Abstract
Accurate perception of the orientation of external objects relative to the body, known as egocentric spatial orientation, is fundamental to performing action. Previously, we found via behavioural and magnetic resonance imaging voxel‐based morphometry studies that egocentric spatial orientation is strongly distorted when the whole body is tilted with respect to gravity, and that the magnitude of this perceptual distortion is correlated with the grey matter volume of the right middle occipital gyrus (rMOG). In the present study, we further validated the association between the neural processing in the rMOG and the perceptual distortion by transiently suppressing neural activity in this region using low‐frequency repetitive transcranial magnetic stimulation (rTMS) and evaluating the consequent effect on perceptual distortion. Our results showed that rTMS over the rMOG significantly reduced perceptual distortions when the body was tilted in the frontal plane, while it did not affect egocentric spatial orientation in the upright position. No significant changes in perceptual distortion were observed when rTMS was applied to another cortical candidate (the right temporo‐parietal junction). These results provide evidence that neural processing in the rMOG is associated with body tilt‐related perceptual distortion, suggesting that the rMOG may be engaged in egocentric spatial orientation related to gravitational information.
Keywords: body axis, egocentric spatial orientation, gravity, middle occipital gyrus, transcranial magnetic stimulation (TMS)
The present study showed that low‐frequency rTMS over the right middle occipital gyrus (rMOG) reduced the perceptual distortion of egocentric spatial orientation induced by whole‐body tilt, while no significant effect was observed when rTMS was applied to the right temporo‐parietal junction as another cortical candidate. We suggest a role of neural processing in the rMOG in egocentric spatial orientation with respect to gravitational information.
List of Abbreviations
- ANOVA
analysis of variance
- EMG
electromyography
- FDI
first dorsal interosseous
- LSD
left‐side‐down
- MEP
motor‐evoked potential
- MNI
Montreal Neurological Institute
- MRI
magnetic resonance imaging
- OCR
oculo‐counter‐rotating
- rMOG
right middle occipital gyrus
- rMT
resting motor threshold
- RSD
right‐side‐down
- rTMS
repetitive transcranial magnetic stimulation
- rTPJ
repetitive temporo‐parietal junction
- SD
standard deviation
- SE
standard error
- SVBA
subjective visual body axis
- TE
tilt‐dependent error
- UR
upright
- VBM
voxel‐based morphometry
1. INTRODUCTION
Accurate perception of the orientation of external objects relative to the body (egocentric spatial orientation) is critical to the planning and execution of goal‐directed actions. Indeed, distorted perception of a target's orientation with respect to body‐centred coordinates can deteriorate the accuracy of body movements such as arm reaching (Tani et al., 2018) and postural control (Barra et al., 2009; Jamal et al., 2018).
The subjective visual body axis (SVBA) task, also referred to as the longitudinal body axis or Z‐axis task, was developed to quantify egocentric spatial orientation (Barra et al., 2008, 2009; Clement et al., 2007; Ceyte et al., 2009; Mars et al., 2005; Tani, Uehara, & Tanaka, 2023b). In the SVBA task, participants are asked to align the direction of a visual line parallel to the longitudinal axis of their own body. Although this task does not require participants to refer to the direction of gravity, SVBA task performance is strongly affected by gravitational information. Specifically, the indicated direction of the visual line is biased towards the side to which the body is laterally tilted (tilt‐dependent error, TE; Barra et al., 2008; Bauermeister, 1964; Ceyte et al., 2007, 2009; McFarland & Clarkson, 1966; Tamura et al., 2017; Tani et al., 2018; Tani & Tanaka, 2021; Tani, Uehara, & Tanaka, 2023a, 2023b). In a recent study (Tani, Uehara, & Tanaka, 2023b), we found that the TE was correlated with the perceived degree of body tilt relative to gravity, independent of the actual body tilt angle. This correlation suggests that the brain may refer to the perceived direction of gravity to compute the egocentric orientation of visual objects (Tani, Uehara, & Tanaka, 2023b; Tani, Iio, et al., 2023; also see Tarnutzer et al., 2012).
Recently, we applied a voxel‐based morphometry approach to demonstrate significant correlations between the grey matter volume in the right middle occipital gyrus (rMOG) with TE across individuals (Tani & Tanaka, 2021). This neuroanatomical finding indicates that the rMOG plays an important role in egocentric spatial orientation related to gravitational information. To build upon this prior study, here, we directly confirmed the association of neural processing in the rMOG with egocentric spatial orientation using low‐frequency (1 Hz) repetitive transcranial magnetic stimulation (rTMS). Given that low‐frequency rTMS can induce transient suppression of regional neural activity (Fitzgerald et al., 2006; Romero et al., 2002), we hypothesized that rTMS over the rMOG would alter TE in the SVBA task.
We further assessed the effects of rTMS over the right middle temporo‐parietal junction (rTPJ). The rTPJ has been found to contribute to bodily information processing (see Blanke & Arzy, 2005, Donaldson et al., 2015 for review) and to the integration of multisensory information, such as visual, vestibular and somatosensory signals, associated with the perception of the gravitational vertical (Fiori et al., 2015; Kheradmand et al., 2015; Santos‐Pontelli et al., 2016). Recent clinical studies have reported that lesions, including rTPJ, are associated with ‘lateropulsion’ behaviour in stroke patients (Babyar et al., 2019; Salazar López et al., 2024). This behaviour, characterized by a whole‐body tilt/fall toward the contralesional side while sitting or standing, is likely due to a misestimation of body orientation relative to gravity (Perennou et al., 2008). These findings suggest that the rTPJ may be a potential candidate for egocentric spatial orientation concerning gravitational information. Indeed, our recent neuropsychological study (Tani, Iio, et al., 2023) have shown that lesions in the rTPJ as well as the rMOG may be associated with abnormal TE.
2. METHODS AND MATERIALS
2.1. Participants
Twenty healthy right‐handed participants (six women) with a mean (±standard errors; SE) age of 20.3 (±0.28) years were recruited via posters and the internet. No participants had a history of psychiatric or neurological disorders, and all were naïve with respect to the rTMS experiment. The number of participants was determined according to a previous rTMS study on spatial orientation by Fiori et al. (2015). Participant handedness was assessed using the FLANDERS handedness questionnaire (Nicholls et al., 2013). Prior to the experiment, written informed consent was provided by all participants in accordance with the Declaration of Helsinki. The experimental protocol was approved by the ethics committee of Otemon Gakuin University. To enhance the transparency and reproducibility of the study (Nosek et al., 2018), the number of participants, protocol and statistical analysis were pre‐registered in the Open Scientific Framework (10.17605/OSF.IO/FEU8T).
2.2. Experimental procedure and task
Each participant underwent three experimental sessions on different days, with at least 5 days between sessions. On each experimental day, rTMS was applied to one location, either rMOG, rTPJ or air (sham), while participants performed the SVBA task both before (pre‐rTMS phase) and after (post‐rTMS phase) rTMS exposure (see below). The order of the three rTMS conditions was randomized across participants.
During the SVBA task sessions in the pre‐ and post‐rTMS phases, the participants sat on a tilted chair (SP‐PS100‐Z, Pair Support, Japan) that could be rotated in the frontal plane with a peak velocity of 4.48°/s and an acceleration of 2.61°/s2. Participants wore a head‐mounted display (HMD; Oculus Rift S, Meta, California, USA) device on which visual stimuli were presented (see Figure 1a for details), and held a numeric keypad (TK‐TCM011SV, Elecom, Osaka, Japan). The right and left thumbs were placed on specific keys to allow pressing without visual cues. The HMD device (including the head), trunk and legs were firmly secured to the seat with bands and seat belts.
FIGURE 1.
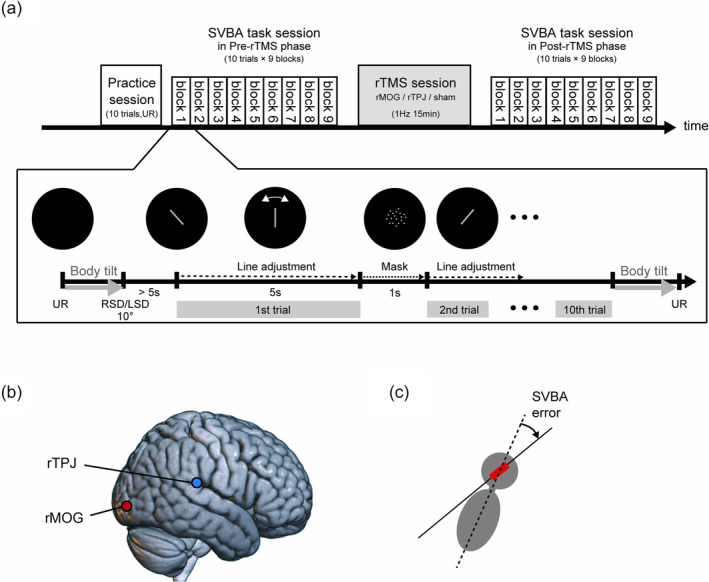
(a) Schematic overview of the experimental procedure on each day. In the subjective visual body axis (SVBA) task sessions in the pre‐/post‐repetitive transcranial magnetic stimulation (rTMS) phase, the participants had 5 s to align the orientation of the presented visual line so that it was parallel to the body longitudinal axis (‘line adjustment’). They performed 10 SVBA task trials in each block. A visual mask (random dots) was presented for 1 s between each SVBA trial (‘mask’). The order of the body positions in the SVBA task was randomized. One of three TMS conditions was assigned for each SVBA task session, and the condition order was randomized across participants. (b) The stimulation sites for the two active rTMS conditions. rMOG, right middle occipital gyrus; rTPJ, right temporo‐parietal junction. (c) SVBA error calculation in each trial. We calculated the angular error between the actual body longitudinal axis (dotted line) and the indicated line (red bar and black solid line).
Figure 1a shows the procedure on each experimental day. The SVBA task session consisted of nine blocks, including three upright (UR), three right‐side‐down (RSD) and three left‐side‐down (LSD) blocks. The block order was randomized. At the beginning of the RSD or LSD block, the chair was tilted 10 degrees to the right or left, respectively (‘Body tilt’ in Figure 1a bottom). In previous studies (Tani, Iio, et al., 2023; Tani & Tanaka, 2021), we confirmed that a lateral body tilt of 10° led to large tilt‐dependent errors in the SVBA task. In the UR block, the chair was not tilted. The participants completed 10 trials of the SVBA task in each body position.
At the beginning of each trial, a grey line segment with a length corresponding to a visual angle of 33.4° was presented in the center of the display. The length of this line was comparable to that used in previous studies (e.g., 43.1°, Tamura et al., 2017; 32.4°, Ceyte et al., 2009). The initial orientation of the line was randomly chosen to be ±30°, ±45° or 90° relative to the vertical axis of the HMD. Participants were asked to use the numeric keypad to adjust this line parallel to their body's longitudinal axis and to respond within 5 s (‘Line adjustment’). The visual line could be moved in 0.5° increments at an angular velocity of approximately 300°/s. The intertrial interval was set to 1 s. During this interval, randomly positioned grey dots were presented within the circular area that would contain the bar (i.e., a circle with a diameter corresponding to a visual angle of 53.1°) to avoid low‐level visual adaptation in the subsequent trial (‘Mask;’ Clifford et al., 2007). During the SVBA task, all visual background information that could provide cues about the direction of gravity and the body's longitudinal axis was excluded. Body tilt with rotational acceleration above the detection threshold of the semicircular canal has been shown to affect the performance of visual adjustment (Jaggi‐Schwarz & Hess, 2003). To avoid this effect on SVBA performance, the first trial was started approximately 5 s following the completion of the body tilt, based on a previous finding, which showed that the post‐rotatory torsional ocular drift led by semicircular canal stimulation was quite small at this time (Tarnutzer et al., 2009). When the participants could not align the line within the time limit (5 s) in a trial, they verbally reported their responses to the experimenter immediately following the trial. After each block was complete, the chair orientation was returned to the UR position (‘Body tilt’). A 5–10 s break was included between the blocks. To minimize the operational error and stabilize the data, each participant completed 10 trials of the SVBA task in the UR position (‘Practice session’ in Figure 1a top) prior to the pre‐rTMS phase on each experimental day. The total duration of each SVBA task session was 9–10 min (1 min per block). This duration was likely sufficient to observe the effects of rTMS, as previous studies on motor‐evoked potential (MEP; Romero et al., 2002; Touge et al., 2001) have indicated that the sustained duration of 1 Hz rTMS at an intensity of 90% resting motor threshold (rMT) is approximately 10 min.
2.3. rTMS
In the rTMS session, low‐frequency (1 Hz) rTMS was applied to the participants for 15 min, while they were seated and relaxed in a reclining chair. According to the rTMS condition on each experimental day, TMS was applied to the rMOG, rTPJ or no brain region (sham TMS). TMS was delivered using a figure‐eight coil with a wing diameter of 70 cm (MCF‐B70; MagVenture, UK) connected to MagPro R20 magnetic stimulator (MagVenture, UK).
The TMS‐coil position and orientation were manually adjusted using the Brainsight neuronavigation system (Rogue Research Inc., Canada; Bashir et al. 2011) and anatomical T1‐weighted brain images (MP‐RAGE; repetition time: 1900 ms; echo time: 2.48 ms; inversion time: 900 ms; flip angle: 9°; matrix: 256 × 256; pixel size: 1.0 × 1.0 mm; slice thickness: 1.0 mm without inter‐slice gap; number of slices: 208), which were obtained using a 3.0‐T SIEMENS scanner (Vida, Germany) with a 64‐channel head/neck coil. For each participant, the anatomical image in native space was co‐registered to Montreal Neurological Institute (MNI) coordinate space using a linear transformation (Rogue Research Inc., 2017) via Brainsight software. Then, the target point for rTMS was identified based on the MNI coordinates. For the rMOG, we targeted the cortical surface using the following MNI coordinates: x = 35, y = −86, z = 6, in accordance with our previous study (Tani & Tanaka, 2021). For the rTPJ, the target coordinates were x = 61, y = −37, z = 22 (Fiori et al., 2015; see Figure 1b). Prior to the rTMS sessions, we visually verified that the rMOG target point was within the area between the intraoccipital and inferior occipital sulcus and that the rTPJ target point was within the area behind the end of the lateral sulcus and below the intraparietal sulcus, for all participants.
The coil was placed tangential to the surface of the scalp and oriented with the handle pointing backward for the rMOG condition (Kassuba et al., 2014) and 45° upward for the rTPJ condition (Fiori et al., 2015). In the sham condition, the coil was placed in a cortical location identical to that in the rMOG condition but held perpendicular to the scalp so that the brain was not stimulated. During the rTMS session, the coil was anchored onto the holder, and the participants were instructed not to move their heads. Thus, the coil location/orientation was fixed relative to the participant's head. When the position/orientation of the coil significantly deviated from the set value as a result of head movement, the experimenter manually adjusted the head position.
The stimulus intensity of the rTMS was set at 90% of the individual rMT measured on the first experimental day before the experiment, as previous studies have shown that the application of rTMS to brain regions to non‐motor cortical areas at this intensity can alter brain activity and behaviours (Gromann et al., 2012; also see Donaldson et al., 2015 for a review). Throughout all three experimental days, we applied rTMS at the identical intensity defined with respect to the first day's rMT, rather than each day's rMT. This is because we have no evidence that intra‐participant daily fluctuations in rMT directly reflect daily variations of the sensitivity of non‐motor cortices, including rMOG and rTPJ, to rTMS. MEP was recorded from the first dorsal interosseous (FDI) muscle of the left hand via Ag/AgCl surface electrodes. Electromyography (EMG) signals were amplified, band‐pass filtered between 16 and 470 Hz and sampled at 3 kHz with a Rogue EMG device. Then, the motor hot spot, the cortical spot where a TMS pulse evoked a MEP in the left FDI muscle with a maximum amplitude, was detected by applying single‐pulse TMS to various positions around the right primary motor cortex. The rMT was defined as the minimum TMS current intensity that produced MEPs >50 μV for at least 5 of 10 stimulation pulses (single‐pulse TMS) applied to the motor hot spot during rest. The coil for the rMT determination procedure was oriented with the handle pointing backwards and laterally at a 45° angle away from the nasion‐inion line (Mills et al., 1992). The mean (±SE) rTMS stimulus intensity was 50.4 (±2.1)% of the maximum stimulator output.
2.4. Data analysis
The present study had a repeated measures design with three within‐subject factors: ‘body position’ with three levels (UR, LSD, RSD), ‘rTMS condition’ with three levels (rMOG, rTPJ, sham) and ‘phases’ with two levels (pre‐, post‐rTMS). The measurement variable was the signed angular deviation of the indicated line from the actual body longitudinal axis in the SVBA task (SVBA error; Figure 1c). Positive and negative values indicated the deviation towards rightward and leftward, respectively.
For each participant, we performed the following analysis. First, we excluded trials in which the participant could not align the line with the target orientation within the time limit (5 s). Subsequently, we averaged the SVBA error for the 30 trials in each body position. Using the averaged data, the effect of leftward or rightward body tilt on the SVBA error (TE to the left and right: , , respectively) was then quantified by subtracting the SVBA error in the UR position (denoted as ) from that in the LSD or RSD position (, ) as follows:
Note that the sign of was set opposite to that of so that a positive indicated that the shift of the indicated line was in the same direction as the body tilt, as well as . The and were then averaged to obtain the . The , and were calculated for each combination of phase and rTMS condition. Finally, for each rTMS condition, we calculated ΔTE by subtracting TE for the pre‐rTMS phase from that for the post‐rTMS phase. Positive and negative ΔTE values indicate increased and decreased TE following rTMS exposure, respectively. We considered ΔTE to be an indicator of the effect of rTMS on the distortion of egocentric spatial orientation induced by body tilt.
We first checked whether the SVBA error was biased in the direction of body tilt in the LSD and RSD positions, as was to be expected according to previous studies (Barra et al., 2008; Ceyte et al., 2007, 2009; McFarland & Clarkson, 1966; Tamura et al., 2017; Tani et al., 2018; Tani, Uehara, & Tanaka, 2023a, 2023b; Tani & Tanaka, 2021; Bauermeister, 1964).
After calculating ΔTE for each rTMS condition and each participant, we evaluated the group effects as follows. We then compared ΔTE in the rMOG or rTPJ condition with that in the control (sham) condition using Dunnett tests (two‐tailed). The difference in ΔTE between the two target conditions (rMOG vs. rTPJ) was not a focus of this study. We did not perform a one‐way repeated measures analysis of variance (ANOVA) prior to the Dunnett test, although this was declared on the pre‐registration form, because theoretical studies have shown that the use of an ANOVA prior to the Dunnett test can inflate the false negative rate (Hothorn, 2016; Howell, 2013; Wilcox, 1987).
We then conducted two additional statistical analyses. First, we confirmed that the TE in the pre‐rTMS phase was not significantly different between the target conditions and sham condition using Dunnett tests. Second, we assessed the effect of rTMS on the SVBA error in the UR position according to Δ, which was obtained by subtracting in the pre‐rTMS phase from that in the post‐rTMS phase, via Dunnett tests (rMOG/rTPJ vs. sham).
The significance level was set at p < 0.05. All statistical analyses were performed using SAS statistical software (version 9.1; SAS, Inc., Cary, NC).
3. RESULTS
All participants completed all three experiments without any discomfort or adverse effects (e.g., seizure) during or after the stimulation trials. One participant (subject ID: 18) was excluded from the analysis because of a large trial‐by‐trial variability (standard deviation: SD) in the data that exceeded three SD from the mean of all participants. As a result, data from 19 participants were included in the group analysis. After excluding the trials in which the participants did not complete the task within the time limit (5 s), data from 98.4% of the total trials were included in the analysis.
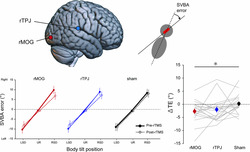
Figure 2a shows the mean SVBA error at each body position for each phase and rTMS condition. The SVBA error was nearly zero (overall mean = −0.80°) when the body was UR. In contrast, as expected according to previous studies (Barra et al., 2008; Ceyte et al., 2007, 2009; McFarland & Clarkson, 1966; Tamura et al., 2017; Tani et al., 2018; Tani, Uehara, & Tanaka, 2023a, 2023b; Tani & Tanaka, 2021; Bauermeister, 1964), the SVBA was strongly biased in the direction of body tilt when the body was laterally tilted (LSD and RSD), regardless of the phase and rTMS condition. The consistency of this trend across individuals was confirmed by the positive values in all conditions in almost all participants (see Figure 2b).
FIGURE 2.
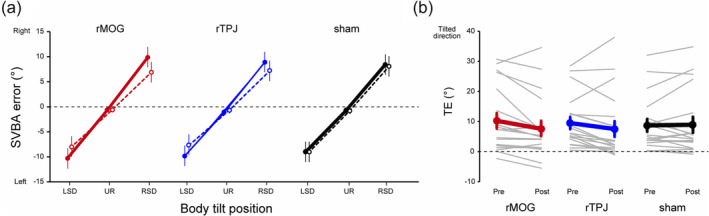
(a) Group‐mean subjective visual body axis (SVBA) error for the pre‐ (solid lines and filled markers) and post‐repetitive transcranial magnetic stimulation (rTMS) (dashed lines and open markers) phases in each rTMS condition. (b) The TE in each phase and rTMS condition. Thin grey and thick coloured (red: right middle occipital gyrus [rMOG], blue: right middle temporo‐parietal junction (rTPJ), black: sham) lines denote the TE for each participant and the mean across participants, respectively. Error bars represent standard errors.
As shown in Figure 2b, the TE tended to decrease after rTMS over the rMOG or rTPJ, while this was not the case in the sham condition. The mean ΔTE was negative for the rMOG (−2.65 ± 0.98°) and rTPJ (−1.99 ± 1.03°) conditions, while nearly zero for the sham condition (0.20 ± 0.84°; Figure 3). The Dunnett test showed that the ΔTE in the rMOG condition was significantly smaller than that in the sham condition (p = 0.04, Cohen's d = −0.69). In contrast, no significant difference was observed between the rTPJ and sham conditions (p = 0.15, Cohen's d = −0.53). Also, the TE in the pre‐rTMS phase (‘Pre’ in Figure 2b) did not significantly differ between the rTMS conditions (rMOG, 10.02 ± 2.51°; rTPJ, 9.33 ± 1.99°; sham, 8.54 ± 2.07°; Dunnett test, rMOG vs. sham, p = 0.78, Cohen's d = 0.15; rTPJ vs. sham, p = 0.80, Cohen's d = 0.08). Furthermore, we confirmed that the reduction in TE induced by rTMS over the rMOG occurred independent of the order of the different rTMS conditions (see Supporting Information for details). These results indicate that rTMS over the rMOG led to a significant reduction of the perceptual distortion of egocentric spatial orientation induced by body tilt.
FIGURE 3.
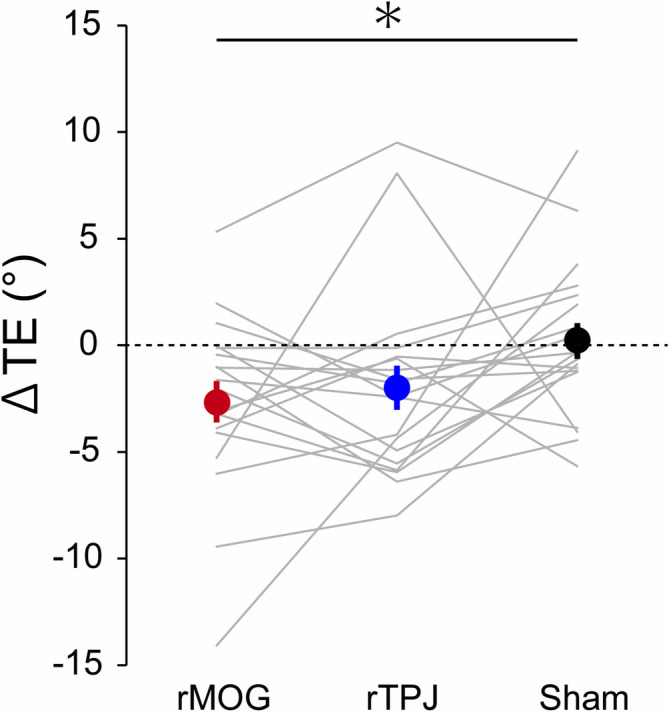
The mean ΔTE across participants in each repetitive transcranial magnetic stimulation (rTMS) condition. Error bars represent standard error. *: p < 0.05.
We evaluated whether the rTMS affected SVBA performance when the body was UR (), and found no significant effect of rTMS on (rMOG, −0.25 ± 0.45°; rTPJ, 0.37 ± 0.36°; sham, 0.06 ± 0.28°; Dunnett tests, rMOG vs. sham, p = 0.78, Cohen's d = −0.2; rTPJ vs. sham, p = 0.80, Cohen's d = 0.19; Figure 4). Also, we examined whether the rTMS affected the trial‐by‐trial variability of . We further calculated the standard deviation of SVBA errors in the UR position (upright standard deviation: ) from 30 trials in each phase and rTMS condition. As with , we calculated Δ by subtracting in the pre‐rTMS phase from that in the post‐rTMS phase in each rTMS condition for each participant and performed Dunnett tests for Δ . We found that Δ did not significantly differ between rMOG/rTPJ and sham conditions (rMOG, 0.04 ± 0.09°; rTPJ, −0.10 ± 0.18°; sham, 0.14 ± 0.07°; rMOG vs. sham, p = 0.62, Cohen's d = −0.16; rTPJ vs. sham, p = 0.37, Cohen's d = −0.43). These results indicate that rTMS over the rMOG did not influence SVBA performance when the body was UR.
FIGURE 4.
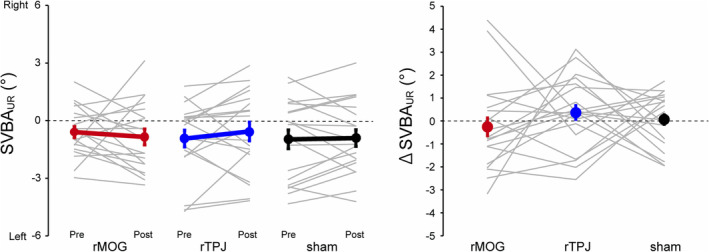
(a) The in each phase and repetitive transcranial magnetic stimulation (rTMS) condition. Thin grey and thick coloured (red: right middle occipital gyrus [rMOG], blue: right middle temporo‐parietal junction (rTPJ), black: sham) lines denote the for each participant and the mean across participants, respectively. Error bars represent standard error. (b) The mean Δ across participants in each repetitive transcranial magnetic stimulation (rTMS) condition. Error bars represent standard error.
4. DISCUSSION
In the present study, we used a whole‐body tilt device and low‐frequency (1 Hz) rTMS to assess the contribution of neural processing in the rMOG and rTPJ in the egocentric spatial orientation when the body is tilted. Overall, we found that perceptual distortions (measured as TE) towards the tilted side of the body in the SVBA task significantly decreased compared to the sham condition following rTMS over the rMOG, but not over the rTPJ.
Previously, we demonstrated a significant association between the grey matter volume in the rMOG and TE in the SVBA task using voxel‐based morphometry (Tani & Tanaka, 2021). Importantly, we found a positive interindividual correlation between smaller rMOG volume and smaller TE. Numerous studies have previously shown that a larger grey matter volume is related to improved functionality in a given brain region (e.g., Maguire et al., 2000), suggesting that lower rMOG functionality leads to smaller TE. This relationship is supported by our recent neuropsychological study of patients with hemispheric stroke, which showed that lesions in the right occipitotemporal cortex, including the rMOG, are associated with an abnormally small TE (Tani, Iio, et al., 2023). Given that 1‐Hz rTMS leads to the suppression of neural processing at the stimulated cortical site (Fitzgerald et al., 2006; Romero et al., 2002), the significant reduction in TE observed in the present study is generally consistent with the results of previous studies.
As noted in the Introduction, when performing the SVBA task, participants are not required to refer to gravity‐related bodily information (i.e., head and body orientation relative to gravity) derived from vestibular and somatosensory signals but rather need to rely on visual (retinal) information. In our previous study, we found that estimation errors were induced depending on the body tilt angles (i.e., TE), which were strongly correlated with perceived body tilt orientation relative to gravity (Tani, Uehara, & Tanaka, 2023b). These results indicate that the TE may not be attributed to visual processing per se but rather to the processing of bodily information and/or the integration process. Tarnutzer et al. (2012) previously proposed that, ignoring the small effect of ocular counter‐roll induced by body tilt (see below), a visual object's orientation relative to the body may be computed by combining two different processes; one of which estimates the orientation using visual information, while the other estimates it by subtracting the body angle and the objects' angle in reference to gravity. If this view is correct, our findings (i.e., rTMS over the rMOG‐reduced TE) indicate that the rMOG may contribute to the latter process. Indeed, neural visual responses in the MOG, which is involved in processing spatial information, such as position, shape and orientation, of visual objects (Cant et al., 2009; Liu et al., 2017; Galati et al., 2001; Saj et al., 2014; Chen et al., 2012; Renier et al., 2010), have been shown to be modulated by vestibular input (Brandt et al., 2002; Della‐Justina et al., 2015; Deutschlander et al., 2002). For example, Deutschlander et al. (2002) reported that simultaneous vestibular stimulation suppressed the neural response of the MOG to visual stimulation, indicating that the MOG receives and somehow integrates vestibular signals, a major modality for conveying gravitational information, with visual signals. Finally, the lack of the TMS effect on performance in the UR position in our experiment (SDUR and ; Figure 4) supports the claim that the rMOG may not play a major role in purely visual processing (i.e., visual processing without an effect of bodily information).
In nonhuman primates, the neural activity in response to visual inputs in the lower visual cortex, including the V1 (Horn & Hill, 1969; Tomko et al., 1981) and V2/3 (Sauvan & Peterhans, 1999), has been shown to be affected by the direction of gravity. Furthermore, higher association areas, such as the caudal intraparietal area (Rosenberg & Angelaki, 2014) and the inferotemporal cortex (Emonds et al., 2023), have been shown to visually encode the tilt orientation of an object with respect to the gravitational vertical. Combining the present results with previous neurophysiological findings, it could be speculated that orientation information about visual objects may be integrated with vestibular‐derived gravitational information at multiple stages of bottom‐up visual processing, including the MOG.
As mentioned in the introduction, we anticipated that rTMS over the rTPJ might influence TE, given the potential role of rTPJ in processing gravitational and gravity‐related bodily information. However, no significant difference in ΔTE was observed between the rTPJ and sham conditions, although a trend towards TE reduction, similar to that seen in the rMOG condition (Figures 2 and 3). We speculate that the contribution of gravitational information processed in the rTPJ to egocentric spatial orientation may be marginal in the current task, where visual contextual cues (e.g., surrounding visual frame; Zoccolotti et al., 1993) are absent. Future research is necessary to determine whether and how neural processing in the rTPJ contributes to egocentric spatial orientation.
One may argue that motor control during manual adjustment of line orientation and/or automatic ocular torsion as a result of head tilt (oculo‐counter‐rotating [OCR]) affects performance in the SVBA task. Although we cannot completely exclude these possibilities, we believe that these factors are unlikely to be the primary causes of TE, as the line could be adjusted with visual feedback within a sufficient time (5 s) and the expected amplitude of the OCR (1°; 10% of the head tilt angle, Miller, 1962) was much smaller than the TE observed in our study (>10°).
In conclusion, our TMS study directly shows, for the first time, that neural processing in the rMOG is associated with the body‐tilt‐induced distortion of egocentric spatial orientation, suggesting a role for the rMOG in the estimation of egocentric orientation of visual objects in reference to gravitational information. However, future behavioural and neurophysiological studies are required to fully understand the computational processes underlying these findings.
AUTHOR CONTRIBUTION
Keisuke Tani: Conceptualization; data curation; formal analysis; investigation; methodology; visualization; writing‐original draft. Eiichi Naito: Conceptualization; investigation; methodology; supervision, writing‐review and editing. Koji Mizobe: Conceptualization; supervision. Satoshi Hirose: Conceptualization; methodology; supervision; writing‐original draft.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer-review/10.1111/ejn.16639.
Supporting information
Data S1. Supporting Information.
ACKNOWLEDGEMENTS
We are grateful to Dr. Satoshi Tanaka from Hamamatsu University School of Medicine for the technical assistance and helpful advice regarding this study. This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (20K19305 and 23K16761) and Otemon Gakuin University (all to K.T.).
Tani, K. , Naito, E. , Mizobe, K. , & Hirose, S. (2025). Right middle occipital gyrus is associated with egocentric spatial orientation during body tilt: Evidence from a repetitive transcranial magnetic stimulation study. European Journal of Neuroscience, 61(1), e16639. 10.1111/ejn.16639
Edited by: Gregor Thut.
DATA AVAILABILITY STATEMENT
The individual data are openly available in the Open Scientific Framework (OSF) at 10.17605/OSF.IO/MBRFV.
REFERENCES
- Babyar, S. R. , Smeragliuolo, A. , Albazron, F. M. , Putrino, D. , Reding, M. , & Boes, A. D. (2019). Lesion Localization of Poststroke Lateropulsion. Stroke, 50(5), 1067–1073. 10.1161/strokeaha.118.023445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra, J. , Benaim, C. , Chauvineau, V. , Ohlmann, T. , Gresty, M. , & Pérennou, D. (2008). Are Rotations in Perceived Visual Vertical and Body Axis After Stroke Caused by the Same Mechanism? Stroke, 39(11), 3099–3101. 10.1161/strokeaha.108.515247 [DOI] [PubMed] [Google Scholar]
- Barra, J. , Oujamaa, L. , Chauvineau, V. , Rougier, P. , & Pérennou, D. (2009). Asymmetric standing posture after stroke is related to a biased egocentric coordinate system. Neurology, 72(18), 1582–1587. 10.1212/wnl.0b013e3181a4123a [DOI] [PubMed] [Google Scholar]
- Bashir, S. , Edwards, D. , & Pascual‐Leone, A. (2011). Neuronavigation Increases the Physiologic and Behavioral Effects of Low‐Frequency rTMS of Primary Motor Cortex in Healthy Subjects. Brain Topography, 24(1), 54–64. 10.1007/s10548-010-0165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauermeister, M. (1964). Effect of body tilt on apparent verticality, apparent body position, and their relation. Journal of Experimental Psychology, 67(2), 142–147. 10.1037/h0046424 [DOI] [PubMed] [Google Scholar]
- Blanke, O. , & Arzy, S. (2005). The Out‐of‐Body Experience: Disturbed Self‐Processing at the Temporo‐Parietal Junction. The Neuroscientist, 11(1), 16–24. 10.1177/1073858404270885 [DOI] [PubMed] [Google Scholar]
- Brandt, T. , Glasauer, S. , Stephan, T. , Bense, S. , Yousry, T. A. , Deutschlander, A. , & Dieterich, M. (2002). Visual‐vestibular and visual‐visual cortex interactions: New insights from fMRI and pets. Proceedings of the New York Academy of Sciences, 956, 230–241. 10.1111/j.1749-6632.2002.tb02822.x [DOI] [PubMed] [Google Scholar]
- Cant, J. S. , Arnott, S. R. , & Goodale, M. A. (2009). fMR‐adaptation reveals separate processing regions for the perception of form and texture in the human ventral stream. Experimental Brain Research, 192(3), 391–405. 10.1007/s00221-008-1573-8 [DOI] [PubMed] [Google Scholar]
- Ceyte, H. , Cian, C. , Nougier, V. , Olivier, I. , & Trousselard, M. (2007). Role of gravity‐based information on the orientation and localization of the perceived body midline. Experimental Brain Research, 176(3), 504–509. 10.1007/s00221-006-0764-4 [DOI] [PubMed] [Google Scholar]
- Ceyte, H. , Cian, C. , Trousselard, M. , & Barraud, P.‐A. (2009). Influence of perceived egocentric coordinates on the subjective visual vertical. Neuroscience Letters, 462(1), 85–88. 10.1016/j.neulet.2009.06.048 [DOI] [PubMed] [Google Scholar]
- Chen, Q. , Weidner, R. , Weiss, P. H. , Marshall, J. C. , & Fink, G. R. (2012). Neural interaction between spatial domain and spatial reference frame in parietal‐occipital junction. Journal of Cognitive Neuroscience, 24(11), 2223–2236. 10.1162/jocn_a_00260 [DOI] [PubMed] [Google Scholar]
- Clement, G. , Arnesen, T. N. , Olsen, M. H. , & Sylvestre, B. (2007). Perception of longitudinal body axis in microgravity during parabolic flight. Neuroscience Letters, 413(2), 150–153. 10.1016/j.neulet.2006.11.047 [DOI] [PubMed] [Google Scholar]
- Clifford, C. W. , Webster, M. A. , Stanley, G. B. , Stocker, A. A. , Kohn, A. , Sharpee, T. O. , & Schwartz, O. (2007). Visual adaptation: Neural, psychological and computational aspects. Vision Research, 47(25), 3125–3131. 10.1016/j.visres.2007.08.023 [DOI] [PubMed] [Google Scholar]
- Della‐Justina, H. M. , Gamba, H. R. , Lukasova, K. , Nucci‐da‐Silva, M. P. , Winkler, A. M. , & Amaro, E. Jr. (2015). Interaction of brain areas of visual and vestibular simultaneous activity with fMRI. Experimental Brain Research, 233(1), 237–252. 10.1007/s00221-014-4107-6 [DOI] [PubMed] [Google Scholar]
- Deutschlander, A. , Bense, S. , Stephan, T. , Schwaiger, M. , Brandt, T. , & Dieterich, M. (2002). Sensory system interactions during simultaneous vestibular and visual stimulation in PET. Human Brain Mapping, 16(2), 92–103. 10.1002/hbm.10030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson, P. H. , Rinehart, N. J. , & Enticott, P. G. (2015). Noninvasive stimulation of the temporoparietal junction: A systematic review. Neuroscience and Biobehavioral Reviews, 55, 547–572. 10.1016/j.neubiorev.2015.05.017 [DOI] [PubMed] [Google Scholar]
- Emonds, A. M. X. , Srinath, R. , Nielsen, K. J. , & Connor, C. E. (2023). Object representation in a gravitational reference frame. eLife, 12, e81701. 10.7554/eLife.81701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori, F. , Candidi, M. , Acciarino, A. , David, N. , & Aglioti, S. M. (2015). The right temporoparietal junction plays a causal role in maintaining the internal representation of verticality. Journal of Neurophysiology, 114(5), 2983–2990. 10.1152/jn.00289.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, P. B. , Fountain, S. , & Daskalakis, Z. J. (2006). A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clinical Neurophysiology, 117(12), 2584–2596. 10.1016/j.clinph.2006.06.712 [DOI] [PubMed] [Google Scholar]
- Galati, G. , Committeri, G. , Sanes, J. N. , & Pizzamiglio, L. (2001). Spatial coding of visual and somatic sensory information in body‐centred coordinates. The European Journal of Neuroscience, 14(4), 737–746. 10.1046/j.0953-816x.2001.01674.x [DOI] [PubMed] [Google Scholar]
- Gromann, P. M. , Tracy, D. K. , Giampietro, V. , Brammer, M. J. , Krabbendam, L. , & Shergill, S. S. (2012). Examining frontotemporal connectivity and rTMS in healthy controls: Implications for auditory hallucinations in schizophrenia. Neuropsychology, 26(1), 127–132. 10.1037/a0026603 [DOI] [PubMed] [Google Scholar]
- Horn, G. , & Hill, R. M. (1969). Modifications of receptive fields of cells in the visual cortex occurring spontaneously and associated with bodily tilt. Nature, 221(5176), 186–188. 10.1038/221186a0 [DOI] [PubMed] [Google Scholar]
- Hothorn, L. A. (2016). The two‐step approach—A significant ANOVA F‐test before Dunnett's comparisons against a control—Is not recommended. Communications in Statistics ‐ Theory and Methods, 45(11), 3332–3343. 10.1080/03610926.2014.902225 [DOI] [Google Scholar]
- Howell, D. C. (2013). Statistical methods for psychology ((8th ed.). ed.). Wadsworth Cengage Learning. [Google Scholar]
- Inc, R. R. (2017). Brainsight user manual. In. Rogue Research Inc. [Google Scholar]
- Jaggi‐Schwarz, K. , & Hess, B. J. (2003). Influence of dynamic tilts on the perception of earth‐vertical. Experimental Brain Research, 149(3), 340–350. 10.1007/s00221-002-1343-y [DOI] [PubMed] [Google Scholar]
- Jamal, K. , Leplaideur, S. , Rousseau, C. , Chochina, L. , Moulinet‐Raillon, A. , & Bonan, I. (2018). Disturbances of spatial reference frame and postural asymmetry after a chronic stroke. Experimental Brain Research, 236(8), 2377–2385. 10.1007/s00221-018-5308-1 [DOI] [PubMed] [Google Scholar]
- Kassuba, T. , Klinge, C. , Holig, C. , Roder, B. , & Siebner, H. R. (2014). Short‐term plasticity of visuo‐haptic object recognition. Frontiers in Psychology, 5, 274. 10.3389/fpsyg.2014.00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradmand, A. , Lasker, A. , & Zee, D. S. (2015). Transcranial magnetic stimulation (TMS) of the supramarginal gyrus: A window to perception of upright. Cerebral Cortex, 25(3), 765–771. 10.1093/cercor/bht267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, N. , Li, H. , Su, W. , & Chen, Q. (2017). Common and specific neural correlates underlying the spatial congruency effect induced by the egocentric and allocentric reference frame. Human Brain Mapping, 38(4), 2112–2127. 10.1002/hbm.23508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire, E. A. , Gadian, D. G. , Johnsrude, I. S. , Good, C. D. , Ashburner, J. , Frackowiak, R. S. , & Frith, C. D. (2000). Navigation‐related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the United States of America, 97(8), 4398–4403. 10.1073/pnas.070039597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars, F. , Vercher, J. L. , & Popov, K. (2005). Dissociation between subjective vertical and subjective body orientation elicited by galvanic vestibular stimulation. Brain Research Bulletin, 65(1), 77–86. 10.1016/j.brainresbull.2004.11.012 [DOI] [PubMed] [Google Scholar]
- McFarland, J. H. , & Clarkson, F. (1966). Perception of orientation—Adaptation to lateral body‐tilt. American Journal of Psychology, 79(2), 265–271. 10.2307/1421133 [DOI] [PubMed] [Google Scholar]
- Miller, E. F. (1962). Counterrolling of the human eyes produced by head tilt with respect to gravity. Acta Oto‐Laryngologica, 54(1–6), 479–501. 10.3109/00016486209126967 [DOI] [PubMed] [Google Scholar]
- Mills, K. R. , Boniface, S. J. , & Schubert, M. (1992). Magnetic brain stimulation with a double coil: The importance of coil orientation. Electroencephalography and Clinical Neurophysiology, 85(1), 17–21. 10.1016/0168-5597(92)90096-t [DOI] [PubMed] [Google Scholar]
- Nicholls, M. E. , Thomas, N. A. , Loetscher, T. , & Grimshaw, G. M. (2013). The Flinders handedness survey (FLANDERS): A brief measure of skilled hand preference. Cortex, 49(10), 2914–2926. 10.1016/j.cortex.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Nosek, B. A. , Ebersole, C. R. , DeHaven, A. C. , & Mellor, D. T. (2018). The preregistration revolution. Proceedings of the National Academy of Sciences of the United States of America, 115(11), 2600–2606. 10.1073/pnas.1708274114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perennou, D. A. , Mazibrada, G. , Chauvineau, V. , Greenwood, R. , Rothwell, J. , Gresty, M. A. , & Bronstein, A. M. (2008). Lateropulsion, pushing and verticality perception in hemisphere stroke: A causal relationship? Brain, 131(Pt 9), 2401–2413. 10.1093/brain/awn170 [DOI] [PubMed] [Google Scholar]
- Renier, L. A. , Anurova, I. , De Volder, A. G. , Carlson, S. , VanMeter, J. , & Rauschecker, J. P. (2010). Preserved functional specialization for spatial processing in the middle occipital gyrus of the early blind. Neuron, 68(1), 138–148. 10.1016/j.neuron.2010.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, J. R. , Anschel, D. , Sparing, R. , Gangitano, M. , & Pascual‐Leone, A. (2002). Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clinical Neurophysiology, 113(1), 101–107. 10.1016/s1388-2457(01)00693-9 [DOI] [PubMed] [Google Scholar]
- Rosenberg, A. , & Angelaki, D. E. (2014). Gravity influences the visual representation of object tilt in parietal cortex. The Journal of Neuroscience, 34(43), 14170–14180. 10.1523/JNEUROSCI.2030-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saj, A. , Cojan, Y. , Musel, B. , Honore, J. , Borel, L. , & Vuilleumier, P. (2014). Functional neuro‐anatomy of egocentric versus allocentric space representation. Neurophysiologie Clinique, 44(1), 33–40. 10.1016/j.neucli.2013.10.135 [DOI] [PubMed] [Google Scholar]
- Salazar López, E. , Krewer, C. , Bergmann, J. , Möhwald, K. , Müller, F. , & Jahn, K. (2024). Lateropulsion in Right‐Sided Stroke: Brain Anatomical Correlates of Severity and Duration. Journal of Neurologic Physical Therapy, 48(1), 38–45. 10.1097/npt.0000000000000446 [DOI] [PubMed] [Google Scholar]
- Santos‐Pontelli, T. E. , Rimoli, B. P. , Favoretto, D. B. , Mazin, S. C. , Truong, D. Q. , Leite, J. P. , Pontes‐Neto, O. M. , Babyar, S. R. , Reding, M. , Bikson, M. , & Edwards, D. J. (2016). Polarity‐dependent misperception of subjective visual vertical during and after transcranial direct current stimulation (tDCS). PLoS ONE, 11(3), e0152331. 10.1371/journal.pone.0152331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvan, X. M. , & Peterhans, E. (1999). Orientation constancy in neurons of monkey visual cortex. Visual Cognition, 6(1), 43–54. 10.1080/713756803 [DOI] [Google Scholar]
- Tamura, A. , Wada, Y. , Inui, T. , & Shiotani, A. (2017). Perceived direction of gravity and the body‐axis during static whole body roll‐tilt in healthy subjects. Acta Oto‐Laryngologica, 137(10), 1057–1062. 10.1080/00016489.2017.1328744 [DOI] [PubMed] [Google Scholar]
- Tani, K. , Iio, S. , Kamiya, M. , Yoshizawa, K. , Shigematsu, T. , Fujishima, I. , & Tanaka, S. (2023). Neuroanatomy of reduced distortion of body‐centred spatial coding during body tilt in stroke patients. Scientific Reports, 13(1), 11853. 10.1038/s41598-023-38751-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani, K. , Shiraki, Y. , Yamamoto, S. , Kodaka, Y. , & Kushiro, K. (2018). Whole‐body roll tilt influences goal‐directed upper limb movements through the perceptual tilt of egocentric reference frame. Frontiers in Psychology, 9, 84. 10.3389/fpsyg.2018.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani, K. , & Tanaka, S. (2021). Neuroanatomical correlates of the perception of body axis orientation during body tilt: A voxel‐based morphometry study. Scientific Reports, 11(1), 14659. 10.1038/s41598-021-93961-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani, K. , Uehara, S. , & Tanaka, S. (2023a). Psychophysical evidence for the involvement of head/body‐centered reference frames in egocentric visuospatial memory: A whole‐body roll tilt paradigm. Journal of Vision, 23(1), 16. 10.1167/jov.23.1.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani, K. , Uehara, S. , & Tanaka, S. (2023b). Association between body tilt and egocentric estimates near upright. Multisensory Research, 36(4), 367–386. 10.1163/22134808-bja10097 [DOI] [PubMed] [Google Scholar]
- Tarnutzer, A. A. , Bockisch, C. J. , Olasagasti, I. , & Straumann, D. (2012). Egocentric and allocentric alignment tasks are affected by otolith input. Journal of Neurophysiology, 107(11), 3095–3106. 10.1152/jn.00724.2010 [DOI] [PubMed] [Google Scholar]
- Tarnutzer, A. A. , Bockisch, C. J. , & Straumann, D. (2009). Head roll dependent variability of subjective visual vertical and ocular counterroll. Experimental Brain Research, 195(4), 621–626. 10.1007/s00221-009-1823-4 [DOI] [PubMed] [Google Scholar]
- Tomko, D. L. , Barbaro, N. M. , & Ali, F. N. (1981). Effect of body tilt on receptive field orientation of simple visual cortical neurons in unanesthetized cats. Experimental Brain Research, 43(3–4), 309–314. 10.1007/BF00238372 [DOI] [PubMed] [Google Scholar]
- Touge, T. , Gerschlager, W. , Brown, P. , & Rothwell, J. C. (2001). Are the after‐effects of low‐frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clinical Neurophysiology, 112(11), 2138–2145. 10.1016/s1388-2457(01)00651-4 [DOI] [PubMed] [Google Scholar]
- Wilcox, R. R. (1987). New designs in analysis of variance. Annual Review of Psychology, 38, 29–60. 10.1146/annurev.ps.38.020187.000333 [DOI] [Google Scholar]
- Zoccolotti, P. , Antonucci, G. , & Spinelli, D. (1993). The gap between rod and frame influences the rod‐and‐frame effect with small and large inducing displays. Perception & Psychophysics, 54, 14–19. 10.3758/BF03206933 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data Availability Statement
The individual data are openly available in the Open Scientific Framework (OSF) at 10.17605/OSF.IO/MBRFV.


