Generalized pustular psoriasis (GPP) is a severe inflammatory cutaneous disease characterized by widespread pustules, edema, erythema, fever, and systemic inflammation. Chinese data indicate that the prevalence and incidence of GPP follow a bimodal age distribution, with peaks in the 0–3 year age group and the 30–39 year age group. 1 In the 0–3 year age group, the prevalence was 0.927 and the incidence rate was 0.742 per 100 000 population‐years. 1 Interleukin (IL)‐36 plays an important role in GPP by activating nuclear factor‐κB (NF‐κB) and mitogen‐activated protein kinase (MAPK) signal pathways. 2 Chemokines (CXCL8, CXCL1, CXCL2, etc.), cytokines (IL‐1β, tumor necrosis factor TNF‐α, IL‐6, IL‐23, IL‐17, etc.), and activated cells (e.g., keratinocyte, neutrophils, dendritic cells, etc.) are also involved. 3 Acitretin, cyclosporine, methotrexate, and etanercept were recommended as first‐line treatments for children with GPP in 2012 by the American National Psoriasis Foundation. 4 However, recent findings suggest that biological agents targeting IL‐36, TNF‐α, IL‐17, IL‐23, or their receptors might be more promising options than conventional drugs. 5 Clinical trials and case series have also demonstrated the superiority of biological agents in adult and older pediatrics with GPP, including spesolimab, etanercept, adalimumab, secukinumab, brodalumab, and others. 6 However, these biological agents, have only been approved by the FDA for children aged 4 years or older, and there is still limited data on their use in GPP patients under 4 years old. 7 , 8
Here, we report on six children under the age of 4 years who were treated for GPP using etanercept from July 2021 to January 2023. The diagnosis of GPP was based on the criteria set by the Japanese Dermatological Association (JDA). 9 A complete blood count, liver function test, kidney function test, antibody test for viral hepatitis B and C, T‐SPOT.TB test, antinuclear antibody (ANA) test, and chest X‐ray were conducted before initiating treatment with etanercept. These tests were repeated every six months following the initial administration of etanercept. The initial dose of etanercept was 0.8 mg/kg per week, which was gradually adjusted to every 10 days, every 2 weeks, and every 3 weeks as the patients achieved stable remission. The severity of GPP was assessed using the JDA severity index for GPP 9 and Generalized Pustular Psoriasis Area and Severity Index (GPPASI). The primary efficacy endpoint was achieving a JDA severity index of 1 or 0 at week 4. The secondary efficacy endpoint was achieving a 75% reduction in baseline GPPASI scores (GPPASI 75) at week 4. Relapse was defined as an increase of 3 points or more on the JDA score.
Six girls with GPP were recruited, with ages ranging from 3 to 47 months and onset ages ranging from 20 days to 17 months (Table 1). One patient (patient 2) was simultaneously diagnosed with Acrodermatitis continua of Hallopeau (ACH). Homozygous or compound heterozygous mutations of the IL‐36RN gene were found in five patients. No variants were detected in the CARD14, IL‐1RN, AP1S3, and MPO genes. Five patients had previously failed to respond to prednisone or acitretin (Table 1).
TABLE 1.
Clinical information and etanercept treatment protocols for the six patients with generalized pustular psoriasis (GPP)
| Patient number | Sex | Onset age (m) | Treatment age (m) | IL‐36RN mutation | Previous therapies | Dose of etanercept † (mg) | Treatment duration (w) | Treatment Protocol |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 17 | 18 | c.115+6T>C hom | Predisone | 10 | 24 | 10 mg QW for 12 weeks, Q10D for 4 weeks, Q2W for 8 weeks |
| 2 | F | 2 | 47 | c.115+6T>C hom/c.227C>T het | Acitretin | 8 | 24 | 8 mg QW for 24 weeks, and then switched to adalimumab |
| 3 | F | 5 | 8 | c.334G>A het/c.115+6T>C het | Predisone | 6.25 | 60 | 6.25 mg QW for 24 weeks, Q10D for 12 weeks, Q2W for 12 weeks, Q3W for 12 weeks |
| 4 | F | 8 | 9 | No | Predisone | 8 | 24 | 8 mg QW for 12 weeks, Q10D for 10 weeks, Q2W for 2 weeks |
| 5 | F | 2 | 3 | c.115+6T>C hom | Predisone | 5 | 28 | 5 mg QW for 16 weeks, Q2W for 12 weeks |
| 6 | F | 20 ‡ | 6 | c.115+6T>C hom | No | 4 | 24 | 4 mg QW for 14 weeks, Q10D for 10 weeks |
The dose was calculated as 0.8 mg/kg for one administration.
The onset age is 20 days.
Abbreviations: hom, homozygous mutation; het, heterozygous mutation; m, months; QW, quaque week; Q10D, quaque 10 days; Q2W, quaque two weeks; Q3W, quaque three weeks; w, weeks.
At baseline, one patient was classified as mild, two as moderate, and three as severe based on the JDA score. After 4 weeks of treatment, all six patients achieved a JDA score of 1 and GPPASI 75 (Table 2). After 12 weeks of treatment, all six patients reached a JDA score of 0 and a 100% reduction in their baseline GPPASI scores. In addition, the response was maintained for 24 weeks.
TABLE 2.
Outcome of etanercept in the six patients with generalized pustular psoriasis (GPP)
| Patient number | JDA severity index score | GPPASI score | Follow‐up period (m) | Relapse | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 w | 12 w | 24 w | Baseline | 4 w | 12 w | 24 w | |||
| 1 | 6 | 1 | 0 | 0 | 27.5 | 3.6 | 0 | 0 | 12 | Yes |
| 2 | 11 | 1 | 0 | 0 | 22.8 | 3.2 | 0 | 0 | − | − |
| 3 | 12 | 1 | 0 | 0 | 22.5 | 3.0 | 0 | 0 | 10 | No |
| 4 | 8 | 1 | 0 | 0 | 32.0 | 3.3 | 0 | 0 | 12 | No |
| 5 | 7 | 1 | 0 | 0 | 18.4 | 1.6 | 0 | 0 | − | − |
| 6 | 12 | 1 | 0 | 0 | 28.0 | 4.0 | 0 | 0 | − | − |
Abbreviations: GPPASI, Generalized Pustular Psoriasis Area and Severity Index; JDA, Japanese Dermatological Association; m, months; w, weeks; −, not available.
The median duration of etanercept treatment was 24 weeks (range: 24–60 weeks). One patient (Patient 2) switched to adalimumab after 24 weeks due to no further improvement in ACH lesions. She achieved almost complete clearance of the ACH lesions with adalimumab over an additional 24 weeks (Figure 1). Two patients (Patient 5 and Patient 6) were still receiving etanercept treatment. The remaining three patients were followed for 10–12 months. One patient (Patient 1) experienced a relapse at 4 months after discontinuing the drug and was subsequently re‐administrated etanercept. However, the 16‐week treatment did not yield satisfactory results. Adalimumab was then applied, resulting in a JDA score of 1 at 4 weeks, which was maintained for 16 weeks. During the treatment period, two patients (Patient 1 and Patient 3) experienced a transient increase in ANA titer, up to 1:100, without any related symptoms. No other adverse events were recorded.
FIGURE 1.
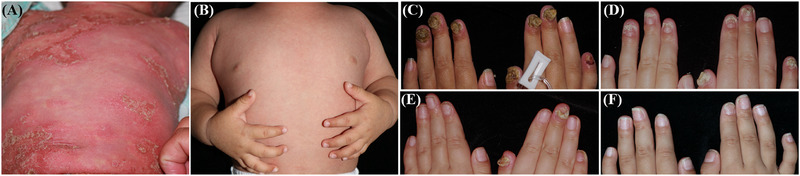
The clinical presentation of Patient 4 and Patient 2. Generalized erythema, papules, and pustules on extremities before treatment (A) and after 8 weeks of treatment (B) with etanercept in Patient 4; erythema, swelling, pustules, and crusts on the fingertips and nails before treatment (C), after 4 weeks of treatment (D), and after 24 weeks of treatment (E) with etanercept, as well as after 24 weeks of treatment with adalimumab (F) in Patient 2.
Etanercept is a widely used TNF receptor inhibitor for treating plaque psoriasis, with demonstrated long‐term efficacy and safety in children aged 4 years and older. 10 However, data on its use in children under 4 years old are limited. In this study, we observed rapid and sustained improvement of GPP in young children treated with etanercept, regardless of IL‐36RN gene mutation status. During the 24–60 week treatment period, no children experienced opportunistic infections, tuberculosis, or demyelination events. Two patients had a transient increase in ANA titer. These findings suggest that etanercept may be effective and safe for children under 4 years old with GPP.
Considering the adverse events and financial burden, the etanercept was gradually tapered and eventually discontinued upon achieving stable complete remission. However, in adults with GPP, Bellinato et al. 11 reported a relapse rate of 62% in the group discontinuing etanercept, compared to only 9% in the group continuing treatment. Fortunately, the median time to relapse was 184 days, and 52% of patients experienced clinical benefit upon re‐administration of etanercept. 11 In our study, we attempted to extend the treatment interval upon achieving stable remission, resulting in no recurrence during treatment. During the long follow‐up period (10–12 months) for three patients, only one experienced a relapse. Thus, tapering and discontinuing etanercept when patients achieve stable remission is worth considering. Unfortunately, the relapsed patient who did not experience satisfactory improvement upon re‐administration of etanercept subsequently required a switch to adalimumab.
ETHICAL APPROVAL
The study was approved by the Beijing Children's Hospital Ethics Review Board ([2024]‐E‐087‐R). Informed consent was obtained from all participants’ parents.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Chen Y, Wang Z, Miao C, Xu Z, Xiang X. Etanercept: A viable treatment option for young children with generalized pustular psoriasis. Pediatr Investig. 2024;8:295–298. 10.1002/ped4.12448
Contributor Information
Zigang Xu, Email: zigangxupek@163.com.
Xin Xiang, Email: 13691145181@163.com.
REFERENCES
- 1. Feng JN, Guo JZ, Zhang Q, Zhuo L, Xu L, Liu LL, et al. Higher prevalence of generalized pustular psoriasis in Asia? A population‐based study using claim data in China and a systematic review. Dermatology. 2023;239:195‐205. DOI: 10.1159/000528850 [DOI] [PubMed] [Google Scholar]
- 2. Bernardo D, Thaçi D, Torres T. Spesolimab for the treatment of generalized pustular psoriasis. Drugs. 2024;84:45‐58. DOI: 10.1007/s40265-023-01988-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iznardo H, Puig L. Exploring the role of IL‐36 cytokines as a new target in psoriatic disease. Int J Mol Sci. 2021;22:4344. DOI: 10.3390/ijms22094344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robinson A, Van Voorhees AS, Hsu S, Korman NJ, Lebwohl MG, Bebo BF Jr, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288. DOI: 10.1016/j.jaad.2011.01.032 [DOI] [PubMed] [Google Scholar]
- 5. Chen Y, Xiang X, Wang Z, Miao C, Xu Z. The update of treatment strategies in pediatrics with generalized pustular psoriasis in China. Pediatr Investig. 2023;7:191‐198. DOI: 10.1002/ped4.12395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krueger J, Puig L, Thaçi D. Treatment options and goals for patients with generalized pustular psoriasis. Am J Clin Dermatol. 2022;23:51‐64. DOI: 10.1007/s40257-021-00658-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cuperus E, Koevoets R, van der Smagt JJ, Toonstra J, de Graaf M, Frenkel J, et al. Juvenile interleukin‐36 receptor antagonist deficiency (DITRA) with c.80T>C (p.Leu27Pro) mutation successfully treated with etanercept and acitretin. JAAD Case Rep. 2018;4:192‐195. DOI: 10.1016/j.jdcr.2017.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pereira TM, Vieira AP, Fernandes JC, Antunes H, Basto AS. Anti‐TNF‐alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350‐352. DOI: 10.1159/000096202 [DOI] [PubMed] [Google Scholar]
- 9. Fujita H, Terui T, Hayama K, Akiyama M, Ikeda S, Mabuchi T, et al. Japanese guidelines for the management and treatment of generalized pustular psoriasis: the new pathogenesis and treatment of GPP. J Dermatol. 2018;45:1235‐1270. DOI: 10.1111/1346-8138.14523 [DOI] [PubMed] [Google Scholar]
- 10. Paller AS, Siegfried EC, Pariser DM, Rice KC, Trivedi M, Iles J, et al. Long‐term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280‐287. DOI: 10.1016/j.jaad.2015.09.056. e1‐3. [DOI] [PubMed] [Google Scholar]
- 11. Bellinato F, Girolomoni G, Gisondi P. Relapse of psoriasis in patients who asked to discontinue etanercept after achieving a stable clinical remission. Br J Dermatol. 2019;181:1319‐1320. DOI: 10.1111/bjd.18225 [DOI] [PubMed] [Google Scholar]


