Abstract
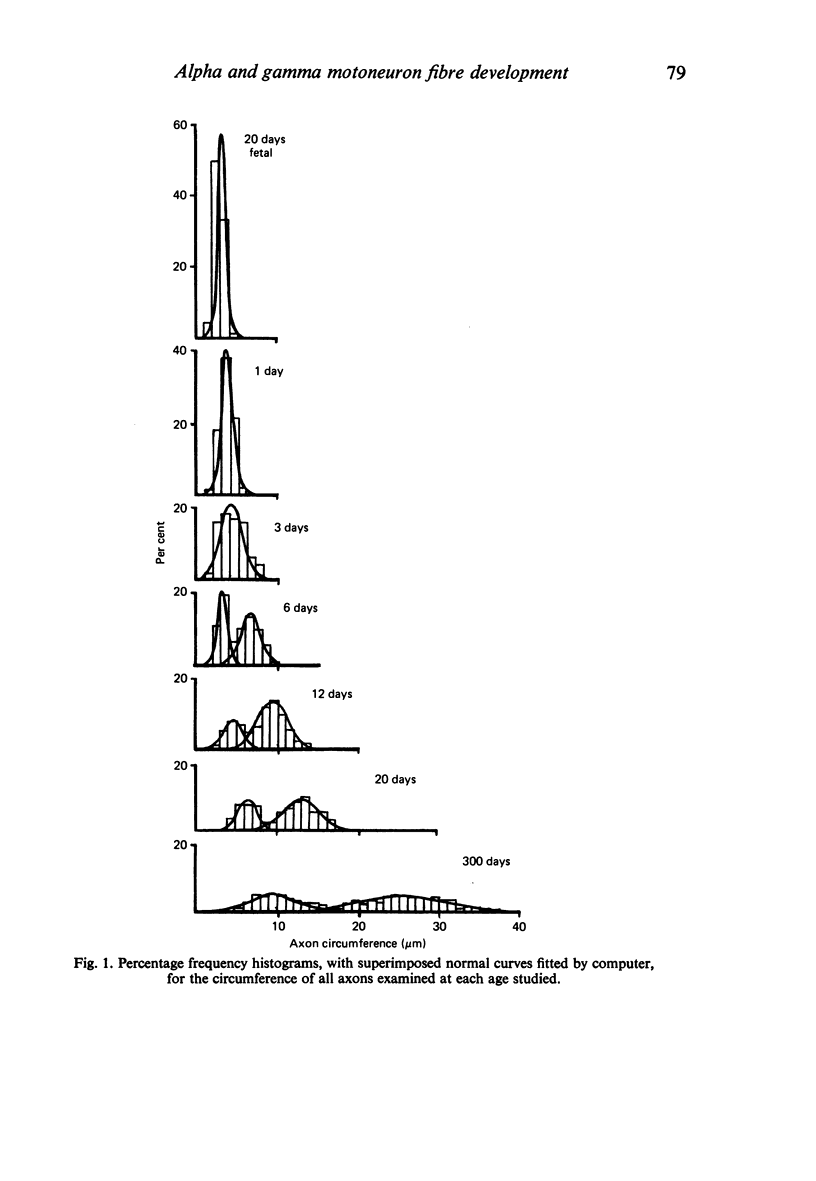
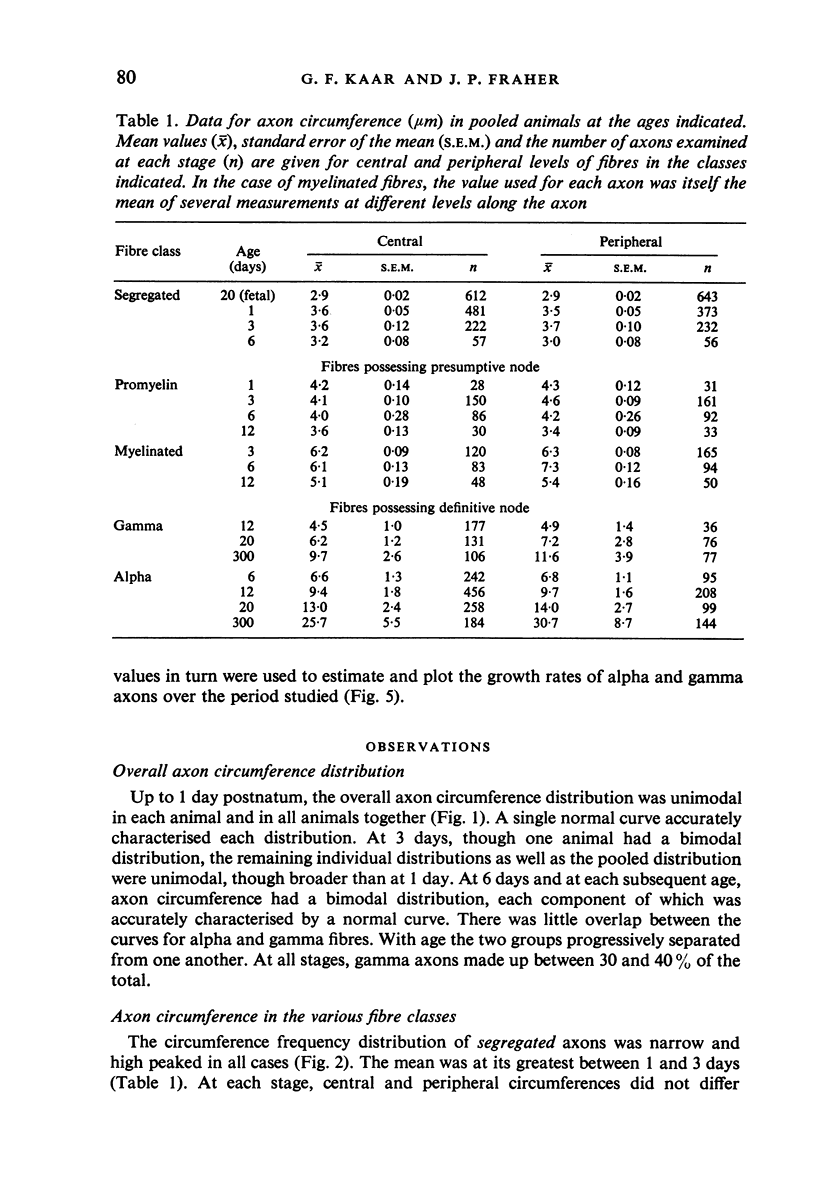
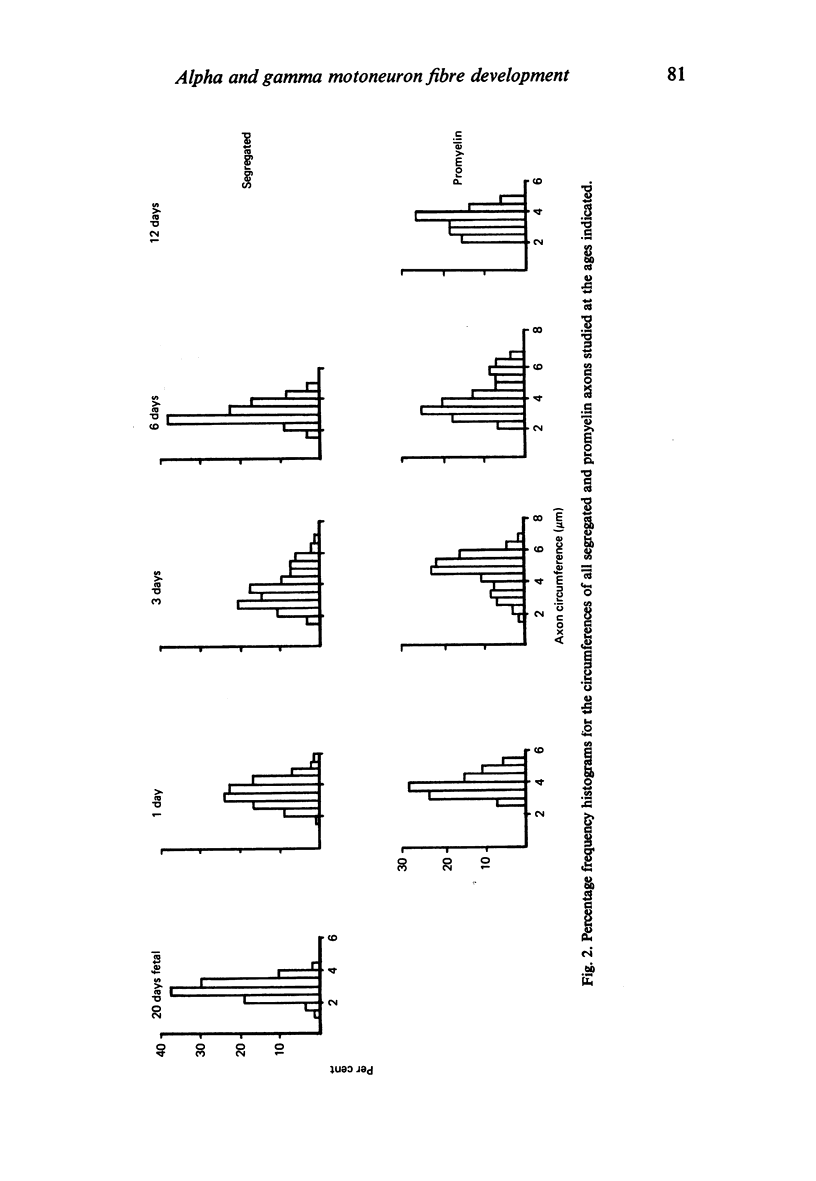
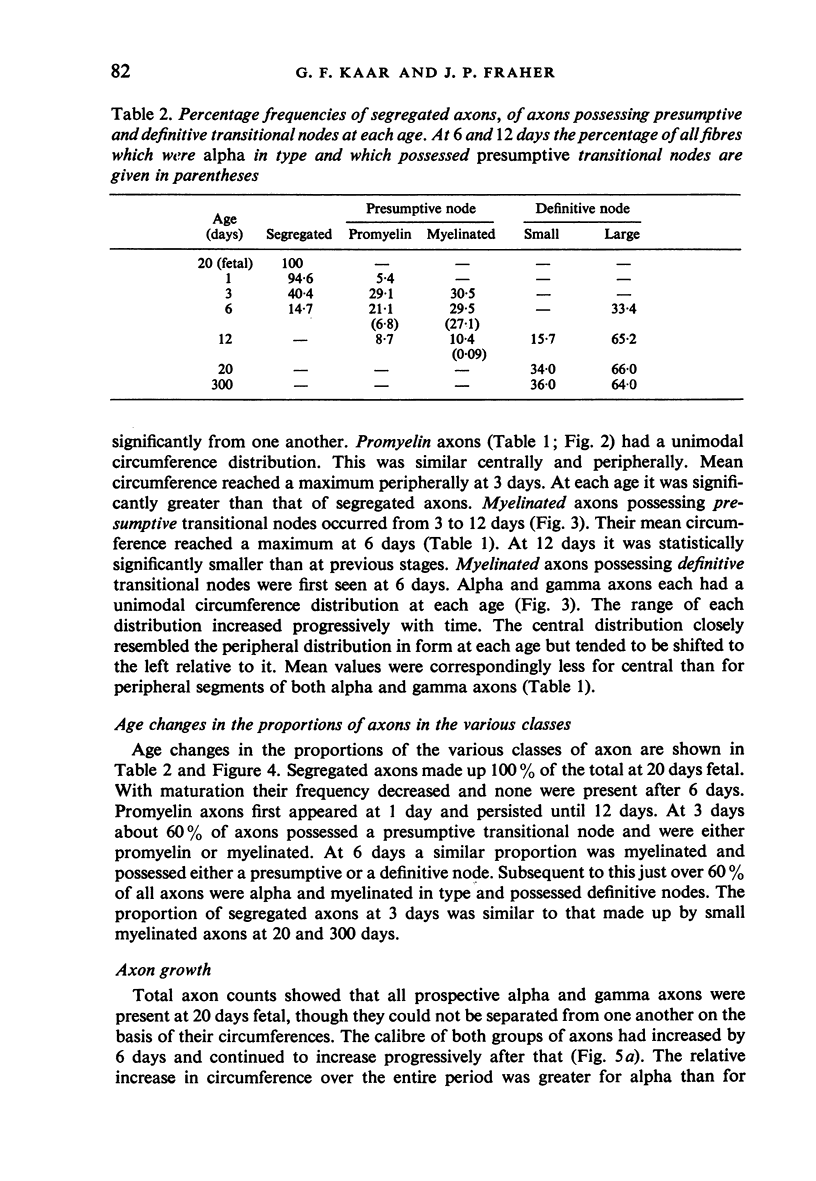
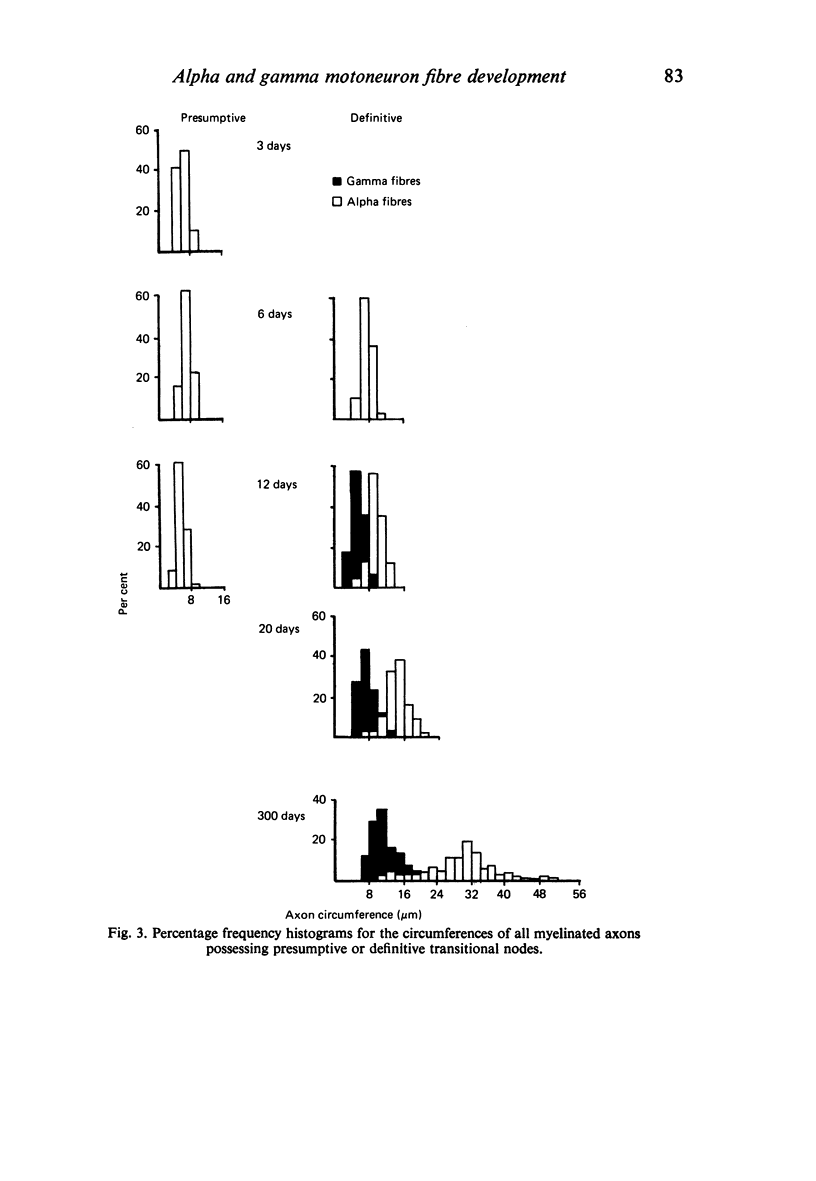
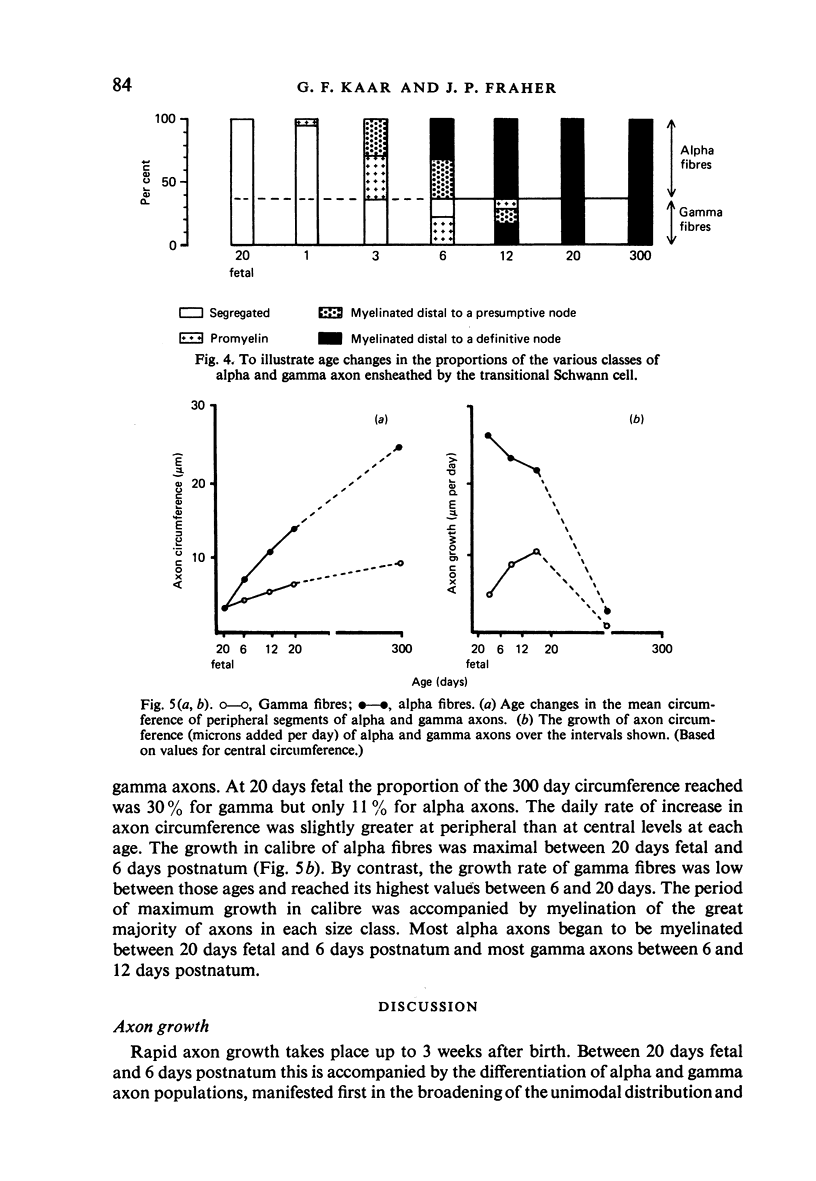
The growth of alpha and gamma motoneuron axons was studied from late fetal life to maturity in the fifth lumbar ventral spinal root of the rat. Overall axon circumference distribution is unimodal up to three days postnatum and bimodal subsequently. Alpha and gamma fibre categories can be distinguished from one another before the bimodal distribution appears, since their degrees of segregation differ. By 3 days postnatum, the great majority of presumptive alpha axons are either promyelin or myelinated in form. The two populations follow separate maturation tracks subsequently, alpha axons making up between 60 and 70% of the total. Alpha axons grow most rapidly during the first week, gamma axons during the second and third weeks postnatum. The onset of myelination in both groups coincides with the most rapid period of growth. The delayed rapid growth of gamma fibres is due to a prolongation of their segregated stage; subsequent stages have a similar duration to those of alpha fibres. The periods of most rapid growth in both alpha and gamma fibres may be related to the formation of their respective terminal connections in the muscles. Myelination commences in relation to a smaller axon circumference in gamma than in alpha fibres. The former are therefore more myelinogenic than the latter.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arees E. A. Growth pattern of axons in the optic nerve of chick during myelogenesis. J Comp Neurol. 1978 Jul 1;180(1):73–84. doi: 10.1002/cne.901800106. [DOI] [PubMed] [Google Scholar]
- Berthold C. H., Carlstedt T. Myelination of S1 dorsal root axons in the cat. J Comp Neurol. 1982 Aug 10;209(3):225–232. doi: 10.1002/cne.902090302. [DOI] [PubMed] [Google Scholar]
- Berthold C. H., Nilsson I., Rydmark M. Axon diameter and myelin sheath thickness in nerve fibres of the ventral spinal root of the seventh lumbar nerve of the adult and developing cat. J Anat. 1983 May;136(Pt 3):483–508. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P. A quantitative study of anterior root fibres during early myelination. J Anat. 1972 May;112(Pt 1):99–124. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P., Kaar G. F. The transitional node of Ranvier at the junction of the central and peripheral nervous systems: an ultrastructural study of its development and mature form. J Anat. 1984 Sep;139(Pt 2):215–238. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P. Quantitative studies on the maturation of central and peripheral parts of individual ventral motoneuron axons. I. Myelin sheath and axon calibre. J Anat. 1978 Aug;126(Pt 3):509–533. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P., Rossiter J. P. Cell clusters on fetal rat ventral roots: prenatal development. J Anat. 1983 Jan;136(Pt 1):111–128. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P. The growth and myelination of central and peripheral segments of ventral motoneurone axons. A quantitative ultrastructural study. Brain Res. 1976 Mar 26;105(2):193–211. doi: 10.1016/0006-8993(76)90421-2. [DOI] [PubMed] [Google Scholar]
- Friede R. L., Bischhausen R. The precise geometry of large internodes. J Neurol Sci. 1980 Dec;48(3):367–381. doi: 10.1016/0022-510x(80)90109-4. [DOI] [PubMed] [Google Scholar]
- Friede R. L., Samorajski T. Relation between the number of myelin lamellae and axon circumference in fibers of vagus and sciatic nerves of mice. J Comp Neurol. 1967 Jul;130(3):223–231. doi: 10.1002/cne.901300304. [DOI] [PubMed] [Google Scholar]
- Hildebrand C., Hahn R. Relation between myelin sheath thickness and axon size in spinal cord white matter of some vertebrate species. J Neurol Sci. 1978 Oct;38(3):421–434. doi: 10.1016/0022-510x(78)90147-8. [DOI] [PubMed] [Google Scholar]
- Kelly A. M., Zacks S. I. The fine structure of motor endplate morphogenesis. J Cell Biol. 1969 Jul;42(1):154–169. doi: 10.1083/jcb.42.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J. Morphogenesis of motor end-plates "in vivo" and "in vitro". Brain Res. 1973 Nov 23;62(2):361–365. doi: 10.1016/0006-8993(73)90697-5. [DOI] [PubMed] [Google Scholar]
- Schröder J. M., Bohl J., Brodda K. Changes of the ratio between myelin thickness and axon diameter in the human developing sural nerve. Acta Neuropathol. 1978 Aug 7;43(1-2):169–178. doi: 10.1007/BF00685012. [DOI] [PubMed] [Google Scholar]
- Skoglund S., Romero C. Postnatal growth of spinal nerves and roots. A morphological study in the cat with physiological correlations. Acta Physiol Scand Suppl. 1965;260:1–50. [PubMed] [Google Scholar]
- Sturrock R. R. A quantitative electron microscopic study of myelination in the anterior limb of the anterior commissure of the mouse brain. J Anat. 1975 Feb;119(Pt 1):67–75. [PMC free article] [PubMed] [Google Scholar]
- ZELENA J. DEVELOPMENT, DEGENERATION AND REGENERATION OF RECEPTOR ORGANS. Prog Brain Res. 1964;13:175–213. [PubMed] [Google Scholar]


