Abstract
Purpose
Intrathecal morphine is increasingly used for pain management in laparoscopic colorectal surgery. While ropivacaine shows advantages of reduced cardiotoxicity and faster motor recovery compared to bupivacaine, the impact of intrathecal morphine-ropivacaine combination on postoperative recovery quality remains unclear. This study aimed to evaluate this combination’s effect on recovery outcomes after laparoscopic colorectal surgery.
Patients and Methods
In this randomized, double-blind, placebo-controlled trial, 78 patients undergoing laparoscopic colorectal surgery received either preservative-free intrathecal morphine 250 μg with ropivacaine 15 mg (Intrathecal group) or a sham subcutaneous saline injection (Control group). The primary outcome was the Quality of Recovery-15 (QoR-15) score 24 hours after surgery. Secondary outcomes included pain scores, opioid consumption, and adverse effects.
Results
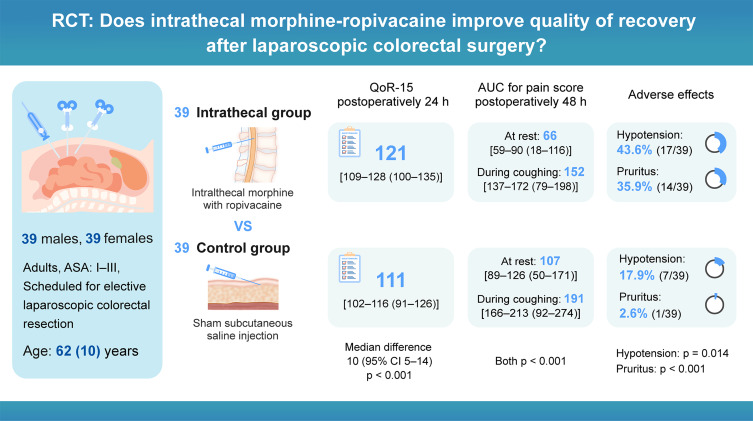
The intrathecal group showed significantly higher QoR-15 scores 24 hours postoperatively compared to the control group (median [IQR]: 121 [109−128] vs 111 [102−116], p < 0.001), with improvements in pain management (p < 0.001), physical comfort (p = 0.001), and physical independence (p = 0.002). The intrathecal group had lower pain scores at rest (area under the curve 0–48 h: 66 [59–90] vs 107 [89–126], p < 0.001) and during coughing (152 [137–172] vs 191 [166–213], p < 0.001), particularly from 0.5 to 24 hours. They also required less postoperative morphine (0–48 h: 10 [6–20] vs 26 [22–36] mg, p < 0.001). While hypotension (43.6% vs 17.9%, p = 0.014) and pruritus (35.9% vs 2.6%, p < 0.001) were more frequent in the intrathecal group, but no respiratory depression occurred in either group.
Conclusion
Intrathecal morphine-ropivacaine administration improves 24-hour postoperative recovery quality and provides superior pain relief after laparoscopic colorectal surgery, despite increased but manageable side effects. Further research should focus on dose optimization and comparative studies of different intrathecal local anesthetic combinations.
Trial Registration
The Chinese Clinical Trial Registry, ChiCTR2100052337.
Keywords: intrathecal morphine, ropivacaine, pain management, quality of recovery, laparoscopic colorectal surgery
Graphical Abstract
Introduction
Colorectal cancer represents a significant global health concern, with 1.9 million new cases and 935,000 deaths worldwide in 2020, accounting for approximately 10% of all cancer diagnoses and related deaths.1 While laparoscopic surgery has emerged as the standard curative treatment, with adoption rates of 40–80% across different countries,2 postoperative pain remains a significant challenge, affecting up to 46% of patients and potentially impeding recovery.3,4
Enhanced Recovery After Surgery (ERAS) protocols have revolutionized perioperative care through their multimodal, multidisciplinary approach to improving clinical outcomes and cost-effectiveness.5,6 Within these protocols, optimal pain management is paramount, particularly the use of multimodal analgesia to reduce perioperative opioid use and their associated complications.7 In this context, intrathecal morphine is increasingly used as an adjuvant for spinal anesthesia in laparoscopic colorectal surgery, demonstrating superior pain relief and reducing opioid requirements.8,9 The combination of intrathecal morphine/ropivacaine may further benefit patients by enhancing muscle relaxation during spinal anesthesia through reduced pain responses.10 However, the impact of this approach on overall postoperative recovery remains unclear.11,12
While previous research has focused primarily on clinical outcomes such as pain scores, opioid consumption, and bowel function recovery,13 contemporary studies increasingly recognize the importance of comprehensive, patient-centered recovery assessment, including emotional and psychological aspects.14 To fill this research gap, we conducted a randomized, controlled trial evaluating the impact of spinal analgesia with intrathecal morphine/ropivacaine on overall postoperative recovery quality. We hypothesized that this approach would improve recovery outcomes, as measured by the Quality of Recovery-15 questionnaire (QoR-15).15
Materials and Methods
Study Setting and Participants
This single-center, randomized, double-blind, placebo-controlled, parallel-group trial was conducted at Fujian Provincial Hospital, Fuzhou, China, from October 26, 2021, to October 20, 2022. The study protocol (No. K2021-06-017) was approved by the Institutional Review Board of Fujian Provincial Hospital, which was registered with the Chinese Clinical Trial Registry (https://www.chictr.org.cn/showproj.html?proj=129307, identifier: ChiCTR2100052337). The study followed the principles of the Declaration of Helsinki and Good Clinical Practice guidelines.16 We report the trial according to the Consolidated Standards of Reporting Trials (CONSORT) statement.17 The protocol remained unchanged throughout the trial.
Eligible participants were adult participants (aged ≥18 years) with American Society of Anesthesiologists physical status I to III, who were scheduled for elective laparoscopic colorectal resection under general anesthesia. Exclusion criteria comprised: (1) allergy or contraindication to trial medication; (2) contraindications to spinal anesthesia; (3) chronic pain syndrome; (4) history of alcohol or substance misuse; (5) analgesic intake within 48 h before surgery; (6) hepatic dysfunction (total bilirubin ≥ 34 µmol L−¹); (7) renal impairment (estimated glomerular filtration rate < 60 mL min−¹ 1.73 m−²); and (8) cognitive impairment or language barriers preventing questionnaire completion. Written informed consent was obtained from all participants before enrollment.
Randomization and Blinding
Participants were randomized in a 1:1 ratio to one of two groups: the Intrathecal group (receiving intrathecal morphine with ropivacaine) or the Control group (receiving a sham subcutaneous saline injection in the lumbar region). An independent research assistant generated the randomization sequence using R software, with randomly permuted blocks of 4 or 6 without stratification. Treatment allocations were concealed in sequentially numbered opaque envelopes, opened only on the day of surgery. To ensure double-blinding, attending anesthesiologists, patients, and data collectors remained unaware of group assignments throughout the study. Additionally, the anesthesiologist performing the intervention had no further involvement in patient care or data collection.
Study Procedures
Intervention
All patients received standard monitoring upon arrival in the operating theatre, including pulse oximetry, non-invasive arterial blood pressure, electrocardiography, and bispectral index. Following positioning in the lateral decubitus position and skin preparation and draping, an experienced anesthesiologist administered 1% lidocaine subcutaneously at the L2–L3 interspace. For the Intrathecal group, a spinal needle was inserted into the subarachnoid space. After confirming cerebrospinal fluid flow, the anesthesiologist injected a 3-mL mixture of preservative-free morphine 250 μg and ropivacaine 15 mg at approximately 0.2 mL/s. In contrast, Control group patients received only a subcutaneous saline injection at the L2–L3 level.
General Anesthesia Procedure
Anesthesia induction comprised intravenous sufentanil 0.5 μg/kg followed by propofol 2.0 mg/kg. After loss of consciousness, rocuronium 0.6 mg/kg was administered to facilitate orotracheal intubation. For PONV prophylaxis, dexamethasone 10 mg and tropisetron 5 mg were administered immediately after intubation. Maintenance of anesthesia was achieved with sevoflurane (0.8 minimal alveolar concentration) and remifentanil infusion, targeting hemodynamic parameters within 80–120% of preoperative values and bispectral index values of 40–60. Additional rocuronium boluses (0.15 mg/kg) were given as needed for neuromuscular blockade maintenance. Hypotension, defined as mean arterial pressure < 65 mmHg or > 30% decrease from preoperative baseline, was managed using a standardized protocol: initial treatment with rapid crystalloid infusion (250–500 mL), followed by vasopressor administration (ephedrine 5–10 mg or phenylephrine 50–100 μg boluses) if needed based on heart rate response. These episodes were most commonly observed after intrathecal injection or following anesthesia induction.
At surgery completion, remifentanil infusion was discontinued immediately before extubation and the surgeon infiltrated the wound with 0.5% ropivacaine 20 mL. When indicated, sugammadex 2 mg/kg was used to reverse neuromuscular blockade. Patients were then transferred to the post-anesthesia care unit (PACU) and moved to the ward upon achieving an Aldrete score ≥ 9.
Postoperative Pain Management
Both groups received standardized postoperative analgesia: oral paracetamol (1 g every 8 hours) and intravenous flurbiprofen axetil (50 mg every 6 hours), beginning 1 hour preoperatively and continuing for 48 hours. Pain intensity was evaluated using a numerical rating scale (NRS, 0 = “no pain”, 10 = “worst pain imaginable”). Rescue analgesia with morphine 2 mg via patient-controlled intravenous analgesia (PCIA) was provided for NRS scores > 3 or upon patient request. For postoperative complications, PONV was treated with tropisetron 5 mg and/or droperidol 0.625 mg, while pruritus received chlorpheniramine 5 mg.
Outcome Measures
The primary outcome was quality of recovery at 24 hours postoperatively, evaluated using the Quality of Recovery-15 (QoR-15) scale. This validated tool requires patients to rate 15 items on an 11-point scale (0–10), with higher scores indicating better recovery. Key secondary outcomes comprised postoperative pain intensity, assessed using NRS scores both at rest and during coughing at multiple timepoints (0.5, 1, 2, 4, 8, 12, 24, and 48 hours post-surgery), along with the area under the curve (AUC) of NRS pain scores over time. Additional pain-related measures included cumulative morphine consumption and patient satisfaction with analgesia at 48 hours postoperatively, evaluated using a 11-point NRS from 0 (very dissatisfied) to 10 (very satisfied). Safety monitoring encompassed the incidence of hypotension and opioid-related adverse effects, including PONV, pruritus, dizziness, and delayed respiratory depression.
The study also evaluated several perioperative parameters. Surgeons assessed the surgical workspace quality using the modified Leiden Surgical Rating Scale (ranging from 1 [extremely poor] to 5 [perfect]).18 Recovery metrics included emergence time, PACU stay duration, time to diet resumption, first flatus, ambulation, and urinary catheter removal, as well as total hospital stay (defined as the interval from surgery completion to discharge). To maintain study integrity, all data were collected by a single research assistant who remained blinded to group assignments.
Sample Size Calculation
The primary outcome, Quality of Recovery-15 (QoR-15) score at 24 hours postoperatively, informed our sample size calculation using PASS software (version 15; NCSS LLC, Kaysville, UT, USA). Based on a pilot study showing a standard deviation of 10.26 for the QoR-15 score, we determined that 35 patients per group would be needed to detect a minimal clinically important difference of 8 points with 90% power at a significance level of 0.05.19 To accommodate an anticipated 10% dropout rate, we increased enrollment to 39 patients per group.
Statistical Analysis
The primary analysis followed the intention-to-treat (ITT) principle, supplemented by a per-protocol (PP) analysis for sensitivity. We addressed missing data through multiple imputation. Data analysis began with normality assessment of continuous variables using the Shapiro–Wilk test and Q-Q plots. For between-group comparisons, we used independent t-tests for normally distributed data, Mann–Whitney U-tests for non-normally distributed data, and chi-squared or Fisher’s exact tests for categorical variables. Analysis of NRS pain scores employed linear mixed models with fixed effects for group, time, and their interaction, using Bonferroni correction for multiple comparisons. For recovery endpoints, we conducted Kaplan-Meier survival analysis with Log-rank tests and calculated hazard ratios with 95% confidence intervals (CIs) using Cox proportional hazards models. All analyses were performed using SPSS 27.0 for Windows and R version 4.4.1, with statistical significance set at p < 0.05.
Results
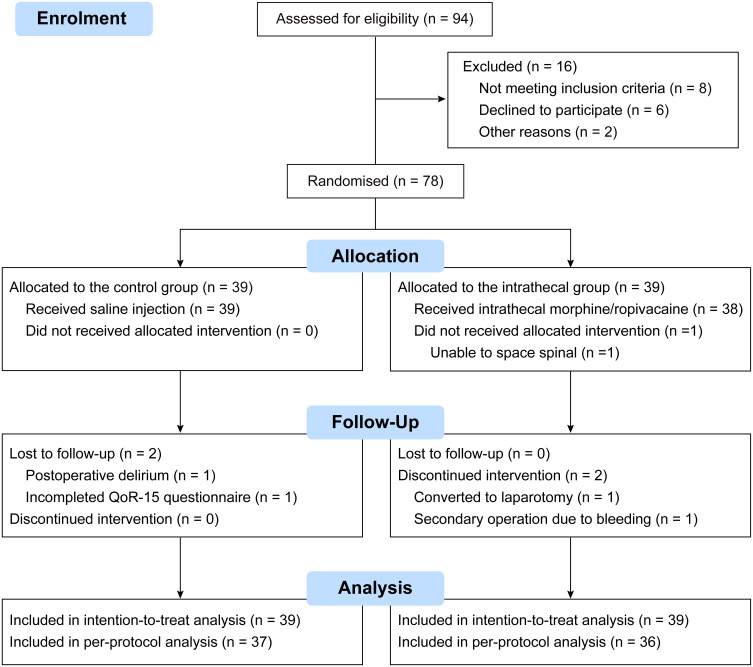
Between October 26, 2021, and October 16, 2022, we recruited 94 participants, with randomly assigned 78 participants to receive the intervention as allocated (n = 39 per group). After excluding one patient due to block failure and four due to protocol violations or loss to follow-up, we analyzed data from 73 participants. Figure 1 details the participant flow, and Table 1 shows the balanced demographics and clinical characteristics between groups.
Figure 1.
CONSORT diagram illustrating patient recruitment, allocation, follow-up, and analysis.
Abbreviation: QoR-15, Quality of Recovery-15.
Table 1.
Preoperative Baseline and Clinical Characteristics
| Control Group n = 39 | Intrathecal Group n = 39 | p value | |
|---|---|---|---|
| Age, years | 66 (57–71) | 60 (55–68]) | 0.056 |
| Height, cm | 165 (7) | 164 (6) | 0.629 |
| Weight, kg | 62 (8) | 64 (11) | 0.421 |
| BMI, kg.m−2 | 22.9 (2.6) | 23.7 (3.4) | 0.236 |
| Sex, n (%) | 0.488 | ||
| Male | 25 (64%) | 22 (56%) | |
| Female | 14 (36%) | 17 (44%) | |
| ASA physical status, n (%) | 0.801 | ||
| 1 | 4 (10%) | 5 (13%) | |
| 2 | 33 (85%) | 33 (85%) | |
| 3 | 2 (5%) | 1 (3%) | |
| Duration of surgery; min | 190 (170–240) | 205 (160–230) | 0.968 |
| Duration of anesthesia; min | 210 (185–265) | 230 (180–265) | 0.900 |
| Surgical procedure, n (%) | 0.481 | ||
| Rectum resection | 14 (36%) | 17 (44%) | |
| Sigmoidoscopy | 13 (33%) | 8 (21%) | |
| Left colectomy | 5 (13%) | 5 (13%) | |
| Transverse colectomy | 0 (0%) | 2 (5%) | |
| Right colectomy | 7 (18%) | 7 (18%) | |
| Comorbidities, n (%) | |||
| Hypertension | 14 (36%) | 9 (23%) | 0.214 |
| Diabetes | 9 (23%) | 6 (15%) | 0.389 |
| Anaemia | 3 (7%) | 4 (10%) | >0.99 |
| Coronary artery disease | 2 (5%) | 3 (7%) | >0.99 |
| COPD | 1 (3%) | 0 (0%) | >0.99 |
| Preoperative QoR-15 score | 136 (129–141) | 135 (127–141) | 0.649 |
| Colorectal cancer stages, n (%) | 0.521 | ||
| Stage I | 11 (28%) | 9 (23%) | |
| Stage II | 16 (41%) | 21 (54%) | |
| Stage III | 12 (31%) | 9 (23%) |
Note: Values are mean (SD), or median (IQR), number (proportion).
Abbreviations: BMI, Body Mass Index; ASA, American Society of Anesthesiologists; COPD, Chronic Obstructive Pulmonary Disease; QoR-15, Quality of Recovery-15.
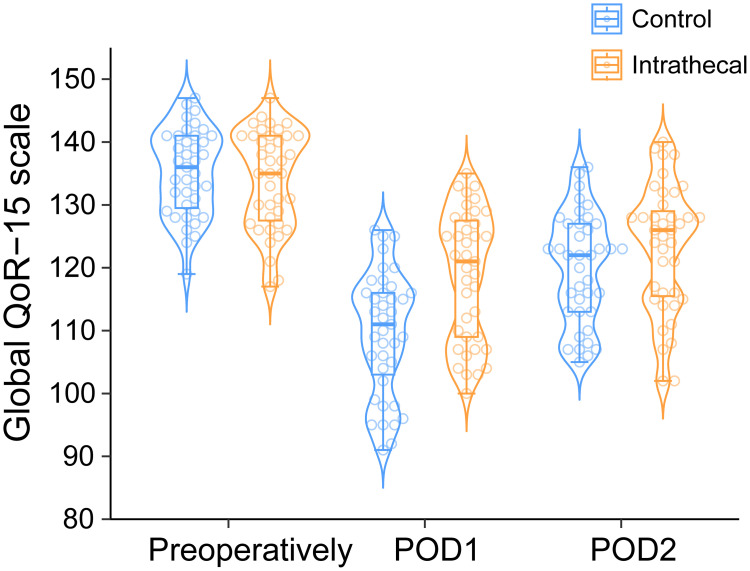
The intrathecal intervention demonstrated several significant advantages. Intraoperative remifentanil requirements were substantially lower (200 [0–400] vs 1000 [600–1500] μg, p < 0.001), and surgical conditions improved (modified L-SRS: 5 [4–5] vs 4 [4–5], p = 0.031). At 24 hours postoperatively, QoR-15 scores were significantly higher in the intrathecal group (121 [109−128] vs 111 [102−116], p < 0.001; difference: 10 [95% CI 5−14]; Figure 2). Per-protocol analyses confirmed these findings (Figure S1). Sub-domain analysis revealed improvements in pain management (difference: 2 [95% CI 2−3], p < 0.001), physical comfort (3 [95% CI 1−5], p = 0.001), and physical independence (2 [95% CI 1−3], p = 0.002), while emotional state and psychological support showed no significant differences (Figure S2A). These benefits diminished by 48 hours, neither global QoR-15 scores (126 [115−130] vs 122 [113−127], p = 0.092, difference: 4 [95% CI −1 to 8]) nor sub-domains showed significant differences between groups (all p > 0.05, Figure S2B).
Figure 2.
Quality of Recovery-15 (QoR-15) score distribution over time.
Notes: Beeswarm-violin plots display QoR-15 scores for control (blue) and intrathecal (orange) groups at pre-operative, 24 h, and 48 h post-surgery timepoints. Individual data points appear as circles. Each plot features median values (horizontal line), interquartile ranges (box), and distribution boundaries (whiskers, 1.5 times IQR). The intrathecal group showed significantly higher scores at 24 h post-surgery (121 [109−128] vs 111 [102−116], p < 0.001), with this difference diminishing at 48 h (126 [115−130] vs 122 [113−127], p = 0.092).
Abbreviations: QoR-15, Quality of Recovery-15; IQR, Interquartile Range.
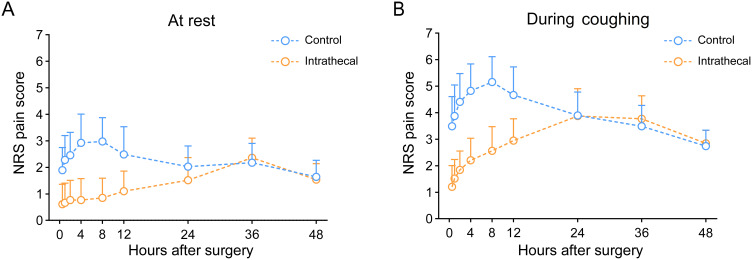
Pain control showed marked improvement in the intrathecal group, as demonstrated by significantly lower AUC for pain scores during the first 48 postoperative hours (Table 2). At rest, the intrathecal group showed median [IQR] scores of 66 [59–90] compared to 107 [82–126] in the control group (Figure 3A, p < 0.001). During coughing, scores were 152 [137–172] versus 191 [166–213], respectively (Figure 3B, p < 0.001). Linear mixed models revealed that the analgesic effect of intrathecal morphine was most pronounced from 0.5 to 24 hours at rest and from 0.5 to 12 hours during coughing (Figure 3, Tables S1–S4). Accordingly, postoperative morphine consumption was markedly lower in the intrathecal group during the first 24 hours (6 [0–8] vs 18 [14–26] mg, p < 0.001), with this difference persisting but diminishing in the 24–48 hour period (8 [2–10] vs 8 [8–12] mg, p = 0.041).
Table 2.
Perioperative Outcomes
| Control Group n = 39 | Intrathecal Group n = 39 | p value | |
|---|---|---|---|
| Perioperative opioid requirements | |||
| Intraoperative remifentanil, μg | 1000 (600–1500) | 200 (0–400) | <0.001 |
| Morphine 0–24h, mg | 18 (14–26) | 6 (0–8) | <0.001 |
| Morphine 24–48h, mg | 8 (8–12) | 8 (2–10) | 0.041 |
| Pain scores (AUC) | |||
| At rest 0–24h | 59 (45–71) | 24 (16–35) | <0.001 |
| During coughing 0–24h | 108 (97–120) | 68 (53–80) | <0.001 |
| Surgical conditions | |||
| Modified L-SRS | 5 (4–5) | 4 (4–5) | 0.031 |
| Rocuronium, mg | 80 (50–100) | 100 (70–130) | 0.024 |
| Adverse events, n (%) | |||
| Hypotension | 7 (17.9%) | 17 (43.6%) | 0.014 |
| PONV | 9 (23.1%) | 16 (41.0%) | 0.089 |
| Dizziness | 11 (28.2%) | 10 (25.6%) | 0.799 |
| Pruritus | 1 (2.6%) | 14 (35.9%) | <0.001 |
| Patient satisfaction | 10 (9–10) | 10 (10–10) | 0.038 |
Note: Values are presented as median (interquartile range) or number (proportion) as appropriate.
Abbreviations: L-SRS, Leiden-Surgical Rating Scale; AUC, Area Under the Curve; PONV, Postoperative Nausea and Vomiting.
Figure 3.
Postoperative pain scores comparison between study groups.
Notes: Line graphs illustrate NRS pain scores for control (blue) and intrathecal (orange) groups at rest (A) and during coughing (B). Measurements were taken at 0.5, 1, 2, 4, 8, 12, 24, and 48 h post-surgery. Data points show means with standard deviation error bars. The intrathecal group demonstrated consistently lower scores, most pronounced from 0.5–24 h post-surgery.
Abbreviations: QoR-15, Quality of Recovery-15; IQR, Interquartile Range; NRS, Numerical Rating Scale (0–10, no pain to worst pain imaginable).
The intrathecal group showed significantly reduced need for rescue analgesia (hazard ratio: 0.18, 95% CI 0.10–0.32; p < 0.001; Figure S3). Despite superior pain control, recovery milestones including resumption of diet, ambulation, flatus, and urinary catheter removal remained similar between groups within the first 72 hours (all p > 0.05, Figures S4–S7). The intrathecal intervention was associated with an increased incidence of hypotension (relative risk: 2.4, 95% CI 1.1–5.2, p = 0.014) and pruritus (relative risk: 14.0, 95% CI 1.9–101.4, p < 0.001). Notably, patient satisfaction scores remained higher in the intrathecal group (10 [10–10] vs 10 [9–10], p = 0.038), and no delayed respiratory depression occurred.
Discussion
This randomized controlled trial demonstrated that intrathecal ropivacaine/morphine significantly improves postoperative recovery following laparoscopic colorectal resection. Our key findings revealed: improved 24-hour QoR-15 scores, reduced pain scores and opioid consumption, and increased incidence of manageable side effects (hypotension and pruritus), although specific recovery milestones remained unchanged between groups.
We selected ropivacaine for its favorable safety profile, including reduced cardio- and neurotoxicity compared to bupivacaine, while maintaining comparable analgesic efficacy.20 Its differential blockade characteristics, with less motor block at equivalent doses, potentially support earlier mobilization.21 The chosen 15 mg dose aimed to achieve effective sensory blockade with minimal motor impairment, aligning with our clinical practice and previous studies.22
The enhanced QoR-15 scores at 24 hours postoperatively support this intervention’s potential for improving early recovery. These results parallel Koning et al’s findings of improved QoR scores following intrathecal morphine in laparoscopic colonic resection.23 Our study extends these findings by demonstrating the combined benefits of intrathecal morphine and ropivacaine, suggesting a more comprehensive approach to perioperative pain management.
The intrathecal intervention demonstrated superior pain control, with patients reporting significantly lower NRS scores during the first 24 hours, particularly notable during coughing—a critical factor for early mobilization and recovery. This enhanced pain management was reflected in substantially reduced consumption of both intraoperative remifentanil and postoperative morphine, demonstrating the strong analgesic efficacy of intrathecal morphine/ropivacaine combination. Our findings validate previous research documenting the superior analgesic effects of intrathecal morphine in both open and laparoscopic colorectal procedures.24,25 The intervention’s success was further confirmed by higher patient satisfaction scores, indicating a more comfortable postoperative experience and improved overall recovery.
Further supporting the efficacy of the intrathecal approach, patients in this group demonstrated a significantly lower need for rescue analgesia. Yet despite enhanced pain control, key recovery milestones—including resumption of diet, ambulation, and urinary catheter removal—showed no significant differences between groups. This finding suggests that while intrathecal morphine with ropivacaine effectively manages pain and enhances overall recovery quality, it does not necessarily expedite specific recovery events. Such results underscore the multifaceted nature of postoperative recovery and emphasize the importance of evaluating outcomes beyond pain management metrics alone.
Three distinct pain patterns characterize laparoscopic colorectal surgery: incisional pain (subsiding within 6–8 hours), visceral pain (primary challenge in first 24 hours), and mild shoulder discomfort.26 The intrathecal morphine-ropivacaine combination targets these through dual mechanisms: ropivacaine blocks voltage-gated sodium channels and nerve impulses, while morphine binds to dorsal horn opioid receptors, modulating potassium and calcium channels.27,28 Additionally, our findings show that ropivacaine’s profound muscle relaxation improved surgical conditions while reducing traditional muscle relaxant requirements, thus minimizing deep neuromuscular blockade side effects.29
The impact of intrathecal morphine-ropivacaine extends beyond pain management. While QoR-15 showed improvements in physical comfort and independence due to effective pain control and reduced motor blockade, emotional and psychological recovery remained unchanged, likely influenced by preoperative anxiety, patient education, and psychological support. Despite enhanced pain control, traditional recovery milestones showed no improvement, highlighting that postoperative recovery depends on multiple factors including surgical stress response, anesthetic technique, and ERAS protocol adherence. Future research should explore combining intrathecal analgesia with early mobilization and psychological support to optimize all aspects of recovery.
Optimizing recovery requires careful balance between pain control and side effect management, including respiratory depression, pruritus, nausea, and urinary retention. Research demonstrates dose-dependent effects of intrathecal morphine (100–400 μg) on postoperative pain control, with pruritus as the primary side effect.30 Based on evidence showing respiratory depression risk above 300 μg,31 we selected a 250 μg dose to maximize analgesic benefits while minimizing complications.
Our findings revealed an increased risk of hypotension and pruritus in the intrathecal group, consistent with previous research.9,32 Notably, we observed no respiratory depression—the most serious potential complication—and PONV rates remained similar between groups, likely attributable to reduced morphine requirements and prophylactic antiemetics. These results demonstrate that careful dosing and management protocols enable safe administration of intrathecal morphine-ropivacaine while maximizing its therapeutic benefits.
This study’s key strengths include its randomized, double-blind design and use of the comprehensive QoR-15 assessment tool. However, several limitations warrant consideration. The absence of sensory testing to verify intrathecal blockade efficacy may have resulted in undetected block failures. Additionally, despite our sham procedure protocol, the possibility of incomplete blinding remains. Finally, as a single-center study, our findings may have limited generalizability.
Conclusion
Single intrathecal morphine-ropivacaine injection significantly improves 24-hour postoperative recovery and pain control after laparoscopic colorectal surgery, despite manageable side effects. Ropivacaine’s safety profile and motor recovery benefits support considering this approach for routine practice in suitable patients. Future research should target optimal dosing strategies and comparative studies of different intrathecal local anesthetic combinations.
Acknowledgments
The authors express special appreciation to Dr Fangqin Xue and Dr Liangxiang Huang for their generous support of this research. We also sincerely thank all the patients and their families for participating in this study.
Funding Statement
This study was supported by the Natural Science Foundation of Xiamen, China (No. 3502Z202374068), the Fujian Medical University Startup Fund for Scientific Research (No. 2020QH1138, 2023QH2075), the Fujian Provincial Health Technology Project (No. 2022CXA007), and the Joint Funds for the Innovation of Science and Technology, Fujian Province (No. 2023Y9275). The funders had no role in study design, data collection and analysis, publication decisions, or manuscript preparation.
Data Sharing Statement
The individual deidentified participant data, the study protocol, and the statistical analysis plan can be accessed from the corresponding author (Yusheng Yao, fjslyys@126.com) upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. doi: 10.3322/caac.21834 [DOI] [PubMed] [Google Scholar]
- 2.Eng C, Yoshino T, Ruíz-García E, et al. Colorectal cancer. Lancet. 2024;404:294–310. doi: 10.1016/S0140-6736(24)00360-X [DOI] [PubMed] [Google Scholar]
- 3.Ekstein P, Szold A, Sagie B, Werbin N, Klausner JM, Weinbroum AA. Laparoscopic surgery may be associated with severe pain and high analgesia requirements in the immediate postoperative period. Ann Surg. 2006;243:41–46. doi: 10.1097/01.sla.0000193806.81428.6f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pirie K, Traer E, Finniss D, Myles PS, Riedel B. Current approaches to acute postoperative pain management after major abdominal surgery: a narrative review and future directions. Br J Anaesth. 2022;129:378–393. doi: 10.1016/j.bja.2022.05.029 [DOI] [PubMed] [Google Scholar]
- 5.Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292–298. doi: 10.1001/jamasurg.2016.4952 [DOI] [PubMed] [Google Scholar]
- 6.Ljungqvist O, de Boer HD, Balfour A, et al. Opportunities and challenges for the next phase of enhanced recovery after surgery: a review. JAMA Surg. 2021;156:775–784. doi: 10.1001/jamasurg.2021.0586 [DOI] [PubMed] [Google Scholar]
- 7.Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152:691–697. doi: 10.1001/jamasurg.2017.0898 [DOI] [PubMed] [Google Scholar]
- 8.Colibaseanu DT, Osagiede O, Merchea A, et al. Randomized clinical trial of liposomal bupivacaine transverse abdominis plane block versus intrathecal analgesia in colorectal surgery. Br J Surg. 2019;106:692–699. doi: 10.1002/bjs.11141 [DOI] [PubMed] [Google Scholar]
- 9.Pirie K, Doane MA, Riedel B, Myles PS. Analgesia for major laparoscopic abdominal surgery: a randomized feasibility trial using intrathecal morphine. Anaesthesia. 2022;77:428–437. doi: 10.1111/anae.15651 [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Jang EA, Chung S, et al. Comparisons of surgical conditions of deep and moderate neuromuscular blockade through multiple assessments and the quality of postoperative recovery in upper abdominal laparoscopic surgery. J Clin Anesth. 2021;73:110338. doi: 10.1016/j.jclinane.2021.110338 [DOI] [PubMed] [Google Scholar]
- 11.Koning MV, Teunissen AJW, van der Harst E, Ruijgrok EJ, Stolker RJ. Intrathecal morphine for laparoscopic segmental colonic resection as part of an enhanced recovery protocol: a randomized controlled trial. Reg Anesth Pain Med. 2018;43:166–173. doi: 10.1097/AAP.0000000000000703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wongyingsinn M, Baldini G, Stein B, Charlebois P, Liberman S, Carli F. Spinal analgesia for laparoscopic colonic resection using an enhanced recovery after surgery programme: better analgesia, but no benefits on postoperative recovery: a randomized controlled trial. Br J Anaesth. 2012;108:850–856. doi: 10.1093/bja/aes028 [DOI] [PubMed] [Google Scholar]
- 13.Irani JL, Hedrick TL, Miller TE, et al. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of colon and rectal surgeons and the Society of American gastrointestinal and endoscopic surgeons. Surg Endosc. 2023;37:5–30. doi: 10.1007/s00464-022-09758-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moonesinghe SR, Jackson AIR, Boney O, et al. Systematic review and consensus definitions for the standardized endpoints in perioperative medicine initiative: patient-centered outcomes. Br J Anaesth. 2019;123:664–670. doi: 10.1016/j.bja.2019.07.020 [DOI] [PubMed] [Google Scholar]
- 15.Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology. 2013;118:1332–1340. doi: 10.1097/ALN.0b013e318289b84b [DOI] [PubMed] [Google Scholar]
- 16.Stephan D, Cordeanu EM, Gaertner S. Real life studies and good clinical practice. Lancet Haematol. 2016;3:e160. doi: 10.1016/S2352-3026(15)00301-4 [DOI] [PubMed] [Google Scholar]
- 17.Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332 [DOI] [PubMed] [Google Scholar]
- 18.Wu L, Wei SW, Xiang Z, Yu EY, Qu SQ, Du Z. Effect of neuromuscular block on surgical conditions during short-duration pediatric laparoscopic surgery involving a supraglottic airway. Br J Anaesth. 2021;127:281–288. doi: 10.1016/j.bja.2021.04.031 [DOI] [PubMed] [Google Scholar]
- 19.Myles PS, Myles DB, Galagher W, Chew C, MacDonald N, Dennis A. Minimal clinically important difference for three quality of recovery scales. Anesthesiology. 2016;125:39–45. doi: 10.1097/ALN.0000000000001158 [DOI] [PubMed] [Google Scholar]
- 20.Mather LE, Copeland SE, Ladd LA. Acute toxicity of local anesthetics: underlying pharmacokinetic and pharmacodynamic concepts. Reg Anesth Pain Med. 2005;30:553–566. doi: 10.1016/j.rapm.2005.07.186 [DOI] [PubMed] [Google Scholar]
- 21.Hansen TG. Ropivacaine: a pharmacological review. Expert Rev Neurother. 2004;4(5):781–791. doi: 10.1586/14737175.4.5.781 [DOI] [PubMed] [Google Scholar]
- 22.Oğün CO, Kirgiz EN, Duman A, et al. Comparison of intrathecal isobaric bupivacaine-morphine and ropivacaine-morphine for Caesarean delivery. Br J Anaesth. 2003;90:659–664. doi: 10.1093/bja/aeg123 [DOI] [PubMed] [Google Scholar]
- 23.Koning MV, de Vlieger R, Teunissen AJW, et al. The effect of intrathecal bupivacaine/morphine on quality of recovery in robot-assisted radical prostatectomy: a randomized controlled trial. Anaesthesia. 2020;75:599–608. doi: 10.1111/anae.14922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong SK, Onsiong SM, Chiu WK, Li MK. Use of intrathecal morphine for postoperative pain relief after elective laparoscopic colorectal surgery. Anaesthesia. 2002;57:1168–1173. doi: 10.1046/j.1365-2044.2002.02873.x [DOI] [PubMed] [Google Scholar]
- 25.Beaussier M, Weickmans H, Parc Y, et al. Postoperative analgesia and recovery course after major colorectal surgery in elderly patients: a randomized comparison between intrathecal morphine and intravenous PCA morphine. Reg Anesth Pain Med. 2006;31:531–538. doi: 10.1016/j.rapm.2006.06.250 [DOI] [PubMed] [Google Scholar]
- 26.Lirk P, Badaoui J, Stuempflen M, et al. PROcedure-SPECific postoperative pain management guideline for laparoscopic colorectal surgery: a systematic review with recommendations for postoperative pain management. Eur J Anaesthesiol. 2024;41:161–173. doi: 10.1097/EJA.0000000000001945 [DOI] [PubMed] [Google Scholar]
- 27.Rawal N. Intrathecal opioids for the management of post-operative pain. Best Pract Res Clin Anaesthesiol. 2023;37:123–132. doi: 10.1016/j.bpa.2023.01.001 [DOI] [PubMed] [Google Scholar]
- 28.Ratnasekara V, Weinberg L, Johnston SA, et al. Multimodal intrathecal analgesia (MITA) with morphine for reducing postoperative opioid use and acute pain following hepato-pancreato-biliary surgery: a multicenter retrospective study. PLoS One. 2023;18:e0291108. doi: 10.1371/journal.pone.0291108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richebé P, Bousette N, Fortier LP. A narrative review on the potential benefits and limitations of deep neuromuscular blockade. Anaesth Crit Care Pain Med. 2021;40:100915. doi: 10.1016/j.accpm.2021.100915 [DOI] [PubMed] [Google Scholar]
- 30.Koning MV, Klimek M, Rijs K, Stolker RJ, Heesen MA. Intrathecal hydrophilic opioids for abdominal surgery: a meta-analysis, meta-regression, and trial sequential analysis. Br J Anaesth. 2020;125:358–372. doi: 10.1016/j.bja.2020.05.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gehling M, Tryba M. Risks and side-effects of intrathecal morphine combined with spinal anesthesia: a meta-analysis. Anaesthesia. 2009;64:643–651. doi: 10.1111/j.1365-2044.2008.05817.x [DOI] [PubMed] [Google Scholar]
- 32.Chapron K, Sleth JC, Capdevila X, Bringuier S, Dadure C. Hyperbaric prilocaine vs. hyperbaric bupivacaine for spinal anaesthesia in women undergoing elective caesarean section: a comparative randomized double-blind study. Anaesthesia. 2021;76:777–784. doi: 10.1111/anae.15342 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The individual deidentified participant data, the study protocol, and the statistical analysis plan can be accessed from the corresponding author (Yusheng Yao, fjslyys@126.com) upon reasonable request.