Abstract
Background
Frailty poses a considerable public health challenge because of its association with negative health consequences. Although obesity is recognized as a contributor to frailty, conventional measures fail to adequately account for the effects of visceral adiposity. The study aimed to investigate the associations between the visceral adiposity index (VAI) or lipid accumulation product (LAP) and frailty.
Methods
This study used data from the National Health and Nutrition Examination Survey (NHANES), which included 5,279 participants aged ≥ 20 years. The VAI and LAP were calculated via recognized formulas, and frailty was evaluated via a deficit accumulation approach. We employed logistic regression and restricted cubic splines to assess the associations among LAP, VAI and frailty.
Results
Out of 5,279 participants, 1,836 individuals were categorized as frail. According to the fully adjusted models, the highest VAI and LAP values were significantly associated with frailty, with adjusted ORs of 1.84 (95% CI: 1.40–2.42) and 2.47 (95% CI: 1.89–3.24), respectively, compared with the lowest values. A nonlinear relationship was identified between the LAP and frailty, with an inflection point of 1.589 (ln-transformed), whereas the VAI was linearly associated with frailty. Sensitivity analyses confirmed the robustness of these associations.
Conclusion
The VAI and LAP are significantly related to frailty, highlighting the importance of visceral adiposity in frailty risk. These results increase the understanding of the metabolic underpinnings of frailty and may guide the development of targeted prevention strategies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02410-8.
Keywords: Frailty, Lipid accumulation product, Visceral adiposity index, NHANES, Public health
Introduction
Frailty, defined as a clinical syndrome with reduced physiological reserves and increased susceptibility to stress, has become a notable public health issue because of its link to falls, disabilities, and mortality, resulting in substantial healthcare costs [1–3]. A review indicated significant variability in frailty prevalence across different studies, with rates ranging from 4 to 59%, which was attributed to differences in study populations, sample sizes, and measurement methods [1]. As populations age globally, understanding the metabolic underpinnings of frailty has become increasingly important for identifying modifiable risk factors and developing targeted interventions [4, 5]. Previous studies showed obesity was one of the key factors influencing frailty [6, 7].
Conventional obesity metrics, including waist circumference (WC) and body mass index (BMI), fail to completely address the functional and metabolic impacts of visceral fat and evaluate fat distribution [8]. To address this limitation, innovative indicators such as the visceral adiposity index (VAI) and the lipid accumulation product (LAP) have been introduced. These indices integrate biochemical and anthropometric parameters, offering improved sensitivity in assessing visceral fat and its associated health risks [9, 10]. These indices have been validated for their ability to predict metabolic disease, obstructive sleep apnea, cardiovascular diseases, and type 2 diabetes [11–16].
Recent findings suggest that visceral adiposity and lipid dysregulation may influence frailty through pathways involving systemic inflammation and metabolic stress [17]. Previous studies also showed pre-frailty was related to visceral fat area with bioelectrical impedance analysis, accompanied by high-cost and time-consuming procedures [18, 19]. The VAI and LAP, which serve as proxies for visceral adiposity, may offer critical insights into this relationship. However, while contemporary research has explored the associations between visceral adiposity and metabolic and inflammatory disorders, limited research has confirmed the relationship between VAI, LAP and frailty [20]. Understanding this relationship is crucial for developing targeted interventions to mitigate frailty in high-risk populations. This research aims to explore the associations of the VAI and LAP with frailty using data from the National Health and Nutrition Examination Survey (NHANES). The results will advance the understanding of metabolic underpinnings of frailty and offer evidence to guide targeted prevention strategies.
Methods
Study population
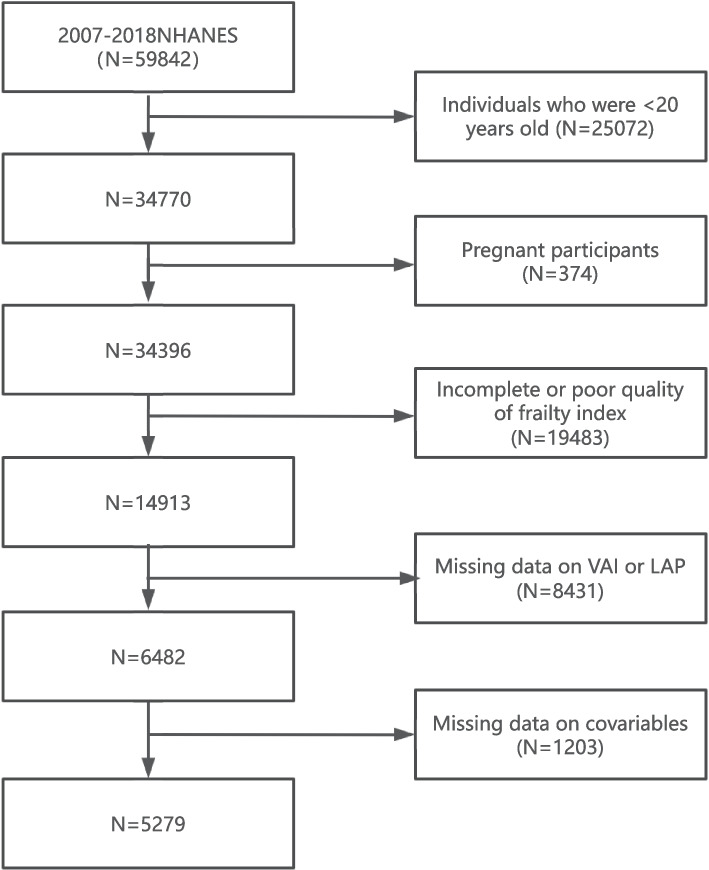
The NHANES, managed by the National Center for Health Statistics (NCHS), is a program designed to assess the health and nutritional status of the U.S. population [21]. In the present study, a total of 34,770 adults from NHANES were included. We excluded pregnant participants (N = 374) and participants whose frailty index (FI) score was incomplete or poor (N = 19,483). Missing data on VAI or LAP (N = 8,431) were also excluded. Additional exclusions were applied to participants with missing covariable data, such as age, sex, race, and other relevant health indicators (N = 1,203). Eventually, 5,279 individuals were enrolled in the study (Fig. 1). The research protocol gained approval from the NCHS Institutional Review Board, and written consent was secured from the participants involved.
Fig. 1.
Flowchart of participant selection
Assessment of VAI and LAP
Laboratory analyses were conducted on blood samples to ascertain triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and total cholesterol (TC) concentrations. VAI was determined through anthropometric and biochemical indicators, following the formulas established previously [12]. The LAP index was assessed on the basis of WC and TG [22].
Within the calculations, WC was measured in cm, BMI in kg/m2, and TG and HDL in mmol/L.
Assessment of frailty
Frailty was evaluated through a deficit accumulation model, with inclusion criteria requiring participants to have completed at least 80% of the 49 items on the FI. The FI was computed by summing individual deficit factors divided by whole deficits assessed, yielding a score from 0 to 1, where 0 indicates no deficits and 1 signifies maximal deficit load. The FI encompasses 49 specific criteria across seven domains: cognitive function, dependence, depressive symptoms, comorbidities, healthcare utilization, physical anthropometric measurements, and laboratory findings (Table S1). For analytical purposes, the continuous frailty score was dichotomized using a threshold value of 0.21, with scores exceeding this cutoff considered indicative of frailty, which is consistent with the established literature [23].
Covariables
Data were gathered through questionnaire interviews and included age, sex, marital status, the family of poverty ratio (PIR), race, and education level. Laboratory analyses were conducted to determine uric acid (UA) and serum creatinine (Scr) levels in blood samples. Alcohol use was categorized as former (≥ 12 drinks ever, no last year but ≥ 12 total, or none last year), never (< 12 drinks ever), heavy (women ≥ 3 drinks/day or ≥ 5 binges/month; men ≥ 4 drinks/day or ≥ 5 binges/month), moderate (women ≥ 2 drinks/day or ≥ 2 binges/month; men ≥ 3 drinks/day or ≥ 2 binges/month), or mild (not meeting other criteria) [24]. The smoking status categories were as follows: current (≥ 100 cigarettes, currently smoking), former (≥ 100 cigarettes, not current) and never (< 100 cigarettes lifetime).
Statistical analysis
Statistical analyses adhered to the NHANES guidelines, considering the complexities of the survey design [21]. The participants were categorized based on frailty status. The VAI and LAP were calculated via ln-transformation because of the skewed distribution. Continuous variables are depicted as the mean ± standard error (SE) or median (quartile 1, quartile 3), whereas categorical variables are depicted as proportions. We performed a weighted chi-square test and a one-way ANOVA, both adjusted for weights, to identify disparities in descriptive statistics. We also compared the included participants and excluded participants. Additionally, we used multivariable logistic regression to calculate the odds ratios (ORs) and 95% confidence intervals (CIs), evaluating the associations between the LAP or VAI and frailty. The initial model accounted for no covariables. Model 1 incorporated age and sex as covariables, whereas Model 2 expanded these variables to include race, smoking status, marital status, educational level, PIR, UA, Scr, and alcohol consumption.
The proportion of missing data in covariables was less than 10%, except for PIR, which had a 10.12% absence. To reduce the likelihood of inferential bias, multiple imputation was employed to address all missing values via the R package MICE (m = 5). The 'pool' function was then applied to integrate the estimates from these five imputed datasets into one comprehensive set of results, employing the Nelson-Aalen method for estimation [25]. A restricted cubic spline employing four knots at the 5th, 35th, 65th and 95th percentiles was utilized to explore nonlinear associations. Piecewise regression and likelihood ratio tests detected disparities in slopes around the inflection point. Additionally, stratified analyses and interaction testing were performed in age, races, PIR, drinking status, smoking status, and marital status. Interactions between subgroups and the VAI or LAP were assessed by likelihood ratio testing. To validate the study's findings, we performed four sensitivity analyses: 1) adjusting the frailty diagnostic threshold to 0.25; 2) performing the multiple imputation; 3) analyzing the associations between the VAI, LAP and pre-frailty, defined as 0.10 < FI < 0.21. All analyses were considered significant at a two-tailed p < 0.05. The analyses were performed with R Studio (version 4.2.2).
Results
Characteristics of participants
This research included 5,279 people with a mean age of 60.53 ± 0.29 years, and 2,677 (52.86%) were female. Table 1 summarizes the participant characteristics by frailty status. In total, 3,443 (69.09%) participants were non-frail, and 1,836 (30.91%) were frail. Table 1 reveals that frail participants were prone to be older, predominantly female, less educated, and had higher poverty rates, smoking prevalence, and UA levels. They were more likely to be separated, former alcohol users, and Non-Hispanic Black compared to non-frail participants. We compared the baseline characteristics between excluded patients and existing patients (Table S2).
Table 1.
Baseline characteristics of participants according to frailty status
| Variable | Total | Non-frailty | Frailty | P value |
|---|---|---|---|---|
| Number(N) | 5279 | 3443 | 1836 | |
| Age (year) | 60.53 ± 0.29 | 60.32 ± 0.35 | 61.02 ± 0.47 | 0.220 |
| Sex, n (%) | < 0.001 | |||
| Female | 2677(52.86) | 1630(48.81) | 1047(61.90) | |
| Male | 2602(47.14) | 1813(51.19) | 789(38.10) | |
| Race, n (%) | < 0.001 | |||
| Non-Hispanic Black | 1007( 8.46) | 617( 7.16) | 390(11.37) | |
| Non-Hispanic White | 2676(76.94) | 1744(79.03) | 932(72.28) | |
| Mexican American | 615( 4.73) | 419(4.68) | 196(4.83) | |
| Others | 981( 9.87) | 663( 9.13) | 318(11.52) | |
| Education level, n (%) | < 0.001 | |||
| Less than high school | 2276(35.20) | 1340(30.75) | 936(45.14) | |
| college or above | 2505(56.77) | 1777(61.51) | 728(46.18) | |
| high school | 498( 8.03) | 326(7.74) | 172(8.68) | |
| Marital, n (%) | < 0.001 | |||
| Married | 2799(57.84) | 1949(61.36) | 850(49.97) | |
| Separated | 1955(32.24) | 1144(28.26) | 811(41.15) | |
| Unmarried | 525( 9.92) | 350(10.38) | 175( 8.87) | |
| Scr, mg/dL | 0.93 ± 0.01 | 0.89 ± 0.00 | 0.99 ± 0.02 | < 0.001 |
| UA, mg/dL | 5.50(4.60,6.50) | 5.50(4.60,6.40) | 5.60(4.60,6.70) | 0.031 |
| Family of poverty ratio, n (%) | < 0.001 | |||
| < 1.3 | 1782(23.21) | 942(17.33) | 840(36.34) | |
| 1.3–3.5 | 2058(37.29) | 1359(36.33) | 699(39.43) | |
| > 3.5 | 1439(39.51) | 1142(46.34) | 297(24.23) | |
| Smoking status, n (%) | < 0.001 | |||
| never | 2461(46.21) | 1706(49.61) | 755(38.60) | |
| former | 1787(34.64) | 1191(35.45) | 596(32.83) | |
| current | 1031(19.15) | 546(14.94) | 485(28.57) | |
| Alcohol status, n (%) | < 0.001 | |||
| never | 820(12.02) | 526(11.59) | 294(12.98) | |
| former | 1159(18.52) | 639(15.04) | 520(26.30) | |
| mild | 1968(41.54) | 1410(45.40) | 558(32.92) | |
| moderate | 638(14.03) | 443(14.66) | 195(12.61) | |
| heavy | 694(13.90) | 425(13.32) | 269(15.20) |
Mean ± standard error (SE) for continuous variables, Percentage (%) for categorical variables
Scr Serum creatinine, UA uric acid
Table S3 categorizes participants into VAI quartiles: Q1 (< − 0.02), Q2 (− 0.02–0.19), Q3 (0.19–0.41), and Q4 (≥ 0.41). Individuals with higher VAI values were generally female, identified as Non-Hispanic White, and prone to be current smokers. They had lower education levels, higher UA levels, and were less likely to have higher family income. Table S4 depicts the characteristics divided by LAP quartiles.
Associations between the VAI or LAP and frailty
The associations between VAI, LAP, and frailty showed a strong dose‒response relationship, with higher quartiles of both indices significantly increasing the odds of frailty (Table 2). For the VAI, individuals in the highest quartile presented markedly elevated ORs for frailty, ranging from 2.45 (95% CI: 1.97–3.05) in Model 1 to 1.84 (95% CI: 1.40–2.42) in Model 3. A similar pattern was observed for LAP, where participants in Q4 demonstrated ORs of 2.69 (95% CI: 2.21–3.28) in Model 1 and 2.47 (95% CI: 1.89–3.24) in Model 3. The continuous measures of the VAI and LAP also consistently showed significant associations with frailty, with adjusted ORs of 2.03 (95% CI: 1.47–2.80) and 2.74 (95% CI: 2.01–3.75), respectively, in Model 3. Across all the models, the dose–response trends for both indices were highly significant (p for trend < 0.001).
Table 2.
The associations of the quartile of VAI or LAP, relative to Quartile 1 with frailty
| Categories | Ranges | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | ||
| Continuous of ln (VAI) | − 0.98–1.96 | 2.78(2.15,3.59) | 2.70(2.09,3.50) | 2.03(1.47,2.80) |
| Quartiles of ln (VAI) | ||||
| Q1 | < − 0.02 | Ref | Ref | Ref |
| Q2 | − 0.02–0.19 | 1.21(0.95,1.54) | 1.18(0.93,1.50) | 1.04(0.83,1.31) |
| Q3 | 0.19–0.41 | 1.63(1.32,2.00) | 1.57(1.27,1.93) | 1.33(1.07,1.65) |
| Q4 | ≥ 0.41 | 2.45(1.97,3.05) | 2.36(1.89,2.94) | 1.84(1.40,2.42) |
| P for trend | < 0.001 | < 0.001 | < 0.001 | |
| Continuous of ln (LAP) | − 0.34–3.42 | 2.93(2.30,3.74) | 2.99(2.33,3.83) | 2.74(2.01,3.75) |
| Quartiles of ln (LAP) | ||||
| Q1 | < 1.47 | Ref | Ref | Ref |
| Q2 | 1.47–1.69 | 1.23(0.98,1.55) | 1.24(0.98,1.57) | 1.20(0.93,1.54) |
| Q3 | 1.69–1.91 | 1.50(1.24,1.82) | 1.51(1.24,1.84) | 1.39(1.10,1.76) |
| Q4 | ≥ 1.91 | 2.69(2.21,3.28) | 2.73(2.24,3.34) | 2.47(1.89,3.24) |
| P for trend | < 0.001 | < 0.001 | < 0.001 | |
Model 1: no cofounder; Model 2: adjusted for age, sex; Model 3: further adjusted for races, education level, marital status, Scr, UA
Alcohol intake, smoking status, and the family of poverty ratio
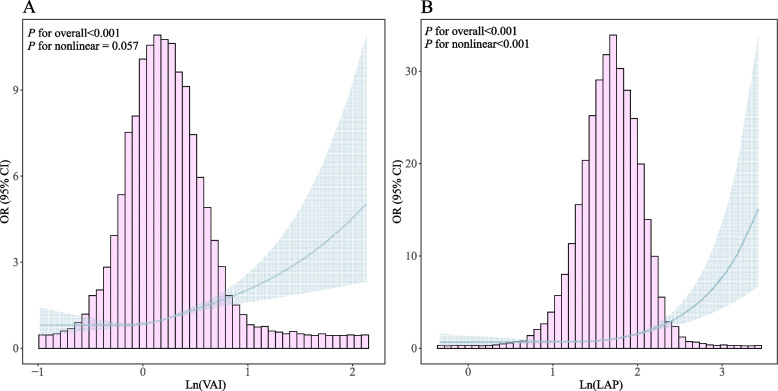
Figure 2 shows that the VAI was linearly related to frailty risk, whereas the LAP showed an inflection point at 1.598 (Ln(LAP)), with frailty risk significantly increasing above this level (OR 4.79, 95% CI: 3.60–6.39; P < 0.001). Log likelihood ratio tests confirmed the nonlinear relationships (P < 0.001 for LAP) (Table 3).
Fig. 2.
Restricted cubic spline analysis between VAI(A), LAP(B) and frailty. The red solid line and gray areas in the figure panels represent ORs and 95% CIs, respectively. ORs were adjusted for age, sex, races, educational level, marital status, PIR, UA, Scr, smoking status, and alcohol intake
Table 3.
Association of LAP and frailty using piece-wise logistic regression
| Threshold effect analysis | OR (95% CI) | P value |
|---|---|---|
| Ln (LAP) | ||
| Model 1 Fitting model by standard logistic regression | 2.836(2.374, 3.395) | < 0.001 |
| Model 2 Fitting model by two-piecewise logistic regression | ||
| Inflection point (K) | 1.598 | |
| < K slope | 1.331(0.936, 1.907) | 0.115 |
| > K slope | 4.791(3.601, 6.388) | < 0.001 |
| Log likelihood ratio test | < 0.001 | |
Adjusted for age, sex, race, education level, marital status, alcohol intake, smoking status, Scr, UA, the family of poverty ratio
According to the sensitivity analyses, the results remained robust when frailty was diagnosed as FI ≥ 0.25 (Table S5). Consistent outcomes were discovered after imputation (Table S6). Similar outcomes were obtained when we also analyzed the associations of the VAI or LAP with pre-frailty (Table S7).
Subgroup analysis
The associations of the VAI and LAP with frailty, including different age groups, races, PIR, drinking, smoking, and marital status, remained stable across subgroups. No significant interactions were found (P for interaction > 0.05) (Table 4).
Table 4.
The associations of frailty with VAI or LAP in various subgroups
| Frailty | Ln (VAI) | Ln (LAP) | ||
|---|---|---|---|---|
| OR (95% CI) | P for interaction | OR (95% CI) | P for interaction | |
| Age | 0.678 | 0.923 | ||
| < 60 | 2.46(1.59,3.81) | 2.90(1.94,4.33) | ||
| ≥ 60 | 2.32(1.66,3.24) | 3.02(2.16,4.23) | ||
| Race | 0.297 | 0.750 | ||
| white | 2.81(1.98,3.99) | 3.07(2.17,4.35) | ||
| non-white | 2.24(1.56,3.22) | 3.45(2.42,4.91) | ||
| Current drinker | 0.569 | 0.216 | ||
| Yes | 2.67(1.91,3.72) | 3.32(2.41,4.56) | ||
| No | 2.34(1.35,4.04) | 2.38(1.38,4.11) | ||
| Current smoker | 0.624 | 0.217 | ||
| Yes | 2.05(1.34,3.12) | 2.38(1.64,3.44) | ||
| No | 2.46(1.81,3.34) | 3.46(2.49,4.80) | ||
| Marital | 0.198 | 0.120 | ||
| Married | 3.13(2.09,4.68) | 3.97(2.70,5.83) | ||
| Unmarried | 1.51(0.75,3.02) | 2.78(1.54,5.02) | ||
| Separated | 2.27(1.50,3.43) | 2.29(1.49,3.53) | ||
| PIR | 0.900 | 0.713 | ||
| < 1.3 | 2.44(1.68,3.54) | 2.62(1.79,3.85) | ||
| 1.3–3.5 | 2.15(1.35,3.41) | 3.08(1.95,4.89) | ||
| ≥ 3.5 | 2.30(1.17,4.53) | 3.30(1.56,6.99) | ||
Adjusted for age, sex, races, education level, marital status, alcohol intake, smoking status, the family of poverty ratio, Scr, UA, if not stratified
Discussion
The study revealed significant associations between the VAI or LAP and frailty in U.S. adults. Higher VAI and LAP values were related to increased frailty risk, independent of demographic and lifestyle factors. The LAP is nonlinearly associated with frailty, but the VAI is linearly associated with frailty. Additionally, no significant interactions were detected in the subgroups. These findings underscore the role of metabolic dysregulation in frailty and highlight the VAI and LAP as potential biomarkers.
Our research confirms a significant association between the VAI or LAP and frailty, supporting the existing scholarly work that underscores adiposity’s contribution to frailty progression. Liao et al. reported that WC, a measure of central obesity, serves as a frailty indicator in elderly Beijing residents [26]. Another study revealed that dynapenic abdominal obesity, calculated with handgrip strength and WC, accelerated frailty progression [27]. The GAZEL study, encompassing people aged 61–76, with ongoing surveillance since 1989, demonstrated that chronic obesity, as indicated by BMI trends, and obesity onset in later life are related to frailty [28]. A Mendelian randomization analysis using data from the UK Biobank and Swedish TwinGene revealed that both overall and abdominal obesity are causally related to frailty [29]. A meta-analysis indicated that obesity is correlated with increased frailty risk among seniors living in the community. Individuals with larger WC presented a 57% greater risk of frailty than individuals with normal WC [7]. Findings from the Women’s Health Initiative, involving 40,657 women aged 65–79 and followed for three years, established that overweight status significantly heightened the likelihood of frailty [30].
However, several studies have shown different effects. A study of 599 community-dwelling women indicated that a BMI within the overweight category (25– < 30 kg/m2) was not related to increased frailty risk [30]. Furthermore, Liao’s study suggested that being overweight elevated the likelihood of frailty solely for those with higher WCs, not for those with WCs within the normal range. This implies that central obesity may be a factor related to frailty [26]. Jayanama et al. noted that BMI values ≥ 25 kg/m2 correlated with elevated frailty levels across both the NHANES and SHARE datasets. Additionally, their research indicated that overweight status may serve as a mortality risk reduction factor among those with moderate to severe frailty [6]. The discrepancy between these studies may be due to different sample sizes, follow-up durations, definitions of frailty, or characteristics of the participants.
Detecting visceral fat with precision often relies on techniques such as magnetic resonance imaging; however, these approaches encounter limitations like costly expenses, time-consuming processes, and exposure to radiation. Consequently, these methodologies are not suitable for large-scale population research. Standard measures such as BMI and WC indicate overall weight excess but have limitations in evaluating fat distribution patterns. In contrast, VAI and LAP are emerging as innovative indicators for the straightforward and non-invasive assessment of visceral fat. Unlike conventional lipid profiles, the VAI and LAP are capable of evaluating a range of metabolic disorders, offering a holistic view of an individual’s metabolic well-being. Unlike many studies that define frailty via the frailty phenotype, which include a combination of only five criteria [31], we use the FI, which includes 49 items, for definition. Compared with the frailty phenotype, the FI has superior performance in identifying frailty status [32].
The complex interplay of various biological factors connecting the VAI, LAP, and frailty is intricate. Visceral obesity is recognized for its secretion of various adipokines and cytokines that promote chronic inflammation, a pivotal element in the development of frailty [33]. Elevated concentrations of interleukin-6 and C-reactive protein, typically observed in those with increased visceral fat, have been implicated in muscle wasting and weakness, cognitive decline, and other frailty components [34–37]. Furthermore, the accumulation of visceral fat is closely related to insulin resistance, which can exacerbate frailty by impairing glucose metabolism and promoting sarcopenia [38–42]. Additionally, lipid accumulation has been shown to impair endothelial function, increasing risks of heart diseases, which are commonly related to frailty [43].
The association between VAI/LAP and frailty may also be mediated by the impact of these indices on hormonal balance. Visceral fat has been shown to influence sex hormone-binding globulin, which impacts the bioavailability of sex steroids, potentially contributing to frailty through their effects on muscle mass, bone health, and cognitive functions [44–46]. Moreover, the potential influence of gut microbiota on frailty cannot be overlooked, as alterations in the microbiome are related to both lipid accumulation and frailty, suggesting a possible process in the pathogenesis of frailty [47, 48].
In summary, the potential mechanisms linking VAI, LAP, and frailty are numerous and interconnected, involving inflammation, insulin resistance, lipid metabolism, hormonal imbalances, and possibly the gut microbiome. These pathways provide biological plausibility to our findings and suggest avenues for future research aimed at understanding and mitigating the frailty associated with obesity. Our findings support the widespread adoption of these tools in clinical practice, improving the ability to manage frailty.
Study strengths and limitations
One strength is the utilization of broad-based NHANES data, ensuring broad generalizability, and the comprehensive control of potential confounders, which enhances the precision of our estimates. We adopted stringent research methodologies to elucidate the distinct influences of the VAI and LAP on the state of frailty. Nevertheless, the observational design of our study restricts our capacity to infer causality, necessitating validation via longitudinal research. Relying on self-reported information could lead to recall bias, and our frailty assessment, while comprehensive, may not encompass all aspects of frailty. Employing logistic regression may lead to an overestimation of the effect size considering the prevalence of frailty. In addition, there remains the potential for residual confounding factors not accounted for in our adjustment for known covariables. Finally, while our findings are representative of U.S. demographics, their relevance to different populations or specific health conditions may be limited.
Conclusion
These findings indicate a notable association between visceral fat and frailty risk among U.S. adults, highlighting the importance of considering metabolic factors in frailty prevention and treatment strategies. Future research should investigate the efficacy of measures to lower the VAI and LAP and delay frailty progression. Further longitudinal analyses are crucial for confirming causal relationships and exploring the potential of these indices as early predictors of frailty, which could inform personalized prevention and management approaches.
Supplementary Information
Supplementary Information 1. Table S1. Variables in the 49-item frailty index and their respective scorings. Table S2. The comparison of characteristics of included participants and excluded participants. Table S3. Baseline characteristics of study participants stratified by VAI quartiles. Table S4. Baseline characteristics of study participants stratified by LAP quartiles. Table S5. The associations of the quartile of VAI or LAP, relative to Quartile 1 with frailty (FI ≥ 0.25). Table S6. The associations of the quartile of VAI or LAP, relative to Quartile 1 with frailty after the imputation. (N = 6482). Table S7. The associations of the quartile of VAI or LAP, relative to Quartile 1 with pre-frailty (0.10 < FI < 0.21, N = 3443).
Acknowledgements
We thank the National Health and Nutrition Examination Surveys for providing the data.
Abbreviations
- VAI
Visceral adiposity index
- LAP
Lipid accumulation product
- NHANES
National Health and Nutrition Examination Survey
- WC
Waist circumference
- BMI
Body mass index
- FI
Frailty index
- TG
Triglyceride
- HDL-C
High-density lipoprotein cholesterol
- TC
Total cholesterol
- PIR
The family of poverty ratio
- UA
Uric acid
- Scr
Serum creatinine
- SE
Standard error
- OR
Odds ratio
- CI
Confidence intervals
Authors’ contributions
Conceptualization, S.Y.; data curation, S.Y. and K.C.; formal analysis, S.Y.; methodology, K.C.; software, S.Y.; supervision, K.C.; writing-original draft, K.C.; writing—review and editing, J.Y. and H.W.; visualization, S.Y.; All authors have read and agreed to the published version of the manuscript.
Funding
No funding.
Data availability
The survey data are publicly available on the internet for data users and researchers throughout the world ( www.cdc.gov/nchs/nhanes/).
Declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by NCHS Ethics Review Board. The participants provided their written informed consent to participate in this study.
Consent for publication
All participants in the NHANES study provided consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–75. [DOI] [PubMed] [Google Scholar]
- 2.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3(7):e323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim DH, Glynn RJ, Avorn J, Lipsitz LA, Rockwood K, Pawar A, et al. Validation of a Claims-Based Frailty Index Against Physical Performance and Adverse Health Outcomes in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2019;74(8):1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veronese N, Custodero C, Cella A, Demurtas J, Zora S, Maggi S, et al. Prevalence of multidimensional frailty and pre-frailty in older people in different settings: A systematic review and meta-analysis. Ageing Res Rev. 2021;72: 101498. [DOI] [PubMed] [Google Scholar]
- 5.Dent E, Hanlon P, Sim M, Jylhävä J, Liu Z, Vetrano DL, et al. Recent developments in frailty identification, management, risk factors and prevention: A narrative review of leading journals in geriatrics and gerontology. Ageing Res Rev. 2023;91: 102082. [DOI] [PubMed] [Google Scholar]
- 6.Jayanama K, Theou O, Godin J, Mayo A, Cahill L, Rockwood K. Relationship of body mass index with frailty and all-cause mortality among middle-aged and older adults. BMC Med. 2022;20(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan L, Chang M, Wang J. Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: a systematic review and meta-analysis. Age Ageing. 2021;50(4):1118–28. [DOI] [PubMed] [Google Scholar]
- 8.Wei J, Liu X, Xue H, Wang Y, Shi Z. Comparisons of visceral adiposity index, body shape index, body mass index and waist circumference and their associations with diabetes mellitus in adults. Nutrients. 2019;11(7):1580. [DOI] [PMC free article] [PubMed]
- 9.Oh JY, Sung YA, Lee HJ. The lipid accumulation product as a useful index for identifying abnormal glucose regulation in young Korean women. Diabet Med. 2013;30(4):436–42. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Shi D, Zhang Q, Wang S, Liu K, Meng Q, et al. Visceral adiposity index (VAI), a powerful predictor of incident hypertension in prehypertensives. Intern Emerg Med. 2018;13(4):509–16. [DOI] [PubMed] [Google Scholar]
- 11.Kouli GM, Panagiotakos DB, Kyrou I, Georgousopoulou EN, Chrysohoou C, Tsigos C, et al. Visceral adiposity index and 10-year cardiovascular disease incidence: The ATTICA study. Nutr Metab Cardiovasc Dis. 2017;27(10):881–9. [DOI] [PubMed] [Google Scholar]
- 12.Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Sun H, Qiu S, Tao H, Yu J, Sun Z. Lipid Accumulation Product Combined With Urine Glucose Excretion Improves the Efficiency of Diabetes Screening in Chinese Adults. Front Endocrinol (Lausanne). 2021;12: 691849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao Q, Li J, Wu Y, Liu X, Wang N, Jiang Y, et al. Enhanced predictive value of lipid accumulation product for identifying metabolic syndrome in the general population of China. Nutrients. 2023;15(14):3168. [DOI] [PMC free article] [PubMed]
- 15.Zhou T, Chen S, Mao J, Zhu P, Yu X, Lin R. Association between obstructive sleep apnea and visceral adiposity index and lipid accumulation product: NHANES 2015–2018. Lipids Health Dis. 2024;23(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai X, Li N, Hu J, Wen W, Yao X, Zhu Q, et al. Nonlinear Relationship Between Chinese Visceral Adiposity Index and New-Onset Myocardial Infarction in Patients with Hypertension and Obstructive Sleep Apnoea: Insights from a Cohort Study. J Inflamm Res. 2022;15:687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B, Li Y, Zhang Y, Liu P, Song Y, Zhou Y, et al. Visceral Fat Obesity Correlates with Frailty in Middle-Aged and Older Adults. Diabetes Metab Syndr Obes. 2022;15:2877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su Y, Yuki M, Ogawa N. Association of visceral fat area with pre-frailty in Japanese community-dwelling older adults: a cross-sectional study. BMC Geriatr. 2022;22(1):686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stranahan AM. Visceral adiposity, inflammation, and hippocampal function in obesity. Neuropharmacology. 2022;205: 108920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National health and nutrition examination survey: sample design, 2011–2014. Vital Health Stat. 2014;162:1–33. [PubMed] [Google Scholar]
- 22.Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hakeem FF, Bernabé E, Sabbah W. Association Between Oral Health and Frailty Among American Older Adults. J Am Med Dir Assoc. 2021;22(3):559-563.e552. [DOI] [PubMed] [Google Scholar]
- 24.Hicks CW, Wang D, Matsushita K, Windham BG, Selvin E. Peripheral Neuropathy and All-Cause and Cardiovascular Mortality in U.S. Adults : A Prospective Cohort Study. Ann Intern Med. 2021;174(2):167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. 2016;4(2):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao Q, Zheng Z, Xiu S, Chan P. Waist circumference is a better predictor of risk for frailty than BMI in the community-dwelling elderly in Beijing. Aging Clin Exp Res. 2018;30(11):1319–25. [DOI] [PubMed] [Google Scholar]
- 27.Sun B, Wang J, Wang Y, Xiao W, Liu Y, Wang Y, et al. Associations of dynapenic abdominal obesity and frailty progression: evidence from two nationwide cohorts. Nutrients. 2024;16(4):518. [DOI] [PMC free article] [PubMed]
- 28.Landré B, Czernichow S, Goldberg M, Zins M, Ankri J, Herr M. Association Between Life-Course Obesity and Frailty in Older Adults: Findings in the GAZEL Cohort. Obesity (Silver Spring). 2020;28(2):388–96. [DOI] [PubMed] [Google Scholar]
- 29.Gu Y, Li Z, Dang A, Zhang W, Liu J, Han X, et al. Obesity, birth weight, and lifestyle factors for frailty: a Mendelian randomization study. Aging (Albany NY). 2023;15(23):14066–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: the Women’s Health and Aging Studies. J Am Geriatr Soc. 2005;53(6):927–34. [DOI] [PubMed] [Google Scholar]
- 31.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-156. [DOI] [PubMed] [Google Scholar]
- 32.Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: Comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60(3):464–70. [DOI] [PubMed] [Google Scholar]
- 33.Dhawan D, Sharma S. Abdominal Obesity, Adipokines and Non-communicable Diseases. J Steroid Biochem Mol Biol. 2020;203: 105737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carbone F, Nulli Migliola E, Bonaventura A, Vecchié A, De Vuono S, Ricci MA, et al. High serum levels of C-reactive protein (CRP) predict beneficial decrease of visceral fat in obese females after sleeve gastrectomy. Nutr Metab Cardiovasc Dis. 2018;28(5):494–500. [DOI] [PubMed] [Google Scholar]
- 35.Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. 2021;320(3):C375-c391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedditzi E, Peters R, Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing. 2016;45(1):14–21. [DOI] [PubMed] [Google Scholar]
- 37.Colleluori G, Villareal DT. Aging, obesity, sarcopenia and the effect of diet and exercise intervention. Exp Gerontol. 2021;155: 111561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heng MWY, Chan AWD, Man REK, Fenwick EK, Chew STH, Tay L, et al. Individual and combined associations of sarcopenia, osteoporosis and obesity with frailty in a multi-ethnic asian older adult population. BMC Geriatr. 2023;23(1):802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ko SH, Jung Y. Energy metabolism changes and dysregulated lipid metabolism in postmenopausal women. Nutrients. 2021;13(12):4556. [DOI] [PMC free article] [PubMed]
- 40.Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. Biomed Pharmacother. 2021;137: 111315. [DOI] [PubMed] [Google Scholar]
- 41.Cai X, Hu J, Zhu Q, Wang M, Liu S, Dang Y, et al. Relationship of the metabolic score for insulin resistance and the risk of stroke in patients with hypertension: A cohort study. Front Endocrinol (Lausanne). 2022;13:1049211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai X, Hu J, Wang M, Wen W, Wang J, Yang W, et al. Association between the sarcopenia index and the risk of stroke in elderly patients with hypertension: a cohort study. Aging (Albany NY). 2023;15(6):2005–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaffe IZ, Karumanchi SA. Lipid droplets in the endothelium: the missing link between metabolic syndrome and cardiovascular disease? J Clin Invest. 2024;134(4):e176347. [DOI] [PMC free article] [PubMed]
- 44.Stangl TA, Wiepjes CM, Smit RAJ, van Hylckama VA, Lamb HJ, van der Velde J, et al. Association Between Low Sex Hormone-Binding Globulin and Increased Risk of Type 2 Diabetes Is Mediated by Increased Visceral and Liver Fat: Results From Observational and Mendelian Randomization Analyses. Diabetes. 2024;73(11):1793–804. [DOI] [PubMed] [Google Scholar]
- 45.Tchernof A, Toth MJ, Poehlman ET. Sex hormone-binding globulin levels in middle-aged premenopausal women. Associations with visceral obesity and metabolic profile. Diabetes Care. 1999;22(11):1875–81. [DOI] [PubMed] [Google Scholar]
- 46.Cai X, Hu J, Wen W, Wang M, Zhu Q, Liu S, et al. Association between the geriatric nutritional risk index and the risk of stroke in elderly patients with hypertension: A longitudinal and cohort study. Front Nutr. 2022;9:1048206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ticinesi A, Nouvenne A, Cerundolo N, Catania P, Prati B, Tana C, et al. Gut microbiota, muscle mass and function in aging: a focus on physical frailty and sarcopenia. Nutrients. 2019;11(7):1633. [DOI] [PMC free article] [PubMed]
- 48.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information 1. Table S1. Variables in the 49-item frailty index and their respective scorings. Table S2. The comparison of characteristics of included participants and excluded participants. Table S3. Baseline characteristics of study participants stratified by VAI quartiles. Table S4. Baseline characteristics of study participants stratified by LAP quartiles. Table S5. The associations of the quartile of VAI or LAP, relative to Quartile 1 with frailty (FI ≥ 0.25). Table S6. The associations of the quartile of VAI or LAP, relative to Quartile 1 with frailty after the imputation. (N = 6482). Table S7. The associations of the quartile of VAI or LAP, relative to Quartile 1 with pre-frailty (0.10 < FI < 0.21, N = 3443).
Data Availability Statement
The survey data are publicly available on the internet for data users and researchers throughout the world ( www.cdc.gov/nchs/nhanes/).