Abstract
Background
Lateral lymph node dissection (LLND) for locally advanced rectal cancer (LARC) is performed widely since it reduces local recurrence. However, there are some disadvantages to LLND, including technical difficulties and association with postoperative urinary dysfunction. Procedures for LARC have also become more minimally invasive: laparoscopic surgery (LS) has become more common, and use of robot-assisted LS (RALS) is increasing. The purpose of this study is to assess differences in postoperative urinary dysfunction after LLND for LARC between LS and RALS, and to identify risk factors for postoperative urinary dysfunction.
Methods
The subjects were 100 patients with LARC (≥ cT3) with the inferior border of the tumor reaching the peritoneal reflection who underwent LS or RALS with LLND between 2009 and 2023 at Juntendo University Hospital. After LLND, the urinary catheter was usually removed on or before postoperative day 5. The duration of urinary catheterization (DUC) was used to evaluate postoperative urinary dysfunction. The standard (S) and long-term (L) groups were defined as cases with urinary catheter removal at ≤ 5 and > 5 days, respectively. DUC was examined for LS vs. RALS and clinicopathological factors were identified that adversely affect DUC.
Results
Of the 100 subjects, 72 underwent LS and 28 received RALS. LLND was bilateral in 65 cases and unilateral in 35 cases. The median DUC was 5 days, with 74 cases in group S and 26 in group L. The most frequent postoperative complication (Clavien-Dindo Grade 2 or higher) was urinary dysfunction, followed by ileus and surgical site infection (SSI), and none differed by procedure (LS vs. RALS). Univariate analysis showed significant differences in LLND laterality (p = 0.02) and SSI (p = 0.04) between groups S and L. In multivariate analysis, bilateral LLND (p < 0.01, HR 7.37) and SSI (p = 0.01, HR 15.36) were independent factors that worsened DUC.
Conclusions
There was no difference in urinary dysfunction after LLND between LS and RALS. Bilateral LLND and SSI were risk factors for lengthening DUC. Compared to bilateral LLND, unilateral LLND can reduce urinary dysfunction; therefore, selective LLND, which is overwhelmingly unilateral LLND, and prevention of perioperative SSI may be important for maintenance of urinary function.
Keywords: Lateral lymph node resection, Urinary dysfunction, Removal of urinary catheter, Duration of urinary catheterization, Robot-assisted laparoscopic surgery, Laparoscopic surgery, Rectal cancer
Background
The incidence of lateral lymph node (LLN) metastasis in locally advanced rectal cancer (LARC) is 14.5–19.9% [1, 2]. The strategy for treatment of LLN metastasis differs between Western countries and Japan. In the West, LLN metastases are considered to be distant metastases outside the rectal region, so the treatment strategy for LARC is neoadjuvant chemoradiotherapy (NACRT) followed by total mesorectal excision (TME). In contrast, lateral lymph node dissection (LLND) is often performed in Japan because the LLNs are considered to be lymph nodes included in the lower rectum [3–5]. Therefore, LLND has become common in advanced rectal cancer surgery in Japan [6, 7].
The advantages of LLND include reduction of local recurrence, by an estimated 5–6% in prophylactic LLND for clinical Stage II/III cases [8, 9], and a possible improvement in prognosis [10, 11]. However, postoperative complications (POCs) associated with LLND cannot be overlooked. In particular, from the viewpoint of quality of life (QOL), urinary dysfunction after LLND is an important POC. Prolonged insertion of a urinary catheter due to urinary dysfunction can decrease QOL, prolong the postoperative hospital stay, and cause adverse economic effects.
Rectal cancer surgery has become more minimally invasive in recent years. Laparoscopic surgery (LS) is now performed in many centers, and the number of cases treated with robot-assisted laparoscopic surgery (RALS) has also increased [12, 13]. With the shift from LS to RALS, it is important to ascertain whether urinary dysfunction after LLND has increased or decreased. Reinsertion of the urinary catheter is a Grade 1 POC in the Clavien-Dindo (CD) classification [14], whereas we focused on the duration of urinary catheterization (DUC) after LLND because the presence of the urinary catheter postoperatively is an important factor for QOL. As far as we are aware, DUC after LLND has not been previously examined.
In this study, a retrospective review of cases of LLND for LARC was performed to examine possible differences in the surgical results and the rates of POCs, including urinary dysfunction, after LLND in LS and RALS. A further aim was to identify clinicopathologic factors that cause urinary dysfunction after LLND, based on their effect on lengthening of DUC.
Methods
Patients
The subjects were 113 patients who underwent primary tumor resection and LLND for LARC via minimally invasive surgery (MIS) (LS and RALS) between December 2009 and December 2023 at Juntendo University Hospital. Bilateral and unilateral LLND cases were included. Cases with duplicate cancers, multiple resections of other organs, LLND without resection of the primary lesion, and total pelvic exenteration were excluded (Fig. 1). Those with a urethral-related urological procedure were also excluded because these patients required a urinary catheter for a long period of time (n = 2). After LLND, the urinary catheter is generally removed on postoperative day (POD) 5, and thus, cases (n = 7) in which the catheter was removed on POD6 or later for no particular reason were also excluded from the study.
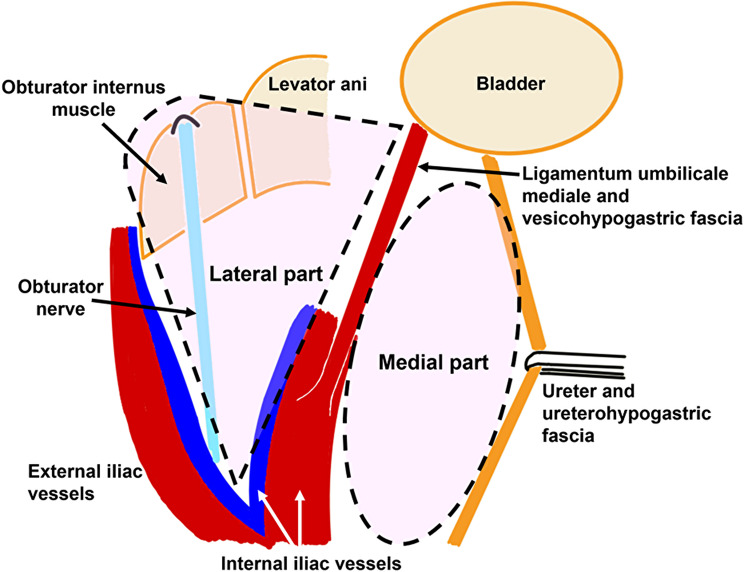
Fig. 1.
Schema of lateral lymph node (left side) is shown. The area to be dissected is recognized as medial and lateral to the internal iliac vessels. In the medial part, the ureterohypogastric fascia and the vesicohypogastric fascia are detached. In the lateral part, obturator internus muscle and obturator nerve are exposed
The indications for LLND have changed over time. Until around 2020, prophylactic bilateral LLND was performed for cases of clinical LARC (≥ cT3) [15], where the lower border of the tumor is located distal to the peritoneal reflection. However, prophylactic LLND was omitted when the risk of developing POCs was judged to be high based on age, physical condition, comorbidities, and frailty. Since around 2021, selective LLND has been performed for cases with clinically positive LLN metastasis. Diagnosis of LN metastasis is based on pelvic computed tomography (CT) with 1-mm slices or pelvic magnetic resonance imaging (MRI) with 3-mm slices; LNs are diagnosed as positive if the short axis diameter is ≥ 7 mm [5]. The indications for preoperative treatment (total neoadjuvant therapy (TNT)/ NACRT/ neoadjuvant chemotherapy (NAC)) include advanced rectal cancer (≥ T3) [15], where the inferior margin of the tumor reaches the peritoneal reflection; LNs in the rectal area clinically suspected to be positive for metastasis; and no contraindications to preoperative treatment. TNT was introduced in our department in 2023.
This retrospective analysis was approved by the Institutional Review Board of Juntendo University (approval number: H19-0214) and was performed in accordance with the Declaration of Helsinki. Due to the retrospective nature of this study, the requirement for informed consent was waived and an opt-out method was used.
Surgical approach
In our department, laparoscopic TME for rectal cancer was started in 1997, and robotic TME in 2015, while laparoscopic TME + LLND began in 2009 and robotic TME + LLND in 2018. Since 2018, RALS (da Vinci Xi surgical system™, Intuitive Surgical, Inc., Sunnyvale, CA, USA) has basically been in use for rectal cancer surgery with TME + LLND, but LS is used if RALS is not available. Since 2018, TME + LLND was performed in 37 RALS cases (77%) and 11 LS cases (23%).
Surgical procedures
LS and RALS were started with a median approach, followed by LN dissection around the inferior mesenteric artery (IMA) and ligation and dissection of the IMA. TME of the rectum was then performed, and the rectum was detached from the pelvis. The autonomic nerves (hypogastric nerve and pelvic nerve) were preserved without ligation or transection. In cases of low anterior resection (LAR) and Hartmann’s procedure, the distal part of the rectum was transected intracorporeally, followed by LLND. For intersphincteric resection (ISR) and abdominoperineal resection (APR), LLND was performed following TME, followed by a perineal procedure. In LLND, we consider the areas of LLND to be two main parts (Fig. 1, left side of LLND): medial part and lateral part of internal iliac vessels. The ureter is first identified and the ureterohypogastric fascia is dissected. The ligamentum umbilicale mediale is then identified and retracted, and the vesicohypogastric fascia is dissected. These maneuvers result in the dissection of the medial part of internal iliac vessels. Next, the dissection proceeds to expose the psoas major and obturator internus muscles, while verifying and preserving the obturator nerve. These maneuvers result in the dissection of the lateral part of internal iliac vessels. As a result, 263P (proximal area comprising internal iliac LNs), 263D (distal area comprising internal iliac LNs), and 283 (comprising obturator LNs) were resected [3, 16]. As mentioned previously, the indications for LLND changed around 2021, but the extent of lymph node dissection did not change. Basically, the obturator nerves were preserved, and the obturator vessels were often resected. If enlarged lymph nodes were close to nearby vessels, including the inferior vesical vessels, they were also resected.
Removal of the urinary catheter after surgery
In our department, the urinary catheter is usually removed on POD1 or POD2 if LLND is not performed, and typically on POD5 if LLND is performed, either bilaterally or unilaterally. The duration of urinary catheterization (DUC) was used as a measure of urinary dysfunction. If the urinary catheter was removed and then reinserted after urinary dysfunction occurred, the DUC was defined as the total catheterization time. Since catheters are typically removed on POD5, cases with catheterization for > 5 and ≤ 5 days were defined as the long-term (L) and standard (S) urinary catheterization groups, respectively.
An algorithm for removal of the urinary catheter is shown in Fig. 2. In principle, the urinary catheter is removed on POD5, and residual urine is measured if urinary dysfunction, such as difficulty in urinating, is subsequently observed. In this study, urinary dysfunction was defined as a condition in which the patient has the urge to urinate, but has difficulty in urinating, and has discomfort, fullness, or pain in the lower abdomen. Residual urine volume measurements were performed on the ward using Yuririn (UriCare Inc., Kanagawa, Japan), which began to be used in 2016. Generally, if the residual urine is ≥ 100 ml, the urinary catheter is reinserted and the patient is examined by a urologist. If necessary, a drug is prescribed, and another attempt is made to remove the catheter after one week. If there are no problems after re-removal, the patient is discharged and has outpatient follow-up of urinary function with a urologist. However, if urinary dysfunction occurs again and the residual urine is ≥ 100 ml, the urinary catheter is reinserted and the patient is referred to a urologist for consultation. Even when residual urine is < 100 ml, the patient is examined by a urologist and followed up with medication if necessary. One week later, a second urinary catheter removal is performed. If further urinary dysfunction occurs, clean intermittent self-catheterization (CISC) is introduced. The period of time until CICS was no longer needed was included in the DUC. Drugs, such as distigmine bromide, urapidil, silodosin, and naftopidil (cholinesterase inhibitors), tamsulosin hydrochloride (an alpha-1 blocker), and bethanechol chloride (a cholinergic) are often administered, with selection at the urologist’s discretion, until symptoms improve.
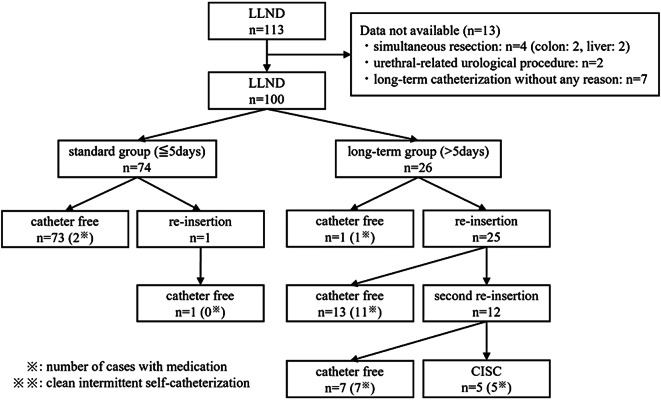
Fig. 2.
Algorithm for duration of urinary catheterization. A total of 113 cases were collected, but after excluding those with concomitant resection of other organs, urethral-related urological procedures, and catheter removal after POD6 for no particular reason (n = 13), 100 cases were included in the study. A total of 74 cases (74.0%) were catheter-free within five days, including one that had the catheter reinserted due to urinary dysfunction, but was removed immediately (≤ POD5). Of the 26 patients with long-term urinary catheterization, 25 underwent initial reinsertion and 12 underwent a second reinsertion, of which 5 patients (5.0%) were switched to clean intermittent self-catheterization (CISC)
Statistical analysis
Data were collected for age, sex, body mass index (BMI), comorbidities, previous abdominal surgery, American Society of Anesthesiologists physical status (ASA), preoperative treatment (TNT, NACRT, NAC, none), tumor location [3], distance from anal verge (AV) to tumor, and pathological stage [15]; for surgical factors including approach, procedure, operative time, blood loss, LLND laterality (bilateral/unilateral), LLND time, number of harvested LNs, inferior vesical artery (IVA) resection, creation of diverting stoma (DS), and intraoperative complications (IOCs); and for postoperative factors including POCs, DUC, and postoperative length of stay. POCs were defined as those of ≥ CD Grade 2 [14]. Benign prostatic hyperplasia (BPH) and prostate-related diseases (including BPH and post-prostate cancer treatment (brachytherapy and intensity modulated radiation therapy (IMRT)) were each recorded separately. Statistical analysis was performed using JMP® v.11 (SAS Institute, Cary, NC, USA). Continuous variables were analyzed by Wilcoxon and Kruskal-Wallis tests, and categorical variables by Pearson χ2 test, Fisher exact test, and likelihood ratio test. Multivariate analysis was performed using logistic regression analysis. Statistical significance was set at p < 0.05.
Results
Patient characteristics
The 100 patients (male: female = 72:28) in the study (Table 1) had a median age of 63 years old. Comorbidities were present in 54.5% of the cases. These were most commonly hypertension and diabetes; however, 9 patients (14.1%) with prostate-related comorbidities (7 patients with BPH and 2 patients after prostate cancer treatment) were also observed. Five of 7 patients with BPH were receiving medication for BPH. Preoperative data on urinary function were not collected. No patients complained of obvious urinary problems during the preoperative medical interview. A history of abdominal surgery was noted in 30.9% of cases. Most cases were ASA 2 (76.3%) and there were no ASA 3 cases. The most common preoperative treatment was NAC (45.0%), followed by none, NACRT, and TNT. The tumor location was more commonly in the lower rectum (Rb) (80%) than the upper rectum (Ra) (20%) [3]. The median AV to tumor distance was 5 cm. Regarding pathological stage, 3 cases were in ypStage 0 (pCR), in which the cancer disappeared after preoperative treatment. (y)pStage II was most common (29.1%), followed by (y)pStage IIIA (23.3%).
Table 1.
Patient characteristics (n = 100)
| Factor | Number |
|---|---|
| Age (years old) | 63 (26–83) |
| Sex (male/female) | 72/28 |
| BMI (kg/m2) | 22.5 (17.1–33.1) |
| Comorbidities (yes/no) | 48/40 |
| ・BPH (yes/no)* | 7/57* |
| Prostate-related comorbidities (yes/no)** | 9/55* |
| ・Previous abdominal surgery (yes/no) | 25/56 |
| ASA (1/2/3) | 23/74/0 |
| Preoperative treatment (TNT/NACRT/NAC/none) | 3/18/45/34 |
| Tumor location (Ra/Rb) | 20/80 |
| Distance from AV to tumor (cm) | 5 (0–11) |
| (y)pStage (0(pCR)/I/II/IIIA/IIIB/IV) | 3/14/25/20/11/13 |
*Only males
**Prostate-related comorbidities involve BPH and prostate cancer after treatment
Abbreviations: BMI: body mass index; BPH: benign prostatic hyperplasia; ASA: American Society of Anesthesiologists Physical Status; TNT: total neoadjuvant therapy; NACRT: neoadjuvant chemoradiotherapy; NAC: neoadjuvant chemotherapy; Ra: upper rectum; Rb: lower rectum; AV: anal verge; (y)pStage: (post preoperative therapy) pathological stage; pCR: pathological complete response
Operative results
Operative results are shown in Table 2. LS was performed more often than RALS (63% vs. 37%), and APR, LAR and ISR were performed at rates of 50.0%, 43.0% and 5.0%, respectively. Bilateral LLND was performed more often than unilateral LLND (65% vs. 35%), and the median times for these procedures were 161.5 and 80 min, respectively. Since 2021, when the indication for LLND changed, 5 of the 20 patients who received preoperative treatment underwent preoperative radiotherapy (RT). Four patients underwent unilateral LLND and one patient underwent bilateral LLND. In bilateral LLND, the median numbers of harvested LLNs and metastatic LLNs were 13.5 (1–40) and 0 (0–6), respectively; while in unilateral LLND, these numbers were 7 (2–20) and 0 (0–1), respectively. LLN metastasis was found in 5 cases (7.7%) in bilateral LLND and in 3 cases (8.6%) in unilateral LLND. Both sides of the IVA were preserved in 90 cases, unilateral resection was performed in 8 cases, and bilateral resection in 2 cases. When performing TME, there was no case of resection of the pelvic nerve plexus. The rate of IOCs was 26.0% and bleeding was most frequent. POCs (≥ CD grade 2) occurred in 59 cases (59.0%), with urinary dysfunction (26%), ileus (12%), and SSI (6.0%) being most common. SSI was observed in 6 patients, all of whom underwent APR, and all 6 SSIs were perineal wound infections. There was one case of conversion to open surgery due to ureteral injury. Most SSIs were superficial, but pelvic abscess was observed. The median time for DUC was 5 days, and the median length of stay after surgery was 16 days.
Table 2.
Operative results (n = 100)
| Factor | Number |
|---|---|
| Surgical approach (LS/RALS) | 63/37 |
| Surgical procedure (APR/LAR/ISR/Hartmann) | 50/43/5/2 |
| Operative time (minutes) | 554.5 (385–833) |
| Blood loss (ml) | 85 (10-2300) |
| LLND laterality (bilateral/unilateral) | 65/35 |
| ・Bilateral LLND time (min) | 161.5 (90–256) |
| ・Unilateral LLND time (min) | 80 (47–143) |
| Harvested LN (total) | 26 (5–76) |
| Harvested LN (LLND) | 11 (1–40) |
| IVA resection (none/unilateral/bilateral) | 90/8/2 |
| LCA preservation (yes/no) | 17/58 |
| DS creation (yes/no) | 47/44 |
| IOCs (yes/no) | 25/71 |
| Conversion to open surgery | 1/99 |
| POCs (≥ CD Grade 2) (yes/no) | 59/41 |
| ・Urinary dysfunction (yes/no) | 26/74 |
| ・Ileus (yes/no) | 12/88 |
| ・SSI (yes/no) | 6/94 |
| ・Anastomotic leakage (yes/no) | 5/95 |
| ・High output syndrome (yes/no) | 4/96 |
| Duration of urinary catheterization (days) | 5 (2-459) |
| Postoperative length of stay (days) | 16 (7–73) |
Abbreviations: LS: laparoscopic surgery; RALS: robot-assisted laparoscopic surgery; APR: abdominoperineal resection; LAR: low anterior resection; ISR: intersphincteric resection; LLND: lateral lymph node dissection; LN: lymph node; IVA: inferior vesical artery; LCA: left colic artery; DS: diverting stoma; IOCs: intraoperative complications; POCs: postoperative complications; CD: Clavien-Dindo classification; SSI: surgical site infection; DUC: duration of urinary catheterization
Comparison of LS and RALS
Patient characteristics and operative results in LS and RALS are shown in Tables 3 and 4. There were slightly higher risk cases in RALS for ASA (p < 0.01) and shorter AV distances in LS (p < 0.01). Preoperative treatment showed an overall difference (p = 0.045), although no difference was found for radiotherapy (p = 0.91). However, other than these factors, there were no differences in patient characteristics. APR was performed more frequently in LS (p < 0.01), but the rate of bilateral LLND was higher in RALS (p = 0.03). Operative time was longer with RALS (p < 0.01), but blood loss was less with RALS (p < 0.01). DUC was one day longer in RALS (p < 0.01), but postoperative length of stay did not differ between the two groups (p = 0.34). There were no significant differences between LS and RALS (p > 0.05) in the total number of POCs, or in any of the individual factors (urinary dysfunction, ileus, SSI, anastomotic leakage (AL), and high output syndrome).
Table 3.
Comparison of patient characteristics between LA and RALS
| Factor | LS n = 63 |
RALS n = 37 |
p value |
|---|---|---|---|
| Age (years old) | 63 (43–80) | 63 (26–83) | 0.86 |
| Sex (male/female) | 43/20 | 29/8 | 0.28 |
| BMI (kg/m2) | 22.5 (18.1–33.1) | 22.5 (17.1–31.9) | 0.87 |
| Comorbidities (yes/no) | 29/29 | 19/11 | 0.23 |
| ・BPH (yes/no)* | 5/36* | 2/21* | 1.00* |
| ・Prostate-related comorbidities (yes/no)** | 6/35* | 3/20* | 1.00* |
| Previous abdominal surgery (yes/no) | 20/37 | 5/19 | 0.29 |
| ASA (1/2/3) | 21/40/0 | 2/34/0 | < 0.01 |
| Preoperative treatment (TNT/NACRT/NAC/none) | 0/13/26/24 | 3/5/19/10 | 0.045 |
| ・Preoperative RT (yes/no) | 13/50 | 8/29 | 0.91 |
| Tumor location (Ra/Rb) | 11/52 | 9/28 | 0.69 |
| Distance from AV to tumor (cm) | 4 (0–10) | 6.5 (2–11) | < 0.01 |
| (y)pStage (0(pCR)/I/II/IIIA/IIIB/IV) | 14/46 | 3/23 | 0.25 |
*Only males
**Prostate-related comorbidities involve BPH and prostate cancer after treatment
Abbreviations: BMI: body mass index; BPH: benign prostatic hyperplasia; ASA: American Society of Anesthesiologists Physical Status; TNT: total neoadjuvant therapy; NACRT: neoadjuvant chemoradiotherapy; NAC: neoadjuvant chemotherapy; RT: radiotherapy; Ra: upper rectum; Rb: lower rectum; AV: anal verge; (y)pStage: (post preoperative therapy) pathological stage; pCR: pathological complete response
Table 4.
Comparison of operative results between LS and RALS
| Factor | LS n = 63 |
RALS n = 37 |
p value |
|---|---|---|---|
| Surgical procedure (APR/LAR, ISR, Hartmann) | 38/25 | 12/25 | < 0.01 |
| Operative time (minutes) | 527 (385–833) | 626 (385–815) | < 0.01 |
| Blood loss (ml) | 100 (10-2300) | 50 (15–560) | < 0.01 |
| LLND laterality (bilateral/unilateral) | 36/27 | 29/8 | 0.03 |
| LLND time (minutes) | |||
| ・Bilateral | 156 (95–256) | 170 (90–230) | 0.51 |
| ・Unilateral | 80 (47–120) | 94 (72–143) | 0.36 |
| Harvested LN (total) | 26 (6–59) | 26.5 (5–76) | 0.66 |
| Harvested LN (LLND) | 11 (2–33) | 11 (1–40) | 0.57 |
| IVA resection (yes/no) | 7/56 | 3/34 | 0.74 |
| LCA preservation (yes/no) | 17/31 | 0/27 | < 0.01 |
| DS creation (yes/no) | 23/33 | 22/11 | 0.03 |
| IOCs (yes/no) | 18/44 | 7/27 | 0.37 |
| Conversion to open surgery (yes/no) | 1/62 | 0/37 | 1.00 |
| POCs (CD ≥ Grade 2) (yes/no) | 33/30 | 26/11 | 0.08 |
| ・Urinary dysfunction (yes/no) | 15/48 | 11/26 | 0.51 |
| ・Ileus (yes/no) | 6/57 | 6/31 | 0.32 |
| ・SSI (yes/no) | 6/57 | 0/37 | 0.08 |
| ・Anastomotic leakage (yes/no) | 3/60 | 2/35 | 1.00 |
| ・High output syndrome (yes/no) | 1/62 | 3/34 | 0.14 |
| Duration of urinary catheterization (days) | 4 (2–55) | 5 (2-459) | < 0.01 |
| Postoperative length of stay (days) | 15 (7–73) | 16 (11–42) | 0.34 |
Abbreviations: LS: laparoscopic surgery; RALS: robot-assisted laparoscopic surgery; APR: abdominoperineal resection; LAR: low anterior resection; ISR: intersphincteric resection; LLND: lateral lymph node dissection; LN: lymph node; IVA: inferior vesical artery; LCA: left colic artery; DS: diverting stoma; IOCs: intraoperative complications; POCs: postoperative complications; CD: Clavien-Dindo classification; SSI: surgical site infection; DUC: duration of urinary catheterization
Duration of urinary catheterization
The cases were divided into groups S (DUC ≤ POD5, n = 74) and L (DUC > POD5, n = 26). There was no significant difference in patient characteristics between the groups (Table 5) with the exception of preoperative RT, which had a significantly higher rate in group S (p = 0.01). However, groups S and L did have significant differences in LLND laterality (p = 0.02), overall POCs (p < 0.01), urinary dysfunction (p < 0.01), SSI (p = 0.04), and DUC (p < 0.01) (Table 6). Two patients in group S with urinary dysfunction (CD ≥ Grade 2) had the urinary catheter removed within 5 days. These patients subsequently had difficulty in urinating, but were not re-catheterized and were given medication, resulting in the CD Grade 2 classification. On the other hand, two patients in group L without urinary dysfunction (CD ≥ Grade 2) had difficulty in urinating, and re-catheterization was the only treatment and no medication was given, so these cases were classified as CD Grade 1.
Table 5.
Univariate analysis of patient characteristics
| Factor | Group S (≤ 5 days) n = 74 |
Group G (> 5 days) n = 26 |
p value |
|---|---|---|---|
| Age (years old) | 63.5 (43–83) | 59.5 (26–79) | 0.55 |
| Sex (male/female) | 57/17 | 15/11 | 0.06 |
| BMI (kg/m2) | 22.5 (17.1–31.9) | 22.4 (18.1–33.1) | 0.31 |
| Comorbidities (yes/no) | 35/29 | 13/11 | 0.97 |
| ・BPH (yes/no)* | 4/46* | 3/11* | 0.17* |
| ・Prostate-related comorbidities (yes/no)** | 6/44* | 3/11* | 0.40* |
| Previous abdominal surgery (yes/no) | 17/44 | 8/12 | 0.31 |
| ASA (1/2) | 17/54 | 6/20 | 0.93 |
| Preoperative treatment (yes/no) | 50/24 | 16/10 | 0.58 |
| ・Preoperative RT (yes/no) | 20/54 | 1/25 | 0.01 |
| Tumor location (Ra/Rb) | 15/59 | 5/21 | 1.00 |
| Distance from AV to tumor (cm) | 5 (0–11) | 4 (0–8) | 0.81 |
| (y)pStage (0(pCR), I/II, IIIA, IIIB, IV) | 13/50 | 4/19 | 1.00 |
*Only males
**Prostate-related comorbidities involve BPH and prostate cancer after treatment
Abbreviations: Group S: standard urinary catheterization group; Group L: long-term urinary catheterization group; BMI: body mass index; BPH: benign prostatic hyperplasia; ASA: American Society of Anesthesiologists Physical Status; RT: radiotherapy; Ra: upper rectum; Rb: lower rectum; AV: anal verge; (y)pStage: (post preoperative therapy) pathological stage; pCR: pathological complete response
Table 6.
Univariate analysis for operative results
| Factor | Group S (≤ 5 days) n = 74 |
Group G (> 5 days) n = 26 |
p value |
|---|---|---|---|
| Surgical approach (LS/RALS) | 48/26 | 15/11 | 0.51 |
| Surgical procedure (APR/LAR, ISR, Hartmann) | 35/39 | 15/11 | 0.36 |
| Operative time (minutes) | 545.5 (399–833) | 602.5 (385–815) | 0.10 |
| Blood loss (ml) | 81 (15-1770) | 104 (10-2300) | 0.80 |
| LLND laterality (bilateral/unilateral) | 43/31 | 22/4 | 0.02 |
| LLND time (minutes) | |||
| ・Bilateral | 160 (94–213) | 163 (90–256) | 0.83 |
| ・Unilateral | 80 (47–143) | 97 (69–133) | 0.67 |
| Harvested LN (total) | 25 (5–63) | 27.5 (12–76) | 0.24 |
| Harvested LN (LLND) | 11 (1–39) | 10.5 (2–40) | 0.98 |
| IVA resection (yes/no) | 7/67 | 3/23 | 0.72 |
| LCA preservation (yes/no) | 14/43 | 3/15 | 0.77 |
| DS creation (yes/no) | 37/34 | 10/10 | 0.87 |
| IOCs (yes/no) | 20/51 | 5/20 | 0.60 |
| Conversion to open surgery | 1/73 | 0/26 | 1.00 |
| POCs (CD ≥ Grade 2) (yes/no) | 34/40 | 25/1 | < 0.01 |
| ・Urinary dysfunction (yes/no) | 2/72 | 24/2 | < 0.01 |
| ・Ileus (yes/no) | 9/65 | 3/23 | 1.00 |
| ・SSI (yes/no) | 2/72 | 4/22 | 0.04 |
| ・Anastomotic leakage (yes/no) | 3/71 | 2/24 | 0.60 |
| ・High output syndrome (yes/no) | 3/71 | 1/25 | 1.00 |
| Duration of urinary catheterization (days) | 4 (2–5) | 14 (7-459) | < 0.01 |
| Postoperative length of stay (days) | 15 (7–73) | 19 (11–52) | 0.050 |
Abbreviations: Group S: standard urinary catheterization group; Group L: long-term urinary catheterization group; LS: laparoscopic surgery; RALS: robotic-assisted laparoscopic surgery; APR: abdominoperineal resection; LAR: low anterior resection; ISR: intersphincteric resection; LLND: lateral lymph node dissection; LN: lymph node; IVA: inferior vesical artery; LCA: left colic artery; DS: diverting stoma; IOCs: intraoperative complications; POCs: postoperative complications; CD: Clavien-Dindo classification; SSI: surgical site infection; DUC: duration of urinary catheterization
Based on this information, a logistic regression multivariate analysis was performed to identify independent factors associated with lengthening of DUC. Six factors were selected for multivariate analysis: age and sex were included because they are important biological factors, the approach (LS/RALS) differed significantly for DUC in univariate analysis (Table 4), APR is reported to be more likely to cause urinary dysfunction [17, 18], and bilateral LLND and SSI differed significantly in univariate analysis (Table 6). Preoperative RT is generally associated with urinary dysfunction, but as mentioned above, DUC was significantly shorter in cases with preoperative RT (Table 6). Since preoperative RT may be more common in unilateral LLND, a χ2 test was performed for preoperative RT and LLND laterality (Table 7). This revealed that preoperative RT was significantly more common in cases with unilateral LLND, and there was a strong correlation between preoperative RT and LLND laterality (p < 0.01). However, since LLND laterality was already included in the multivariate analysis, preoperative RT was not included in this analysis. Since the analysis was conducted to identify factors related to reduced urinary function, urinary dysfunction and DUC were excluded as confounding factors, despite the significant differences found in univariate analysis. Overall POCs were also excluded because these are related to urinary dysfunction and SSI (Table 6). In multivariate analysis, bilateral LLND (hazard ratio (HR) 7.37, p < 0.01) and SSI (HR 15.36, p = 0.01) were identified as independent factors for lengthening of DUC after LLND (Table 8).
Table 7.
Correlation between preoperative RT and LLND laterality
| Factor | Bilateral LLND (n = 65) |
Unilateral LLND (n = 35) |
p value |
|---|---|---|---|
| Preoperative RT (+) | 6 | 15 | < 0.01 |
| Preoperative RT (−) | 59 | 20 |
Abbreviations: RT: radiotherapy; LLND: lateral lymph node dissection
Table 8.
Multivariate analysis for duration of urinary catheterization
| Factor | p value | HR | 95%CI |
|---|---|---|---|
| Age (years old) | 0.61 | ||
| Sex (male/female) | 0.18 | ||
| Surgical approach (LS/RALS) | 0.37 | ||
| Surgical procedure (APR/LAR, ISR, Hartmann) | 0.37 | ||
| LLND laterality (bilateral/unilateral) | < 0.01 | 7.37 | 1.89–49.27 |
| SSI (yes/no) | 0.01 | 15.36 | 1.68-210.54 |
Abbreviations: HR: hazard ratio; CI: confidence interval; LS: laparoscopic surgery; RALS: robot-assisted laparoscopic surgery; APR: abdominoperineal resection; LAR: low anterior resection; ISR: intersphincteric resection; LLND: lateral lymph node dissection; SSI: surgical site infection
Algorithm for duration of urinary catheterization
The algorithm for DUC used in our department is shown in Fig. 1. A total of 74 patients (74.0%) were catheter-free within 5 days (group S). In one of these cases, the catheter was re-inserted because of urinary dysfunction, but this improved quickly and removal occurred within 5 days without medication. Of the other 26 patients (group L), 25 (25.0%) had catheters reinserted, 12 (12.0%) then had reinsertion for the second time, and 5 (5.0%) were finally switched to CISC. However, 4 of these 5 cases were catheter-free within 2 months, but one patient did not have the catheter removed until one year and 3 months after surgery. Medication for urinary dysfunction was used in 26 cases, indicating ≥ CD Grade 2 urinary dysfunction in these cases (26.0%).
Discussion
There are multiple definitions of urinary dysfunction. In this study, we chose to use DUC and urinary dysfunction ≥ CD Grade 2. The incidence of urinary dysfunction after LLND has been reported to range from 10.8 to 35.2% [11, 19–22], and thus, the rate of 26.0% of patients with urinary dysfunction ≥ CD Grade 2 found in the current study is consistent with previous findings. Several papers have reported that urinary dysfunction occurs during LLND for rectal cancer surgery [17–19, 21–23], but few have examined DUC after LLND. In cases with urinary dysfunction, if the only treatment is reinsertion of the urinary catheter, the event is defined as CD Grade 1, unlike the introduction of drugs. However, because urinary catheterization has a significant impact on QOL, we focused on DUC as a novel endpoint in this study.
A comparison of LS and RALS was used to assess possible differences in POCs, including urinary dysfunction, after the two procedures. However, the results showed no significant differences, suggesting that safety has been maintained in the shift from LS to RALS. In recent years, there have been several studies of urinary function after LLND. Significantly less urinary dysfunction has been found after LS compared to open surgery (OS) [24, 25], while LS and RALS have been reported to be comparable [26, 27], or urinary function may even be better after RALS than after LS [20, 28–30], indicating that MIS contributes to maintenance of urinary function. Multiple joint dexterity specific to RALS may contribute to nerve preservation in the narrow pelvis [28], and RALS provides a higher number of harvested LLNs [31].
The significance of performing LLND for LARCs has also been widely examined in previous studies. From an oncological perspective, prophylactic bilateral LLND contributes to reduction of local recurrence [8, 9, 32], but does not improve prognosis [33]. In contrast, selective LLND, in which LLND is performed on the side diagnosed as positive for metastasis preoperatively, has been suggested to have the potential to improve prognosis [11]. The 2024 Japanese treatment guidelines state that if pre- or intraoperative diagnosis is positive for LLN metastasis, LLND is strongly recommended [34]. Thus, preoperative LLN diagnosis is important for selective LLND, and Kawai et al. found that a combination of the long axis and short/long ratio measured preoperatively on MRI permits diagnosis with a sensitivity of > 90% [35]. However, LLND is strongly associated with postoperative urinary dysfunction [11, 19, 22, 23]. This may be because TME and LLND procedures affect the hypogastric nerve and pelvic nerve, which are responsible for urinary function, and because the pudendal nerve, which passes through the Alcock canal, is also responsible for urinary function [21, 36].
In multivariate analysis in this study, bilateral LLND and SSI were identified as independent factors associated with worsening urinary catheter removal. As mentioned above, several nerves are related to urinary function [17, 33]. TME alone can cause urinary dysfunction, but LLND is a more likely cause. In addition, because part of the blood flow to the bladder is provided by the IVA, dissecting the IVA also damages the nearby autonomic nervous system, which is associated with bladder ischemia and urinary dysfunction [21, 37]. If the IVA is not resected, the neurovascular periphery associated with urinary function may still be affected by the LLND manipulation. Although our data showed no association of IVA resection with DUC, several reports from Japan have found that IVA resection causes dysfunction [21, 38]. Furthermore, bilateral LLND is reported to cause more long-term urinary dysfunction than unilateral LLND. These findings indicate that bilateral LLND has a more detrimental effect on urinary function than unilateral LLND. In selective LLND, unilateral LLND is performed in 83.0–86.0% of cases, while bilateral LLND is used for only 14.0–17.0% [39–41]. Given that oncologically, bilateral prophylactic LLND does not have a significant prognostic impact [33], selective unilateral LLND over bilateral LLND may contribute to a reduction in urinary dysfunction. In this study, of the 5 patients who underwent preoperative RT after 2021, when the indication for LLND changed, 4 underwent unilateral LLND and one underwent bilateral LLND, but all 5 of these patients were in group S and experienced no urinary dysfunction.
SSI was also found to be a risk factor for DUC. Cytokines elicited by inflammation have the potential to cause microcirculatory damage and dysfunction to other organs [42]. Spreading of these cytokines to the bladder and surrounding nerves and blood vessels could cause urinary dysfunction. During TME, the pelvic autonomic nerves are protected from inflammatory factors by the nerve plane. Thus, inflammation, including SSI, may damage nerves associated with urinary function [43]. In fact, all of our SSI cases (n = 6) were APR cases, and all SSIs were perineal wound infections, supporting the possibility of inflammatory spread to the nerves. the SSIs included perineal wound infection and pelvic abscess. These events were considered to be related to APR, suggesting that urinary dysfunction occurred due to the spread of inflammation to nearby nerves and blood vessels. Rachid et al. also found a significant relationship between postoperative SSI and ureteral catheter use (p < 0.01) [44], and SSI after lower gastrointestinal surgery significantly prolongs the hospital stay [45], indicating that perioperative SSI control is critical to QOL.
APR, blood loss, and preoperative treatment have previously been associated with urinary dysfunction after LLND, but were not identified as risk factors in the current study. APR is a risk factor for urinary function after rectal cancer surgery [17, 18], which is probably due to the performance in APR of surgical manipulations around the pelvic nerves, which are related to urinary function. In this study, APR was not significant in univariate analysis (p = 0.36) and was not identified as a risk factor in the multivariate analysis of urinary dysfunction (p = 0.37). These results may be due to LLND being more important than the procedure (APR) with reference to its effects on urinary dysfunction, or because of the relatively small number of cases.
Concerning blood loss, Sadakari et al. found that excessive bleeding (≥ 400 ml) can cause urinary dysfunction, and theorized that in cases of LLND, the operation tends to be longer than in cases without LLND, potentially leading to increased damage to the nervous system and bleeding. In this study, bleeding of ≥ 400 ml occurred in 8 cases (8.0%), but was not significantly associated with DUC [21].
The effect on the nerves may be influenced by preoperative treatment, as well as surgical manipulation. Among preoperative treatments, NACRT can cause tissue degeneration of the nerves around the rectum [46, 47]. However, as noted above, preoperative RT was significantly more common in group S (Table 5, p = 0.01), and preoperative RT and LLND laterality were strongly correlated (Table 7, p < 0.01). The reason for the shorter DUC in cases with preoperative RT may have been that laterality (bilateral) had a greater effect on DUC than the effect of RT.
Furthermore, of the 35 patients who underwent unilateral LLND, 25 had the catheter removed within 4 days after surgery, and none had the catheter reinserted (0%). This suggests that unilateral LLND should be considered prospectively for an earlier catheter removal date than POD5.
There are some limitations in this study. Since the robotic system is used by many departments in our hospital, the laparoscopic system was used when the robotic system was not available. As a result, there were more LS cases in the first half of the study period and more RALS in the second half, which may have affected the results and should be considered as a limitation. There has also been a shift from LS to RALS due to technological developments in surgical instruments, and this change is also a limitation due to the retrospective design of the study. The indications for LLND have changed over time, as mentioned above, which also may have affected the outcome, and we plan to accumulate cases and reevaluate the results in a study with a unified indication for LLND. Also, sexual function, as well as urinary function, should be considered after autonomic nerve preservation surgery with LLND for rectal cancer. This was not examined in this study, but we plan to evaluate both functions in further studies. A further limitation is that the timing of removal of the urinary catheter is typically set at POD5, but can vary depending on the patient’s condition and other reasons. This may be due partly to the fact that the clinical path was not operational and there was no compliance with the removal of the urinary catheter at POD5. If the catheter was removed before POD5, the urinary status after removal was checked and the case was classified into group S. In fact, none of those removed before POD4 were re-catheterized. However, some patients had catheters removed after POD6 for no reason and did not subsequently develop urinary dysfunction, and these cases were excluded from the study. In addition, there were times when the residual urine measuring device was not available, which is a further limitation. Also, epidural catheter anesthesia has been associated with postoperative urinary dysfunction [48], but epidural catheters have rarely been used at our center in recent years. If an epidural catheter was used, it was removed on POD1 or POD2, so we did not include this in the study because its impact on urinary catheter removal was considered to be minimal. Finally, this study is a single-center, retrospective study with a limited number of cases. In the future, we aim to gather a larger number of cases from multiple centers and conduct a prospective investigation.
Conclusions
The findings of this study indicate that there is no difference in POCs, including urinary dysfunction, between LS and RALS, indicating the safety of both procedures. Bilateral LLND and SSI were independent factors for lengthening of DUC after LLND, thus selective LLND may result in less urinary dysfunction than prophylactic bilateral LLND. Preventing SSI is also likely to reduce development of urinary dysfunction.
Acknowledgements
We express our deepest gratitude to all colleagues from the Department of Coloproctological Surgery, Juntendo University Faculty of Medicine, who managed the patients before and after surgery and assisted with surgeries.
Abbreviations
- LLND
Lateral lymph node dissection
- LARC
Locally advanced rectal cancer
- DUC
Duration of urinary catheterization
- LS
Laparoscopic surgery
- RALS
Robot-assisted laparoscopic surgery
Author contributions
KS, HR, and MT performed data collection. MT and HR contributed to statistical analyses. MT and KS drafted and revised the manuscript. KS supervised writing of the manuscript. SK, MT, HT, TI, HM, KA, YT, RT, KH, MK, SI, and KS contributed to preoperative examinations, surgeries, and perioperative patient management. All authors have read and approved the final manuscript and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work.
Funding
The authors received no funding for this study.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Juntendo University (approval number: H19-0214).
Consent for publication
Due to the retrospective nature of this study, the requirement for informed consent was waived.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kinugasa T, Akagi Y, Ochi T, Ishibashi Y, Tanaka N, Oka Y, et al. Lateral lymph-node dissection for rectal cancer: meta-analysis of all 944 cases undergoing surgery during 1975–2004. Anticancer Res. 2013;33(7):2921–7. [PubMed] [Google Scholar]
- 2.Ishibe A, Ota M, Watanabe J, Suwa Y, Suzuki S, Kanazawa A, et al. Prediction of lateral pelvic lymph-node metastasis in low rectal cancer by magnetic resonance imaging. World J Surg. 2016;40(4):995–1001. [DOI] [PubMed] [Google Scholar]
- 3.Rectum, JSfCotCa. Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: 3rd English Edition [Secondary Publication], 2019;3:20191030 edn. [DOI] [PMC free article] [PubMed]
- 4.Inoue H, Sasaki K, Nozawa H, Kawai K, Murono K, Emoto S, et al. Therapeutic significance of D3 dissection for low rectal cancer: a comparison of dissections between the lateral pelvic lymph nodes and the lymph nodes along the root of the inferior mesenteric artery in a multicenter retrospective cohort study. Int J Colorectal Dis. 2021;36(6):1263–70. [DOI] [PubMed] [Google Scholar]
- 5.Otero de Pablos J, Mayol J. Controversies in the management of lateral pelvic lymph nodes in patients with advanced rectal cancer: East or West? Front Surg. 2019;6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iv AA, Koprowski MA, Nabavizadeh N, Tsikitis VL. The evolution of rectal cancer treatment: the journey to total neoadjuvant therapy and organ preservation. Ann Gastroenterol. 2022;35(3):226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boublikova L, Novakova A, Simsa J, Lohynska R. Total neoadjuvant therapy in rectal cancer: the evidence and expectations. Crit Rev Oncol Hematol. 2023;192:104196. [DOI] [PubMed] [Google Scholar]
- 8.Fujita S, Mizusawa J, Kanemitsu Y, Ito M, Kinugasa Y, Komori K, et al. Mesorectal excision with or without lateral lymph node dissection for clinical stage II/III lower rectal cancer (JCOG0212): a multicenter, randomized controlled, noninferiority trial. Ann Surg. 2017;266(2):201–7. [DOI] [PubMed] [Google Scholar]
- 9.Tsukamoto S, Fujita S, Ota M, Mizusawa J, Shida D, Kanemitsu Y, et al. Long-term follow-up of the randomized trial of mesorectal excision with or without lateral lymph node dissection in rectal cancer (JCOG0212). Br J Surg. 2020;107(5):586–94. [DOI] [PubMed] [Google Scholar]
- 10.Ozawa H, Kotake K, Hosaka M, Hirata A, Sugihara K. Impact of lateral pelvic lymph node dissection on the survival of patients with T3 and T4 low rectal cancer. World J Surg. 2016;40(6):1492–9. [DOI] [PubMed] [Google Scholar]
- 11.Hida K, Nishizaki D, Sumii A, Okamura R, Sakai Y, Konishi T, et al. Prognostic impact of lateral pelvic node dissection on the survival of patients in low rectal cancer subgroups based on lymph node size. Ann Surg Oncol. 2021;28(11):6179–88. [DOI] [PubMed] [Google Scholar]
- 12.Erozkan K, Gorgun E. Robotic colorectal surgery and future directions. Am J Surg. 2024;230:91–8. [DOI] [PubMed] [Google Scholar]
- 13.Hamabe A, Takemasa I, Kotake M, Nakano D, Hasegawa S, Shiomi A, et al. Feasibility of robotic-assisted surgery in advanced rectal cancer: a multicentre prospective phase II study (VITRUVIANO trial). BJS Open. 2024;8(3):zrae048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.TNM. Classification of Malignant Tumours, 8th edition; 2016.
- 16.Wang L, Hirano Y, Heng G, Ishii T, Kondo H, Hara K, et al. The significance of lateral lymph node metastasis in low rectal cancer: a propensity score matching study. J Gastrointest Surg. 2021;25(7):1866–74. [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Martínez D, Rodríguez-Infante A, Otero-Díez JL, Baldonedo-Cernuda RF, Mosteiro-Díaz MP, García-Flórez LJ. Is my life going to change? A review of quality of life after rectal resection. J Gastrointest Oncol. 2020;11(1):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wani RA, Bhat IU, Parray FQ, Chowdri NA. Quality of life after total mesorectal excision (TME) for rectal carcinoma: a study from a tertiary care hospital in Northern India. Indian J Surg Oncol. 2017;8(4):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou S, Mei S, Feng B, Yang Y, Wang X, Wang Q, et al. Mesorectal excision with or without lateral lymph node dissection for elderly patients with mid-low rectal cancer: safety and feasibility analysis. Jpn J Clin Oncol. 2023;53(1):26–34. [DOI] [PubMed] [Google Scholar]
- 20.Chaouch MA, Hussain MI, Carneiro da Costa A, Mazzotta A, Krimi B, Gouader A, et al. Robotic versus laparoscopic total mesorectal excision with lateral lymph node dissection for advanced rectal cancer: a systematic review and meta-analysis. PLoS ONE. 2024;19(5):e0304031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadakari Y, Hisano K, Sada M, Mizuuchi Y, Nagayoshi K, Fujita H, et al. Long-term effects of laparoscopic lateral pelvic lymph node dissection on urinary retention in rectal cancer. Surg Endosc. 2022;36(2):999–1007. [DOI] [PubMed] [Google Scholar]
- 22.Shigaki T, Fujita F, Obara J, Nakane H, Ogata S, Yomoda T, et al. Examination of postoperative voiding and sexual dysfunction in cases of nerve preservation with lateral lymph node dissection for lower rectal cancer. J Jpn Soc Coloproctol. 2019;72(6):373–80. [Google Scholar]
- 23.Zou B, Ning N, Yan Y, Zhang Y. Lateral lymph node dissection can increase overall survival and 5–year survival rate of rectal cancer patients: a meta–analysis. Oncol Lett. 2024;27(2):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe J, Ishibe A, Suwa Y, Ozawa M, Nakagawa K, Suwa H, et al. Short- and long-term outcomes of laparoscopic versus open lateral lymph node dissection for locally advanced middle/lower rectal cancer using a propensity score-matched analysis. Surg Endosc. 2021;35(8):4427–35. [DOI] [PubMed] [Google Scholar]
- 25.Du R, Zhou J, Li D, Zhang Q, Liu J, Ma C, et al. Postoperative morbidity and mortality after mesorectal excision with laparoscopic versus conventional open lateral lymph node dissection for advanced rectal cancer: a meta-analysis. Asian J Surg. 2021;44(1):26–35. [DOI] [PubMed] [Google Scholar]
- 26.Morohashi H, Sakamoto Y, Miura T, Kagiya T, Ogasawara K, Takahashi Y, et al. Short-term outcomes of robotic-assisted laparoscopic versus laparoscopic lateral lymph node dissection for advanced lower rectal cancer. Surg Endosc. 2021;35(9):5001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obatake M, Hotchi M, Ishimura N, Kanzaki M, Yoshikawa M, Tokuda K, et al. Propensity score-matched analysis of the short-term outcomes of robotic versus laparoscopic surgery for rectal cancer. Asian J Endosc Surg. 2023;16(3):455–64. [DOI] [PubMed] [Google Scholar]
- 28.Chen YC, Tsai YY, Ke TW, Shen MY, Fingerhut A, Chen WT. Robotic versus laparoscopic pelvic lateral lymph node dissection in locally advanced rectal cancer: a systemic review and meta-analysis. Surg Endosc. 2024;38(7):3520–30. [DOI] [PubMed] [Google Scholar]
- 29.Song SH, Choi GS, Kim HJ, Park JS, Park SY, Lee SM, et al. Long-term clinical outcomes of total mesorectal excision and selective lateral pelvic lymph node dissection for advanced low rectal cancer: a comparative study of a robotic versus laparoscopic approach. Tech Coloproctol. 2021;25(4):413–23. [DOI] [PubMed] [Google Scholar]
- 30.Kim HJ, Choi GS, Park JS, Park SY, Lee HJ, Woo IT, et al. Selective lateral pelvic lymph node dissection: a comparative study of the robotic versus laparoscopic approach. Surg Endosc. 2018;32(5):2466–73. [DOI] [PubMed] [Google Scholar]
- 31.Shi H, Yi X, Yan X, Wu W, Ouyang H, Ou C et al. Meta-analysis of the efficacy and safety of robot-assisted comparative laparoscopic surgery in lateral lymph node dissection for rectal cancer. Surg Endosc. 2024; Aug 1. 10.1007/s00464-024-11111-3. Epub ahead of print. [DOI] [PubMed]
- 32.Kawamura H, Miyakawa T, Tsujimoto Y, Yamamoto R, Watanabe N, Honda M. The clinical effect of total mesorectal excision with lateral lymph node dissection for lower rectal cancer: a systematic review and meta-analysis. Ann Cancer Res Ther. 2022;30(2):106–14. [Google Scholar]
- 33.Kanemitsu Y, Shida D, Tsukamoto S, Moritani K, Sakamoto R. Japanese evidences on nerve-preserving lateral pelvic lymh node dissection for rectal cancer: Major historical milestones and clinical impact: the past, present and future. Clin Colon Rectal Surg. 2020;33(6):349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rectum, JSfCotCa. JSCCR guidelines 2024 for the treatment of Colorectal Cancer. Kanehara Shuppan; 2024.
- 35.Kawai K, Shiomi A, Miura T, Uehara K, Watanabe J, Kazama S, et al. Optimal diagnostic criteria for lateral lymph node dissection using magnetic resonance imaging: a multicenter prospective study. ANZ J Surg. 2023;93(1–2):206–13. [DOI] [PubMed] [Google Scholar]
- 36.Tsuchida S. Nervous control of micturition. Jpn J Urol. 1989;80(9):1257–77. [DOI] [PubMed] [Google Scholar]
- 37.Davies MR. Anatomy of the nerve supply of the rectum, bladder, and internal genitalia in anorectal dysgenesis in the male. J Pediatr Surg. 1997;32(4):536–41. [DOI] [PubMed] [Google Scholar]
- 38.Manabe T, Koga Y, Kubo H, Baba K, Nagayoshi K, Nagai S, et al. Adverse effects on the postoperative urinary function after combined resection of inferior vesical artery in laparoscopic lateral pelvic lymph node dissection: retrospective analysis of consecutive 95 series. Surg Laparosc Endosc Percutan Tech. 2019;29(6):493–7. [DOI] [PubMed] [Google Scholar]
- 39.Akiyoshi T, Toda S, Tominaga T, Oba K, Tomizawa K, Hanaoka Y, et al. Prognostic impact of residual lateral lymph node metastasis after neoadjuvant (chemo)radiotherapy in patients with advanced low rectal cancer. BJS Open. 2019;3(6):822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JN, Liu Z, Wang ZJ, Mei SW, Shen HY, Li J, et al. Selective lateral lymph node dissection after neoadjuvant chemoradiotherapy in rectal cancer. World J Gastroenterol. 2020;26(21):2877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogura A, Akiyoshi T, Nagasaki T, Konishi T, Fujimoto Y, Nagayama S, et al. Feasibility of laparoscopic total mesorectal excision with extended lateral pelvic lymph node dissection for advanced lower rectal cancer after preoperative chemoradiotherapy. World J Surg. 2017;41(3):868–75. [DOI] [PubMed] [Google Scholar]
- 42.Wakabayashi G, Shimazu M, Yamamoto S, Morisue A, Tamagawa E, Harada H, et al. Cytokine and post-operative organ failure. J Clin Surg. 1997;52:575–80. [Google Scholar]
- 43.Li K, He X, Zheng Y. An optimal surgical plane for laparoscopic functional total mesorectal excision in rectal cancer. J Gastrointest Surg. 2021;25(10):2726–7. [DOI] [PubMed] [Google Scholar]
- 44.Flouchi R, El Far M, Hibatallah A, Elmniai A, Rhbibou I, Touzani I, et al. Incidence of surgical site infections and prediction of risk factors in a hospital center in Morocco. J Infect Dev Ctries. 2022;16(7):1191–8. [DOI] [PubMed] [Google Scholar]
- 45.Nishi H, Ishizaki M. The incidence and risk factors for surgical site infection in upper and lower digestive surgery of workers aged seventy years or younger. Jpn J Occup Med Traumatol. 2021;69:216–24. [Google Scholar]
- 46.Nishizawa Y, Fujii S, Saito N, Ito M, Ochiai A, Sugito M, et al. The association between anal function and neural degeneration after preoperative chemoradiotherapy followed by intersphincteric resection. Dis Colon Rectum. 2011;54(11):1423–9. [DOI] [PubMed] [Google Scholar]
- 47.Koushi K, Nishizawa Y, Kojima M, Fujii S, Saito N, Hayashi R, et al. Association between pathologic features of peripheral nerves and postoperative anal function after neoadjuvant therapy for low rectal cancer. Int J Colorectal Dis. 2016;31(12):1845–52. [DOI] [PubMed] [Google Scholar]
- 48.Darrah DM, Griebling TL, Silverstein JH. Postoperative urinary retention. Anesthesiol Clin. 2009;27(3):465–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.