ABSTRACT
Importance
Gastrointestinal complications are common perioperative complications in children with congenital heart disease (CHD), and as near‐infrared reflectance spectroscopy (NIRS) provides a non‐invasive, real‐time monitoring of regional tissue oxygenation, we envisioned monitoring and preventing the development of gastrointestinal complications through the use of NIRS.
Objective
To assess the utility of NIRS for predicting gastrointestinal complication risks and determining optimal initial feeding times in infants post‐cardiac surgery.
Methods
This retrospective study included 65 infants with CHD treated at our hospital from January 2021 to January 2022. We collected and analyzed data on mesenteric regional venous and arterial oxygen saturation, arterial partial pressure of oxygen, first lactic acid levels, timing of initial enteral feeding, and incidence of gastrointestinal complications.
Results
Out of 65, 61 infants were eligible for inclusion (four cases were excluded). Infants with gastrointestinal complications post‐surgery showed significantly lower mesenteric NIRS values and earlier feeding times compared to those without complications (55.5 ± 3.3 vs. 59.6 ± 6.3, P = 0.029; and 59.8 ± 6.7 vs. 66.9 ± 5.7, P = 0.002, respectively). Multivariable binary logistic regression analysis revealed that mesenteric NIRS readings at the time of initial feeding independently predicted gastrointestinal complications (odds ratio, 0.802; 95% confidence interval, 0.693–0.928; P = 0.003). receiver operating characteristic curve analysis indicated a significant predictive value of mesenteric NIRS at initial feeding time (area under the curve: 0.799), with a suggested critical threshold of 63.1% (93% sensitivity, 70% specificity). Pearson correlation test confirmed a significant association between mesenteric NIRS at initial feeding time and the establishment of enteral feeding.
Interpretation
Mesenteric NIRS measurements at the time of initial feeding provide a reliable method for identifying infants at risk of gastrointestinal complications following cardiac surgery and can inform decisions regarding the timing of initial postoperative feeding.
Keywords: Congenital cardiac disease, Mesenteric NIRS, Gastrointestinal complications, Initial feeding
Gastrointestinal complications are common perioperative complications in children with congenital heart disease, and as near‐infrared reflectance spectroscopy (NIRS) provides a non‐invasive, real‐time monitoring of regional tissue oxygenation, we envisioned monitoring and preventing the development of gastrointestinal complications through the use of NIRS.
INTRODUCTION
The incidence of congenital heart disease (CHD) is approximately 6 per 1000 live births, necessitating surgical or percutaneous intervention for many patients during infancy or childhood. 1 Gastrointestinal complications are notably prevalent in infants undergoing cardiac surgery, with necrotizing enterocolitis incidence ranging from 3.3% to 13% in neonates with cardiac conditions. 2 Feeding intolerance is especially common in children with single ventricle physiology. 3 Contributing factors to these gastrointestinal complications include abnormal blood shunting and ischemia‐reperfusion injury, reduced mesenteric blood flow, and tissue hypoxia, potentially occurring pre‐, intra‐, or post‐operatively. 4 , 5 Common gastrointestinal complications include necrotizing enterocolitis, gastrointestinal bleeding, feeding intolerance, intestinal ischemia, intestinal perforation, and pancreatitis. 6 Ferguson et al. 2 documented a high incidence of gastrointestinal complications following surgical intervention for CHD in infants and children.
Monitoring the balance between tissue oxygen supply and consumption is vital, and methods such as central or mixed venous oxygen saturation, jugular or superior vena cava oxygen saturation, and arterial oxygen saturation are commonly employed. 7 However, many of these techniques are invasive and unsuitable for continuous monitoring, posing risks of missing hemodynamic instabilities or critical events. Near‐infrared reflectance spectroscopy (NIRS) offers a non‐invasive, real‐time monitoring of regional tissue oxygenation, capable of detecting early changes in organ perfusion. 8 , 9 , 10 The estimation of mesenteric venous oxygen saturation by NIRS correlates with intestinal perfusion markers (gastric tension measurements) in postoperative children with CHD. 11 NIRS measurements of venous oxygen saturation in the brain and kidneys post‐cardiac surgery are associated with prognosis and complications. 12 , 13 , 14 , 15
Despite increasing use and acceptance in gastrointestinal research, specific diagnostic or intervention thresholds (critical values) for mesenteric venous oxygen saturation remain undefined in clinical practice, leading to significant uncertainty in NIRS‐based intensive care decision‐making. 16 This study aims to estimate NIRS values in infants with postoperative gastrointestinal complications following cardiac surgery and utilize these values to guide feeding decisions.
METHODS
Ethical approval
The study was approved by the Fujian Children's Hospital Ethics Committee and adhered to the Declaration of Helsinki (Ethical Approval Number: 2022ETKLR12025). The informed consent was waived.
Participants
This retrospective case‐control study focused on infants who underwent cardiac surgery from January 2021 to January 2022. Inclusion criteria were infants successfully operated on using cardiopulmonary bypass, weighing ≤10 kg, and aged under 1 year. We excluded premature and low birth weight infants, those with congenital extracardiac abnormalities (including digestive system anomalies), and infants with preexisting gastrointestinal diseases like gastroesophageal reflux disease. A total of 65 infants met these criteria. Data collected included age, weight, gender, CHD type, initial lactic acid levels, arterial oxygen saturation (SaO2), and arterial partial pressure of oxygen (PaO2). Patients were also evaluated using the Risk Adjustment in Congenital Heart Surgery‐1 (RACHS‐1) classification system.
Data collection
Two researchers independently conducted data collection. Continuous monitoring of mesenteric venous oxygen saturation in the abdomen was achieved using USB storage devices. We extracted demographic data, medical variables, and gastrointestinal complications from the patients’ medical records. SaO2, PaO2, and initial lactic acid levels were sourced from nursing records and clinical data. The first lactic acid measurement was defined as the lactic acid level measured when the patient was admitted to the ICU ward. 17 The first‐time lactic acid levels > 5 mmol/L were defined as high lactic acid levels. 18 Bedside nurses documented physiological and clinical variables hourly.
Near‐infrared reflectance spectroscopy
The INVOS 5100C device (Medtronic Inc.) is utilized for monitoring regional tissue perfusion. This device operates by emitting near‐infrared light at two wavelengths, 730 and 805 nm, which align with the absorption spectra of oxygenated and deoxygenated hemoglobin. Mesenteric NIRS operates based on the principle that near‐infrared light can penetrate biological tissues to a certain depth. Different molecules in the tissue, such as oxygenated and deoxygenated hemoglobin, have unique absorption spectra in the near‐infrared range. When near‐infrared light is emitted into the tissue, these molecules absorb specific wavelengths of the light, altering the light's intensity as it passes through and reflects back from the tissue. The sensor then detects the light that has been reflected back from the tissue. Based on the differential absorption of oxygenated and deoxygenated hemoglobin, the device calculates the relative concentrations of each within the blood flowing through the mesenteric tissue. The NIRS device uses the detected light intensities to compute the ratio of oxygenated to deoxygenated hemoglobin, providing a measure of regional oxygen saturation (rSO2) in the mesenteric tissue. The conversion of the detected light signal into rSO2 value is achieved through a formula that accounts for the absorption coefficients of oxygenated and deoxygenated hemoglobin at different wavelengths. The rSO2 is calculated as the ratio of oxygenated hemoglobin to the total hemoglobin (oxygenated plus deoxygenated), typically expressed as a percentage. This value reflects the balance between oxygen delivery and consumption in the tissue, providing a real‐time, continuous measure of tissue oxygenation. 19 , 20 , 21
Patients commenced NIRS monitoring via the INVOS system within 30 min of ICU admission post‐surgery. Given that the infra‐umbilical abdomen is the most reliable site for sensor placement to optimally assess tissue oxygenation in areas supplied by the mesenteric arteries, specially trained nurses positioned the self‐adhesive sensor 1–2 cm below the patient's umbilicus, linking it to the NIRS monitor. 22 The near‐infrared spectroscopy values we obtain are continuously measured and displayed as a graph. Yet, for recording purposes, readings are taken every 5 min, and these readings reflect the average value of changes during each 5‐min interval.
In our ICU, attending physicians evaluate the appropriate timing for postoperative initial feeding. Feeding via a nasogastric tube is initiated as soon as possible once the patient's hemodynamics stabilize and peripheral perfusion is satisfactory, taking into account intestinal peristalsis and blood flow (the peripheral perfusion was evaluated by measuring skin perfusion such as capillary refilling time, peripheral limb temperature and lactic acid level). However, enteral feeding is deferred if the patient exhibits high lactate levels or requires high doses of vasoactive drugs (e.g., epinephrine > 0.03 µg·kg−1·min−1, norepinephrine, vasopressin), indicating unstable hemodynamics. 23 The initial feeding rate is typically set at 0.5 mL·kg−1·h−1 and is increased by 0.5 mL·kg−1·h−1 every 6 or 12 h based on the patient's digestive and absorptive capacity. All infants received sufentanil for pain relief within 24 h post‐surgery and midazolam for sedation (2–3 µg·kg−1·min−1) during endotracheal intubation. For infants, particularly those intubated or with poor voluntary sucking, continuous pumping is employed for enteral feeding. We start with formula or breast milk, prioritizing the latter. The feeding amount and mode are tailored to each infant's specific needs, transitioning from continuous pumping to transoral feeding as the patient's condition improves, with the goal of achieving complete enteral nutrition. Low cardiac output, cardiac arrest, and cardiac dysfunction are considered risk factors impacting feeding rate decisions. Feeding intolerance, currently without a uniform definition, is considered if one or more of the following occur: frequent vomiting (≥3 times/day), significant abdominal distension, stagnation or reduction of food intake for over 3 days, excessive gastric retention, repeated unplanned feeding discontinuations, or diarrhea (defecation frequency > 6 times/day). Diagnosis of feeding intolerance requires at least two of these symptoms or signs and is made by a physician, with documentation in the medical record. For cases where feeding intolerance halts feeding for over 4 h, we recommence at a lower rate (0.5 mL·kg−1·h−1) and gradually increase it every 6 or 12 h, based on the infant's condition.
Mesenteric venous blood oxygen saturation was monitored from ICU admission until either 7 days post‐establishment of stable total enteral nutrition or transfer out of the ICU. Postoperative mesenteric NIRS was calculated as the mean value recorded within 1–4 h after ICU admission. Initial feeding NIRS was determined as the average mesenteric NIRS value within 3 h of initiating feeding. The mesenteric arteriovenous difference in oxygen (mAVDO2) was estimated by subtracting mesenteric venous oxygen saturation from arterial oxygen saturation. The time required to establish enteral feeding was defined as the duration from ICU admission until the patient tolerated enteral feeds at a rate of >2 mL·kg−1·h−1. Necrotizing enterocolitis was diagnosed by attending physicians based on clinical, biochemical, and radiological evidence, following the revised Bell staging criteria. 24 Gastrointestinal complications encompassed both necrotizing enterocolitis and feeding intolerance. 25 All data, collected according to these definitions, were sourced from clinical records by the research team.
Statistical analysis
All statistical analyses were performed using SPSS software, version 23.0 (SPSS Inc.). For measurement data, a normality test was first conducted. Data conforming to a normal distribution were analyzed using the t‐test, while those not conforming were subjected to a nonparametric test (Mann‐Whitney test). Univariate analysis was employed to identify potential factors associated with gastrointestinal complications. Factors with a P‐value less than 0.15 in the univariate analysis were further analyzed using multivariate logistic regression. The diagnostic capability of initial feeding time mesenteric NIRS for gastrointestinal complications was assessed using the area under the receiver operating characteristic (ROC) curve. Additionally, a Pearson correlation test was conducted to explore the relationship between mesenteric NIRS at initial feeding time and other clinical variables. A P‐value of less than 0.05 was considered statistically significant for all analyses.
RESULTS
In this study, 65 infants were initially included, but the final analysis was conducted on 61 infants. Four cases were excluded: two developed necrotizing small bowel colitis in the neonatal period, one was complicated with hypertrophic pyloric stenosis, and one had incomplete data. Of the 61 infants analyzed, 36 (59.0%) were male, and 20 (32.8%) were neonates (This term refers to the period from the delivery of the fetus from the mother up to 28 days post‐birth 26 ). The mean value of body weight was 4.4 ± 1.5 kg. Detailed clinical features are presented in Table 1. Regarding the RACHS‐1 categorization of the cardiac surgeries performed, the distribution was as follows: category 1 (4.9%), category 2 (86.9%), category 3 (4.9%), and category 4 (3.3%), with no cases in categories 5 and 6. Specific surgeries included atrial septal defect repairs and partial anomalous pulmonary venous connection corrections in category 1; ventricular septal defect repairs, pulmonary valve stenosis corrections, total anomalous pulmonary venous drainage corrections, and tetralogy of Fallot corrections in category 2; Cor triatriatum corrections and switch procedures in category 3; and aortic arch dysplasia corrections in category 4. Gastrointestinal complications occurred in 14 cases (23.0%), with 3 cases (4.9%) diagnosed as necrotizing enterocolitis and 11 cases (18.0%) exhibiting feeding intolerance.
TABLE 1.
General characteristics of the participants
| Variable | n = 61 |
|---|---|
| Male | 36 (59.0) |
| Neonate | 20 (32.8) |
| Weight (kg) | 4.4 ± 1.5 |
| PaO2 | 96.2 ± 10.5 |
| mAVDO2 | 31.3 ± 4.9 |
| Operation time (h) | 3.3 ± 0.9 |
| CPB time (min) | 81.5 ± 26.0 |
| Aortic clamping time (min) | 37.9 ± 5.4 |
| Mesenteric rSO2 (Postoperative) | 58.6 ± 6.0 |
| Mesenteric rSO2 (Initial feeding) | 65.2 ± 6.6 |
| First‐time lactic acid (mmol/L) | 2.8 ± 0.7 |
| Establishment time of enteral feeding (h) | 44.3 ± 12.8 |
| Mechanical ventilation time (days) | 3.0 ± 1.1 |
| Intensive care unit stay (days) | 4.0 ±1.9 |
| RACHS‐1 | |
| 1 | 3 (4.9) |
| 2 | 53 (86.9) |
| 3 | 3 (4.9) |
| 4 | 2 (3.3) |
| 5 | 0 (0) |
| 6 | 0 (0) |
Note: Data are shown as n (%) or mean ± standard deviation.
Abbreviations: CPB, cardiopulmonary bypass; mAVDO2, mesenteric arteriovenous difference of oxygen (It was estimated by subtracting mesenteric NIRS saturation from arterial oxygen saturation); mesenteric rSO2, mesenteric regional venous oxygen saturation (the value obtained from the NIRS monitor); PaO2, arterial oxygen partial pressure; RACHS‐1, Risk Adjustment for Congenital Heart Surgery‐1 classification system.
Univariate analysis identified several factors associated with gastrointestinal complications in infants, including body weight, postoperative and initial feeding time mesenteric NIRS, RACHS‐1 category, mAVDO2, and first‐time lactic acid level (P < 0.15). Subsequently, multivariate binary logistic regression analysis, adjusting for confounding factors, revealed that mesenteric NIRS at initial feeding time independently correlated with gastrointestinal complications (odds ratio: 0.802; 95% confidence interval: 0.693–0.928; P = 0.003). (Table 2)
TABLE 2.
Logistic regression analysis of risk factors for gastrointestinal (GI) complications
| Variable |
GI complication (n = 14) |
No GI complication (n = 47) |
P † | Adjusted OR | 95%CI | P ‡ |
|---|---|---|---|---|---|---|
| Gender (male) | 7 (52.4) | 28 (60.4) | 0.525 | |||
| Neonate | 5 (23.8) | 15 (31.9) | 0.791 | |||
| Weight (kg) | 3.8 ± 0.9 | 4.6 ± 1.5 | 0.094 | 0.312 | 0.090–1.081 | 0.066 |
| RACHS‐1 >3 | 1 (21.4) | 1 (6.6) | 0.118 | |||
| PaO2 | 93.1 ± 12.5 | 97.2 ± 9.8 | 0.207 | |||
| mAVDO2 | 33.3 ± 4.9 | 30.7 ± 4.8 | 0.091 | 0.873 | 0.655–1.165 | 0.356 |
| Mesenteric rSO2 (Postoperative) | 55.5 ± 3.3 | 59.6 ± 6.3 | 0.029 | 0.850 | 0.710–1.019 | 0.079 |
| Mesenteric rSO2 (Initial feeding) | 59.8 ± 6.7 | 66.9 ± 5.7 | 0.002 | 0.802 | 0.693–0.928 | 0.003 |
| First‐time lactic acid (mmol/L) | 3.1 ± 0.9 | 2.7 ± 0.7 | 0.063 | 0.847 | 0.697–1.030 | 0.096 |
| CPB time (min) | 88.0 ± 26.5 | 78.3 ± 25.3 | 0.218 | |||
| Aortic clamping time (min) | 39.4 ± 5.8 | 37.4 ± 5.2 | 0.223 |
Note: Data are shown as n (%) or mean ± standard deviation.
Univariate regression
Multivariate logistic regression.
Abbreviations: CPB, cardiopulmonary bypass; GI, gastrointestinal; mAVDO2, mesenteric arteriovenous difference of oxygen (It was estimated by subtracting mesenteric NIRS saturation from arterial oxygen saturation); mesenteric rSO2, mesenteric regional venous oxygen saturation (the value obtained from the NIRS monitor); OR, odds ratio; PaO2, arterial oxygen partial pressure; RACHS‐1, Risk Adjustment for Congenital Heart Surgery‐1 classification system.
The ROC curve analysis demonstrated the predictive value of mesenteric NIRS at initial feeding time in identifying gastrointestinal complications. The area under the ROC curve was 0.799, indicating a significant predictive ability (Figure 1). Notably, with a cutoff value of 63.1%, the sensitivity for predicting complications was 93%, and the specificity was 70%. These results suggest that mesenteric NIRS at initial feeding time is an effective indicator for assessing gastrointestinal outcomes in infants.
FIGURE 1.
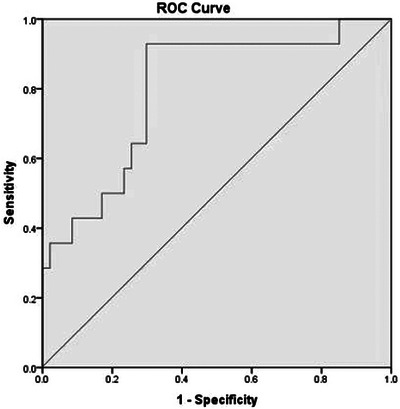
Receiver operating characteristic (ROC) curve for mesenteric near‐infrared reflectance spectroscopy (NIRS) predicting gastrointestinal complications. The area under the ROC curve for mesenteric NIRS of 0.799 suggests that mesenteric NIRS is a reasonably good discriminator of adverse gastrointestinal complications.
Furthermore, our study investigated factors associated with mesenteric NIRS at initial feeding time. Employing the Pearson correlation test, we found a significant relationship only with the time taken to establish enteral feeding (r = −0.363, P = 0.004). Specifically, higher mesenteric NIRS values at initial feeding time were associated with shorter times to establish enteral feeding. In contrast, mesenteric NIRS at the time of initial feeding did not correlate with ICU length of stay (r = −0.145, P = 0.266) and duration of mechanical ventilation (r = −0.066, P = 0.614).
DISCUSSION
In our study, the strongest correlation was found between mesenteric NIRS and gastrointestinal complications. Further analysis indicated that mesenteric NIRS at initial feeding time independently predicted the occurrence of these complications. This aligns with findings from Iliopoulos et al., who suggested that mesenteric NIRS upon admission could inform feeding strategies and intestinal protection in children post‐cardiopulmonary bypass. 16 However, our study did not assess mesenteric NIRS preoperatively due to resource constraints, and none of our subjects had preoperative gastrointestinal complications, highlighting a need for further research to establish reliability. Additionally, Dave et al. 27 reported that in premature infants without CHD, NIRS is useful for monitoring visceral area oxygenated tissue changes to guide feeding. Considering that children with CHD post‐surgery have a high incidence of gastrointestinal complications due to factors like reduced cardiac function and blood redistribution, postoperative mesenteric NIRS monitoring could be pivotal in predicting and preemptively addressing these complications. 6 Furthermore, intraoperative factors such as cardiopulmonary bypass and hypothermia may adversely affect gastrointestinal mucosa, elevating the risk of complications. 28 Hence, future studies should explore the relationship between intraoperative mesenteric NIRS and clinical outcomes to enhance our understanding and management of these risks.
For infants exhibiting mesenteric NIRS values below 63.1% at initial feeding time, we recommend either delayed feeding or initiating with a minimal amount of continuous feeding, while closely monitoring their abdominal condition. If no signs of feeding intolerance are observed, gradual increases in feeding may be considered. Our study also indicated that early postoperative mesenteric NIRS values do not reliably predict the onset of gastrointestinal complications. This may be attributed to reduced cardiac function shortly after surgery and the impact of high doses of vasoactive drugs, leading to gastrointestinal vasoconstriction and subsequently diminished gastrointestinal perfusion.
In our multifactor regression analysis, mesenteric NIRS at initial feeding time emerged as a strong predictor of gastrointestinal complications, with an area under the curve of 0.799. Specifically, a mesenteric NIRS value below 63.1% at initial feeding time proved to be an excellent indicator of potential adverse gastrointestinal outcomes. Consequently, future clinical practices should incorporate mesenteric NIRS measurements to assess gastrointestinal function as part of the feeding management protocol. Additionally, integrating these measurements with assessments of gastrointestinal peristalsis and intestinal blood flow could further enhance the accuracy and effectiveness of gastrointestinal function evaluation and feeding guidance.
Other research has established a link between initial feeding time mesenteric NIRS and the timing for establishing enteral feeding. 29 These studies highlight gastrointestinal function as a crucial prognostic indicator in critically ill patients, with intestinal dysfunction often precipitating multiple organ dysfunction. This is corroborated by our findings that infants with lower mesenteric NIRS values exhibit compromised gastrointestinal function and reduced ability to adapt to enteral nutrition. Our study also determined that mesenteric NIRS at the initial feeding time did not correlate with the length of ICU stay. However, we observed that infants with gastrointestinal complications generally had longer ICU stays. This suggests that factors influencing ICU stay duration are multifaceted, and mesenteric NIRS alone is not an independent predictor of this outcome. Given that our study cohort predominantly comprised infants, the durations of postoperative mechanical ventilation and ICU stays were notably longer compared to older children. The administration of sedative and analgesic medications during mechanical ventilation could impact the gastrointestinal motility in these patients, potentially influencing the study outcomes. However, the uniformity in the type and dosage of sedative and analgesic medications for all patients during their ICU stay mitigated the impact of these confounding factors to a certain extent. This highlights the need for more standardized criteria in future studies to minimize errors caused by such variables, thereby enhancing the comparability of study results.
Gastrointestinal complications are a common and serious concern in congenital cardiac surgery. 2 , 3 , 4 , 5 Traditionally, postoperative gastrointestinal function monitoring and feeding decisions have been based largely on clinical observations, lacking routine, safe, and continuous monitoring tools. Assessing intestinal mucosal perfusion offers a direct method to identify patients at risk for gastrointestinal complications, thereby aiding clinicians in formulating objective feeding and intestinal protection strategies. 11 Mesenteric NIRS stands out as a simple, noninvasive technique providing real‐time, continuous data on gastrointestinal blood perfusion. These attributes render it an ideal tool for postoperative care guidance. Our study underscored the utility of mesenteric NIRS at initial feeding time in effectively identifying infants at risk for postoperative gastrointestinal complications, thereby informing decisions on the timing of initial feeding.
There are some limitations in the monitoring process of mesenteric NIRS. If there is variability loss in mesenteric readings or a decrease in rSO2 values, other factors must be considered. For example, gases or liquids in the abdominal cavity or intestinal lumen may lengthen the infrared path length and distort rSO2 readings, as the penetration depth of most near‐infrared sensors is approximately 1.5 cm. The presence of feces, especially meconium, may act as chromophores and reduce the accuracy of rSO2 readings. In addition, intestinal structure and function may affect the trend of rSO2. For instance, intestinal motility caused by enteral feeding, changes in intestinal wall thickness, or alterations in vascular and tissue blood flow can also lead to fluctuations and variability in rSO2. 30 Therefore, a cautious evaluation of potential pathologies or related events is crucial when assessing changes in mesenteric oxygenation trends while ensuring the correct probe placement and accurate skin adhesion.
Additionally, our study faced several other limitations. Firstly, it was a single‐institution, retrospective study with a relatively small sample size. Despite a complication rate of approximately 23% and a power value (1‐β) around 0.75 at an α of 0.05, suggesting reasonable statistical efficiency, these findings warrant further validation through large‐scale, randomized trials. Secondly, there was considerable variability in disease types among our study cohort. Future research would benefit from including more patients with similar characteristics, ideally those with single anatomical lesions, to ensure greater homogeneity. Thirdly, the majority of our patients underwent ventricular septal defect repair, and the number of neonates—who are at the highest risk for necrotizing enterocolitis—was limited. Furthermore, due to the predominance of infants and toddlers among our center's patients and the scarcity of samples from older children, the study was limited to infants with a body weight below 10 kg.
Gastrointestinal complications in infants following cardiac surgery show a significant independent correlation with mesenteric NIRS measurements at the initial feeding time. The assessment of mesenteric NIRS at this critical juncture is effective in identifying infants at risk for postoperative gastrointestinal complications, which has a guiding value for determining the initial feeding time.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
We highly acknowledge the following researchers’ contributions: Xiuxuan Ruan, Qiliang Zhang, Zewei Lin, Wangsheng Dai, Liwen Wang, Zengchun Wang, and Lingshan Yu.
Xie W, Liu Y, Zeng Y, Zheng Y, Chen Q. Utilizing mesenteric near‐infrared reflectance spectroscopy to predict gastrointestinal complication risks and optimize feeding strategies in infants undergoing cardiac surgery. Pediatr Investig. 2024;8:287–294. 10.1002/ped4.12437
[Correction added on 10 July 2024, after first online publication: Author affiliation order changed]
REFERENCES
- 1. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890‐1900. DOI: 10.1016/s0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- 2. Ferguson LP, Gandiya T, Kaselas C, Sheth J, Hasan A, Gabra HO. Gastrointestinal complications associated with the surgical treatment of heart disease in children. J Pediatr Surg. 2017;52:414‐419. DOI: 10.1016/j.jpedsurg.2016.10.052 [DOI] [PubMed] [Google Scholar]
- 3. Weiss SL, Gossett JG, Kaushal S, Wang D, Backer CL, Wald EL. Comparison of gastrointestinal morbidity after Norwood and hybrid palliation for complex heart defects. Pediatr Cardiol. 2011;32:391‐398. DOI: 10.1007/s00246-010-9864-9 [DOI] [PubMed] [Google Scholar]
- 4. Reilly PM, Wilkins KB, Fuh KC, Haglund U, Bulkley GB. The mesenteric hemodynamic response to circulatory shock: an overview. Shock. 2001;15:329‐343. DOI: 10.1097/00024382-200115050-00001 [DOI] [PubMed] [Google Scholar]
- 5. Luce WA, Schwartz RM, Beauseau W, Giannone PJ, Boettner BL, Cheatham JP, et al. Necrotizing enterocolitis in neonates undergoing the hybrid approach to complex congenital heart disease. Pediatr Crit Care Med. 2011;12:46‐51. DOI: 10.1097/PCC.0b013e3181e3250c [DOI] [PubMed] [Google Scholar]
- 6. Mirzaei M, Mirzaei S, Sepahvand E, Rahmanian Koshkaki A, Kargar Jahromi M. Evaluation of complications of heart surgery in children with congenital heart disease at Dena Hospital of Shiraz. Glob J Health Sci. 2015;8:33‐38. DOI: 10.5539/gjhs.v8n5p33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tweddell JS, Ghanayem NS, Mussatto KA, Mitchell ME, Lamers LJ, Musa NL, et al. Mixed venous oxygen saturation monitoring after stage 1 palliation for hypoplastic left heart syndrome. Ann Thorac Surg. 2007;84:1301‐1310. discussion 1310‐1311. DOI: 10.1016/j.athoracsur.2007.05.047 [DOI] [PubMed] [Google Scholar]
- 8. Wong JJ, Chen CK, Moorakonda RB, Wijeweera O, Tan TYS, Nakao M, et al. Changes in near‐infrared spectroscopy after congenital cyanotic heart surgery. Front Pediatr. 2018;6:97. DOI: 10.3389/fped.2018.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mintzer JP, Moore JE. Regional tissue oxygenation monitoring in the neonatal intensive care unit: evidence for clinical strategies and future directions. Pediatr Res. 2019;86:296‐304. DOI: 10.1038/s41390-019-0466-9 [DOI] [PubMed] [Google Scholar]
- 10. Weber F, Scoones GP. A practical approach to cerebral near‐infrared spectroscopy (NIRS) directed hemodynamic management in noncardiac pediatric anesthesia. Paediatr Anaesth. 2019;29:993‐1001. DOI: 10.1111/pan.13726 [DOI] [PubMed] [Google Scholar]
- 11. Kaufman J, Almodovar MC, Zuk J, Friesen RH. Correlation of abdominal site near‐infrared spectroscopy with gastric tonometry in infants following surgery for congenital heart disease. Pediatr Crit Care Med. 2008;9:62‐68. DOI: 10.1097/01.PCC.0000298640.47574.DA [DOI] [PubMed] [Google Scholar]
- 12. Carra G, Flechet M, Jacobs A, Verstraete S, Vlasselaers D, Desmet L, et al. Postoperative cerebral oxygen saturation in children after congenital cardiac surgery and long‐term total intelligence quotient: a prospective observational study. Crit Care Med. 2021;49:967‐976. DOI: 10.1097/CCM.0000000000004852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim MJ, Baek JS, Kim JA, Cha SG, Yu JJ. Cerebral and somatic oxygen saturation in neonates with congenital heart disease before surgery. J Clin Med. 2021;10:2455. DOI: 10.3390/jcm10112455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang D, Ouyang C, Zhao X, Cui B, Dai F, Meng L, et al. Renal tissue desaturation and acute kidney injury in infant cardiac surgery: a prospective propensity score‐matched cohort study. Br J Anaesth. 2021;127:620‐628. DOI: 10.1016/j.bja.2021.06.045 [DOI] [PubMed] [Google Scholar]
- 15. Adams PS, Vargas D, Baust T, Saenz L, Koh W, Blasiole B, et al. Associations of perioperative renal oximetry via near‐infrared spectroscopy, urinary biomarkers, and postoperative acute kidney injury in infants after congenital heart surgery: should creatinine continue to be the gold standard? Pediatr Crit Care Med. 2019;20:27‐37. DOI: 10.1097/PCC.0000000000001767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iliopoulos I, Branco RG, Brinkhuis N, Furck A, LaRovere J, Cooper DS, et al. Mesenteric near‐infrared spectroscopy and risk of gastrointestinal complications in infants undergoing surgery for congenital heart disease. Cardiol Young. 2016;26:772‐780. DOI: 10.1017/S1047951115001365 [DOI] [PubMed] [Google Scholar]
- 17. Ganushchak YM, Maessen JG, de Jong DS. The oxygen debt during routine cardiac surgery: illusion or reality? Perfusion. 2002;17:167‐173. DOI: 10.1191/0267659102pf561oa [DOI] [PubMed] [Google Scholar]
- 18. Huang JS, Chen YK, Lin SH, Chen Q, Cao H, Zheng YR. A comparison of the changes in serum lactate between surgical repair and transthoracic device closure of ventricular septal defects in pediatric patients. Front Cardiovasc Med. 2023;9:961997. DOI: 10.3389/fcvm.2022.961997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marin T, Moore J. Understanding near‐infrared spectroscopy: an update. Crit Care Nurs Clin North Am. 2024;36:41‐50. DOI: 10.1016/j.cnc.2023.08.001 [DOI] [PubMed] [Google Scholar]
- 20. Martini S, Corvaglia L. Splanchnic NIRS monitoring in neonatal care: rationale, current applications and future perspectives. J Perinatol. 2018;38:431‐443. DOI: 10.1038/s41372-018-0075-1 [DOI] [PubMed] [Google Scholar]
- 21. Guo Y, Wang Y, Marin T, Easley K, Patel RM, Josephson CD. Statistical methods for characterizing transfusion‐related changes in regional oxygenation using near‐infrared spectroscopy (NIRS) in preterm infants. Stat Methods Med Res. 2019;28:2710‐2723. DOI: 10.1177/0962280218786302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ledo A, Aguar M, Núñez‐Ramiro A, Saénz P, Vento M. Abdominal near‐infrared spectroscopy detects low mesenteric perfusion early in preterm infants with hemodynamic significant ductus arteriosus. Neonatology. 2017;112:238‐245. DOI: 10.1159/000475933 [DOI] [PubMed] [Google Scholar]
- 23. del Castillo SL, McCulley ME, Khemani RG, Jeffries HE, Thomas DW, Peregrine J, et al. Reducing the incidence of necrotizing enterocolitis in neonates with hypoplastic left heart syndrome with the introduction of an enteral feed protocol. Pediatr Crit Care Med. 2010;11:373‐377. DOI: 10.1097/PCC.0b013e3181c01475 [DOI] [PubMed] [Google Scholar]
- 24. Bell RS, Graham CB, Stevenson JK. Roentgenology and clinical manifestations of neonatal necrotizing enterocolitis. Experience with 43 cases. Am J Roentgenol Radium Ther Nucl Med. 1971;112:123‐134. DOI: 10.2214/ajr.112.1.123 [DOI] [PubMed] [Google Scholar]
- 25. Ghanayem NS, Dearani JA, Welke KF, Béland MJ, Shen I, Ebels T. Gastrointestinal complications associated with the treatment of patients with congenital cardiac disease: consensus definitions from the multi‐societal database committee for pediatric and congenital heart disease. Cardiol Young. 2008;18:240‐244. DOI: 10.1017/S1047951108002989 [DOI] [PubMed] [Google Scholar]
- 26. Beune IM, Bloomfield FH, Ganzevoort W, Embleton ND, Rozance PJ, van Wassenaer‐Leemhuis AG, et al. Consensus based definition of growth restriction in the newborn. J Pediatr. 2018;196:71‐76.e1. DOI: 10.1016/j.jpeds.2017.12.059 [DOI] [PubMed] [Google Scholar]
- 27. Dave V, Brion LP, Campbell DE, Scheiner M, Raab C, Nafday SM. Splanchnic tissue oxygenation, but not brain tissue oxygenation, increases after feeds in stable preterm neonates tolerating full bolus orogastric feeding. J Perinatol. 2009;29:213‐218. DOI: 10.1038/jp.2008.189 [DOI] [PubMed] [Google Scholar]
- 28. Booker PD, Romer H, Franks R. Gut mucosal perfusion in neonates undergoing cardiopulmonary bypass. Br J Anaesth. 1996;77:597‐602. DOI: 10.1093/bja/77.5.597 [DOI] [PubMed] [Google Scholar]
- 29. Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post‐injury multiple organ failure: the role of the gut. Shock. 2001;15:1‐10. DOI: 10.1097/00024382-200115010-00001 [DOI] [PubMed] [Google Scholar]
- 30. Marin T, Moore JE. Mesenteric oxygenation changes associated with necrotizing enterocolitis and pneumoperitoneum after multiple blood transfusions: a case report. Adv Neonatal Care. 2018;18:121‐127. DOI: 10.1097/ANC.0000000000000461 [DOI] [PubMed] [Google Scholar]


