Highlights
-
•
The global control of SHS exposure is far from optimal, as the downward trend of SHS exposure has slowed or even reversed in recent years.
-
•
The SHS exposure and disease burden of CMDs caused by SHS is especially severe in underdeveloped areas.
-
•
Among females aged 25–29 years old, SHS exposure accounts for 16.12 % and 13.30 % of IHD- and T2DM-related deaths, respectively, in 2019.
-
•
The number of deaths caused by CMDs related to SHS exposure is expected to rise globally in the next 20 years.
Keywords: Second-hand smoke, Global disease burden, Cardiometabolic disease, Bayesian age-period-cohort
Abstract
Objective
Secondhand smoke (SHS) is a strong but comparatively controllable cardiometabolic risk factor. This study aims to assess the present and future burden of cardiometabolic diseases (CMDs) from SHS exposure.
Methods
Using the Global Burden of Disease (GBD) framework, we examined mortality and disability-adjusted life year (DALY) from CMDs attributable to SHS, by age, sex, and year, including cardiovascular disease [CVD, ischemic heart disease (IHD) and/or stroke], and/or Type 2 Diabetes Mellitus (T2DM) from 1990 to 2019. The predicted death number and age-standardized mortality rate (ASMR) from 2020 to 2040 were estimated by the Bayesian age-period cohort (BAPC) model.
Results
SHS exposure declined until 2016 but stabilized or increased thereafter. From 1990 to 2019, CMD-related deaths and DALYs due to SHS are continuously increasing, particularly in low-middle and middle Sociodemographic Index (SDI) regions. In 2019, a significant proportion of CMD-related deaths and DALYs among females under 65 were attributed to SHS exposure. In females aged 25–29, SHS contributed to 16.12 % and 13.30 % of IHD and T2DM deaths, respectively. Surprisingly, forecasts show that annual deaths from IHD, stroke, and T2DM related to SHS exposure are anticipated to rise over the next 20 years.
Conclusions
SHS exposure has stopped declining in recent years. CMD-related deaths from controlled SHS have increased and are predicted to rise substantially over the next 20 years. Reducing SHS exposure could have major benefits for cardiometabolic health worldwide, especially for women under 65 years in less developed regions.
Non-standard abbreviations and acronyms
ASDR age-standardized disability-adjusted life year
ASMR age-standardized mortality rate
ASR Age-Standardized Rate
BAPC Bayesian Age-Period-Cohort
DALY disability-adjusted life year
EAPC estimated annual percentage change
IHD heart disease
SDI Sociodemographic Index
SHS Secondhand smoke
ST-GPR spatiotemporal Gaussian process regression
TMRELs theoretical minimum risk exposure levels
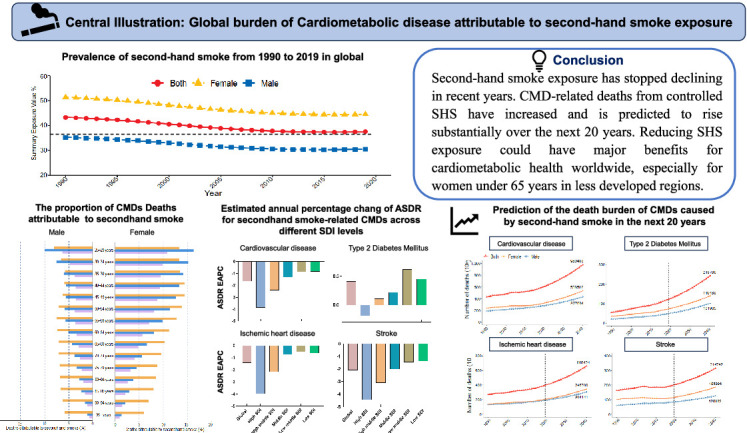
Central IIIustration: Global burden of Cardiometabolic disease attributable to second-hand smoke exposure
1. Introduction
Cardiometabolic diseases (CMDs), encompassing type 2 diabetes (T2D), heart disease, and stroke, collectively represent a major global cause of morbidity and mortality due to shared risk factor profiles [1]. Identifying risk factors to mitigate CMD incidence and improving patient prognosis remain imperative.
Secondhand smoke (SHS) contributes substantially to the disease burden, causing an estimated 1.3 million deaths and 37.0 million disability-adjusted life years (DALYs) in 2019 [2]. SHS exposes individuals to higher concentrations of certain harmful compounds compared to mainstream smoke with epidemiological studies showing that SHS exposure elevates cardiovascular disease (CVD) risk by 30 % [3] and correlates significantly with poor glycemic control in household settings [4]. Passive smoking often has nearly as large an impact as chronic active smoking [5].
Given these risks, the World Health Organization (WHO) has unequivocally established the health risks associated with SHS and advocated for the global implementation of smoke-free environments to protect individuals from SHS exposure [6]. Behavioral modifications in personal lifestyle factors, such as smoking cessation, dietary improvements, and increased physical activity, often face challenges [7,8]. In contrast, reducing SHS exposure through public policies, such as smoke-free regulations, presents a more feasible approach to managing CMD risk.
Despite this, limited research has examined global trends in CMD burden attributable to SHS across diverse socioeconomic contexts and healthcare resources. While prior analyses of the global burden of disease (GBD) have reported SHS-related stroke burden, they have not comprehensively assessed the full scope of the CMD burden attributable to SHS [9]. This study aims to quantify SHS prevalence and CMD burden across 204 countries, stratified by economic status, and to project the CMD burden due to SHS over the next 20 years. This analysis offers critical insights for public health policy, quantifying the cardiometabolic benefits of previous actions, informing future smoke-free initiatives, and reinforcing global advocacy efforts to protect public health.
2. Methods
2.1. Study data
A repository of global health and population data, the GHDx included census data, surveys, registries, indicators, estimates, and administrative health data. The Global Burden of Disease 2019 project estimated the incidence, age-standardized mortality rate (ASMR), and age-standardized disability-adjusted life year (ASDR) associated with 369 diseases and injuries for the SDI in 204 countries and regions from 1990 to 2019. The GHDx data are openly available and have been used in numerous studies [10].
Mortality data were sourced from vital registration records, classified according to the International Classification of Diseases (ICD) system, or from household mortality surveys, commonly referred to as verbal autopsies. For each health state, DALYs were calculated by adding years of life lost (YLLs), calculated using the maximum observed life expectancy with years lived with disability (YLDs), and determined using standardized disability weights. The 95 % uncertainty intervals (UIs) for each estimate were derived by generating 1000 draws from the posterior distribution, with the 2.5th and 97.5th percentiles representing the intervals. Age standardization was performed using the direct method, applying a global age structure of 2019 [11].
2.2. Definition of secondhand smoke exposure
SHS exposure was defined as current exposure to tobacco smoke in home, work, or public places [2]. Household composition served as a proxy for non-occupational SHS exposure, assuming that all individuals living with a daily smoker are exposed to tobacco smoke. Surveys were used to estimate the proportion of individuals exposed to SHS in the workplace. Only non-smokers were considered exposed to SHS, with non-smokers defined as individuals who do not smoke daily. Ex-smokers and occasional smokers were categorized as non-smokers for this analysis. SHS exposure was measured through self-report, with data sourced from major representative survey series containing household composition modules, including the Demographic Health Surveys (DHS), Multiple Indicator Cluster Surveys (MICS), and Living Standards Measurement Surveys (LSMS), as well as national and subnational censuses, including those from the IPUMS project, all identified using the Global Health Data Exchange catalog (GHDx). Studies have demonstrated a strong correlation between self-reported SHS exposure and cotinine measurements [12].
Relative risks for each disease endpoint associated with SHS exposure were obtained from recent dose-response meta-analyses of prospective observational studies, and, where available, randomized controlled trials. To estimate the theoretical minimum risk exposure levels (TMRELs) for SHS, the exposure levels corresponding to the lowest risk of mortality for each disease endpoint were calculated based on the studies included in the meta-analyses of SHS-related relative risks. The TMREL was then computed as a weighted average, utilizing global death data for each disease outcome. SHS exposure was modeled within the GBD framework using a spatiotemporal Gaussian process regression (ST-GPR) approach, which integrates hierarchical linear mixed models with weighted residuals to estimate SHS exposure by space, time, and age [13,14].
2.3. Estimation of the disease burden of cardiometabolic diseases in the global burden of disease study 2019
CMDs encompass a range of conditions, including cardiovascular disease (CVD), Type 2 Diabetes Mellitus (T2DM), ischemic heart disease (IHD), and stroke. The GBD study collects data on SHS exposure and its association with CVD, including IHD and stroke. IHD is defined to include acute myocardial infarction, chronic stable angina, chronic IHD, and heart failure resulting from IHD, using standard case definitions. Stroke fatalities were classified according to WHO clinical criteria, which described it as: "a clinical manifestation (typically focal) of rapidly progressing brain dysfunction persisting for >24 h or resulting in death."
T2DM was defined in the GBD Study 2019 as a fasting plasma glucose concentration ≥126 mg/dL (7mmol/l) or as a reported treatment for diabetes in the Global Burden of Disease Study 2019. Data sourced from a systematic review of published literature, surveys, longitudinal studies, and other relevant sources were incorporated into DisMod MR-2.1, a Bayesian meta-regression model, to estimate the nonfatal burden of diabetes comprehensively. This model encompasses various types of diabetes [10].
To calculate the burden of CMDs caused by SHS, GBD estimated the population attributable fractions (PAFs) for these outcomes by comparing the risk of this distribution with the expected risk at the theoretical lowest risk exposure level, based on continuous risk-outcome curves derived using a nonparametric Bayesian spline approach, according to the provided SHS exposure values. We calculated the number of cause-specific deaths attributable to each risk factor by multiplying the estimated PAFs by the total number of deaths from that disease in each stratum [15].
2.4. Statistical analysis
Absolute numbers and age-standardized rates (ASRs) per 100,000 persons with 95 % Uis, were used to quantify the burden and mortality associated with SHS-related CMDs. To facilitate temporal and regional comparisons, age-standardized mortality rates (ASMR) and age-standardized disability rates (ASDR) were also employed. Trends in ASMR and ASDR were assessed using the estimated annual percentage change (EAPC). The natural logarithm of the Age-Standardized Rate (ASR) was modeled using the regression equation: ln (ASR) = α + βx + ɛ, where x represents the calendar year. The EAPC and its 95 % confidence Intervals (CIs) were derived from the regression model: y = 100 × (exp (β) - 1), where y denotes the EAPC [16]. The proportional contribution of SHS exposure (PAF) was calculated by dividing the attributable deaths and DALYs for this factor by the total deaths and DALYs for each disease. Additionally, the correlation between ASDR and Sociodemographic Index (SDI) by region was assessed using the Spearman correlation coefficient, with statistical significance defined as a two-sided p-value < 0.05 [17].
The sample was spatially stratified using three GBD splitting methods. The first method categorized countries and territories into five superregions based on the SDI: high SDI, high-middle SDI, middle SDI, and low SDI [18]. The SDI serves as a comprehensive indicator of each country's or region's level of social development. The second method divides the world into 21 geographic regions based on epidemiological similarity and geographic proximity. The third method separates countries and territories into 204 distinct entities. Additionally, the sample population aged 25 to 95+ was stratified into 5-year age groups, resulting in a total of 15 age groups.
This study applied the Bayesian Age-Period-Cohort (BAPC) method to predict disease burden trends. By applying Bayesian formulas based on age, period, and cohort factors, it derived probability distributions [19,20]. Compared to methods reliant solely on sample statistics, BAPC provides greater flexibility in parameter selection and prior probability distributions, thereby yielding more robust predictions. Forecasted deaths and ASMR from CMDs over the next 20 years were generated using R program version 4.1.3, for statistical analysis.
3. Results
3.1. The age-standardized prevalence of SHS in global
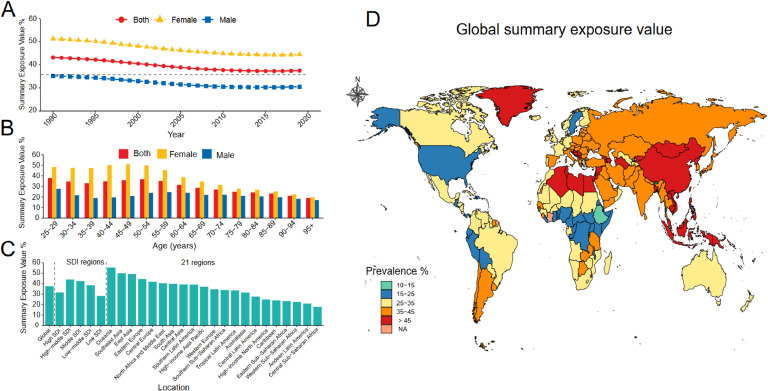
The prevalence of SHS exposure has exhibited a declining trend since 1990; however, this decline plateaued after 2010 and began to reverse starting in 2016 (Fig. 1A). Compared to males, females have consistently constituted the majority of those exposed to SHS over the past three decades, with SHS prevalence among females nearly twice as high as that of males for individuals under 65 years of age (Fig. 1B). In 2019, over one-third of the global population was affected by SHS exposure, with an overall age-standardized prevalence of 37.51 % (95 % UI: 37.00–38.09), including 30.50 % for males and 44.53 % for females (Table S1). Regions with a middle SDI exhibited the highest SHS prevalence, surpassing areas with either high or low SDI. Specifically, Oceania had the highest SHS prevalence at 55.40 % (95 % UI: 53.37–57.47), followed by Southeast Asia at 49.95 % (95 % UI: 49.17–50.70) (Fig. 1C). The prevalence of SHS in specific countries is shown in Fig. 1D.
Fig. 1.
The prevalence of secondhand smoke was grouped by sex, age, region, and country.
A. The prevalence of secondhand smoke was grouped by sex.
B. The prevalence of secondhand smoke was grouped by age.
C. The prevalence of secondhand smoke was grouped by region.
D. The prevalence of secondhand smoke was grouped by country.
3.2. Global trend in cardiometabolic disease burden attributable to secondhand smoke in 2019 and trend analysis, 1990–2019
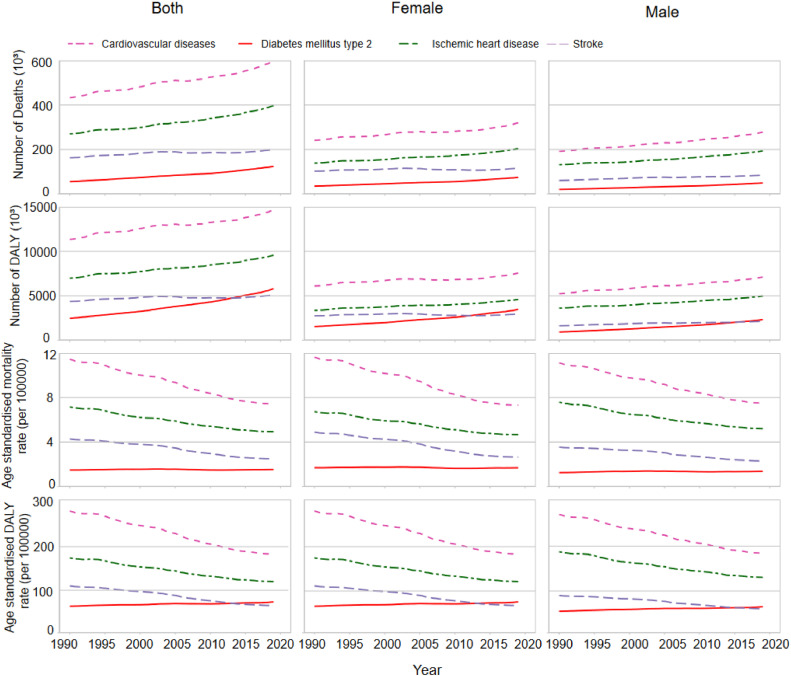
The global disease burden of CMDs attributable to SHS from 1990 to 2019 is illustrated in Fig. 2. Overall, the annual burden of CMD-related deaths and DALYs attributable to SHS has increased over time. CVD-related annual deaths due to SHS rose from 432,558 (357,386 to 508,294) in 1990 to 598,475 (489,749 to 713,482) in 2019, representing a 38.4 %. DALYs increased from 13,274,636 (9286,654 to 13,274,636) in 1990 to 14,657,667 (12,020,285 to 17,496,914) in 2019, a 29.2 % increase. IHD-related deaths increased by 47.2 %, and DALYs rose by 37.2 %. Stroke-related deaths increased by 23.6 %, while DALYs increased by 16.6 % (Table S2, Table S3, Table S4).
Fig. 2.
The burden of Cardiometabolic diseases caused by secondhand smoke grouped by sex from 1990 to 2019.
DALY, disability-adjusted life year.
Notably, T2DM-related deaths and DALYs attributed to SHS increased by 122.58 % and 135.5 %, respectively. Global T2DM-related deaths increased from 55,512 in 1990 to 123,455 in 2019, while DALY increased from 2461,899 in 1990 to 5802,501 in 2019 (Table S5). The ASMR and ASDR for CVD decreased between 1990 and 2019, with an EAPC of −1.63 % (−1.69 % to −1.57 %) and −1.67 % (−1.73 % to −1.60 %), respectively. Similar downward trend was observed for IHD and stroke caused by SHS. However, a slight increase was observed in ASDR for T2DM was observed over the past three decades (EAPC, 0.41 %; 95 % CI, 0.37 % - 0.45 %) (Fig. 3). Additionally, the burden of CMDs attributable to SHS is higher in females than in males (Fig. 1).
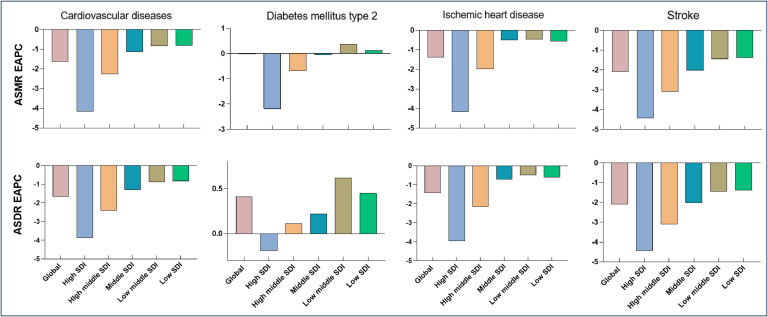
Fig. 3.
Estimated Annual Percentage Change in different SDI regions.
ASMR, age-standardized mortality rate. ASDR, age-standardized disability-adjusted life-year rate. SDI, Social Development Index EAPC, estimated annual percentage change.
3.3. The burden of CMD is attributable to SHS exposure by SDI region, 21 regions, and country
A Pearson correlation analysis revealed a significant negative correlation between the SDI and the ASDR for CMDs attributable to SHS (Figure S1). With the exception of high SDI regions, both CMD-related deaths and DALYs attributable to SHS have demonstrated an upward trend in other regions, with the most pronounced increase observed in the middle SDI region. In 2019, the low-middle and middle SDI regions exhibited the highest ASMR and ASDR for CMDs attributable to SHS in 2019 (Figure S2, Figure S3).
Among the 21 GBD regions, Central Asia, Oceania, and North Africa had the highest ASMR and ASDR for CVD due to SHS in 2019. Between 1990 and 2019, Oceania exhibited the sharpest upward trend in SHS-related CVD, with an increase of 0.18 % (0.1 % to 0.26 %) in ASMR and 0.23 % (0.14 % to 0.32 %) in ASDR (Table S2). Similar trends were observed for IHD and stroke caused by SHS (Table S4, Table S5). For T2DM attributable to SHS, Oceania, Southern Sub-Saharan Africa, and Southeast Asia reported the highest ASMR and ASDR in 2019. From 1990 to 2019, Central Asia showed the largest upward trend in SHS-related T2DM, with an increase of 2.56 % (2.21 % to 2.92 %) in ASMR and 2.26 % (2.05 % to 2.48 %) in ASDR (Table S5).
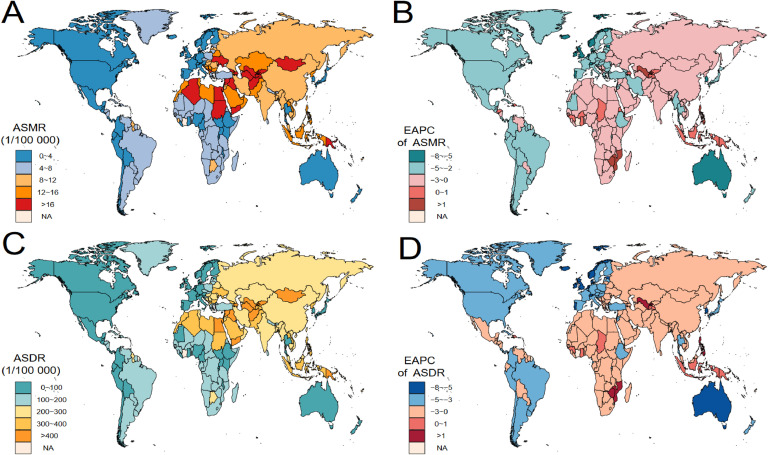
The burden of CMDs caused by SHS varies widely across countries. An upward trend in the ASMR of CVD was observed in 22 countries over the past 30 years, the upward trends in the ASDR of CVD were observed in 15 countries, with the strongest trends observed in the Netherlands, Bosnia, Herzegovina, and the Netherlands, Bosnia (Fig. 4 and Table S2). An upward trend in the ASMR of T2DM attributable to SHS was observed in 95 countries over time, the upward trends in the ASDR of T2DM were observed in 125 countries, with the strongest trends observed in Rwanda, Venezuela (the Bolivarian Republic of), Zambia, Lesotho and Uzbekistan (Figure S4 and Table S5).
Fig. 4.
Spatial distribution of cardiovascular disease burden attributable to secondhand smoke in 204 Countries and Territories.
ASMR, age-standardized mortality rate. ASDR, age-standardized disability-adjusted life-year rate. EAPC, estimated annual percentage change
A. The global distribution of ASMRs of cardiovascular diseases attributable to secondhand smoke in 2019.
B. The global distribution of ASDRs of cardiovascular diseases attributable to secondhand smoke in 2019.
C. EAPCs in the ASMR of cardiovascular diseases attributable to secondhand smoke by country from 1990 to 2019.
D. EAPCs in the ASDR of cardiovascular diseases attributable to secondhand smoke by country from 1990 to 2019.
3.4. Differences between gender and age groups
For CVD, the ASMR due to SHS increased with increasing age, except for deaths in the 84–95-year age group (Figure S5). The ASMR for SHS-related CVD was higher in females than males for age groups older than 85 years, whereas it was lower in females than in males for age groups ≤85 years. The ASDR for CVD is higher in females over 75 years compared to males. A similar trend was observed for IHD, which followed the same pattern as CVD. For SHS-related T2DM, both ASMR and ASDR increased with age, with the peak ASDR occurring in the 80–84 year age range for females and the 85–89 year age range for males. However, for individuals over 85 years, the ASMR and ASDR of SHS-related T2DM were lower in females compared to males.
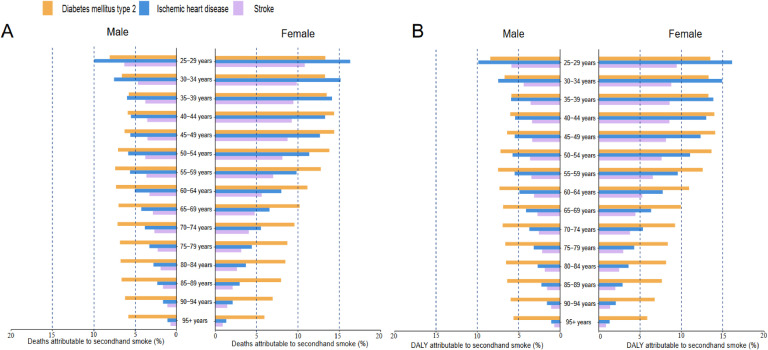
Notable differences were observed in the proportions of deaths and DALYs attributable to SHS across sex and age subgroups. In 2019, the burden of CMDs caused by SHS had a more significant impact on individuals under 65 years of age, particularly females. The proportion of deaths due to SHS attributable to T2DM IHD, and stroke exceeded 10 % among females aged 25–29 years old (Fig. 5A). Specifically, the PAFs for IHD and T2DM were 16.12 % and 13.30 %, respectively. A similar trend was observed for DALYs (Fig. 5B). In economically developed regions such as Western Europe, Australia, and high-income North America, the proportion of CMDs attributed to SHS was lower in females. Conversely, in regions with uneven economic development, such as Central Asia, Southeast Asia, and Oceania, the burden of CMDs due to SHS was higher in females (Figure S6).
Fig. 5.
The proportion of Cardiometabolic diseases Deaths and DALYs attributable to secondhand smoke by sex and age in 2019.
DALY, disability-adjusted life year.
3.5. The predicted burden of cardiometabolic diseases attributable to SHS, 2020–2040
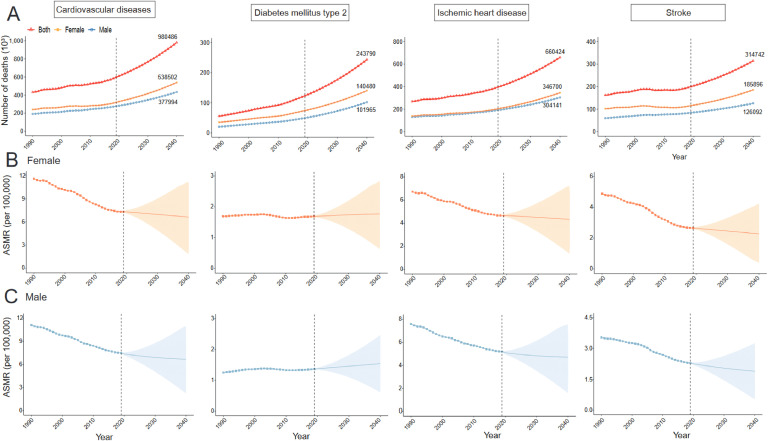
Prediction analysis using the BAPC model indicated a global increase in the number of CMD deaths attributable to SHS from 2020 to 2040 (Fig. 6A). Specifically, the number of CVD-related deaths attributable to SHS, which rose from 432,558 in 1990 to 598,475 in 2019, is expected to increase to 980,487 in 2040, marking a 63.8 % increase over the next two decades. The number of IHD-related deaths is projected to rise from 269,968 in 2019 to 397,420 in 2019 and is anticipated further increase to 660,424 in 2040, an increase of 66.2 %. The number of stroke deaths attributable to SHS increased from 162,590 in 1990 to 201,055 in 2019 and is expected to further rise to 314,742 in 2040, representing a 56.5 % increase. The number of T2DM-related deaths due to SHS is anticipated to increase significantly over the next 20 years, from 55,511 in 1990 to 123,455 in 2019, with an anticipated rise to 298,999 in 2040, reflecting a 142.2 % increase. Projections also suggest that the ASMR for CVD due to SHS will continue to decline from 2020 to 2040, including for IHD and stroke. However, the ASMR for SHS-related T2DM is anticipated to continue increasing over the next two decades (Fig. 6B and C).
Fig. 6.
Prediction of cardiometabolic disease deaths burden from 2020 to 2040 caused by secondhand smoke global.
ASMR: age-standardized mortality rate
A. Prediction of CMD deaths caused by secondhand smoke by sex in global.
B. Prediction of CMDs ASMR caused by secondhand smoke in females.
C. Prediction of CMDs ASMR caused by secondhand smoke in males.
4. Discussion
This study highlights the suboptimal global control of SHS exposure. Although the global prevalence of SHS has declined over recent decades, this downward trend has decelerated or even reversed in recent years. The burden of CMDs, including associated deaths and DALYs attributable to SHS, is predicted to continue rising, despite reductions in ASMR and ASDR driven by increased life expectancy. Significant disparities exist in both SHS exposure and the associated CMD burden across different age groups, sexes, and regions. CMD-related deaths and DALY attributable to SHS are particularly pronounced among women under 65 years old. The burden of SHS and related CMDs is disproportionately high in less developed regions, such as Central Asia, North Africa, Southeast Asia, and Oceania. Our forecast research shows that the number of CMD-related deaths attributable to SHS is anticipated to rise over the next two decades.
In contrast to managing other traditional cardiometabolic risk factors, controlling SHS exposure presents a relatively more feasible approach. While public education can enhance awareness of cardiometabolic risk factors, adherence to recommended changes, such as quitting active smoking, improving dietary habits, and increasing physical activity, remains inadequate in practice [7,21]. To our knowledge, this study represents the first comprehensive investigation into global trends and their contribution to the CMD burden, both past and future. These findings offer an up-to-date reference for informing global health policies and investments aimed at reducing SHS exposure and improving cardiometabolic health.
Previous studies have demonstrated that smoking prevalence is lower among females than in males globally [22], yet the prevalence of SHS exposure is significantly higher in females than in males, which accounts for the greater burden of SHS-related CMDs among women in our analysis. Physiological, women differ from men in cardiovascular and metabolic characteristics [23] and their more fluctuating hormone levels may render them more susceptible to the harmful effects of SHS [24]. The proportion of CMD-related deaths attributable to SHS is higher in females than in males in less economically developed regions. However, it is lower in economically developed regions. In contrast, this proportion is lower in economically developed regions, potentially due to gender inequalities in lower-income countries, where women face greater challenges in accessing healthcare, health education, and preventive interventions.
Our study demonstrates that the prevalence of SHS and the associated CMD burden is higher among young people. Previous research has also shown that SHS is more harmful to children and young people [2,25]. This may be due to their frequent exposure to SHS in social and occupational settings. This highlights the urgent need for policymakers and government agencies to enhance the enforcement of smoking bans in public places. While secondhand e-cigarette use has not been considered a confounding factor in the current GBD analysis, it represents a critical issue. Studies have shown that harmful substances, such as aerosols from e-cigarettes, can also contribute to cardiometabolic dysfunction [26,27]. The use of e-cigarettes has increased exponentially over the past decade, especially among young people [28]. Existing smoke-free policies do not address e-cigarettes, and the potential health risks associated with their use remain inadequately evaluated [29]. Future public health policies should take into account the harm caused by secondhand e-cigarette exposure and incorporate appropriate regulatory measures.
Consistent with previous research [30], our correlation analysis results reveal a negative relationship between the SDI and the burden of CMD burden due to SHS over the past three decades. High-income countries have made significant progress in reducing tobacco use and its related disease burden, largely due to their effective regulatory frameworks [31,32]. However, tobacco control policies in developing countries face considerable challenges, as tobacco revenues are a key economic driver [33]. The CMD burden in low-middle and middle SDI regions warrants greater attention. Our findings suggest that the disease burden of CMDs attributable to SHS is inversely correlated with the SDI, but the highest burden is not observed in the lowest-SDI areas. This discrepancy can be attributed to two factors. First, underreporting in less developed regions due to limited disease surveillance networks may lead to an underestimation of SHS-related diseases [10,34]. For instance, a study found that 59.7 % of diabetics in Africa remain undiagnosed [35]. Second, previous studies have shown that the introduction of smoke-free policies, such as raising tobacco taxes and prices, has effectively reduced tobacco consumption in low-SDI areas [36].
Our findings indicate that regions such as Oceania, Central Asia, Southeast Asia, South Africa, and North Africa exhibit relatively high SHS prevalence and associated CMD burden. Previous studies have highlighted that Southeast Asia and South Africa are major tobacco producers and consumers, with approximately 250 million smokers and a substantial number of smokeless tobacco users [37], which likely contributes to the significant SHS-related CMD burden. Consistent with earlier research, Oceania shows the highest mortality and DALY rates linked to SHS exposure [38]. Specifically, some Pacific Island countries (e.g., Fiji, Kiribati, Nauru, and the Solomon Islands) also bear a heavy burden of SHS-related CMDs. In these lower SDI-ranking nations, weak SHS protection policies are also a contributing factor [39]. Therefore, comprehensive control measures for CMD-related disease, along with early disease screening, are essential in these regions.
Since the adoption of the WHO Framework Convention on Tobacco Control (FCTC) in 2005, a notable reduction in SHS-related disease burden has been observed, with the number of deaths remaining stable, suggesting improvements in nonsmokers protection through smoke-free laws and enhanced healthcare access [40,41]. However, in recent years, the promotion of "smoke-free" environments has diminished, and the prevalence of SHS exposure has plateaued. Women and young people continue to be exposed to higher levels of SHS exposure in domestic settings and social gatherings, potentially due to reduced concern over the harms of inhalable smoke following the enactment of smoke-free laws and improved protections for nonsmokers. In addition to strengthening public awareness campaigns, advocating for smoking bans in public spaces, and increasing tobacco taxes, it is critical to integrate gender considerations into tobacco control research, policies, and interventions. In economically disadvantaged regions and countries where the WHO FCTC policies have been enacted but not fully implemented, targeted rapid response funding and technical support could accelerate tobacco control efforts.
5. Strength and limitations
This study provides comprehensive estimates of the spatial and temporal trends in the burden of CMDs attributable to respirable SHS at global, regional, and national levels, while also projecting the disease burden for the next two decades. Several limitations should be noted. Firstly, research indicates that public spaces such as bars, restaurants, and workplaces are particulary vulnerable to the effects of SHS. More research is needed to SHS exposure in various settings, as considerable unmeasured exposure remains, contributing to the disease burden. Strengthening SHS-related health intervention policies is crucial [42]. Second, certain factors influencing SHS exposure at home, such as tobacco type, intensity of exposure, natural ventilation, and household crowding, were not considered in this study. Third, the reliance on self-reported data, rather than objective biomarkers, may result in misclassification due to inaccurate reporting. Finally, caution is needed when interpreting the predictive estimates based on the model, as potential biases could influence the results. Despite these limitations, our findings underscore the significance of controlling SHS exposure as a key strategy for reducing the burden of CMDs in the future.
6. Conclusion
This study revealed that global control of SHS exposure remains far from optimal. The number of CMD deaths and DALYs attributable to SHS continues to rise, particularly in recent years. Even more conserning is that CMD-related deaths from SHS exposure have been increasing steadily over the past three decades and are projected to rise further over the next 20 years. Given the trends in SHS exposure globally, policymakers should prioritize the development of enforceable strategies to strengthen smoking bans in public spaces. A proactive approach to controlling SHS exposure has the potential to significantly reduce global CMD-related DALYs and deaths, particularly for women under 65 years old in less economically developed regions.
Funding
This research was funded by the National Natural Science Foundation of China (82,200,396), Natural Science Foundation of Heilongjiang Province of China (YQ2022H006), New era Longjiang outstanding doctoral key project (No. LJYXL2022–013); Cultivation Project of Second Affiliated Hospital of Harbin Medical University (No. PYMS2023–3). Gout Etiology and Functional Food Research Innovation Team, the North Medicine and Functional Food CharacteristicSubject Project in Heilongjiang Province (HLJTSXK-2022–03), National Fund Cultivation Program of Jiamusi University (JMSUGPZR2022–022), Scientific and Technological Innovation Team of Jiamusi University (cxtd202101), and Innovation Project of Harbin Medical University (2022-KYYWF-0281), Foundation of Heilongjiang Educational Committee (2023-KYYWF-0620), and Project of Jiamusi University (JMSUQP23027).
Contributions
YL and SJW conceived and designed the study. YYZ and BY developed the protocols, and YG, and WT organized all the data. GYL and CGY analyzed and visualized the results. YL contributed to the manuscript. Reviewed and edited the manuscript, SJW, YYZ, and BY take responsibility for the integrity and accuracy of this analysis. All authors reviewed the manuscript.
Ethics approval and consent to participate
Not applicable.
Data sharing
Data used in the analyses can be obtained from the Global Health Data Exchange Global Burden of Disease Results Tool (https://ghdx.healthdata.org/gbd-results-tool).
CRediT authorship contribution statement
Yan Liu: Writing – review & editing, Writing – original draft, Conceptualization. Yi Gao: Visualization, Project administration, Data curation. Guangcan Yan: Project administration, Investigation. Yige Liu: Resources, Methodology. Wei Tian: Software, Resources, Project administration. Yiying Zhang: Validation, Supervision. Shanjie Wang: Writing – review & editing, Validation, Supervision, Resources. Bo Yu: Writing – review & editing, Project administration, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We highly appreciate the work of the GBD 2019 collaborators.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2024.100902.
Contributor Information
Yiying Zhang, Email: zhangyiying@jmsu.edu.cn.
Shanjie Wang, Email: shanjie_wang@hrbmu.edu.cn.
Appendix. Supplementary materials
References
- 1.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai C., Hu D., Yu G., et al. Global, regional, and national deaths, disability-adjusted life years, years lived with disability, and years of life lost for the global disease burden attributable to second-hand smoke, 1990-2019: a systematic analysis for the global burden of disease study. Sci Total Environ. 2023;862 doi: 10.1016/j.scitotenv.2022.160677. [DOI] [PubMed] [Google Scholar]
- 3.Tong E.K., Glantz S.A. Tobacco industry efforts undermining evidence linking secondhand smoke with cardiovascular disease. Circulation. 2007;116:1845–1854. doi: 10.1161/CIRCULATIONAHA.107.715888. [DOI] [PubMed] [Google Scholar]
- 4.Kim D., Choy Y.S., Park E.C. Association between secondhand smoke and glycemic control in adult diabetes patients. Prev Med. 2017;94:48–54. doi: 10.1016/j.ypmed.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Barnoya J., Glantz S.A. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 6.Flor L.S., Anderson J.A., Ahmad N., et al. Health effects associated with exposure to secondhand smoke: a burden of proof study. Nat Med. 2024;30:149–167. doi: 10.1038/s41591-023-02743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roth G.A., Mensah G.A., CO Johnson, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the gbd 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S., Liu Y., Cai H., et al. Decreased risk of all-cause and heart-specific mortality is associated with low-fat or skimmed milk consumption compared with whole milk intake: a cohort study. Clin Nutr. 2021;40:5568–5575. doi: 10.1016/j.clnu.2021.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Yang X., Sun J., Zhang W. Global burden of stroke attributable to secondhand smoke in 204 countries and territories from 1990 to 2019: analysis of the global burden of disease study. Front Neurol. 2024;15 doi: 10.3389/fneur.2024.1320033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C., Wang B., Liu S., et al. Type 2 diabetes attributable to pm(2.5): a global burden study from 1990 to 2019. Environ Int. 2021;156 doi: 10.1016/j.envint.2021.106725. [DOI] [PubMed] [Google Scholar]
- 11.Fan J., Li X., Yu X., et al. Global burden, risk factor analysis, and prediction study of ischemic stroke, 1990-2030. Neurology. 2023;101:e137–e150. doi: 10.1212/WNL.0000000000207387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avila-Tang E., Elf J.L., Cummings K.M., et al. Assessing secondhand smoke exposure with reported measures. Tob Control. 2013;22:156–163. doi: 10.1136/tobaccocontrol-2011-050296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yakoob M.Y., Micha R., Khatibzadeh S., et al. Impact of dietary and metabolic risk factors on cardiovascular and diabetes mortality in south asia: analysis from the 2010 global burden of disease study. Am J Public Health. 2016;106:2113–2125. doi: 10.2105/AJPH.2016.303368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hankey B.F., Ries L.A., Kosary C.L., et al. Partitioning linear trends in age-adjusted rates. Cancer Causes Control. 2000;11:31–35. doi: 10.1023/a:1008953201688. [DOI] [PubMed] [Google Scholar]
- 17.Bo Y., Zhu Y., Zhang X., et al. Spatiotemporal trends of stroke burden attributable to ambient pm(2.5) in 204 countries and territories, 1990-2019: a global analysis. Neurology. 2023;101:e764–e776. doi: 10.1212/WNL.0000000000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan R., Ren F., Xie Y., Li K., Tong Z. The global, regional, and national burdens of cervical cancer attributable to smoking from 1990 to 2019: population-based study. Jmir Public Health Surveill. 2022;8:e40657. doi: 10.2196/40657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jurgens V., Ess S., Cerny T., Vounatsou P. A bayesian generalized age-period-cohort power model for cancer projections. Stat Med. 2014;33:4627–4636. doi: 10.1002/sim.6248. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z., Xu K., Jiang Y., et al. Global trend of aetiology-based primary liver cancer incidence from 1990 to 2030: a modelling study. Int J Epidemiol. 2021;50:128–142. doi: 10.1093/ije/dyaa196. [DOI] [PubMed] [Google Scholar]
- 21.Liu J., Wang S., Cui C., et al. The association between glucose-related variables and plaque morphology in patients with st-segment elevated myocardial infarction. Cardiovasc Diabetol. 2020;19:109. doi: 10.1186/s12933-020-01074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the global burden of disease study 2019. Lancet. 2021;397:2337–2360. doi: 10.1016/S0140-6736(21)01169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi Y., Yamagishi K., Muraki I., et al. Secondhand smoke and the risk of incident cardiovascular disease among never-smoking women. Prev Med. 2022;162 doi: 10.1016/j.ypmed.2022.107145. [DOI] [PubMed] [Google Scholar]
- 24.Merklinger-Gruchala A., Jasienska G., Thune I., Kapiszewska M. Joint effect of particulate matter and cigarette smoke on women's sex hormones. Bmc Womens Health. 2022;22:3. doi: 10.1186/s12905-021-01586-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shastri S.S., Talluri R., Shete S. Disparities in secondhand smoke exposure in the united states: national health and nutrition examination survey 2011-2018. Jama Intern Med. 2021;181:134–137. doi: 10.1001/jamainternmed.2020.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon J., He X., Shinde A., et al. The role of puff volume in vaping emissions, inhalation risks, and metabolic perturbations: a pilot study. Sci Rep. 2024;14:18949. doi: 10.1038/s41598-024-69985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schweitzer K.S., Chen S.X., Law S., et al. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am J Physiol Lung Cell Mol Physiol. 2015;309:L175–L187. doi: 10.1152/ajplung.00411.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose J.J., Krishnan-Sarin S., Exil V.J., et al. Cardiopulmonary impact of electronic cigarettes and vaping products: a scientific statement from the american heart association. Circulation. 2023;148:703–728. doi: 10.1161/CIR.0000000000001160. [DOI] [PubMed] [Google Scholar]
- 29.Zhai C., Hu D., Yu G., et al. Global, regional, and national deaths, disability-adjusted life years, years lived with disability, and years of life lost for the global disease burden attributable to second-hand smoke, 1990-2019: a systematic analysis for the global burden of disease study. Sci Total Environ. 2023;862 doi: 10.1016/j.scitotenv.2022.160677. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L., Tong Z., Han R., et al. Global, regional, and national burdens of ischemic heart disease attributable to smoking from 1990 to 2019. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.028193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith C.E., Hill S.E., Amos A. Impact of population tobacco control interventions on socioeconomic inequalities in smoking: a systematic review and appraisal of future research directions. Tob Control. 2020;30:e87–e95. doi: 10.1136/tobaccocontrol-2020-055874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo L., Jiang J., Zhang G., et al. Stroke mortality attributable to ambient particulate matter pollution from 1990 to 2015 in china: an age-period-cohort and spatial autocorrelation analysis. Int J Environ Res Public Health. 2017;14 doi: 10.3390/ijerph14070772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otanez M., Glantz S.A. Social responsibility in tobacco production? Tobacco companies' use of green supply chains to obscure the real costs of tobacco farming. Tob Control. 2011;20:403–411. doi: 10.1136/tc.2010.039537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the global burden of disease study 2015. Lancet. 2017;389:1885–1906. doi: 10.1016/S0140-6736(17)30819-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saeedi P., Petersohn I., Salpea P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 36.Ngo A., Drope J., Guerrero-Lopez C.M., Siu E., Chaloupka F.J. As countries improve their cigarette tax policy, cigarette consumption declines. Tob Control. 2024;33:e91–e96. doi: 10.1136/tc-2022-057486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rani M., Thamarangsi T., Agarwal N. Youth tobacco use in south-east asia: implications for tobacco epidemic and options for its control in the region. Indian J Public Health. 2017;61:S12–S17. doi: 10.4103/ijph.IJPH_241_17. [DOI] [PubMed] [Google Scholar]
- 38.He H., Pan Z., Wu J., et al. Health effects of tobacco at the global, regional, and national levels: results from the 2019 global burden of disease study. Nicotine Tob Res. 2022;24:864–870. doi: 10.1093/ntr/ntab265. [DOI] [PubMed] [Google Scholar]
- 39.Buksh S.M., de Wit J., Hay P. Sociocultural influences contribute to overeating and unhealthy eating: creating and maintaining an obesogenic social environment in indigenous communities in urban fiji. Nutrients. 2022:14. doi: 10.3390/nu14142803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flor L.S., Reitsma M.B., Gupta V., Ng M., Gakidou E. The effects of tobacco control policies on global smoking prevalence. Nat Med. 2021;27:239–243. doi: 10.1038/s41591-020-01210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semple S., Dobson R., O'Donnell R., et al. Smoke-free spaces: a decade of progress, a need for more? Tob Control. 2022;31:250–256. doi: 10.1136/tobaccocontrol-2021-056556. [DOI] [PubMed] [Google Scholar]
- 42.Buettner-Schmidt K., Boursaw B., Lobo M.L. Place and policy: secondhand smoke exposure in bars and restaurants. Nurs Res. 2018;67:324–330. doi: 10.1097/NNR.0000000000000286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.