Abstract
Introduction
The FGFR3-TACC3 fusion gene exists in a variety of malignant tumors, including bladder cancer. In our ongoing research on the CRISPR-Cas13a gene-editing system, we reported the use of CRISPR-Cas13a gene-editing system to knockout FGFR3-TACC3 and inhibit the proliferation of bladder tumor cells.
Purpose
This study aimed to use the CRISPR-Cas13a gene-editing system to target the FGFR3-TACC3 fusion gene in bladder cancer cells, which has the potential to be a new and effective treatment for bladder cancer.
Materials and Methods
The efficacy of the CRISPR-Cas13a gene-editing system was analysed by qRT-PCR. The inhibitory effects of Cas13a-mediated knockdown of the FGFR3-TACC3 fusion gene on the proliferation of RT4 and RT112 cell lines were assessed utilizing CCK-8, EdU, and organoid formation assays. Subsequently, the comparative tumorigenic capability of RT4 cells with FGFR3-TACC3 knockdown achieved by Cas13a was examined in a nude mouse model.
Results
At the cellular level, the comparative analysis of FGFR3-TACC3 knockdown efficacy between CRISPR-Cas13a and shRNA revealed a more pronounced reduction with the former. This knockdown effectively curtailed cellular proliferation, with CRISPR-Cas13a-mediated knockdown exhibiting a superior inhibitory effect over shRNA-mediated knockdown. In organoid cultures derived from RT4 cells, a similar trend was observed, with Cas13a-mediated knockdown of FGFR3-TACC3 leading to a more substantial suppression of proliferation compared to shRNA-mediated knockdown. In vivo tumor models corroborated these findings, demonstrating a significantly diminished tumor volume in the Cas13a-treated cohort relative to both the control and shRNA-treated groups.
Conclusion
The CRISPR-Cas13a gene-editing system has been demonstrated to significantly suppress tumor proliferation both in vitro and in vivo, thereby presenting itself as a promising candidate for a novel and efficacious therapeutic intervention in bladder cancer treatment.
Keywords: FGFR3-TACC3, oncogenic mutation, CRISPR-Cas13a, mRNA knockdown, bladder cancer
Introduction
As early as 2012, researchers from CUMC (Columbia University Medical Center) found that 3% of patients with glioblastoma had the FGFR3-TACC3 fusion gene.1 In 2018, this same group discovered that FGFR3-TACC3 causes the overactivation of mitochondrial metabolism and increased energy directed at cell proliferation, leading to cancer. In tumor cells from affected humans and a mouse model of brain cancer, targeted knockout of FGFR3-TACC3 prevented tumor growth.2 At the same time, other studies discovered the same gene fusion in non-small cell lung cancer, oesophageal cancer, triple-negative breast cancer, and cervical cancer.3–7 These results suggested that the fusion gene may be common to multiple types of tumors and acts as an oncogene.
To determine whether bladder cancer could contain the gene fusion, 42 pairs of tissue samples were studied through transcriptome sequencing. It was found that 32 gene rearrangements had the potential to lead to gene fusion and including FGFR3-TACC3 fused. The study also found that the increased expression of TACC3 was regulated by the transcriptional regulator FGFR3, rather than by amplification of the TACC3 gene itself.8 Recently, researchers revisit the pivotal role of fibroblast growth factor receptor 3 (FGFR3) in bladder cancer (BLCA), underscoring its prevalence in both non-muscle-invasive and muscle-invasive forms of the disease. FGFR3 mutations in up to half of BLCAs play a well-established role in tumorigenesis.9 The fusion of these two genes leads to the constitutive activation of the FGFR3 tyrosine kinase domain, resulting in the activation of downstream signaling pathways such as RAS/MAPK, PI3K/Akt, and STAT3, which are crucial for tumor cell proliferation and survival. This fusion gene has since been recognized as a key oncogenic driver in various solid tumors, including bladder cancer.10
Mechanistically, FGFR3-TACC3 phosphorylates the PIN4 protein and this phosphopeptide is an intermediate step in the activation signal pathway for mitochondrial metabolism. Therefore, it can cause the multiplication of peroxisomes, release large amounts of oxidants, and induce the key regulator of mitochondrial metabolism (PGC1-α) to promote excessive movement of mitochondria, thereby supplying the large amount of energy required for the rapid division and growth of cancer cells. In short, the FGFR3-TACC3-PIN4 pathway induces peroxisome biosynthesis and new protein synthesis, enhances mitochondrial activity, and promotes energy production, thereby regulating cell proliferation.11
Bladder cancer is a malignant tumor that occurs in the mucosa. It is the most common malignant tumor of the urinary tract and one of the ten most common tumors.12 However, current treatment results are poor, with easy relapse and poor prognosis,13 warranting an urgent need to develop new and effective treatments. We speculated that FGFR3-TACC3 can be targeted to inhibit cell proliferation as a treatment for bladder cancer.
The CRISPR-Cas gene-editing system was developed from enzymes found in ancient archaebacteria. This is a naturally-occurring, genome-editing immune mechanism used to identify and destroy the defence systems of invading bacteriophages and other pathogens.14 In recent years, the application of the CRISPR-Cas system has become a hotspot in the field of life sciences.15 The CRISPR-Cas9 system targets DNA for specific identification and knockdown. However, this targeting may destroy the stability of the target genome and produce undesirable genetic changes.16–19 The biological safety of treatments using CRISPR-Cas9 is increasingly being questioned; however, targeting mRNA can greatly reduce the occurrence of undesirable genetic changes without destroying the target genome.
RNA interference (RNAi), using small interfering RNA (siRNA) and/or short hairpin RNA (shRNA), has proven to be a useful approach for inhibiting gene expression and received considerable study for possible cancer therapeutics.20 shRNA can be integrated into the genome through viral vectors and processed into siRNA to stably inhibit gene expression in mammals.21 siRNA is a double-stranded RNA with a length of 20–25 nucleotides, and has many different uses in biology.22 siRNA silences the expression of certain genes by promoting the assembly of the RNA-induced silencing complex (RISC) and cutting mRNA molecules encoding target genes. However, RNAi knockout efficiency is low, and the off-target rate is high.23 Therefore, there is an urgent need to develop a new type of mRNA-targeting tool.
Studies have confirmed that in human cell lines, Cas13a, another Cas enzyme, only targets RNA specified by guide RNA (gRNA), while keeping other RNAs intact.24 It was confirmed that Cas13a (previously named C2c2) is a type VI effector protein with RNase activity guided by RNA, which can specifically reduce mRNA levels in mammalian cells. After recognising the target sequence, the HEPN catalytic site is activated, which triggers Cas13a to degrade non-specific RNA. However, the application of CRISPR-Cas13a in eukaryotic organisms (especially mammals) is still in the research and exploration stages.25 We report here for the first time that the use of CRISPR-Cas13a gene-editing system to target fusion genes in bladder cancer cells. Compared to traditional targeted mRNA tools (RNAi), this method has a higher knockout efficiency. Taken together, the CRISPR-Cas13a gene-editing system more significantly inhibits tumor proliferation using multiple experimental approaches.
Materials and Methods
Cell Lines and Culture Conditions
Human bladder cancer cell lines RT4 and RT112 were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Ham’s F12 medium (Gibco) supplemented with 10% heat-inactivated foetal bovine serum (FBS; Gibco; Thermo Fisher Scientific Inc)., 1% antibiotic-antimycotic mixture (100 µg/mL streptomycin, 100 U/mL penicillin), and 2 mM glutamine (Sigma-Aldrich, Saint Quentin Fallavier, France). Cell cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2. They were tested for the absence of mycoplasma before the start of the experiments.
Construction of the Recombinant Lentiviral Vector
The FGFR3-TACC3 shRNA lentiviral expression vector was constructed by the Ji Kai Gene Company (Shanghai, China). Both RT4 and RT112 cell lines were used as targets. The day before transduction, the cells were seeded in 6-well plates at a density of 1×106 cells per well. On the day of induction, 10 µL lentiviral particles were added to target cells according to the MOI. The experiment was terminated 12 h after infection. Stable clones were selected using a liquid medium containing 2 ng/µL polybrene.
The target sequences of FGFR3-TACC3 for constructing lentiviral shRNAs are presented in Table 1.
Table 1.
Designing shRNA to the FGFR3-TACC3 Fusion Breakpoint in the Present Study
| shRNA Set | Target Sequence of FGFR3-TACC3 |
|---|---|
| shRNA- FGFR3-TACC3-1#(Sh1) | CGTCCACCGAC/GTAAAGGCGA |
| shRNA- FGFR3-TACC3-2#(Sh2) | CACCGAC/GTAAAGGCGACACA |
| shRNA-NC(Sh-NC) | ATAAAGGAGACACACACCGAC |
Abbreviations: shRNA, short hairpin RNA; NC, negative control.
CRISPR-Cas13a Vector Transfections
CRISPR-Cas13a targeting FGFR3-TACC3 (Cas13a-FGFR3-TACC3#1, #2, #3) and negative control (Cas13a-NC) were constructed by the Ji Kai Gene Company (Shanghai, China). Bladder cancer cells, RT4 and RT112, were seeded in 6-well plates and transfected with 10 μL Cas13a-FGFR3-TACC3 or Cas13a-NC at 80–90% confluency. The sequence of the CRISPR-Cas13a vector and schematic diagram of the plasmid constructions are listed in Supplementary Figure S1 and Supplementary Material 1.
RNA Extraction and Quantitative Real-Time Reverse Transcription-PCR (qRT-PCR)
RT4 and RT112 cells transfected with siRNA or processed by CRISPR-Cas13a were seeded in 12-well plates at a density of 1×105 cells per well. After 12–24 h, total RNA was extracted using the Trizol kit (Qiagen, Valencia, CA, USA). cDNA was synthesised using a Thermo-Script RT kit (Life Technologies, Rockville, MD, USA). Quantitative real-time PCR was performed using the CFX96™ Real-Time System (Bio-Rad, Hercules, CA, USA), using SYBR PCR reagent (Takara, Shiga, Japan). The cycling conditions were as follows: 40 cycles of 15s at 95°C, 15s at 60°C, and 45s at 75°C. β-actin was used as the loading control. Fold-changes in the relative expression of target genes were calculated using the 2−ΔΔCq method.26 All experiments were performed in duplicate and repeated three times. Primers used for quantitative PCR are presented in Table 2.
Table 2.
Primers Used for Quantitative PCR
| Primer Set | Sequence |
|---|---|
| FGFR3-TACC3 | Forward: 5′-AGAGGCCCACCTTCAAGCA-3′ |
| Reverse: 5′-TCCTCAGCTCCCGGTTCTC-3′ | |
| β-actin | Forward: 5′- CCCTCCCCAGTCCTCATGTA −3′ |
| Reverse: 5′- TCAGGCAGCTCGTAGCTCTT −3′ |
CCK-8 Assay
Cell proliferation was assessed using CCK-8 assay (CCK-8, Dojindo, Japan). Cells were seeded in a 96-well plate (3×103 cells/well) for 24 h and then transiently transfected with CRISPR-Cas13a or siRNA. A total of 10 µL of CCK-8 (5 mg/mL) was added to each well. Absorbance at 450 nm was measured at 0, 24, and 48 h after transfection using a microplate reader (Bio-Rad, Hercules, CA, USA). Each test was performed at least thrice.
Ethynyl-2′-Deoxyuridine (EdU) Assay
The EdU assay was performed using an EdU kit (BeyoClickTM, EDU-488, China). Cells were co-cultured with EdU working solution (1:1000) at 37 °C in a humidified 5% CO2 atmosphere for 2–4 h, followed by fixation with 4% paraformaldehyde for 30 min and treatment with 0.3% Triton X-100 for 30 min at room temperature. Then, according to the manufacturer’s protocol, cells were co-incubated with the click reaction solution for 30 min at room temperature in a dark environment, after which cells were treated with Hoechst solution for 10 min. A fluorescence microscope (Olympus Corporation, Japan) was used to capture images at a magnification of 200×, and cell counting was performed using ImageJ (http://imagej.net/ImageJ).
Culture of Human Bladder Cancer Cell Organoids
Briefly, RT-4 sh1/ RT-4Cr2 cells (10,000 per well) were seeded in a 24-well plate and combined with 10 µL basement membrane extract (BME, Type 2, Pathclear). Following BME solidification, the cells were cultured in human bladder cancer organoid expansion medium [kindly provided by Lei Yu,25 consisting of Advanced EDME/F12 (500 mL) with antibiotics (AA16, 5 mL), Gluta MAX (5 mL), HEPE (5 mL), B2T (1 mL), NAC (125 μL), EGG (5 μL), Noggin (50 μL), R-spondin-1 (250 μL), A8S-01 (1 µL), FGF1 (5 μL), FGF2 (5 μL), Nicotinamide (500 μL), and SP202190 (16.7 µL)]. Human bladder organoids underwent biweekly passaging, either through mechanical shearing with a glass pipet or by dissociation using TrypLE (ThermoFisher 12605036). Following passaging, ROCK inhibitor (Y-27632, 10 µM) was introduced to the media to prevent cell death. Organoids were cryopreserved in a freezing medium (50% FBS, 10% DMSO, and 40% Advanced DMEM/F-12) and demonstrated efficient recovery.
In vivo Xenograft Experiments
Male BALB/c nude mice (4–6 weeks old) were purchased from the Guangdong Experimental Animal Center (Guangzhou, China). The protocol for animal experiments was approved by the Ethics Committee of Guangzhou University of Chinese Medicine, and all mice were kept under strict pathogen-free conditions. To establish the xenograft model, approximately 5.0×106 each of four RT4 cell lines (FGFR3-TACC3-shRNA, FGFR3-TACC3-shRNA-control; FGFR3-TACC3-Cas13a, FGFR3-TACC3- Cas13a -control) were subcutaneously injected into the left and right sides of the nude mice. Tumor volume was measured every 3 days. The tumor volume was calculated as follows: tumor volume (mm3) = length × width2× 0.52. At the endpoint, all mice were euthanised, tumors were removed, and the volume was measured.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 8.0 software (GraphPad Software, Inc). All experiments were performed in triplicate, except for the in vivo experiments. Data are presented as the mean ± standard deviation (SD). Differences between the two groups were analysed using paired-sample t-tests. Multiple comparisons were performed using ANOVA. Post hoc Bonferroni corrections were applied. Statistical significance was set at p <0.05. Statistical probabilities (p) were expressed as * p < 0.05, ** p < 0.01, and *** p < 0.001.
Results
CRISPR-Cas13a-Mediated Knockdown of FGFR3-TACC3 is More Effective Than That by siRNA in Bladder Cancer Cells
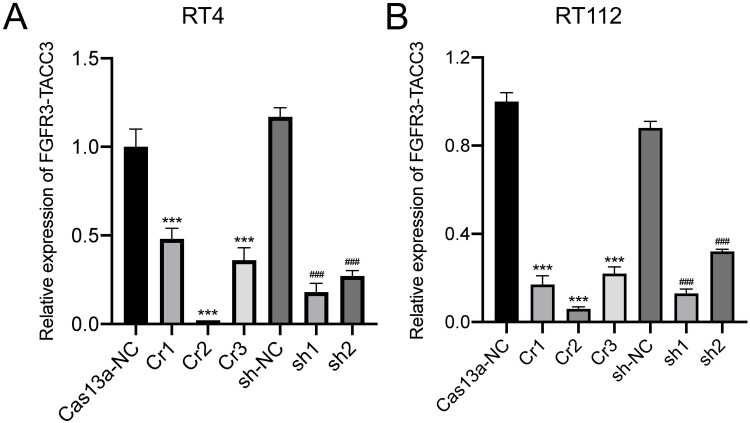
qRT-PCR was used to measure the relative expression of FGFR3-TACC3 knockdown by CRISPR-Cas13a (#1, #2, #3) or Cas13a-NC and shRNA (#1, #2) or shRNA-NC in bladder cancer cells RT4 and RT112. In RT4 cells after Cas13a- FGFR3-TACC3 or Cas13a-NC and shRNA- FGFR3-TACC3 (#1, #2) or shRNA-NC transfection for 24 h, the relative expression levels of FGFR3-TACC3 knockdown by CRISPR-Cas13a were significantly reduced compared to shRNA. The relative expression levels of FGFR3-TACC3 knockdown by CRISPR-Cas13a- FGFR3-TACC3#2 (Cr2) were more significantly decreased than that of Cr1 or Cr3. The relative expression level of FGFR3-TACC3 knockdown by shRNA- FGFR3-TACC3#1 (sh1) was more significantly decreased than that of sh2 in RT4 and RT112 cells. (Figure 1A) The above results for RT112 were consistent with the results in RT4. (Figure 1B) Therefore, Cr2 and sh1 with more effective knockdown were selected for the subsequent experiments.
Figure 1.
Analysis of FGFR3-TACC3 mRNA expression in bladder cancer cells by qRT-PCR. RT4 and RT112 were transfected with CRISPR-Cas13a (#1, #2, #3) or Cas13a-NC and shRNA (#1, #2) or shRNA-NC for 24 h. (A and B). mRNA expression of FGFR3-TACC3 was reduced after knockdown of FGFR3-TACC3 by CRISPR-Cas13a and shRNA in RT4 and RT112 cells. The Cr2 and Si1 groups with more effective knockdown were selected for subsequent experiments. Significant results are presented as ***p < 0.001, ###p < 0.001.
Knockdown of FGFR3-TACC3 Suppresses Proliferation in Bladder Cancer Cells
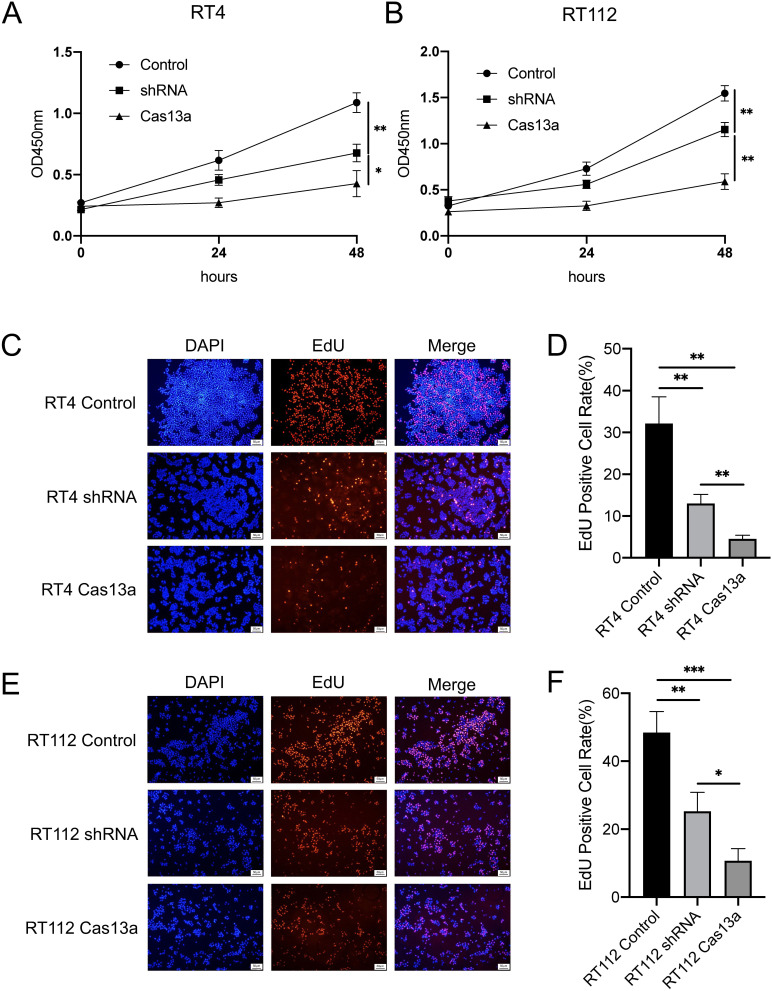
To evaluate proliferation of RT4 and RT112 cells following FGFR3-TACC3-knockdown by Cas13a or shRNA, growth curves were established using a CCK-8 assay. The proliferative activity of the shRNA group was significantly higher than that of the Cas13a group in RT4 and RT112 cells (Figure 2A and B).
Figure 2.
Knockdown of FGFR3-TACC3 suppresses proliferation in bladder cancer cells. (A and B). CCK-8 assay results show the effects of FGFR3-TACC3 knockdown by Cas13a or shRNA on proliferation in RT4 and RT112 cells. The Cas13a group was inhibited proliferation compared with the shRNA group. (C-F). EdU assay results show the effects of FGFR3-TACC3 knockdown by Cas13a or shRNA on proliferation in RT4 and RT112 cells. The Cas13a group was obviously inhibited compared with the shRNA group. Significant results are presented as *p < 0.05, **p < 0.01, and ***p< 0.001.
To further evaluate the proliferative activity of RT4 and RT112 with FGFR3-TACC3 knockdown by Cas13a or shRNA, we also examined cell proliferation. EdU assays showed results similar to those of the CCK-8 assay. We found that the proportion of EdU-positive cells was significantly decreased with Cas13a-mediated knockdown of FGFR3-TACC3 compared to shRNA in RT4 and RT112 cells (Figure 2C–F).
These results showed that knockdown of FGFR3-TACC3 suppressed proliferation and that the suppression was greater with Cas13a-mediated knockdown of FGFR3-TACC3 as compared to that in shRNA-mediated knockdown. This suggests that knockdown of FGFR3-TACC3 by Cas13a was more effective in inhibiting proliferation than that by shRNA at the cellular level.
Knockdown of FGFR3-TACC3 Suppresses Proliferation in Cell-Derived Organoids
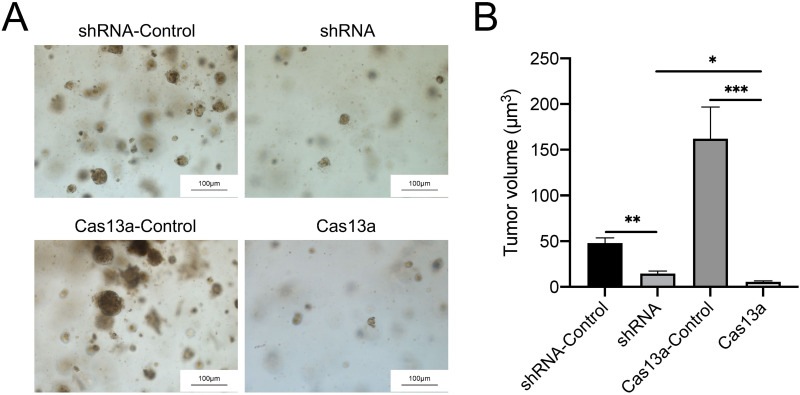
To evaluate RT4 proliferation with FGFR3-TACC3 knockdown by Cas13a or shRNA, tumor volume curves in cell-derived organoids were established. The tumor volumes of RT4 cells with FGFR3-TACC3-knockdown by Cas13a or shRNA were both lower than those of the control group. In the Cas13a-mediated knockdown group, the tumor volume decreased to less than 10 μm3 (Figure 3B). The difference between the two groups was significant (Figure 3A and B).
Figure 3.
Knockdown of FGFR3-TACC3 suppresses proliferation in cell-derived organoids. The volume of cell-derived organoids showing the effects of FGFR3-TACC3 knockdown by Cas13a or shRNA on proliferation in RT4 cells. Knockdown of FGFR3-TACC3 decreased the volume of cell-derived organoids. The Cas13a group obviously inhibited the volume of cell-derived organoids compared with the shRNA group (A). Images of cells derived from organoid (B). Volume of cells derived from organoid. The determination of cell-derived organoid volume involved the measurement of organoid diameter (in micrometers) across distinct groups (n = 20, obtained from four independent batches). Significant results are presented as *p < 0.05, **p < 0.01, and ***p< 0.001.
These results showed that knockdown of FGFR3-TACC3 suppressed proliferation and that the suppression by Cas13a-mediated knockdown was higher compared to shRNA-mediated knockdown in RT4-derived organoids. In summary, knockdown of FGFR3-TACC3 by Cas13a was more effective in inhibiting proliferation than that by shRNA at the organoid level.
Knockdown of FGFR3-TACC3 Suppresses Proliferation in vivo
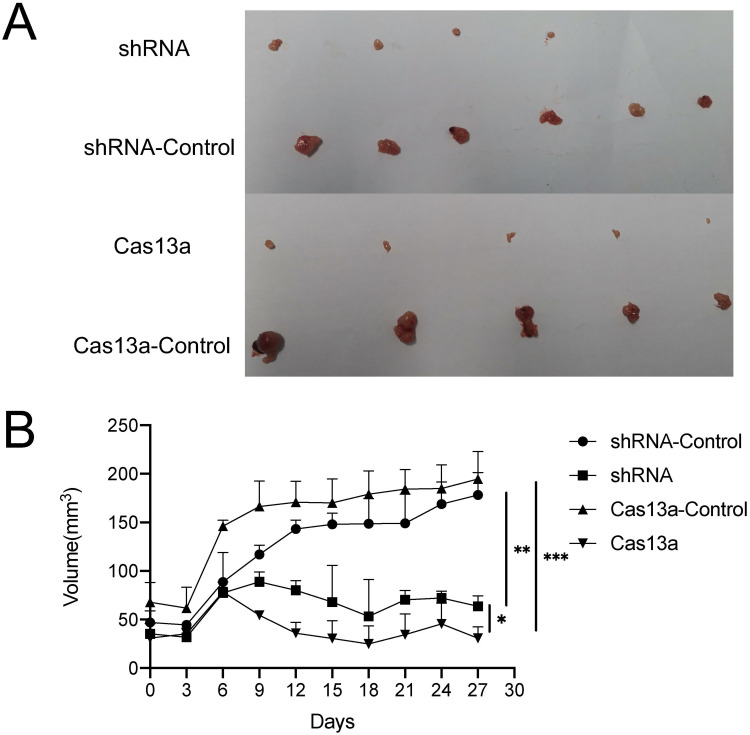
To determine whether our in vitro data can be extrapolated to in vivo situations, we compared the differences in the tumorigenic capability of RT4 cells with FGFR3-TACC3 knockdown by Cas13a or shRNA using the nude mouse subcutaneous tumor cell transplantation model. BALB/c nude mice were subcutaneously inoculated with RT4 cells with FGFR3-TACC3 knocked down by either Cas13a or shRNA or control cells. Tumors were harvested after 30 days. Compared with the control group, tumors from the Cas13a or shRNA group displayed significantly reduced volumes (Figure 4A). Compared with the shRNA group, tumors from the Cas13a group displayed a significantly reduced volume (Figure 4B). Thus, FGFR3-TACC3 is needed to promote bladder cancer cell tumorigenesis in vivo.
Figure 4.
Knockdown the FGFR3-TACC3 inhibits bladder cancer cell tumorigenesis in mice. BALB/c nude mice (n = 6 per group) were subcutaneously inoculated with RT4 cells stably expressing FGFR3-TACC3 shRNA or shRNA-control or FGFR3-TACC3 knockdown by Cas13a or Cas13a-control. The shRNA group had two non-tumor-forming mice. The Cas13a and Cas13a-control groups each had a non-tumor-forming mouse. Tumors were harvested on day 30. (A). Tumor image.(B). Tumor volume. *p <0.05, **p <0.01, ***p <0.005.
Discussion
It is estimated that up to 550,000 new cases of bladder cancer are reported worldwide per year.27 In the absence of tumor metastasis, cystectomy is the first choice of treatment for bladder cancer. However, the recurrence rate of bladder cancer after surgery can be as high as 17%.28 Therefore, there is an urgent need to develop new methods to facilitate early diagnosis and effective treatment.
Gene fusion is a hybrid gene formed by the fusion of two unrelated genes, which generates a completely new function or a function different from the two individual genes before fusion.29 It is usually a fusion of a strong promoter with a downstream functional gene (a proto-oncogene).30 In most cases, fused genes encode proteins with abnormal sequences or functions or cause gene expression disorders, leading to or promoting tumors.31 The fusion gene exists uniquely in tumors and is not expressed in normal tissues and, therefore, has the potential to become a safe and specific target for tumor therapy.
FGFR3-TACC3 fusion plays an important role in tumor metabolism. It can cause excessive mitochondrial metabolism, thereby promoting mitochondrial oxidative phosphorylation and providing increased energy for rapid cell growth2. This fusion gene also provides insights into ways of destroying the energy supply of cancer cells and suggesting new gene fusion-mediated therapeutic targets for cancers. Studies have found that FGFR3-TACC3 also exists in bladder cancer8. However, there has been no research to verify the mechanism of FGFR3-TACC3 knockdown in bladder cancer. For the first time, we knocked out FGFR3-TACC3 while comparing two different gene-editing technologies, and then demonstrated that it can inhibit the proliferation of bladder cancer cells both in vitro and in vivo.
Gene therapy specifically targets disease-causing genes, making treatment more precise and personalised. At present, RNAi and newer gene-editing tools (such as CRISPR) are used to correct the expression of target genes. In 2001, Tuschl et al used chemically synthesised RNAi to silence the expression of target genes in mammalian cells.32 However, RNAi has many problems, such as poor stability, poor specificity, and the need for special delivery technologies.33 These drawbacks also limit the use of RNA in clinical applications.
Many studies have been conducted to address the shortcomings of RNAi. Some studies have found that Cas13a edits genes by targeting mRNA. Its focus on targeting RNA complements the DNA-targeting CRISPR-Cas9 system.34 Therefore, the high-throughput regulation and modification of mRNA through the Cas13a system can achieve a broader level of regulation of the target gene. This has far-reaching significance in the research and prevention of diseases. This research later confirmed that Cas13a can specifically reduce mRNA levels in mammalian cells.35
In clinical applications, the CRISPR-Cas13a system can trigger random shearing effects in U87 cells overexpressing glioma-specific mutations, particularly epidermal growth factor receptor variant III (EGFRvIII), through CRISPR RNA degradation, leading to the death of tumor cells.25 This discovery revealed the potential of the CRISPR-Cas13a system for inhibiting tumor proliferation.
To verify the application potential and advantages of this treatment in bladder cancer, we applied CRISPR-Cas13a to knockdown FGFR3-TACC3 in bladder cancer cells. Through a full range of experiments, from cells to animals, we found that CRISPR-Cas13a has the advantage of high efficiency compared with shRNA.
To demonstrate this, we also used tumor cell organoids to verify the therapeutic advantages of CRISPR-Cas13. Organoids are three-dimensional (3D) cell cultures that contain key characteristics of their representative organs. Organoids have unique advantages over flat cultures and animals and can serve as a bridge between traditional 2D cell culture and animal models. Compared with cells cultured by traditional methods, organoids are closer to the biological characteristics of the human body and can reproduce to a greater degree the complexity of an organism. Moreover, compared with the mammalian tumor-bearing model, organoids are intuitive and visual, and real-time effect observations can be performed, which provides experimental reproducibility.36 Therefore, this technology is considered a breakthrough for guiding treatment of patients with cancer. We cultured bladder cancer cell organoids and then compared the tumor inhibition by shRNA and CRISPR-Cas13a. We found that both can significantly inhibit tumor volume, and that CRISPR-Cas13a has a stronger inhibitory effect out of the two methods. These results further illustrate the extensive advantages of CRISPR-Cas13a-mediated therapy.
In summary, we compared the efficiency of CRISPR-Cas13a with that of shRNA and demonstrated that the CRISPR-Cas13a gene-editing system is a novel tool for the efficient knockdown of FGFR3-TACC3 mRNA, which inhibited bladder tumor growth in vitro and in vivo. To the best of our knowledge, our study is the first to employ the CRISPR-Cas13a gene-editing system to knock down fusion genes for possible bladder cancer treatment. Knocking down FGFR3-TACC3 has the potential to become a new and efficient treatment option for bladder cancer.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Funding Statement
This research was funded by the Science, Technology & Innovation Commission of Shenzhen Municipality, grant numbers JCYJ20190806165209164 and JCYJ20170818161642912, and the Shenzhen High-level Hospital Construction Fund.
Data Sharing Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The animal study protocol was approved by the Ethics Committee of Guangzhou University of Chinese Medicine (protocol number 20220310019). The study was conducted in strict accordance with the guidelines in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Singh D, Chan JM, Zoppoli P, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science. 2012;337(6099):1231–1235. doi: 10.1126/science.1220834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frattini V, Pagnotta SM, Tala, Fan JJ, et al. A metabolic function of FGFR3-TACC3 gene fusions in cancer. Nature. 2018;553(7687):222–227. doi: 10.1038/nature25171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamura R, Yoshihara K, Saito T, et al. Novel therapeutic strategy for cervical cancer harboring FGFR3-TACC3 fusions. Oncogenesis. 2018;7(1):4. doi: 10.1038/s41389-017-0018-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chew NJ, Nguyen EV, Su SP, et al. FGFR3 signaling and function in triple negative breast cancer. Cell Commun Signal. 2020;18(1):13. doi: 10.1186/s12964-019-0486-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Best SA, Harapas CR, Kersbergen A, Rathi V, Asselin-Labat ML, Sutherland KD. FGFR3-TACC3 is an oncogenic fusion protein in respiratory epithelium. Oncogene. 2018;37(46):6096–6104. doi: 10.1038/s41388-018-0399-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizukami T, Sakai K, Naruki S, et al. Identification of a FGFR3-TACC3 fusion in esophageal cancer. Ann Oncol. 2017;28(2):437–438. doi: 10.1093/annonc/mdw550 [DOI] [PubMed] [Google Scholar]
- 7.Ou SI, Horn L, Cruz M, et al. Emergence of FGFR3-TACC3 fusions as a potential by-pass resistance mechanism to EGFR tyrosine kinase inhibitors in EGFR mutated NSCLC patients. Lung Cancer. 2017;111:61–64. doi: 10.1016/j.lungcan.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo G, Sun X, Chen C, et al. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nat Genet. 2013;45(12):1459–1463. doi: 10.1038/ng.2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noeraparast M, Krajina K, Pichler R, et al. FGFR3 alterations in bladder cancer: sensitivity and resistance to targeted therapies. Cancer Commun. 2024;44(10):1189–1208. doi: 10.1002/cac2.12602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly CC C, Zhang W, Zhang Q, et al. FGFR3-TACC3 fusion proteins act as naturally occurring drivers of tumor resistance by functionally substituting for EGFR/ERK signaling. Oncogene. 2017;36:471–481. doi: 10.1038/onc.2016.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Association for Cancer Research. FGFR3-TACC3 activates mitochondrial respiration via PIN4 phosphorylation. Cancer Discov. 2018;8(2):139. doi: 10.1158/2159-8290.CD-RW2018-008 [DOI] [PubMed] [Google Scholar]
- 12.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 13.Grayson M. Bladder cancer. Nature. 2017;551(7679):S33. doi: 10.1038/551S33a [DOI] [PubMed] [Google Scholar]
- 14.Makarova KS, Haft DH, Barrangou R, et al. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol. 2011;9(6):467–477. doi: 10.1038/nrmicro2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knott GJ, Doudna JA. CRISPR-Cas guides the future of genetic engineering. Science. 2018;361(6405):866–869. doi: 10.1126/science.aat5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rath J. Ethics Dumping. Cham: Springer; 2018:107–113. [Google Scholar]
- 17.Xu S, Kim J, Tang Q, et al. CAS9 is a genome mutator by directly disrupting DNA-PK dependent DNA repair pathway. Protein Cell. 2020;11(5):352–365. doi: 10.1007/s13238-020-00699-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haapaniemi E, Botla S, Persson J, Schmierer B, Taipale J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med. 2018;24(7):927–930. doi: 10.1038/s41591-018-0049-z [DOI] [PubMed] [Google Scholar]
- 19.Kosicki M, Bradley A, Bradley A. Repair of CRISPR–Cas9-induced double-stranded breaks leads to large deletions and complex rearrangements. Nat Biotechnol. 2018;36:765–771. doi: 10.1038/nbt.4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xin Y, Huang M, Guo WW, Huang Q, Zhang LZ, Jiang G. Nano-based delivery of RNAi in cancer therapy. Mol Cancer. 2017;16(1):134. doi: 10.1186/s12943-017-0683-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen P, Gu WL, Gong MZ, Wang J, Li DQ. shRNA-mediated silencing of hTERT suppresses proliferation and promotes apoptosis in osteosarcoma cells. Cancer Gene Ther. 2017;24(8):325–332. doi: 10.1038/cgt.2017.22 [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Lu Z, Wientjes MG, Au JL-S. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010;12(4):492–503. doi: 10.1208/s12248-010-9210-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–138. doi: 10.1038/nrd2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gootenberg JS, Abudayyeh OO, Lee JW, et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017;356(6336):438–442. doi: 10.1126/science.aam9321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, Liu X, Zhou J, et al. The CRISPR‐cas13a gene‐editing system induces collateral cleavage of RNA in glioma cells. Adv. Sci. 2019;6(20):1901299. doi: 10.1002/advs.201901299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 27.Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries (vol 68, pg 394, 2018). Ca-a Cancer J Clin. 2020;70(4):313. [DOI] [PubMed] [Google Scholar]
- 28.Lenis AT, Lec PM, Chamie K, Mshs M. Bladder cancer: a review. JAMA. 2020;324(19):1980–1991. doi: 10.1001/jama.2020.17598 [DOI] [PubMed] [Google Scholar]
- 29.Snel B, Bork P, Huynen M. Genome evolution: gene fusion versus gene fission. Trends Genet. 2000;16(1):9–11. doi: 10.1016/S0168-9525(99)01924-1 [DOI] [PubMed] [Google Scholar]
- 30.Mitelman F, Johansson B, Mertens F. The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer. 2007;7(4):233–245. doi: 10.1038/nrc2091 [DOI] [PubMed] [Google Scholar]
- 31.Kummerfeld SK, Teichmann SA. Relative rates of gene fusion and fission in multi-domain proteins. Trends Genet. 2005;21(1):25–30. doi: 10.1016/j.tig.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 32.Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15(2):188–200. doi: 10.1101/gad.862301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson AL, Burchard J, Schelter J, et al. Widespread siRNA “off-target” transcript silencing mediated by seed region sequence complementarity. RNA. 2006;12(7):1179–1187. doi: 10.1261/rna.25706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abudayyeh OO, Gootenberg JS, Konermann S, et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 2016;353(6299):aaf5573. doi: 10.1126/science.aaf5573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abudayyeh OO, Gootenberg JS, Essletzbichler P, et al. RNA targeting with CRISPR-Cas13. Nature. 2017;550(7675):280–284. doi: 10.1038/nature24049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18(7):407–418. doi: 10.1038/s41568-018-0007-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.