Abstract
Objective
In this cross‐sectional study, we aim to investigate the interactions between obesity, siesta behavior, and the genetic propensity for siesta in a Mediterranean population, in whom siesta is deeply rooted.
Methods
We applied a previously generated Siesta‐Polygenic Score (PGS) in the ONTIME study (n = 1278). Siesta and other Mediterranean lifestyle behaviors were characterized using questionnaires. We further determined obesity grade. Secondarily, we measured weight loss during treatment as well as long‐term weight‐loss maintenance. Logistic regression analyses were performed to address our aim.
Results
A total of 42.4% of the population usually took siesta. A significant genetic influence on siesta propensity was found, with a higher genetic predisposition linked to taking siesta more frequently (odds ratio [OR] = 1.17, 95% CI: 1.03–1.32; p = 0.015). Participants with a higher genetic propensity for siesta showed poorer dietary habits (p < 0.05). Among individuals with a high genetic propensity for siesta, we found that those who usually take siesta have lower odds of having obesity (p = 0.038) compared with those who do not. Similarly, in exploratory analysis, among individuals with a high genetic propensity for siesta, we found that those who usually take siesta have higher odds of weight‐loss success (p = 0.007) compared with those who do not.
Conclusions
Considering the ongoing debate regarding whether siesta is beneficial or detrimental, our findings suggest that individual genetic predisposition to siesta might influence the association between siesta and health.
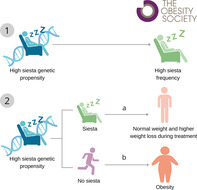
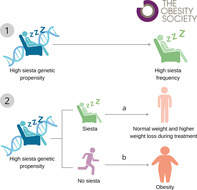
Study Importance.
What is already known?
Siesta is a deeply rooted habit in the Mediterranean culture that occurs in a postprandial state.
A siesta habit might be a marker of related diseases such as obesity.
What does this study add?
Siesta genetics may interact with siesta behavior to influence the deleterious relationship between siesta and obesity.
This study contributes to a deeper understanding of the genetic basis of siesta and its associations with obesity in Mediterranean populations.
How might these results change the direction of research or the focus of clinical practice?
Further research is necessary to elucidate the mechanisms underlying the observed potential role of genetics in the associations between siesta and obesity.
Our results shed light on targeted and personalized interventions aimed at promoting healthier sleep and lifestyle habits in diverse populations.
INTRODUCTION
Siesta is a short daytime sleep episode that generally occurs in a postprandial state [1, 2]. Siesta is heritable [3], evolutionarily conserved across species ranging from flies to mammals [4, 5], and is deeply rooted in the Mediterranean culture. However, many other cultures now take naps for reasons other than climatic reasons, such as to combat fatigue and sleepiness from excessive working hours [6, 7]. In Spain, siesta is a social norm that is deeply embedded in the culture; more than 40% of Spaniards habitually take 1‐h siestas, generally at post‐lunch times during a break from work (between 3 p.m. and 5 p.m.) [8].
We, among others, have speculated about a siesta habit as a marker of related diseases such as obesity [1, 9, 10, 11, 12]. We have previously found, in a Mediterranean population from Southeast Spain (i.e., the Obesity, Nutrigenetics, Timing, and Mediterranean [ONTIME] study) [13], that long siestas (>30 min) were associated with higher values of body mass index (BMI), waist circumference, fasting glucose, systolic blood pressure (SBP), and diastolic blood pressure (DBP), along with a higher prevalence of metabolic syndrome (MetS) [13]. In contrast, the probability of having elevated SBP was lower among individuals with short siesta duration (≤30 min) than in those who do not take siesta [13]. In addition, a beneficial effect of siesta on metabolic health has been suggested in countries where siesta is a common practice, but it might be detrimental in those with no regular siesta habit [11].
Genetic factors have been shown to contribute to siesta [1, 3, 14, 15]. Our previous study involving twins found that the heritability of siesta was similar to or even higher than other sleep characteristics such as nighttime sleep duration and timing [3]. In addition, we previously studied the genetic architecture of daytime napping in a large population (i.e., the UK Biobank; n = 452,633 with 44% who nap) and discovered 123 genetic variants associated with napping frequency, and we developed a napping propensity genome‐wide polygenic score (PGS) herein referred to as Siesta‐PGS [1]. We also showed that daytime napping was associated with both obesity and central adiposity, with Mendelian randomization showing a causal effect of napping on increasing waist circumference [1] in this older (aged 40–69 years) UK population. However, this population is from the UK, where daytime napping (or siesta) is not embedded in its culture. Also, no study, to our knowledge, has yet explored whether napping genetics associate with other aspects of napping behavior. In addition, previous literature has shown that genetic predisposition can influence behavior, and engaging in or abstaining from such behaviors based on individual genetic makeup can significantly impact health outcomes [16]. A conflict between individual internal tendency to take siesta and actual siesta behavior might influence adverse outcomes.
Based on these results, we consider that it is important to try to replicate this Siesta‐PGS in a younger population and in a country where siesta is part of the culture, as is the case in a Mediterranean population from Southeast Spain. Furthermore, we hypothesize that genetic propensity for siesta is associated with more frequent siesta and other Mediterranean lifestyle behaviors such as summer siesta or higher energy intake. We also expect that, among individuals with a high genetic propensity for siesta, not taking siesta is associated with obesity, but that this is not the case for those with a high genetic propensity for siesta who usually take siesta.
The primary aims of this study are as follows: 1) to determine whether the previous Siesta‐PGS discovered in the UK Biobank is generalizable to a highly relevant population in whom siesta is culturally embedded, such as our Mediterranean population (i.e., the ONTIME study) and test for associations between Siesta‐PGS and siesta behavior (frequency in times per week); and 2) to test for potential associations between this Siesta‐PGS and obesity traits. We further tested the interaction of Siesta‐PGS with siesta frequency for obesity risk.
In exploratory analyses, we aimed to test the associations between Siesta‐PGS and other siesta characteristics such as siesta duration, seasonality, place of siesta (sofa/bed), choosing siesta if you could, and feeling hungry after siesta; blood pressure, weight loss during the dietary program, and long‐term weight‐loss maintenance (WLM); and other Mediterranean lifestyle behaviors (dietary intake, physical activity, nighttime sleep, etc.).
METHODS
Participants
For this observational cross‐sectional study, we collected genetic data from 1278 participants enrolled in the ONTIME study (ClinicalTrials.gov: NCT02829619) who were recruited from six weight‐loss clinics in Spain. All participants were adults and generally had overweight or obesity. Sample size was selected based on a previous study [17]. Participant exclusion from the study has been previously described [13]. All participants provided written informed consent before entering the study. The study procedures were approved by the Committee of Research Ethics of the University of Murcia (identifier: 632/2017) before recruitment, and the protocol followed good clinical practice. A flowchart of participants is presented in Figure 1. For further information, see the Extended Methods section in online Supporting Information.
FIGURE 1.
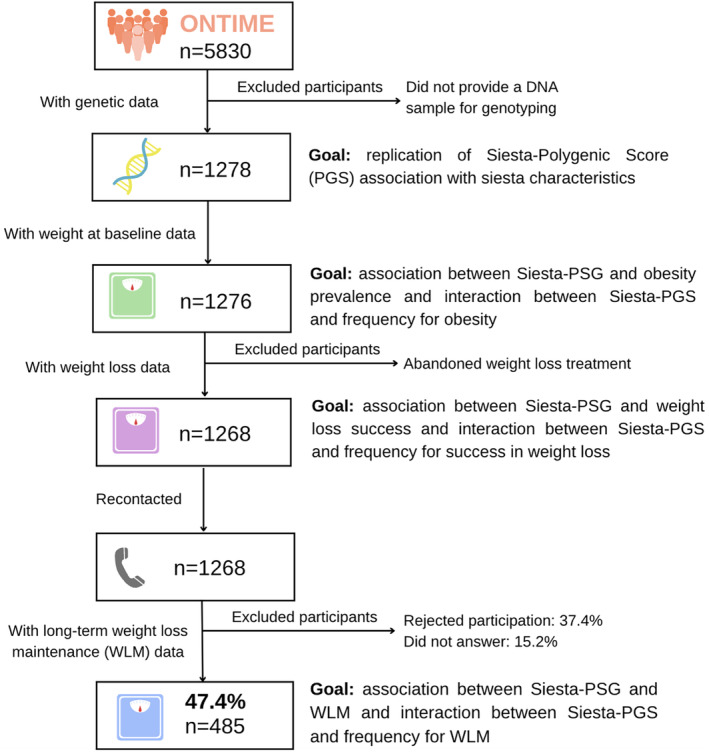
Participant flowchart. [Color figure can be viewed at wileyonlinelibrary.com]
General characteristics, obesity, and metabolic trait variables
Age, sex, and other general characteristics were recorded at the clinical center.
BMI was calculated as weight in kilograms divided by height in meters squared. Having obesity was categorized as “yes” when BMI was ≥30 kg/m2 and “no” when BMI was <30 kg/m2. As previously described [13], total body fat was measured, and high body fat percentage was considered >37.1%, according to the median. Central adiposity was assessed as previously described [13]. To assess metabolic health, we determined fasting plasma concentrations of glucose, cholesterol, triglycerides, and lipoproteins. MetS score was also calculated as previously described [13]. Further MetS details are provided in the Extended Methods section in online Supporting Information.
Weekly weight loss was evaluated during the mean (SD) ∼16 (9)‐week behavioral‐based dietary program for weight loss. The characteristics of the weight‐loss program have been described elsewhere in detail [18]; we calculated the percentage of weight loss as total weight loss in kilograms with respect to the weight at baseline (weight at the first visit of the weight‐loss treatment). Next, we divided participants into a group of those with success in weight loss (≥5% weight loss) and a group of those with non‐success (<5% weight loss). The average kilograms of body weight lost per week during the participants' intervention period was considered as rate or speed of weight loss.
Those participants who attended the clinical center to lose weight were recontacted to assess their current weight in the follow‐up, which was measured in similar conditions to baseline and final body weight at the end of the treatment. WLM percentage (WLM%) was calculated as follows: (follow‐up weight − end‐of‐treatment weight) × (100/end‐of‐treatment weight). WLM was categorized as “yes” when WLM% was ≤0 and “no” when WLM% was >0.
Siesta genetics
Genotyping, imputation, and quality control were performed in 1278 ONTIME participants as previously described [17]. A genome‐wide PGS for siesta was calculated for each participant using Polygenic Risk Score of Continuous Shrinkage (PRS‐CS) [19], using our previously developed daytime napping PGS in the UK Biobank population [1]. The Siesta‐PGS was standardized with a mean of 0 and an SD of 1. For further explanation of Siesta‐PGS, see the Extended Methods section in online Supporting Information.
Mediterranean lifestyle behaviors
Every individual included in the study had the opportunity to take siesta daily. Daytime napping was characterized using the siesta characteristics questionnaire that was administered to the participants at baseline (Table S1). The siesta questionnaire evaluated weekly siesta frequency, duration in minutes (to categorize siestas as short, i.e., ≤30 min, and long, i.e., >30 min, based on previous studies) [13, 20, 21, 22], and other dimensions of siesta behavior as previously described [13].
We also evaluated other sleep characteristics, including weekly duration of nighttime sleep, which was computed as the interval between its onset and offset. None of the participants was a shift worker. We used the validated 19‐item scale Morningness‐Eveningness Questionnaire (MEQ) scale to determine participants' morning or evening preference [23]. Both nighttime sleep and MEQ were categorized according to the median because the median value reflects the typical sleep/chronotype distribution for this specific group and makes the analyses more relevant to the specific population being studied. Furthermore, using the median allows for equal comparison groups and, thereby, robust statistical analysis and easier interpretation.
We performed a single 24‐h dietary recall to evaluate participants' dietary intake at baseline. An extended explanation of the 24‐h dietary recall is provided in the Extended Methods section in online Supporting Information. Participants also reported their typical mealtimes on weekdays and weekends. Evaluation of energy intake and macronutrient composition was performed using the GRUNUMUR nutritional evaluation software program, using Spanish food composition tables [24]. A Mediterranean dietary score ranging from 0 to 9 was also calculated as previously described [25]. Food intake variables were categorized as high or low according to the median.
Regular physical activity over the past 7 days was evaluated using the International Physical Activity Questionnaire (IPAQ), and a total activity score was calculated [26]. We divided participants into low (<2012 metabolic equivalents [Met]‐min/week) and high physical activity level groups (≥2012 Met‐min/week) according to the median.
Statistical analyses
Associations
The primary outcomes in the current study were the association between Siesta‐PGS and siesta behavior and the association between Siesta‐PGS and obesity.
For these outcomes, logistic regression analyses were performed between Siesta‐PGS and categorical variables (i.e., siesta frequency and obesity). We divided Siesta‐PGS in tertiles, and ANOVA was used to determine differences in siesta frequency. In addition, χ2 analysis was performed to determine significant differences between a high and low genetic propensity for siesta (divided by median) and siesta frequency categories (i.e., always, sometimes/usually, and never/rarely). We considered these siesta frequency categories following the same approach that has been previously published in the UK Biobank [1]. Never/rarely included frequency of siesta of never or once or twice per week; sometimes/usually included frequency of siesta three to six times per week; and always included frequency of siesta every day, i.e., seven times per week.
Interactions
For these primary outcomes, logistic regression models were used to test for the interaction between Siesta‐PGS and siesta frequency for obesity. A likelihood ratio test was used to compare the model that includes the interaction with the model without the interaction. Siesta‐PGS cutoffs were established when statistical significance appeared.
Within the exploratory outcomes, logistic regression analyses were used (see further details in the Extended Methods section in online Supporting Information). Given the exploratory nature of these analyses, multiple comparison corrections were not applied.
Primary and exploratory outcomes classification is shown in Table S2.
Sex, age, and 10 principal components of ancestry were included as covariates in all analyses. For the primary outcomes, we further included nighttime sleep duration as a covariate in primary sensitivity analyses and added diet (daily energy intake in kilocalories) and physical activity (total activity score in Met‐min per week) in secondary sensitivity analyses. Regression analyses were performed using Stata (StataCorp LLC). A Hosmer–Lemeshow test in Stata did not show lack of fit for any regression model (p > 0.05). Descriptive data, χ2 tests, and ANOVA were performed using SPSS Statistics (IBM Corp.). P < 0.05 was considered statistically significant for all analyses. We chose not to impute the missing values given the nature of our study and to avoid bias of imputation methods. For all analyses, we used the available data, and missing values were not included.
RESULTS
The general characteristics of the population divided by siesta frequency (never/rarely, sometimes/usually, and always) are presented in Table 1. Those participants with a higher siesta frequency were significantly older than those who never/rarely took siesta (p < 0.05). A total of 81% of the population was female, and 53% had obesity. Participants had a mean BMI of 31.2 kg/m2 at baseline and lost an average of 7.97 kg (9.26% of baseline body weight) during the weight‐loss intervention. Success in long‐term WLM was seen in 24.1% of the population.
TABLE 1.
Descriptive data of general characteristics and obesity traits in the population studied.
| Total population | Never/rarely siesta frequency | Sometimes/usually siesta frequency | Always siesta frequency | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | ||
| Age | 1276 | 40.6 (13.12) | 813 | 39.17 (12.72)a | 241 | 41.17 (12.51)a | 160 | 46.58 (14.01)b | <0.001 |
| Sex (% woman) | 1028 | 80.60 | 671 | 82.5 | 184 | 76.3 | 125 | 78.1 | 0.068 |
| Race (% White) | 1272 | 99.60 | 809 | 99.5 | 240 | 99.6 | 160 | 100.0 | 0.798 |
| Study level (% university studies) | 338 | 66.90 | 209 | 67.2 | 75 | 69.4 | 35 | 68.4 | 0.721 |
| Weight (kg) | 1276 | 84.77 (17.60) | 813 | 84.55 (17.54) | 241 | 85.16 (17.57) | 160 | 85.41 (18.84) | 0.797 |
| Height (m) | 1276 | 1.64 (0.08) | 813 | 1.64 (0.08) | 241 | 1.65 (0.08) | 160 | 1.64 (0.08) | 0.616 |
| BMI (kg/m2) | 1276 | 31.24 (5.49) | 813 | 31.17 (5.60) | 241 | 31.21 (5.26) | 160 | 31.56 (5.44) | 0.711 |
| Obesity (% yes) | 681 | 53.40 | 424 | 52.2 | 129 | 53.5 | 92 | 57.5 | 0.460 |
| Waist circumference (cm) | 1274 | 102.61 (14.64) | 811 | 102.24 (14.57) | 241 | 102.66 (14.77) | 160 | 104.71 (15.19) | 0.152 |
| Total weight loss (kg) | 1275 | 7.97 (5.23) | 813 | 7.75 (5.22) | 240 | 8.43 (5.37) | 160 | 8.08 (5.01) | 0.196 |
| Total weight loss (% of baseline body weight) | 1275 | 9.26 (5.33) | 813 | 9.05 (5.43) | 240 | 9.73 (5.46) | 160 | 9.19 (4.50) | 0.221 |
| Weight loss (% of success) | 992 | 77.8 | 616 | 75.8a | 195 | 81.3a | 133 | 83.1a | 0.044 |
| Rate of weight loss (kg/wk) | 1275 | 0.59 (0.43) | 813 | 0.59 (0.46) | 240 | 0.62 (0.38) | 160 | 0.56 (0.35) | 0.409 |
| Long‐term WLM (% of body weight) | 485 | 9.05 (13.57) | 324 | 9.21 (14.30) | 85 | 8.86 (13.23) | 57 | 7.55 (10.14) | 0.699 |
| Long‐term WLM (% of success) | 117 | 24.1 | 84 | 25.9 | 16 | 18.8 | 14 | 24.6 | 0.399 |
| MetS score | 1116 | 2.11 (1.17) | 704 | 2.08 (1.19) | 212 | 2.13 (1.13) | 146 | 2.25 (1.06) | 0.232 |
| SBP (mm Hg) | 1118 | 117.14 (14.92) | 708 | 116.30 (14.92) | 209 | 118.55 (13.78) | 148 | 118.62 (16.07) | 0.062 |
| DBP (mm Hg) | 1118 | 71.27 (10.17) | 708 | 71.84 (10.37)a | 209 | 74.79 (9.27)b | 148 | 73.35 (9.75)a,b | <0.001 |
| Glucose (mg/dL) | 1258 | 86.54 (13.26) | 803 | 86.31 (13.43) | 238 | 86.12 (11.34) | 158 | 88.02 (14.37) | 0.291 |
| Total cholesterol (mg/dL) | 1272 | 192.38 (37.77) | 812 | 191.97 (38.98) | 241 | 193.05 (34.04) | 160 | 191.78 (36.24) | 0.918 |
| LDL cholesterol (mg/dL) | 1270 | 114.21 (32.39) | 811 | 113.80 (32.78) | 241 | 114.77 (30.98) | 160 | 114.59 (31.83) | 0.900 |
| HDL cholesterol (mg/dL) | 1272 | 57.74 (15.53) | 812 | 57.89 (15.71) | 241 | 57.46 (15.52) | 160 | 56.30 (15.06) | 0.492 |
| Triglycerides (mg/dL) | 1271 | 101.59 (51.91) | 811 | 100.74 (53.86) | 241 | 104.04 (50.03) | 160 | 104.17 (45.74) | 0.574 |
Note: Values are mean (SD) for numerical variables and percentage for categorical variables for each characteristic in the total sample and in siesta frequency categories. P value from ANOVA for numerical variables and χ2 test for categorical variables. Bold values indicate statistically significant results (p < 0.05); italicized values indicate those with borderline significance (p < 0.1). Superscript letters indicate differences among groups according to Bonferroni post hoc. Given the exploratory nature of these analyses, multiple comparison corrections were not applied.
Abbreviations: DBP, diastolic blood pressure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MetS, metabolic syndrome; SBP, systolic blood pressure; WLM, weight‐loss maintenance.
In this Mediterranean population, 42% of the participants usually took siesta, with an average frequency of siesta of four times per week and the highest frequency among those who took siesta every day (7 days/week; 13.2% of the total population; Table 2). The main cause of siesta was to relax (43%), followed by tiredness (39%), need (11%), and to disconnect from work (6%).
TABLE 2.
Descriptive data of Mediterranean lifestyle characteristics in the population studied.
| Total population | Never/rarely siesta frequency | Sometimes/usually siesta frequency | Always siesta frequency | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | ||
| Siesta subjective characteristics | |||||||||
| Siesta frequency (d/wk) | 568 | 4.24 (2.15) | 167 | 1.65 (0.48)a | 241 | 4.20 (1.02)b | 160 | 7.00 (0.00)c | <0.001 |
| Do you usually take siesta during the week? (yes, %) | 507 | 42.40 | 82 | 10.6a | 241 | 100b | 160 | 100b | <0.001 |
| Siesta duration (total week, min) | 479 | 39.97 (26.11) | 88 | 42.36 (24.65) | 232 | 38.60 (24.59) | 156 | 40.55 (29.17) | 0.487 |
| Long siesta (% of population) | 201 | 16.80 | 37 | 4.6a | 94 | 40.5b | 68 | 43.6b | <0.001 |
| Causes or motives of siesta (%) | 0.005 | ||||||||
| Tiredness | 170 | 39.4 | 68 | 40.2a | 64 | 41.3a | 33 | 37.5a | |
| Relax | 187 | 43.3 | 84 | 49.7a | 59 | 38.1a | 33 | 37.5a | |
| Disconnect from work | 27 | 6.3 | 9 | 5.3a | 13 | 8.4a | 4 | 4.5a | |
| Need | 48 | 11.1 | 8 | 4.7a | 19 | 12.3b | 18 | 20.5b | |
| If you could choose between taking siesta or not? (yes, %) | 383 | 59.2 | 154 | 40.6a | 134 | 84.8b | 77 | 86.5b | <0.001 |
| If you could choose the duration, how long would it be? (%) | 0.104 | ||||||||
| <30 min | 153 | 39.4 | 71 | 44.7 | 57 | 42.9 | 22 | 28.2 | |
| 30–60 min | 190 | 49 | 74 | 46.5 | 62 | 46.6 | 43 | 55.1 | |
| >60 min | 45 | 11.6 | 14 | 8.8 | 14 | 10.5 | 13 | 16.7 | |
| If you could not take siesta, how would you feel? (%) | <0.001 | ||||||||
| Irritated | 35 | 7.8 | 8 | 4.3a | 12 | 7.5a,b | 11 | 12.8b | |
| Fatigue | 200 | 44.3 | 65 | 35.1a | 86 | 53.8b | 39 | 45.3a,b | |
| No effect | 216 | 47.9 | 112 | 60.5a | 62 | 38.8b | 36 | 41.9b | |
| Associated feelings with siesta | |||||||||
| How do you feel after a siesta if it is short (≤30 min; bad, %) | 77 | 16.2 | 27 | 13.6 | 27 | 16.7 | 19 | 20.7 | 0.302 |
| How do you feel after a siesta if it is long (>30 min; bad, %) | 207 | 46 | 89 | 49.7 | 78 | 48.8 | 32 | 34.8 | 0.046 |
| When you wake up from a siesta, are you hungry? (yes, %) | 187 | 40.4 | 69 | 37.5 | 64 | 39 | 44 | 46.8 | 0.308 |
| What do you feel like eating after a siesta? (%) | 0.032 | ||||||||
| Sweet | 113 | 69.8 | 38 | 66.7a | 43 | 81.1a | 28 | 63.6a | |
| Salty | 11 | 6.8 | 3 | 5.3a | 5 | 9.4a | 1 | 2.3a | |
| Indifferent | 38 | 23.5 | 16 | 28.1a | 5 | 9.4b | 15 | 34.1a | |
| Bed/sofa as place for siesta (%) | 67/273 | 19.7/80.3 | 24/95 | 20.2a,b/79.8a,b | 16/108 | 12.9b/87.1b | 23/58 | 28.4a/71.6a | 0.023 |
| Seasonal siesta (only summer, %) | 104 | 20.2 | 61 | 21.3 | 30 | 21.3 | 9 | 12.2 | 0.192 |
| Nighttime sleep characteristics | |||||||||
| Nighttime sleep onset (hh:mm) | 1203 | 23:54 (00:52) | 786 | 23:51 (00:51) | 221 | 23:58 (00:54) | 154 | 00:00 (00:52) | 0.096 |
| Nighttime sleep offset (hh:mm) | 1200 | 07:28 (00:57) | 785 | 07:27 (00:57) | 219 | 07:30 (00:58) | 154 | 07:36 (00:58) | 0.200 |
| Nighttime sleep duration (hh:mm) | 1200 | 07:34 (01:01) | 785 | 07:35 (01:01) | 219 | 07:32 (01:03) | 154 | 07:36 (00:58) | 0.798 |
| Dietary intake | |||||||||
| 24‐h energy intake (kcal/d) | 1033 | 1973.89 (716.28) | 669 | 1951.05 (731.63) | 203 | 2016.00 (699.13) | 124 | 2020.08 (661.69) | 0.388 |
| Carbohydrate intake (kcal/d) | 961 | 805.83 (356.76) | 622 | 789.46 (351.80) | 188 | 825.66 (385.34) | 119 | 845.03 (344.15) | 0.195 |
| Fat intake (kcal/d) | 957 | 861.46 (401.23) | 620 | 853.93 (411.42) | 186 | 890.64 (394.22) | 119 | 847.69 (358.20) | 0.513 |
| Protein intake (kcal/d) | 961 | 336.80 (132.10) | 622 | 335.66 (136.60) | 188 | 337.78 (120.07) | 119 | 339.46 (119.43) | 0.959 |
| Mediterranean diet score | 989 | 3.43 (1.52) | 642 | 3.40 (1.51) | 195 | 3.48 (1.62) | 116 | 3.53 (1.51) | 0.592 |
| Physical activity | |||||||||
| Physical activity level (Met‐min/wk) | 1137 | 3673.13 (6309.18) | 742 | 3683.25 (6031.33) | 211 | 3543.21 (8258.26) | 148 | 3942.87 (4643.17) | 0.841 |
Note: Values are mean (SD) for numerical variables and percentage for categorical variables for each characteristic in the total sample and in the siesta frequency categories. P value from ANOVA for numerical variables and χ2 test for categorical variables. Bold values indicate statistically significant results (p < 0.05); italicized values indicate those with borderline significance (p < 0.1). Superscript letters indicate differences among groups according to Bonferroni post hoc. The Mediterranean diet score ranges from 0 to 9. Given the exploratory nature of these analyses, multiple comparison corrections were not applied.
Abbreviation: Met, metabolic equivalents.
Association of siesta genetics and siesta frequency
Our Siesta‐PGS was associated with a higher frequency of siesta per week, indicating that those individuals who had a greater genetic propensity for siesta took siesta more frequently (Figure 2). Indeed, logistic regression analyses showed that an increase of 1 SD in Siesta‐PGS was associated with higher odds of taking siesta (odds ratio [OR] = 1.17, 95% confidence interval [CI]: 1.03–1.32; p = 0.015). Interestingly, in those individuals with a higher Siesta‐PGS (≥mean), the proportion of individuals who took siesta was significantly higher (22% sometimes/usually and 15% always) than in those with lower genetic scores (<mean; 18% sometimes/usually and 12% always; χ2 p = 0.029; Figure 2A). Furthermore, there were significant differences in the frequency of siesta among the three tertiles of Siesta‐PGS, with a higher frequency in the highest Siesta‐PGS tertile (p = 0.044; Figure 2B). Further exploratory analyses showed that no significant associations were found between Siesta‐PGS and siesta duration (p = 0.494) or other characteristics of siesta, such as the place for siesta (p = 0.442), desire to always take siesta if they could (p = 0.120), or being hungry after siesta (p = 0.894; all data shown in Table S3). Figure S1 shows the distribution of Siesta‐PGS.
FIGURE 2.
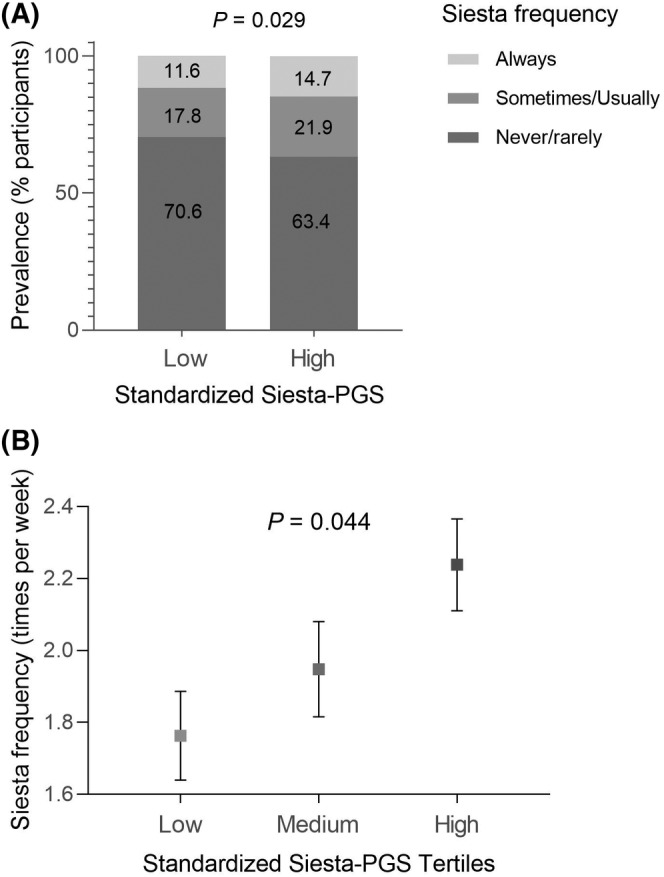
(A) Differences in the prevalence of siesta (always, sometimes/usually, and never/rarely) in participants with low genetic propensity for siesta (siesta‐polygenic score [PGS] ≤ 0) and high genetic propensity for siesta (Siesta‐PGS > 0). (B) Average siesta frequency (times per week) in each Siesta‐PGS tertile (standardized). Error bars represent SEM.
Genetic propensity for siesta and obesity
As a primary outcome, no significant association was found between Siesta‐PGS and obesity (OR = 0.98, 95% CI: 0.87–1.11 kg/m2; p = 0.771).
Obesity distribution in each Siesta‐PGS decile and in each siesta frequency group (0–7 times per week) is presented in Figure S2A,B. As an exploratory analysis, we further selected only those participants who were aged ≥40 years to compare them with the UK Biobank population (aged 40–69 years), and no significant association was found between Siesta‐PGS and BMI (β = −0.110, 95% CI: −0.531 to 0.311; p = 0.609).
Furthermore, exploratory analyses showed no significant associations between Siesta‐PGS and body fat percentage or central adiposity (measured as waist circumference and waist‐hip ratio; all p > 0.05). Based on our previous Mendelian randomization findings of a causal effect of more frequent daytime napping on higher DBP and SBP [1], we also secondarily studied the association between genetic propensity for siesta and SBP and DBP. However, the association was not significant (p = 0.699 and p = 0.689, respectively), nor was the association between siesta frequency and these variables (all p > 0.05) in our population.
As a secondary outcome, we studied the associations between Siesta‐PGS and success in weight loss (≥5% weight loss) during the dietary treatment and between Siesta‐PGS and long‐term WLM (yes/no). Results showed no significant associations in both cases (p = 0.403 and p = 0.943, respectively). The distribution of success in weight loss in each Siesta‐PGS decile is presented in Figure S2C and in each siesta frequency group (0–7 times per week) in Figure S2D.
In addition to sex, age, and 10 principal components of ancestry, we performed further sensitivity analyses using nighttime sleep duration as a covariate in primary analyses, but significance remained present or absent, as was the case without adjusting for nighttime sleep, in all association analyses (p = 0.026 for siesta frequency, p = 0.938 for obesity, p = 0.406 for success in weight loss, and p = 0.967 for WLM [yes/no]). We further adjusted for diet (daily energy intake) and physical activity (Met‐min per week), and significance was lost for siesta frequency (p = 0.473) and remained nonsignificant for obesity (p = 0.270) and for WLM (yes/no; p = 0.631; Table S3A). Interestingly, when adding diet and physical activity to the model, a significant association was found between a higher Siesta‐PGS and less probability of success in weight loss (p = 0.048). These new results are shown in Table S3A.
We also explored associations (logistic regression) of siesta behavior (siesta frequency) with obesity, success in weight loss, and WLM. None of these associations was significant when adjusting for sex, age, and 10 principal components of ancestry or when further adjusting for nighttime sleep. Nevertheless, when adding diet and physical activity to the model, a significant association was found between a higher siesta frequency and a higher probability of success in weight loss (p = 0.026) as previously shown with ANOVA (Table 1). These results are shown in Table S3B.
Interaction between genetic propensity for siesta and siesta frequency for obesity traits
Our primary analyses results showed that there was a significant interaction between Siesta‐PGS and frequency of siesta regarding the presence of obesity (obesity yes/no; likelihood ratio test, p = 0.038) when dividing the frequency of siesta into three categories (never/rarely, sometimes/usually, and always). These results suggest that the effect of siesta on the probability of having obesity is modified by genetics.
Those participants with a higher genetic propensity for siesta who took siesta sometimes/usually compared with no siesta (i.e., never/rarely) had a lower probability of having obesity. However, for those participants with a lower genetic propensity for siesta, no significant differences were found in the probability of having obesity between those participants taking siesta sometimes/usually or those who never/rarely take siesta. The association between siesta behavior and a lower probability of having obesity started to be significant in those participants with Siesta‐PGS ≥ 1 (Figure 3). Interestingly, for any Siesta‐PGS cutoff, we did not find statistically significant differences between never/rarely taking siesta and always taking siesta (7 days/week) regarding the risk of obesity (all p values in the range of 0.405–0.981; Figure S3). We secondarily tested for an interaction between Siesta‐PGS and frequency of siesta regarding the presence of high central adiposity measured as waist circumference and waist‐hip ratio and for higher SBP and DBP. None of the interactions was significant (all p > 0.05).
FIGURE 3.
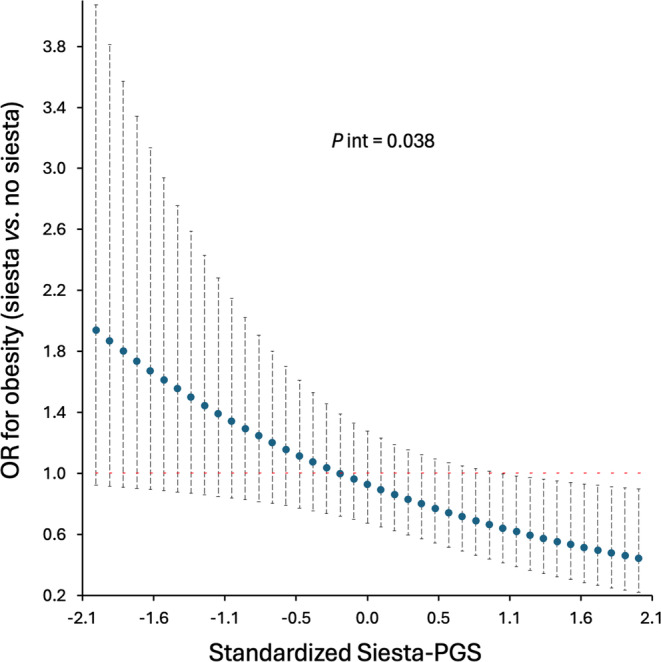
Association between siesta (sometimes/usually) compared with no siesta (never/rarely) and obesity as a function of Siesta‐Polygenic Score (Siesta‐PGS; primary result). Odds ratio (OR) and 95% CI. Interaction p value (P int) from likelihood ratio test. The never/rarely category includes frequency of siesta of never or once or twice per week. The sometimes/usually category includes frequency of siesta three to six times per week. For example, for the lowest Siesta‐PGS standardized value (−2.1; i.e., lower siesta genetic propensity), those who usually take siesta have an OR of having obesity 1.9 higher than those who do not take siesta, whereas, among those participants with the highest genetic propensity for siesta (Siesta‐PGS value 2.1), those who usually take siesta have an OR of having obesity 0.6 lower than those who do not take siesta. [Color figure can be viewed at wileyonlinelibrary.com]
Another exploratory analysis that we performed was the potential interaction between Siesta‐PGS and frequency of siesta regarding the success in weight loss by dividing the frequency of siesta into three categories, but no significance was found. However, when we categorized siesta into two groups, i.e., never/sometimes (0–3 days/week) and usually/always (4–7 days/week), we found a significant interaction between genetic propensity for siesta and siesta frequency for the success of weight loss during the treatment (≥5% weight loss; p = 0.007).
Among those participants with a higher genetic propensity for siesta, the probability of success in weight loss was significantly higher when participants reported taking siesta usually/always than when they reported never/sometimes siesta (significance was reached for Siesta‐PGS ≥ 1.1; p < 0.05; Figure 4). No significant interaction between Siesta‐PGS and siesta frequency was found for long‐term WLM (p = 0.875).
FIGURE 4.
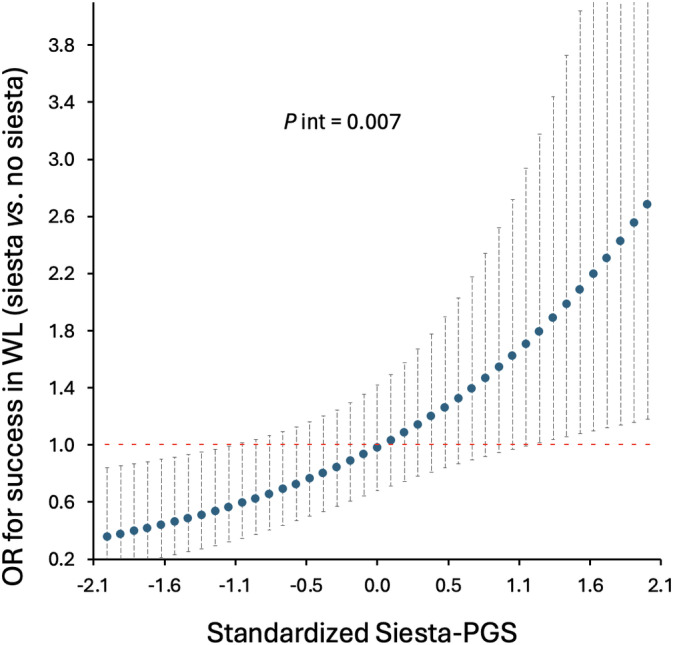
Association between siesta (usually/always) compared with no siesta (sometimes/never) and success in weight loss (WL) as a function of Siesta‐Polygenic Score (Siesta‐PGS; secondary result). Odds ratio (OR) and 95% CI. Interaction p value (P int) from likelihood ratio test. After exploratory analyses, the sometimes/never category includes frequency of siesta of never or once, twice, or three times per week, and the usually/always category includes frequency of siesta four to seven times per week. For example, for the lowest Siesta‐PGS standardized value (−2.1; i.e., lower siesta genetic propensity), those who usually take siesta have an OR of success in WL 0.2 lower than those who do not take siesta, whereas, among those participants with the highest genetic propensity for siesta (Siesta‐PGS value 2.1), those who usually take siesta have an OR of success in WL 2.6 higher than those who do not take siesta. [Color figure can be viewed at wileyonlinelibrary.com]
Association of genetic propensity for siesta and other Mediterranean lifestyle behaviors (exploratory)
Descriptive data of Mediterranean lifestyle behaviors in the population studied and differences in each characteristic among siesta frequency groups are shown in Table 2.
We secondarily aimed to test for potential associations between Siesta‐PGS and different lifestyle factors of this Mediterranean population (Table S4). A higher Siesta‐PGS was significantly associated with higher energy intake, higher protein intake, and higher saturated fat intake and a trend to higher carbohydrate and total fat intake (Figure 5; Table S2). We also observed a trend to seasonal summer siesta with a higher Siesta‐PGS (p = 0.064). No significant associations were observed between Siesta‐PGS and other lifestyle factors (Table S2).
FIGURE 5.
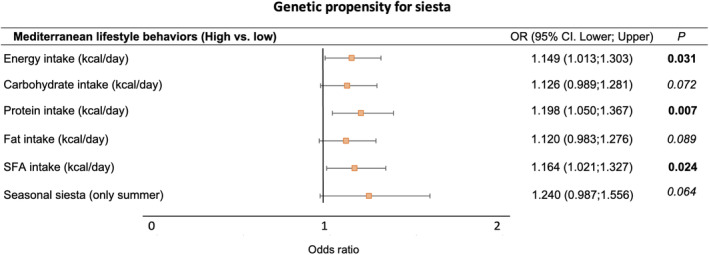
Associations between genetic propensity for siesta and Mediterranean lifestyle behaviors (secondary results). Logistic regression analyses between standardized Siesta‐PGS (as continuous variable) and each Mediterranean lifestyle categorized into high and low. High and low categories in every lifestyle variable are defined considering the median value of the population as the cutoff point. Given the exploratory nature of these analyses, multiple comparison corrections were not applied. SFA, saturated fatty acids. [Color figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
Our results showed that siesta behavior and siesta genetics interact to have an influence on obesity risk. This confirms our initial hypothesis supporting that those individuals who have a genetic propensity for siesta and, in fact, take siesta frequently are less likely to develop obesity and more likely to lose weight during the treatment than those who have a genetic propensity for siesta and do not. We further found a possible interaction between siesta behavior and genetics to influence success in weight loss.
Currently, multiple genome‐wide PGSs have been generated to determine the individual genetic propensity for a certain trait or illness. One example is the interaction between the circadian genetic risk and sleep quality in determining the risk for myocardial infarction [16]. Our results suggest that characterizing genetic propensity for siesta of the individual may be beneficial for obesity and metabolic health management because people with a high genetic propensity for siesta who usually take siesta had a lower probability of having obesity and a higher probability of losing body weight during a dietary treatment compared with those who did not take siesta.
In the current population, surprisingly, those who took siesta 7 days/week and had genetic propensity for siesta had similar associations with the probability of developing obesity to those who never took siesta. Both situations may be physiologically comparable because both are constant habits; therefore, the body may not perceive relevant changes across the 7 days of the week [27]. Both of these situations are consistent with our hypothesis that misalignment of a high genetic propensity for siesta and infrequent siesta behavior is associated with adverse obesity outcomes.
Although siesta has been previously associated with obesity and other metabolic alterations [1, 9, 10, 11, 12], it has recently been shown that a siesta habit is potentially positive for higher alertness [28]. Lou et al. concluded their study with the need for a targeted intervention focused on maximizing siesta benefits [28]. We propose here that, among other factors, the beneficial or detrimental effects of siesta could be modulated by genetics, potentially explaining the controversial results found. Our genetic instrument was developed from a UK population and, in this study, applied to the Spanish cohort. About 42% to 44% of participants in both the UK Biobank and ONTIME populations, respectively, reported daytime napping or siesta. The prevalence of a high genetic propensity for siesta is expected to be similar across European populations given that allele frequencies of nap‐associated alleles are similar across these populations [1].
This novel consideration of misalignment between genetic predisposition for siesta and siesta behavior could enhance our understanding of the controversial effects of siesta on health in different populations. Studies regarding interactions between siesta genetic propensity and siesta behavior for obesity traits in a non‐Spanish population are needed to test the generalizability of our discovery to the global human population.
The genetic component of siesta found is in agreement with the results that we previously obtained from a classical twin study [3], which showed the relative contribution of genetic factors to daytime napping [3], and also with previous studies in animals [29, 30, 31]. A genetic influence on midday napping has been shown in humans [1, 14, 15] and rodents [30, 31]. This genetic propensity for siesta could explain why certain individuals who have the opportunity to take siesta choose not to take it; however, other individuals who do not have the opportunity to take siesta or do not have an optimal place for siesta fall asleep at the slightest opportunity. This is common even among members of the same family. An anti‐siesta gene, i.e., daywake (dyw), has been found in flies, which may regulate this behavior [32].
Although previous research in the UK Biobank has suggested a link between siesta genetics and obesity, in the context of this Mediterranean population in whom siesta is habitual, we did not find a significant association between our Siesta‐PGS and obesity. This lack of association is not surprising, taking into account that the current Siesta‐PGS is capturing siesta frequency, whereas siesta duration seems to be a stronger factor for obesity, with higher BMI values relating to longer siestas [13, 20, 21]; no significant association has been previously found between a higher frequency of siesta and obesity in this Mediterranean population [13]. Additionally, in the current study, we did not find associations between a higher genetic propensity for siesta and higher central adiposity or higher blood pressure (both SBP and DBP) or between a higher siesta frequency and these variables.
Contextual factors may be involved in these differences between Mediterranean and British populations. For example, the current Mediterranean population is relatively younger (mean age 40.60 [SD 13.12] years) than the UK Biobank population, with a more precise assessment of siesta [1]. Another potential reason for the lack of association between genetic propensity for siesta and obesity could be that, different from the UK Biobank population, in the current Mediterranean population, 53% of the participants had obesity. Another factor that may be involved in the reason why we did not find an association between our Siesta‐PGS and obesity in our Mediterranean population is that genetic propensity for siesta was associated with siesta but did not necessarily imply less sleep at night. Previous studies have shown that late nocturnal bedtime and short nocturnal sleep have been associated with increased risk of developing obesity [33]. However, in the current Mediterranean population, we observed no associations between siesta genetic propensity and nighttime sleep duration and timing, individual chronotype, and physical activity level. This is in agreement with a previous study in flies which showed that the anti‐siesta dyw gene did not affect nighttime sleep levels [32].
It is also worth highlighting the fact that siesta is highly embedded in the Mediterranean culture; therefore, as opposed to the UK, a high frequency of siesta in this population from the Mediterranean coast may not be as deleterious and may be favorable in those individuals with a high genetic propensity for siesta. Spanish siesta typically occurs in a postprandial state, after lunch, as it is thought that food consumption may induce sleepiness [34]. Thus, taking siesta in this Mediterranean area may be a consequence of this postprandial sleepiness and not due to aging, illness, or problematic work schedules [6, 7]. Timing of siesta is also characteristic because it is on a protected timing (from ~3 p.m. to 5 p.m.) and occurs during a time of day when work schedules allow being at home, making it an intentioned decision that is preferred over other activities.
Our results showed that genetic propensity for siesta was associated with worse lifestyle habits in this Mediterranean population, and a previous study showed that subjective siesta was positively correlated with higher intake of energy, total fat, and saturated fat, among other factors, in 459 postmenopausal women [35].
As we mentioned earlier, Mediterranean siesta is a cultural habit embedded in the Spanish population. The differences between worldwide daytime napping and Mediterranean siesta underscore the importance of considering cultural and contextual factors when interpreting genetic influences on health outcomes. Because long‐term environmental and cultural processes might affect human evolution, gene–culture interactions are a growing field of study [36]. The relevance of studying Siesta‐PGS in other populations and its interaction with siesta behavior is that it could provide evidence on health implications of siesta (or other forms of daytime naps) globally. Indeed, the previously detrimental association between genetic propensity for siesta/napping and obesity in the British population might be influenced by the less frequent siesta habit in Northern European countries.
Our study has some limitations. The results are derived from a cross‐sectional study focused on a specific population of adults with overweight and obesity participating in the ONTIME study. Consequently, we need to be careful about generalizing these findings to other populations. Additionally, the exploratory nature of some analyses may incur type I error, and further analyses considering multiple comparison correction are recommended. Importantly, causality cannot be inferred from our results because this is an observational study.
CONCLUSION
Overall, this study sheds light on a deeper understanding of the genetic basis of daytime napping and the potential association with a siesta habit. Importantly, those participants who have a genetic propensity for siesta and, in fact, take siesta frequently are less likely to develop obesity and more likely to lose weight during the treatment than those who have a genetic propensity to take siesta and do not. These findings are clinically relevant for the pursuit of personalized lifestyle recommendations depending on genetic background.
AUTHOR CONTRIBUTIONS
Marta Garaulet conceptualized and designed the study, acquired funding, designed the research, managed the project, and helped to write and critically review the final version of the paper. María Rodríguez‐Martín contributed to data analyses and literature research, figure and table design, and manuscript writing and final review. Diego Salmerón contributed to data interpretation and analysis and writing and review of the final manuscript. Frank A. J. L. Scheer, Hassan S. Dashti, and Richa Saxena conceived and designed the study and contributed to final review of the manuscript. Ana Isabel Cascales contributed to the recruitment of patients and collected data and samples. Aurora Aragón‐Alonso contributed to intellectual advice and reviewed the paper. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
CONFLICT OF INTEREST STATEMENT
Frank A. J. L. Scheer served on the Board of Directors for the Sleep Research Society and has received consulting fees from the University of Alabama at Birmingham and Morehouse School of Medicine. The interests of Frank A. J. L. Scheer were reviewed and managed by Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict‐of‐interest policies. Frank A. J. L. Scheer consultancies are not related to the current work. The other authors declared no conflicts of interest.
CLINICAL TRIAL REGISTRATION
ClinicalTrials.gov identifier: NCT02829619.
Supporting information
Data S1.
ACKNOWLEDGMENTS
This study was funded by the Spanish Ministry of Science and Innovation (MCINN/AEI) under grant PID2020‐112768RB‐I00. María Rodríguez‐Martín was supported by the MICINN grant PID2020‐112768RB‐I00. Frank A. J. L. Scheer was supported in part by the National Institutes of Health under grants R01 HL140574 and R01 HL153969.
Rodríguez‐Martín M, Salmerón D, Dashti HS, et al. Siesta behavior and genetics interact to influence obesity risk. Obesity (Silver Spring). 2025;33(1):164‐176. doi: 10.1002/oby.24173
María Rodríguez‐Martín and Diego Salmerón contributed equally as first authors.
DATA AVAILABILITY STATEMENT
Data will be available on request to Marta Garaulet (garaulet@um.es)
REFERENCES
- 1. Dashti HS, Daghlas I, Lane JM, et al. Genetic determinants of daytime napping and effects on cardiometabolic health. Nat Commun. 2021;12(1):900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dinges DF, Orne MT, Whitehouse WG, Orne EC. Temporal placement of a nap for alertness: contributions of circadian phase and prior wakefulness. Sleep. 1987;10(4):313‐329. [PubMed] [Google Scholar]
- 3. Lopez‐Minguez J, Morosoli JJ, Madrid JA, Garaulet M, Ordoñana JR. Heritability of siesta and night‐time sleep as continuously assessed by a circadian‐related integrated measure. Sci Rep. 2017;7(1):12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Capellini I, Nunn CL, McNamara P, Preston BT, Barton RA. Energetic constraints, not predation, influence the evolution of sleep patterning in mammals. Funct Ecol. 2008;22(5):847‐853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gradisar M, Wolfson AR, Harvey AG, Hale L, Rosenberg R, Czeisler CA. The sleep and technology use of Americans: findings from the National Sleep Foundation's 2011 Sleep in America poll. J Clin Sleep Med. 2013;9(12):1291‐1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruggiero JS, Redeker NS. Effects of napping on sleepiness and sleep‐related performance deficits in night‐shift workers: a systematic review. Biol Res Nurs. 2014;16(2):134‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faraut B, Andrillon T, Vecchierini MF, Leger D. Napping: a public health issue. From epidemiological to laboratory studies. Sleep Med Rev. 2017;35:85‐100. [DOI] [PubMed] [Google Scholar]
- 8. FUNDADEPS . Estudio sobre salud y descanso. https://fundadeps.org/recursos/estudio-sobre-salud-y-descanso/
- 9. Hartzler BM. Fatigue on the flight deck: the consequences of sleep loss and the benefits of napping. Accid Anal Prev. 2014;62:309‐318. [DOI] [PubMed] [Google Scholar]
- 10. Vgontzas AN, Pejovic S, Zoumakis E, et al. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin‐6 secretion. Am J Physiol Endocrinol Metab. 2007;292(1):E253‐E261. [DOI] [PubMed] [Google Scholar]
- 11. Mohammad Y. Siesta and risk for ischemic stroke: results from a case‐control study. Medicina (Kaunas). 2020;56(5):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Celis‐Morales C, Lyall DM, Guo Y, et al. Sleep characteristics modify the association of genetic predisposition with obesity and anthropometric measurements in 119,679 UK Biobank participants. Am J Clin Nutr. 2017;105(4):980‐990. [DOI] [PubMed] [Google Scholar]
- 13. Vizmanos B, Cascales AI, Rodríguez‐Martín M, et al. Lifestyle mediators of associations among siestas, obesity, and metabolic health. Obesity (Silver Spring). 2023;31(5):1227‐1239. [DOI] [PubMed] [Google Scholar]
- 14. Fei CJ, Li ZY, Ning J, et al. Exome sequencing identifies genes associated with sleep‐related traits. Nat Hum Behav. 2024;8(3):576‐589. [DOI] [PubMed] [Google Scholar]
- 15. Spada J, Scholz M, Kirsten H, et al. Genome‐wide association analysis of actigraphic sleep phenotypes in the LIFE Adult Study. J Sleep Res. 2016;25(6):690‐701. [DOI] [PubMed] [Google Scholar]
- 16. Lane JM, Qian J, Mignot E, Redline S, Scheer FAJL, Saxena R. Genetics of circadian rhythms and sleep in human health and disease. Nat Rev Genet. 2023;24(1):4‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dashti HS, Scheer FAJL, Saxena R, Garaulet M. Impact of polygenic score for BMI on weight loss effectiveness and genome‐wide association analysis. Int J Obes (Lond). 2024;48(5):694‐701. [DOI] [PubMed] [Google Scholar]
- 18. Corbalán MD, Morales EM, Canteras M, Espallardo A, Hernández T, Garaulet M. Effectiveness of cognitive‐behavioral therapy based on the Mediterranean diet for the treatment of obesity. Nutrition. 2009;25(7‐8):861‐869. [DOI] [PubMed] [Google Scholar]
- 19. Ge T, Chen CY, Ni Y, Feng YA, Smoller JW. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun. 2019;10(1):1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gribble AK, Sayón‐Orea C, Bes‐Rastrolle M, et al. Risk of developing metabolic syndrome is affected by length of daily siesta: results from a prospective cohort study. Nutrients. 2021;13(11):4182. 10.3390/nu13114182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Papandreou C, Díaz‐López A, Babio N, et al. Long daytime napping is associated with increased adiposity and type 2 diabetes in an elderly population with metabolic syndrome. J Clin Med. 2019;8(7):1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hilditch CJ, Dorrian J, Banks S. A review of short naps and sleep inertia: do naps of 30 min or less really avoid sleep inertia and slow‐wave sleep? Sleep Med. 2017;32:176‐190. [DOI] [PubMed] [Google Scholar]
- 23. Horne JA, Ostberg O. A self‐assessment questionnaire to determine morningness‐eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97‐110. [PubMed] [Google Scholar]
- 24. Perez‐Llamas F, Garaulet M, Herrero F, et al. Multivalent informatics application for studies of the nutritional status of the population. Assessment of food intake. Nutr Hosp. 2004;19(3):160‐166. [PubMed] [Google Scholar]
- 25. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599‐2608. [DOI] [PubMed] [Google Scholar]
- 26. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381‐1395. [DOI] [PubMed] [Google Scholar]
- 27. Gardner B, Lally P, Wardle J. Making health habitual: the psychology of 'habit‐formation' and general practice. Br J Gen Pract. 2012;62(605):664‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lou S, Hu S, Chen Y, et al. Benefits of napping habits in healthy adults: maintaining alerting performance and cortisol levels change within 90 minutes of habitual napping time. Sleep Med. 2024;119:214‐221. [DOI] [PubMed] [Google Scholar]
- 29. Low KH, Chen WF, Yildirim E, Edery I. Natural variation in the Drosophila melanogaster clock gene period modulates splicing of its 3′‐terminal intron and mid‐day siesta. PLoS One. 2012;7(11):e49536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001;21(8):2610‐2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ehlen JC, Hesse S, Pinckney L, Paul KN. Sex chromosomes regulate nighttime sleep propensity during recovery from sleep loss in mice. PLoS One. 2013;8(5):e62205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang Y, Edery I. Daywake, an anti‐siesta gene linked to a splicing‐based thermostat from an adjoining clock gene. Curr Biol. 2019;29(10): 1728‐1734.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tse LA, Wang C, Rangarajan S, et al. Timing and length of nocturnal sleep and daytime napping and associations with obesity types in high‐, middle‐, and low‐income countries. JAMA Netw Open. 2021;4(6):e2113775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murphy KR, Deshpande SA, Yurgel ME, et al. Postprandial sleep mechanics in Drosophila. Elife. 2016;5:e19334. doi: 10.7554/eLife.19334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grandner MA, Kripke DF, Naidoo N, Langer RD. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 2010;11(2):180‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laland KN, Odling‐Smee J, Myles S. How culture shaped the human genome: bringing genetics and the human sciences together. Nat Rev Genet. 2010;11(2):137‐148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
Data will be available on request to Marta Garaulet (garaulet@um.es)


