ABSTRACT
Bronchopulmonary dysplasia (BPD) is a chronic lung disease that arises during the neonatal period, and its underlying mechanisms are still not fully understood. The disorder of microvascular development plays a significant role in the development of BPD. This article presents a comprehensive review of the advancements made in understanding the mechanisms and treatment approaches related to microvascular development in the pathogenesis of BPD.
Keywords: Bronchopulmonary dysplasia, Microvascular, Therapy
This review discusses how factors such as oxidative stress, inflammation, gender, and aberrant angiogenesis lead to pulmonary microvascular development disorders, ultimately causing bronchopulmonary dysplasia. Among these factors, oxidative stress is identified as the most significant risk factor.

INTRODUCTION
Bronchopulmonary dysplasia (BPD) is a chronic lung disease characterized by impaired lung development due to exposure of immature lungs to various prenatal and postnatal factors. 1 The new diagnostic criteria of BPD require confirmation of persistent lung parenchymal disease by imaging in preterm infants with gestational age <32 weeks, and the need for oxygen therapy support (for at least three consecutive days) to maintain arterial blood oxygen saturation between 90% and 95% at postmenstrual age (PMA) 36 weeks. These diagnostic criteria further delineate the classification of BPD based on oxygen requirement and categorize preterm infants who die from respiratory failure between postnatal age >14 days and PMA <36 weeks as having severe BPD. 2 The field of perinatal management and neonatal intensive care medicine has witnessed significant progress in the supportive treatment of extremely premature infants, leading to a notable reduction in the mortality rate associated with BPD. However, the incidence of BPD remains high, with nearly 10 000 new cases of BPD occurring in neonates in the United States each year. 3 Therefore, research into the pathogenic mechanisms of this disease becomes particularly important. While previous research has primarily focused on issues related to alveolar development in BPD, where various prenatal and postnatal factors lead to simplification of distal lung acini, resulting in the formation of large cystic alveolar structures, reduced ventilation, and gas exchange function, cessation of alveolar and surrounding vascular microenvironment development, leading to reshaping of lung structure, and ultimately resulting in BPD. 3 However, recent experimental and clinical data have provided us with new insights into the pathological mechanisms of BPD. One of the important findings is that pulmonary angiogenesis is essential for alveolar development and that disorders of pulmonary angiogenesis may contribute to BPD development; this has been termed the “vascular hypothesis”. 4 Although experimental animal models have contributed considerably to our understanding of the involved molecular mechanisms, 5 and there is a lack of conclusive clinical evidence that these mechanisms also play a pathogenic role in human disease, there are exciting developments in BPD treatment by promoting pulmonary angiogenesis and stabilization. This article focuses on factors contributing to impaired pulmonary microvascular development in BPD, including oxidative stress, inflammation, and gender, and summarizes changes in cytokines, proteins, and key signaling pathways. Finally, we review recent research advances targeting pulmonary microvascular development to treat BPD.
OXIDATIVE STRESS AND PULMONARY MICROVASCULAR DEVELOPMENT
Except for the low‐concentration oxygen therapy strategy, for fetuses adapted to a low oxygen environment (4% O2), the normal atmospheric oxygen concentration (21% O2) is considered a hyperoxic environment. 6 Therefore, even exposure to ambient oxygen levels may potentially result in hyperoxic lung injury in premature infants. Conclusive evidence has revealed that premature exposure to hyperoxia and reactive oxygen species (ROS) is a significant risk factor in the development of BPD. 7 Prolonged exposure to hyperoxia disrupts the normal structure of lung tissue microvasculature, resulting in alveolar capillary collapse and changes in pulmonary microcirculation. 8 Increased generation of ROS may affect lung development by inducing DNA damage repair, inhibiting apoptosis, and activating oncogenes through the initiation of signal transduction pathways. 9 Studies based on stereoscopic 3D reconstruction techniques have shown that the reduction in the number of pulmonary capillary endothelial cells begins much earlier than that of alveolar epithelial cells in a hyperoxic BPD mouse model, 10 suggesting that damage to the pulmonary vasculature may be the initiating factor in alveolar immaturity. The formation of a pulmonary vascular system is an extremely complex and precise process that involves several cytokines, signaling pathways, and various precursor cells. Abnormalities in any link may lead to the abnormal development of pulmonary blood vessels and alveoli, thereby affecting lung function. 11
Anti‐/proangiogenic gene imbalance
Short‐term hyperoxia (≥40% O2) exposure (lasting only 2 h) reduces the expression of the biomarkers vascular endothelial growth factor (VEGF) and soluble vascular endothelial growth factor receptor 1 (VEGFR1), which regulate pulmonary angiogenesis. 12 Moreover, prolonged hyperoxia (≥60% O2) stimulation significantly reduces VEGF levels in the blood, thereby affecting the growth and development of other organs. 13 Transcriptomic and proteomic analyses of lung tissues from mice with hyperoxia‐induced BPD by using high‐throughput sequencing technology revealed that hyperoxia leads to a significant decrease in the expression of the proangiogenic genes, namely platelet‐derived growth factor A (PDGFA) and platelet‐derived growth factor receptor β (PDGFRb). 14 Diminished PDGF signaling decreased VEGF expression, which subsequently caused apoptosis of pulmonary endothelial cells (ECs) and decreased microvessel density 15 ; simultaneously, a significant increase in the expression of the antiangiogenic factor pigment epithelium‐derived factor counteracted the stimulatory effect of VEGF on lung microvascular endothelial cells (LMVECs), leading to a decrease in platelet endothelial cell adhesion molecule (PECAM) positive cells, thereby disrupting pro‐ and antiangiogenic factors and inhibiting vascular proliferation, migration, and capillary formation. 16 Hyperoxia has been observed to hinder the tubular growth of ECs through a reduction in PECAM‐1 expression and substantial inhibition of proliferating cell nuclear antigen translation which governs the cell cycle. 17 This effect may be attributed to the upregulation of short endoglin within the transforming growth factor‐β (TGF‐β) receptor family, activating the TGF‐β‐activin receptor‐like kinase (ALK) 1‐mothers against decapentaplegic homolog (Smad)1/5 signaling pathway in ECs, while concurrently downregulating long endoglin and impeding the TGF‐β‐ALK5‐Smad2/3 signaling pathway. 18 Nevertheless, conflicting evidence exists concerning the involvement of stromal‐derived factor‐1 (SDF‐1), an angiogenic chemokine, in the context of hyperoxia. A study has reported a significant amelioration of hyperoxia‐induced impairment in pulmonary vascular development by targeting the blockade of chemokine receptor 4 (CXCR4) and inhibiting the SDF‐1/CXCR4 axis. 19 In contrast, some researchers suggest that increasing the expression levels of SDF‐1 and CXCR4 and activating the SDF‐1/CXCR4 axis could promote pulmonary angiogenesis and reduce lung injury. 20 , 21 , 22 Hyperoxia may also inhibit cell migration and proliferation by decreasing the levels of microRNAs such as miR‐150 and miR‐185–5p and attenuating the expression of the angiogenic factors glycoprotein nonmetastatic melanoma protein B (GPNMB) and cyclin‐dependent kinase 6 in their target genes, thereby resulting in impaired angiogenesis. 23 , 24 These studies suggest that an imbalance in the expression of pulmonary antiangiogenic and proangiogenic genes is an important factor in impaired angiogenesis in a hyperoxic environment.
Oxidative stress induces lung inflammation
Apart from the direct impact of oxidative stress on vascular development, exposure to hyperoxia can also initiate lung inflammation. 7 Firstly, hyperoxia is known to downregulate the expression levels of Twist1 and angiopoietin 1 (Ang1) protein, as well as its receptor Tie2. Concurrently, it upregulates angiopoietin 2 (Ang2), which acts as an antagonist of Ang1. This perturbation leads to the attenuation of the Twist1/Tie2 signaling pathway, subsequently inhibiting the activation of the downstream Akt/Foxo1 signaling pathway. Consequently, vascular stability is reduced, and vascular permeability is increased. 25 Next, myeloperoxidase plays an initiating role by amplifying hyperoxia‐induced oxidative stress, resulting in the release of high mobility group protein B1 upon the death of LMVECs; this further increases the expression of the inflammatory markers cyclooxygenase‐1/cyclooxygenase‐2, receptor for advanced glycation end products, and toll‐like receptor 4, while attenuating the activation of nuclear factor E2‐related factor 2 (Nrf2) and leading to LMVECs damage. 26 Simultaneously, a large influx of neutrophils and macrophages impairs vascular neogenesis, while the elevated level of G protein‐coupled receptor formyl peptide receptor 1 and the decreased level of formyl peptide receptor 2 not only increases the expression of inflammatory factors interleukin (IL)‐1α and IL‐6, which exacerbate inflammation and injury in hyperoxic BPD lung tissues but also may decrease the expression of VEGF and hepatocyte growth factor. 27 , 28 This inhibits the proliferation and lumen formation capacity of ECs, thereby leading to impaired vascular development. 28 , 29 Hyperoxia can also enhance the transcriptional activity of nuclear factor kappa B (NF‐κB) in the classical inflammatory signaling pathway by phosphorylating the p65 protein and activating the downstream target gene monocyte chemotactic protein 1 (MCP‐1) to induce lung inflammation which subsequently affects pulmonary vascular development 30 ; in contrast, tumor necrosis factor inducible gene 6 protein can ameliorate hyperoxia‐induced inflammation in the lung by decreasing the expression of pNF‐κB and inflammatory markers (MCP and IL‐6) to ameliorate the impaired angiogenesis caused by hyperoxia. 31 Inhibition of aryl hydrocarbon receptor activation decreases the activity of the NF‐κB subunit RelB and reduces the expression of antioxidant enzymes, which then increases hyperoxia‐induced ROS accumulation, thereby causing damage to LMVECs and leading to impaired pulmonary microvascular development. 32 , 33
Disruption of signaling pathways
Hyperoxia conditions significantly alter biological processes such as cellular metabolism, stress response, signal transduction, cell cycle, and immune regulation, in addition to regulating inflammatory signaling pathways. 33 One crucial metabolic regulatory pathway involved in these mechanisms is the adenosine 5‐monophosphate‐activated protein kinase (AMPK) signaling pathway, which is ubiquitously present in diverse organisms and primarily governs intracellular energy balance. The AMPK‐α1 subunit plays a vital role in promoting pulmonary angiogenesis, as demonstrated by the significant reduction in pulmonary angiogenesis observed in the absence of AMPK‐α1 in ECs. 34 Extracellular signal‐regulated kinase (ERK) is a branch of the mitogen‐activated protein kinase (MAPK) signaling pathway; it plays an important role in cell growth, differentiation, proliferation, apoptosis, and cell cycle regulation, and other life activities, and it is also a key intracellular signaling pathway. The inhibition of ERK1 alone does not exacerbate hyperoxia‐induced angiogenic disorders in BPD, as ERK2 is compensated by activation 35 ; however, the knockdown of the ERK2 gene in ECs significantly exacerbates hyperoxia‐induced pulmonary angiogenic disorders, 36 suggesting that the ERK2 gene may be a key factor in angiogenesis inhibition by hyperoxia.
Furthermore, the absence of adrenomedullin not only diminishes the expression of nitric oxide synthase 3 (NOS3), nitric oxide (NO) production, and angiogenesis by inhibiting calcitonin receptor‐like receptors and receptor activity modifying protein 2 but also reduces ERK1/2 activity, inhibiting the MAPK signaling pathway and disrupting vascular tubular structure formation, resulting in impaired pulmonary angiogenesis. 37 , 38 Sphingosine kinase 1 (SPHK1) is a crucial enzyme involved in the metabolism of sphingosine, a neurosphingolipid metabolite, into sphingosine 1 phosphate (S1P), which plays a pivotal role in regulating vascular tone, endothelial function and integrity, and lymphocyte transport. Imbalances in SPHK1 production and signaling are closely associated with the development of diseases such as endothelial dysfunction and abnormal angiogenesis. 39 In LMVECs, exposure to hyperoxia not only promotes the expression of NADPH oxidase 2 and 4 proteins, increasing ROS production and damaging blood vessels by upregulating S1P protein expression, 40 but also activates the SPHK1/S1P signaling pathway while decreasing the expression of proangiogenic factors such as Ang1, Tie2, and VEGF proteins, thereby inhibiting the Ang1/Tie2 signaling pathway, 41 leading to impaired pulmonary angiogenesis.
The activation of the SPHK1/S1P signaling axis also promotes signal transducer and activator of transcription 3 phosphorylation, increases lysyl oxidase (LOX) expression and activity, and disrupts angiogenesis and stabilization. 42 Enhanced LOX activity further disrupts extracellular matrix structure, leading to lung tissue sclerosis in young mice and activation of the LRP5/Tie2 signaling pathway, eventually disrupting pulmonary angiogenesis. 43 Additionally, the lack of the antiapoptotic factor Bcl‐2 also exacerbates hyperoxia‐induced reduction in pulmonary angiogenesis and impairment of lung development. 44
Damage of vascular endothelial precursor cells
Vascular endothelial precursor cells comprise a group of cells that possess the potential to differentiate into ECs. These cells are considered progenitors or precursors of ECs and include endothelial progenitor cells (EPCs) and mesenchymal stem cells (MSCs). Among these, endothelial colony‐forming cells (ECFCs) represent a specific subpopulation of EPCs that demonstrate self‐renewal capabilities and play a role in neovascularization. Notably, ECFCs derived from the lungs of premature infants and EPCs are vulnerable to the effects of hyperoxia. 45 This exposure results in decreased expression of critical transcription factor forkhead box F1 and the tyrosine kinase receptor protein c‐KIT. 46 Consequently, their development and functionality are compromised, leading to impaired pulmonary vascular and alveolar development.
ECs are mainly composed of general capillary endothelial cells (gCap) and aerosol capillary endothelial cells (aCap). Among these cells, gCap cells mainly regulate vasodilation and function as progenitor cells in capillary homeostasis and repair, whereas aCap cells are mainly involved in gas exchange and transport. 47 Under hyperoxia, an increased expression of fibroblast growth factor receptor 1 in aCap cells of neonatal mice activated the downstream ERK/MAPK signaling pathway and the PI3K/Akt signaling pathway, which led to a significant decrease and increase in the number of gCap and aCap cells, respectively 48 ; this not only decreased the expression of genes in angiogenesis‐related pathways but also increased the expression of antiangiogenic genes (e.g., cyclin‐dependent kinase inhibitor 1A and so forth) and the expression of insulin‐like growth factor binding protein 7, leading to impaired pulmonary angiogenesis. 49 Hyperoxia exposure can also activate axon guidance and cell division cycle 42 signaling pathways, thereby leading to an increased expression of antiangiogenic genes (e.g., Semaphorin 3A and so forth), inhibition of the JAK/STAT signaling pathway with anti‐inflammatory effects and the fibroblast growth factor signaling pathway genes with proangiogenic effects in lung‐derived mesenchymal stem cells (L‐MSCs), 50 and ultimately an increase in this type of L‐MSCs. 51 Hyperoxia exposure also leads to increased phosphorylation of Smad2 and Smad3 and decreased Smad7 protein levels; this overactivates the TGF‐β signaling pathway and contributes to endothelial‐to‐mesenchymal transition in LMVECs, thereby inhibiting pulmonary angiogenesis. 52 In conclusion, exposure to a hyperoxic environment disrupts the development and functionality of vascular endothelial precursor cells and L‐MSCs, thereby leading to impaired pulmonary vascular and alveolar development. This understanding enhances our comprehension of the mechanisms underlying the impact of hyperoxia on pulmonary angiogenesis and establishes a crucial theoretical foundation for the prevention and treatment of associated disorders.
INFLAMMATION AND PULMONARY MICROVASCULAR DEVELOPMENT
Although there is a close and complex interaction between inflammation and angiogenesis in BPD, the current study suggests that pulmonary inflammation plays an inhibitory role in the proliferation and migration of vascular ECs. In addition to hyperoxia‐induced lung inflammation, lung inflammation triggered by prenatal or postnatal infections is an important factor that contributes to the inhibition of pulmonary angiogenesis and impaired pulmonary microcirculation. First, infection leads to the upregulation of proinflammatory factors and reduces the expression of ECs proangiogenic factors such as insulin‐like growth factor 1, vascular endothelial growth factor A (VEGFA), and its receptors VEGFR1 and VEGFR2, thereby hindering the development of pulmonary microvasculature. 53 , 54 Second, inflammatory factors can promote the expression of angiogenesis inhibitory proteins such as soluble fms like tyrosine kinase 1, 53 soluble endoglin, 55 and thrombospondin 1, 56 , 57 which inhibit pulmonary angiogenesis. Inflammation can also disrupt the VEGF/NO signaling pathway by activating the MAPK and NF‐κB signaling pathways with the TLR signaling pathway, thereby mediating the activation of proinflammatory signaling in human LMVECs and leading to impaired pulmonary vascular development. 58 , 59 , 60 , 61 Inflammation also leads to decreased levels of short‐chain fatty acid sodium propionate, which inhibits Nrf2 expression, increases kelch‐like ECH‐associated protein 1 levels, decreases Nrf2 nuclear translocation, and inhibits angiogenesis. 62 In contrast, prenatal endotoxin exposure leads to a significant decrease in the expression of pulmonary vitamin D receptors and 1α‐hydroxylase and cytochrome P450 family 24 subfamily A member 1 involved in the vitamin D metabolic pathway, thus decreasing pulmonary artery ECs growth and lumen formation, which later develops into BPD. 63 , 64 However, some investigators suggest that during early lung development, inflammation instead inhibits the expression of plasma macrophage inflammatory protein 2 by increasing the level of activation of the NF‐кB signaling pathway and disrupting angiogenesis. 65 In clinical studies, similar “paradoxical” have also been observed. Preterm infants with chorioamnionitis have higher concentrations of disaturated‐phosphatidylcholine, surfactant protein B, and myeloperoxidase activity in the epithelial lining fluid compared to those without chorioamnionitis, while extremely preterm newborns show similar amounts of disaturated‐phosphatidylcholine, surfactant protein A, and surfactant protein B in the epithelial lining fluid compared to more mature infants. 66 Overall, lung inflammation induces BPD by affecting pulmonary angiogenesis.
GENDER AND PULMONARY MICROVASCULAR DEVELOPMENT
Gender differences exist in the incidence of BPD and the degree of impaired lung function. A clinical multicenter cohort study showed that preterm male infants had significantly higher mortality rates in the neonatal period and infancy than female infants and were at a higher risk for BPD. 67 , 68 Although the mechanism of this sex difference in BPD incidence is unknown, it has been determined that sex differences in angiogenesis could be a potential cause of male BPD inferiority. 69 Male LMVECs show significant differences in outgrowth efficiency and response to different sex hormones as compared to female LMVECs, and these differences could be further exacerbated by hyperoxia exposure. 70 Compared to males, LMVECs of females may attenuate hyperoxia‐induced vascular development impairment by increasing the expression level of proangiogenic miR‐30a and promoting hypoxia‐inducible factor‐1α (HIF‐1α) expression while suppressing the expression of Snai1 protein. 71 , 72 In contrast, male LMVECs exhibited higher α‐SMA levels 73 ; this finding is consistent with the result that endothelial‐to‐mesenchymal transition was more pronounced in male LMVECs during hyperoxia exposure. 52 Additionally, the reduced expression of HIF‐1α in male ECs may be associated with an increase in growth differentiation factor 15. 74 In a hyperoxic BPD model, the expression of the angiogenic pathway‐related genes such as VEGF, VEGFR2, and PDGFR was significantly suppressed in male mice as compared to that in female mice 69 ; the expression of the angiogenic marker PECAM‐1 was significantly reduced 73 , 75 ; C‐X‐C motif chemokine ligand 4 was significantly upregulated in lung tissue; and fatty acid binding protein 4 expression was significantly decreased. 76 The expression level of IKKβ and p‐IKbα and the activation level of p65 are higher in female human umbilical vein endothelial cells (HUVECs) after hyperoxia exposure, and the activation of the proangiogenic NF‐κB signaling pathway is stronger. 77 The differences in these factors and signaling pathways could be responsible for the greater susceptibility of males to hyperoxic lung injury.
ABNORMAL OR ABERRANT ANGIOGENESIS
BPD is suggested to be caused by abnormal or aberrant vascular development in the lung rather than by impaired angiogenesis. A hyperoxic environment can lead to a hyperoxia‐induced increase in histone deacetylase 3 expression, which activates the EZH1‐p65‐PGF axis by inhibiting miR‐17; this subsequently increases abnormal angiogenesis in BPD model mice. 78 In neonatal mice, hyperoxia also stimulates the pentose phosphate pathway through enhanced overexpression of phosphogluconate dehydrogenase in ECs, which induces their abnormal proliferation and leads to aberrant angiogenesis and alveolar simplification. 79 FOSL1 protein is a crucial and early mediator of inflammation‐induced angiogenesis in ECs and LMVECs. Its activation can trigger the expression of transcriptional regulators, consequently promoting the upregulation of angiogenic genes. This process contributes to the onset of excessive and abnormal angiogenesis during the initial phases of inflammation. 80 Notably, the activation of the NF‐кB inflammatory signaling pathway is necessary in the early stages of lung development. The transforming growth factor‐β‐induced protein can promote pulmonary vascular development by activating the NF‐кB signaling pathway through αvβ3, which further increases the expression of granulocyte colony‐stimulating factor and IL‐1β to mediate NO and VEGFA production. 81 , 82 However, limited evidence exists regarding this matter, and the majority of studies suggest that excessive and abnormal angiogenesis occurs during the early stages of lung development. The early disruption of various signaling molecules in the lung may indeed trigger the anomalous proliferation of ECs, resulting in irregular vascular development. However, with the persistent influence of oxidative stress, inflammation, and other factors, the overall number of pulmonary vessels in the BPD model ultimately remains lower than that observed in normal mice. It is also undeniable that excessive or aberrant vascular development could serve as a new concept for BPD research.
OTHER RISK FACTORS
Undoubtedly, premature is one of the most significant risk factors for BPD, 83 a higher incidence of BPD observed in infants born at younger gestational ages. 84 Studies have indicated that prematurity not only leads to significant dysregulation of inflammatory responses but also disrupts relevant pathways involved in normal lung development, such as vascular morphogenesis and epithelial‐mesenchymal transition. 85 However, it remains unclear whether prematurity itself directly causes BPD, if factors leading to prematurity are the direct cause, or if there are other yet unidentified factors contributing to BPD. 86 In addition to common factors such as mechanical ventilation, 87 oxidative stress, and inflammation, there are other potential risk factors that may trigger BPD. For example, exposure of pregnant women and fetuses to passive smoking decreases the expression of VEGF and VEGF receptors, leading to reduced angiogenesis and triggering a BPD‐like disease. 88 Passive smoking also hinders the synthesis of hydrogen sulfide, a messenger of angiogenesis, and impairs the epithelial‐mesenchymal transition required for normal angiogenesis in the lung, 89 thereby leading to BPD. Cigarette exposure during pregnancy may also reduce the levels of antiapoptotic factors (PI3K/Akt, NF‐κB, and Bcl‐2) by decreasing the levels of nicotinic acetylcholine receptors, which subsequently reduces HIF‐1α expression and affects pulmonary angiogenesis. 90 Therefore, reducing cigarette exposure is essential for fetal lung development. Perfluorooctane sulfonates, commonly found in household products, may also cause lung inflammation while suppressing the expression of HIF‐1α and VEGFA, which are the key factors that promote lung development, thereby increasing pathological features similar to those of BPD. 91 Intrauterine growth restriction in preterm infants also inhibits the NF‐κB signaling pathway that promotes vascular growth in pulmonary artery ECs, increases the expression of fatty acid‐binding protein 4, and dysregulates the peroxisome proliferator‐activated receptor signaling pathway, thereby causing impaired pulmonary microvascular development and eventual development of BPD. 92 , 93 In clinical studies, compared to preterm infants with normal birth weight, those with low birth weight may exhibit certain deficiencies in pulmonary oxygen diffusion, potentially increasing the risk of respiratory system‐related issues and a higher likelihood of developing BPD. 94 The accumulation of the connective tissue mast cell subpopulation in the lung may inhibit the stabilization of LMVECs, disrupt interendothelial cell junctions, and directly lead to disturbed angiogenesis, thereby triggering BPD. 95 Ibuprofen, a frequently administered antipyretic in clinical settings, has been found to decrease the plasma levels of PDGF‐BB, VEGFA, and HIF‐2α in preterm infants. 96 It exerts inhibitory effects on the S phase of the cell cycle, promotes apoptosis, and interferes with lumen formation, migration, and cell proliferation in HUVECs. These actions disrupt angiogenesis and lead to a reduction in pulmonary vascular density during lung development. However, it is worth noting that ibuprofen can also alleviate symptoms associated with experimental BPD by mitigating pulmonary inflammation, reducing alveolar enlargement, decreasing alveolar septal thickness, and attenuating small artery wall thickening. 97 Therefore, the adverse effects on angiogenesis need to be carefully weighed against the benefits of alveolarization and inflammation when using ibuprofen for treating BPD in preterm infants. The pathological mechanism of pulmonary microvascular dysplasia is shown in Figure 1.
FIGURE 1.
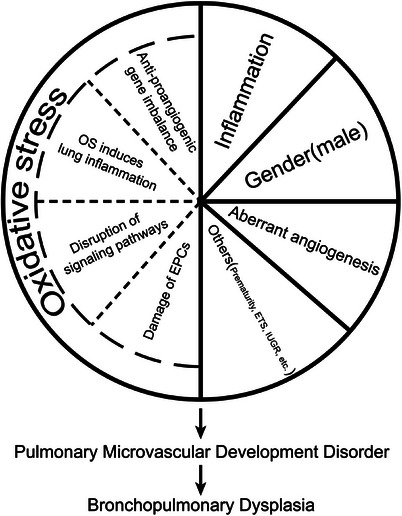
Pathological mechanism of pulmonary microvascular dysplasia. EPCs, endothelial progenitor cells; OS, oxidative stress; ETS, environmental tobacco smoke; IUGR, intrauterine growth restriction.
THERAPY
In recent years, following intensive research on pulmonary microvascular development in BPD, an increasing number of novel medications and approaches for reducing lung injury by improving pulmonary vascular development have emerged and have yielded some results. Therapeutic approaches are divided into two main areas: (i) treatment with small‐molecule proteins or medications for the identified pathogenesis and (ii) use of different types of vascular endothelial precursor cells for treatment.
Restoring the balance of anti‐/proangiogenic genes and signaling pathways reduces lung inflammation
Because an imbalance in the expression of antiangiogenic and proangiogenic genes is an important factor in angiogenic disorders, maintaining the balance of angiogenic factors is crucial for treating angiogenic disorders. The inhibitors of small‐molecule proteins smooth muscle protein 22 alpha and glutaredoxin 1 can significantly promote the expression of Ang2, VEGF, and VEGFA by increasing the stability of HIF‐1α to maintain the proliferation and lumen formation of pulmonary ECs. 98 , 99 , 100 Caffeine, which is commonly used clinically, not only increases the expression of HIF‐2α and VEGFR1 proteins and promotes microangiogenesis but also induces Ang1 to enhance the effect of VEGF. 101 Retinoic acid and vitamin A treatment can partially reverse the vascular development disorder caused by VEGF deficiency. 102 Adrenomedullin promotes pulmonary angiogenesis and tubular structure formation through the activation of the ERK1/2 and MAPK signaling pathways. 38 Knockdown of miR‐150, attenuating its inhibition of the angiogenic factor GPNMB, or the use of soluble GPNMB significantly increased the microvascular network in the lung. 23 Inhibition of LOX activity attenuates LRP5/Tie2 signaling, reduces damage to extracellular matrix structures, and increases pulmonary angiogenesis. 43 Although the use of soluble Klotho protein, 103 recombinant adiponectin protein, 104 and the small‐molecule compound β‐naphthoflavone 105 can promote pulmonary angiogenesis and reduce injury, the precise mechanism has not been fully elucidated, and further in‐depth studies are required. In addition to maintaining the balance of anti‐/proangiogenic factors, the reduction of vascular damage caused by lung inflammation is one of the important therapeutic approaches. In lung tissues of young rats, maternal supplementation with polyunsaturated fatty acids omega 3 (PUFAω3) reduced the expression of proinflammatory cytokines; inhibited leukocyte infiltration; and increased the levels of VEGF, Ang1, Tie2, NOS3, and NO, thereby significantly improving impaired pulmonary angiogenesis. 106 Vitamin D treatment increased pulmonary angiogenesis in neonatal rats, alleviated BPD due to prenatal inflammatory exposure, and increased neonatal survival. 64 Therefore, supplementation with PUFAω3 and vitamin D during pregnancy could be a promising intervention to improve fetal pulmonary vascular development and maturation and enhance neonatal survival through multiple pathways. Glycogen synthase kinase‐3β and TNFα stimulated protein 6 reduce lung inflammation and increase pulmonary angiogenesis by attenuating the transcriptional activity of NF‐κB and inhibiting the downstream target gene MCP‐1. 30 , 31 The use of sodium propionate, a short‐chain fatty acid, increases Nrf2 expression, promotes Nrf2 nuclear translocation, exerts a proangiogenic effect, and ameliorates the impaired angiogenesis caused by inflammation. 62 Iloprost and heparin can also reduce lung inflammation and improve impaired microvascular development by inhibiting inflammatory factors. 107 , 108 The Rho kinase inhibitor Y‐27632 can promote pulmonary microangiogenesis by inhibiting the expression of the antiangiogenic protein thrombospondin 1. 57 In addition, the formyl peptide receptor 2 agonist WKYMVm, leukadherin 1, and semaphorin 3C not only significantly reduce the infiltration of inflammatory cells in the lung by decreasing the expression of inflammatory factors but also increase the expression of proangiogenic factors, thereby protecting the formation of vascular networks in LMVECs. 27 , 29 , 109 The activation of the aryl hydrocarbon receptor also reduces hyperoxia‐induced ROS generation while functioning as an anti‐inflammatory molecule. 32 The small molecule derivative AVR‐48 also attenuates hyperoxia‐induced lung inflammation and promotes pulmonary angiogenesis. 110 In summary, the above‐mentioned medications or proteins promote pulmonary angiogenesis by promoting the expression and stabilization of proangiogenic growth factors such as HIF, VEGF, Ang1, and Tie2; restoring the balance of anti‐/proangiogenic factors; or by inhibiting the activation of inflammatory signaling pathways such as NF‐κB to attenuate pulmonary vascular injury and significantly improve impaired pulmonary angiogenesis.
Therapy with stem cells and its derivatives
Stem cell therapy has shown a high potential for treating BPD. However, there are some differences between different sources of MSCs used for treating experimental BPD. MSCs from umbilical cord tissue can better inhibit lung macrophage infiltration and promote the healing of lung cells. 111 Amniotic fluid MSCs not only facilitate autologous transplantation therapy but also increase VEGF expression and have better efficacy in promoting vascular neogenesis. 112 , 113 Stem cell therapy can exert effects through multiple pathways, such as reducing hyperoxia‐induced multiorgan injury by regulating heme oxygenase 1 and the JAK/STAT pathway or improving hyperoxia‐induced impaired pulmonary angiogenesis by promoting ECs proliferation through cyclin‐dependent kinase 6 upregulation by miR‐185‐5p in exosomes. 24 , 114 , 115 Small extracellular vesicles of human umbilical cord MSC origin can promote pulmonary ECs generation and tubular structure formation in HUVECs by activating the PTEN/Akt signaling pathway. 116 Both intratracheal or transvenous injection of extracellular vesicles of MSCs and transperitoneal injection of exosomes of MSCs promote pulmonary angiogenesis in hyperoxia‐exposed young rats. 117 , 118 The combined application of erythropoietin and stem cells and the intratracheal injection of naked plasmids expressing SDF‐1 can significantly reduce hyperoxia‐induced lung injury, possibly by activating the SDF‐1/CXCR4 axis and increasing VEGF expression levels. 20 , 22 These studies suggest that MSCs and their derivatives are promising BPD therapeutic approaches. In addition to MSCs, ECFCs, and EPCs from umbilical cord blood are also potential sources of cells for treating BPD. The intravenous injection of cord blood‐derived ECFCs can promote alveolar and pulmonary vascular growth 45 , 119 and restore hyperoxic‐ and inflammation‐impaired lung function, while the transplantation of EPCs can prevent alveolar simplification under hyperoxia exposure. 46 Exosomes from EPCs can enhance the biological activity of LMVECs and alleviate hyperoxia‐induced impairment of pulmonary microvascular development. 120 These investigations indicate that ECFCs and EPCs have the potential to address BPD through distinct mechanisms, highlighting their significance as valuable cellular sources for BPD treatment. In recent years, innovative medication delivery approaches like shell‐nucleus aerosol particles have emerged, enabling the targeted release of effective medications into the alveolar interstitium. This method utilizes nebulization, a painless delivery technique, to facilitate pulmonary microvascular revascularization. 121 Nanoparticles can also deliver proangiogenic transcription factors to the lungs more precisely through the circulatory system, thereby enhancing the medication effect and stimulating pulmonary angiogenesis and alveolarization more effectively. 122 The current therapeutic approaches for pulmonary microvascular dysplasia are shown in Figure 2. Notably, their approaches do not have distinct boundaries; instead, they synergistically enhance each other and improve pulmonary vascular development through various mechanisms.
FIGURE 2.
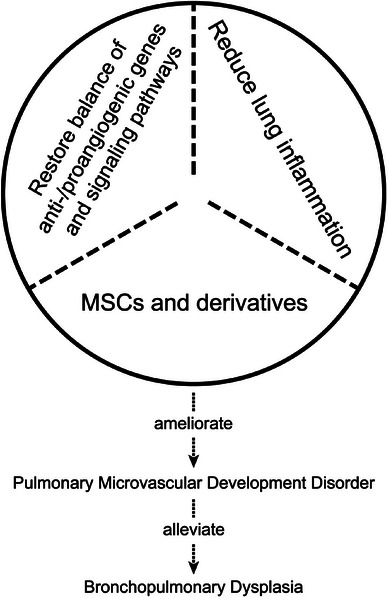
Therapies of pulmonary microvascular dysplasia. MSCs, mesenchymal stem cells.
FUTURE PROSPECTS
In order to advance precision medicine in the field of BPD, exploration of potential research avenues to enhance our understanding of BPD is warranted. Future research should focus on comprehensive phenotypic characterization of BPD, which involves utilizing advanced imaging techniques, biomarker analysis, and genetic sequencing to identify different BPD subtypes and their underlying mechanisms. For instance, three‐dimensional visualization of pulmonary vascular development in BPD patients can be achieved using computer‐generated images obtained from micro‐CT scans, allowing for clearer analysis of pulmonary vascular features and quantitative comparisons. 123 Furthermore, large‐scale genome‐wide association studies and transcriptome analyses can be employed to identify genetic variations and gene expression patterns associated with different BPD phenotypes. With the evolving landscape of novel drug delivery methods, further investigation is warranted into the differential therapeutic effects of various sources of miRNA and other bioactive molecules delivered to the lungs via exosomes through different pathways. In the treatment of pulmonary hypertension, the release of drugs via implanted pumps through intravenous infusion has become relatively mature. 124 Similarly, implantable devices can also be applied in the treatment of BPD. Furthermore, with the advancement of artificial intelligence, future endeavors may involve precise control of drug or signaling molecule release within implantable devices, thereby timely restoring the balance of genes regulating pulmonary anti‐/proangiogenesis, in conjunction with the role of stem cells, to achieve a cure for BPD. Future directions in BPD research should aim to deepen our understanding of the disease's heterogeneity and underlying mechanisms, promote early diagnosis and personalized treatment, and ultimately improve patient outcomes. Through the adoption of multidisciplinary and precision medicine approaches, we can come closer to providing optimal treatment for patients with BPD. However, it is acknowledged that there is still a long journey ahead in these endeavors.
LIMITATIONS
It is necessary to acknowledge the limitations of this paper, the vast majority of relevant molecular mechanisms in the text are derived from animal models. However, due to differences in anatomical structure, physiological functions, and metabolic pathways between animals and humans, it is challenging to fully replicate human pathophysiology, especially in simulating rare diseases such as BPD. Even if animal experiments yield certain research outcomes, translating these findings into clinical treatment modalities still faces numerous challenges. Moreover, the similarity between animal models and human diseases as well as the effectiveness of treatment methods require validation through clinical trials, a process that is often time‐consuming, labor‐intensive, and costly. Therefore, researchers need to comprehensively utilize various research methods, including cell models, tissue engineering techniques, and ex vivo organ models, to better understand the pathogenesis of BPD and to seek more effective treatment approaches.
CONCLUSION
In summary, risk factors such as hyperoxia and inflammation may induce BPD by disrupting the balance of anti‐/proangiogenic genes, leading to the disruption of key signaling pathways of pulmonary vascular development and causing impaired pulmonary microvascular development. The alleviation of microvascular development has also become a new research direction for treating BPD, and various small‐molecule proteins or medications, stem cell therapies, and novel medication delivery technologies have been gradually developed; however, there is still no perfect treatment plan, and further research and exploration are required to bring more hope to BPD patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Zhang J, Du W, Zhang Z, Li T, Li X, Xi S. Research progress of microvascular development in bronchopulmonary dysplasia. Pediatr Investig. 2024;8:299–312. DOI: 10.1002/ped4.12441
REFERENCES
- 1. Sahni M, Bhandari V. Recent advances in understanding and management of bronchopulmonary dysplasia. F1000Res. 2020;9:703. DOI: 10.12688/f1000research.25338.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Higgins RD, Jobe AH, Koso‐Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. 2018;197:300‐308. DOI: 10.1016/j.jpeds.2018.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalikkot Thekkeveedu R, Guaman MC, Shivanna B. Bronchopulmonary dysplasia: a review of pathogenesis and pathophysiology. Respir Med. 2017;132:170‐177. DOI: 10.1016/j.rmed.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am J Respir Crit Care Med. 2001;164:1755‐1756. DOI: 10.1164/ajrccm.164.10.2109111c [DOI] [PubMed] [Google Scholar]
- 5. Hilgendorff A, Reiss I, Ehrhardt H, Eickelberg O, Alvira CM. Chronic lung disease in the preterm infant. Lessons learned from animal models. Am J Respir Cell Mol Biol. 2014;50:233‐245. DOI: 10.1165/rcmb.2013-0014TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maltepe E, Saugstad OD. Oxygen in health and disease: regulation of oxygen homeostasis‐clinical implications. Pediatr Res. 2009;65:261‐268. DOI: 10.1203/PDR.0b013e31818fc83f [DOI] [PubMed] [Google Scholar]
- 7. Wang J, Dong W. Oxidative stress and bronchopulmonary dysplasia. Gene. 2018;678:177‐183. DOI: 10.1016/j.gene.2018.08.031 [DOI] [PubMed] [Google Scholar]
- 8. Nakanishi H, Morikawa S, Kitahara S, Yoshii A, Uchiyama A, Kusuda S, et al. Morphological characterization of pulmonary microvascular disease in bronchopulmonary dysplasia caused by hyperoxia in newborn mice. Med Mol Morphol. 2018;51:166‐175. DOI: 10.1007/s00795-018-0182-2 [DOI] [PubMed] [Google Scholar]
- 9. Rosanna DP, Salvatore C. Reactive oxygen species, inflammation, and lung diseases. Curr Pharm Des. 2012;18:3889‐3900. DOI: 10.2174/138161212802083716 [DOI] [PubMed] [Google Scholar]
- 10. Appuhn SV, Siebert S, Myti D, Wrede C, Surate Solaligue DE, Pérez‐Bravo D, et al. Capillary changes precede disordered alveolarization in a mouse model of bronchopulmonary dysplasia. Am J Respir Cell Mol Biol. 2021;65:81‐91. DOI: 10.1165/rcmb.2021-0004OC [DOI] [PubMed] [Google Scholar]
- 11. Galambos C, deMello DE. Molecular mechanisms of pulmonary vascular development. Pediatr Dev Pathol. 2007;10:1‐17. DOI: 10.2350/06-06-0122.1 [DOI] [PubMed] [Google Scholar]
- 12. Keenaghan M, Cai CL, Kumar D, Valencia GB, Rao M, Aranda JV, et al. Response of vascular endothelial growth factor and angiogenesis‐related genes to stepwise increases in inspired oxygen in neonatal rat lungs. Pediatr Res. 2013;73:630‐638. DOI: 10.1038/pr.2013.21 [DOI] [PubMed] [Google Scholar]
- 13. Greco F, Wiegert S, Baumann P, Wellmann S, Pellegrini G, Cannizzaro V. Hyperoxia‐induced lung structure‐function relation, vessel rarefaction, and cardiac hypertrophy in an infant rat model. J Transl Med. 2019;17:91. DOI: 10.1186/s12967-019-1843-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shrestha AK, Gopal VY, Menon RT, Hagan JL, Huang S, Shivanna B. Lung omics signatures in a bronchopulmonary dysplasia and pulmonary hypertension‐like murine model. Am J Physiol Lung Cell Mol Physiol. 2018;315:L734‐L741. DOI: 10.1152/ajplung.00183.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oak P, Pritzke T, Thiel I, Koschlig M, Mous DS, Windhorst A, et al. Attenuated PDGF signaling drives alveolar and microvascular defects in neonatal chronic lung disease. EMBO Mol Med. 2017;9:1504‐1520. DOI: 10.15252/emmm.201607308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chetty A, Bennett M, Dang L, Nakamura D, Cao GJ, Mujahid S, et al. Pigment epithelium‐derived factor mediates impaired lung vascular development in neonatal hyperoxia. Am J Respir Cell Mol Biol. 2015;52:295‐303. DOI: 10.1165/rcmb.2013-0229OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xing Y, Fu J, Yang H, Yao L, Qiao L, Du Y, et al. MicroRNA expression profiles and target prediction in neonatal Wistar rat lungs during the development of bronchopulmonary dysplasia. Int J Mol Med. 2015;36:1253‐1263. DOI: 10.3892/ijmm.2015.2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee Y, Lee J, Nam SK, Hoon Jun Y. S‐endoglin expression is induced in hyperoxia and contributes to altered pulmonary angiogenesis in bronchopulmonary dysplasia development. Sci Rep. 2020;10:3043. DOI: 10.1038/s41598-020-59928-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drummond S, Ramachandran S, Torres E, Huang J, Hehre D, Suguihara C, et al. CXCR4 blockade attenuates hyperoxia‐induced lung injury in neonatal rats. Neonatology. 2015;107:304‐311. DOI: 10.1159/000371835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guerra K, Bryan C, Dapaah‐Siakwan F, Sammour I, Drummond S, Zambrano R, et al. Intra‐tracheal administration of a naked plasmid expressing stromal derived factor‐1 improves lung structure in rodents with experimental bronchopulmonary dysplasia. Respir Res. 2019;20:255. DOI: 10.1186/s12931-019-1224-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reiter J, Drummond S, Sammour I, Huang J, Florea V, Dornas P, et al. Stromal derived factor‐1 mediates the lung regenerative effects of mesenchymal stem cells in a rodent model of bronchopulmonary dysplasia. Respir Res. 2017;18:137. DOI: 10.1186/s12931-017-0620-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun C, Zhang S, Wang J, Jiang W, Xin Q, Chen X, et al. EPO enhances the protective effects of MSCs in experimental hyperoxia‐induced neonatal mice by promoting angiogenesis. Aging (Albany NY). 2019;11:2477‐2487. DOI: 10.18632/aging.101937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Narasaraju T, Shukla D, More S, Huang C, Zhang L, Xiao X, et al. Role of microRNA‐150 and glycoprotein nonmetastatic melanoma protein B in angiogenesis during hyperoxia‐induced neonatal lung injury. Am J Respir Cell Mol Biol. 2015;52:253‐261. DOI: 10.1165/rcmb.2013-0021OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhong XQ, Wang D, Chen S, Zheng J, Hao TF, Li XH, et al. Umbilical cord blood‐derived exosomes from healthy term pregnancies protect against hyperoxia‐induced lung injury in mice. Clin Transl Sci. 2023;16:966‐977. DOI: 10.1111/cts.13502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruan Y, Dong W, Kang L, Lei X, Zhang R, Wang F, et al. The changes of twist1 pathway in pulmonary microvascular permeability in a newborn rat model of hyperoxia‐induced acute lung injury. Front Pediatr. 2020;8:190. DOI: 10.3389/fped.2020.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Teng RJ, Jing X, Martin DP, Hogg N, Haefke A, Konduri GG, et al. N‐acetyl‐lysyltyrosylcysteine amide, a novel systems pharmacology agent, reduces bronchopulmonary dysplasia in hyperoxic neonatal rat pups. Free Radic Biol Med. 2021;166:73‐89. DOI: 10.1016/j.freeradbiomed.2021.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jagarapu J, Kelchtermans J, Rong M, Chen S, Hehre D, Hummler S, et al. Efficacy of leukadherin‐1 in the prevention of hyperoxia‐induced lung injury in neonatal rats. Am J Respir Cell Mol Biol. 2015;53:793‐801. DOI: 10.1165/rcmb.2014-0422OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim YE, Park WS, Ahn SY, Sung DK, Chang YS. Intratracheal transplantation of mesenchymal stem cells attenuates hyperoxia‐induced lung injury by down‐regulating, but not direct inhibiting formyl peptide receptor 1 in the newborn mice. PLoS One. 2018;13:e0206311. DOI: 10.1371/journal.pone.0206311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim YE, Park WS, Ahn SY, Sung DK, Sung SI, Kim JH, et al. WKYMVm hexapeptide, a strong formyl peptide receptor 2 agonist, attenuates hyperoxia‐induced lung injuries in newborn mice. Sci Rep. 2019;9:6815. DOI: 10.1038/s41598-019-43321-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hummler SC, Rong M, Chen S, Hehre D, Alapati D, Wu S. Targeting glycogen synthase kinase‐3β to prevent hyperoxia‐induced lung injury in neonatal rats. Am J Respir Cell Mol Biol. 2013;48:578‐588. DOI: 10.1165/rcmb.2012-0383OC [DOI] [PubMed] [Google Scholar]
- 31. Bryan C, Sammour I, Guerra K, Sharma M, Dapaah‐Siakwan F, Huang J, et al. TNFα‐stimulated protein 6 (TSG‐6) reduces lung inflammation in an experimental model of bronchopulmonary dysplasia. Pediatr Res. 2019;85:390‐397. DOI: 10.1038/s41390-018-0250-2 [DOI] [PubMed] [Google Scholar]
- 32. Zhang S, Patel A, Chu C, Jiang W, Wang L, Welty SE, et al. Aryl hydrocarbon receptor is necessary to protect fetal human pulmonary microvascular endothelial cells against hyperoxic injury: mechanistic roles of antioxidant enzymes and RelB. Toxicol Appl Pharmacol. 2015;286:92‐101. DOI: 10.1016/j.taap.2015.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shivanna B, Maity S, Zhang S, Patel A, Jiang W, Wang L, et al. Gene expression profiling identifies cell proliferation and inflammation as the predominant pathways regulated by aryl hydrocarbon receptor in primary human fetal lung cells exposed to hyperoxia. Toxicol Sci. 2016;152:155‐168. DOI: 10.1093/toxsci/kfw071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elsaie A, Menon RT, Shrestha AK, Gowda SH, Varghese NP, Barrios RJ, et al. Endothelial adenosine monophosphate‐activated protein kinase‐alpha1 deficiency potentiates hyperoxia‐induced experimental bronchopulmonary dysplasia and pulmonary hypertension. Antioxidants (Basel). 2021;10:1913. DOI: 10.3390/antiox10121913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Menon RT, Thapa S, Shrestha AK, Barrios R, Shivanna B. Extracellular signal‐regulated kinase 1 alone is dispensable for hyperoxia‐mediated alveolar and pulmonary vascular simplification in neonatal mice. Antioxidants (Basel). 2022;11:1130. DOI: 10.3390/antiox11061130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Menon RT, Shrestha AK, Barrios R, Reynolds C, Shivanna B. Tie‐2 cre‐mediated deficiency of extracellular signal‐regulated kinase 2 potentiates experimental bronchopulmonary dysplasia‐associated pulmonary hypertension in neonatal mice. Int J Mol Sci. 2020;21:2408. DOI: 10.3390/ijms21072408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Menon RT, Shrestha AK, Reynolds CL, Barrios R, Caron KM, Shivanna B. Adrenomedullin is necessary to resolve hyperoxia‐induced experimental bronchopulmonary dysplasia and pulmonary hypertension in mice. Am J Pathol. 2020;190:711‐722. DOI: 10.1016/j.ajpath.2019.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Menon RT, Shrestha AK, Shivanna B. Hyperoxia exposure disrupts adrenomedullin signaling in newborn mice: implications for lung development in premature infants. Biochem Biophys Res Commun. 2017;487:666‐671. DOI: 10.1016/j.bbrc.2017.04.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jozefczuk E, Guzik TJ, Siedlinski M. Significance of sphingosine‐1‐phosphate in cardiovascular physiology and pathology. Pharmacol Res. 2020;156:104793. DOI: 10.1016/j.phrs.2020.104793 [DOI] [PubMed] [Google Scholar]
- 40. Harijith A, Pendyala S, Reddy NM, Bai T, Usatyuk PV, Berdyshev E, et al. Sphingosine kinase 1 deficiency confers protection against hyperoxia‐induced bronchopulmonary dysplasia in a murine model: role of S1P signaling and Nox proteins. Am J Pathol. 2013;183:1169‐1182. DOI: 10.1016/j.ajpath.2013.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sudhadevi T, Jafri A, Ha AW, Basa P, Thomas JM, Fu P, et al. Hyperoxia‐induced S1P1 signaling reduced angiogenesis by suppression of TIE‐2 leading to experimental bronchopulmonary dysplasia. Cell Biochem Biophys. 2021;79:561‐573. DOI: 10.1007/s12013-021-01014-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ha AW, Bai T, Ebenezer DL, Sethi T, Sudhadevi T, Mangio LA, et al. Sphingosine kinase 1 regulates lysyl oxidase through STAT3 in hyperoxia‐mediated neonatal lung injury. Thorax. 2022;77:47‐57. DOI: 10.1136/thoraxjnl-2020-216469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mammoto T, Jiang E, Jiang A, Mammoto A. Extracellular matrix structure and tissue stiffness control postnatal lung development through the lipoprotein receptor‐related protein 5/Tie2 signaling system. Am J Respir Cell Mol Biol. 2013;49:1009‐1018. DOI: 10.1165/rcmb.2013-0147OC [DOI] [PubMed] [Google Scholar]
- 44. MasoudiMotlagh M, Sepehr R, Sheibani N, Sorenson CM, Ranji M. Optical cryoimaging of mitochondrial redox state in bronchopulmonary‐dysplasia injury models in mice lungs. Quant Imaging Med Surg. 2015;5:159‐162. DOI: 10.3978/j.issn.2223-4292.2014.12.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alphonse RS, Vadivel A, Fung M, Shelley WC, Critser PJ, Ionescu L, et al. Existence, functional impairment, and lung repair potential of endothelial colony‐forming cells in oxygen‐induced arrested alveolar growth. Circulation. 2014;129:2144‐2157. DOI: 10.1161/CIRCULATIONAHA.114.009124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ren X, Ustiyan V, Guo M, Wang G, Bolte C, Zhang Y, et al. Postnatal alveologenesis depends on FOXF1 signaling in c‐KIT+ endothelial progenitor cells. Am J Respir Crit Care Med. 2019;200:1164‐1176. DOI: 10.1164/rccm.201812-2312OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gillich A, Zhang F, Farmer CG, Travaglini KJ, Tan SY, Gu M, et al. Capillary cell‐type specialization in the alveolus. Nature. 2020;586:785‐789. DOI: 10.1038/s41586-020-2822-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Long Y, Chen H, Deng J, Ning J, Yang P, Qiao L, et al. Deficiency of endothelial FGFR1 alleviates hyperoxia‐induced bronchopulmonary dysplasia in neonatal mice. Front Pharmacol. 2022;13:1039103. DOI: 10.3389/fphar.2022.1039103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hurskainen M, Mižíková I, Cook DP, Andersson N, Cyr‐Depauw C, Lesage F, et al. Single cell transcriptomic analysis of murine lung development on hyperoxia‐induced damage. Nat Commun. 2021;12:1565. DOI: 10.1038/s41467-021-21865-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Collins JJ, Lithopoulos MA, Dos Santos CC, Issa N, Möbius MA, Ito C, et al. Impaired angiogenic supportive capacity and altered gene expression profile of resident CD146+ mesenchymal stromal cells isolated from hyperoxia‐injured neonatal rat lungs. Stem Cells Dev. 2018;27:1109‐1124. DOI: 10.1089/scd.2017.0145 [DOI] [PubMed] [Google Scholar]
- 51. Mižíková I, Lesage F, Cyr‐Depauw C, Cook DP, Hurskainen M, Hänninen SM, et al. Single‐cell RNA sequencing‐based characterization of resident lung mesenchymal stromal cells in bronchopulmonary dysplasia. Stem Cells. 2022;40:479‐492. DOI: 10.1093/stmcls/sxab023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gong J, Feng Z, Peterson AL, Carr JF, Vang A, Braza J, et al. Endothelial to mesenchymal transition during neonatal hyperoxia‐induced pulmonary hypertension. J Pathol. 2020;252:411‐422. DOI: 10.1002/path.5534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Seedorf G, Kim C, Wallace B, Mandell EW, Nowlin T, Shepherd D, et al. rhIGF‐1/BP3 preserves lung growth and prevents pulmonary hypertension in experimental bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2020;201:1120‐1134. DOI: 10.1164/rccm.201910-1975OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hogmalm A, Bry M, Strandvik B, Bry K. IL‐1β expression in the distal lung epithelium disrupts lung morphogenesis and epithelial cell differentiation in fetal mice. Am J Physiol Lung Cell Mol Physiol. 2014;306:L23‐L34. DOI: 10.1152/ajplung.00154.2013 [DOI] [PubMed] [Google Scholar]
- 55. Kim SK, Romero R, Savasan ZA, Xu Y, Dong Z, Lee DC, et al. Endoglin in amniotic fluid as a risk factor for the subsequent development of bronchopulmonary dysplasia. Am J Reprod Immunol. 2013;69:105‐123. DOI: 10.1111/aji.12046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ruschkowski BA, Esmaeil Y, Daniel K, Gaudet C, Yeganeh B, Grynspan D, et al. Thrombospondin‐1 plays a major pathogenic role in experimental and human bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2022;205:685‐699. DOI: 10.1164/rccm.202104-1021OC [DOI] [PubMed] [Google Scholar]
- 57. Lee AH, Dhaliwal R, Kantores C, Ivanovska J, Gosal K, McNamara PJ, et al. Rho‐kinase inhibitor prevents bleomycin‐induced injury in neonatal rats independent of effects on lung inflammation. Am J Respir Cell Mol Biol. 2014;50:61‐73. DOI: 10.1165/rcmb.2013-0131OC [DOI] [PubMed] [Google Scholar]
- 58. Delaney C, Wright RH, Tang JR, Woods C, Villegas L, Sherlock L, et al. Lack of EC‐SOD worsens alveolar and vascular development in a neonatal mouse model of bleomycin‐induced bronchopulmonary dysplasia and pulmonary hypertension. Pediatr Res. 2015;78:634‐640. DOI: 10.1038/pr.2015.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Menden H, Xia S, Mabry SM, Noel‐MacDonnell J, Rajasingh J, Ye SQ, et al. Histone deacetylase 6 regulates endothelial MyD88‐dependent canonical TLR signaling, lung inflammation, and alveolar remodeling in the developing lung. Am J Physiol Lung Cell Mol Physiol. 2019;317:L332‐L346. DOI: 10.1152/ajplung.00247.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. You Y, Guo C, Zhang H, Deng S, Tang J, Xu L, et al. Effect of intranasal instillation of lipopolysaccharide on lung development and its related mechanism in newborn mice. J Interferon Cytokine Res. 2019;39:684‐693. DOI: 10.1089/jir.2019.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Menden H, Tate E, Hogg N, Sampath V. LPS‐mediated endothelial activation in pulmonary endothelial cells: role of Nox2‐dependent IKK‐β phosphorylation. Am J Physiol Lung Cell Mol Physiol. 2013;304:L445‐L455. DOI: 10.1152/ajplung.00261.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen D, Gao ZQ, Wang YY, Wan BB, Liu G, Chen JL, et al. Sodium propionate enhances Nrf2‐mediated protective defense against oxidative stress and inflammation in lipopolysaccharide‐induced neonatal mice. J Inflamm Res. 2021;14:803‐816. DOI: 10.2147/JIR.S303105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mandell E, Seedorf GJ, Ryan S, Gien J, Cramer SD, Abman SH. Antenatal endotoxin disrupts lung vitamin D receptor and 25‐hydroxyvitamin D 1α‐hydroxylase expression in the developing rat. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1018‐L1026. DOI: 10.1152/ajplung.00253.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mandell E, Seedorf G, Gien J, Abman SH. Vitamin D treatment improves survival and infant lung structure after intra‐amniotic endotoxin exposure in rats: potential role for the prevention of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2014;306:L420‐L428. DOI: 10.1152/ajplung.00344.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hou Y, Liu M, Husted C, Chen C, Thiagarajan K, Johns JL, et al. Activation of the nuclear factor‐κB pathway during postnatal lung inflammation preserves alveolarization by suppressing macrophage inflammatory protein‐2. Am J Physiol Lung Cell Mol Physiol. 2015;309:L593‐L604. DOI: 10.1152/ajplung.00029.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Verlato G, Simonato M, Giambelluca S, Fantinato M, Correani A, Cavicchiolo ME, et al. Surfactant components and tracheal aspirate inflammatory markers in preterm infants with respiratory distress syndrome. J Pediatr. 2018;203:442‐446. DOI: 10.1016/j.jpeds.2018.08.019 [DOI] [PubMed] [Google Scholar]
- 67. Stevenson DK, Verter J, Fanaroff AA, Oh W, Ehrenkranz RA, Shankaran S, et al. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed. 2000;83:F182‐F185. DOI: 10.1136/fn.83.3.f182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zysman‐Colman Z, Tremblay GM, Bandeali S, Landry JS. Bronchopulmonary dysplasia – trends over three decades. Paediatr Child Health. 2013;18:86‐90. DOI: 10.1093/pch/18.2.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Coarfa C, Zhang Y, Maity S, Perera DN, Jiang W, Wang L, et al. Sexual dimorphism of the pulmonary transcriptome in neonatal hyperoxic lung injury: identification of angiogenesis as a key pathway. Am J Physiol Lung Cell Mol Physiol. 2017;313:L991‐L1005. DOI: 10.1152/ajplung.00230.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hayward‐Piatkovskyi B, Gonyea CR, Pyle SC, Lingappan K, Gleghorn JP. Sex‐related external factors influence pulmonary vascular angiogenesis in a sex‐dependent manner. Am J Physiol Heart Circ Physiol. 2023;324:H26‐H32. DOI: 10.1152/ajpheart.00552.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang Y, Coarfa C, Dong X, Jiang W, Hayward‐Piatkovskyi B, Gleghorn JP, et al. MicroRNA‐30a as a candidate underlying sex‐specific differences in neonatal hyperoxic lung injury: implications for BPD. Am J Physiol Lung Cell Mol Physiol. 2019;316:L144‐L156. DOI: 10.1152/ajplung.00372.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang Y, Dong X, Lingappan K. Role of HIF‐1α‐miR30a‐Snai1 axis in neonatal hyperoxic lung injury. Oxid Med Cell Longev. 2019;2019:8327486. DOI: 10.1155/2019/8327486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang Y, Dong X, Shirazi J, Gleghorn JP, Lingappan K. Pulmonary endothelial cells exhibit sexual dimorphism in their response to hyperoxia. Am J Physiol Heart Circ Physiol. 2018;315:H1287‐H1292. DOI: 10.1152/ajpheart.00416.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang Y, Jiang W, Wang L, Lingappan K. Sex‐specific differences in the modulation of Growth Differentiation Factor 15 (GDF15) by hyperoxia in vivo and in vitro: role of Hif‐1α. Toxicol Appl Pharmacol. 2017;332:8‐14. DOI: 10.1016/j.taap.2017.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lingappan K, Jiang W, Wang L, Moorthy B. Sex‐specific differences in neonatal hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol. 2016;311:L481‐L493. DOI: 10.1152/ajplung.00047.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cheng H, Wang H, Wu C, Zhang Y, Bao T, Tian Z. Proteomic analysis of sex differences in hyperoxic lung injury in neonatal mice. Int J Med Sci. 2020;17:2440‐2448. DOI: 10.7150/ijms.42073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang Y, Lingappan K. Differential sex‐specific effects of oxygen toxicity in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2017;486:431‐437. DOI: 10.1016/j.bbrc.2017.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang D, Hong H, Li XX, Li J, Zhang ZQ. Involvement of Hdac3‐mediated inhibition of microRNA cluster 17‐92 in bronchopulmonary dysplasia development. Mol Med. 2020;26:99. DOI: 10.1186/s10020-020-00237-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gong J, Feng Z, Peterson AL, Carr JF, Lu X, Zhao H, et al. The pentose phosphate pathway mediates hyperoxia‐induced lung vascular dysgenesis and alveolar simplification in neonates. JCI insight. 2021;6:e137594. DOI: 10.1172/jci.insight.137594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nitkin CR, Xia S, Menden H, Yu W, Xiong M, Heruth DP, et al. FOSL1 is a novel mediator of endotoxin/lipopolysaccharide‐induced pulmonary angiogenic signaling. Sci Rep. 2020;10:13143. DOI: 10.1038/s41598-020-69735-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu M, Iosef C, Rao S, Domingo‐Gonzalez R, Fu S, Snider P, et al. Transforming growth factor‐induced protein promotes NF–κB‐mediated angiogenesis during postnatal lung development. Am J Respir Cell Mol Biol. 2021;64:318‐330. DOI: 10.1165/rcmb.2020-0153OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bui CB, Kolodziej M, Lamanna E, Elgass K, Sehgal A, Rudloff I, et al. Interleukin‐1 receptor antagonist protects newborn mice against pulmonary hypertension. Front Immunol. 2019;10:1480. DOI: 10.3389/fimmu.2019.01480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ambalavanan N, Van Meurs KP, Perritt R, Carlo WA, Ehrenkranz RA, Stevenson DK, et al. Predictors of death or bronchopulmonary dysplasia in preterm infants with respiratory failure. J Perinatol. 2008;28:420‐426. DOI: 10.1038/jp.2008.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cao Y, Jiang S, Sun J, Hei M, Wang L, Zhang H, et al. Assessment of neonatal intensive care unit practices, morbidity, and mortality among very preterm infants in China. JAMA Netw Open. 2021;4:e2118904. DOI: 10.1001/jamanetworkopen.2021.18904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Storti M, Faietti ML, Murgia X, Catozzi C, Minato I, Tatoni D, et al. Time‐resolved transcriptomic profiling of the developing rabbit's lungs: impact of premature birth and implications for modelling bronchopulmonary dysplasia. Respir Res. 2023;24:80. DOI: 10.1186/s12931-023-02380-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hwang JS, Rehan VK. Recent advances in bronchopulmonary dysplasia: pathophysiology, prevention, and treatment. Lung. 2018;196:129‐138. DOI: 10.1007/s00408-018-0084-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kroon AA, Wang J, Post M. Alterations in expression of elastogenic and angiogenic genes by different conditions of mechanical ventilation in newborn rat lung. Am J Physiol Lung Cell Mol Physiol. 2015;308:L639‐L649. DOI: 10.1152/ajplung.00293.2014 [DOI] [PubMed] [Google Scholar]
- 88. Singh SP, Gundavarapu S, Smith KR, Chand HS, Saeed AI, Mishra NC, et al. Gestational exposure of mice to secondhand cigarette smoke causes bronchopulmonary dysplasia blocked by the nicotinic receptor antagonist mecamylamine. Environ Health Perspect. 2013;121:957‐964. DOI: 10.1289/ehp.1306611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Singh SP, Devadoss D, Manevski M, Sheybani A, Ivanciuc T, Exil V, et al. Gestational exposure to cigarette smoke suppresses the gasotransmitter H2S biogenesis and the effects are transmitted transgenerationally. Front Immunol. 2020;11:1628. DOI: 10.3389/fimmu.2020.01628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Singh SP, Chand HS, Gundavarapu S, Saeed AI, Langley RJ, Tesfaigzi Y, et al. HIF‐1α plays a critical role in the gestational sidestream smoke‐induced bronchopulmonary dysplasia in mice. PLoS One. 2015;10:e0137757. DOI: 10.1371/journal.pone.0137757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhang H, Lu H, Yu L, Yuan J, Qin S, Li C, et al. Effects of gestational exposure to perfluorooctane sulfonate on the lung development of offspring rats. Environ Pollut. 2021;272:115535. DOI: 10.1016/j.envpol.2020.115535 [DOI] [PubMed] [Google Scholar]
- 92. Dodson RB, Powers KN, Gien J, Rozance PJ, Seedorf G, Astling D, et al. Intrauterine growth restriction decreases NF‐κB signaling in fetal pulmonary artery endothelial cells of fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2018;315:L348‐L359. DOI: 10.1152/ajplung.00052.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zana‐Taieb E, Pham H, Franco‐Montoya ML, Jacques S, Letourneur F, Baud O, et al. Impaired alveolarization and intra‐uterine growth restriction in rats: a postnatal genome‐wide analysis. J Pathol. 2015;235:420‐430. DOI: 10.1002/path.4470 [DOI] [PubMed] [Google Scholar]
- 94. Correani A, Lanciotti L, Giorgetti C, Palazzi ML, Monachesi C, Antognoli L, et al. Reduced pulmonary oxygen diffusion at 36 weeks of postmenstrual age in small‐for‐gestational‐age preterm infants of less than 32 weeks without bronchopulmonary dysplasia. Pediatr Pulmonol. 2023;58:3054‐3062. DOI: 10.1002/ppul.26620 [DOI] [PubMed] [Google Scholar]
- 95. Ren Y, Lyu Y, Mereness JA, Wang S, Pang J, Mariani TJ. Rare pulmonary connective tissue type mast cells regulate lung endothelial cell angiogenesis. Am J Pathol. 2020;190:1763‐1773. DOI: 10.1016/j.ajpath.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Huang X, Han D, Wei Y, Lin B, Zeng D, Zhang Y, et al. Decreased plasma levels of PDGF‐BB, VEGF‐A, and HIF‐2α in preterm infants after ibuprofen treatment. Front Pediatr. 2022;10:919879. DOI: 10.3389/fped.2022.919879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chen X, Han D, Wang X, Huang X, Huang Z, Liu Y, et al. Vascular and pulmonary effects of ibuprofen on neonatal lung development. Respir Res. 2023;24:39. DOI: 10.1186/s12931-023-02342-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ito R, Barnes EA, Che X, Alvira CM, Cornfield DN. SM22α cell‐specific HIF stabilization mitigates hyperoxia‐induced neonatal lung injury. Am J Physiol Lung Cell Mol Physiol. 2022;323:L129‐L141. DOI: 10.1152/ajplung.00110.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu X, Li K, Zhang F, Zhang Y, Deng C, Guo C. Ablation of glutaredoxin 1 promotes pulmonary angiogenesis and alveolar formation in hyperoxia‐injured lungs by modifying HIF‐1α stability and inhibiting the NF‐κB pathway. Biochem Biophys Res Commun. 2020;525:528‐535. DOI: 10.1016/j.bbrc.2020.02.129 [DOI] [PubMed] [Google Scholar]
- 100. Han W, Zhang F, Mo D, Zhang X, Chen B, Ding X, et al. Involvement of HIF1 stabilization and VEGF signaling modulated by Grx‐1 in murine model of bronchopulmonary dysplasia. Cell Biol Int. 2023;47:796‐807. DOI: 10.1002/cbin.11985 [DOI] [PubMed] [Google Scholar]
- 101. Dumpa V, Nielsen L, Wang H, Kumar V. Caffeine is associated with improved alveolarization and angiogenesis in male mice following hyperoxia induced lung injury. BMC Pulm Med. 2019;19:138. DOI: 10.1186/s12890-019-0903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yun EJ, Lorizio W, Seedorf G, Abman SH, Vu TH. VEGF and endothelium‐derived retinoic acid regulate lung vascular and alveolar development. Am J Physiol Lung Cell Mol Physiol. 2016;310:L287‐L298. DOI: 10.1152/ajplung.00229.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Batlahally S, Franklin A, Damianos A, Huang J, Chen P, Sharma M, et al. Soluble Klotho, a biomarker and therapeutic strategy to reduce bronchopulmonary dysplasia and pulmonary hypertension in preterm infants. Sci Rep. 2020;10:12368. DOI: 10.1038/s41598-020-69296-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shah D, Sandhu K, Das P, Bhandari V. Adiponectin ameliorates hyperoxia‐induced lung endothelial dysfunction and promotes angiogenesis in neonatal mice. Pediatr Res. 2022;91:545‐555. DOI: 10.1038/s41390-021-01442-5 [DOI] [PubMed] [Google Scholar]
- 105. Lingappan K, Maturu P, Liang YW, Jiang W, Wang L, Moorthy B, et al. β‐Naphthoflavone treatment attenuates neonatal hyperoxic lung injury in wild type and Cyp1a2‐knockout mice. Toxicol Appl Pharmacol. 2018;339:133‐142. DOI: 10.1016/j.taap.2017.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhong Y, Catheline D, Houeijeh A, Sharma D, Du L, Besengez C, et al. Maternal omega‐3 PUFA supplementation prevents hyperoxia‐induced pulmonary hypertension in the offspring. Am J Physiol Lung Cell Mol Physiol. 2018;315:L116‐L132. DOI: 10.1152/ajplung.00527.2017 [DOI] [PubMed] [Google Scholar]
- 107. Olave N, Lal CV, Halloran B, Bhandari V, Ambalavanan N. Iloprost attenuates hyperoxia‐mediated impairment of lung development in newborn mice. Am J Physiol Lung Cell Mol Physiol. 2018;315:L535‐L544. DOI: 10.1152/ajplung.00125.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sun Y, Chen C, Zhang X, Wang S, Zhu R, Zhou A, et al. Heparin improves alveolarization and vascular development in hyperoxia‐induced bronchopulmonary dysplasia by inhibiting neutrophil extracellular traps. Biochem Biophys Res Commun. 2020;522:33‐39. DOI: 10.1016/j.bbrc.2019.11.041 [DOI] [PubMed] [Google Scholar]
- 109. Vadivel A, Alphonse RS, Collins JJ, van Haaften T, O'Reilly M, Eaton F, et al. The axonal guidance cue semaphorin 3C contributes to alveolar growth and repair. PLoS One. 2013;8:e67225. DOI: 10.1371/journal.pone.0067225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Das P, Acharya S, Prahaladan VM, Kumova OK, Malaeb S, Behera S, et al. Chitin‐derived AVR‐48 prevents experimental bronchopulmonary dysplasia (BPD) and BPD‐associated pulmonary hypertension in newborn mice. Int J Mol Sci. 2021;22:8547. DOI: 10.3390/ijms22168547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Benny M, Courchia B, Shrager S, Sharma M, Chen P, Duara J, et al. Comparative effects of bone marrow‐derived versus umbilical cord tissue mesenchymal stem cells in an experimental model of bronchopulmonary dysplasia. Stem Cells Transl Med. 2022;11:189‐199. DOI: 10.1093/stcltm/szab011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Xie Y, Chen F, Jia L, Chen R, Zhang VW, Zhong X, et al. Mesenchymal stem cells from different sources show distinct therapeutic effects in hyperoxia‐induced bronchopulmonary dysplasia in rats. J Cell Mol Med. 2021;25:8558‐8566. DOI: 10.1111/jcmm.16817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Grisafi D, Pozzobon M, Dedja A, Vanzo V, Tomanin R, Porzionato A, et al. Human amniotic fluid stem cells protect rat lungs exposed to moderate hyperoxia. Pediatr Pulmonol. 2013;48:1070‐1080. DOI: 10.1002/ppul.22791 [DOI] [PubMed] [Google Scholar]
- 114. Dong N, Zhou PP, Li D, Zhu HS, Liu LH, Ma HX, et al. Intratracheal administration of umbilical cord‐derived mesenchymal stem cells attenuates hyperoxia‐induced multi‐organ injury via heme oxygenase‐1 and JAK/STAT pathways. World J Stem Cells. 2022;14:556‐576. DOI: 10.4252/wjsc.v14.i7.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhong XQ, Yan Q, Chen ZG, Jia CH, Li XH, Liang ZY, et al. Umbilical cord blood‐derived exosomes from very preterm infants with bronchopulmonary dysplasia impaired endothelial angiogenesis: roles of exosomal MicroRNAs. Front Cell Dev Biol. 2021;9:637248. DOI: 10.3389/fcell.2021.637248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. You J, Zhou O, Liu J, Zou W, Zhang L, Tian D, et al. Human umbilical cord mesenchymal stem cell‐derived small extracellular vesicles alleviate lung injury in rat model of bronchopulmonary dysplasia by affecting cell survival and angiogenesis. Stem Cells Dev. 2020;29:1520‐1532. DOI: 10.1089/scd.2020.0156 [DOI] [PubMed] [Google Scholar]
- 117. Sharma M, Bellio MA, Benny M, Kulandavelu S, Chen P, Janjindamai C, et al. Mesenchymal stem cell‐derived extracellular vesicles prevent experimental bronchopulmonary dysplasia complicated by pulmonary hypertension. Stem Cells Transl Med. 2022;11:828‐840. DOI: 10.1093/stcltm/szac041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Braun RK, Chetty C, Balasubramaniam V, Centanni R, Haraldsdottir K, Hematti P, et al. Intraperitoneal injection of MSC‐derived exosomes prevent experimental bronchopulmonary dysplasia. Biochem Biophys Res Commun. 2018;503:2653‐2658. DOI: 10.1016/j.bbrc.2018.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Baker CD, Seedorf GJ, Wisniewski BL, Black CP, Ryan SL, Balasubramaniam V, et al. Endothelial colony‐forming cell conditioned media promote angiogenesis in vitro and prevent pulmonary hypertension in experimental bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2013;305:L73‐L81. DOI: 10.1152/ajplung.00400.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhang X, Lu A, Li Z, Sun J, Dai D, Qian L. Exosomes secreted by endothelial progenitor cells improve the bioactivity of pulmonary microvascular endothelial cells exposed to hyperoxia in vitro. Ann Transl Med. 2019;7:254. DOI: 10.21037/atm.2019.05.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chen Y, Chen W, Xiang X, Deng L, Qian J, Cui W, et al. Pollen‐inspired shell‐core aerosol particles capable of brownian motion for pulmonary vascularization. Adv Mater. 2023;35:e2207744. DOI: 10.1002/adma.202207744 [DOI] [PubMed] [Google Scholar]
- 122. Bolte C, Ustiyan V, Ren X, Dunn AW, Pradhan A, Wang G, et al. Nanoparticle delivery of proangiogenic transcription factors into the neonatal circulation inhibits alveolar simplification caused by hyperoxia. Am J Respir Crit Care Med. 2020;202:100‐111. DOI: 10.1164/rccm.201906-1232OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Aydin E, Levy B, Oria M, Nachabe H, Lim FY, Peiro JL. Optimization of pulmonary vasculature tridimensional phenotyping in the rat fetus. Sci Rep. 2019;9:1244. DOI: 10.1038/s41598-018-37906-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Richter MJ, Harutyunova S, Bollmann T, Classen S, Gall H, et al. Long‐term safety and outcome of intravenous treprostinil via an implanted pump in pulmonary hypertension. J Heart Lung Transplant. 2018;37:1235‐1244. DOI: 10.1016/j.healun.2018.06.006 [DOI] [PubMed] [Google Scholar]


