Abstract
Introduction
To identify prognostic biomarkers that could predict how well patients will respond to lenvatinib/pembrolizumab (LEN/PEM). The utility of certain inflammatory biomarkers in endometrial liquid‐based cytology (LBC) or peripheral blood samples, such as neutrophil counts, lymphocyte counts, and neutrophil‐to‐lymphocyte ratio (NLR) were explored.
Methods
The study included 25 patients with advanced or recurrent endometrial cancer who had received LEN/PEM between August 2018 and March 2024. Predictors for overall response (OR), disease control, and progression‐free survival, based on neutrophil/lymphocyte counts, NLR scores of the endometrial LBC prior to initial treatment, and peripheral blood prior to initial treatment and prior to LEM/PEM treatment were compared using a receiver operating characteristic curve. Significant predictors were evaluated using the log‐rank test, and multivariate analysis.
Results
Although neutrophil counts and NLR score in endometrial LBC prior to initial treatment were better effective predictors for OR, the most accurate predictor of a progression‐free status was NLR score in peripheral blood prior to LEM/PEM (0.722, 95% CI: 0.45–0.99, sensitivity: 57.1%, specificity: 94.4%). In peripheral blood prior to LEN/PEM, the lower NLR (NLR <5.39) group had a significantly longer PFS than the higher NLR (5.39 ≤ NLR) group (p = 0.023, median survival: 13.5 vs. 3.0 months), and tended to be independently correlated with PFS (hazard ratio = 2.571; 95% CI = 0.857–7.719; p = 0.092).
Conclusion
Inflammatory biomarkers in endometrial LBC failed to predict the efficacy of LEN/PEM, while peripheral blood NLR score sampled prior to LEN/PEM potentially could be a significant predictor.
Keywords: endometrial cancer, lenvatinib, liquid‐based cytology, lymphocytes, neutrophil‐to‐lymphocyte ratio, pembrolizumab
INTRODUCTION
The prevalence of endometrial cancer has been increasing, both steadily and significantly, making it the most common gynecological cancer in Japan. 1 Most adjuvant therapies for endometrial cancer involve chemotherapy (88% to 96%), yet the reported outcomes for advanced disease have been unfavorable, with a survival rate of only 17%. 1 , 2
Although the clinical benefits of immune checkpoint inhibitors (ICIs) including pembrolizumab (PEM) have been established in patients with microsatellite instability‐high (MSI‐H) or mismatch repair‐deficient (dMMR) advanced endometrial cancer, pembrolizumab administered as a single agent has demonstrated limited efficacy in patients with microsatellite‐stable or mismatch repair‐proficient (pMMR) disease. 3 , 4 Lenvatinib (LEN) is a tyrosine kinase inhibitor with antiangiogenic properties that has demonstrated an objective response rate of 14.3% in the treatment of second‐line recurrent endometrial cancer as a monotherapy.
Based on findings of the KEYNOTE‐775/309 study, ICI consisting of LEN/PEM have become the widely accepted standard treatment for advanced or recurrent endometrial cancer following the failure of platinum‐based chemotherapy. 2 However, adverse events of grade 3 or higher occurred in 88.9% of patients who received LEN/PEM. Most patients experienced dose reductions and interruptions (66.5% and 69.2%) due to these events, and a considerable number discontinued the trial drug (33.0%). 2
The identification of reliable biomarkers for ICI is essential for making treatment decisions and predicting patient responses to LEN/PEM treatment, which can ultimately enhance patient outcomes. 5 Nevertheless, a prognostic marker incorporating ICI along with LEN/PEM has not been identified. Biomarkers that may indicate a response to PEM may comprise TMB‐high, which can be identified through next‐generation sequencing (NGS), as well as MSI‐H status and dMMR. Implementing these tests in routine clinical practice is associated with substantial costs and is complicated and time‐consuming.
Liquid‐based cytology (LBC) is a beneficial morphological diagnostic tool, similar to conventional smears or cell blocks, and it also plays an essential role in genomic analysis using NGS for providing diagnostic support, as previously documented. 6 , 7 , 8 The Cancer Genome Atlas (TCGA) has developed a classification system for endometrial cancers that identifies four distinct molecular subtypes. This molecular classification system, known as the TCGA classification system, which classifies endometrial cancer as POLE (polymerase epsilon) mutation (POLEmut)‐subtype, and MMR‐deficient (MMR‐d), might be more sensitive to ICIs with anti‐PD1/PDL1. 9 We previously demonstrated that lymphocytes are predominantly increased in endometrial LBC in these groups. 10 , 11 In particular, patients with high lymphocyte counts in LBC, with a “surface epithelial slackening” pattern, and immunohistochemically proficient‐MMR are more likely to have a POLEmut prediction. 10 , 11 , 12
Recent gynecological studies have indicated that pretreatment C‐reactive protein (CRP), NLR, platelet‐to‐lymphocyte ratio (PLR), and hemoglobin, albumin, lymphocyte, and platelet (HALP) scores are correlated with clinical prognosis. 13 , 14 NLR scores were significantly elevated in endometrial cancer patients, and NLR score increased in advanced stage disease. 15 Although these markers are indicative of systemic inflammation, they do not provide a direct reflection of the local tumor environment in endometrial cancer. The identification of predictors for patients who may benefit from ICIs through routine clinical examination with endometrial cytology may result in cost and time savings. Endometrial LBC can provide a more accurate assessment of lymphocyte and neutrophil counts compared to conventional techniques. 16 This is because LBC can eliminate blood and mucus and disperse cells uniformly. 16
In patients with high neutrophil‐to‐lymphocyte ratio (NLR), patients may have a high frequency of POLEmut and MMR‐subtypes. 10 , 11 Lenvatinib reduces immunosuppressive cells and improves the immune environment, and it has been shown to enhance anti‐tumor immune responses by promoting T‐cell activation. 2 , 17 Therefore, we hypothesized that NLR may be a prognostic biomarker for LEN/PEM therapy.
In the present study, we examined whether inflammatory biomarkers including neutrophil counts, lymphocyte counts, and NLR in endometrial LBC could be used as prognostic predictors for LEN/PEM.
METHODS
A retrospective analysis of a clinical database was conducted to identify patients with advanced or recurrent endometrial cancer who had received treatment with LEN/PEM between August 2018 and March 2024. Clinical data were collected by reviewing inpatient medical records, and the disease was classified according to the 2008 FIGO staging system.
The study involved 40 patients who underwent LEN/PEM treatment for endometrial cancer that had advanced or returned following at least one previous platinum‐based chemotherapy regimen. A total of 15 cases were excluded as follows: cytological diagnosis of atypical gland cells (n = 1); received initial treatment prior to 2018 and had no LBC performed (n = 14). Finally, the study included 25 patients with endometrial cancer. Pathological data were obtained from histological reports in the outpatient clinic for patients who did not undergo surgery or from surgically resected endometrial cancers in patients who received primary treatment. This trial was conducted in accordance with the Declaration of Helsinki and the study protocol was approved by the Institutional Review Board (IRB) of Kagoshima University Graduate School of Medical Sciences (approval # 230081). This study was a retrospective observational study, therefore written informed consent from participants was not required in accordance with our IRB. Instead, information about the research was made available to the treatment patients with LEM/PEM, guaranteeing the opportunity to reject the research.
Procedure for calculating neutrophil and lymphocyte counts in endometrial LBC
Cytological samples were collected from outpatients via endometrial biopsy by scraping the endometrium, hereafter referred to as “cytology,” using a Soft‐cyte® device (Soft Medical, Bunkyo‐ku, Japan) prior to initial treatment in the outpatient clinic. Endometrial cytology specimens were prepared with a liquid‐based method and subsequently examined by both a cytopathologist and a cytotechnologist, each blinded to patient clinical information.
The protocol for assessing neutrophil and lymphocyte counts in cytological examinations was as follows: initially, the entire slide was examined using a low power field (LPF) microscope at a magnification of 100×, with a 10× objective lens, to assess the whole image, including the distribution and cell agglomerations. Next, the neutrophils and lymphocytes were scrutinized under a high power field (HPF) using a 400× (10× objective lens) from three different fields within the area exhibiting the highest concentration of inflammatory cells. Individual values were calculated as an average of the three fields.
Study guideline
A flowchart summarizing the study is shown in Figure 1. First, the prognostic predictors of neutrophil and lymphocyte counts, NLR scores of the endometrial LBC prior to initial treatment, peripheral blood prior to initial treatment, and peripheral blood prior to LEM/PEM were compared using receiver operating characteristic (ROC) curve analysis. Pre‐treatment peripheral blood values of lymphocyte and neutrophil counts were obtained immediately prior to the administration of LEN/PEM. Chemotherapy was administered as prior treatment in all patients, and the bone marrow suppression was fully recovered before LEN/PEM therapy was administered.
FIGURE 1.
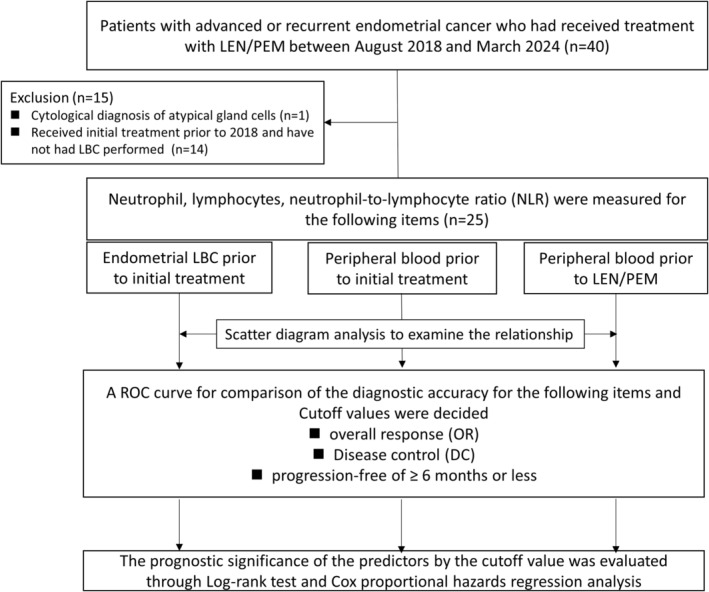
Flow chart summarizing the retrospective cohort analysis of our study. LEN/PEM, lenvatinib and pembrolizumab; NLR, neutrophil to lymphocyte ratio; LBC, Liquid‐based cytology; ROC, receiver operating characteristic curve.
A ROC curve was generated for comparison of the diagnostic accuracy of each item to predict those patients with maximum overall response (OR), maximum disease control (DC) and progression‐free for ≥6 months or less. Cutoff values for the predictor with the highest level of discrimination for OR, DC, and progression‐free survival for clinical benefits were determined using the Youden index. The prognostic significance of the predictors was evaluated using the log‐rank test. Scatter diagrams were plotted to examine the relationship prior to initial treatment between endometrial LBC and peripheral blood neutrophils or lymphocytes.
All patients fully recovered from the bone suppression caused by chemotherapeutic agents when they were administered ICI, and no patients received immunosuppressants, including steroids, which can impact complete blood counts. NLR scores were defined as neutrophils (L)/lymphocytes (L). The period from the start date of immunotherapy to the confirmation of tumor progression or death was defined as PFS or OS. The median follow‐up duration was determined as the period between the last confirmed survival or death date and the initiation date of LEM/PEM treatment. The commonly used definition of OR was the proportion of patients who achieved either partial response (PR) or complete response (CR). DC was achieved with PR, CR, or stable disease (SD).
Statistics
Survival curves were calculated using the Kaplan–Meier method, and both PFS and OS were evaluated using the log‐rank test to compare the groups. The Cox proportional hazards regression model was employed to determine independent variables in the multivariate analysis. Baseline factors with p < 0.20 in univariate analysis were included in multivariate analyses. Differences were considered statistically significant at p < 0.05. All statistical analyses were performed on a personal computer using SPSS for Windows, v.29 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient information is listed in Table 1. The median follow‐up duration was 15 months (range: 1–26 months). Grades 1–2 endometrioid carcinoma were the most common (60%) histology, and FIGO stage IV was the most common (48%) stage. No patients were found to have had prior radiotherapy, except one patient with local irradiation of a recurrent vaginal tumor. Although no dose reduction in pembrolizumab was observed, the patient with 10/25 (40%) of lenvatinib underwent dose reduction from the standard of 20 mg/day.
TABLE 1.
Baseline characteristic of 25 patients in advanced endometrial cancer.
| n = 25 | ||
|---|---|---|
| Median age (range) | 62 (35–79) | |
| Performance status | ||
| 0 | 11 | |
| 1≤ | 14 | |
| Histological type | ||
| Endometrioid | Grade 1–2 | 9 |
| Grade 3 | 3 | |
| Carcinosarcoma | 3 | |
| Serous | 4 | |
| Clear | 2 | |
| Others | 4 | |
| FIGO stage (FIGO 1988) | ||
| I | 4 | |
| II | 1 | |
| III | 8 | |
| IV | 12 | |
| Treatment | ||
| No surgery | 5 | |
| TAH + BSO ± Omentectomy | 6 | |
| TAH + BSO ± LNBx(SNNS) ± Omentectomy | 2 | |
| TAH + BSO ± RPLD±Omentectomy | 8 | |
| Semi‐RAH* + BSO ± RPLD±Omentectomy | 4 | |
| Adjuvant therapy | ||
| None | 1 | |
| Paclitaxel + Carboplation | 24 | |
| Recurrent sites | ||
| Only local | 3 | |
| Distant ± Local | 22 | |
| MSI | ||
| Not measured | 12 | |
| dMMR | 1 | |
| pMMR | 12 | |
| Number of prior chemotherapy regimen | ||
| 1 | 21 | |
| 2 | 2 | |
| 3 | 2 | |
| Lenvatinib | ||
| Without dose reduction | 15 | |
| Dose reduction | 10 | |
| 1 level | 8 | |
| 2 level | 1 | |
| 3 level | 1 | |
| Dose interruption | 7 | |
| Pembrolizumab | ||
| Administering delay | 2 | |
| Dose interruption | 1 | |
| Best overall response | ||
| CR | 1 | |
| PR | 10 | |
| SD | 8 | |
| PD | 6 | |
| Disease progression | ||
| No | 11 | |
| Yes | 14 | |
| Death | ||
| No | 15 | |
| Yes | 10 | |
Abbreviations: BSO, bilateral salpingo‐oophorectomy; CR, complete response; dMMR, mismatch repair deficient; FIGO, International Federation of Gynecology and Obstetrics; LNBx, lymph node biopsy; MSI, microsatellite instability; PR, partial response; pMMR, proficent mismatch repair proficient; PD, progressive disease; RPLD, retroperitoneal lymphadenectomy; SD, stable disease; SNNS, sentinel navigation surgery; TAH, total abdominal hysterectomy.
Results of area under the ROC curve in predictors are listed in Table 2. Although neutrophil counts and NLR score in endometrial LBC prior to initial treatment were superior effective predictors for OR, the most accurate prediction of PFS was NLR score in peripheral blood prior to LEM/PEM (0.722, 95% CI: 0.45–0.99, sensitivity: 57.1%, specificity: 94.4%); the ROC curve is shown in Figure 2a. According to limited to assessment prior to initial treatment, endometrial LBC was considered a more precise predictor of the impact of LEN/PEM than peripheral blood measurements in OR, DC, and progression‐free survival. Neutrophil and lymphocyte counts showed a mild positive correlation between endometrial LBCs prior to initial treatment and peripheral blood prior to LEN/PEM in the scatter diagrams (Figure 2b,c).
TABLE 2.
Area under the ROC Curve in predictors.
| Area under the ROC curve | ||||
|---|---|---|---|---|
| Neutrophil | Lymphocyte | NLR | ||
| Endometrial LBC prior to initial treatment | ||||
| Overall response | 0.707 | 0.533 | 0.673 | |
| Disease control | 0.575 | 0.566 | 0.561 | |
| Progression‐free | 0.548 | 0.647 | 0.635 | |
| Peripheral blood prior to initial treatment | ||||
| Overall response | 0.567 | 0.600 | 0.533 | |
| Disease control | 0.518 | 0.509 | 0.544 | |
| Progression‐free | 0.611 | 0.532 | 0.548 | |
| Peripheral blood prior to LEM/PEM | ||||
| Overall response | 0.700 | 0.643 | 0.540 | |
| Disease control | 0.649 | 0.579 | 0.640 | |
| Progression‐free | 0.659 | 0.667 | 0.722 | |
Note: Bold indicates 0.65 ≤ Area under the ROC curve.
Abbreviations: LBC, liquid‐based cytology; LEM/PEM, lenvatinib and pembrolizumab; NLR, neutrophil to lymphocyte ratio; ROC, receiver operating characteristic.
FIGURE 2.
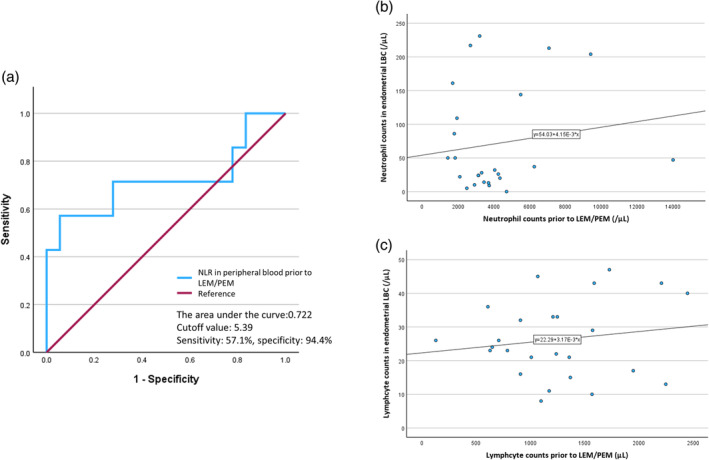
(a) ROC curve of the NLR in peripheral blood prior to LEN/PEM treatment. (b) Scatter diagrams of the neutrophils between endometrial LBC prior to initial treatment and peripheral blood prior to LEN/PEM treatment. (c) Scatter diagrams of the lymphocytes between endometrial LBC prior to initial treatment and peripheral blood prior to LEN/PEM treatment. ROC, receiver operating characteristic; NLR, neutrophil to lymphocyte ratio; LEN/PEM, lenvatinib and pembrolizumab; LBC, Liquid‐based cytology.
The median progression‐free survival and overall survival outcomes of our research were 8.0 and 13.5 months, respectively. The analysis of PFS outcomes in relation to clinicopathological factors was conducted using the log‐rank test (Table 3) and demonstrated using a Kaplan–Meier curve (Figure 3). Regarding the NLR score in peripheral blood prior to LEN/PEM, which was the most accurate predictor in the ROC curves, the lower NLR (NLR <5.39) group had a significantly longer PFS than the higher NLR (5.39 ≤ NLR) group (p = 0.023, median survival: 13.5 vs. 3.0 months). Cox multivariate analysis was used to identify independent effects on PFS. Two models were created for the three factors, to account for confounding of the inflammatory biomarkers. Of the risk factors assessed, NLR score in peripheral blood prior to LEN/PEM (Hazard Ratio = 2.571; 95% CI = 0.857–7.719; p = 0.092) tended to independently correlate with PFS, but were not significant.
TABLE 3.
Univariate and multivariate analysis of progression‐free survival for all patients by clinicopathological factors.
| Factors | n | Progression‐free survival | ||||
|---|---|---|---|---|---|---|
| Multivariate | ||||||
| Univariate | Model 1 | Model 2 | ||||
| p‐Value | Hazard Ratio (95%CI) | p‐Value | Hazard Ratio (95%CI) | p‐Value | ||
| Age | 0.514 | |||||
| <62 years | 15 | |||||
| 62 years ≤ | 10 | |||||
| Performance status | 0.882 | |||||
| 0 | 11 | |||||
| 1≤ | 14 | |||||
| Histological type | 0.160 | 0.180 | 0.176 | |||
| Endometrioid Grade 1–2 | 10 | 1 (ref) | 1 (ref) | |||
| Others | 15 | 2.219 | 2.229 | |||
| FIGO stage | 0.620 | |||||
| I–II | 5 | |||||
| III–IV | 20 | |||||
| Lenvatinib dose reduction | 0.451 | |||||
| None | 15 | |||||
| Present | 10 | |||||
| Number of prior chemotherapy regimen | 0.677 | |||||
| 1 | 21 | |||||
| 2≤ | 4 | |||||
| Recurrent site | 0.199 | 0.216 | 0.295 | |||
| Only local | 3 | 1 (ref) | 1 (ref) | |||
| Distant ± local | 22 | 2.857 | 2.539 | |||
| Inflammatory markers | ||||||
| Endometrial LBC | ||||||
| Neutrophil counts <23.0 HPF | 8 | 0.410 | ||||
| 23.0 HPF ≤ Neutrophil counts | 17 | |||||
| Lymphocyte counts < 27.5 HPF | 6 | 0.955 | ||||
| 27.5 HPF ≤ Lymphocyte counts | 19 | |||||
| NLR <1.17 | 12 | 0.123 | 1 (ref) | 0.170 | ||
| 1.17 ≤ NLR | 13 | 0.464 | ||||
| Peripheral blood prior to initial treatment | ||||||
| Neutrophil counts <5775/μL | 12 | 0.332 | ||||
| 5775/μL ≤ Neutrophil counts | 13 | |||||
| Lymphocyte counts < 1605/μL | 13 | 0.444 | ||||
| 1605/μL ≤ Lymphocyte counts | 12 | |||||
| NLR <2.74 | 17 | 0.962 | ||||
| 2.74 ≤ NLR | 8 | |||||
| Peripheral blood prior to LEM/PEM | ||||||
| Neutrophil counts <3730/μL | 15 | 0.433 | ||||
| 3730/μL < neutrophil counts | 10 | |||||
| Lymphocyte counts < 1470/μL | 17 | 0.118 | ||||
| 1470/μL ≤ Lymphocyte counts | 8 | |||||
| NLR <5.39 | 12 | 0.023 | 1 (ref) | 0.092 | ||
| 5.39 ≤NLR | 13 | 2.571 | ||||
Note: Bold indicates 0.65 ≤ Area under the ROC curve.
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; HPF, high‐power field; LEM/PEM, lenvatinib and pembrolizumab; LBC, Liquid‐based cytology; NLR, neutrophil to lymphocyte ratio.
FIGURE 3.
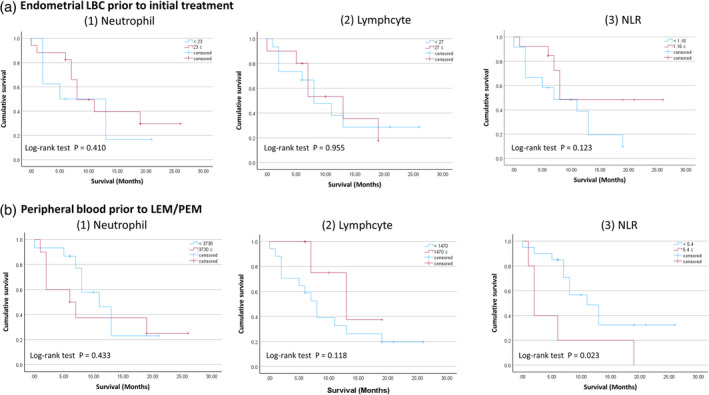
(a) Progression‐free survival in the lower and higher groups for each item in endometrial LBC prior to initial treatment. (b) Progression‐free survival in the lower and higher groups for each item in peripheral blood prior to LEN/PEM treatment. LBC, Liquid‐based cytology; NLR, neutrophil to lymphocyte ratio; LEN/PEM, lenvatinib and pembrolizumab.
Figure 4 contains a representative photograph from only one patient with dMMR, showing highly atypical scattered lymphocytes in the background. The patient's neutrophil and lymphocyte counts in endometrial LBC were 50 HPF and 29 HPF (NLR, 1.72), respectively. The patient's NLR score in peripheral blood prior LEN/PEM was 1.17. The treatment maximum efficacy was PR and LEN/PEM was maintained for 8 months without disease progression.
FIGURE 4.
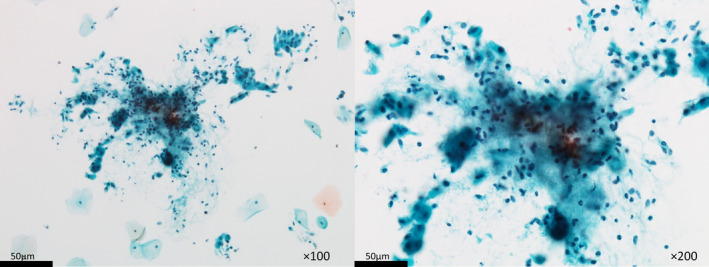
Representative LBC photographs of a patient with dMMR, with highly atypical scattered lymphocytes in the background. The patient's neutrophil and lymphocyte counts in endometrial LBC were 50 HPF and 29 HPF (NLR, 1.72), respectively (Left: ×100, Right: ×200).
DISCUSSION
Our research suggested that measuring inflammatory biomarkers via endometrial LBC prior to initial treatment for endometrial cancer patients failed to predict those who would benefit from treatment with LEN/PEM. On the other hand, peripheral blood NLR score which has traditionally been reported as a useful biomarker, sampled prior to LEN/PEM, could potentially be a useful prognostic predictor for the therapeutic effect of LEN/PEM.
In the TCGA classification system for endometrial cancers, patients with POLEmut and MMR‐d subtypes acquire high levels of neoantigens due to their high mutational burden. 18 It could be that POLEmut and MMR‐d subtypes are potentially more sensitive to ICIs that incorporate anti‐PD1/PDL1 antibodies. 9 Histologically, prominent tumor‐infiltrating lymphocytes (TILs), peri‐tumoral lymphocytes, and tumor giant cells are typical histopathological items of POLEmut and MMR‐d tumors. 19 These findings could potentially result in elevated levels of lymphocytes in endometrial LBC. 10 , 11 We formulated the hypothesis that higher lymphocyte counts in endometrial LBC could serve as a predictor for the efficacy of LEN/PEM treatment due to the potential presence of POLEmut and dMMR, which would benefit from the ICI regimen. However, this hypothesis was not confirmed in the current study. One reason was that the local tumor environment differs when collecting LBCs prior to initial treatment, in contrast to when LEN/PEM is administered following one or more regimens of chemotherapy. In the peripheral blood which represents the systemic environment, neutrophil and lymphocyte counts prior to initial treatment, at the same time as endometrial LBC sampling, were clearly different from those prior to LEN/PEM. Secondly, there was only a single dMMR patient in this cohort, which suggested that this population had a limited impact on ICI. The expected synergistic activation of T‐cells in LEN/PEM was not realized effectively. 17 Third, the importance of LEN in the effectiveness of this treatment regimen was greater than we initially anticipated. 17
Another important outcome of this study suggests that higher lymphocyte levels and lower NLR (NLR <5.39) in peripheral blood prior to LEN/PEM may be candidate predictors of a therapeutic response in LEN/PEM treatment, and therefore be a candidate biomarker in future personalized therapy. The Youden Index which was the method of determining the cutoff value for this study, is commonly used as an indicator to summarize ROC curves, and is used to measure the effectiveness of diagnostic markers, while also enabling the selection of the optimal threshold value for the inflammatory biomarker. 20 Limitations of this study include the relatively small sample size, while the role of endometrial LBC in LEN/PEM has already been shown to be limited. Secondly, the duration of the observation period was limited, which may be attributed to the inclusion of patients with poor prognosis who had undergone multiple treatment regimens.
In conclusion, relationships between inflammatory biomarkers based on lymphocytes or NLR in endometrial LBC prior to initial treatment and the efficacy of LEN/PEM have not been conclusively established.
AUTHOR CONTRIBUTIONS
Shintaro Yanazume: Conceptualization; data curation; formal analysis; writing – original draft. Yusuke Kobayashi: Data curation; writing – review and editing. Yukari Kirita: Conceptualization; data curation; investigation. Ikumi Kitazono: Formal analysis; supervision; writing – review and editing. Ayumi Kozai: Data curation; formal analysis. Chikako Nagata: Data curation; formal analysis; writing – review and editing. Akihide Tanimoto: Data curation; formal analysis; supervision; writing – review and editing. Hiroaki Kobayashi: Conceptualization; supervision; writing – review and editing.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest.
Yanazume S, Kobayashi Y, Kirita Y, Kitazono I, Nagata C, Kozai A, et al. A potential inflammatory biomarker for advanced endometrial cancer treated with lenvatinib plus pembrolizumab. J Obstet Gynaecol Res. 2025;51(1):e16182. 10.1111/jog.16182
Contributor Information
Akihide Tanimoto, Email: akit09@m3.kufm.kagoshima-u.ac.jp.
Hiroaki Kobayashi, Email: hirokoba@m2.kufm.kagoshima-u.ac.jp.
DATA AVAILABILITY STATEMENT
The data for this study are shown in tables and figures; no other datasets were generated or analyzed during the current study.
REFERENCES
- 1. Yamagami W, Nagase S, Takahashi F, Ino K, Hachisuga T, Aoki D, et al. Clinical statistics of gynecologic cancers in Japan. J Gynecol Oncol. 2017;28(2):e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Makker V, Colombo N, Casado Herraez A, Santin AD, Colomba E, Miller DS, et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386(5):437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus‐Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair‐deficient cancer: results from the phase II KEYNOTE‐158 study. J Clin Oncol. 2020;38(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ott PA, Bang YJ, Berton‐Rigaud D, Elez E, Pishvaian MJ, Rugo HS, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1‐positive endometrial cancer: results from the KEYNOTE‐028 study. J Clin Oncol. 2017;35(22):2535–2541. [DOI] [PubMed] [Google Scholar]
- 5. Hao Z, Wang P. Lenvatinib in management of solid tumors. Oncologist. 2020;25(2):e302–e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roy‐Chowdhuri S, Pisapia P, Salto‐Tellez M, Savic S, Nacchio M, de Biase D, et al. Invited review‐next‐generation sequencing: a modern tool in cytopathology. Virchows Arch. 2019;475(1):3–11. [DOI] [PubMed] [Google Scholar]
- 7. Akahane T, Kitazono I, Yanazume S, Kamio M, Togami S, Sakamoto I, et al. Next‐generation sequencing analysis of endometrial screening liquid‐based cytology specimens: a comparative study to tissue specimens. BMC Med Genet. 2020;13(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akahane T, Kitazono I, Kobayashi Y, Nishida‐Kirita Y, Yamaguchi T, Yanazume S, et al. Direct next‐generation sequencing analysis using endometrial liquid‐based cytology specimens for rapid cancer genomic profiling. Diagn Cytopathol. 2021;49(9):1078–1085. [DOI] [PubMed] [Google Scholar]
- 9. Piulats JM, Guerra E, Gil‐Martin M, Roman‐Canal B, Gatius S, Sanz‐Pamplona R, et al. Molecular approaches for classifying endometrial carcinoma. Gynecol Oncol. 2017;145(1):200–207. [DOI] [PubMed] [Google Scholar]
- 10. Yanazume S, Kirita Y, Kobayashi Y, Kitazono I, Akahane T, Mizuno M, et al. Can endometrial cytology identify patients who would benefit from immunotherapy? Acta Cytol. 2024;68(2):128–136. [DOI] [PubMed] [Google Scholar]
- 11. Yanazume S, Iwakiri K, Kobayashi Y, Kitazono I, Akahane T, Mizuno M, et al. Cytopathological features associated with POLE mutation in endometrial cancer. Cytopathology. 2023;34(3):211–218. [DOI] [PubMed] [Google Scholar]
- 12. Kitazono I, Akahane T, Yokoyama S, Kobayashi Y, Togami S, Yanazume S, et al. “Surface epithelial slackening” pattern in endometrioid carcinoma: a morphological feature for differentiating the POLE mutation‐subtype from the no specific molecular profile subtype. Pathol Res Pract. 2023;247:154563. [DOI] [PubMed] [Google Scholar]
- 13. Nishio S, Murotani K, Yamagami W, Suzuki S, Nakai H, Kato K, et al. Pretreatment systemic inflammatory markers predict survival in endometrial cancer: a Japanese gynecologic oncology group 2043 exploratory data analysis. Gynecol Oncol. 2024;181:46–53. [DOI] [PubMed] [Google Scholar]
- 14. Njoku K, Ramchander NC, Wan YL, Barr CE, Crosbie EJ. Pre‐treatment inflammatory parameters predict survival from endometrial cancer: a prospective database analysis. Gynecol Oncol. 2022;164(1):146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pergialiotis V, Oikonomou M, Damaskou V, Kalantzis D, Chrelias C, Tsantes AE, et al. Platelet to lymphocyte and neutrophil to lymphocyte ratio as predictive indices of endometrial carcinoma: findings from a retrospective series of patients and meta‐analysis. J Gynecol Obstet Hum Reprod. 2018;47(10):511–516. [DOI] [PubMed] [Google Scholar]
- 16. Norimatsu Y, Yanoh K, Hirai Y, Kurokawa T, Kobayashi TK, Fulciniti F. A diagnostic approach to endometrial cytology by means of liquid‐based preparations. Acta Cytol. 2020;64(3):195–207. [DOI] [PubMed] [Google Scholar]
- 17. Jeffrey How SNW, Sims TT, Lito K, Celestino J, Rangel KM, Peng W, et al. Uncovering mechanisms of response of pembrolizumab and lenvatinib for the treatment of platinum‐resistant high grade serous ovarian cancer. J Clin Oncol. 2023;41, Number 16_suppl:TPS5619. [Google Scholar]
- 18. Howitt BE, Shukla SA, Sholl LM, Ritterhouse LL, Watkins JC, Rodig S, et al. Association of polymerase e‐mutated and microsatellite‐instable endometrial cancers with neoantigen load, number of tumor‐infiltrating lymphocytes, and expression of PD‐1 and PD‐L1. JAMA Oncol. 2015;1(9):1319–1323. [DOI] [PubMed] [Google Scholar]
- 19. McAlpine J, Leon‐Castillo A, Bosse T. The rise of a novel classification system for endometrial carcinoma; integration of molecular subclasses. J Pathol. 2018;244(5):538–549. [DOI] [PubMed] [Google Scholar]
- 20. Fluss R, Faraggi D, Reiser B. Estimation of the Youden index and its associated cutoff point. Biom J. 2005;47(4):458–472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this study are shown in tables and figures; no other datasets were generated or analyzed during the current study.


