Abstract
This study aimed to investigate the effects of maturity on the changes in major lipid metabolites of coffee and their associated pathways. UPLC-ESI-MS/MS was used to compare the lipidomic profiles of coffee beans at five different maturity stages. A total of 516 lipid metabolites across 26 subclasses were identified, with 111 showing significant differences. Glycerolipids (GL) and fatty acyls (FA) were the most abundant, followed by glycerophospholipids (GP), sphingolipids (SP) and prenol lipids (PR). PCA and OPLS-DA analyses demonstrated significant changes in coffee lipids during maturation. Glycerophospholipid metabolism and glycerolipid metabolism were identified as key metabolic pathways, with phosphatidic acid (PA), lysophosphatidic acid (LPA) and diacylglycerol (DG) as key lipid metabolites in these pathways during coffee maturation. Lipids in immature and overripe beans were significantly different from those in mature coffee beans. This study provides a foundational understanding of lipid transformation and flavor profile formation during coffee maturation.
Keywords: Coffee, Maturation, Lipidomics, Glycerophospholipid metabolism, Glycerolipid metabolism
Graphical abstract
Highlights
-
•
516 lipids identified across 26 subclasses in coffee beans at five maturity stages.
-
•
Glycerolipids (GL) and fatty acyls (FA) were the most abundant lipid classes.
-
•
Immature and overripe beans had significantly different lipids compared to mature coffee beans.
-
•
Glycerophospholipid metabolism and glycerolipid metabolism are critical metabolic pathways.
1. Introduction
In the ‘third wave coffee’ era, the demand for higher quality coffee has transformed the industry, turning coffee from a traditional commodity into a product rich in characteristics (Sittipod, Schwartz, Paravisini, & Peterson, 2019; Wang et al., 2024). Arabica coffee (Coffea arabica L.), the most widely produced and commercialized coffee species globally, is recognized for its outstanding drinking quality, rich aroma and unique flavor (Agnoletti et al., 2022; Cheng, Furtado, Smyth, & Henry, 2016). Improving the flavor quality of coffee can tap into high-value markets and increase profits for growers (Wang et al., 2023). However, achieving this is a complex process involving many stages from seed to cup (Wang et al., 2022). Factors such as species/cultivars, geographical origin, maturity, processing, roasting and storage all influence coffee flavor (Hu et al., 2020; Wang et al., 2023). Ensuring uniform and appropriate cherry maturity to optimize the accumulation of flavor precursor compounds is one of the key prerequisites for producing high-quality coffee.
Coffee cherry maturity is commonly assessed by color, a widely accepted classification method, although other indicators like firmness, acidity, sugar content, and density are also relevant but harder to measure in the field (Velásquez, Peña, Bohórquez, Gutierrez, & Sacks, 2019). As coffee cherries ripen, their color changes from green to red, yellow, or orange, reflecting chemical composition changes specific to each variety, most of which are red-fruiting (de Melo Pereira et al., 2019). As coffee cherries ripen, phenolic compounds and astringency decrease, while flavor precursors accumulate and volatile compounds (e.g., aldehydes, ketones, and higher alcohols) increase, enhancing the aroma of coffee (Yeretzian, Jordan, Badoud, & Lindinger, 2002). However, the lack of an automatic abscission mechanism in coffee cherries can result in overripening, leading to nutritional deficiencies and a higher risk of parasite invasion (Aristizábal, Johnson, Shriner, & Wall, 2023). These issues not only affect coffee bean quality but also negatively impact the sensory attributes of the final beverage (Hu et al., 2020). Lipid composition in coffee beans changes during ripening, greatly influencing the flavor, body and fragrance/aroma during roasting and brewing (Jham, Velikova, Muller, Nikolova-Damyanova, & Cecon, 2001; Pazmino-Arteaga, Gallardo, Gonzalez-Rodriguez, & Winkler, 2022). These changes highlight the critical role of lipids in defining coffee quality parameters. However, the role of lipids in metabolic transformation and flavor formation during ripening remains underexplored, emphasizing the importance of targeted studies on lipid profile changes. Understanding these transformations can provide insights into optimizing harvest timing and post-harvest processing, ultimately contributing to enhanced coffee quality.
Lipidomics, a branch of metabolomics, offers comprehensive molecular insights into lipid metabolism and pathways at the system level. Recent applications of lipidomic analysis have significantly advanced our understanding of lipid profiles in green and roasted coffee beans. Silva, da Silva, Garrett, and Rezende (2020) used LC-HRMS/MS technology to profile lipids in green coffee beans, discovering that the Matyash extraction method identified a greater variety of lipids than the traditional Folch and Bligh-Dyer methods. Silva, Garrett, Rezende, and Meckelmann (2022) further showed that LC-IM-qTOF-MS enhanced lipidomic characterization, revealing that triacylglycerols were predominantly associated with Arabica coffee, while diacylglycerols and phospholipids were more prevalent in Robusta. Aurum et al. (2022) used LC-MS/MS for lipid profiling, successfully determining the geographical origins of coffee from six major Indonesian regions through multivariate analysis. Additionally, Zhu et al. (2023) investigated dynamic changes in lipid composition during coffee roasting, providing detailed insights into how lipid levels vary throughout the process. Despite these advances, comprehensive studies on lipid profiles during coffee ripening remain lacking, highlighting a significant gap in current research.
This study aims to monitor key lipid content during coffee ripening and explore lipid transformation mechanisms using an extensive targeted lipidomic analysis (UPLC-ESI-MS/MS(MRM)). Unlike previous studies focusing solely on general chemical changes during coffee maturation, our work uniquely integrates lipid profiling with maturity stages, revealing key metabolic pathways that drive flavor and aroma development. This understanding provides a scientific basis for developing targeted harvesting strategies that align with optimal maturity levels, ensuring higher quality coffee with optimized flavor profiles to meet consumer satisfaction.
2. Materials and methods
2.1. Chemicals and standards
In this study, the following HPLC-grade chemicals were utilized: acetonitrile (ACN, CAS 75–05-8, Merck, Germany); methanol (MeOH, CAS 67–56-1, Merck, Germany); isopropyl alcohol (IPA, CAS 67–63-0, Merck, Germany); dichloromethane (CH2Cl2, CAS 75–09-2, Merck, Germany); methyl tert-butyl ether (MTBE, CAS 1634-04-4, Merck, Germany); formic acid (FA, CAS 64–18-6, Sigma-Aldrich, USA); and ammonium formate (AmFA, CAS 540–69-2, Sigma-Aldrich, USA). Lipid standards were sourced from Sigma-Aldrich and Avanti Polar Lipids (Alabaster, AL). Ultrapure water was produced using a Milli-Q system (Millipore, Billerica, MA).
2.2. Samples collection
The study took place during the 2023/2024 harvest season at the experimental field of the Dehong Tropical Agriculture Research Institute, Yunnan, China (97o86′10″ E, 24o02′57″ N, 796.4 m above sea level). The coffee trees, belonging to the Sarchimor series of C. arabica L., were planted in 2017 with a spacing of 2.5 m × 2 m (2000 trees/ha). The region has a subtropical humid monsoon climate, with an average annual temperature of 21 °C and annual precipitation of 1394.8 mm. The soil at the test site is derived from granite parent material and is brick red, with a pH of 4.9.
This study sampled coffee cherries at five distinct maturity stages from the same vigorous coffee tree, based on appearance (Marín-López, Arcila-Pulgarín, Montoya-Restrepo, & Oliveros-Tascón, 2003). Each maturity stage was sampled replicates, with 20 fresh coffee cherries collected for each replicate (Fig. 1). Maturity stage 1 (M1) featured yellow-green cherries (i.e., 203 days), stage 2 (M2) corresponds to nearly ripe or “pintón” cherries (i.e., 210 days), stage 3 (M3) to ripe cherries (i.e., 217 days), stage 4 (M4) to overripe cherries (i.e., 224 days), and stage 5 (M5) to withered cherries (i.e., 231 days). All coffee cherries were immediately flash-frozen in liquid nitrogen after collection, then freeze-dried and stored at −80 °C until analysis.
Fig. 1.
Coffee samples were collected from different stages of maturity (M1 - M5). M1, yellow-green cherries; M2, nearly ripe or “pintón” cherries; M3, ripe cherries; M4, overripe cherries; M5, withered cherries. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.3. Total lipid extraction
Green coffee beans total lipid extraction was performed using a MeOH and MTBE solvent system, as outlined by Song et al. (2020). Initially, the sample was pulverized, and 20 mg was placed into a 2 mL centrifuge tube. The sample was then homogenized with a 4 mm diameter steel ball at a 30 Hz frequency for 20 s using a mixer mill (MM400, Retsch, Germany). Subsequently, 1 mL of a MTBE: MeOH solution (3:1, v/v), containing an internal standard mix, was added and the mixture was vigorously shaken for 30 min using a vortex mixer (MIX-200, Shanghai Jingxin, China). After adding 300 μL of ultra-pure water, the tube was agitated for one minute and then allowed to stand at 4 °C for 10 min. The contents were then centrifuged at 12,000 rpm in a centrifuge (5424R model, Eppendorf, Germany) at 4 °C for 3 min. From this, 400 μL of the upper layer was transferred into a fresh 1.5 mL centrifuge tube and dried under a CentriVap system (LABCONCO, USA) at −20 °C. Once dried, the residue was reconstituted in 200 μL of an ACN: IPA solution (1:1, v/v), vortexed for 3 min, and centrifuged at −20 °C at 12,000 rpm for 3 min. Finally, 120 μL of this solution was collected for further analysis by liquid chromatography-mass spectrometry.
2.4. Lipid analysis
The sample extracts were analyzed using a UPLC-ESI-MS/MS system (UPLC, ExionLC ADˈ https://sciex.com.cn/; MS, QTRAP® 6500+ System, https://sciex.com/) by MetWare Biotechnology Co., Ltd. (Wuhan, China), based on the AB Sciex QTRAP 6500 LC-MS/MS platform. To monitor the stability of the system, pooled quality control (QC) samples were injected every 10 samples throughout the running batch.
The UPLC system utilized a Thermo Accucore™ C30 column (2.6 μm, 2.1 mm × 100 mm i.d.), with an injection volume of 2 μL and a column temperature set at 45 °C. A flow rate of 0.35 mL/min was maintained throughout the analysis. Mobile phase A consisted of acetonitrile/water (60/40 v/v) with 0.1 % formic acid and 10 mmol/L ammonium formate, while mobile phase B was acetonitrile/water (10/90 v/v) with the same additives. The elution gradient included steps transitioning from an initial A/B ratio of 80:20 (v/v) at t = 0 to 70:30 (v/v) at t = 2 min, 40:60 (v/v) at t = 4 min, 15:85 (v/v) at t = 9 min, 10:90 (v/v) at t = 14 min, and finally 5:95 (v/v) at t = 15.5 min and t = 17.3 min, before returning to 80:20 (v/v) at t = 17.5 min and maintaining this ratio until t = 20.0 min. The effluent from the UPLC system was directed to an ESI-triple quadrupole-linear ion trap (QTRAP)-MS instrument.
A QTRAP-MS (QTRAP® 6500+ LC-MS/MS System) equipped with an ESI Turbo Ion-Spray interface was used for both linear ion trap (LIT) and triple quadrupole (QQQ) scans. Analyst 1.6.3 software (Sciex) controlled the instrument in positive and negative ion mode, with the ESI source set to turbo spray ion source at a temperature of 500 °C, and ion spray voltages of 5500 V (Positive) and − 4500 V (Negative). Gas pressures for gas 1 (GS1), gas 2 (GS2), and curtain gas (CUR) were set at 45, 55, and 35 psi, respectively. Instrument calibration used 10 μmol/L and 100 μmol/L polypropylene glycol solutions for QQQ and LIT mode, respectively. QQQ scans were conducted as an MRM experiment with nitrogen collision gas set to 5 psi. Optimization of declustering potential (DP) and collision energy (CE) facilitated individual MRM transitions. Specific MRM transitions were monitored based on eluting metabolites, with the MRM metabolite detection multi-peak diagram illustrating detectable substances in the sample.
2.5. Metabolites identification and quantification
The mass spectrometry data were processed using Analyst 1.6.3 software (AB Sciex Pte. Ltd., Singapore). Qualitative analysis relied on the Metware Database (MWDB) developed by Metware Biotechnology Co., Ltd., utilizing retention times (RT), daughter ions, and parent ions of detected substances for identification.
For lipid quantification, QQQ MS analysis in MRM mode was utilized. Chromatographic peaks detected for each substance across different samples were initially corrected to ensure accuracy prior to quantitative analysis. Subsequently, peak areas for all substances were integrated. Quantitative analysis employed the internal standard method, with lipid concentrations (C) calculated using the formula:
Here, R for the ratio of the analyte's peak area to that of the internal standard, F for correction factors specific to each substance, c for the internal standard's concentration in μmol/L, V for the sample extraction solution volume in μL, m for the sample's weight in grams, and 0.001 as a unit conversion factor.
2.6. Statistical analysis
Chord plots were generated using Origin 2022. Additionally, heatmaps and volcano plots were created on the Metware Cloud platform (https://cloud.metware.cn), which offers free online data analysis tools. Heatmaps were generated using R version 3.5.1 (pheatmap 1.0.12), and volcano plots using R version 3.5.1 (ggplot2 3.3.0). Unsupervised principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were performed using R version 3.5.1 (MetaboAnalystR 1.0.1) on the same platform. A permutation test with 200 permutations was conducted to mitigate overfitting risks. Metabolites showing significant changes between groups were identified using Variable Importance in Projection (VIP) scores (VIP ≥ 1) and fold changes (FC ≥ 2.0 or FC ≤ 0.5).
2.7. KEGG annotation and enrichment analysis
Differential lipids were annotated using the Kyoto Encyclopedia of Genes and Genomes (KEGG) compound database (https://www.kegg.jp/kegg/compound/), and these annotated metabolites were mapped to the KEGG Pathway database (https://www.kegg.jp/kegg/pathway.html). Pathway analysis was then performed to elucidate changes in the metabolic pathways of coffee bean lipids during ripening. Additionally, metabolite set enrichment analysis (MSEA) was employed to identify significantly modulated pathways, with significance determined by the P-value from the hypergeometric test.
3. Result and discussion
3.1. Lipidomics profiling of of green coffee beans
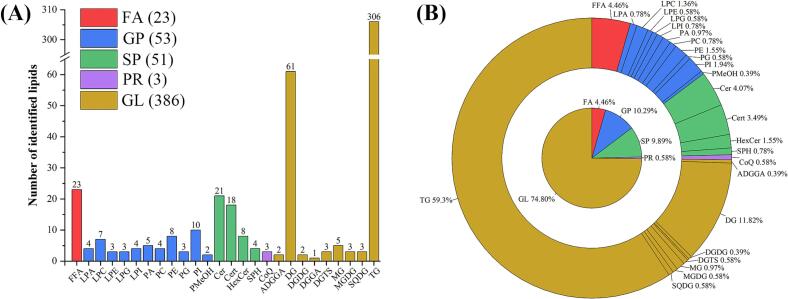
The lipid composition of coffee samples at different maturity stages was analyzed using HPLC-ESI-MS/MS (MRM) technology to monitor changes during maturation. To ensure the reliability of the lipidomics analysis, the repeatability of quality control (QC) samples was assessed. The TIC curve overlap, Pearson correlation, and CV (coefficient of variation) value distribution of QC samples (Fig. S1) indicated that mass spectrometry analysis of the same sample at different time points produced stable signals with high repeatability and reliability. These results confirm the robust analytical performance of the lipidomics methodology used. Through qualitative and quantitative analysis of mass spectrometry data, 516 lipid molecules were identified, spanning five major lipid classes: fatty acyls (FA; N = 23; 4.46 %), glycerophospholipids (GP; N = 53; 10.29 %), sphingolipids (SP; N = 51; 9.89 %), glycerolipids (GL; N = 386; 74.80 %), and prenol lipids (PR; N = 3; 0.58 %) (Fig. 2a, Table S1). Among them, GP, SP, and GL were predominant in green coffee beans based on the number of lipid molecules.
Fig. 2.
Composition of lipid profile of coffee beans with different maturity. (A) Quantities of lipid categories and subclasses. (B) Percentages of lipid categories and subclasses. M1, yellow-green cherries; M2, nearly ripe or “pintón” cherries; M3, ripe cherries; M4, overripe cherries; M5, withered cherries. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To better understand the complex pathways of lipid molecular transformation at different stages of coffee maturity, these lipid molecules were further divided into 26 subclasses. Our results revealed one subclass in the FA category (free fatty acids [FFA; N = 23; 4.46 %]), 12 subclasses in the GP category (phosphatidic acid [PA; N = 5; 0.97 %], phosphatidylcholine [PC; N = 4; 0.78 %], phosphatidylethanolamine [PE; N = 8; 1.55 %], phosphatidylglycerol [PG; N = 3; 0.58 %], phosphatidylinositol [PI; N = 10; 1.94 %], lysophosphatidic acid [LPA; N = 4; 0.78 %], lysophosphatidylcholine [LPC; N = 7; 1.36 %], lysophosphatidylethanolamine [LPE; N = 3; 0.58 %], lysophosphatidylglycerol [LPG; N = 3; 0.58 %], lysophosphatidylinositol [LPI; N = 4; 0.78 %], and phosphatidylmethanol [PMeOH; N = 2; 0.39 %]), four subclasses in the SP category (sphingine [SPH; N = 4; 0.78 %], ceramide [Cer; N = 21; 4.07 %], hexosylceramide [HexCer; N = 8; 1.55 %], and phytoceramide [Cert; N = 18; 3.49 %]), one subclass in the PR category (coenzyme Q [CoQ; N = 3; 0.58 %]), and nine subclasses in the GL category (acyldiacylglyceryl glucuronide [ADGGA; N = 2; 0.39 %], diacylglycerol [DG; N = 61; 11.82 %], digalactosyldiacylglycerol [DGDG; N = 2; 0.39 %], diacylglyceryl glucuronide [DGGA; N = 1; 0.19 %], diacylglyceryltrimethylhomoserine [DGTS; N = 3; 0.58 %], monoacylglycerol [MG; N = 5; 0.97 %], monogalactosyldiacylglycerol [MGDG; N = 3; 0.58 %], sulfoquivonosyldiacylglycerol [SQDG; N = 3; 0.58 %], and triacylglycerol [TG; N = 306; 59.30 %]).
3.2. Dynamic changes of lipid during coffee maturity stages
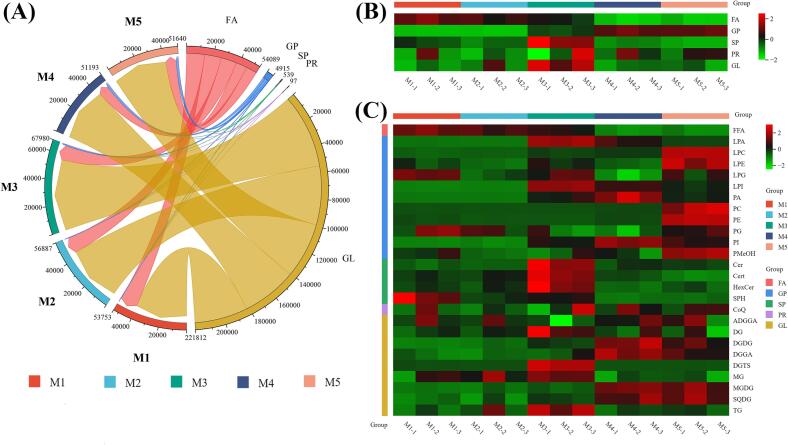
In this study, the internal standard method was used to analyze and compare different lipids in green coffee beans across various maturation stages. A chord diagram (Fig. 3a) and heat maps (Fig. 3b, c) were employed to visualize the dynamic changes in lipid content during maturation. The lipids with the highest content in green coffee beans were GL and FA, followed by GP, SP, and PR (Fig. 3a). GL levels increased from the M1 to M3 stages, decreased significantly at the M4 stage, and increased slightly at the M5 stage, though they remained significantly lower than at the M3 stage. GP levels showed little change between the M1 and M2 stages, increased significantly at the M3 stage, and further increased at the M4 and M5 stages. GP, which contains more unsaturated bonds, is more prone to oxidation (Liu et al., 2023), possibly explaining the substantial variation in its content in overripe coffee beans. FA levels decreased significantly from the M1 to M4 stages and increased slightly at the M5 stage, a trend also reflected in Fig. 3b. Throughout the maturation process, the relative content of FA exhibited a downward trend due to the generation of GP and GL. Additionally, SP levels were significantly higher at the M3 stage than at other stages.
Fig. 3.
(A) Dynamic changes of lipid contents during coffee maturation. Heatmap visualization of the metabolic variations of lipid classes (B) and subclasses (C) during coffee maturation. M1, yellow-green cherries; M2, nearly ripe or “pintón” cherries; M3, ripe cherries; M4, overripe cherries; M5, withered cherries. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Next, a heat map was generated to visually display the differences in lipid subclasses of coffee beans at different maturity stages (Fig. 3c). The lipid profile of coffee beans continued to change throughout the maturation process. In the M1 and M2 stages, the overall lipid composition of coffee beans showed minimal variation, except for a significant decrease in the content of the lipid subclass SPH. SPH and its derivatives play a crucial role in seed development and maturation by regulating cell membrane structure, participating in signal transduction, stress response, and the formation of storage substances (Sperling & Heinz, 2003). Hu et al. (2020) and Velásquez et al. (2019) observed that the cupping score of immature green cherries was significantly lower than that of other maturity stages, and these results indicate that flavor precursor compounds are still accumulating and transforming at this stage. This finding also explains the substantial changes in SPH content during early maturation. As the beans further mature, the lipid composition undergoes more pronounced changes, with the relative content of lipid metabolites shifting most significantly at the M3 stage. At this time, the coffee beans are in the fully mature stage, lipids continue to participate in the corresponding metabolic reactions, metabolite accumulation may be optimal here, and lipid content reaches a peak here (Fig. S2). Additionally, due to the absence of a ripening and shedding mechanism, the lipid components of overripe coffee cherries (M4 and M5) may undergo further oxidation and degradation, leading to a significant decrease in lipid content and major changes in lipid subclass composition.
3.3. Multivariate statistical analysis
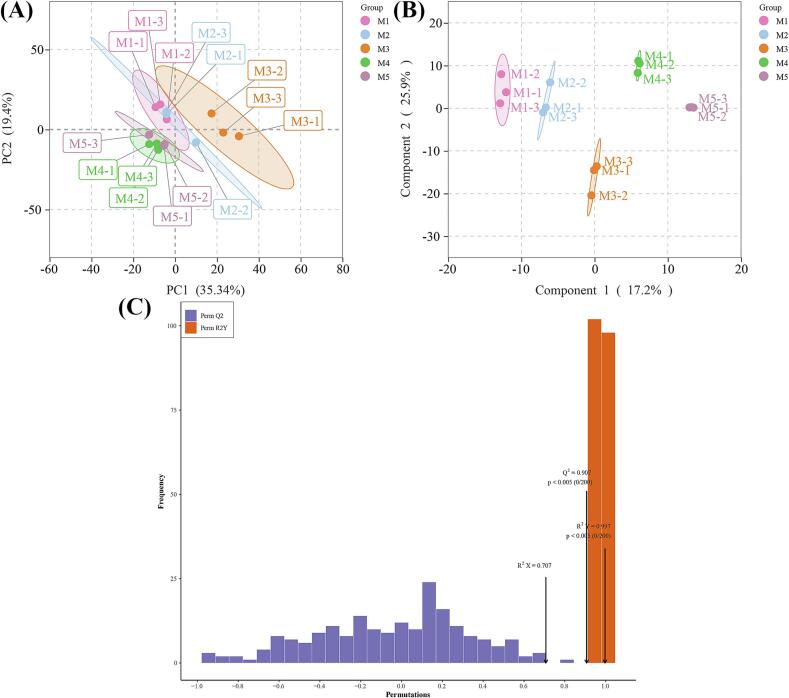
To further compare lipidomic characteristics and identify differences in lipid content across coffee maturity stages, we performed multivariate statistical analysis on the five sample groups. PCA, primarily used as a dimensionality reduction method, is a tool for visualizing and analyzing complex data sets by revealing data groupings, trends, and outliers (Rodionova, Kucheryavskiy, & Pomerantsev, 2021). Unsupervised PCA was applied to reveal the separation trends of lipid metabolites at each maturity stage. Similar approaches have been used to evaluate lipid datasets from other foods, such as during egg yolk storage, dry-cured mutton ham processing and Mongolian sheep postmortem chilled aging (Guo et al., 2022; Liu, Guo, et al., 2023; Zhang et al., 2023). The PCA score plot (Fig. 4a) shows a clear separation trend among the five groups, indicating significant differences between these sample groups. The percentage variance explained by the first two principal components (PC1 and PC2) accounted for 35.34 % and 19.40 % of the total variance, respectively. As shown in Fig. 4a, the M3 stage exhibited the most pronounced lipid changes and the greatest separation from other stages, likely due to more significant lipid transformation and accumulation during this stage.
Fig. 4.
The PCA score plot (A), OPLS-DA score plot (B) and permutation test of the OPLS-DA model (C) were based on overall lipid molecules during coffee maturation. M1, yellow-green cherries; M2, nearly ripe or “pintón” cherries; M3, ripe cherries; M4, overripe cherries; M5, withered cherries. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
These dynamic lipid shifts revealed by PCA were further validated by OPLS-DA, which enhanced group separation and identified key lipid metabolites critical for coffee quality. These analyses provided insights into the biochemical processes underlying the formation of flavor and aroma precursors, thus supporting the optimization of harvesting and processing strategies. Based on the PCA results, an OPLS-DA model was established to enhance group separation. OPLS-DA, a supervised learning method, combines partial least squares regression with discriminant analysis to improve the accuracy and interpretability of high-dimensional data classification (Kang et al., 2022). The OPLS-DA model validated the PCA findings (Fig. 4b), showing that the lipid content in green coffee bean samples changes dynamically during ripening. A permutation test was conducted to further assess the model's validity (Fig. 4c). The classification metrics, R2Y and Q2, were 0.997 and 0.907, respectively, indicating that the OPLS-DA model was reliable with good fit and predictability.
3.4. Screening of key lipids
Metabolomics data are typically high-dimensional and massive, and requiring a combination of univariate and multivariate statistical methods to accurately identify differential metabolites (Guo et al., 2022). Based on the OPLS-DA results for adjacent maturation stages (M1 vs M2, M2 vs M3, M3 vs M4 and M4 vs M5), variable importance in projection (VIP) values were obtained through multivariate analysis (Fig. S3). Additionally, univariate analysis was employed to calculate Fold Change (FC) values for lipid content between adjacent maturation stages (Fig. S4). By integrating these data, highly differential lipids were identified using criteria of VIP ≥ 1.0 and FC ≥ 2.0 or FC ≤ 0.5. A total of 111 highly differential lipids were identified across the maturation stages (Table S2), distributed among five major lipid classes (FA, N = 1; GP, N = 43; SP, N = 17; GL, N = 48; PR, N = 2), indicating that different maturation stages significantly impact lipid diversity.
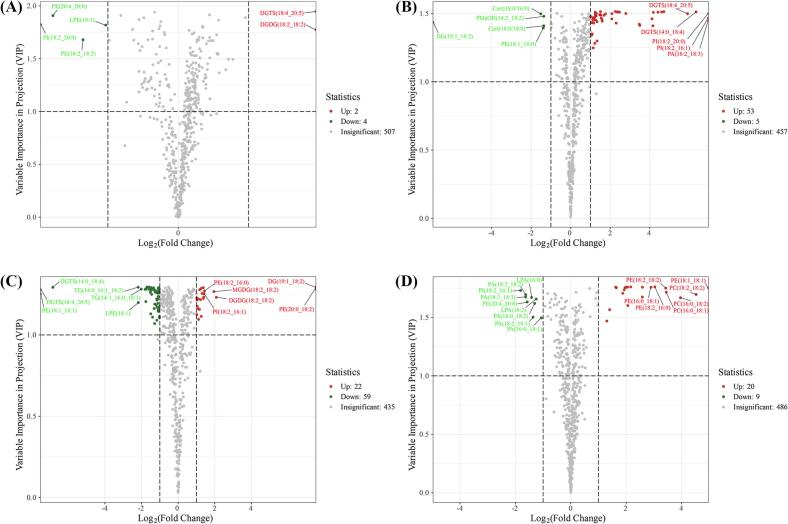
The statistical significance of lipid content changes between consecutive treatment stages was visualized using a volcano plot (Fig. 5). The x-axis represents the logarithm of the FC of a metabolite between the two samples, while the y-axis represents the VIP value. Higher values on both axes indicate more significant changes in lipid content. In the volcano plot, each point represents a lipid molecule: green points indicate downregulation, red points indicate upregulation, and gray points indicate no significant difference. From stage M1 to M2, minimal lipid changes were observed, with 6 lipids showing significant changes (2 upregulated, 4 downregulated); from M2 to M3, 58 lipids exhibited significant changes (53 upregulated, 5 downregulated); from M3 to M4, 81 lipids were significantly altered (22 upregulated, 59 downregulated); and from M4 to M5, 29 lipids showed significant changes (20 upregulated, 9 downregulated). Around the full maturity stage (M3), coffee beans underwent significant lipid synthesis and decomposition, as evidenced by the greater distance between M3 and adjacent groups in the PCA and OPLS-DA score plots (Fig. 4a, b), which explains the substantial lipid content changes between M3 and adjacent stages (Fig. S2).
Fig. 5.
Volcano plot of the differential lipids of M1 vs M2 (A), M2 vs M3 (B), M3 vs M4 (C) and M4 vs M5 (D), the criteria set at VIP ≥ 1, FC ≥ 2 or ≤ 0.5. M1, yellow-green cherries; M2, nearly ripe or “pintón” cherries; M3, ripe cherries; M4, overripe cherries; M5, withered cherries. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
From stage M1 to M2, the color of coffee cherries transitions from yellow-green to a mixture of red and yellow, signaling the coffee's progression to the mature stage. Hu et al. (2020) found that starting from immature green cherries (1#), as the maturity increases (1# to 7#), the flavor, acidity and sweetness in the cupping flavor attributes all show an upward trend, confirming that the flavor compounds in coffee beans begin to synthesize and accumulate during this period. Fig. 5a illustrates the lipid composition changes at this stage, where DGDG (18:2_18:2) and DGTS (18:4_20:5) are significantly upregulated, while LPE (18:1), PE (18:2_18:2), PE (20:4_20:0), and PI (18:2_20:0) are significantly downregulated. As coffee beans are still developing at this stage, these lipid changes suggest that lipid synthesis lags slightly behind the synthesis of other major compounds such as proteins and carbohydrates.
As maturation progressed, significant changes in lipid content were observed in coffee beans at stage M3 compared with stage M2 (Fig. 5b), with most differential lipids being upregulated. Among the differential lipids, Cer (t18:0/16:0), PMeOH (18:2_18:2), DG (19:1_18:2), Cer (t18:0/18:0), and PE (18:1_18:0) were significantly downregulated, while the remaining 53 lipids (GL: 18; GP: 26; SP: 9) were significantly upregulated. At this stage, the synthesis rate of GL and GP lipids increased markedly, resulting in a significant rise in lipids such as DGTS (14:0_18:4), DGTS (18:4_20:5), LPA (18:1), LPA (16:0), and LPA (18:0). Concurrently, the large-scale synthesis of GP and GL led to a significant decrease in FA content, which serves as a precursor. These lipids accumulate in mature seeds as essential components for energy storage and cell membrane structure. SP lipids, which confer unique properties to cell membranes, regulate plant water loss or absorption, maintain water balance within seeds, and protect plants from physical, chemical, and biological damage (Heredia, 2003; Liu, Hou, Bao, Wang, & Chen, 2021). The large-scale upregulation of SP during stage M3 corresponds with the full maturation of coffee seeds.
From stage M3 to M4 (Fig. 5c), coffee beans undergo further maturation, marked by deepening color and increased sugar accumulation in the pulp. During this stage, lipid composition changes more actively: 22 lipids (GL: 8; GP: 12; PR: 2) are significantly upregulated, while 59 lipids (FA: 1; GL: 40; GP: 7; SP: 11) are significantly downregulated. These findings suggest that lipids in mature coffee seeds are unstable, with overripeness potentially leading to the oxidation or decomposition of GL and GP. Additionally, the absence of a dormancy mechanism in coffee seeds may trigger early germination within the cherry fruit (Waters, Arendt, & Moroni, 2017). During seed germination or early growth, FA is transported to the mitochondria for β-oxidation, generating energy for seed growth and development (Xiang et al., 2023). The significant decrease in FA content in overripe coffee seeds could indicate the onset of germination.
Due to the absence of an abscission mechanism, withered coffee cherries remain on the tree, prompting further investigation into lipid transformations during the M4-M5 stage (Fig. 5d). Among the differential lipids, 9 GPs were significantly downregulated, while 20 lipids (18 GP and 2 SP) were significantly upregulated. At this stage, the high water content in coffee seeds and ongoing germination lead to significant changes in GP levels. These changes can be attributed to two main factors: first, GP is broken down during germination to provide the energy required for initial growth (Liu et al., 2023); second, as a key component of the cell membrane, GP stabilizes and regulates membrane structure and function by forming a bilayer lipid structure, playing a crucial role in membrane reorganization and new membrane formation during seed germination (Qin, Zhang, Wang, & Su, 2023).
3.5. Lipid metabolism pathways analysis
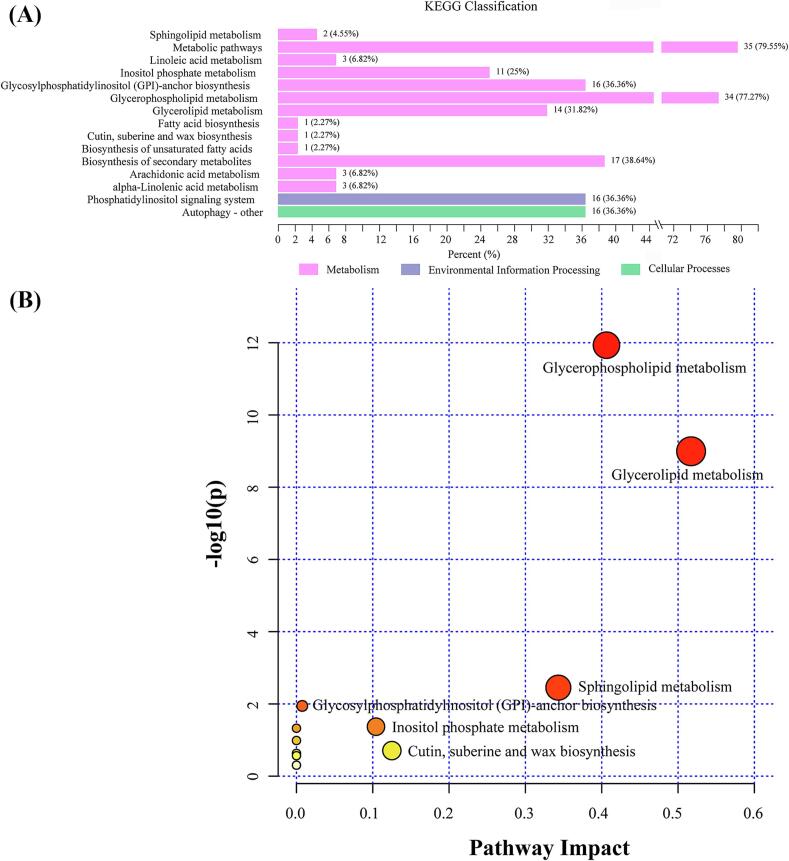
To further analyze lipid changes during coffee maturation, the 111 major differential lipids identified were mapped to the KEGG database for metabolic pathway analysis. These lipids were primarily involved in 15 metabolic pathways (Fig. 6a), including glycerophospholipid metabolism, alpha-linolenic acid metabolism, linoleic acid metabolism, and the biosynthesis of unsaturated fatty acids. Seven key KEGG pathways with an average relative abundance greater than 30 % were identified in the ripening samples, including metabolic pathways, glycerophospholipid metabolism, biosynthesis of secondary metabolites, glycosylphosphatidylinositol (GPI)-anchor biosynthesis, phosphatidylinositol signaling system, autophagy - other, and glycerolipid metabolism. These metabolic pathways are likely the most critical in the coffee maturation process.
Fig. 6.
KEGG pathway analysis of 111 differentially expressed lipids in coffee beans at different maturity stages. (A) KEGG pathway annotations, with the x-axis representing the proportion and number of annotated metabolites, and the y-axis listing pathway names. (B) KEGG enrichment statistics, where the x-axis shows the rich factor for each pathway, and the y-axis lists the KEGG metabolic pathways. Bubble size and color indicate the number of different lipids and the degree of enrichment, respectively.
As shown in Fig. 6a, metabolic pathways have the highest relative abundance among all pathways, at 79.55 %. These pathways involve a series of complex biochemical reactions in organisms, including energy production and utilization, synthesis and decomposition of organic matter, material transport and transformation, and signal transduction (Guo et al., 2022). This indicates that coffee seeds undergo vigorous biochemical reactions during maturation. Glycerophospholipid metabolism, with an enrichment of 77.27 %, is the second most abundant pathway. This pathway maintains cell membrane stability and supports signal transduction during coffee maturation, enabling the accumulation of key flavor precursors like volatile aromatics and lipid intermediates, which shape coffee's aroma and mouthfeel (Silva et al., 2020). GP also play a crucial role in the release of arachidonic acid, promoting membrane fusion, and protection cells from oxidation (Zitouni, Wewer, Dörmann, Abdelly, & Ben Youssef, 2016). Due to its unique physiological characteristics, coffee undergoes both maturation and germination stages during its maturation process, which contributes to its vigorous metabolism.
A dataset of 111 significantly different lipids was analyzed using MetaboAnalyst 6.0 to identify the most significant metabolic pathways. The results are visualized as a bubble chart, where larger and darker bubbles indicate greater pathway enrichment and impact. As shown in Fig. 6b, glycerolipid metabolism and glycerophospholipid metabolism have the largest and darkest bubbles, highlighting them as the most critical pathways, followed by sphingolipid metabolism. Glycerolipid metabolism, in particular, is essential for energy storage and the biosynthesis of key lipids, which act as precursors for Maillard reaction products during roasting, a process essential for the development of coffee's characteristic flavors and aromas (Wang et al., 2025; Yeretzian et al., 2002). From stages M1 to M4, FA content decreased, likely due to the synthesis of GP and GL, while in stage M5, FA content increased as GP and GL were decomposed during germination. In summary, glycerolipid metabolism and glycerophospholipid metabolism are the pathways with the greatest impact during coffee maturation.
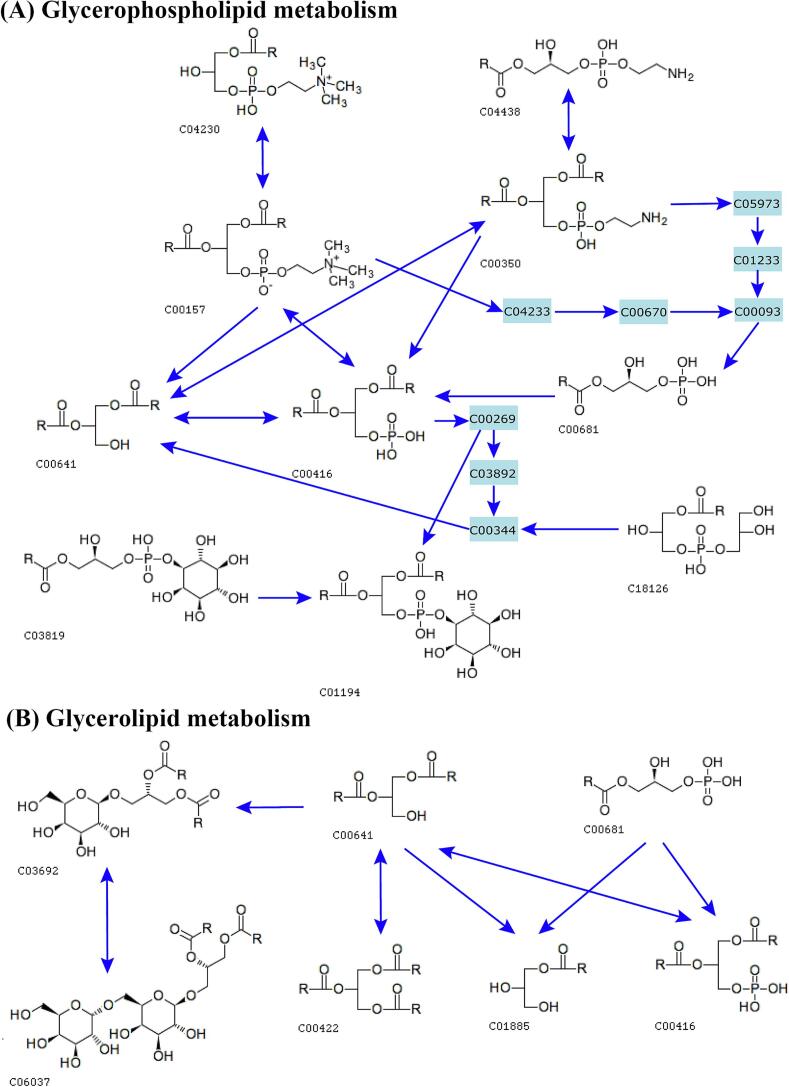
To further explore lipid metabolism changes during coffee maturation, we annotated the KEGG pathways of glycerophospholipid metabolism and glycerolipid metabolism. Fig. 7 illustrates the transformation of lipid molecules during coffee maturation. Specifically, PI (C00416), PE (C00157), PC (C00350), PA (C01194), LPA (C00681), LPC (C04230), LPE (C04438), LPG (C18126), LPI (C03819) and DG (C00641) are primarily involved in glycerophospholipid metabolism (Fig. 7a), while LPA, PA, DG, DGDG (C06037), MG (C01885), MGDG (C03692) and TG (C00422) are mainly associated with glycerolipid metabolism (Fig. 7b). Coffee maturation encompasses two key physiological functions: seed maturation and germination. In these processes, glycerophospholipid metabolism and glycerolipid metabolism serve different roles. Lipid accumulation is crucial during seed maturation, where GP maintain the stability of cell membranes, support seed integrity, participate in signal transduction, and provide antioxidant protection. Simultaneously, GL act as energy reserves, supplying the necessary energy through hydrolysis to facilitate germination (Cao et al., 2023; Huang et al., 2022).
Fig. 7.
Overview of significant lipid metabolic pathways in coffee during maturation. (A) Glycerophospholipid metabolism. (B) Glycerolipid metabolism. The numbers correspond to the ID of each lipid subclass.
Lipid degradation plays a vital role in seed germination and seedling formation. GP help rebuild cell membranes and regulate energy utilization, while GL release energy through hydrolysis, supporting the germination process and related metabolic pathways, thereby promoting smooth seed germination and growth (Cao et al., 2023). During coffee bean maturation, DG, PA, and LPA emerge as key lipid metabolites (Fig. 7). PA and LPA, as precursors for GP synthesis, form the foundation for the production of essential GP. Additionally, these lipids function as phospholipid signaling molecules, regulating membrane stability, signal transduction, cell behavior, and energy metabolism (Liu, Guo, et al., 2023). Notably, DG plays a critical role in membrane assembly, energy storage and supply, signal transduction, and metabolic regulation. DG and PA can be interconverted under certain conditions, further participating in glycerophospholipid metabolism and glycerolipid metabolism (Fig. 7). Thus, during coffee maturation, the interaction between glycerophospholipid metabolism and glycerolipid metabolism is ongoing.
4. Conclusion
This study conducted a comprehensive lipidomic analysis to reveal differences in lipid profiles of coffee beans at various stages of maturity. A total of 516 lipid molecules were identified throughout the maturation process, categorized into five major lipid classes: FA, GP, SP, GL, and PR. These lipids were further subdivided into 26 subclasses, with TG having the highest number of species, followed by DG, FFA, Cer, and Cert. A detailed analysis of lipid content changes during maturation identified 111 lipids with significant differences. KEGG pathway analysis of these differential lipids highlighted glycerophospholipid and glycerolipid metabolism as the most critical metabolic pathways. Further annotation identified PA, LPA, and DG as key lipid metabolites involved in these pathways during maturation. To our knowledge, this is the first lipidomic study on coffee maturation. These findings are significant for understanding lipid transformations and flavor profile formation during coffee maturation, offering practical guidance for optimizing harvest timing and enhancing coffee quality to meet high-value market demands.
CRediT authorship contribution statement
Yanbing Wang: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation. Xiaoyuan Wang: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation. Xiaogang Liu: Writing – review & editing, Project administration, Methodology, Funding acquisition. Xiaoqiong Liu: Writing – review & editing, Project administration, Methodology, Funding acquisition, Conceptualization. Lirong Li: Writing – review & editing, Methodology. Zhiqing Sun: Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study was supported financially by the Yunnan Fundamental Research Projects (NO. 202301AS070030), Yunnan Province Innovative Talent Project (No. 202405 AD350051).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.102062.
Contributor Information
Yanbing Wang, Email: wongyb@kust.edu.cn.
Xiaoyuan Wang, Email: wongxiaoyuan@163.com.
Xiaogang Liu, Email: liuxiaogangjy@126.com.
Xiaoqiong Liu, Email: fluidliu99@163.com.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
Data availability
Data will be made available on request.
References
- Agnoletti B.Z., Folli G.S., Pereira L.L., Pinheiro P.F., Guarconi R.C., Oliveira E.C.D., Filgueiras P.R. Multivariate calibration applied to study of volatile predictors of arabica coffee quality. Food Chemistry. 2022;367 doi: 10.1016/j.foodchem.2021.130679. [DOI] [PubMed] [Google Scholar]
- Aristizábal L.F., Johnson M.A., Shriner S., Wall M. Frequent and efficient harvesting as an economically viable strategy to regulate coffee berry borer on commercial farms in Hawaii. Journal of Economic Entomology. 2023;116(2):513–519. doi: 10.1093/jee/toad041. [DOI] [PubMed] [Google Scholar]
- Aurum F.S., Imaizumi T., Thammawong M., Suhandy D., Praseptiangga D., Tsuta M.…Nakano K. Lipidomic profiling of Indonesian coffee to determine its geographical origin by LC-MS/MS. European Food Research and Technology. 2022;248(12):2887–2899. doi: 10.1007/s00217-022-04098-5. [DOI] [Google Scholar]
- Cao D., Ma Y.Z., Cao Z.H., Hu S.S., Li Z., Li Y.Z.…Yin D.M. Coordinated lipid mobilization during seed development and germination in peanut (Arachis hypogaea L.) Journal of Agricultural and Food Chemistry. 2023;72(6):3218–3230. doi: 10.1021/acs.jafc.3c06697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B., Furtado A., Smyth H.E., Henry R.J. Influence of genotype and environment on coffee quality. Trends in Food Science & Technology. 2016;57:20–30. doi: 10.1016/j.tifs.2016.09.003. [DOI] [Google Scholar]
- Guo X., Shi D., Liu C.J., Huang Y.L., Wang Q.L., Wang J.Y.…Lu S.L. UPLC-MS-MS-based lipidomics for the evaluation of changes in lipids during dry-cured mutton ham processing. Food Chemistry. 2022;377 doi: 10.1016/j.foodchem.2021.131977. [DOI] [PubMed] [Google Scholar]
- Heredia A. Biophysical and biochemical characteristics of cutin, a plant barrier biopolymer. Biochimica et Biophysica Acta-General Subjects. 2003;1620(1–3):1–7. doi: 10.1016/S0304-4165(02)00510-X. [DOI] [PubMed] [Google Scholar]
- Hu G., Peng X., Wang X., Li X., Li X., Qiu M. Excavation of coffee maturity markers and further research on their changes in coffee cherries of different maturity. Food Research International. 2020;132 doi: 10.1016/j.foodres.2020.109121. [DOI] [PubMed] [Google Scholar]
- Huang R., Liu M., Gong G., Wu P., Bai M., Qin H., Wang G., Liao H., Wang X., Li Y., Wu H., Wang X., Yang C., Schubert D., Zhang S. BLISTER promotes seed maturation and fatty acid biosynthesis by interacting with WRINKLED1 to regulate chromatin dynamics in arabidopsis. The Plant Cell. 2022;34(6):2242–2265. doi: 10.1093/plcell/koac083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jham G.N., Velikova R., Muller H.V., Nikolova-Damyanova B., Cecon P.R. Lipid classes and triacylglycerols in coffee samples from Brazil: Effects of coffee type and drying procedures. Food Research International. 2001;34(2–3):111–115. doi: 10.1016/S0963-9969(00)00137-X. [DOI] [Google Scholar]
- Kang C., Zhang Y., Zhang M., Qi J., Zhao W., Gu J., Guo W., Li Y. Screening of specific quantitative peptides of beef by LC-MS/MS coupled with OPLS-DA. Food Chemistry. 2022;387 doi: 10.1016/j.foodchem.2022.132932. [DOI] [PubMed] [Google Scholar]
- Liu G., Yan L., Wang S., Yuan H., Zhu Y., Xie C., Wang P., Yang R. A novel type of sprout food development: Effects of germination on phytic acid, glucosinolates, and lipid profiles in rapeseed. Food Bioscience. 2023;55 doi: 10.1016/j.fbio.2023.102893. [DOI] [Google Scholar]
- Liu N., Hou L., Bao J., Wang L., Chen X. Sphingolipid metabolism, transport, and functions in plants: Recent progress and future perspectives. Plant Communications. 2021;2(5) doi: 10.1016/j.xplc.2021.100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Guo X., Wang N., Lu S., Dong J., Qi Z., Zhou J., Wang Q. Evaluation of changes in egg yolk lipids during storage based on lipidomics through UPLC-MS/MS. Food Chemistry. 2023;398 doi: 10.1016/j.foodchem.2022.133931. [DOI] [PubMed] [Google Scholar]
- Marín-López S., Arcila-Pulgarín J., Montoya-Restrepo E., Oliveros-Tascón C. Relación entre el estado de madurez del fruto del café y las características de beneficio, rendimiento y calidad de la bebida. Cenicafé. 2003;54(4):297–315. [Google Scholar]
- de Melo Pereira G.V., de Carvalho Neto D.P., Magalhães Júnior A.I., Vásquez Z.S., Medeiros A.B.P., Vandenberghe L.P.S., Soccol C.R. Exploring the impacts of postharvest processing on the aroma formation of coffee beans – a review. Food Chemistry. 2019;272:441–452. doi: 10.1016/j.foodchem.2018.08.061. [DOI] [PubMed] [Google Scholar]
- Pazmino-Arteaga J., Gallardo C., Gonzalez-Rodriguez T., Winkler R. Loss of sensory cup quality: Physiological and chemical changes during green coffee storage. Plant Foods for Human Nutrition. 2022;77(1):1–11. doi: 10.1007/s11130-022-00953-8. [DOI] [PubMed] [Google Scholar]
- Qin Y., Zhang B., Wang Y., Su R. Characterization of SEC14 family in wheat and the function of TaSEC14-7B in salt stress tolerance. Plant Physiology and Biochemistry. 2023;202 doi: 10.1016/j.plaphy.2023.107926. [DOI] [PubMed] [Google Scholar]
- Rodionova O., Kucheryavskiy S., Pomerantsev A. Efficient tools for principal component analysis of complex data— a tutorial. Chemometrics and Intelligent Laboratory Systems. 2021;213 doi: 10.1016/j.chemolab.2021.104304. [DOI] [Google Scholar]
- Silva A.C.R., da Silva C.C., Garrett R., Rezende C.M. Comprehensive lipid analysis of green Arabica coffee beans by LC-HRMS/MS. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109727. [DOI] [PubMed] [Google Scholar]
- Silva A.C.R., Garrett R., Rezende C.M., Meckelmann S.W. Lipid characterization of arabica and robusta coffee beans by liquid chromatography-ion mobility-mass spectrometry. Journal of Food Composition and Analysis. 2022;111 doi: 10.1016/j.jfca.2022.104587. [DOI] [Google Scholar]
- Sittipod S., Schwartz E., Paravisini L., Peterson D.G. Identification of flavor modulating compounds that positively impact coffee quality. Food Chemistry. 2019;301 doi: 10.1016/j.foodchem.2019.125250. [DOI] [PubMed] [Google Scholar]
- Song L., Liu Z., Hu H., Yang Y., Li T., Lin Z., Ye J., Chen J., Huang X., Liu D., Zhou J., Shi Y., Zhao H., Xie C., Chen L., Song E., Lin S., Lin S. Proto-oncogene Src links lipogenesis via lipin-1 to breast cancer malignancy. Nature Communications. 2020;11(1):5842. doi: 10.1038/s41467-020-19694-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling P., Heinz E. Plant sphingolipids: Structural diversity, biosynthesis, first genes and functions. Biochimica et Biophysica Acta-Molecular and Cell Biology of Lipids. 2003;1632(1–3):1–15. doi: 10.1016/S1388-1981(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Velásquez S., Peña N., Bohórquez J.C., Gutierrez N., Sacks G.L. Volatile and sensory characterization of roast coffees - effects of cherry maturity. Food Chemistry. 2019;274:137–145. doi: 10.1016/j.foodchem.2018.08.127. [DOI] [PubMed] [Google Scholar]
- Wang X., Quan C., Liu X., Wang Y., Bai X., Li Y., Liu X. Quantitative lipidomics reveals the effects of roasting degree on arabica coffee beans lipid profiles. Food Control. 2025;169 doi: 10.1016/j.foodcont.2024.111015. [DOI] [Google Scholar]
- Wang X., Wang Y., Hu G., Hong D., Guo T., Li J.…Qiu M. Review on factors affecting coffee volatiles: From seed to cup. Journal of the Science of Food and Agriculture. 2022;102(4):1341–1352. doi: 10.1002/jsfa.11647. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang X., Hu G., Al-Romaima A., Peng X., Li J., Bai X., Li Z., Qiu M. Anaerobic germination of green coffee beans: A novel strategy to improve the quality of commercial Arabica coffee. Current Research in Food Science. 2023;6 doi: 10.1016/j.crfs.2023.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang X., Hu G., Zhang Z., Al-Romaima A., Bai X., Li J., Zhou L., Li Z., Qiu M. Comparative studies of fermented coffee fruits post-treatments on chemical and sensory properties of roasted beans in Yunnan, China. Food Chemistry. 2023;423 doi: 10.1016/j.foodchem.2023.136332. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang X., Quan C., Al-Romaima A., Hu G., Peng X., Qiu M. Optimizing commercial Arabica coffee quality by integrating flavor precursors with anaerobic germination strategy. Food Chemistry: X. 2024;23 doi: 10.1016/j.fochx.2024.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters D.M., Arendt E.K., Moroni A.V. Overview on the mechanisms of coffee germination and fermentation and their significance for coffee and coffee beverage quality. Critical Reviews in Food Science and Nutrition. 2017;57(2):259–274. doi: 10.1080/10408398.2014.902804. [DOI] [PubMed] [Google Scholar]
- Xiang F., Liu W., Liu X., Song Y., Zhang Y., Zhu X., Wang P., Guo S., Song C. Direct balancing of lipid mobilization and reactive oxygen species production by the epoxidation of fatty acid catalyzed by a cytochrome P450 protein during seed germination. New Phytologist. 2023;237(6):2104–2117. doi: 10.1111/nph.18669. [DOI] [PubMed] [Google Scholar]
- Yeretzian C., Jordan A., Badoud R., Lindinger W. From the green bean to the cup of coffee: Investigating coffee roasting by on-line monitoring of volatiles. European Food Research and Technology. 2002;214(2):92–104. doi: 10.1007/s00217-001-0424-7. [DOI] [Google Scholar]
- Zhang M., Su R., Corazzin M., Hou R., Zhang Y., Sun L., Hu G., Dou L., Guo Y., Su L., Zhao L., Jin Y. Lipid transformation during postmortem chilled aging in Mongolian sheep using lipidomics. Food Chemistry. 2023;405 doi: 10.1016/j.foodchem.2022.134882. [DOI] [PubMed] [Google Scholar]
- Zhu J., Zhou L., Zhao M., Wei F., Fu H., Marchioni E. Revealing the dynamic changes of lipids in coffee beans during roasting based on UHPLC-QE-HR-AM/MS/MS. Food Research International. 2023;174 doi: 10.1016/j.foodres.2023.113507. [DOI] [PubMed] [Google Scholar]
- Zitouni M., Wewer V., Dörmann P., Abdelly C., Ben Youssef N. Quadrupole time-of-flight mass spectrometry analysis of glycerophospholipid molecular species in the two halophyte seed oils: Eryngium maritimum and Cakile maritima. Food Chemistry. 2016;213:319–328. doi: 10.1016/j.foodchem.2016.06.083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Data Availability Statement
Data will be made available on request.