Abstract
Objective
The aim of this study was to investigate the dynamic changes in QTc interval duration among patients with COVID-19 infection before, during, and after infection, in order to assess the short- and potential long-term impact of COVID-19 on cardiac electrophysiology.
Methods
A retrospective analysis was conducted on 303 inpatients diagnosed with COVID-19 who visited a tertiary Grade A hospital in China between August 2022 and December 2023. Inclusion criteria required patients to have at least two electrocardiogram (ECG) recordings at three specific time points: before COVID-19 infection, during acute infection, and after recovery (more than one month post-infection).
Results
The mean age of participants was 72.8 ± 14.7 years, with a male preponderance (62%, n = 188). A significant prolongation of QTc interval was observed during COVID-19 infection compared to pre-infection levels (438.3 ± 26.7 ms vs. 433.9 ± 26.6 ms, p = 0.025). QTc interval was positively correlated with age both before (r = 0.23, p = 0.001) and during infection (r = 0.19, p = 0.001). In short-term follow-up (≤ 6 months), QTc interval remained unchanged from the infectious period (p > 0.05), whereas it significantly decreased during long-term follow-up (> 6 months; 429.6 ± 32.5 ms vs. 437.5 ± 28.2 ms, p = 0.002). Additionally, P-wave duration significantly decreased from the infectious period to long-term follow-up (99.5 ± 14.8 ms to 96.4 ± 15.2 ms, p = 0.024).
Conclusions
COVID-19 infection demonstrated a significant correlation with prolonged QTc interval, persisting in the short term but gradually returning to normal in the long term. Similarly, P-wave duration shortened over time, suggesting potential cardiac electrophysiological recovery.
Clinical trial number
Not applicable.
Keywords: COVID-19, QTc interval, Cardiac arrhythmia, Dynamic changes, Electrocardiogram
Introduction
The global pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), commonly known as COVID-19, has posed unprecedented challenges to healthcare systems worldwide. Beyond its primary respiratory manifestations, COVID-19 has emerged as a multi-systemic disease with a wide array of cardiovascular complications [1, 2]. Among these, arrhythmias have been recognized as a significant contributor to morbidity and mortality in affected patients [3, 4].
The pathophysiological basis for arrhythmia in COVID-19 remains multifactorial. Direct viral injury to cardiomyocytes, inflammatory responses, electrolyte imbalances, and drugs known to prolong QTc can increase the risk for arrhythmia [5]. Previous investigations have highlighted the association between COVID-19 infection and alterations in electrocardiographic parameters, particularly the corrected QT interval (QTc) [6, 7]. Prolongation of the QTc interval, an established surrogate marker for arrhythmias, has been implicated in the occurrence of malignant arrhythmias and increased risk of death among COVID-19 patients [8]. This observation suggests that monitoring QTc could serve as a valuable tool in assessing cardiac risk and guiding therapeutic interventions. Not only does the risk of cardiac arrhythmias increase during acute infection, but studies have also reported that this risk may persist after COVID-19 infection. A multicenter prospective cohort study has revealed that cardiac arrhythmias were prevalent three months post-hospitalization for COVID-19, with premature ventricular beats observed in 20% of patients and nonsustained ventricular tachycardia in 5% [9].
Despite the accumulating evidence linking COVID-19 to cardiovascular complications, the dynamic changes in electrocardiographic indices—specifically the QTc interval—across different stages of the disease (pre-infection, acute infection, and post-infection) remain inadequately characterized. Understanding these temporal variations is crucial for elucidating the natural history of cardiac involvement in COVID-19, identifying high-risk individuals, and informing long-term management strategies. Furthermore, the recognition of “long COVID,” characterized by persistent symptoms and functional impairments long after the acute phase of infection [10], raises concerns about the potential for lasting cardiac sequelae, including sustained QTc alterations. Therefore, the present study aims to systematically investigate the alterations in electrocardiographic parameters, focusing on QTc intervals, in patients at three distinct time points: prior to COVID-19 infection, during acute infection, and following recovery. By doing so, we seek to provide insights into the short- and potential long-term impact of COVID-19 on cardiac electrophysiology.
Methods
Patients
This study was conducted among patients who visited our hospital, a tertiary Grade A hospital in China, between August 2022 and December 2023. This retrospective study has been approved by the institutional academic ethics committee, and as it does not involve patient intervention or information disclosure, informed consent is not required. We retrieved patients with a discharge diagnosis of ‘COVID-19 infection’ through the hospital information system. Patients were identified as having COVID-19 through nucleic acid testing. The inclusion criteria stipulated that patients must have had at least two electrocardiogram (ECG, Medix Electrocardiographic System, Shanghai, China)) recordings at three specific time points: before COVID-19 infection, during acute infection, and after recovery (more than one month post-infection). Patients who had only one ECG at these three time points could not meet the requirements for paired comparison and were excluded. To mitigate the influence of atrial fibrillation (AF) on ECG parameters in this study, ECGs indicating AF were excluded.
Endpoints
We utilized the standard 12-lead resting ECGs, which included a computerized interpretation function. The ECG parameters were analyzed by a computer and subsequently saved in the Hospital Information System (HIS). To obtain the corrected QT interval (QTc), the Bazett formula was adopted to adjust the QT interval. Furthermore, every ECG was reviewed and interpreted manually by two independent cardiology residents as part of a retrospective analysis. Considering that the QTc interval increases with age, yet the disparity between males and females diminishes [11], and given the relatively high average age of the patients in our study, we did not stratify QTc prolongation by gender. The primary endpoints for comparison were: changes in P-wave duration, QT interval, and QTc between pre-infection and acute infection; and changes in these parameters between acute infection and the period more than one month after infection. In cases where multiple ECG recordings were available before and after infection, we selected the most recent pre-infection recording and the longest follow-up ECG record post-infection for analysis. Based on the follow-up duration, we categorized follow-ups exceeding six months as long-term follow-ups and those less than or equal to six months as short-term follow-ups. Baseline patient data were collected, including gender, age, body mass index (BMI), history of hypertension, diabetes, and chronic obstructive pulmonary disease (COPD).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD), while dichotomous variables were presented as counts (%). Paired t-tests were employed to compare ECG parameters at different time points, when the data follows a normal distribution. For correlation analysis, Pearson’s correlation coefficient was utilized. Between-group comparisons were conducted using independent sample t-tests. A p-value less than 0.05 was considered statistically significant. All statistical analyses and graphical representations were performed using SPSS version 22.0 and GraphPad Prism software.
Results
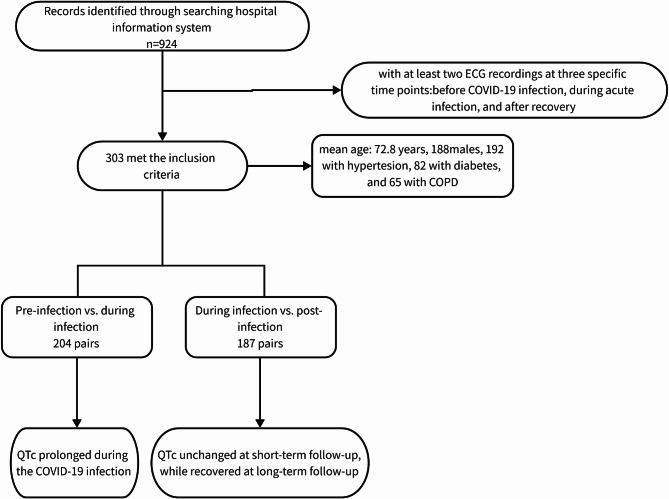
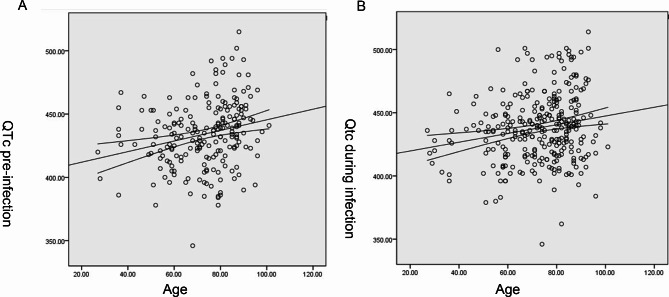
In an initial electronic search, a total of 924 patients were identified. After applying our predefined inclusion and exclusion criteria, 303 patients met the eligibility requirements and were subsequently included in the analysis (Fig. 1). The mean age of these patients was 72.8 ± 14.7 years, with a male preponderance of 188 individuals (62%). Among them, 203 patients had available BMI data, yielding an average BMI of 23.2 ± 4.1 kg/m². Comorbidity analysis revealed that 192 patients (63.6%) had hypertension, 82 (27.2%) had diabetes mellitus, and 65 (21.5%) had a history of COPD. Both the QTc before (r = 0.23, p = 0.001) and during infection (r = 0.19, p = 0.001) showed a positive correlation with age (Fig. 2). Notably, 36 patients (11.9%) succumbed to their illness during the acute infectious period. The age of the deceased population (81.3 ± 11.4 years), was significantly higher than that of the survivors (71.7 ± 14.8 years, p < 0.001). The average heart rates of the included patients were 75.9 ± 14.6, 83.0 ± 18.9, and 80.6 ± 18.6 beats per minute (bpm) before infection, during infection, and after infection, respectively. No patients were administered hydroxychloroquine or chloroquine.
Fig. 1.
Study flow chart
Fig. 2.
Correlation between QTc and age before (A) and during (B) COVID-19 infection
Electrocardiogram parameters before and during COVID-19 infection
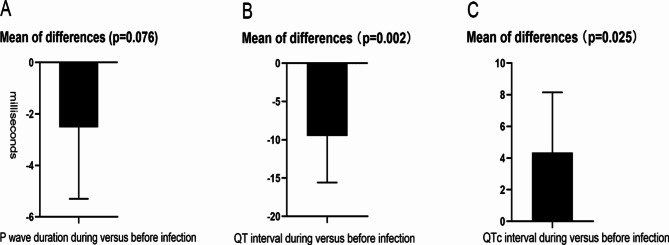
In a comparative analysis of 204 paired ECG parameters obtained before and during hospitalization for infection, the mean P-wave duration was found to be 101.4 ± 14.9 ms before infection and 98.8 ± 15.6 ms during the infectious period. A paired comparison did not reveal a significant difference between these values (p = 0.076). The mean QT interval was 390.2 ± 37.8 ms before infection, which decreased to 380.6 ± 45.9 ms during hospitalization for infection, demonstrating a statistically significant difference (p = 0.002). Additionally, when considering the corrected QT interval (QTc), it was prolonged during the infectious period compared to before infection (438.3 ± 26.7 ms vs. 433.9 ± 26.6 ms, p = 0.025). The results were displayed in Fig. 3. In our investigation of the association between the magnitude of QTc interval changes prior to and during infection relative to baseline data, we categorized patients into two groups: those with a significant increase (SI group) defined as a QTc prolongation of 30 ms or more, and those without such a significant increase (NSI group). The selection of this cutoff value was based on previous literatures [12, 13]. Although the SI group exhibited a higher mean age (76.5 ± 11.8 years) compared to the NSI group (72.6 ± 15.2 years), this difference did not reach statistical significance (p = 0.18). Additionally, no significant differences were observed between the two groups in terms of BMI (p = 0.84), gender distribution (p = 0.61), prevalence of hypertension (p = 0.76), diabetes (p = 0.06), or COPD (p = 0.86). However, the baseline QTc in the NSI group was significantly higher than that in the SI group (Table 1).
Fig. 3.
Difference plots of electrocardiogram parameters during versus before COVID-19 infection. Paired T-test showed that (A) P-wave duration was not significantly changed, (B) QT interval decreased and (C) QTc significantly prolonged during COVID-19 infection compared with those before infection
Table 1.
Comparison of baseline characteristics between the group with significant increase in QTc during infection and the group without significant increase
| SI group | NSI group | p | |
|---|---|---|---|
| Number | 31 | 173 | - |
| Age (years) | 76.5 ± 11.8 | 72.6 ± 15.2 | 0.18 |
| Gender (male, %) | 67.7 | 63.0 | 0.61 |
| BMI (kg/m2)* | 22.9 ± 3.1 | 22.7 ± 4.1 | 0.84 |
| HTN (%) | 61.3 | 64.2 | 0.76 |
| Diabetes (%) | 12.9 | 28.9 | 0.06 |
| COPD (%) | 25.8 | 24.3 | 0.86 |
| Baseline QTc | 412.9 ± 26.7 | 437.8 ± 24.9 | < 0.001 |
SI group: the group with significant increase in QTc (≥ 30msec) during infection; NSI: the group without significant increase in QTc during infection
* In the SI and NSI groups, only 21 and 114 individuals had BMI data available, respectively
Electrocardiogram parameters during and post COVID-19 infection
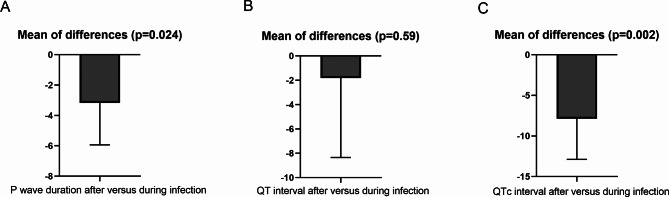
In a paired analysis of ECG parameters involving 187 patients, who had ECG recordings both during hospitalization for infection and at least once during the follow-up period exceeding one month post-infection, several notable findings emerged. The mean follow-up duration was 5.4 ± 2.7 months, with a range of 1 to 11 months. The average P-wave duration during the infectious period was 99.5 ± 14.8 ms, which decreased significantly to 96.4 ± 15.2 ms during the follow-up period (p = 0.024). Regarding the QT interval, no significant difference was observed between the infectious period (378.9 ± 40.2 ms) and the follow-up period (377.1 ± 43.5 ms) (p = 0.59). However, a significant decrease in the QTc interval was noted during the follow-up period compared to the infectious period (429.6 ± 32.5 ms vs. 437.5 ± 28.2 ms, p = 0.002). The results were shown in Fig. 4. When conducting paired comparisons between pre- and post-infection QTc intervals (n = 101), we found no significant difference during the follow-up period after infection compared to before infection (434.3 ± 27.1ms vs. 428.5 ± 36.9ms, p = 0.113).
Fig. 4.
Difference plots of electrocardiogram parameters after versus during COVID-19 infection. Paired T-test showed that (A) P-wave duration was significantly decreased, (B) QT interval unchanged and (C) QTc significantly reduced after COVID-19 infection compared with those during infection
Subgroup analysis
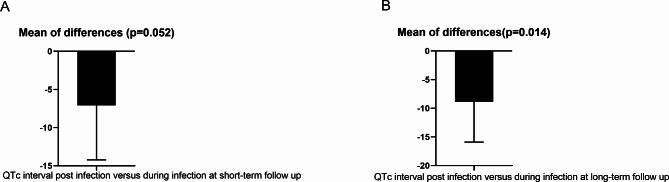
In order to gain deeper insights into the long-term trends of QTc interval changes following COVID-19 infection and their potential correlation with follow-up duration, we stratified our study participants into two subgroups based on the length of follow-up: a short-term follow-up group (comprising 104 pairs with a duration of ≤ 6 months) and a long-term follow-up group (consisting of 83 pairs with a duration > 6 months). In the short-term follow-up group, the post-infection QTc intervals remained comparable to those observed during the active infection period, with no statistically significant difference detected (p > 0.05) (Fig. 5A). Conversely, within the long-term follow-up group, a significant decrease in QTc intervals was observed post-infection compared to during the infection phase (p = 0.014) (Fig. 5B).
Fig. 5.
Changes of QTc post COVID-19 infection at short and long-term follow up. During short-term follow-up, QTc did not show significant changes when compared to that during acute COVID-19 infection; however, it was notably decreased during long-term follow-up
Discussion
The present study conducted a comprehensive analysis of ECG parameter alterations, specifically focusing on QTc interval, across distinct temporal phases in patients with COVID-19 infection: pre-infection, acute infection, and post-recovery. Our findings offer valuable insights into the short- and long-term impact of COVID-19 infection on cardiac electrophysiology, which may have significant implications for patient management and outcomes.
Firstly, we documented a positive correlation between QTc interval and age both at baseline (pre-infection) and during acute COVID-19 infection. This observation aligns with established knowledge that QTc prolongation is associated with advancing age [11, 14], potentially due to age-related declines in myocardial repolarization and other cardiac factors. A study based on 733 COVID-19 infected individuals and 232 non-infected individuals found that COVID-19 infection is an independent risk factor for prolonged QTc interval on electrocardiograms at 2 and 5 days after hospitalization [15]. In this study, patients aged over 80 years had a significantly prolonged QTc interval of 11.91 milliseconds compared to those under 50 years old [15]. In the present study, we demonstrated that QTc interval was significantly prolonged during acute COVID-19 infection compared to pre-infection levels, corroborating previous reports of QTc prolongation as a prevalent ECG abnormality in COVID-19 patients [7]. In our analysis, while the difference in QTc before and during COVID-19 infection reached statistical significance, the magnitude of prolongation was not substantial. Possible reasons for this could be as follows: The patients in our study were relatively older, hence their baseline QTc was already elevated. A relatively higher baseline QTc implies limited room for further increase, which was also confirmed by our subgroup analysis. We found that the baseline QTc in the group with significant QTc elevation was lower than that in the group without significant elevation. However, even a small average increase in mean QTc may be significant in the overall population. A study by Akhtar et al. reported that among 293 COVID-19 patients, the QTc interval was significantly prolonged in those who died, but the magnitude of the prolongation was only around 15ms, and when averaged across the entire COVID-19 population, this increase is even lower [16].
Since QTc increases during COVID-19 acute infection, will this condition persist or recover, and if so, how long will it take to recover? There are potential mechanisms underlying arrhythmias in the post-COVID phase, including inflammatory cardiac channelopathies, cardiac remodeling, and autoimmune responses [17]. These mechanisms contribute to long-term cardiovascular alterations following COVID-19 infection and can serve as the foundation for the development of cardiac arrhythmias in post-COVID patients [18]. Interestingly, our data revealed that while QTc prolongation persisted in the short-term post-infection period (up to 6 months), it significantly decreased in the long-term follow-up (beyond 6 months). This result indicates that COVID-19 infection may acutely correlate with disruptions in cardiac electrophysiology, the associated QTc prolongation may be reversible over time, potentially reflecting gradual improvements in the cardiac environment and resolution of inflammatory responses. However, the persistence of QTc prolongation in the short-term underscores the importance of continued monitoring and potential therapeutic interventions during this period to mitigate the risk of arrhythmias.
COVID-19 infection can promote the occurrence of atrial fibrillation [19], and atrial fibrillation can also increase the risk of death during COVID-19 infection [20]. An examination of electronic medical records from the Veterans Health Administration showed that, compared to matched controls, non-hospitalized patients had a 1.7-fold increased likelihood of developing atrial fibrillation six months after a previous SARS-CoV-2 infection [21]. Given the relationship between COVID-19 and atrial arrhythmias, particularly atrial fibrillation [22], our study also compared changes in P-wave duration. Although no significant difference was observed between pre-infection and acute infection, which might be attributed to the limited sample size of eligible ECG recordings for this comparison. A notable decrease in P-wave duration was detected in the long-term follow-up compared to the acute infectious period. P-wave duration is a potential predictor of atrial arrhythmias, particularly atrial fibrillation [23, 24]. Therefore, the observed decrease in P-wave duration post-COVID-19 recovery may indicate a reduced risk of atrial arrhythmias over time. Future studies with larger sample sizes are necessary to validate these findings.
Limitations
Several limitations must be acknowledged and considered when interpreting the findings. Firstly, the sample size is constrained by the availability of patients who fulfilled the inclusion criteria of having multiple ECG recordings across pre-infection, acute infection, and post-infection time points. Importantly, the participants in this study were likely to have more severe COVID-19 illness, given their requirement for hospitalization and high mortality, potentially limiting the generalizability of our results to a broader, less ill population. The advanced age of our patient cohort (mean age 72.8 years) further emphasizes this caveat, as the impact of COVID-19 on cardiac electrophysiology may differ in younger individuals. Secondly, we have used the Bazzett formula for QTc calculation. It is known that in sinus tachycardia other formulae perform better [25]. It must be acknowledged that a small subset of COVID-19 patients had heart rates that met the criteria for sinus tachycardia, which could potentially affect the correction of QT intervals. However, in this study, the average heart rates of the included patients were 75.9 ± 14.6, 83.0 ± 18.9, and 80.6 ± 18.6 bpm before infection, during infection, and after infection, respectively. Although the heart rate during infection was faster than before infection, it remained within the normal range overall. Therefore, the impact of this correction formula is relatively small. Thirdly, the multifaceted nature of QTc interval prolongation in COVID-19 poses a challenge in disentangling the independent contributions of the virus itself and concomitant medication use, particularly antiviral therapies known to prolong the QT interval. The most commonly reported COVID-19 drugs that prolong QTc are hydroxychloroquine or chloroquine [26, 27], but the patients included in this study did not use these drugs. Instead, most patients used Azvudine, and a few used Nirmatrelvir/Ritonavir. However, the impact of these drugs on QTc has not been established [27, 28]. On the other hand, our study design aimed to describe the temporal trends in QTc, rather than elucidating underlying mechanisms, thus the specific impact of antiviral therapy on QTc dynamics remains unaddressed. Future studies incorporating detailed medication histories and pharmacogenomic data may provide further insights into this issue. Additionally, the utilization of standard 12-lead ECGs as the primary data source limited our ability to comprehensively evaluate cardiac arrhythmic burden. While ECGs offer a snapshot of cardiac electrical activity, dynamic ECG monitoring (e.g., Holter monitoring) would have provided a more robust assessment of clinically significant arrhythmias and their impact on patient outcomes. Unfortunately, the scarcity of patients with paired Holter recordings at multiple time points precluded their inclusion as primary endpoints in this study. Nonetheless, our findings based on 12-lead ECGs remain valuable in highlighting the potential cardiac electrophysiological changes associated with COVID-19. Lastly, the duration of follow-up, though extending beyond the acute phase of infection, was still relatively short, particularly for assessing long-term sequelae. The emergence of “long COVID” underscores the need for longitudinal studies with extended follow-up periods to fully elucidate the potential lasting effects of COVID-19 on cardiac electrophysiology.
Conclusions
The QTc interval before and during COVID-19 infection shows a positive correlation with age. During COVID-19 infection, the QTc interval of patients is prolonged compared to pre-infection levels. This prolongation may persist in the short term after recovery from infection, but typically returns to normal in the long term (six months). Similarly, in long-term follow-up after infection, the P-wave duration is also shortened compared to during infection. The observed QTc prolongation during acute infection and its persistence in the short-term post-infection period highlight the need for ongoing vigilance and potential interventions to prevent arrhythmias. Conversely, the gradual normalization of QTc and P-wave duration in the long-term suggests that cardiac electrophysiological function may eventually recover from the acute insult of COVID-19. However, further research is required to fully elucidate the underlying mechanisms of these changes and their clinical implications.
Acknowledgements
None.
Author contributions
DS.L. contributed to the design and implementation of the research. JC.H., JH.Q. and FF.C contributed to the data collection and consolidation. All authors agree the submission.
Funding
Anhui Province provided financial support in the form of the Outstanding Young Talents Support Program in Colleges and Universities (gxyq2022048) and Natural Science Research Project of Higher Education Institutions (2024AH051940). This work was also supported by the Humanities and Social Sciences Research Planning Foundation of Ministry of Education, China (NO. 22YJAZH134).
Data availability
Data is provided within the manuscript or supplementary information files. The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This retrospective study has been approved by the institutional academic ethics committee of The Second Affiliated Hospital of Wannan College, and as it does not involve patient intervention or information disclosure, informed consent is not required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Farshidfar F, Koleini N, Ardehali H. Cardiovascular complications of COVID-19. JCI Insight 2021; 6. [DOI] [PMC free article] [PubMed]
- 2.Lo YA, Jok C, Tse H. Cardiovascular complications of COVID-19. Hong Kong Med J. 2022;28:249. [DOI] [PubMed] [Google Scholar]
- 3.Peltzer B, Manocha KK, Ying X, et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J Cardiovasc Electrophys. 2020;31:3077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Wang Z, Tse G, et al. Cardiac arrhythmias in patients with COVID-19. J Arrhythmia. 2020;36:827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawakami R, Sakamoto A, Kawai K, et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC Review topic of the Week. J Am Coll Cardiol. 2021;77:314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Liu T, Liu M, Li Y, Yang Y, Zhao J. Electrocardiogram abnormalities in patients with COVID-19. Zhong Hua Xin Lv Shi Chang Xue Za Zhi. 2020;24:128–32. [Google Scholar]
- 7.Santoro F, Monitillo F, Raimondo P, et al. QTc interval prolongation and life-threatening arrhythmias during hospitalization in patients with coronavirus disease 2019 (COVID-19): results from a multicenter prospective registry. Clin Infect Dis. 2021;73:e4031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulletta S, Della Bella P, Pannone L et al. QTc interval prolongation, inflammation, and mortality in patients with COVID-19. J Interventional Cardiac Electrophysiol 2022:1–8. [DOI] [PMC free article] [PubMed]
- 9.Ingul CB, Grimsmo J. Cardiac dysfunction and Arrhythmias 3 months after hospitalization for COVID-19. J Am Heart Association. 2022;11:e023473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis. 2021;53:737–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heemskerk CP, Pereboom M, van Stralen K, et al. Risk factors for QTc interval prolongation. Eur J Clin Pharmacol. 2018;74:183–91. [DOI] [PubMed] [Google Scholar]
- 12.Kloth J, Pagani A, Verboom M, et al. Incidence and relevance of QTc-interval prolongation caused by tyrosine kinase inhibitors. Br J Cancer. 2015;112:1011–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bednar MM, Harrigan EP, Anziano RJ, Camm AJ, Ruskin JN. The QT interval. Prog Cardiovasc Dis. 2001;43:1–45. [DOI] [PubMed] [Google Scholar]
- 14.Rabkin SW, Cheng X-BJ, Thompson DJ. Detailed analysis of the impact of age on the QT interval. J Geriatric Cardiology: JGC. 2016;13:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin GA, Desai AD, Chai Z, et al. Cardiac corrected QT interval changes among patients treated for COVID-19 infection during the early phase of the pandemic. JAMA Netw open. 2021;4:e216842–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akhtar Z, Gallagher MM, Yap YG, et al. Prolonged QT predicts prognosis in COVID-19. Pacing Clin Electrophysiol. 2021;44:875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huseynov A, Akin I, Duerschmied D, Scharf RE. Cardiac arrhythmias in Post-COVID Syndrome: Prevalence, Pathology, diagnosis, and treatment. Viruses 2023; 15. [DOI] [PMC free article] [PubMed]
- 18.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in patients recently recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gawałko M, Kapłon-Cieślicka A, Hohl M, Dobrev D, Linz D. COVID-19 associated atrial fibrillation: incidence, putative mechanisms and potential clinical implications. IJC Heart Vasculature. 2020;30:100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romiti GF, Corica B, Lip GY, Proietti M. Prevalence and impact of atrial fibrillation in hospitalized patients with COVID-19: a systematic review and meta-analysis. J Clin Med. 2021;10:2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–64. [DOI] [PubMed] [Google Scholar]
- 22.Stone E, Kiat H, McLachlan CS. Atrial fibrillation in COVID-19: a review of possible mechanisms. FASEB J. 2020;34:11347–54. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen JB, Kühl JT, Pietersen A, et al. P-wave duration and the risk of atrial fibrillation: results from the Copenhagen ECG Study. Heart Rhythm. 2015;12:1887–95. [DOI] [PubMed] [Google Scholar]
- 24.Magnani JW, Johnson VM, Sullivan LM, et al. P wave duration and risk of longitudinal atrial fibrillation in persons ≥ 60 years old (from the Framingham Heart Study). Am J Cardiol. 2011;107:917–21. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandenberk B, Vandael E, Robyns T, et al. Which QT correction formulae to use for QT monitoring? J Am Heart Association. 2016;5:e003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Changal K, Paternite D, Mack S, et al. Coronavirus disease 2019 (COVID-19) and QTc prolongation. BMC Cardiovasc Disord. 2021;21:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz-Arocutipa C, Brañez‐Condorena A, Hernandez AV. QTc prolongation in COVID‐19 patients treated with hydroxychloroquine, chloroquine, azithromycin, or lopinavir/ritonavir: a systematic review and meta‐analysis. Pharmacoepidemiol Drug Saf. 2021;30:694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin B, Jin L, Li L, Ke J, Lin J. Relationship between ultra-short heart rate variability and short-term mortality in hospitalized COVID-19 patients. J Electrocardiol. 2024;84:32–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript or supplementary information files. The data supporting the findings of this study are available from the corresponding author upon reasonable request.