Abstract
Cervical anastomotic leakage (AL) is a severe complication following esophageal cancer surgery, leading to significant morbidity and risk of mortality. This case report describes the successful application of negative pressure wound therapy with instillation (NPWTi) in managing AL after esophageal surgery. A 61-year-old patient developed an anastomotic leak on postoperative day 7, accompanied by persistent neck pain and leakage of nutritional fluids. Treatment involved a dual-tube NPWTi system with continuous saline instillation to clean and prevent infection, maintain wound moisture, and promote tissue granulation. Within 15 days, the leakage was substantially controlled, and a barium swallow test confirmed complete closure by day 20. This case suggests NPWTi as a promising and less invasive approach to managing AL post-esophagectomy, warranting further research on its clinical efficacy and safety.
Keywords: Esophageal cancer, Anastomotic leakage, Negative pressure with instillation, Wound therapy
Introduction
Esophageal cancer is a highly aggressive and lethal gastrointestinal malignancy, with approximately 511,000 new cases and 445,000 deaths reported globally in 2022.1 In China, esophageal cancer ranks as the fifth leading cause of cancer-related mortality, resulting in approximately 224,000 new cases and 188,000 deaths every year.2 Surgery is the primary treatment for patients with early to mid-stage disease.3,4 Following the surgical removal of the tumor, the digestive tract must be reconstructed through anastomoses. However, anastomotic leakage (AL) is a severe complication post-surgery, with an incidence ranging from 3% to 25%, and a mortality rate from 7% to 35%.5, 6, 7
AL refers to a full-thickness defect in the anastomosis used to reconstruct the gastrointestinal tract.8 When there is a defect in the anastomosis, the contents of the gastrointestinal tract can leak through the defect into the thoracic cavity or other tissue spaces. Food and corrosive digestive fluids can damage surrounding organs and tissues, leading to pain, difficulty swallowing, and severe infection. Leakage from an anastomosis site markedly diminishes quality of life, can delay adjuvant treatments thereby impacting long-term survival, and in severe cases the leakage can lead to death.9, 10, 11
The choice of treatment for AL depends on factors such as the location of the defect and the amount of fluid flowing through the defect, and symptom severity. The primary treatments include conservative management, surgical intervention, endoscopic treatment, and stent drainage.8 A crucial aspect of managing cervical AL is ensuring effective drainage, which continuously removes corrosive digestive fluids, prevents the accumulation of infected fluids, and maintains relative dryness at the anastomotic site. Effective drainage is essential for promoting granulation tissue growth and facilitating healing of the anastomosis.12
Negative pressure wound therapy (NPWT) is a widely recognized wound management technique that effectively promotes the healing of acute and chronic wounds. NPWT increases blood flow at the wound site and promotes angiogenesis, and has been demonstrated to be efficacy in treating open wounds, healing of tissue compromised post-operatively, and diabetic foot ulcers.13, 14, 15, 16 Negative pressure wound therapy with instillation (NPWTi) aids in cleaning wound surfaces and dissolving necrotic tissue by actively and continuously removing exudates, dissolved tissues, and bacteria, and the process controls infection and accelerates healing.17, 18, 19 Reports indicate that NPWTi has been successfully applied to a variety of complex wounds, but there are relatively few cases documenting its use in treating cervical AL following esophageal cancer surgery. In this report, we present a case where NPWTi was successfully used to treat a patient with AL following esophageal cancer surgery.
Case report
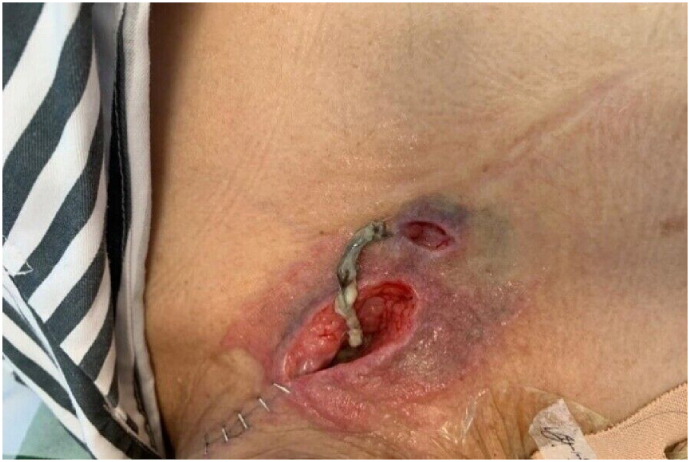
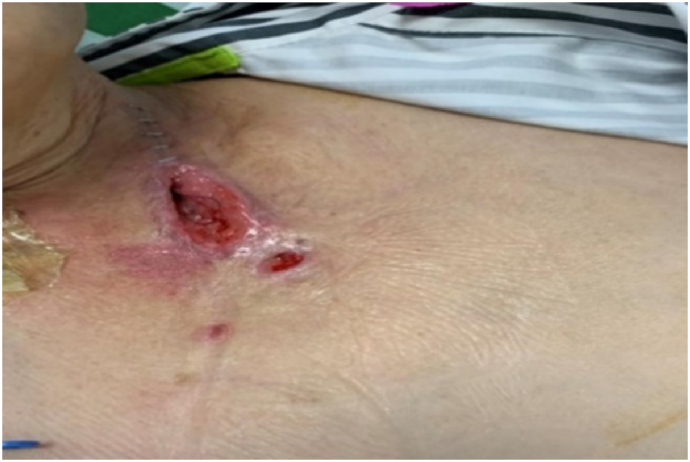
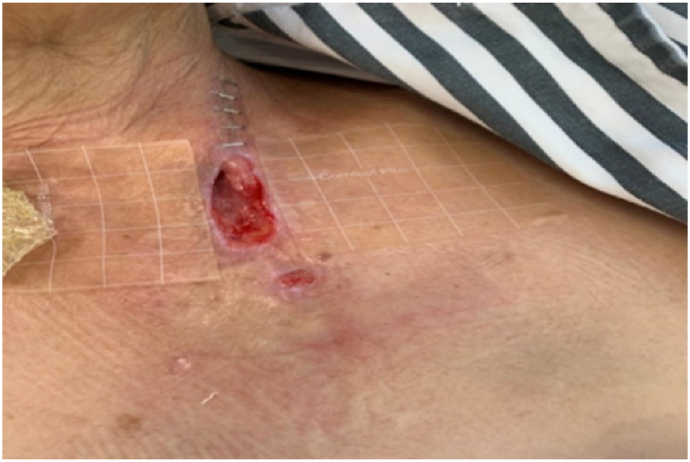
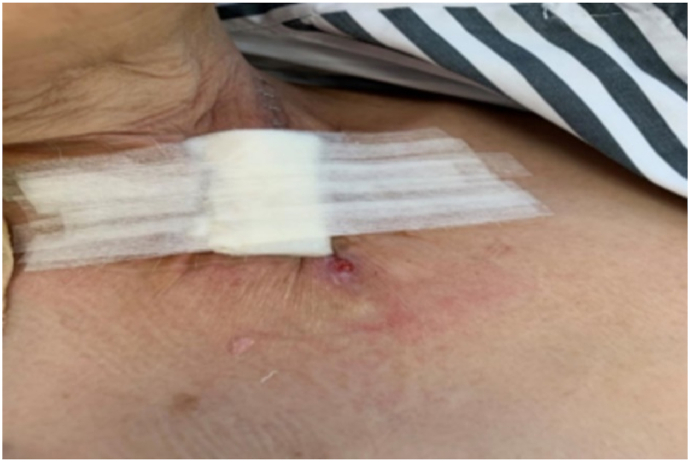
A 61-year-old woman with esophageal cancer underwent partial esophagectomy with esophagogastric anastomosis and jejunostomy. By postoperative day 7, the neck wound had deteriorated, with a 5 cm × 3 cm open wound (Fig. 1) observed near the anastomosis. Pale yellow nutritional fluid was noted leaking from the wound during enteral feeding (Fig. 2). A barium swallow test showed deposits along the anastomosis, confirming the presence of an AL. The patient experienced continuous neck pain and distress, causing significant anxiety for her and her family due to the potential life-threatening complications.
Fig. 1.
Anastomotic leakage on the left side of the neck, accompanied by necrotic tissue.
Fig. 2.
Leakage of nutritional fluid from the fistula during enteral feeding.
Treatment considerations
The anastomotic defect posed a significant infection risk and contributed to continuous pain and anxiety. Our treatment goals included effective drainage of the leak, wound decontamination, and promoting rapid closure of the anastomosis to restore the structure and function of the digestive tract.
Wound management
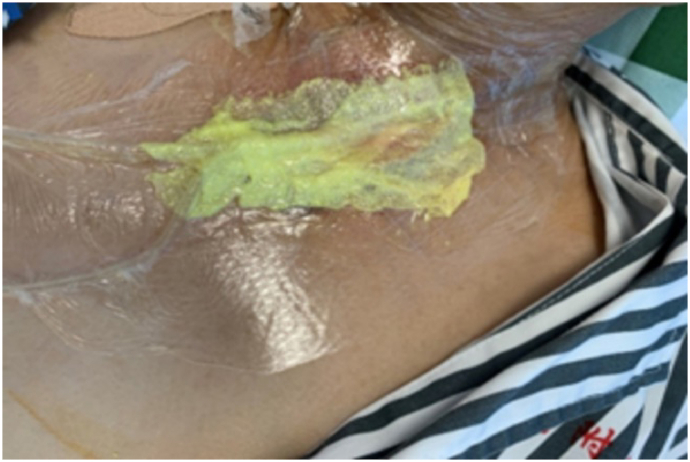
The presence of excessive mucous leakage from the fistula resulted in frequent saturation of the wound dressing, rendering standard wound dressings ineffective. On the 10th postoperative day, a stoma therapist treated the fistula using NPWTi. Initially, the wound was irrigated with a 0.9% saline solution to remove naturally detached necrotic tissue. Subsequently, a dual-tube system that consisted of a suction catheter fitted into a soft tube was applied. Based on the size of the fistula, a size 14 suction catheter was selected, and its original side holes were slightly enlarged to about 0.5 cm. Additional holes of the same size were cut every 0.5 cm on the opposite side. A soft tube with a diameter of approximately 1.5 mm was inserted into the suction catheter and aligned with the tip of the catheter. The dual-tube system was then wrapped with iodine-soaked gauze and placed into the wound. A layer of iodine-soaked gauze was used to cover the wound surface, and a transparent protective film was applied to seal the wound and device (Fig. 3). A continuous infusion of 0.9% saline at 40 ml/h was administered to dilute the wound exudate and leakage. The opposite end of the tube was connected to a vacuum device, and a central negative pressure of 90–120 mm Hg was applied to aspirate the instillation fluid, wound exudate, and bacteria. Dressings were changed every 2–3 days, with each treatment course lasting for 7 days, during which the wound's condition was monitored.
Fig. 3.
Iodine-soaked gauze covers the wound bed and is secured with a transparent protective film to ensure a sealed environment.
After 3 days of NPWTi , the patient reported a reduction in neck pain and the frequency of dressing changes was reduced. To determine the status of the defect, the patient ingested 10 mg of methylene blue which resulted in the appearance of blue fluid at the fistula site, indicating that the fistula had not yet closed (Fig. 4). NPWTi and dressing changes were continued.
Fig. 4.
The wound dressing was discolored blue by the methylene blue that leaked from the fistula.
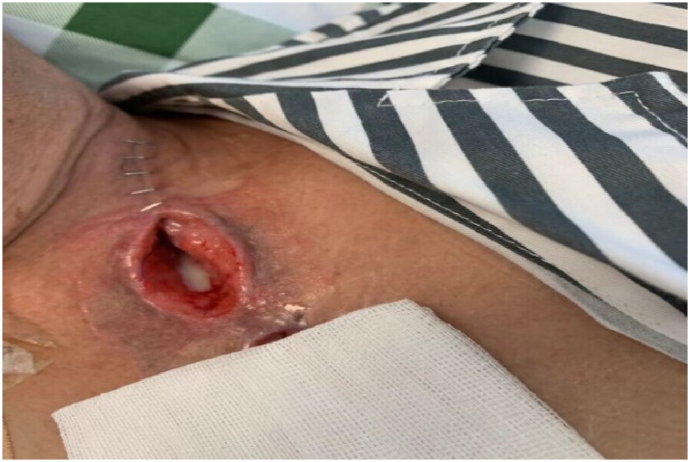
After an additional week of NPWTi the wound was visible reduced in size, and no fluid leakage was observed in the wound bed (Fig. 5). Since there were no signs of infection, the treatment was adjusted to include regular gauze for wrapping the suction tube and covering the wound, and NPWTi was continued (Fig. 6).
Fig. 5.
Wound size reduction observed after 1 week of NPWTi. NPWTi, negative pressure wound therapy with instillation.
Fig. 6.
Ongoing NPWTi and saline irrigation were performed using regular gauze. NPWTi, negative pressure wound therapy with instillation.
After 2 more days, the negative-pressure drainage device was removed and the patient again ingested 10 mg of methylene blue. No blue fluid was observed leaking from the wound. The wound was closed with the closure technique. Hydrocolloid dressings were applied to both edge of the wound to protect the skin (Fig. 7), and adhesive tape was used to pull and secure the wound edges toward the center (Fig. 8).
Fig. 7.
After 12 days of NPWTi hydrocolloid dressings were applied to protect the skin surrounding the wound. NPWTi, negative pressure wound therapy with instillation.
Fig. 8.
The wound was dressed with gauze and stabilized through a tension adhesive technique.
Results
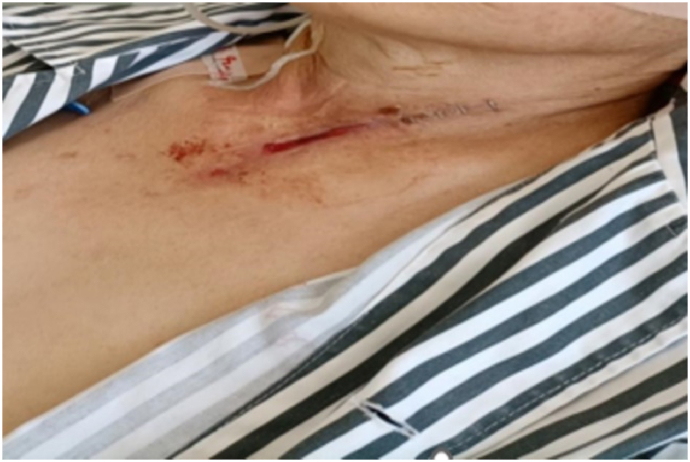
Three days after traction closure, the wound dressing was removed and the postoperative esophageal fistula had almost completely healed (12 days of NPWTi and 3 days of traction closure) (Fig. 9). At 20 days after beginning NPWTi, a barium swallow test revealed no barium leakage, confirming closure of the fistula.
Fig. 9.
Three days after the traction closure, the neck wound had almost healed.
Discussion
For esophageal cancer patients, complications from anastomotic leaks following surgery can severely impact their quality of life. Anastomotic leaks can lead to infection, sepsis, and multiple organ failure, which are associated with high mortality.20 A stepwise approach is required for treating postoperative anastomotic leaks after esophageal cancer surgery that is tailored to the specific condition of the leak, and may include conservative treatment of additional surgical procedures.21 Conservative treatments include temporarily withholding oral intake, administering intravenous antibiotics, gastrointestinal decompression, ensuring drainage patency, and providing nutritional support.22 Invasive treatments include the placement of endoscopic stents, vacuum-assisted closure devices, and surgical re-anastomosis.23, 24, 25 Endoscopic esophageal stent placement involves using covered metal stents to seal the leak; however, neck esophageal leaks often require suturing or nasal anchoring to prevent stent displacement and ensure effectiveness.23 Vacuum-assisted closure devices, while effective, pose the risk of displacement within the esophagus.24 Re-anastomosis surgery is a viable treatment only when appropriate surgical indications are met.25,26 While they may be effective in select patients, invasive procedures can significantly heighten the physical, psychological, and financial burdens on patients.27, 28, 29
The etiology of the AL in this case is likely multifactorial. The tumor was located 19–25 cm from the incisors, which required a mid-to-low esophagectomy with a retrosternal pull-up of a tubularized stomach to the cervical region. This may have increased the tension and/or reduced the blood supply at the anastomotic site, contributing to impaired healing. Additionally, the patient received chemotherapy 3 months prior to surgery (sintilimab, albumin-bound paclitaxel, and cisplatin), and the cytotoxic effects of the chemotherapy likely compromised healing of the anastomosis. In addition, the patient's preoperative nutritional status was poor, which likely weakened tissue repair capacity. The esophageal tumor occupied 80% of the lumen, causing significant dysphagia for 5 months before surgery, and during this period the patient relied primarily on a liquid diet and experienced a weight loss of 5% of her body weight in the 3 months before surgery. The patient's Nutrition Risk Screening 2002 (NRS-2002) score of 5 indicated a high risk of malnutrition. On postoperative day 7 her hemoglobin was 85 g/L and her red blood cell count was 2.55 × 1012/L, confirming anemia and malnutrition, which are both known to hinder wound healing. Moreover, infection and exposure to digestive fluids likely exacerbated the anastomotic dehiscence. It was on postoperative day 7 that there were signs that the anastomosis had dehisced; there was redness, swelling, and a foul odor at the wound. Inflammatory markers were elevated: her C-reactive protein (CRP) was 117.71 mg/L and procalcitonin (PCT) wat 0.28 ng/mL, indicating a significant inflammatory response.
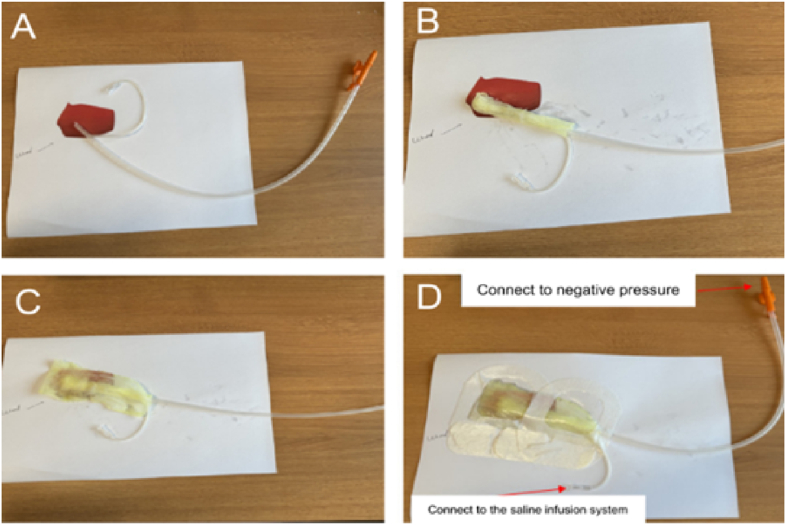
Identifying an effective treatment method for early intervention after the detection of an anastomotic leak is essential for improving quality of life and alleviating financial burdens. The NPWTi technique employs a comprehensive approach to prevent wound maceration and manage the challenges associated with the viscosity of saliva and gastric secretions. A schematic representation of the NPWTi system is shown in Fig. 10. Continuous negative pressure effectively removes fluids from around the wound, including high-viscosity saliva and gastric secretions, thereby preventing their accumulation and infiltration into the surrounding tissue. To enhance drainage, a dual-tube system with increased side holes was used, thus facilitating suction from both inside the wound and any overflow. This modification reduces the risk of tissue maceration and improves the durability of the NPWTi system. Additionally, precise control over negative pressure and instillation rate allows for balanced fluid drainage and replenishment, avoiding excessive drying or fluid buildup. The continuous instillation of saline not only dilutes the viscosity of saliva and gastric secretions, but also aids in wound cleansing. Regular monitoring of the NPWTi system ensures its effective operation, preventing fluid accumulation around the wound site. This multifaceted approach manages fluid dynamics effectively and reduces the risk of infection, promoting rapid wound healing. In our case, we used iodine-soaked gauze as an additional anti-infective measure. Continuous removal of secretions and necrotic tissue and the use of iodine-soaked gauze markedly decreased the chances of infection. Negative pressure drainage also approximates the wound edges, enhances blood perfusion, and promotes the development of granulation tissue, thereby accelerating the healing of a wound.30
Fig. 10.
Schematic representation of the negative pressure wound therapy with instillation (NPWTi) system. (A) A soft tube with an approximate diameter of 1.5 mm was inserted into the suction catheter, ensuring alignment with its tip. (B) The dual-tube system was encased in iodine-soaked gauze and positioned within the wound. (C) An additional layer of iodine-soaked gauze was applied to cover the wound surface. (D) A transparent protective film was used to maintain a sealed environment.
The effectiveness of NPWTi is influenced by multiple factors, including the size and location of the wound, infection status, tissue viability, and the patient's overall health. NPWTi is most effective for smaller to moderate-sized wound and anastomotic leaks, as these are more likely to close under negative pressure. In contrast, larger wounds and anastomotic leaks, and those near major blood vessels, such as arteries, require careful consideration due to the risk of bleeding. While NPWTi is effective in managing mild local infections, more severe infections may necessitate additional, more aggressive interventions. The condition of the surrounding tissue is also critical—healthy tissue with an adequate blood supply enhances NPWTi outcomes, whereas necrotic tissue or poor circulation may require debridement before NPWTi can be initiated. As previously mentioned, the patient's overall health plays a key role in treatment success; patients who are severely malnourished, immunocompromised, or suffering from multi-organ failure may not respond well to NPWTi.
NPWTi is considered an effective initial treatment for AL following esophageal cancer surgery; however, it is not universally the first-line option. For small to moderate leaks in stable patients, NPWTi is typically the preferred method. In contrast, larger or more complex leaks, or those in patients with deteriorating conditions, may necessitate immediate surgical intervention. If NPWTi is unsuccessful, alternative treatment options such as endoscopic clipping, stent placement, or surgical revision should be considered. The treatment strategy must be dynamic and individualized, involving regular reassessment of healing progress, drainage volume, infection markers, and the patient's overall condition.
Reevaluation of NPWTi is warranted if specific indicators suggest the therapy is not progressing effectively. Key criteria include minimal reduction in leak size after 2–3 weeks or persistently high or increasing drainage volumes, which may indicate a suboptimal response to treatment. Additionally, elevated infection markers, such as white blood cell count or CRP levels, signal the need for reassessment. If NPWTi becomes ineffective, complications such as hemorrhage or deep infections occur, or the patient is experiencing marked discomfort, then more aggressive interventions need to be considered.
Although there is currently limited research specifically focused on applying NPWTi to treat cervical anastomotic leaks after esophageal cancer surgery. However, this method is well-established for treating burn wounds, diabetic ulcers, and venous leg ulcers.31, 32, 33, 34, 35, 36 Based on its successful application for these conditions, we hypothesized that it may offer potential benefits in managing cervical anastomotic leaks after esophageal cancer surgery, and this case demonstrates its effectiveness. Thus, based on this case report further research is warranted to validate the effectiveness and safety of NPWTi in treating cervical anastomotic leaks after esophageal cancer surgery.
Conclusions
This case illustrates the successful use of NPWTi in managing cervical AL after esophageal cancer surgery. NPWTi offers a cost-effective, minimally invasive treatment option with low patient discomfort, supporting its further investigation in esophageal AL management.
CRediT authorship contribution statement
Dr. Baojia Luo Conceived and designed the analysis, Drs. Rongrong Jiang, Xiaoping Chen, Yonglan Ge and Yanyan Fang Collected the data, Drs. Mengxiao Jiang, Wenguang Liang, and Huiting Zhang wrote and revised the article. All authors approved the final version of the manuscript. All authors were granted complete access to all the data in the study, with the corresponding author bearing the final responsibility for the decision to submit for publication. The corresponding author affirms that all listed authors fulfill the authorship criteria and that no others meeting the criteria have been omitted.
Ethics statement
The study was approved by the Ethics Committee of the Sun Yat-sen University Cancer Center (IRB No. GYX2019-011). The patient has given her written informed consent for this case report to be published.
Funding
This study received no external funding.
Data availability statement
The data that support the findings of this study are available from the corresponding authors, upon reasonable request.
Declaration of generative AI and AI-assisted technologies in the writing process
No AI tools/services were used during the preparation of this work.
Declaration of competing interest
The authors declare no conflict of interest.
Contributor Information
Huiting Zhang, Email: Zhanght@sysucc.org.cn.
Rongrong Jiang, Email: Jiangrr@sysucc.org.cn.
Baojia Luo, Email: luobj@sysucc.org.cn.
References
- 1.Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. Published online April 4, 2024. 10.3322/caac.21834. [DOI] [PubMed]
- 2.Han B, Zheng R, Zeng H, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. Published online February 2, 2024. 10.1016/j.jncc.2024.01.006. [DOI] [PMC free article] [PubMed]
- 3.van Geffen EGM, Neelis KJ, Putter H, et al. Esophagectomy after definitive chemoradiation in esophageal cancer: a safe therapeutic strategy. Dis Esophagus Off J Int Soc Dis Esophagus. Published online August 8, 2024:doae059. 10.1093/dote/doae059. [DOI] [PMC free article] [PubMed]
- 4.Ronellenfitsch U., Klose J., Kleeff J. Multimodal therapy of upper gastrointestinal malignancies. Cancers. 2021;13(4):793. doi: 10.3390/cancers13040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascari F., De Pascale S., Rosati R., et al. Multicenter study on the incidence and treatment of mediastinal leaks after esophagectomy (MuMeLe 2) J Gastrointest Surg Off J Soc Surg Aliment Tract. 2024;28(7):1072–1077. doi: 10.1016/j.gassur.2024.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Murray W., Davey M.G., Robb W., Donlon N.E. Management of esophageal anastomotic leaks, a systematic review and network meta-analysis. Dis Esophagus Off J Int Soc Dis Esophagus. 2024;37(7) doi: 10.1093/dote/doae019. [DOI] [PubMed] [Google Scholar]
- 7.Verstegen M.H.P., Slaman A.E., Klarenbeek B.R., et al. Outcomes of patients with anastomotic leakage after transhiatal, McKeown or Ivor Lewis esophagectomy: a nationwide cohort study. World J Surg. 2021;45(11):3341–3349. doi: 10.1007/s00268-021-06250-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabbi M., Hagens E.R.C., van Berge Henegouwen M.I., Gisbertz S.S. Anastomotic leakage after esophagectomy for esophageal cancer: definitions, diagnostics, and treatment. Dis Esophagus Off J Int Soc Dis Esophagus. 2021;34(1) doi: 10.1093/dote/doaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugawara K., Yagi K., Aikou S., Yamashita H., Seto Y. Impacts of complications after esophageal cancer surgery on health-related quality of life and nutritional status. Gen Thorac Cardiovasc Surg. 2022;70(12):1048–1057. doi: 10.1007/s11748-022-01846-y. [DOI] [PubMed] [Google Scholar]
- 10.Schuring N., Jezerskyte E., van Berge Henegouwen M.I., et al. Influence of postoperative complications following esophagectomy for cancer on quality of life: a European multicenter study. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2023;49(1):97–105. doi: 10.1016/j.ejso.2022.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Jezerskyte E., van Berge Henegouwen M.I., van Laarhoven H.W.M., et al. Postoperative complications and long-term quality of life after multimodality treatment for esophageal cancer: an analysis of the prospective observational cohort study of esophageal-gastric cancer patients (POCOP) Ann Surg Oncol. 2021;28(12):7259–7276. doi: 10.1245/s10434-021-10144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauge T, Dretvik T, Johnson E, Mala T. Treatment of anastomotic leakage following Ivor Lewis esophagectomy-10 year experience from a Nordic center. Dis Esophagus Off J Int Soc Dis Esophagus. Published online May 14, 2024:doae040. 10.1093/dote/doae040. [DOI] [PMC free article] [PubMed]
- 13.Ma Z., Li Z., Shou K., et al. Negative pressure wound therapy: regulating blood flow perfusion and microvessel maturation through microvascular pericytes. Int J Mol Med. 2017;40(5):1415–1425. doi: 10.3892/ijmm.2017.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H., Si S., Liu X., Geng H., Liang J. Evaluation of a new low-cost negative pressure wound therapy in the treatment of diabetic foot ulcers. J Wound Care. 2024;33(Sup2a):xli–xlvii. doi: 10.12968/jowc.2024.33.Sup2a.xli. [DOI] [PubMed] [Google Scholar]
- 15.Lin X., Su J., Yang Z. Optimising wound care for patients with cirrhosis: a study of the effect of combination therapy on wound healing. Int Wound J. 2024;21(2) doi: 10.1111/iwj.14727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh P.K., Sethi M.K., Mishra T.S., et al. Comparison of surgical site infection (SSI) between negative pressure wound therapy (NPWT) assisted delayed primary closure and conventional delayed primary closure in grossly contaminated emergency abdominal surgeries: a randomized controlled trial. Langenbecks Arch Surg. 2023;409(1):19. doi: 10.1007/s00423-023-03202-x. [DOI] [PubMed] [Google Scholar]
- 17.De Pellegrin L., Feltri P., Filardo G., et al. Effects of negative pressure wound therapy with instillation and dwell time (NPWTi-d) versus NPWT or standard of care in orthoplastic surgery: a systematic review and meta-analysis. Int Wound J. 2023;20(6):2402–2413. doi: 10.1111/iwj.14072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim P.J., Attinger C.E., Constantine T., et al. Negative pressure wound therapy with instillation: international consensus guidelines update. Int Wound J. 2020;17(1):174–186. doi: 10.1111/iwj.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujisawa K, Miyamoto S, Saito Y, Okazaki M. Negative pressure wound therapy with instillation and dwell time for pharyngocutaneous fistula. Laryngoscope. Published online June 1, 2024. 10.1002/lary.31549. [DOI] [PubMed]
- 20.Hauge T., Amdal C.D., Falk R.S., Johannessen H.O., Johnson E. Long-term outcome in patients operated with hybrid esophagectomy for esophageal cancer - a cohort study. Acta Oncol Stockh Swed. 2020;59(7):859–865. doi: 10.1080/0284186X.2020.1750694. [DOI] [PubMed] [Google Scholar]
- 21.Ubels S., Lubbers M., Verstegen M.H.P., et al. Treatment of anastomotic leak after esophagectomy: insights of an international case vignette survey and expert discussions. Dis Esophagus Off J Int Soc Dis Esophagus. 2022;35(12):doac020. doi: 10.1093/dote/doac020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manghelli J.L., Ceppa D.P., Greenberg J.W., et al. Management of anastomotic leaks following esophagectomy: when to intervene? J Thorac Dis. 2019;11(1):131–137. doi: 10.21037/jtd.2018.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosianu C.G., Hoara P., Achim F., et al. The use of esophageal stents in the management of postoperative fistulas-current status, clinical outcomes and perspectives-review. Life Basel Switz. 2023;13(4):966. doi: 10.3390/life13040966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lange J., Kähler G., Bernhardt J., et al. The VACStent trial: combined treatment of esophageal leaks by covered stent and endoscopic vacuum therapy. Surg Endosc. 2023;37(5):3657–3668. doi: 10.1007/s00464-023-09861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamarajah S.K., Markar S.R. Navigating complexities and considerations for suspected anastomotic leakage in the upper gastrointestinal tract: a state of the art review. Best Pract Res Clin Gastroenterol. 2024;70 doi: 10.1016/j.bpg.2024.101916. [DOI] [PubMed] [Google Scholar]
- 26.Seo H.W., Jeon Y.J., Cho J.H., et al. Treatment patterns and outcomes of anastomotic leakage after esophagectomy for esophageal cancer. J Chest Surg. 2024;57(2):152–159. doi: 10.5090/jcs.23.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamoto K., Ninomiya I., Fujiwara Y., et al. Use of esophageal stent for the treatment of postoperative gastrointestinal-airway fistula after esophagectomy. Esophagus Off J Jpn Esophageal Soc. 2019;16(4):413–417. doi: 10.1007/s10388-019-00673-0. [DOI] [PubMed] [Google Scholar]
- 28.Li Y., Wang Y., Chen J., et al. Management of thoracogastric airway fistula after esophagectomy for esophageal cancer: a systematic literature review. J Int Med Res. 2020;48(5) doi: 10.1177/0300060520926025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng B., Zeng T., Yang H., et al. The clinical characteristics, treatments and prognosis of post-esophagectomy airway fistula: a multicenter cohort study. Transl Lung Cancer Res. 2022;11(3):331–341. doi: 10.21037/tlcr-22-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman R.P. Negative pressure wound therapy with instillation and dwell time: mechanisms of action literature review. Eplasty. 2023;23 [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J., Shi K., Zhang N., Hong L., Yu J. Assessment between antiseptic and normal saline for negative pressure wound therapy with instillation and dwell time in diabetic foot infections. Sci Rep. 2024;14(1) doi: 10.1038/s41598-024-58900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afzal H., Dawson E., Fonseca R., et al. Negative pressure wound therapy with and without instillation in necrotizing soft tissue infections. Surg Infect. 2024;25(3):199–205. doi: 10.1089/sur.2023.299. [DOI] [PubMed] [Google Scholar]
- 33.Saini R., Jeyaraman M., Jayakumar T., Iyengar K.P., Jeyaraman N., Jain V.K. Evolving role of negative pressure wound therapy with instillation and dwell time (NPWTi-d-) in management of Trauma and Orthopaedic wounds: mechanism, applications and future perspectives. Indian J Orthop. 2023;57(12):1968–1983. doi: 10.1007/s43465-023-01018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nuhiji E. Trends and innovation in negative pressure wound therapy: a review of burn wound management. Adv Wound Care. 2024;13(8):391–399. doi: 10.1089/wound.2023.0114. [DOI] [PubMed] [Google Scholar]
- 35.Fernández L.G. Treatment of complex thoracic and abdominal trauma patients: a review of literature and negative pressure wound therapy treatment options. Adv Wound Care. 2024;13(8):416–423. doi: 10.1089/wound.2023.0113. [DOI] [PubMed] [Google Scholar]
- 36.Leow K., Tey J., Tan A., Tan K., Wong K.L. Negative pressure wound therapy with instillation and dwell time modifications for lower limb wounds with the waterfall technique: a case series. Wounds Compend Clin Res Pract. 2020;32(12):E120–E125. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors, upon reasonable request.