Summary
The endocannabinoid system (ECS), which is composed of endocannabinoids (eCBs), cannabinoid receptors (CBRs), and associated signaling molecules, has been identified within the brain. In neuropathic pain animal models and patients, long-lasting alterations in the ECS have been observed. These changes of neurons and glial cells in the ECS contribute to the modulation of neuropathic pain. Intervention strategies such as the activation of CBRs, the enhancement of hydrolytic enzyme function, and the inhibition of synthetizing enzymes typically alleviate neuropathic pain through CBR-dependent mechanisms. Additionally, emotions such as fear, anxiety, and depression are frequently experienced with neuropathic pain. Exogenous cannabinoids can mitigate these mood disorders via CBR signaling pathways. Therefore, the targeting of long-lasting ECS alterations represents a potential therapeutic approach for both neuropathic pain and emotional disorders. In this review, the long-lasting variations in neurons and glial cells in the ECS related to neuropathic pain and the accompanying emotional comorbidities are elucidated. Furthermore, the cellular and molecular mechanisms underlying synaptic plasticity and neural circuit activities in the brain are reviewed.
Subject areas: Natural sciences, Biological sciences, Neuroscience, Systems neuroscience, Sensory neuroscience
Graphical abstract
Natural sciences; Biological sciences; Neuroscience; Systems neuroscience; Sensory neuroscience
Introduction
Lesions in or dysfunction of the central and peripheral nervous systems can lead to neuropathic pain. Maladaptive changes in central regions such as the anterior cingulate cortex (ACC), thalamus, and periaqueductal gray (PAG) may be critical for central pain regulation.1 These brain areas also play important roles in regulating emotions such as depression and anxiety.2 Research has shown that neuropathic pain can cause unpleasant sensations and emotional experiences, negatively impacting quality of life and increasing economic burdens.1,3 Common treatments such as anticonvulsants, opiates, and tricyclic antidepressants are sometimes ineffective for treating neuropathic pain. The analgesic effect of cannabis was recognized as early as the 4th century BCE.4 Cannabinoid receptor type 1/2 (CB1R/CB2R), endocannabinoids (eCBs), and enzymes involved in eCB synthesis or degradation are implicated in neuropathic pain modulation.5 The endocannabinoid system (ECS) is a complex system that includes eCBs, cannabinoid receptors, and metabolizing enzymes. Under neuropathic pain conditions, alterations in neurons and glial cells in the ECS are closely linked to both pain perception and emotional disorders. These changes affect neuronal excitability and synaptic plasticity within individual brain regions and activities across different neural circuits. In this review, changes in the ECS associated with neuropathic pain and emotional disorders are explored.
Endocannabinoid system
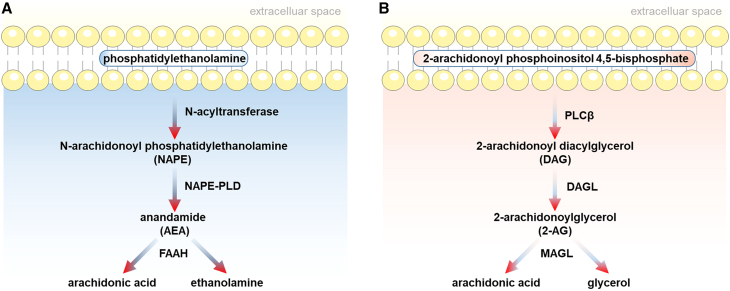
Endogenous ligands of cannabinoid receptors are known as eCBs. Endocannabinoid ligands include N-arachidonoylethanolamide or anandamide (AEA), 2-arachidonoyl glycerol (2-AG), palmitoyl ethanolamide (PEA), and oleoyl ethanolamide (OEA). They are synthesized via an activity-driven “on-demand” pattern following strong neuronal activation. The increase in calcium levels for postsynaptic neurons results in the synthesis of eCBs. The levels of eCBs can be regulated by various synthetizing and degradative/oxidative enzymes (Figure 1). To generate 2-AG, phosphatidylinositol 4, 5-bisphosphate in the membrane is catalyzed into diacylglycerol (DAG) via the phosphoinositide-specific PLCβ. Subsequently, diacylglycerol lipase (DAGL) catalyzes DAG to produce 2-AG (Figure 1A). In the biosynthesis of AEA, N-acetyltransferase catalyzes phosphatidylethanolamine in the membrane and arachidonyl, resulting in the formation of N-arachidonoyl phosphatidylethanolamine (NAPE). AEA is produced during the hydrolysis process of NAPE to phosphoanandamide via phospholipase D (NAPE-PLD) (Figure 1B). During the decomposition process, arachidonic acid (AA) and ethanolamine are derived from AEA through fatty acid amide hydrolase (FAAH). Moreover, 2-AG is degraded into AA and glycerol via monoacylglycerol lipase (MAGL).
Figure 1.
Endocannabinoid synthesis and degradation pathways
Synthesis and degradation of two major endocannabinoids, anandamide (A) and 2-AG (B). DAGL, diacylglycerol lipase; FAAH, fatty acid amide hydrolase; MAGL, monoacylglycerol lipase; NAPE-PLD, N-acylphosphatidylethanolamine-hydrolyzing phospholipase D; PLC, phospholipase C.
In contrast to neuronal transmitters, eCBs cannot be stored in vesicles. Instead, eCBs are released by postsynaptic neurons and bind to presynaptic cannabinoid receptors.6 The binding sites of eCBs have been verified to be cannabinoid receptors 1 and 2 (CB1Rs and CB2Rs).7 Both cannabinoid receptors belong to the superfamily of G protein–coupled receptors (GPCRs). The distribution of CBRs has been revealed through numerous studies, with the results demonstrating that CB1Rs are abundantly expressed in the brain. CB2Rs have also been found in the brain but at a lower density. The localization of CBRs in presynaptic neuronal terminals strongly suggests their roles in regulating synaptic transmission. CB1Rs mediate retrograde signals that result in short-term synaptic plasticity, known as depolarization-induced suppression of inhibition (DSI) or depolarization-induced suppression of excitation (DSE), and long-term depression (LTD) at excitatory and inhibitory synapses.8,9
The ECS participates in neuropathic pain
The changes in the ECS associated with neuropathic pain are summarized in Table 1. For example, the levels of AEA and 2-AG are elevated in pain-related brain regions such as the PAG, rostral ventral medulla (RVM), and lateral entorhinal cortex in chronic neuropathic pain models.10 The increased eCB levels might be related to increased biosynthesis or decreased metabolism and serve as endogenous neuroprotective mechanisms. The neuroprotective effects of eCB alterations may be related to changes in neuronal excitability and transmission. For example, endocannabinoid retrograde signaling relies on presynaptic CBR activation. As illustrated in Figure 2, presynaptic stimulation enhances the coupling of postsynaptic metabotropic glutamate receptors (mGluRs) with phospholipase C-β (PLCβ) and DAGLα. As a result, 2-AG is formed, which facilitates the interaction between the CB1R of excitatory neurons, subsequently resulting in reduced adenylyl cyclase (AC) and protein kinase A (PKA) activities, which decreases glutamate release. The activities of the vesicle-associated protein Rab3B and downstream Rab3-interacting molecule 1α (RIM1α) are inhibited following the reduction in PKA activity, leading to decreased GABA release.11 DSE and DSI modulate presynaptic neurotransmitter release by suppressing either inhibitory or excitatory neuron excitability. The short-term influence of the ECS in balancing excitatory and inhibitory transmission might underlie the hyperexcitability of the brain following neuropathic pain. Moreover, the ECS can regulate long-term synaptic transmission in neuropathic pain states. The ECS-mediated reductions in glutamate and GABA levels can ultimately lead to LTD.12
Table 1.
Changes of neuronal ECS in different brain regions under various neuropathic pain conditions
| Brain Area | Model | ECS changes | Reference |
|---|---|---|---|
| Thalamus | SNL | The levels of AEA, 2-AG, PEA, and OEA in SNL rats remained unchanged. | Jhaveri et al.13 |
| SNI | The levels of AEA were significantly reduced in the older SNI mice. In contrast, the levels of 2AG remained unchanged in these animals. The enzyme FAAH exhibited increased activity in the younger SNI mice, while it did not change in the older mice. The CB1Rs in the contralateral thalamus of SNI rats were found to be upregulated. |
Siegling et al.14 and Bishay et al.15 | |
| SCI | The CB1R expression levels in the ventral posterolateral (VPL) and ventral posteromedial (VPM) thalamic nuclei were elevated in rats with moderate and severe spinal cord injury (SCI) at 7 days post-operation. In the later phase, at 42 days post-operation, CB1R expression in the VPL and VPM was increased in the moderate injury group but decreased in the severe injury group. | Knerlich-Lukoschus et al.16 | |
| Prefrontal Cortex | SNI | The activity of FAAH was observed to be elevated in both young and aged mice subjected to Spared Nerve Injury (SNI). The levels of 2-AG were elevated in the mPFC three days post SNI surgery. The expression of DAGLα increased seven days following the injury. However, there were no changes observed in MAGL and NAPE-PLD within 35 days after nerve injury. |
Mecca et al.17 and Bishay et al.15 |
| SNL | The CB1 receptors in the medial prefrontal cortex (mPFC) exhibited upregulation at 14 days post-surgery. | Bushlin et al.18 | |
| CCI | The expression of CB2Rs was observed to be increased at day 28 after surgery. | Bai et al.19 | |
| Anterior Cingulate Cortex | SCI | The expression of CB1Rs in the anterior cingulate cortical areas, specifically in the Cg1 region and cingulate gyrus (cg), was reduced in both moderately and severely injured spinal cord injury (SCI) rats at 7 days post-operation. However, at 42 days post-operation, the CB1Rs levels in the Cg1 and cg were increased in the moderately injured group but remained unchanged in the severely injured group. | Knerlich-Lukoschus et al.16 |
| CCI | The levels of AEA and 2-AG remained unchanged in the rACC at day 1 and day 10 post-surgery. The expression of CB1Rs was elevated in the ACC at day 24 after surgery. | Hoot et al.20 and Silva-Cardoso et al.21 | |
| Insular Cortex | CPN | In common peroneal nerve injury rats, protein and mRNA expressions of CB1R and NAPE-PLD were observed to be upregulated at day 14 post-surgery. Contrarily, the FAAH level remained unaltered at this time point. Furthermore, in common peroneal nerve injury mice, CB1R levels in the rostral agranular insular cortex did not show any significant change at days 7 and 14 post-operation. | Zhang et al.22 and Jee Kim et al.23 |
| CCI | The CB1R expression was significantly increased in rats 24 days post-injury. | Silva-Cardoso et al.21 | |
| Amygdala | SNI | FAAH levels remained unchanged in both young and old SNI mice. Levels of 2-AG and precursor DAGs were increased in the basolateral amygdala (BLA) at 10 day post-injury. |
Patel et al.24 Bishay et al.15 |
| SCI | The CB1Rs levels of BLA were decreased in both moderately and severely injured SCI rats at 7 days and 42 days post-operation. | Knerlich-Lukoschus et al.16 | |
| CCI | The upregulation of CB1Rs was observed in rats 24 days post-injury. | Silva-Cardoso et al.21 | |
| Hippocampus | CCI | The expression of CB1Rs was elevated in the CA1, CA3, and Dentate Gyrus (DG) regions at 24 days post-injury. Conversely, the expression of CB2Rs remained unchanged at day 28 after surgery. | Silva-Cardoso et al.21 and Bai et al.19 |
| SCI | The CB1R densities in the CA1 region did not alter at 7 days post-operation in both moderately and severely injured SCI rats, but significantly decreased in the severe injury group at 42 days post-operation. The CB1R levels in the CA3 region increased at 7 days post-operation in severely injured SCI rats and further escalated at 42 days post-operation in both moderate and severe injury groups. The CB1R expression in the DG was diminished at 7 days and 42 days post-operation in moderately injured SCI rats. | Knerlich-Lukoschus et al.16 | |
| Periaqueductal Gray | CCI | The levels of both AEA and 2-AG significantly increased at three days post-operation. This enhancement continued and became more pronounced at seven days post-operation. The CB1R levels in vlPAG was reduced in CCI rats at 7 days post-operation. The CB2Rs expression was not changed at day 28 after surgery. |
Petrosino et al.,10 Bai et al.19 and Palazzo et al.25 |
| SCI | The densities of CB1Rs were observed to be downregulated in both moderately and severely injured spinal cord injury (SCI) rats at 7 days post-operation. On day 42, the density of CB1Rs was found to be lower in the severely injured SCI rats. | Knerlich-Lukoschus et al.16 | |
| Rostral Ventromedial Medulla | CCI | Both AEA and 2-AG levels were significantly elevated in rats subjected to chronic constriction injury (CCI) at 7 day after CCI. Concurrently, the level of PEA was notably reduced at this time point. | Petrosino et al.10 |
Abbreviations: ECS endocannabinoid system, AEA N-arachidonoylethanolamide, 2-AG 2-arachidonoyl glycerol, PEA palmitoyl ethanolamide, OEA oleoyl ethanolamide, CBR cannabinoid receptor, DAGL diacylglycerol lipase, MAGL monoacylglycerol lipase, NAPE-PLD N-arachidonoylphosphatidyl ethanolamine phospholipase D, FAAH fatty acid amide hydrolase, SNL spinal nerve ligation, SNI spared nerve selected injury, CCI chronic constriction injury, SCI spinal cord injury, CPN common peroneal nerve injury.
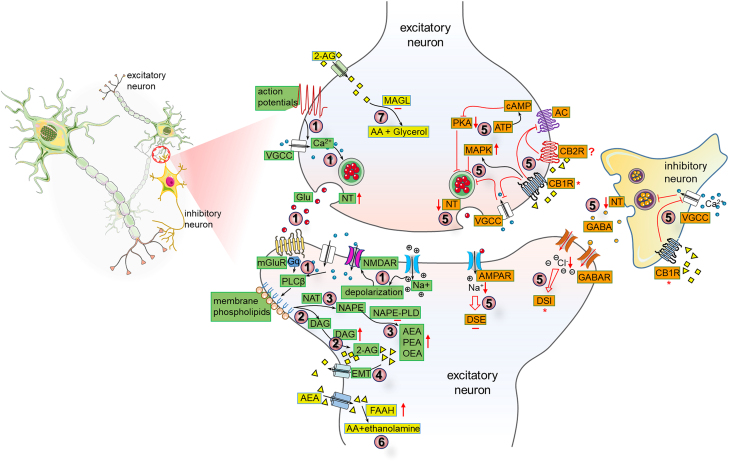
Figure 2.
Long-lasting changes in the ECS including CB1Rs mediating short-term neuronal plasticity in neuropathic pain states
In neuropathic pain conditions, endocannabinoids (eCBs) are synthesized as needed (in green). The calcium concentration in postsynaptic cells increases through voltage-gated calcium channels (VGCC) and N-methyl-D-aspartate receptors (NMDARs), which also heighten the action potentials in presynaptic terminals. The activation of synthetizing substrate plasma membrane phospholipids via calcium and/or Gq of metabotropic glutamate receptors (mGluRs) stimulated phospholipase C-β (PLCβ) (①). A key pathway for 2-AG synthesis involves PLC and diacylglycerol lipase (DAGL) enzymes (②). Other eCB such as AEA, the anti-inflammatory palmitoylethanolamide (PEA), and the anorexigenic oleoyl ethanolamide (OEA) are synthesized through N-acyltransferase (NAT) and NAPE-phospholipase D (NAPE-PLD) (③). Endocannabinoids are then transported to the synaptic cleft by the endocannabinoid membrane transporter (EMT) (④). As described in orange, CB1Rs in the presynaptic terminal of excitatory neurons are activated by these eCBs, leading to reduced activity of protein kinases like PKA, increased activity of MAP kinases (MAPK), and decreased Ca2+ influx. This results in diminished neurotransmitter (NT) release through effectors like adenylate cyclase (AC) and VGCC, ultimately influencing short-term plasticity such as depolarization-induced suppression of inhibition (DSI) and excitation (DSE) in postsynaptic cells (⑤). Once they have performed their physiological functions, eCBs are degraded by specific enzymes (in yellow). AEA is broken down by fatty acid amide hydrolase (FAAH) into arachidonic acid (AA) and ethanolamine in the postsynaptic cell (⑥), while 2-AG is converted into arachidonic acid (AA) and glycerol by monoacylglycerol lipase (MAGL) in the presynaptic cell (⑦). Symbols are used to denote increases (↑), decreases (↓), no change (−), and controversial changes (∗).
In addition to CB1Rs and CB2Rs receptors, endocannabinoid ligands bind to other receptors. The G protein-coupled receptor 55 (GPR55) and transient receptor potential subfamily V member 1 (TRPV1) receptors have been widely investigated. AEA activates TRPV1 and GPR55, whereas PEA and 2-AG potentially activate GPR55.26 The role of GPR55 in neuropathic pain has been investigated with GPR55 knockout mice in which mechanical hyperalgesia disappears after partial nerve ligation.27 TRPV1, a modestly selective calcium channel gated by AEA, exhibits in neurons and astrocytes.28 TRPV1 and CB1 receptors are coexpressed in the same brain region.16 Moreover, coactivation of CB1Rs and TRPV1 receptors by AEA may influence neuropathic pain via the cannabinoid pathway in the central nervous system. TRPV1 receptors, also known as thermoTRP channels, are involved in sex-related differences in neuropathic pain. For example, estrogen has been shown to induce hyperalgesia through the activation of the IL-23/IL-17A/TRPV1 axis and increase in PKC-ε levels in patients with chemotherapy-induced peripheral neuropathy.29 Moreover, testosterone has been shown to reduce TRPV1 receptor expression levels in an inflammatory pain model.30 Thus, hormone differences have been suggested to influence sex-related differences in neuropathic pain. Numerous animal and human studies have revealed that sex-related differences affect ECS components and their influence on neuropathic pain. For example, sex-related differences in cannabinoid metabolism, cannabinoid receptor expression levels, eCB synthesis, and cannabinoid signaling pathways might lead to differences in pain modulation related to sex. These findings support the significant role of ECS changes in the context of neuropathic pain. Considering the characteristics and physiological features of the ECS and the neuroanatomical connections throughout the brain, the ECS may exert diverse effects on various brain areas, such as the midcingulate cortex and motor cortex, which are involved in pain behavior, and the prefrontal cortex (PFC) and hippocampus, which are associated with pain sensation and related emotions.
The ECS in different brain areas
Nociceptive signals, originating from peripheral sensory neurons, are transmitted to the spinal cord dorsal horn and subsequently relayed to supraspinal structures.31 Clinical human brain imaging and animal studies both suggest that synaptic plasticity in cortical regions, such as the ACC and the insular cortex (IC), contributes to neuropathic pain.31 Additionally, the top-down descending pain modulatory system maintains a balance between pain facilitation and inhibition.32 Long-lasting changes in the ECS within the brain contribute to the persistence and modulation of neuropathic pain. Interventions that reverse these ECS changes in various brain regions have become effective treatments for neuropathic pain. For example, AEA and CB1R mRNA levels are decreased in the hippocampi of male CCI model rats.33 A reduction in depressive-like behaviors and neuropathic pain in male CCI model rats was accompanied by corrections in AEA and CB1R mRNA levels after the inhibition of FAAH.33
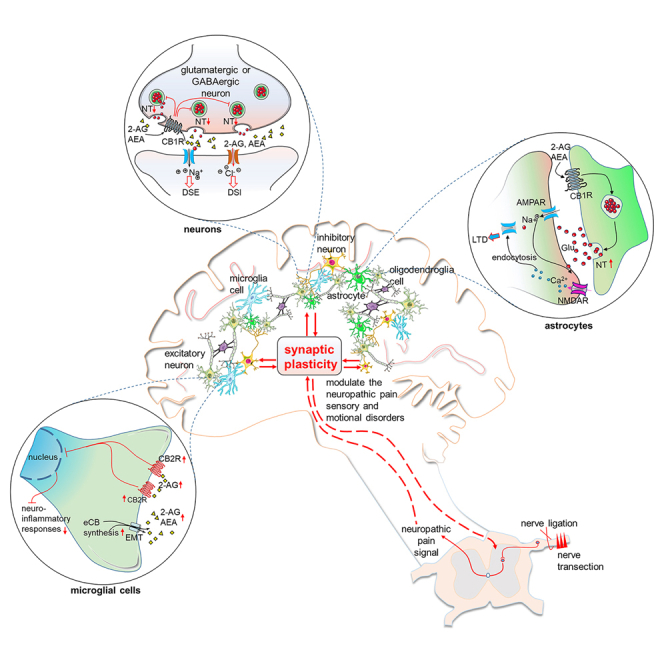
Microglias, as parts of the immune system, continually monitor the brain in a “resting” state. When neuronal injury occurs, microglias rapidly transition to activated phenotypes, facilitating neuronal repair and detoxifying the affected area.34 Endocannabinoids, which are released by microglias, play crucial roles in modulating neuropathic pain (Figure 3). Notably, 2-AG is released by microglias in a calcium-dependent manner and is hydrolyzed by b-hydrolase 6 rather than MAGL.35 In the resting state, microglias and astrocytes exhibit low CB2R levels, but the levels of these receptors for glial cells significantly increase following neuropathic pain.36 In fact, CB2Rs of microglias and astrocytes are overexpressed, whereas those of spinal cord neurons are not in SNI model mice. These elevated CB2Rs and 2-AG levels promotes the transition of microglias from proinflammatory phenotypes to anti-inflammatory phenotypes37 (Figure 3). Unpleasant emotions such as anxiety can help individuals prepare for dangerous situations, and depression can reduce distractions from threats. Anxiety- and depression-like behaviors are common in neuropathic pain models. However, when these emotions become long-lasting or are triggered by nonthreatening stimuli, individuals lose their normal function. Increased anxiety-like behavior can increase sensitivity to pain.38 These emotional disorders increase the likelihood of social isolation, increase threat responsiveness, and reduce physical movement, leading to severe pain that hinders daily life.38 These findings demonstrate the comorbidity of anxiety, depression accompanying with neuropathic pain.
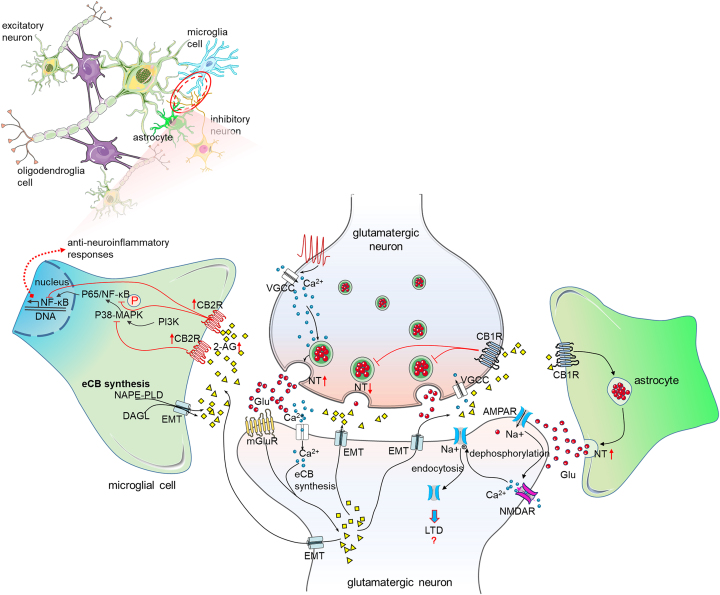
Figure 3.
Long-lasting changes of the ECS in glial cells following neuropathic pain
In neuropathic pain conditions, eCBs are produced in postsynaptic cells and microglial cells. The phosphorylated P65-NF-κB mediated inflammatory response is inhibited by eCBs. The activation of CB2Rs on microglial cells produces analgesia through the anti-inflammatory response of ECS. In astrocytes, the activation of CB1Rs results in the release of glutamates. The long-term depression (LTD) is induced through the dephosphorylation and endocytosis of α-amino-3-hydroxy-5-methyl-4-isox-azolepropionic acid receptors (AMPARs). A red upward arrow indicates an increase, a red downward arrow indicates a decrease, and a red question mark indicates an unclear change.
Thalamus
The spinothalamic tract is a classic pathway that transmits nociceptive information from the spinal cord to the brain. Noxious stimuli are encoded by neurons in the spinal cord, which then transmit this information to the thalamus.39 There are tissue-specific differences in CB1Rs expression levels in the thalamus. Higher CB1Rs expression levels were detected in the thalamic reticular nucleus than in the ventrobasal nucleus of the thalamus.40 Thus, DSI is enhanced at intra-thalamic reticular nucleus synapses via the calcium-dependent release of 2-AG.40 The oscillatory activity in the thalamic reticular nucleus depends on reciprocal connections between thalamic relay cells and neurons. Therefore, the degree of thalamic reticular nucleus-dependent synchronization could be regulated by the ECS.
The components of the ECS in the thalamus do not consistently change across different neuropathic pain models (Table 1). The expression levels of CB1Rs of ventral posterolateral and ventral posteromedial thalamic nuclei and reticular thalamic (RT) nuclei were increased in the early stage of spinal cord injury (SCI).16 In the delayed stage, CB1Rs expression levels were elevated in the moderate injury group but significantly decreased in the severe injury group.16 The reduction in CB1R expression may promote the formation of allodynia, resulting in severe neuropathic pain. Notably, CB1R expression peaked within the first two days and gradually returned to a normal level. In severe SCI model rats, CB1R-positive neurons were also shown to be expressed TRPV1-p receptors.16 However, changes in the expression levels of genes related to neuropathic pain and their influence on SCI pain have not been investigated. Additionally, CB1R expression levels are increased only in the contralateral thalamus and not in the ipsilateral thalamus.14 It has been hypothesized that this transient increase in CB1Rs expression might increase the antinociceptive efficacy of eCBs under neuropathic pain conditions. The ECS in the contralateral thalamus plays an important role in the modulation of neuropathic pain.
In the thalamus, the ECS of microcircuits that project to different subregions modulates neuropathic pain. The posterior complex (Po) of the thalamus is an important region of the thalamic nucleus. Nociceptive information is delivered to the Po of the thalamus via the zona incerta (ZI) in the subthalamic nucleus (Figure 4A). Parvalbumin-positive neurons in the ZI act as extrathalamic GABAergic inputs that control the Po of the thalamus.41 The obvious hypoexcitability of the ventral ZI with the Po of the thalamus (ZIv-Po) might cause neuropathic pain.41 CB1Rs are specifically expressed at parvalbumin-positive terminals in the ZIv-Po pathway. For example, local microinjection of the CB1R agonist WIN 55,212-2 resulted in increased pain thresholds. Selective inhibition of the ZIv-Po circuit or administration of cannabinoids in the Po of the thalamus ameliorated pathological pain.41 In addition, thalamic reticular nucleus projections to the ventroposterior region of the thalamus are responsible for hyperalgesia caused by chronic sleep disruption-induced chronic pain.42 More importantly, inhibitory effects have been observed in this pathway. For example, neuron activity in the thalamic reticular nucleus decreased significantly after chronic sleep disruption. However, the activation of neurons in the ventroposterior region increased with increased c-Fos expression levels. Metabolomic analysis revealed that the levels of N-arachidonoyl dopamine, an endocannabinoid, and CB1R activity both decreased in the thalamic reticular nucleus following chronic sleep disruption-induced chronic pain.43 Thus, CB1Rs expression levels in the thalamic reticular nucleus are much greater than those in other regions. Synchronization in the thalamus could be modulated by the ECS. In neuropathic pain states, the activity of the ECS varies across different periods and degrees of injury within the same neuropathic pain model. Moreover, CB1Rs expression levels in thalamic subregions varied temporally and spatially. The Po of the thalamus and the thalamic reticular nucleus are two important areas for regulating neuropathic pain that rely on ECS-mediated changes in synaptic plasticity.
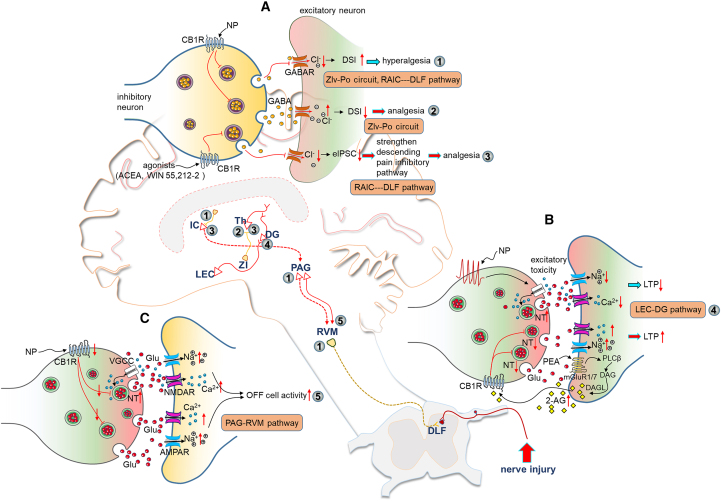
Figure 4.
Alterations of the ECS in pain pathways of the brain in neuropathic pain states
(A) In neuropathic pain conditions, the enhancement of CB1R-DSI is accompanied by disinhibition on postsynaptic cells. The increased hyperexcitability in the posterior complex of the thalamus (Po) and rostral agranular insular cortexes (RAIC), which are parts of the subthalamic nucleus zona incerta (ZI) to the Po circuit (ZIv-Po) and the RAIC to the dorsolateral fasciculus (DLF) of the spinal dorsal horn (RAIC-DLF pathway) respectively, causes hyperalgesia in neuropathic pain models (①). The activation of CB1Rs increases the disinhibition on the RAIC and produces analgesia via strengthening the descending pain inhibitory pathway (②). Meanwhile, activation of CB1Rs in the ZIv-Po circuit, accompanied by an increase in the DSI amplitude produce analgesic effect (③).
(B) Excitatory toxicity of glutamates impairs long-term potential (LTP) in the lateral entorhinal cortex (LEC)-dentate gyrus (DG) pathway under neuropathic pain conditions. The concentration of 2-AG is increased by the palmitoylethanolamide (PEA). Subsequently, CB1R-mediated inhibition of glutamate release reverses LTP damage (④).
(C) In SNI mice, downregulation of CB1Rs in the PAG enhances OFF cell activity of the RVM. As a result, the inhibitory action of the PAG-RVM pathway on nociceptive signals is strengthened (⑤). A red upward arrow represents an increase, while a red downward arrow represents a decrease.
Prefrontal cortex (PFC)
Strong functional connections exist in the medial prefrontal cortex (mPFC) and the thalamus. Nociceptive information, which originates from the thalamus, is amplified in the mPFC.17 A direct relationship between spontaneous pain and mPFC activation was shown in patients via functional imaging.44 Moreover, the dorsolateral prefrontal cortex (DLPFC) has been demonstrated to play an important role in neuropathic pain.45 Most CB1Rs are expressed in interneurons, whereas fewer CB1Rs are located in glutamatergic neurons in human PFC tissues.46 Furthermore, AEA- and 2-AG-mediated plasticity in the PFC shows both layer and neuronal specificities.47 Dysfunction of the ECS has been associated with emotional disorders. For example, increased anxiety-like behaviors were reported after 2-AG levels increased in the PFC.48
Under neuropathic pain conditions, changes in synthetizing enzymes affect endocannabinoid levels (Table 1). For example, the levels of DAGL, a 2-AG synthesizing enzyme, were transiently increased in the mPFC of SNI model rats.17 Consequently, 2-AG levels were increased in the mPFC after nerve injury. Interestingly, functional enhancement in DAGL expression was found in the first seven days, after which it recovered to normal levels in SNI model rats. For degraded enzymes such as MAGL and FAAH, inconsistent changes were detected in the PFC in the context of neuropathic pain (Table 1). Differences in neuropathic pain models and brain areas might account for these variations. The majority of CB1Rs are expressed in the terminals of GABAergic neurons in the mPFC.49 The inhibitory effect of GABAergic neurons on excitatory neurons is suppressed by the ECS through presynaptic CB1Rs. For example, a double immunoelectron microscopy study on the rodent mPFC revealed that CB1Rs in GABAergic neurons are associated with postsynaptic mGluR5 in pyramidal neurons.50 The activation of mGluR5 plays an important role in the change in glutamatergic signaling into retrograde eCB signaling. For example, VU0360172 (a positive allosteric modulator of mGluR5) increases the synaptic output of pyramidal cells by increasing the amplitude of CB1R-mediated DSI in the mPFC.50 The PLC-DAG pathway for eCB biosynthesis might be responsible for this enhanced DSI following mGluR5 activation.50 In contrast, the DSI amplitude was decreased in the mPFC on day 35 after SNI.17 Furthermore, excessive activation of CB1Rs might result in reduced functionality of the ECS. Therefore, the CB1R-mediated DSI of GABAergic neurons was not observed in the mPFC of SNI model mice (Figure 1). The mechanisms underlying various changes in DSI amplitude in different neuropathic pain models, brain regions, and experimental protocols should be explored in the future.
Moreover, mGluR5 activation is needed for LTD in the prelimbic area of the PFC.47 The activation of phospholipase C, a primary intracellular effector of mGluR5, facilitates inositol triphosphate-mediated release of Ca2+ for postsynaptic neurons. Inhibitory transmission may be decreased in a short- or long-term manner when mGluR5 interacts with presynaptic CB1Rs. In general, the suppression of GABAergic neurons supports the excitability of mPFC pyramidal neurons. The DSI amplitude increases, and the LTD of GABAergic neurons contributes to the hyperexcitability of pyramidal neurons in the mPFC. However, with the accumulation of 2-AG, the CB1Rs function loss of GABAergic neurons leads to uncontrollable inhibition of inputs to pyramidal neurons in the later stage of SNI model rats. The desensitized state of CB1Rs might be the reason for the LTD of pyramidal neurons (Figure 3). Thus, long-lasting changes in the ECS regulate the synaptic plasticity of the PFC in both directions. Both synaptic transmission in the PFC and the PFC-led top-down control of pain sensation are controlled by the ECS. In the chronic phase in SNI model rats, mechanical hyperalgesia and the action potentials of wide-dynamic-range neurons in the spinal cord dorsal horn were both inhibited by the activation of CB1Rs in the mPFC.51 Furthermore, the decreased mechanical threshold and the increased activity of dorsal root ganglion neurons and wide-dynamic-range neurons in the early phase of SNI were reversed by the inhibition of CB1Rs in the mPFC.51 This research suggested that the top-down control of neuropathic pain in the mPFC–spinal cord dorsal horn–dorsal root ganglion pathway could be regulated by blocking or activating CB1Rs in different phases. These studies suggest that interactions in the ECS in different phases of neuropathic pain models are challenging to be investigated.
Glutamatergic TRPV1 receptors also participate in allodynia in SNI rats through the basolateral amygdala complex (BLA)-mPFC pathway. The overexpression of TRPV1 receptor is associated with increased glutamate release.52 AEA-induced excitatory responses of the BLA-mPFC pathway rely on TRPV1 receptors. This excitatory effect is inhibited with increases in FAAH levels in SNI model rats.52 Nevertheless, the increase in 2-AG expression in the mPFC might provide negative feedback control on the TRPV1-mediated increase in glutamate release by presynaptic CB1Rs.52
Emotional dysfunction owing to neuropathic pain is also regulated by the mPFC. In the long-lasting phase of neuropathic pain, increased eCB levels lead to CB1R dysfunction, breaking the feedback loop and resulting in uncontrolled inhibitory inputs from GABAergic neurons, suppressing mPFC function and leading to depression.17 Glutamatergic neurons in the mPFC and serotonergic neurons in the prelimbic mPFC are involved in mood disorders.53 Exogenous cannabinoids such as cannabidiol (CBD) produce antidepressive effects in the prelimbic mPFC through CB1Rs and serotonergic receptors.54 These findings suggest that cannabinoid and non-cannabinoid receptors of different neurons contribute to regulating anxiety and depression following neuropathic pain.
These findings reveal that CB1Rs of GABAergic neurons in the mPFC play critical roles in neuropathic pain. The increase in the DSI amplitude in the early period and the loss of function of CB1Rs in the later period contributed to hyperalgesia and depression, respectively. The mechanism underlying neuropathic pain in the mPFC in these later stages is fascinating. It is not clear whether this phenomenon occurs in other neuropathic pain models. Moreover, AEA, 2-AG, PEA and OEA levels in the PFC did not show sex-related differences in SNI model rats.55 More research is needed to confirm sex-related differences in the ECS in the PFC.
Anterior cingulate cortex (ACC)
Our previous studies indicated that neuronal plasticity within the ACC is essential for the manifestation of neuropathic pain behavior and emotional disorders.56 Anatomical evidence has shown that noxious inputs are transferred to the ACC from the spinothalamic tract and the thalamic nuclei.57 In addition to this medial stream, the somatosensory cortex, which represents the lateral stream, transmits the sensory aspects of pain to the ACC.58 Reductions in the functional connectivity of the ACC and somatosensory cortex after sublingual tetrahydrocannabinol (THC) administration result in the inhibition of chronic radicular neuropathic pain.59 Additionally, the ACC has been shown to activate spinal neurons through a direct descending pathway.60 Consequently, the ACC serves as a crucial integrative center for pain-related sensory processing and emotional responses. Through immunohistochemistry, moderate CB1R-positive cell bodies and fibers were discovered in the ACC.61 CB1Rs are associated with the regulation of the excitability of pyramidal neurons.62 Changes in the levels of 2-AG within the ACC impact pain behavior and conditioned fear via 2-AG-CB2R signaling.63
Significant reductions in CB1R densities have been found in anterior cingulate cortical area 1 (Cg1) and the cingulate gyrus (cg) in the early phase of SCI model rats (Table 1). Moreover, CB1R-mediated G protein activity in the ACC diminished after 10 days of CCI.20 This decrease in both the density and activity of CB1Rs mirrored the loss of function of CB1Rs in the mPFC. The underlying mechanisms for these alterations in CB1Rs may be associated with excessive eCB and CB1R desensitization. Notably, the loss of function of CB1Rs and the corresponding negative effects in CCI model rats could be corrected with a CB1R agonist.20 This CB1R desensitization appears to be mediated by elevated levels of NAEs—Nhomo-c-linolenoylethanolamine and N-docosatetraenoylethanolamine—in the ACC of CCI mice rather than AEA or 2-AG levels.20 However, CB1 and TRPV1 receptors levels were both increased in the ACC of male CCI model rats on the 24th experimental day.21 Changes in the ECS of the ACC may vary during different periods of neuropathic pain.
Pain-related emotional information is transmitted to higher cortex areas such as the ACC and the anterior insula cortex (AIC) during pain memory consolidation.64 Notably, CB1Rs levels were increased in the ACC after CBD application in CCI model rats, potentially facilitating the analgesic effect of CBD. The ACC also receives input from the basolateral amygdala (BLA), which encodes negative emotions.65 CBD produces analgesic and anxiolytic effects by inhibiting the ACC-AIC-BLA circuit after CB1Rs are activated in the BLA.21 Overall, the activity of the ECS in the ACC is first decreased, followed by an increase in later stages under neuropathic pain conditions. It is speculated that the loss of function of CB1Rs in the ACC might be involved in emotional disorders. In addition, DSE, CB2Rs levels, and GPR55 receptors levels in the ACC have been reported to be associated with inflammatory pain.63,66,67 Future studies should clarify their roles in neuropathic pain.
Insular cortex (IC)
Cortico-cortical interactions between the IC and other structures, such as the ACC and thalamus, indicate that the IC serves as a crucial interface for both top-down perception and bottom-up regulation of neuropathic pain.68 Electrophysiological experiments have shown that enhanced excitatory synaptic transmission in the IC contributes to the generation of neuropathic pain.22 Additionally, CB1Rs, NAPE-PLD, FAAH and TRPV1 receptors in the IC modulate neuropathic pain.23 Cannabinoid receptor 1 in the IC plays an important role in the acquisition, extinction and reconsolidation of memory in rats.69
The deactivation of FAAH or MAGL leads to an increase in eCB levels. For example, URB597, an antagonist of FAAH, elevates the AEA concentration in the IC, thereby inhibiting the excitability of IC neurons.23 The increased eCB levels lead to analgesia production by reducing the excitability of IC neurons. Although there is no change in FAAH levels in the IC, the levels of FAAH signaling-related factors and NAPE-PLD were elevated in common peroneal nerve ligation rats.23 These findings suggest that AEA levels in the IC may increase due to the increased activity of its synthetizing enzyme NAPE-PLD. Consequently, the increased AEA levels result in enhanced CB1Rs expression and activity.23 Furthermore, CB1Rs levels were increased in the anterior IC of CCI rats (Table 1).21 However, CB1Rs levels remained unchanged in the bilateral rostral agranular insular cortex (RAIC) of common peroneal nerve ligation mice.22 The activation of CB1Rs by ACEA, a selective agonist, in the RAIC induced specific analgesic effects. This analgesic effect was negated upon the deletion of CB1Rs of GABAergic neurons but not those of glutamatergic neurons.22 These findings suggest that the activation of CB1Rs of GABAergic interneurons plays an important role in the analgesic effect of the ECS. The antinociceptive effect of activated CB1Rs in the RAIC depend on the disinhibition of GABAergic neurons and the enhanced function of the descending pain inhibitory pathway via the dorsolateral fasciculus (Figure 4A).22 Despite these varying changes in CB1Rs, the neural mechanisms underlying the analgesic effects of activated CB1Rs in the IC warrant further investigation.
Since TRPV1 receptors and CB1Rs are colocalized, TRPV1 receptors might modulate neuropathic pain through the ECS. NAPE-PLD expression was attenuated by the TRPV1 receptor antagonist I-RTX in the IC of neuropathic pain model rats. The number of TRPV1 channels increased significantly in male rats subjected to ligation and transection of sciatic nerve branches.23 The calcium ion influx mediated by TRPV1 channels may prompt NAPE-PLD synthesis, leading to the production of AEA and a reduction in pain.70 In conclusion, the positive feedback loop in which AEA increases CB1Rs expression in the IC provides an endogenous analgesic strategy for addressing neuropathic pain. Analgesic targets in the ECS in the IC may include GABAergic neurons. This may lead to the development of more focused targets for analgesic drugs.
Amygdala
The amygdala, which is composed of multiple nuclei, including lateral, basolateral, central, and medial nuclei, has been recognized as a primary brain center for pain modulation.71 CB1Rs are expressed in the amygdala, with higher densities in the basolateral amygdala than in the centromedial amygdala.72 In addition to receptors, enzymes such as FAAH and MAGL are expressed in the amygdala.73 The levels of eCBs, AEA, and 2-AG in the amygdala are associated with stress and anxiety behavior.74
In SNI mice, the reduction in AEA levels in the amygdala is attributed to increased FAAH activity.15 The increase in 2-AG levels in the amygdala of SNI mice is believed to be associated with neuroinflammation.75 This increase in 2-AG levels could be due to the decrease in MAGL expression in the amygdala.24 CB1Rs have been detected in the basolateral amygdala complex (BLA) and amygdalohippocampal area (AHip) but not in the anterior amygdaloid area, the central nucleus, the medial nuclei, or the intercalated nuclei of the amygdala.16 Under neuropathic pain conditions, CB1Rs levels in the BLA and AHip decreased in the SCI group (Table 1).16 However, the levels of CB1Rs and TRPV1 receptors in the BLA were significantly increased in CCI model rats.21 This increase in CB1Rs levels in the BLA were strengthened after sub-chronic systemic treatment with CBD.21 CBD may also be associated with the increase in CB1R levels in the ACC, AIC, BLA, and ventral hippocampus (VH). It has been proposed that CBD-mediated analgesia in the BLA depends on corticolimbic circuits such as ACC–AIC–BLA and BLA-VH.21 In addition to these intercortical connections, the activity of the central nucleus of the amygdala (CeA)-PAG pathway is modulated by the ECS. The CeA inhibits PAG neurons through GABAergic inputs.76 Importantly, this direct extrinsic GABAergic input from the CeA to the PAG projection neurons innervating the RVM is selectively inhibited by cannabinoids via CB1Rs under physiological conditions.76 These results imply that the descending output of the PAG could be gated by the ECS. Thus, the influence of the ECS on the direct extrinsic CeA-PAG circuit is an intriguing topic under neuropathic pain conditions.
In summary, there are contradictions in the changes of the ECS, especially changes in CB1Rs. Moreover, the interaction between TRPV1 receptors and eCBs under neuropathic pain conditions should be investigated further. Moreover, the levels of AEA, 2-AG, PEA and OEA in the amygdala did not differ between male and female SNI rats.55 The sex-related differences in the ECS in the amygdala also need to be investigated.
Hippocampus
The hippocampal cortex is known for its critical roles in memory and cognition. In the hippocampus, the ECS triggers a reduction in GABA or glutamate release in a long-lasting manner (i.e., LTD).77 Disinhibition can facilitate LTP at Schaffer collaterals to CA1 synapses.78 Synapse plasticity mediated by the ECS could play an important role in learning and memory. In addition to retrograde eCBs signaling, AEA can activate TRPV1 receptors in a nonretrograde manner.77 The activation of TRPV1 receptors induces postsynaptic LTD of medial prefrontal path inputs to dentate granule cells.79 This synaptic activity reflects the physiological role of the ECS in modulating learning and memory. In addition, young mice lacking the CB1 receptor exhibited mild anxiety-related behavior.80
The hippocampus is typically divided into three subregions: CA1, CA3, and the dentate gyrus (DG). CB1Rs have been found in these hippocampal subregions in SCI and CCI model rats. In the CA1 and CA3 regions, CB1Rs levels were significantly increased in CCI model rats and SCI model rats in the delayed phase (42 days).16 Evidence of colocalization between CB1Rs and TRPV1 receptors was found in the CA1 and CA3 hippocampal areas of male sham and SCI model rats.16 Interestingly, the phosphorylated form of TRPV1 (TRPV1-p) receptors colocalized with CB1Rs was found only in the severe injury group 42 days post-operation.16 A notable increase in the levels of CB1Rs and TRPV1 receptors in the DG has also been observed in male CCI model rats.21 However, no significant difference in CB1R levels in the DG between SCI model and sham rats was found.16 This variation in CB1R levels may be attributed to the use of different neuropathic pain models (Table 1). Research on changes in CB2Rs levels is limited, and only an increasing trend in CB2R levels in the ventral hippocampus (vHIP) has been reported in CCI mice.19 Furthermore, the activity of the ECS in the hippocampus can be regulated through exogenous drugs. Cold hypersensitivity was inhibited by the MAGL antagonist JNJ-42226314, with a corresponding increase in 2-AG levels in the hippocampus.81 Interestingly, 2-AG levels in the DG increased after the application of PEA.82 Following this, the impaired excitatory toxicity-induced LTP in the lateral entorhinal cortex (LEC)-DG pathway in SNI mice is corrected (Figure 4B).82 Furthermore, evoked field excitatory postsynaptic potentials in the CA1 were decreased by JNJ-42226314 in a dose-dependent manner.81 These studies suggest that synaptic transmission in the hippocampus is influenced by the ECS.
The central and peripheral manifestations of anxiety and depression are mediated by CB1R and CB2R signaling, indicating that the ECS is a crucial regulator of these conditions in neuropathic pain.83 Emotional aspects of neuropathic pain, such as fear and anxiety, are processed through a neural circuit from the ventral hippocampus (VH) to the BLA.84 AEA levels in the hippocampus increased after systemic injection of the FAAH inhibitor URB597, which inhibited both depressive-like behaviors and neuropathic pain.33 This antidepressant effect is enhanced by the activation of CB2Rs and TRPV1 receptors by AEA.85 The application of PEA can indirectly increase AEA levels by acting as a false substrate for FAAH, potentially having a positive cooperative effect on neuropathic pain and related emotional disorders.86 Through double immunostaining and electron microscopy, CB1Rs of hippocampal astrocytes were identified and quantified.87 Acute application of exogenous cannabinoids triggered LTD at hippocampal CA3‒CA1 synapses. This cannabinoid-induced LTD was absent in mice lacking astrocyte CB1Rs, indicating that these receptors are essential for inducing LTD at these synapses.87 The activation of astroglial CB1Rs increased glutamate concentrations, subsequently promoting the internalization of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors (AMPARs) at CA3–CA1 synapses through NR2B-containing NMDARs (Figure 3).87 The interaction between glial CBRs and glutamate receptors of hippocampal astrocytes could be a meaningful future research direction.
As mentioned above, eCB signaling in the hippocampus is associated with synaptic plasticity, anxiety-related behavior, learning and memory through CBRs and TRPV1 receptors. TRPV1 receptors and CB1Rs are colocalized in the hippocampus. TRPV1 receptors, especially their phosphorylated form in the hippocampus, might affect neuropathic pain in a nonretrograde manner. In addition to those of neurons, CB1Rs of astrocytes regulate the excitability of hippocampal neurons in normal state. Changes in the function and expression of astrocyte CB1Rs under neuropathic pain are important to investigated.
Periaqueductal gray (PAG)
The descending pathway comprising the PAG-rostral ventromedial medulla (RVM)-dorsal horn (DH) serves as an endogenous pain modulator.88 AEA and 2-AG are the two main endocannabinoids in the PAG. AEA inhibits anxiety-like responses through the activation of CB1Rs in the dorsolateral periaqueductal gray (dlPAG).89 However, 2-AG induces anxiety-like responses through the activation of CB1Rs and CB2Rs in the dlPAG.89
The changes in the ECS within the PAG and the RVM are summarized in Table 1. These changes in the ECS may arise owing to different testing time points and the use of various neuropathic pain models. For example, the density of CB1Rs in the PAG significantly decreased in rats after moderate and severe spinal cord lesions at early and delayed phases, and in rats on the 7th day following CCI.16,25 However, no significant changes in CB1Rs in the PAG of CCI model rats have been found.10 CB1R and TRPV1 receptors are coexpressed in the PAG neurons of male SCI model rats.16 The analgesic effects of AA-5-HT, a FAAH and TRPV1 receptor blocker, are alleviated by CB1R and TRPV1 receptor antagonists.90 The colocalization of these receptors in the PAG could enable AA-5-HT-mediated antinociception. The AA-5-HT-mediated analgesic effect on formalin-induced pain relies on the PAG-locus coeruleus (LC)-spinal cord pathway.90 Moreover, the levels of 2-AG but not those of AEA increased after AA-5-HT application.90 The cooperative effects of the ECS and TRPV1 receptors on neuropathic pain have not been investigated. The changes in TRPV1 receptors in the PAG during neuropathic pain are also unclear. Pain perception may be modulated by GPR55 through the PAG-RVM-spinal cord dorsal horn axis. The antinociceptive and pronociceptive effects of GPR55 in the PAG have been investigated in inflammatory pain models but not in neuropathic pain models.91 Moreover, an endogenous ligand of GPR55 lysophosphatidylinositol, but not 2-AG, was used in these studies.
The rational approach to pain reduction involves enhancing the function of this endogenous antinociceptive descending pathway. Activating CB1Rs and CB2Rs in the PAG with WIN 55,212-2 produced an antinociceptive effect. This analgesic effect was reversed by mGluR5 and mGluR2-3 receptor antagonists.92 These findings suggest that alterations in glutamatergic synaptic transmission impact the ECS-mediated analgesic effects. Moreover, GABAergic synaptic transmission along the PAG–RVM axis is inhibited following the activation of CB1Rs in the PAG. This ECS-mediated disinhibition of PAG-projecting neurons enhances the excitability of RVM neurons.
These studies revealed that variations in CB1Rs in the PAG may occur due to different degrees of nerve damage or different time stages. The participation of TRPV1 receptors and GPR55 in the PAG in pain modulation has been demonstrated in inflammatory pain models. Analgesic effects owing to the activation of TRPV1 receptors and GPR55 does not depend on the ECS. However, whether this phenomenon is consistent with neuropathic pain is unknown.
Rostral ventromedial medulla (RVM)
The RVM serves as a crucial relay site in the descending pathway. There are two types of pain-responding neurons in the RVM: ON cells and OFF cells. ON cells become activated with bursts of activity, whereas OFF cells are inhibited before nociceptive stimulation.93 The inhibition of pain transmission is associated with the enhancement of OFF cell activity and a reduction or delay in the onset of the associated pauses.94
The levels of AEA, PEA and oleoylethanolamide (OEA) decreased, accompanied by an increase in FAAH levels in the RVM of male streptozocin-diabetic model rats.95 An increased TRPV1 mRNA expression was observed in the RVM. A FAAH-mediated decrease in eCB levels in the RVM might represent negative feedback control of TRPV1-mediated stimulation of glutamate release and neuronal excitotoxicity.96 Tail flick-induced ON cell firing and the OFF cell pause duration decreased after microinjection of WIN 55,212-2 in CCI rats (Figure 4C). This antinociceptive effect can be blocked by SR141716A (a CB1R antagonist) and MPEP (an mGluR5 antagonist) but not by CPCCOEt (an mGluR1 antagonist).25 These findings suggest that the functional interaction between mGluRs and the ECS is essential for PAG-RVM-spinal cord dorsal horn axis-mediated pain inhibition. Compared with male SNI rats, female SNI rats not only presented earlier onset but also greater sensitivity to mechanical and cold allodynia.97 As described in the review, sex-related differences in ECS ligand levels have been found.55 However, endogenous cannabinoid ligand (e.g., AEA, 2-AG, PEA, and OEA) levels in the PAG and RVM of SNI model rats do not differ between the sexes.55 The authors of this study reported that endocannabinoid levels might be altered at other time points following SNI surgery.
Motor cortex
Intracortical recordings and functional neuroimaging studies have shown that the primary motor cortex (M1) is involved in the perception of neuropathic pain.98 M1 layer V-restricted activation leads to pain relief in SNI mice through projections from the layer V to the ZI and the PAG.99 The activation of M1 layer VI neurons alleviates negative affective valence in neuropathic model mice through the M1-lateral domain of the mediodorsal thalamus-nucleus accumbens pathway.99 Thus, in the M1, distinct, layer-specific pathways are used to modulate pain and emotional disorders associated with neuropathic pain. ECS-mediated plasticity is associated with producing motion via connatural neural circuits.100 The long-term plasticity induced by the ECS acts as a long-term buffer for stabilizing the organization and activity of motor neurons.100
Through immunostaining and Western blotting, no changes in the levels of FAAH, MAGL enzymes or CB1Rs were found in the M1.101 Although CB2Rs are expressed in both astroglial cells and neurons, elevated CB2R levels are found only for astroglial cells.101 In addition, ECS components are regulated after stimulation of the M1. The CB2R density in the dorsal horn of the spinal cord was increased after M1 stimulation in CCI model rats.102 The levels of proinflammatory factors such as IL-1β and TNF-α, which are released by astrocytes and microglia, are decreased following the activation of CB2Rs.103 M1 stimulation produces analgesic effects via CB2R-mediated inhibition of glial activity and downregulation of IL-1β and TNF-α activity.102 On the basis of this evidence, neuropathic pain is modulated by the M1. The synaptic plasticity of the M1 is influenced by the ECS. However, changes in the ECS in the M1 associated with neuropathic pain remain unknown.
Midcingulate cortex (MCC)
In addition to the ACC, the MCC plays important roles in emotion, action, memory and pain.104 Nociceptive information from the anteromedial thalamic nucleus arrives at the MCC.105 In naive mice, chemogenetic activation of the MCC aggravates pain sensitivity.106 Moreover, hypersensitivity to pain is associated with the MCC-IC pathway.107 In addition, the connections between the MCC and the ZI lead to inhibitory effects.108 Poor episodic memory performance, executive function, speed and mental flexibility are associated with the MCC in Parkinson’s disease patients.109 This poor performance is associated with lower CB1R levels in the MCC.109 Phytocannabinoids such as THC and CBD significantly reduce connectivity between the DLPFC and the IC.110 The distribution and physiological function of the ECS in the MCC should be explored. Moreover, the changes in the components of the ECS are still unclear. THC and CBD affect the endocannabinoid system. This indirect evidence suggests that the activity of the MCC and its corresponding circuit might be reduced after the activation of the ECS.
Pharmaceutical development
Cannabis has been used for pain treatment for millennia. THC is the main active constituent of cannabis and shows high binding affinity for CB1Rs, with effects such as reducing anxiety and pain.111 Cannabis-based medicines contain THC, BCD, or a mixture of these medicines; analogues include THC and CBD extracts.112 In fact, a wide range of concentrations have been reported for single and mixed cannabis-related medicines. For example, THC dosages range from 1.6 to 96 mg for everyday use. Decreases in attention, learning and memory are aggravated with increasing THC doses.113 To identify side effects, the dosage or concentration of cannabis-based medicines should be standardized. Inhalation/smoking-based methods, oromucosal sprays, and oral sprays are three main clinical applications.112 The pharmacokinetics and pharmacodynamics of cannabinoids vary among these three applications.112 It is reasonable to suggest that inhalation methods and oromucosal sprays are more suited for treating acute pain, whereas oral formulations are appropriate for treating patients with long-term pain. Thus, on the basis of the characteristics of the given application, different methods should be chosen for each patient in clinical settings.
Cannabis-based medicines are derived from plants and act as agonists to CBRs. The activation of CBRs by phytocannabinoids or synthetic cannabinoids may help relieve neuropathic pain.114 Pharmacological approaches such as the use of CBRs agonists, eCB-regulating enzyme inhibitors, and other interventions that modulate the ECS have been shown to relieve neuropathic pain.115 Short-term and long-term changes in ECS components in the brain can occur after neuropathic pain. There are many possible therapeutic targets of natural and synthetic cannabinoids, and others can modulate the transmission and perception of neuropathic pain.
Nonmedicinal and recreational cannabis has been legalized in Luxembourg and other European countries.116 Medicinal, nonmedicinal, and recreational cannabis use has become increasingly widespread. The changes in the ECS of the brain area which is associated with neuropathic pain, following the cannabis use is highly desirable. This may also inspire the development of ECS agents that do not produce unwanted side effects. Positive allosteric modulators (PAMs) of the ECS have attracted increasing attention. PAMs such as GAT229 reduce neuropathic pain without psychoactive effects.117 Similarly, ZCZ011 effectively relieves hyperalgesia without the development of tolerance.114 Thus, the use of PAMs is a promising therapeutic strategy for treating neuropathic pain.
Conclusions and implications
The ECS in the brain is involved in the modulation of neuropathic pain and emotional disorders. Long-lasting changes in the ECS components have been observed in various neuropathic pain models. However, the directions of these changes are not always consistent across different brain regions. For example, CB1Rs expression is increased in the ACC, AIC, BLA, DH, and VH but is decreased in the PAG of CCI rats.21,25 Conversely, CB1Rs expression is stable in the IC of common peroneal nerve ligation model mice.22 These differences could be attributed to variations in pain models and brain nuclei. Further research is needed to understand the mechanisms governing changes in the ECS under different neuropathic pain conditions.
The ECS is composed of endocannabinoids, synthetizing and degrading enzymes, cannabinoid receptors, and downstream signaling pathways. Methods that increase the expression of eCB, cannabis sativa, or analogs or activate cannabinoid and other high-affinity receptors are thought to relieve neuropathic pain.17,23,112 In most studies, systemic injections of these agents have been used, but direct evidence of their effects on specific brain regions is limited. Additionally, the sensitivity of central neurons to endocannabinoids should be considered. For example, 2-AG has various effects on pain when provided at different doses in the prelimbic cortex (PrL). Microglias in the brain also participate in endocannabinoid metabolism and express both CB1Rs and CB2Rs. The activation of spinal dorsal horn microglial CB2Rs in SNI rats has been shown to reduce mechanical and cold hyperalgesia.118 Moreover, CB2Rs of microglias and astrocytes have been shown to modulate neuropathic pain at the spinal cord level, but their roles in supraspinal regions are less clear.119
Overall, anxiety and depression are pathological responses that can lead to highly disabling and severely limiting pain when they are exacerbated. These emotional disorders are related to dysfunctions of the ECS and can be modulated via interventions that influence this system. The neuronal mechanisms underlying the interactions between ECS dysfunction and emotional disorders are not fully understood. Future research should explore the specific roles of different subsets of neurons in brain regions involved in emotional disorders.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 32271056 to H.X., No. 82221001 to S.W. and No. 82373102 to Y.H.), Research project of Clinical medicine and X Research Center (LHJJ24JH13 to H.X. and Z.S.), Clinical research projects at Air Force Medical University (2021LC2109) to Y.H. and Quality Department of Anesthesiology Fund Planning of The People's Liberation Army Joint Logistic Support Force (No. 41C41B26 to M.Z.).
Author contributions
H.X. designed the outline and guided the manuscript. S.W. and Y.H. supported the manuscript. M.Z. and T.W. wrote the original draft. F.M. and M.J. took part in the discussion and revision about the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Shengxi Wu, Email: shengxi@fmmu.edu.cn.
Hui Xu, Email: xubz@fmmu.edu.cn.
References
- 1.Zhang Y.N., Xing X.X., Chen L., Dong X., Pan H.T., Hua X.Y., Wang K. Brain Functional Alteration at Different Stages of Neuropathic Pain With Allodynia and Emotional Disorders. Front. Neurol. 2022;13:843815. doi: 10.3389/fneur.2022.843815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bushnell M.C., Ceko M., Low L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen S.P., Vase L., Hooten W.M. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397:2082–2097. doi: 10.1016/S0140-6736(21)00393-7. [DOI] [PubMed] [Google Scholar]
- 4.Fine P.G., Rosenfeld M.J. Cannabinoids for neuropathic pain. Curr. Pain Headache Rep. 2014;18:451–459. doi: 10.1007/s11916-014-0451-2. [DOI] [PubMed] [Google Scholar]
- 5.Lowe H., Toyang N., Steele B., Bryant J., Ngwa W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021;22:9472. doi: 10.3390/ijms22179472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egertova M., Elphick M.R. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J. Comp. Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Munro S., Thomas K.L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 8.Kreitzer A.C., Regehr W.G. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 9.Gerdeman G.L., Ronesi J., Lovinger D.M. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 10.Petrosino S., Palazzo E., de Novellis V., Bisogno T., Rossi F., Maione S., Di Marzo V. Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats. Neuropharmacology. 2007;52:415–422. doi: 10.1016/j.neuropharm.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Chevaleyre V., Heifets B.D., Kaeser P.S., Sudhof T.C., Castillo P.E. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piette C., Cui Y., Gervasi N., Venance L. Lights on Endocannabinoid-Mediated Synaptic Potentiation. Front. Mol. Neurosci. 2020;13:1–10. doi: 10.3389/fnmol.2020.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jhaveri M.D., Elmes S.J., Richardson D., Barrett D.A., Kendall D.A., Mason R., Chapman V. Evidence for a novel functional role of cannabinoid CB(2) receptors in the thalamus of neuropathic rats. Eur. J. Neurosci. 2008;27:1722–1730. doi: 10.1111/j.1460-9568.2008.06162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegling A., Hofmann H.A., Denzer D., Mauler F., De Vry J. Cannabinoid CB(1) receptor upregulation in a rat model of chronic neuropathic pain. Eur. J. Pharmacol. 2001;415 doi: 10.1016/s0014-2999(01)00798-1. R5–7. [DOI] [PubMed] [Google Scholar]
- 15.Bishay P., Haussler A., Lim H.Y., Oertel B., Galve-Roperh I., Ferreiros N., Tegeder I. Anandamide deficiency and heightened neuropathic pain in aged mice. Neuropharmacology. 2013;71:204–215. doi: 10.1016/j.neuropharm.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Knerlich-Lukoschus F., Noack M., von der Ropp-Brenner B., Lucius R., Mehdorn H.M., Held-Feindt J. Spinal cord injuries induce changes in CB1 cannabinoid receptor and C-C chemokine expression in brain areas underlying circuitry of chronic pain conditions. J. Neurotrauma. 2011;28:619–634. doi: 10.1089/neu.2010.1652. [DOI] [PubMed] [Google Scholar]
- 17.Mecca C.M., Chao D., Yu G., Feng Y., Segel I., Zhang Z., Rodriguez-Garcia D.M., Pawela C.P., Hillard C.J., Hogan Q.H., et al. Dynamic Change of Endocannabinoid Signaling in the Medial Prefrontal Cortex Controls the Development of Depression After Neuropathic Pain. J. Neurosci. 2021;41:7492–7508. doi: 10.1523/JNEUROSCI.3135-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bushlin I., Gupta A., Stockton S.D., Jr., Miller L.K., Devi L.A. Dimerization with cannabinoid receptors allosterically modulates delta opioid receptor activity during neuropathic pain. PLoS One. 2012;7:1–14. doi: 10.1371/journal.pone.0049789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai X., Batalle G., Martinez-Martel I., Pol O. Hydrogen Sulfide Interacting with Cannabinoid 2 Receptors during Sciatic Nerve Injury-Induced Neuropathic Pain. Antioxidants (Basel) 2023;12:1–19. doi: 10.3390/antiox12061179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoot M.R., Sim-Selley L.J., Poklis J.L., Abdullah R.A., Scoggins K.L., Selley D.E., Dewey W.L. Chronic constriction injury reduces cannabinoid receptor 1 activity in the rostral anterior cingulate cortex of mice. Brain Res. 2010;1339:18–25. doi: 10.1016/j.brainres.2010.03.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva-Cardoso G.K., Lazarini-Lopes W., Hallak J.E., Crippa J.A., Zuardi A.W., Garcia-Cairasco N., Leite-Panissi C.R.A. Cannabidiol effectively reverses mechanical and thermal allodynia, hyperalgesia, and anxious behaviors in a neuropathic pain model: Possible role of CB1 and TRPV1 receptors. Neuropharmacology. 2021;197:1–12. doi: 10.1016/j.neuropharm.2021.108712. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M., Li C., Xue Q., Lu C.B., Zhao H., Meng F.C., Zhang Y., Wu S.X., Zhang Y., Xu H. Activation of Cannabinoid Receptor 1 in GABAergic Neurons in the Rostral Anterior Insular Cortex Contributes to the Analgesia Following Common Peroneal Nerve Ligation. Neurosci. Bull. 2023;1348-1362 doi: 10.1007/s12264-023-01029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jee Kim M., Tanioka M., Woo Um S., Hong S.K., Hwan Lee B. Analgesic effects of FAAH inhibitor in the insular cortex of nerve-injured rats. Mol. Pain. 2018;14:1–16. doi: 10.1177/1744806918814345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel S., Kingsley P.J., Mackie K., Marnett L.J., Winder D.G. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34:2699–2709. doi: 10.1038/npp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palazzo E., Luongo L., Bellini G., Guida F., Marabese I., Boccella S., Rossi F., Maione S., de Novellis V. Changes in cannabinoid receptor subtype 1 activity and interaction with metabotropic glutamate subtype 5 receptors in the periaqueductal gray-rostral ventromedial medulla pathway in a rodent neuropathic pain model. CNS Neurol. Disord. Drug Targets. 2012;11:148–161. doi: 10.2174/187152712800269731. [DOI] [PubMed] [Google Scholar]
- 26.Morales P., Jagerovic N. Advances Towards The Discovery of GPR55 Ligands. Curr. Med. Chem. 2016;23:2087–2100. doi: 10.2174/0929867323666160425113836. [DOI] [PubMed] [Google Scholar]
- 27.Staton P.C., Hatcher J.P., Walker D.J., Morrison A.D., Shapland E.M., Hughes J.P., Chong E., Mander P.K., Green P.J., Billinton A., et al. The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain. 2008;139:225–236. doi: 10.1016/j.pain.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Toth A., Boczan J., Kedei N., Lizanecz E., Bagi Z., Papp Z., Edes I., Csiba L., Blumberg P.M. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res. Mol. Brain Res. 2005;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Luo X., Chen O., Wang Z., Bang S., Ji J., Lee S.H., Huh Y., Furutani K., He Q., Tao X., et al. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron. 2021;109:2691–2706.e5. doi: 10.1016/j.neuron.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiaofeng B.X.Z., Zhou Q. Effect of Testosterone on TRPV1 Expression in a Model of Orofacial Myositis Pain in the Rat. J. Mol. Neurosci. 2018;64:93–101. doi: 10.1007/s12031-017-1009-7. [DOI] [PubMed] [Google Scholar]
- 31.Zhuo M. Neuronal mechanism for neuropathic pain. Mol. Pain. 2007;3:14. doi: 10.1186/1744-8069-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.You H.J., Lei J., Sui M.Y., Huang L., Tan Y.X., Tjølsen A., Arendt-Nielsen L. Endogenous descending modulation: spatiotemporal effect of dynamic imbalance between descending facilitation and inhibition of nociception. J. Physiol. 2010;588:4177–4188. doi: 10.1113/jphysiol.2010.196923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang H.X., Ke B.W., Liu J., Ma G., Hai K.R., Gong D.Y., Yang Z., Zhou C. Inhibition of Fatty Acid Amide Hydrolase Improves Depressive-Like Behaviors Independent of Its Peripheral Antinociceptive Effects in a Rat Model of Neuropathic Pain. Anesth. Analg. 2019;129:587–597. doi: 10.1213/ANE.0000000000003563. [DOI] [PubMed] [Google Scholar]
- 34.Garden G.A., Möller T. Microglia biology in health and disease. J. Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- 35.Marrs W.R., Blankman J.L., Horne E.A., Thomazeau A., Lin Y.H., Coy J., Bodor A.L., Muccioli G.G., Hu S.S.J., Woodruff G., et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat. Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duffy S.S., Hayes J.P., Fiore N.T., Moalem-Taylor G. The cannabinoid system and microglia in health and disease. Neuropharmacology. 2021;190:108555. doi: 10.1016/j.neuropharm.2021.108555. [DOI] [PubMed] [Google Scholar]
- 37.Luongo L., Maione S., Di Marzo V. Endocannabinoids and neuropathic pain: focus on neuron-glia and endocannabinoid-neurotrophin interactions. Eur. J. Neurosci. 2014;39:401–408. doi: 10.1111/ejn.12440. [DOI] [PubMed] [Google Scholar]
- 38.de Heer E.W., Gerrits M.M.J.G., Beekman A.T.F., Dekker J., van Marwijk H.W.J., de Waal M.W.M., Spinhoven P., Penninx B.W.J.H., van der Feltz-Cornelis C.M. The association of depression and anxiety with pain: a study from NESDA. PLoS One. 2014;9:e106907. doi: 10.1371/journal.pone.0106907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giesler G.J., Menétrey D., Guilbaud G., Besson J.M. Lumbar cord neurons at the origin of the spinothalamic tract in the rat. Brain Res. 1976;118:320–324. doi: 10.1016/0006-8993(76)90718-6. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y.G., Wu C.S., Lu H.C., Beierlein M. Target-dependent control of synaptic inhibition by endocannabinoids in the thalamus. J. Neurosci. 2011;31:9222–9230. doi: 10.1523/JNEUROSCI.0531-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H., Dong P., He C., Feng X.Y., Huang Y., Yang W.W., Gao H.J., Shen X.F., Lin S., Cao S.X., et al. Incerta-thalamic Circuit Controls Nocifensive Behavior via Cannabinoid Type 1 Receptors. Neuron. 2020;107:538–551.e7. doi: 10.1016/j.neuron.2020.04.027. [DOI] [PubMed] [Google Scholar]
- 42.Liu J., Zhang M.Q., Wu X., Lazarus M., Cherasse Y., Yuan M.Y., Huang Z.L., Li R.X. Activation of Parvalbumin Neurons in the Rostro-Dorsal Sector of the Thalamic Reticular Nucleus Promotes Sensitivity to Pain in Mice. Neuroscience. 2017;366:113–123. doi: 10.1016/j.neuroscience.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Ding W., Yang L., Shi E., Kim B., Low S., Hu K., Gao L., Chen P., Ding W., Borsook D., et al. The endocannabinoid N-arachidonoyl dopamine is critical for hyperalgesia induced by chronic sleep disruption. Nat. Commun. 2023;14:6696. doi: 10.1038/s41467-023-42283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baliki M.N., Geha P.Y., Fields H.L., Apkarian A.V. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Posa L., De Gregorio D., Gobbi G., Comai S. Targeting Melatonin MT2 Receptors: A Novel Pharmacological Avenue for Inflammatory and Neuropathic Pain. Curr. Med. Chem. 2018;25:3866–3882. doi: 10.2174/0929867324666170209104926. [DOI] [PubMed] [Google Scholar]
- 46.Long L.E., Lind J., Webster M., Weickert C.S. Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neurosci. 2012;13:87. doi: 10.1186/1471-2202-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lafourcade M., Elezgarai I., Mato S., Bakiri Y., Grandes P., Manzoni O.J. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imperatore R., Morello G., Luongo L., Taschler U., Romano R., De Gregorio D., Belardo C., Maione S., Di Marzo V., Cristino L. Genetic deletion of monoacylglycerol lipase leads to impaired cannabinoid receptor CB(1)R signaling and anxiety-like behavior. J. Neurochem. 2015;135:799–813. doi: 10.1111/jnc.13267. [DOI] [PubMed] [Google Scholar]
- 49.Hill M.N., McLaughlin R.J., Pan B., Fitzgerald M.L., Roberts C.J., Lee T.T.Y., Karatsoreos I.N., Mackie K., Viau V., Pickel V.M., et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J. Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiritoshi T., Sun H., Ren W., Stauffer S.R., Lindsley C.W., Conn P.J., Neugebauer V. Modulation of pyramidal cell output in the medial prefrontal cortex by mGluR5 interacting with CB1. Neuropharmacology. 2013;66:170–178. doi: 10.1016/j.neuropharm.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tran H., Feng Y., Chao D., Liu Q.S., Hogan Q.H., Pan B. Descending mechanism by which medial prefrontal cortex endocannabinoid signaling controls the development of neuropathic pain and neuronal activity of dorsal root ganglion. Pain. 2024;165:102–114. doi: 10.1097/j.pain.0000000000002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Novellis V., Vita D., Gatta L., Luongo L., Bellini G., De Chiaro M., Marabese I., Siniscalco D., Boccella S., Piscitelli F., et al. The blockade of the transient receptor potential vanilloid type 1 and fatty acid amide hydrolase decreases symptoms and central sequelae in the medial prefrontal cortex of neuropathic rats. Mol. Pain. 2011;7:7–19. doi: 10.1186/1744-8069-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drevets W.C., Price J.L., Simpson J.R., Jr., Todd R.D., Reich T., Vannier M., Raichle M.E. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 54.Malvestio R.B., Medeiros P., Negrini-Ferrari S.E., Oliveira-Silva M., Medeiros A.C., Padovan C.M., Luongo L., Maione S., Coimbra N.C., de Freitas R.L. Cannabidiol in the prelimbic cortex modulates the comorbid condition between the chronic neuropathic pain and depression-like behaviour in rats: The role of medial prefrontal cortex 5-HT(1A) and CB(1) receptors. Brain Res. Bull. 2021;174:323–338. doi: 10.1016/j.brainresbull.2021.06.017. [DOI] [PubMed] [Google Scholar]
- 55.Blanton H.L., Barnes R.C., McHann M.C., Bilbrey J.A., Wilkerson J.L., Guindon J. Sex differences and the endocannabinoid system in pain. Pharmacol. Biochem. Behav. 2021;202 doi: 10.1016/j.pbb.2021.173107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao H., Xue Q., Li C., Wang Q., Han S., Zhou Y., Yang T., Xie Y., Fu H., Lu C., et al. Upregulation of Beta4 subunit of BK(Ca) channels in the anterior cingulate cortex contributes to mechanical allodynia associated anxiety-like behaviors. Mol. Brain. 2020;13:22. doi: 10.1186/s13041-020-0555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao X., Ding M., Zhang Y.Q. Role of the Anterior Cingulate Cortex in Translational Pain Research. Neurosci. Bull. 2021;37:405–422. doi: 10.1007/s12264-020-00615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rainville P., Duncan G.H., Price D.D., Carrier B., Bushnell M.C. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 59.Weizman L., Dayan L., Brill S., Nahman-Averbuch H., Hendler T., Jacob G., Sharon H. Cannabis analgesia in chronic neuropathic pain is associated with altered brain connectivity. Neurology. 2018;91:e1285–e1294. doi: 10.1212/WNL.0000000000006293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen T., Taniguchi W., Chen Q.Y., Tozaki-Saitoh H., Song Q., Liu R.H., Koga K., Matsuda T., Kaito-Sugimura Y., Wang J., et al. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat. Commun. 2018;9:1886. doi: 10.1038/s41467-018-04309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moldrich G., Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21:1735–1742. doi: 10.1016/s0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- 62.Wu J., Hua L., Liu W., Yang X., Tang X., Yuan S., Zhou S., Ye Q., Cui S., Wu Z., et al. Electroacupuncture Exerts Analgesic Effects by Restoring Hyperactivity via Cannabinoid Type 1 Receptors in the Anterior Cingulate Cortex in Chronic Inflammatory Pain. Mol. Neurobiol. 2024;61:2949–2963. doi: 10.1007/s12035-023-03760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corcoran L., Mattimoe D., Roche M., Finn D.P. Attenuation of fear-conditioned analgesia in rats by monoacylglycerol lipase inhibition in the anterior cingulate cortex: Potential role for CB(2) receptors. Br. J. Pharmacol. 2020;177:2240–2255. doi: 10.1111/bph.14976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huff M.L., Emmons E.B., Narayanan N.S., LaLumiere R.T. Basolateral amygdala projections to ventral hippocampus modulate the consolidation of footshock, but not contextual, learning in rats. Learn. Mem. 2016;23:51–60. doi: 10.1101/lm.039909.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corder G., Ahanonu B., Grewe B.F., Wang D., Schnitzer M.J., Scherrer G. An amygdalar neural ensemble that encodes the unpleasantness of pain. Science. 2019;363:276–281. doi: 10.1126/science.aap8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo B., Wang J., Yao H., Ren K., Chen J., Yang J., Cai G., Liu H., Fan Y., Wang W., Wu S. Chronic Inflammatory Pain Impairs mGluR5-Mediated Depolarization-Induced Suppression of Excitation in the Anterior Cingulate Cortex. Cereb. Cortex. 2018;28:2118–2130. doi: 10.1093/cercor/bhx117. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y., Qin Y., Gao Y., Wang S., Wang Q., Tang X., Rong Z., Cheng C., Li L., Xu Y., et al. Linderane Attenuates Complete Freund's Adjuvant-Induced Pain and Anxiety in Mice by Restoring Anterior Cingulate Cortex Microglia M2 Polarization through Activating Cannabinoid 2 Receptor. ACS Pharmacol. Transl. Sci. 2024;7:797–808. doi: 10.1021/acsptsci.3c00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu C., Yang T., Zhao H., Zhang M., Meng F., Fu H., Xie Y., Xu H. Insular Cortex is Critical for the Perception, Modulation, and Chronification of Pain. Neurosci. Bull. 2016;32:191–201. doi: 10.1007/s12264-016-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kobilo T., Hazvi S., Dudai Y. Role of cortical cannabinoid CB1 receptor in conditioned taste aversion memory. Eur. J. Neurosci. 2007;25:3417–3421. doi: 10.1111/j.1460-9568.2007.05561.x. [DOI] [PubMed] [Google Scholar]
- 70.Kathuria S., Gaetani S., Fegley D., Valiño F., Duranti A., Tontini A., Mor M., Tarzia G., Rana G.L., Calignano A., et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2002;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 71.Allen H.N., Bobnar H.J., Kolber B.J. Left and right hemispheric lateralization of the amygdala in pain. Prog. Neurobiol. 2021;196:101891. doi: 10.1016/j.pneurobio.2020.101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katona I., Rancz E.A., Acsady L., Ledent C., Mackie K., Hajos N., Freund T.F. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J. Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bisogno T., Berrendero F., Ambrosino G., Cebeira M., Ramos J.A., Fernandez-Ruiz J.J., Di Marzo V. Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem. Biophys. Res. Commun. 1999;256:377–380. doi: 10.1006/bbrc.1999.0254. [DOI] [PubMed] [Google Scholar]
- 74.Lisboa S.F., Gomes F.V., Terzian A.L.B., Aguiar D.C., Moreira F.A., Resstel L.B.M., Guimarães F.S. The Endocannabinoid System and Anxiety. Vitam. Horm. 2017;103:193–279. doi: 10.1016/bs.vh.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 75.Witting A., Walter L., Wacker J., Möller T., Stella N. P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc. Natl. Acad. Sci. USA. 2004;101:3214–3219. doi: 10.1073/pnas.0306707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winters B.L., Lau B.K., Vaughan C.W. Cannabinoids and Opioids Differentially Target Extrinsic and Intrinsic GABAergic Inputs onto the Periaqueductal Grey Descending Pathway. J. Neurosci. 2022;42:7744–7756. doi: 10.1523/JNEUROSCI.0997-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castillo P.E., Younts T.J., Chávez A.E., Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Araque A., Castillo P.E., Manzoni O.J., Tonini R. Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology. 2017;124:13–24. doi: 10.1016/j.neuropharm.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chávez A.E., Chiu C.Q., Castillo P.E. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat. Neurosci. 2010;13:1511–1518. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maccarrone M., Valverde O., Barbaccia M.L., Castañé A., Maldonado R., Ledent C., Parmentier M., Finazzi-Agrò A. Age-related changes of anandamide metabolism in CB1 cannabinoid receptor knockout mice: correlation with behaviour. Eur. J. Neurosci. 2002;15:1178–1186. doi: 10.1046/j.1460-9568.2002.01957.x. [DOI] [PubMed] [Google Scholar]
- 81.Wyatt R.M., Fraser I., Welty N., Lord B., Wennerholm M., Sutton S., Ameriks M.K., Dugovic C., Yun S., White A., et al. Pharmacologic Characterization of JNJ-42226314, [1-(4-Fluorophenyl)indol-5-yl]-[3-[4-(thiazole-2-carbonyl)piperazin-1-yl]azetidin-1-yl]methanone, a Reversible, Selective, and Potent Monoacylglycerol Lipase Inhibitor. J. Pharmacol. Exp. Ther. 2020;372:339–353. doi: 10.1124/jpet.119.262139. [DOI] [PubMed] [Google Scholar]
- 82.Boccella S., Cristiano C., Romano R., Iannotta M., Belardo C., Farina A., Guida F., Piscitelli F., Palazzo E., Mazzitelli M., et al. Ultra-micronized palmitoylethanolamide rescues the cognitive decline-associated loss of neural plasticity in the neuropathic mouse entorhinal cortex-dentate gyrus pathway. Neurobiol. Dis. 2019;121:106–119. doi: 10.1016/j.nbd.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 83.Ruehle S., Remmers F., Romo-Parra H., Massa F., Wickert M., Wörtge S., Häring M., Kaiser N., Marsicano G., Pape H.C., Lutz B. Cannabinoid CB1 receptor in dorsal telencephalic glutamatergic neurons: distinctive sufficiency for hippocampus-dependent and amygdala-dependent synaptic and behavioral functions. J. Neurosci. 2013;33:10264–10277. doi: 10.1523/JNEUROSCI.4171-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Herry C., Ciocchi S., Senn V., Demmou L., Müller C., Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 85.Facci L., Dal Toso R., Romanello S., Buriani A., Skaper S.D., Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc. Natl. Acad. Sci. USA. 1995;92:3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ho W.S.V., Barrett D.A., Randall M.D. 'Entourage' effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br. J. Pharmacol. 2008;155:837–846. doi: 10.1038/bjp.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han J., Kesner P., Metna-Laurent M., Duan T., Xu L., Georges F., Koehl M., Abrous D.N., Mendizabal-Zubiaga J., Grandes P., et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012;148:1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 88.Palazzo E., Luongo L., Novellis V.d., Rossi F., Maione S. The Role of Cannabinoid Receptors in the Descending Modulation of Pain. Pharmaceuticals. 2010;3:2661–2673. doi: 10.3390/ph3082661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Almeida-Santos A.F., Gobira P.H., Rosa L.C., Guimaraes F.S., Moreira F.A., Aguiar D.C. Modulation of anxiety-like behavior by the endocannabinoid 2-arachidonoylglycerol (2-AG) in the dorsolateral periaqueductal gray. Behav. Brain Res. 2013;252:10–17. doi: 10.1016/j.bbr.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 90.de Novellis V., Palazzo E., Rossi F., De Petrocellis L., Petrosino S., Guida F., Luongo L., Migliozzi A., Cristino L., Marabese I., et al. The analgesic effect of N-arachidonoyl-serotonin, a FAAH inhibitor and TRPV1 receptor antagonist, associated with changes in rostral ventromedial medulla and locus coeruleus cell activity in rats. Neuropharmacology. 2008;55:1105–1113. doi: 10.1016/j.neuropharm.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 91.Blanton H., Armin S., Muenster S., Abood M., Benamar K. Contribution of G Protein-Coupled Receptor 55 to Periaqueductal Gray-Mediated Antinociception in the Inflammatory Pain. Cannabis Cannabinoid Res. 2022;7:274–278. doi: 10.1089/can.2022.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palazzo E., Marabese I., de Novellis V., Oliva P., Rossi F., Berrino L., Rossi F., Maione S. Metabotropic and NMDA glutamate receptors participate in the cannabinoid-induced antinociception. Neuropharmacology. 2001;40:319–326. doi: 10.1016/s0028-3908(00)00160-x. [DOI] [PubMed] [Google Scholar]
- 93.Heinricher M.M., Barbaro N.M., Fields H.L. Putative nociceptive modulating neurons in the rostral ventromedial medulla of the rat: firing of on- and off-cells is related to nociceptive responsiveness. Somatosens. Mot. Res. 1989;6:427–439. doi: 10.3109/08990228909144685. [DOI] [PubMed] [Google Scholar]
- 94.Heinricher M.M., Tortorici V. Interference with GABA transmission in the rostral ventromedial medulla: disinhibition of off-cells as a central mechanism in nociceptive modulation. Neuroscience. 1994;63:533–546. doi: 10.1016/0306-4522(94)90548-7. [DOI] [PubMed] [Google Scholar]
- 95.Silva M., Martins D., Charrua A., Piscitelli F., Tavares I., Morgado C., Di Marzo V. Endovanilloid control of pain modulation by the rostroventromedial medulla in an animal model of diabetic neuropathy. Neuropharmacology. 2016;107:49–57. doi: 10.1016/j.neuropharm.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 96.Zimov S., Yazulla S. Vanilloid receptor 1 (TRPV1/VR1) co-localizes with fatty acid amide hydrolase (FAAH) in retinal amacrine cells. Vis. Neurosci. 2007;24:581–591. doi: 10.1017/S095252380707054X. [DOI] [PubMed] [Google Scholar]
- 97.Boullon L., Finn D.P., Llorente-Berzal Á. Sex Differences in a Rat Model of Peripheral Neuropathic Pain and Associated Levels of Endogenous Cannabinoid Ligands. Front. Pain Res. 2021;2 doi: 10.3389/fpain.2021.673638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peyron R., Schneider F., Faillenot I., Convers P., Barral F.G., Garcia-Larrea L., Laurent B. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology. 2004;63:1838–1846. doi: 10.1212/01.wnl.0000144177.61125.85. [DOI] [PubMed] [Google Scholar]
- 99.Gan Z., Gangadharan V., Liu S., Körber C., Tan L.L., Li H., Oswald M.J., Kang J., Martin-Cortecero J., Männich D., et al. Layer-specific pain relief pathways originating from primary motor cortex. Science. 2022;378:1336–1343. doi: 10.1126/science.add4391. [DOI] [PubMed] [Google Scholar]
- 100.El Manira A., Kyriakatos A. The role of endocannabinoid signaling in motor control. Physiology. 2010;25:230–238. doi: 10.1152/physiol.00007.2010. [DOI] [PubMed] [Google Scholar]
- 101.Espejo-Porras F., Fernández-Ruiz J., de Lago E. Analysis of endocannabinoid receptors and enzymes in the post-mortem motor cortex and spinal cord of amyotrophic lateral sclerosis patients. Amyotroph. Lateral Scler. Frontotemporal Degener. 2018;19:377–386. doi: 10.1080/21678421.2018.1425454. [DOI] [PubMed] [Google Scholar]
- 102.Silva G.D., Lopes P.S.S., Fonoff E.T., Pagano R.L. The spinal anti-inflammatory mechanism of motor cortex stimulation: cause of success and refractoriness in neuropathic pain? J. Neuroinflammation. 2015;12:10. doi: 10.1186/s12974-014-0216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stella N. Endocannabinoid signaling in microglial cells. Neuropharmacology. 2009;56:244–253. doi: 10.1016/j.neuropharm.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perini I., Bergstrand S., Morrison I. Where pain meets action in the human brain. J. Neurosci. 2013;33:15930–15939. doi: 10.1523/JNEUROSCI.3135-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peyron R., Quesada C., Fauchon C. Cingulate-mediated approaches to treating chronic pain. Handb. Clin. Neurol. 2019;166:317–326. doi: 10.1016/B978-0-444-64196-0.00017-0. [DOI] [PubMed] [Google Scholar]
- 106.Qiu Y., Lian Y.N., Wu C., Liu L., Zhang C., Li X.Y. Coordination between midcingulate cortex and retrosplenial cortex in pain regulation. Front. Mol. Neurosci. 2024;17 doi: 10.3389/fnmol.2024.1405532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tan L.L., Pelzer P., Heinl C., Tang W., Gangadharan V., Flor H., Sprengel R., Kuner T., Kuner R. A pathway from midcingulate cortex to posterior insula gates nociceptive hypersensitivity. Nat. Neurosci. 2017;20:1591–1601. doi: 10.1038/nn.4645. [DOI] [PubMed] [Google Scholar]
- 108.Hu T.T., Wang R.R., Du Y., Guo F., Wu Y.X., Wang Y., Wang S., Li X.Y., Zhang S.H., Chen Z. Activation of the Intrinsic Pain Inhibitory Circuit from the Midcingulate Cg2 to Zona Incerta Alleviates Neuropathic Pain. J. Neurosci. 2019;39:9130–9144. doi: 10.1523/JNEUROSCI.1683-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ceccarini J., Casteels C., Ahmad R., Crabbé M., Van de Vliet L., Vanhaute H., Vandenbulcke M., Vandenberghe W., Van Laere K. Regional changes in the type 1 cannabinoid receptor are associated with cognitive dysfunction in Parkinson's disease. Eur. J. Nucl. Med. Mol. Imag. 2019;46:2348–2357. doi: 10.1007/s00259-019-04445-x. [DOI] [PubMed] [Google Scholar]
- 110.Ertl N., Freeman T.P., Mokrysz C., Ofori S., Borissova A., Petrilli K., Curran H.V., Lawn W., Wall M.B. Acute effects of different types of cannabis on young adult and adolescent resting-state brain networks. Neuropsychopharmacology. 2024;49:1640–1651. doi: 10.1038/s41386-024-01891-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wilson R.I., Nicoll R.A. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 112.Sharon H., Brill S. Cannabis-based medicines for chronic pain management: current and future prospects. Curr. Opin. Anaesthesiol. 2019;32:623–628. doi: 10.1097/ACO.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 113.Andreae M.H., Carter G.M., Shaparin N., Suslov K., Ellis R.J., Ware M.A., Abrams D.I., Prasad H., Wilsey B., Indyk D., et al. Inhaled Cannabis for Chronic Neuropathic Pain: A Meta-analysis of Individual Patient Data. J. Pain. 2015;16:1221–1232. doi: 10.1016/j.jpain.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Quintero J.M., Diaz L.E., Galve-Roperh I., Bustos R.H., Leon M.X., Beltran S., Dodd S. The endocannabinoid system as a therapeutic target in neuropathic pain: a review. Expert Opin. Ther. Targets. 2024;28:739–755. doi: 10.1080/14728222.2024.2407824. [DOI] [PubMed] [Google Scholar]
- 115.Donvito G., Nass S.R., Wilkerson J.L., Curry Z.A., Schurman L.D., Kinsey S.G., Lichtman A.H. The Endogenous Cannabinoid System: A Budding Source of Targets for Treating Inflammatory and Neuropathic Pain. Neuropsychopharmacology. 2018;43:52–79. doi: 10.1038/npp.2017.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Editorial Debating the legalisation of recreational cannabis: The Lancet Regional Health - Europe. Lancet Reg. Health Eur. 2021;10 doi: 10.1016/j.lanepe.2021.100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bagher A.M., Binmahfouz L.S., Shaik R.A., Eid B.G. Cannabinoid receptor 1 positive allosteric modulator (GAT229) attenuates cisplatin-induced neuropathic pain in mice. Saudi Pharmaceut. J. 2023;31:255–264. doi: 10.1016/j.jsps.2022.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou Y., Xu Y., Yang J., Yu Z., Wang W., Yuan M., Wang Y., Bai Q., Li Z. Spinal cannabinoid receptor 2 activation alleviates neuropathic pain by regulating microglia and suppressing P2X7 receptor. Front. Mol. Neurosci. 2023;16:1061220. doi: 10.3389/fnmol.2023.1061220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Luongo L., Palazzo E., Tambaro S., Giordano C., Gatta L., Scafuro M.A., Rossi F.S., Lazzari P., Pani L., de Novellis V., et al. 1-(2',4'-dichlorophenyl)-6-methyl-N-cyclohexylamine-1,4-dihydroindeno[1,2-c]pyrazole-3-carboxamide, a novel CB2 agonist, alleviates neuropathic pain through functional microglial changes in mice. Neurobiol. Dis. 2010;37:177–185. doi: 10.1016/j.nbd.2009.09.021. [DOI] [PubMed] [Google Scholar]