Abstract
Dermatomyositis (DM) is an autoimmune idiopathic inflammatory myopathy with characteristic dermatologic manifestations. Myositis-specific autoantibodies (MSAs) delineate DM subtypes and their prognoses. Uncommonly, patients present with distinct clinical features of DM, including photosensitive dermatitis, heliotrope rash, Gottron’s papules, and nailfold changes; however, their autoimmune serology is negative for expected MSAs. Herein, we describe two unconventional cases of seronegative, amyopathic MDA5-DM and offer potential explanations, including fluctuating antibody levels, non-MSA pathophysiology, and limitations in current immunoassays.
Keywords: Dermatomyositis, seronegative, MDA5, autoimmune
Introduction
Dermatomyositis (DM) is an autoimmune disorder characterized by progressive muscle weakness and distinct dermatologic manifestations. Hallmark skin lesions include Gottron’s papules, heliotrope rash, and photosensitive dermatitis, with nailfold changes such as dilated capillary loops and capillary dropout. Systemic manifestations can include interstitial lung disease (ILD), cardiac anomalies, and dysphagia. 1 The pathogenesis of DM involves genetic predispositions, environmental triggers, and immune-mediated responses targeting the dermoepidermal junction and perifascicular areas of muscles, manifesting as skin and muscle pathology. 1
The diagnosis of DM integrates clinical evaluation with laboratory tests, electromyography, and biopsies of skin and muscle. 2 Myositis-specific autoantibody (MSA) serology provides critical insight into phenotype and prognosis. 3 For instance, the presence of anti-MDA5 protein is associated with an increased risk of rapidly progressive ILD. 1
The treatment of DM is immunosuppression, which aims to manage symptoms and prevent disease progression. Modern advancements include biologic therapies targeting specific immune pathways. 3
Uncommonly, patients can present with clinically evident DM, yet have no serologic evidence of disease. With patient consent, we reviewed medical records from 2017 to 2024 and presented two such cases of seronegative DM.
Patient one
A 70-year-old female presented with a six-month history of pruritic, erythematous, scaly, and ill-defined plaques on the face, eyelids, arms, and trunk. Accompanying symptoms included subjective bilateral leg muscle weakness and persistent dyspnea. A previous 5-day course of prednisone 50 mg and topical corticosteroids was ineffective.
Skin biopsy revealed lichenoid lymphocytic infiltration with vacuolar interface dermatitis, however, initial autoimmune serology was negative, including anti-nuclear antibodies (ANA) and MSA panels. C-reactive protein (CRP), creatine kinase (CK), free light chains (FLC), and IgG were normal, with slightly low IgM. Despite the absence of confirmatory serology, the clinical presentation was consistent with DM. (Figure 1a).
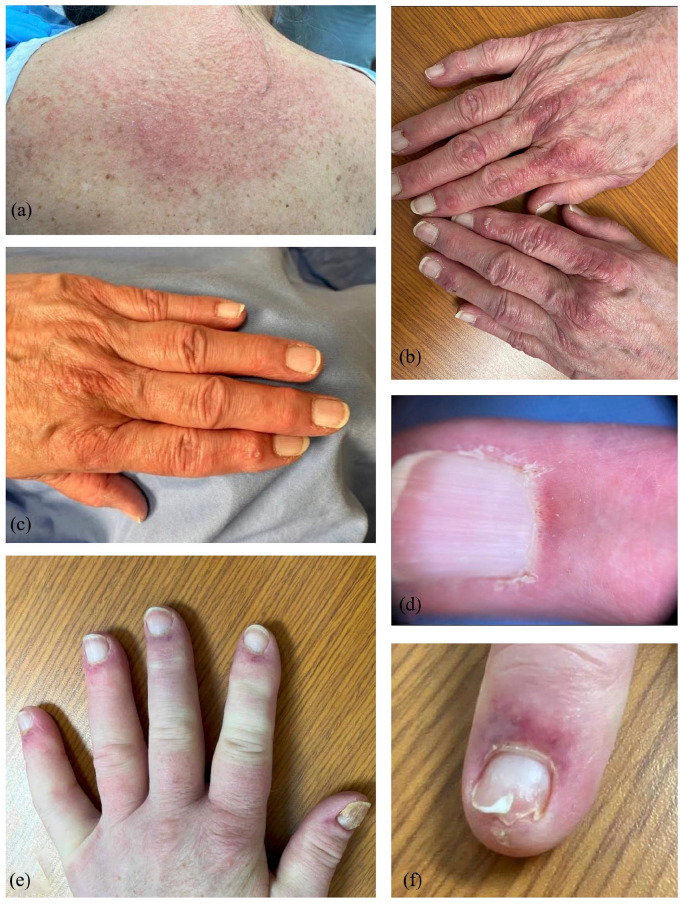
Figure 1.
Clinical presentation of seronegative dermatomyositis in two patients. (a–d) shows (a) shawl sign, (b) Gottron’s papules, (c) digital ulcerations, and (d) capillary dropout in patient one, (e) and (f) show periungual erythema with onycholysis and edema in patient two.
Distinct treatments with methotrexate, azathioprine, and hydroxychloroquine were not tolerated, and her condition advanced to include digital ulcerations. Her hands exhibited bilateral Gottron’s papules, dystrophic cuticles, and periungual erythema, with nailfold examination revealing dilated capillaries and capillary dropout. Her photodistributed skin findings, heliotrope rash, dyspnea, and proximal leg weakness also persisted. (Figure 1(b)–(d)).
Repeat autoimmune serology returned positive for speckled ANA and weak positive double-stranded DNA (dsDNA), but extractable nuclear antigen (ENA), MSA, and scleroderma panels remained negative. Consultations were sought by rheumatology, neurology, and pulmonology. Pelvic magnetic resonance imaging (MRI), CT chest, pulmonary function tests (PFT), and electromyography (EMG) were non-contributory, suggesting osteoarthritis as the explanation; therefore, muscle biopsy was not pursued. She was diagnosed with early, amyopathic MDA5-DM, and achieved remission with mycophenolate mofetil 1 g twice daily, and a tapered course of prednisone 30 mg daily.
Patient two
A 65-year-old female with a history of mixed connective tissue disease, Sjogren’s syndrome, and Raynaud’s phenomenon presented with a 2-year history of pruritic, tender, periorbital erythema and edema. Management included hydroxychloroquine and azathioprine. A previous skin biopsy showed vacuolar interface dermatitis with increased dermal mucin, consistent with cutaneous lupus. Autoimmune workup had thus far been positive for RF, ENA (anti-SSA), and intermittently positive for anti-dsDNA.
Hand examination revealed bilateral edema and erythema, dystrophic nails, thin nail plates, and onycholysis affecting all digits. Dermoscopy showed periungual erythema with dilated capillary loops. Her nails partially responded to itraconazole; however, the swelling and erythema of her digits and her nailfold signs persisted. Her periorbital rash initially responded to doxycycline but eventually returned. She denied both a change in exercise tolerance and localized muscle weakness, with no muscle weakness on examination.
She later developed digital synovitis, prompting a rheumatology referral and a diagnosis of possible psoriatic arthritis. Azathioprine was discontinued, and methotrexate was initiated; however, both methotrexate and hydroxychloroquine were poorly tolerated and later replaced with increasing doses of prednisone, from 20 to 75 mg, at various intervals over 2 years. Repeat hand examination displayed erythematous, edematous fingers, and evidence of a resolving digital ulceration. Microvascular examination showed enlarged, bushy capillaries associated with microhemorrhages and severe disorganization (Figure 1(e) and (f)).
Subsequent workup returned positive for ENA, anti-SSA/SSB, ANCA, and anti-dsDNA. IgG, beta-2 microglobulin, and FLC were elevated, while C3 and C4 were mildly decreased, consistent with overlap syndromes and Sjogren’s, despite a previously negative RNP. Scleroderma and MSA panels were entirely negative, with the exception of elevated Ro52. She was diagnosed with amyopathic MDA5-DM overlap syndrome and started on Rituximab. PFT was normal; further investigations for underlying malignancy, including CT chest and whole-body PET, are pending.
Discussion
Seronegative DM poses a unique diagnostic challenge due to the absence of MSAs typically associated with the disease. This report describes two patients with clinically convincing evidence of DM, yet no biochemical, imaging, or biopsy confirmation. Patient One, a 70-year-old female who exhibited Gottron’s papules, heliotrope rash, and nailfold pathology, achieved remission with prednisone and MMF. Patient Two, a 65-year-old female, displayed digital ulceration, bushy capillaries with severe disorganization and microhemorrhages, and was treated with Rituximab.
Similar cases of seronegative DM and their diagnostic challenges have been documented.4–6 Comparative studies within seronegative cohorts 7 and between seropositive counterparts 8 suggest that seronegativity might confer a more favorable prognosis, although the risk of infection, malignancy, and death remains.9–12 Continued awareness, diligent follow-up, and tailored management of seronegative cases remain crucial.
A possible explanation for seronegative DM describes fluctuations in antibody levels, both intrinsically during active and quiescent disease phases, 4 and extrinsically due to immunosuppressive treatment.4,13 The timing of investigations may not align with a seropositive window.
In addition, non-MSA pathophysiology may contribute to disease but is not captured by currently available methods. 14 These include vascular injury, interferon pathways, 4 and complement pathways distinct from detectable antibody-mediated pathways. 14
Furthermore, the identification of various MSAs has helped define subsets of DM, 15 but there are still complexities. Some MSA-positive patients never develop myositis,15,16 while others exhibit characteristic DM phenotypes without the corresponding MSA. It’s possible that an undetected autoantibody contributes to specific DM manifestations or that known MSAs may have a broader range of associated phenotypes than currently recognized. Further research on DM serum samples is needed to explore these possibilities.
Finally, deficiencies in immunoassays, such as insufficient expression of target antigens in cultured cell lines, 4 are also possible. Our institution uses the Myositis Panel Plus (MitogenDx, Calgary, Canada) a line immunoassay that includes Jo-1, Mi2-α, Mi2-β, MDA5, NXP2, TIF1γ, PL7, PL12, PM/Scl75, PM/Scl100, Ku, SRP, EJ, OJ, Ro52/TRIM21, SAE1, HMGCR, and NT5C1A/Mup44. 17 However, assay procedure and composition vary across sites, hindering direct comparisons between research groups and further complicating the study of seronegative DM.
This report illustrates the complexities of seronegative presentations of classically seropositive diseases, specifically in the context of DM, and underscores the need for a multidisciplinary approach in clinically suspected diagnoses. Despite advancements in serologic markers of DM, the presentation, investigative results, and outcomes remain on a spectrum.
Acknowledgments
The authors would like to acknowledge Dr. Mohamed Osman who performed nailfold video capillaroscopy for these patients.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Patient consent: Written informed consent for patient information and images to be published was provided by the patients.
ORCID iDs: Lauren C Balogh  https://orcid.org/0009-0005-3418-640X
https://orcid.org/0009-0005-3418-640X
Robert Gniadecki  https://orcid.org/0000-0002-2310-8300
https://orcid.org/0000-0002-2310-8300
References
- 1. DeWane ME, Waldman R, Lu J. Dermatomyositis: clinical features and pathogenesis. J Am Acad Dermatol 2020; 82: 267–281. [DOI] [PubMed] [Google Scholar]
- 2. Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017; 76(12): 1955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Waldman R, DeWane ME, Lu J. Dermatomyositis: diagnosis and treatment. J Am Acad Dermatol 2020; 82: 283–296. [DOI] [PubMed] [Google Scholar]
- 4. Ahmed A, Scarborough R, Gabriela R, et al. Seronegative dermatomyositis presenting with features of anti-MDA5 subtype. J Cutan Pathol 2018; 45: 851–854. [DOI] [PubMed] [Google Scholar]
- 5. Harazeen A, Walter B, Li X, et al. When a painful rash keeps recurring: a case of seronegative amyopathic dermatomyositis without neurological sequelae. Cureus 2023; 15(7):e42727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamjani MH, Forbes J, Issac L, et al. A report of seronegative clinically amyopathic dermatomyositis. Int J Res Dermatol 2021; 7(1): 123–125. [Google Scholar]
- 7. Li S, Gao S, Chen Q, et al. Clinical heterogeneities and prognoses of patients with myositis-specific antibody negative dermatomyositis: a retrospective study in China. Clin Exp Rheumatol 2022; 40: 284–291. [DOI] [PubMed] [Google Scholar]
- 8. Xing X, Gan Y, Mo W, et al. Clinical and immunological characteristics and prognosis of patients with autoantibody negative dermatomyositis: a case-control study. Clin Rheumatol 2024; 43: 1145–1154. [DOI] [PubMed] [Google Scholar]
- 9. Ng SN, Ng CM, Ciang CO, et al. Anti-MDA5 idiopathic inflammatory myositis (IIM) confers poor prognosis but negative myositis-specific antibody (MSA) is not benign either. Ann Rheum Dis 2019; 78(Suppl 2): 851. [Google Scholar]
- 10. Pye A, Goenaga-Vazquez Y, Hebert AA. Dermatomyositis associated with ovarian cancer, negative screening, and negative antibodies. J Am Acad Dermatol 2024; 90(1). AB149 (abstract). [Google Scholar]
- 11. High WA, Cohen JB, Murphy BA, et al. Fatal interstitial pulmonary fibrosis in anti-Jo-1–negative amyopathic dermatomyositis. J Am Acad Dermatol 2003; 49(2): 295–298. [DOI] [PubMed] [Google Scholar]
- 12. De Jesus AV, De Souza JM. Clinically amyopathic dermatomyositis associated with cutaneous ulcerations: a case-based review. Ann Med Surg 2024; 86: 1210–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cross LS, Aslam A, Misbah SA. Antinuclear antibody-negative lupus as a distinct diagnostic entity—does it no longer exist?. QJM 2004; 97(5): 303–308. [DOI] [PubMed] [Google Scholar]
- 14. Kamperman RG, van der Kooi AJ, de Visser M, et al. Pathophysiological mechanisms and treatment of dermatomyositis and immune-mediated necrotizing myopathies: a focused review. Int J Mol Sci 2022; 23(8): 4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tansley S, Gunawardena H. The evolving spectrum of polymyositis and dermatomyositis—moving towards clinicoserological syndromes: a critical review. Clin Rev Allergy Immunol 2014; 47: 264–273. [DOI] [PubMed] [Google Scholar]
- 16. Mourot A, Bourré-Tessier J, Nadon V, et al. Seronegative Polyarthritis in association with anti-NXP2 antibodies: a case series. J Rheumatol 2023; 50: 153–155. [DOI] [PubMed] [Google Scholar]
- 17. MitogenDx. Autoimmune myopathy/myositis test panel plus. MitogenDx. https://mitogendx.com/diagnostic-tests/autoimmune-myopathy-myositis-test-panel-plus/ (accessed 20 August 2024). [Google Scholar]