Abstract
Background
We aimed to investigate the potential of altered levels of various acute phase proteins (APPs) in the plasma, either used alone or in combination with ultrasound-, clinical-, and conventional blood-based tests, for predicting the risk of intra-amniotic inflammation (IAI), microbial invasion of the amniotic cavity (MIAC), histologic chorioamnionitis (HCA), and funisitis in women with preterm premature rupture of membranes (PPROM).
Methods
A total of 195 consecutive pregnancies involving singleton women with PPROM (at 23 + 0–34 + 0 weeks) who underwent amniocentesis and from whom plasma samples were obtained at amniocentesis were retrospectively included in this study. Amniotic fluid (AF) was cultured to assess the MIAC and analyzed for interleukin (IL)-6 levels to define IAI (AF IL-6 level of ≥2.6 ng/mL). The plasma concentrations of hepcidin, mannose-binding lectin (MBL), pentraxin-2, retinol-binding protein 4 (RBP4), serum amyloid A1 (SAA1), and serpin A1 were determined using ELISA. Ultrasonographic cervical length (CL), neutrophil-to-lymphocyte ratio (NLR), and C-reactive protein levels were measured. IAI/MIAC was defined as IAI, MIAC, or both.
Results
Multivariate logistic regression analyses showed the following: (1) elevated plasma levels of hepcidin and SAA1 and decreased levels of RBP4 in the plasma were independently associated with IAI/MIAC and (2) decreased plasma RBP4 levels were independently associated with funisitis; however, (3) none of the plasma APPs investigated were associated with acute HCA when adjusted for baseline covariates. Using stepwise regression analysis, noninvasive prediction models comprising plasma RBP4 levels, CL, NLR, and gestational age at sampling were proposed, which provided a good prediction of IAI/MIAC and funisitis (area under the curve: 0.80 and 0.72, respectively).
Conclusions
Hepcidin, RBP4, and SAA1 were identified as potential APP biomarkers in the plasma predictive of IAI/MIAC or funisitis in patients with PPROM. In particular, combination of these APP biomarkers with ultrasound-, clinical-, and conventional blood-based markers can significantly support the diagnosis of IAI/MIAC and funisitis.
Keywords: Acute phase proteins, funisitis, intra-amniotic inflammation, microbial invasion of the amniotic cavity, plasma, prediction model, preterm premature rupture of membranes
Introduction
Preterm premature rupture of membranes (PPROM) is a condition with multiple causes that affects 2–3% of pregnancies and contributes to 30–40% of preterm births (PTB); thus, is one of the leading causes of infant morbidity, mortality, and long-term neurological disability.1–4 Accumulating evidence indicates that subclinical infectious and inflammatory conditions in utero significantly contribute to the pathogenesis of PPROM, including microbial invasion of the amniotic cavity (MIAC), intra-amniotic inflammation (IAI), and acute histologic chorioamnionitis (HCA).2,5–7 In particular, the presence of these PPROM-related complications poses additional risks for mothers and their fetuses or infants, including increased risks of impending PTB, clinical chorioamnionitis, and neonatal morbidity (e.g., sepsis and neurological morbidity) and mortality.6,8–12 Hence, it is clinically important to promptly and accurately identify high-risk patients for inflammation-related complications, especially via noninvasive means, in the context of PPROM.
An early and precise identification of PPROM-related complications (IAI, MIAC, or acute HCA) has been traditionally based on the measurement of inflammatory and infectious molecules in amniotic fluid (AF) obtained via amniocentesis.7,13–16 However, the amniocentesis procedure is invasive and technically challenging, especially when a small amount of residual AF is present, both of which limit their clinical use. In this scenario, measuring relevant markers in maternal blood samples, which can be obtained via less invasive, affordable, and repeatable methods, may represent a feasible alternative to invasive amniocentesis for detecting MIAC, IAI, and acute HCA. This hypothesis is even more convincing as evidence shows that (i) placental and fetal exosomes and fetal cells migrate into maternal blood and organs during pregnancy, in particular, during MIAC, IAI, or acute HCA;17–19 (ii) macrophages/neutrophils/monocytes in the AF, as IAI markers, are predominantly of fetal or maternal origin, or a mixture of both in women with IAI or MIAC;20,21 and (iii) neutrophils in the chorio-decidua during acute HCA are predominantly of maternal origin22,23 and reflected in the maternal circulation. Indeed, previous studies have shown significant associations between plasma levels of inflammatory molecules and IAI, MIAC, or acute HCA in women with PPROM.15,24–28 Nevertheless, the abovementioned associations between circulating molecules and in utero inflammation/infection are insufficiently robust to warrant their routine utilization as single biomarkers in the context of PPROM.15,24–28 Of note, recent evidence indicates that the combined use of serum inflammatory biomarkers with different properties (e.g., acute phase proteins [APPs], neutrophil-to-lymphocyte ratio [NLR], and interleukin [IL]-6) and clinical, demographic, and ultrasound data can significantly increase the diagnostic efficiency for pregnancy-related complications, such as IAI, MIAC, HCA, preeclampsia, and fetal growth restriction, as compared with each factor alone.29–32
APPs are significantly involved in mediating the innate immune response to infectious agents and systemic inflammation.33–36 They are mainly produced by the liver in response to pro-inflammatory cytokines and include C-reactive protein (CRP), hepcidin, mannose-binding lectin (MBL), pentraxin-2, retinol-binding protein 4 (RBP4), serum amyloid A1 (SAA1), complement proteins, and serpin A1.33–36 Several APPs are useful in the diagnosis and monitoring of inflammation-related diseases, despite the drawbacks of low specificity and high sensitivity.33,34 However, in the context of PPROM, to date, sufficient data (except for CRP) to determine whether these proteins could act as useful plasma markers for predicting PPROM-related complications remain lacking. Thus, the aim of this study was to determine whether altered levels of various APPs in plasma are independently associated with IAI, MIAC, acute HCA, and funisitis in women with PPROM. Additionally, we aimed to determine whether using such APP biomarkers in combination with ultrasound-, clinical-, and conventional blood-based markers could improve the potential to predict the risk of PPROM-related complications.
Materials and methods
Study population
A retrospective cohort study was conducted in consecutive singleton pregnant women with a diagnosis of PPROM at 23 + 0 to 34 + 0 weeks of gestation who were admitted to Seoul National University Bundang Hospital (Seongnamsi, Republic of Korea) from June 2004 through August 2021 and underwent an amniocentesis for the assessment of the inflammatory and microbiological status of the amniotic cavity. PPROM was defined as amniorrhexis occurring before the onset of spontaneous labor and at <37 weeks of gestation. It was diagnosed visually using a sterile speculum examination to confirm the pooling of AF in the vagina (or leakage of fluid through the cervix), along with a positive nitrazine test (and/or a positive AmniSure ROM test [Qiagen, Hilden, Germany]). Gestational ages (GAs) were established based on the last menstrual period and the first-trimester fetal biometry of patients. This study received approval from the Institutional Review Board of the Ethics Committee of Seoul National University Bundang Hospital (approval no. B-1105/128-102), and written informed consent was obtained from all patients for the amniocentesis, as well as for the collection and use of biological specimens and clinical data for research purposes.
Patients with PPROM (1) who delivered a live fetus, (2) whose plasma were collected at the time of amniocentesis, and (3) who had an aliquot of plasma sample available for analysis were included in the study. Women with (1) multiple gestations, (2) active labor on recruitment (defined as cervical dilation >3 cm measured via sterile speculum examination), (3) major congenital anomalies, and (4) clinical evidence of chorioamnionitis at admission were excluded from the study. The primary endpoint of the study was IAI/MIAC (defined as IAI, MIAC, or both) as it is important to detect and treat this condition early for improving pregnancy outcomes related to PPROM. 37 The secondary endpoints were acute HCA and funisitis. Most clinical and demographic data contained in this manuscript, except for the plasma levels of various APPs assessed herein, have been previously reported in papers published in Am J Reprod Immunol, Reprod Sci, J Korean Med Sci, and Sci Rep.24,26–28
Biological samples and analysis
Ultrasound-guided transabdominal amniocentesis was performed under aseptic conditions at the time of admission. The AF samples were then immediately transported to the microbiology laboratory for conventional culture [i.e., aerobic/anaerobic bacteria, fungus, and genital mycoplasmas (U. urealyticum and M. hominis)] performed according to the methods previously described in detail elsewhere. 38 The remaining AF was centrifuged at 1500 × g for 10 min, and the supernatant was collected and stored at −70°C until further analysis. The managing physicians had access to the AF culture results. To define IAI, IL-6 levels were measured in the stored AF samples using ELISA Human IL-6 DuoSet Kit (R&D System, Minneapolis, MN, USA). The measurement of IL-6 levels in the AF is described in detail in the Supplementary Materials. AF IL-6 data were collected for research purposes only, and their results were not made available to the managing physicians.
According to the hospital protocol for hospitalized patients with PPROM, the concentrations of CRP and white blood cell (WBC) counts (total and differential) were measured at the time of amniocentesis using previously reported methods. 38 Any remaining blood samples were collected in ethylenediaminetetraacetic acid tubes and centrifuged at 1500 × g for 10 min. The supernatant was aliquoted and stored frozen at −70°C until further use. The NLR was calculated from the complete blood count as the ratio of the absolute neutrophil count to the absolute lymphocyte count. Cervical length (CL) was also measured by transvaginal ultrasonography using an ultrasound transducer covered with a sterile condom. These measurements were performed as previously described.39,40 Briefly, ultrasound CL was measured in the midsagittal plane of the uterine cervix as a straight-line distance from the internal os to the external os. At least three measurements were performed and the shortest value was used for analysis.
The prematurely delivered placentas were histologically evaluated for the presence of acute HCA. Placental tissue samples used for the histopathological evaluation were collected and processed as previously described.41,42 Clinical information was not disclosed to the pathologists.
Immunoassay of plasma proteins
The levels of hepcidin, MBL, pentraxin-2, RBP4, SAA1, and serpin A1 were assessed in the stored plasma samples using ELISA kits (DuoSet ELISA; R&D Systems, Minneapolis, MN) according to the manufacturer's instruction. The dynamic ranges for each ELISA assay and their corresponding dilution ratios are described in detail in the Supplementary Material. The intra- and inter-assay coefficients of variation (CVs) were below 10% for all the analyzed proteins, except for the inter-assay CVs of hepcidin (12.7%), RBP4 (16.2%), and SAA1 (15.1%). These APP markers were selected for the present study because of the following reasons: i) They are considered to be valuable as blood markers of local inflammatory lesions and systemic inflammatory reactions;34,35,43 however, whether their altered expression in plasma/serum is associated with in utero inflammation/infection in the setting of PPROM remains unelucidated; ii) Their plasma levels increased more rapidly and showed higher peaks under infectious and inflammatory conditions, which may contribute to identification of robust biomarkers associated with the inflammatory disease; 35 and iii) Good assay performance of ELISA kits in plasma samples was confirmed using linearity-of-dilution and spike-and-recovery experiments.
Clinical management of PPROM and definition of various factors
The procedure for the management of PPROM in our hospital has been previously described.14,28,44 Briefly, prophylactic broad-spectrum antibiotics with ampicillin plus macrolides (clarithromycin, azithromycin, or erythromycin) were administered to all women with PPROM. However, the attending physician had the autonomy to determine the type and duration of antibiotic treatment. Corticosteroid and tocolytic (atosiban, magnesium sulfate, or ritodrine) therapy was administered at the discretion of the attending physician to women with PPROM at < 34 weeks of gestation. Culture-proven MIAC in women with PPROM at < 34 weeks of gestation was not generally considered an indication for delivery. These patients with PPROM received antibiotic treatment tailored to the results of AF culture. The treatment was administered with close monitoring for clinical signs of chorioamnionitis and fetal compromise up to 34 + 0 weeks gestation, at which point delivery was conducted. MIAC was defined as the presence of microorganisms (i.e., bacteria, Ureaplasma spp./ Mycoplasma hominis, and fungi) detected by conventional AF culture analysis. IAI was defined as AF IL-6 levels ≥ 2.6 ng/mL, based on previous reports.14,45 Acute HCA was defined as the presence of neutrophil infiltration in fetal membranes (chorion-decidua or amnion), umbilical cord, or chorionic plate, in accordance with previously published criteria.42,46 Acute funisitis was defined as the presence of neutrophil infiltration into the wall of umbilical cord vessels and/or Wharton's jelly, according to previously published criteria. 42
Statistical analyses
Bivariate analyses for demographics, clinical data, and plasma levels of the APPs were performed using the Chi-squared test, Fisher's exact test, Student's t-test, or Mann-Whitney U test, as appropriate. Multivariate logistic regression analyses were further performed to determine the independent association of the plasma levels of each APP with the endpoints of interest, after adjusting for baseline variables (i.e., GA at sampling and parity) that had a P-value < 0.1 in univariate analysis. Additionally, noninvasive combined models for predicting PPROM-related endpoints were constructed using multivariate logistic analyses with forward selection comprising the identified plasma APP biomarkers (hepcidin, RBP4, and SAA1) and ultrasound- (CL), clinical- (GA at sampling and parity), and conventional blood-based markers (CRP, WBC, and NLR), which were selected based on P < 0.1 in univariate analysis. The linearity of continuous variables was assessed using the Box-Tidwell test and variables that failed to meet the linearity assumption were categorized into “high” and “low” categories using the cutoff point derived from the receiver operating characteristic (ROC) curves. ROC curve analyses were conducted to determine the area under the curves (AUCs), optimal cutoff values, and predictive ability of each significant APP and noninvasive combined models in association with each corresponding endpoint. The optimal cutoff values were determined using the maximum Youden index (which provides the maximum sum of sensitivity and specificity) or the point on the ROC curve closest to (0, 1). 47 AUCs of the newly identified APP markers and the noninvasive combined models were compared in a pairwise fashion using the method proposed by Delong et al. 48 All prediction models were internally validated using the two-stage bootstrap methods with 200 replications to estimate in-sample optimism-corrected performance. 49 The diagnostic potential of the APP markers was defined by their AUC values, specifically as fair if 0.5–0.75, good if 0.76–0.92, very good if 0.93–0.97, and excellent if 0.98–1.00, in accordance with Brubaker et al. 50 The correlation among APP levels in the plasma/serum was analyzed using the Spearman rank correlation test. SPSS version 25.0 (IBM Corp., Armonk, NY, USA) was used for data analyses, and a two-tailed P-value < 0.05 was considered to be statistically significant.
Results
Clinical characteristics of the study population
Overall, 195 consecutive women with PPROM were recruited for this study, among whom 47.7% (93/195), 40.5% (79/195), and 33.8% (66/195) had IAI/MIAC, IAI, and MIAC, respectively. The prevalence of concomitant IAI and MIAC, IAI without MIAC, and MIAC without IAI was 26.6% (52/195), 13.8% (27/195), and 7.2% (14/195), respectively. The microbes isolated from the AF of 66 women with MIAC included U. urealyticum (n = 50), M. hominis (n = 35), Peptostreptococcus spp. (n = 3), Streptococci viridans (n = 3), Lactobacillus spp. (n = 2), Streptococcus agalactiae (n = 2), Staphylococcus aureus (n = 2), unidentified gram-positive cocci (n = 1), Haemophilus influenzae (n = 1), Escherichia coli (n = 1), Streptococcus mitis (n = 1), Candida glabrata (n = 1), and unidentified gram-negative rods (n = 1). Polymicrobial invasion was present in 33.3% [36/66]) of women with MIAC. Patients with IAI/MIAC were more parous and had a significantly lower median GA at sampling and delivery than those without this condition. The median serum CRP levels, WBC count, and NLR were significantly higher and the median CL was significantly shorter in patients with IAI/MIAC (Table 1).
Table 1.
Demographic and clinical characteristics of the study population according to the presence or absence of intra-amniotic inflammation (IAI) and/or microbial invasion of the amniotic cavity (MIAC), acute histologic chorioamnionitis (HCA), and funisitis in women with preterm premature rupture of membranes.
| IAI and/or MIAC | P-value | Acute HCA | P-value | Funisitis | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Positive (n = 93) | Negative (n = 102) | Positive (n = 88) | Negative (n = 84) | Positive (n = 42) | Negative (n = 130) | ||||
| Maternal age (years) | 32.1 ± 3.8 | 31.2 ± 4.1 | 0.113 | 32.0 ± 3.6 | 31.2 ± 4.2 | 0.294 | 31.0 ± 3.5 | 31.8 ± 4.0 | 0.193 |
| Nulliparity | 39.8% (37/93) | 56.9% (58/102) | 0.017 | 39.8% (35/88) | 52.4% (44/84) | 0.097 | 52.4% (22/42) | 43.8% (57/130) | 0.335 |
| Gestational age at sampling (weeks) | 29.2 ± 3.2 | 31.1 ± 2.4 | <0.001 | 29.4 ± 3.0 | 31.3 ± 2.7 | <0.001 | 29.1 ± 2.9 | 30.7 ± 2.9 | <0.001 |
| Gestational age at delivery (weeks) | 30.9 ± 2.6 | 33.5 ± 2.2 | <0.001 | 31.0 ± 2.7 | 33.2 ± 1.8 | <0.001 | 30.7 ± 2.7 | 32.5 ± 2.3 | <0.001 |
| Cervical length by ultrasound (cm) | 2.12 ± 1.31 | 2.63 ± 1.18 | 0.005 | 2.19 ± 1.33 | 2.47 ± 1.17 | 0.155 | 2.12 ± 1.39 | 2.39 ± 1.21 | 0.184 |
| Serum CRP (mg/dL) | 1.3 ± 1.7 | 0.5 ± 0.6 | <0.001 | 1.29 ± 1.70 | 0.49 ± 0.62 | 0.010 | 1.04 ± 1.55 | 0.86 ± 1.29 | 0.316 |
| Maternal WBC count (103/mm3) | 12.09 ± 3.67 | 10.39 ± 3.02 | <0.001 | 12.20 ± 3.85 | 10.28 ± 2.91 | 0.001 | 11.87 ± 3.88 | 11.07 ± 3.43 | 0.300 |
| Maternal blood NLR | 8.54 ± 5.61 | 5.69 ± 4.38 | <0.001 | 8.26 ± 6.31 | 6.21 ± 3.94 | 0.009 | 8.46 ± 7.01 | 6.88 ± 4.71 | 0.182 |
| Use of tocolytic agents | 61.3% (57/93) | 56.9% (58/102) | 0.530 | 61.4% (54/88) | 59.5% (50/84) | 0.805 | 61.9% (26/42) | 60.0% (78/130) | 0.826 |
| Use of antibiotics | 95.7% (89/93) | 95.1% (97/102) | 1.000 | 97.7% (86/88) | 95.2% (80/84) | 0.436 | 100% (42/42) | 95.4% (124/130) | 0.338 |
| Use of antenatal corticosteroids | 90.3% (84/93) | 87.3% (89/102) | 0.499 | 95.5% (84/88) | 83.3% (70/84) | 0.012 | 95.2% (40/42) | 87.7% (114/130) | 0.247 |
| Clinical chorioamnionitis | 8.6% (8/93) | 8.8% (9/102) | 0.956 | 15.9% (14/88) | 3.6% (3/84) | 0.009 | 26.2% (11/42) | 4.6% (6/130) | <0.001 |
| Histologic chorioamnionitisa | 69.0% (58/84) | 34.1% (30/88) | <0.001 | ||||||
Significant findings (P < 0.05) are highlighted in bold fonts.
CRP, C-reactive protein; WBC, white blood cell; NLR, neutrophil-to-lymphocyte ratio.
Data are given as mean ± standard deviation or % (n/N).
Data for the histologic evaluation of the placenta were only available in 172 of the 195 women because in 18 cases, delivery took place at another institution and in 5 cases, histologic evaluation of the placenta was not performed because of our institutional policy that only the placentas in cases of preterm delivery are to be sent for histopathologic examination or because of missing data for the histologic chorioamnionitis.
Association of plasma APPs with IAI/MIAC
The median plasma levels of SAA1 were significantly higher and those of RBP4 were significantly lower in women with IAI/MIAC than in women without this condition (Table 2). Plasma hepcidin levels tended to be higher in women with IAI/MIAC compared to those without this condition (P = 0.087). Owing to the baseline difference in parity and GA at sampling, we further performed multivariate logistic regression analysis. The results revealed that high plasma levels of hepcidin and SAA1 and low plasma levels of RBP4 were significantly and independently associated with IAI/MIAC (Table 3). However, based on the univariate analyses, no differences in plasma levels of MBL, pentraxin-2, and serpine A1 were found in association with IAI/MIAC in women with PPROM.
Table 2.
Various acute phase proteins levels in plasma of the study population according to the presence or absence of intra-amniotic inflammation (IAI) and/or microbial invasion of the amniotic cavity (MIAC), acute histologic chorioamnionitis (HCA), and funisitis in women with preterm premature rupture of membranes.
| IAI and/or MIAC | P-value | Acute HCA | P-value | Funisitis | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Positive (n = 93) | Negative (n = 102) | Positive (n = 88) | Negative (n = 84) | Positive (n = 42) | Negative (n = 130) | ||||
| Plasma hepcidin (ng/mL) | 8.38 ± 14.54 | 4.15 ± 5.51 | 0.087 | 7.22 ± 13.29 | 5.20 ± 8.87 | 0.299 | 7.78 ± 17.01 | 5.72 ± 8.79 | 0.774 |
| Plasma MBL (µg/mL) | 1.05 ± 0.57 | 1.05 ± 0.61 | 0.893 | 1.09 ± 0.61 | 1.03 ± 0.61 | 0.510 | 1.04 ± 0.64 | 1.07 ± 0.60 | 0.698 |
| Plasma pentraxin-2 (µg/mL) | 79.26 ± 20.74 | 76.62 ± 18.95 | 0.197 | 76.42 ± 19.22 | 78.90 ± 20.77 | 0.239 | 73.14 ± 22.47 | 79.09 ± 18.96 | 0.113 |
| Plasma RBP4 (µg/mL) | 15.78 ± 4.99 | 17.97 ± 4.25 | <0.001 | 16.14 ± 4.83 | 17.72 ± 4.69 | 0.071 | 14.81 ± 4.22 | 17.60 ± 4.82 | 0.006 |
| Plasma SAA1 (µg/mL) | 4.08 ± 3.58 | 2.15 ± 2.12 | <0.001 | 3.72 ± 3.41 | 2.43 ± 2.59 | 0.009 | 3.54 ± 3.47 | 2.94 ± 2.96 | 0.499 |
| Plasma serpin A1 (µg/mL) | 1658.31 ± 845.39 | 1887.48 ± 1322.19 | 0.308 | 1819.04 ± 1278.03 | 1659.97 ± 765.84 | 0.697 | 1759.76 ± 797.16 | 1734.76 ± 1132.46 | 0.722 |
Significant findings (P < 0.05) are highlighted in bold fonts.
MBL, mannose-binding lectin; RBP4, retinol-binding protein 4; SAA1, serum amyloid A1.
Data are given as mean ± standard deviation.
Table 3.
Relationship of various acute phase proteins in plasma with the presence of intra-amniotic inflammation (IAI) and/or microbial invasion of the amniotic cavity (MIAC), acute histologic chorioamnionitis (HCA), and funisitis analyzed using multiple logistic regression.
| Predictors | IAI and/or MIAC a | Acute HCAb | Funisitis c | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Plasma hepcidin (ng/mL) | 1.058 (1.015–1.102) | 0.008 | ||||
| Plasma RBP4 (µg/mL) | 0.891 (0.829–0.958) | 0.002 | 0.935 (0.869–1.006) | 0.072 | 0.875 (0.802–0.954) | 0.003 |
| Plasma SAA1 (µg/mL) | 1.231 (1.097–1.382) | <0.001 | 1.113 (0.992–1.248) | 0.068 | ||
| Serum CRP (mg/dL) | 2.020 (1.340–3.043) | 0.001 | 1.835 (1.191–2.826) | 0.006 | ||
Significant findings (P < 0.05) are highlighted in bold fonts.
OR, odds ratio; CI, confidence interval; RBP4, retinol-binding protein 4; SAA1, serum amyloid A1; CRP, C-reactive protein.
Adjustment for gestational age at sampling and parity.
Adjustment for gestational age at sampling, use of corticosteroids, and parity.
Adjustment for gestational age at sampling.
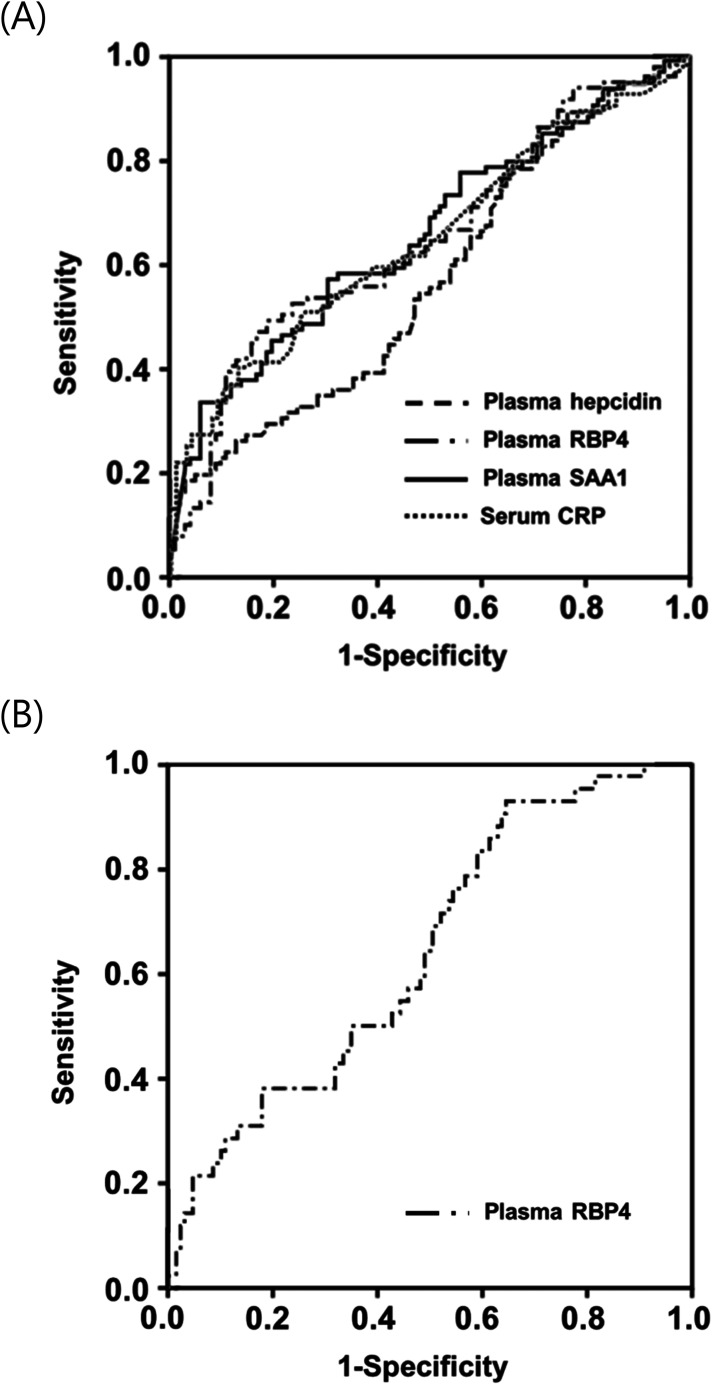
The AUC values for plasma hepcidin, RBP4, and SAA1 in identifying IAI/MIAC were 0.57, 0.65, and 0.66, respectively. These values did not exhibit significant differences among themselves (P = 0.088–0.841) or when compared to the AUC values of serum CRP (P = 0.153–0.921), as shown in Table 4 and Figure 1A.
Table 4.
Diagnostic indices of various plasma acute phase proteins for prediction of intra-amniotic inflammation and/or microbial invasion of the amniotic cavity, acute histologic chorioamnionitis, and funisitis.
| Variables | Area (±SE) under the ROC curve | 95% CI | Cut-off valuea | Sensitivityb (95% CI) | Specificityb (95% CI) | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Intra-amniotic inflammation and/or microbial invasion of the amniotic cavity | |||||||
| Plasma hepcidin (ng/mL) | 0.57 ± 0.04 f | 0.49–0.65 | ≥ 1.023 | 60.9 (50.1–70.8) | 45.1 (35.2–55.2) | 50.0 | 56.1 |
| Plasma RBP4 (µg/mL) | 0.65 ± 0.04 f | 0.57–0.72 | ≤ 16.953 | 62.0 (51.2–71.9) | 53.9 (43.8–63.8) | 54.8 | 61.1 |
| Plasma SAA1 (µg/mL) | 0.66 ± 0.04 f | 0.58–0.73 | ≥ 2.045 | 58.1 (47.4–68.2) | 67.6 (57.7–76.6) | 62.1 | 63.9 |
| Serum CRP (mg/dL) | 0.65 ± 0.04 f | 0.57–0.72 | ≥ 0.335 | 61.3 (50.6–71.2) | 55.0 (44.7–65.0) | 55.9 | 60.4 |
| Maternal blood NLR | 0.72 ± 0.04f | 0.64–0.79 | ≥ 5.196 | 76.7 (66.6–84.9) | 61.9 (51.4–71.5) | 65.1 | 74.1 |
| Combined model A c | 0.80 ± 0.03 | 0.74–0.87 | ≥ 0.383 | 85.5 (76.1–92.3) | 63.4 (52.8–73.2) | 67.6 | 83.1 |
| Acute histologic chorioamnionitis | |||||||
| Serum CRP (mg/dL) | 0.61 ± 0.04g | 0.53–0.69 | ≥ 0.305 | 60.2 (49.2–70.5) | 48.8 (37.6–60.1) | 55.8 | 53.3 |
| Maternal blood NLR | 0.62 ± 0.04g | 0.53–0.70 | ≥ 5.196 | 65.9 (54.8–75.8) | 51.9 (40.4–63.3) | 59.6 | 58.6 |
| Combined model B d | 0.75 ± 0.04 | 0.68–0.83 | ≥ 0.402 | 77.3 (67.1–85.5) | 62.2 (50.8–72.7) | 68.7 | 71.8 |
| Funisitis | |||||||
| Plasma RBP4 (µg/mL) | 0.65 ± 0.05h | 0.56–0.74 | ≤ 16.619 | 64.3 (48.0–78.5) | 51.2 (42.2–60.1) | 30.0 | 81.5 |
| Combined model C e | 0.72 ± 0.04 | 0.64–0.81 | ≥ 0.226 | 71.4 (55.4–84.3) | 62.8 (53.8–71.1) | 38.5 | 87.1 |
SE, standard error; ROC, receiver-operating characteristic; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; RBP4, retinol-binding protein 4; SAA1, serum amyloid A1; CRP, C-reactive protein.
Cut-off values corresponding to the highest sum of sensitivity and specificity or the point on the ROC curve closest to (0, 1).
Values are given as % (95% CI).
Combined model A consists of plasma RBP4 levels, cervical length, maternal blood NLR (≥5.19), gestational age at sampling, and parity.
Combined model B consists of gestational age at sampling and serum CRP levels.
Combined model C consists of gestational age at sampling and plasma RBP4 levels.
P < 0.05 compared with combined model A by the method of DeLong et al.
P < 0.05 compared with combined model B by the method of DeLong et al.
P < 0.05 compared with combined model C by the method of DeLong et al.
Figure 1.
Receiver-operating characteristic curves (A) of plasma hepcidin, RBP4, SAA1, and serum CRP in detecting IAI/MIAC (Hepcidin: AUC, 0.57 ± 0.04; P = 0.089. RBP4: AUC, 0.65 ± 0.04; P < 0.001. SAA1: AUC, 0.66 ± 0.04; P < 0.001. CRP: AUC, 0.65 ± 0.04; P < 0.001); (B) of plasma RBP4 in detecting funisitis (RBP4: AUC, 0.65 ± 0.05; P = 0.006). AUC, area under the curve ± standard error; RBP4, retinol-binding protein 4; SAA1, serum amyloid A1; CRP, C-reactive protein; IAI/MIAC, intra-amniotic inflammation and/or microbial invasion of the amniotic cavity.
Among the measured serum/plasma APPs showing statistically significant associations with outcomes (CRP, hepcidin, RBP4, and SAA1), plasma concentrations of hepcidin (r = 0.154, P = 0.044), RBP4 (r = −0.268, P < 0.001), and SAA1 (r = 0.498, P < 0.001) were significantly correlated with serum CRP concentrations. Furthermore, plasma RBP4 concentrations were also significantly correlated with plasma SAA1 concentrations (r = −0.292, P < 0.001) (Table S1).
Association of plasma APPs with acute HCA and funisitis
Out of the 195 patients with PPROM included in the study, 172 had accessible data for placental histopathology. This subset of 172 patients constituted the cohort used to evaluate plasma APP concentrations concerning acute HCA and funisitis. Acute HCA and funisitis were detected in 51.1% (88/172) and 24.4% (42/172) of these patients analyzed, respectively.
Similar to the results observed for IAI/MIAC, the median serum CRP levels, WBC count, and NLR were significantly higher in patients with acute HCA compared with women without this condition, whereas the median CL did not differ between the two groups (Table 1). The median plasma levels of SAA1 were significantly higher in women with acute HCA than in those without acute HCA (Table 2). Moreover, the plasma RBP4 levels tended to be lower in women with acute HAC, but this difference was not statistically significant (P = 0.071). Due to baseline significant differences or tendency for initial disparities in corticosteroid administration rates, nulliparity, and GA at sampling, we conducted additional multivariate analyses. These analyses indicated that reduced plasma RBP4 levels and elevated plasma SAA1 levels showed no statistical significance in their association with HCA after adjusting for these three variables (P = 0.072 and 0.068, respectively) (Table 3).
Univariate analyses showed that the median plasma RBP4 levels were significantly lower in women with funisitis than in those without this condition (Table 2). Because of baseline differences in GA at sampling between the funisitis and non-funisitis groups, we further performed additional multivariate-adjusted analyses. The results showed that decreased plasma RBP4 levels were significantly associated with funisitis upon adjusting for GA at sampling (Table 3). The AUC for the plasma RBP4 levels was 0.65 for the identification of funisitis (Table 4 and Figure 1B).
Development of noninvasive combined models for predicting IAI/MIAC and funisitis based on significant plasma APPs biomarkers and ultrasound-, clinical-, and conventional blood-based markers
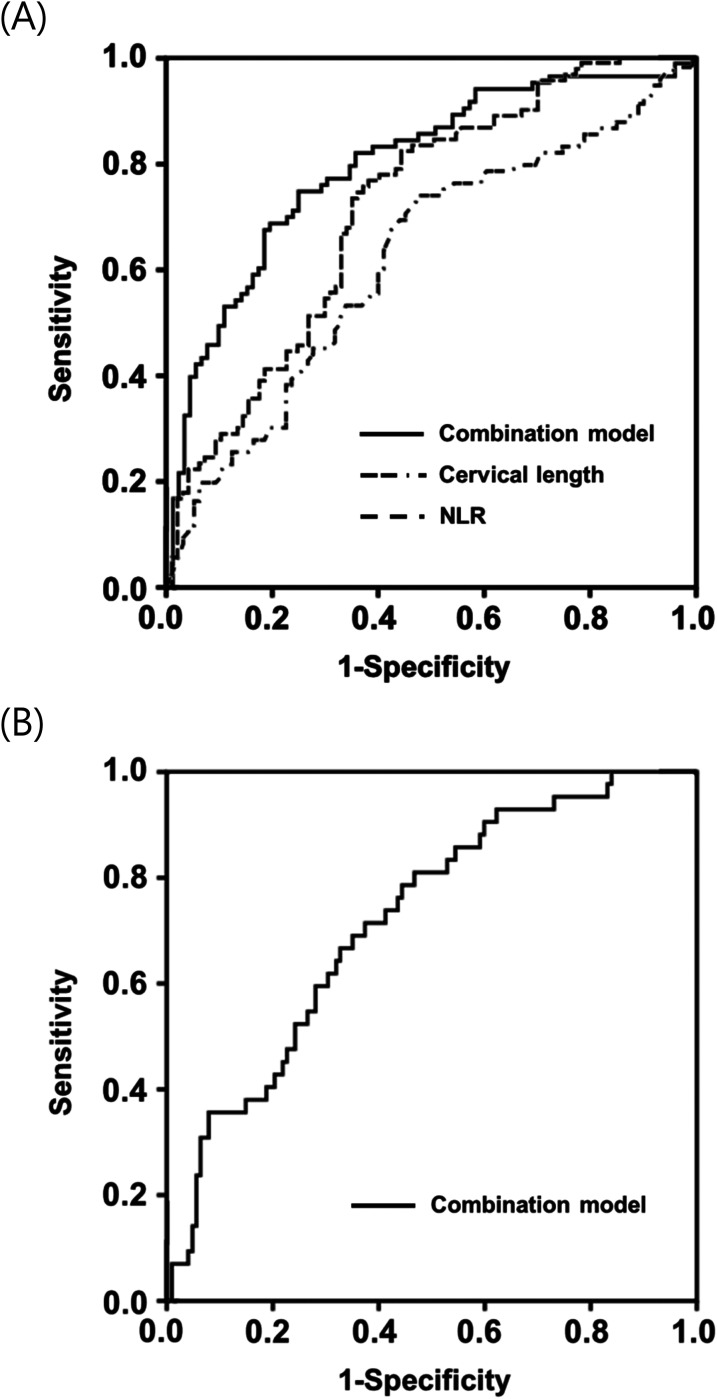
None of the continuous predictors used to develop models, except for NLR and hepcidin levels, failed to meet the linearity assumption. Thus, NLR and hepcidin were categorized as high or low using 5.19 and 1.02 ng/mL, respectively, as cutoff values according to ROC curve data. To develop a noninvasive combined predictive model for IAI/MIAC, the newly identified plasma proteins (high hepcidin level [≥1.02 ng/mL], RBP4, and SAA), ultrasound- (CL), clinical- (GA at sampling and parity), and conventional blood-based markers (high NLR [≥5.19], CRP, and WBC) associated with IAI/MIAC (P < 0.1 in univariate analyses) were included in a stepwise regression analysis (Table 5). Plasma RBP4 levels, high NLR (≥ 5.19), GA at sampling, CL, and parity were chosen as the best combination for IAI/MIAC prediction, with an AUC value of 0.806 (95% confidence interval [CI]: 0.741–0.870; P = 0.500 by Hosmer-Lemeshow test) (Table 5). The AUC value of this noninvasive combined model was significantly greater than those of the covariates alone included in the model (P < 0.01 for each) (Table 4; Figure 2A).
Table 5.
Regression coefficients, odds ratios, and 95% confidence intervals of the best noninvasive models for predicting intra-amniotic inflammation (IAI) and/or microbial invasion of the amniotic cavity (MIAC), acute histologic chorioamnionitis (HCA), and funisitis.
| Predictor | Beta-coefficient | SE | OR (95% CI) | P-value |
|---|---|---|---|---|
| IAI and/or MIAC a | ||||
| Cervical length (cm) | −0.412 | 0.156 | 0.662 (0.488–0.900) | 0.008 |
| High blood NLR (≥5.19) | 1.301 | 0.366 | 3.671 (1.791–7.526) | <0.001 |
| Plasma RBP4 (µg/mL) | −0.092 | 0.041 | 0.912 (0.841–0.989) | 0.025 |
| Gestational age at sampling (weeks) | −0.230 | 0.064 | 0.795 (0.701–0.901) | <0.001 |
| Nulliparity | −0.710 | 0.361 | 0.492 (0.242–0.998) | 0.049 |
| Constant | 8.978 | 2.263 | 7930.0 | <0.001 |
| Acute HCA b | ||||
| Gestational age at sampling (weeks) | −0.220 | 0.061 | 0.803 (0.712–0.905) | <0.001 |
| Serum CRP (mg/dL) | 0.534 | 0.208 | 1.706 (1.136–2.563) | 0.010 |
| Constant | 5.306 | 1.946 | 201.565 | 0.006 |
| Funisitis c | ||||
| Gestational age at sampling (weeks) | −0.173 | 0.061 | 0.841 (0.746–0.949) | 0.005 |
| Plasma RBP4 (µg/mL) | −0.134 | 0.044 | 0.875 (0.802–0.954) | 0.003 |
| Constant | 6.206 | 1.987 | 495.801 | 0.002 |
SE, standard error; OR, odds ratio; CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; RBP4, retinol binding protein 4; SAA1, serum amyloid A1.
Final model resulting from a forward regression analysis including the following predictive parameters: gestational age at sampling, parity, high plasma hepcidin level (≥1.02 ng/mL), RBP4, and SAA1 levels, cervical length, serum CRP levels, maternal WBC counts, and high blood NLR (≥5.19).
Final model resulting from a forward regression analysis including the following predictive parameters: gestational age at sampling, plasma SAA1 levels, serum CRP levels, maternal WBC counts, and high blood NLR (≥5.19).
Final model resulting from a forward regression analysis including the following predictive parameters: gestational age at sampling and plasma RBP4.
Figure 2.
Receiver-operating characteristic curves (A) of combination model, cervical length, and blood NLR in detecting IAI/MIAC (Combination model: AUC, 0.80 ± 0.03; P < 0.001. cervical length: AUC, 0.62 ± 0.04; P = 0.005. NLR: AUC, 0.72 ± 0.04; P < 0.001); (B) of combination model in detecting funisitis (Combination model: AUC, 0.72 ± 0.04; P < 0.001). AUC, area under the curve ± standard error; NLR, neutrophil-to-lymphocyte ratio; IAI/MIAC, intra-amniotic inflammation and/or microbial invasion of the amniotic cavity.
To develop a noninvasive combined model for acute HCA, five parameters were included in the stepwise regression analysis (Table 5). Of note, only GA at sampling and serum CRP (but none of the biomarkers investigated in the study) were selected for the best combination, with an AUC value of 0.751 (95% CI: 0.678–0.825; P = 0.049 by Hosmer-Lemeshow test) (Table 5). For funisitis, a noninvasive combined model was also developed using two variables, namely plasma RBP4 levels and GA at sampling (Table 5). Plasma RBP4 levels and GA at sampling were finally selected as the best combination, with an AUC value of 0.722 (95% CI: 0.638–0.806; P = 0.893 by Hosmer-Lemeshow test). The AUC for this funisitis model was significantly greater than that of plasma RBP4 (P = 0.029), but not of GA at sampling (P = 0.315) (Table 4; Figure 2B).
Validation of the model with the bootstrap technique
Internal validation showed that the optimism-corrected AUCs for the IAI/MIAC, acute HCA, and funisitis predictions were of 0.768 (95% CI: 0.727–0.857), 0.724 (95% CI: 0.575–0.781), and 0.710 (95% CI: 0.602–0.769), respectively, with optimisms ranging from 0.038 to 0.012.
Discussion
The principal findings of this study are as follows: (i) in women with PPROM, elevated levels of hepcidin and SAA1 and decreased levels of RBP4 in the plasma were independently associated with increased risks of IAI/MIAC; (ii) decreased plasma RBP4 levels were independently associated with funisitis; and (iii) in particular, combination of these APP biomarkers (RBP4) with ultrasound-, clinical-, and conventional blood-based markers (CL, NLR, GA at sampling, and parity) can significantly enhance the predictive ability for IAI/MIAC or funisitis as compared with each biomarker alone (AUC: 0.72–0.80 vs. 0.57–0.66). However, (iv) plasma MBL, pentraxin-2, and serpin A1 were not significantly associated with IAI/MIAC, acute HCA, or funisitis. To our knowledge, this is the first study to investigate the potential role of several important mediators found in plasma in acute-phase protein responses in women with PPROM in association with in utero inflammation/infection.
Despite the significant associations between the altered plasma levels of hepcidin, RBP4, and SAA1 and IAI/MIAC or funisitis, the corresponding AUC values showed only a fair diagnostic capacity (range, 0.57–0.66). 51 Similar findings are also reported for other inflammation-related biomarkers discovered in the plasma/serum from PPROM patients, such as IL-6, haptoglobin, FCGR3A, LBP, E-selectin, and kallistatin.15,26–28 Indeed, the identification of blood-based biochemical markers for in utero inflammation/infection in cases of PPROM that could find utility in clinical practice may pose challenges. Blood represents a complex biological sample characterized by the presence of high-abundance proteins, a broad spectrum of proteins with varying concentrations, and inherent biological variability.52,53 In particular, the aforementioned blood-based biomarkers are sensitive but not specific for local in utero as well as systemic inflammation/infection.15,26–28,33,34 Consequently, the clinical utility of the single blood-based biomarkers identified in this study and in previous studies may be limited in the context of PPROM.
Thus, we further investigated the combined predictive effects of APP biomarkers, inflammatory markers with different properties in a given sample (blood), and other clinically relevant characteristics (e.g., ultrasound and demographic-clinical factors) on the studied outcomes of interest. CL, WBC count, NLR, and CRP levels were proposed as potent predictive markers for IAI, MIAC, and acute HCA/funisitis in the context of PPROM.25,28,29,54 The present study showed that combining plasma RBP4 along with CL, NLR, parity, and GA at sampling can significantly enhanced the predictive ability for in utero inflammation/infection as compared with these markers alone. These observations concur with the findings of previous studies that evaluated the combined effect of different markers in maternal serum/plasma and maternal clinical profile data for the noninvasive identification of in utero inflammation/infection,24,29,30,55 collectively suggesting that the underlying pathogenesis of in utero inflammation/infection is multifactorial (e.g., danger signals, sterile IAI, and colonization). 6 From a practical point of view, our noninvasive combined model for detecting in utero inflammation/infection can be useful in the following clinical settings of PPROM: (i) initial screening to stratify patients according to their risk status and consequently deliver the most adequate interventions (e.g., amniocentesis or clarithromycin use), 37 (ii) pregnancies complicated by anhydramnios or severe oligohydramnios (resulting from ruptured membrane), and (iii) the need for serial evaluation of the development of in utero inflammation/infection and/or to access treatment efficacy.
Hepcidin is a type II (positive) acute-phase protein that is mainly produced by the liver via I L-6 stimulation and principally regulates the homeostasis of iron concentration (iron metabolism). 56 This protein plays an important role in the innate antibacterial immunity by reducing availability of iron to invading microorganism. Furthermore, it also acts as antimicrobial peptide. 56 In line with host defense role of hepcidin, a previous study on cord blood and AF from a mixed cohort of women with PTL or PPROM showed that hepcidin levels are significantly elevated in cord blood or AF from patients with IAI/MIAC compared with those without this condition. 57 However, to date, no data has been reported concerning the altered expression of plasma hepcidin associated with IAI, MIAC, or acute HCA in the PPROM setting. In the context of PTL, only one study on maternal serum reported a significant association between elevated serum hepcidin levels and the risk of spontaneous PTB (SPTB) in women with threatened PTL. 58 The present study also demonstrates for the first time in the context of PPROM that high plasma hepcidin levels are independently associated with IAI/MIAC. In general, this is in line with the results of the aforementioned PTL study, 58 given the reported associations between MIAC/IAI/acute HCA and SPTB risk in the PTL or PPROM.54,59–61
RBP4 is a member of the lipocalin family. It acts as a specific carrier for retinol (vitamin A) in the blood and is primarily produced in the liver and adipose tissue (the liver is the major source of plasma RBP4). 62 RBP4, also referred to as an adipokine, belongs to a group of cytokines derived from adipocytes. These adipokines play a pivotal role in establishing a connection between insulin resistance, obesity, and inflammatory diseases associated with obesity. 63 In particular, RBP4 acts as a negative acute-phase protein, whose expression in the liver/macrophages is rapidly reduced after stimulation with the endotoxin lipopolysaccharide (LPS) and tumor necrosis factor (TNF)-alpha, and hence, its levels in plasma are decreased during the inflammatory response.33,64,65 In the context of PTL, a previous proteomic study on pooled AF samples showed a significant upregulated expression of the AF RBP4 in a preterm delivery group compared to that in a term delivery group. 66 Similarly, elevated AF RBP4 levels were also found to be associated with IAI/MIAC in women with PTL. 67 Contrary to the aforementioned reports on AF samples, the present study demonstrates for the first time that RBP4 levels are significantly decreased in the plasma of women with PPROM complicated by IAI/MIAC or funisitis, which is in agreement with the commonly accepted biological role of RBP4 as a negative acute phase reactant. Similar to our findings, Vaisbuch et al. reported that RBP4 levels are significantly decreased in the maternal plasma of patients with acute pyelonephritis during pregnancy (acute infectious disease), 68 supporting the herein reported RBP4 data in plasma samples.
Although we cannot explain the exact pathophysiological mechanism by which decreased plasma RBP4 levels correlate with the in utero inflammation/infection reported, several multifactorial mechanisms may be involved.69–72 In particular, (i) increased RBP4 turn-over and loss due to rapid kidney clearance, gut function impairment, increased vascular permeability, and/or increased catabolism; (ii) decreased production (rare) by the liver in response to inflammatory cytokines; and (iii) counter-action response to insulin resistance (hypermetabolic/catabolic state) associated with infection can result in low circulating RBP4 levels in women with in utero inflammation/infection. However, these exact mechanisms are beyond the scope of the present study and further investigations are needed to explore these hypotheses.
SAA1 is an acute phase reactant that is mainly produced by the liver in response to increased levels of inflammatory cytokines (e.g., IL-1β/6 and TNF-alpha) and endotoxin secreted during inflammation, infection, and trauma. 73 This protein plays an important role in the regulation of inflammatory and immune responses as well as the propagation of the primordial acute phase response. 73 SAA1 is also expressed locally in all gestational tissues, including fetal membranes, placenta, and uterus. 74 Notably, this protein (particularly produced locally in these gestational tissues and secreted into the circulation) may function as a damage-associated molecular pattern molecule, resulting in the activation of the innate immune system.73,74 In line with the known biological role and expression site of SAA1, in the context of in vivo experiments, several previous studies revealed that SAA1 mRNA and protein expression were upregulated in (i) human myometrium tissues during term labor, (ii) placenta (delivered at term from women with labor), and (iii) amnion tissue following spontaneous labor, all of which suggest that SAA1 may be involved in the process of parturition and spontaneous rupture of membranes.75–77 In a mouse model of PTL, Yang et al. found that plasma SAA1 and 2 were significantly elevated in LPS-treated mice compared to that in controls and in LPS-treated mice that delivered preterm compared to those that delivered at term. 78 However, in the available human studies, the expression of the SAA1 subtype has not yet been investigated in maternal blood derived from women with PPROM with respect to IAI, MIAC, and acute HCA. Previous studies, most of which comprise a small sample size, have shown that SAA levels (but not SAA subtype) were significantly elevated in the maternal blood samples collected from women with PPROM and HCA and women with PTL followed by preterm delivery as compared with those without this condition.79,80 Similar to the results of total SAA levels studied above, this study demonstrated for the first time that SAA1 levels were significantly or nearly significantly elevated in the plasma of women with PPROM complicated by IAI/MIAC and acute HCA.
In contrast to hepcidin, RBP4, and SAA1, no associations were observed between altered plasma levels of MBL, pentraxin-2, and serpin A1 and IAI/MIAC in pregnancies complicated by PPROM, despite the fact that they are also mainly produced in the liver upon stimulation by TNF-alpha, IL-1, and IL-6.35,36 We were unable to locate any additional studies corroborating or linked to this discovery. Consequently, we are unable to provide a definitive explanation for the mechanisms underlying the utility of the studied APPs in the detection of IAI or MIAC and why certain APPs studied do not exhibit the same utility. Nonetheless, we posit that this phenomenon is possibly associated with (i) the extent of elevation in APP levels in the bloodstream, (ii) the interval elapsed before detecting an APP in circulation following exposure to in utero infection or inflammation, and (iii) the duration required for subsequent clearance of this APP from the circulation. For example, SAA and CRP levels in plasma are rapidly upregulated, and within 24–48 h after the start of inflammatory stimulus, their levels in plasma are more than 1000–3000 times higher than normal. 35 Inversely, once the origin of disease is removed, their plasma levels quickly decrease due to their short plasma half-life (SAA: 34.9 ± 28.7 h; CRP: 46.4 ± 21.7 h). 81 Hence, these conditions rendered SAA1 and CRP as valuable APP markers in plasma for the detection of IAI/MIAC in the present study.
From a clinical point of view, good biomarkers should be minimally invasive, cost-effective, rapidly assessed, and easily obtainable. In this regard, the herein identified plasma biomarkers may be of value for practical applications as they comply with all these requirements. Indeed, immunoassays (e.g., ELISA) have been largely automated and are relatively simple, rapid (results within 24 h), cost-effective, sensitive, and robust analysis techniques that are widely used in clinical laboratory routine. 82 Nevertheless, it should be pointed out that analysis of the herein proposed plasma biomarkers may not be available in a short time, especially in resource-limited settings. Thus, further research is needed to develop the point-of-care testing (POC) of these biomarkers (particularly for RBP4) so it can support clinical decision-making in resource-poor settings. Notably, a recent study has reported that POC CRP levels correlate well with those of standard laboratory CRP and have good diagnostic accuracy for predicting sepsis in neonates. 83
This study had several limitations that should be considered. First, molecular (polymerase chain reaction)-based methods were not used for the detection of microorganisms from the AF, despite the fact that such methods are considered complementary to traditional culture-based methods in the identification of MIAC. 84 As a result, actual cases of MIAC may not have been diagnosed when AF cultures were falsely negative. Second, we used a retrospective study design and obtained the data from a single institution. Moreover, the potential biomarkers and the noninvasive prediction models identified in the present study were not validated in an independent dataset. These may limit the generalizability of our findings. Third, APP levels were measured using plasma samples that were stored at −70°C for up to 18 years before being processed. This may have impacted the results of immunoassay analyses owing to protein degradation caused by long-term storage.85,86 Fourth, we did not perform multiple comparison corrections (e.g., Bonferroni method for false-discovery rate, P< 0.0083 [0.05 divided by 6]) due to the exploratory nature of this study as well as to avoid false-negative rather than false-positive errors. Fifth, the diagnostic potential of the herein identified plasma markers showed only fair performance (AUC: 0.57–0.66) for detecting in utero inflammation/infection, 51 which suggests that a single plasma marker alone is not sufficient for diagnostic assessment in clinical practice. Sixth, despite the moderate discriminatory ability (AUC = 0.751) of the herein described prediction model for acute HCA, its calibration was poor (Hosmer-Lemeshow test P = 0.049), indicating that the model may overestimate or underestimate the probability of acute HCA occurrence. Thus, further studies are needed to develop noninvasive prediction models with good calibration and discrimination potential for acute HCA. Notwithstanding these limitations, this study had several strengths: (i) it comprised a relatively large, homogenous (concerning ethnicity) study population; and (ii) it compared the diagnostic accuracy of novel plasma APPs with that of serum CRP (a prototype marker of inflammation), which allows clinicians to determine whether the plasma APPs detected in this study can be readily translated in current clinical practice for patients with PPROM. In the current study, the plasma APP biomarkers identified (hepcidin, RBP4, and SAA1) do not show superior diagnostic performance as compared with conventional blood-based markers (CRP and NLR) for diagnosing IAI/MIAC; thus, their clinical utility as a single marker may be limited in the PPROM setting. Nonetheless, these plasma APPs may be useful to improve the prediction of in utero inflammation/infection in combination with ultrasound, clinical, and routine blood parameters.
Conclusion
Hepcidin, RBP4, and SAA1 (but not MBL, pentraxin-2, and serpin A1) were identified as potential APP biomarkers in the plasma predictive of IAI/MIAC or funisitis in patients with PPROM. In particular, combination of these APP biomarkers (RBP4) with ultrasound- (CL), clinical- (GA at sampling and parity), and conventional blood-based markers (NLR) can significantly support the diagnosis of IAI/MIAC and funisitis. Owing to their less-invasive nature and repeatability, these biomarkers and combined prediction models may aid in risk stratification and monitoring of women with PPROM-related complications. Additional research is necessary to elucidate the mechanisms within plasma that determine the involvement of specific APPs in the pathogenesis of in utero infection and inflammation and to differentiate which APPs are not implicated.
Supplemental Material
Supplemental material, sj-docx-1-ini-10.1177_17534259241306237 for Plasma acute phase proteins as potential predictors of intra-amniotic inflammation and infection in preterm premature rupture of membranes by Hee Young Cho, Kyo Hoon Park, Eunji Oh, Min Jung Lee, Bo Young Choi and Eun Mi Im in Innate Immunity
Supplemental material, sj-docx-2-ini-10.1177_17534259241306237 for Plasma acute phase proteins as potential predictors of intra-amniotic inflammation and infection in preterm premature rupture of membranes by Hee Young Cho, Kyo Hoon Park, Eunji Oh, Min Jung Lee, Bo Young Choi and Eun Mi Im in Innate Immunity
Supplemental material, sj-sav-3-ini-10.1177_17534259241306237 for Plasma acute phase proteins as potential predictors of intra-amniotic inflammation and infection in preterm premature rupture of membranes by Hee Young Cho, Kyo Hoon Park, Eunji Oh, Min Jung Lee, Bo Young Choi and Eun Mi Im in Innate Immunity
Acknowledgments
This work was presented as abstract at the 2023 meeting of the International Federation of Placenta Associations (IFPA), Rotorua, New Zealand, September, 5–8, 2023 and this Abstract was published in Placenta, the official journal of IFPA.
Footnotes
Authors’ contributions: HY Cho: data collection or management, data analysis, manuscript writing/editing; KH Park: conceptualization, protocol/project development, supervision, funding acquisition, data analysis, manuscript writing/editing; E Oh: data collection or management, data analysis, manuscript writing; MJ Lee: data collection or management, data analysis; BY Choi: data collection or management, data analysis; EM Im: data collection or management, data analysis, ELISA assay. All authors approved the final version of the manuscript before submission
Availability of data and materials: The datasets used and analyzed in the current study are available in the Supplementary File.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval and consent to participate: The ethics committee at Seoul National University Bundang Hospital approved the study (IRB no. B-1105/128-102). Patients provided written informed consent for the collection and use of the blood and AF samples and for the use of their clinical information for research purposes.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the The Seoul National University Bundang Hospital Research Fund, (grant number 13-2022-0011).
ORCID iD: Kyo Hoon Park https://orcid.org/0000-0003-3550-9686
Supplemental material: Supplemental material for this article is available online.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet 2008; 371: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menon R, Richardson LS. Preterm prelabor rupture of the membranes: a disease of the fetal membranes. Semin Perinatol 2017; 41: 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sae-Lin P, Wanitpongpan P. Incidence and risk factors of preterm premature rupture of membranes in singleton pregnancies at Siriraj Hospital. J Obstet Gynaecol Res 2019; 45: 573–577. [DOI] [PubMed] [Google Scholar]
- 4.Manuck TA, Varner MW. Neonatal and early childhood outcomes following early vs later preterm premature rupture of membranes. Am J Obstet Gynecol 2014; 211: 308.e1–308.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menon R, Fortunato SJ. Infection and the role of inflammation in preterm premature rupture of the membranes. Best Pract Res Clin Obstet Gynaecol 2007; 21: 467–478. [DOI] [PubMed] [Google Scholar]
- 6.Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015; 28: 1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SM, Park KH, Joo E, et al. High-throughput analysis of amniotic fluid proteins associated with histological chorioamnionitis in preterm premature rupture of membranes using an antibody-based microarray. Am J Reprod Immunol 2022; 88: e13595. [DOI] [PubMed] [Google Scholar]
- 8.Ogunyemi D, Murillo M, Jackson U, et al. The relationship between placental histopathology findings and perinatal outcome in preterm infants. J Matern Fetal Neonatal Med 2003; 13: 102–109. [DOI] [PubMed] [Google Scholar]
- 9.Kacerovsky M, Musilova I, Andrys C, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol 2014; 210: 325.e1–325.e10. [DOI] [PubMed] [Google Scholar]
- 10.Wu YW, Colford JM, Jr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA 2000; 284: 1417–1424. [DOI] [PubMed] [Google Scholar]
- 11.Park JW, Park KH, Lee SY. Noninvasive prediction of intra-amniotic infection and/or inflammation in women with preterm labor: various cytokines in cervicovaginal fluid. Reprod Sci 2013; 20: 262–268. [DOI] [PubMed] [Google Scholar]
- 12.Kibel M, Asztalos E, Barrett J, et al. Outcomes of pregnancies complicated by preterm premature rupture of membranes between 20 and 24 weeks of gestation. Obstet Gynecol 2016; 128: 313–320. [DOI] [PubMed] [Google Scholar]
- 13.Cobo T, Kacerovsky M, Palacio M, et al. A prediction model of histological chorioamnionitis and funisitis in preterm prelabor rupture of membranes: analyses of multiple proteins in the amniotic fluid. J Matern Fetal Neonatal Med 2012; 25: 1995–2001. [DOI] [PubMed] [Google Scholar]
- 14.Park KH, Kim SN, Oh KJ, et al. Noninvasive prediction of intra-amniotic infection and/or inflammation in preterm premature rupture of membranes. Reprod Sci 2012; 19: 658–665. [DOI] [PubMed] [Google Scholar]
- 15.Lee SM, Park KH, Jung EY, et al. Inflammatory proteins in maternal plasma, cervicovaginal and amniotic fluids as predictors of intra-amniotic infection in preterm premature rupture of membranes. PLoS One 2018; 13: e0200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cobo T, Kacerovsky M, Holst RM, et al. Intra-amniotic inflammation predicts microbial invasion of the amniotic cavity but not spontaneous preterm delivery in preterm prelabor membrane rupture. Acta Obstet Gynecol Scand 2012; 91: 930–935. [DOI] [PubMed] [Google Scholar]
- 17.Dawe GS, Tan XW, Xiao ZC. Cell migration from baby to mother. Cell Adh Migr 2007; 1: 19–27. [PMC free article] [PubMed] [Google Scholar]
- 18.Sheller-Miller S, Choi K, Choi C, et al. Cyclic-recombinase-reporter mouse model to determine exosome communication and function during pregnancy. Am J Obstet Gynecol 2019; 221: 502.e1–502.e12. [DOI] [PubMed] [Google Scholar]
- 19.Jin J, Menon R. Placental exosomes: a proxy to understand pregnancy complications. Am J Reprod Immunol 2018; 79: e12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Lopez N, Romero R, Xu Y, et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol 2017; 217: 693.e1–693.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez-Lopez N, Romero R, Leng Y, et al. The origin of amniotic fluid monocytes/macrophages in women with intra-amniotic inflammation or infection. J Perinat Med 2019; 47: 822–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNamara MF, Wallis T, Qureshi F, et al. Determining the maternal and fetal cellular immunologic contributions in preterm deliveries with clinical or subclinical chorioamnionitis. Infect Dis Obstet Gynecol 1997; 5: 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steel JH, O'Donoghue K, Kennea NL, et al. Maternal origin of inflammatory leukocytes in preterm fetal membranes, shown by fluorescence in situ hybridisation. Placenta 2005; 26: 672–677. [DOI] [PubMed] [Google Scholar]
- 24.Park JW, Park KH, Lee JE, et al. Antibody microarray analysis of plasma proteins for the prediction of histologic chorioamnionitis in women with preterm premature rupture of membranes. Reprod Sci 2019; 26: 1476–1484. [DOI] [PubMed] [Google Scholar]
- 25.Musilova I, Kacerovsky M, Stepan M, et al. Maternal serum C-reactive protein concentration and intra-amniotic inflammation in women with preterm prelabor rupture of membranes. PLoS One 2017; 12: e0182731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joo E, Park KH, Kim YM, et al. Maternal plasma and amniotic fluid LBP, pentraxin 3, resistin, and IGFBP-3: biomarkers of microbial invasion of amniotic cavity and/or intra-amniotic inflammation in women with preterm premature rupture of membranes. J Korean Med Sci 2021; 36: e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Back JH, Kim SY, Gu MB, et al. Proteomic analysis of plasma to identify novel biomarkers for intra-amniotic infection and/or inflammation in preterm premature rupture of membranes. Sci Rep 2023; 13: 5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho I, Lee KN, Joo E, et al. Plasma E-selectin and kallistatin as predictive markers of histologic chorioamnionitis in women with preterm premature rupture of membranes. Am J Reprod Immunol 2022; 88: e13584. [DOI] [PubMed] [Google Scholar]
- 29.Shi H, Sun L, Wang Z, et al. Non-invasive prediction of histologic chorioamnionitis using maternal serum markers in women with preterm prelabour rupture of membranes. Am J Reprod Immunol 2022; 88: e13594. [DOI] [PubMed] [Google Scholar]
- 30.Cobo T, Burgos-Artizzu XP, Collado MC, et al. Noninvasive prediction models of intra-amniotic infection in women with preterm labor. Am J Obstet Gynecol 2023; 228: 78.e1–78.e13. [DOI] [PubMed] [Google Scholar]
- 31.Rolnik DL, Wright D, Poon LCY, et al. ASPRE Trial: performance of screening for preterm pre-eclampsia. Ultrasound Obstet Gynecol 2017; 50: 492–495. [DOI] [PubMed] [Google Scholar]
- 32.Crovetto F, Triunfo S, Crispi F, et al. Differential performance of first-trimester screening in predicting small-for-gestational-age neonate or fetal growth restriction. Ultrasound Obstet Gynecol 2017; 49: 349–356. [DOI] [PubMed] [Google Scholar]
- 33.Jain S, Gautam V, Naseem S. Acute-phase proteins: as diagnostic tool. J Pharm Bioallied Sci 2011; 3: 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrodl W, Buchler R, Wendler S, et al. Acute phase proteins as promising biomarkers: perspectives and limitations for human and veterinary medicine. Proteomics Clin Appl 2016; 10: 1077–1092. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani A, Garlanda C. Humoral innate immunity and acute-phase proteins. N Engl J Med 2023; 388: 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehlting C, Wolf SD, Bode JG. Acute-phase protein synthesis: a key feature of innate immune functions of the liver. Biol Chem 2021; 402: 1129–1145. [DOI] [PubMed] [Google Scholar]
- 37.Kacerovsky M, Romero R, Stepan M, et al. Antibiotic administration reduces the rate of intraamniotic inflammation in preterm prelabor rupture of the membranes. Am J Obstet Gynecol 2020; 223: 114.e1–114.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SY, Park KH, Jeong EH, et al. Intra-amniotic infection/inflammation as a risk factor for subsequent ruptured membranes after clinically indicated amniocentesis in preterm labor. J Korean Med Sci 2013; 28: 1226–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suh YH, Park KH, Hong JS, et al. Prediction of prolonged pregnancy in nulliparous women by transvaginal ultrasonographic measurement of cervical length at 20–24 weeks and 37 weeks. J Korean Med Sci 2007; 22: 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park KH. Transvaginal ultrasonographic cervical measurement in predicting failed labor induction and cesarean delivery for failure to progress in nulliparous women. J Korean Med Sci 2007; 22: 722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park JW, Park KH, Jung EY, et al. Short cervical lengths initially detected in mid-trimester and early in the third trimester in asymptomatic twin gestations: association with histologic chorioamnionitis and preterm birth. PLoS One 2017; 12: e0175455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung EY, Choi BY, Rhee J, et al. Relation between amniotic fluid infection or cytokine levels and hearing screen failure in infants at 32 wk gestation or less. Pediatr Res 2017; 81: 349–355. [DOI] [PubMed] [Google Scholar]
- 43.Gruys E, Toussaint MJ, Niewold TA, et al. Acute phase reaction and acute phase proteins. J Zhejiang Univ Sci B 2005; 6: 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryu A, Park KH, Oh KJ, et al. Predictive value of combined cervicovaginal cytokines and gestational age at sampling for intra-amniotic infection in preterm premature rupture of membranes. Acta Obstet Gynecol Scand 2013; 92: 517–524. [DOI] [PubMed] [Google Scholar]
- 45.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med 2016; 29: 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim CJ, Romero R, Chaemsaithong P, et al. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015; 213: S29–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 2006; 163: 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 49.Noma H, Shinozaki T, Iba K, et al. Confidence intervals of prediction accuracy measures for multivariable prediction models based on the bootstrap-based optimism correction methods. Stat Med 2021; 40: 5691–5701. [DOI] [PubMed] [Google Scholar]
- 50.Obuchowski NA. Receiver operating characteristic curves and their use in radiology. Radiology 2003; 229: 3–8. [DOI] [PubMed] [Google Scholar]
- 51.Brubaker PH. Do not be statistically cenophobic: time to roc and roll!. J Cardiopulm Rehabil Prev 2008; 28: 420–421. [DOI] [PubMed] [Google Scholar]
- 52.Thambisetty M, Lovestone S. Blood-based biomarkers of Alzheimer's disease: challenging but feasible. Biomark Med 2010; 4: 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ebert MP, Korc M, Malfertheiner P, et al. Advances, challenges, and limitations in serum-proteome-based cancer diagnosis. J Proteome Res 2006; 5: 19–25. [DOI] [PubMed] [Google Scholar]
- 54.Lee SM, Park KH, Jung EY, et al. Frequency and clinical significance of short cervix in patients with preterm premature rupture of membranes. PLoS One 2017; 12: e0174657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinez-Portilla RJ, Hawkins-Villarreal A, Alvarez-Ponce P, et al. Maternal serum interleukin-6: a non-invasive predictor of histological chorioamnionitis in women with preterm-prelabor rupture of membranes. Fetal Diagn Ther 2019; 45: 168–175. [DOI] [PubMed] [Google Scholar]
- 56.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003; 102: 783–788. [DOI] [PubMed] [Google Scholar]
- 57.Fisher AL, Sangkhae V, Presicce P, et al. Fetal and amniotic fluid iron homeostasis in healthy and complicated murine, macaque, and human pregnancy. JCI Insight 2020; 5: e135321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akkaya Firat A, Alici Davutoglu E, Ozel A, et al. Hypoxia-inducible factor-1alpha, hepcidin and interleukin-6 levels in pregnancies with preterm labour. J Obstet Gynaecol 2020; 40: 813–819. [DOI] [PubMed] [Google Scholar]
- 59.Kim HJ, Park KH, Kim YM, et al. A protein microarray analysis of amniotic fluid proteins for the prediction of spontaneous preterm delivery in women with preterm premature rupture of membranes at 23 to 30 weeks of gestation. PLoS One 2020; 15: e0244720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee KN, Park KH, Ahn K, et al. Extracellular matrix-related and serine protease proteins in the amniotic fluid of women with early preterm labor: association with spontaneous preterm birth, intra-amniotic inflammation, and microbial invasion of the amniotic cavity. Am J Reprod Immunol 2023; 90: e13736. [DOI] [PubMed] [Google Scholar]
- 61.Galletta MAK, Schultz R, Sartorelli M, et al. Clinical characteristics, complications, and predictive model of histological chorioamnionitis in women with preterm premature rupture of membranes. PLoS One 2023; 18: e0283974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steinhoff JS, Lass A, Schupp M. Biological functions of RBP4 and its relevance for human diseases. Front Physiol 2021; 12: 659977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005; 436: 356–362. [DOI] [PubMed] [Google Scholar]
- 64.Abd Eldaim MA, Kamikawa A, Soliman MM, et al. Retinol binding protein 4 in dairy cows: its presence in colostrum and alteration in plasma during fasting, inflammation, and the peripartum period. J Dairy Res 2010; 77: 27–32. [DOI] [PubMed] [Google Scholar]
- 65.Broch M, Ramirez R, Auguet MT, et al. Macrophages are novel sites of expression and regulation of retinol binding protein-4 (RBP4). Physiol Res 2010; 59: 299–303. [DOI] [PubMed] [Google Scholar]
- 66.Bujold E, Romero R, Kusanovic JP, et al. Proteomic profiling of amniotic fluid in preterm labor using two-dimensional liquid separation and mass spectrometry. J Matern Fetal Neonatal Med 2008; 21: 697–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, et al. Retinol binding protein 4: an adipokine associated with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med 2010; 23: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vaisbuch E, Romero R, Mazaki-Tovi S, et al. Maternal plasma retinol binding protein 4 in acute pyelonephritis during pregnancy. J Perinat Med 2010; 38: 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ingenbleek Y, Bernstein LH. Plasma transthyretin as a biomarker of lean body mass and catabolic states. Adv Nutr 2015; 6: 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cray C. Acute phase proteins in animals. Prog Mol Biol Transl Sci 2012; 105: 113–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gounden V, Vashisht R, Jialal I. Hypoalbuminemia. In: StatPearls. edn. Treasure Island FL ineligible companies. Disclosure: Rishik Vashisht declares no relevant financial relationships with ineligible companies. Disclosure: Ishwarlal Jialal declares no relevant financial relationships with ineligible companies.: © 2024, StatPearls Publishing LLC.; 2024.
- 72.Chakraborty S, Bhattacharyya R, Banerjee D. Infections: a possible risk factor for type 2 diabetes. Adv Clin Chem 2017; 80: 227–251. [DOI] [PubMed] [Google Scholar]
- 73.Sack GH, Jr. Serum amyloid A - a review. Mol Med 2018; 24: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin YK, Zhu P, Wang WS, et al. Serum amyloid A, a host-derived DAMP in pregnancy? Front Immunol 2022; 13: 978929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang Y, Pin L, Shi W, et al. SAA1 Regulates pro-labour mediators in term labour by activating YAP pathway. Mol Cell Biochem 2021; 476: 2791–2801. [DOI] [PubMed] [Google Scholar]
- 76.Gan XW, Wang WS, Lu JW, et al. De novo synthesis of SAA1 in the placenta participates in parturition. Front Immunol 2020; 11: 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li W, Wang W, Zuo R, et al. Induction of pro-inflammatory genes by serum amyloid A1 in human amnion fibroblasts. Sci Rep 2017; 7: 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Q, Whitin JC, Ling XB, et al. Plasma biomarkers in a mouse model of preterm labor. Pediatr Res 2009; 66: 11–16. [DOI] [PubMed] [Google Scholar]
- 79.Cekmez Y, Cekmez F, Ozkaya E, et al. Proadrenomedullin and serum amyloid A as a predictor of subclinical chorioamnionitis in preterm premature rupture of membranes. J Interferon Cytokine Res 2013; 33: 694–699. [DOI] [PubMed] [Google Scholar]
- 80.Ibrahim MI, Ellaithy MI, Hussein AM, et al. Measurement of maternal serum amyloid A as a novel marker of preterm birth. J Matern Fetal Neonatal Med 2021; 34: 2467–2472. [DOI] [PubMed] [Google Scholar]
- 81.Takata S, Wada H, Tamura M, et al. Kinetics of c-reactive protein (CRP) and serum amyloid A protein (SAA) in patients with community-acquired pneumonia (CAP), as presented with biologic half-life times. Biomarkers 2011; 16: 530–535. [DOI] [PubMed] [Google Scholar]
- 82.Wauthier L, Plebani M, Favresse J. Interferences in immunoassays: review and practical algorithm. Clin Chem Lab Med 2022; 60: 808–820. [DOI] [PubMed] [Google Scholar]
- 83.Goyal M, Mascarenhas D, Rr P, et al. Diagnostic accuracy of point-of-care testing of C-reactive protein, interleukin-6, and procalcitonin in neonates with clinically suspected sepsis: a prospective observational study. Med Princ Pract 2024; 33: 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014; 210: 125.e1–125.e15. [DOI] [PubMed] [Google Scholar]
- 85.Mitchell BL, Yasui Y, Li CI, et al. Impact of freeze-thaw cycles and storage time on plasma samples used in mass spectrometry based biomarker discovery projects. Cancer Inform 2005; 1: 98–104. [PMC free article] [PubMed] [Google Scholar]
- 86.Keustermans GC, Hoeks SB, Meerding JM, et al. Cytokine assays: an assessment of the preparation and treatment of blood and tissu samples. Methods 2013; 61: 10–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ini-10.1177_17534259241306237 for Plasma acute phase proteins as potential predictors of intra-amniotic inflammation and infection in preterm premature rupture of membranes by Hee Young Cho, Kyo Hoon Park, Eunji Oh, Min Jung Lee, Bo Young Choi and Eun Mi Im in Innate Immunity
Supplemental material, sj-docx-2-ini-10.1177_17534259241306237 for Plasma acute phase proteins as potential predictors of intra-amniotic inflammation and infection in preterm premature rupture of membranes by Hee Young Cho, Kyo Hoon Park, Eunji Oh, Min Jung Lee, Bo Young Choi and Eun Mi Im in Innate Immunity
Supplemental material, sj-sav-3-ini-10.1177_17534259241306237 for Plasma acute phase proteins as potential predictors of intra-amniotic inflammation and infection in preterm premature rupture of membranes by Hee Young Cho, Kyo Hoon Park, Eunji Oh, Min Jung Lee, Bo Young Choi and Eun Mi Im in Innate Immunity