [DOI] [PubMed] [Google Scholar]
- 79.Poulin SP, Dautoff R, Morris JC, Barrett LF, Dickerson BC. Amygdala atrophy is prominent in early Alzheimer’s disease and relates to symptom severity. Psychiatry Res Neuroimaging. (2011) 194:7–13. doi: 10.1016/j.pscychresns.2011.06.014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elst LT, Groffmann M, Ebert D, Schulze-Bonhage A. Amygdala volume loss in patients with dysphoric disorder of epilepsy. Epilepsy Behav. (2009) 16:105–12. doi: 10.1016/j.yebeh.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 81.Wright CI, Dickerson BC, Feczko E, Negeira A, Williams D. A functional magnetic resonance imaging study of amygdala responses to human faces in aging and mild Alzheimer’s disease. Biol Psychiatry. (2007) 62:1388–95. doi: 10.1016/j.biopsych.2006.11.013, PMID: [DOI] [PubMed] [Google Scholar]
- 82.Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. (2010) 167:61–9. doi: 10.1176/appi.ajp.2009.09010043, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deveney CM, Connolly ME, Haring CT, Bones BL, Reynolds RC, Kim P, et al. Neural mechanisms of frustration in chronically irritable children. Am J Psychiatry. (2013) 170:1186–94. doi: 10.1176/appi.ajp.2013.12070917, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klingbeil J, Brandt ML, Stockert A, Baum P, Hoffmann KT, Saur D, et al. Associations of lesion location, structural disconnection, and functional diaschisis with depressive symptoms post stroke. Front Neurol. (2023) 14:1144228. doi: 10.3389/fneur.2023.1144228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gregory S, Scahill RI, Seunarine KK, Stopford C, Zhang H, Zhang J, et al. Neuropsychiatry and white matter microstructure in Huntington’s disease. J Huntington’s Dis. (2015) 4:239–49. doi: 10.3233/JHD-150160, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tighe SK, Oishi K, Mori S, Smith GS, Albert M, Lyketsos CG, et al. Diffusion tensor imaging of neuropsychiatric symptoms in mild cognitive impairment and alzheimer’s dementia. J Neuropsychiatry Clin Neurosci. (2012) 24:484–8. doi: 10.1176/appi.neuropsych.11120375, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deng L, Sui R, Zhang L. Diffusion tensor tractography characteristics of white matter tracts are associated with post-stroke depression. Neuropsychiatr Dis Treat. (2021) 17:167–81. doi: 10.2147/NDT.S274632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang X, Shi Y, Fan T, Wang K, Zhan H, Wu W. Analysis of correlation between white matter changes and functional responses in post-stroke depression. Front Aging Neurosci. (2021) 13:728622. doi: 10.3389/fnagi.2021.728622, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang F, Ping Y, Jin X, Hou X, Song J. White matter hyperintensities and post-stroke depression: a systematic review and meta-analysis. J Affect Disord. (2023) 320:370–80. doi: 10.1016/j.jad.2022.09.166, PMID: [DOI] [PubMed] [Google Scholar]
- 90.Akil M, Brewer GJ. Psychiatric and behavioral abnormalities in Wilson's disease. Adv Neurol. (1995) 65:171–8. PMID: [PubMed] [Google Scholar]
- 91.Rosenblatt A, Leroi I. Neuropsychiatry of Huntington’s disease and other basal ganglia disorders. Psychosomatics. (2000) 41:24–30. doi: 10.1016/S0033-3182(00)71170-4, PMID: [DOI] [PubMed] [Google Scholar]
- 92.Etemadifar M, Abtahi S-H, Abtahi S-M, Mirdamadi M, Sajjadi S, Golabbakhsh A, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. (2012) 2012:1–4. doi: 10.1155/2012/768580, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wiggins JL, Brotman MA, Adleman NE, Kim P, Oakes AH, Reynolds RC, et al. Neural correlates of irritability in disruptive mood dysregulation and bipolar disorders. Am J Psychiatry. (2016) 173:722–30. doi: 10.1176/appi.ajp.2015.15060833, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mukherjee P, Vilgis V, Rhoads S, Chahal R, Fassbender C, Leibenluft E, et al. Associations of irritability with functional connectivity of amygdala and nucleus accumbens in adolescents and young adults with ADHD. J Atten Disord. (2021) 26:1040–50. doi: 10.1177/10870547211057074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pan C, Li G, Sun W, Miao J, Wang Y, Lan Y, et al. Psychopathological network for early-onset post-stroke depression symptoms. BMC Psychiatry. (2023) 23:114. doi: 10.1186/s12888-023-04606-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang Y, Chen A, Zhu S, Yang L, Zhou J, Pan S, et al. Repetitive transcranial magnetic stimulation for depression after basal ganglia ischaemic stroke: protocol for a multicentre randomised double-blind placebo-controlled trial. BMJ Open. (2018) 8:e018011. doi: 10.1136/bmjopen-2017-018011, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Medeiros GC, Roy D, Kontos N, Beach SR. Post-stroke depression: a 2020 updated review. Gen Hosp Psychiatry. (2020) 66:70–80. doi: 10.1016/j.genhosppsych.2020.06.011, PMID: [DOI] [PubMed] [Google Scholar]
- 98.Daum I, Ackermann H. Frontal-type memory impairment associated with thalamic damage. Int J Neurosci. (1994) 77:187–98. doi: 10.3109/00207459408986030 [DOI] [PubMed] [Google Scholar]
- 99.Mutarelli EG, Omuro AMP, Adoni T. Hypersexuality following bilateral thalamic infarction: case report. Arq Neuropsiquiatr. (2006) 64:146–8. doi: 10.1590/S0004-282X2006000100032, PMID: [DOI] [PubMed] [Google Scholar]
- 100.Benejam B, Sahuquillo J, Poca MA, Frascheri L, Solana E, Delgado P, et al. Quality of life and neurobehavioral changes in survivors of malignant middle cerebral artery infarction. J Neurol. (2009) 256:1126–33. doi: 10.1007/s00415-009-5083-9 [DOI] [PubMed] [Google Scholar]
- 101.Tang WK, Chan SS, Chiu HF, Ungvari GS, Wong KS, Kwok TC, et al. Poststroke depression in Chinese patients: frequency, psychosocial, clinical, and radiological determinants. J Geriatr Psychiatry Neurol. (2005) 18:45–51. doi: 10.1177/0891988704271764, PMID: [DOI] [PubMed] [Google Scholar]
- 102.Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. (1989) 20:864–70. doi: 10.1161/01.STR.20.7.864, PMID: [DOI] [PubMed] [Google Scholar]
- 103.Leung VP, Lam LC, Chiu HF, Cummings JL, Chen QL. Validation study of the Chinese version of the neuropsychiatric inventory (CNPI). Int J Geriatr Psychiatry. (2001) 16:789–93. doi: 10.1002/gps.427, PMID: [DOI] [PubMed] [Google Scholar]
- 104.Mok VCT, Wong A, Wong K, Chu WCW, Xiong Y, Chan AYY, et al. Executive dysfunction and left frontal white matter hyperintensities are correlated with neuropsychiatric symptoms in stroke patients with confluent white matter hyperintensities. Dement Geriatr Cogn Disord. (2010) 30:254–60. doi: 10.1159/000318744, PMID: [DOI] [PubMed] [Google Scholar]
- 105.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. (1965) 14:61–5. PMID: [PubMed] [Google Scholar]
- 106.Shek DT. Reliability and factorial structure of the Chinese version of the Beck depression inventory. J Clin Psychol. (1990) 46:35–43., PMID: [DOI] [PubMed] [Google Scholar]
- 107.Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. (2007) 28:1194–205. doi: 10.1002/hbm.20345, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun M, Zhan Z, Chen B, Xin J, Chen X, Yu E, et al. Development and application of a Chinese version of the language screening test (CLAST) in post-stroke patients. Medicine. (2020) 99:e22165. doi: 10.1097/MD.0000000000022165, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yeung P, Wong L, Chan C, Leung JL, Yung C. A validation study of the Hong Kong version of Montreal cognitive assessment (HK-MOCA) in Chinese older adults in Hong Kong. Hong Kong Med J. (2014). doi: 10.12809/hkmj144219, PMID: [DOI] [PubMed] [Google Scholar]
- 110.Costa PT, McCrae RR. Professional manual for the revised NEO personality inventory and NEO five-factor inventory. Odessa, FL: Psychological Assessment Resources; (1992). [Google Scholar]
- 111.Yang J, McCrae RR, Costa PT, Dai X, Yao S, Cai T, et al. Cross-cultural personality assessment in psychiatric populations: the NEO-PI—R in the People’s republic of China. Psychol Assess. (1999) 11:359–68. doi: 10.1037/1040-3590.11.3.359 [DOI] [Google Scholar]
- 112.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. (1998) 17:87–97. doi: 10.1109/42.668698, PMID: [DOI] [PubMed] [Google Scholar]
- 113.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. (1994) 18:192–205. doi: 10.1097/00004728-199403000-00005, PMID: [DOI] [PubMed] [Google Scholar]
- 114.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. (2002) 17:143–55. doi: 10.1002/hbm.10062, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cocosco CA, Zijdenbos AP, Evans AC. A fully automatic and robust brain MRI tissue classification method. Med Image Anal. (2003) 7:513–27. doi: 10.1016/S1361-8415(03)00037-9, PMID: [DOI] [PubMed] [Google Scholar]
- 116.Han X, Fischl B. Atlas renormalization for improved brain Mr image segmentation across scanner platforms. IEEE Trans Med Imaging. (2007) 26:479–86. doi: 10.1109/TMI.2007.893282, PMID: [DOI] [PubMed] [Google Scholar]
- 117.Thirion J-P. Image matching as a diffusion process: an analogy with Maxwell’s demons. Med Image Anal. (1998) 2:243–60. doi: 10.1016/S1361-8415(98)80022-4, PMID: [DOI] [PubMed] [Google Scholar]
- 118.Tang WK, Chen Y, Lam WWM, Mok V, Wong A, Ungvari GS, et al. Emotional incontinence and executive function in ischemic stroke: a case-controlled study. J Int Neuropsychol Soc. (2009) 15:62–8. doi: 10.1017/S1355617708090061, PMID: [DOI] [PubMed] [Google Scholar]
- 119.Tang WK, Chen Y, Lu J, Liang H, Chu WC, Tong Mok VC, et al. Frontal infarcts and anxiety in stroke. Stroke. (2012) 43:1426–8. doi: 10.1161/STROKEAHA.111.640482, PMID: [DOI] [PubMed] [Google Scholar]
- 120.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, New Jersey: Lawrence Erlbaum Associates; (1988). [Google Scholar]