Abstract
Polymeric biomaterials have important applications in aiding clinical disease treatment, including drug delivery, bioimaging, and tissue engineering. Currently, conventional tumor chemotherapy faces obstacles such as poor solubility/stability, inability to target, and uncontrolled drug release in clinical trials, for which the emergence of supramolecular material therapeutics combining non-covalent interactions with conventional therapies is a very promising candidate. Due to their molecular recognition abilities with a range of biomolecules, cucurbit[n]uril (CB[n]), a type of macrocyclic receptors with robust backbones, hydrophobic cavities, and carbonyl-binding channels, have garnered a lot of attention. Therefore, this paper reviews recent advances in CB[n] material-based supramolecular therapeutics for clinical treatments, including targeted delivery applications and related imaging and sensing systems. This study also covers the distinctive benefits of CB materials for biological applications, as well as the trends and prospects of this interdisciplinary subject, based on numerous state-of-the-art research findings.
Graphical Abstract
Keywords: Cucurbiturils, Targeted therapy, Host–guest chemistry
Background
Cancer is the second primary cause of death in the United States and is a main global public health issues [1]. Solid tumor subgroups present a significant medical challenge because of their distinct physiological traits and the huge variations in the ecological niche fingerprint of each tumor microenvironment [2]. While there are many approaches to treating solid tumors, most current cancer treatment strategies are deficient in one or more respects.
Although many promising anti-cancer drugs have been identified, suitable carrier systems for targeted therapies are still lacking. A developing nanotechnology-based therapy is gaining popularity since it can get around the conventional problems outlined above. Nanomaterials enhance drug performance by exploiting the pathophysiological uniqueness of solid tumors. Due to the improved permeability and retention effect (EPR) of nanoparticles, it allows for better drug delivery requirements with few side effects and offers excellent passive targeting capabilities (for solid tumors) [3, 4]. Additionally, adequate medicinal drugs, visualization agents, and targeting ligands can be physically or covalently loaded onto nanomaterials [5].
Among these, simple biomolecular supramolecular nanomedicines have promising applications in cancer therapy, and those developed based on non-covalent interactions often exhibit dynamic properties and stimulatory responses; [6–9]. CBs distinguish out among the several synthetic macrocyclic bodies for their versatility in molecular apprehension and self-assembly. CBs was first discovered in 1999, [10] and people bind it with DNA at first [11]. By 2001, people gradually understand the unique traits CBs’ have, its prominent binding properties and solubility in water and organic solvents [12–14] provide a new idea of CBs’ application and offer a new way of treatment. At the same time, CBs also facing the challenge of their functionalization, which shows their poor solubility in common solvents, and difficulty in introducing functional groups on their surfaces [15]. Since 2003, a myriad of CB[n] derivatives has been described in order to answer the long-standing problem in cucurbit[n]uril chemistry [15, 16]. CB[n] is composed of n glycylurea molecules arranged in a ring structure via methylene bridges. The number of repeating units determines the size of the entrance and the volume of the cavity. The arrangement of the hydroxyurea units creates a hydrophobic cavity with a carbonyl-lined entrance. The stiffness of the molecular backbone allows the CB to encapsulate a wide variety of inorganic and organic guest molecules with highly tunable binding affinities [17]. In comparison to other materials, the carbonyl group at the entrance of CB enables charge-dipole interactions and hydrogen bonding with the guest, and is capable of coordinating with metal ions, thereby allowing CB to bind preferentially to positively charged guests in contrast to materials such as cyclodextrins. Furthermore, the high structural rigidity of CB enables a highly selective recognition process. The macromolecular CB also allows for convenient access to ultra-stable homocysts and heterocysts, which provides a convenient method for the preparation of multidimensional nanostructures [17–19]. They have consequently recently drawn more attention in the area of cancer therapy. In particular, co-assembly using biomolecules such as peptides, nucleic acids, and proteins has proven to be a successful method for creating simplified but accurate biological systems, figuring out molecular binding patterns, creating novel biomaterials, and creating novel disease therapeutics [20]. For example, because the host–guest bond's binding affinity can vary depending on the surroundings of the tumor and normal tissue (such as pH, redox, and enzymes), this allows for precise regulation of the loaded drug or prodrug’s release in the tumor [21]. Supramolecular chemotherapy is more adaptable than traditional chemotherapy and nanomedicines that lack stimulus response due to the non-enzymatic dynamic nature of covalent bonds. Additionally, the portal of CB[n] is markedly electronegative, and the cavity of the macrocycle is encircled by a thick ring of the glycuronium substituent, which contains no functional groups or electron pairs accessible to the inner cavity. Consequently, the inner cavity of CB[n]s is notably hydrophobic. This significantly enhances the solubility and stability of poorly soluble anticancer drugs in physiological environments by forming host–guest complexes, which allows anticancer drugs to be highly accumulated in tumors. This significantly improves the efficacy of supramolecular chemotherapeutic drugs while minimizing the side effects of anticancer drugs on healthy tissues [17, 22, 23]. Additionally, by merely altering structural components, targeting ligands, visualization aids, or even therapeutic medications for example, a more adaptable strategy for the treatment of tumors can be created. Supramolecular chemotherapy is more adaptable than conventional chemotherapy and nanodrugs that lack stimulus response due to the non-enzymatic dynamic nature of covalent bonds. Furthermore, supramolecular chemotherapeutic systems can be easily integrated by simply changing structural components, such as targeting ligands, visualizers, or even therapeutic drugs, to give them versatile therapeutic diagnostic properties. These structural modifications can be made to targeting ligands, visualizers, or even therapeutic drugs [24, 25]. Due to cucurbiturils’ prominent traits like its stability, water solubility and excellent binding capacity, [16] it has a huge potential to applicate in several areas for example biochemistry, medical and biological [16]. Although CB[n] may facing several challenges, as more research goes forward, we can keep our expectations of CB[n]’s application in biology, medical and more areas (Table 1).
Table 1.
Types of cucurbit[n]uril in Supramolecular active-targeted therapy based on host–guest interactions
| Cucurbit[n]uril | Targeted molecules | Reference | |
|---|---|---|---|
| acyclic cucurbit[n]uril (CB[n]) | organic compound | Alkylamines | [23] |
| Spermidine | [26] | ||
| Polymers | CPT-SS-TPP/CPT-mPEG | [114] | |
| medicines | Doxorubicin | [46] | |
| complexes of POD and VP-16 | [53] | ||
| NMBAs | [125] | ||
| Fentanyl | [141] | ||
| Cucurbit[6]uril | organic compound | Alkylamines | [77] |
| Spermidine (SP) /spermidine (SPD) | [98] | ||
| Polymers | 1,4-bis[2-(4-pyridyl)ethenyl]-benzene (BPEB) derivatives | [80] | |
| SP–folate (FA–SP) | [118] | ||
| medicines | Doxorubicin | [47] | |
| Cucurbit[7]uril | organic compound | 4-(4-dimethylaminostyryl) quinoline (DSQ) | [92] |
| Polymers | eight-arm polyethylene glycol (PEG8-Fc) | [29] | |
| [68 Ga]Ga-NOTA-PEG3-NMe2-Fc | [30] | ||
| triphenylamine derivative (vinyl-pyridinium triphenylamines) | [61] | ||
| hyaluronic acid (HA)−4-(4-bromophenyl)pyridin-1-ium bromide (BrBP)(HA-BrBP) | [69] | ||
| Polyethylenimide (PEI) | [140] | ||
| medicines | OxPt | [22] | |
| carboplatin/oxaliplatin | [25] | ||
| Oximes | [31] | ||
| Avidin | [93] | ||
| paraquat (PQ) | [131] | ||
| sorafenib (SO) | [134] | ||
| Bedaquinoline (BDQ) | [135] | ||
| trazodone (TZ) | [136] | ||
| oxaliplatin (OxPt) | [140] | ||
| Chloride | Asoxime | [127] | |
| nickelbutyl chloride (NC) | [137] | ||
| Nanomaterials | GQDs-OH | [104] | |
| Photomolecule | diarylethene (DAE) | [87] | |
| Cucurbit[8]uril | Medicines | acridine orange (AO) /oxaliplatin (OxPt) | [111] |
| ketamine/morphine | [120] | ||
| Polymers | Phe-Gly-Gly (FGG) | [37] | |
| Pt·pentapeptides Phe-(Gly)3-Cys | [38] | ||
| dithienylethene derivative (DTE-MPBT) | [56] | ||
| triphenylamine derivative (vinyl-pyridinium triphenylamines) | [61] | ||
| cyanogenic astragalus derivatives | [73] | ||
| diarylethene phenyl pyridine derivatives (DTE-TP) | [68] | ||
| hyaluronic acid (HA)−4-(4-bromophenyl)pyridin-1-ium bromide (BrBP)(HA-BrBP) | [69] | ||
| HA-CD/G | [81] | ||
| tris-bipyridine derivative (1) and azobenzene quaternary ammonium salt (E-2) | [117] | ||
| Nanomaterials | gold nanorods(GNR) | [86] | |
| Tubulin | microtubules (MTs) | [40] | |
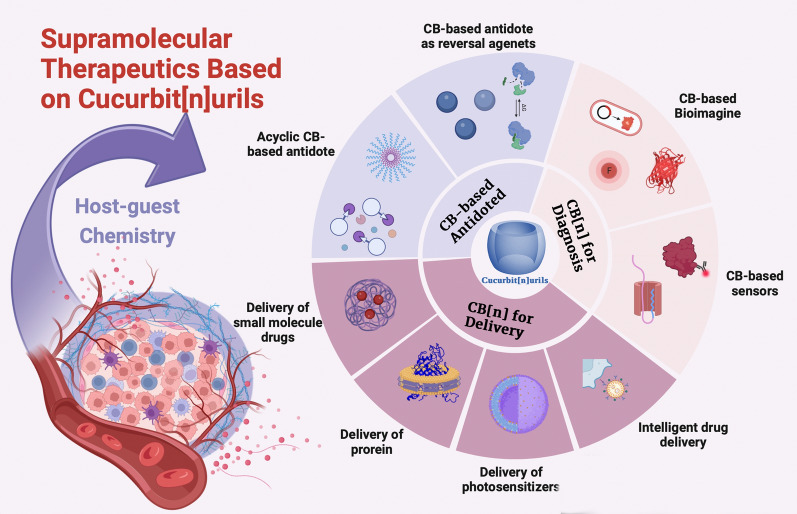
In this paper, inspired by the continuing interest in targeted therapy, we review recent advances in cucurbit[n]uril materials and its nanosystems for cancer diagnosis and therapy, focusing on supramolecular delivery systems and their potential for biosensing, fluorescence imaging, supramolecular probes. We also show some interesting studies on the biocompatibility and toxicity of CB[n], which offer promising strategies and for the precise and collaborative treatment of cancer.
CB[n] delivery system
Overview of CB[n]
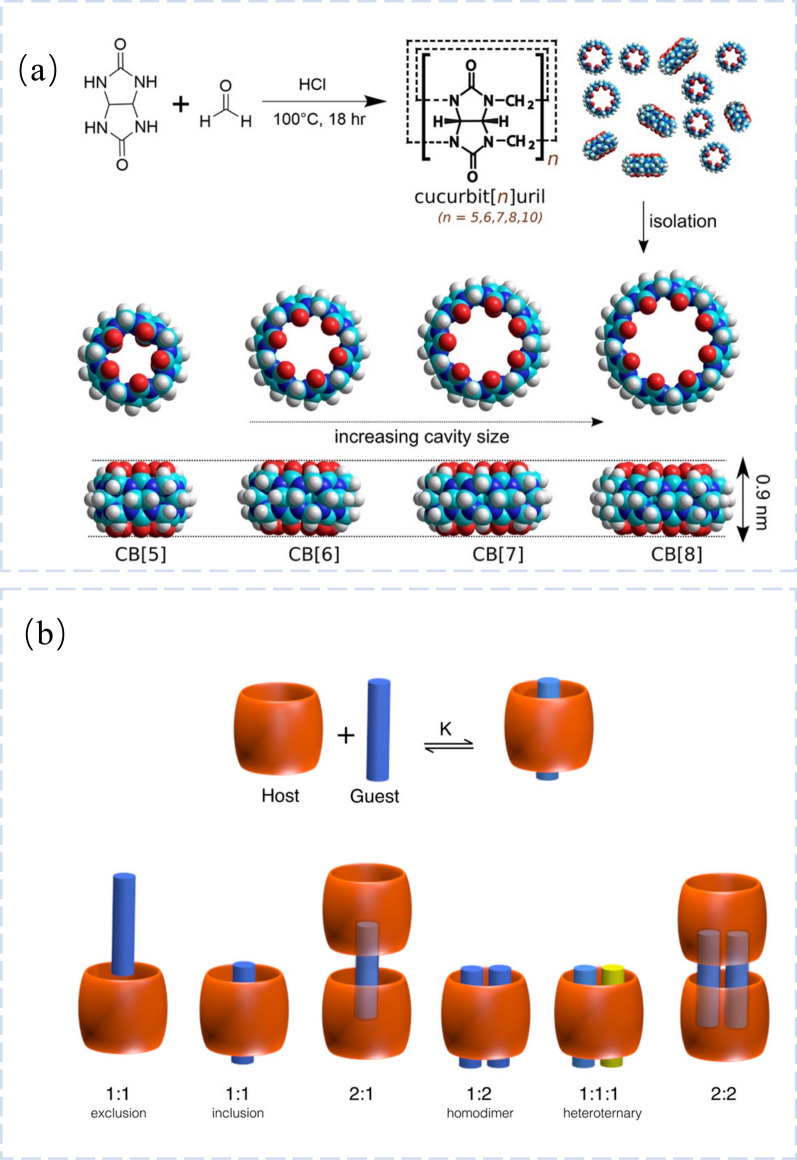
CB[n] is a substantial cyclic polymer that resembles a pumpkin as in Fig. 1a [17]. It has polar portals around the hydrophobic cavity and methyl groups that act as bridges connecting several glyoxal units. CB portals can be at either end of the structure, depending on the molecular structure [26]. The CB family’s carbonyl chemistry produces a lot of lone electron pairs. Through ionic dipole interactions, van der Waals forces, and hydrophobic effects, this peculiarity makes the formation of stable complexes by using CB with guest molecules possible. Additionally, when CB is employed as a carrier, wrapping CB can increase the drug’s solubility and stability, and further modifying CB can increase the drug.s targeting and controlled release. Multiple CB molecules can be assembled into various complexes by the joint action of numerous non-covalent bonds, and thus, they can serve as dynamic nanoplatforms. Larger CB[n]s are able to combine aromatic chemicals to generate 1:1 binary complex (CB [7]) or even 1:1:1 hetero-ternary complexes (CB [8]) (Fig. 1b) [17]. Since noncovalent bonds can be affected by temperature, light, pH, redox, and other variables, CB-directed nanoplatforms have the potential for a variety of stimulated reactions [27].
Fig. 1.
Structures of CB[n]. a (Top) The reaction between glycoluril and formaldehyde. (Bottom) Space-filling models of CB [5]-CB [8] demonstrating the increasing size yet constant height of the CB[n] macrocycles. Reprinted with permission from ref 13.
Copyright 2015 Chem Rev. b CB[n] inclusion and exclusion complexes. Reprinted with permission from ref 13. Copyright 2015 Chem Rev
Additionally, the fundamental interactions between individual CB molecules are unaffected by their individual alterations, CB-based nanoplatforms can go through several modifications before being used to build more intricate drug delivery systems. Due to these characteristics, the development of targeted drug delivery is undoubtedly made possible by the emergence of CB in a time when smart pharmaceuticals are of considerable interest.
CB[n] deliver inorganic small molecules drugs
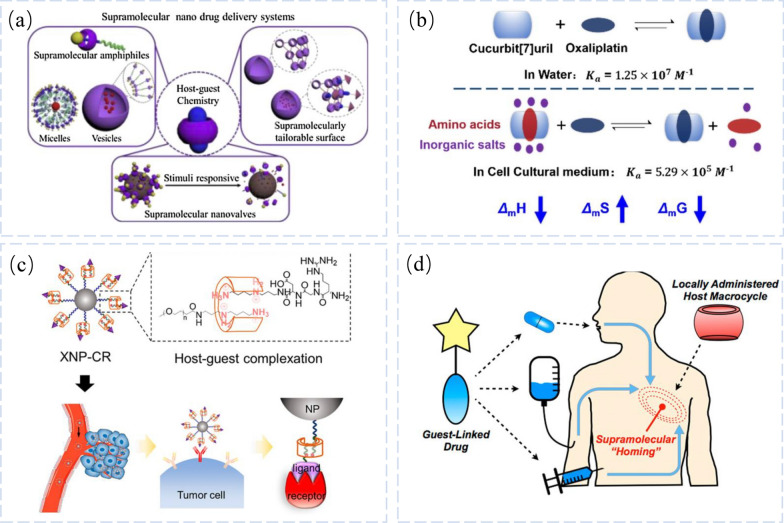
Designing drug delivery systems (DDS) is a crucial part of molecular engineering because it shows how to use formulation techniques to avoid drug adverse effects, increase the therapeutic effectiveness of pharmaceuticals, and give them greater functionality [28]. However, it is difficult to reconcile the controlled release of the medications from conventional DDS in the tumor environment with its stability along the circulatory process. The noncovalent host–guest contact is extremely stable, and DDS based on this interaction has special benefits like excellent selectivity, flexible binding capacity, and controlled drug release (Fig. 2a) [27, 29].
Fig. 2.
Supramolecular Nano Drug Delivery. a CB [6] and CB [7] based supramolecular nano drug delivery systems through formation of supramolecular amphiphiles, supramolecular nanovalves as well as supramolecular tailorable surface. Reprinted with permission from ref 22.
Copyright 2021 Chinese Chemical Letters. b Host–Guest Interactions between Oxaliplatin and Cucurbit[7]uril/Cucurbit[7]uril Derivatives under Pseudo-Physiological Conditions. Reprinted with permission from ref 24. Copyright 2020 Langmuir. c Supramolecular Container-Mediated Surface Engineering Approach for Regulating the Biological Targeting Effect of Nanoparticles. Reprinted with permission from ref 28. Copyright 2020 Nano Lett. d Schematic of approach for supramolecular homing of guest-appended small molecules on the basis of affinity for locally applied host macrocycles. Reprinted with permission from ref 30. Copyright 2019 ACS Cent Sci
However, due to the environment's many components, host–guest interactions in DDS may be impacted in physiological situations. For the development of DDSs, it is crucial to research the host–guest interactions between carriers and medications under physiological circumstances. In this study, Xi Zhang's group examined the host–guest interaction of cucurbit[7] uril/cucurbit[7]uril with OxPt, a popular clinical antitumor drug, under pseudo-physiological conditions. They discovered that amino acids could occupy the cavities of CB[7], resulting the host–guest interaction’s enthalpy change as decrease. (Fig. 2b) [29]. Additionally, a decrease in the binding enthalpy changes and an increase in the binding entropy change were brought about by the pre-assembly of inorganic cations and the pore structure of CB[7]. As a result, in 1640 medium, CB[7]’s binding constant with OxPt is 1/20 of what it is in pure water. The molecular weight and structure of the PEG polymer backbone have no appreciable impact on the host–guest interaction when CB[7] is adjusted at the end of the star PEG. This shows the direction which contains high loading and high release efficiency for the development of innovative DDSs.
For drug delivery devices, precisely delivering hydrophobic medications has proven to be quite difficult. One of them, supramolecular hierarchical self-assembly, produces nanoparticles efficiently and has a variety of intriguing biological applications. Because of their remarkable features such as their excellent bioavailability, compatibility, and stability, micelles have been the subject of the most comprehensive research into nano-drug delivery systems for a long time. Bo Yang’s group created a novel supramolecular host biotin, acyclic cucurbitacin (ACBB), for the first time [30]. They also created a nanoparticle-based on ACBB and alkylamine immobilized amphiphilic host–guest complexes of cannabinoids that self-assemble to create functionalized supramolecular micelles (FSMs) for cell-targeted drug delivery. It has been shown that FSMs promote apoptosis of tumor cells and exhibit potent antitumor activity. Clearly, FSMs have excellent drug delivery prospects, high bioavailability, stability, good delivery efficiency, and accurate targeting.
The poor solubility and stability of the majority of anticancer drugs/prodrugs in the physiological milieu significantly diminishes their effectiveness [7, 31]. In typical subject-guest inclusion complexes, the subject molecule provides cavities to encapsulate the guest molecule through noncovalent interactions [9]. Such subject-guest complexes provide a strong and dependable coupling to produce supramolecular medicines due to their great stability, solubility, and bioavailability. Among them, cucurbit[7]uril (CB[7]) is a molecular container that joins with platinum-based anticancer medications to form a host–guest complex, modifying their effectiveness and safety (Fig. 3a) [32]. Ekaterina Pashkina’s group [33] evaluated the effects of two tumor cell lines (B16 and K562) in melanoma animal models on their viability and proliferation as well as their activity. They also reported the effects of CB[7]-oxaliplatin complexes and a mixture of CB[7] and carboplatin (1:1) on primary cell cultures (peripheral blood mononuclear cells). Additionally, in vitro research was done on the effects of CB-containing platinum (II) analogs [7] on T and B cells. Despite the absence of stable CB[7]-carboplatin complexes, CB[7] had an impact on the biological effects of carboplatin. In vivoCB[7] boosted carboplatin’s anticancer activity but also its acute toxicity. On the tumor cell lines B16 and K562, it’s complex with CB[7] shown stronger cytotoxic effects compared to free oxaliplatin, whereas in vivo, the effects of free oxaliplatin and encapsulated medications were nearly in same level. However, oxaliplatin encapsulation in CB[7] decreased the drug’s toxicity has also been confirmed in vivo research. Despite of the fact that carboplatin and CB are unable to form stable inclusion complexes, the presence of such tiny voids alters the biological characteristics of carboplatin. This fact can be explained by the complex of carboplatin metabolites with CB[7] and the improvement of their biological properties, as well as the ability of CB[7] to induce carboplatin hydration or the encapsulation of certain mediator components that may affect the biological activity of carboplatin. Therefore, on the mouse B16 melanoma cell line, the combination of carboplatin and CB[7] had a more notable antitumor impact than free carboplatin.
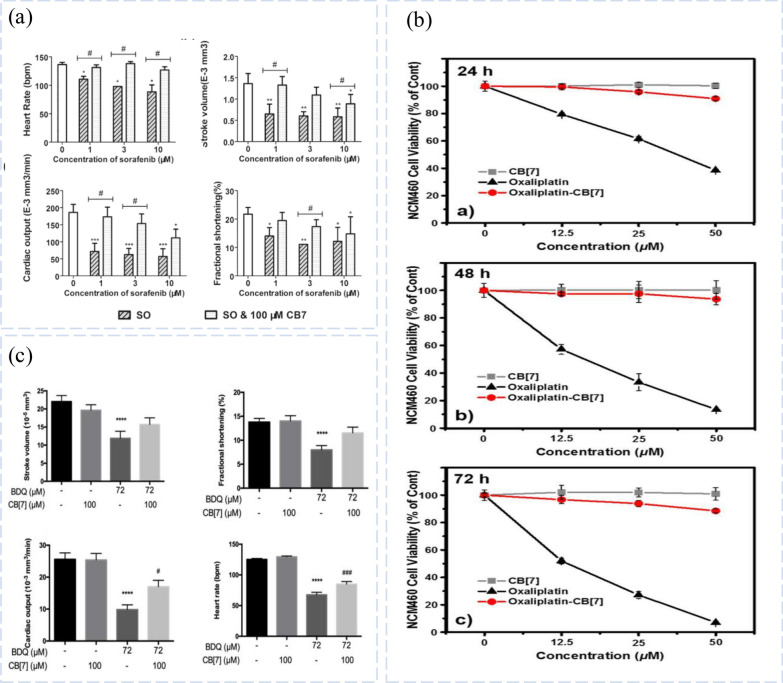
Fig. 3.
The toxic effect of CB complexes. a Effects of SO and SO@CB complexes on cardiac function in zebrafish. Reprinted with permission from ref 151. Copyright 2017, Royal Society of Chemistry. b Cytotoxicity of OxPt, CB[7] and oxaliplatin-CB[7] complexes. Reprinted with permission from ref 42. Copyright 2017, American Chemical Society. c Effects of BDQ, CB[7] and CB[7]-BDQ complexes on zebrafish cardiac function. Reprinted with permission from ref 48. Copyright 2018, Elsevier Ltd.
It has been demonstrated that macrocyclic molecules can only bind to targeting ligands or nanoparticles through unilateral subject-object complexation, which is not conducive to further modifying targeting ligands or functionalized nanoparticles, leading to uncontrollable targeting ligand orientation or lack of binding affinity to nanoparticles. The (Acyclic Cucurbituril, aCB) surface molecular container works as a bridge for bidirectional host–guest complexation to change the targeting ligand to the nanoparticle (Fig. 2c) [34]. Better biological targeting effectiveness is the outcome of this considerably improving the binding between the targeting ligand and the related receptor with a regulated orientation.
Supramolecular vascular-engineered nanoparticles improve the targeting ability for sensitive disease diagnosis, as demonstrated in successful in vivo tumor MRI using ion pcr. It is well established that non-covalent linkages based on bilateral host–guest complexation are responsible for making surface-engineered nanoparticles more effective in targeting biological targets.
In order to route medications to specific areas, drug delivery devices often rely on the biological recognition of antibodies or other proteins. Unfortunately, local accumulation of nanoparticles using the gold standard antibody for targeting (Herceptin) is less than 1%, and only 14 out of every million sick cells are successfully targeted (0.0014%) [35]. Strategies to improve drug delivery to their targets while limiting concomitant side effects still require further discovery. One option being explored to overcome the limitations of traditional targeting requires "pre-targeting" the desired site for subsequent placement of drug administration, using antibodies or reagents for biorthogonal “click” chemistry to facilitate recognition and accumulation at the pre-targeting site. In contrast, CB[7], coupled with a high-affinity supramolecular bond offered by a small number of guests, offers a unique in vivo drug targeting approach. Matthew J. Webber’group used injectable CB[7] enriched hydrogels for pre-targeting in order to spatially identify therapeutically targeted regions of action based on supramolecular affinity targeting and maintaining small compounds attached to the guest for systemic administration (Fig. 2d) [36]. This supramolecular seeking axis enables customized biomaterials by extending the localization of small molecular loads beyond injectable hydrogels. In addition, it shows promise as a therapeutic to increase the effectiveness of guest-modified chemotherapeutic agents in tumor models. Most importantly, supramolecular affinity does not irreversibly deplete the target site. Theoretically, the same host site could be repositioned in the future to extend the life cycle of the device. This possibility is not offered by traditional in situ chemical ligation methods and offers great promise for future drug-targeting studies based on host–guest supramolecular affinity.
To overcome the limitations of existing pre-targeting platforms in terms of in vivo stability and modularity of pre-targeting components, Jacob L. Houghton’s group proposed a new pre-targeting method for pre-targeting platforms [37]. 68 Ga-labeled ferrocene guest radioligands ([68 Ga] Ga-NOTA-PEG3NMe2Fc) and CB[7]host molecularly modified anti-cancer embryonic antigen–antibody (M5A; CB7-M5A) were investigated as promising host–guest chemical pre-targeting agents in BxPC3 xenograft nude mice for positron emission tomography studies. The proposed pre-targeting approach (leading to moderate tumor uptake (%ID/g)) can be made more effective by adjusting various pre-targeting parameters (e.g. interval time and pre-targeting agent dose) and further improving the structure of the radioligand. This optimization enhances the context of tumor uptake and other pre-targeting techniques for tumors.
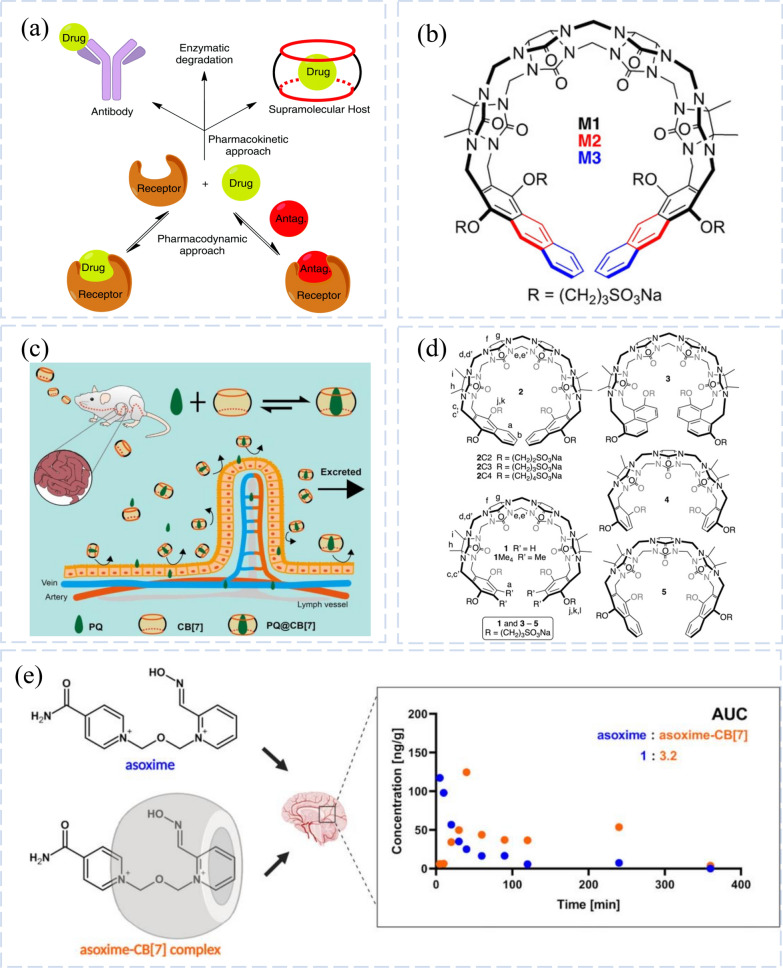
Although the possibility of CB[7] for drug administration and other biomedical purposes has been studied in vitro, there are still a lack of clinically significant in vivo results. In vitro studies typically focus on a single drug in a controlled environment. However, in vivo models are far from straightforward. In one study, Jana Zdarova Karasova’group identified the advantages and disadvantages of this drug delivery method by elucidating the link between oxime KO27, atropine, Paraoxon, and CB[7] [38]. Studies have shown that CB[7] affects multifactorial OP intoxication and its treatment by (i) clearing propoxyphene and preventing the free fraction of this toxin from entering the brain, (ii) enhancing the availability of atropine in the CNS, and (iii) increase the entry of oxime into the brain. In terms of therapeutic outcomes, the benefits of the combination of CB[7] and oxime may outweigh any potential dangers.
Xi Zhang groups have been exploring the potential value of CB[n] in supramolecular antitumor drugs. In 2016, he proposed a cytotoxic supramolecular modulation strategy based on host–guest interactions and performed a proof-of-concept using CB[7] with dimethyl viologen [39]. In the proof-of-concept dimethyl viologen (MV) was used as a model antitumor agent and CB[7] was used as a water-soluble macrocyclic host to form a MV-CB host–guest complex with MV. The MV-CB host–guest complex exhibited lower cytotoxicity than both MV and CB[7] alone in the BEAS-2B cell model, while exhibiting greater cytotoxicity than MV alone in HT-29 intestinal tumor cells overexpressing spermine and in the A549 lung tumor cell line. This study reveals the potential value of CB [7] in supramolecular chemotherapy.
In 2017, their group further exploited the host–guest complex formed by the clinical antitumor drug oxaliplatin and CB [7] to explore the potential application value of supramolecular chemotherapy in clinical cancer therapy (Fig. 3b) [40]. This study provides further evidence that CB [7] achieves targeted drug release in the setting of overexpressed spermine. Consistent with previous studies, oxaliplatin embedded in CB[7] showed greatly reduced cytotoxicity to normal colon cells, while exhibiting enhanced cytotoxicity to colon cancer cells.
Based on this study, their group designed a backbone polymer polyCB[7] with CB[7] and PEG motifs to serve as a drug carrier for oxaliplatin [41]. Like the previous design, polyCB[7] exhibited low cytotoxicity to normal cells and showed stronger cytotoxicity to cancer cells overexpressing spermine. Compared to the previous design, polyCB[7] had a longer in vivo residence time, which provides a potential application of supramolecular chemotherapy in the clinic.
In subsequent studies, Yueyue Chen’s team further explored the supramolecular chemotherapeutic potential of other antitumor drugs encapsulated in CB[7] [42–44]. Doxorubicin-ZnO, heptaplatin, and lobaplatin all have potential supramolecular chemotherapeutic applications. When combined with CB[7], these three drugs showed greatly reduced cytotoxicity to normal cells and exhibited stronger cytotoxicity to tumor cells overexpressing spermine. This means that the combination of antitumor drugs with CB[7] is not an isolated case, and a large number of other more cytotoxic drugs have the potential to become anticancer drugs by combining with CB[7].
Recently, her team also included CB[8] in the study of potential carriers for chemotherapeutic drugs [45]. They used CB[8] to encapsulate two chemotherapeutic drugs, lobaplatin and eptaplatin, to form the LP + EP@CB[8] complex (CLE), and analyzed the nanomechanical properties of the surface of colorectal tumor cells after the action of the CLE using bioscope AFM. This study suggests that CLE enhances Pt infiltration into the colorectal tumor microenvironment, such that metastasis of colorectal tumor cells may be reduced.
In addition to the above-described delivery applications of CB[n] in the field of cancer, the delivery action of CB[n] has also been used in the treatment of other diseases. Bedaquinoline (BDQ), as an anti-tuberculosis drug, has irreducible cardiotoxicity. Through the host–guest interaction between CB[7] and BDQ, Kuok and colleagues were able to synthesis the CB[7]-BDQ complex with a molar ratio of 1:1. CB[7]-BDQ combination demonstrated less cardiotoxicity than BDQ in vivo zabrafish models, and CB[7] increased BDQ's water solubility and enhanced its antibacterial effects (Fig. 3c) [46]. Trazodone (TZ) is often used to treat depression, but its hepatotoxicity has limited its clinical use. Huang and colleagues came up with a plan to encapsulate TZ in the supramolecular macrocyclic host to lessen its hepatotoxicity. They discovered that CB[7] exhibited a strong affinity for TZ and its metabolite chlorphenypiperazine, which significantly decreased the liver toxicity brought on by TZ, by describing the physicochemical characteristics and toxicity of the host–guest complex formed by CB[7] and TZ at a molar ratio of 1:1. As a result, CB[n] compounds became more widely used as pharmaceutical excipients [47]. Polyethylenimide (PEI) is a gene delivery vector with good transfection efficiency [48], but high cytotoxicity limits its popularization and application. Huang and colleagues obtained lower cytotoxicity by simple encapsulation of PEI with CB[7], while retaining the high transfection efficiency of PEI [49], which provided guidance for further clinical studies.
CB[n] deliver proteins
Proteins are an inexhaustible source of creativity. Thanks to nature's careful tuning of protein amino acid sequences, proteins have evolved from primary structures to quaternary structures, a feature unique to protein oligomers and often necessary to achieve their functions [50]. Recent advances in research have focused on manipulating protein independence to create novel nanomaterials with desired functions and multiple applications [51]. Proteins are intrinsically fertile ground for inter- and intramolecular interactions; thus, they present attractive multifunctional building blocks for manipulation [52]. Based on this understanding, Elisavet Ioannou et al. proposed a scheme for the development of supramolecular protein complexes, which are formed through subject-guest interactions in the presence of macrocyclic subject CB[8] [53]. Based on this Elisavet Ioannou and Nikolaos E. Labrou provided a scheme for the development of supramolecular protein complexes based on subject-guest interactions in the presence of macrocyclic subject CB[8] [54]. CB[8] has a significant effect on the high-affinity tripeptide-ph-gly-gly (FGG); therefore, CB [8] can act as a selective adherent for protein molecules when FGG is fused to the protein surface. Depending on the application, the binding of the appropriate recognition motifs can be applied directly to the building blocks of the protein or spliced before the complex is formed[4]. The morphology of the derived complexes depends on the location of the recognition motif on the building block of the protein along its general structure.
However, CB[n] have excellent water-based recognition capabilities, and increasingly (body)n:(guest) n supramolecular polymers have been described. By using Pt/peptide/CB[8] assembly sites to specifically target secondary hosts CB[7] and CB[8]. H. Barbero and E. Massonfound that it is possible to functionalize CB[8]-immobilized Pt dimers in situ and quantitatively with a pair of cysteine-containing polypeptides [55]. This opens the way for CB[8]-safe Pt dimeric protein logical design for recognition and restriction opens the door. In addition, Adam R. Urbach’group showed that high-affinity cucurbitacin-mediated peptide recognition is not dependent on aromatic residues. These findings suggest that the synthetic receptor CB[8] can be used to target unaltered peptides containing n-terminal methionines in addition to the known ability to target n-terminal and disordered loops in folded proteins [56].
Reconfigurable protein assemblies are undoubtedly crucial in life processes and are one of the major areas of research in supramolecular chemistry. Microtubules (MT) are important protein filaments that have a considerable impact on mitosis and are the subject of interest for Y. M. Zhang and coworkers. Briefly, they showed in their study that intertubular aggregation of microtubules can be efficiently regulated by peptide-microtubule protein interactions and synergistic effects of host–guest complexes [57]. When bound to CB[7] and CB[8] at different stoichiometric ratios, benzimidazole-modified antisecretory peptides (BPs) recognize MTs. significant apoptosis and tumor ablation in vivo are caused by the extensive conversion of BP from fibrillogenic to nanoparticle-like aggregates CB[8], which cross-link the self-assembled morphology of MTs. Ultimately, it inhibits tumor proliferation. This study shows that a combined approach combining biocompatible microtubule-directed drugs and modulating supramolecular complexes (BPCB[8])@MT assembly has significant benefits and can be used to treat cancer. Strategies based on centralized and managed protein–protein interactions may offer new opportunities for the clinical translation of supramolecular therapies.
CB[n] deliver photosensitizers
One of the more well-known forms of phototherapy is called photodynamic therapy (PDT), which is used to treat cancer. PDT involves the use of photosensitizers to produce large quantities of reactive oxygen species (ROS) under specific laser wavelengths, which are involved in cellular autophagic death by activating the death receptor pathway and the apoptotic mitochondrial pathway. It is non-invasive, not poisonous to the system, and very selective.
Combining both forms of “chemo phototherapy” has been described as a developing treatment strategy for solid tumors in order to increase the efficacy of cancer treatment [58]. It is believed that the combination of these highly selective therapies will increase the effectiveness of tumor suppression by encouraging tumor regression and preventing medication resistance [58–60]. Like chemotherapy, supramolecular photosensitization is an effective treatment option for solid tumors. chemotherapy, supramolecular photosensitizers (photoactivated drugs) are the counterparts of chemotherapeutic agents in phototherapy [61, 62]. With the extra benefit of regulating their photoactivity and photostability, CB[n]s supramolecular encapsulation of photosensitized pharmaceuticals enhances their solubility and biodistribution [63].
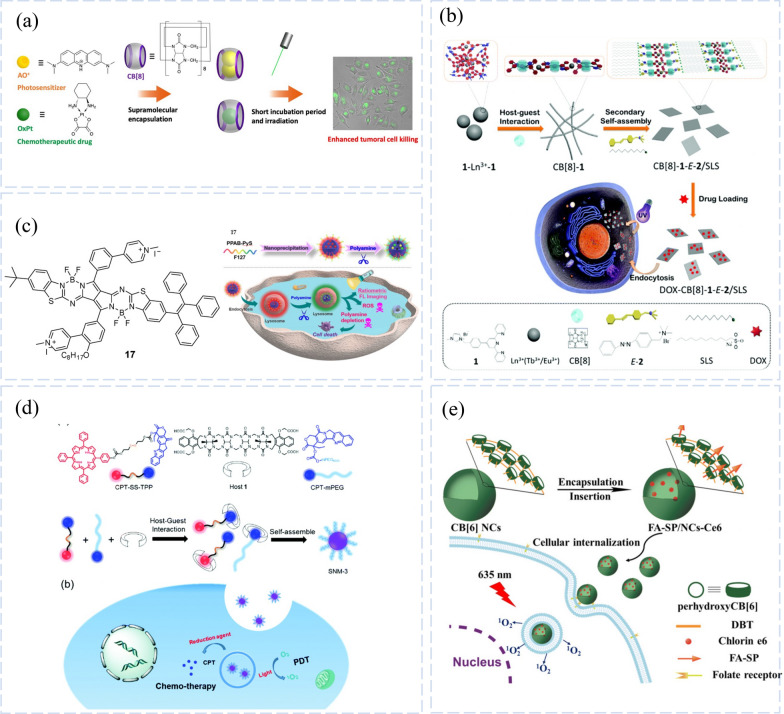
For combined chemical-photodynamic therapy, a variety of drug delivery systems (DDS) have been developed, including inorganic nanoparticles, organic self-assemblies, [64] and polymers [65]. These DDS have demonstrated less adverse effects, increased anticancer effectiveness, and high yields. However, host–guest interactions-based combination therapeutic nanomedicines have hardly ever been studied. A new supramolecular nanomedicine (SNM-3) described by Da Ma’s group is based on host–guest interactions between therapeutic drugs and acyclic CB[n] [66]. Enhanced chemical-photodynamic combination therapy it can be used for Tetraphenyl porphyrin (TPP), a photodynamic compound, camptothecin (CPT), and PEG were combined to create composite supramolecular nanomedicines (Fig. 4d). A redox-reactive disulfide bond linker (CPT-SS-TPP) was used to join CPT and TPP. CPT-mPEG, which was added into the supramolecular nanomedicine to improve its physiological stability, was created by coupling methyl polyethylene glycol to CPT.
Fig. 4.
CB-based luminescent Systems for Photodynamic Therapy. a Supramolecular co-encapsulation of a photosensitizer and chemotherapeutic drug in cucurbit [8luril for potential chemo phototherapy. Reprinted with permission from ref 107.
Copyright 2022 Photoch Photobio Sci. b Illustration of the formation and therapeutic action of the DOX-CB [8]−1-E-2/SLS supramolecular nanosheets. Reprinted with permission from ref 114. Copyright 2019 Journal of Materials Chemistry B. c The combination of photodynamic therapy with a putative depletion of polyamines on cancer treatment with compound 17. Reprinted with permission from ref 108. Copyright 2021 Commun Biol. d Chemical structures of CPT-SS-TPP, host 1, CPT-mPEG and a schematic illustration of the mechanism of chemo-photodynamic combination therapy. Reprinted with permission from ref 111. Copyright 2020 Chem Commun (Camb). e Facile Preparation of Cucurbit [6] uril-Based Polymer Nanocapsules for Targeted Photodynamic Therapy. Reprinted with permission from ref 115. Copyright 2019 ACS Appl Mater Interfaces
Acyclic CB[n] host 1 facilitated the supramolecular assembly of CPT-SSTPP and CPT-mPEG to generate a three-component supramolecular nanomedicine (SNM-3). To maximize the nanoscale morphology and therapeutic benefits, it can also be controlled by varying the ratio of the three components. The synthesis of intracellular singlet oxygen is more effectively boosted by the super-optimized supramolecular nanomedicine, which also has good stability. In the lowering intracellular environment, SNM-3 can distribute and release CPT and TPP. Cytotoxicity tests revealed that SNM-3 increases the combined chemotherapy-photodynamic therapy's ability to kill the three tumor cells.
In photodynamic treatment, supramolecular nanodrug delivery devices with stimulus-responsive qualities have drawn a lot of interest. CB[8] is a powerful macrocyclic compound that easily forms charge transfer complexes with a variety of guest molecule ratios. It has been extensively employed to create a range of molecular machines, photoluminescent materials, and topological nanostructures [19, 67, 68]. Cucurbit-based 2D supramolecular nano assemblies, which serve as direct drug transporters, have, however, only occasionally been documented. With a morphological transition based on secondary self-assembly of supramolecules in ternary complexes with a tris-bipyridine derivative [1] and a water-soluble azobenzene quaternary ammonium salt (E-2) as functional guests, Ting Zhang et al. reported a light-controlled, reversible, and recyclable 2D supramolecular nanosheet (Fig. 4b). [69]. Under UV light irradiation, CB[8] −1-E-2/SLS (SLS-sodium dodecyl sulfonate) assemblies show quick, reversible morphological alterations. The anticancer drug doxorubicin (DOX) is carried by several common hydrophobic alkyl chains in the composition of the nanosheet through hydrophobic interactions. The nanosheets disintegrate into erratic fragments with a dramatic size reduction when exposed to light, causing the medication to flow out gradually. Based on the rapid absorption of cancer cells by photosensitive supramolecular nanosheets and the increase in cytotoxicity after light irradiation, it is expected that light-sensitive supramolecular nanosheets will become effective drug carriers for cervical cancer. The nanosheets exhibit quick light-controlled drug release and a high drug loading capacity. This two-dimensional nanosheet with shape-tunable properties is thought to hold promise as a material for intelligent light-controlled medication delivery.
The encapsulation of the photosensitizer acridine orange (AO) and the chemotherapeutic medication oxaliplatin (OxPt) in CB[8] was investigated by the research team of Denis Fuentealba, and their individual and combined effects on in vitro-cultured tumor cells were evaluated (Fig. 4a) [70]. It was demonstrated that the OxPt@CB[8] complex was only cytotoxic at very lengthy incubation durations (24 h), but the AO@CB[8] complex was well absorbed into the cells and displayed considerable phototoxicity. Any photodynamic effects of subsequent treatment with the AO@CB[8] combination were reduced by pretreating cells for 24 h with the OxPt@CB[8] complex. However, after 90 min of co-incubation between the two complexes, the combined cytotoxicity/phototoxicity outperformed each treatment alone. A synergistic impact was discovered, increasing cytotoxicity/phototoxicity by an extra 30%. These findings suggest a novel approach for the development of photochemotherapy applications, wherein photosensitizers and chemotherapeutic drugs are co-encapsulated in a sizable ring. Here, CB[8] demonstrates a few standout benefits: [1] it can encapsulate up to two photosensitizer molecules, doubling the payload; and [71] some photosensitizers have a higher binding affinity than CB[7]. These findings are exciting for the potential use of supramolecular complexes based on CB[7]in chemo phototherapeutic applications, but further research will need to show synergy by adjusting the doses of the drug and light as well as demonstrating it in vivo.
Cancer therapy possibilities have been looked at that alter polyamine levels to block biosynthesis, transport, and breakdown. With good targeting, high tumor suppression, and little side effects, supramolecular chemotherapy can be utilized to treat spermine high-expression cancers. However, because spermine and the host have reversible host–guest interactions, apoptosis cannot be effectively induced. A promising approach would involve combining PDT with polyamine depletion.
A novel PPAB-based photosensitizer (compound 17) that can irreversibly create two photosensitizers and synergistically deplete polyamines was investigated by the team of Ruibing Wang [72]. A promising approach for polyamine depletion is combining it with PDT by synergistically damaging tumor cells (Fig. 4c). Compound 17 has ability to target lysosomes, perform ratiometric fluorescence imaging, deplete polyamines, and increase ROS production. Consequently, giving an irreversible polyamine depletion method that, when paired with PDT, increases anticancer activity.
For payload distribution and bioimaging applications, previously created covalent self-assembled polymer nanocapsules (NCs) based on CB[6] offer great potential. However, the multi-step process required to create these NCs is often arduous and time-consuming. Direct alkylation of per hydroxylated CB[6] with a dihydroxy linker not only saves a lot of time and effort but, more importantly, does not require UV irradiation or post-processing of the preparation, making non-covalent modification of the inclusions and nanocarbon surfaces possible. This approach was reported by Qian Cheng and Rui-Bing Wang’group [73]. This could result in a sharp rise in the quantity of biomedical research investigations conducted on these unique materials. For improving the ability to target cancer cells for targeted photodynamic therapy, the employment of photosensitive therapeutic payloads encapsulated within NCs also lends support (Fig. 4e).
CB [n] deliver pH-responsive nanodrugs
Incentive-response nanomedicine is an important "smart" drug delivery system (DDS) for targeted administration of chemotherapy drugs. PH-reacting vectors are considered one of the most promising tumor-oriented delivery systems targeting local diseases with low pH such as tumor tissue (pH = 5.7 ~ 7.8) and infectious/inflammatory sites (pH = 6.5), instead of targeting normal tissues and blood with a high pH (pH = 7.4) [74, 75]. This allows for the controlled release of anticancer drugs in the tumor environment, thereby increasing the specificity of chemotherapy. Specificity of chemotherapy treatment. It also contributed significantly to reducing the potentially serious side effects of doxorubicin and other chemotherapeutic agents due to their nonspecific interactions with normal and tumor tissues.
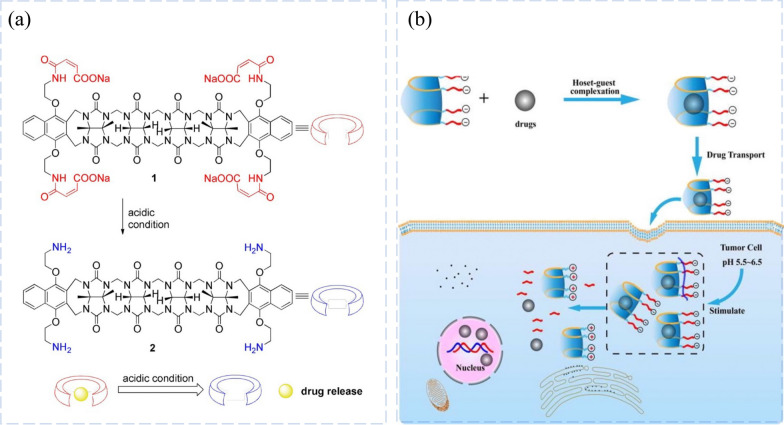
Because of the dynamic nature of host–guest interactions, supramolecular vesicles have great potential to develop into pH-sensitive targeted transmission of DDS translation. The Da Ma’group reported intelligent supramolecular vesicles constructed from host–guest interaction between the “acid-degrading” acyclic CB[n] and the doxorubicin pre-drug (Fig. 5a) [76]. “Acid-degradable” acyclic CB[n] is a high-affinity host for several common antitumor drugs, and its degradation leads to a more dramatic decrease in binding affinity than that observed for “acid-sensitive” hosts. The resulting supramolecular vesicles react to the acidity of the tumor (pH = 6.5). Under mild acidic conditions (pH = 6.5–5.5), acid-decomposed acyclic CB[n] can decompose, resulting in the transition of vesicles to micelles to form positively charged micelles. This shift results in pH-dependent changes in size and surface charge, which improves the uptake of doxorubicin tumor cells.
Fig. 5.
pH-responsive Drug Release Systems. a pH-Induced degradation of anionic container 1 to form cationic container 2, and a cartoon depicting acid-triggered drug release from the cavity of container 1. Reprinted with permission from ref 43.
Copyright 2019 Chemistry. b Host-1 and drugs combination and in vivo release concept. Reprinted with permission from ref 50. Copyright 2019 Bioorg Med Chem
Then, with CB[6] and doxorubicin as guests, the Da Ma'group created a supramolecular complex with slow dissociation properties for controlled release of the pH response [77]. CB[6] encapsulation significantly changed the chemical and biological properties and helped to achieve responsive controlled release to a weakly acidic pH (≈6.5) in cancer cells. By regulating pH responsiveness and transmembrane efficacy, CB[6] encapsulation allows for optimal controlled release and targeted drug administration. It is noteworthy that CB[6] encapsulation has been observed to inhibit the binding of doxorubicin to serum proteins, primarily albumin and immunoglobulins. This may potentially contribute to an improved biodistribution of doxorubicin in vivo.
Similarly, pH-responsive drug release systems (DDS) target the acidic tumor extracellular environment (pH = 6.5–7.0) [78]. Enabling the controlled release of other chemotherapeutic agents in the tumor environment also contributes significantly to reducing potentially serious side effects due to their non-specific interactions with normal and tumor tissues.
POD (POD, Fig. 4b) is a natural compound extracted from Polygonum multiflorum that has been shown in numerous studies to have good antitumor effects against various tumor cell lines (e.g., P-388 mouse leukemia, A-549 human lung cancer, HT29 human colon cancer, MEL-28 human melanoma) [79–81]. Etoposide (VP-16, Fig. 5b) is a synthetic derivative of POD. Since its discovery, derivatives have been used in the treatment of human malignant tumors and have become important antitumor drugs [82]. However, the poor water solubility of Pod, high toxicity to normal tissues, and various side effects that can cause liver and kidney dysfunction and diarrhea have hindered its further application in clinical treatment [79]. To improve the water solubility, the absolute bioavailability of POD and VP-16, and tumor targeting, Bo Yang’group designed and prepared acid-controlled release complexes of POD and VP-16 with pH-unstable acyclic cucurbit rings and validated their acid-controlled properties and encapsulation behavior [83]. The synthesis of stimulated-responsive acyclic CB[n] for increased drug solubility and targeted drug delivery is shown in Fig. 5. Under weakly acidic conditions (pH = 5.5–6.5), host-1 can be degraded to host-2, and when host-1 is delivered to the surface of tumor cells, anticancer drugs will be released for active action due to the weakly acidic conditions of tumor tissue. These studies provide strategies for better anticancer activity and clinical application of low cytotoxic anticancer drugs for targeting and response systems.
Although scientists have developed many “smart” nano-carrier systems for targeted release of drugs to reduce harmful side effects and improve therapeutic efficacy, only a limited amount of cargo can reach diseased tissues [84]. Therefore, the use of exogenous or endogenous triggers to achieve the release of cargo that responds to a stimulus can be a remedial option.
CB[n] for diagnosis applications
Nanomedicine represents an innovative direction with the potential to improve cancer diagnosis and treatment compared to conventional approaches. As a diagnostic platform, nanosystems can combine multiple modalities on a single nanosystem, which is a major advantage in conferring greater sensitivity and deeper insight into in vivo processes. In this regard, CB[n]s have been found to encapsulate a variety of guest molecules such as fluorescent dyes, drugs, and biomolecules. When using CB encapsulations, their performance varies to varying degrees. Therefore, the application of CB in the medical industry is gradually developing, and the diagnostic and imaging role of therapeutic supramolecular drugs can report the presence, location, status and response of tumors to a specific treatment, which is essential for accurate treatment. With the combination of imaging and therapeutic modalities, nanosystems for therapeutic diagnostics have the potential to change the current medical paradigm to one of imaging-guided therapy and personalized treatment. In this regard, we highlight the research progress of CB-based luminescent molecules or systems for imaging, probes, sensing systems.
CB[n]-based bioimaging
CB[n] probes
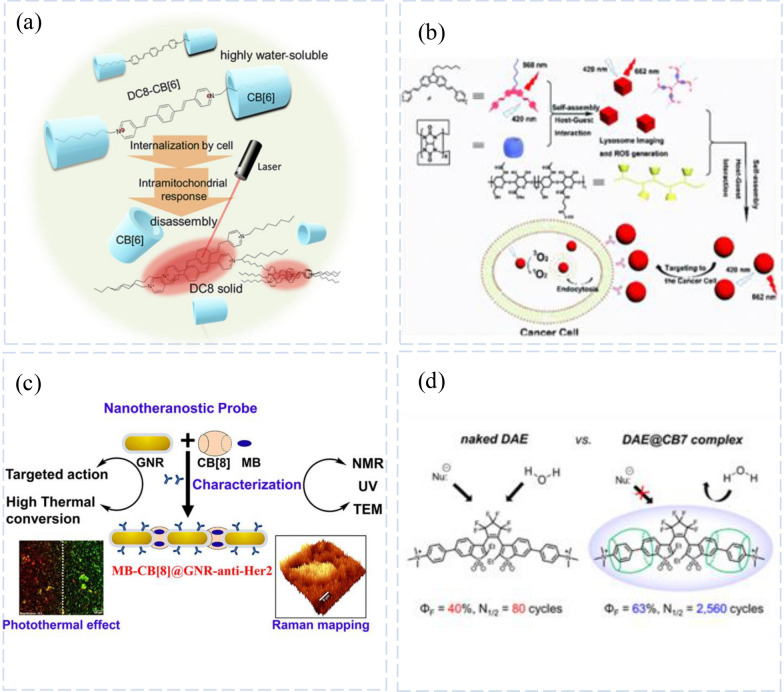
Due to their flexible stimulus–response properties, supramolecular probe adducts are also used in targeted drug administration and therapeutic diagnostic studies [67, 85]. Shuping Xu’group used BPEB derivatives (DCn: n = 8,12,16) and CB[6] based on the advantages of simple, rapid, and very stable complex formation of CB-encapsulated fluorescent dyes (CB[6]) couples, a supramolecular fluorescent imaging probe based on host–guest combinations was created for mitochondria-specific imaging (Fig. 6a) [86]. Due to their water solubility, these host–guest conjugates can be effectively inserted into cells and targeted mitochondria based on positive charge. In response to the intracellular microenvironment, these spliceosomes begin to disassemble dynamically. The released BPEBs display highly hydrophobic characteristics, which can crystallize to form fluorescent solids that illuminate the mitochondria. In situ fluorescence lifetime imaging confirms the intracellular disassembly of the host–guest probes. These smart mitochondria-targeted fluorescence imaging probes can be used to study the structure and function of mitochondria and are important for the dynamic transformation of supramolecular assemblies in the cell.
Fig. 6.
CB[n]-based Luminescent Systems for Supramolecular Probes. a Fluorescent Imaging Probe Targeting Mitochondria Based on Supramolecular Host- Guest Assembly and Disassembly. Reprinted with permission from ref 76.
Copyright 2022 ACS Omega. b Schematic Illustration of the Construction of the Targeting PDT System. Reprinted with permission from ref 77. Copyright 2019 Chem Commun (Camb). c Nanotheranostic Probe Built on Methylene Blue Loaded CB [8] and Gold Nanorod: Targeted Phototherapy in Combination with SERS Imaging on Breast Cancer Cells. Reprinted with permission from ref 82. Copyright 2021 J Phys Chem B. d Schemagtic Representation of Synergistic Phototherapies Exhibited by Targeted Theranostic Nanoprobe on Single Laser Irradiation. Reprinted with permission from ref 83. Copyright 2022 J Am Chem Soc
CB[8]’s ability to limit the rotation of the guest molecule and form an extended conjugated system made it possible for Xuan Wu and Yu Liu’s group to successfully assemble G, CB[8], and HA-CD ternary nano supramolecular assemblies using a multi-step assembly method. This extended conjugated system can be used as a targeted NIR lysosomal probe and PDT agent (Fig. 6b) [87]. This ternary construction dramatically decreases the light cytotoxicity of the G CB[8] complex to normal cells (293 T) while maintaining the light cytotoxicity to cancer cells (A549) because of the overexpression of the receptor on the surface of cancer cells. A complex system with NIR imaging capability and improved targeting PDT efficiency was successfully built in a relatively simple manner using orthogonal host–guest recognition of different macrocyclic molecules, offering a new method for building efficient cancer diagnostic and therapeutic systems by carefully designing the basic building blocks.
Localized cancer treatment absolutely requires the most recent developments in nano-architectural platforms for safe and efficient tailored phototherapy in a synergistic approach. Photothermal and photodynamic therapy (PTT and photodynamic therapy) is considered the most promising local treatment strategy for cancer as it has no long-term side effects, minimal invasiveness and reasonable price [88, 89]. This strategy necessitates the use of two separate laser light wavelengths in order to achieve the synergistic PDT and PTT phenomenon [90]. Therefore, it is quite difficult to establish a straightforward and efficient plan to investigate both PDT and PTT in a single laser [91].
Kaustabh Kumar Maiti’s group. Demonstrated an excellent nanotherapeutic diagnostic probe for solid tumor treatment (Fig. 6c) [92]. In order to study human breast cancer cells, a macrocyclic subject called CB[8] is used as glue between two gold nanorods (GNR) in a linear nano assembly with a spacing of about 3 nm. This method also uses phototherapy to study the cells. The development of human breast cancer cells during treatment. On a single laser trigger, the GNR is in the charge of the PTT and the photosensitizer methylene blue is responsible for the PDT on the CB[8], ensuring simultaneous phototherapy. In order to localize PTT and PDT on Her 2-positive SKBR 3 cells, nanoconstructures were also labeled with a targeted anti-Her 2 monoclonal antibody (MB-CB[8]@GNR-anti-Her 2). Cells were then identified using a surface-enhanced Raman spectroscopy (SERS) platform, and combined intracellular phototherapy was further assessed. To achieve effective local treatment of breast tumor cells using dual phototherapy, current technology is clearly a direct approach to proof of concept.
The research team of Stefan W. Hell created and implemented a straightforward photo switching system based on the supramolecular guest–host concept using the fluorescent molecules diarylethene (DAE) and CB[7] (referred to as DAE@CB[7]).Due to its excellent water solubility, low cytotoxicity, and significantly better binding affinity than alternative host molecules like cyclodextrins and calixarenes, [93] CB[7] was chosen as the host molecule for this supramolecular probe. The DAE molecule is shielded from the environment in this assembly by CB[7], which increases the fluorescence intensity and fatigue resistance of the DAE molecules in pure water. Additionally, complexation can preserve or enhance a substance's physical characteristics, including its capacity for photoisomerization, photostability, and fluorescence quantum yield (Fig. 6d).
The results of the experiment demonstrate that the fluorescence quantum yield increases and that the DAE@CB[7] complex can be switched on and off 2560 times in aqueous solution whereas the free DAE molecules can only be switched on and off 80 times. Biological conjugations of DAE@CB[7] and antibodies can be made by adding reactive groups to the system. According to experimental findings, under 355/485 nm light irradiation, intracellular proteins can be specifically labeled, and probes can be switched in and out of the cellular environment.
Others
An exciting feature of CB-based luminescent materials is that they come in a wide variety of ways, from small complexes to large supramolecular polymer systems documented in the literature. This is a direct result of the variable chemometry of the CB body-guest complex [94]. Due to their smaller size, CB[6] and CB[7] are preferably formed as 1:1 binary complexes, while CB[8] is usually obtained by simultaneously encapsulating two identical guest molecules in a 1:2 way or as two different guest molecules in a 1:1:1 way. In general, CB[6] and CB[7] binary complexing capabilities have been used to construct small luminescent complexes, while 1D supramolecular polymers or higher-order nanostructures are designed to take advantage of CB[8]’s three-component complex formation properties. One of the common features of all these materials is that their luminescence is tunable to respond to a variety of external stimuli and competing guests.
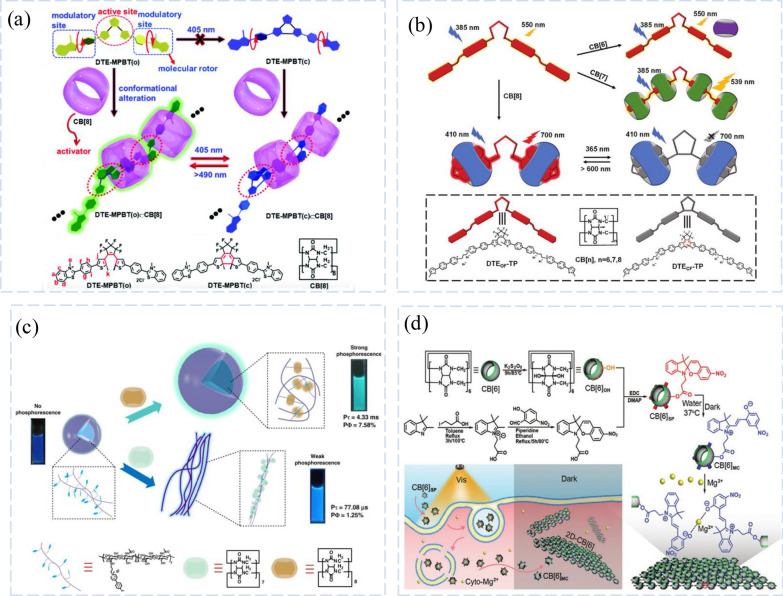
In order to construct for the first time an inverted fluorescence supramolecular set of two visible lights for directed lysosomal cell imaging and two-dimensional information recognition controlled by visible light, the team of Lei Lu and Yu Liu simulated the structure and function of natural enzymes [95]. Combined activated photochromic and combined activated emission enhancement were selected. Where CB acts as a macrocyclic host and activator. After a complex design and synthesis process, water-soluble DTE-MPBT containing thiophene ethylene and a fluorescent molecular rotor were generated. In addition, CB[8] acted as a modulator to limit the rotation of the MPBT slices (molecular rotor), resulting in a partially developed intermolecular charger transfer that tends to flatten out. By the AAEE method, the intervention of CB[8] also leads to efficient fluorescence enhancement of FF from 0.5 to 46.2% of DTE-MPBT.DTE-MPBTCCB[8] also demonstrates novel dual visible light-driven solid-state fluorescence switching properties with intelligent features for optically manipulated data storage and counterfeit detection. Notably, the generated bright fluorescence is highly fatigue-resistant and can be efficiently switched between two independent visible light sources (of different wavelengths) (Fig. 7a).
Fig. 7.
CB-based Luminescent Systems for Imaging. a Chemical Structures, Assembling Pattern and Assembly-activated Photochromism of the Host and Guest. The Proposed Assembling Pattern and Visible-light-driven Switching Mechanism of the Assembly DTE-MPBT CB [5], and the Chemical Structures of DTE-MPBT and CB [5]. Reprinted with permission from ref 53.
Copyright 2021 Mater Horiz. b Schematic Representation Structures of Guest Molecule 1 and CBs and Photographs of the Complex Solution. Reprinted with permission from ref 58. Copyright 2021Chem Commun (Camb). c The Construction and Behavior of CBs/HA-BrBP Supramolecular Pseudorotaxane Polymers in Aqueous Solution. Reprinted with permission from ref 66. Copyright 2020 Nat Commun. d Schematic Illustration and Chemical Structures of the Photo-controlled NIR Phosphorescence of DTE-TP/CB [5]. Reprinted with permission from ref 66. Copyright 2022 Small
In the case of fluorescent guest molecules, the subject-guest complex can often affect charge transfer and aggregation processes in the CB cavity of the guest molecule and result in tunable photophysical properties such as wavelength displacement and emission increase [96]. By using this strategy, the UV–vis of the dye molecule as a guest and the red shift in the fluorescence spectrum can be used for bioimaging and photothermal therapy applications [97–99].
Based on this, Yingjie Zhao’s group obtained two different complexes of the tri-thematic guest molecule 1 with CB[7] and CB[8] in water [100]. X-ray single crystal diffraction and 1H NMR spectra gave the supramolecular structures of the complexes. In particular, the fluorescence spectrum of 1_CB[8] was red-shifted by 142 nm. different cell staining behavior was observed due to the different structures. 1_CB[7] could stain both nucleus and cytoplasm, while 1_CB[8] stayed mainly in the cytoplasm. This simple complex formation process not only regulates the photophysical properties of the dye, but also allows the paint to selectively color different areas of the cell.
In conclusion, the presence of CB[n]s results in a significant red-shift in the UV and fluorescence spectra of the host–guest complex compared to the guest molecule itself, making it an ideal candidate for biomarkers.
Encapsulation in the CB cavity causes significant changes in the photophysical properties of the chromophore and often leads to increased emissions. This is due to the combination of hydrophobicity of the cavity and mechanical shielding of the guest in an aqueous medium with harmful self-aggregation and quenching [94]. In addition, the hardening of the encapsulated chromophores in the CB cavity facilitates access to pure organic-based room-temperature phosphorescent materials, which has attracted extensive attention regarding bioimaging [101]. The majority of reported NIR phosphorescent systems are currently based on transition metal complexes [102, 103]. In such complexes, metal–ligand charge transfer (MLCT) can occur, which promotes spin–orbit bonding, where modification of the chelating ligand can lead to a red-shift in the emission, resulting in NIR phosphorescence [104, 105]. In contrast to inorganic NIR phosphorescence, pure organic NIR room-temperature phosphorescence (RTP) is rare [106].
Thus, Yu Liu’s group reported the construction of macrocyclic-restricted pure organic room-temperature phosphorescent (RTP) supramolecular assemblies using diarylethene phenyl pyridine derivatives (DTE-TP) and CB[8] bicarbonate (Fig. 7b) [107]. The results showed that CB[8] induced the folding of DTE-TP to form supramolecular assemblies and promoted the photochromism of the diaryl ethylene fraction. In addition, CB[8] in the process of holding the body's guest molecule also increases the degree of charge transfer interaction in the molecule, resulting in a near-infrared (NIR) RTP emission of 700 nm, followed by a red shift to 817 nm with phosphorescent resonance energy transfer. Due to the excellent photochromic properties of the guest diaryl ethylene nucleus, light at wavelengths of 365 nm and > 600 nm could be the NIR phosphorescence emission can be effectively modulated by irradiation at 365 and 600 nm. The targeting and photocontrol capabilities of this device as a supramolecular phosphorescence switch are also demonstrated in cell imaging investigations. In conclusion, this macrocyclic confinement technique's improved phosphorescence offers a simple path to materials with controllable functionality.
While the research group of Jing-Jing Li and Yu Liu revealed a water-soluble ultra-long organic supramolecular polymer that combines hyaluronic acid (HA) and CB[n]s (n = 7,8) as tumor-targeting ligands with 4-(4-bromobiphenyl) pyridine-1-ammonium bromide (BrBP) phosphor ether (Fig. 7c) [108]. The findings revealed spherical CB[8]/HA-BrBP pseudo-multi-UV photophore polymers with a phosphorescent quantum yield of 7.58% and an incredibly long RTP lifetime (4.33 ms) when compared to nanofibrous CB[7]/HA-BrBP compositions. The biaxial pseudo-poly (paclitaxel) polymer CB[8]/HA-BrBP has been successfully used for targeted mitochondrial phosphorescent imaging of tumor cells due to a combination of phosphorescent emission and the targeting ability of tumor cells. These properties are attributed to the synergistic effect of BrBP, along with strong host–guest interaction with CB[8] and HA chain hydrogen bonds.
Supramolecular organic backbones (SOFs) constructed using host–guest systems have received much attention due to their synthesis and morphological control by simple design methods. Among them, cucurbit[8]uril (CB[8]), a water-soluble host molecule, is effective in inducing SOF in aqueous systems [17, 109]. However, despite the demand for organic fluorescence such as biosensors or bioimaging, the implementation of fluorescent SOF is very limited because most organic chromophores suffer from concentration burst effects in the concentrated state, including in the nanochannels of SOF in the concentrated state [110]. In order to achieve luminescence enhancement in the nanochannels of SOF, Prof. Soo Young Park's group proposed using cyanogenic astragalus derivatives with aggregation-induced enhanced emission (AIEE) [111]. And using CB[8] as the main molecule, a bent cyanogenic astragalus derivative was designed to achieve zero-dimensional cyclic SOF in an aqueous solution preparation. This intriguing guest molecule, unlike other cyano stilbene derivatives, exhibits rotational flexibility and a bent shape in the NP condensed state, partially sliding and stacking but not securely packed. The monomer, on the other hand, gave off very little fluorescence (ΦF = 2%), while the hexameric ring complex produced enhanced fluorescence (ΦF = 68%) with a diameter of a few nanometers when CB[8] was added. The fact that this is the first instance of a high emission 0D SOF being effectively produced down to 0.1 mM, a very high concentration in comparison to previous SOF, is most significant. This opens new possibilities for bioimaging, drug administration, biocompatible dispersion, and other uses that call for uniformly dispersed, highly fluorescent nanoscale media.
Some 2D materials are becoming an emerging platform for intracellular bioimaging, biosensing, or disease treatment diagnostics due to their unique structural advantages and physicochemical characteristics. Despite promising developments in this young field, overcoming the endocytic absorption barrier to enable the ingestion of very large 2D materials remains a significant obstacle [112, 113]. Regardless of their chemical makeup, 2D materials may always be seen as assemblies made up of faceted monomer units that are laterally coupled and extend in two orthogonal directions [114]. Because of their typically smaller than 1 nm size, these monomers are not difficult to internalize and enrich in living cells. The Yi Chen group reported the creation of the first molecularly designed monomer that satisfies these criteria. (Fig. 7d) [115]. This monomer easily moves into the cytoplasmic lysate where, in reaction to light, it polymerizes in place to form a 2D substance with a single monomer thickness. The resulting 2D material can be produced in place to lateral dimensions as large as 0.8–1.2 m, which is larger than what the endocytic absorption pathway permits. The surface of the resulting 2D materials can be further tailored to host contrast agents, biorecognition components, or therapeutic agents because CB[6] forms stable complexes with positively charged alkylamines through subject-guest interactions. This creates a robust platform for intracellular signaling or therapeutic diagnostic agents with potency that may be greater than that of conventional platforms acting on endocytosis prerequisites.
CB[n]-based sensors
By identifying and removing precancerous lesions, screening helps prevent colorectal and cervical cancers, according to Global Cancer Facts & Figures. Additionally, screening can lower mortality from breast, colorectal, cervical, and lung cancers (in heavy or long-term smokers) and can identify some tumors early, when treatment is frequently more effective [116]. This makes early detection of cancer critical to both patient survival and treatment outcomes. In contrast to conventional cancer detection techniques, such as positron emission tomography (PET)2, magnetic resonance imaging (MRI), and computed tomography (CT), sensors come into play here.
A new class of highly sensitive, reasonably priced, and non-invasive sensors that can more accurately identify diseases has been made possible through CB-based dye encapsulation.
Varying l -o-amino acids with varying pH values exhibit different affinities for DSQ@iQ[7] sensors. Since amino acids are the fundamental structural constituents and constituent parts of proteins and peptides, they play a variety of roles in physiological processes [117]. Coordination of metal complexes, shear indicators, as well as specific amino acids and sensors makes it possible to detect amino acids using fluorescent sensors and colorimetric chemical sensors [118, 119]. Among these, host–guest interactions and their possible use in chemical sensors based on cucurbituronium supramolecules’ ability to self-assemble and recognize amino acids are progressing quickly. For the purpose of detecting L-amino acids in aqueous solutions, Mei-Xiang Yang and his team have developed a pH-stimulated responsive 4-(4-dimethylaminostyryl)quinoline (DSQ) and reversed-phase CB[7] complex-based supramolecular sensor [120]. At various pHs, DSQ complexation within CB[7] results in increased fluorescence and the creation of CB[7]-DSQ complexes of various colors. The CB[7]-DSQ sensor responded differently to the addition of amino acids in terms of fluorescence and color alterations. Differentiating L-amino acids with comparable structural characteristics is made possible using principal component analysis. This amino acid sensing makes it possible to track the structure of proteins and helps to identify amino acids in various side chains.
Advances in hyperpolarized 129Xe MRI technology in the clinical setting have proven to be of considerable interest for this diagnostic imaging modality. In this regard, Prof. Alexander Pine ‘group reported for the first time the direct functionalization of cucurbituril for a xenon biosensor [121]. When biotinylated CB[7] (btCB[7]) is attached to a protein target with significant CEST contrast, a 129Xe hyper-CEST response is generated at a unique shift of 28 ppm. Enabling multiple biological targets through affinity coupling, this structure combines the advantages of a directly functionalized xenon-loaded macrocyclic compound with a non-covalently functionalized structural biosensor. The ability of cucurbiturate-based xenon biosensors to selectively, prudently, and maybe simultaneously report several disease signals during a single 129Xe imaging session is thus supported.
Because their high levels in urine reflect faulty biological processes connected to this lethal disease, spermidine (SP) and spermidine (SPD) are biomarkers for early identification of human malignancies [122]. The robust complexation of macrocyclic hosts with spermine in recent years has drawn increased scientific interest to supramolecular methods for spermine detection [123]. In other instances, spermine has modulated the supramolecular system morphology and generated strong fluorescence signals [124]. Due to its great capacity for binding polyamines, water-soluble CB [7] has been chosen as a preferred host. As a supramolecular sensor, a highly emissive “dumbbell” super-amphiphilic molecule based on perylene diimine (compound 1) and CB[7] was utilized to detect spermine quickly and extremely sensitively [125]. Spermine has a strong affinity for CB[7] and binds to CB[7] well (binding constant K = 2.6 × 107 M−1), causing the top amphiphile to dissociate and resulting in a fluorescence burst. Compared to other structural analogs (such as spermidine, putrescine, l-arginine, and l-lysine), the higher amphiphiles are more selective for spermine. This great sensitivity persisted even in the presence of spermine at low doses (10 nM) [122].
To create a fast, sensitive, and targeted supramolecular sensation platform to detect SP and SPD in water, Amrita Chatterjee's team developed a solid-backed amplification strategy for the polymerization-induced emission effects of water-soluble tetrastylbene (TPE) probes [126]. Unmeasurable TPE derivatives (TPEHP) react electrostatically with the solid surface of hydroxyapatite nanoparticles (HAp NPs) distributed in aqueous solutions to generate a less emissive conjugate that is several times more effective at emitting light. Due to their increased binding affinity for CB[6], SP and SPD invade the CB[6] portal and disrupt the matching three-component supramolecular assembly, making them more sensitive to disabling the fluorescence sensor. Limits of detection (LOD) for SP and SPD, respectively, from the sensing system were 1.4 × 10–8 and 3.6 × 10–8 M, which fall well short of the range needed for early cancer diagnosis. Additionally, both SP and SPD showed acceptable linearity. With minimal intervention of various metal ions, anions, common compounds, amino acids and other biogenic amines, this sensor platform is suitable for real-time, low-level evaluation of SP and SPD in human urine and blood samples.
Due to non-specific interactions between amine functional groups, the fabrication of dyes on nanomaterials to detect biogenic amines frequently lacks selectivity and calls for the use of hazardous organic solvents for sensing experiments. These probes have not been completely validated under physiological circumstances. Conversely, supramolecular approaches have demonstrated potential for improving selectivity for SP or SPD through supramolecular interactions.
By displacing the reporter unit through stronger subject-object interactions, [127]. In comparison to other cavity ligands, CB[6] or CB[7], which have a high affinity for SP and SPD, clearly have an advantage. More recently, it has given rise to supramolecular systems for complex applications such as the detection of metabolites [128] and supramolecular chemotherapy [44]. These systems, despite having sensitive probes, are less selective for specific BPA [129] or created to only detect spermine, [130] which makes it difficult to calculate the total concentration of SP and SPD in biological samples. According to Anjan Chattopadhyay and Amrita Chatterjee’s research team, this discovery spurred the development of supramolecular sensors to detect subppb levels of cancer biomarkers symin and spermine [131]. The donor–acceptor combination of red-emitting dyes with pyridinium units (BPBP) as energy acceptors with CB[7] and hydroxy graphene quantum dots as energy donors was employed for an affinity-driven dynamic exchange of the guest. The strategic introduction of cavity ligand CB[7] allows for highly selective and sensitive detection of spermidine and spermidine through proportional metrological reactions under physiological conditions, the first step in the use of GQD-based donor–acceptor pairs in affinity-based supramolecular sensation. By transferring energy, GQDs-OH excites the red dye in the binary conjugate while maintaining its own off state. The dye is removed from the surface of GQDs-OH, causing it to glow in addition to CB[7]. The red dye begins to settle back onto the surface of the GQD, reducing the fluorescence emission of GQDs-OH in response to the injection of spermine or spermidine. Other neutral compounds such as amino acids, common metal ions, anions, and polyamines are less influenced by this supramolecular sensing assembly. Notably, the two-component BPBP@CB[7] system may be employed as an SP and SPD turn-on sensor with respectably good LODs of 20.2 and 36.3 ppb. Therefore, a good method for analyte ratiometric detection using cavity ligands as their acceptable recognition units is the combination of cavity ligands with GQD-based charge-transfer adducts.
CB[n] for antidote
Even though numerous CB[n]-based nanoplatforms have been developed, and while early success has been attained using these materials in a range of prospective biomedical applications, it is still difficult to expand these systems to real-world clinical therapeutic applications. The actual cytotoxicity and detoxification of CB[n] as a medicinal excipient in this particular circumstance.
The antidotes should not only have good biocompatibility and biosafety, but also be able to recognize the poison specifically and have high affinity, to realize the effective detoxification of drugs. CB[n] have been developed as antidotes for some poisons due to the good biocompatibility and high drug loading capacity. As drug isolation chambers, supramolecular hosts have higher stability and longer in vivo maintenance time than traditional bio-antidotes such as enzymes and antibodies. In addition, the structures of supramolecular compounds are adjustable, so that CB[n] can be designed and synthesized to meet functional needs. However, the selectivity of these bio-macromolecular hosts for drugs remains to be enhanced, which is the focus of the future research.
In this section, we mainly introduced the application strategies of CB[n] as anesthetic agents, neuromuscular blocking agents, paraquat and other toxic reversal agents, as well as the research progress of CB[n] in reducing biological toxicity of clinical drugs.
CB[n]-based antidote as reversal agents
The pharmacodynamic methods of antagonism and pharmacokinetic methods of drug concentration reduction both alleviate the toxicity of drugs (Fig. 8a) [132]. According to the theory of pharmacokinetics, catalyzing the breakdown of poison or isolating it from the blood can reduce the concentration of drugs in the circulation system, which is one of the mechanisms of detoxification therapy. Supramolecular macrocyclic substances have been used as an alternative to traditional detoxification therapy, for they can bind to guest compounds and isolate them in the cavity, thus achieving the separation of drugs from blood. Murkli et al. confirmed the excellent performance of CB[n] as the drug isolator, which had high affinity for neuromuscular blocking agents, narcotic drugs and abuse drugs [133, 134]. In addition, they explored the isolation effect of CB[8] derivatives on drugs in another study to expand the detoxification range of CB[n]. The results showed that water-soluble Me4CB[8] showed strong affinity for ketamine, morphine and other commonly abused drugs, which had low cytotoxicity and could be used as a safe and efficient bioantidote, and was expected to be promoted to treat drug abuse [135].
Fig. 8.
Chemical structure and mechanism of CB[n]. a Process of pharmacodynamics and pharmacokinetic detoxification. Reprinted with permission from ref 133.
Copyright 2020 Chemical Society Reviews. b The structures of M1, M2 and M3 CB[n]-type receptors. Reprinted with permission from ref 139. Copyright 2020 Chemistry. c Detoxification process of CB[7] complexed with PQ. Reprinted with permission from ref 145. Copyright 2019 Theranostics. d The structures of C2, C3 and C4 CB[n]-type receptors. Reprinted with permission from ref 140. Copyright 2019 Croatica Chemica Acta. e The AUC of asoxime-CB [7] is 3.2 times to that of oxime. Reprinted with permission from ref 142. Copyright 2021 Molecular Pharmaceutics
Neuromuscular blocking agents (NMBAs), which cause temporary paralysis of skeletal muscle by specifically binding to acetylcholine (ACh) receptors at neuromuscular junctions, are commonly used anesthetic agents currently [136]. The use of NMBAs in medical procedures may cause adverse effects such as respiratory depression and bradycardia [137], and to undo the blocking effects of NMBAs, efficient detoxifying techniques must be developed. The M1 CB[n]-type receptor, which is the most prevalent, and the M2 CB[n]-type receptor, which is made by extending the aromatic sidewalls of M1 in a linear plane, have both been shown to be in vivo reversal agents for NMBAs and abused substances. Murkli and colleagues further extended the aromatic sidewalls of the M2 variant to prepare M3-type CB[n]. It had been found that M3 had a stronger reversal effect on NMBAs than M2, that is, the linear π-extension of the aromatic sidewalls of the macrocyclic host effectively enhanced its affinity for NMBAs, enhancing its great potential as the reversal agent for NMBAs (Fig. 8b) [138]. Shaya and Isaacs explored host affinity for guest compounds by combining CB[n] with ACh and 4 NMBAs in vitro. Results showed that 2C3 and its analogues 2C2 and 2C4 had high affinity, good binding effect, and high selectivity for ACh, which could be further used to develop in vivo reversal agents for NMBAs (Fig. 8d) [139].
Oxime reactivates the function of acetylcholinesterase and is commonly used in the detoxification of organophosphorus (OP) poisoning. However, due to the instability and low permeability of oxime, it is easily eliminated in vivo and difficult to penetrate the blood–brain barrier, which greatly reduces the detoxification efficiency of oxime [140]. Andrys et al. encapsulated asoxime with CB[7] and measured the pharmacokinetics of asoxime-CB[7] complex in vivo in mice models. The results of liquid chromatography-mass spectrometry showed that compared with asoxime alone, the content of oxime in the brain was higher with asoxime-CB[7] complex, and the area under the curve was increased by more than 3 times. At the same time, CB[7] was detected in the brain. In addition, data showed that by increasing the content of cerebral oxime, CB[7] improved the brain function by up to 11%. All the above results indicated that the encapsulation of CB[7] effectively improved the detoxification process of oxime and increased the detoxification efficiency of OP poisoning. This may owe that the encapsulation of supramolecular macrocyclic host CB[7], which concealed the hydrophilicity of oxime and enhanced its ability to penetrate the biofilm barrier, thus improving its bioavailability (Fig. 8e) [141]. In a similar study, Karasova et al. pointed out that CB[7] assisted oxime to enter the brain, in addition to removing blood oxyphosphorus and enhancing the effect of atropine. In other words, CB[7] significantly improved the overall therapeutic effect from 3 aspects: reducing the circulating concentration of toxicants, enhancing the bioavailability of antagonists and improving the targeting of antidotes [38].
As one of the most lethal pesticides, paraquat (PQ) causes rapid failure of multiple organs, and there is a lack of effective detoxification methods currently [142]. In view of the high affinity of paraquat with CB[7], which can form a stable complex [143], several studies have explored the feasibility of CB[7] as an antidote for PQ. In addition to determining the pharmacokinetics and in vivo distribution of the PQ@CB[7] complex in cell lines and mouse models, respectively (Fig. 8c), Zhang and colleagues also described the affinity of PQ with CB[7] in vitro. The outcomes demonstrated that both could be strongly complexed in vivo and in vitro under any circumstances; in other words, CB[7] considerably lessened PQ’s toxicity (Fig. 9a, b) [144]. Another study combined PQ and CB[7] in water in equal parts to create PQ@CB[7], a promising macromolecular medication for lowering PQ toxicity. According to in vitro investigations, CB[7] dramatically decreased the interference of PQ on cells by lowering the quantity of ROS and the degree of apoptosis in cells. In vivo tests revealed that PQ@CB[7] considerably increased the survival rate of zebrafish and mice by reducing tissue abnormalities and organ failure brought on by PQ (Fig. 9c)145.
Fig. 9.
The detoxification effect of CB [7]. a The detoxification effect of CB [7] on paraquat poisoning in mouse lung cells. Reprinted with permission from ref 145.
Copyright 2019 Theranostics. b The detoxification effect of CB [7] on PQ poisoning at the cellular level. Reprinted with permission from ref 145. Copyright 2019 Theranostics. c Effects of PQ@CB [7] on survival rate and physiological parameters of mice. Reprinted with permission from ref 151. Copyright 2019 Journal Agricultural and Food Chemistry. d The detoxification effect of CB [4] on paraquat poisoning in a mouse model in vivo. Reprinted with permission from ref 145. Copyright 2019 Theranostics
Acyclic CB[n]-based antidote
Cheng and colleagues reported a chimeric synthesis strategy of acyclic cucurbituril and cyclodextrin that significantly enhanced the host–guest affinity. It had been demonstrated that the acyclic CB[n]–cyclodextrin chimeric host increased fentanyl's affinity and isolated it from the circulatory system, making it a possible supramolecular antidote [146]. Thevathasan and colleagues tested the reversal effect of acyclic CB[n], Calabadion 1, on fentanyl in mice models. Pneumotachography and blood gas analysis indicated that acyclic CB[n] effectively reversed fentanyl-induced hypoventilation, acidosis, and hypercapnia. And 15.6 mg·kg-1 acyclic CB[n] could reverse respiratory depression by up to 90%, and there was no tendency of respiratory depression recurrence. They pointed out that compared with competitive antagonists, acyclic CB[n] was more efficient and safer in alleviating adverse drug reactions for the encapsulation of fentanyl [134]. Ganapati et al. confirmed that Calabadion 1 and 2 had good affinity for a variety of illicit drugs, with the highest affinity for fentanyl, which could be further optimized as antidotes for opioid abuse poisoning.
The detoxification effect of Calabadion 2 was similarly reflected in the reversal of NMBAs. Haerter and colleagues evaluated the detoxification effect of Calabadion 2 by establishing dose–response curves of NMBAs in vitro and in vivo. In vitro experiments had shown that Calabadion 2 had a high affinity for rocuronium bromide and a high selectivity for ACh, and that all types of NMBAs could be effectively bound by Calabadion 2 and eliminated through the renal pathway [147]. Diaz-Gil and colleagues evaluated the detoxification effect of Calabadion 2 on narcotic drugs. They examined the detoxification effects of Calabadion 2 by electroencephalogram (EEG), blood pressure and circulatory toxicity after intravenous and intramuscular injections of the narcotic ketamine to rats. The results showed that Calabadion 2 effectively reversed the deep anesthetic effects of ketamine and could be excreted via the renal pathway after encapsulation of the drug. Meanwhile, Calabadion 2 showed low cytotoxicity and could be used as a safe and reliable antidote for narcotic drugs [148].
Summary and perspectives
CB[n]s based on host–guest chemistry show promising potential in the fields of targeted delivery of drug therapy, diagnostic imaging, and detoxification. CB[n] acts as a cross-linker that combines different molecules and functional components to form macromolecular host–guest complexes, thereby producing supramolecular polymers with multiple properties, such as targeting, imaging, or stimulus sensitivity. In addition, CB[n] can also be used as a cross clutch or stimulus response; As a functional group, it provides the non-covalent supramolecular encapsulation capacity of polymeric materials, thereby CB have ability to utilize a variety of molecules and functional components to form macromolecular host–guest complexes [149]. Through cleverly designed host–guest complexes, CB[n]s provides excellent in vivo stability, solubility, and bioavailability of drugs. They reduce or even eliminate toxicity to normal tissues, synergistically enhance drug effects, and achieve targeted drug delivery. Besides targeted delivery, the protective effect of CB[n] host–guest complexes on the guest, make CB-based luminescent molecules or systems promising for cancer diagnosis and therapy. CB[n] utilizes its targeted delivery effect and high affinity for specific molecules to achieve detoxification functions by reducing the circulating concentration of toxicants, increasing the bioavailability of antagonists, and improving the targeting of antitoxins.
Normally, CB[8] functions as a “glue” to join two molecules that don't have any affinity for one another. Targeted medication delivery is less prevalent when using CB[8]-based nanoplatforms than controlled release. Soluble CB[7] serves as a modulator to hide drug activity and facilitate the attachment of nanocarriers. They have the properties of a large number of guest molecules, a stable host–guest complex and high safety of small changes and have a high potential for application in the medical field. When creating drug carriers, researchers should choose the CB that best fits the application (Table 1).
The supramolecular nanotherapy of CB is springing up rapidly, and the disruptive perspective for host–guest chemistry in clinical application is motivating. Design functionalized CB and guest molecules is not difficult for chemists, but it is still a challenge to communicate with biological system. CB-based clinical biological therapy is the hope for advanced cancer patients. One is that CB systems driven the cell therapy. Through the host–guest interactions, manipulate the functions of immune cells (eg. natural killer cells, macrophage, dendritic cells) to cure specific solid tumor as CB-engineering immune cell therapy. The other one is CB-based gene therapy. As a carrier, CB delivery targeted therapeutic DNA (or DNA hydrogel) inside solid tumor. Importantly, fabricate multiple stimuli-responsive (eg. light or/and electrical stimulation) response to assist CB-based biological therapy. All the CB-based biological therapy is to anticipate to attain high efficiency for solid tumor, but with no side effect to normal tissues. The nanobiotechnology of CB-based biological therapy is to be envisioned in clinic, pharmaceutical science and functional materials science.
Current investigations have been on creating and testing CB[n]-based supramolecular nanoplatforms in vitro. More in vivo research, particularly with big animal models, is urgently required to confirm the therapeutic promise of particular platforms and to make it easier for prospective clinical translation. Furthermore, many therapeutic techniques should be applied at once since malignancies, especially hypoxic solid tumors, are challenging to treat with a single therapeutic strategy due to their intrinsic disadvantages. Therefore, one of the most important factors to take into account is the combined use of various therapeutic techniques to achieve additive therapeutic effects by fusing the benefits of each treatment strategy. On the other hand, although numerous CB[n]-based nanoplatforms have been developed over the past few years and employing these materials in a number of prospective biomedical applications have initially been successful, scaling these systems to real-world clinical applications has proven to be difficult. Low yields, exorbitant costs, and actual harmful effects as drug excipients are a few of these reasons. However, given past advances and ongoing efforts to develop and validate these systems in preclinical models, it is expected that CB can provide an important therapeutic or diagnostic platform for accurate synergistic treatment of cancer to address important medical challenges in the future.
Acknowledgements
The authors would like to express our gratitude to the Beijing Municipal Natural Science Foundation, the National Natural Science Foundation of China, and the Capital Medical University program for their invaluable support.
Abbreviations
- CB[n]
Cucurbit[n]uril
- DDS
Drug delivery systems
- ACBB
Acyclic cucurbitacin
- FSMs
Functionalized supramolecular micelles
- aCB
Acyclic Cucurbituril
- MV
Dimethyl viologen
- CLE
LP + EP@CB[8] complex
- BDQ
Bedaquinoline
- TZ
Trazodone
- PEI
Polyethylenimide
- FGG
High-affinity tripeptide-ph-gly-gly
- MT
Microtubules
- BPs
Benzimidazole-modified antisecretory peptides
- PDT
Photodynamic therapy
- ROS
Reactive oxygen species
- CPT
Camptothecin
- CT
Charge transfer
- DOX
Doxorubicin
- AO
Acridine orange
- NCs
Nanocapsules
- GNR
Gold nanorods
- DAE
Diarylethene
- SERS
Surface-enhanced Raman spectroscopy
- MLCT
Metal–ligand charge transfer
- RTP
Room-temperature phosphorescence
- DTE-TP
Diarylethene phenyl pyridine derivatives
- NIR
Near-infrared
- HA
Hyaluronic acid
- SOFs
Supramolecular organic backbones
- AIEE
Aggregation-induced enhanced emission
- PET
Positron emission tomography
- MRI
Magnetic resonance imaging
- CT
Computed tomography
- DSQ
Dimethylaminostyryl)quinoline
- TPE
Tetrastylbene
- HAp NPs
Hydroxyapatite nanoparticles
- LOD
Limits of detection
- BPBP
Pyridinium units
- NMBAs
Neuromuscular blocking agents
- Ach
Acetylcholine
- PQ
Paraquat
- EEG
Electroencephalogram
Author contributions
M.M., as a major contributor, wrote the original manuscript and draw the figures. G.W., Y.L. and Z.P. participated in part writing the manuscript. Y.C. provided the funding and reviewed for this manuscript. All the authors revised the final manuscript.
Funding
This work was financially supported by Beijing Natural Science Foundation (No. L223015), National Natural Science Foundation of China (No. 21805197), Beijing Natural Science Foundation (No.2212026), and the Project of Capital Medical University (No. CXZDS2024).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Veeranarayanan S, Azam AH, Kiga K, Watanabe S, Cui LZ. Bacteriophages as solid tumor theragnostic agents. Int J Mol Sci. 2022. 10.3390/ijms23010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71(3):409–19. 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Li WP, Little N, Park J, Foster CA, Chen JW, Lu JQ. Tumor-associated fibroblast-targeting nanoparticles for enhancing solid tumor therapy: progress and challenges. Mol Pharmaceut. 2021;18(8):2889–905. 10.1021/acs.molpharmaceut.1c00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DE, Koo H, Sun IC, Ryu JH, Kim K, Kwon IC. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem Soc Rev. 2012;41(7):2656–72. 10.1039/c2cs15261d. [DOI] [PubMed] [Google Scholar]
- 6.Yu G, Chen X. Host-guest chemistry in supramolecular theranostics. Theranostics. 2019;9(11):3041–74. 10.7150/thno.31653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo C, Sun J, Sun B, He Z. Prodrug-based nanoparticulate drug delivery strategies for cancer therapy. Trends Pharmacol Sci. 2014;35(11):556–66. 10.1016/j.tips.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Ma X, Tian H. Stimuli-responsive supramolecular polymers in aqueous solution. Acc Chem Res. 2014;47(7):1971–81. 10.1021/ar500033n. [DOI] [PubMed] [Google Scholar]
- 9.Hu J, Liu S. Engineering responsive polymer building blocks with host-guest molecular recognition for functional applications. Acc Chem Res. 2014;47(7):2084–95. 10.1021/ar5001007. [DOI] [PubMed] [Google Scholar]
- 10.Heo J, Kim SY, Whang D, Kim K. Shape-induced, hexagonal, open frameworks: rubidium ion complexed cucurbituril. Angew Chem Int Ed Engl. 1999;38(5):641–3. 10.1002/(sici)1521-3773(19990301)38:5%3c641::Aid-anie641%3e3.0.Co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Isobe H, Tomita N, Lee JW, Kim HJ, Kim K, Nakamura E. Ternary complexes between DNA, polyamine, and cucurbituril: a modular approach to DNA-binding molecules. Angew Chem Int Ed Engl. 2000;39(23):4257–60. 10.1002/1521-3773(20001201)39:23%3c4257::Aid-anie4257%3e3.0.Co;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Kornmüller A, Karcher S, Jekel M. Cucurbituril for water treatment. Part II: ozonation and oxidative regeneration of cucurbituril. Water Res. 2001;35(14):3317–24. 10.1016/s0043-1354(01)00039-2. [DOI] [PubMed] [Google Scholar]
- 13.Marquez C, Nau WM. two mechanisms of slow host-guest complexation between cucurbit[6]uril and cyclohexylmethylamine: pH-responsive supramolecular kinetics. Angew Chem Int Ed Engl. 2001;40(17):3155–60. 10.1002/1521-3773(20010903)40:17%3c3155::Aid-anie3155%3e3.0.Co;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Kim HJ, Oh J, Kim SY, Lee JW, Sakamoto S, et al. Cucurbit[n]uril derivatives soluble in water and organic solvents. Angew Chem Int Ed Engl. 2001;40(22):4233–5. 10.1002/1521-3773(20011119)40:22%3c4233::Aid-anie4233%3e3.0.Co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Jon SY, Selvapalam N, Oh DH, Kang JK, Kim SY, Jeon YJ, et al. Facile synthesis of cucurbit[n]uril derivatives via direct functionalization: expanding utilization of cucurbit[n]uril. J Am Chem Soc. 2003;125(34):10186–7. 10.1021/ja036536c. [DOI] [PubMed] [Google Scholar]
- 16.Yin H, Cheng Q, Bardelang D, Wang R. Challenges and opportunities of functionalized cucurbiturils for biomedical applications. JACS Au. 2023;3(9):2356–77. 10.1021/jacsau.3c00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrow SJ, Kasera S, Rowland MJ, del Barrio J, Scherman OA. Cucurbituril-based molecular recognition. Chem Rev. 2015;115(22):12320–406. 10.1021/acs.chemrev.5b00341. [DOI] [PubMed] [Google Scholar]
- 18.Yang XR, Liu FB, Zhao ZY, Liang F, Zhang HJ, Liu SM. Cucurbit[10]uril-based chemistry. Chin Chem Lett. 2018;29(11):1560–6. 10.1016/j.cclet.2018.01.032. [Google Scholar]
- 19.Lee JW, Samal S, Selvapalam N, Kim HJ, Kim K. Cucurbituril homologues and derivatives: new opportunities in supramolecular chemistry. Acc Chem Res. 2003;36(8):621–30. 10.1021/ar020254k. [DOI] [PubMed] [Google Scholar]
- 20.Liu YH, Zhang YM, Yu HJ, Liu Y. Cucurbituril-based biomacromolecular assemblies. Angew Chem Int Ed Engl. 2021;60(8):3870–80. 10.1002/anie.202009797. [DOI] [PubMed] [Google Scholar]
- 21.Yan X, Wang F, Zheng B, Huang F. Stimuli-responsive supramolecular polymeric materials. Chem Soc Rev. 2012;41(18):6042–65. 10.1039/c2cs35091b. [DOI] [PubMed] [Google Scholar]
- 22.Haag R. Supramolecular drug-delivery systems based on polymeric core-shell architectures. Angew Chem Int Ed Engl. 2004;43(3):278–82. 10.1002/anie.200301694. [DOI] [PubMed] [Google Scholar]
- 23.Cabral H, Nishiyama N, Kataoka K. Supramolecular nanodevices: from design validation to theranostic nanomedicine. Acc Chem Res. 2011;44(10):999–1008. 10.1021/ar200094a. [DOI] [PubMed] [Google Scholar]
- 24.Rodell CB, Mealy JE, Burdick JA. Supramolecular guest-host interactions for the preparation of biomedical materials. Bioconjugate Chem. 2015;26(12):2279–89. 10.1021/acs.bioconjchem.5b00483. [DOI] [PubMed] [Google Scholar]
- 25.Webber MJ. Engineering responsive supramolecular biomaterials: toward smart therapeutics. Bioeng Transl Med. 2016;1(3):252–66. 10.1002/btm2.10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagona J, Mukhopadhyay P, Chakrabarti S, Isaacs L. The cucurbit[n]uril family. Angew Chem Int Edit. 2005;44(31):4844–70. 10.1002/anie.200460675. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Gao Y, Ding Y, Xu A, Tan H. Supramolecular nano drug delivery systems mediated via host-guest chemistry of cucurbit[n]uril (n = 6 and 7). Chin Chem Lett. 2021;32(1):313–8. 10.1016/j.cclet.2020.04.049. [Google Scholar]
- 28.Langer R. New methods of drug delivery. Science. 1990;249(4976):1527–33. 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 29.Wu H, Chen H, Tang B, Kang Y, Xu JF, Zhang X. Host-guest interactions between oxaliplatin and cucurbit[7]uril/cucurbit[7]uril derivatives under pseudo-physiological conditions. Langmuir. 2020;36(5):1235–40. 10.1021/acs.langmuir.9b03325. [DOI] [PubMed] [Google Scholar]
- 30.Zhu P, Zhang Y, Lv P, Liao X, Zhao Y, Yang B. Functional supramolecular micelles driven by the amphiphilic complex of biotin-acyclic cucurbituril and cannabidiol for cell-targeted drug delivery. Int J Pharm. 2022;625:122048. 10.1016/j.ijpharm.2022.122048. [DOI] [PubMed] [Google Scholar]
- 31.Sun T, Wang Q, Bi Y, Chen X, Liu L, Ruan C, et al. Supramolecular amphiphiles based on cyclodextrin and hydrophobic drugs. J Mater Chem B. 2017;5(14):2644–54. 10.1039/c6tb03272a. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Huang Q, Bardelang D, Wang C, Lee SMY, Wang R. Supramolecular alleviation of cardiotoxicity of a small-molecular kinase inhibitor. Org Biomol Chem. 2017;15 (38):8046–53. [DOI] [PubMed]
- 33.Pashkina E, Aktanova A, Mirzaeva I, Kovalenko E, Andrienko I, Knauer N, et al. The effect of cucurbit[7]uril on the antitumor and immunomodulating properties of oxaliplatin and carboplatin. Int J Mol Sci. 2021. 10.3390/ijms22147337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao M, Liang Z, Zhang B, Wang Q, Lee J, Li F, et al. Supramolecular container-mediated surface engineering approach for regulating the biological targeting effect of nanoparticles. Nano Lett. 2020;20(11):7941–7. 10.1021/acs.nanolett.0c02701. [DOI] [PubMed] [Google Scholar]
- 35.Dai Q, Wilhelm S, Ding D, Syed AM, Sindhwani S, Zhang Y, et al. Quantifying the ligand-coated nanoparticle delivery to cancer cells in solid tumors. ACS Nano. 2018;12(8):8423–35. 10.1021/acsnano.8b03900. [DOI] [PubMed] [Google Scholar]
- 36.Zou L, Braegelman AS, Webber MJ. Spatially defined drug targeting by in situ host-guest chemistry in a living animal. ACS Cent Sci. 2019;5(6):1035–43. 10.1021/acscentsci.9b00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jallinoja VIJ, Carney BD, Zhu M, Bhatt K, Yazaki PJ, Houghton JL. Cucurbituril-ferrocene: host-guest based pretargeted positron emission tomography in a xenograft model. Bioconjug Chem. 2021;32(8):1554–8. 10.1021/acs.bioconjchem.1c00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zdarova Karasova J, Mzik M, Kucera T, Vecera Z, Kassa J, Sestak V. Interaction of cucurbit[7]uril with oxime K027, atropine, and paraoxon: risky or advantageous delivery system? Int J Mol Sci. 2020. 10.3390/ijms21217883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Huang Z, Xu JF, Sun Z, Zhang X. Cytotoxicity regulated by host-guest interactions: a supramolecular strategy to realize controlled disguise and exposure. ACS Appl Mater Interfaces. 2016;8(35):22780–4. 10.1021/acsami.6b08295. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Huang Z, Zhao H, Xu JF, Sun Z, Zhang X. Supramolecular chemotherapy: cooperative enhancement of antitumor activity by combining controlled release of oxaliplatin and consuming of spermine by cucurbit[7]uril. ACS Appl Mater Interfaces. 2017;9(10):8602–8. 10.1021/acsami.7b01157. [DOI] [PubMed] [Google Scholar]
- 41.Chen H, Chen Y, Wu H, Xu JF, Sun Z, Zhang X. Supramolecular polymeric chemotherapy based on cucurbit[7]uril-PEG copolymer. Biomaterials. 2018;178:697–705. 10.1016/j.biomaterials.2018.02.051. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Sun Z. Supramolecular chemotherapy based on the host-guest complex of lobaplatin-cucurbit[7]uril. ACS Appl Bio Mater. 2020;3(4):2449–54. 10.1021/acsabm.0c00172. [DOI] [PubMed] [Google Scholar]
- 43.Huang X, Zhou H, Jiao R, Liu H, Qin C, Xu L, et al. Supramolecular chemotherapy: host-guest complexes of heptaplatin-cucurbit[7]uril toward colorectal normal and tumor cells. Langmuir. 2021;37(18):5475–82. 10.1021/acs.langmuir.0c03603. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Jing L, Meng Q, Li B, Chen R, Sun Z. Supramolecular chemotherapy: noncovalent bond synergy of cucurbit[7]uril against human colorectal tumor cells. Langmuir. 2021;37(31):9547–52. 10.1021/acs.langmuir.1c01422. [DOI] [PubMed] [Google Scholar]
- 45.Zhou H, Meng Q, Li B, Liu Y, Li Z, Li X, et al. Supramolecular combination chemotherapy: cucurbit[8]uril complex enhanced platinum drug infiltration and modified nanomechanical property of colorectal cancer cells. Langmuir. 2022;38(46):14326–34. 10.1021/acs.langmuir.2c02388. [DOI] [PubMed] [Google Scholar]
- 46.Kuok KI, In Ng PC, Ji X, Wang C, Yew WW, Chan DPC, et al. Supramolecular strategy for reducing the cardiotoxicity of bedaquiline without compromising its antimycobacterial efficacy. Food Chem Toxicol. 2018;119:425–9. 10.1016/j.fct.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 47.Huang Q, Li S, Yin H, Wang C, Lee SMY, Wang R. Alleviating the hepatotoxicity of trazodone via supramolecular encapsulation. Food Chem Toxicol. 2018;112:421–6. 10.1016/j.fct.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 48.Jerzykiewicz J, Czogalla A. Polyethyleneimine-based lipopolyplexes as carriers in anticancer gene therapies. Materials. 2021. 10.3390/ma15010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Q, Li S, Ding YF, Yin H, Wang LH, Wang R. Macrocycle-wrapped polyethylenimine for gene delivery with reduced cytotoxicity. Biomater Sci. 2018;6(5):1031–9. 10.1039/c8bm00022k. [DOI] [PubMed] [Google Scholar]
- 50.Yang X, Wang R, Kermagoret A, Bardelang D. Oligomeric cucurbituril complexes: from peculiar assemblies to emerging applications. Angew Chem Int Ed Engl. 2020;59(48):21280–92. 10.1002/anie.202004622. [DOI] [PubMed] [Google Scholar]
- 51.Chen R, Li H, Cai J, Wang C, Lin Z, Liu C, et al. Fine particulate air pollution and the expression of microRNAs and circulating cytokines relevant to inflammation, coagulation, and vasoconstriction. Environ Health Perspect. 2018;126(1):017007. 10.1289/ehp1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuan SL, Bergamini FRG, Weil T. Functional protein nanostructures: a chemical toolbox. Chem Soc Rev. 2018;47(24):9069–105. 10.1039/c8cs00590g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang Z, Qin K, Deng G, Wu G, Bai Y, Xu JF, et al. Supramolecular chemistry of cucurbiturils: tuning cooperativity with multiple noncovalent interactions from positive to negative. Langmuir. 2016;32(47):12352–60. 10.1021/acs.langmuir.6b01709. [DOI] [PubMed] [Google Scholar]
- 54.Ioannou E, Labrou NE. Rational design of self-assembling supramolecular protein nanostructures utilizing the cucurbit[8]uril macrocyclic host. Methods Mol Biol. 2022;2487:177–87. 10.1007/978-1-0716-2269-8_11. [DOI] [PubMed] [Google Scholar]
- 55.Barbero H, Masson E. Design and recognition of cucurbituril-secured platinum-bound oligopeptides. Chem Sci. 2021;12(29):9962–8. 10.1039/d1sc02637b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirani Z, Taylor HF, Babcock EF, Bockus AT, Varnado CD Jr, Bielawski CW, et al. Molecular recognition of methionine-terminated peptides by cucurbit[8]uril. J Am Chem Soc. 2018;140(38):12263–9. 10.1021/jacs.8b07865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang YM, Liu JH, Yu Q, Wen X, Liu Y. Targeted polypeptide-microtubule aggregation with cucurbit[8]uril for enhanced cell apoptosis. Angew Chem Int Ed Engl. 2019;58(31):10553–7. 10.1002/anie.201903243. [DOI] [PubMed] [Google Scholar]
- 58.Luo D, Carter KA, Miranda D, Lovell JF. Chemophototherapy: an emerging treatment option for solid tumors. Adv Sci. 2017;4(1):1600106. 10.1002/advs.201600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dabrowski JM, Arnaut LG. Photodynamic therapy (PDT) of cancer: from local to systemic treatment. Photochem Photobiol Sci. 2015;14(10):1765–80. 10.1039/c5pp00132c. [DOI] [PubMed] [Google Scholar]
- 60.O’Connor AE, Gallagher WM, Byrne AT. Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem Photobiol. 2009;85(5):1053–74. 10.1111/j.1751-1097.2009.00585.x. [DOI] [PubMed] [Google Scholar]
- 61.Rajora MA, Lou JWH, Zheng G. Advancing porphyrin’s biomedical utility via supramolecular chemistry. Chem Soc Rev. 2017;46(21):6433–69. 10.1039/C7CS00525C. [DOI] [PubMed] [Google Scholar]
- 62.Yang K, Zhang Z, Du J, Li W, Pei Z. Host-guest interaction based supramolecular photodynamic therapy systems: a promising candidate in the battle against cancer. Chem Commun. 2020;56(44):5865–76. 10.1039/d0cc02001j. [DOI] [PubMed] [Google Scholar]
- 63.Wang XQ, Lei Q, Zhu JY, Wang WJ, Cheng Q, Gao F, et al. Cucurbit[8]uril regulated activatable supramolecular photosensitizer for targeted cancer imaging and photodynamic therapy. ACS Appl Mater Interfaces. 2016;8(35):22892–9. 10.1021/acsami.6b07507. [DOI] [PubMed] [Google Scholar]
- 64.Ji C, Gao Q, Dong X, Yin W, Gu Z, Gan Z, et al. A size-reducible nanodrug with an aggregation-enhanced photodynamic effect for deep chemo-photodynamic therapy. Angew Chem Int Ed Engl. 2018;57(35):11384–8. 10.1002/anie.201807602. [DOI] [PubMed] [Google Scholar]
- 65.Wei X, Liu L, Guo X, Wang Y, Zhao J, Zhou S. Light-activated ROS-responsive nanoplatform codelivering apatinib and doxorubicin for enhanced chemo-photodynamic therapy of multidrug-resistant tumors. ACS Appl Mater Interfaces. 2018;10(21):17672–84. 10.1021/acsami.8b04163. [DOI] [PubMed] [Google Scholar]
- 66.Mao W, Liao Y, Ma D. A supramolecular assembly mediated by host-guest interactions for improved chemo-photodynamic combination therapy. Chem Commun. 2020;56(30):4192–5. 10.1039/d0cc01096k. [DOI] [PubMed] [Google Scholar]
- 67.Ni XL, Chen S, Yang Y, Tao Z. Facile cucurbit[8]uril-based supramolecular approach to fabricate tunable luminescent materials in aqueous solution. J Am Chem Soc. 2016;138(19):6177–83. 10.1021/jacs.6b01223. [DOI] [PubMed] [Google Scholar]
- 68.Ghale G, Nau WM. Dynamically analyte-responsive macrocyclic host-fluorophore systems. Acc Chem Res. 2014;47(7):2150–9. 10.1021/ar500116d. [DOI] [PubMed] [Google Scholar]
- 69.Zhang T, Zhang C-H. Photo-controlled reversible secondary self-assembly of supramolecular nanosheets and their drug delivery behavior. J Mater Chem B. 2019;7(48):7736–43. 10.1039/C9TB02017A. [DOI] [PubMed] [Google Scholar]
- 70.Solis-Egana F, Lavin-Urqueta N, Diaz DG, Marino-Ocampo N, Faundez MA, Fuentealba D. Supramolecular co-encapsulation of a photosensitizer and chemotherapeutic drug in cucurbit[8]uril for potential chemophototherapy. Photoch Photobio Sci. 2022;21(3):349–59. 10.1007/s43630-022-00174-7. [DOI] [PubMed] [Google Scholar]
- 71.Balmain A. Cancer as a complex genetic trait: tumor susceptibility in humans and mouse models. Cell. 2002;108(2):145–52. 10.1016/S0092-8674(02)00622-0. [DOI] [PubMed] [Google Scholar]
- 72.Li W, Wang L, Sun T, Tang H, Bui B, Cao D, et al. Characterization of nanoparticles combining polyamine detection with photodynamic therapy. Commun Biol. 2021;4(1):803. 10.1038/s42003-021-02317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun C, Zhang H, Yue L, Li S, Cheng Q, Wang R. Facile preparation of cucurbit[6]uril-based polymer nanocapsules for targeted photodynamic therapy. ACS Appl Mater Interfaces. 2019;11(26):22925–31. 10.1021/acsami.9b04403. [DOI] [PubMed] [Google Scholar]
- 74.Angelos S, Khashab NM, Yang YW, Trabolsi A, Khatib HA, Stoddart JF, et al. pH clock-operated mechanized nanoparticles. J Am Chem Soc. 2009;131(36):12912–4. 10.1021/ja9010157. [DOI] [PubMed] [Google Scholar]
- 75.Wike-Hooley JL, Haveman J, Reinhold HS. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984;2(4):343–66. 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- 76.Mao W, Mao D, Yang F, Ma D. Transformative supramolecular vesicles based on acid-degradable acyclic cucurbit[n]uril and a prodrug for promoted tumoral-cell uptake. Chemistry. 2019;25(9):2272–80. 10.1002/chem.201804835. [DOI] [PubMed] [Google Scholar]
- 77.Mao WP, Wang SY, Mao DK, Liu YM, Li LB, Ma D. Supramolecular complexation with kinetic stabilization: cucurbit[6]uril encapsulated doxorubicin-based prodrugs for pH-responsive controlled release. New J Chem. 2022;46(11):5355–60. 10.1039/d1nj06237a. [Google Scholar]
- 78.Kanamala M, Wilson WR, Yang M, Palmer BD, Wu Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: a review. Biomaterials. 2016;85:152–67. 10.1016/j.biomaterials.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 79.Zhang X, Rakesh KP, Shantharam CS, Manukumar HM, Asiri AM, Marwani HM, et al. Podophyllotoxin derivatives as an excellent anticancer aspirant for future chemotherapy: a key current imminent needs. Bioorg Med Chem. 2018;26(2):340–55. 10.1016/j.bmc.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 80.Kamal A, Hussaini SMA, Rahim A, Riyaz S. Podophyllotoxin derivatives: a patent review (2012–2014). Expert Opin Ther Pat. 2015;25(9):1025–34. 10.1517/13543776.2015.1051727. [DOI] [PubMed] [Google Scholar]
- 81.Antunez-Mojica M, Rodriguez-Salarichs J, Redondo-Horcajo M, Leon A, Barasoain I, Canales A, et al. Structural and biochemical characterization of the interaction of tubulin with potent natural analogues of podophyllotoxin. J Nat Prod. 2016;79(8):2113–21. 10.1021/acs.jnatprod.6b00428. [DOI] [PubMed] [Google Scholar]
- 82.Aisner J, Lee EJ. Etoposide. Current and future status. Cancer. 1991;67(1 Suppl):215–9. 10.1002/1097-0142(19910101)67:1+%3c215::aid-cncr2820671302%3e3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 83.Li F, Liu D, Liao X, Zhao Y, Li R, Yang B. Acid-controlled release complexes of podophyllotoxin and etoposide with acyclic cucurbit[n]urils for low cytotoxicity. Bioorg Med Chem. 2019;27(3):525–32. 10.1016/j.bmc.2018.12.035. [DOI] [PubMed] [Google Scholar]
- 84.Xing P, Zhao Y. Supramolecular vesicles for stimulus-responsive drug delivery. Small Methods. 2018. 10.1002/smtd.201700364. [Google Scholar]
- 85.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2008;417(1):1–13. 10.1042/bj20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chu N, Cong L, Yue J, Xu W, Xu S. Fluorescent imaging probe targeting mitochondria based on supramolecular host-guest assembly and disassembly. ACS Omega. 2022;7(38):34268–77. 10.1021/acsomega.2c03766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu X, Chen Y, Yu Q, Li FQ, Liu Y. A cucurbituril/polysaccharide/carbazole ternary supramolecular assembly for targeted cell imaging. Chem Commun. 2019;55(30):4343–6. 10.1039/c9cc01601e. [DOI] [PubMed] [Google Scholar]
- 88.Vaidya A, Sun Y, Ke T, Jeong EK, Lu ZR. Contrast enhanced MRI-guided photodynamic therapy for site-specific cancer treatment. Magn Reson Med. 2006;56(4):761–7. 10.1002/mrm.21009. [DOI] [PubMed] [Google Scholar]
- 89.Tian B, Wang C, Zhang S, Feng L, Liu Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano. 2011;5(9):7000–9. 10.1021/nn201560b. [DOI] [PubMed] [Google Scholar]
- 90.Lin J, Wang S, Huang P, Wang Z, Chen S, Niu G, et al. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano. 2013;7(6):5320–9. 10.1021/nn4011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao L, Fei J, Zhao J, Li H, Cui Y, Li J. Hypocrellin-loaded gold nanocages with high two-photon efficiency for photothermal/photodynamic cancer therapy in vitro. ACS Nano. 2012;6(9):8030–40. 10.1021/nn302634m. [DOI] [PubMed] [Google Scholar]
- 92.Narayanan N, Kim JH, Santhakumar H, Joseph MM, Karunakaran V, Shamjith S, et al. Nanotheranostic probe built on methylene blue loaded cucurbituril [8] and gold nanorod: targeted phototherapy in combination with sers imaging on breast cancer cells. J Phys Chem B. 2021;125(49):13415–24. 10.1021/acs.jpcb.1c08609. [DOI] [PubMed] [Google Scholar]
- 93.Kim D, Aktalay A, Jensen N, Uno K, Bossi ML, Belov VN, et al. Supramolecular complex of photochromic diarylethene and cucurbit[7]uril: fluorescent photoswitching system for biolabeling and imaging. J Am Chem Soc. 2022;144(31):14235–47. 10.1021/jacs.2c05036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhaumik SK, Biswas R, Banerjee S. Cucurbituril based luminescent materials in aqueous media and solid state. Chem Asian J. 2021;16(16):2195–210. 10.1002/asia.202100594. [DOI] [PubMed] [Google Scholar]
- 95.Liu G, Xu X, Dai X, Jiang C, Zhou Y, Lu L, et al. Cucurbituril-activated photoreaction of dithienylethene for controllable targeted lysosomal imaging and anti-counterfeiting. Mater Horiz. 2021;8(9):2494–502. 10.1039/d1mh00811k. [DOI] [PubMed] [Google Scholar]
- 96.Yu Y, Li Y, Wang X, Nian H, Wang L, Li J, et al. Cucurbit[10]uril-based [2]rotaxane: preparation and supramolecular assembly-induced fluorescence enhancement. J Org Chem. 2017;82(11):5590–6. 10.1021/acs.joc.7b00400. [DOI] [PubMed] [Google Scholar]
- 97.Biedermann F, Scherman OA. Cucurbit[8]uril mediated donor-acceptor ternary complexes: a model system for studying charge-transfer interactions. J Phys Chem B. 2012;116(9):2842–9. 10.1021/jp2110067. [DOI] [PubMed] [Google Scholar]
- 98.Ziganshina AY, Ko YH, Jeon WS, Kim K. Stable pi-dimer of a tetrathiafulvalene cation radical encapsulated in the cavity of cucurbit[8]uril. Chem Commun. 2004;7:806–7. 10.1039/b316651a. [DOI] [PubMed] [Google Scholar]
- 99.Song Q, Jiao Y, Wang Z, Zhang X. Tuning the energy gap by supramolecular approaches: towards near-infrared organic assemblies and materials. Small. 2016;12(1):24–31. 10.1002/smll.201501661. [DOI] [PubMed] [Google Scholar]
- 100.Liu H, Lin M, Cui Y, Gan W, Sun J, Li B, et al. Single-crystal structures of cucurbituril-based supramolecular host-guest complexes for bioimaging. Chem Commun. 2021;57(79):10190–3. 10.1039/d1cc04823f. [DOI] [PubMed] [Google Scholar]
- 101.Xiang H, Cheng J, Ma X, Zhou X, Chruma JJ. Near-infrared phosphorescence: materials and applications. Chem Soc Rev. 2013;42(14):6128–85. 10.1039/c3cs60029g. [DOI] [PubMed] [Google Scholar]
- 102.Wang S, Gu K, Guo Z, Yan C, Yang T, Chen Z, et al. Self-assembly of a monochromophore-based polymer enables unprecedented ratiometric tracing of hypoxia. Adv Mater. 2019;31(3):e1805735. 10.1002/adma.201805735. [DOI] [PubMed] [Google Scholar]
- 103.Schulze M, Steffen A, Wurthner F. Near-IR phosphorescent ruthenium(II) and iridium(III) perylene bisimide metal complexes. Angew Chem Int Ed Engl. 2015;54(5):1570–3. 10.1002/anie.201410437. [DOI] [PubMed] [Google Scholar]
- 104.Leung MY, Tang MC, Cheung WL, Lai SL, Ng M, Chan MY, et al. Thermally stimulated delayed phosphorescence (TSDP)-based gold(III) complexes of tridentate pyrazine-containing pincer ligand with wide emission color tunability and their application in organic light-emitting devices. J Am Chem Soc. 2020;142(5):2448–59. 10.1021/jacs.9b12136. [DOI] [PubMed] [Google Scholar]
- 105.Tang MC, Leung MY, Lai SL, Ng M, Chan MY, Wing-Wah YV. Realization of thermally stimulated delayed phosphorescence in arylgold(III) complexes and efficient gold(III) based blue-emitting organic light-emitting devices. J Am Chem Soc. 2018;140(40):13115–24. 10.1021/jacs.8b09205. [DOI] [PubMed] [Google Scholar]
- 106.Sun S, Ma L, Wang J, Ma X, Tian H. Red-light excited efficient metal-free near-infrared room-temperature phosphorescent films. Natl Sci Rev. 2022;9(2):nwab085. 10.1093/nsr/nwab085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang C, Liu YH, Liu Y. Near-infrared phosphorescent switch of diarylethene phenylpyridinium derivative and cucurbit[8]uril for cell imaging. Small. 2022;18(21):e2201821. 10.1002/smll.202201821. [DOI] [PubMed] [Google Scholar]
- 108.Zhou WL, Chen Y, Yu Q, Zhang H, Liu ZX, Dai XY, et al. Ultralong purely organic aqueous phosphorescence supramolecular polymer for targeted tumor cell imaging. Nat Commun. 2020;11(1):4655. 10.1038/s41467-020-18520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Uzunova VD, Cullinane C, Brix K, Nau WM, Day AI. Toxicity of cucurbit[7]uril and cucurbit[8]uril: an exploratory in vitro and in vivo study. Org Biomol Chem. 2010;8(9):2037–42. 10.1039/b925555a. [DOI] [PubMed] [Google Scholar]
- 110.Cornil J, Beljonne D, Calbert J-P, Brédas J-L. Interchain interactions in organic π-conjugated materials: impact on electronic structure, optical response, and charge transport. Adv Mater. 2001;13(14):1053–67. 10.1002/1521-4095(200107)13:14%3c1053::AID-ADMA1053%3e3.0.CO;2-7. [Google Scholar]
- 111.Il Kim S, Ju Kim H, Young PS. Highly fluorescent supramolecular nanoring composed of bent-shaped cyanostilbene derivatives and cucurbit[8]urils. Chemistry. 2023;29(17):e202203828. 10.1002/chem.202203828. [DOI] [PubMed] [Google Scholar]
- 112.Zhang X, Chen X, Song J, Zhang J, Ren X, Zhao Y. Size-transformable nanostructures: from design to biomedical applications. Adv Mater. 2020;32(48):e2003752. 10.1002/adma.202003752. [DOI] [PubMed] [Google Scholar]
- 113.Li Y, Kroger M, Liu WK. Shape effect in cellular uptake of PEGylated nanoparticles: comparison between sphere, rod, cube and disk. Nanoscale. 2015;7(40):16631–46. 10.1039/c5nr02970h. [DOI] [PubMed] [Google Scholar]
- 114.Zhuang X, Mai Y, Wu D, Zhang F, Feng X. Two-dimensional soft nanomaterials: a fascinating world of materials. Adv Mater. 2015;27(3):403–27. 10.1002/adma.201401857. [DOI] [PubMed] [Google Scholar]
- 115.Hou D, Pu L, Zhou S, Wang R, Xu Y, Zhang W, et al. Spiropyran-appended cucurbit[6]uril enabling direct generation of 2D materials inside living cells. Small. 2021;17(52):e2102392. 10.1002/smll.202102392. [DOI] [PubMed] [Google Scholar]
- 116.Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, et al. Cancer screening in the United States, 2019: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2019;69(3):184–210. 10.3322/caac.21557. [DOI] [PubMed] [Google Scholar]
- 117.Shahrokhian S. Lead phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode. Anal Chem. 2001;73(24):5972–8. 10.1021/ac010541m. [DOI] [PubMed] [Google Scholar]
- 118.Zhou Y, Yoon J. Recent progress in fluorescent and colorimetric chemosensors for detection of amino acids. Chem Soc Rev. 2012;41(1):52–67. 10.1039/C1CS15159B. [DOI] [PubMed] [Google Scholar]
- 119.Li SH, Yu CW, Xu JG. A cyclometalated palladium-azo complex as a differential chromogenic probe for amino acids in aqueous solution. Chem Commun. 2005;4:450–2. 10.1039/b414131h. [DOI] [PubMed] [Google Scholar]
- 120.Yang MX, Tang Q, Yang M, Wang Q, Tao Z, Xiao X, et al. pH-stimulus response dye-cucurbituril sensor for amino acids in aqueous solution. Spectrochim Acta A Mol Biomol Spectrosc. 2020;230:118076. 10.1016/j.saa.2020.118076. [DOI] [PubMed] [Google Scholar]
- 121.Truxal AE, Cao L, Isaacs L, Wemmer DE, Pines A. Directly functionalized cucurbit[7]uril as a biosensor for the selective detection of protein interactions by (129) Xe hyperCEST NMR. Chemistry. 2019;25(24):6108–12. 10.1002/chem.201900610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu B, Wang L, Ran X, Tang H, Cao D. Recent advances in fluorescent methods for polyamine detection and the polyamine suppressing strategy in tumor treatment. Biosensors. 2022. 10.3390/bios12080633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bhamore JR, Murthy ZVP, Kailasa SK. Fluorescence turn-off detection of spermine in biofluids using pepsin mediated synthesis of gold nanoclusters as a probe. J Mol Liq. 2019;280:18–24. 10.1016/j.molliq.2019.01.132. [Google Scholar]
- 124.Tawfik SM, Shim J, Biechele-Speziale D, Sharipov M, Lee Y-I. Novel, “turn off-on” sensors for highly selective and sensitive detection of spermine based on heparin-quenching of fluorescence CdTe quantum dots-coated amphiphilic thiophene copolymers. Sens Act, B Chem. 2018;257:734–44. 10.1016/j.snb.2017.10.172. [Google Scholar]
- 125.Kim TI, Kim Y. Analyte-directed formation of emissive excimers for the selective detection of polyamines. Chem Commun. 2016;52(70):10648–51. 10.1039/c6cc05761f. [DOI] [PubMed] [Google Scholar]
- 126.Naik VG, Kumar V, Bhasikuttan AC, Kadu K, Ramanan SR, Bhosle AA, et al. Solid-supported amplification of aggregation emission: a tetraphenylethylene-cucurbit[6]uril@hydroxyapatite-based supramolecular sensing assembly for the detection of spermine and spermidine in human urine and blood. ACS Appl Bio Mater. 2021;4(2):1813–22. 10.1021/acsabm.0c01527. [DOI] [PubMed] [Google Scholar]
- 127.Singh P, Mittal LS, Bhargava G, Kumar S. Ionic self-assembled platform of perylenediimide-sodium dodecylsulfate for detection of spermine in clinical samples. Chem Asian J. 2017;12(8):890–9. 10.1002/asia.201700120. [DOI] [PubMed] [Google Scholar]
- 128.Zhong C, Hu C, Kumar R, Trouillet V, Biedermann F, Hirtz M. Cucurbit[n]uril-immobilized sensor arrays for indicator-displacement assays of small bioactive metabolites. ACS Appl Nano Mater. 2021;4(5):4676–87. 10.1021/acsanm.1c00293. [Google Scholar]
- 129.Mallick S, Chandra F, Koner AL. A ratiometric fluorescent probe for detection of biogenic primary amines with nanomolar sensitivity. Analyst. 2016;141(3):827–31. 10.1039/C5AN01911G. [DOI] [PubMed] [Google Scholar]
- 130.Tu J, Sun S, Xu Y. A novel self-assembled platform for the ratiometric fluorescence detection of spermine. Chem Commun. 2016;52(5):1040–3. 10.1039/C5CC07861J. [DOI] [PubMed] [Google Scholar]
- 131.Bhosle AA, Banerjee M, Hiremath SD, Sisodiya DS, Naik VG, Barooah N, et al. A combination of a graphene quantum dots-cationic red dye donor-acceptor pair and cucurbit[7]uril as a supramolecular sensor for ultrasensitive detection of cancer biomarkers spermine and spermidine. J Mater Chem B. 2022;10(40):8258–73. 10.1039/d2tb01269c. [DOI] [PubMed] [Google Scholar]
- 132.Deng CL, Murkli SL, Isaacs LD. Supramolecular hosts as in vivo sequestration agents for pharmaceuticals and toxins. Chem Soc Rev. 2020;49(21):7516–32. 10.1039/d0cs00454e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ganapati S, Grabitz SD, Murkli S, Scheffenbichler F, Rudolph MI, Zavalij PY, et al. Molecular containers bind drugs of abuse in vitro and reverse the hyperlocomotive effect of methamphetamine in rats. ChemBioChem. 2017;18(16):1583–8. 10.1002/cbic.201700289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thevathasan T, Grabitz SD, Santer P, Rostin P, Akeju O, Boghosian JD, et al. Calabadion 1 selectively reverses respiratory and central nervous system effects of fentanyl in a rat model. Br J Anaesth. 2020;125(1):e140–7. 10.1016/j.bja.2020.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Murkli S, Klemm J, Brockett AT, Shuster M, Briken V, Roesch MR, et al. In Vitro and in vivo sequestration of phencyclidine by Me(4) cucurbit[8]uril*. Chemistry. 2021;27(9):3098–105. 10.1002/chem.202004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stäuble CG, Blobner M. The future of neuromuscular blocking agents. Curr Opin Anaesthesiol. 2020;33(4):490–8. 10.1097/aco.0000000000000891. [DOI] [PubMed] [Google Scholar]
- 137.Zafirova Z, Dalton A. Neuromuscular blockers and reversal agents and their impact on anesthesia practice. Best Pract Res Clin Anaesthesiol. 2018;32(2):203–11. 10.1016/j.bpa.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 138.Murkli S, Klemm J, King D, Zavalij PY, Isaacs L. Acyclic cucurbit[n]uril-type receptors: aromatic wall extension enhances binding affinity, delivers helical chirality, and enables fluorescence sensing. Chemistry. 2020;26(66):15249–58. 10.1002/chem.202002874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shaya D, Isaacs L. Acyclic cucurbit[n]uril-type containers as receptors for neuromuscular blocking agents: structure-binding affinity relationships. Croat Chem Acta. 2019;92(2):163–71. 10.5562/cca3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Worek F, Thiermann H, Wille T. Organophosphorus compounds and oximes: a critical review. Arch Toxicol. 2020;94(7):2275–92. 10.1007/s00204-020-02797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Andrýs R, Klusoňová A, Lísa M, Kassa J, Karasová J. Effect of oxime encapsulation on acetylcholinesterase reactivation: pharmacokinetic study of the asoxime-cucurbit[7]uril complex in mice using hydrophilic interaction liquid chromatography-mass spectrometry. Mol Pharm. 2021;18(6):2416–27. 10.1021/acs.molpharmaceut.1c00257. [DOI] [PubMed] [Google Scholar]
- 142.Eizadi-Mood N, Jaberi D, Barouti Z, Rahimi A, Mansourian M, Dorooshi G, et al. The efficacy of hemodialysis on paraquat poisoning mortality: a systematic review and meta-analysis. J Res Med Sci. 2022;27:74. 10.4103/jrms.jrms_235_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ling Y, Mague JT, Kaifer AE. Inclusion complexation of diquat and paraquat by the hosts cucurbit[7]uril and cucurbit[8]uril. Chemistry. 2007;13(28):7908–14. 10.1002/chem.200700402. [DOI] [PubMed] [Google Scholar]
- 144.Zhang X, Xu X, Li S, Li L, Zhang J, Wang R. A synthetic receptor as a specific antidote for paraquat poisoning. Theranostics. 2019;9(3):633–45. 10.7150/thno.31485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang X, Huang Q, Zhao ZZ, Xu X, Li S, Yin H, Li L, Zhang J, Wang R. An Eco- and User-Friendly Herbicide. J Agric Food Chem. 2019;67(28):7783–92. [DOI] [PubMed]
- 146.Cheng M, Isaacs L. Acyclic cucurbituril featuring pendant cyclodextrins. Supramol Chem. 2021;33(3):53–62. 10.1080/10610278.2021.1927033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Haerter F, Simons JC, Foerster U, Moreno Duarte I, Diaz-Gil D, Ganapati S, et al. Comparative effectiveness of calabadion and sugammadex to reverse non-depolarizing neuromuscular-blocking agents. Anesthesiology. 2015;123(6):1337–49. 10.1097/aln.0000000000000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Diaz-Gil D, Haerter F, Falcinelli S, Ganapati S, Hettiarachchi GK, Simons JC, et al. A novel strategy to reverse general anesthesia by scavenging with the acyclic cucurbit[n]uril-type molecular container calabadion 2. Anesthesiology. 2016;125(2):333–45. 10.1097/aln.0000000000001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Z, Sun C, Yang K, Chen X, Wang R. Cucurbituril-based supramolecular polymers for biomedical applications. Angew Chem Int Ed Engl. 2022;61(38):e202206763. 10.1002/anie.202206763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.