Abstract
Background:
Fluoroquinolones (FQs) are associated with potential tendon injury but comparative risk versus other antibiotic (non-FQ) options for the same indication has rarely been evaluated.
Objective
Describe the incidence (relative risk) of any tendon injury in patients receiving FQs compared with other (non-FQ) antibiotics for treatment of urinary tract infections (UTIs).
Methods
A retrospective propensity score-weighted cohort study was performed to evaluate the association between FQ antibiotics and tendon injury at two time points (within one month and within six months of use) compared with non-FQ regimens for treatment of UTI. The evaluation was performed using the Merative™ MarketScan® Research Databases from 2014 to 2020. Adult patients with International Classification of Diseases (ICD)-9/10 coding for UTI were included. Patients with a history of tendon injury or those who received both FQ and non-FQ regimens during the study period were excluded. Propensity score weighting was used to adjust for selection bias due to contributing risk factors, including demographics (age, sex), comorbidities (diabetes mellitus, chronic kidney disease), and concurrent medications (corticosteroids).
Results
Both the 1-month and 6-month cohorts were predominately female and less than 50 years of age. At one month, the incidence of tendon injury was 0.2% in the FQ group and 0.1% in the non-FQ group, and the odds of tendon injury were not estimated to be significantly different between groups (odds ratio [OR] = 1.03, 95% confidence interval [CI] 0.93, 1.32). Odds of tendon injury were also not estimated to be significantly different in the 6-month cohort (OR = 0.98, 95% CI 0.84, 1.05).
Conclusion and Relevance
In this population of predominantly young female patients without high incidence of potentially contributing comorbidities, increased risk of tendon injury was not associated with FQ use. Future research is needed to determine whether demographic differences between this and other previously studied populations account for this discordant result
Keywords: fluoroquinolone, tendon injury, urinary tract infection
Fluoroquinolone (FQ) antibiotics are commonly used for the treatment of urinary tract infections (UTIs). According to data from the Center for Disease Control (CDC), FQs accounted for 14.8 million outpatient prescriptions in the United States in 2022. 1 Although they are frequently used, the exact place in UTI management has been a point of discussion. The Infectious Disease Society of America (IDSA) guideline statement for the Management of Uncomplicated Cystitis and Pyelonephritis lists FQ agents as a second line for cystitis due to “collateral damage,” but they remain a first-line option for pyelonephritis. The term “collateral damage” used by the IDSA guidelines refers to the potentially broader-than-necessary spectrum for uncomplicated, localized bladder infections, as well as the long list of potential adverse effects associated with these agents. 2 Fluoroquinolones have acquired numerous black-box warnings for their serious potential adverse effects.3 -5 A black-box warning was issued in 2008 specifically for the risk of tendinitis and tendon rupture. 6 This warning was further emphasized by the Food and Drug Administration (FDA) in 2016 when it recommended that this antibiotic class only be used when the benefits outweigh the risks for cystitis, sinusitis, and bronchitis. 4 Even after these warnings, FQs continue to be commonly used to treat UTIs.7 -11
Many mechanisms have been proposed to explain the risk of tendon injury associated with FQ use. In an article by Bidell and Lodise, both direct tissue injury to tendon components and indirect effects that impair the integrity of tendon structure or impact the repair processes have been described. 12 In light of the known risk of tendon injury from FQ therapy, our study aimed to evaluate the occurrence of this adverse event when used for a single indication relative to other antibiotic options used for this same indication.
While it seems clear from the literature that FQ antibiotics are associated with tendon injury, very few studies have evaluated the relative risk (or increased odds) of this adverse effect explicitly compared with other available treatment options for the same, single indication. Previous studies have primarily either compared FQ recipients with a hodge-podge group of “other non-FQ antibiotics” (used for a variety of indications, some not even potential alternatives for the indication of interest) or to a single non-FQ antibiotic agent (but not in a group of patients being treated for the same indication) or to patients not receiving any antibiotic (non-antibiotic users).13 -20 While these studies have helped to quantify the risk of tendon injury associated with FQ use, they do not provide the prescriber a relative risk versus other antibiotic options when choosing therapy for an individual patient for a specific indication. Our study evaluates the risk of any tendon injury in patients treated for UTIs with FQ versus other non-FQ treatment options. Many studies have focused evaluation solely on the Achilles tendon and often capture only rupture rather than any level of tendon injury. There is evidence that the effect of FQs is not limited to the Achilles tendon or only rupture.19,21
Most studies have evaluated the risk window of 30 days, while some have extended evaluation up to 6 months or 1-year postinitiation of FQ therapy.14,22 -25 Evaluation of FQ dose, duration of therapy, or cumulative exposure has been accounted for in some studies and risk appears to increase as the dose/duration/exposure increases. 26 Risk factors and concurrent conditions that increase the risk of tendon injury in patients receiving FQ agents continue to be a topic of interest. 26 We evaluated the risk of any tendon injury of any severity within one month and six months FQ exposure, specifically in a population of patients treated for UTI. We believe that this adds critical knowledge to the literature about the risk of this significant adverse drug event when treating patients for a single condition.
In our study, tendon injury is defined broadly, including tendon rupture, tendinitis and other injuries (see supplemental data for relevant International Classification of Disease (ICD)-9 and ICD-10 codes). This study builds upon a previous study conducted using the Merative™ MarketScan® Research Databases (hereafter, “MarketScan®”) to evaluate the risk of tendon injury in adult patients treated for a separate single indication. We previously reported the incidence of tendon injury in patients being treated for community-acquired pneumonia (CAP) with either FQ or non-FQ guideline-based antibiotic regimens and found a significantly increased risk associated with FQ use. 27 We aimed to evaluate a separate population treated for another single indication, UTI, to compare the risk of tendon injury in patients treated with FQs versus other non-FQ regimens recommended for this indication. The purpose of comparing alternative guideline-based recommendations for a single indication is to allow clinicians to gauge risk associated with FQ versus other regimens that would be appropriate for the same indication (rather than a heterogeneous list of non-FQ agents that might not all be options for treatment of the specific condition/same indication)—which has been the case in many of the published studies that evaluated the risk of tendon injury with the FQ antibiotic class.
Materials and Methods
Study Design and Data Source
We conducted a retrospective, propensity score (PS)-weighted cohort study to evaluate the association between the use of FQ antibiotics and the risk of tendon injury in patients treated for UTI using the Merative™ MarketScan® Commercial Claims and Encounters (CCAE) and Medicare Supplemental and Coordination of Benefits (COB) databases. MarketScan comprises one of the largest collections of de-identified claims data in the United States; it includes adjudicated claims data on outpatient and inpatient health care visits and outpatient pharmacy dispensing data. Health care service visit and pharmaceutical claims in the CCAE and COB databases from 2014 through 2020 were used for this study. This study was conducted in accordance with Investigational Review Board Committee requirements for Human Subjects.
Cohort Definition and Exposures
Subjects were identified who had an antibiotic prescription claim within 10 days of health care services claim with a diagnosis of bacterial UTI (see supplemental data for the corresponding ICD-9 and ICD-10 codes).28,29 Initial health care visits could have occurred in the inpatient or outpatient setting as long as patients had a corresponding prescription drug claim for oral antibiotic therapy. The date of the first such prescription claim was established as the index date for the patient. Patients were required to be 18 years or older at the index date and to be evaluated during a 6-month washout period prior to the index date. Patients were required to have continuous enrollment during the post-index date evaluation period. Patients evaluated at the 6-month endpoint had the same inclusion criteria as the 1-month cohort plus no additional health care visits coded for a subsequent UTI nor any additional antibiotic therapy in months two through six. Patients were classified into two groups: FQ and non-FQ, based on whether they received FQ antibiotics or a non-FQ containing antibiotic regimen for treatment of UTI. Patients receiving a combination of FQ and non-FQ antibiotics were excluded from the sample. Additional exclusion criteria were use of antibiotics during the washout period, history of previous tendon injury documented in the washout period, and use of multiple (crossover) regimens of antibiotic treatment during the follow-up period. In part, these restrictions were chosen to ensure that the exposures in the FQ and non-FQ groups were homogeneous and to reduce the number of potentially confounding variables. As described below, additional protection against confounding was achieved through PS weighting.
Information regarding antibiotic agents and duration of therapy was collected using National Drug Code (NDC) number documentation from prescriptions captured in the outpatient drug claims database for the following agents: FQ therapy group (levofloxacin, ciprofloxacin, moxifloxacin) and non-FQ-based regimens (amoxicillin, amoxicillin-clavulanate, ceftriaxone, cephalexin, cefuroxime, cefdinir, cefpodoxime, cefixime, doxycycline, nitrofurantoin, sulfamethoxazole/trimethoprim, fosfomycin, tobramycin, gentamicin, amikacin, and linezolid). Moxifloxacin was only included because it was determined that some patients were prescribed this agent for the treatment of UTI, even though this agent is not approved and should not be used for this indication.
Outcomes and Covariates
Patients were followed for up to six months (180 days) following the index date to detect tendon injuries (see Supplemental Data Table X for the relevant ICD-9 and ICD-10 codes). The risk of tendon injury was evaluated for all patients meeting inclusion criteria at each of two time points: within 30 days and within 180 days of the index date. Potential baseline confounders were identified a priori, including age [AGE], gender [SEX], concurrent receipt of systemic steroid (corticosteroid), concurrent conditions, such as diabetes mellitus (E11), chronic kidney disease (N18), organ transplantation (heart/lung/kidney), and history of tendon injury (identified by ICD-9/10 coding)28,29 during the 6-month washout period). We considered statin use as input into the PS model; however, since the link between statins and tendon injury is weaker than the other included variables, we chose not to include it in our current study.
Statistical Analysis
To control for baseline confounders, PS weighting was used with inverse probability of treatment weighting to estimate the average treatment effect among the treated (ATT). Propensity scores were estimated via logistic regression of FQ use on age, sex, previous, concurrent, or subsequent corticosteroid therapy, and the presence of concurrent long-term conditions. Covariate balance in the PS-weighted sample was established to a threshold of 0.1 for standardized bias and 0.05 for Kolmogorov-Smirnov distance for all potential confounders. The ATT was estimated as an odds ratio (OR) via PS-weighted logistic regression of occurrence of tendon injury on FQ use with all baseline confounders included as additive covariates to control for residual confounding. A 95% confidence interval (CI) for the OR quantifying the ATT of FQ use was estimated with the nonparametric bootstrap (percentile method).
As our statistical methods are focused on unconfounding the causal effect of FQ use, we report the ATT for this exposure but omit effects of confounders in the PS-weighted logistic model. This approach follows work of Westreich and Greenland 30 and others 31 who argue that such effects can remain confounded and are not amenable to straight-forward interpretation.
Demographic and clinical characteristics were described using standard descriptive statistics. Differences between FQ and non-FQ cohorts were expected, and justified the use of propensity weighting, but we also report demographics to characterize the sample.
Data management was done using SAS 9.4 (SAS Institute, Cary, NC) using PROC SQL. Additional statistical analyses were done in R 32 using the WeightIt, 33 cobalt, 34 and boot 35 packages for PS weighting, covariate balance checking, and nonparametric bootstrap, respectively.
Results
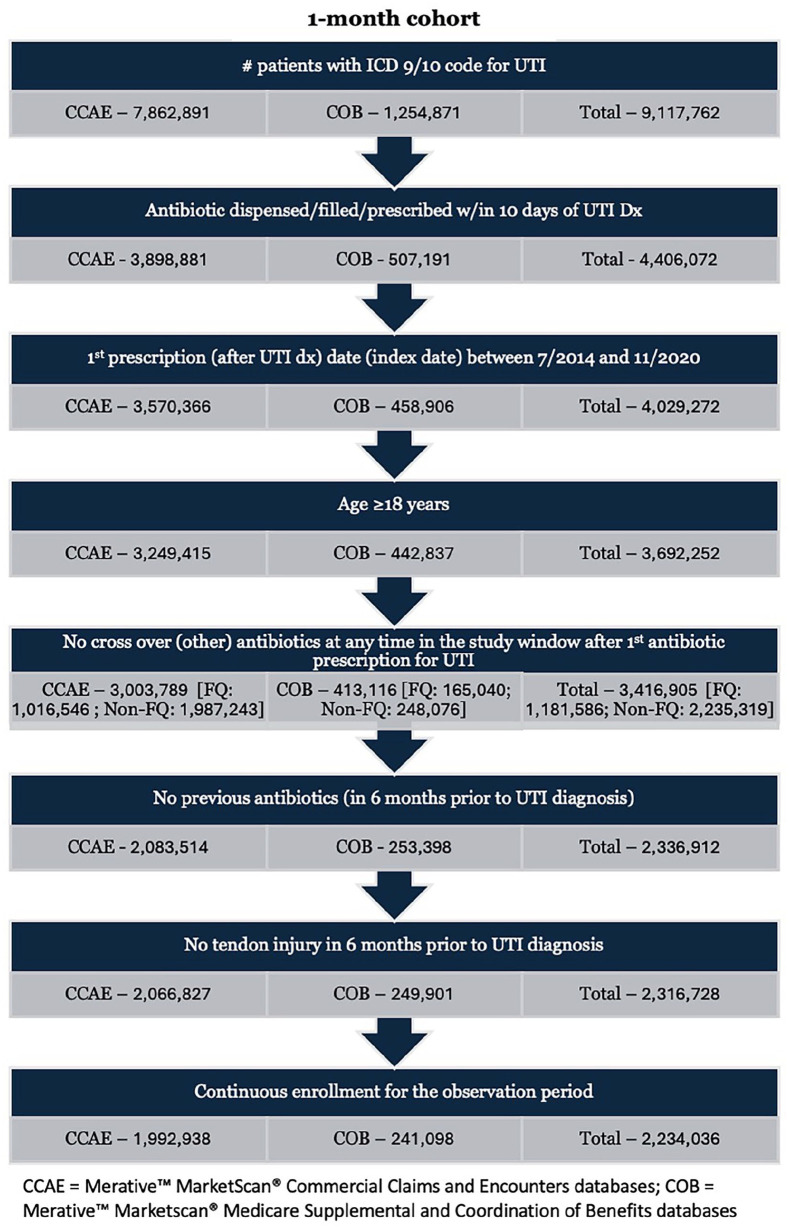
Determination of patient cohorts for the 1-month and 6-month analyses is described in Figures 1 and 2. A total of 2,234,036 patients from the combined MarketScan CCAE and COB databases were included for the analysis of tendon injury at 1 month. Of the total 1-month study cohort, 774,767 patients (34.7%) received FQ agents for treatment of UTI (FQ group) and 1,459,269 (65.3%) received a non-FQ-based regimen (non-FQ group). Baseline demographics of the two groups are shown in Table 1. Mean age of patients in the FQ and non-FQ groups were 48.4 and 43.8 years, respectively. The FQ group had a larger percentage of patients aged > 60 years (22.7% vs 17%) and male gender (25.9% vs 13.8%). The percentage of patients with diabetes mellitus (6.6% vs 5.7%) and chronic kidney disease were similar between groups (0.2% FQ group vs 0.2% for non-FQ group). Prior corticosteroid use, defined as any steroid use from the beginning of the 6-month washout period to the index date, was documented in 7.5% of FQ and 7% of non-FQ patients. Concomitant corticosteroid use, defined as any steroid use within 10 days of the index date, was documented in 0.8% of the FQ and 0.9% of the non-FQ group. Receipt of corticosteroids in the postantibiotic course period, from > 10 days after index date until the end of the 6-month follow-up period, occurred in 1.3% and 1.4% of the FQ and non-FQ groups, respectively.
Figure 1.
One-month cohort.
Abbreviations: CCAE, Merative™ MarketScan® Commercial Claims and Encounters database; COB, Merative™ MarketScan® Medicare Supplemental and Coordination of Benefits database.
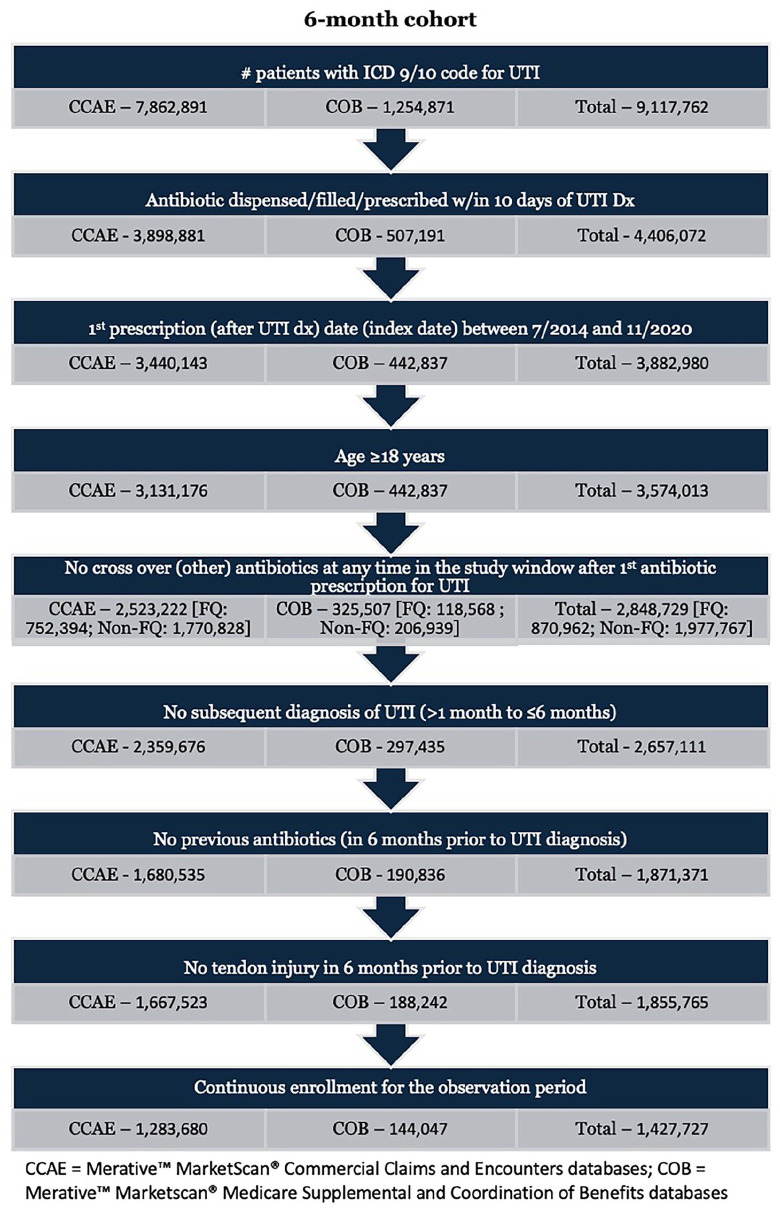
Figure 2.
Six-month cohort.
Abbreviations: CCAE, Merative™ MarketScan® Commercial Claims and Encounters database; COB, Merative™ MarketScan® Medicare Supplemental and Coordination of Benefits database.
Table 1.
Patient Demographics (1 Month).
| FQ-treatment group (FQ) | Non-FQ treatment group (Non-FQ) | |
|---|---|---|
| Merative™ MarketScan® Commercial Claims and Encounters (CCAE) and Medicare Claims and Encounters (MCAE) | ||
| • Number | 774 767 | 1,459,269 |
| • CCAE | 675 007 (87.1) | 1,317,931 (90.3) |
| • Age at index date (mean [SD]) | 48.4 (16.7) | 43.8 (17.3) |
| • Age > 60 years (N [%)) | 175 867 (22.7) | 247,535 (17.0) |
| • Male sex (N [%]) | 200 872 (25.9) | 201,403 (13.8) |
| • Length of antibiotic treatment (mean [SD]) | 8.1 (5.4) | 8.6 (6.7) |
| • Diabetes mellitus (N [%]) | 51 096 (6.6) | 82,730 (5.7) |
| • Chronic kidney disease [N [%]] | 1241 (0.2) | 2,323 (0.2) |
| • Prior corticosteroid therapy [N [%]] | 58 209 (7.5) | 102,414 (7.0) |
| • Concomitant corticosteroid therapy [N [%]] | 5955 (0.8) | 13,215 (0.9) |
| • Postcorticosteroid therapy [N [%]] | 10 241 (1.3) | 20,911 (1.4) |
| • Specific FQ agent | ||
| ° Ciprofloxacin [N [%]] | 710 977 (91.8) | |
| ° Levofloxacin [N [%]] | 68 494 (8.8) | |
| ° Moxifloxacin [N [%]] | 801 (0.1) | |
| • Non-FQ treatment | ||
| ° Nitrofurantoin | 568,468 (39.0) | |
| ° Trimethoprim/sulfamethoxazole | 548,683 (37.6) | |
| ° Cephalexin | 191,256 (13.1) | |
| ° Amoxicillin-clavulanate | 69,021 (4.7) | |
| ° Amoxicillin | 54,572 (3.7) | |
| ° Azithromycin | 43,248 (3.0) | |
| ° Doxycycline | 43,444 (3.0) | |
| ° Cefuroxime | 35,758 (2.5) | |
| ° Cefdinir | 33,398 (2.3) | |
| ° Cefpodoxime | 6,293 (0.4) | |
| ° Cefixime | 2,091 (0.1) | |
| ° Minocycline | 1,772 (0.1) | |
| ° Linezolid | 230 (0.0) | |
| • Tendon injury [N [%]] | 229 (0.2%) | 321 (0.1%) |
The most prescribed FQ for the 1-month study population was ciprofloxacin (91.8%), followed by levofloxacin (8.8%) and moxifloxacin (0.1%). The most frequently prescribed non-FQ regimens in the 1-month data set were nitrofurantoin (39%), SMX/TMP (37.6%), cephalexin (13.1%), amoxicillin-clavulanate (4.7%), amoxicillin (3.7%), azithromycin (3%), doxycycline (3%), cefuroxime (2.5%), cefdinir (2.3%), and cefuroxime (0.4%). Mean antibiotic durations of therapy were similar between groups at 8.1 days in the FQ group and 8.6 days in the non-FQ group.
We also performed this evaluation over a 6-month postantibiotic exposure window. A total of 1,427,727 patients were included in the 6-month cohort with 452,635 (31.7%) receiving FQ therapy (FQ group) and 975,092 (68.3%) receiving non-FQ antibiotics (non-FQ group). Male patients accounted for 26.9% and 13.8% of the 6-month FQ and non-FQ groups, respectively. The mean age of the groups was 48.4 (FQ) and 43.5 (non-FQ) years, and, similarly to the 1-month cohort, the FQ group had a larger portion of patients aged > 60 years (21.8% vs 15.7%). Diabetes was documented for 6.3% of FQ patients versus 5.3% of non-FQ patients, while chronic kidney disease was identified for 0.1% of both groups. Prior corticosteroid therapy was documented for 6.9% of FQ patients and 6.8% of non-FQ patients. Concomitant corticosteroid use was reported in approximately 0.7% and 0.9% of FQ and non-FQ patients, respectively. Postantibiotic therapy corticosteroid therapy in the follow-up period was recorded for 7.7% of FQ patients and 8.4% of non-FQ patients. Mean antibiotic duration of therapy for the 6-month cohort was 8.1 versus 8.5 days for the FQ and non-FQ groups, respectively.
As with the 1-month population, ciprofloxacin was the most commonly prescribed FQ agent (92.2%) in the 6-month sample, followed by levofloxacin (9.7%) and moxifloxacin (0.2%). The most common non-FQ agents in the 6-month data set were SMX/TMP (39.5%), nitrofurantoin (39%), cephalexin (14.8%), amoxicillin (8.6%), amoxicillin-clavulanate (8.5%), azithromycin 7.0%), doxycycline (4.8%), cefdinir (3.1%), cefuroxime (2.8%), cefpodoxime (0.4%), and cefixime (0.2%).
In the 1-month cohort, tendon injury occurred in 0.2% of the FQ group compared with 0.1% of the non-FQ group. For the 6-month cohort, tendon injury occurred in 0.8% of the FQ group compared with 0.7% in the non-FQ group. Neither the 1-month or 6-month data set showed a significantly increased odds of any tendon injury in patients receiving an FQ-based regimen compared with those receiving a non-FQ-based regimen (OR = 1.03, 95% CI 0.93, 1.32 for 1-month and OR = 0.98, CI 0.84, 1.05 for 6-months).
In both the four-month and 6-month cohorts, the four most common tendon injuries identified by ICD coding were disorders of bursae and tendons in shoulder region, unspecified (72610), radial styloid tenosynovitis (de Quevain) (M654), synovitis and tenosynovitis, unspecified (M654), and other synovitis and tenosynovitis, right/left ankle and foot (M65871, M65872). Other specified disorders of bursa and tendons in shoulder region (72619) and complete rupture of rotator cuff (72761) were the next most common. Achilles tendon injury was not commonly reported in our evaluation, similarly to our previous evaluation of tendon injuries in patients with FQs for CAP. In that evaluation, shoulder injuries were also the most common type reported in patients receiving FQ therapy.
Discussion
We evaluated the risk of tendon injury associated with use of FQ antibiotics in patients being treated for UTI using the MarketScan® database population. We previously found an increased risk of tendon injury within one month of therapy associated with use of FQ antibiotics in patients treated for pneumonia in the outpatient setting. 36 In this evaluation of patients treated with FQ or non-FQ antibiotic regimens for UTI, there was not a significantly increased risk of tendon injury associated with FQ use. We hypothesize that because the UTI patients represented a slightly younger, predominantly female, and healthier population, they potentially had fewer contributing risk factors or comorbid conditions. Between the pneumonia and UTI data sets, the UTI population was predominantly female (compared with a more balanced gender distribution in the pneumonia population), had fewer patients > 60 years of age and lower percentages of patients with diabetes mellitus and/or chronic kidney disease (see Tables 1 and 2 for demographic information).
Table 2.
Patient Demographics (6 Months).
| FQ treatment group (FQ) | Non-FQ treatment group (non-FQ) | |
|---|---|---|
| Merative™ MarketScan® Commercial Claims and Encounters (CCAE) and Medicare Claims and Encounters (MCAE) | ||
| • Number | 452 635 | 975 092 |
| • CCAE | 395 881 (87.5) | 887 799 (91.0) |
| • Age at index date (mean [SD]) | 48.4 (16.4) | 43.5 (17.0) |
| • Age > 60 years (N [%]) | 98 505 (21.8) | 153 064 (15.7) |
| • Male sex (N [%]) | 121 549 (26.9) | 135 038 (13.8) |
| • Length of antibiotic treatment (mean [SD]) | 8.1 (5.4) | 8.5 (6.5) |
| • Diabetes mellitus (N [%]) | 28 314 (6.3) | 52 099 (5.3) |
| • Chronic kidney disease (N [%)] | 563 (0.1) | 1163 (0.1) |
| • Prior corticosteroid therapy (N [%]) | 31 171 (6.9) | 66 564 (6.8) |
| • Concomitant corticosteroid therapy (N [%]) | 3228 (0.7) | 8717 (0.9) |
| • Postcorticosteroid therapy (N [%]) | 34 812 (7.7) | 81 598 (8.4) |
| • Specific FQ agent | ||
| ° Ciprofloxacin (N [%]) | 417 149 (92.2) | |
| ° Levofloxacin (N [%]) | 43 875 (9.7) | |
| ° Moxifloxacin (N [%]) | 696 (0.2) | |
| • Non-FQ treatment | ||
| ° Trimethoprim/sulfamethoxazole | 385 517 (39.5) | |
| ° Nitrofurantoin | 381 490 (39.1) | |
| ° Cephalexin | 144 578 (14.8) | |
| ° Amoxicillin | 83 966 (8.6) | |
| ° Amoxicillin-clavulanate | 82 983 (8.5) | |
| ° Azithromycin | 68 081 (7.0) | |
| ° Doxycycline | 47 262 (4.8) | |
| ° Cefdinir | 30 219 (3.1) | |
| ° Cefuroxime | 27 657 (2.8) | |
| ° Cefpodoxime | 4137 (0.4) | |
| ° Cefixime | 1635 (0.2) | |
| ° Minocycline | 3069 (0.3) | |
| ° Linezolid | 157 (0.0) | |
| • Tendon injury (N [%]) | 699 (8%) | 1644 (7%) |
Our results showed this risk to most frequently occur at the wrist, ankle/foot, shoulder, and rotator cuff rather than the Achilles tendon that has historically been the most often reported in the literature.
This study follows our original study evaluating risk of FQ-associated tendon injury in patients treated for pneumonia and adds another to evaluate FQ-associated tendon injury risk in patients treated for single, specific indications and to use a comparator group of patients being treated with other (non-FQ) alternative therapies for the same indication. Previous studies have evaluated this association in populations of patients receiving FQs for multiple indications (a group of patients with FQ use for any indication), which does not provide prescribers the information to weigh the comparative risk of tendon injury when choosing between antibiotic options for the same indication. To our knowledge, we are also one of the first to use the MarketScan® Databases to evaluate the risk of tendon injury in an adult population.
Most published studies that have evaluated the risk of FQ-associated tendon injury to date have found a positive association or an increased risk or odds of this adverse event with FQ use, but there have also been a few studies that showed no difference in risk (or varying results between the various FQ agents). Baik et al 17 found that as a class, FQs were not associated with increased risk of tendon ruptures within 30 days in a Medicare (> 65 years of age) beneficiary population. Seven antibiotics were evaluated (ciprofloxacin, levofloxacin, moxifloxacin vs amoxicillin, amoxicillin-clavulanate, azithromycin, and cephalexin). Of the FQs, only levofloxacin (but not ciprofloxacin or moxifloxacin) showed a significant increased risk of tendon rupture (16% for rotator cuff, 120% for Achilles). Cephalexin also exhibited an increased risk of tendon rupture (all types combined with hazard ratios [HRs] of 1.19-1.93 across all anatomic sites) in the 30-day window. The risk of tendon rupture with levofloxacin never exceeded the risk seen with cephalexin in any comparison. No data were provided about the dosing or duration of therapy for the antibiotic therapies that were included in the analysis.
A study by Chinen et al 37 used 2 large administrative databases in Japan (the National Health Insurance and Elderly Health Insurance) to evaluate the risk of tendon rupture between third-generation compared with both earlier generation FQ agents and non-FQ agents. Levofloxacin and ciprofloxacin were considered “early” (second generation) FQs and moxifloxacin, along with several agents not available in the United States, were considered third generation. In this analysis, risk was not significantly increased with third generation FQs (interrater reliability [IRR] = 1.05, 95% CI 0.33, 3.37) or non-FQs (IRR = 1.08, 95% CI 0.80, 1.47), but was elevated in patients with exposure to first-generation or second-generation FQs (IRR = 2.94, 95% CI 1.90, 4.54). No information was provided on dosing regimens, duration of therapy, and patient could have received other antibiotic therapies or multiple antibiotic courses during the study period without being excluded. This study only evaluated tendon rupture and not any/other tendon injury types.
The association may, however, be even more nuanced and require a more detailed assessment to fully elucidate the risk of tendon injury associated with FQ agents in various populations and scenarios. In the studies that have looked at either total dose, cumulative dose, or duration of therapy, higher doses and especially longer duration of therapy (or repeated course of FQ therapy over a time periods) appear to increase risk of this adverse effect.26,38,39
In a retrospective cohort study of adult patients hospitalized and treated for CAP in the Upstate New York Veterans Health Care Administration, Patel et al 26 evaluated the incidence of three common FQ-associated adverse effects, one being adverse tendon events, and identified patient-level factors that correlated with increased risk. Over a six-year period (2011-2016), 1071 patients were included and evaluated for a period of 90-day post initiation of FQ therapy. Adverse tendon events included tendinopathy, tendon pain/rupture, torn rotator cuff, tendinitis, and Achilles heel pain/tear/torn/rupture. The study population was predominantly male (97.7%) with a mean age of 73.2+/–12.9 years. Moxifloxacin was the predominant FQ agent used (55.9%), followed by levofloxacin (36.6%), and ciprofloxacin (7.5%). Overall incidence of adverse tendon events was 1.8% and was significantly higher with levofloxacin (3.1%) versus moxifloxacin (1.2%) or ciprofloxacin (0%). In addition to finding an increased incidence of tendon injury with FQ therapy, variables independently associated with an adverse tendon event were heart failure, use of levofloxacin, and treatment duration ≥ 7 days.
Rasmussen et al 38 conducted a nested case-control study of patients in the Danish nationwide registers and databases who experienced Achilles tendon lesion/rupture over a 19-year study period (2003-2021). The exposure of interest was claimed prescriptions for oral FQ (ciprofloxacin and moxifloxacin, the 2 FQs available options in the Danish Health Care system) with an active comparator of amoxicillin for controls. Cumulative defined daily doses (cDDDs) were compared with evaluate an association between FQ exposure and tendon adverse events. Overall, a clear signal of increased rates of Achilles tendon lesions was seen in patients who received FQs compared with those who received amoxicillin (60-day HR = 3.6 [95% CI 2.09, 6.19], 90-day HR = 2.74 [95% CI 1.87, 4.02]; 1-year HR = 1.49 [95% CI 1.29, 1.73]). The authors reported that increasing cumulative daily doses were associated with increased rates of Achilles tendon rupture, though only statistically significant for those receiving > 10 days of therapy (1-day to 5-day reference, 6-day to 10-day HR = 1.12 [95% CI 0.76, 1.63], > 10 HR = 1.68 [95% CI 1.05, 2.70]).
Morales et al 16 performed a nested case-control study using the UK Health Improvement Network primary care database to evaluate the relative and absolute risk of tendon rupture in adult patients treated with FQs vs amoxicillin-clavulanate. 39 The risk of any tendon rupture increased in patients who received FQs (adjusted incidence rate ratio [aIRR] = 1.61, 95% CI 1.25, 2.09) as did Achilles tendon rupture (aIRR = 3.14, 95% CI 2.11, 4.65). This increased risk remained for up to 60-day post initiation of FQ therapy. This study also assessed the impact of cumulative FQ exposure, which was measured as total number of days of therapy. Mean duration of therapy was 10.6 days (SD = 8.9) for FQ patients and 8.6 days (SD = 7.4) for amoxicillin-clavulanate patients. Increasing cumulative days of FQ therapy was found to be significantly associated with an increased risk of Achilles tendon rupture (IRR = 1.06, 95% CI 1.03, 1.09) and risk was estimated to increase by nearly 6% with each additional day of therapy.
It is possible that shorter durations of therapy (relative to pneumonia) may also have contributed to our results since durations of therapy for uncomplicated UTI/cystitis can be as short as 3 days with FQs and even 5 days for pyelonephritis and on average, for our study population, were less than 10 days. There was not a significant difference in duration of therapy received between the UTI (8.1-day FQ vs 8.6-day non-FQ) and CAP (8.3-day FQ vs 7.8-day non-FQ) populations in our 2 evaluations, however.
A combination of these things likely contributed to the lack of a significant associated found been FQ use and tendon injury in our study and in this specific population. Effect of dose, duration, specific FQ agent, indication, comorbidities, risk factors, and patient populations should be the focus of future study to elucidate what variables produce the greatest risk of tendon injury in patients who receive agents in the FQ class.
Our study was a retrospective, claims-based evaluation conducted using the MarketScan Databases for data collection and patient identification. MarketScan does not contain patient-level or case-specific information, and our data collection was limited to health care visit coding and prescription drug claim information. We were reliant on accurate coding and documentation for claims contained in the MarketScan Databases and this is a limitation of our study. In addition, inclusion of the Medicare database limited the ability to report information related to race or ethnicity. We included patients treated with oral antibiotics who may have been treated initially as inpatients in addition to those seen only in the ambulatory setting. However, patients were required to have a prescription for oral antibiotic therapy associated with the health care visit and were evaluated for other antibiotic therapy in the prestudy washout period and at any time during the study period. Moxifloxacin was included because there were patients who received this agent for treatment of UTI, but this is a limitation since this FQ is not indicated for treatment of UTI. However, since we were not evaluating clinical outcomes of UTI treatment and instead evaluating risk of tendon injury upon exposure, we believe that it was acceptable to include these patients in our results. Based on the finding that azithromycin and doxycycline were prescribed as antibiotic regimens received by patients in the non-FQ cohort, it also appears that patients intended to be treated for a sexually transmitted infection (who also probably were ordered urine specimens as part of the diagnostic workup) were included in patient identification though based on ICD-9/10 coding designation. This was an unanticipated cofounder that we would have ideally controlled for in our methods (exclusions), though we believe that it did not significantly affect the results as most of these patients were also likely young, relatively healthy individuals. We believe that our patient populations accurately represent FQ versus non-FQ antibiotic treatment regimens for outpatient/ambulatory treatment of UTI as best we could control for using the MarketScan Databases.
It is also possible that more patients experienced tendon injury than was captured by diagnosis code identification, particularly if they did not report or seek follow-up for the tendon issue or if the incident was delayed (and thought to be separate) from the antibiotic course. If the individual did not have an associated visit for a tendon diagnosis, they could have been underrepresented. There is no obvious reason to suspect that such underreporting of injuries would affect the FQ and non-FQ groups differentially, however, we also acknowledge the possibility of uncontrolled confounders undermining the causal link between FQ use and tendon injury.
We believe that our evaluation adds important information to the body of literature surrounding this topic and will help give clinicians more specific data regarding the risk of tendon injury for patient with UTI treated with FQ versus non-FQ antibiotic regimens.
Conclusion and Relevance
In our claims-based data set of patients treated for UTI, the risk of any tendon injury in the 1-month period following antibiotic exposure was not significantly higher for FQ antibiotics when compared with non-FQ therapies. The risk of tendon injury was also not significantly different at six months. This finding differs from our previous evaluation of tendon injury risk in patients treated for CAP over the same time period. Differences in the populations in terms of age, comorbidities, and health status or drug-specific factors (agent, dose, duration of therapy) may have contributed to the discordant results. Our findings provide prescribers with risk assessment data for drug therapy options used to treat a specific illness (UTI). Prescribers should still consider the risk of tendon injury with FQ use, particularly in patients who have higher risk when considering treatment regimens for UTI and use alternative options with lower risk whenever possible, but if used, shorter course and lower cumulative exposure of FQs may help to lessen the risk (but this remains to be fully elucidated and should be a focus of future research).
Supplemental Material
Supplemental material, sj-docx-1-pmt-10.1177_87551225241303848 for Risk of Tendon Injury in Patients Treated With Fluoroquinolone (FQ) Vs Non-Fluoroquinolone Antibiotics for Urinary Tract Infection (UTI) by Virginia H. Fleming, Jianing Xu, Xianyan Chen, Daniel Hall and Robin L Southwood in Journal of Pharmacy Technology
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Virginia H. Fleming  https://orcid.org/0009-0001-6826-8711
https://orcid.org/0009-0001-6826-8711
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Centers for Disease Control and Prevention. Fluoroquinolones. Centers for disease control—antimicrobial resistance & patient safety portal web site. Updated 2022. Accessed July 13, 2024. https://arpsp.cdc.gov/profile/antibiotic-use/fluoroquinolones
- 2. Gupta K, Hooton TM, Naber KG, et al. Executive summary: international clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the infectious diseases society of America and the European society for microbiology and infectious diseases. Clin Infect Dis. 2011;52(5):561-564. doi: 10.1093/cid/cir102 [DOI] [PubMed] [Google Scholar]
- 3. Tanne JH. FDA adds “black box“ warning label to fluoroquinolone antibiotics. BMJ. 2008;337(7662):816. doi: 10.1136/bmj.a816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arias DC. US Food and Drug Administration. FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. Published 2016. Accessed May 14, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-advises-restricting-fluoroquinolone-antibiotic-use-certain
- 5. US Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. Updated 2017. Accessed July 13, 2024. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics
- 6. Food and Drug Administration. Information for healthcare professionals: fluoroquinolone antimicrobial drugs [ciprofloxacin (marketed as Cipro and generic ciprofloxacin), ciprofloxacin extended-release (marketed as Cipro XR and Proquin XR), gemifloxacin (marketed as Factive), levofloxacin (marketed as Levaquin), moxifloxacin (marketed as Avelox), norfloxacin (marketed as Noroxin), and ofloxacin (marketed as Floxin)]. Accessed May 14, 2023. http://wayback.archive-it.org/7993/20161022101528/http:/www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126085.htm
- 7. Bratsman A, Mathias K, Laubscher R, Grigoryan L, Rose S. Outpatient fluoroquinolone prescribing patterns before and after US FDA boxed warning. Pharmacoepidemiol Drug Saf. 2020;29(6):701-707. [DOI] [PubMed] [Google Scholar]
- 8. Schmiemann G, Hoffmann F, Hamprecht A, Jobski K. Patterns and trends of antibacterial treatment in patients with urinary tract infections, 2015–2019: an analysis of health insurance data. BMC Primary Care. 2022;23(1):1-11. doi: 10.1186/s12875-022-01816-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Langner JL, Chiang KF, Stafford RS. Current prescribing practices and guideline concordance for the treatment of uncomplicated urinary tract infections in women. Am J Obstet Gynecol. 2021;225(3):272.e1-272.e11. doi: 10.1016/j.ajog.2021.04.218 [DOI] [PubMed] [Google Scholar]
- 10. Daneman N, Chateau D, Dahl M, et al. Fluoroquinolone use for uncomplicated urinary tract infections in women: a retrospective cohort study. Clin Microbiol Infect. 2020;26(5):613-618. doi: 10.1016/j.cmi.2019.10.016 [DOI] [PubMed] [Google Scholar]
- 11. Cowart K, Worley M, Rouby NE, Sando K. Evaluation of FDA boxed warning on prescribing patterns of fluoroquinolones for uncomplicated urinary tract infections. Ann Pharmacother. 2019;53(12):1192-1199. doi: 10.1177/1060028019865224 [DOI] [PubMed] [Google Scholar]
- 12. Bidell MR, Lodise TP. Fluoroquinolone-associated tendinopathy: does levofloxacin pose the greatest risk? Pharmacotherapy. 2016;36(6):679-693. doi: 10.1002/phar.1761 [DOI] [PubMed] [Google Scholar]
- 13. Khaliq Y, Zhanel GG. Fluoroquinolone-associated tendinopathy: a critical review of the literature. Clin Infect Dis. 2003;36(11):1404-1410. doi: 10.1086/375078 [DOI] [PubMed] [Google Scholar]
- 14. Stephenson AL, Wu W, Cortes D, Rochon PA. Tendon injury and fluoroquinolone use: a systematic review. Drug Saf. 2013;36(9):709-721. [DOI] [PubMed] [Google Scholar]
- 15. Yu X, Jiang DS, Wang J, et al. Fluoroquinolone use and the risk of collagen-associated adverse events: a systematic review and meta-analysis. Drug Saf. 2019;42(9):1025-1033. [DOI] [PubMed] [Google Scholar]
- 16. Morales DR, Flynn R, Kurz X. Addendum to: relative and absolute risk of tendon rupture with fluoroquinolone and concomitant fluoroquinolone/corticosteroid therapy: population-based nested case–control study. Clin Drug Investig. 2019;39(6):591-594. 10.1007/s40261-019-00792-7 [DOI] [PubMed] [Google Scholar]
- 17. Baik S, Lau J, Huser V, McDonald CJ. Association between tendon ruptures and use of fluoroquinolone, and other oral antibiotics: a 10-year retrospective study of 1 million US senior Medicare beneficiaries. BMJ Open. 2020;10(12):e034844. doi: 10.1136/bmjopen-2019-034844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang CK, Chien WC, Hsu WF, et al. Positive association between fluoroquinolone exposure and tendon disorders: a nationwide population-based cohort study in Taiwan. Front Pharmacol. 2022;13:814333. doi: 10.3389/fphar.2022.814333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jupiter DC, Fang X, Ashmore Z, Shibuya N, Mehta HB. The relative risk of Achilles tendon injury in patients taking quinolones. Pharmacotherapy. 2018;38(9):878-887. doi: 10.1002/phar.2162 [DOI] [PubMed] [Google Scholar]
- 20. Ross RK, Kinlaw AC, Herzog MM, Jonsson Funk M, Gerber JS. Fluoroquinolone antibiotics and tendon injury in adolescents. Pediatrics. 2021;147(6):e2020033316. doi:10.1542/peds.2020–033316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wise BL, Peloquin C, Choi H, Lane NE, Zhang Y. Impact of age, sex, obesity, and steroid use on quinolone-associated tendon disorders. Am J Med. 2012;125(12):1228.e23-1228.e28. doi: 10.1016/j.amjmed.2012.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seeger JD, West WA, Fife D, Noel GJ, Johnson LN, Walker AM. Achilles tendon rupture and its association with fluoroquinolone antibiotics and other potential risk factors in a managed care population. Pharmacoepidemiol Drug Saf. 2006;15(11):784-792. doi: 10.1002/pds.1214 [DOI] [PubMed] [Google Scholar]
- 23. Corrao G, Zambon A, Bertù L, et al. Evidence of tendinitis provoked by fluoroquinolone treatment: a case-control study. Drug Saf. 2006;29(10):889-896. doi: 10.2165/00002018-200629100-00006 [DOI] [PubMed] [Google Scholar]
- 24. van der Linden PD, Sturkenboom MCJM, Herings RMC, Leufkens HMG, Rowlands S, Stricker BHC. Increased risk of Achilles tendon rupture with quinolone antibacterial use, especially in elderly patients taking oral corticosteroids. Arch Intern Med. 2003;163(15):1801-1807. doi: 10.1001/archinte.163.15.1801 [DOI] [PubMed] [Google Scholar]
- 25. van der Linden PD, Sturkenboom M, Herings R, Leufkens H, Stricker BC. Fluoroquinolones and risk of Achilles tendon disorders: case-control study. BMJ. 2002;324(7349):1306-1307. doi: 10.1136/bmj.324.7349.1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patel N, Gorseth A, Belfiore G, Stornelli N, Lowry C, Thomas L. Fluoroquinolone-associated adverse events of interest among hospitalized veterans affairs patients with community-acquired pneumonia who were treated with a fluoroquinolone: a focus on tendonitis, Clostridioides difficile infection, and aortic aneurysm. Pharmacotherapy. 2024;44(1):49-60. doi: 10.1002/phar.2877 [DOI] [PubMed] [Google Scholar]
- 27. Fleming VH, White BP, Southwood R. Resistance of Escherichia coli urinary isolates in ED-treated patients from a community hospital. Am J Emerg Med. 2014;32(8):864-870. doi: 10.1016/j.ajem.2014.04.033 [DOI] [PubMed] [Google Scholar]
- 28. Centers for Disease Control. ICD-9 code look up. Centers for disease control—ICD-9 code look up web site. Accessed April 14, 2022. https://www.cdc.gov/nchs/icd/icd9cm.htm
- 29. Centers for Disease Control. ICD—10—code look up. Centers for disease control—ICD—10—code look up web site. Accessed April 14, 2022. https://www.cdc.gov/nchs/icd/icd-10-cm.htm
- 30. Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177(4):292-298. doi: 10.1093/aje/kws412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hünermund P, Louw B. On the nuisance of control variables in regression analysis. arXiv preprint arXiv:2005.10314, 2020. [Google Scholar]
- 32. R Core Team. R: a language and environment for statistical computing. R foundation for statistical computing. Updated 2021. https://wwwR-project.org/
- 33. Noah Greifer. WeightIt software—WeightIT: weighting for covariate balance in observational studies. R package version 0.13.1. Accessed April 12, 2022. https://CRAN.R-project.org/package=WeightIt
- 34. Noah Greifer. Cobalt: covariate balance tables and plots. R package version 4.4.0. Accessed April 14, 2022. https://CRAN.R-project.org/package=WeightIt Web site. https://CRAN.R-project.org/package=cobalt
- 35. Canty A, Brian R. Boot: bootstrap R (S-Plus) functions. R package version 1.3-28. Updated 2021. Accessed April 12, 2022. https://cran.r-project.org/web/packages/boot/index.html
- 36. Fleming VH, Xu J, Chen X, Hall D, Southwood RL. Risk of tendon injury in patients treated with fluoroquinolone (FQ) versus non-fluoroquinolone antibiotics for community-acquired pneumonia (CAP). Ann Pharmacother. 2024;58(8):771-780. doi: 10.1177/10600280231210275 [DOI] [PubMed] [Google Scholar]
- 37. Chinen T, Sasabuchi Y, Matsui H, Yasunaga H. Association between third-generation fluoroquinolones and achilles tendon rupture: a self-controlled case series analysis. Ann Fam Med. 2021;19(3):212-216. doi: 10.1370/afm.2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rasmussen PV, Strange JE, Holt A. Oral fluoroquinolones and risk of achilles tendon rupture. J Sport Health Sci. 2024;13(6):749-750. doi: 10.1016/j.jshs.2024.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morales DR, Slattery J, Pacurariu A, Pinheiro L, McGettigan P, Kurz X. Relative and absolute risk of tendon rupture with fluoroquinolone and concomitant fluoroquinolone/corticosteroid therapy: population-based nested case-control study. Clin Drug Investig. 2019;39(2):205-213. doi: 10.1007/s40261-018-0729-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-pmt-10.1177_87551225241303848 for Risk of Tendon Injury in Patients Treated With Fluoroquinolone (FQ) Vs Non-Fluoroquinolone Antibiotics for Urinary Tract Infection (UTI) by Virginia H. Fleming, Jianing Xu, Xianyan Chen, Daniel Hall and Robin L Southwood in Journal of Pharmacy Technology