Abstract
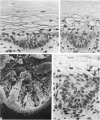
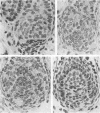
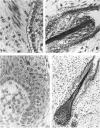
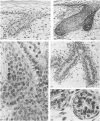
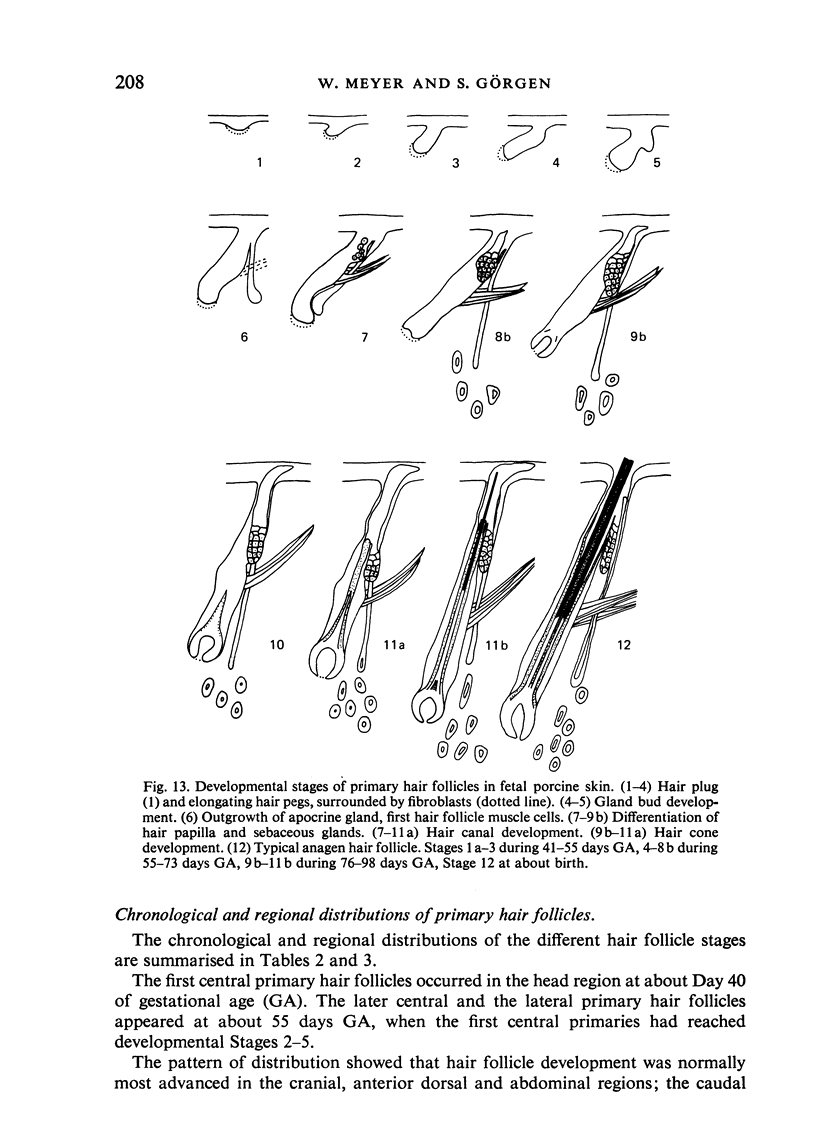
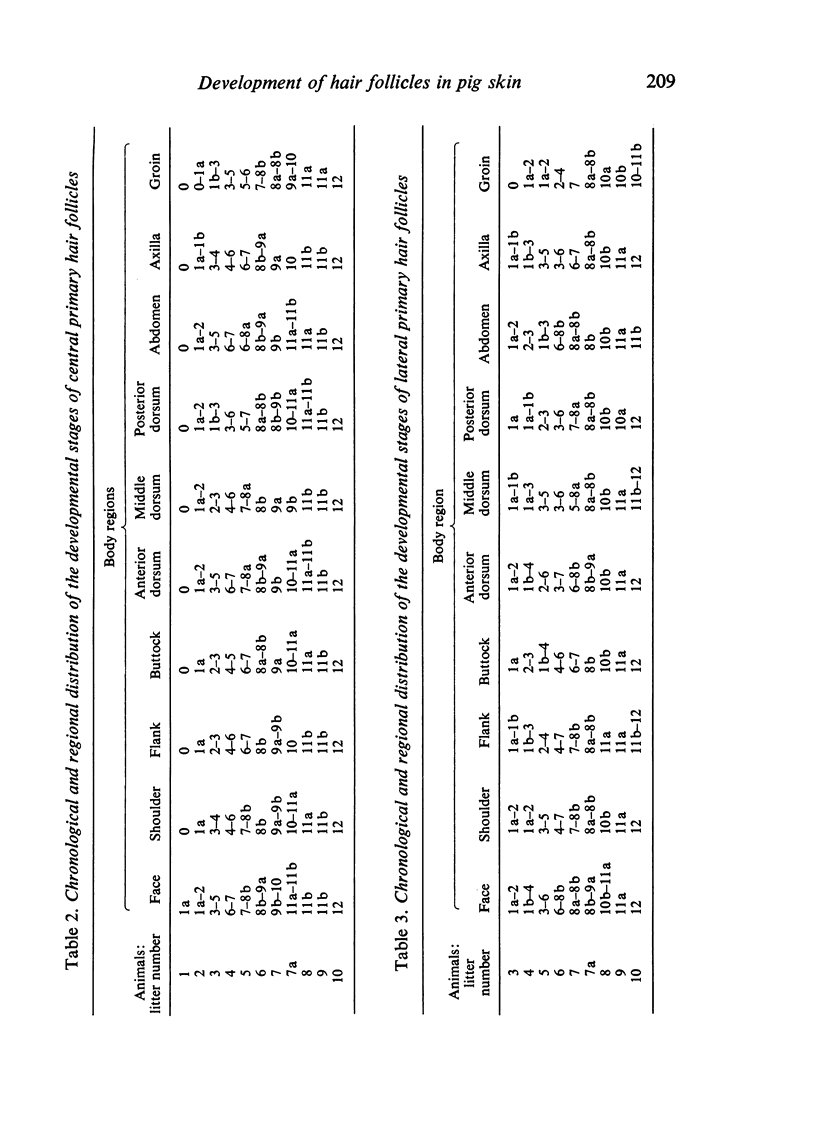
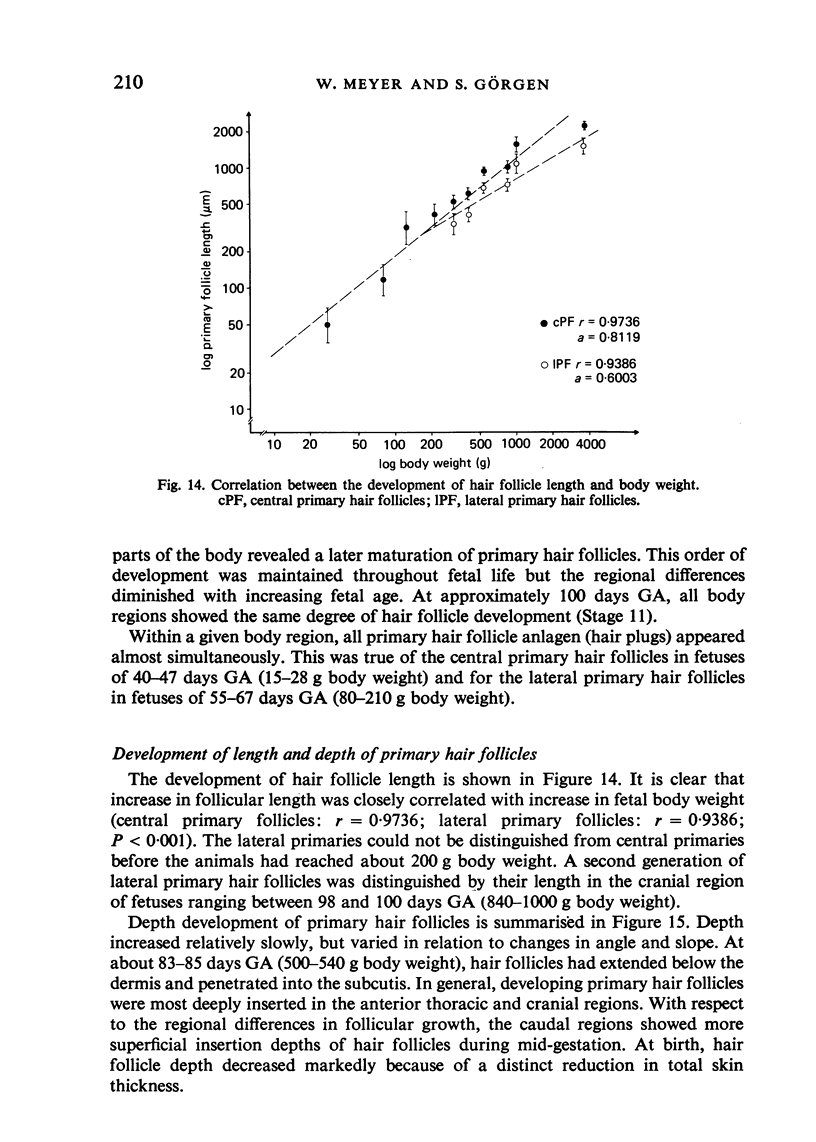
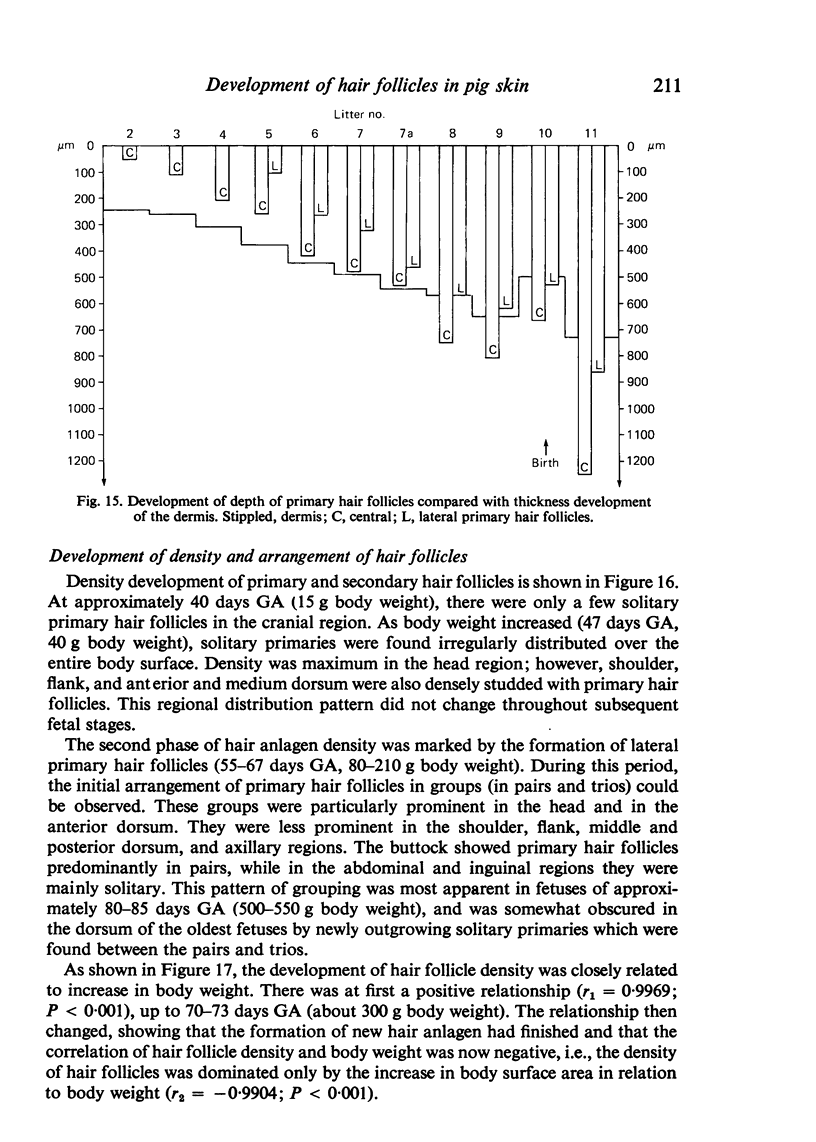
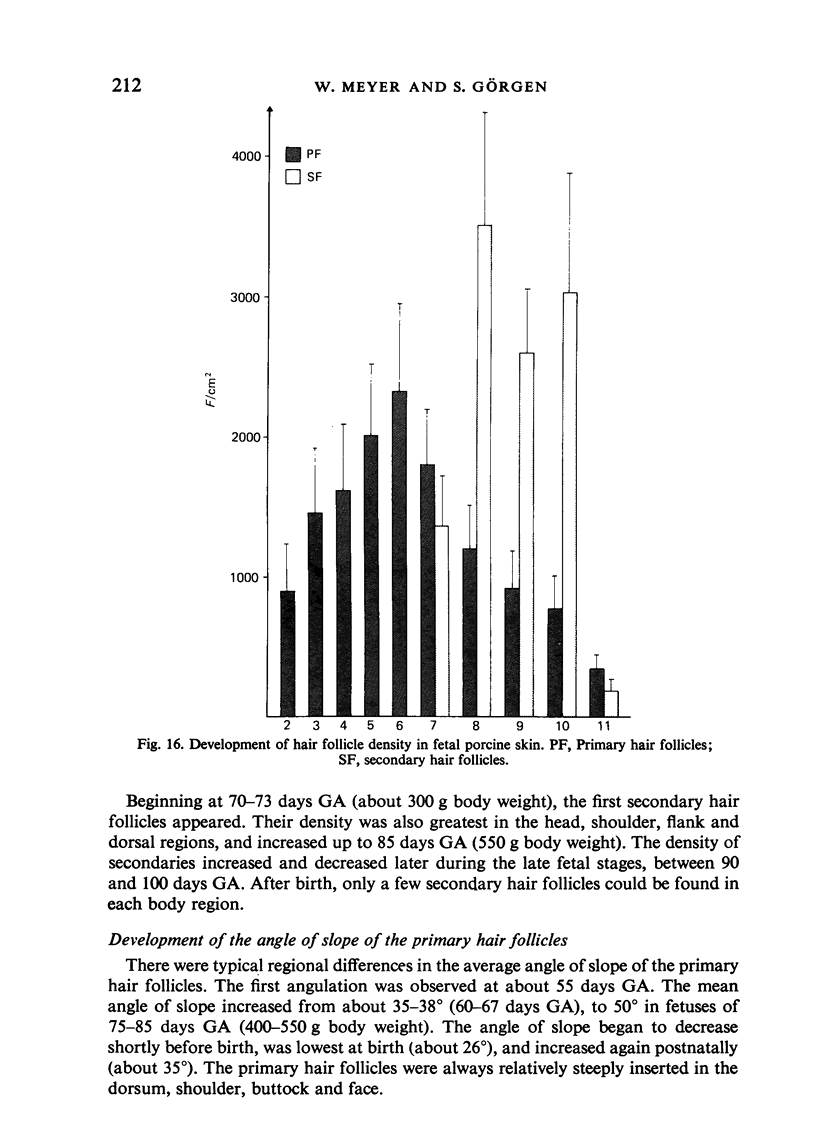
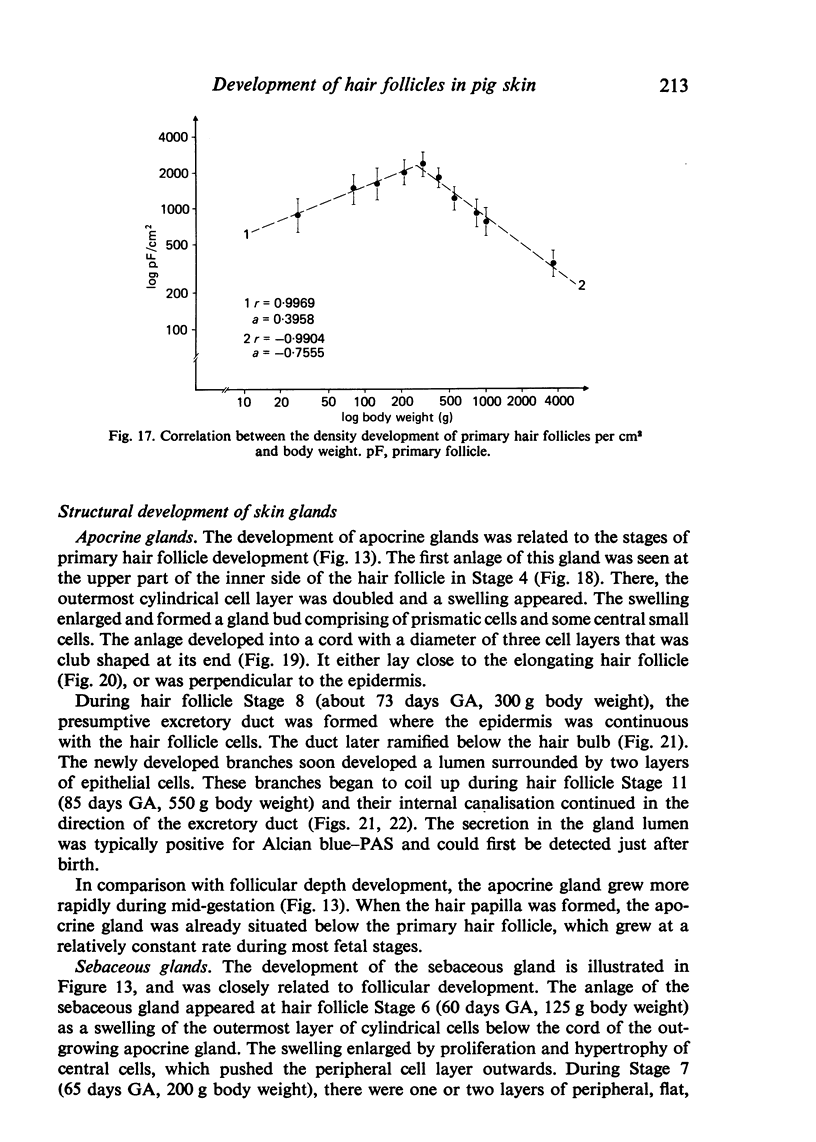
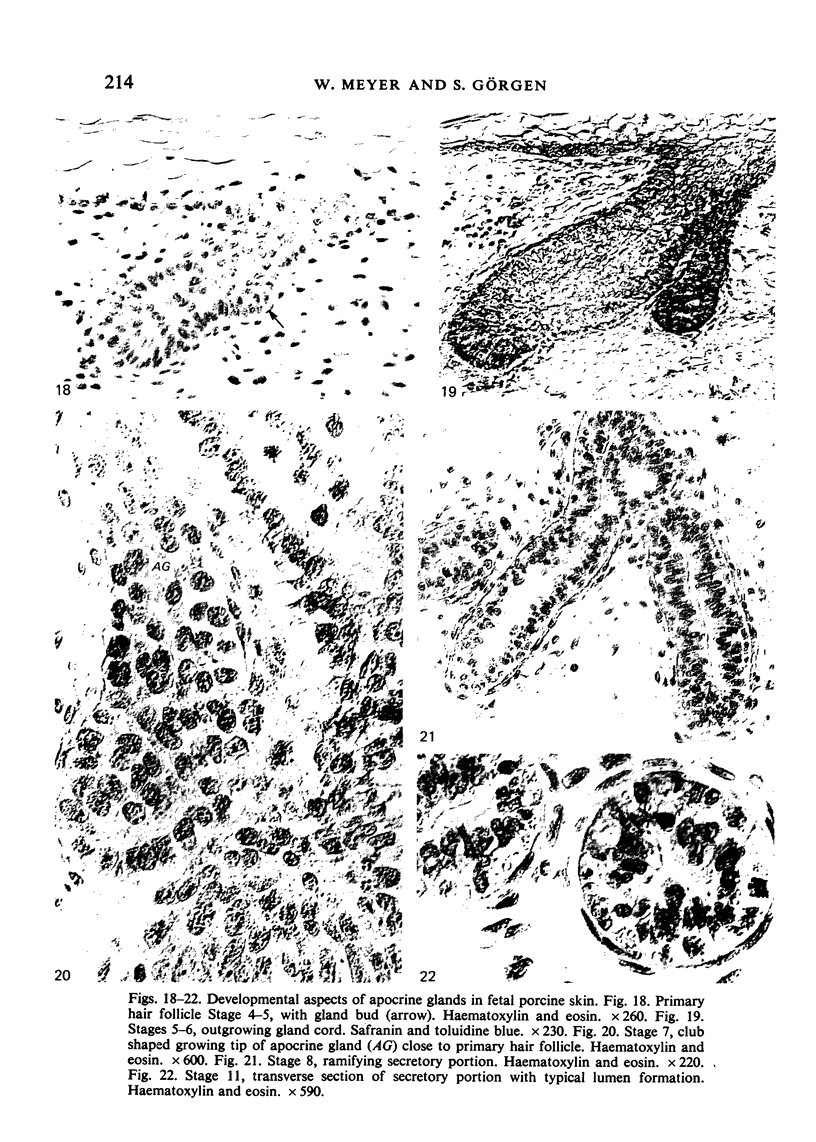
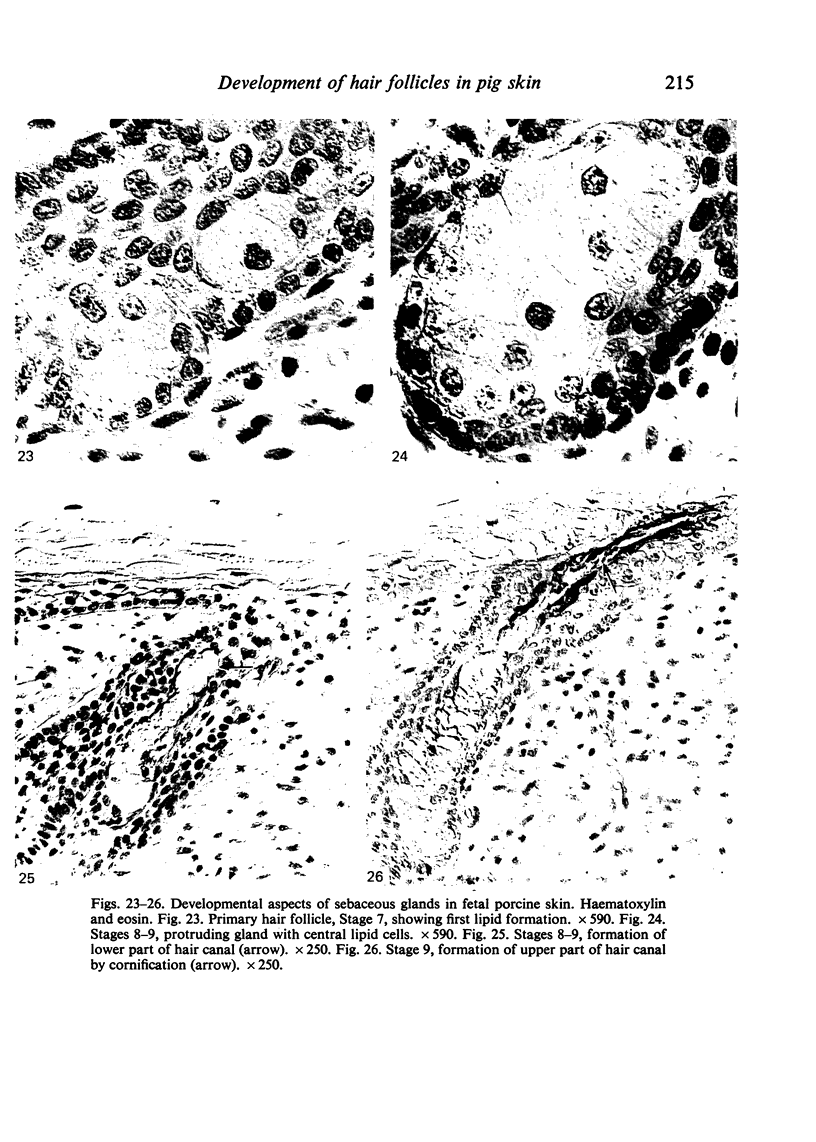
The prenatal development of the hair coat and skin glands was studied in skin samples of ten body regions of porcine fetuses (German Landrace) ranging between 40 and 114 days (birth) of gestation (15-1000 g body weight), using light microscopy and scanning electron microscopy. During the development of hair follicles, central and lateral primary hair follicles as well as secondary hair follicles can be distinguished. Initiation of primary hair follicles begins at 40-41 days of gestational age (15 g BW) and has finished at 73 days (300 g BW). The structural development of the primary hair follicle is described in detail, and is divided into twelve stages, in general agreement with those of other mammals. The secondary hair follicles formed later remain rudimentary and disappear during the birth period. The length, depth, density and angle of slope of the primary hair follicles, and the depth of the apocrine skin glands were measured. The development of hair follicle length is closely correlated with the increase in body weight. There is a similar but changing correlation for the development of hair follicle density, especially in connection with hair follicle initiation. In the development of the apocrine skin glands, a branching of the secretory tubule is conspicuous. Secretion does not begin before birth. The development of the sebaceous glands is closely related to hair follicle maturation, i.e. the formation of the first hair canal. The results are discussed in relation to findings from corresponding studies in small laboratory animals, other domestic mammals and man.
Full text
PDF
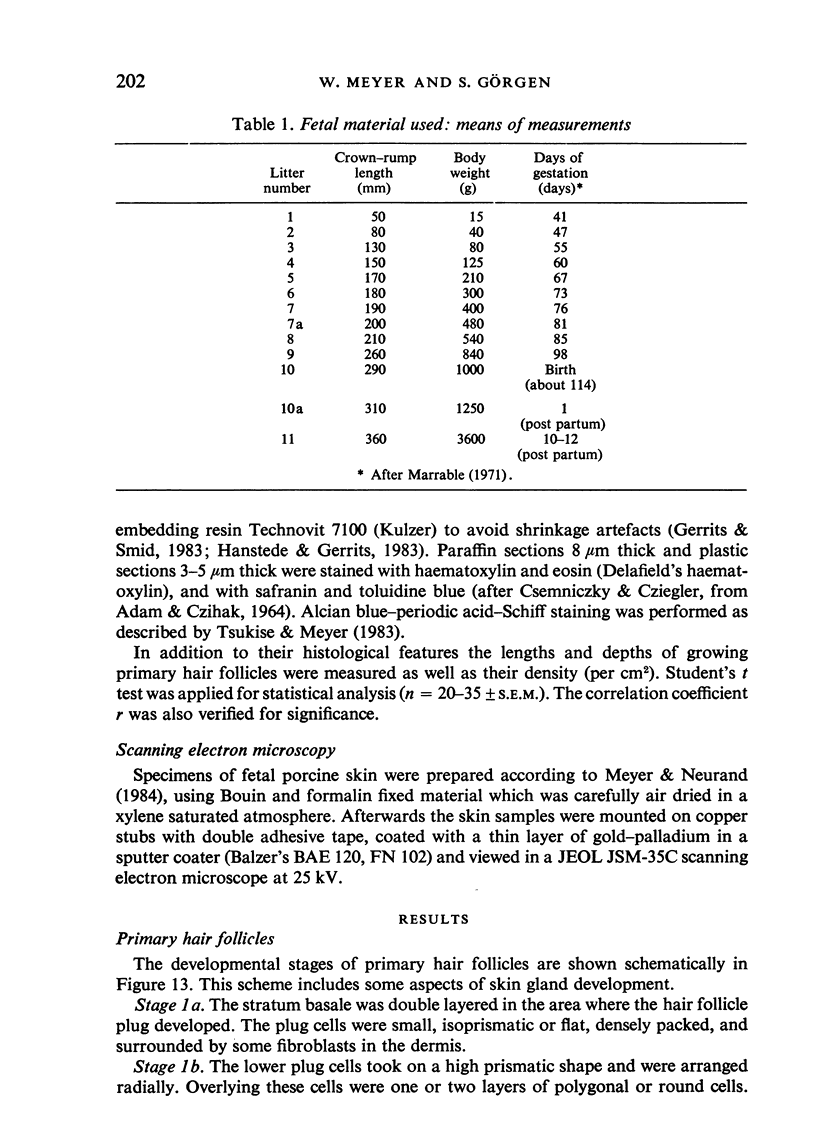
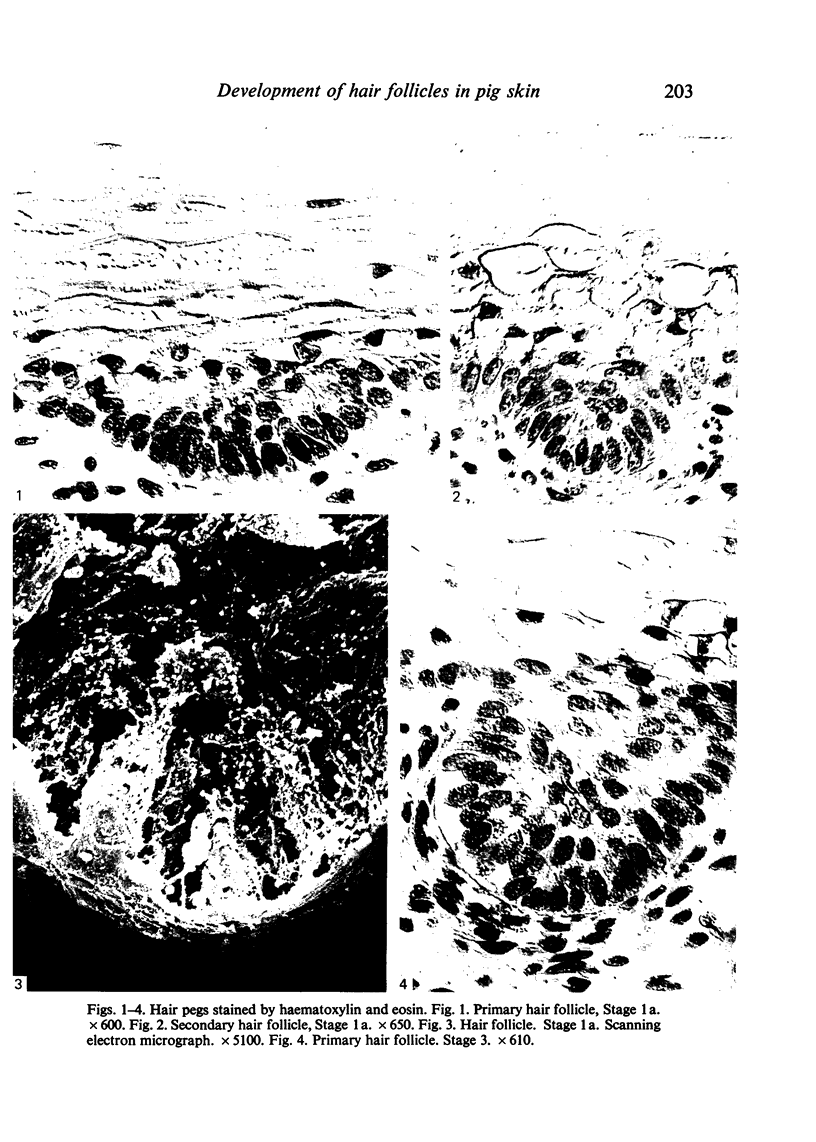
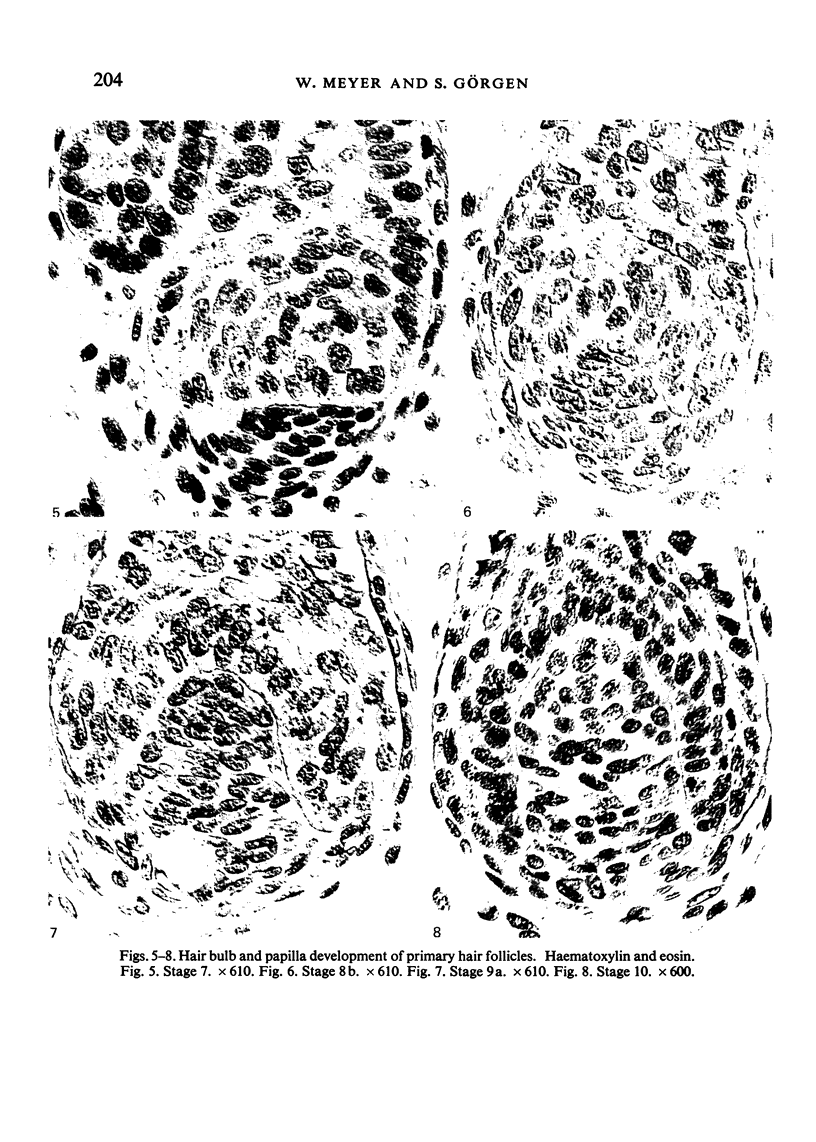








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER E. O. Development of the piliary system and the replacement of hair in mammals. Ann N Y Acad Sci. 1951 Mar;53(3):508–516. doi: 10.1111/j.1749-6632.1951.tb31953.x. [DOI] [PubMed] [Google Scholar]
- DAVIDSON P., HARDY M. H. The development of mouse vibrissae in vivo and in vitro. J Anat. 1952 Oct;86(4):342–356. [PMC free article] [PubMed] [Google Scholar]
- Dougbag A. S., Berg R. The development of the sweat and sebaceous glands in the skin of the one-humped camel (Camelus dromedarius). Z Mikrosk Anat Forsch. 1983;97(5):894–902. [PubMed] [Google Scholar]
- Dougbag A. S., Berg R. The prenatal development of hair follicles and hair in the one-humped camel (Camelus dromedarius). Z Mikrosk Anat Forsch. 1983;97(5):886–893. [PubMed] [Google Scholar]
- FLEISCHHAUER K. Uber die Entstehung der Haaranordnung und das Zustandekommen räumlicher Beziehungen zwischen Haaren und Schweissdrüsen. Z Zellforsch Mikrosk Anat. 1953;38(4):328–355. [PubMed] [Google Scholar]
- FOWLER E. H., CALHOUN M. L. THE MICROSCOPIC ANATOMY OF DEVELOPING FETAL PIG SKIN. Am J Vet Res. 1964 Jan;25:156–164. [PubMed] [Google Scholar]
- Gerrits P. O., Smid L. A new, less toxic polymerization system for the embedding of soft tissues in glycol methacrylate and subsequent preparing of serial sections. J Microsc. 1983 Oct;132(Pt 1):81–85. doi: 10.1111/j.1365-2818.1983.tb04711.x. [DOI] [PubMed] [Google Scholar]
- Hanstede J. G., Gerrits P. O. The effects of embedding in water-soluble plastics on the final dimensions of liver sections. J Microsc. 1983 Jul;131(Pt 1):79–86. doi: 10.1111/j.1365-2818.1983.tb04233.x. [DOI] [PubMed] [Google Scholar]
- Holbrook K. A., Odland G. F. Structure of the human fetal hair canal and initial hair eruption. J Invest Dermatol. 1978 Dec;71(6):385–390. doi: 10.1111/1523-1747.ep12556818. [DOI] [PubMed] [Google Scholar]
- Lyne A. G., Hollis D. E. The structure and development of the epidermis in sheep fetuses. J Ultrastruct Res. 1972 Mar;38(5):444–458. doi: 10.1016/0022-5320(72)90082-2. [DOI] [PubMed] [Google Scholar]
- MONTAGNA W. Histology and cytochemistry of human skin. XIX. The development and fate of the axillary organ. J Invest Dermatol. 1959 Sep;33:151–161. doi: 10.1038/jid.1959.135. [DOI] [PubMed] [Google Scholar]
- Robins E. J., Breathnach A. S. Fine structure of bulbar end of human foetal hair follicle at stage of differentiation of inner root sheath. J Anat. 1970 Jul;107(Pt 1):131–146. [PMC free article] [PubMed] [Google Scholar]
- TAKAGI S., TAGAWA M. A cytological and cytochemical study of the sweat gland of the horse. Jpn J Physiol. 1959 Jun 25;9(2):153–159. doi: 10.2170/jjphysiol.9.153. [DOI] [PubMed] [Google Scholar]
- Tsukise A., Meyer W. Histochemistry of complex carbohydrates in the hairy skin of the domestic pig. Histochem J. 1983 Sep;15(9):845–860. doi: 10.1007/BF01011825. [DOI] [PubMed] [Google Scholar]