Abstract
Background
Atopic dermatitis (AD) affects individuals of all ages, and the first‐line treatment are emollients and topical corticosteroids. There is insufficient knowledge about factors possibly affecting the drug utilization of young adults with AD.
Objectives
To describe the drug utilization of young adults with AD in relation to sex, socio‐economic status and disease severity.
Methods
A cross‐sectional study based on the 24‐year follow‐up from the population‐based BAMSE (Children, Allergy, Milieu, Stockholm, Epidemiology Survey) birth cohort linked with dispensing data from the National Drug Register (n = 2912). Self‐reported AD and socio‐economic status were defined from questionnaire data and disease severity was determined through the clinical examination and Patient‐Oriented Eczema Measure questionnaire.
Results
The prevalence of AD in young adults was 17.7% (n = 516) and 45.5% of them were dispensed at least one drug for the treatment of AD during the study period (January 2016 to June 2019). Topical corticosteroids (TCS) were the most common drugs (32.9%) followed by emollients (21.7%). A larger proportion of men were dispensed TCS than women (39.0% vs. 29.1%: p‐value = 0.020). A larger proportion of young adults with moderate‐to‐severe AD were dispensed TCS than those with mild AD (52.6% vs. 35.3%: p‐value = 0.026). No one was dispensed the recommended amount of emollients and less than five individuals were dispensed the recommended amount of TCS for mild disease. Male sex (adj.OR 1.54, 95% CI 1.06–2.34) and moderate‐to‐severe AD (adj.OR 2.62, 95% CI 1.59–4.31) were associated with dispensation of TCS.
Conclusions
A large proportion of young adults with AD was undertreated or untreated. Sex and disease severity did affect the dispensing patterns of investigated drugs.
INTRODUCTION
Atopic dermatitis (AD), commonly known as atopic eczema, is a recurrent inflammatory skin disease characterized by dryness, itchiness and soreness. 1 , 2 , 3 Up to one‐fifth of young adults (age 16–32 years) in Europe and the United States is estimated to experience AD during a 12‐month period. 4 , 5 , 6 , 7 , 8 , 9 , 10 AD commonly affect the hands and face of adults and the flexor surfaces of children. 9 , 11
The standard treatment regime according to European 12 , 13 , 14 , 15 , 16 and national 17 guidelines are application of emollients twice a day and the use of topical corticosteroids (TCS) or topical calcineurin inhibitors (TCI) as a reactive (for flare‐ups) or proactive (for prevention of flares) treatment. 13 , 16 TCS are available in different potencies (Class I least potent, Class IV very potent). Patient's age, severity and localization of the AD all influence the choice of TCS potency. Class III (potent) TCS are most often used for treatment of AD among adults 12 , 13 , 14 , 15 , 16 and Class IV (very potent) are generally not used unless the AD is very difficult to treat. 13 , 16 However, for treatment of sensitive areas such as the face or genitalia a lower potency (Class I or II) TCS or TCIs (secondarily) can be used. International guidelines recommend 1000 g of emollient and 60–90 g of TCS per month for proactive treatment of adults with mild AD. 16 Emollients and TCS class I in small package sizes can be bought over‐the‐counter in Sweden. Systemic treatments such as systemic corticosteroids (SCS) and other immunosuppressants (methotrexate, azathioprine, cyclosporine and mycophenolic acid) can be used for severe AD. Biological drugs such as dupilumab (monoclonal antibody) 18 and abrocitinib (Janus kinase inhibitor) 19 has also been approved for the treatment of moderate or severe AD when topical treatment is inadequate.
Previous studies focusing on drug utilization has shown limited use of drugs for AD among adults with AD. 20 , 21 , 22 , 23 , 24 Factors such as sex or level of education have seldom been investigated in relation to the drug utilization of individuals with AD. A longitudinal observational study using population‐based register data showed that socio‐economic status (level of education and disposable income) affected the use of inhaled corticosteroids among young adults with asthma. 25 Higher education and higher income were independently associated with higher use of inhaled corticosteroids. Sex has also been reported to effect dermatological treatments and one retrospective Swedish study showed that less women receive phototherapy treatments for their psoriasis or AD than men. 26 A quantitative study from Scotland showed that men with moderate‐to‐severe AD received larger amounts of TCS than women of the same severity. 27 It has previously been reported that more severe AD is associated with a higher use of TCS. 21 The aim was therefore to describe the drug utilization of young adults with AD in relation to sex, socio‐economic status and disease severity.
MATERIALS AND METHODS
Study design and study population
This was a cross‐sectional study based on individual‐level data obtained from the ongoing longitudinal population‐based birth cohort BAMSE (Children, Allergy, Milieu, Stockholm, Epidemiology Survey) project. 28 The BAMSE project recruited infants at health care centres born between 1994 and 1996 in the Stockholm County. A total of 4089 infants (75% of all eligible) were recruited and have been followed up at ages 1, 2, 4, 8, 12, 16 and 24 with questionnaires and clinical examinations at ages 4, 8, 16 and 24. 29
The 24‐year follow‐up was conducted between 2017 and 2019 and contained an extensive questionnaire and clinical examination. A total of 3064 individuals (74.9% of original cohort) completed the 24‐year questionnaire and 9 were excluded because of incompletion of answers defining AD and 143 because of not giving consent for register linkage, leaving a study population of 2912 individuals (71.2% of original cohort) (Figure 1). The questionnaire included questions about the participant's occupational status, self‐assessment of AD symptoms and comorbidities. The mean age of the participants at the 24‐year follow‐up was 22.7 (range 19.7–25.2) years.
FIGURE 1.
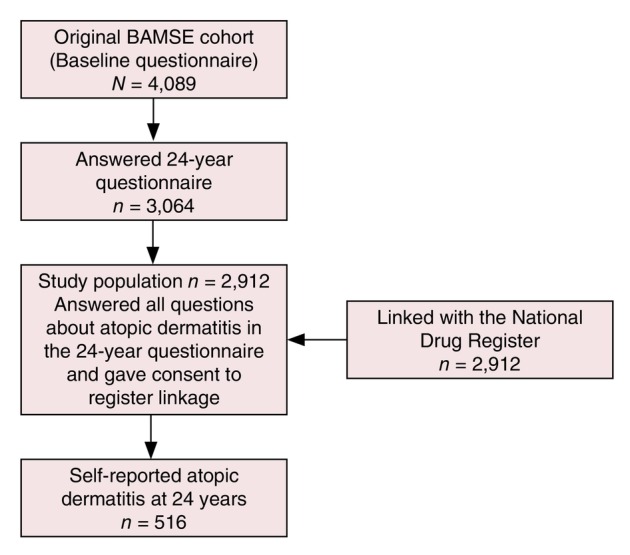
Flowchart of the study procedure. BAMSE: Children, Allergy, Milieu, Stockholm, Epidemiology Survey.
Record linkage with the National Drug Register
Data on the participant's dispensed drugs were collected between January 2016 and June 2019 from the National Drug Register. The National Drug Register covers more than 99% of the population who have had any drug dispensed but do not cover over‐the‐counter drugs. 30 The drugs included for the treatment of AD were chosen based on international guidelines for treatment of AD. 12 , 13 , 14 , 15 , 16 Investigated drugs included five groups of drugs based on the Anatomical Therapeutic Chemical code (ATC): Emollients (D02AE and D02AX); Topical corticosteroids (TCS; D07); Topical calcineurin inhibitors (TCI; D11AH01 and D11AH02); Systemic corticosteroids (SCS; H02AB01, H02AB06); and Systemic immunosuppressants (L04AA06, L04AD01, L04AX01 and L04AX03). An initial analysis of the utilization of the investigated drugs was done on the entire study population, but further analyses were limited to the young adults with AD.
Health measurements from 24‐year questionnaire data
Atopic dermatitis was defined according to the William's 31 criteria with modifications and included an itchy skin rash in the 12 months preceding the 24‐year follow‐up in combination with three of the following four criteria:
Dry skin in the last 12 months.
History of AD at age 1 and/or 2 years based on questionnaire data (onset below age 2 years). 32
History of flexural dermatitis.
Personal history of asthma or hay fever defined as reported asthma and/or rhinitis at any BAMSE follow‐up from age 4.
Higher parental socio‐economic status was defined as at least one parent with a white‐collar employment at baseline questionnaire. The classification system MIS 1982:4 defined by Statistics Sweden was used for determination of the employment category. 33
Occupational status among the young adults was divided into three groups. Working defined as current employment when the 24‐year questionnaire was answered. Studying defined as current pursuit of degree in higher studies (community college, college and university). Other defined as other occupation status such as parental leave, unemployment or sick leave.
Atopic heredity was defined as a mother and/or father with doctor's diagnosis of asthma and/or asthma drug and/or doctor's diagnosis of hay fever in combination with furred pets and/or pollen allergy and/or doctor's diagnosis of eczema which was assessed from baseline questionnaire. 34
Asthma was assessed through the 24‐year follow‐up questionnaire and defined as doctor's diagnosis of asthma and symptoms of asthma in the last 12 months. 34
Allergic rhinitis was defined as symptoms from eyes or nose because of furred animals or pollen in the las 12 months prior to answering the 24‐year follow‐up questionnaire. 34
Food allergy was defined from the 24‐year follow‐up as answering yes to the question ‘do you avoid food due to previous reactions or allergy tests?’
Health outcomes from clinical examination
To assess severity of AD, the participants were asked to fill in Patient‐Oriented Eczema Measure (POEM) 35 questionnaire if they showed visible AD during the 24‐year clinical examination. POEM is a validated scale used for the patient to self‐evaluate the severity of AD in the last week. 35 POEM includes questions about how many days in the last week the patient experienced the following because of their AD: dryness of the skin, bleeding of the skin, weeping/oozing of the skin, sleep disturbances, itchy skin, flaky skin and rough skin. There are five response alternatives to each question: no days (0 points), 1–2 days (1 point), 3–4 days (2 points), 5–6 days (3 points) and every day (4 points). In this study two levels of severity; mild (1–7 points) and moderate‐to‐severe (8–28 points) AD were used in accordance with previous definitions from the BAMSE study. 10
Presence of IgE antibodies was determined by a positive Phadiatop (Phadiatop ≥0.35 kU/L) and/or Fx5 (Fx5 ≥ 0.35 kU/L) at 24‐year follow‐up, and is further described elsewhere. 36
Statistics
The background characteristics were presented as percentages and numbers. Chi‐squared test was applied to the comparison of variables between groups. Means for continuous variables were compared between two groups using a two‐tailed two sample t‐test. A significance level of 0.05 indicated statistically significant differences.
Logistic regression was used to estimate the odds ratios (ORs) with 95% confidence intervals (CIs) for the associations between factors identified from the literature and dispensations of investigated AD drugs with adjustment for possible confounders. STATA Statistical Software (release 16:0; Stata‐Corp, College Station, TX, USA) was used for all analysis and statistical calculations.
RESULTS
In the study population of 2912 young adults, 516 (17.7%) fulfilled the study definition of AD, a larger proportion of women than men (20.4% vs. 14.6%; p < 0.001). Larger proportions of individuals with AD had atopic heredity, asthma, allergic rhinitis and presence of IgE antibodies compared to the study population (Table 1).
TABLE 1.
Background characteristics of the study population and young adults with atopic dermatitis (AD).
| Characteristics | Study population (N = 2912) | Young adults with AD (n = 516) | p‐Values* |
|---|---|---|---|
| Gender | |||
| Male | 1366 (46.9) | 200 (38.8) | <0.001 |
| Female | 1546 (53.1) | 316 (61.2) | |
| Parental socio‐economic status a | |||
| Blue‐collar worker | 435 (15.2) | 78 (15.4) | 0.889 |
| White‐collar worker | 2430 (84.8) | 429 (84.6) | |
| Occupational status b | |||
| Studying | 1493 (51.4) | 265 (51.6) | 0.021 |
| Working | 1187 (40.9) | 224 (43.6) | |
| Other | 224 (7.7) | 25 (4.9) | |
| Atopic heredity c | |||
| No | 1612 (55.8) | 246 (48.0) | <0.001 |
| Yes | 1276 (44.2) | 267 (52.0) | |
| Asthma d | |||
| No | 2598 (89.2) | 390 (75.6) | <0.001 |
| Yes | 314 (10.8) | 126 (24.4) | |
| Allergic rhinitis e | |||
| No | 1999 (69.3) | 193 (37.7) | <0.001 |
| Yes | 887 (30.7) | 319 (62.3) | |
| Food allergy f | |||
| No | 1936 (66.5) | 258 (50.0) | <0.001 |
| Yes | 823 (28.3) | 231 (44.8) | |
| IgE antibodies g | |||
| No | 1194 (55.4) | 128 (32.0) | <0.001 |
| Yes | 962 (44.6) | 272 (68.0) | |
Higher parental socio‐economic status was defined as at least one parent who is a white‐collar worker (Socio‐economic Division (SEI); Reports on Statistical Coordination 1982:4) at baseline questionnaire.
Occupational status was divided into three groups. Working defined as current employment when questionnaire was answered. Studying defined as current pursuit of degree in higher studies (community college, college, university). Other defined as other occupation such as parental leave, unemployment or sick leave.
Atopic heredity was defined as a mother and/or father with doctor's diagnosis of asthma and asthma drug, and/or diagnosis or hay fever because of furred pets and/or pollen allergy and/or doctor's diagnosis of eczema (contact allergy among parents is excluded) at baseline questionnaire.
Asthma was determined from the 24‐year follow‐up questionnaire and includes questions such as symptoms of asthma in the last 12 months, use of asthma medicines in the last 12 months and a doctor's diagnosis of asthma ever.
Allergic rhinitis was defined from the 24‐year follow‐up as symptoms from nose or eyes because of furred animal or pollen.
Food allergy was defined from the 24‐year follow‐up as answering yes to the question ‘do you avoid food due to previous reactions or allergy tests?’
Presence of IgE antibodies was determined through positive Phadiatop and/or food mix (fx1, fx5 or fx22) test from a blood sample taken at the 24‐year follow‐up clinical examination.
Comparison between the study population and the young adults with atopic dermatitis.
Dispensation of investigated drugs and sex
In all, 635 (21.8%) out of the study population were dispensed at least one of the investigated drugs during the study period. Among those that did not fulfil the criteria of AD (n = 2396), 402 individuals (16.8%) were dispensed at least one of the investigated drugs. Further analysis will focus on the 516 participants who fulfilled the criteria for AD. Overall, 233 (45.2%) of those were dispensed any of the investigated drugs during the study period, and there was no difference between sex (48.0% men vs. 43.4% women; p‐value = 0.302). In analyses of groups of drugs, 21.7% were dispensed at least one emollient and 32.9% were dispensed TCS (Table 2). A larger proportion of men (39.0%) than women (29.1%) were dispensed TCS (p‐value 0.020). There were no differences in average amount of dispensed emollient (p‐value = 0.929) or TCS (p‐value = 0.613). Among the young adults with AD, 4.1% were dispensed a TCI. A larger proportion of women than men (18.4% vs. 11.5%: p‐value = 0.037) were dispensed SCS during the study period and the women also retrieved a larger number of prescriptions, on average, than men (2.29 vs. 1.30; p‐value = 0.023). No one received the recommended monthly amount of emollient and less than 5 of the young adults received the recommended amount of TCS.
TABLE 2.
Dispensation of investigated drugs January 2016 to June 2019 among young adults with atopic dermatitis (n = 516) by sex. Each individual could have had dispensed drugs from several categories and was then included each category.
| Dispensed drug category | Parameter | All | Men | Women | p‐Value* |
|---|---|---|---|---|---|
| Emollients | No. individuals (%) | 112 (21.7) | 50 (25.0) | 62 (19.6) | 0.149 |
| No. prescriptions (average per person) | 428 (3.82) | 184 (3.68) | 244 (3.94) | 0.819 | |
| Average amount a per person in grams (range) | 40.0 (24–393) | 40.5 (2.4–248) | 39.6 (2.4–398) | 0.929 | |
| Topical corticosteroids | No. individuals (%) | 170 (32.9) | 78 (39.0) | 92 (29.1) | 0.020 |
| No. prescriptions (average per person) | 512 (301) | 259 (3.32) | 253 (2.75) | 0.400 | |
| Average amount a per person (range) | 5.65 (>1–94) | 6.05 (>1–47) | 5.31 (>1–94) | 0.613 | |
| Topical calcineurin inhibitors | No. individuals (%) | 21 (4.07) | 9 (4.50) | 12 (3.80) | 0.694 |
| No. prescriptions (average per person) | 40 (1.90) | 16 (1.78) | 24 (2.00) | 0.700 | |
| Average amount per person in grams (range) | – | – | – | – | |
| Systemic corticosteroids | No. individuals (%) | 81 (15.7) | 23 (11.5) | 58 (18.4) | 0.037 |
| No. prescriptions (average per person) | 163 (2.01) | 30 (1.30) | 133 (2.29) | 0.023 | |
| Average amount per person in grams (range) | – | – | – | – |
Average amount per month.
Comparison between males and females.
Among the systemic drugs, 81 individuals were dispensed SCS during the study period (see Table 2) and less than 10 individuals were dispensed systemic immunosuppressants. A majority (73) of the individuals with dispensed SCS also reported having asthma, allergic rhinitis or food allergy.
None of the participants were dispensed the recommended amount of emollients (see Table 2). Less than 5 individuals were dispensed a sufficient amount of TCS based on the recommendations for maintenance treatment of AD. 16
Dispensed drugs in relation to AD severity
Among the 516 young adults with AD, 163 were assessed with ongoing AD at the clinical examination and filled in POEM (score ≥1). Among them, 52.1% (n = 85) individuals had mild AD, 47.9% (n = 78) had moderate‐to‐severe AD. Men and women had similar average POEM‐score of 8.0 respective 8.4 (p = 0.83) and the highest score was 26.0.
Among the individuals with moderate‐to‐severe AD, 66.7% (n = 52) were dispensed an investigated drug compared to 45.9% (n = 39) of the individuals with mild AD (p‐value = 0.008). A larger proportion of individuals with moderate‐to‐severe AD were dispensed emollients than those with mild AD (46.2% vs. 21.2%: p‐value<0.001; Table 3) and the same pattern was seen for TCS (52.6% vs. 35.3%: p‐value = 0.026).
TABLE 3.
Dispensation of the investigated drugs January 2016 and June 2019 among young adults with atopic dermatitis (AD; n = 516) by disease severity. Each individual could have had dispensed drugs from several categories and was then included in each category. Mild atopic dermatitis corresponded to a Patient‐Oriented Eczema Measure (POEM) score 1–7 and moderate‐to‐severe AD to POEM score 8–26.
| Dispensed drug category | Parameter | Mild AD (n = 85) | Moderate‐to‐severe AD (n = 78) | p‐Value* |
|---|---|---|---|---|
| Emollients | No. individuals (%) | 18 (21.2) | 36 (46.2) | <0.001 |
| No. prescriptions (average per person) | 76 (4.22) | 150 (4.17) | 0.976 | |
| Average amount a per person in grams (range) | 52 (8–226) | 47 (2–393) | 0.829 | |
| Topical corticosteroids | No. individuals (%) | 30 (35.3) | 41 (52.6) | 0.026 |
| No. prescriptions (average per person) | 88 (2.93) | 170 (4.15) | 0.180 | |
| Average amount a per person in grams (range) | 5.63 (>1–31) | 8.10 (>1–43) | 0.194 |
Per month.
Comparison between mild and moderate‐to‐severe atopic dermatitis.
Factors associated with dispensing of the investigated drugs
In the multivariate analysis, other occupational status such as being on parental leave or unemployed (adj. OR 4.64, 95% CI 1.66–12.94), and moderate‐to‐severe AD (adj. OR 2.07, 95% CI 1.08–3.96) were associated with higher odds of having any of the investigated drugs dispensed. In addition, dispensation of TCS was positively associated with male sex (adj. OR 1.54, 95% CI 1.06–2.34), other occupational status (adj. OR 3.85, 95% CI 1.56–9.52), moderate‐to‐severe AD (adj. OR 2.62, 95% CI 1.59–4.31) and TCI (adj. OR 21.9, 95% CI 5.01–95.7; Figure 2). For emollients (data not shown), atopic heredity (adj. OR 1.67, 96% CI 1.08–2.57) and moderate‐to‐severe AD (adj. OR 4.27, 95% CI 2.54–7.16) were associated with increased odds of dispensation.
FIGURE 2.
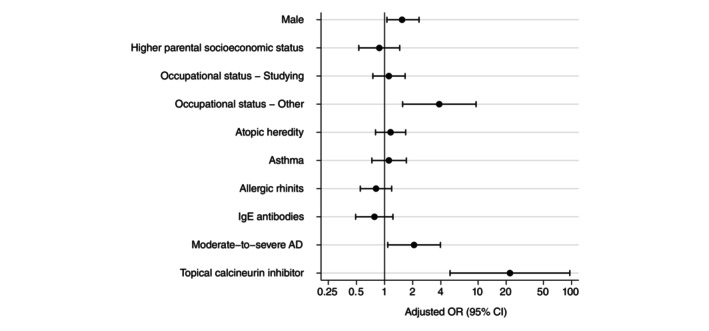
Factors associated with dispensing of topical corticosteroids among young adults with atopic dermatitis (n = 516). Odds ratios (OR) are adjusted for sex and higher parental socio‐economic status (i.e. at least one parent who is a white‐collar worker).
DISCUSSION
This cross‐sectional study indicates that young adults with AD are undertreated or untreated for their AD with the investigated prescription drugs. TCS was the most common treatment followed by emollients. Male sex, other occupational status and moderate‐to‐severe AD were associated with having first‐line treatment of TCS dispensed although only around half of the individuals with moderate‐to‐severe AD was dispensed emollients or TCS during the study period. None of the young adults were dispensed the recommended monthly amount of emollient and less than five individuals were dispensed the recommended amount of TCS.
Undertreatment of patients with AD have been observed before. 20 , 21 , 22 , 23 , 24 Studies aimed at explaining lack of adherence among AD patients has identified key reasons such as tedious application processes, 37 pain during application and uncertainty about the application process and side effects of the TCSs 38 as reasons to why topical treatments are often not followed as intended. In the same qualitative study 38 of young adult's experience with AD the individuals also reported difficulties with getting into contact with health care providers, which might have contributed to lack of prescriptions and therefore an undertreatment.
Guidelines emphasize the importance of treatment education to ensure that patients with AD know how to treat their AD, but uncertainty of how to treat AD have been reported. 38 Reported difficulties to get into contact with health care could also influence the adherence as the patient is unable to ask questions about their treatment regime. Topical corticophobia has been shown to be present among both patients 39 , 40 and health care providers 41 , 42 and is a possible cause of undertreatment of AD.
Another aspect of undertreatment are the economic aspects. In Sweden, a high‐cost reimbursement of approximately 220 EUR per year is in place to limit the individual's medicine costs. In 2016, medicine within the reimbursement system became free for all children in Sweden. A report 43 from the National Board of Health and Welfare showed a high increase in dispensed emollients indicating that emollients are drugs people either do not want to or are unable to spend money on.
This study also showed that a larger proportion of men with AD are dispensed TCS than women. A previous study from Scottland showed that men with moderate‐to‐severe AD were prescribed larger amounts of TCS than women with the same severity. 27 A health register based study from Italy 44 have shown that women do not receive preventative treatment as often as men, which could be part of the explanation why a smaller proportion of women were dispensed TCS, as it can be used as prevention for flare‐ups of AD. 12 , 13 , 14 , 15 , 16
A larger proportion of women compared to men were dispensed SCS. SCS is also used for plenty of other conditions such as asthma and allergies 45 and the majority of the dispensation of SCS in this study could possibly be explained by multiple comorbidities also eligible for SCS such as asthma, allergic rhinitis and food allergy. However, a larger proportion of women did get dispensed SCS and it has previously been observed that women receive more anti‐inflammatory drugs such as SCS than men. 44 , 46
A minority of participants with AD were dispensed TCI during the study period and dispensation of TCI was positively associated with dispensation of TCS. The finding was expected as TCI is commonly used for fewer areas (sensitive areas) than TCS. 12 , 13 , 14 , 16 Other studies have also shown lower use of TCI than TCS. 22 , 47
Other occupational status (i.e. parental leave, unemployment and sick leave) was associated with having any of the investigated drugs dispensed, but based on a small number of individuals, hence this result should be interpreted with caution. A previous study from the same cohort investigating the adherence of asthma medication did not find a relation between socio‐economy and adherence. 34
Strengths and limitations
This study had several strengths. We utilized data from the BAMSE project which still obtains high response rates (about 75% of the original cohort 28 ). The design of the BAMSE project is population‐based and thereby reduces selection biases. This study also uses the National Drug Register for the estimation of drug utilization which captures over 99% of the Swedish population with dispensed drugs.
Three different types of data (questionnaire data, clinical data and register data) were utilized and allowed for unexplored research questions such as if socio‐economic status and sex impact drug utilization among young adults with AD. This study uses self‐reported data for AD definition, which means it also captures milder cases of AD which might have been excluded if, for instance, medical records of doctor's diagnosis had been used. This study showed that 16.8% of those that did not fulfil the questionnaire criteria of AD were dispensed some of the investigated drugs during the study period. It is possible that the definition of AD in the BAMSE project might have excluded some AD cases, and it is also possible that some of the participants lacking fulfilment of AD criteria had other indications (e.g. psoriasis) that made them eligible for the investigated drugs. Another limitation of this study is the use of POEM as an assessment of disease severity. Although the study managed to assess the severity of AD, POEM only assesses the disease severity of the previous week which might result in some misclassifications affecting the estimation of drug utilization in the two severity groups. Some individuals with more severe AD might be satisfactory treated at the moment of the POEM questionnaire with could result in misclassification.
This study only displayed the dispensed investigated drugs and did not investigate whether or not the study population had received prescriptions from health care professionals that they had not retrieved. A qualitative Swedish study about young adults´ perceptions of living with AD showed that the participants had difficulties getting into contact with health care regarding their AD. 38 It is hard to say if the lack of dispensed investigated drugs depended on the participant not filling their prescription's or the health care's unwillingness to prescribe. In addition, we used dispensing data which may overestimate the actual use among the young adults.
Pharmaceutical graded emollients such as carbamide and Class I TCS (low potency) are available OTC in Sweden. We were not able to take the OTC sales into account in this study therefore the estimates of total drug use might have been underrated. However, among individuals with moderate‐to‐severe AD, 33.3% had no dispensation of any of the investigated drugs. Even though emollients and Class I TCS (low potency) are available as OTC and might relieve symptoms they are insufficient for treatment of moderate‐to‐severe AD. International guidelines recommend Class III TCS for treatment of acute flare‐ups and Class II–III for proactive treatment of patients with moderate‐to‐severe AD. 16
In this population‐based cohort a large proportion of young adults with AD were undertreated or untreated. Male sex and moderate‐to‐severe AD were associated with dispensation of TCS. However, a third of those with moderate‐to‐severe AD had none of the investigated drugs dispensed.
FUNDING INFORMATION
This study was supported by grants from the Swedish Research Council (grant agreements 2016‐03086; 2018‐02524; 2020‐02170), the Swedish Research Council for Health, Working Life and Welfare (2017‐00526), Formas (2016‐01646), the Swedish Heart‐Lung Foundation, the Swedish Asthma and Allergy research foundation and Region Stockholm (ALF projects, and for cohort and database maintenance). Thermo Fisher Scientific kindly provided reagents for IgE analyses.
CONFLICT OF INTEREST STATEMENT
SL has received lecture fees from Sanofi and AbbVie. NB has received speaker honoraria and/or been a consultant for Pfizer, Sanofi and Galenica. EM has received lecture fees from Airsonett, ALK, AstraZeneca and Sanofi outside the submitted work. PC, MÖ, EK, AB and ED declare no conflict of interest.
ETHICS STATEMENT
The BAMSE project has been approved by the regional ethics committee in Stockholm (No. 2016/1380‐31/2). The BAMSE project participants has given informed consent to participate in the BAMSE project.
ACKNOWLEDGEMENTS
We thank the children and parents participating in the BAMSE cohort and all staff involved in the study through the years.
Carmanius PL, Lundin S, Ödling M, Kimland E, Ballardini N, Melén E, et al. Drug utilization among young adults with atopic dermatitis: Influence of sex, socio‐economic status and disease severity. J Eur Acad Dermatol Venereol. 2025;39:145–153. 10.1111/jdv.20076
Linked article: M. Trzeciak et al. J Eur Acad Dermatol Venereol 2025;39:31–32. https://doi.org/10.1111/jdv.20437.
DATA AVAILABILITY STATEMENT
According to Swedish Law, the data cannot be placed in a public available repository. Researchers can after ethical approval from the Swedish Ethical Review Authority (https://etikprovningsmyndigheten.se) apply for data from the National Board of Health and Welfare, Sweden (www.socialstyrelsen.se). We will consider proposals for research collaborations. Enquiries can be submitted to the corresponding author (elin.dahlen@ki.se).
REFERENCES
- 1. Leung DYM, Guttman‐Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134(4):769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150(6):593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johansson S, Bieber T, Dahl B, Friedmann P, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the nomenclature review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113(5):832–836. [DOI] [PubMed] [Google Scholar]
- 4. Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: A US population–based study. J Allergy Clin Immunol. 2013;132(5):1132–1138. [DOI] [PubMed] [Google Scholar]
- 5. Rönmark EP, Ekerljung L, Lötvall J, Wennergren G, Rönmark E, Torén K, et al. Eczema among adults: prevalence, risk factors and relation to airway diseases. Results from a large‐scale population survey in Sweden. Br J Dermatol. 2012;166(6):1301–1308. [DOI] [PubMed] [Google Scholar]
- 6. Vinding GR, Zarchi K, Ibler KS, Miller IM, Ellervik C, Jemec GBE. Is adult atopic eczema more common than we think? – a population‐based study in Danish adults. Acta Derm Venereol. 2014;94(4):480–482. [DOI] [PubMed] [Google Scholar]
- 7. de Lusignan S, Alexander H, Broderick C, Dennis J, McGovern A, Feeney C, et al. The epidemiology of eczema in children and adults in England: a population‐based study using primary care data. Clin Exp Allergy. 2021;51(3):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan LN, Magyari A, Ye M, Al‐Alusi NA, Langan SM, Margolis D, et al. The epidemiology of atopic dermatitis in older adults: a population‐based study in the United Kingdom. PLoS One. 2021;16(10):e0258219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mortz CG, Andersen KE, Dellgren C, Barington T, Bindslev‐Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70(7):836–845. [DOI] [PubMed] [Google Scholar]
- 10. Johansson EK, Bergström A, Kull I, Melén E, Jonsson M, Lundin S, et al. Prevalence and characteristics of atopic dermatitis among young adult females and males—report from the Swedish population‐based study BAMSE. J Eur Acad Dermatol Venereol. 2022;36(5):698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–360. [DOI] [PubMed] [Google Scholar]
- 12. Wollenberg A, Christen‐Zäch S, Taieb A, Paul C, Thyssen JP, de Bruin‐Weller M, et al. ETFAD/EADV eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34(12):2717–2744. [DOI] [PubMed] [Google Scholar]
- 13. Wollenberg A, Barbarot S, Bieber T, Christen‐Zaech S, Deleuran M, Fink‐Wagner A, et al. Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–682. [DOI] [PubMed] [Google Scholar]
- 14. Wollenberg A, Barbarot S, Bieber T, Christen‐Zaech S, Deleuran M, Fink‐Wagner A, et al. Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850–878. [DOI] [PubMed] [Google Scholar]
- 15. Wollenberg A, Kinberger M, Arents B, Aszodi N, Avila Valle G, Barbarot S, et al. European guideline (EuroGuiDerm) on atopic eczema: part I – systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9):1409–1431. [DOI] [PubMed] [Google Scholar]
- 16. Wollenberg A, Kinberger M, Arents B, Aszodi N, Avila Valle G, Barbarot S, et al. European guideline (EuroGuiDerm) on atopic eczema – part II: non‐systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol. 2022;36(11):1904–1926. [DOI] [PubMed] [Google Scholar]
- 17. Atopic dermatitis – treatment recommendation [Internet]. Uppsala: Swedish Medical Products Agency; 2023. [cited 2023 Sep 10]. Available from: https://www.lakemedelsverket.se/49f165/globalassets/dokument/behandling‐och‐forskrivning/behandlingsrekommendationer/behandlingsrekommendation/atopisk‐dermatit‐behandlingsrekommendation.pdf [Google Scholar]
- 18. EMA . Dupixent [Internet]. Amsterdam: European Medicines Agency; 2018. [cited 2022 Dec 19]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent. [Google Scholar]
- 19. EMA . Cibinqo [Internet]. Amsterdam: European Medicines Agency; 2021. [cited 2022 Dec 1]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo [Google Scholar]
- 20. von Kobyletzki L, Ballardini N, Henrohn D, Neary MP, Ortsäter G, Geale K, et al. Care pathways in atopic dermatitis: a retrospective population‐based cohort study. J Eur Acad Dermatol Venereol. 2022;36(9):1456–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campanati A, Bianchelli T, Gesuita R, Foti C, Malara G, Micali G, et al. Comorbidities and treatment patterns in adult patients with atopic dermatitis: results from a nationwide multicenter study. Arch Dermatol Res. 2022;314(6):593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hagenström K, Sauer K, Mohr N, Dettmann M, Glaeske G, Petersen J, et al. Prevalence and medications of atopic dermatitis in Germany: claims data analysis. Clin Epidemiol. 2021;13:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lundin S, Wahlgren CF, Bergström A, Johansson EK, Dahlén E, Andersson N, et al. Use of emollients and topical glucocorticoids among adolescents with eczema: data from the population‐based birth cohort BAMSE. Br J Dermatol. 2018;179(3):709–716. [DOI] [PubMed] [Google Scholar]
- 24. Kleyn CE, Barbarot S, Reed C, Losi S, von Arx LB, Robert C, et al. Burden of moderate to severe atopic dermatitis in adults from France, Italy, and the UK: patient‐reported outcomes and treatment patterns. Dermatol Ther. 2022;12(8):1947–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davidsen JR, Søndergaard J, Hallas J, Siersted HC, Knudsen TB, Lykkegaard J, et al. Impact of socioeconomic status on the use of inhaled corticosteroids in young adult asthmatics. Respir Med. 2011;105(5):683–690. [DOI] [PubMed] [Google Scholar]
- 26. Nyberg F, Osika I, Evengård B. “The laundry bag project”– unequal distribution of dermatological healthcare resources for male and female psoriatic patients in Sweden. Int J Dermatol. 2008;47(2):144–149. [DOI] [PubMed] [Google Scholar]
- 27. Choi JY, Dawe R, Ibbotson S, Fleming C, Doney A, Foerster J. Quantitative analysis of topical treatments in atopic dermatitis: unexpectedly low use of emollients and strong correlation of topical corticosteroid use both with depression and concurrent asthma. Br J Dermatol. 2020;182(4):1017–1025. [DOI] [PubMed] [Google Scholar]
- 28. Wickman M, Kull I, Pershagen G, Nordvall SL. The BAMSE project: presentation of a prospective longitudinal birth cohort study. Pediatr Allergy Immunol. 2002;13(s15):11–13. [DOI] [PubMed] [Google Scholar]
- 29. BAMSE Projektet | Karolinska Institutet [Internet]. [cited 2022 Sep 2]. Available from: https://ki.se/imm/bamse‐projektet
- 30. Wettermark B, Hammar N, MichaelFored C, Leimanis A, Otterblad Olausson P, Bergman U, et al. The new Swedish prescribed drug register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2006;16(7):726–735. [DOI] [PubMed] [Google Scholar]
- 31. Williams HC. Diagnostic criteria for atopic dermatitis. Lancet. 1996;348:1391–1392. [DOI] [PubMed] [Google Scholar]
- 32. Böhme M, Lannerö E, Wickman M, Nordvall SL, Wahlgren CF. Atopic dermatitis and concomitant disease patterns in children up to two years of age. Acta Derm Venereol. 2002;82:98–103. [DOI] [PubMed] [Google Scholar]
- 33. Almqvist C, Pershagen G, Wickman M. Low socioeconomic status as a risk factor for asthma, rhinitis and sensitization at 4 years in a birth cohort. Clin Exp Allergy. 2005;35(5):612–618. [DOI] [PubMed] [Google Scholar]
- 34. Dahlén E, Bergström A, Ödling M, Ekström S, Melén E, Kull I. Non‐adherence and sub‐optimal treatment with asthma medications in young adults: a population‐based cohort study. J Asthma. 2022;59(8):1661–1669. [DOI] [PubMed] [Google Scholar]
- 35. Grinich EE, Schmitt J, Küster D, Spuls PI, Williams HC, Chalmers JR, et al. Standardized reporting of the eczema area and severity index (EASI) and the patient‐oriented eczema measure (POEM): a recommendation by the Harmonising outcome measures for eczema (HOME) initiative. Br J Dermatol. 2018;179(2):540–541. [DOI] [PubMed] [Google Scholar]
- 36. Melén E, Bergström A, Kull I, Almqvist C, Andersson N, Asarnoj A, et al. Male sex is strongly associated with IgE‐sensitization to airborne but not food allergens: results up to age 24 years from the BAMSE birth cohort. Clin Transl Allergy. 2020;10(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zschocke I, Mrowietz U, Lotzin A, Karakasili E, Reich K. Assessing adherence factors in patients under topical treatment: development of the topical therapy adherence questionnaire (TTAQ). Arch Dermatol Res. 2014;306(3):287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lundin S, Jonsson M, Wahlgren CF, Johansson E, Bergstrom A, Kull I. Young adults' perceptions of living with atopic dermatitis in relation to the concept of self‐management: a qualitative study. BMJ Open. 2021;11(6):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aubert‐Wastiaux H, Moret L, Le Rhun A, Fontenoy AM, Nguyen JM, Leux C, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165(4):808–814. [DOI] [PubMed] [Google Scholar]
- 40. Gerner T, Haugaard JH, Vestergaard C, Deleuran M, Jemec GB, Mortz CG, et al. Healthcare utilization in Danish children with atopic dermatitis and parental topical corticosteroid phobia. Pediatr Allergy Immunol. 2021;32(2):331–341. [DOI] [PubMed] [Google Scholar]
- 41. Lambrechts L, Gilissen L, Morren MA. Topical corticosteroid phobia among healthcare professionals using the TOPICOP score. Acta Derm Venereol. 2019;99(11):1004–1008. [DOI] [PubMed] [Google Scholar]
- 42. Smith SD, Lee A, Blaszczynski A, Fischer G. Pharmacists' knowledge about use of topical corticosteroids in atopic dermatitis: pre and post continuing professional development education. Australas J Dermatol. 2016;57(3):199–204. [DOI] [PubMed] [Google Scholar]
- 43. Follow‐up of the reform on free medicines for children – Final report [Internet]. Stockholm: National Board of Health and Welfare; 2019. p. 70 [cited 2022 Dec 12]. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint‐dokument/artikelkatalog/ovrigt/2019‐10‐6383.pdf [Google Scholar]
- 44. Orlando V, Mucherino S, Guarino I, Guerriero F, Trama U, Menditto E. Gender differences in medication use: A drug utilization study based on real world data. Int J Environ Res Public Health. 2020;17(11):3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Drug treatment in asthma [Internet]. Uppsala: Swedish Medical Products Agency; 2015. [cited 2022 Nov 24]. Available from: https://www.lakemedelsverket.se/48d6d0/globalassets/dokument/behandling‐och‐forskrivning/behandlingsrekommendationer/behandlingsrekommendation/behandlingsrekommendation‐astma.pdf [Google Scholar]
- 46. Equal care [Internet]. Stockholm: National board of Health and Welfare; 2004. [cited 2023 Jan 15]. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint‐dokument/artikelkatalog/ovrigt/2004‐103‐3_20041033.pdf [Google Scholar]
- 47. Andersen YMF, Egeberg A, Skov L, Thyssen JP. Demographics, healthcare utilization and drug use in children and adults with atopic dermatitis in Denmark: a population‐based cross‐sectional study. J Eur Acad Dermatol Venereol. 2019;33(6):1133–1142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
According to Swedish Law, the data cannot be placed in a public available repository. Researchers can after ethical approval from the Swedish Ethical Review Authority (https://etikprovningsmyndigheten.se) apply for data from the National Board of Health and Welfare, Sweden (www.socialstyrelsen.se). We will consider proposals for research collaborations. Enquiries can be submitted to the corresponding author (elin.dahlen@ki.se).


