Abstract
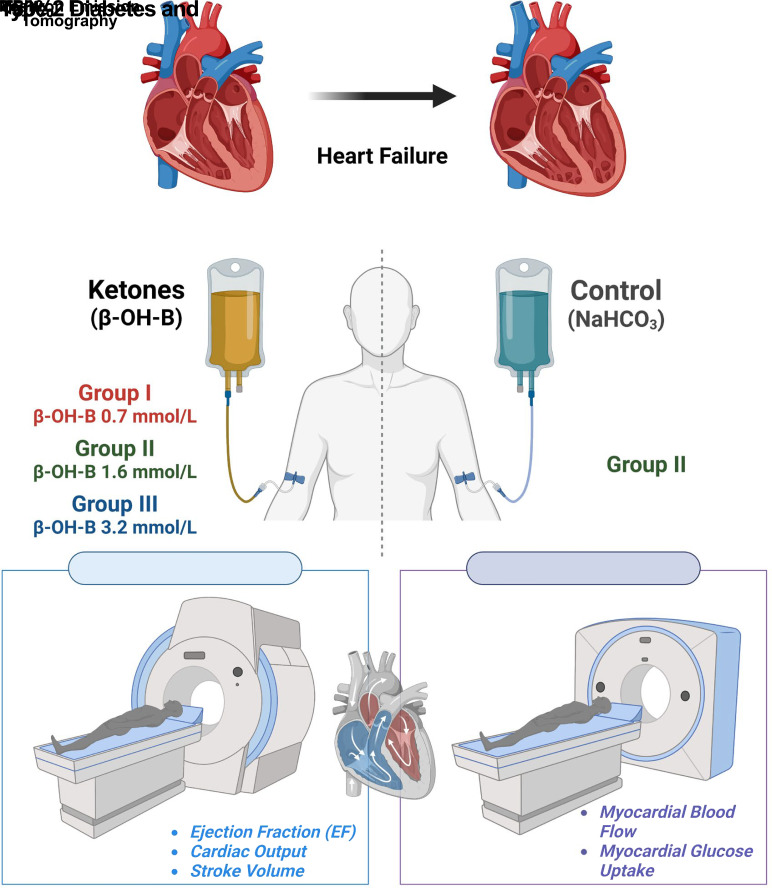
We examined the effect of increased levels of plasma ketones on left ventricular (LV) function, myocardial glucose uptake (MGU), and myocardial blood flow (MBF) in patients with type 2 diabetes (T2DM) with heart failure. Three groups of patients with T2DM (n = 12 per group) with an LV ejection fraction (EF) ≤50% received incremental infusions of β-hydroxybutyrate (β-OH-B) for 3–6 h to increase the plasma β-OH-B concentration throughout the physiologic (groups I and II) and pharmacologic (group III) range. Cardiac MRI was performed at baseline and after each β-OH-B infusion to provide measures of cardiac function. On a separate day, group II also received a sodium bicarbonate (NaHCO3) infusion, thus serving as their own control for time, volume, and pH. Additionally, group II underwent positron emission tomography study with 18F-fluoro-2-deoxyglucose to examine effect of hyperketonemia on MGU. Groups I, II, and III achieved plasma β-OH-B levels (mean ± SEM) of 0.7 ± 0.3, 1.6 ± 0.2, 3.2 ± 0.2 mmol/L, respectively. Cardiac output (CO), LVEF, and stroke volume (SV) increased significantly during β-OH-B infusion in groups II (CO, from 4.54 to 5.30; EF, 39.9 to 43.8; SV, 70.3 to 80.0) and III (CO, from 5.93 to 7.16; EF, 41.1 to 47.5; SV, 89.0 to 108.4), and did not change with NaHCO3 infusion in group II. The increase in LVEF was greatest in group III (P < 0.001 vs. group II). MGU and MBF were not altered by β-OH-B. In patients with T2DM and LVEF ≤50%, increased plasma β-OH-B level significantly increased LV function dose dependently. Because MGU did not change, the myocardial benefit of β-OH-B resulted from providing an additional fuel for the heart without inhibiting MGU.
Article Highlights
Sodium–glucose cotransporter 2 inhibitor (SGLT2i) therapy is associated with an increase in plasma ketone concentration.
In patients with type 2 diabetes and heart failure with reduced ejection fraction, we examined the effect of β-hydroxybutyrate (β-OH-B) infusion, spanning the physiologic and pharmacologic ranges of plasma β-OH-B concentrations (0.7, 1.6, and 3.2 mmol/L) on myocardial function (using MRI), myocardial blood flow (measured with positron emission tomography [PET]/15O-labeled water), and myocardial glucose uptake (PET/18F-FDG).
β-OH-B caused a dose-response increase in left ventricular ejection fraction and myocardial blood flow without altering myocardial glucose uptake.
These results suggest that the SGLT2i-induced increase in plasma ketone concentration may contribute to its beneficial effects on myocardial function.
Graphical Abstract
Introduction
Type 2 diabetes mellitus (T2DM) is a cardiometabolic disorder in which both microvascular and macrovascular complications contribute to morbidity and mortality (1). Cardiovascular (CV) events continue to be the leading cause of death in patients with T2DM. Additionally, heart failure (HF) affects ∼6.5 million adults in the U.S. (2) and ∼26 million worldwide (3). The incidence of HF in patients with T2DM is ∼22%. T2DM also increases the risk for hospitalization for HF (HHF) by ∼50% and decreases overall 5-year survival by ∼80% (4–6).
Although hyperglycemia is the major risk factor for microvascular complications, it is a weak risk factor for macrovascular complications (e.g., myocardial infarction, stroke) (7) compared with the more classic CV risk factors (hypertension, dyslipidemia, obesity, endothelial dysfunction, insulin resistance, inflammation) (8). Nine large placebo-controlled trials (9) have examined the effect of sodium–glucose cotransporter 2 inhibitors (SGLT2is) on CV outcomes in patients with T2DM, with eight of these trials demonstrating a CV benefit. Although SGLT2i treatment reduced both major adverse CV events (MACE) and HHF, the magnitude of decrease in HHF produced by SGLT2is was markedly greater than MACE. In contrast to MACE, SGLT2is consistently and equally reduced HHF in participants with (by 30%) and without (by 37%) established CV disease (9,10). Notably, the separation of Kaplan-Meier curves for CV mortality and HHF occurred within 3 months, suggesting nonatherogenic mechanism(s).
In the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (11) and the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (12), SGLT2i treatment decreased HHF plus CV mortality in patients with HFrEF by 24%, and 25%, respectively. A similar reduction was observed in patients with HFrEF and without T2DM, indicating that the benefit of SGLT2is on HF is independent of diabetes and decrease in plasma glucose concentration. It also is unlikely that the modest reductions in body weight and blood pressure can explain these CV benefits. Multiple mechanisms have been invoked to explain the beneficial effects of SGLT2is on HHF and CV mortality (13–17). Hemodynamic factors (specifically, simultaneous reduction in preload secondary to intravascular volume depletion and afterload secondary to reduced blood pressure and improved aortic distensibility) most commonly have been cited. The “ketone hypothesis” (18,19) (i.e., a shift from glucose oxidation to ketone utilization as a more efficient and less oxygen-consumptive substrate for the heart) also has been proposed to explain the CV benefits of SGLT2i therapy (19). However, the effect of ketones on the heart has not been well studied.
Ketones are avidly taken up by multiple tissues in the body, including skeletal and cardiac muscle, via the monocarboxylate transporter, in proportion to their delivery (i.e. blood flow multiplied by arterial ketone concentration) (20,21). The fractional extraction of ketones by muscle, both skeletal and cardiac, is ∼40%, which is comparable to that of pyruvate and much greater than that of glucose (∼2%) or free fatty acid (FFA; ∼15–20%) (18). Hyperketonemia, thus, provides a glycolysis-independent pool of acetyl CoA and, ultimately, energy in the form of ATP (18,19,22,23). Furthermore, oxidation of ketones yields an amount of ATP per mole of oxygen consumed that is similar to that of glucose and much greater than FFA and does not lead to uncoupling in the mitochondrial membrane, which can occur with increased FFA oxidation (18,24,25). The heart has a high energy demand, which primarily is met by oxidation of glucose and FFA (24,26). The contribution of ketone oxidation to myocardial energy demand is modest (10–20%) (19,26) but can increase markedly at higher plasma ketone concentrations (25). Thus, increasing ketone delivery to the heart augments ATP production but does not increase myocardial efficiency (i.e., cardiac work per oxygen consumed) (19,25). Multiple studies have demonstrated that the failing heart relies upon ketones as an oxidative fuel (25–28).
We and others have shown that SGLT2i treatment of patients with T2DM consistently increases the fasting plasma ketone concentration two- to fourfold (29,30), and ketone levels >1 mmol/L have been reported in 15–20% of patients with diabetes treated with an SGLT2i for 52 weeks (31). In patients with HF, cardiac muscle uptake of β-hydroxybutyrate (β-OH-B) is increased (19,32,33), although skeletal muscle ketone uptake has been reported to be decreased (34). HF, independent of T2DM, is an insulin-resistant state (35). However, ketone uptake by cardiac and skeletal muscle is insulin independent (16,36). It is possible, therefore, that the SGLT2i-induced hyperketonemia in patients with HF contributes to the beneficial CV effects of this class of glucose-lowering medication. As a first step in considering this hypothesis, it must be demonstrated that physiologic hyperketonemia can augment myocardial function in patients with HF.
In this study, we examined, in patients with T2DM with an ejection fraction (EF) ≤50%, the effect of β-OH-B infusion, spanning the physiologic and pharmacologic ranges of plasma β-OH-B concentrations, on myocardial function, myocardial glucose uptake (MGU), and myocardial blood flow (MBF), with cardiac MRI and positron emission tomography (PET).
Research Design and Methods
Study Participants
Thirty-six participants with New York Heart Association Class II-III HF (EF ≤50%) and T2DM (HbA1c >6.5%) were studied (Table 1). Participants were recruited from the HF clinic of UT Health San Antonio and from the Texas Diabetes Institute. Before the screening visit, patients had an established diagnosis of HF documented by an acceptable imaging modality within 6 months. If this was not available, transthoracic echocardiography (Phillips Equipment Corp, Bothell, WA) was performed at UT Health San Antonio Echocardiology Core Laboratory by a certified echocardiography cardiologist to confirm EF ≤50%.
Table 1.
Baseline physiological characteristics of study participants
| Group I | Group II | Group II | |
|---|---|---|---|
| Plasma β-OH-B | (0.7 mmol/L) | (1.6 mmol/L) | (3.2 mmol/L) |
| n | 12 | 12 | 12 |
| Age (years) | 57 ± 2 | 60 ± 2 | 57 ± 3 |
| Sex (M/F) | 11/1 | 9/3 | 8/4 |
| BMI (kg/m2) | 31 ± 1 | 32 ± 1 | 34 ± 2 |
| EF (%) | 31 ± 3 | 39 ± 2 | 39 ± 3 |
| HbA1c (%) | 7.4 ± 0.3 | 7.7 ± 0.3 | 76.9 ± 0.3 |
| eGFR (mL/min/1.73 m2) | 64 ± 7 | 77 ± 8 | 73 ± 7 |
Data are reported as mean ± SEM. One-way ANOVA was used. P values were not statistically significant.
Other inclusion criteria included age range from 18 to 80 years; BMI 23–38 kg/m2; HbA1c 6.5–10.0%; blood pressure <145/85 mmHg; and estimated glomerular filtration rate (eGFR) >30 mL/min/1.73 m2. Participants had to be taking a stable dose of medications (e.g., ACE inhibitor or angiotensin receptor blocker, diuretic, β-blocker, calcium channel blocker) for HF. Participants were treated with diet plus metformin or metformin/sulfonylurea. Patients treated with SGLT2is, glucagon-like peptide-1 receptor agonists, or thiazolidinediones were excluded. Body weight was stable (±4 pounds [1.8 kg]) over the past 3 months.
Study Design
At the screening visit, patients arrived at the Clinical Research Center of the Texas Diabetes Institute at 0800 h, after a 10- to 12-h overnight fast for determination of lean body mass and fat mass with DEXA (Discovery Wi; Hologic, Bedford, MA). After eligibility was confirmed, participants underwent a medical history and physical exam to exclude major organ system diseases other than diabetes and cardiac disease. Routine blood chemistry tests, complete blood cell count, complete metabolic panel, urinalysis, lipid profile, thyrotropin level, HbA1c, and electrocardiogram results were obtained. Blood pressure was measured with an automated sphygmomanometer after the participant had been reclining for 5 min.
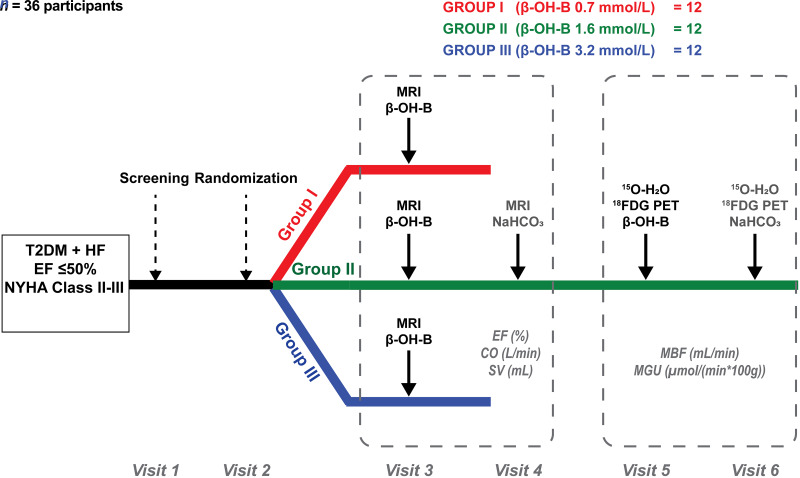
Patients with diabetes who met entry criteria were randomized (1:1:1 ratio) to receive one of three different infusion rates of β-OH-B (Fig. 1): group I (mean values: age, 57 years; BMI, 31 kg/m2; HbA1c, 7.4%; EF, 31%) received a 6-h β-OH-B infusion (prime dose was 0.4 mg/kg/min for 20 min followed by constant rate of 0.2 mg/kg/min until study end). A 6-h period was chosen on the basis of a previous study (37) that demonstrated a significant effect on myocardial function within 6 h in patients with HFrEF. Group II (mean values: age, 60 years; BMI, 32 kg/m2; HbA1c, 7.7%; EF, 39%) received a 6-h β-OH-B infusion (prime dose was 1.5 mg/kg/min for 20 min followed by a constant rate of 0.75 mg/kg/min). The randomization was performed blindly by a pharmacist not involved in the study, but the investigators performing the study were aware of who received the low, intermediate, and high β-OH-B infusion rates. The two individuals who performed the data analyses were blinded to the randomization code.
Figure 1.
Study design. NYHA, New York Heart Association.
On a separate day and in random order, group II received a 6-h sodium bicarbonate (NaHCO3) infusion (0.12 mol/L solution) with matching volume to the β-OH-B study and thus served as their own controls. The NaHCO3 infusion was designed to mimic the expected increase in plasma bicarbonate (HCO3) concentration and pH observed with the β-OH-B infusion. Group III (mean values: age, 57 years; BMI, 34 kg/m2; HbA1c, 6.9%; EF, 39%) received a 3-h β-OH-B infusion (prime dose of 4.0 mg/kg/min for 20 min followed by constant rate of 2.0 mg/kg/min until study end). Cardiac MRI was performed before and at end of each infusion. Naβ-OH-B (d/l) was obtained from Gold Bio (St. Louis, MO). Within 10 days, group II also underwent, in random order, two PET studies with 18F-fluoro-2-deoxyglucose (18FDG)–15O-labeled water (15O-H2O) before and after β-OH-B and NaHCO3 infusion (as described) to examine whether β-OH-B replaced glucose as a metabolic fuel for the heart. All MRI and PET studies were performed in the morning after an overnight fast. A detailed description of the MRI and PET procedure is described in the following two subsections.
The three β-OH-B infusion rates were chosen to cover the range of increments in plasma β-OH-B concentrations observed in patients with T2DM who were treated with an SGLT2i (29–31). Groups I, II, and III achieved plasma β-OH-B levels of 0.7, 1.6, and 3.2 mmol/L, respectively. Studies were performed at the Clinical Research Center of the Texas Diabetes Institute, University Health System and the Research Imaging Institute, UT Health San Antonio.
Cardiac MRI
Participants reported for cardiac MRI to the UT Health San Antonio Research Imaging Institute (RII) at 0700 h after an overnight fast (38,39) to confirm EF ≤50% and obtain quantitative measures of baseline cardiac function (cardiac output [CO], stroke volume [SV], peak left ventricular [LV] EF). After the participant was comfortably reclining in the MRI machine, three blood samples were collected from an antecubital vein catheter for β-OH-B, glucose, insulin, C-peptide, glucagon, and HCO3 concentrations. Cardiac MRI was performed with a Siemens TIM TRIO 3T whole-body MRI (Siemens Medical Systems, Malvern, PA). The instrumentation, acquisition characteristics, and derived parameters of LV function have been described in detail in a previous publication (38). Participants then were randomized (1:1:1 ratio) to receive one of three infusion rates of β-OH-B, as described. Each group received a prime-continuous infusion of β-OH-B (4 mol/L solution; pH adjusted to 7.24) to increase plasma β-OH-B concentration. The total volume infused was in compliance with standard of care and did not exceed 300 mL, to avoid volume overload (40). Baseline blood sample collection was repeated at 270, 285, and 300 min after start of β-OH-B infusion. After 6 h of β-OH-B infusion, cardiac MRI was repeated in groups I and II. In group III, the cardiac scan was repeated after 3 h, to be compliant with recommendations concerning acute volume loading in patients with congestive HF (40).
When infused with sodium, β-OH-B is a HCO3 equivalent and is associated with a small increase in plasma HCO3 concentration (36). Therefore, as a control for time and increase in plasma HCO3, participants in group II returned 7–10 days after β-OH-B infusion and received a 6-h infusion of NaHCO3 to mimic the increase in plasma HCO3 concentration observed with Na–β-OH-B infusion. The total volumes of β-OH-B and NaHCO3 infused were the same to obviate any effect of volume on cardiac function.
MRI data were analyzed blindly by two investigators using a commercial postprocessing package (CMR42; Circle Cardiovascular Imaging Inc., Calgary, AB, Canada). The CMR42 flow module computes velocity, flow, regurgitant volumes, and CO. The EF was computed using end diastolic volumes and end systolic volumes. Dimensional parameters were normalized to body surface area, using the Mosteller formula. Ejection and filling functions were assessed from the respective maximal and average downslope and upslope of volume time curves to determine peak LV ejection and filling rates.
Cardiac PET
Participants in group II also underwent a cardiac PET study to examine the effect of hyperketonemia on MGU and MBF (38,41). Participants reported to the RII at 0700 h after 10-h overnight fast 7–10 days after the NaHCO3 infusion study. Catheters were placed in both antecubital veins, one for β-OH-B (or NaHCO3) and radiotracer administration and another for blood withdrawal. PET scans were performed in two-dimensional imaging mode using an ECAT 931–08/12 PET scanner (CTI, Knoxville, TN) with a 10.5-cm axial field of view and resolution of 8.4 × 8.3 × 6.6 mm3 full width at half-maximum. After optimization of the participant’s position, a 20-min transmission scan was performed after exposure to a retractable 68Ge ring source to correct emission data for tissue attenuation of γ photons. Then, 15O-H2O (10.5 MBq/kg) was administered intravenously through the antecubital vein catheter over 20 s, and a PET scan was performed to measure MBF (38,41). A representative cardiac PET image is shown in Supplementary Fig. 1.
As with the cardiac MRI study, participants underwent a 6-h β-OH-B infusion (prime dose was 1.5 mg/kg/min for 20 min followed by constant rate of 0.75 mg/kg/min) or 6-h NaHCO3 infusion (0.12 mol/L solution with matching volume) carried out in random order on two separate days, with 18FDG/PET performed at the end of each infusion visit. At 280 min after the start of β-OH-B or NaHCO3 infusions, 15O-H2O was injected for repeated measurement of MBF. At 300 min, 18F-FDG (185 MBq) was injected, followed by a dynamic PET scan (12 × 10 s, 3 × 20 s, 4 × 40 s, 5 × 60 s, 4 × 150 s, 4 × 300 s, and 2 × 600 s) for the measurement of MGU. The NaHCO3 infusion study was not performed in groups I and III, because there was no effect on any parameter of myocardial function (as determined from MRI) or MGU and MBF (as determined from PET scans) in group II, and the cost of performing the NaHCO3 infusion study was felt to outweigh the benefit.
PET sinograms were corrected for tissue attenuation and reconstructed through standard reconstruction algorithms. Regional time-activity curves were obtained from the left ventricle, using Carimas 2.9 software (Turku PET Centre; https://carimas.fi/). The input function for 18F-FDG was derived from continuous monitoring of left ventricle blood radioactivity and arterialized plasma samples. Whole blood was converted into plasma input, and delay correction was performed. Arterial input for 15O-H2O was obtained from the left ventricle time activity curve. Fractional uptake rate of 18F-FDG was calculated using fractional uptake concept, and MGU was quantified by multiplying fractional uptake rate by mean plasma glucose concentration during the imaging period. Washout rate (represented by the rate constant k2) for 15O-H2O was calculated using the one-tissue compartment analysis, and MBF was quantified by multiplying k2 by the partition coefficient of water (0.9464).
Analytical Procedures
Plasma glucose was measured with a glucose oxidase method (Analox Technologies, Toronto, ON, Canada). Plasma insulin (IBL America, Minneapolis, MN) and C-peptide (MP Biomedicals, Santa Ana, CA) levels were determined with immunoradiographic assays and plasma glucagon (MilliporeSigma, Burlington, MA) by radioimmunoassay (42). Plasma β-OH-B concentration was measured using hydrophilic interaction liquid chromatography–tandem mass spectrometry (37).
Statistics
Change from baseline (before versus after infusion of β-OH-B or NaHCO3) in LV function, MBF, MGU, and plasma metabolite and hormone levels was compared using a two-sided paired Student t test. Between-group comparisons for the change from baseline to study end for all preceding variables were made using one-way or two-way ANOVA. Post hoc testing was done with Tukey correction using GraphPad Prism software (La Jolla, CA), with P ≤ 0.05 considered statistically significant. All data are presented as the mean ± SEM. There were no significant sex differences between the three groups.
Based on a study by Nielsen et al. (37) in patients with HFrEF, we determined that 12 participants per group would be sufficient to achieve power of 90%. The testing procedure was carried out at all three infusion rates (I, II, and III) and with three outcome variables (LV function, MBF, and plasma metabolite/hormone levels). Tukey correction was used for multiple comparisons, assuming blood flow and glucose uptake are normally distributed with the same effect size as EF and α = 0.017 (0.05/3).
Study Approval
Protocols were approved by UT Health San Antonio Institutional Review Board (approval no. 18-077H). Informed written consent was obtained from each patient before participation. The study is registered with Clinical trials.gov (identifier NCT03560323).
Data and Resource Availability
Data sharing will be available upon request to the corresponding author.
Results
Study Participants
Participants in the three groups were well matched for age, sex, BMI, HbA1c, eGFR, and EF at baseline (Table 1).
Hemodynamic and Hormone Concentration Changes
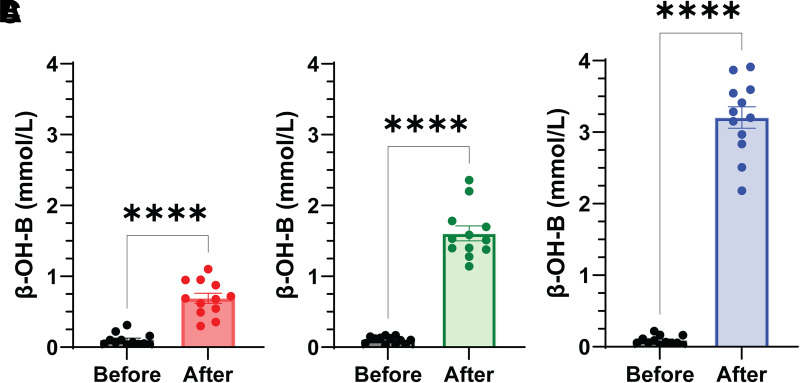
In response to the β-OH-B infusion, groups I, II, and III achieved plasma β-OH-B concentrations of 0.7 ± 0.3, 1.6 ± 0.2, and 3.2 ± 0.2 mmol/L, respectively (P < 0.0001 for group I vs. group II and group II vs. III); the fasting β-OH-B concentration was 0.1 ± 0.02 mmol/L (Fig. 2). Blood pressure increased in all three groups, and heart rate changed variably (Table 2). Plasma HCO3 concentration and pH increased dose dependently in all three groups. As expected, plasma glucose concentration decreased during the length of study (3–6 h) after the overnight fast, and plasma insulin and C-peptide concentrations declined in parallel to the decrease in plasma glucose. Plasma glucagon decreased modestly in all three groups.
Figure 2.
Plasma β-OH-B concentration within each group before and after β-OH-B infusion. The mean plasma ketone level for group I was 0.7 ± 0.3 (A); for group II, 1.6 ± 0.2 (B); and for group III, 3.2 ± 0.2 mmol/L. Results shown are mean ± SEM. ****P ≤ 0.0001, paired t test; n = 12/group.
Table 2.
Hemodynamic measures and hormone concentrations before and after β-OH-B
| Group I (0.7 mmol/L) | Group II (1.6 mmol/L) | Group III (3.2 mmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | P value | Before | After | P value | Before | After | P value | |
| HR (bpm) | 68 ± 3 | 65 ± 2 | 0.04 | 68 ± 4 | 69 ± 4 | NS | 70 ± 3 | 76 ± 4 | 0.01 |
| SBP (mmHg) | 118 ± 4 | 125 ± 4 | 0.02 | 123 ± 4 | 154 ± 4 | 0.001 | 130 ± 4 | 137 ± 5 | NS |
| DBP (mmHg) | 72 ± 3 | 73 ± 2 | NS | 72 ± 2 | 78 ± 3 | NS | 74 ± 3 | 76 ± 3 | NS |
| Glucose (mg/dL) | 162 ± 22 | 124 ± 13 | 0.006 | 138 ± 13 | 108 ± 9 | 0.001 | 143 ± 16 | 114 ± 13 | <0.001 |
| Insulin (μU/mL) | 8.1 ± 0.87 | 6.2 ± 0.46 | 0.05 | 19 ± 3 | 12 ± 2 | 0.002 | 20 ± 2 | 18 ± 1 | 0.003 |
| C-peptide (ng/mL) | 6 ± 1.5 | 4 ± 0.7 | 0.05 | 5 ± 0.4 | 4 ± 0.3 | <0.001 | 6 ± 0.8 | 4 ± 0.6 | 0.01 |
| Glucagon (pg/mL) | 65 ± 10 | 48 ± 7 | 0.01 | 84 ± 11 | 65 ± 10 | 0.04 | 76 ± 10 | 61 ± 9 | NS |
| FFA (mEq/mL) | 0.43 ± 0.09 | 0.55 ± 0.06 | NS | 0.47 ± 0.06 | 0.43 ± 0.05 | NS | 0.45 ± 0.07 | 0.22 ± 0.07 | 0.01 |
| HCO3 (mEq/L) | 27 ± 1 | 30 ± 1 | <0.001 | 27 ± 1 | 33 ± 7 | <0.001 | 24 ± 2 | 32 ± 7 | <0.001 |
| pH | 7.38 ± 0.01 | 7.40 ± 0.01 | NS | 7.38 ± 0.01 | 7.43 ± 0.01 | <0.001 | 7.39 ± 0.01 | 7.46 ± 0.01 | <0.001 |
Data are reported as mean ± SEM; analysis was by paired t test.
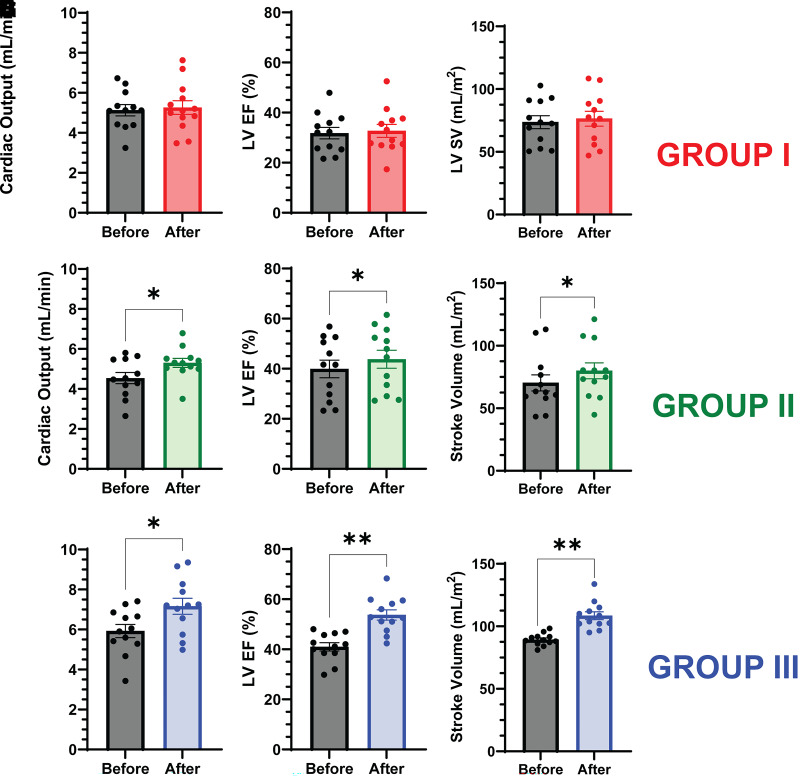
Cardiac Response to β-OH-B Infusion
The increase in plasma ketone concentration (0.7 ± 0.3 mmol/L) in group I had no effect on any parameter of LV function. However, in groups II and III, the increase in plasma ketone concentration (1.6 ± 0.2 and 3.2 ± 0.2 mmol/L, respectively) significantly increased all parameters of LV function (Fig. 3). In group II, CO (4.54 ± 0.28 to 5.30 ± 0.22; P = 0.02), EF (39.9 ± 3.5 to 43.8 ± 3.6; P = 0.02), and SV (70.3 ± 6.5 to 80.0 ± 6.4; P = 0.04) all increased. In group III, the increases in CO (5.93 ± 0.36 to 7.16 ± 0.44; P = 0.04), EF (41.1 ± 4.2 to 47.5 ± 4.9; P = 0.001), and SV (89.0 ± 5.4 to 108.4 ± 7.0; P = 0.007) were greater than in group II. Infusion of NaHCO3 in group II (the control study) had no effect on any parameters of LV function (Fig. 4).
Figure 3.
Effect of β-OH-B infusion on LV function in group I (A–C), group II (D–F), and group III (G–I). CO, LV EF, and SV were measured with cardiac MRI. Results shown are mean ± SEM. *P ≤ 0.05, **P ≤ 0.01, paired t test; n = 12/group.
Figure 4.
Effect of NaHCO3 infusion on LV function in group II (A–C). CO, LV EF, and SV were measured with cardiac MRI. Results shown are mean ± SEM; paired t test; n = 12. *P ≤ 0.05; **P ≤ 0.01; ****P ≤ 0.001.
Cardiac Metabolic Response to β-OH-B Infusion
The same 12 participants in group II also underwent cardiac PET with 18F-FDG and [15O]-H2O on two separate days with the β-OH-B and NaHCO3 infusions, respectively (Fig. 4). Compared with NaHCO3, β-OH-B infusion had no effect on MGU [9.60 ± 1.01 vs. 8.56 ± 0.74 µmol/(min × 100 g); P = 0.42] during β-OH-B and NaHCO3 infusion, respectively, or MBF (1.23 ± 0.09 vs. 1.18 ± 0.11 mL/min; P = 0.76).
Safety
No serious adverse events were observed in any of the three treatment groups.
Discussion
The major novel observation of the present study is that β-OH-B caused a dose-response increase in LV systolic function (EF, CO, and SV) in participants with T2DM whose LVEF was ≤50% without decreasing MGU. Only two previous studies, to our knowledge, have examined the effect of Na–β-OH-B infusion on myocardial function and coronary blood flow. In the study by Gormsen et al. (21), eight healthy participants without diabetes received a ketone infusion for 390 min to increase the plasma β-OH-B concentration to a mean of 3.8 mmol/L. This resulted in a 51% decline in MGU (as determined with 18FDG) without change in myocardial palmitate uptake or oxidation (11C-palmitate), whereas MBF increased by 75%. In the study by Nielsen et al. (37), 16 patients without diabetes but with HFrEF received a 3-h infusion of Na–β-OH-B, which increased the mean plasma β-OH-B level from 0.4 to 3.3 mmol/L. CO, SV, and EF significantly increased by ∼8%; coronary blood flow was not measured in this study (37). There was a dose-response increase in CO of 0.3 L/min at a threshold plasma β-OH-B concentration of 0.7 mmol/L. In general, the findings of these two studies (21,37), conducted with participants without diabetes, are consistent with the present results we report here for patients with T2DM. However, unlike the study of Gormsen et al. (21), we did not observe any decrease in MGU after β-OH-B. This is an important difference and mostly likely is explained by the different patient populations (i.e., healthy participants without diabetes with normal cardiac function in the Gormsen et al. (21) study versus individuals with T2DM with HFrEF in the present study) or by the different experimental conditions [e.g., insulin clamp in the Gormsen et al. (21) study versus overnight fast in our study]. Our results regarding improved cardiac function after β-OH-B infusion in patients with diabetes with HFrEF are similar to those of Nielsen et al. (37), who studied patients without diabetes but with HFrEF.
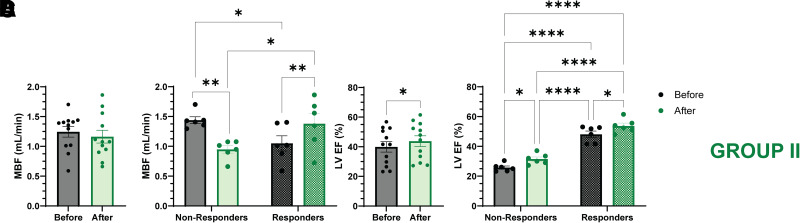
Although we did not observe any increase in mean MBF in group II patients who were studied with PET/15O-H2O, there appeared to be a bimodal response, with 6 of 12 participants showing no change in MBF (1.23 ± 0.09 vs. 1.18 ± 0.11 mL/min; P = NS) and MBF in the other six increasing (1.05 ± 0.13 to 1.38 ± 0.17; P = 0.003). With multivariate analysis including age, sex, BMI, HbA1c, eGFR, all measured myocardial functional parameters, MGU, plasma HCO3, and blood pH, only the baseline EF (31.4 ± 5.1 vs. 48.6 ± 2.6%; P < 0.05) showed any difference between the nonresponders and responders, respectively. This suggests the impact of β-OH-B infusion on MBF is dependent on the baseline EF (i.e., participants with high baseline EF experienced an increase in MBF). This observation is consistent with the β-OH-B–induced stimulation of MBF observed in participants without diabetes who have normal cardiac function (21) and is deserving of further follow up in a larger prospective study.
The present study demonstrates that elevation of the plasma β-OH-B concentration to a mean of 1.6 mmol/L significantly augments myocardial function. Because CV function is enhanced by ketone infusion, this opens the door to the development of novel therapeutic interventions for patients with diabetes, as well as those without diabetes, hospitalized with HF to improve myocardial contractility while reducing oxygen consumption. Consistent with this, in a recent study, Berg-Hansen et al. (43) demonstrated that infusion of exogenous ketone ester to increase the plasma β-OH-B concentration to 2.9 ± 0.3 mmol/L (i.e., similar to that in group III participants in our study) in 12 patients with cardiogenic shock significantly augmented CO and increased EF by 4% in association with decreased right ventricular and LV filling pressures and increased mixed venous oxygen saturation. In the associated editorial (44), Lopaschuk and Karwi postulated that the robust acute effect of ketone esters is mediated by increased myocardial ketone uptake and oxidation, leading to enhanced ATP production. This postulation is entirely consistent with the results of our present study. Furthermore, our results demonstrate that a physiologic increase (1.6 mmol/L) in plasma β-OH-B concentration does not replace glucose as a substrate for the heart, as originally suggested by Ferrannini et al. (18), but adds an additional fuel to that of glucose that can be used to generate ATP for the energy-starved failing heart.
With regard to the beneficial effects of SGLT2i on cardiovascular function in patients with HF and with or without diabetes (14–17), multiple mechanisms have been proposed, of which one is the “ketone hypothesis” (18). Although our findings are consistent with this hypothesis, studies that examined the relationship between changes in plasma ketone concentration, myocardial substrate utilization, cardiac function, and MBF after treatment with SGLT2i in humans have yielded conflicting results (45–47). In the dapagliflozin heart failure trial (DAPAHEART) (45), eight patients with T2DM without HF were treated with dapagliflozin for 4 weeks. No change in MGU (as determined by PET) was observed, whereas MBF (corrected for cardiac workload) at rest declined; plasma ketone levels were not reported. Similarly, in 25 patients with T2DM and without HF who were treated with dapagliflozin for 6 weeks, there was no change in CO, EF, or myocardial FFA uptake; however, no increase in plasma ketone concentration was observed in this study (46). In a study by Lauritsen et al. (47), 13 patients with T2DM and without HF were treated with empagliflozin for 4 weeks. Plasma β-OH-B levels increased from a mean of 49 to 92 umol/L, MGU decreased by 57%, and myocardial FFA uptake remained unchanged; neither EF nor MBF changed significantly. Unfortunately, none of these studies included patients with diabetes with HF, the duration of SGLT2i treatment was short (<6 weeks), myocardial substrate utilization was not systematically examined, and, surprisingly, no increase in plasma ketone level was observed in several studies. Therefore, the impact of SGLT2i-induced hyperketonemia on myocardial function and substrate utilization in patients with T2DM with HFrEF remains unresolved and awaits a long-term, prospective, placebo-controlled study with MRI and PET/18F-FDG and 11C-β-OH-B.
In summary, the present study demonstrates, for the first time, to our knowledge, that in patients with T2DM with HF and reduced EF, acute physiologic increase in plasma β-OH-B concentration with exogenous β-OH-B increases CO, EF, and SV.
This article contains supplementary material online at https://doi.org/10.2337/figshare.27244935.
Article Information
Acknowledgments. We appreciate the participation of the research participants, who made this study possible.
Funding. This research was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (grant R01DK24092 to R.A.D.), the Max and Minnie Tomerlin Voelcker Fund (grant to C.S.-H.), and Merck & Co.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. C.S.-H. receives grant support from AstraZeneca; is on the advisory board of Novo Nordisk, Bayer, and Mankind; and is a member of the speakers’ bureau for Novo Nordisk. R.A.D. receives grant support from AstraZeneca, Merck, 89 Bio, and CORCEPT; is a member of the advisory boards of AstraZeneca, Intarcia, CORCEPT, and Novo Nordisk; and is a member of the speakers’ bureau of AstraZeneca and CORCEPT. E.C. receives grant support from AstraZeneca and Janssen Pharmaceuticals; is a member of the advisory boards of VeroScience, the Boehringer Ingelheim and Lilly Diabetes Alliance, and Sanofi; and is a member of the speakers’ bureaus of AstraZeneca, Janssen Pharmaceuticals, and the Boehringer Ingelheim and Lilly Diabetes Alliance. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.A.D. designed the study. C.S.-H., Y.Q., H.H., E.C., and G.C. contributed to conducting the study. H.H., F.M.A., A.J.M., S.N., J.R., P.I., P.F., and G.C. were involved in analysis of the data. C.S.-H. wrote the first draft of the manuscript, which was revised by R.A.D. and subsequently reviewed by all authors. R.A.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement
This research was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (grant R01DK24092 to R.A.D.), the Max and Minnie Tomerlin Voelcker Fund (grant to C.S.-H.), and Merck & Co.
Footnotes
Clinical trial reg. no. NCT03560323, clinicaltrials.gov
References
- 1. Di Angelantonio E, Kaptoge S, Wormser D, et al.; Emerging Risk Factors Coalition . Association of cardiometabolic multimorbidity with mortality. JAMA 2015;314:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Virani SS, Callaway CW, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018;137:e67–e492 [DOI] [PubMed] [Google Scholar]
- 3. Evans M, Cogan KE, Egan B.. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol 2017;595:2857–2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB.. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 2004;27:1879–1884 [DOI] [PubMed] [Google Scholar]
- 5. Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis: a community perspective. J Am Coll Cardiol 2009;54:1695–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lawson CA, Jones PW, Teece L, et al. Association between type 2 diabetes and all-cause hospitalization and mortality in the UK general heart failure population: stratification by diabetic glycemic control and medication intensification. JACC Heart Fail 2018;6:18–26 [DOI] [PubMed] [Google Scholar]
- 7. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [published correction appears in Lancet 1998;352:1558]. Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 8. Ferrannini E, DeFronzo RA.. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J 2015;36:2288–2296 [DOI] [PubMed] [Google Scholar]
- 9. McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021;6:148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arnott C, Li Q, Kang A, et al. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc 2020;9:e014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McMurray JJV, Solomon SD, Inzucchi SE, et al.; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008 [DOI] [PubMed] [Google Scholar]
- 12. Packer M, Anker SD, Butler J, et al.; EMPEROR-Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424 [DOI] [PubMed] [Google Scholar]
- 13. Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA.. SGLT2 inhibitors and cardiovascular risk lessons learned from the EMPA-REG OUTCOME study. Diabetes Care 2016;39:717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verma S, McMurray JJV.. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 2018;61:2108–2117 [DOI] [PubMed] [Google Scholar]
- 15. Kaplan A, Abidi E, El-Yazbi A, Eid A, Booz GW, Zouein FA.. Direct cardiovascular impact of SGLT2 inhibitors: mechanisms and effects. Heart Fail Rev 2018;23:419–437 [DOI] [PubMed] [Google Scholar]
- 16. Abdul-Ghani M, DeFronzo RA, Del Prato S, Chilton R, Singh R, Ryder REJ.. Cardiovascular disease and type 2 diabetes: has the dawn of a new era arrived? [published correction appears in Diabetes Care 2017;40:1606]. Diabetes Care 2017;40:813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeFronzo RA, Norton L, Abdul-Ghani M.. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 2017;13:11–26 [DOI] [PubMed] [Google Scholar]
- 18. Ferrannini E, Mark M, Mayoux E.. CV Protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care 2016;39:1108–1114 [DOI] [PubMed] [Google Scholar]
- 19. Honka H, Solis-Herrera C, Triplitt C, Norton L, Butler J, DeFronzo RA.. Therapeutic manipulation of myocardial metabolism: JACC state-of-the-art review. J Am Coll Cardiol 2021;77:2022–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mikkelsen KH, Seifert T, Secher NH, Grøndal T, van Hall G.. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-d-β-hydroxybutyratemia in post-absorptive healthy males. J Clin Endocrinol Metab 2015;100:636–643 [DOI] [PubMed] [Google Scholar]
- 21. Gormsen LC, Svart M, Thomsen HH, et al. Ketone body infusion with 3-hydroxybutyrate reduces myocardial glucose uptake and increases blood flow in humans: a positron emission tomography study. J Am Heart Assoc 2017;6:e005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stanley WC, Meadows SR, Kivilo KM, Roth BA, Lopaschuk GD.. beta-Hydroxybutyrate inhibits myocardial fatty acid oxidation in vivo independent of changes in malonyl-CoA content. Am J Physiol Heart Circ Physiol 2003;285:H1626–31 [DOI] [PubMed] [Google Scholar]
- 23. Sato K, Kashiwaya Y, Keon CA, et al. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J 1995;9:651–658 [DOI] [PubMed] [Google Scholar]
- 24. Lopaschuk GD, Dyck JRB.. Ketones and the cardiovascular system. Nature Cardio Res 2023;2:425–437 [DOI] [PubMed] [Google Scholar]
- 25. Ho KL, Zhang L, Wagg C, et al. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc Res 2019;115:1606–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC.. Myocardial fatty acid metabolism in health and disease. Physiol Rev 2010;90:207–258 [DOI] [PubMed] [Google Scholar]
- 27. Aubert G, Martin OJ, Horton JL, et al. The failing heart relies on ketone bodies as a fuel. Circulation 2016;133:698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horton JL, Davidson MT, Kurishima C, et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019;4:e124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Inagaki N, Goda M, Yokota S, Maruyama N, Iijima H.. Safety and efficacy of canagliflozin in Japanese patients with type 2 diabetes mellitus: post hoc subgroup analyses according to body mass index in a 52-week open-label study. Expert Opin Pharmacother 2015;16:1577–1591 [DOI] [PubMed] [Google Scholar]
- 32. Murashige D, Jang C, Neinast M, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 2020;370:364–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Voros G, Ector J, Garweg C, et al. Increased cardiac uptake of ketone bodies and free fatty acids in human heart failure and hypertrophic left ventricular remodeling. Circ Heart Fail 2018;11:e004953. [DOI] [PubMed] [Google Scholar]
- 34. Janardhan A, Chen J, Crawford PA.. Altered systemic ketone body metabolism in advanced heart failure. Tex Heart Inst J 2011;38:533–538 [PMC free article] [PubMed] [Google Scholar]
- 35. Kostis JB, Sanders M.. The association of heart failure with insulin resistance and the development of type 2 diabetes. Am J Hypertens 2005;18:731–737 [DOI] [PubMed] [Google Scholar]
- 36. Bratusch-Marrain PR, DeFronzo RA.. Failure of hyperketonemia to alter basal and insulin-mediated glucose metabolism in man. Horm Metab Res 1986;18:185–189 [DOI] [PubMed] [Google Scholar]
- 37. Nielsen R, Møller N, Gormsen LC, et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation 2019;139:2129–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clarke GD, Solis-Herrera C, Molina-Wilkins M, et al. Pioglitazone improves left ventricular diastolic function in subjects with diabetes. Diabetes Care 2017;40:1530–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hartiala JJ, Mostbeck GH, Foster E, et al. Velocity-encoded cine MRI in the evaluation of left ventricular diastolic function: measurement of mitral valve and pulmonary vein flow velocities and flow volume across the mitral valve. Am Heart J 1993;125:1054–1066 [DOI] [PubMed] [Google Scholar]
- 40. Tomiyama H, Nishikawa E, Watanabe G, et al. Hormonal and cardiorenal responses to acute saline loading in mild congestive heart failure–the effect of angiotensin converting enzyme inhibition. Jpn Circ J 1998;62:29–35 [DOI] [PubMed] [Google Scholar]
- 41. Gastaldelli A, Gaggini M, Daniele G, et al. Exenatide improves both hepatic and adipose tissue insulin resistance: a dynamic positron emission tomography study. Hepatology 2016;64:2028–2037 [DOI] [PubMed] [Google Scholar]
- 42. Qin Y, Adams J, Solis-Herrera C, Triplitt C, DeFronzo R, Cersosimo E.. Clinical parameters, fuel oxidation, and glucose kinetics in patients with type 2 diabetes treated with dapagliflozin plus saxagliptin. Diabetes Care 2020;43:2519–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berg-Hansen K, Christensen KH, Gopalasingam N, et al. Beneficial effects of ketone ester in patients with cardiogenic shock: a randomized, controlled, double-blind trial. JACC Heart Fail 2023;11:1337–1347 [DOI] [PubMed] [Google Scholar]
- 44. Lopaschuk GD, Karwi QG.. Jump starting the heart: ketone esters improve cardiac function in patients with cardiogenic shock. JACC Heart Fail 2023;11:1348–1350 [DOI] [PubMed] [Google Scholar]
- 45. Leccisotti L, Cinti F, Sorice GP, et al. Dapagliflozin improves myocardial flow reserve in patients with type 2 diabetes: the DAPAHEART Trial: a preliminary report. Cardiovasc Diabetol 2022;21:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oldgren J, Laurila S, Åkerblom A, et al. Effects of 6 weeks of treatment with dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on myocardial function and metabolism in patients with type 2 diabetes: a randomized, placebo-controlled, exploratory study. Diabetes Obes Metab 2021;23:1505–1517 [DOI] [PubMed] [Google Scholar]
- 47. Lauritsen KM, Nielsen BRR, Tolbod LP, et al. SGLT2 inhibition does not affect myocardial fatty acid oxidation or uptake, but reduces myocardial glucose uptake and blood flow in individuals with type 2 diabetes: a randomized double-blind, placebo-controlled crossover trial. Diabetes 2021;70:800–808 [DOI] [PubMed] [Google Scholar]