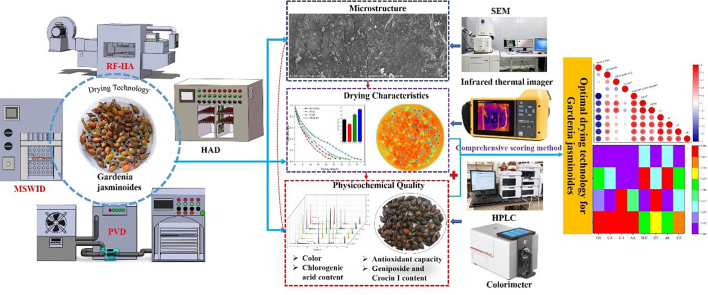
Abstract
Gardenia jasminoides Ellis is widely used as healthy food and herbal medicine for its anti-inflammatory, analgesic, antihypertensive, and antiviral functions. The drying behavior and physicochemical quality of Gardenia jasminoides Ellis were studied to evaluate its adaptability under four drying techniques: hot air drying (HAD), medium-and short-wave infrared drying (MSWID), pulsed vacuum drying (PVD), and radio frequency-HAD (RF-HAD). Compared with HAD and MSWID, PVD and RF-HAD can form beneficial microporous channels for moisture migration inside Gardenia jasminoides Ellis, thus shortening drying time by 32.56–42.51 % and increasing geniposide content by 3.31–13.77 %, while better preserving the brightness and redness. In addition, Pearson correlation analysis confirmed the RF-HAD dried samples showed the best antioxidant activity with the highest content of active ingredients (chlorogenic acid, geniposide), and there was a significant positive correlation between sample color and yellow pigment content. After comprehensive comparison, RF-HAD is proposed to be the most suitable method for Gardenia jasminoides Ellis drying. This research could provide scientific basis and technical support for promoting the high quality development of industrial processing of Gardenia jasminoides Ellis.
Keywords: Bioactive chemicals, Drying kinetics, Gardenia jasminoides Ellis, Microstructure, Physicochemical quality
Graphical abstract
Highlights
-
•
RF-HAD is the most applicable technique for Gardenia jasminoides Ellis drying.
-
•
PVD had higher drying rate and crocin I content.
-
•
RF-HAD showed better color, bioactive compound and antioxidant properties.
-
•
The porous structure improved the drying rate and bioactive compounds content.
-
•
Color parameters were correlated with cardenia yellow pigment.
1. Introduction
Gardenia jasminoides Ellis is a medicinal and edible plant from the Sycamore family, predominantly grown in tropical and subtropical areas worldwide, such as China, Japan, Korea, Cambodia, and North America (Dai et al., 2019; Wang et al., 2019). As a health food, Gardenia jasminoides Ellis is commonly consumed as a tea, an appealing spice, and herbal medicine. It has been reported to have gallbladder, anti-inflammatory, analgesic, antihypertensive, antiviral, and other pharmacological effects. These beneficial effects are largely because of its key chemical components, such as geniposide, chlorogenic acid, and crocin I (Luo et al., 2021; Rao et al., 2023).
Seasonally, Gardenia jasminoides Ellis is typically harvested from September to November with a moisture content of up to 65 % on a wet basis, which makes it difficult to store after harvest, even under low-temperature storage. The seasonal nature and quick spoilage of fresh Gardenia jasminoides Ellis restrict its consumption and further processing. Under such conditions, drying has been proven to be an effective method to extend shelf life and enhance the availability of fresh agricultural materials (Sun et al., 2023; Xue et al., 2024). Dried Gardenia jasminoides Ellis can be obtained under various drying effects, such as hot air (HAD), microwave, infrared, vacuum, vacuum freezing, heat pump, and sun rays (Cheng et al., 2019; Dai et al., 2021; Liang et al., 2018; Tan et al., 2012; Zhao et al., 2009). Among various methods, HAD is the traditional and the most widely used method. However, the appearance, microstructure and bioactive ingredients may be negatively impacted by the extended drying time and high surface temperature during HAD (Qin et al., 2022). Especially, fresh Gardenia jasminoides Ellis has a distinct epidermal structure covered with a waxy hydrophobic layer that hinders moisture diffusion during drying. The presence of epicuticular waxes (a lipid-soluble fraction) restricts moisture diffusion through the surface, extending drying duration under HAD (Liu et al., 2022). In addition, the space between the seed mass and the shell creates an air barrier, resulting in poor thermal conductivity and inadequate heat transfer. This impedes the movement of moisture from within the seed mass to the shell's exterior. Consequently, the drying process becomes inefficient, leading to various quality issues. These problems may include shell rupturing, discoloration, and the degradation of valuable bioactive compounds (Ai, Mowafy, & Liu, 2022; Ai, Ren, et al., 2022; Ai, Xiao, et al., 2022; Ai, Zhu, et al., 2022; Wang, Liu, et al., 2020; Wang, Wang, et al., 2020). Therefore, in view of the unique material characteristics of Gardenia jasminoides Ellis, it is urgent to choose a suitable drying technology to improve the end product quality and the drying efficiency, while reducing energy consumption.
Pulsed vacuum drying (PVD) is a novel drying technology that utilizes the cyclic pulsation of the pressure in the drying chamber between vacuum and atmospheric pressure to break the equilibrium of the partial pressure of water vapor between the material surface and the drying medium, thus accelerating the migration of moisture (Geng et al., 2023; Li, Guo, et al., 2024; Li, Tian, et al., 2024). Additionally, the extended periods of vacuum exposure can help prevent oxidation reactions, thereby minimizing product quality degradation. Given its numerous benefits, PVD has been successfully used in the processing of different categories of agricultural products, such as garlic sprouts (Li, Guo, et al., 2024; Li, Tian, et al., 2024), chrysanthemum (Xu, Feng, et al., 2022; Xu, Wu, et al., 2022), and blueberries (Liu et al., 2022). Medium-short wave infrared drying (MSWID) is a kind of non-contact drying method that directly converts infrared energy into heat energy by utilizing the electromagnetic wave generated by infrared emitter onto the material. Since it is not required to pass through the medium and can directly make the surface layer of the material to heat up quickly, it has the advantages of fast drying rate, high energy efficiency. Moreover, it has obvious advantages in improving the drying efficiency of Chinese herbal medicines and retaining the content of active ingredients (Ai, Mowafy, & Liu, 2022; Ai, Ren, et al., 2022; Ai, Xiao, et al., 2022; Ai, Zhu, et al., 2022). Radio frequency-hot air drying (RF-HAD) is an innovative drying method where electrical energy directly interacts with materials, generating internal heat through the friction of polar water molecules. This process significantly shortens the heating time and increases the heating rate, thereby preventing the quality degradation often seen with prolonged heat treatment in HAD (Chen et al., 2021). Recently, RF-HAD has been examined for processing various agricultural products, including tiger nuts (Li, Guo, et al., 2024; Li, Tian, et al., 2024), Amomi fructus (Ai, Mowafy, & Liu, 2022; Ai, Ren, et al., 2022; Ai, Xiao, et al., 2022; Ai, Zhu, et al., 2022), jujube slices (Niu et al., 2022), and in-shell hazelnuts (Chen et al., 2021). It can be seen that the application effect of RF-HAD on different categories of materials is different, mainly depending on the structure and chemical composition of the materials. Based on the content of the previous study, Gardenia jasminoides Ellis contain about 60 % of non-polar oil with heat sensitive bioactive compounds, RF-HAD drying may help retain Gardenia jasminoides Ellis quality with a differential heating effect. However, it is not known whether RF-HAD is suitable for materials with waxy skin layers such as Gardenia jasminoides Ellis, and the effects of different drying techniques on the drying efficiency and product quality were different. It is necessary to systematically evaluate the applicability of different drying technologies for drying Gardenia jasminoides Ellis.
Drying involves interlinked heat and mass transfer mechanisms, accompanied by physical, chemical, and phase change transformations. Due to this complexity, it's challenging to accurately depict the drying process using only experimental measurement methods and empirical formulas. Drying models, however, offer a valid approach to characterize the heat and mass transfer parameters throughout the drying process. These models can effectively illustrate drying kinetics and how various drying technologies influence them. Lewis, Weibull, and page models have been applied with better data fitting to predict the drying process of numerous fruits and vegetables (An et al., 2022; Lin et al., 2023). In addition, the macro-phenomena observed during drying is closely linked to the microstructure of the material being dried. Changes in this microstructure can impact the texture and rehydration properties of dried food products (Liu, Xie, et al., 2021; Liu, Zhang, et al., 2021). Consequently, different drying technologies can influence the moisture migration behavior within the material, thereby affecting the quality of the final product. Thus, examining the microstructure of the drying material is valuable for understanding moisture migration patterns and variations in the product quality.
Therefore, the main research objectives of this research were to: 1) systematically evaluate the effects of different drying technologies (HAD, PVD, MSWID and RF-HAD) on the drying kinetics, energy consumption and quality parameters of Gardenia jasminoides Ellis to obtain the suitable drying method for it; 2) analyze the relationship between the physical attributes and bioactive compounds of Gardenia jasminoides Ellis to enable visual assessment of internal quality through external appearance characteristics; 3) explore how different drying technologies affect the drying kinetics and quality attributes of Gardenia jasminoides Ellis through microstructural changes and mathematical models. Ultimately, this study aimed at determining the best drying method that could potentially have large-scale industrial application. Hence, the information obtained from this could help inform the decision of the food scientist, and the agriculturist on the right and appropriate drying method for Gardenia jasminoides Ellis.
2. Materials and methods
2.1. Raw materials and chemicals
Fresh Gardenia jasminoides Ellis were collected in October 2023 from a local farm in Nanchang City, Jiangxi Province, China, and maintained at 4 °C until drying experiments. The Gardenia jasminoides Ellis samples with uniform size, color, and freshness were obtained, ensuring homogeneous physical attributes. The original water content of Gardenia jasminoides Ellis was 61.40 ± 0.31 % on a wet basis (w.b.), following the toluene method as specified in General rule no. 0832 of Chinese Pharmacopoeia (Commission, 2015). All chemical reagents and reference standards for chemical assays, such as chlorogenic acid, geniposide, crocin I, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and the total antioxidant capacity (T-AOC) assay kit, were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Drying experimental design
Before drying, Gardenia jasminoides Ellis samples were pretreated for 30 s by steam blanching. This pretreatment was established based on the outcome of a previous study and our good preliminary results (unpublished data), which showed that steam blanching for 30 s of Gardenia jasminoides Ellis could increase surface brightness and bioactive substance content while reducing drying time by destroying waxy layer structure (Luo et al., 2021). After the blanching pretreatment, Gardenia jasminoides Ellis was subjected to four different drying methods (HAD, RF-HAD, MSWID, and PVD (Fig. 1)) until 8.5 % final moisture content (w.b.). For each drying method, approximately 200 g of sample was used consistently. All drying techniques were conducted in triplicate, and the mean results of these trials were utilized for subsequent analysis. The process parameters for each drying technique were selected based on pre-experiments and our team's prior research experience, with the goal of enhancing drying efficiency and preserving product quality.
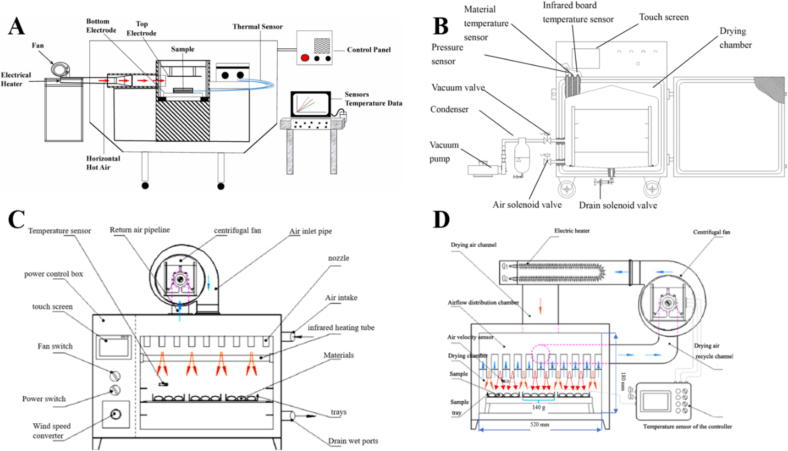
Fig. 1.
Schematic diagram of drying equipment. A: Radio frequency-hot air dryer (adapted from Mahmood et al., 2022); B: Pulsed vacuum dryer (adapted from Xie et al., 2017); C: Medium-and short-wave infrared dryer (adapted from Wang, Liu, et al., 2020; Wang, Wang, et al., 2020); D: Hot air dryer (adapted from Wang, Liu, et al., 2020; Wang, Wang, et al., 2020).
For the RF-HAD, the samples were placed in a perforated round plastic container (22 cm in diameter and 1 cm in height) and dried using a 6-kW, 27-MHz pilot-scale RF heating system (COMBI 6-S, Strayfield International, Wokingham, U.K.) fitted with an auxiliary hot air circulating system (Fig. 1A). The maximum capacity of the RF-HAD equipment is 4000 g. An intermittent RF heating mode was chosen to keep the sample temperature at 60 ± 2 °C, achieved by automatically toggling the RF heating switch. An electrode gap of 110 mm was selected to optimize the drying time, heating uniformity, and quality attributes. A forced hot air (55 °C) blew horizontally over the material surface from the electrode side at 1.5 m/s velocity, to ensure the final temperature of Gardenia jasminoides Ellis does not exceed 60 °C. During RF-HAD, three fiber optic temperature thermocouples (TempSens, Opsens Inc., Sainte-Foy, Quebec, Canada) were used to monitor the internal temperature changes of the samples at three key locations within the container: the corner, center, and edge.
In PVD, the samples were arranged in a single layer on a stainless steel tray and dried in laboratory-scale PVD equipment (Fig. 1B), which was described by Liu, Xie, et al. (2021) and Liu, Zhang, et al. (2021). The far-infrared radiation heating panel's temperature was monitored and regulated using thermal sensors. It was found that the actual material temperature was 10 °C lesser than that of the PVD system. Therefore, for a reliable comparison between the four drying techniques, and achieving a similar material temperature (60 °C), the far-infrared radiation plate was set at 70 °C with vacuum to atmospheric pressures pulsation of 12 to 3 min, respectively, based on literature (Xie et al., 2022).
During the MSWID process, the MSWID system described by Ai, Mowafy, and Liu (2022), Ai, Ren, et al. (2022), Ai, Xiao, et al. (2022) and Ai, Zhu, et al. (2022) (Fig. 1C) was utilized for the samples' dehydration after arranging them on the tray inside the dryer. A thermal sensor (UT320, UNI-T Group Co., Ltd., China) having ±0.1 °C of accuracy was positioned on the upper surface of the material to regulate the temperature. This high-precision thermometer was used to ensure precise temperature control at around 60 °C. Based on previous research findings (Ai, Mowafy, & Liu, 2022; Ai, Ren, et al., 2022; Ai, Xiao, et al., 2022; Ai, Zhu, et al., 2022), a 10 cm gap between the radiation source and the material was selected to ensure even heat distribution. This distance was determined to be optimal for maintaining uniform heating across the sample.
For the HAD, a DHG-9073BS-III lab-scale hot air dryer manufactured by Shanghai Yiheng Scientific Instrument Co., Ltd. in Shanghai City, China (Fig. 1D) was utilized to dry the samples after arranging them in one layer inside the system. The drying process was conducted at a setting temperature of 60 °C and air velocity of 2 m/s.
The dried Gardenia jasminoides Ellis products were ground by a JYL-C012 crusher manufactured by Jiuyang Co., Ltd. in China and then sifted through an 80-mesh sieve, resulting in particles with a size of 178 μm. The resulting powder was sealed in airtight aluminum zip-lock bags and stored frozen (−20 °C) for subsequent analyses of physicochemical properties.
2.3. Analysis of drying characteristics
2.3.1. Drying kinetics and modeling
The samples' weight was tracked during drying by measuring it every 30 min for the first 2 h of drying and then every 60 min until the process ended. This weight tracking process was conducted utilizing an SP402 electronic balance, manufactured by Ohaus Co. in New Jersey, USA, that has a sensitivity of 0.01 g. The drying process was evaluated by examining how the moisture ratio (MR) of Gardenia jasminoides Ellis changed over time. The MR was determined using Eq. (1):
| (1) |
where Mt is the samples' dry-based moisture content (d.b.) after a specific time t, g/g, and M0 is their initial moisture content (d.b.), g/g.
The Gardenia jasminoides Ellis drying rate (DR) within time t1 and t2 under different drying systems was calculated by Eq. (2) (Sharma & Dash, 2019):
| (2) |
where Mt1 and Mt2 represent the moisture contents (d.b.) at times t1 and t2, respectively, g/g.
Three mathematical models (Lewis model, Weibull model, Page model) were conducted to predict the drying characteristics of Gardenia jasminoides Ellis under different drying technologies. The details of each mathematical model were described by An et al. (2022). To assess the model's accuracy, three statistical measures were employed: the coefficient of determination (R2), reduced chi-square (λ2), and root mean square error (RMSE). These were calculated by comparing predicted values with experimental data. The model's fit is considered better when it has a higher R2 value and lower λ2 and RMSE values.
2.3.2. Effective moisture diffusivity (Deff)
The effective moisture diffusivity (Deff) was determined using the linearity ( (MR) versus drying time (t)) of the simplified equation of Fick's second law (Eq. 3) (Ai, Mowafy, & Liu, 2022; Ai, Ren, et al., 2022; Ai, Xiao, et al., 2022; Ai, Zhu, et al., 2022):
| (3) |
where r is the Gardenia jasminoides Ellis radius (5.21 ± 0.35 mm), which was assumed to be constant, and t is the dehydration time (s).
2.3.3. Specific energy consumption (SEC)
The specific energy consumption (SEC) was calculated according to Eq. (4)
| (4) |
where W represents the energy consumed during drying (kW·h), m0 and mi denote the sample's initial and final weights (g), and stands for the equipment load utilization factor.
2.4. Analysis of physical properties
2.4.1. Microstructure
Dried Gardenia jasminoides Ellis shell (5 mm × 5 mm × 5 mm) and kernel (2.5 mm × 2.5 mm × 2.5 mm) were sputtered with a golden thin layer (10 nm) during 60 s duration. Then, an SU3500 scanning electron microscope, manufactured by Hitachi Ltd. in Japan, with 15 kV acceleration voltage and 10 Pa vacuum pressure was utilized to scan the samples' microstructure morphology. The texture micro-images were captured at a × 300 magnification level.
2.4.2. Color
The color characteristics of the dried Gardenia jasminoides Ellis were measured using a colorimeter (model SMY-2000SF, manufactured by Shengmingyang Co., Beijing, China). This device assessed three color parameters: L* (indicating lightness from black to white), a* (representing the range from green to red), and b* (showing the spectrum from blue to yellow). The overall color change, denoted as ΔE, was then computed using Eq. (5):
| (5) |
where, ,refer to the fresh values of color coordinates.
2.5. Analysis of chemical properties
2.5.1. Bioactive components content
The extraction parameters of bioactive components were performed following Luo et al. (2021) with slight modifications. Briefly, a 0.1 g Gardenia jasminoides Ellis powder was precisely weighed and subjected to ultrasonic extraction (300 W, 80 kHz) at 50 °C for 40 min using 25 mL of a 50 % aqueous methanol solution. Following this, the mixture was centrifuged at 10000 r/min and 4 °C for 2 min. The resulting suspension was then collected, filtered through a 0.22 μm-membrane, and stored at 4 °C for subsequent analyses.
The chlorogenic acid, geniposide, and crocin I analyses were conducted using an Agilent 1290 series high-performance liquid chromatography (HPLC) system manufactured by Agilent Technologies in Santa Clara, CA, USA. The separation was performed on an Agilent Zorbax SB-C18 column with dimensions of 250 mm × 4.6 mm and a particle size of 5 μm. The analytical method was based on the procedure described by Wang et al. (2019). Throughout the analysis, the column temperature was kept constant at 30 °C. The mobile phase consisted of two components: mobile phase A, which was 0.3 % formic acid solution in water, and mobile phase B, which was acetonitrile. The gradient program of phase A (%)/phase B (%) was: 95/5 at time 0, 80/50 at time 15 (min), 70/30 at time 17, 60/40 at time 25, 10/90 at time 26, and 5/95 at time 30, respectively. Flow rate: 0.40 mL/min. The injection volume was 20 uL. The detection wavelengths of chlorogenic acid, geniposide, and crocin I were set at 328, 238, and 440 nm, respectively. The chlorogenic acid, geniposide, and crocin I contents were evaluated based on their calibration curves ((y = 40.17028x – 22.50662, R2 = 0.9994), (y = 38.22021x – 152.05603, R2 = 0.9984), and (y = 48.57699x – 103.67149, R2 = 0.9993), respectively). The content of bioactive components in Gardenia jasminoides Ellis was indicated based on the peak area.
2.5.2. Antioxidant capacity
To assess the antioxidant properties of dried Gardenia jasminoides Ellis affected by different drying technologies, two methods were employed: the DPPH free radical scavenging activity test and ferric reducing antioxidant power (FRAP) assay. DPPH assay was determined following An et al. (2022) with some modifications. A 1 mL extract was combined with 2 mL of 0.1 mmol/L DPPH solution, incubating the mixture in darkness at 37 °C for 30 min. Following incubation, the absorbance of the resulting solution was measured at 593 nm using a spectrophotometer manufactured by Beijing Purkinje General Instrument Co. Ltd. in Beijing, China. Then, the spectrophotometer results were used to present the DPPH radical scavenging capacity in percentage (%) and calculated according to Eq. (6):
| (6) |
where Acontrol and Asample are absorbance values of the “DPPH with methanol solution” and “DPPH with extract solution”, respectively.
The FRAP assay was evaluated utilizing the T-AOC assay kit in accordance with the manufacturer's instructions, and the output results were presented in μmol Trolox equivalent per 100 g of sample (μmol TE/100 g).
2.5.3. Cardenia yellow pigment content
The cardenia yellow pigment was determined by ultraviolet spectrophotometry method. Determination of cardenia yellow pigment was performed on basis of the procedure described by Zhao et al. (2009) with some modifications. A sample of 2.0 g dried Gardenia jasminoides Ellis powder was combined with 20 mL of an ethanol-water solution (60 % ethanol by volume). This mixture underwent ultrasonic extraction (300 W, 80 kHz) at 40 °C for 30 min. Following extraction, the mixture was collected and centrifuged for 10 min at 4500 r/min and 25 °C. The supernatant was then diluted with 60 % ethanol aqueous solution. This extraction process was dually performed. The final extract was filtered and collected for further analysis. The filtrate absorbance was evaluated at 440 nm utilizing a UV spectrophotometer fabricated by Pursee General Instrument Co. in Beijing, China. The cardenia yellow pigment content was expressed as the absorbance value (A440).
2.6. Selection of optimal drying technology
To standardize the diverse evaluation metrics and eliminate discrepancies between them, a comprehensive normalization approach was implemented. This process involved two distinct formulas: one for positive indicators (Eq. 7), which was applied for antioxidant capacity, geniposide content, crocin I, and chlorogenic acids, and another for negative indicators (Eq. 8), which was conducted for drying time, ΔE value, and SEC.
| (7) |
| (8) |
where and represent the negative and positive normalized indicators, respectively; , , and present each indicator's actual, maximum, and minimum values, respectively.
Comprehensive scores (CS) for each drying technique were calculated by applying weights to the indices, as outlined in Eq. (9):
| (9) |
where,,,, , , and are the normalization results of antioxidant capacity, geniposide content, crocin I content, chlorogenic acids content, drying time, ΔE value, and SEC, respectively. , , , , , , and are the corresponding weight of the indexes, and their values are defined as 0.1, 0.3, 0.1, 0.1, 0.1, 0.1, and 0.2, respectively. These weights were determined through hierarchical analysis (Pushpalatha et al., 2018) and based on each indicator's contribution to the final quality of Gardenia jasminoides Ellis.
2.7. Statistical analysis
All the quantitative results were expressed as mean ± standard deviation (SD) (n = 3). Statistical analyses were conducted using IBM SPSS statistics (version 20.0, SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was performed, and the significant differences between groups were determined using Duncan's multiple range test, with a significant level set at p<0.05.
3. Results and discussion
3.1. Effects of different drying techniques on drying characteristics of Gardenia jasminoides Ellis
3.1.1. Drying kinetics and modeling
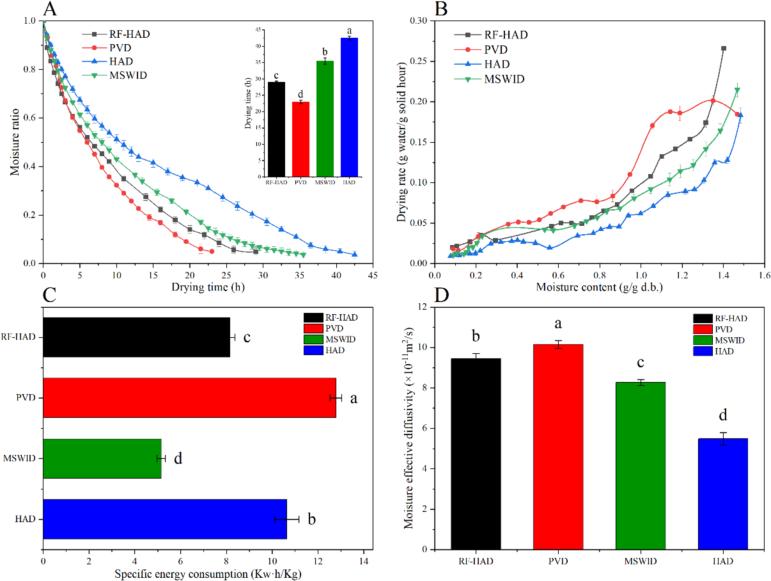
The impacts of different drying techniques on the drying kinetics of Gardenia jasminoides Ellis are shown in Fig. 2. The moisture ratio steadily diminished as the drying time increased, eventually reaching 8.5 % moisture content (w.b.), corresponding to a moisture ratio of 0.04 (Fig. 2A). For various drying techniques, the process extended from 23 to 43 h. When compared to traditional HAD, the primary advantage of the innovative drying techniques (RF-HAD and PVD) is their reduced drying time. As illustrated in Fig. 2A, HAD required the longest drying duration (43 h), followed by MSWID (36 h), RF-HAD (29 h), and PVD (23h), respectively. Among all drying methods, PVD and RF-HAD were the fastest methods to dry Gardenia jasminoides Ellis, and their drying times were 46.51 % and 32.56 %, respectively, shorter than HAD at the exact material temperature. The results agree with the data of Panax Notoginseng roots and blueberries under PVD reported by Liu, Xie, et al. (2021), Liu, Zhang, et al. (2021) and Jiang et al. (2021), who found that PVD could shorten drying time by 22.90 % of Panax Notoginseng roots and 32.14 % of blueberry compared to HAD at a matched temperature. The shortened drying duration for PVD could be attributed to the alternative pressure that creates a more porous material microstructure, enhancing water movement from the interior to the exterior patterns (Jiang et al., 2021). Additionally, the pressure fluctuation of the drying environment could expel the air between the kernel and the shell of Gardenia jasminoides Ellis, allowing them to fit tightly together without air gaps, thereby improving mass transfer efficiency (Ai, Mowafy, & Liu, 2022; Ai, Ren, et al., 2022; Ai, Xiao, et al., 2022; Ai, Zhu, et al., 2022). The difference in the drying duration between RF-HAD and HAD was mainly caused by different heating principles. HAD necessitated a longer warm-up period to reach the desired temperature at the material's core, due to the poor thermal conductivity of the air spaces between the shell and kernel, which hindered heat transfer from the surface to the center. In contrast, RF heating rapidly elevated the material's core temperature to the required level through volumetric heating, which induces molecular motion, thus expediting the moisture migration from inside to outside. Compared to RF-HAD, the thermal effect and pressure pulsation during PVD advocated the material's moisture to evaporate and diffuse (Zheng et al., 2023).
Fig. 2.
Moisture ratio (A), drying rate (B), specific energy consumption (C) and moisture effective diffusivity (D) of Gardenia jasminoides Ellis under different drying techniques. Note: RF-HAD, Radio frequency-hot air drying; MSWID, Medium-and short-wave infrared drying; PVD, Pulsed vacuum drying; HAD, Hot air drying. Different letters on the different columns indicate statistically significant difference at p < 0.05 according to the Duncan test.
Fig. 2B illustrates the relationship between the drying rate and moisture content. It was noted that all the drying processes performed a falling-rate trend, suggesting that aqueous phase diffusion was the primary mechanism for moving the moisture from the interior parts of Gardenia jasminoides Ellis. Among the various drying technologies, PVD exhibited the highest drying rate. The superior drying rate under PVD could be attributed to the combined thermal and mechanical effect on the material texture, such as microscopic channels, pores, and cell structure changes. Previous studies have shown that the pulsating pressure difference and thermal effect during PVD can change the cell wall structure and porosity, which is conducive to promoting the mass transfer process of drying (Liu et al., 2022). Therefore, To better understand how microstructure affects water movement, it is essential to examine the microstructural modifications that occur during Gardenia jasminoides Ellis dehydration.
Table 1 presents a summary of the four applied models' fitting results and their factor values, encompassing model constants (k, α, β, and n) as well as model excellence criteria (R2, RMSE, and χ2). For the Page model, the n value varied between different drying techniques. This outcome was anticipated, as the n value is primarily associated with the microstructural characteristics of the sample (Li et al., 2022), which was differently altered by various drying techniques (Fig. 3). For the Lewis and Page models, at the exact processing temperature, their k values under PVD were greater than that of other drying methods, which agreed with the drying behavior shown in Fig. 2A. While the k value can be relevant to the drying rate in the forehead stage, the higher k value associated with the higher drying rate technology. For the Weibull model, the α value of PVD was the smallest, followed by RF-HAD, MSWID, and HAD, which is in agreement with the drying kinetics curve and total drying duration in Fig. 2A. The shape parameter (β) represents the shape of the drying rate curve. In Table 1, the β value is between 0.3 and 1, indicating that Gardenia jasminoides Ellis performed a reduced drying rate along the process. The Page model denoted the greatest main values of R2 (0.998) as well as the lowest main values of RMSE (0.0117) and χ2 (1.5096 × 10−4) compared to other models, followed by the Weibull and Lewis models. As a result, the Page model can be highlighted as the best for fitting the dying process of Gardenia jasminoides Ellis. Wang et al. (2019) reported a similar conclusion that the Page model had a better fitting to describe the fruit Chinese medicinal materials drying.
Table 1.
Fitting results of drying kinetics for Gardenia jasminoides Ellis under different drying techniques.
| Model | Parameters | RF-HAD | PVD | MSWID | HAD |
|---|---|---|---|---|---|
| Page model | k | 0.1279 | 0.1701 | 0.1119 | 0.1062 |
| n | 0.7499 | 0.9591 | 0.7723 | 0.9315 | |
| χ2(×10−4) | 2.8058 | 1.5096 | 1.8620 | 3.5664 | |
| R2 | 0.9966 | 0.9983 | 0.9972 | 0.9968 | |
| RMSE | 0.0159 | 0.0117 | 0.0131 | 0.0182 | |
| Weibull model | α | 10.6067 | 8.5345 | 11.2752 | 17.0403 |
| β | 0.6700 | 0.8991 | 0.7624 | 0.9066 | |
| χ2(×10−4) | 2.3102 | 1.4376 | 1.5789 | 3.5708 | |
| R2 | 0.9934 | 0.9873 | 0.9852 | 0.9964 | |
| RMSE | 0.0178 | 0.0157 | 0.0145 | 0.0281 | |
| Lewis model | k | 0.1056 | 0.1175 | 0.0874 | 0.0628 |
| χ2(×10−4) | 17.9000 | 2.0710 | 6.2170 | 17.1000 | |
| R2 | 0.9793 | 0.9976 | 0.9935 | 0.9795 | |
| RMSE | 0.0037 | 0.0013 | 0.0015 | 0.0017 |
Note: RF-HAD, Radio frequency-hot air drying; MSWID, Medium-and short-wave infrared drying; PVD, Pulsed vacuum drying; HAD, Hot air drying.
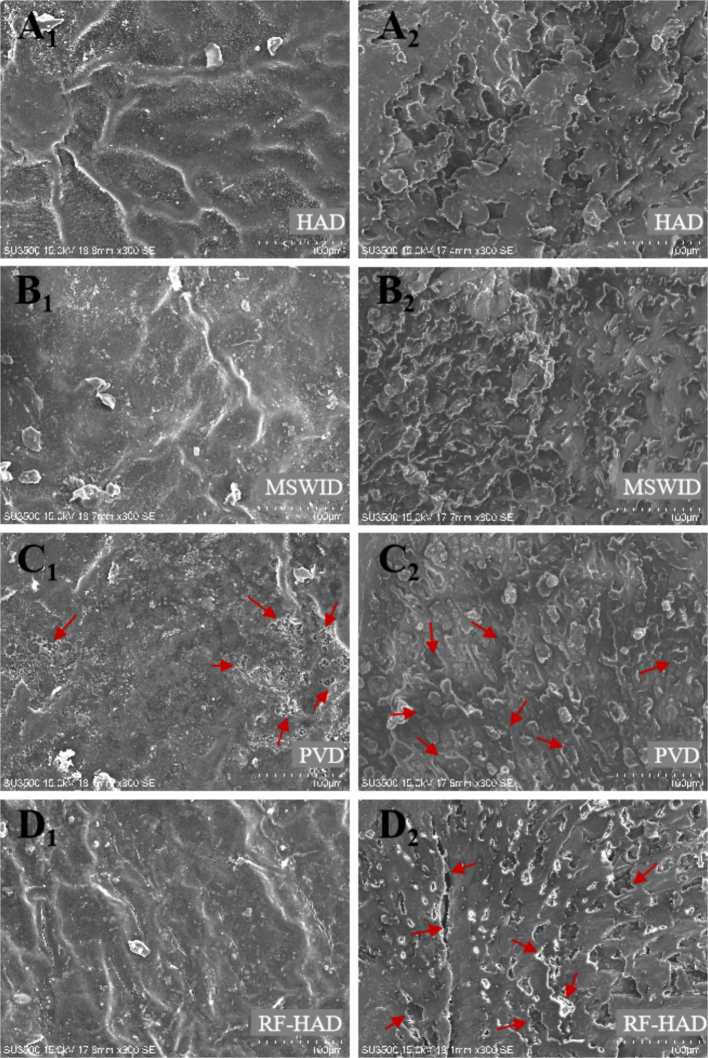
Fig. 3.
Scanning electron microscope images of shell (A1-D1) and kernel (A2-D2) of Gardenia jasminoides Ellis under different drying techniques.
Note: RF-HAD, Radio frequency-hot air drying; MSWID, Medium-and short-wave infrared drying; PVD, Pulsed vacuum drying; HAD, Hot air drying.
3.1.2. Effective moisture diffusivity (Deff)
The Deff serves as a crucial parameter for assessing a material's dehydration capacity during drying. A higher Deff value indicates a more vital dehydration ability and suggests that less activation energy is needed for moisture diffusion. The outcomes of Deff values (×10−11 m2/s) of Gardenia jasminoides Ellis for RF-HAD, PVD, MSWID, and HAD process were 9.45 ± 0.25, 10.15 ± 0.19, 8.27 ± 0.15, and 5.48 ± 0.31, respectively (Fig. 2D). Our Deff outcomes were in agreement with those of Niu et al. (2022), where their Deff values of agro products ranged from 10−12 to 10−8. Also, Wang et al. (2019) revealed the Deff of Gardenia jasminoides Ellis between 1.765 and 5.749 × 10−8 m2/s during vacuum drying and HAD. The Deff values under PVD at the same temperature were 1.07–1.85 times greater than those of the remaining drying techniques, revealing that PVD implemented superior mass transfer efficiency. This is because the vacuum environment and pressure pulsation corresponded to higher mass transfer efficiency, highly activating water molecules, and boosting moisture diffusivity.
3.1.3. Specific energy consumption (SEC)
Drying is among the most energy-intensive processes in various industrial fields, characterized by a significant carbon footprint and relatively low thermal efficiency, typically ranging from 25 % to 50 %. This process consumes about 10 % of the total energy used in the entire food industry, making energy efficiency a crucial area of research. Improving energy efficiency not only reduces environmental impact but also indirectly enhances the product trade value. Under a similar setting temperature (60 °C), the SEC values of Gardenia jasminoides Ellis during the four drying technologies were compared, presenting the outcomes in Fig. 2C. PVD revealed the highest SEC value (12.78 kW·h/kg), followed by HAD (10.64 kW·h/kg), the lowest SEC value was achieved by MSWID (5.15 kW·h/kg) and RF-HAD (8.15 kW·h/kg). Although PVD had the shortest drying time, continuous vacuum operation during the vacuum holding stage consumed much energy, resulting in higher energy consumption than other drying methods. The MSWID and RF-HAD process achieved approximately 23.40–51.60 % energy savings in comparison with HAD. These findings could be attributed to the shorter processing duration and higher Deff of MSWID and RF-HAD than HAD. The MSWID and RF-HAD generated heat directly inside the material without being transferred by air, supporting moisture movement from inside to outside, shortening drying time, and decreasing SEC.
3.2. Effects of different drying techniques on the microstructure of Gardenia jasminoides Ellis
The shell and kernel of Gardenia jasminoides Ellis have different physical properties, such as porosity, moisture content, and density, leading to inconsistent microstructure characteristics during drying (Dai et al., 2019). The impacts of drying treatments on the microstructural changes in the shell and kernel of Gardenia jasminoides Ellis were SEM-identified and presented in Fig. 3. The HAD-dried samples (Fig. 3A1 and A2) exhibited a more compacted and cellularly contracted structure in both shell and kernel. This was due to the prolonged and significant imbalance between internal and external pressure during the drying process. Compared to other drying methods, the surface of Gardenia jasminoides Ellis shell visualized a more porous structure with minor deformation under PVD (Fig. 3C1). Similar results also were observed in the dried sea buckthorn berries and peanuts Geng et al. (2023) and Xie et al. (2022). The porosity and microstructure of the dried samples are related to the diversity in medium pressure and water diffusion mechanisms. Impetuous moisture gradients within the material led to microstructural stress, distracting most of the capillaries and ultimately causing irreparable structural changes (Qin et al., 2022). Therefore, the formation of pore structures in PVD samples can be attributed to the significant pressure difference between the interior and exterior of the material. This difference arises from the vacuum effect, which depressed the water boiling point in Gardenia jasminoides Ellis. The substantial vapor pressure gradient from the center to the surface of Gardenia jasminoides Ellis forced the water to diffuse to the surface. This eliminated the surface resistance to moisture diffusion (mass transfer), thereby shortening the drying process (Tabibian et al., 2020). Differing from other drying systems, the internal tissue arrangement of the RF-HAD-dried Gardenia jasminoides Ellis kernel was regular and showed an obvious honeycomb structure (Fig. 3D2). Also, deformed and expanded tiny tunnels and pores in some patterns of the kernel were realized, matching the drying impacts on the peanut structure reported by Xie et al. (2022). This effect could partly stem from the insignificant temperature diversity across the material, caused by the parallel impacts of hot air and RF energy, which promotes steady moisture migration. The porous structure produced by RF-HAD and PVD encouraged the moisture transfer in Gardenia jasminoides Ellis while drying. This also probably enhances the phytochemical extracting process, potentially leading to more concentration of bioactive compounds (Zheng et al., 2021).
3.3. Effects of different drying techniques on the physicochemical quality of Gardenia jasminoides Ellis
3.3.1. Color
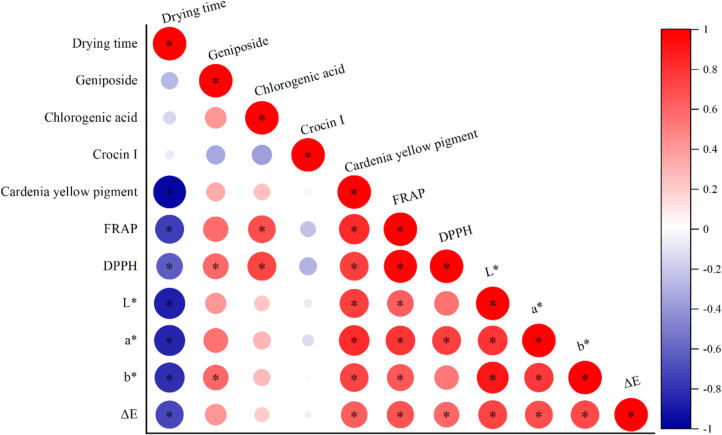
Color stands as a key quality indicator for dried Gardenia jasminoides Ellis, significantly influencing consumer approval and the marketability of dried products. The change of color in Chinese herbs is linked to pigments in the form of flavonoids, phenols, chlorophyll and yellow pigments (Chen et al., 2020). The color indicators for both fresh and dried Gardenia jasminoides Ellise were provided in Table 2. The analysis revealed that drying techniques markedly (p < 0.05) affected color parameters of Gardenia jasminoides Ellis. During drying, enzymatic browning and non-enzymatic browning typically lead to a reduction in L* level and a rise in a* and b* values. The samples dried by HAD showed lower L* values than that of fresh samples and those dried by other methods. This may be attributed to the higher temperature and ample oxygen, which create a favorable environment for the browning of Gardenia jasminoides Ellis, consistent with the findings of Liu et al. (2024). Among the dried products, the highest brightness levels were achieved by PVD (38.52 ± 1.30) and RF-HAD (37.67 ± 0.65), while HAD and MSWID came next. These outcomes could be attributed to the shorter processing period under PVD, thereby limiting the available time for browning reactions. In addition, previous studies have also shown that RF treatment can cause structural damage and inactivation of major enzymes responsible for enzymatic browning, such as polyphenol oxidase (Xie et al., 2022). The a* levels of dried products, which represent their reddish coloring, ranging from 10.70 ± 1.26 to 15.88 ± 0.34, which were markedly higher than that of the fresh Gardenia jasminoides Ellis (9.88 ± 0.27). This is because drying may assist the reformation of certain components, promoting the red coloration of Gardenia jasminoides Ellis. Pearson correlation analysis in Fig. 4 showed that a* and b* color values revealed a positive correlation with the content of cardenia yellow pigment, indicating that cardenia yellow pigment may be the main pigment affecting the color of gardenia, and reducing the loss of this component is conducive to the retention of sample color. Among all the drying technologies, PVD and RF-HAD treatments resulted in the highest a* values, revealing a more vibrant red color in the samples. This brighter red appearance is generally considered desirable and associated with higher quality in the marketplace. These high a* values may be ascribed to less enzymatic browning and cardenia yellow pigment denaturation affected by the shortened processing time under PVD or RF-HAD, as shown in Table 3. Sea buckthorn berries and Amomi fructus revealed similar results during studies by Geng et al. (2023), Ai, Mowafy, and Liu (2022), Ai, Ren, et al. (2022), Ai, Xiao, et al. (2022) and Ai, Zhu, et al. (2022), respectively. The total color difference (ΔE) of dried Gardenia jasminoides Ellis ranged from 8.89 ± 0.14 to 11.86 ± 0.81. This metric is commonly used to assess the overall color variation between dried and fresh materials. Superior to other drying techniques, PVD and RF-HAD achieved the best color quality with the highest ΔE value. The previous reports about ΔE revealed that the lower the ΔE value, the better color the appearance of most agricultural products. However, in the Gardenia jasminoides Ellis case, the marked increase in redness during drying is favorable, resulting in a higher ΔE value.
Table 2.
Color parameters of Gardenia jasminoides Ellis under different drying techniques.
| Drying method | L⁎ | a⁎ | b⁎ | ΔE | |
|---|---|---|---|---|---|
| Fresh | 41.45 ± 0.79a | 9.88 ± 0.27c | 7.66 ± 0.18b |  |
|
| RF-HAD | 37.67 ± 0.65b | 15.88 ± 0.34a | 16.99 ± 0.50a | 11.72 ± 0.34a |  |
| PVD | 38.52 ± 1.30b | 15.74 ± 1.79a | 17.55 ± 0.43a | 11.86 ± 0.81a |  |
| MSWID | 37.21 ± 0.88b | 13.33 ± 1.17b | 16.60 ± 2.07a | 10.48 ± 0.99a |  |
| HAD | 32.74 ± 0.83c | 10.70 ± 1.26c | 6.09 ± 0.93b | 8.89 ± 0.14b |  |
Note: RF-HAD, Radio frequency-hot air drying; MSWID, Medium-and short-wave infrared drying; PVD, Pulsed vacuum drying; HAD, Hot air drying. The different lowercase letter in the same row indicates that the means are significantly different at p < 0.05.
Fig. 4.
Pearson correlation analysis of various parameters in Gardenia jasminoides Ellis dried by different drying techniques. * indicates significant correlation at p < 0.05.
Table 3.
Bioactive components content and antioxidant capacity of Gardenia jasminoides Ellis under different drying techniques.
| Drying method | Geniposide (mg/g) |
Chlorogenic acid (mg/g) | Crocin I (mg/g) |
Cardenia yellow pigment (A440) | DPPH (%) |
FRAP (μmol/g) |
|---|---|---|---|---|---|---|
| RF-HAD | 68.01 ± 1.00a | 1.54 ± 0.16a | 21.90 ± 0.76a | 0.77 ± 0.02b | 15.14 ± 0.71a | 8.41 ± 0.30a |
| PVD | 61.76 ± 0.68bc | 1.07 ± 0.03b | 22.10 ± 0.35a | 0.83 ± 0.02a | 10.55 ± 0.89b | 6.60 ± 0.43b |
| MSWID | 64.83 ± 3.56ab | 1.19 ± 0.16b | 19.39 ± 0.63b | 0.58 ± 0.12c | 7.69 ± 0.28c | 4.82 ± 0.17c |
| HAD | 59.78 ± 3.44c | 1.12 ± 0.12b | 19.41 ± 1.44b | 0.51 ± 0.03d | 7.20 ± 0.26c | 4.16 ± 0.20d |
Note: RF-HAD, Radio frequency-hot air drying; MSWID, Medium-and short-wave infrared drying; PVD, Pulsed vacuum drying; HAD, Hot air drying. The different lowercase letter in the same row indicates that the means are significantly different at p < 0.05.
3.3.2. Chlorogenic acid content
Chlorogenic acid, as an important phenolic nutrient, is highly susceptible to heat and oxygen exposure, readily breaking down into phenol compounds under harsh drying environments (Shi et al., 2017). Moreover, during drying, a portion of chlorogenic acids can undergo hydrolysis and degradation, resulting in lower molecular weight compounds. For instance, 5-caffeylquinic acid, which is particularly unstable during thermal processing, can decompose into 3-caffeylquinic acid and 4-caffeylquinic acid when subjected to temperatures above 37 °C and pH levels between 5 and 9 (Kulapichitr et al., 2022). The results of the chlorogenic acid content for dried Gardenia jasminoides Ellis are presented in Table 3. Among the four drying methods, RF-HAD revealed the highest chlorogenic acid content (1.54 ± 0.16 mg/g), followed by MSWID and HAD. The smallest chlorogenic acid amount was 1.07 ± 0.03 mg/g indicated under PVD, which was 30.52 % lower than that of RF-HAD samples. The better retention of chlorogenic acid in RF-HAD samples can be primarily attributed to the unique properties of radio frequency energy. During the drying process, this energy is rapidly converted into heat through molecular vibration and is swiftly and uniformly absorbed into the sample's core. This mechanism leads to the breakdown of the covalent bonds of polymeric polyphenols by radio frequency waves. Consequently, during the extraction process, a greater quantity of small molecular phenolic compounds, including chlorogenic acid, is released (Li, Guo, et al., 2024; Li, Tian, et al., 2024). In addition, the retention of chlorogenic acid was ultimately linked with the drying time of Gardenia jasminoides Ellis. The shorter drying duration of RF-HAD shortened the material exposure duration to oxygen, eliminating the chlorogenic acid decomposition. However, HAD revealed the least retention of chlorogenic acid, which broke down under the long-term processing effect.
3.3.3. Geniposide and crocin I content
Geniposide and crocin I are the primary chemical constituents available in the Gardenia jasminoides Ellis. Crocin I, the main carotenoid in Gardenia jasminoides Ellis, contains unsaturated bonds in its molecular structure, making it vulnerable to oxygen and high temperatures. This susceptibility leads to chemical reactions, like oxidation-reduction and polymerization hydrolysis (Tabibian et al., 2020). As shown in Fig. 5 and Table 3, all drying techniques had notable (p < 0.05) effects on the contained geniposide and crocin I, and their contents ranged from 59.78 ± 3.44 mg/g to 68.01 ± 1.00 mg/g and 19.39 ± 0.63 mg/g to 22.10 ± 0.35 mg/g, respectively. For the content of geniposide, RF-HAD obtained the highest content, followed by MSWID, PVD and HAD, which could be ascribed to their drying duration, temperature and stress. Geniposide is easily decomposed under the action of protease and β-glucosidase (Wang & Fan, 2012). During RF-HAD drying, the rapid increase of core temperature inhibited β-glucosidase activity, thereby preventing enzymatic degradation of geniposide and playing a good effect on enzyme elimination and glycoside preservation, so a higher content of geniposide was obtained. Previous studies have also shown that RF technology can eliminate enzymes at a similar drying duration and has better efficiency than other heat treatment methods to inhibit enzyme activity due to its own volume heating mode (Yao et al., 2021).
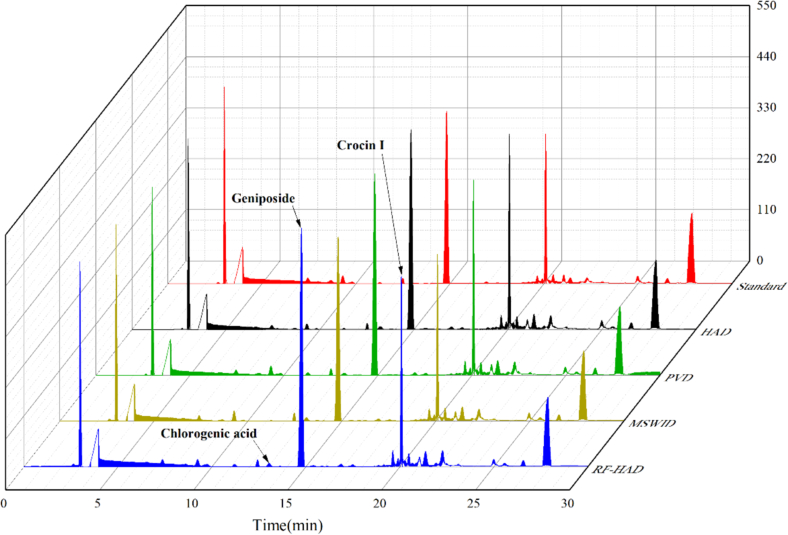
Fig. 5.
HPLC chromatograms of Gardenia jasminoides Ellis with different drying techniques.
For the crocin I content, there was not much deviation between RF-HAD (21.90 ± 0.76 mg/g) and PVD (22.10 ± 0.35 mg/g), they were all relatively high, while MSWID (19.39 ± 0.63 mg/g) and HAD (19.41 ± 1.44 mg/g) had lower contents, without notable variation between them. Compared with the other two drying methods, the higher retention of crocin I in RF-HAD and PVD may be ascribed to their short-term and milder intensity of drying. For PVD, the low-temperature conditions prohibited the thermal breakdown of crocetin I, which can be decomposed into crocetin under the action of temperature and moisture (Chen et al., 2020). Additionally, the vacuum condition under PVD can avoid long-term material exposure to oxygen, thus preventing the occurrence of oxidation reactions. Regarding MSWID, the relatively high drying stress and extended processing duration accelerated the crocin I degradation, presenting the lowest level.
3.3.4. Antioxidant capacity analysis
The presence of high levels of antioxidants can amplify the therapeutic efficacy and immune-boosting properties of functional components. When assessing the quality of Gardenia jasminoides Ellis, the antioxidation activity of its dried products serves as a crucial indicator. During this research, we evaluated the antioxidation capacity of the Gardenia jasminoides Ellis extract using two ways: DPPH and FRAP radical scavenging assays. The outputs of these analyses are presented in Table 3. The RF-HAD samples exhibited the highest DPPH and FRAP values, closely followed by PVD and MSWID samples. However, HAD-treated samples demonstrated the lowest DPPH and FRAP radical scavenging capacities. This change can be attributed to the reduced drying time and oxygen exposure during the RF-HAD and PVD processes, which minimized the antioxidants' degradation under light, heat and oxygen (Xu, Feng, et al., 2022; Xu, Wu, et al., 2022). These findings align with a study by Liu, Xie, et al. (2021) and Liu, Zhang, et al. (2021), which affirmed that the RF-HAD had the most beneficial impact on the antioxidant activity of Sichuan pepper, while HAD presented the least favorable effect. The possible reason was that the formation of microporous channels during the RF-HAD process contributed to the extraction of antioxidants, resulting in more accumulation of antioxidant-active ingredients.
The correlation analysis results, as depicted in Fig. 4, clarified significant positive associations between DPPH scavenging capacity and both chlorogenic acid content (r = 0.74, p<0.05) and geniposide content (r = 0.59, p<0.05). Also, the FRAP scavenging capacity demonstrated a high positive correlation with chlorogenic acid content (r = 0.69, p<0.05). These findings suggest that chlorogenic acid and geniposide were the primary contributors to the antioxidant capacity in this research. Furthermore, radio frequency treatment may have the ability to disrupt covalent links between antioxidants and insoluble polymers, thereby releasing natural antioxidants in the forms of polyphenols, chlorogenic acid, flavonoids and geniposide (Li, Guo, et al., 2024; Li, Tian, et al., 2024). This mechanism may explain why RF-HAD-dried material exhibited higher antioxidant capacity compared to those dried using HAD.
3.4. Drying technology optimization of Gardenia jasminoides Ellis based on comprehensive evaluation
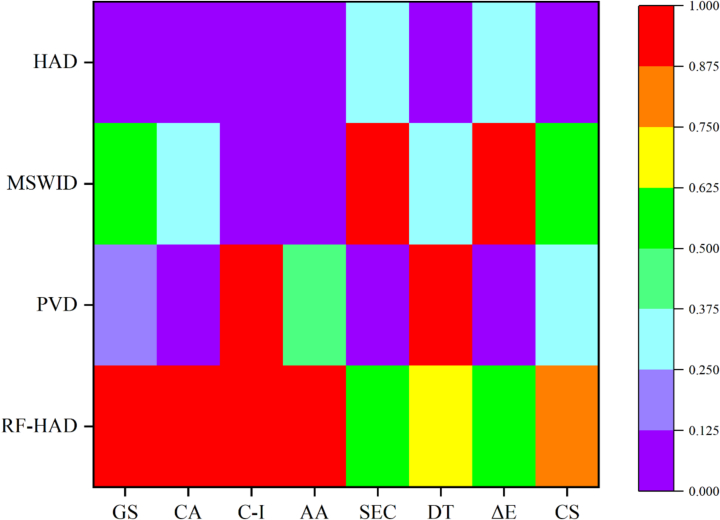
The analysis of the results demonstrates that drying technologies exert complex and interrelated effects on various quality indicators of Gardenia jasminoides Ellis. To address this multifaceted impact, we employed a comprehensive evaluation methodology in this study. This approach aimed to identify the best drying technique and process conditions that would yield high-quality Gardenia jasminoides Ellis products while maintaining worthy drying efficiency and reduced energy consumption. The outcomes of this comprehensive assessment, which considered both drying behavior and product quality, were heat-mapped and are presented in Fig. 6. This heat map utilizes a color gradient from purple to red, with purple indicating lower composite scores and red signifying higher scores. Of all the drying technologies, RF-HAD obtained the highest composite score (0.8439), followed by MSWID (0.5550) and PVD (0.3197), HAD obtained the lowest composite score (0.0955). Under the RF-HAD drying technology, Gardenia jasminoides Ellis had better color and antioxidant activity, as well as the retention of geniposide and chlorogenic acid content, reduced drying duration and energy usage. As a result, RF-HAD was selected as the optimal drying technology to realize worthier drying behavior and impressing Gardenia jasminoides Ellis quality.
Fig. 6.
Heat map of comprehensive scoring of Gardenia jasminoides Ellis dried by different drying techniques.
Note: GS, Geniposide; CA, Chlorogenic acid; C—I, Crocin I; AA, Antioxidant capacity; SEC, Specific energy consumption; DT, Drying time; CS, Comprehensive score.
4. Conclusions
Drawing from the present study results, it can be concluded that both the drying technique employed and the specific parameters applied significantly influenced the drying kinetics and quality attributes of Gardenia jasminoides Ellis. Regarding drying characteristics, PVD and RF-HAD had the shortest drying duration when compared with HAD and MSWID, and the Deff value for PVD was 1.07–1.85 times greater than those of the rest drying technologies, which suggests that PVD demonstrated the highest efficiency in terms of mass transfer. The Page model provided a superior fit for the drying curves compared to both the Lewis and Weibull models. The cell structure of dried samples was irreversibly changed, and a significant loose porous structure was observed in the shell obtained by PVD and the kernel obtained by RF-HAD, which accelerated the drying process and increased the bioactive components content of the Gardenia jasminoides Ellise. As for quality, PVD and RF-HAD obtained the highest a* values due to less cardenia yellow pigment degradation caused by shorter drying time. RF-HAD exhibited the highest chlorogenic acid and gardenin content as well as antioxidant capacity. PVD obtained the highest crocin I content. On the whole, RF-HAD is the best drying technology to enhance both drying performance and product quality, with the highest overall score. Moving forward, research efforts could be directed towards exploring pretreatment techniques to enhance the visual appeal, improve physicochemical quality, and increase drying efficiency.
CRediT authorship contribution statement
Ziping Ai: Validation, Software, Resources. Zhifeng Xiao: Funding acquisition, Formal analysis, Data curation. Muhua Liu: Visualization, Validation, Supervision. Lingqu Zhou: Validation, Methodology, Investigation. Lingjian Yang: Writing – original draft, Software, Formal analysis. Yijie Huang: Visualization, Project administration, Investigation. Qiangqiang Xiong: Validation, Investigation, Data curation. Tao Li: Writing – review & editing, Investigation, Conceptualization. Yanhong Liu: Visualization, Project administration, Formal analysis. Hongwei Xiao: Writing – review & editing, Software, Conceptualization. Jiale Guo: Software, Methodology, Formal analysis. Wenling Sun: Validation, Project administration, Investigation. Samir Mowafy: Validation, Software, Data curation. Honghui Rao: Writing – review & editing, Resources, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was financially supported by Jiangxi Provincial Natural Science Foundation (20242BAB20330 and 20242BAB25363), and Jiangxi Province Key R&D Program project (No.20232BBF60016).
Data availability
Data will be made available on request.
References
- Ai Z., Mowafy S., Liu Y. Comparative analyses of five drying techniques on drying attributes, physicochemical aspects, and flavor components of Amomum villosum fruits. LWT- Food Science and Technology. 2022;154 doi: 10.1016/j.lwt.2021.112879. [DOI] [Google Scholar]
- Ai Z., Ren H., Lin Y., Sun W., Yang Z., Zhang Y.…Liu Y. Improving drying efficiency and product quality of Stevia rebaudiana leaves using innovative medium-and short-wave infrared drying (MSWID) Innovative Food Science and Emerging Technologies. 2022;81 doi: 10.1016/j.ifset.2022.103154. [DOI] [Google Scholar]
- Ai Z., Zhu G., Zheng Z., Xiao H., Mowafy S., Liu Y. Successive two-stage hot air drying with humidity control combined radio frequency drying improving drying efficiency and nutritional quality of Amomi fructus. Food and Bioprocess Technology. 2022;16:149–166. doi: 10.1007/s11947-022-02928-8. [DOI] [Google Scholar]
- Ai Z.P., Xiao H., Zhang Y., Lei D., Peng Z., Li M., Liu Y. Effect of hot air impingement drying on drying behavior, volatile components profile, shell burst ratio, flavonoid contents, microstructure of Amomum villosum fruits. Drying Technology. 2022;41:107–121. doi: 10.1080/07373937.2022.2087184. [DOI] [Google Scholar]
- An N., Sun W., Li B., Wang Y., Shang N., Lv W.…Wang L. Effect of different drying techniques on drying kinetics, nutritional components, antioxidant capacity, physical properties and microstructure of edamame. Food Chemistry. 2022;373 doi: 10.1016/j.foodchem.2021.131412. [DOI] [PubMed] [Google Scholar]
- Chen D., Xing B., Yi H., Li Y., Zheng B., Wang Y., Shao Q. Effects of different drying methods on appearance, microstructure, bioactive compounds and aroma compounds of saffron (Crocus sativus L.) Lwt. 2020;120 doi: 10.1016/j.lwt.2019.108913. [DOI] [Google Scholar]
- Chen L., Subbiah J., Jones D., Zhao Y., Jung J. Development of effective drying strategy with a combination of radio frequency (RF) and convective hot-air drying for inshell hazelnuts and enhancement of nut quality. Innovative Food Science and Emerging Technologies. 2021;67 doi: 10.1016/j.ifset.2020.102555. [DOI] [Google Scholar]
- Cheng M., An C., Yang C., Zhuang Y., Su Z., Huang Z. Effects of different drying methods on 4 iridoid glycosides in Gardenia root. Journal of Chinese Medicinal Materials. 2019;42:1533–1536. doi: 10.13863/j.issn1001-4454.2019.07.015. [DOI] [Google Scholar]
- Commission C.P. China Medical Science and Technology Press; Beijing: 2015. Pharmacopoeia of the people's Republic of China. [Google Scholar]
- Dai Y., Deng K., Zhou W., Li J., Cao Y. Research progress in functional components and drying technology in the fruit of Gardenia jasminoides Ellis. Science and Technology of Food Industry. 2019;40:300–306. doi: 10.13386/j.issn1002-0306.2019.21.049. [DOI] [Google Scholar]
- Dai Y., Deng K., Zhou W., Li J., Cao Y. Drying characteristics and kinetics of fruit of Gardenia jasminoides Ellis with solar assisted heat pump drying. Chinese Journal of Tropical Crops. 2021;42:2405–2412. doi: 10.3969/j.issn.1000-2561.2021.08.037. [DOI] [Google Scholar]
- Geng Z., Zhu L., Wang J., Yu X., Li M., Yang W.…Yang X. Drying Sea buckthorn berries (Hippophae rhamnoides L.): Effects of different drying methods on drying kinetics, physicochemical properties, and microstructure. Frontiers in Nutrition. 2023;10:1106009. doi: 10.3389/fnut.2023.1106009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Xiao H., Zheng Z. Effects of different drying methods on drying characteristics, microstructure, quality, and energy consumption of Panax Notoginseng roots (Araliaceae) Drying Technology. 2021;40:1247–1261. doi: 10.1080/07373937.2020.1863978. [DOI] [Google Scholar]
- Kulapichitr F., Borompichaichartkul C., Fang M., Suppavorasatit I., Cadwallader K.R. Effect of post-harvest drying process on chlorogenic acids, antioxidant activities and CIE-lab color of Thai Arabica green coffee beans. Food Chemistry. 2022;366 doi: 10.1016/j.foodchem.2021.130504. [DOI] [PubMed] [Google Scholar]
- Li M., Ai Z., Xiao H., Samir M., Pei Y., Liu Y. Improvement of drying efficiency and physicochemical quality of kiwifruit slices using infrared-assisted tilted tray air impingement drying. Drying Technology. 2022;41:1159–1170. doi: 10.1080/07373937.2022.2125526. [DOI] [Google Scholar]
- Li M., Tian Y., Jiang L., Xu J., Li R., Wang S. Developing effective radio frequency drying processes for tiger nuts: Dynamic analysis of moisture state, dielectric properties and quality. Journal of Food Engineering. 2024;375 doi: 10.1016/j.jfoodeng.2024.112058. [DOI] [Google Scholar]
- Li W., Guo J., Ai Z., Mowafy S., Jia Z., Zhang Y.…Liu Y. Evaluation of pulsed vacuum drying efficiency and quality of garlic sprout based on infrared radiation heating and electronic panel conduction heating. Drying Technology. 2024;42:318–333. doi: 10.1080/07373937.2023.2289159. [DOI] [Google Scholar]
- Liang X., Wang Y., Lei J., Xie C., Tang W., Du T. Effect of different initial processing methods on quality of gardenia. China Journal of Chinese Materia Medica. 2018;43:3285–3290. doi: 10.19540/j.cnki.cjcmm.20180611.004. [DOI] [PubMed] [Google Scholar]
- Lin Q., Zong X., Lin H., Huang X., Wang J., Nie S. Based on quality, energy consumption selecting optimal drying methods of mango slices and kinetics modelling. Food Chemistry: X. 2023;17 doi: 10.1016/j.fochx.2023.100600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Hu L., Deng N., Cai Y., Li H., Zhang B., Wang J. Effects of different hot-air drying methods on the dynamic changes in color, nutrient and aroma quality of three chili pepper (capsicum annuum l.) varieties. Food Chemistry. 2024;X 22 doi: 10.1016/j.fochx.2024.101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang Y., Wei X., Wu D., Dai J., Liu S., Qin W. Effect of radio frequency-assisted hot-air drying on drying kinetics and quality of Sichuan pepper (Zanthoxylum bungeanum maxim.) LWT- Food Science and Technology. 2021;147 doi: 10.1016/j.lwt.2021.111572. [DOI] [Google Scholar]
- Liu Z., Xie L., Zielinska M., Pan Z., Deng L., Zhang J.…Xiao H. Improvement of drying efficiency and quality attributes of blueberries using innovative far-infrared radiation heating assisted pulsed vacuum drying (FIR-PVD) Innovative Food Science and Emerging Technologies. 2022;77 doi: 10.1016/j.ifset.2022.102948. [DOI] [Google Scholar]
- Liu Z., Xie L., Zielinska M., Pan Z., Wang J., Deng L., Wang H., Xiao H. Pulsed vacuum drying enhances drying of blueberry by altering micro-, ultrastructure and water status and distribution. LWT- Food Science and Technology. 2021;142 doi: 10.1016/j.lwt.2021.111013. [DOI] [Google Scholar]
- Luo J., Zhang X., Zeng J., Xie H., Lu Y. Effects of different maturity and initial processing methods on the content of four kinds of active ingredients in Gardenia. Science and Technology of Food Industry. 2021;42:241–246. doi: 10.13386/j.issn1002-0306.2020110228. [DOI] [Google Scholar]
- Mahmood N., Liu Y., Munir Z., Zhang Y., Niazi B.M.K. Effects of hot air assisted radio frequency drying on heating uniformity, drying characteristics and quality of paddy. LWT- Food Science and Technology. 2022;158 doi: 10.1016/j.lwt.2022.113131. [DOI] [Google Scholar]
- Niu Y., Yao X., Xiao H., Wang D., Zheng X., Wang Q., Zhu R., Zang Y., Liu H. Effects of radio frequency assisted hot air drying on the texture and microstructure of jujube slices. Transactions of the Chinese Society of Agricultural Engineering. 2022;38:296–306. doi: 10.11975/j.issn.1002-6819.2022.02.033. [DOI] [Google Scholar]
- Pushpalatha R., Selvamuthukumar S., Kilimozhi D. Hierarchy analysis of different cross-linkers used for the preparation of cross-linked cyclodextrin as drug nanocarriers. Chemical Engineering Communications. 2018;205:759–771. doi: 10.1080/00986445.2017.1416354. [DOI] [Google Scholar]
- Qin H., Yang T., Yang S., Yang M., Wang Y., Zhang J. Effects of different pre-drying and drying methods on volatile compounds in the pericarp and kernel of Amomum tsao-ko. Frontiers in Plant Science. 2022;13 doi: 10.3389/fpls.2022.803776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Z., Zhang F., Dong Y., Wei Y. Research progress on "quality evaluation through morphological identification" and cause of quality formation in Gardeniae Fructus. Chinese Traditional and Herbal Drugs. 2023;54:1998–2004. doi: 10.7501/j.issn.0253-2670.2023.06.032. [DOI] [Google Scholar]
- Sharma M., Dash K.K. Effect of ultrasonic vacuum pretreatment on mass transfer kinetics during osmotic dehydration of black jamun fruit. Ultrasonics Sonochemistry. 2019;58 doi: 10.1016/j.ultsonch.2019.104693. [DOI] [PubMed] [Google Scholar]
- Shi X., Chu J., Zhang Y., Liu C., Yao X. Nutritional and active ingredients of medicinal chrysanthemum flower heads affected by different drying methods. Industrial Crops and Products. 2017;104:45–51. doi: 10.1016/j.indcrop.2017.04.021. [DOI] [Google Scholar]
- Sun W., Li M., Zhang Y., Ai Z., Lei D., Pei Y., Liu Y. Effect of different drying techniques on drying characteristics, physical quality, and active components of Citri reticulatae pericarpium, and the correlation between physiochemical quality. Industrial Crops and Products. 2023;204 doi: 10.1016/j.indcrop.2023.117350. [DOI] [Google Scholar]
- Tabibian S.A., Labbafi M., Askari G.H., Rezaeinezhad A.R., Ghomi H. Effect of gliding arc discharge plasma pretreatment on drying kinetic, energy consumption and physico-chemical properties of saffron (Crocus sativus L.) Journal of Food Engineering. 2020;270 doi: 10.1016/j.jfoodeng.2019.109766. [DOI] [Google Scholar]
- Tan W., Liao H., Lin Z., Fang Y., Guo S. Influence of diferent dehydrations on active components in Gardenia. Asia-Pacific Traditional Medicine. 2012;8:18–19. doi: 10.3969/j.issn.1673-2197.2012.03.009. [DOI] [Google Scholar]
- Wang H., Liu Z., Vidyarthi S.K., Wang Q., Gao L., Li B.…Xiao H. Effects of different drying methods on drying kinetics, physicochemical properties, microstructure, and energy consumption of potato (Solanum tuberosum L.) cubes. Drying Technology. 2020;39:418–431. doi: 10.1080/07373937.2020.1818254. [DOI] [Google Scholar]
- Wang W., Fan L. Effects of harvest time and drying methods on geniposide content in Gardeniae Fructus. China Pharmacist. 2012;15:811–813. doi: 10.3969/j.issn.1008-049X.2012.06.022. [DOI] [Google Scholar]
- Wang W., Wang W., Wang Y., Yang R., Tang J., Zhao Y. Hot-air assisted continuous radio frequency heating for improving drying efficiency and retaining quality of inshell hazelnuts (Corylus avellana L. cv. Barcelona) Journal of Food Engineering. 2020;279 doi: 10.1016/j.jfoodeng.2020.109956. [DOI] [Google Scholar]
- Wang X., Zhu S., Gu W., Cao J., Liu Q., Tian R., Zhou C., Wang F. Modelling of the moisture dynamic process and effects on multiple functional components of Gardenia jasminoides Ellis with different drying methods. Science and Technology of Food Industry. 2019;40:51–57. doi: 10.13386/j.issn1002-0306.2019.12.00. [DOI] [Google Scholar]
- Xie Y., Gao Z., Liu Y., Xiao H. Pulsed vacuum drying of rhizoma dioscoreae slices. LWT- Food Science and Technology. 2017;80:237–249. doi: 10.1016/j.lwt.2017.02.016. [DOI] [Google Scholar]
- Xie Y., Lin Y., Li X., Yang H., Han J., Shang C., Li A., Xiao H., Lu F. Peanut drying: Effects of various drying methods on drying kinetic models, physicochemical properties, germination characteristics, and microstructure. Information Processing in Agriculture. 2022 doi: 10.1016/j.inpa.2022.04.004. [DOI] [Google Scholar]
- Xu B., Feng M., Chitrakar B., Wei B., Wang B., Zhou C.…Duan X. Selection of drying techniques for Pingyin rose on the basis of physicochemical properties and volatile compounds retention. Food Chemistry. 2022;385 doi: 10.1016/j.foodchem.2022.132539. [DOI] [PubMed] [Google Scholar]
- Xu H., Wu M., Zhang T., Gao F., Zheng Z., Li Y. Effects of different pulsed vacuum drying strategies on drying kinetics, phenolic composition, and antioxidant capacity of chrysanthemum (Imperial chrysanthemum) International Journal of Agricultural and Biological Engineering. 2022;15:236–242. doi: 10.25165/j.ijabe.20221504.7359. [DOI] [Google Scholar]
- Xue T., Ruan K., Xu H., Liu H., Tang Z., Yang Y., Duan J., Sun X., Wang M., Song Z. Effect of different drying methods on the drying characteristics, chemical properties and antioxidant capacity of Ziziphus jujuba var. Spinosa fruit. LWT- Food Science and Technology. 2024;196 doi: 10.1016/j.lwt.2024.115873. [DOI] [Google Scholar]
- Yao Y., Sun Y., Cui B., Fu H., Chen X., Wang Y. Radio frequency energy inactivates peroxidase in stem lettuce at different heating rates and associate changes in physiochemical properties and cell morphology. Food Chemistry. 2021;342 doi: 10.1016/j.foodchem.2020.128360. [DOI] [PubMed] [Google Scholar]
- Zhao C., Zhang H., Chen Y., Chen C. Effects of different drying methods on the content of two components in Fructus Gardeniae. West China Journal of Pharmaceutical Sciences. 2009;24:149–151. doi: 10.13375/j.cnki.wcjps.2009.02.015. [DOI] [Google Scholar]
- Zheng J., Li H., Wang D., Li R., Wang S., Ling B. Radio frequency assisted extraction of pectin from apple pomace: Process optimization and comparison with microwave and conventional methods. Food Hydrocolloids. 2021;121 doi: 10.1016/j.foodhyd.2021.107031. [DOI] [Google Scholar]
- Zheng Z., Wang S., Wang H., Xiao H., Liu Z., Pan Y., Gao L. Comparative study on the influence of various drying techniques on drying characteristics and physicochemical quality of garlic slices. Foods. 2023;12:1314. doi: 10.3390/foods12061314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.