Abstract
Background
Antimicrobial resistance (AMR) presents a serious threat to health, highlighting the urgent need for more effective antimicrobial agents with innovative mechanisms of action. Nanotechnology offers promising solutions by enabling the creation of nanoparticles (NPs) with antibacterial properties. This study aimed to explore the antibacterial, anti-biofilm, and anti-virulence effects of eco-friendly synthesized α-Fe₂O₃ nanoparticles (α-Fe₂O₃-NPs) against pathogenic bacteria.
Methods
The α-Fe2O3-NPs were synthesized using a green synthesis method that involved Bacillus sp. GMS10, with iron sulfate as a precursor. The NPs were characterized through ultraviolet-visible (UV-Vis) spectroscopy, Field Emission Scanning Electron Microscopy (FESEM), Energy Dispersive X-ray Spectroscopy (EDX), Dynamic Light Scattering (DLS), Zeta Potential Analysis, X-ray Diffraction (XRD), and Fourier Transform Infrared Spectroscopy (FT-IR). Their antimicrobial activity was assessed against Gram-positive and Gram-negative bacteria. The study also evaluated the effect of the α-Fe2O3-NPs on bacterial cell membrane disruption, biofilm formation, efflux pump inhibition, and swarming motility.
Results
The UV-Visible spectrum showed a peak at 228 nm, indicating plasmon absorbance of the α-Fe2O3-NPs. FESEM revealed spherical NPs (~ 30 nm), and DLS confirmed a hydrodynamic size of 36.3 nm with a zeta potential of -25.1 mV, indicating good stability. XRD identified the rhombohedral α-Fe2O3 phase, and FTIR detected O-H, C-H, C = O, and Fe-O functional groups, suggesting organic capping for stability. Antibacterial assays demonstrated that the α-Fe2O3-NPs had MIC values ranging from 0.625 to 5 µg/mL and MBC values between 5 and 20 µg/mL, with a strong effect against Gram-positive bacteria. The NPs significantly increased membrane permeability, inhibited biofilm formation in S. aureus and E. coli, and disrupted efflux pumps in S. aureus SA-1199B (a fluoroquinolone-resistant strain overexpressing norA). Additionally, the α-Fe2O3-NPs inhibited P. aeruginosa swarming motility.
Conclusion
The bacteria-synthesized α-Fe2O3-NPs demonstrated significant antimicrobial activity, particularly against Gram-positive bacteria, and exhibited strong potential for inhibiting biofilm formation and efflux pump activity, offering a promising strategy to address AMR. Focus on further evaluating their therapeutic potential in clinical settings and conducting comprehensive assessments of their safety profiles to ensure their applicability in medical treatments.
Clinical trial number
Not applicable.
Keywords: α-Fe2O3-nanoparticles, Green method, Antibacterial, Anti-virulence
Introduction
Antimicrobial resistance (AMR) is an escalating global challenge among pathogens, including bacteria, viruses, and fungi, significantly impact both personal health and the global economy [1]. Recent data indicate that bacterial AMR causes approximately 4.95 million deaths each year [2]. Given the urgent challenge of antimicrobial resistance, there is a critical need for novel drugs with innovative mechanisms of action that combat resistant strains. Researchers across various biological fields are actively pursuing the development of these new antimicrobial agents [3].
A potential solution to antimicrobial resistance challenges is provided by nanotechnology, which provides novel approaches to synthesizing NPs with antimicrobial properties [4]. NPs have garnered significant interest in recent decades owing to their distinctive properties, diverse synthesis methods, and wide-ranging applications [5].
Among iron oxide NPs, α-Fe₂O₃-NPs are distinguished by their unique rhombohedral crystal structure and specialized physical and chemical characteristics. Compared to other forms, such as maghemite, α-Fe₂O₃-NPs demonstrate superior chemical and thermodynamic stability, enhanced biocompatibility, and a larger surface area, making them particularly suitable for biomedical applications. These attributes, combined with their high surface area-to-volume ratio and superparamagnetic properties, offer significant potential for developing effective antimicrobial agents, distinguishing α-Fe₂O₃ from other iron oxide NPs [6–8].
Traditional nanoparticle synthesis methods often rely on harsh chemicals and extreme conditions, leading to hazardous waste generation and high production costs. While these methods are effective, they have notable drawbacks, including high energy consumption and toxic reagents [6]. In contrast, green synthesis techniques employing biological resources like plants, bacteria, and fungi provide a more sustainable and eco-friendly alternative. These methods use natural biomolecules to reduce metal ions and stabilize NPs, enhancing their biocompatibility, stability, and biological activity [9, 10].
Bacteria can synthesize NPs by either accumulating metal ions within their cells or using their metabolic products to reduce these ions into NPs. This biologically mediated synthesis is considered a promising green approach, offering benefits such as low cost, environmental sustainability, scalability, and more precise control over nanoparticle synthesis compared to traditional chemical methods [11]. While prior studies have primarily focused on the bactericidal effects of green-synthesized α-Fe₂O₃-NPs [12, 13], this study uniquely examines their anti-biofilm and anti-virulence properties. By targeting both biofilm formation and virulence factors, it offers an innovative approach to addressing the challenges of antimicrobial resistance in clinical infections.
In this study, we aimed to green-synthesize α-Fe₂O₃-NPs using bacteria and evaluate their efficacy in reducing biofilm formation and inhibiting virulence traits in multi-drug-resistant pathogens.
Materials and methods
Bacterial strains and culture conditions
All chemical reagents were sourced from Sigma-Aldrich (USA). Bacillus sp. GMS10 (GenBank accession number MW362307), which was previously isolated from soil near the Sari Gunay Gold Mine in Qorveh, Kurdistan Province, as reported by Ashengroph et al. [14], was employed for -Fe2O3-NP synthesis. The bacterial strains used for testing included Staphylococcus aureus ATCC 25,923, Staphylococcus aureus SA1199B (a norA-overexpressing strain used for the ethidium bromide efflux pump inhibition test), Bacillus cereus ATCC 11,778, Escherichia coli ATCC 25,922, and Pseudomonas aeruginosa ATCC 27,853. These strains were obtained from the Persian Type Culture Collection (PTCC) in Iran. All bacterial culture media were obtained from Merck (Darmstadt, Germany), and the bacteria were cultured under aerobic conditions at 37 °C for 24 to 36 h.
Extracellular synthesis of α-Fe₂O₃-NPs
Bacillus sp. GMS10 was used for the green synthesis of α-Fe₂O₃-NPs. A high-purity (> 99%) iron sulfate (FeSO₄·7 H₂O) stock solution was prepared by dissolving the salt in sterile deionized water, then filtered through a 0.22 μm membrane and stored at 4 °C. Bacillus sp. GMS10 culture was grown in sterile nutrient broth (NB), consisting of 5 g peptone and 3 g meat extract per liter. Cultures were incubated overnight at 35 °C with shaking at 150 rpm to promote optimal bacterial growth. After incubation, the cultures were centrifuged at 5000 ×g for 10 min. The supernatant was collected while discarding the bacterial pellets. This supernatant served as a bacterial extract for nanoparticle synthesis. To synthesize the NPs, 50 mL of FeSO₄·7 H₂O solution (concentrations ranging from 0.2 M to 1 M) was mixed with 50 mL of the bacterial supernatant and incubated at 150 rpm for 24 h at room temperature. To purify the NPs, the mixture was centrifuged at 10,000 g for 30 min after incubation. Afterward, sterile deionized water was used to wash the pellets three times to remove any residual chemicals. For further characterization, the samples were freeze-dried (Alpha 1-2Dplus, Christ, Germany) for 12 h.
Characterization of Fe2O3-NPs
The synthesized α-Fe₂O₃-NPs were characterized using various spectroscopic and microscopic techniques to evaluate their physical and chemical properties. Ultraviolet-visible (UV-Vis) spectroscopy (Specord 210 Plus, Analytik Jena, Germany) was employed to determine the shape, size, and size distribution of the NPs based on their surface plasmon resonance (SPR) properties within the 200 to 800 nm wavelength range. Measurements were performed at room temperature, with a scan rate of 1 nm/s, and the intensity of absorption was recorded at 1 nm intervals.
The morphology and elemental composition were confirmed using field emission scanning electron microscopy (FESEM) and energy dispersive X-ray spectroscopy (EDX) (MIRA3, TESCAN, Czech Republic). The FESEM was operated at an accelerating voltage of 30.0 kV, and the samples were gold-coated to enhance conductivity. The acquired images were analyzed using ImageJ software to measure particle size and distribution.
Dynamic light scattering (DLS) and zeta potential analysis (Malvern, UK) were employed to measure the particle size distribution in aqueous dispersion and assess the surface charge and stability of the NPs, respectively. X-ray diffraction (XRD) (Philips PW1730, Netherlands) was utilized to investigate the crystallinity and phase composition of the NPs, using CuKα radiation (λ = 1.5406 Å) over a 2θ range of 5 to 80°, with a scan rate of 0.05°/min. Additionally, Fourier transform infrared spectroscopy (FTIR) (Bruker, USA) was conducted to identify the organic and inorganic functional groups present in the NPs, covering a spectral range of 400 to 4000 cm⁻¹. The samples were prepared by mixing the NPs with KBr and pressing them into a pellet.
Antibacterial activity of the α-Fe2O3-NPs
The antibacterial potential of the α-Fe₂O₃-NPs was tested against two Gram-positive S. aurous and B. cereus, and two Germ-negative E. coli, and P. aeruginosa bacteria. The microdilution broth method was applied to determine the MIC of the α-Fe2O3-NPs [15]. To achieve this, α-Fe₂O₃-NPs concentrations ranging from 0.312 to 160 µg/mL were prepared in a 96-well plate using Müller Hinton broth (MHB). Bacterial suspensions were adjusted to a turbidity of 0.5 McFarland and added to the wells, resulting in a final concentration of 4 × 10⁵ to 5 × 10⁵ CFU/mL. Additionally, MHB without Fe2O3-NPs and with bacteria was used as a growth control. The plates were incubated at 37 °C for 18–24 h under aerobic conditions. In order to find the MBC, 10 µL of culture samples from wells with no growth (which is considered the MIC) were transferred onto freshly prepared Mueller-Hinton Agar (MHA) plates. The plates were placed in an incubator at 37 °C for 18–24 h. After incubation, colony counts were used to assess bacterial viability. The MBC was defined as the lowest compound concentration that resulted in the death of 99.9% of the bacterial inoculum. The vancomycin and gentamicin antibiotics were used as controls for Gram-positive and Germ-negative bacteria, respectively [16].
Bacterial cell membrane disruption assay
Cell membrane disruption was assessed using crystal violet uptake assays at sub-MIC concentrations, as previously described [17]. Bacterial suspensions were prepared by inoculating bacteria (10⁷ CFU/mL) in Luria-Bertani (LB) broth. The suspensions were then centrifuged at 10,000× g for 5 min. The pellet was washed twice with phosphate-buffered saline (PBS) (pH 7.4) and resuspended in 50 mM PBS. α-Fe₂O₃-NPs were added to the bacterial suspension at MIC/2 concentration and incubated at 37 °C for 30 min. Control samples were prepared in the same manner but without NP treatment. Following incubation, the samples were centrifuged at 10,000× g for 5 min, and the bacterial pellet was resuspended in PBS containing 10 µg/mL of crystal violet stain. The suspension was incubated at 37 °C for 10 min to allow crystal violet uptake. After incubation, the samples were centrifuged again at 10,000× g for 5 min, and the absorbance of the supernatant was measured at 570 nm. The initial absorbance value of the crystal violet solution, without bacterial treatment, was considered to be 100%. The percentage of crystal violet uptake was calculated using the following formula:
 |
Biofilm formation inhibition
The effect of the α-Fe2O3-NPs on the bacterial biofilm formation was investigated using Micro titer plate (MtP) assay [18]. The α-Fe2O3-NPs were prepared in MHB at three different sub-MIC concentrations based on established MIC values: MIC/2, MIC/4, and MIC/8 (Table 1). Cultures of each bacterial strain (S. aureus, B. cereus, E. coli, and P. aeruginosa) were grown overnight in MHB and adjusted to match the turbidity of a 0.5 McFarland standard. The standardized culture was diluted 1:100 in fresh MHB to achieve a final inoculum density of approximately 5 × 10⁵ CFU/mL in each well. Subsequently, 50 µL of the diluted bacterial inoculum was added to each well of the microtiter plate. Following this, 50 µL of α-Fe₂O₃-NP solutions, prepared at concentrations of MIC/2, MIC/4, and MIC/8, were added to all wells. A growth control was included, consisting of wells with bacterial inoculum and MHB but without α-Fe₂O₃-NPs, to assess the initial biofilm formation. The micro plate was incubated at 37 °C for 18–24 h. In the following step, crystal violet staining assays were used to quantify biofilm formation. Therefore, planktonic cells were removed, and surface-adhered cells were treated with 200 µl of crystal violet (0.1%) for 20 min. Thereafter, the excess dye was removed, and the wells washed three times with PBS. Crystal violet of the stained cells was solubilized with 100 µl of ethanol (95%), and optical density (OD) was measured using a microplate reader (ELx808, BioTek, USA) at 570 nm. Each assay was performed in triplicate, with results presented as the mean ± standard deviation (SD). As a measure of efficacy, the percentage of inhibition was calculated using the mean absorbance of the negative control wells by the following formula:
Table 1.
The values of MIC, sub-MIC concentrations and MBC for α-Fe2O3-NPs against tested bacteria
| Bacteria | MIC (µg/mL) | sub-MIC concentrations (µg/mL) | MBC (µl/ml) | ||
|---|---|---|---|---|---|
| MIC/2 | MIC/4 | MIC/8 | |||
| S. aureus | 1.25 | 0.625 | 0.312 | 0.156 | 5 |
| B. cereus | 0.625 | 0.312 | 0.156 | 0.078 | 2.5 |
| E. coli | 2.5 | 1.25 | 0.625 | 0.312 | 10 |
| P. aeruginosa | 5 | 2.5 | 1.25 | 0.625 | 20 |
Percentage of inhibition = 100 - [(OD570 nm of the treated wells) / (mean OD570 nm of the negative control wells contained no antimicrobial agent) × 100)]
Ethidium bromide efflux pump inhibition assay
Specifically, this test was performed on Staphylococcus aureus SA-1199B, a strain that overexpresses norA. Tests were conducted as described previously [16]. The S. aureus SA‐1199B cells were grown overnight in MHB until an optical density at 600 nm of 0.6 was reached, followed by centrifugation at 5000× g for 5 min. Pellets were suspended in 2 ml of PBS (pH = 7). Bacterial suspensions were vortexed and transferred to 96-well plates followed, by addition of a saline solution including Et-Br (8 µg/ml) and the α-Fe2O3-NPs (at MIC/2 concentration). Plates were placed in a Corbett Life Science Rotor-Gene 6000 Cycler (Qiagen, Germany) with excitation at 518 nm and emission at 605 nm. Differences in relative final fluorescence between the α-Fe2O3-NPs-containing samples and control (samples containing only Et-Br and no α-Fe₂O₃-NPs) were indicative of the activity of the α-Fe2O3-NPs to inhibit Et-Br efflux.
Anti-swarming motility
The swarming motility of P. aeruginosa was assessed using swim plates containing the following composition: 1% tryptone, 0.5% NaCl, 0.5% glucose, and 0.5% agar. Two sets of plates were used in the experiment. The first group contained α-Fe₂O₃-NPs at MIC/2 concentration, while the second set served as the control, with no α-Fe₂O₃-NPs added. The plates were air-dried for 10 min prior to inoculation. Bacterial cells were then gently inoculated at the center of the agar surface using a toothpick for both the treated and control groups. The plates were incubated at 30 °C for 20–24 h, after which bacterial swarming and motility on the agar surface were observed [19].
Statistically analysis
All experiments were performed in triplicate and repeated three times to ensure the reliability and reproducibility of the results. The data is presented as the mean ± SD. To compare the effects between the treated and control groups, an independent two-sample t-test was conducted. For the analysis of multiple groups, one-way ANOVA (Analysis of Variance) was used to determine if there were significant differences among the groups. When ANOVA indicated significant differences, post-hoc analysis was performed. A significance level of p < 0.05 was considered statistically significant for all tests. All statistical analyses were carried out using GraphPad Prism software (version 6).
Results
The α-Fe2O3-NPs characterization and confirmation
Ultraviolet -Visible Spectroscopy
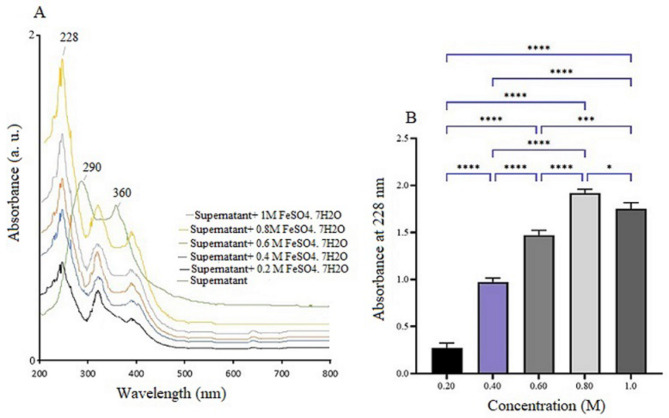
The UV-visible absorption spectrum of the synthesized Fe₂O₃-NPs, shown in Fig. 1A and B, spans the range from 200 to 700 nm. In Fig. 1A, a prominent absorption peak at 228 nm is observed across different iron sulfate concentrations, confirming the formation of ferric oxide NPs. This peak corresponds to the characteristic absorption range for iron oxide NPs (220–230 nm), validating their successful synthesis [20]. The control sample, which lacks the precursor, shows no absorption peaks, confirming that nanoparticle synthesis occurs only in the treated sample. The control exhibits peaks at 290 and 360 nm, likely due to natural organic compounds, such as proteins, present in the bacterial supernatant. In the treated sample, these peaks shift to 320 and 380 nm, suggesting possible chemical interactions during nanoparticle formation. Figure 1B illustrates the effect of varying iron sulfate concentrations (0.2 to 1.0 M) on the absorption spectrum after 24 h of incubation at 25 °C with shaking at 100 rpm. The absorbance increases progressively from 0.2 M to 0.8 M, reaching a peak at 0.8 M, indicating optimal nanoparticle production. However, concentrations above 0.8 M lead to a decline in synthesis, possibly due to iron ion toxicity or reduced biomolecular activity. Statistical analysis confirms that 0.8 M is the optimal concentration for efficient nanoparticle synthesis.
Fig. 1.
(A) The UV-Vis absorption spectrum of α-Fe₂O₃-NPs synthesized using Bacillus sp. GMS10 supernatant, with varying concentrations of FeSO₄·7 H₂O, shows a distinct peak at 228 nm, which is absent in the control. (B) The effect of iron sulfate concentration (0.2–1.0 M) on nanoparticle absorbance at 228 nm reveals the highest production at 0.8 M. Statistical significance is denoted by **** (p < 0.0001) and * (p < 0.05)
Field emission scanning electron microscope with EDX elemental analysis
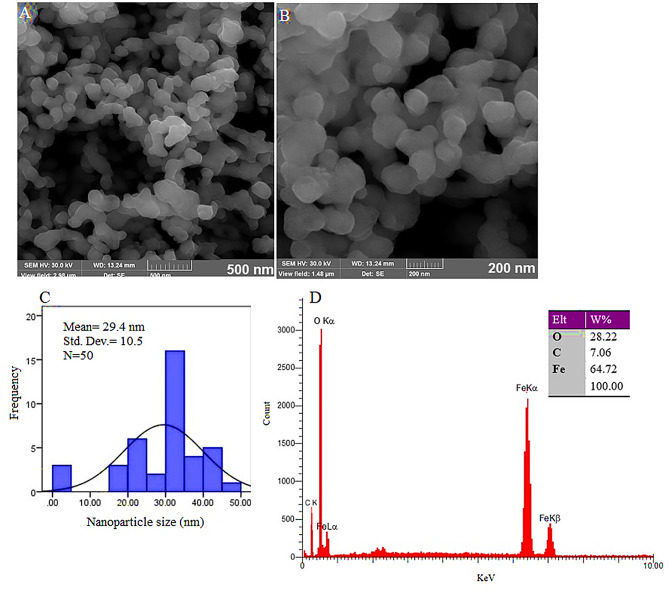
The α-Fe₂O₃-NPs were synthesized using the bacterial supernatant with 0.8 M iron sulfate and predominantly exhibit spherical shapes, as shown in Fig. 2A and B. FESEM images (Fig. 2A and B) and the size histogram (Fig. 2C) reveal well-dispersed NPs with an average size of 29.4 ± 10.5 nm, demonstrating consistent nanoparticle production. The EDX analysis (Fig. 2D) verifies the elemental composition of the NPs, with distinct peaks for iron and oxygen. The weight composition comprises 64.72% iron and 28.22% oxygen, with a small amount of carbon (7.06%). The presence of carbon is likely due to biological macromolecules serving as capping agents, stabilizing NPs. In summary, these results confirm the successful synthesis of spherical α-Fe₂O₃-NPs, with the expected size and elemental composition.
Fig. 2.
FESEM images (A and B) and the size histogram (C) display well-dispersed nanoparticles with an average size of 29.4 ± 10.5 nm, indicating consistent nanoparticle production
DLS analysis and zeta potential analysis
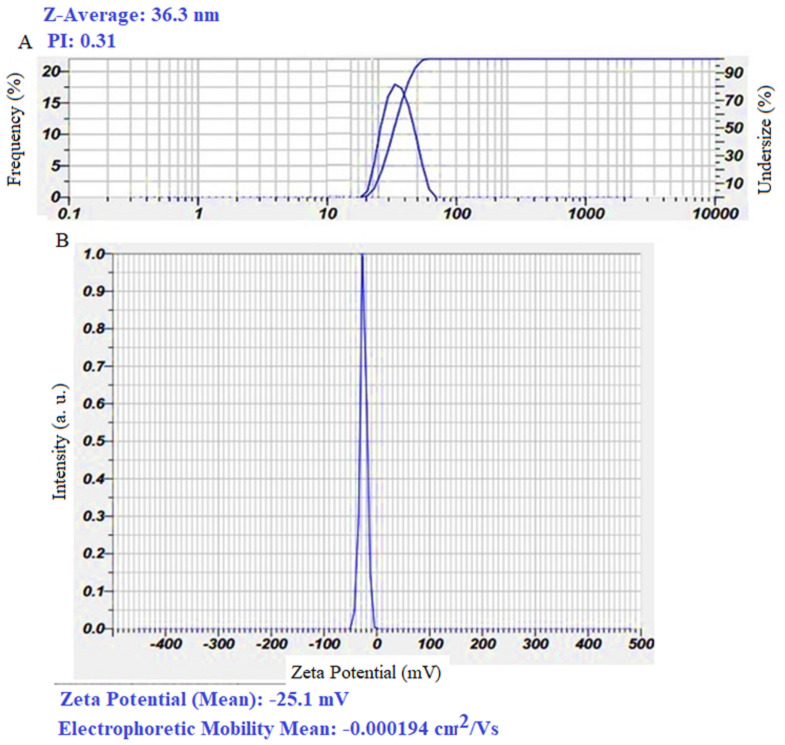
As shown in Fig. 3A, the α-Fe₂O₃-NPs in an aqueous solution (hydrodynamic state) exhibit an average diameter of 36.3 nm, with a polydispersity index (PDI) of 0.31, suggesting a high degree of monodispersity and uniform size distribution. Zeta potential analysis (Fig. 3B) measured a value of -25.1 mV, indicating moderate colloidal stability that helps prevent nanoparticle aggregation [21]. This stability, coupled with the capping effect of biological macromolecules, further enhances nanoparticle efficacy in biological environments. These attributes highlight the promising role of α-Fe₂O₃-NPs in treating bacterial infections in both medical and environmental applications.
Fig. 3.
Dynamic Light Scattering (DLS) analysis (A) and Zeta potential measurement (B) of α-Fe₂O₃-NPs synthesized using the supernatant of Bacillus sp. GMS10
XRD and FT-IR analysis
Figure 4 presents the XRD (Figure A) and FTIR (Figure B) analyses of Fe₂O₃-NPs synthesized using Bacillus sp. GMS10. The XRD pattern displays distinct peaks at 2θ angles of 24.9°, 33.18°, 35.7°, 49.52°, 54.13°, and 75.47°, which correspond to the (012), (104), (110), (113), (024), and (220) planes, respectively. These peaks closely align with the rhombohedral hematite phase (α-Fe₂O₃), as identified in the JCPDS card number 33–0664 [22]. The sharp and intense peaks indicate high crystallinity, confirming that hematite is the predominant phase. The FTIR spectrum (Fig. 4B) shows the presence of various functional groups on the surface of the synthesized NPs, indicative of both organic capping agents and the iron oxide structure. The broad peak at 3396.59 cm⁻¹ corresponds to O-H stretching vibrations, suggesting the presence of hydroxyl groups, potentially from water molecules or hydroxyl groups attached to the nanoparticle surface. The peak observed at 2924.77 cm⁻¹ is attributed to C-H stretching vibrations, likely arising from aliphatic chains in organic compounds used as capping agents. Additionally, the peak at 1631.46 cm⁻¹ is associated with C = O stretching, which may originate from carboxyl groups in biomolecules such as amino acids or peptides that serve as stabilizers for the NPs. The 1405.13 cm⁻¹ peak represents C-O stretching vibrations, further indicating the presence of organic compounds, such as esters or carboxylates, in the nanoparticle coating. The peak at 1006.94 cm⁻¹ corresponds to C-H bending vibrations, pointing to hydrocarbon chains within the capping agents. Furthermore, the peaks at 523.46 cm⁻¹ and 456.10 cm⁻¹ are attributed to Fe-O stretching vibrations, confirming the iron oxide composition of the NPs. These Fe-O bonds are characteristic of the iron oxide structure, providing stability to the NPs framework. Overall, the presence of hydroxyl, carboxyl, C-H, and Fe-O groups suggests that the NPs are effectively capped with biomolecules such as amino acids and peptides. These organic compounds not only stabilize the NPs but also enhance their dispersion in various environments, which is essential for their potential applications. This comprehensive analysis addresses all the vibrations observed in the FTIR spectrum and supports the successful synthesis and stabilization of iron oxide NPs with organic capping agents [23]. The biomolecules act as stabilizers for the Fe₂O₃-NPs in biological environments. The analysis confirms the crystalline hematite structure, which is coated with biomolecules, as indicated by the FTIR spectrum. This biomolecular capping, along with the crystalline structure, enhances the potential of the NPs for applications in biological systems.
Fig. 4.
XRD (A) and FT-IR (B) analyses of α-Fe₂O₃-NPssynthesized using the supernatant of Bacillus sp. GMS10
Antibacterial activity of the synthesized α-Fe2O3-NPs
The antimicrobial activity test revealed that the synthesized compound exhibited antibacterial properties against all four bacterial strains (MIC = 0.625–5 µg/mL and MBC = 5–20 µg/mL). Table 1 presents the MIC, sub-MIC concentrations and MBC for α-Fe2O3-NPs against S. aureus, B. cereus, E. coli, and P. aeruginosa. Notably, the synthesized NPs demonstrated a stronger effect against Gram-positive bacteria than Gram-negative bacteria.
Bacterial cell membrane disruption
The impact of α-Fe₂O₃-NPs at MIC/2 concentration on the bacterial cell membrane was assessed using a crystal violet uptake assay. As presented in Table 2, the synthesized α-Fe2O3-NPs significantly increased membrane permeability in all four studied bacteria.
Table 2.
The influence of synthesized α-Fe₂O₃-NPs on crystal violet uptake by tested bacterial strains
| Bacteria | Ccrystal violet uptake (%) | P value | |
|---|---|---|---|
| Treated | Control | ||
| S. aureus | 33.4 ± 2.3 | 21.3 ± 1.9 | 0.007* |
| B. cereus | 28.9 ± 2 | 22.6 ± 2.7 | 0.024* |
| E. coli | 19.6 ± 1.5 | 13 ± 0.9 | 0.008* |
| P. aeroginosa | 17.7 ± 1.1 | 12.2 ± 2.7 | 0.036* |
*: Statistically significant
Anti-biofilm potential of α-Fe2O3-NPs
The anti-biofilm assay results revealed that the synthesized α-Fe₂O₃-NPs demonstrated significant anti-biofilm activity against S. aureus and E. coli at sub-MIC concentrations. Notably, they inhibited S. aureus biofilm formation at MIC/2 concentration and E. coli biofilm at both MIC/2 and MIC/4 concentrations (Fig. 5).
Fig. 5.
Biofilm inhibition activity of the α-Fe2O3-NPs at various concentrations (MIC/2, MIC/4 and MIC/8) against S. aureus (A), B. cereus (B), E.coli (C) and P. aeruginosa (D); (*: statistically significant, NS: statistically not significant)
Ethidium bromide efflux pump inhibition
The results indicated that α-Fe₂O₃-NPs at MIC/2 concentration significantly increased the accumulation of ethidium bromide (Et-Br) in S. aureus SA-1199B, a norA overexpressing strain (Fig. 6). This suggests that the α-Fe₂O₃-NPs effectively disrupt the efflux pump activity in S. aureus.
Fig. 6.

Effect of the α-Fe2O3-NPs on the ethidium bromide accumulation (*: statistically significant)
Anti-swarming motility
This test was designed to examine the effect of α-Fe₂O₃-NPs on P. aeruginosa swarming motility at MIC/2 concentration. As presented in Fig. 7, the α-Fe₂O₃-NPs inhibit P. aeruginosa at MIC/2 concentration.
Fig. 7.

Effect of the α-Fe2O3-NPs on the P. aeruginosa swimming at MIC/2 concentration
Discussion
The increasing prevalence of bacteria resistant to traditional antibiotics poses a significant threat to global health [1]. Metallic NPs offer a promising alternative to conventional antibiotics. NPs can interact with various cellular components, disrupting cell membrane permeability, inducing oxidative stress, and affecting gene expression and protein activation [24].
In this study, α-Fe₂O₃-NPs were synthesized using an extracellular, supernatant-based method with Bacillus sp. GMS10 and tested for their antibacterial, anti-biofilm, and anti-virulence effects. This eco-friendly, cost-effective, and scalable method avoids toxic chemicals, producing high-purity, monodisperse NPs that are easily purified and visualized. Unlike plant-based methods, it provides consistent, year-round production, and its bio-coating of non-toxic biomolecules enhances nanoparticle stability and biocompatibility. This supernatant-based approach is versatile, adaptable to various metal ions, and simplifies the synthesis process compared to intracellular methods, aligning well with sustainable nanotechnology goals [25]. The NPs were characterized using various techniques. UV-Vis spectroscopy showed an absorption peak at 228 nm, which is consistent with the presence of iron oxide NPs. FESEM and DLS analyses revealed that the NPs were predominantly spherical, with an average size ranging from 30 to 36 nm. Additionally, FT-IR analysis identified functional groups associated with the α-Fe₂O₃-NPs. These characteristics confirm the successful synthesis of the NPs and highlight their potential for biological applications. Although some variations in size and other properties were observed compared to previous studies [26, 27], such differences are expected and can be attributed to variations in synthesis conditions, including the organism used, growth environment, and other physical parameters [28]. A key aspect of this study is the use of a " green " method for nanoparticle synthesis, which offers several advantages over traditional physical and chemical methods. Biological techniques have garnered significant attention due to their cost-effectiveness, non-toxicity, eco-friendliness, and enhanced stability [6, 7]. Due to the beneficial properties of NOs synthesized using green methods, researchers across in various fields are increasingly focused on producing NPs using these eco-friendly approaches and evaluating their biological activities. For example, in a study utilizing a green synthesis method with Komagataeibacter intermedius, silver NPs (AgNPs) were produced and shown to have antimicrobial effects against S. aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa [29]. In a recent study, zinc oxide NPs (ZnO-NPs) were synthesized using Streptomyces baarnensis, and their bactericidal properties were confirmed against drug-resistant strains of Klebsiella pneumoniae and S. aureus [30]. In conclusion, the findings of this study, along with those from similar research, demonstrate that various microorganisms can produce metal NPs using different methods. These NPs not only exhibit high safety but also possess a wide range of biological properties, including antibacterial activity against pathogens, making them promising candidates for future research and applications.
In the subsequent experiment, we assessed the antibacterial potential of the synthesized α-Fe₂O₃-NPs against pathogenic bacteria. The results demonstrated that the α-Fe₂O₃-NPs exhibited effective antibacterial activity against all tested bacterial strains, with MIC values ranging from 0.625 to 5 µg/mL. Several previous studies have also reported the antibacterial activity of iron-based NPs (α-Fe₂O₃-NPs and Fe₃O₄-NPs) against various bacterial strains [13, 31–34]. For example, a study by Muhammad et al. (2019) demonstrated the effectiveness of α-Fe₂O₃-NPs synthesized from Papaver somniferum L. against B. subtilis, S. epidermidis, Klebsiella pneumoniae, and P. aeruginosa [35]. Similarly, a study by Yoonus et al. (2020) reported the antibacterial activity of α-Fe₂O₃-NPs derived from Piper betel leaves against both Gram-positive and Gram-negative bacteria [36]. In another similar study, the antibacterial activity of α-Fe₂O₃-NPs synthesized using Sida cordifolia plant extract was checked against various Gram-positive and Gram-negative bacteria. The antibacterial potential of these NPs was confirmed against B. subtilis, S. aureus, E. coli, and K. pneumoniae [37].
It is important to note that varying MIC values and growth inhibition zones have been reported in previous studies on the antibacterial properties of iron-based NPs. These differences can be attributed to factors such as particle size, shape, surface chemistry, synthesis method, bacterial strain, growth conditions, iron concentration, and exposure time [38]. The antibacterial activity of NPs is believed to involve several mechanisms, including oxidative stress, disruption of cell membranes, interference with cellular processes, and adsorption to the bacterial cell surface [39]. Interestingly, our findings indicate that the α-Fe₂O₃-NPs increase bacterial cell membrane permeability by damaging it. This damage could facilitate greater antibiotic penetration, enhancing antibiotic efficacy by reducing MICs through a dual effect: direct bacterial harm and increased antibiotic uptake [1]. Regarding the mechanism of action of α-Fe₂O₃-NPs, their antibacterial activity is primarily attributed to the generation of reactive oxygen species (ROS). Unlike other metal NPs, hematite NPs are highly stable and release minimal metal ions. Under UV or visible light irradiation, hematite NPs can produce ROS through defect-mediated or photocatalytic mechanisms. The generated ROS can penetrate the bacterial cell membrane and cause oxidative damage, leading to cell death [37]. Additionally, various intermolecular interactions contribute to the disruption of cellular functions and membrane organization, further enhancing the antibacterial activity of hematite NPs [40]. Antibiotic resistance presents a significant challenge in treating bacterial infections, as bacteria evolve mechanisms to evade the effects of antimicrobial agents. This often leads to the emergence of multidrug-resistant (MDR) strains, complicating treatment options [41]. To address this problem, researchers have explored alternative strategies, including the use of compounds with anti-virulence properties in addition to traditional antibacterial effects. Anti-virulence agents target virulence factors of pathogens instead of killing or stopping their growth and consequently disarm infectious pathogens. Moreover, these compounds can be used in combination with other antimicrobial agents, allowing for the targeting of different bacterial mechanisms and helping to reduce the likelihood of resistance development [16, 42]. Effective motility in motile bacteria, biofilm formation, and the activation of various efflux pumps are considered three major virulence/survival factors contributing to bacterial pathogenicity [43, 44]. Among these, biofilm formation is especially crucial as it creates a protective barrier against environmental stressors and antimicrobial agents, resulting in a dramatic increase in resistance-often by up to 1,000-fold [45]. Biofilm inhibition is a critical focus for developing new therapeutic interventions [46, 47]. Accordingly, in this study, we investigated the anti-virulence effects of the α-Fe2O3-NPs against three highlighted bacterial virulence factors: biofilm formation, bacterial motility, and efflux pump activity. Our findings showed that the tested agent effectively inhibited all three virulence factors at sub-MIC concentrations.
In relation to the anti-biofilm properties of NPs, although iron is essential for biofilm formation and some studies have reported that iron NPs increase biofilm formation in bacteria [48], we observed that bacterial cells treated with sub-MIC concentrations of α-Fe₂O₃-NPs formed fewer biofilms than the control. This may be due to several factors, including the cytotoxic effects of α-Fe₂O₃-NPs on bacterial growth, which subsequently decreases bacterial accumulation, activates ROS, and causes damage to bacterial cells. Additionally, since biofilm formation is regulated by the quorum sensing (QS) system in bacteria, some of the anti-biofilm activity of α-Fe₂O₃-NPs may be attributed to its anti-QS activity [18, 19]. The disruption of bacterial biofilms is crucial in combating microbial resistance, as biofilms provide protective and suitable survival conditions for bacteria in harsh environments and in the presence of antibiotics, posing a serious health threat [18].
The α-Fe2O3-NPs significantly inhibited the swarming motility of P. aeruginosa, a pathogen known for its advanced motility and virulence. Although the exact molecular mechanism of this inhibition remains unclear, it is hypothesized that NPs exert their anti-motility effects through multiple potential pathways. These mechanisms may include the disruption of key enzymes essential for bacterial movement, disrupting and damaging cell membranes, ROS generation, and potential alterations in bacterial metabolism [9, 47]. By interfering with these processes, α-Fe₂O₃-NPs can impair bacterial motility and, consequently, inhibit successful colonization [9].
Based on the anti-biofilm assay, the synthesized α-Fe2O3-NPs demonstrated notable anti-biofilm properties against S. aureus and E. coli at sub-MIC concentrations. This reduction in biofilm formation is particularly significant, given the high resistance of biofilms to conventional antibiotics. Although other studies have reported the anti-biofilm properties of various NPs, this research is among the first to specifically highlight iron NP efficacy in this regard. NPs may disrupt biofilm formation through multiple mechanisms, including preventing bacterial adhesion, targeting QS genes, or interfering with biofilm-associated enzymes [49].
We also reported the anti-NorA efflux pump inhibitory activity of α-Fe₂O₃-NPs against S. aureus. The NorA efflux pump is known for its role in mediating resistance to a wide range of antibiotics, particularly fluoroquinolones, by actively pumping out the drug from the bacterial cell, reducing its intracellular concentration and effectiveness [50]. In our study, the α-Fe₂O₃-NPs demonstrated the ability to inhibit the activity of this efflux pump, thus enhancing the susceptibility of S. aureus to fluoroquinolone antibiotics. This finding suggests that α-Fe₂O₃-NPs could potentially serve as a valuable adjunct in overcoming antibiotic resistance mediated by the NorA efflux pump, which is a significant challenge in the treatment of infections caused by S. aureus [50].
Conclusion
The study investigated the antibacterial, anti-biofilm and anti-virulence potential of the α-Fe2O3-NPs synthesized through a green method using Bacillus sp. GMS10. The α-Fe2O3-NPs demonstrated strong antibacterial activity, with MIC values ranging from 0.015 to 0.032 µg/mL against pathogenic bacteria, including both Gram-positive and Gram-negative strains. Additionally, the study highlighted their anti-virulence effects, specifically targeting key bacterial mechanisms such as motility, biofilm formation, and efflux pump activity. While the promising results indicate the potential of α-Fe₂O₃-NPs as effective antimicrobial agents, further studies are needed to explore their mechanisms of action. Future research should focus on clinical trials to assess their in vivo efficacy and safety, as well as their potential applications in infection control, wound healing, cancer therapy, and drug delivery.
Author contributions
H.J.F performed the laboratory experiments and collected the data, M.A designed the study, A.S.H wrote the manuscript; M.A and A.S.H drew the graphs and performed the analysis. M.M.Z contributed to bacterial strain isolation and nanoparticle analysis.
Funding
The authors are grateful to the research Council of the University of Kurdistan for their financial support under Grant Agreement Number 00/9/34027/2021.
Data availability
The data that support the findings of this study are available from the corresponding authors as the corresponding author, upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Morahem Ashengroph, Email: m.ashengroph@uok.ac.ir.
Aram Sharifi, Email: a.sharifi@uok.ac.ir.
References
- 1.Larsson D, Flach C-F. Antibiotic resistance in the environment. Nat Rev Microbiol. 2022;20(5):257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. lancet. 2022;399(10325):629–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abushaheen MA, Fatani AJ, Alosaimi M, Mansy W, George M, Acharya S, et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis Mon. 2020;66(6):100971. [DOI] [PubMed] [Google Scholar]
- 4.Gupta N, Rai DB, Jangid AK, Kulhari H. Use of nanotechnology in antimicrobial therapy. Methods in microbiology. Elsevier; 2019. pp. 143–72.
- 5.Thorley AJ, Tetley TD. New perspectives in nanomedicine. Pharmacol Ther. 2013;140(2):176–85. [DOI] [PubMed] [Google Scholar]
- 6.Naz S, Islam M, Tabassum S, Fernandes NF, Carcache de Blanco EJ, Zia M. Green synthesis of hematite (α-Fe2O3) nanoparticles using Rhus punjabensis extract and their biomedical prospect in pathogenic diseases and cancer. J Mol Struct. 2019;1185:1–7. [Google Scholar]
- 7.Saqib S, Zaman W, Ayaz A, Habib S, Bahadur S, Hussain S, Muhammad S, Ullah F. Postharvest disease inhibition in fruit by synthesis and characterization of chitosan iron oxide nanoparticles. Biocatal Agric Biotechnol. 2020;28:101729. [Google Scholar]
- 8.Saqib S, Munis MF, Zaman W, Ullah F, Shah SN, Ayaz A, Farooq M, Bahadur S. Synthesis, characterization and use of iron oxide nano particles for antibacterial activity. Microsc Res Tech. 2019;82(4):415–20. [DOI] [PubMed] [Google Scholar]
- 9.Ghasemi S, Harighi B, Ashengroph M. Biosynthesis of silver nanoparticles using Pseudomonas canadensis, and its antivirulence effects against Pseudomonas tolaasii, mushroom brown blotch agent. Sci Rep. 2023;13(1):3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan F, Jeong G-J, Singh P, Tabassum N, Mijakovic I, Kim Y-M. Retrospective analysis of the key molecules involved in the green synthesis of nanoparticles. Nanoscale. 2022;14(40):14824–57. [DOI] [PubMed] [Google Scholar]
- 11.Nadeem M, Khan R, Shah N, Bangash IR, Abbasi BH, Hano C, et al. A review of microbial mediated iron nanoparticles (IONPs) and its biomedical applications. Nanomaterials. 2021;12(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandelli A, Ritter AC, Veras FF. Antimicrobial activities of metal nanoparticles. Metal Nanopart Pharma. 2017:337–63.
- 13.Zouari Ahmed R, Laouini SE, Salmi C, Bouafia A, Meneceur S, Mohammed HA et al. Green synthesis of α-Fe2O3 and α-Fe2O3@ Ag NC for degradation of rose Bengal and antimicrobial activity. Biomass Convers Biorefinery. 2023:1–15.
- 14.Ashengroph M, Daj S. Green synthesis and characterization of silver sulfide nanoparticles using Bacillus safensis strain GMS10 isolated from contaminated soil of gold mine. Appl Biology. 2023;35(4):7–23. [Google Scholar]
- 15.Asadi S, Nayeri-Fasaei B, Zahraei-Salehi T, Yahya-Rayat R, Shams N, Sharifi A. Antibacterial and anti-biofilm properties of carvacrol alone and in combination with cefixime against Escherichia coli. BMC Microbiol. 2023;23(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharifi A, Mohammadzadeh A, Salehi TZ, Mahmoodi P, Nourian A. Cuminum cyminum L. essential oil: A promising antibacterial and antivirulence agent against multidrug-resistant Staphylococcus aureus. Front Microbiol. 2021;12:667833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devi KP, Nisha SA, Sakthivel R, Pandian SK. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J Ethnopharmacol. 2010;130(1):107–15. [DOI] [PubMed] [Google Scholar]
- 18.Sharifi A, Mohammadzadeh A, Zahraei Salehi T, Mahmoodi P. Antibacterial, antibiofilm and antiquorum sensing effects of Thymus daenensis and Satureja hortensis essential oils against Staphylococcus aureus isolates. J Appl Microbiol. 2018;124(2):379–88. [DOI] [PubMed] [Google Scholar]
- 19.Sharifi A, Nayeri Fasaei B. Selected plant essential oils inhibit biofilm formation and luxS-and pfs‐mediated quorum sensing by Escherichia coli O157: H7. Lett Appl Microbiol. 2022;74(6):916–23. [DOI] [PubMed] [Google Scholar]
- 20.Chauhan S, Upadhyay LSB. Biosynthesis of iron oxide nanoparticles using plant derivatives of Lawsonia inermis (Henna) and its surface modification for biomedical application. Nanatechnol Environ Eng. 2019;4:1–10. [Google Scholar]
- 21.Gharari Z, Hanachi P, Sadeghinia H, Walker TR. Eco-friendly green synthesis and characterization of silver nanoparticles by Scutellaria multicaulis leaf extract and its biological activities. Pharmaceuticals. 2023;16(7):992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geng B, Tao B, Li X, Wei W. Ni 2+/surfactant-assisted route to porous α-Fe₂O₃ nanoarchitectures. Nanoscale. 2012;4(5):1671–6. [DOI] [PubMed] [Google Scholar]
- 23.Lozano M, Rodríguez-Ulibarri P, Echeverría J, Beruete M, Sorolla M, Beriain M. Mid-infrared spectroscopy (MIR) for simultaneous determination of fat and protein content in meat of several animal species. Food Anal Methods. 2017;10(10):3462–70. [Google Scholar]
- 24.Hoseinzadeh E, Makhdoumi P, Taha P, Hossini H, Stelling J, Amjad Kamal M, et al. A review on nano-antimicrobials: metal nanoparticles, methods and mechanisms. Curr Drug Metab. 2017;18(2):120–8. [DOI] [PubMed] [Google Scholar]
- 25.Campaña AL, Saragliadis A, Mikheenko P, Linke D. Insights into the Bacterial Synthesis of Metal Nanoparticles. Front Nanotechnol. 2023;5:1216921. [Google Scholar]
- 26.Benhammada A, Trache D, Kesraoui M, Tarchoun AF, Chelouche S, Mezroua A. Synthesis and characterization of α-Fe2O3 nanoparticles from different precursors and their catalytic effect on the thermal decomposition of nitrocellulose. Thermochimica acta. 2020;686:178570. [Google Scholar]
- 27.Hao C, Shen Y, Wang Z, Wang X, Feng F, Ge C, et al. Preparation and characterization of Fe2O3 nanoparticles by solid-phase method and its hydrogen peroxide sensing properties. ACS Sustain Chem Eng. 2016;4(3):1069–77. [Google Scholar]
- 28.Titus D, Samuel EJJ, Roopan SM. Nanoparticle characterization techniques. Green synthesis, characterization and applications of nanoparticles. Elsevier; 2019. pp. 303–19.
- 29.Kumar M, Dhiman SK, Bhat R, Saran S. In situ green synthesis of AgNPs in bacterial cellulose membranes and antibacterial properties of the composites against pathogenic bacteria. Polym Bull. 2024;81(8):6957–78. [Google Scholar]
- 30.Kalaba MH, El-Sherbiny GM, Ewais EA, Darwesh OM, Moghannem SA. Green synthesis of zinc oxide nanoparticles (ZnO-NPs) by Streptomyces baarnensis and its active metabolite (Ka): a promising combination against multidrug-resistant ESKAPE pathogens and cytotoxicity. BMC Microbiol. 2024;24(1):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philip S, Kuriakose S. Synthesis, characterization and antimicrobial properties of superparamagnetic α-Fe2O3 nanoparticles stabilized by biocompatible starch. J Cluster Sci. 2021;32(5):1339–49. [Google Scholar]
- 32.Rana P, Sharma S, Sharma R, Banerjee K. Apple pectin supported superparamagnetic (γ-Fe2O3) maghemite nanoparticles with antimicrobial potency. Mater Sci Energy Technol. 2019;2(1):15–21. [Google Scholar]
- 33.Al-Tememe E, Algalal HMAA, Abodood AAF, Mohammed KA, Khamees EJ, Zabibah RS, et al. Anticancer and Antimicrobial activity of PVA/Fe2O3/TiO2 hybrid nanocomposite. Int J Nanosci. 2022;21(03):2250018. [Google Scholar]
- 34.Vihodceva S, Šutka A, Sihtmäe M, Rosenberg M, Otsus M, Kurvet I, et al. Antibacterial activity of positively and negatively charged hematite (α-Fe2O3) nanoparticles to Escherichia coli, Staphylococcus aureus and Vibrio fischeri. Nanomaterials. 2021;11(3):652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muhammad W, Khan MA, Nazir M, Siddiquah A, Mushtaq S, Hashmi SS, et al. Papaver somniferum L. mediated novel bioinspired lead oxide (PbO) and iron oxide (Fe2O3) nanoparticles: In-vitro biological applications, biocompatibility and their potential towards HepG2 cell line. Mater Sci Engineering: C. 2019;103:109740. [DOI] [PubMed] [Google Scholar]
- 36.Yoonus J, Resmi R, Beena B. Evaluation of antibacterial and anticancer activity of green synthesized iron oxide (α-Fe2O3) nanoparticles. Mater Today: Proc. 2021;46:2969–74. [Google Scholar]
- 37.Pallela PNVK, Ummey S, Ruddaraju LK, Gadi S, Cherukuri CS, Barla S et al. Antibacterial efficacy of green synthesized α-Fe2O3 nanoparticles using Sida cordifolia plant extract. Heliyon. 2019;5(11). [DOI] [PMC free article] [PubMed]
- 38.Ezealigo US, Ezealigo BN, Aisida SO, Ezema FI. Iron oxide nanoparticles in biological systems: Antibacterial and toxicology perspective. JCIS Open. 2021;4:100027. [Google Scholar]
- 39.Yılmaz GE, Göktürk I, Ovezova M, Yılmaz F, Kılıç S, Denizli A. Antimicrobial nanomaterials: a review. Hygiene. 2023;3(3):269–90. [Google Scholar]
- 40.Rufus A, Sreeju N, Philip D. Synthesis of biogenic hematite (α-Fe2O3) nanoparticles for antibacterial and nanofluid applications. RSC Adv. 2016;6(96):94206–17. [Google Scholar]
- 41.Beović B. The issue of antimicrobial resistance in human medicine. Int J Food Microbiol. 2006;112(3):280–7. [DOI] [PubMed] [Google Scholar]
- 42.Dehbanipour R, Ghalavand Z. Anti-virulence therapeutic strategies against bacterial infections: recent advances. Germs. 2022;12(2):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leitão JH. Microbial virulence factors. Volume 21. MDPI; 2020. p. 5320. [DOI] [PMC free article] [PubMed]
- 44.Rahmanian N, Moulavi P, Ashrafi F, Sharifi A, Asadi S. Surface-functionalized UIO-66-NH2 for dual-drug delivery of vancomycin and amikacin against vancomycin-resistant Staphylococcus aureus. BMC Microbiol. 2024;24(1):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kowalska J, Maćkiw E, Stasiak M, Kucharek K, Postupolski J. Biofilm-forming ability of pathogenic bacteria isolated from retail food in Poland. J Food Prot. 2020;83(12):2032–40. [DOI] [PubMed] [Google Scholar]
- 46.Slobodníková L, Fialová S, Rendeková K, Kováč J, Mučaji P. Antibiofilm activity of plant polyphenols. Molecules. 2016;21(12):1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saeki EK, Yamada AY, De Araujo LA, Anversa L, Garcia DO, De Souza RLB, et al. Subinhibitory concentrations of biogenic silver nanoparticles affect motility and biofilm formation in Pseudomonas aeruginosa. Front Cell Infect Microbiol. 2021;11:656984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borcherding J, Baltrusaitis J, Chen H, Stebounova L, Wu CM, Rubasinghege G, Mudunkotuwa IA, Caraballo JC, Zabner J, Grassian VH, Comellas AP. Iron oxide nanoparticles induce Pseudomonas aeruginosa growth, induce biofilm formation, and inhibit antimicrobial peptide function. Environ Sci Nano. 2014;1(2):123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Lacerda Coriolano D, de Souza JB, Bueno EV, Medeiros SMdFRdS, Cavalcanti IDL, Cavalcanti IMF. Antibacterial and antibiofilm potential of silver nanoparticles against antibiotic-sensitive and multidrug-resistant Pseudomonas aeruginosa strains. Brazilian J Microbiol. 2021;52:267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaatz GW, Seo SM, Ruble CA. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1993;(5):1086–94. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors as the corresponding author, upon reasonable request.