Abstract
Background:
Early biologic intervention after diagnosis has shown improved clinical and endoscopic outcomes in patients with Crohn’s disease (CD), while very little is known about the effectiveness of early versus late administration of Ustekinumab (UST).
Objectives:
We aimed to compare early versus late UST use in managing CD and identify potential predictors associated with clinical and endoscopic outcomes.
Design:
This was a retrospective observational study.
Methods:
This study included patients with CD who started UST treatment from 2020 to 2023 in our center. Clinical and endoscopic outcomes were compared between early stage (⩽24 months) and later-stage (>24 months) groups at 6 months after starting UST therapy, and clinical predictors associated with any of the outcomes were assessed by logistic regression model. Furthermore, time-to-event analyses were applied to observe CD-related prognosis during follow-up.
Results:
This study included 237 patients with CD, with 44.3% (n = 105) starting UST at the early stage and 55.7% (n = 132) at the later stage. Patients with early UST use demonstrated significantly higher rates of clinical and endoscopic remissions as compared to those with late UST use at 6 months after treatment. After adjusting for disease-related factors using multivariate logistic regression analysis, active perianal disease and severe disease were negatively associated with clinical and endoscopic remission in both early and late UST use groups. Finally, early UST administration was associated with a more favorable long-term outcome in terms of overall hospitalization and treatment escalation during follow-up.
Conclusion:
Starting UST therapy in the early stage of CD especially within the first 6 months was associated with high rates of clinical and endoscopic remission and a low rate of CD-related complications.
Keywords: clinical remission, Crohn’s disease, endoscopic remission, Ustekinumab
Plain language summary
Early Ustekinumab intervention in CD
What is already known? Early biological intervention (early anti-tumor necrosis factor and anti-integrins) had demonstrated improved clinical and endoscopic results in patients suffering from CD. What is new here? Starting UST therapy in the early stage of CD, especially within the first 6 months was associated with high rates of clinical and endoscopic remissions and a low rate of CD-related complications. What do the findings mean? These findings emphasized the significance of initiating UST therapy at an early stage in managing CD, aiding clinicians in making informed decisions about the timing of UST treatment within the disease trajectory.
Introduction
Crohn’s disease (CD) is a chronic, relapsing, and remitting inflammatory disease of the gastrointestinal tract with a progressive and disabling course. 1 Due to the progressive nature of this disease, life-long medical treatment is necessary to reverse the natural history of CD.2,3 The medical management of CD has dramatically changed over the past few decades with the advent of biological agents (anti-tumor necrosis factor (TNF), anti-integrins, and anti-cytokines), and they have been reported to be associated with improved clinical outcomes.4 –7 Conventional “step-up” therapy potentially resulted in long delays to effective medical therapies and the consequent development of chronic, irreversible complications such as stenosis and fistula.8,9 Recent consensus supports the “top‑down” strategy, advocating for the earlier use of biologics, which might witness superior clinical outcomes in CD patients with shorter disease duration in clinical practice.10 –13
The concept of early CD has been defined according to the “Paris definition” as a disease duration ⩽18 months from diagnosis and no prior or current treatment with immunomodulators or biologics. 14 However, early intervention of biological therapy was defined as initiation within 24 months after CD diagnosis in multiple clinical trials and meta-analyses.11,15 –18 Indeed, previous studies have shown that early initiation of anti-TNF (infliximab and adalimumab) in the course of CD (within 24 months) may improve clinical outcomes in terms of significantly less stricture formation, decreased need for surgery, and greater success in maintaining disease remission and promoting fistula healing.16,17 Besides, another study found that patients with CD for 24 months or less are significantly more likely to achieve a complete response, corticosteroid-free remission or endoscopic response to vedolizumab than patients with longer disease duration. 18 However, it is unclear whether the benefits associated with early anti-TNF or anti-integrin use in CD could be applied to the new anti-IL12/23 (Ustekinumab) therapeutic biological agent. 19 More importantly, the optimal timing for early Ustekinumab (UST) therapy initiation in CD is still controversial, and the definition of the window of opportunity within this period remains unclear.
This study utilized the prospectively acquired data from our center to explore whether initiating UST within the first 24 months after CD diagnosis yields positive effects on predefined outcome parameters (clinical and endoscopic remission) compared to patients who started UST treatment after the initial 24 months since diagnosis. Additionally, we investigated the impact of early UST initiation on CD-related prognosis (hospitalization, treatment escalation, disease behavior progression, and intestinal surgeries) and assessed potential factors that predict these clinical outcomes using multivariate regression analysis.
Materials and methods
Study design and population
This retrospective, observational, single-center study involved a review of electronic medical records of patients aged ⩾18 years, diagnosed with CD and treated at the IBD center of the Gastroenterology Department of the First Affiliated Hospital, Sun Yat-sen University (China) between January 2020 and December 2023. CD was definitively diagnosed based on a comprehensive evaluation that included clinical presentation, laboratory findings, endoscopic features, radiologic imaging, histologic assessment, and surgical findings. Patients were included if they started UST therapy before or after the 24 months after diagnosis and had at least one clinical and/or endoscopic follow-up of 6 months after UST initiation. UST was administered intravenously at a dosage of approximately 6 mg/kg (260 mg for patients weighing under 55 kg; 390 mg for patients weighing between 55 and 85 kg; 520 mg for patients weighing over 85 kg) at week 0; patients who respond to intravenous induction subsequently receive 90 mg subcutaneously every 8 weeks or every 12 weeks. Contrarily, patients without an established diagnosis of CD or with insufficient medical records before referrals were excluded, although previous use of steroids, 5-aminosalicylates, or immunomodulators was permitted.
The reporting of our study conformed to the STROBE statement (Supplemental Material), and the details of all patients were de-identified to ensure confidentiality. 20 In addition, this study was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University, and a waiver of informed consent was granted for this retrospective study.
Data extraction
The medical records of all patients were reviewed, and informative data were collected including patient characteristics (gender, body mass index, smoking status at diagnosis, age at diagnosis of CD, and age at UST initiation); disease characteristics (phenotype classified according to the Montreal classifications for CD at diagnosis, disease-related complications, extra-intestinal manifestations, and prior surgeries); treatment history (immunomodulators, steroids, and prior biologics exposure); concomitant treatments (steroids and/or immunomodulators); laboratory tests (C-reactive protein (CRP), white blood cell, hemoglobin (Hb), platelet (PLT), and albumin (ALB)); disease severity (clinical and endoscopic assessments); follow-up outcomes (hospitalization, treatment escalation, intestinal surgery, and behavioral progression).
Outcomes and definitions
The primary outcomes of interest were to compare the cumulative rates of clinical remission and endoscopic remission at 6 months after the start of UST treatment between the early UST use group and the late UST use group. Early UST use was defined as ⩽24 months and late UST use as >24 months at the time of UST initiation, and this definition is consistent with most prior data on the impact of disease duration on the efficacy of biologics. These two groups of patients received UST therapy based on their disease activity, previous medication history, and treatment availability. The secondary outcomes of interest were the timing assessments for hospitalizations, behavioral progression, treatment escalation, and intestinal surgeries during the follow-up period. CD-related hospitalization was defined as any hospital admission for complications including infections, fistula, strictures, abscess, massive lower gastrointestinal bleeding, or exacerbations. CD-related surgeries were referred to as intestinal, perianal disease surgeries, and stricturoplasty. Treatment escalation was defined as switching to other biological agents or small molecule drugs or combination with another biological agent and/or small molecule drug. Behavioral progression was defined as the development of stricturing (B2) or penetrating (B3) complications in patients with non-stricturing, non-penetrating behavior (B1). Composite outcome was defined as the occurrence of at least one positive result among the secondary outcomes listed above. Clinical assessments were performed based on the Crohn’s disease activity index (CDAI), where remission was defined as the complete resolution of all CD-related symptoms as assessed by treating physicians. Endoscopic remission was defined as the absence of ulcers and/or erosions in CD according to the Simple Endoscopic Score for Crohn’s Disease (SES-CD) or Rutgeerts score done by local study investigators and was reverified by a coordinating study investigator with any discrepancies resolved through consensus between the study sites and the coordinating site. Severe disease was referred to CDAI >450, and/or SES-CD >15 or Rutgeerts score ⩾i3.
Statistical analysis
Demographic and baseline characteristics were summarized using descriptive statistics. Continuous variables were described as means and standard deviations, or median (interquartile range (IQR)) based on data distribution. Categorical or nominal variables were presented as numbers and percentages. Differences between the two groups were compared using independent sample t-test (two group comparisons) or Pearson chi-square or Fisher exact tests. Univariate and multivariate analyses were performed to express results as hazard ratios with 95% confidence intervals (CIs) using Cox proportional hazard regression to evaluate risk factors associated with outcome events. The Kaplan-Meier method was used to estimate times from starting drug use to the development of outcome events, with groups compared by log-rank tests. Statistical significance was defined as a p value <0.05. All statistical analyses were performed using SPSS statistics version 23.0 for Windows (IBM, New York, NY, USA).
Results
Study population
A total of 237 patients with CD were included, of whom 105 were early initiators of UST (⩽24 months) and 132 were late initiators of UST (>24 months) therapy, separately. Most of the patients enrolled were male (73.3% in the early UST group vs 66.7% in the late UST group, p = 0.268). Notably, patients who received early UST therapy were younger (median age 26.9 years vs 33.4 years, p < 0.001), but the median age at diagnosis showed no significant differences between these two groups. In addition, patients with late UST therapy had a higher fraction of B3 phenotype (34.1% vs 21.0%, p = 0.026), while those early initiators had a higher proportion of B1 phenotype (59.0% vs 37.9%, p = 0.001). In terms of medication history, the rates of an immunomodulator (IM) and steroid use before first UST use were higher in late initiators than those in early initiators (72.0% and 37.1% vs 16.2% and 8.6%, respectively; p < 0.001 for both comparisons), while the rate of concomitant IM and steroid use were not different between these two groups. Furthermore, a higher percentage of patients with late UST use had previously received other biologics, including TNF antagonists and vedolizumab inefficiently as compared to those early initiators (70.5% vs 31.4%, p < 0.001). For CD-related complications at baseline, there were no significant differences between groups in perianal disease and extra-intestinal manifestation, except for the presence of intestinal resection history, which was significantly higher in those patients with late UST use when compared with those who started within 24 months (42.4% vs 8.6%, p < 0.001) shown in Table 1.
Table 1.
Comparison of baseline characteristics between early and late UST use groups.
| Characteristics | Early UST use (n = 105) | Late UST use (n = 132) | p Value |
|---|---|---|---|
| Male gender, n (%) | 77 (73.3) | 88 (66.7) | 0.268 |
| Age at diagnosis, years, median (IQR) | 26.7 (22.1, 33.1) | 26.6 (21.3, 35.8) | 0.776 |
| Age at UST initiation, years, median (IQR) | 26.9 (22.4, 33.5) | 33.4 (27.4, 43.0) | <0.001 |
| Disease location, n (%) | |||
| Ileal | 19 (18.1) | 31 (23.5) | 0.312 |
| Colonic | 1 (1.0) | 5 (3.8) | 0.335 |
| Ileocolonic | 85 (81.0) | 96 (72.7) | 0.139 |
| Upper GI tract | 10 (9.5) | 9 (6.8) | 0.446 |
| Disease behavior, n (%) | |||
| Non-stricturing, non-penetrating (B1) | 62 (59.0) | 50 (37.9) | 0.001 |
| Stricturing (B2) | 21 (20.0) | 37 (28.0) | 0.153 |
| Penetrating (B3) | 22 (21.0) | 45 (34.1) | 0.026 |
| Active perianal disease, n (%) | 60 (57.1) | 71 (53.8) | 0.606 |
| Extra-intestinal manifestations, n (%) | 4 (3.8) | 6 (4.5) | 1.000 |
| Previous intestinal resection, n (%) | 9 (8.6) | 56 (42.4) | <0.001 |
| Previous or current smoking, n (%) | 9 (8.6) | 14 (10.6) | 0.599 |
| Prior steroid, n (%) | 9 (8.6) | 49 (37.1) | <0.001 |
| Prior IM, n (%) | 17 (16.2) | 95 (72.0) | <0.001 |
| Biologics exposure, n (%) | 33 (31.4) | 93 (70.5) | <0.001 |
| Concomitant steroids, n (%) | 3 (2.9) | 2 (1.5) | 0.796 |
| Concomitant IM, n (%) | 1 (1.0) | 7 (5.3) | 0.139 |
GI, Gastrointestinal; IM, Immunomodulator; IQR, Interquartile range; UST, Ustekinumab.
Clinical and endoscopic remission
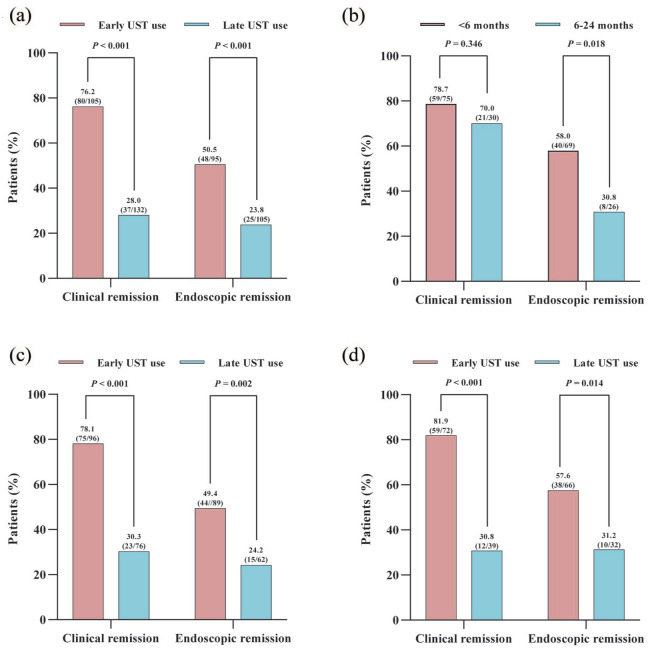
As shown in Table 2 and Figure 1(a), a total of 76.2% (80/105) patients with CD reached clinical remission and 50.5% (48/95) achieved endoscopic remission in the early UST group within 6 months of initiation of UST treatment. In the late UST initiators, 28.0% (37/132) of the patients achieved clinical remission and 23.8% (25/105) achieved endoscopic remission. Significant differences were observed in both clinical and endoscopic remission rates when comparing early UST use group to late UST use group (both p < 0.001). Besides, a significant difference in CDAI scores between early initiators and late initiators (137.0, IQR: 117.0–148.0 vs 168.0, IQR: 147.8–199.1; p < 0.001) was observed in the 6th month following the UST induction treatment. Additionally, clinical remission rates in the early treatment group stratified according to the timing of initiation of the UST therapy (⩽6 months vs >6 and ⩽24 months; 78.7% vs 70.0%, p = 0.346) remained high, but there was a significant difference between groups in term of endoscopic remission (⩽6 months vs >6 and ⩽24 months; 58.0% vs 30.8%, p = 0.018) (Figure 1(b)). Moreover, significantly decreased CDAI scores, CRP levels, and PLT count were observed in both early- and late-UST-treated groups, while only early initiators of UST achieved recovery in the levels of Hb and ALB. Furthermore, there were significant differences in SES-CD scores (2.5, IQR: 1.0–6.0 vs 4.0, IQR: 3.0–10.0; p < 0.001) between those with early UST use and those with late UST use at 6 months of follow-up. Considering surgical induction of remission and prior exposure to biological agents could potentially influence the outcomes of UST treatment, we further performed a sensitivity analysis. For those without a history of intestinal surgery, early UST use contributed to significantly higher rates of clinical and endoscopic remission as compared to late UST use (p < 0.001 and p = 0.002) (Figure 1(c)). In addition, early UST use was associated with significantly higher rates of clinical and endoscopic remission than late UST use in those patients without prior exposure to biologics (p < 0.001 and p = 0.014) (Figure 1(d)).
Table 2.
Comparative analysis of clinical and endoscopic characteristics in early and late UST use groups at different time points.
| Characteristics | Early UST use (n = 105) | Late UST use (n = 132) | Q value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 6-month follow-up | p Value | Baseline | 6-month follow-up | p Value | ||
| Clinical characteristics | |||||||
| BMI, kg/m2, median (IQR) | 18.4 (17.0, 20.2) | 19.5 (18.1, 21.1) | 0.008 | 19.4 (17.7, 21.7) | 19.8 (17.9, 21.8) | 0.290 | 0.472 |
| CRP, mg/L, median (IQR) | 6.3 (2.4, 27.9) | 3.0 (0.8, 6.9) | <0.001 | 8.0 (2.2, 20.5) | 2.7 (0.9, 8.9) | <0.001 | 0.643 |
| WBC, ×109/L, median (IQR) | 6.5 (5.1, 8.5) | 6.3 (5.0, 7.3) | 0.115 | 6.3 (4.9, 7.5) | 6.0 (5.0, 7.3) | 0.460 | 0.572 |
| Hemoglobin, g/L, median (IQR) | 122.0 (102.0, 136.0) | 130.0 (117.0, 143.0) | 0.006 | 123.5 (104.0, 138.0) | 128.0 (112.0, 139.3) | 0.177 | 0.106 |
| PLT, ×109/L, median (IQR) | 314 (255, 388) | 264 (220, 318) | <0.001 | 290.5 (247, 360) | 257.0 (212, 317) | 0.003 | 0.898 |
| Albumin, g/L, median (IQR) | 38.4 (33.3, 42.0) | 39.7 (37.3, 42.2) | 0.021 | 38 (34.6, 41.4) | 38.0 (35.8, 40.3) | 0.726 | 0.002 |
| CDAI, median (IQR) | 226.4 (191.0, 270.6) | 137.0 (117.0, 148.0) | <0.001 | 230.0 (198.8, 272.3) | 168.0 (147.8, 199.1) | <0.001 | <0.001 |
| Clinical remission, n (%) | 0 (0.0) | 80 (76.2) | – | 0 (0.0) | 37 (28.0) | – | <0.001 |
| Endoscopic characteristics | |||||||
| SES-CD, median (IQR) a | 8.5 (5.0, 15.0) | 2.5 (1.0, 6.0) | <0.001 | 10.0 (5.0, 15.0) | 4.0 (3.0, 10.0) | <0.001 | 0.003 |
| Rutgeerts score ⩾i2, n (%) b | 5 (100.0) | 2 (40.0) | – | 40 (90.9) | 34 (77.3) | 0.080 | – |
| Endoscopic remission, n (%) c | 4 (4.2) | 48 (50.5) | – | 6 (5.7) | 25 (23.8) | – | <0.001 |
Q value refers to the statistics comparisons between early and late UST use groups at 6 months follow-up.
Data available in 90 patients in early UST use group and 61 patients in the late UST use group.
Data available in 5 patients in the early UST use group and 44 patients in the late UST use group.
Data available in 95 patients in the early UST use group and 105 patients in the late UST use group at baseline.
BMI, body mass index; CDAI, Crohn’s disease activity index; CRP, C-reactive protein; IQR, interquartile range; PLT, blood platelet; SES-CD, Simple Endoscopic Score for Crohn’s disease; UST, Ustekinumab; WBC, white blood cell.
Figure 1.
Percentages of CD patients achieving clinical remission and endoscopic remission: (a) comparison of early UST use versus late UST use in overall enrolled patients; (b) comparison of <6 months versus 6–24 months in patients with early UST use; (c) comparison of early UST use versus late UST use in patients without a history of intestinal surgery; (d) comparison of early UST use versus late UST use in patients without prior exposure to biologics.
CD, Crohn’s disease; UST, Ustekinumab.
Logistic regression analysis for factors associated with clinical and endoscopic outcomes
In univariate logistic regression evaluating protective factors for clinical remission of all enrolled patients with UST treatment, the odds ratio (OR) for early initiation of UST therapy was 0.122 (95% CI 0.068–0.219, p < 0.001), and other univariate risk predictors of clinical remission included stricturing/penetrating phenotype, active perianal disease, previous intestinal resection, biologics exposure, and high CRP/ALB value. In multivariate regression, early UST use (OR 0.105, 95% CI 0.049–0.225, p < 0.001), active perianal disease (OR 4.742, 95% CI 2.357–9.538, p < 0.001) and high CRP/ALB value (OR 1.884, 95% CI 1.182–3.001, p = 0.008) retained statistical significance for clinical remission. In terms of endoscopic remission for all enrolled patients, only early UST use at diagnosis was a significant positive predictor for the achievement of this outcome in both univariate and multivariate regression analysis (OR 0.400, 95% CI 0.205–0.781, p = 0.007), but severe disease (OR 3.312, 95% CI 1.541–7.116, p = 0.002) was the independent negative factor for achieving endoscopic remission at 6 months after treatment initiation (Table 3).
Table 3.
Predictors of clinical remission and endoscopic remission in all patients with CD undergoing UST use.
| Predictors of treatment outcomes | Univariate analysis | p Value | Multivariable analysis | p Value |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Clinical remission | ||||
| Male gender | 0.590 (0.337–1.035) | 0.066 | 0.527 (0.260–1.067) | 0.075 |
| Early UST use | 0.122 (0.068–0.219) | <0.001 | 0.105 (0.049–0.225) | <0.001 |
| Stricturing/penetrating phenotype | 1.690 (1.011–2.828) | 0.045 | 0.732 (0.332–1.617) | 0.441 |
| Active perianal disease | 2.783 (1.639–4.725) | <0.001 | 4.742 (2.357–9.538) | <0.001 |
| Previous intestinal resection | 3.206 (1.735–5.924) | <0.001 | 2.382 (0.927–6.121) | 0.071 |
| Biologics exposure | 3.087 (1.816–5.248) | <0.001 | 1.364 (0.692–2.688) | 0.370 |
| Severe disease | 1.705 (0.949–3.064) | 0.074 | 1.024 (0.472–2.221) | 0.953 |
| CRP/ALB | 1.501 (1.052–2.141) | 0.025 | 1.884 (1.182–3.001) | 0.008 |
| Endoscopic remission | ||||
| Male gender | 0.548 (0.282–1.067) | 0.077 | 0.604 (0.295–1.239) | 0.169 |
| Early UST use | 0.306 (0.167–0.559) | <0.001 | 0.400 (0.205–0.781) | 0.007 |
| Biologics exposure | 2.957 (1.622–5.389) | <0.001 | 1.761 (0.894–3.470) | 0.102 |
| Severe disease | 3.782 (1.818–7.870) | <0.001 | 3.312 (1.541–7.116) | 0.002 |
Variables entered in the multivariable models before backward selection was performed included those with p < 0.1. For clinical and endoscopic remission, variables included gender, early UST use, disease location, stricturing/penetrating phenotype, active perianal disease, extra-intestinal manifestations, previous intestinal resection, previous or current smoking, biologics exposure, severe disease, body mass index, and CRP/ALB.
ALB, albumin; CD, Crohn’s disease; CRP, C-reactive protein; UST, Ustekinumab.
Subsequently, a stratified analysis was performed to assess differences according to the time of initiation of UST therapy. In the group of early UST use shown in Table 4, active perianal disease, previous biologics exposure, and high CRP/ALB value were negatively associated with the achievement of clinical remission. Even after adjusting for clinically relevant predictors in multivariate analysis, active perianal disease (OR 4.809, 95% CI 1.329–17.396, p = 0.017) and high CRP/ALB value (OR 3.350, 95% CI 1.663–6.750, p < 0.001) at baseline were similarly negative factors for this outcome. In the group of late UST use shown in Table 5, active perianal disease was the only independent negative factor associated with the achievement of any of the outcomes in both univariate and multivariate analysis. In terms of endoscopic remission, severe disease at diagnosis was an independent negative factor for achieving endoscopic remission in both early and late UST use groups.
Table 4.
Predictors of clinical remission and endoscopic remission in CD patients with early UST use.
| Predictors of treatment outcomes | Univariate analysis OR (95% CI) | p Value | Multivariate analysis OR (95% CI) | p Value |
|---|---|---|---|---|
| Clinical remission | ||||
| Active perianal disease | 3.012 (1.089–8.330) | 0.034 | 4.809 (1.329–17.396) | 0.017 |
| Biologics exposure | 2.593 (1.024–6.569) | 0.044 | 2.374 (0.833–6.768) | 0.106 |
| CRP/ALB | 2.665 (1.474–4.819) | 0.001 | 3.350 (1.663–6.750) | <0.001 |
| Endoscopic remission | ||||
| Biologics exposure | 2.579 (1.040–6.394) | 0.041 | 2.230 (0.874–5.690) | 0.093 |
| Severe disease | 3.319 (1.223–9.006) | 0.018 | 2.930 (1.057–8.118) | 0.039 |
Variables entered in the multivariable models before backward selection was performed included those with p < 0.1. For clinical and endoscopic remission, variables included gender, disease location, stricturing/penetrating phenotype, active perianal disease, extra-intestinal manifestations, previous intestinal resection, previous or current smoker, biologics exposure, severe disease, body mass index, and CRP/ALB.
ALB, albumin; CD, Crohn’s disease; CRP, C-reactive protein; UST, Ustekinumab.
Table 5.
Predictors of clinical remission and endoscopic remission in CD patients with late UST use.
| Predictors of treatment outcomes | Univariate analysis OR (95% CI) | p Value | Multivariate analysis OR (95% CI) | p Value |
|---|---|---|---|---|
| Clinical remission | ||||
| Disease location | ||||
| Ileal | Reference | Reference | ||
| Colonic | 2.375 (0.345–16.357) | 0.380 | 2.516 (0.314–20.124) | 0.385 |
| Ileocolonic | 0.471 (0.198–1.118) | 0.088 | 0.759 (0.291–1.978) | 0.573 |
| Active perianal disease | 1.171 (1.072–1.405) | <0.001 | 1.195 (1.078–1.489) | <0.001 |
| Body mass index | 1.151 (1.013–1.308) | 0.031 | 1.129 (0.985–1.293) | 0.081 |
| Endoscopic remission | ||||
| Severe disease | 3.880 (1.220–12.347) | 0.022 | 3.880 (1.220–12.347) | 0.022 |
Variables entered in the multivariable models before backward selection was performed included those with p < 0.1. For clinical and endoscopic remission, variables included gender, disease location, stricturing/penetrating phenotype, active perianal disease, extra-intestinal manifestations, previous intestinal resection, previous or current smoker, biologics exposure, severe disease, body mass index, and CRP/ALB.
ALB, albumin; CD, Crohn’s disease; CRP, C-reactive protein; UST, Ustekinumab.
Disease course and complications
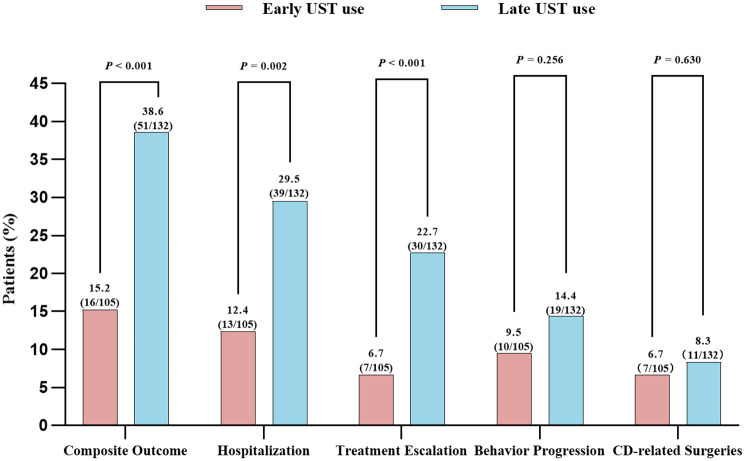
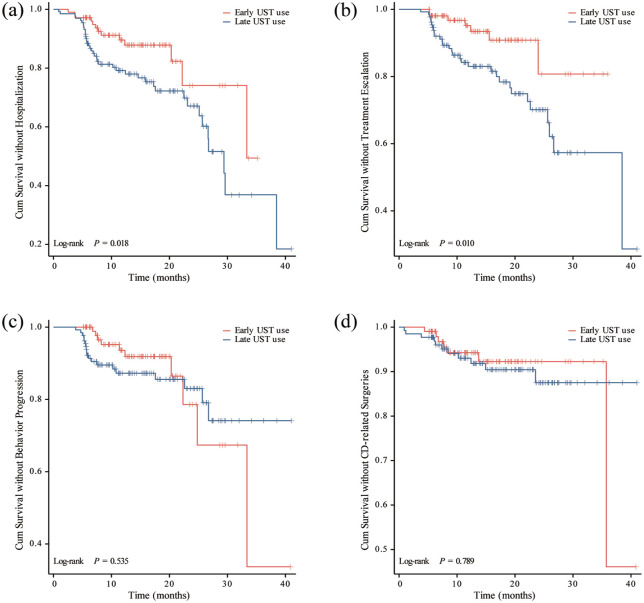
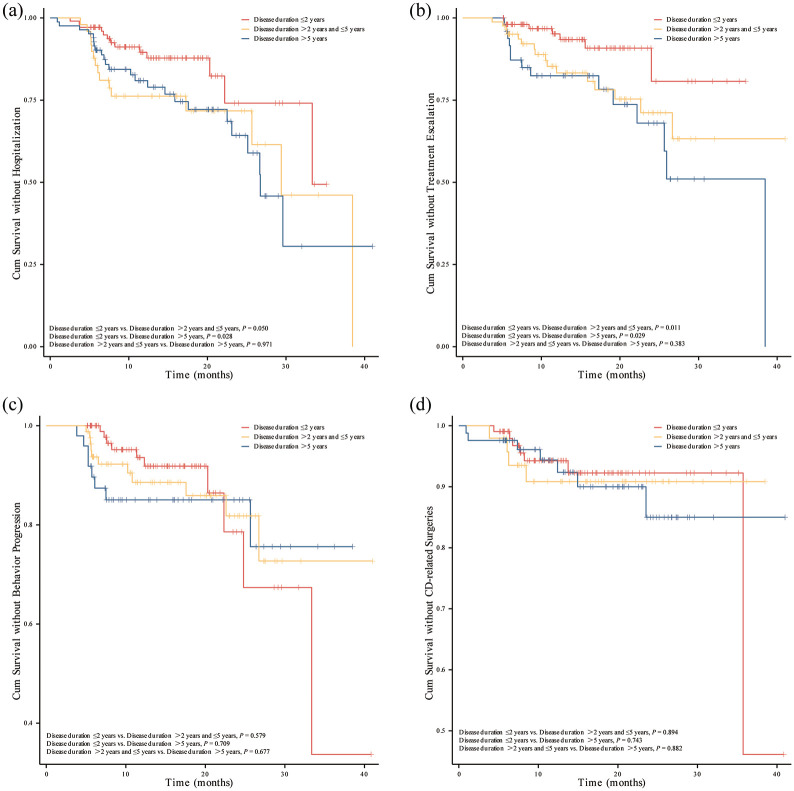
For all included patients, the median follow-up time was 13.9 months (IQR 8.1–20.7 months). Overall, during the follow-up period, 7 patients (6.7%) required treatment escalation, 10 patients (9.5%) experienced progression in Montreal classification of disease behavior, 13 patients (12.4%) were hospitalized, and 7 patients (6.7%) underwent intestinal resection in early UST group. However, patients with late UST use showed higher rates of developing disease complications, showing 30 patients (22.7%) required treatment escalation, 19 patients (14.4%) experienced progression in Montreal classification of disease behavior, 39 patients (29.5%) were hospitalized, and 11 patients (8.3%) underwent intestinal resection. Furthermore, the overall percentage of composite outcome among patients who initiated UST therapy early was significantly lower than those who started UST treatment later (15.2% vs 38.6%, p < 0.001) (Table 6; Figure 2). Kaplan-Meier analysis further revealed significantly reduced risks of hospitalization and treatment escalation (log-rank test: p = 0.018 and p = 0.010) in the early UST group compared to the late UST group during the follow-up period (Figure 3). However, there were no significant differences in the cumulative probabilities of behavior progression and CD-related surgeries between patients with early versus late UST administration. We further categorized the late UST group into two distinct subgroups: those treated between 2–5 years after CD diagnosis (>2 to ⩽5 years group) and those treated after more than 5 years (>5 years group). Compared to the early UST use (⩽2 years group) group, both distinct subgroups derived from the late UST use cohort also demonstrated significantly higher rates of hospitalization (log-rank test: p = 0.050 and p = 0.028) and treatment escalation (log-rank test: p = 0.011 and p = 0.029) (Figure 4).
Table 6.
New-onset clinical outcomes following UST treatment during the follow-up period.
| Clinical outcomes | Early UST use (n = 105) | Late UST use (n = 132) | p Value |
|---|---|---|---|
| Composite outcome, n (%) | 16 (15.2) | 51 (38.6) | <0.001 |
| Hospitalization, n (%) | 13 (12.4) | 39 (29.5) | 0.002 |
| Treatment escalation, n (%) | 7 (6.7) | 30 (22.7) | <0.001 |
| Behavior progression, n (%) | 10 (9.5) | 19 (14.4) | 0.256 |
| CD-related surgeries, n (%) | 7 (6.7) | 11 (8.3) | 0.630 |
CD, Crohn’s disease; UST, Ustekinumab.
Figure 2.
Comparison of clinical outcomes between early UST use and late UST use in patients with CD.
CD, Crohn’s disease; UST, Ustekinumab.
Figure 3.
Kaplan-Meier curve for CD-related complications stratified by early UST use and late UST use. (a) CD-related hospitalization. (b) CD-related treatment escalation. (c) Progression in disease behavior. (d) CD-related intestinal surgeries.
CD, Crohn’s disease; UST, Ustekinumab.
Figure 4.
Kaplan-Meier curve for CD-related complications stratified by UST-enabled disease duration: ⩽2 years, >2 years and ⩽5 years, >5 years. (a) CD-related hospitalization. (b) CD-related treatment escalation. (c) Progression in disease behavior. (d) CD-related intestinal surgeries.
CD, Crohn’s disease; UST, Ustekinumab.
Safety
The adverse-event analysis captured for maintenance periods across the early and late UST groups was shown in Table 7, and there were no significant differences in adverse events between these two different treatment groups (21.0% in early initiators vs 18.2% in late initiators). Additionally, no patients in either the early or late UST treatment groups discontinued therapy due to opportunistic infections or other adverse events.
Table 7.
Rates of adverse events during the period of UST treatment.
| Adverse events | Early UST use (n = 105) | Late UST use (n = 132) | p Value |
|---|---|---|---|
| All adverse events, n (%) | 22 (21.0) | 24 (18.2) | 0.592 |
| Infection, n (%) | 9 (8.6) | 12 (9.1) | 0.889 |
| Community-acquired pneumonia | 2 (1.9) | 7 (5.3) | 0.309 |
| Clostridioides difficile colitis | 6 (5.7) | 2 (1.5) | 0.157 |
| CMV colitis | 2 (1.9) | 1 (0.8) | 0.842 |
| Urinary tract infection | 2 (1.9) | 0 (0.0) | 0.380 |
| Cellulitis | 0 (0.0) | 2 (1.5) | 0.581 |
| Vulvitis | 0 (0.0) | 1 (0.8) | 1.000 |
| Otitis media | 0 (0.0) | 1 (0.8) | 1.000 |
| Dermatological lesions, n (%) | 6 (5.7) | 3 (2.3) | 0.301 |
| Rash | 5 (4.8) | 2 (1.5) | 0.280 |
| Eczema | 0 (0.0) | 1 (0.8) | 1.000 |
| Herpes zoster | 1 (1.0) | 0 (0.0) | 0.443 |
| Arthralgia, n (%) | 1 (1.0) | 3 (2.3) | 0.782 |
| Headache, n (%) | 0 (0.0) | 1 (0.8) | 1.000 |
| Peripheral neuropathy, n (%) | 2 (1.9) | 1 (0.8) | 0.842 |
| Massive lower gastrointestinal bleeding, n (%) | 1 (1.0) | 0 (0.0) | 0.443 |
| Infusion reaction and hypersensitivity, n (%) | 2 (1.9) | 2 (1.5) | 1.000 |
| Malignancy, n (%) | 1 (1.0) | 1 (0.8) | 1.000 |
CMV, cytomegalovirus; UST, Ustekinumab.
Discussion
In this study, we have shown that initiation of biological therapy with UST within the first 24 months after diagnosis is associated with high rates of clinical and endoscopic remission at 6 months after treatment initiation, and low rates of CD-related complications during follow-up. Despite this, we found no differences in clinical remission at different times of UST initiation within this 24-month window of opportunity, but endoscopic remission was only attained in CD patients with early UST intervention within the first 6 months. In addition, early UST use was associated with clinical and endoscopic remission in patients with CD at the 6th month, while active perianal disease and severe disease showed opposite correlations with these predefined outcomes regardless of UST initiation timing. Furthermore, an association was also demonstrated between early UST administration and a reduced risk of developing CD-related complications, including hospitalization and treatment escalation. Notably, our results contributed to the growing literature on early intervention in CD and demonstrated that improved outcomes with early treatment were not restricted to the effect of anti-TNF or anti-integrin therapy.
Biological agents have ushered in a transformative era in the treatment of CD, and these innovative agents have emerged as the first-line option in clinical practice and lead to enhanced clinical outcomes for patients. 21 However, a substantial proportion of patients, approximately 40%, exhibit primary non-response to TNF inhibitors, and 23%–46% of patients encountered secondary loss of response, underscoring the pressing need for precision treatment of biological agents personalized to individual patient needs.22,23 Currently, new biological agents such as anti-IL-12/IL-23 inhibitors have been developed and are emerging as promising alternative therapies in the clinical management of CD. 24 Real-world evidence has demonstrated that UST achieved notable success in both inducing and sustaining remission in individuals with IBD who are refractory to anti-TNF agents or conventional therapy.6,25,26 In addition, UST also demonstrated its efficacy in treating perianal refractory CD and other extra-intestinal manifestations, showcasing its versatility in addressing a range of CD-related complications.27,28 Importantly, UST has been linked to a reduced risk of infection and other adverse events, which further enhances its appeal as a safer and more tolerable therapeutic option for patients with complex or treatment-resistant CD.27,28 Despite UST offers significant advantages in enhancing clinical and endoscopic remission for patients who have not responded to primary anti-TNF therapy, serving as a valuable secondary treatment option, there remains a dearth of information regarding the definition of the window of opportunity and the optimal timing for initiating UST therapy. The precise definition of the window of opportunity in early CD treatment suggests that early effective treatment within a certain period soon after disease diagnosis may contribute to the prevention of bowel damage and disability. 14 Substantial evidence is increasingly endorsing the early use of biologic therapy for patients with CD, and a recent meta-analysis demonstrated that the use of biologics within the first 2 years after diagnosis or in a top-down strategy is associated with significantly higher rates of clinical and endoscopic remission and with lower relapse rates compared with conventional step-up management.10,29 A recent randomized controlled trial (RCT), PROFILE, also found that in patients newly diagnosed with active CD who had well-balanced clinical characteristics and disease activity at baseline, top-down therapy using a combination of infliximab and an immunomodulator was more effective than accelerated step-up therapy, resulting in higher rates of steroid-free and surgery-free remission, as well as endoscopic remission. 8 Notably, the patients in this study had a median disease duration of only 12 days, which suggested that the benefits of intervention with biologics may be maximized in the early stages of the disease. In this study, our results supported the benefit of initiating UST therapy within 24-month window of opportunity to achieve favorable clinical outcomes, with particular emphasis on the first 6 months following the initial diagnosis. We found high clinical and endoscopic remission rates of 76.2% and 50.5% in early UST initiators at 6 months after treatment, which were in agreement with previous clinical trials that started infliximab treatment in patients with a median disease duration of <2 years and that demonstrated one of the highest endoscopic remission rates of 44% at week 26. 15 It is worth noting that our results exhibited significant advantages in improved clinical outcomes for CD patients in early UST use group as compared to those with vedolizumab treatment (38% in clinical remission and 29% in endoscopic remission). 18 Nevertheless, our research did not yield conclusive comparative data regarding the clinical outcomes between early intervention with anti-TNF agents and UST, and this absence of direct comparison appeals to the need for further studies to elucidate the relative efficacy of these two therapeutic approaches when initiated promptly after diagnosis. In addition, there were very clear differences between the early UST use cohort and the late UST use cohort in terms of previous medication history (steroids, immune modulators, and biologics), prior surgery, and the presence of a B3 phenotype. Even though the multivariable analysis identified shorter disease duration as independently associated with treatment response, it is likely that the demographic and therapeutic differences at baseline may explain this association. Therefore, cohorts with matched baseline characteristics as detailed above should be constructed to further verify our conclusions.
Predicting the response to biologics in IBD is a critical aspect, and personalized medicine holds the promise of enhancing treatment efficacy, minimizing the risk of adverse drug events, and curtailing healthcare costs by establishing the most suitable therapy for a selected patient.30,31 Ileocolonic disease, no prior surgery, and uncomplicated phenotype had been reported to be associated with better responses to UST in CD. 32 However, no specific biomarker for UST response has been identified in clinical practice of IBD, as noted in another review. 30 In the present study, the initiation of UST therapy in the early stages emerged as the sole positive predictor associated with achieving both clinical and endoscopic remission in the included patients with CD. In addition, multivariate logistic regression analyses have uncovered that the presence of active perianal disease and high disease severity at baseline are independent factors that may significantly predict a diminished response to UST treatment even in CD patients with short-duration disease. Although in a large real-life multicenter cohort study, roughly 40% of the patients with active perianal CD failing multiple biologic agents reached success on perianal disease at 6 months with clinical success, clinical and endoscopic outcomes could not be evaluated simultaneously.28,33 Similar results to our research were found in another study that perianal disease, Harvey-Bradshaw Index, current opioid use, and current corticosteroid use are associated with UST failure after dose intensification in CD. 34 Further prospective research is essential to refine the application of early UST therapy for patients with CD, particularly those with active perianal disease or high disease severity.
Despite UST has consistently demonstrated its effectiveness in achieving clinical and endoscopic remission, the evidence regarding its early intervention’s impact on the prevention of long-term complications in CD remains sparse. 35 In our study, newly diagnosed CD patients who started biological therapy within 24 months of the diagnosis, 15.2% (16/105) of the patients developed CD-related complications during follow-up, while 38.6% (51/132) of the patients in late UST group reached the predefined complications. In terms of long-term outcomes, previous research has focused on treatment discontinuation, clinical and endoscopic remission, health-related quality of life, adverse events, and so on, but few studies reported CD-related complications such as disease progression, hospitalization rate, and intestinal resection.26,35 –37 Interestingly, in contrast to the outcomes observed with anti-TNF therapies, our study revealed that early administration of UST was associated with a reduced number of overall hospitalizations and instances of treatment escalation. However, this did not translate to a significant difference in colectomy rates or the progression of disease behavior between patients who received early versus late UST treatment. This distinction suggested that while early UST intervention may influence certain aspects of management in CD, its impact on the overall disease course and the need for surgical interventions may be more nuanced and require further exploration. Furthermore, our study reported no deaths, and no new safety concerns were identified during the safety monitoring phase. These findings emphasized the significance of initiating UST therapy at an early stage in the management of CD, aiding clinicians in making informed decisions about the timing of UST treatment within the disease trajectory. Further investigation is necessary to fully understand the extent to which early UST therapy can mitigate the development of chronic issues associated with CD, such as strictures, fistulas, and the need for surgical interventions.
Strengths and limitations
The strengths of our study are notable, particularly the inclusion of a substantial patient cohort and the extended follow-up periods, both of which reflect real-world clinical scenarios. Standardizing treatment strategies were implemented among study subjects, and clinical data were sourced from a well-established IBD patient registry of our center. Our data are concordant with studies of TNF antagonist medications in CD showing a benefit in early intervention, and this extension broadens the scope of our understanding and offers new perspectives on the timing and selection of biologic therapies for CD. It is important to acknowledge the limitations of our study, which include its retrospective design, the absence of standardized follow-up assessments, and the presence of some missing data, particularly in the areas of endoscopic and radiological evaluations, and these factors could potentially limit the power of our findings. While our results showed active perianal disease and severe disease were negatively associated with clinical and endoscopic remission in both early and late UST use groups, we were unable to characterize fistula response as perianal examinations were not universally documented for all patients. Consequently, there is a clear need for rigorous, RCTs with extended follow-up periods to yield more robust evidence that can inform early treatment decisions involving UST in the management of CD.
Conclusions
In summary, patients with CD who were treated with UST and had a disease duration of 24 months or less showed significantly enhanced outcomes, with notably higher rates of both clinical and endoscopic remission compared to those with longer disease duration. In contrast, patients with CD featuring active perianal complications and severe disease might not exhibit the same level of improvement when treated with UST. The combined safety and efficacy of UST positions it as a compelling candidate for early, proactive intervention, with the aim of achieving sustainable disease modification in CD. Further research is essential to investigate the potential long-term benefits of initiating UST early in the treatment process, particularly its capacity to halt or delay the progression of CD.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848241307596 for Early intervention with Ustekinumab is associated with higher rates of clinical and endoscopic remission in patients with Crohn’s disease by Tong Tu, Mengqi Chen, Manying Li, Linxin Liu, Zihan Chen, Jianming Lin, Baili Chen, Yao He, Minhu Chen, Zhirong Zeng and Xiaojun Zhuang in Therapeutic Advances in Gastroenterology
Acknowledgments
None.
Footnotes
ORCID iDs: Tong Tu  https://orcid.org/0009-0008-3461-9692
https://orcid.org/0009-0008-3461-9692
Yao He  https://orcid.org/0000-0002-5530-5624
https://orcid.org/0000-0002-5530-5624
Minhu Chen  https://orcid.org/0000-0001-8181-0846
https://orcid.org/0000-0001-8181-0846
Xiaojun Zhuang  https://orcid.org/0000-0001-9361-3911
https://orcid.org/0000-0001-9361-3911
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Tong Tu, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Mengqi Chen, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Manying Li, Department of Medical Ultrasonics, Institute of Diagnostic and Interventional Ultrasound, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China.
Linxin Liu, Boji Pharmaceutical Research Center, Boji Medical Biotechnological Co. Ltd., Guangzhou, Guangdong, China.
Zihan Chen, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Jianming Lin, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Baili Chen, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Yao He, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Minhu Chen, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Zhirong Zeng, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, No. 58 Zhongshan Road 2, Guangzhou 510080, China.
Xiaojun Zhuang, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, No. 58 Zhongshan Road 2, Guangzhou 510080, China.
Declarations
Ethics approval and consent to participate: This study was reviewed and approved by The Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (Number of approval: [2024]464), and a waiver of informed consent was permitted in the retrospective study.
Consent for publication: Not applicable.
Author contributions: Tong Tu: Data curation; Formal analysis; Investigation; Writing – original draft; Writing – review & editing.
Mengqi Chen: Data curation; Formal analysis; Writing – review & editing.
Manying Li: Data curation; Formal analysis; Writing – review & editing.
Linxin Liu: Formal analysis; Methodology; Writing – review & editing.
Zihan Chen: Data curation; Writing – review & editing.
Jianming Lin: Data curation; Writing – review & editing.
Baili Chen: Project administration; Writing – review & editing.
Yao He: Project administration; Writing – review & editing.
Minhu Chen: Project administration; Writing – review & editing.
Zhirong Zeng: Conceptualization; Project administration; Writing – review & editing.
Xiaojun Zhuang: Conceptualization; Funding acquisition; Methodology; Project administration; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (82100576).
The authors declare that there is no conflict of interest.
Availability of data and materials: The data underlying this study will be shared on reasonable request to the corresponding author.
References
- 1. Dolinger M, Torres J, Vermeire S. Crohn’s disease. Lancet 2024; 403(10432): 1177–1191. [DOI] [PubMed] [Google Scholar]
- 2. Ley D, Leroyer A, Dupont C, et al. New therapeutic strategies have changed the natural history of pediatric Crohn’s disease: a two-decade population-based study. Clin Gastroenterol Hepatol 2022; 20(11): 2588–2597. [DOI] [PubMed] [Google Scholar]
- 3. Geem D, Hercules D, Pelia RS, et al. Progression of pediatric Crohn’s disease is associated with anti-tumor necrosis factor timing and body mass index Z-score normalization. Clin Gastroenterol Hepatol 2024; 22(2): 368–376. [DOI] [PubMed] [Google Scholar]
- 4. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010; 362(15): 1383–1395. [DOI] [PubMed] [Google Scholar]
- 5. Dulai PS, Singh S, Jiang X, et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US victory consortium. Am J Gastroenterol 2016; 111(8): 1147–1155. [DOI] [PubMed] [Google Scholar]
- 6. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016; 375(20): 1946–1960. [DOI] [PubMed] [Google Scholar]
- 7. Gisbert JP, Chaparro M. Anti-TNF agents and new biological agents (Vedolizumab and Ustekinumab) in the prevention and treatment of postoperative recurrence after surgery in Crohn’s disease. Drugs 2023; 83(13): 1179–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Noor NM, Lee JC, Bond S, et al. A biomarker-stratified comparison of top-down versus accelerated step-up treatment strategies for patients with newly diagnosed Crohn’s disease (PROFILE): a multicentre, open-label randomised controlled trial. Lancet Gastroenterol Hepatol 2024; 9(5): 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoekman DR, Stibbe JA, Baert FJ, et al. Long-term outcome of early combined immunosuppression versus conventional management in newly diagnosed Crohn’s disease. J Crohns Colitis 2018; 12(5): 517–524. [DOI] [PubMed] [Google Scholar]
- 10. Ben-Horin S, Novack L, Mao R, et al. Efficacy of biologic drugs in short-duration versus long-duration inflammatory bowel disease: a systematic review and an individual-patient data meta-analysis of randomized controlled trials. Gastroenterology 2022; 162(2): 482–494. [DOI] [PubMed] [Google Scholar]
- 11. Ungaro RC, Aggarwal S, Topaloglu O, et al. Systematic review and meta-analysis: efficacy and safety of early biologic treatment in adult and paediatric patients with Crohn’s disease. Aliment Pharmacol Ther 2020; 51(9): 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Attauabi M, Steenholdt C, Poulsen A, et al. Network meta-analysis: comparative onset of early effect of biologics and small molecules in moderately to severely active luminal Crohn’s disease. Aliment Pharmacol Ther 2024; 60(2): 124–143. [DOI] [PubMed] [Google Scholar]
- 13. Klomberg RCW, van der Wal HC, Aardoom MA, et al. Improved clinical outcomes with early anti-tumour necrosis factor alpha therapy in children with newly diagnosed Crohn’s disease: real-world data from the international prospective PIBD-SET quality inception cohort study. J Crohns Colitis 2024; 18(5): 738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peyrin-Biroulet L, Billioud V, D’Haens G, et al. Development of the Paris definition of early Crohn’s disease for disease-modification trials: results of an international expert opinion process. Am J Gastroenterol 2012; 107(12): 1770–1776. [DOI] [PubMed] [Google Scholar]
- 15. Revés J, Mascarenhas A, José Temido M, et al. Early intervention with biologic therapy in Crohn’s disease: how early is early? J Crohns Colitis 2023; 17(11): 1752–1760. [DOI] [PubMed] [Google Scholar]
- 16. Frei R, Fournier N, Zeitz J, et al. Early initiation of anti-TNF is associated with favourable long-term outcome in Crohn’s disease: 10-year-follow-up data from the swiss IBD cohort study. J Crohns Colitis 2019; 13(10): 1292–1301. [DOI] [PubMed] [Google Scholar]
- 17. Schreiber S, Reinisch W, Colombel JF, et al. Subgroup analysis of the placebo-controlled CHARM trial: increased remission rates through 3 years for adalimumab-treated patients with early Crohn’s disease. J Crohns Colitis 2013; 7(3): 213–221. [DOI] [PubMed] [Google Scholar]
- 18. Faleck DM, Winters A, Chablaney S, et al. Shorter disease duration is associated with higher rates of response to vedolizumab in patients with Crohn’s disease but not ulcerative colitis. Clin Gastroenterol Hepatol 2019; 17(12): 2497–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vuyyuru SK, Shackelton LM, Hanzel J, et al. Targeting IL-23 for IBD: rationale and progress to date. Drugs 2023; 83(10): 873–891. [DOI] [PubMed] [Google Scholar]
- 20. von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335(7624): 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Derkx B, Taminiau J, Radema S, et al. Tumour-necrosis-factor antibody treatment in Crohn’s disease. Lancet 1993; 342(8864): 173–174. [DOI] [PubMed] [Google Scholar]
- 22. Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol 2009; 104(3): 760–767. [DOI] [PubMed] [Google Scholar]
- 23. Qiu Y, Chen BL, Mao R, et al. Systematic review with meta-analysis: loss of response and requirement of anti-TNFα dose intensification in Crohn’s disease. J Gastroenterol 2017; 52(5): 535–554. [DOI] [PubMed] [Google Scholar]
- 24. Verstockt B, Salas A, Sands BE, et al. IL-12 and IL-23 pathway inhibition in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2023; 20(7): 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Onali S, Pugliese D, Caprioli FA, et al. An objective comparison of Vedolizumab and Ustekinumab effectiveness in Crohn’s disease patients’ failure to TNF-alpha inhibitors. Am J Gastroenterol 2022; 117(8): 1279–1287. [DOI] [PubMed] [Google Scholar]
- 26. García MJ, Rivero M, Fernández-Clotet A, et al. Comparative study of the effectiveness of Vedolizumab versus Ustekinumab after anti-TNF failure in Crohn’s disease (versus-CD): data from the ENEIDA registry. J Crohns Colitis 2024; 18(1): 65–74. [DOI] [PubMed] [Google Scholar]
- 27. Johnson AM, Barsky M, Ahmed W, et al. The real-world effectiveness and safety of Ustekinumab in the treatment of Crohn’s disease: results from the SUCCESS consortium. Am J Gastroenterol 2023; 118(2): 317–328. [DOI] [PubMed] [Google Scholar]
- 28. Chapuis-Biron C, Kirchgesner J, Pariente B, et al. Ustekinumab for perianal Crohn’s disease: the BioLAP multicenter study from the GETAID. Am J Gastroenterol 2020; 115(11): 1812–1820. [DOI] [PubMed] [Google Scholar]
- 29. Zhang L, Jin Z, Hao J. Efficacy of early biologic therapy versus late/conventional therapy in children and adolescents with Crohn’s disease: a systematic review and meta-analysis. Saudi J Gastroenterol 2023; 29(5): 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gisbert JP, Chaparro M. Predictors of primary response to biologic treatment [anti-TNF, Vedolizumab, and Ustekinumab] in patients with inflammatory bowel disease: from basic science to clinical practice. J Crohns Colitis 2020; 14(5): 694–709. [DOI] [PubMed] [Google Scholar]
- 31. Verstockt B, Noor NM, Marigorta UM, et al. Results of the seventh scientific workshop of ECCO: precision medicine in IBD-disease outcome and response to therapy. J Crohns Colitis 2021; 15(9): 1431–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barré A, Colombel JF, Ungaro R. Review article: predictors of response to Vedolizumab and Ustekinumab in inflammatory bowel disease. Aliment Pharmacol Ther 2018; 47(7): 896–905. [DOI] [PubMed] [Google Scholar]
- 33. Singh S, Proctor D, Scott FI, et al. AGA technical review on the medical management of moderate to severe luminal and perianal fistulizing Crohn’s disease. Gastroenterology 2021; 160(7): 2512–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dalal RS, Njie C, Marcus J, et al. Predictors of Ustekinumab failure in Crohn’s disease after dose intensification. Inflamm Bowel Dis 2021; 27(8): 1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sandborn WJ, Rebuck R, Wang Y, et al. Five-year efficacy and safety of Ustekinumab treatment in Crohn’s disease: the IM-UNITI trial. Clin Gastroenterol Hepatol 2022; 20(3): 578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Forss A, Clements M, Myrelid P, et al. Ustekinumab is associated with real-world long-term effectiveness and improved health-related quality of life in Crohn’s disease. Dig Dis Sci 2023; 68(1): 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Solitano V, Facciorusso A, Jess T, et al. Comparative risk of serious infections with biologic agents and oral small molecules in inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2023; 21(4): 907–921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848241307596 for Early intervention with Ustekinumab is associated with higher rates of clinical and endoscopic remission in patients with Crohn’s disease by Tong Tu, Mengqi Chen, Manying Li, Linxin Liu, Zihan Chen, Jianming Lin, Baili Chen, Yao He, Minhu Chen, Zhirong Zeng and Xiaojun Zhuang in Therapeutic Advances in Gastroenterology