Abstract
Objectives:
In this study, the Kellgren and Lawrence system was used as the most common radiographic grading system for diagnosis of osteoarthritis in patients defined as Grade III and IV. It is aimed to reveal oxidative stress and cellular immunity status. In this context, the aim is to discuss possible risk parameters regarding disease process and treatment effectiveness.
Methods:
Twenty-five patients and 25 healthy individuals were included in the study. Total antioxidant, total oxidative stress, and thiol-disulfide balance values were determined spectrophotometrically in blood samples taken from individuals in the study groups. Neopterin levels were determined by the HPLC method.
Results:
In our study, total antioxidant status (TAS) values were found to be lower in the healthy control group than in the patient group (p=0.000). There was no statistically significant difference between the groups in total oxidant status (TOS) (p=0.815). The oxidative stress index (OSI) value evaluated based on TAS and TOS values did not show a statistical difference between the groups (p=0.065). The native thiol levels were determined to be statistically significantly lower in the patient group (p=0.000). But, disulfide and neopterin values were statistically significantly higher in the patient group (p=0.001 and p=0.000).
Conclusion:
According to the findings of the current study; It is observed that the oxidant balance of individuals with osteoarthritis is disrupted in favor of free radicals, and as a result, cellular immunity decreases due to inflammation and the disease process. It is observed that these parameters change in direct proportion to the staging of the disease, especially in patients with stage III-IV knee osteoarthritis.
Key Words: Cellular immunity, Osteoarthritis, Oxidative stress
Introduction
Oteoarthritis (OA) is a degenerative disease defined via damage to joint cartilage, erosion, deterioration of cartilage structure, osteophytes, subchondral sclerosis, and adverse biochemical/morphological changes in the synovial and joint capsule. 1,2 It is seen most often in the knee, hip, foot, spine, and hand joints.3 Hip and knee osteoarthritis are the main forms of arthritis with an overall prevalence of approximately 300 million worldwide in recent years. 4,5 Knee osteoarthritis is the most common arthritis lesion in orthopaedics practice, accounting for 85% of osteoarthritis worldwide.6,7 Osteoarthritis is more common in females than males and has an increasing incidence with age, being more common in individuals over 50 years of age.7 According to the Turkish Statistical Institute (TUIK) 2022 Turkey Health Research Report, the prevalence of arthrosis, which includes osteoarthritis, was determined as 4.9% in males and 11.1% in females, with 8% overall prevalence.8 Osteoarthritis affects the articular cartilage and the subchondral bone, adjacent connective tissue, and synovial membrane. The chondrocyte cells that make up the structure of cartilage are immobile and cannot self-renew. Therefore, chondrocyte death is a major factor in the pathogenesis of osteoarthritis.9 Although pain is the most prominent symptom in patients with OA, it is accompanied by symptoms such as limitation of movement, stiffness, swelling, locking, numbness, etc. 6,10 In the American College of Rheumatology (ACR) diagnostic criteria, updated in 2016, there are three categories for the diagnosis of knee osteoarthritis: clinical, clinical/radiological, and clinical/laboratory. According to the clinical symptoms, knee osteoarthritis is diagnosed in the presence of at least three of the six parameters of age over 50 years, short-term morning stiffness, crepitation in joint movements, bone tenderness, bone enlargement, and non-obvious temperature increase in the joint.11Although the cause of the emergence and development of knee osteoarthritis is not known exactly, it is thought to be affected by many factors, including systemic, local, and genetic factors, and is thought to be caused by a multifactorial combination.7,12
Osteoarthritis is linked to multiple risk factors such as age, obesity, variable mechanics, and joint trauma.13,14 The identification of biomarkers associated with OA may reveal the etiology and pathogenesis of the disease. Biochemical analysis of degenerative cartilage in patients with OA has shown an association with the down-regulation of SOD2 in the genesis and development of osteoarthritis. This suggests that SOD2-centered redox reactions play a significant role in the pathogenesis of knee osteoarthritis.15,16
Redox is a process that can promote physiological signalling reactions as well as lead to pathophysiological conditions. Oxidative stress refers to a state of imbalance that reduces the body's natural antioxidant defenses and leads to the production of more reactive oxygen species (ROS).17 High oxidative stress may increase the inflammatory response observed in patients with low antioxidant capacity.18,19 The inflammatory process of osteoarthritis begins in the synovial membrane with the activation of humoral and cellular immune. Damage-associated molecular patterns (DAMPs) released from the extracellular matrix into the joint space stimulate the production of inflammatory mediators produced by macrophages.20-22 in osteoarthritis patients, ROS production is associated with the oxidation and damage development of numerous joint components.20 Neutrophils, one of the immune cells, are widely present in inflammatory synovial fluid and can induce the production of mediators, which are highly involved in cartilage damage. In addition, ROS generated by neutrophils stimulate the production of proteolytic enzymes, one of the main actors in cartilage destruction. 2,3 Monocytes are involved in osteoarthritis as they can stimulate subchondral bone and synovium through their ability to produce cytokines and chemokines. Similarly, macrophages are another immune cell that affects the course of osteoarthritis. With both proinflammatory and anti-inflammatory activity, macrophages can inhibit chondrocyte apoptosis. 24,25
In our study, the most commonly used radiographic grading system for the diagnosis of osteoarthritis was the Kellgren and Lawrence system, in patients defined as Grades III and IV. It is aimed to reveal oxidative stress and cellular immunity status. In this context, the aim is to discuss possible risk parameters regarding disease process and treatment effectiveness.
Materials and Methods
Study Population
The number of samples was determined in line with G-power analysis, in our study.23-25 According to the power analysis performed to calculate the sample size required, 25 patients and 25 healthy individuals, were included in the study (Effect size d = 0.94, α err prob = 0.05, Power (1-β err prob) = 0.95). The patients included were those with stage III and IV knee OA diagnosed according to the Kellgren-Lawrence system (Grades III and IV) and diagnosed with primary knee osteoarthritis according to the American College of Rheumatology (ACR) criteria who presented at the Department of Orthopedics and Traumatology of Gülhane Training and Research Hospital between November and December 2023. Patients were excluded from the study if they had a history of physical therapy, pregnancy, knee surgery and prosthesis use, cancer, mental illness, received an intra-articular injection (steroid, hyaluronic acid, PRP) in the last 6 months, cognitive impairment, using immunosuppressive drugs, and obesity were aged <18 years of age. None of the patients smoked or drank alcohol.27
Blood Samples
The blood samples used in the study were serum samples taken for routine diagnosis and leftover serum samples from the triggers when the patients presented at the hospital for diagnosis and treatment. Approximately 10 mL of blood from the inner forearm (normal blood collection site) was withdrawn into an anticoagulant-free tube. The samples were centrifuged at 4000 g, and the resulting supernatant plasma was separated into centrifuge tubes using a micropipette. These were stored at - 80 °C until the study was performed.
Measurement of Cellular Immunity Marker
Neopterin values in the serum of subjects were determined using the HPLC (High-Performance Liquid Chromatography) method used by Avci et al. 2014.28
Measurement of Total Antioxidant Status (TAS) and Total Oxidant Status (TOS)
Serum TAS and TOS levels were determined with the colorimetric measurement methods of Erel et al. (2004 and 2005).29The TAS data were reported as micromole Trolox equivalents per liter (mmol Trolox Eq/L). The TOS assay was calibrated with hydrogen peroxide and the results were reported as mmol H2O2 Eq/L.30
Determination of Oxidative Stress Index (OSI)
The OSI ratio is a biomarker of the oxidative stress degree. The OSI data was calculated using the following formula:
OSI (arbitrary unit)=(TOS (µmolH2O2EQ/L)) / (TAS (µmolTroloxWq/L))×100
Determination of Thiol / Disulfide Balance
Thiol-disulfide homeostasis level as native thiol (NTL), total thiol (TTL), and disulfide was measured by the automated spectrophotometric method described by Erel and Neselıoglu (2014).30
Statistical Analysis
The data were examined using IBM SPSS 20 software. The values are given as mean and standard deviation values in the table. The conformity of the data to normal distribution
was examined with Shapiro-Wilk and Kolmogorov-Smirnov tests. Data that conformed to normal distribution were evaluated with the parametric-ANOVA test, and data not showing normal distribution with the non-parametric-Mann-Whitney U test. A value of p<0.05 was accepted as the level of statistical significance.
Results
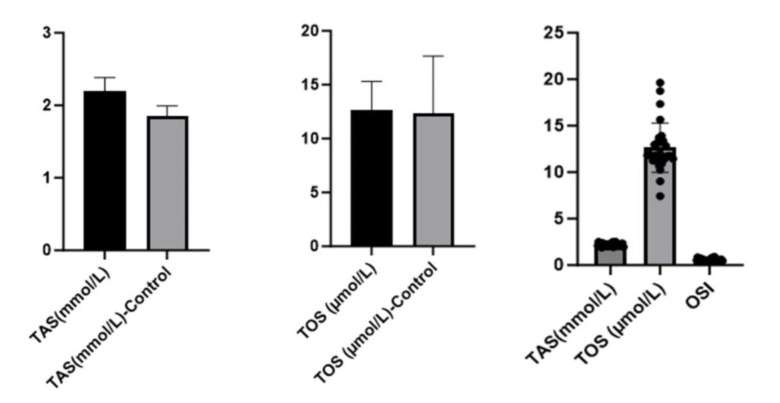
TAS values were found to be lower in the healthy control group than in the patient group (p=0.000). There was no statistically significant difference between the groups in TOS values (p=0.815). The OSI value evaluated based on TAS and TOS values did not show a statistical difference between the groups (p = 0.065) [Table 1, Figure 1].
Table 1.
Oxidative Stress and Cellular Immunity in Individuals with Knee Osteoarthritis and Healthy Control Subjects
| Parameters | Control (n=25) | Patients (n=25) | p |
|---|---|---|---|
| TAS (mmol/L) | 1.84±0.14* | 2.20±0.18* | .000*** |
| TOS (µmol/L) | 12.35±5.30 | 12.63±2.67 | .815** |
| OSI | 0.67±0.28 | 0.57±0.12 | .065** |
| TTL (µmol/L) | 577.5±78.6* | 400.4±162.3* | .000*** |
| NTL (µmol/L) | 515.81±88.89 | 259.6±124.7 | .000*** |
| Disulfide | 29.09±7.03* | 70.40±43.59* | .001*** |
| Neopterin (µmol/L) | 4.65±2.0 | 8.75±3.28 | .000*** |
*p>0.05, Parameters do not follow normal distribution according to Shapiro-Wilk test and Kolmogorov-Smirnov test (in intra-group distribution)
** p>0.05 No statistically significant difference was observed between groups
***A statistically significant difference was observed between groups (p<0.05)
Figure 1.
TAS, TOS and OSI values in both groups
In thiol studies, native thiol levels (NTL) were determined to be statistically significantly lower in the patient group (p = 0.000). The total thiol (TTL) value was found to be high in the control group and a statistically significant decrease was observed in the patient group (p=0.000). The disulfide value was statistically significantly higher in the patient group (p = 0.001) [Table 1, Figure 2]. While neopterin values were low in the control group (4.65±2.0), they were statistically significantly higher in the patient group (8.75±3.28) (p = 0.000) [Table 1, Figure 3].
Figure 2.
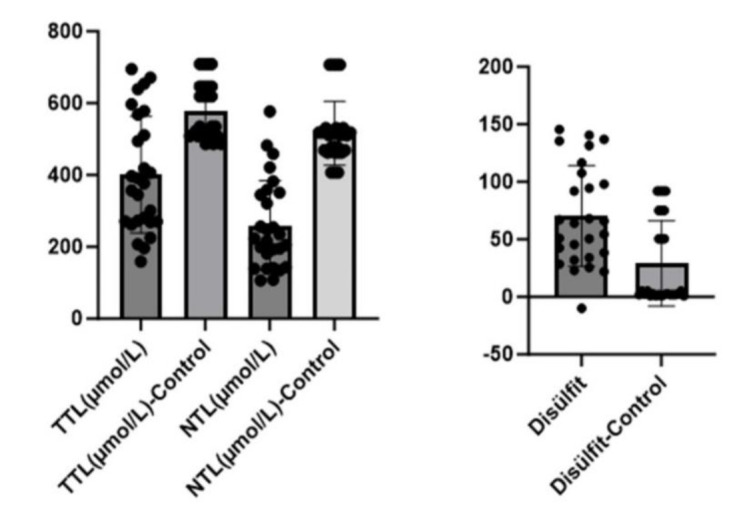
TTL, NTL and disulfide in both groups
Figure 3.
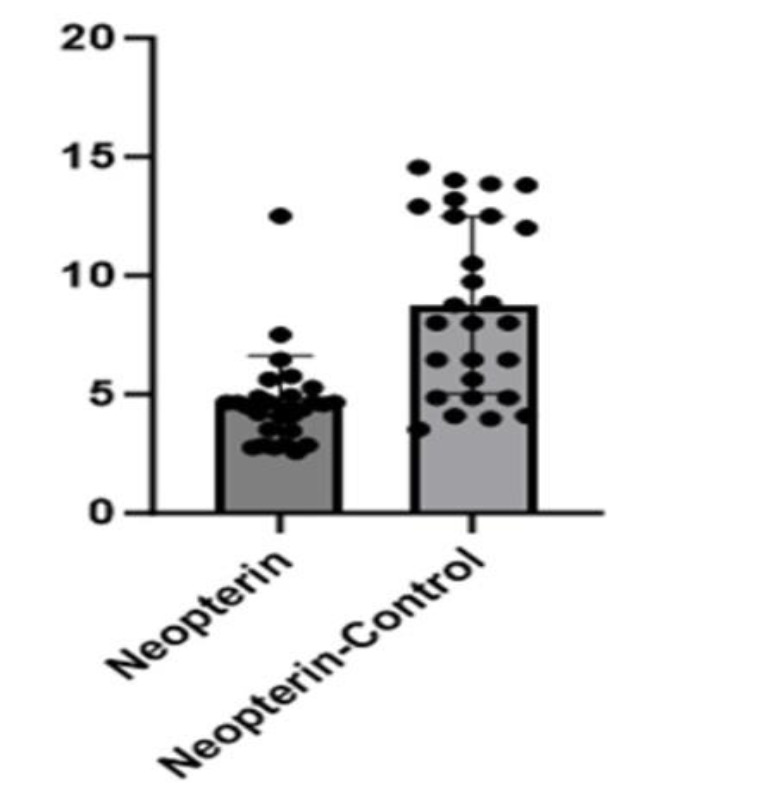
Neopterin concentration in both groups
Discussion
Osteoarthritis is a degenerative joint disease characterized by damage to articular cartilage, erosion, deterioration of cartilage structure, osteophytes, subchondral sclerosis, and biochemical/morphological changes in the synovial and joint capsule.1,2 When studies on oxidative stress and antioxidant status in osteoarthritis patients are examined, it is noteworthy that the studies are mostly in a compilation style and contain general information. In addition, there is limited information in the literature showing the relationship between oxidative stress and antioxidant status, especially in knee osteoarthritis. This study aimed to reveal the balance of TAS, TOS, thiol, and disulfide in patients with knee osteoarthritis. Concentrations of all parameters except TOS were significantly different in patients compared to healthy
controls, indicating that oxidative stress plays a role in the pathogenesis of OA. In previous studies, when oxidative stress parameters were examined in OA patients, an increase in the NO level, especially in the synovial fluid, was reported.9,10 Kariminezhad et al. (2024) showed an increase in oxidant levels and a decrease in antioxidant values in patients with knee osteoarthritis.31 In studies conducted by Ertürk et al. (2012) and Altay et al. (2015) using the same method, serum TAS and TOS measurements in knee osteoarthritis patients showed that oxidative parameters were higher than those of the control group, while antioxidative parameters did not changes.32,33 Gundogdu et al. (2024) reported that TOS and OSI levels were significantly higher and TAS values were significantly lower in patients with knee osteoarthritis.34 Although not completely in parallel with the literature, the TAS levels in the current study were significantly higher in patients with knee osteoarthritis, while no statistically significant difference was observed in TOS and OSI values. Thiols are one of the important antioxidants in the body that are sensitive to oxidative stress and organic compounds containing sulfhydryl (-SH) groups.35 ROS can transfer their excess electrons to thiol-containing compounds and cause the sulfhydryl groups contained in thiol molecules to form disulfide bonds. The bonds formed can undergo oxidation, so thiol-disulfide equilibrium has a dynamic structure. This is important in the regulation of antioxidant protection, detoxification mechanisms, syncyl transmission, apoptosis, and cellular signaling mechanisms.36 Devrimsel et al. (2021) showed that disulfide/natural thiol, disulfide, and disulfide/total thiol ratios were higher in patients with knee osteoarthritis compared to the control group.37 Ozler et al. (2022) showed a correlation between decreased native and total thiol levels and OA progression, suggesting that thiol/disulfide homeostasis can be used as a potentially useful marker for the diagnosis of early and advanced OA.38 Kariminezhad et al.(2024) reported that oxidant parameters increased while antioxidant parameters decreased in patients with osteoarthritis, indicating a relationship between knee osteoarthritis and oxidative stress.31 Consistent with the literature, a significant decrease was determined in thiol and non-thiol groups and a significant increase in disulfide levels in the current study. Considering the pathogenesis of the disease, cartilage damage increases in tissue exposed to oxidative stress more than in normal physiological conditions. To cope with oxidative stress, antioxidant mechanisms should be activated, and antioxidant molecules should be increased. This flowchart of the disease is consistent with the results of this study.
Neopterin is a biomarker produced by monocytes and macrophages in response to interferon-gamma released from T lymphocytes at the site of inflammation. Therefore, it is a molecule that can be directly associated with cellular immunity. In clinical practice, plasma, and urine neopterin concentrations are usually measured to assess the level of inflammation.39 in the present study, serum neopterin values were seen to be significantly higher in the patient group compared to the control group, suggesting that serum neopterin levels may provide valuable information about the patient's inflammation status. The data obtained in this study differ from the results of some studies in the literature. Studentova et al. (2024)found that synovial fluid neopterin levels were lower in patients with osteoarthritis.40
The limitation of our study is that the small sample size used may limit our ability to generalize the results to patients with osteoarthritis.
Conclusion
According to the findings of the current study; it is observed that the oxidant balance of individuals with osteoarthritis is disrupted in favor of free radicals, and as a result, cellular immunity decreases due to inflammation and the disease process. It is observed that these parameters change in direct proportion to the staging of the disease, especially in patients with stage III-IV knee osteoarthritis. However, it should be taken into consideration that the data obtained cannot be generalized to all osteoarthritis cases.
Acknowledgement
N/A
Authors Contribution:
Mustafa Aydın: Conceived, designed, and performed analysis; collected data, wrote paper. Gulcin Alp Avcı: Collected data, contributed data or analysis tools, performed analysis. Ulku Irem Yılmaz: Collected data, contributed data or analysis tools. Tugba Aydın: Literature search. Emre Avcı: Supervised study, conceived and designed analysis, wrote paper, reviewed final draft of manuscript.
Declaration of Conflict of Interest:
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Declaration of Funding:
The authors received no financial support for the research and/or authorship of this article.
Declaration of Ethical Approval for Study:
This study was approved by the Clinical Research Ethics Committee of Gülhane Training and Research Hospital (decision no: 2023/275, dated: 22.11.2023), and all the study subjects participated voluntarily. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Declaration of Informed Consent:
The authors declared that there is no information (names, initials, hospital identification numbers, or photographs) in the submitted manuscript that can be used to identify patients.
References
- 1.Ansari MY, Ahmad N, Haqqi TM. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed Pharmacother. 2020;129:110452. doi: 10.1016/j.biopha.2020.110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yazdi MM, Jamalaldini MH, Sobhan MR, et al. Association of ESRα Gene Pvu II T>C, XbaI A>G and BtgI G>A Polymorphisms with Knee Osteoarthritis Susceptibility: A Systematic Review and Meta-Analysis Based on 22 Case-Control Studies. Arch Bone Jt Surg. 2017;5(6):351–362. [PMC free article] [PubMed] [Google Scholar]
- 3.Gierman LM, van El B, van der Ham F, et al. Profiling the Secretion of Soluble Mediators by End Stage Osteoarthritis Synovial Tissue Explants Reveals a Reduced Responsiveness to an Inflammatory Trigger. PLoS One. 2013;8:5. doi: 10.1371/journal.pone.0062634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cieza A, Causey K, Kamenow K, Wulf Hansen S, Chatterji S, Vos T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10267):2006–2017. doi: 10.1016/S0140-6736(20)32340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safiri S, Kolahi AA, Smith E, et al. Global, regional and national burden of osteoarthritis 1990-2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79(6):819–828. doi: 10.1136/annrheumdis-2019-216515. [DOI] [PubMed] [Google Scholar]
- 6.Abramoff B, Caldera FE. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med Clin North Am. 2020;104(2):293–311. doi: 10.1016/j.mcna.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Du X, Liu Z, Tao X, et al. Research Progress on the Pathogenesis of Knee Osteoarthritis. Orthop Surg. 2023;15(9):2213–2224. doi: 10.1111/os.13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turkish Statistical Institute (TUIK) Turkey Health Research Report. Available at: https://www.tuik.gov.tr/Home/Index.2022.
- 9.Zahan OM, Serban O, Gherman C, Fodor D. The evaluation of oxidative stress in osteoarthritis. Med Pharm Rep. 2020;93(1):12–22. doi: 10.15386/mpr-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perumal S, Mayilsamy S, Thangaraj S. Rheumatological Perspective of Osteoarthritis and Their Common Clinical Presentations from Patients Who Are Attending Teaching Hospital. Naturalista Campano. 2024;28(1):1999–2025. [Google Scholar]
- 11.Abari IS, Salehi-Abari I. 2016 ACR Revised Criteria for Early Diagnosis of Knee Osteoarthritis Autoimmune Diseases and Therapeutic 2016 ACR Revised Criteria for Early Diagnosis of Knee Osteoarthritis. Autoimmune Dis Ther Approaches. 2016;3(1):118 . [Google Scholar]
- 12.Perico DA, Uribe AC, Niño SJ, et al. A proposed modification to the Kellgren and Lawrence classification for knee osteoarthritis using a compartment-specific approach. J ExpOrthop. 2024;11(1):e12008. doi: 10.1002/jeo2.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukherjee A, Das B. The role of inflammatory mediators and matrix metalloproteinases (MMPs) in the progression of osteoarthritis. Biomater Biosyst. 2024;13:100090. doi: 10.1016/j.bbiosy.2024.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Merchan EC, De la Corte-Rodriguez H, Roman-Belmonte JM. The Effect of Biomechanical Footwear on Pain from Knee Osteoarthritis. Arch Bone Jt Surg. 2022;10(5):381–384. doi: 10.22038/ABJS.2021.55417.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grässel S, Muschter D. Recent advances in the treatment of osteoarthritis. F1000Res. 2020;9:F1000 Faculty Rev–325. [Google Scholar]
- 16.He Y, Li Z, Alexander PG, et al. Pathogenesis of Osteoarthritis: Risk Factors, Regulatory Pathways in Chondrocytes, and Experimental Models. Biology (Basel) 2020;9(8):194. doi: 10.3390/biology9080194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Luo P, Yang M, Wang J, Hou W, Xu P. The role of oxidative stress in the development of knee osteoarthritis: A comprehensive research review. Front Mol Biosci. 2022;9:1001212. doi: 10.3389/fmolb.2022.1001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scarian E, Viola C, Dragoni F, et al. New Insights into Oxidative Stress and Inflammatory Response in Neurodegenerative Diseases. Int J Mol Sci. 2024;25(5):2698. doi: 10.3390/ijms25052698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung HY, Kim DH, Lee EK, et al. Redefining chronic inflammation in aging and age-related diseases: Proposal of the senoinflammation concept. Aging Dis. 2019;10(2):367–382. doi: 10.14336/AD.2018.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan Z, Jiang D, Yang M, et al. Emerging Roles of Macrophage Polarization in Osteoarthritis: Mechanisms and Therapeutic Strategies. Orthop Surg. 2024;16(3):532–550. doi: 10.1111/os.13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manukyan G, Gallo J, Mikulkova Z, et al. Phenotypic and functional characterisation of synovial fluid-derived neutrophils in knee osteoarthritis and knee infection. Osteoarthritis Cartilage. 2023;31(1):72–82. doi: 10.1016/j.joca.2022.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Lee HR, Lee S, Yoo IS, et al. CD14+ monocytes and soluble CD14 of synovial fluid are associated with osteoarthritis progression. Arch Rheumatol. 2022;37(3):335–343. doi: 10.46497/ArchRheumatol.2022.9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Chu Y, Zhang P, et al. Targeting macrophage polarization as a promising therapeutic strategy for the treatment of osteoarthritis. Int Immunopharmacol. 2023;116:109790. doi: 10.1016/j.intimp.2023.109790. [DOI] [PubMed] [Google Scholar]
- 25.Maneesh M, Jayalekshmi H, Suma T, et al. Evidence for oxidative stress in osteoarthritis. Indian J Clin Biochem. 2005;20(1):129–30. doi: 10.1007/BF02893057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suantawee T, Tantavisut S, Adisakwattana S, et al. Oxidative Stress, Vitamin E, and Antioxidant Capacity in Knee Osteoarthritis. J Clin Diagn Res. 2013;7(9):1855–9. doi: 10.7860/JCDR/2013/5802.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paździor M, Kiełczykowska M, Kurzepa J, Luchowska-Kocot D, Kocot J, Musik I. The Oxidative Stress in Knee Osteoarthritis Patients An Attempt of Evaluation of Possible Compensatory Effects Occurring in the Disease Development. Medicina (Kaunas) 2019;55(5):150. doi: 10.3390/medicina55050150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avci E, Çakir E, Cevher SC, Yaman H, Agilli M, Bilgi C. Determination of oxidative stress and cellular inflammation in patients with diabetic nephropathy and non-diabetic nephropathy being administered hemodialysis treatment due to chronic renal failure. Ren Fail. 2014;36(5):767–773. doi: 10.3109/0886022X.2014.890841. [DOI] [PubMed] [Google Scholar]
- 29.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37(4):277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Erel O, Neselioglu s. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47(18):326–32. doi: 10.1016/j.clinbiochem.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 31.Kariminezhad Z, Rahimi M, Fernandes J, et al. Development of New Resolvin D1 Analogues for Osteoarthritis Therapy: Acellular and Computational Approaches to Study Their Antioxidant Activities. Antioxidants (Basel) 2024;13(4):386. doi: 10.3390/antiox13040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ertürk C, Altay MA, Selek Ş, Koçyigit A. Paraoxonase-1 activity and oxidative status in patients with knee osteoarthritis and their relationship with radiological and clinical parameters. Scand J Clin Lab Invest. 2012;72(5):433–439. doi: 10.3109/00365513.2012.687116. [DOI] [PubMed] [Google Scholar]
- 33.Altay MA, Ertürk C, Bilge A, Yaptı M, Levent A, Aksoy N. Evaluation of prolidase activity and oxidative status in patients with knee osteoarthritis: relationships with radiographic severity and clinical parameters. Rheumatol Int. 2015;35(10):1725–1731. doi: 10.1007/s00296-015-3290-5. [DOI] [PubMed] [Google Scholar]
- 34.Gundogdu G, Kilic-Erkek O, Gundogdu K. The impact of sericin on inflammation, oxidative stress, and lipid metabolism in female rats with experimental knee osteoarthritis. Clin Rheumatol. 2024;43(7):2307–2316. doi: 10.1007/s10067-024-06987-4. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Zhang Y, Tang Q, Zhang Y, Yin Y, Chen L. Application of Antioxidant Compounds in Bone Defect Repair. Antioxidants. 2024;13(7):789. doi: 10.3390/antiox13070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Q, Yin W, Wang H, Gao J, Gu Y, Wang W, Liu C, Pan G, Li B. Dynamic Proteinaceous Hydrogel Enables In-Situ Recruitment of Endogenous TGF-β1 and Stem Cells for Cartilage Regeneration. Advanced Functional Materials. 2024:2403055 . [Google Scholar]
- 37.Devrimsel G, Arpa M, Beyazal S, Erel O. Assessment of Thiol/Disulphide Homeostasis in Patients with Knee Osteoarthritis. Indian J Biochem Bioph. 2021 :21–26. [Google Scholar]
- 38.Ozler K, Erel O, Gokalp O, Avcioglu G, Neselioglu S. Is there a relationship between dynamic thiol/disulfide homeostasis and osteoarthritis progression? Arch Physiol Biochem. 2022;128(2):431–437. doi: 10.1080/13813455.2019.1689274. [DOI] [PubMed] [Google Scholar]
- 39.Inno G, Takahashi Y, Naruko T, et al. Enhanced expression of neopterin in valve tissue of bicuspid aortic stenosis. J Thorac Dis. 2024;16(1):191–200. doi: 10.21037/jtd-23-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Studentova H, Hola K, Melichar B, Spisarova M. Neopterin as a potential prognostic and predictive biomarker in metastatic renal cell carcinoma treated with immune checkpoint inhibitors. Expert Rev Anticancer Ther. 2024;24(6):339–345. doi: 10.1080/14737140.2024.2341734. [DOI] [PubMed] [Google Scholar]