ABSTRACT
Importance
The diagnosis of congenital auricular deformity often relies on the clinical experience of clinicians, leading to a high incidence of misdiagnosis and missed diagnosis due to the lack of quantitative diagnostic criteria.
Objective
To characterize auricle morphology in newborns from southern China and explore the underlying etiology of congenital auricle deformity.
Methods
A total of 636 neonates (1272 ears) with less than seven days old were included. The auricles of each infant were measured and photographed. The relationship between maternal factors and the occurrence of congenital auricle deformity was analyzed.
Results
The incidence of auricular deformity in southern China was 79.87%. Helical rim deformity and mixed deformity had the highest incidence (17.30% each), while cryptotia had the lowest incidence (0.31%). Among mixed deformities, lop ear with conchal crus ear was the most common (22.73%). Each type of auricle deformity had distinct measurement indicators: the vertical distance of cephalo‐auricular was 73.97% longer and cephalo‐superaurale was 70.00% longer in protruding ears compared to normal auricle; the vertical distance of cephalo‐auricular was 10.96% less in lop ears, 15.07% less in conchal crus ears, and 41.1% longer in cup ears; the distance between helix and antihelix was 22.35% less in constricted ear, 12.94% greater in helical rim deformity, and 43.53% greater in Stahl's ear. Family history of hereditary ear deformity and paternal smoking were significant factors associated with ear deformity in southern China.
Interpretation
The incidence of auricle deformities is high in southern China, with significant differences in the morphometric structures of different auricle types.
Keywords: Auricle deformity, Southern China, Newborns, Incidence, Risk factor
This study characterizes newborn ear morphology in southern China, revealing a 79.87% incidence of auricular deformities, with distinct morphometric differences among types and associations with family history and paternal smoking.
INTRODUCTION
The incidence of auricle deformities in newborns varies greatly in different regions, ranging from 29% in the US to 57.63% in southern China. 1 , 2 , 3 Auricle deformities in children may lead to psychological and behavioral problems and social maladjustment. 4 , 5 Fortunately, non‐invasive correction of ear deformities at an early age is available. 6 , 7 , 8 Accurate diagnosis and classification of auricle deformity therefore is vital for early treatment. At present, the diagnosis of congenital auricular deformity is largely based on the clinical experience of clinicians. The lack of quantitative diagnostic criteria inevitably leads to a high incidence of misdiagnosis and missed diagnosis. The insufficient awareness of congenital auricular deformity by obstetricians and pediatricians also hinders early identification. On this basis, we conducted an observational study on one‐week‐old infants, aiming to provide a measurement basis for the description of neonatal auricle morphology and to correlate various abnormal factors of the mother and fetus throughout pregnancy with the deformities.
METHODS
Ethical approval
This clinical trial was approved by the Ethics Committee of Zhujiang Hospital and the General Hospital of the Southern Theater Command (ethics batch number: 2022‐KY‐289‐02). Informed consent from the legal guardians of all the infants recruited in this study has been obtained.
Research objects
Newborns within one week of age in southern China from March 2023 to July 2023 were recruited. The participants were mainly from the Department of Obstetrics and Gynecology of the General Hospital of the Southern Theater Command and the Department of Obstetrics, Gynecology and Neonatology of Zhujiang Hospital of Southern Medical University. The auricles of each infant were measured and photographed (Photography and measurement of all infants were conducted from day 2 to day 5 postpartum, with deliberate avoidance of immediate post‐delivery documentation due to potential ear pressure during pregnancy or passage through a narrow birth canal). A total of 637 neonates with a total of 1274 ears were measured. One newborn had microtia and was excluded from the group. Therefore, a total of 636 cases were enrolled, with a total of 1272 ears.
Measurement methods
The participants took the supine position, fully exposing both auricles. The measurer stood on the side of the subject's head and used a medical beauty measuring ruler and a protractor to measure the appearance of the physiognomic ear length in centimeters (cm), physiognomic ear breadth (cm), morphologic ear length (cm), morphologic ear breadth (cm), the vertical distance of cephalo‐auricular (cm), antihelix angle (°), the distance between helix and antihelix (cm), and the vertical distance of cephalo‐superaurale (cm) (Figure 1). According to our clinical observation, different auricle deformities have different effects on the shape of the auricle. In order to describe the shape of the auricle more comprehensively, we made several adjustments to the auricle measurement indicators and added three new indicators as follows: The antihelix angle – the angle formed by the antihelix at the extension of crus of helix; the distance between helix and antihelix – the length from the deepest point above tragion to the junction between superior crura of the antihelix and inferior crura of antihelix; the vertical distance of cephalo‐superaurale – the vertical distance from the superaurale to cephalo.
FIGURE 1.
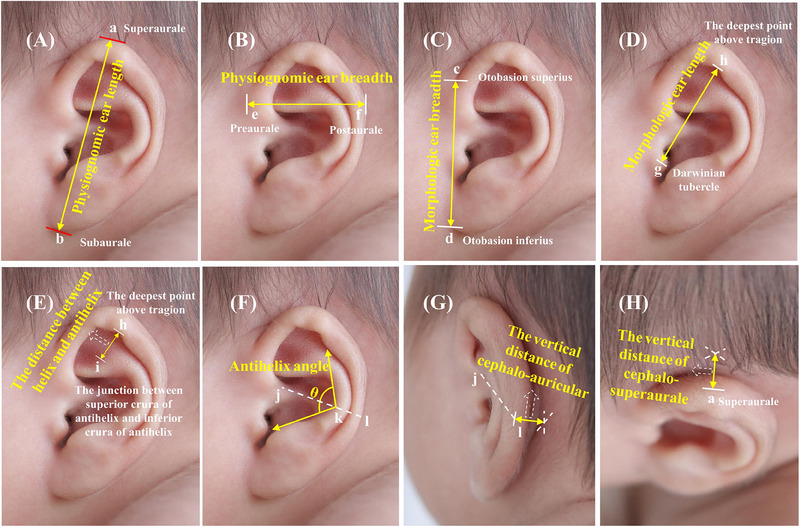
Measurements of the ear (left). (A) Physiognomic ear length: the distance from the superaurale to the subaurale. (B) Physiognomic ear breadth: the distance from the preaurale to the postaurale. (C) Morphologic ear breadth: the distance from the otobasion superius to the otobasion inferius. (D) Morphologic ear length: the distance from the Darwinian tubercle to the deepest point above the tragion. (E) Distance between helix and antihelix. (F) Antihelix angle. (G) The vertical distance of cephalo‐auricular: the vertical distance from the point where the extension of the crus of the helix intersects with the helix to cephalo. (H) The vertical distance of cephalo‐superaurale.
The precision of data retention is maintained at 0.1 mm, while angle measurements are preserved to an accuracy of 0.1°. A pixel grid with a side length of 1 cm × 1 cm was placed in front of the ear as a reference for image acquisition, so as to facilitate the reference ratio of subsequent two‐dimensional measurement. In order to avoid artificial error, all data collection was carried out by XQZ on unified standard photographs according to standardized data collection specifications and was repeated twice. The camera was kept parallel to the side of the newborn's head, so that the auricle is in the middle position, and the height of the auricle accounts for 1/2 of the height of the photo taken. The width of the auricle was in the middle and accounted for 1/3 of the width of the entire photo. One picture was taken for each ear, and the pixel grid is attached. The data were saved on a fixed hard disk after being checked by 3 researchers.
Technical measurement error and reliability coefficient analysis
Two measurements were taken for measurement indicators. Relative and absolute technical error of measurement (TEM) was calculated. 9 In the first stage, the difference between the first and second measurements was determined (deviation between them) for each anthropometric point measured by the same investigator. In the second stage, the deviations obtained were raised to the second power. In the third stage, the results of the second stage were summed (Σd2) and applied to equation 1 in order to obtain the absolute TEM.
| (1) |
In the fourth stage, the absolute TEM was transformed into relative TEM in order to obtain the error expressed as a percentage corresponding to the total average of the variable to be analyzed. Equation 2 was then used. The variable average value (VAV) was calculated as follows: the arithmetic means of the mean between two measurements obtained of each volunteer for the same measurement index was first calculated. This procedure was performed for all objects and the obtained averages were summed up and divided by 636 (number of subjects), generating VAV.
| (2) |
The TEM of physiognomic ear length, physiognomic ear breadth, morphologic ear length, morphologic ear breadth, the vertical distance of cephalo‐auricular, the distance between helix and antihelix, and the vertical distance of cephalo‐superaurale < 1 mm was within the acceptable range. The absolute TEM ranged from 0.1 to 1.0 mm. These technical errors are all below 1 mm, indicating the reliability and repeatability of the measurement. The TEM of the antihelix angle, which remained smaller than 3°, was within the acceptable range. The absolute TEM range was 0.5 to 3°. The technical errors of the antihelix angle were all below 3°. The relative TEM for all measurements was below 9.63%, indicating negligible subjectivity in evaluating measurements using this method.
Statistical methods
Statistical analysis was performed using SPSS 26.0, and the test level was α = 0.05. In order to avoid intraobserver variability, all relevant data were measured by a single investigator (XQZ). Data were represented as the mean± standard deviation or n (%). The Student's t‐test was used to compare the difference between the normal auricle and the deformed auricle. Univariate analysis was performed on each variable, and a chi‐square test was used, with a statistical significance level of α = 0.05. Odds ratio (OR) and 95% confidence intervals (CI) were estimated using stepwise logistic regression analysis. P < 0.05 was considered statistically significant.
RESULTS
Data overview
Participants were diagnosed by an expert group, consisting of 3 professors with over 20 years of experience in plastic surgery or otolaryngology, based on photos. The diagnoses were compared with the results from the EarWell Neonatal Ear Shape Correction System (Becon Medical Ltd.). The expert group made the same diagnoses as the Earwell system. Among 636 neonates (1272 ears), 128 exhibited normal auricular structures, while 508 (79.87%) displayed abnormal auricular morphology (827 ears). Additionally, bilateral auricular abnormalities were observed in 319 participants. A specific diagnosis for each pinna morphology was obtained (Figure 2). Helical rim deformity and mixed deformity had the highest incidence (17.30% each), while cryptotia had the lowest incidence (0.31%). There were many subtypes of mixed deformity. The most common was lop ear with conchal crus ear (22.73%), helical rim deformity with conchal crus ear (13.18%), protruding ear with constricted ear (8.18%), constricted ear with cup ear (8.18%), and protruding ear with cup ear (5.91%).
FIGURE 2.
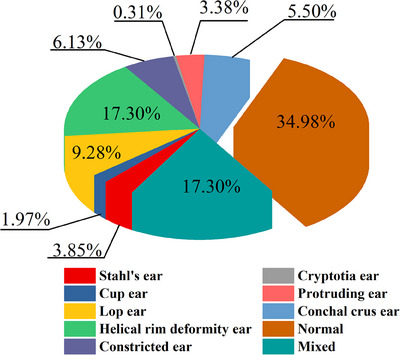
Distribution of 1272 ears.
We did not find a statistically significant difference (P > 0.05) in the incidence or types of ear deformities between the left and right ears (Figure S1). Furthermore, there was no significant difference (P > 0.05) in the incidence or types of ear deformities between males and females (Figure S2).
Morphological description of normal pinnae and ear deformities in newborns
We measured the normal auricle shape of newborns using various indicators (Table S1) and compared them to deformed auricles (Table 1). The deformities exhibited diverse manifestations (Figure 3), and each specific deformity showed distinct characteristics on different measurement indicators (Figure S3 and Table 1).
TABLE 1.
The measurement results for various auricle types
| Variable | Normal auricle | Stahl's ear | Cup ear | Lop ear | Helical rim deformity | Conchal crus ear | Constricted ear | Cryptotia | Protruding ear |
|---|---|---|---|---|---|---|---|---|---|
| Morphologic ear length (cm) | 2.22 ±0.22 | 2.50 ±0.23*** | 2.12 ±0.21* | 2.15 ±0.27* | 2.28 ±0.28** | 2.19 ±0.20 | 1.99 ±0.25*** | 2.15 ±0.31 | 2.28 ±0.29 |
| Morphologic ear breadth (cm) | 2.56 ±0.22 | 2.60 ±0.19 | 2.52 ±0.28 | 2.56 ±0.21 | 2.53 ±0.20 | 2.54 ±0.21 | 2.52 ±0.23 | 2.68 ±0.32 | 2.52 ±0.23 |
| Physiognomic ear length (cm) | 3.56 ±0.26 | 3.72 ±0.24*** | 3.44 ±0.24* | 3.39 ±0.25*** | 3.58 ±0.24 | 3.52 ±0.30 | 3.44 ±0.25*** | 3.63 ±0.22 | 3.57 ±0.27 |
| Physiognomic ear breadth (cm) | 2.12 ±0.20 | 2.22 ±0.22** | 1.98 ±0.25** | 2.13 ±0.22 | 2.13 ±0.26 | 2.18 ±0.27* | 1.92 ±0.27*** | 2.08 ±0.05 | 1.92 ±0.24*** |
| Antihelix angle (°) | 86.71 ±13.73 | 78.73 ±20.76* | 74.52 ±20.23* | 85.42 ±19.46 | 80.13 ±20.68*** | 83.73 ±20.91 | 83.28 ±18.39 | 76.25 ±17.50 | 88.47 ±21.03 |
| Vertical distance of cephalo‐auricular (cm) | 0.73 ±0.22 | 0.61 ±0.21** | 1.03 ±0.30*** | 0.65 ±0.18*** | 0.66 ±0.22*** | 0.62 ±0.20*** | 0.83 ±0.24*** | 0.63 ±0.17 | 1.27 ±0.24*** |
| Vertical distance of cephalo‐superaurale (cm) | 0.50 ±0.16 | 0.41 ±0.17*** | 0.68 ±0.23*** | 0.40 ±0.16*** | 0.48 ±0.16 | 0.47 ±0.14 | 0.44 ±0.15*** | 0.48 ±0.05 | 0.85 ±0.26*** |
| Distance between helix and antihelix (cm) | 0.85 ±0.22 | 1.22 ±0.24*** | 0.94 ±0.25* | 0.79 ±0.22** | 0.96 ±0.25*** | 0.87 ±0.24 | 0.66 ±0.20*** | 0.80 ±0.20 | 0.94 ±0.27* |
| Ratio of morphologic ear breadth to morphologic ear length | 1.16 ±0.12 | 1.05 ±0.12*** | 1.20 ±0.18 | 1.21 ±0.18** | 1.12 ±0.16** | 1.17 ±0.13 | 1.28 ±0.16*** | 1.26 ±0.22 | 1.12 ±0.13* |
| Ratio of physiognomic ear breadth to physiognomic ear length | 0.60 ±0.06 | 0.60 ±0.07 | 0.58 ±0.07 | 0.63 ±0.07*** | 0.60 ±0.07 | 0.63 ±0.14 | 0.56 ±0.09*** | 0.57 ±0.03 | 0.54 ±0.07*** |
*P < 0.05 vs. normal auricle; ** P < 0.01 vs. normal auricle; *** P < 0.001 vs. normal auricle.
FIGURE 3.
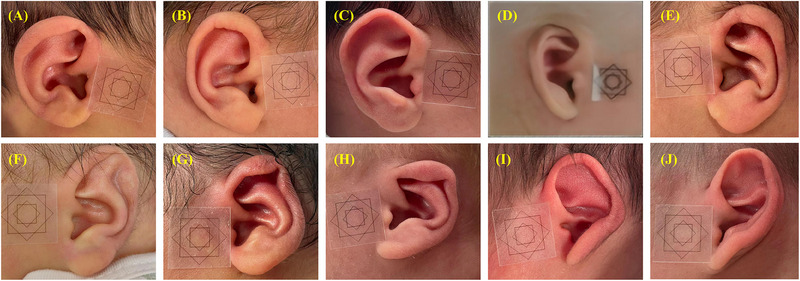
Morphology of various types of neonatal auricular within 1 week of birth. (A) Protruding ear; (B) Normal ear; (C) Helical rim deformity; (D) Cryptotia; (E) Conchal crus ear; (F) Stahl's ear; (G) Constricted ear; (H) Lop ear; (I) Cup ear; (J) Mixed deformity.
The feature of Stahl's ear is the increased morphological ear length (2.50 ± 0.23 vs. 2.22 ± 0.22, P < 0.001) and the increased distance between the helix and the antihelix (1.22 ± 0.24 vs. 0.85 ± 0.22, P < 0.001). The vertical distance of cephalo‐auricular, vertical distance of cephalo‐superaurale, and the ratio of the morphologic ear breadth to the morphological ear length were reduced (all P < 0.05).
The cup ear is characterized by a reduced antihelix angle and the increased vertical distance of cephalo‐auricular, cephalo‐superaurale, and the distance between the helix and the antihelix. The upper and lower ends of the auricle fold in half along the middle, making it a cup‐shaped side view.
For the lop ear, the vertical distance of the cephalo‐auricular was 10.96% less than in normal auricle, and the ratio of physiognomic ear breadth to physiognomic ear length was 5% larger. The upper end of the auricle hangs down like a curtain, making the whole auricle appear stubby.
Helical rim deformity showed a 12.94% greater distance between helix and antihelix (0.96 ± 0.25 vs. 0.85 ± 0.22, P < 0.001) and the vertical distance of cephalo‐auricular was 9.59% less. This is consistent with the features like flatness, non‐curling, folding, depression, and protrusion.
Conchal crus ear is characterized by a shortened vertical distance of cephalo‐auricular due to the excessive development of the crus of the helix, which extends to and merges with the antihelix a, making the antihelix abnormally hypertrophic and protruding.
The constricted ear is marked by a shortened distance between the helix and antihelix (0.66 ± 0.20 vs. 0.85 ± 0.22 cm, P < 0.001), resulting from a contracted and curled helix rim.
Cryptotia is characterized by a significantly reduced vertical distance of cephalo‐superaurale, caused by the herniation of part of the auricle at the connection between the top of the auricle and the craniofacial region, leading to the top of the auricle tilting toward the anterior point of the ear.
For protruding ear, the vertical distance of the cephalo‐auricular was 73.97% longer (1.27 ± 0.24 vs. 0.73 ± 0.22, P < 0.001) and the vertical distance of the cephalo‐superaurale was 70.00% longer (0.85 ± 0.26 vs. 0.50 ± 0.16, P < 0.001) than in normal auricle.
Etiological analyis of congenital auricle deformity
To understand the underlying etiology of the neonatal auricular deformity, a univariate analysis was performed to analyze the association between maternal factors and the neonatal auricular deformity. The results showed that familial history of ear deformity, parental smoking status, and maternal anemia disease were associated with ear malformations (P < 0.05), whereas birth weight, amniotic fluid status, mode of conception, and maternal obesity were not associated with ear malformations (P > 0.05) (Table 2).
TABLE 2.
Comparison between normal ears and deformed ears
| Variable | Normal ears (n = 445) | Deformed ears (n = 827) | χ2 | P |
|---|---|---|---|---|
| Fetal position | 1.942 | 0.379 | ||
| Shoulder presentation | 13 (2.92) | 21 (2.54) | ||
| Pillow presentation | 422 (94.83) | 776 (93.83) | ||
| Breech presentation | 10 (2.25) | 30 (3.63) | ||
| Fetal congenital diseases | 0.703 | 0.402 | ||
| Yes | 18 (4.04) | 26 (3.14) | ||
| No | 427 (95.96) | 801 (96.86) | ||
| Amniotic fluid condition | 1.445 | 0.485 | ||
| Amniotic fluid muddy | 10 (2.25) | 22 (2.66) | ||
| Polyhydramnios | 11 (2.47) | 13 (1.57) | ||
| Normal amniotic fluid | 424 (95.28) | 792 (95.77) | ||
| Gestational age | 1.459 | 0.227 | ||
| 28–36 weeks | 23 (5.17) | 57 (6.89) | ||
| 37–41 weeks | 422 (94.83) | 770 (93.11) | ||
| ≥ 42 weeks | 0 | 0 | ||
| Neonatal weight | 5.488 | 0.064 | ||
| < 2500 g | 28 (6.29) | 54 (6.53) | ||
| 2500–4000 g | 406 (91.24) | 766 (92.62) | ||
| ≥ 4000 g | 11 (2.47) | 7 (0.85) | ||
| Mode of Conception | 0.010 | 0.920 | ||
| Assisted | 15 (3.37) | 27 (3.26) | ||
| Natural | 430 (96.63) | 800 (96.74) | ||
| Delivery method | 0.632 | 0.427 | ||
| Natural birth | 308 (69.21) | 590 (71.34) | ||
| Cesarean delivery | 137 (30.79) | 237 (28.66) | ||
| Mother's age | 0.910 | 0.635 | ||
| <31 years | 218 (48.99) | 410 (49.58) | ||
| 31‒35 years | 152 (34.16) | 294 (35.55) | ||
| ≥ 36 years | 75 (16.85) | 123 (14.87) | ||
| Familial history | 8.904 | 0.003 | ||
| Yes | 49 (11.01) | 143 (17.29) | ||
| No | 396 (88.99) | 684 (82.71) | ||
| Paternal smoking history | 7.898 | 0.005 | ||
| Yes | 31 (6.97) | 99 (11.97) | ||
| No | 414 (93.03) | 728 (88.03) | ||
| Maternal anemia disease | 7.945 | 0.019 | ||
| Mild | 88 (19.77) | 126 (15.24) | ||
| Moderate | 10 (2.25) | 38 (4.59) | ||
| Never | 347 (77.98) | 663 (80.17) | ||
| Gestational diabetes mellitus | 1.913 | 0.167 | ||
| Yes | 80 (17.98) | 124 (14.99) | ||
| No | 365 (82.02) | 703 (85.01) | ||
| Maternal obesity | 0.019 | 0.890 | ||
| Yes | 8 (1.80) | 14 (1.69) | ||
| No | 437 (98.20) | 813 (98.31) | ||
| Maternal hepatitis | 0.595 | 0.440 | ||
| Yes | 26 (5.84) | 40 (4.84) | ||
| No | 419 (94.16) | 787 (95.16) | ||
| Thyroid disease | ||||
| Yes | 65 (14.61) | 137 (16.57) | 0.831 | 0.362 |
| No | 380 (85.39) | 690 (83.43) | ||
| Maternal neurological disorders | 2.680 | 0.102 | ||
| Yes | 5 (1.12) | 3 (0.36) | ||
| No | 440 (98.88) | 824 (99.64) | ||
| Maternal cardiovascular disease | 2.451 | 0.117 | ||
| Yes | 36 (8.09) | 48 (5.80) | ||
| No | 409 (91.91) | 779 (94.20) | ||
| Maternal reproductive system disease | 0.417 | 0.519 | ||
| Yes | 18 (4.04) | 40 (4.84) | ||
| No | 427 (95.96) | 787 (95.16) | ||
| Other systemic diseases | 5.863 | 0.015 | ||
| Yes | 14 (3.15) | 10 (1.21) | ||
| No | 431 (96.85) | 817 (98.79) |
Data were shown as n (%).
Multivariate logistic regression analysis was used to identify significant factors contributing to the occurrence of neonatal ear deformity. Maternal anemia was not an independent factor for neonatal ear deformity [OR (95% CI): 1.489 (0.501−4.429), P = 0.474]. Father's smoking history [OR (95% CI): 9.058 (1.657–49.503), P = 0.011], and the newborn's family history of ear deformity [OR (95% CI): 5.901 (1.284−27.116), P = 0.023] were identified as risk factors.
DISCUSSION
There is a lack of epidemiological and quantitative diagnostic criteria for congenital auricular deformities. This causes delayed diagnosis and missed opportunities for non‐invasive correction. Our study aims to raise awareness of the high incidence of auricular deformities in newborns and assist in early diagnosis and intervention by quantifying normative data for medical practitioners.
Morbidity and malformation progression
This study has found that the incidence of congenital auricle morphological deformities in southern China is 79.87%, with the highest incidence being helix rim deformities and mixed deformities. The most common subtype of mixed deformities is lop ears combined with conchal crus ear, accounting for 22.73% of mixed deformities. In a study of the Pearl River Delta population, the incidence of neonatal auricle deformity is 57.47%. 10 Charipova et al. 11 showed that the most common subtype of mixed deformity is helical rim deformity combined with protruding ears, accounting for about 39.1% of mixed deformities. The difference in the findings of mixed deformity may be related to age, as Charipova's study included participants aged 3‒156 days. Previous studies have shown that some auricle deformities can heal spontaneously with age, while the incidence of protruding ears increases. This may explain the high proportion of protruding ears in mixed deformities in Charipova's study. 2 , 10 , 12 , 13 Zhao et al. 14 found that the self‐healing rate of neonatal auricle deformity was 31.55% after a 30‐day follow‐up, and the incidence of protruding ears also increased. In a study of Se‐Joon Oh, 12 the self‐correction rate of ear deformity was about 50% in a one‐year follow‐up of newborns, with the incidence of protruding ears increasing from 14% to 21%, showing an increasing trend over time. Although these studies indicate that congenital auricle deformity can self‐heal, the outcomes vary greatly and are unpredictable. Therefore, it is difficult to reach a definitive conclusion on how each baby's ear shape changes after birth.
Morphology
The EarWell and InfantEar (TalexMedical) devices have been designed and developed based on the average auricle size of newborns from foreign countries. There are variations in ear shape amongst newborns of different ancestries, resulting in poorer fit and suboptimal correction. Hence, we want to establish data foundations for the development of ear mold correctors in China. This study aimed to provide a descriptive diagnosis of each type of congenital auricle morphological deformity from various measurement indicators, and a quantitative description of the characteristic manifestations of various deformities. Given that conchal crus ear, cup ear, protruding ear, and other deformities can impact the curvature of the antihelix, we have developed a new measurement index: the antihelix angle. Deformities like lop ear, Stahl's ears, and constricted ear can affect the shape of both the antihelix and the helix. To account for this, we have included measurements for the distance between the helix and the antihelix. Additionally, cryptotia and helical rim deformity often manifest as abnormalities at the upper end of the auricle. Therefore, we have introduced a measurement index: the vertical distance of cephalo‐superaurale. Protruding ears, constricted ears, lop ears, conchal crus ear, helical rim deformity, cryptotia, cup ear, Stahl's ear, and mixed deformities all manifest as abnormal helix or antihelix, resulting in changes in the relationship between the helix and antihelix. The vertical distance of cephalo‐superaurale, the distance between the helix and antihelix, and the antihelix angle are indispensable indicators for describing and measuring the relationship between the helix and the antihelix.
Etiology
There are many causes of neonatal ear deformity, among which genetics and embryonic development play an important role. 15 , 16 , 17 This study analyzed the relationship between neonatal ear deformities and maternal factors. The widely adopted artificial‐assisted reproductive technology raises concerns about pregnancy outcomes and offspring safety. 18 Our study is the first to explore the effect of artificial‐assisted reproduction on auricle deformities, finding no statistically significant difference in the incidence of auricle deformities between natural conception and artificial insemination.
In terms of embryonic conditions and developmental environment, this study evaluated congenital diseases and amniotic fluid conditions, finding no statistical significance. Previous studies have shown an increased incidence of auricular deformities in newborns of elderly primiparous women, those born naturally through the birth canal, and overweight infants. 14 , 19 However, we did not observe a significant difference in the incidence of auricle deformity related to maternal age, mode of delivery, or birth weight. The optimization and popularization of prenatal education have led to a sharp drop in the proportion of overweight children (none were overweight in this study).
The incidence of auricle deformity is higher in newborns with a family history of genetic diseases compared to those without. When parents have helical rim deformities, conchal crus ears, constricted ears, cup ears, lop ears, or Stahl's ears, the probability of the offspring having the same deformity significantly increases. One exception is that the types of auricle deformities in the offspring of parents with protruding ears are diverse, possibly due to the complexity of protruding ear deformities. Long‐term follow‐up shows that other types of deformities may evolve into protruding ears.
Logistic regression analysis showed that paternal smoking is an independent factor for the occurrence of neonatal auricle deformity. The rate of smoking fathers is 9.058 times that of non‐smoking fathers, making paternal smoking the most significant factor affecting the occurrence of neonatal ear deformities.
There are both surgical and non‐surgical interventions available for children with ear deformities that cannot self‐correct. 7 However, variations in anesthesia risk, surgical stage, and outcome further contribute to parental hesitation towards opting for surgical correction. 20 Traditional ear mold correction has a success rate (distinguished achiever ratio) of 92.6%. 21 But the prevailing view among Chinese parents is that neonatal ear deformities will correct themselves over time. Coupled with a lack of understanding of ear mold correction methods and the high cost of imported ear mold materials, many children miss the window for non‐invasive correction by the time of correct diagnosis. We recommend that non‐invasive corrective treatment be promptly administered for all types of malformations upon diagnosis, rather than adopting a wait‐and‐see approach for natural healing.
In conclusion, the incidence of auricle deformities in southern China is high, with a diverse range of types. There are great differences in the morphometric structures of various auricle deformities, but all exhibit changes in the distance between the helix and the antihelix. Additionally, newborns with a family history of protruding ears can develop other types of auricle deformities.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supporting Information
Zhou X, Peng X, Ding Y, Zhu Y, Tian D, Wu M, et al. Investigation of morphometric features of auricle in newborns and etiology of auricle deformity. Pediatr Investig. 2024;8:278–286. 10.1002/ped4.12445
Contributor Information
Bin Zhang, Email: drzhangbin06118@163.com.
Xiangdong Qi, Email: qixiangdong@smu.edu.cn.
REFERENCES
- 1. Chen P, Yang J, Yang L, Liu Y, Gao M, Li S, et al. One‐year outcomes of ear molding for infants with constricted ear. Plast Reconstr Surg. 2023;151:159‐166. DOI: 10.1097/PRS.0000000000009781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Byrd HS, Langevin CJ, Ghidoni LA. Ear molding in newborn infants with auricular deformities. Plast Reconstr Surg. 2010;126:1191‐1200. DOI: 10.1097/PRS.0b013e3181e617bb [DOI] [PubMed] [Google Scholar]
- 3. Wu S, Qi X, Zhao H, Qin J, Zhong S. Preliminary research on the morphological classification of neonatal auricle (in Chinese). Chin J Clin Anat. 2013;31:384‐388. DOI: 10.13418/j.issn.1001-165x.2013.04.004 [DOI] [Google Scholar]
- 4. Horlock N, Vögelin E, Bradbury ET, Grobbelaar AO, Gault DT. Psychosocial outcome of patients after ear reconstruction: a retrospective study of 62 patients. Ann Plast Surg. 2005;54:517‐524. DOI: 10.1097/01.sap.0000155284.96308.32 [DOI] [PubMed] [Google Scholar]
- 5. Songu M, Kutlu A. Long‐term psychosocial impact of otoplasty performed on children with prominent ears. J Laryngol Otol. 2014;128:768‐771. DOI: 10.1017/S0022215114001662 [DOI] [PubMed] [Google Scholar]
- 6. Joukhadar N, McKee D, Caouette‐Laberge L, Bezuhly M. Management of congenital auricular anomalies. Plast Reconstr Surg. 2020;146:205e‐216e. DOI: 10.1097/PRS.0000000000006997 [DOI] [PubMed] [Google Scholar]
- 7. Daniali LN, Rezzadeh K, Shell C, Trovato M, Ha R, Byrd HS. Classification of newborn ear malformations and their treatment with the EarWell Infant Ear Correction System. Plast Reconstr Surg. 2017;139:681‐691. DOI: 10.1097/PRS.0000000000003150 [DOI] [PubMed] [Google Scholar]
- 8. Anstadt EE, Johns DN, Kwok AC, Siddiqi F, Gociman B. Neonatal ear molding: timing and technique. Pediatrics. 2016;137:e20152831. DOI: 10.1542/peds.2015-2831 [DOI] [PubMed] [Google Scholar]
- 9. Perini TA, de Oliveira GL, Ornellas JDS, de Oliveira FP. Technical error of measurement in anthropometry. Rev Bras Med Esporte. 2005;11:81‐85. [Google Scholar]
- 10. Zhao H, Lin G, Seong YH, Shi J, Xu J, Huang W. Anthropometric research of congenital auricular deformities for newborns. J Matern Fetal Neonatal Med. 2019;32:1176‐1183. DOI: 10.1080/14767058.2017.1402877 [DOI] [PubMed] [Google Scholar]
- 11. Charipova K, Rogers A, Barra C, Baker SB. Evolution of anomaly‐specific techniques in infant ear molding: a 10‐year retrospective study. Plast Reconstr Surg. 2022;150:394‐404. DOI: 10.1097/PRS.0000000000009335 [DOI] [PubMed] [Google Scholar]
- 12. Kim M, Lee HM, Choi SW, Lee S, Kim C, Kong SK, et al. A longitudinal study of changes of congenital auricular deformity regarding self‐correction. J Plast Reconstr Aesthet Surg. 2021;74:2705‐2711. DOI: 10.1016/j.bjps.2021.03.023 [DOI] [PubMed] [Google Scholar]
- 13. Matsuo K, Hayashi R, Kiyono M, Hirose T, Netsu Y. Nonsurgical correction of congenital auricular deformities. Clin Plast Surg. 1990;17:383‐395. [PubMed] [Google Scholar]
- 14. Zhao H, Ma L, Qi X, Qin J, Yin B, Zhong M, et al. A morphometric study of the newborn ear and an analysis of factors related to congenital auricular deformities. Plast Reconstr Surg. 2017;140:147‐155. DOI: 10.1097/PRS.0000000000003443 [DOI] [PubMed] [Google Scholar]
- 15. Yotsuyanagi T, Yamauchi M, Yamashita K, Sugai A, Gonda A, Kitada A, et al. Abnormality of auricular muscles in congenital auricular deformities. Plast Reconstr Surg. 2015;136:78e‐88e. DOI: 10.1097/PRS.0000000000001383 [DOI] [PubMed] [Google Scholar]
- 16. Schultz K, Guillen D, Maricevich RS. Newborn ear deformities: early recognition and novel nonoperative techniques. Semin Plast Surg. 2017;31:141‐145. DOI: 10.1055/s-0037-1603958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chitkara U, Lee L, El‐Sayed YY, Holbrook RH Jr, Bloch DA, Oehlert JW, et al. Ultrasonographic ear length measurement in normal second‐ and third‐trimester fetuses. Am J Obstet Gynecol. 2000;183:230‐234. DOI: 10.1067/mob.2000.105737 [DOI] [PubMed] [Google Scholar]
- 18. Yan J, Huang G, Sun Y, Zhao X, Chen S, Zou S, et al. Birth defects after assisted reproductive technologies in China: analysis of 15,405 offspring in seven centers (2004 to 2008). Fertil Steril. 2011;95:458‐460. DOI: 10.1016/j.fertnstert.2010.08.024 [DOI] [PubMed] [Google Scholar]
- 19. Harris J, Källén B, Robert E. The epidemiology of anotia and microtia. J Med Genet. 1996;33:809‐813. DOI: 10.1136/jmg.33.10.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang W, Wang J, Zhang C. Advances in the application of non‐surgical therapy for congenital auricular malformations (in Chinese). Chin J Aesth Plast Surg. 2021;32:208‐210. DOI: 10.3969/j.issn.1673-7040.2021.04.007 [DOI] [Google Scholar]
- 21. Kim J, Jo T, Choi J, Kim J, Jeong W. Efficacy of classic ear molding for neonatal ear deformity: case series and literature review. J Clin Med. 2022;11:5751. DOI: 10.3390/jcm11195751 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


