Abstract
Background
The aim of this study is to investigate the effect of soil water stability on maize (Zea mays L.) yield, water use, and its photosynthetic physiological mechanisms, and to innovate the relationship between maize and soil water, which currently only considers soil water content and neglects soil water stability.
Methods
An organized water experiment was conducted on maize. The effects of stable soil water (SW) at two water content levels were examined, with fluctuating soil water (FW) as a control. The assessed effects included leaf water, chlorophyll, gas exchange, leaf water use efficiency (WUE), stable carbon isotope ratio (δ13C), and yield of maize.
Results
Soil water stability had a significant effect on maize yield, yet it was slightly smaller than soil water content. Compared with FW, SW increased the maximum net photosynthetic rate, saturated light intensity, stomatal conductance, SPAD, leaf water content, and leaf WUE, and decreased δ13C, promoting dry matter assimilation and conversion into grain yield, ultimately increasing yield by 100.8%. Under the same soil water stability, 55% FC versus 75% FC weakened photosynthetic capacity and exacerbated stomatal limitation of maize leaves, making them more susceptible to light inhibition, which decreased photoassimilate accumulation, resulting in a significant decrease in yield. And the δ13C under 75% FC conditions decreased by 4.7–7.7% compared with 55% FC.
Conclusion
In conclusion, SW exhibits a positive effect on maize leaf water content, photosynthetic carbon assimilation, and grain yields, regardless of soil water content. Compared to FW, SW increased leaf WUE and maize yield by enhancing photosynthesis, and SW has stronger discrimination against 13C during photosynthetic CO2 assimilation, thus decreasing leaf δ13C. This study fills a gap in understanding how soil water stability influences maize yield and gas exchange, and provides a fresh perspective on how to improve crop yield and WUE by managing soil water stability.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05942-4.
Keywords: Soil water stability, Photosynthetic traits, Water use efficiency, Light response curve, Stable carbon isotopes, Maize
Introduction
Maize (Zea mays L.) is one of the most widely cultivated crops, and its production directly influences the development of the food industry and global food security [1]. The overwhelming majority of food production in China depends on irrigation water [2, 3]. Insufficient water cannot sustain normal crop development, and excessive water decreases agricultural yields and depletes groundwater, introduces salts such as nitrates into groundwater, and devastates ecosystems [4, 5]. Improving soil water-maize management strategies can conserve water resources, safeguard food production, and mitigate ecological damage; scientific water management is critical for ensuring future water supply and food security [4, 6].
Soil water is a crucial regulator of crop growth, development, and yield [7, 8]. As soil water information is difficult to obtain in agricultural production, it is frequently defined indirectly by easily accessible irrigation indicators, such as irrigation methods, irrigation amounts, and irrigation frequencies [9–11]. However, there is no accurate correspondence between irrigation indicators and soil water [12–15]. In addition, previous studies on soil water paid more attention to its spatial variability [16–19] but ignored its change in temporal dimension, that is, soil water stability. Although there is always some variation in soil water, it is difficult to maintain absolute stability. For example, Wang et al. (2020) and Niu et al. (2022) used the temporal variation of soil water to quantitatively characterize the soil water stability, and classified the soil water as fluctuating soil water (FW) if the temporal variation was greater than 0.1 and stable soil water (SW) if it was less than or equal to 0.1 [20, 21]. Li et al. (2023) adopted the fluctuation of soil water to quantitatively characterize the soil water stability, and the fluctuation coefficient of 0.01–0.02 was categorized as SW, and the fluctuation coefficient of 0.07–0.10 was called FW [22]. In addition to the quantitative properties of soil water (soil water content, SWC) [23, 24], new studies indicate directly or indirectly that soil water stability has a considerable impact on plant growth and development [20–22, 25]. Wang et al. (2020) suggested that SW conditions were more conducive to the growth and development of maize plants [20]. Li et al. (2023) discovered that SW alleviated water stress and improved the morphogenesis of tomato seedlings [22]. In another study on cherry radish, Li et al. (2024) demonstrated that irrigation with a stable moisture increased radish yield by 35–94% compared to fluctuating moistures [25]. At present, the relationship between maize and soil water stability is very limited, especially in terms of yield, water use efficiency (WUE), photosynthesis, and stable carbon isotope ratio (δ13C), and the underlying mechanism remains unknown.
Photosynthesis is the fundamental physiological process for producing maize material, which is susceptible to environmental changes [26]. Severe water stress significantly decreased the leaf area index, relative chlorophyll content, and net photosynthetic rate (Pn) of maize leaves, leading to a reduction in the growth rate and consequently the yield [27]. Meanwhile, excessive SWC was detrimental to maize growth by increasing oxidative damage, reducing photosynthetic ability and chlorophyll concentration, and destroying chloroplast structure and root anatomy [24]. Although many studies have examined the relationships between drought stress and the light response curve [28–31], it is currently unclear how maize plants adapt and self-regulate in response to soil water stability from a photosynthetic physiological perspective. Gas exchange is environmentally responsive and temporally dynamic, which directly reflects the instantaneous state of photosynthesis [32]. Since plant syntheses rely on photosynthetic assimilates, the stable carbon isotope ratio (δ13C) of leaf dry matter is considered to be a time-integrated measure during tissue growth [33, 34]. The variation of δ13C can be used to explain the carbon sequestration process in photosynthesis, which is related to gas exchange and biomass under different water conditions [34, 35]. Extensive research has been conducted on the correlation between leaf δ13C and SWC [36–38]. However, little is known regarding the relationship between soil water stability and the leaf δ13C of maize.
Our hypothesis argues that leaf gas exchange and the stable carbon isotopic composition of maize may exhibit variations in response to varying soil water conditions. The aim of this study was to investigate the impact of soil water stability on leaf water content, gas exchange characteristics, fitting parameters of light response curves, stable carbon isotopes, and grain yield at various stages of maize.
Materials and methods
Experimental site
The pot experiment was conducted from June to September 2021 in a rainproof shelter located at the Chinese Academy of Agricultural Sciences in Beijing, China (39.6°N, 116.2°E). The study site has a typical continental climate that is characterized as warm-temperate and semi-humid; the annual mean temperature was 10–12 °C and there was an annual frost-free period of 180–220 d. During the test period, Fig. S1 [see Additional file 1] depicts the daily changes in temperature, humidity, and evaporation from a standard reference water surface. The test soil came from 0 to 20 cm cultivated soil in Fangshan District, Beijing, and the soil texture was loam with a: field capacity (FC) of 35% (v/v), total nitrogen content of 0.8 g kg–1, total phosphorus content of 0.6 g kg–1, total potassium content of 12 g kg–1, alkali hydrolyzed nitrogen of 81 mg kg–1, available phosphorus of 14.8 mg kg–1, available potassium of 125 mg kg–1, organic matter content of 13.3 g kg–1, and pH (soil: water, 1:5) was 8.3.
Experimental device
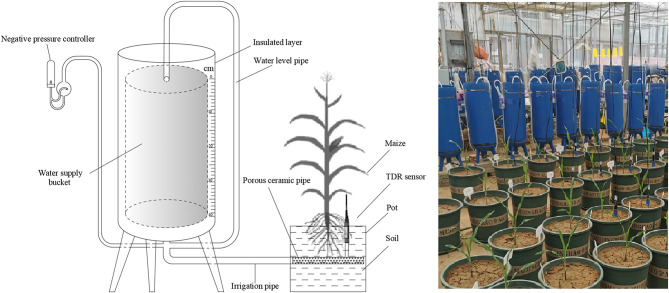
To achieve precise control over soil water conditions, we implemented a method known as pressure potential difference-crop initiate drawing water device (P-CIDW), namely negative pressure irrigation technology. This method allows for the continuous and stable supply of water to soil-plant systems. It has been successfully utilized in various crop studies, as evidenced by works [22, 39–42]. The application of P-CIDW has facilitated a more accurate examination of the relationship between soil water and maize. The P-CIDW was designed by the Chinese Academy of Agricultural Sciences (Chinese Patents. ZL201110093923.2 and ZL201310554433.7) (Fig. 1), which consisted of a negative pressure controller, a water supply bucket (height: 80 cm, inner radius: 13.1 cm, capacity: 22 L), and an irrigator (porous ceramic pipe). The irrigator was 250 mm long, with an outer diameter of 18 mm and an inner diameter of 10 mm.
Fig. 1.
The schematic diagram and physical photo of the pressure potential difference-crop initiate drawing water device
Experimental design
The soil water stability factor was set to two levels of stable soil water (abbreviated as “S”) and fluctuating soil water (abbreviated as “F”), and the soil water content factor was set to two levels of 55% FC (low soil water, abbreviated as “L”) and 75% FC (high soil water, abbreviated as “H”), for a total of 4 treatments (SL, SH, FL, and FH) with 5 replicates per treatment. A total of 20 pots, at a spacing of 0.45 × 0.50 m, were set up. Based on previous experimental results [20], the SL and SH treatments were established by − 9 kPa and − 3 kPa of P-CIDW, respectively. The FL and FH treatments were established by watering with the lower and upper limits of irrigation at 40–70% FC and 60–90% FC, respectively. The volumetric SWC was recorded every 30 min during the experiment using the ENVIdata-DT soil water monitoring system (ENVIdata-DT, IMKO, Germany). The SL and SH treatments were irrigated using the P-CIDW system, and the irrigation amount was recorded at 17:00 daily according to the water level pipe. The FL and FH treatments triggered irrigation as soon as the SWC approached or was below the lower limit of irrigation and irrigated to the upper limit of irrigation.
The widely-grown maize cultivar cv. Zhengdan 958 (Zea mays L.) used in this experiment was purchased from Beijing Zhongnong Fenglian Technology Development Co., Ltd., China. The pot, which had a height of 35 cm and a radius of 17 cm, was used to grow maize plants. Each pot was filled with 30.0 kg of air-dried soil, and the soil bulk density was 1.4 g cm–3. The fertilizer amount was the same (0.25 g N, 0.13 g P2O5 and 0.13 g K2O per 1 kg of soil) for every replicate. Nitrogen fertilizer was applied in accordance with a base-topdressing ratio of 4:6 (topdressing at the eleven-leaf stage), and phosphorus and potassium fertilizers were used as base fertilizers at one time. Before sowing, the base fertilizers were thoroughly mixed into the soil, and all pots were irrigated to 100% FC. Each pot was sown with three seeds on 1 June in 2021. After emergence, the pots were thinned to one seedling for a follow-up study on 10 June in 2021. The seedling stage was uniformly irrigated to maintain the SWC at 70–80% FC. The water control experiment began on 15 June in 2021 (the three-leaf stage) and ended on 27 September in 2021 (the harvest stage).
Sampling and measurements
Determination of soil water stability parameters
The fluctuation coefficient (δ) of soil water was determined using Eq. (1) [20]:
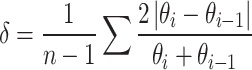 |
1 |
where θi is the mean SWC on the ith d, θi–1 is the mean SWC on the (i–1)th d, and n is the number when SWC was observed. The magnitude of δ reflects the soil water stability, with a smaller value indicating a more stable SWC.
The coefficient of soil water temporal variability (CV) was calculated as follows [20]:
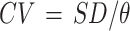 |
2 |
where SD is the standard deviation of SWC at different times, and θ is the mean SWC at various periods. If CV ≤ 0.1, the soil water belongs to weak variability; if 0.1 < CV < 1, it belongs to medium variability; and if CV ≥ 1, it belongs to strong variability. The smaller the CV value, the more stable the soil water is.
Determination of leaf relative water content (LRWC) and relative electrical conductivity (LREC)
The latest fully expanded leaf/ear-leaf of maize plants were sampled at the ten-leaf stage (V10), milk stage (R3), and physiological maturity stage (R6) for the determination of LRWC and LREC [43], and the calculation formulas were as follows:
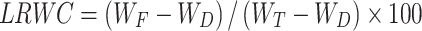 |
3 |
where WF is the leaf fresh weight (g), WD is the leaf dry weight (g), and WT is the leaf saturated weight (g).
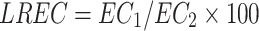 |
4 |
where EC1 is the initial leaf electrical conductivity (µs cm–1) and EC2 is the final leaf electrical conductivity (µs cm–1).
Leaf SPAD measurements
The SPAD value of maize leaves was measured every 7 days after treatment using a SPAD-502 portable chlorophyll meter (Minolta Camera Co. Ltd., Japan). The indicator leaf was the latest fully expanded leaf/ear-leaf, and 6–10 sites were selected at equal intervals throughout the leaf to determine its mean SPAD value.
Determination of gas exchange
At the six-leaf stage (V6), V10, silking stage (R1), R3, and R6 stages of maize, the light response curves were measured by a portable photosynthesis system (Li-6400XT, LI-Cor, NE, USA) on sunny and windless days. The photosynthetically active radiation (PAR) was taken at 2000, 1500, 1000, 700, 500, 300, 200, 100, 50, 20, and 0 µmol m–2 s–1, and the maximum and minimum waiting times were 200 s and 120 s, respectively. Under each specified PAR, Pn, stomatal conductance (Gs), and transpiration rate (Tr) of the latest fully expanded leaf/ear-leaf were determined. The light response curve was fitted using a modified rectangular hyperbolic model [44], which was depicted as follows:
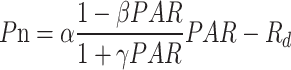 |
5 |
where Pn is the net photosynthetic rate (µmol CO2 m–2 s–1), α is the initial slope of the light response curve, PAR is the photosynthetically active radiation (µmol m–2 s–1), Rd is the dark respiration rate (µmol m–2 s–1), and β and γ are coefficients.
In the meantime, the saturated light intensity (Isat) and the maximum Pn (Pnmax) of maize leaves were estimated using the formulas:
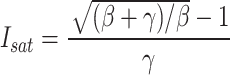 |
6 |
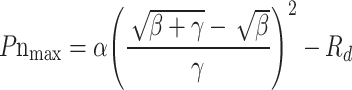 |
7 |
At the leaf level, instantaneous water use efficiency (WUEins) was calculated as follows [45]:
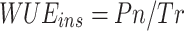 |
8 |
where WUEins is the instantaneous water use efficiency (µmol CO2 mmol–1 H2O), Pn is the net photosynthetic rate (µmol CO2 m–2 s–1), and Tr is the transpiration rate (mmol H2O m–2 s–1).
Measurement of stable carbon isotopes
After the leaf samples were ground with a ball mill, the stable carbon isotope ratio (δ13C) in the samples was determined by a stable isotope ratio mass spectrometer (Thermo Fisher Inc., USA). The δ13C value was expressed relative to the Pee Dee Belemnite (PDB) [46].
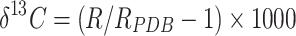 |
9 |
where δ13C is the ratio expressed in parts per thousand (‰), R is the molar abundance ratio of the sample (13C/12C), and RPDB is the molar abundance ratio of PDB.
Grain yield
At the R6 stage, five maize plants were harvested per treatment, and the grain yield (14% moisture content) was measured [47].
Statistical analysis
Microsoft Excel 2010 software (Microsoft Crop, Redmond, WA, USA) was used for data processing, SAS 9.0 (SAS Institute, Cary, NC, USA) was used for analysis of variance (ANOVA) (two-way ANOVA), and Duncan’s multiple-range test was used for multiple comparisons (P < 0.05). Origin Pro 2021 software (OriginLab Corporation, Northampton, MA, USA) was used for correlation analysis and graph construction.
Results
Soil water parameters
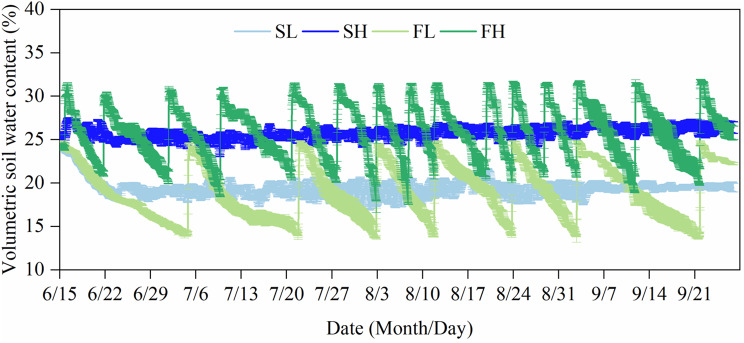
The average soil water content (SWC) for the SL, SH, FL, and FH treatments were 19.4% (55.5% FC), 25.8% (73.8% FC), 19.1% (54.5% FC), and 25.9% (73.9% FC), respectively. The fluctuation coefficients for these treatments were 0.01, 0.01, 0.06, and 0.08, while the temporal variability coefficients were 0.05, 0.02, 0.16, and 0.12, respectively. The SL and SH treatments could be classified as stable soil water (SW) due to their minimal changes in SWC, very minor fluctuation coefficients observed during the treatment, and temporal variability coefficients below 0.1 (Fig. 2). The FL and FH treatments could be classified as fluctuating soil water (FW) due to their significant changes in SWC, which showed a “sawtooth” pattern. Additionally, these treatments exhibited relatively substantial fluctuation coefficients and temporal variability coefficients exceeding 0.1. The observed SWC of the SL and FL treatments closely approximated the target value of 55% FC, whereas the observed SWC of the SH and FH treatments closely approximated the target value of 75% FC. These observed values fell within the acceptable margin of error for the experimental design, which was ± 2%.
Fig. 2.
Changes in volumetric soil water content of different treatments. Values are the mean ± SD (n = 4). SL: stable soil water with 55% FC, SH: stable soil water with 75% FC, FL: fluctuating soil water with 55% FC, and FH: fluctuating soil water with 75% FC
Variation of LRWC and LREC
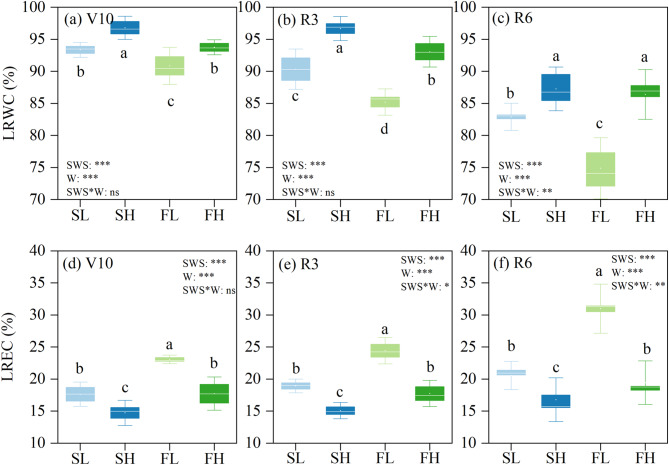
The LRWC and LREC were shown to be highly influenced by soil water stability and water content. Additionally, it was observed that the interaction between these factors had a considerable impact on LRWC during the R6 stage and LREC during the R3 and R6 stages, as depicted in Fig. 3. In comparison to FW, SW showed enhancements in the LRWC at the V10, R3, and R6 stages, with improvements of 3.0, 5.0, and 5.9%, respectively. Conversely, the LREC experienced a drop of 20.3, 18.8, and 23.7% at the same stage. Compared to the low water (LW), the high water (HW) showed an increase in LRWC of 3.4, 8.1, and 10.3% at the V10, R3, and R6 stages, respectively. Conversely, the LREC exhibited a drop of 19.8, 23.8, and 27.8% at the same stages.
Fig. 3.
Dynamic changes in leaf relative water content (LRWC) and relative electrical conductivity (LREC) of maize plants. Values are the mean ± SD (n = 4–5). Duncan’s multiple-range test was used to test differences among treatments at the P < 0.05 level. Different lowercase letters above the columns indicate significant differences among treatments at the same stage. SL: stable soil water with 55% FC, SH: stable soil water with 75% FC, FL: fluctuating soil water with 55% FC, FH: fluctuating soil water with 75% FC, V10: ten-leaf stage, R3: milk stage, R6: physiological maturity stage, SWS: soil water stability, W: soil water content, SWS*W: the interaction between soil water stability and soil water content. *, **, and *** indicate significance levels at P < 0.05, P < 0.01, and P < 0.001, respectively, and ns indicates that the difference is not significant
Dynamic changes in the leaf SPAD
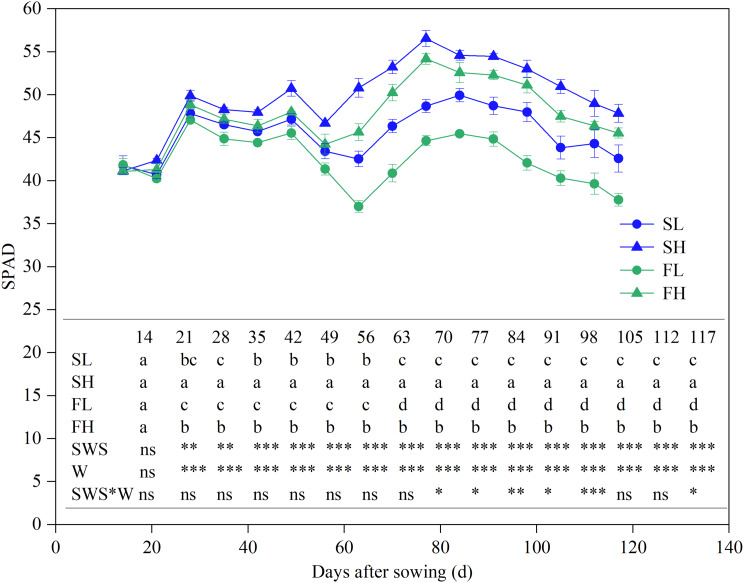
The observed pattern of variation in maize leaf SPAD remained consistent across different treatments (Fig. 4). The soil water stability and water content exerted a notable influence on the leaf SPAD after a week of water treatment. However, their interaction was found to have a meaningful effect only during the late reproductive growth stages. Overall, the ranking of SPAD could be summarized as follows: SH > FH > SL > FL. The SL increased leaf SPAD by 7.6% compared to the FL. Similarly, the SH increased leaf SPAD by 4.6% compared to the FH. Additionally, the SW exhibited an average increase of 6.1% in leaf SPAD compared to the FW. The SH increased leaf SPAD by 9.5% compared to the SL. Similarly, the FH led to a 12.7% increase in leaf SPAD compared to the FL. Furthermore, the HW showed an average increase of 11.1% in leaf SPAD compared to the LW. The effect of SW on enhancing leaf SPAD was shown to be more significant in situations when SWC was low, whereas the influence of increasing SWC on leaf SPAD was seen to be more prominent under FW conditions.
Fig. 4.
Dynamic changes in maize leaf SPAD over time under different treatments. Values are the mean ± SD (n = 4–5). Duncan’s multiple-range test was used to test differences among treatments at the P < 0.05 level. Different lowercase letters in the same column indicate significant differences among treatments within the same day. SL: stable soil water with 55% FC, SH: stable soil water with 75% FC, FL: fluctuating soil water with 55% FC, FH: fluctuating soil water with 75% FC, SWS: soil water stability, W: soil water content, SWS*W: the interaction between soil water stability and soil water content. *, **, and *** indicate significance levels at P < 0.05, P < 0.01, and P < 0.001, respectively, and ns indicates that the difference is not significant
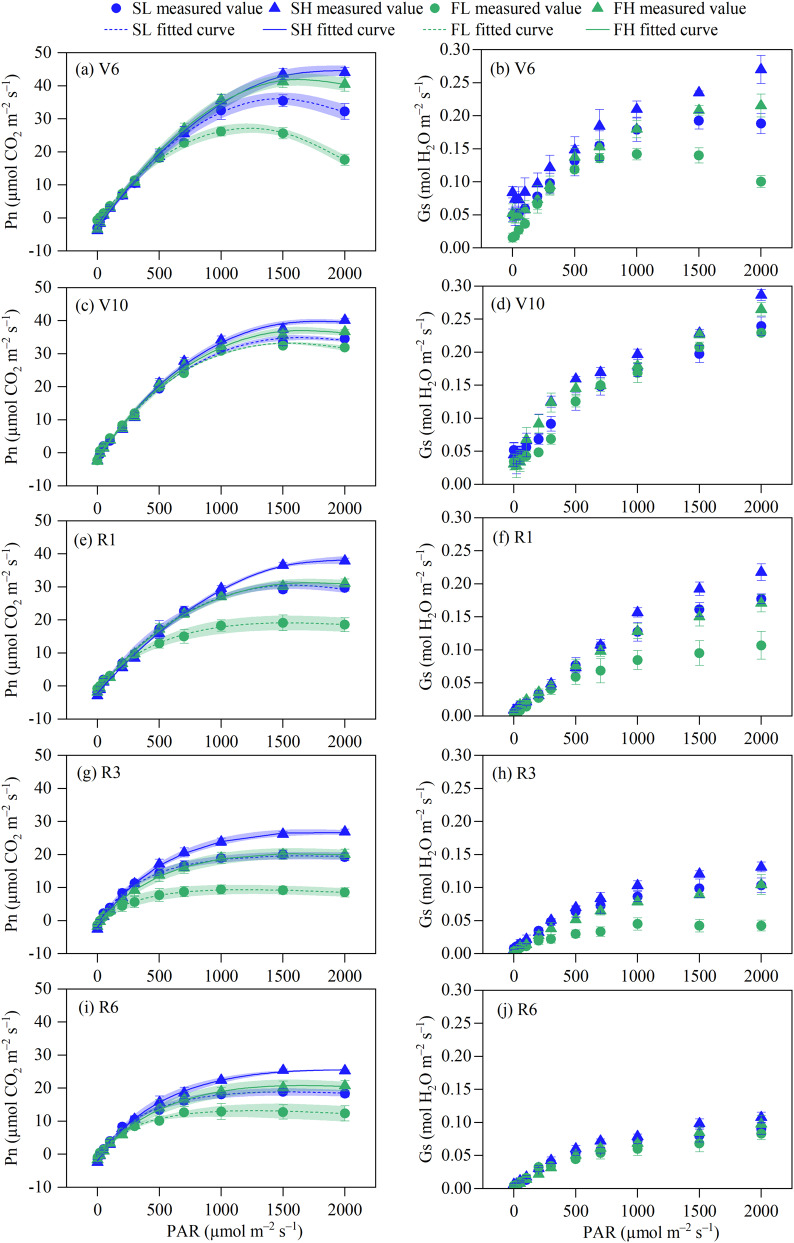
Response of leaf gas exchange
The order of the Pn at every stage was as follows: SH > FH > SL > FL, as depicted in Fig. 5. The observed trends in the light response curves per treatment at the R1, R3, and R6 stages were largely consistent. There was no significant difference between the SL and FH treatments. Additionally, the Pn showed a decling trend as maize maturity progressed. Under similar SWC, maize plants subjected to SW exhibited higher Pn compared to those subjected to FW. This difference in Pn suggested that the SW created a more favorable environment for the production of photosynthetic products. Under similar soil water stability, the HW exhibited a greater propensity for enhancing the Pn of maize leaves compared to the LW. Furthermore, the impact of maize growth stages on the effectiveness of the SWC was shown to be negligible.
Fig. 5.
Dynamic changes in net photosynthetic rate (Pn) and stomatal conductance (Gs) of maize leaves. Values are the mean ± SD (n = 3). SL: stable soil water with 55% FC, SH: stable soil water with 75% FC, FL: fluctuating soil water with 55% FC, FH: fluctuating soil water with 75% FC, PAR: photosynthetically active radiation, V6: six-leaf stage, V10: ten-leaf stage, R1: silking stage, R3: milk stage, and R6: physiological maturity stage
The Gs curve of maize leaves indicated that the Gs was higher with the SH during the V6 stage. Conversely, the Gs was lower with the FL. However, there was no significant difference between the SL and FH. The study revealed that the Gs under SW conditions exhibited a greater value compared to those under FW, given similar SWC levels. Under similar soil water stability, HW was shown to have a more positive impact on the Gs of maize leaves compared to the LW. At the V10 stage, there was a minimal disparity observed among the SL, SH, FL, and FH treatments. During the R1 stage, there was no apparent distinction observed among the four treatments under weak light conditions. However, when the light intensity increased, the Gs of maize leaves subjected to the SH exhibited a quick increase, followed by the SL and FH, while the FL showed a slower increase. During the R3 stage, there was a collective decline in the Gs values of the four treatments in comparison to the R1 stage. The variation of Gs in each treatment was similar to that of R1, but the gap between SW and FW increased. During the R6 stage, there was a drop observed in the Gs values as the maize plants matured.
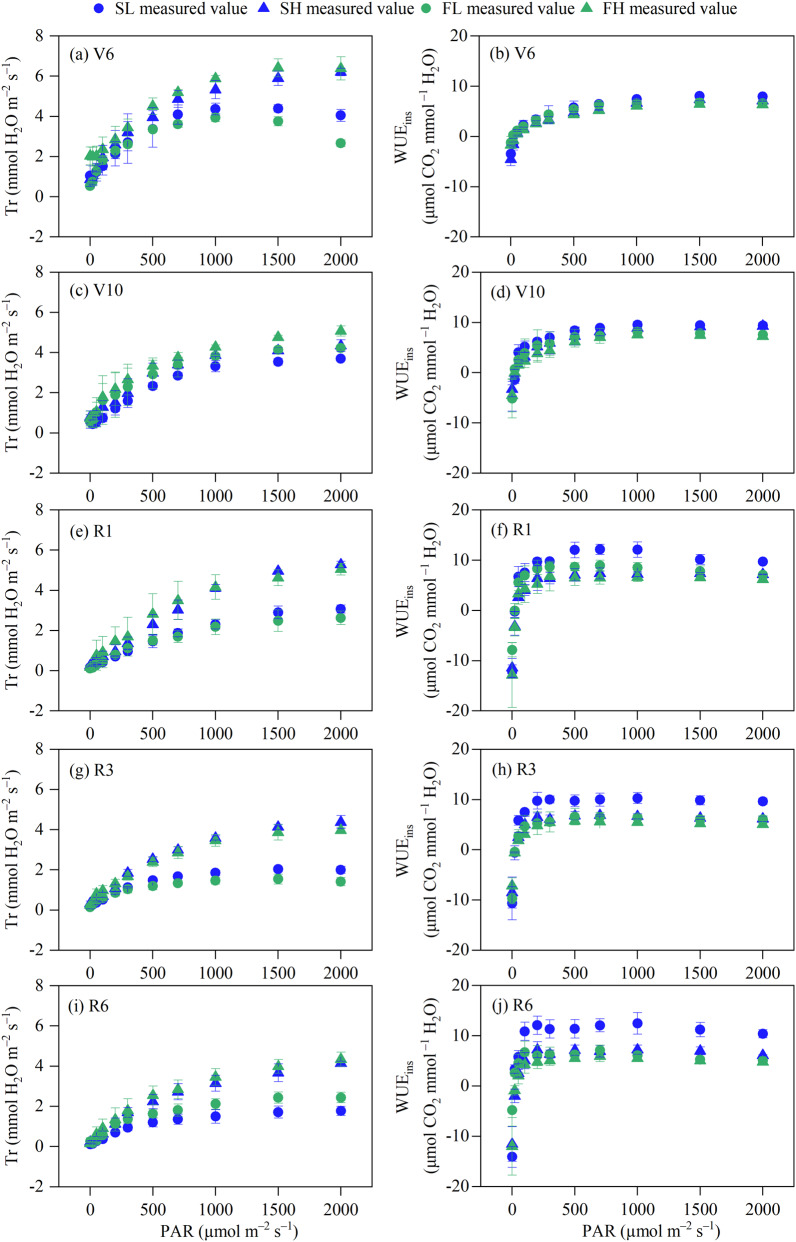
The Tr curve of maize leaves showed that at the V6 stage, the leaf Tr of maize plants under FH treatment was higher than that under SH treatment, while the Tr of SL treatment was higher than that of FL treatment under strong light. At the V10 stage, the Tr of FH treatment was larger and the Tr of SL treatment was minor, while there was no significant difference between SH and FL treatment. At the R1 and R3 stages, the Tr under HW treatment was greater than that under LW treatment, but there was no substantial difference between Tr under stable and fluctuating moisture conditions with the same SWC. At the R6 stage, the ranking of Tr was FH > SH > FL > SL (Fig. 6).
Fig. 6.
Dynamic changes in transpiration rate (Tr) and instantaneous water use efficiency (WUEins) of maize leaves. Values are the mean ± SD (n = 3). SL: stable soil water with 55% FC, SH: stable soil water with 75% FC, FL: fluctuating soil water with 55% FC, FH: fluctuating soil water with 75% FC, PAR: photosynthetically active radiation, V6: six-leaf stage, V10: ten-leaf stage, R1: silking stage, R3: milk stage, R6: physiological maturity stage
The WUEins curve of maize leaves indicated that there was no discernible disparity in WUEins among the treatments throughout the V6 stage. At the V10 stage, the leaf WUEins in maize plants of SW were found to be better than those of FW when subjected to identical SWC constitutions. Conversely, HW exhibited lower WUEins than LW when exposed to equibalent levels of soil water stability. During the R1 stage, the WUEins of plants subjected to the SL treatment were found to be higher compared to the FL treatment. However, no significant difference in WUEins was seen between the SH and FH treatments under weak light conditions. Furthermore, the WUEins of plants exposed to the SH treatment under strong light conditions were found to be higher than those subjected to the FH treatment. During the R3 and R6 stages, the application of SW resulted in an increase in WUEins when compared to the use of FW. Furthermore, the impact of SW on WUEins of maize leaves was particularly pronounced under LW conditions.
Maximum net photosynthetic rate, saturated light intensity, light compensation point, and dark respiration rate
The Pnmax, Isat, and Rd of maize leaves were strongly influenced by the soil water stability throughout all stages. Additionally, the light compensation point (Ic) during the V6 and R1 stages, as well as the α during the R3 stage, were also affected by the stability of soil water (Table 1). Throughout all stages, the SWC had a notable impact on the Pnmax, Isat, Ic, and Rd, but no discernible influence was observed on the α. The interaction between the stability and content of soil water had a notable impact on Pnmax and Rd during the V6 stage, Pnmax during the R1 stage, and Pnmax and Ic during the R3 stage.
Table 1.
Effects of different treatments on the fitting parameters of light response curves of maize leaves
| Stage | Treatment | α | Pnmax (µmol CO2 m–2 s–1) | Isat (µmol m–2 s–1) | Ic (µmol m–2 s–1) | Rd (µmol m–2 s–1) | |
|---|---|---|---|---|---|---|---|
| V6 | SL | 0.053a | 36.14b | 1500c | 48.01a | 2.45a | |
| SH | 0.051a | 44.94a | 1877a | 56.20a | 2.82a | ||
| FL | 0.049a | 27.10c | 1223d | 19.69b | 0.96b | ||
| FH | 0.053a | 42.22a | 1683b | 49.86a | 2.62a | ||
| V10 | SL | 0.056a | 34.98c | 1674bc | 33.05a | 1.80a | |
| SH | 0.056a | 40.00a | 1796a | 35.39a | 1.96a | ||
| FL | 0.056a | 33.26d | 1584c | 24.32b | 1.34b | ||
| FH | 0.056a | 37.19b | 1698ab | 34.23a | 1.86a | ||
| R1 | SL | 0.049a | 30.61b | 1610bc | 31.18b | 1.48b | |
| SH | 0.042a | 38.32a | 1924a | 50.08a | 2.09a | ||
| FL | 0.046a | 19.10c | 1565c | 17.87c | 0.82c | ||
| FH | 0.048a | 31.37b | 1748b | 34.48b | 1.64b | ||
| R3 | SL | 0.070a | 19.73b | 1621b | 21.51b | 1.42b | |
| SH | 0.060ab | 26.89a | 1825a | 34.87a | 2.01a | ||
| FL | 0.043b | 9.44c | 1192c | 26.76b | 1.03c | ||
| FH | 0.050ab | 20.34b | 1648ab | 28.97ab | 1.37bc | ||
| R6 | SL | 0.064a | 18.85b | 1549b | 19.84b | 1.21bc | |
| SH | 0.059a | 25.53a | 1949a | 35.27a | 1.97a | ||
| FL | 0.060a | 13.23c | 1219c | 17.63b | 0.94c | ||
| FH | 0.049a | 20.83b | 1684ab | 30.24a | 1.43b | ||
| ANOVA (F values) | |||||||
| V6 | SWS | 0.05 | 35.38*** | 28.40*** | 11.40** | 14.07** | |
| W | 0.37 | 146.26*** | 89.60*** | 13.96** | 19.99** | ||
| SWS*W | 1.71 | 10.22* | 0.88 | 4.58 | 8.13* | ||
| V10 | SWS | 0.01 | 23.67** | 9.46* | 4.86 | 10.45* | |
| W | 0.00 | 92.26*** | 14.82** | 7.46* | 14.87** | ||
| SWS*W | 0.06 | 1.36 | 0.02 | 2.85 | 4.10 | ||
| R1 | SWS | 0.33 | 109.69*** | 6.26* | 49.80*** | 17.50** | |
| W | 0.57 | 128.52*** | 31.54*** | 75.12*** | 28.97*** | ||
| SWS*W | 1.80 | 6.69* | 2.19 | 0.31 | 0.68 | ||
| R3 | SWS | 6.22* | 128.43*** | 23.83** | 0.02 | 20.08** | |
| W | 0.06 | 147.65*** | 28.27*** | 10.70* | 15.95** | ||
| SWS*W | 1.28 | 6.38* | 4.14 | 5.50* | 1.20 | ||
| R6 | SWS | 1.18 | 36.55*** | 1330** | 2.55 | 20.11** | |
| W | 1.42 | 70.00*** | 28.17*** | 38.34*** | 47.69*** | ||
| SWS*W | 0.23 | 0.29 | 0.16 | 0.39 | 2.21 | ||
Note: Duncan’s multiple-range test was used to test differences among treatments at the P < 0.05 level. Different lowercase letters in the same column indicate significant differences among treatments at the same stage. SL: stable soil water with 55% FC, SH: stable soil water with 75% FC, FL: fluctuating soil water with 55% FC, FH: fluctuating soil water with 75% FC, SWS: soil water stability, W: soil water content, SWS*W: the interaction between soil water stability and soil water content, α: the initial slope of the light response curve, Pnmax: maximum net photosynthetic rate, Isat: saturated light intensity, Ic: light compensation point, Rd: dark respiration rate, V6: six-leaf stage, V10: ten-leaf stage, R1: silking stage, R3: milk stage, and R6: physiological maturity stage. *, **, and *** indicate significance levels at P < 0.05, P < 0.01, and P < 0.001, respectively
At the V6 stage, there was no significant difference in Pnmax between the SH and FH treatments; however, it did differ from the Pnmax observed in the SL and FL treatments (Table 1). At the V10 stage, there was a small difference observed in the light response curves across all treatments. However, there was a significant difference in Pnmax between the different treatments. There was no significant difference in Pnmax between the SL and FH treatments at the R1, R3, and R6 stages. However, this difference was considerably different from the SH and FL treatments.
At all stages, the ranking of Isat of maize leaves was as follows: SH > FH > SL > FL. However, it is pertinent to note that only at the V6 stage did the four treatments exhibit a statistically significant difference. At the V6, V10, R1, R3, and R6 stages, the Pnmax of SW increased by 19.9, 6.4, 41.2, 70.6, and 32.5%, respectively, compared to FW. Additionally, Isat of SW increased by 17.1, 5.7, 6.5, 23.3, and 21.4%, respectively, compared to FW. When comparing the performance of LW at five different stages, it was shown that HW increased Pnmax by 40.1, 13.1, 44.7, 75.9, and 46.5%, respectively. Additionally, HW increased Isat by 31.4, 7.2, 15.6, 25.4, and 32.0%, respectively.
The Ic and Rd increased at all stages when the SWC and soil water stability increased, with the exception of the Ic at the R3 stage. Under the similar SWC, SW raised the Ic by 0.4–78.3% and the Rd by 20.0–81.4% compared to FW. Under similar soil water stability, the HW increased Ic by 23.9–85.1% and Rd by 23.6–93.4% compared to the LW.
Stabilize carbon isotopes and grain yield
The soil water stability and water content significantly affected leaf δ13C and grain yield of maize, while their interaction had no significant effects on δ13C or yield (Table 2). Under similar SWC conditions, compared to FW, the δ13C of SW was reduced by 5.3, 8.4, and 10.3% at the V10, R3, and R6 stages, respectively. Under the same soil water stability, compared with LW, the δ13C of HW decreased by 4.7, 7.7, and 7.6% at the V10, R3, and R6 stages, respectively. All water treatments showed clear carbon isotope fractionation; more carbon was assimilated when fractionation was stronger in the treatment of SW or HW. The δ13C fluctuation trend was consistent across all treatments across the three stages, and the fractionation at the V6 stage was more pronounced, suggesting that the carbon assimilation during this time was stronger than in other periods.
Table 2.
Effects of different treatments on leaf δ13C and grain yield of maize
| Treatment | δ13C | Grain yield (g plant–1) |
||
|---|---|---|---|---|
| Ten-leaf stage | Milk stage | Physiological maturity stage | ||
| SL | –11.08b | –10.81b | –11.90b | 99.49c |
| SH | –11.57c | –11.56c | –12.97c | 194.45a |
| FL | –10.49a | –9.91a | –10.93a | 37.79d |
| FH | –11.02b | –10.73b | –11.61ab | 140.60b |
| ANOVA (F values) | ||||
| SWS | 13.97** | 18.93*** | 19.90*** | 288.40*** |
| W | 11.09** | 15.88** | 11.08** | 844.88*** |
| SWS*W | 0.01 | 0.04 | 0.54 | 1.33 |
Note: Duncan’s multiple-range test was used to test differences among treatments at the P < 0.05 level. Different lowercase letters in the same column indicate significant differences among treatments at the same stage. SL: stable soil water with 55% FC, SH: stable soil water with 75% FC, FL: fluctuating soil water with 55% FC, FH: fluctuating soil water with 75% FC, SWS: soil water stability, W: soil water content, SWS*W: the interaction between soil water stability and soil water content. *, **, and *** indicate significance levels at P < 0.05, P < 0.01, and P < 0.001, respectively
The treatments significantly affected maize yield, following SH > FH > SL > FL. Compared with FL treatment, the yield of SL treatment was increased by 163.3%; compared with FH treatment, the yield of SH treatment was increased by 38.3%; SW improved yield by an average of 100.8% compared to FW under similar SWC conditions. Compared with SL treatment, the yield of SH treatment was increased by 95.5%; compared with FL treatment, the yield of FH treatment was increased by 272.1%; HW, compared with LW, increased yield by an average of 183.8% under the same soil water stability.
Discussion
Stable soil water improves the leaf WUE of maize plants by increasing Pn rather than decreasing Tr
The soil water plays a significant role in influencing various aspects of crop growth, yield, and water use efficiency (WUE). Several reports have shown that leaf WUE increased with the decrease of soil water content (SWC) [48, 49], and the same phenomenon was found in our study: lower SWC resulted in lower plant transpiration, thus increasing instantaneous water use efficiency (WUEins), regardless of being in a stable soil water (SW) or fluctuating soil water (FW) condition. Our study confirms that stable water conditions are beneficial for improving photosynthesis in crops [50]. The gradual decrease in soil water may leave a “memory” for plants. In a prior study, stomatal conductance of maize leaves could not return to the control level following rehydration [51]. Compared with a stable water state, stomate pore length became smaller under fluctuating water conditions, which was a change in anatomical structure, and even if soil water fluctuated to a peak again, the developed stomate pore length could not be changed [52]. These were the reasons why stomatal conductance of maize leaves under SW was greater than that under FW in this study. A study in tomato plants showed that the activities of superoxide dismutase, peroxidase, and catalase increased continuously during the gradual drought, and their activities decreased to close to the control level after rehydration but still higher than the control level [53], resulting in drought memory. It could be inferred that the process of gradually decreasing soil water might affect the antioxidant protection system and thus affect the damage of cell membranes (Fig. 3) and produce memory. Research has indicated that there was a notable decrease in the rate of chlorophyll synthesis in leaves during rehydration [54]. It was observed in this study that the SPAD under FW conditions was, in fact, lower than that under SW conditions (Fig. 4). At 96 h after rehydration, the maximum quantum efficiency, photosystem II operating efficiency, and Rubisco (Ribulose-1,5-bisphosphate carboxylase) activity showed a gradual recovery process [54], but Rubisco activity was slower to recover [55]. As a result, Rubisco activity might not have recovered sufficiently yet and has dropped again. Therefore, Rubisco activity was consistently higher in stable soil water than in fluctuating soil water (Fig. S3 [see Additional file 1]). Concisely, the combination of steady and elevated stomatal conductance, SPAD value, and Rubisco activity in SW led to an increased photosynthetic rate.
Another important finding was that SW, versus FW, resulted in an increase in the WUEins of maize leaves, and increasing Pn played a more fundamental role than decreasing Tr in WUEins improvement (Figs. 5 and 6). Furthermore, the present study demonstrated that FW exacerbated stomatal limitations due to isohydric behavior in response to frequent dry and wet al.ternations (Figs. 2 and 5), which was aligned with that of Morabito et al. (2022), who found that isohydric behavior protected Vitis vinifera from a sudden increase in tension in response to a fast-developing drought [56]. It is widely acknowledged that the parameters of gas exchange in plant leaves have the ability to promptly respond to changes in soil water levels, and there is often a strong relationship between these two variables [48]. Nevertheless, it was found that there was not a good correlation between the Pn and Gs of maize leaves with the instantaneous SWC (Fig. S2 [see Additional file 1]), which might be due to the changed leaf stomatal morphology under FW compared with SW. Xu et al. (2024) showed that when the median value of soil water was the same, the stomate pore length of soil water with larger fluctuation was smaller than that of soil water with smaller fluctuation [52], indicating that Pn and Gs were not only affected by SWC but also affected by stomatal morphological differences. In combination with Fig. 4, it was evident that the relationship between the response of the SPAD and soil water stability, long-term average SWC, and their interaction became increasingly apparent as the growth stage progressed, rather than being influenced by instantaneous SWC. These findings indicated that the observed variations in Pn, Gs, and SPAD of maize leaves were not solely influenced by the instantaneous SWC but rather by the specific water treatment applied. Here, we provide a new perspective for improving leaf WUE through managing soil water stability.
Stable soil water promotes maize yield via enhancing photosynthetic capacity
The process of photosynthesis serves as the foundation for the accumulation of dry matter, while soil water has an important impact on plants photosynthetic activity, growth, and yield [57]. Previous studies have demonstrated that the application of mulching ridges and furrows in maize fields resulted in a notable increase in soil water storage. This improved the light response curve and increased photosynthetic capacity, chlorophyll content, and crop yield [58]. Our study corroborated these findings, showing a suitable SWC level can increase the photosynthetic and grain yield of maize plants. The most interesting findings were that soil water stability had a significant effect on maize yield; specifically, maize plants grown under SW conditions exhibited considerably higher yields compared to those grown under FW settings, which was confirmed by a two-way ANOVA (Table 2).
In order to further analyze the mechanism of yield variation, we simulated the light response curves of maize leaves subjected to various treatments using a modified rectangular hyperbolic model. According to a study conducted by Xing et al. (2020), an elevation in soil water stress during the maize silking stage resulted in a reduction in Pnmax, Isat, and Rd [29]. Here, it was noted that potential photosynthetic capacity of maize leaves was diminished during various growth stages due to low SWC. Based on the observed increase rates of Pnmax by HW relative to LW, it could be inferred that SWC exerted varying influences on Pnmax at different stages, with the order of impact being R3 > R6 > R1 > V6 > V10, which suggested that SWC had stage-dependent effects on Pnmax of maize leaves and that the effect of SWC on leaf Pnmax during the reproductive growth stage was greater than that during the vegetative growth stage. This was due to the fact that maize plants had considerably more leaf area during their reproductive growth stage than during their vegetative growth stage, together with increased transpiration [59, 60]. Eom et al. (2013) calculated crop water requirements through potential evapotranspiration and crop coefficient and also found that the water requirements in the maize reproductive growth stage were greater than those in the vegetative growth stage [59]. Additionally, during the reproductive growth stage, the SPAD of different SWC treatments varied substantially, but not during the vegetative growth stage (Fig. 4). Lower SWC appeared to have significantly accelerated the degradation of photosynthetic pigments in leaves at the reproductive growth stage. As a result, SWC had a greater effect on Pnmax during the reproductive growth stage than the vegetative growth stage. Moreover, it was evident that soil water stability exerted varying influences on Pnmax at different growth stages. Specifically, the impact on Pnmax followed the order R3 > R1 > R6 > V6 > V10. It was worth noting that these findings have not been previously documented in existing literature. These findings will doubtless be much scrutinized, but there are some immediately dependable conclusions that the application of SW, as compared to FW, resulted in a notable enhancement in the Pnmax and Isat (Table 1), indicating that SW increased the photosynthetic capacity of maize leaves and expanded the range of available light intensity. The potential reason for this outcome could be attributed to the fact that SW enhanced the Rubisco activity of maize leaves (Fig. S3 [see Additional file 1]). When the soil water returned from its valleys to its peaks, the recovery of Rubisco activity under FW conditions was comparatively delayed. Previous research revealed that the activity of Rubisco was still significantly below the control level even after one day of rehydration and that some varieties needed eight days to regain the control level [55]. Nevertheless, in this study, the SWC was lower than the SW around 4 days after the FW peaked (Fig. 2). Consequently, Rubisco activity might not have rebounded fully and might have declined once more. Therefore, Rubisco activity under the FW condition was consistently lower than that of SW, and Rubisco significantly enhanced the carbon carboxylation ability of maize leaves and then improved its energy conversion efficiency [61]. Another potential factor may be a long-term variation in soil water levels resulting from the FW treatment, which induced significant water stress in maize plants [21]. Water fluctuation subsequently impaired the leaf photosynthetic efficiency, resulting in a decrease in the capacity for photosynthetic carbon assimilation. Consequently, FW had an adverse impact on the crop biomass accumulation, ultimately leading to a substantial reduction in maize yield.
Additionally, the findings suggested that soil water stability and water content had minimal impact on the photosynthetic capacity of maize leaves under conditions of low light intensity (Table 1), but the growth and development of maize plants in FW were susceptible to inhibition under conditions of high light intensity. In agricultural production, summer maize plants commonly thrive in an area characterized by ample light availability [62]. The results of this study indicated that the potential photosynthetic capacity of maize leaves was improved by managing the SW mode, increasing the tolerance of maize plants to strong light, avoiding photosynthesis inhibition by intense light, and promoting maize plant growth and development, corroborating a previous study [50]. Furthermore, previous research has indicated a strong correlation between the photosynthetic capacity of plants and many anatomical characteristics of plant leaves, including stomatal density, vein density, and palisade tissue thickness [63, 64]. The observed variations in the photosynthetic physiology of maize leaves in response to water treatments at different stages may be attributed to alterations in leaf anatomical traits, and this will be thoroughly and systematically analyzed in future studies.
Stable soil water decreased leaf δ13C by altering the maize plants carbon assimilation
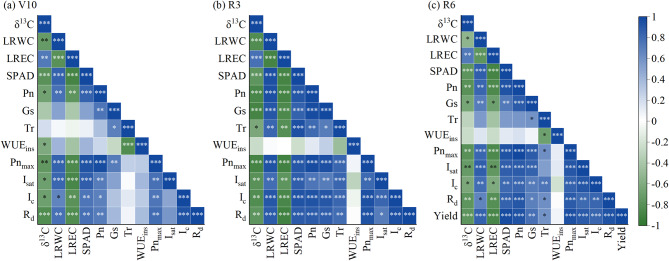
Plants discriminate against the heavier isotope, and this discrimination happens mainly during the process of photosynthesis fixing carbon [65]. The δ13C reflects the preferential assimilation in C3 crops of the lighter carbon isotope 12C over 13C [46]. However, C4 crops have a more complex process; the Kranz anatomy determined the carbon concentrating mechanism, which spatially separated the initial carbon fixation from the Rubisco-catalyzed carbon assimilation in mesophyll and bundle sheath cells, respectively [65]. The δ13C of cotton plants under a limited water supply was higher than in crops grown under well-watered conditions [36]. Similarly, in this study, higher δ13C values were usually obtained in the LW treatment relative to the HW treatment (Table 2), indicating that C3 and C4 crops have similar responses to SWC. Interestingly, δ13C of plant material in SW showed more negative values than that in FW, showing that the discrimination against 13C was stronger for maize plants grown in SW during photosynthetic CO2 assimilation (Table 2). On the one hand, studies have shown that soil water with a large fluctuation will reduce stomate pore length, which may lead to a decrease in intercellular carbon dioxide concentration [52, 66]. In the process of gradually decreasing soil water content in FW, the decrease in CO2 conductivity in leaves resulted in a decrease in the availability of the gaseous substrate of Rubisco. In such adverse conditions, δ13C increased and leaves’ usage ratio of 13CO2 increased (Table 2). On the other hand, along with the ratio of CO2 assimilation rate and Gs, δ13C is determined as an extra contributing component by the leakage of CO2 from the bundle sheath cells back to the mesophyll [67]. The effectiveness of photosynthesis is reduced by this leakage, which is influenced by the coordination of several photosynthetic enzymes. In the process of decreasing soil water, Rubisco activity also gradually decreased. Research has exhibited that there was a positive correlation between Rubisco activity and chloroplastic CO2 concentration [68], and the recovery of Rubisco activity was relatively slow after soil water reached the peaks [55], which may be reduced after insufficient recovery. Therefore, lower Rubisco activity and higher δ13C were observed in FW treatment, and the two were negatively correlated (Table 2, Figs. S3 and S4 [see Additional file 1]). In addition, soil water stability significantly affected the leaf LRWC and LREC (Fig. 3), which were closely related to proteins, lipids, and sugars in leaves [22, 69], and these substances carried different isotopic signatures. The relative composition of compounds caused by post-photosynthetic fractionations may also contribute to differences in leaf δ13C of maize plants grown in SW and FW [65, 70]. Alternatively, δ13C was negatively correlated with the leaf relative water content, SPAD, net photosynthetic rate, maximum net photosynthetic rate, saturated light intensity, light compensation point, and dark respiration rate, and positively correlated with the leaf relative conductivity, while the relationship between δ13C and leaf WUE varied with the growth of maize plants (Fig. 7). This indicated that δ13C may serve as a comprehensive indicator of the water physiology and water status of maize plants. Further research should be undertaken to investigate the influence of soil water stability on the values of fractionation factors, including fractionation during diffusion of CO2 in the liquid phase, carboxylation of Rubisco, and the combined fractionation of CO2 dissolution and PEPC carboxylation [34].
Fig. 7.
Relationship between stable carbon isotope ratio (δ13C), photosynthetic parameters, and water use efficiency of maize plants. LRWC: leaf relative water content, LREC: leaf relative electrical conductivity, SPAD: relative chlorophyll content, Pn: net photosynthetic rate, Gs: stomatal conductance, Tr: transpiration rate, WUEins: instantaneous water use efficiency, Pnmax: maximum net photosynthetic rate, Isat: saturated light intensity, Ic: light compensation point, Rd: dark respiration rate, V10: ten-leaf stage, R3: milk stage, and R6: physiological maturity stage. Pn, Gs, Tr, and WUEins were the data when photosynthetically active radiation was 1500 µmol m–2 s–1. *, **, and *** indicate significance levels at P < 0.05, P < 0.01, and P < 0.001, respectively
Conclusion
In this study, the effects of soil water stability and water content on the leaf water content, SPAD, photosynthetic physiology, leaf water use efficiency, δ13C, and yield of maize at various stages were studied using a two-factor experimental design. The main results were as follows:
Both soil water stability and soil water content were shown to have an impact on maize grain yield; however, it was observed that soil water content had a greater impact than soil water stability. Furthermore, their interaction did not have a significant effect on yield. In contrast to the fluctuating soil water, the stable soil water resulted in a significant increase in yield. Specifically, the stable soil water led to a yield increase of 163.3% under 55% FC and 38.3% under 75% FC. When comparing the yield of stable soil water with fluctuating soil water under similar water content, it was shown that the average yield increase was 100.8%.
Stable soil water improved the maximum net photosynthetic rate, saturated light intensity, stomatal conductance, leaf SPAD, leaf water content, and leaf water use efficiency of maize plants compared to fluctuating soil water, which promoted the assimilation and conversion of dry matter into grain yield and ultimately increased the maize yield, while its effect depended on soil water content.
Low soil water content of 55% FC, as opposed to high water content of 75% FC, weakened photosynthetic capacity and exacerbated stomatal limitation of maize leaves, rendering them more susceptible to light inhibition, which decreased photoassimilate accumulation, resulting in a significant decrease in yield.
The δ13C of maize leaves was influenced by soil water stability and water content. The maize leaf δ13C under stable soil water decreased by 5.3–10.3% relative to fluctuating soil water with the same water level, indicating that stable soil water has stronger discrimination against 13C during photosynthetic CO2 assimilation. And the δ13C under 75% FC fell by 4.7–7.7% in comparison to that under 55% FC under similar soil water stability.
This work applies a two-dimensional perspective to the stability and content of soil water for understanding the relationship between maize and soil water. It also provides new ideas on how to manage agricultural water resources.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Ge Li, Huaiyu Long, and Renlian Zhang. Aiguo Xu and Li Niu supervised the study and contributed to reviewing and editing the manuscript. The first draft of the manuscript was written by Ge Li, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Third Xinjiang Scientific Expedition Program (2021xjkk0200), the National Key Research and Development Program of China (2018YFE0112300), the Science and Technology Project of Henan Province (242102110197), and the Central Public-interest Scientific Institution Basal Research Fund (No. IFI2024-16).
Data availability
All data supporting the findings of this study are available within this article and within its additional files published online.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Huaiyu Long, Email: longhuaiyu@caas.cn.
Li Niu, Email: niulipipi@163.com.
References
- 1.Zilberman D, Lefler J. Biotechnology for African food security. Nat Food. 2021;2:79. [DOI] [PubMed] [Google Scholar]
- 2.Ministry of water resources of the People’s Republic of China. Statistic bulletin on China water activities. Beijing, China: China Water & Power; 2022. [Google Scholar]
- 3.Kang S, Hao X, Du T, Tong L, Su X, Lu H, et al. Improving agricultural water productivity to ensure food security in China under changing environment: from research to practice. Agric Water Manag. 2017;179:5–17. [Google Scholar]
- 4.Leghari SJ, Hu K, Wei Y, Wang T, Bhutto TA, Buriro M. Modelling water consumption, N fates and maize yield under different water-saving management practices in China and Pakistan. Agric Water Manag. 2021;255:107033. [Google Scholar]
- 5.Bwambale E, Abagale FK, Anornu GK. Smart irrigation monitoring and control strategies for improving water use efficiency in precision agriculture: a review. Agric Water Manag. 2022;260:107324. [Google Scholar]
- 6.Shen Z, Zhang Q, Singh VP, Pokhrel Y, Li J, Xu C-Y, et al. Drying in the low-latitude Atlantic Ocean contributed to terrestrial water storage depletion across Eurasia. Nat Commun. 2022;13:1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nam S, Kang S, Kim J. Maintaining a constant soil moisture level can enhance the growth and phenolic content of sweet basil better than fluctuating irrigation. Agric Water Manag. 2020;238:106203. [Google Scholar]
- 8.Liang J, Liang G, Zhao Y, Zhang Y. A synergic method of Sentinel-1 and Sentinel-2 images for retrieving soil moisture content in agricultural regions. Comput Electron Agric. 2021;190:106485. [Google Scholar]
- 9.Mehmood F, Wang G, Gao Y, Liang Y, Chen J, Si Z, et al. Nitrous oxide emission from winter wheat field as responded to irrigation scheduling and irrigation methods in the North China Plain. Agric Water Manag. 2019;222:367–74. [Google Scholar]
- 10.Siakou M, Bruggeman A, Eliades M, Zoumides C, Djuma H, Kyriacou MC, et al. Effects of deficit irrigation on ‘Koroneiki’ olive tree growth, physiology and olive oil quality at different harvest dates. Agric Water Manag. 2021;258:107200. [Google Scholar]
- 11.Attia A, El-Hendawy S, Al-Suhaibani N, Alotaibi M, Tahir MU, Kamal KY. Evaluating deficit irrigation scheduling strategies to improve yield and water productivity of maize in arid environment using simulation. Agric Water Manag. 2021;249:106812. [Google Scholar]
- 12.Hokam EM, El-Hendawy SE, Schmidhalter U. Drip irrigation frequency: the effects and their interaction with nitrogen fertilization on maize growth and nitrogen use efficiency under arid conditions. J Agron Crop Sci. 2011;197:186–201. [Google Scholar]
- 13.Sarker KK, Hossain A, Timsina J, Biswas SK, Malone SL, Alam MK, et al. Alternate furrow irrigation can maintain grain yield and nutrient content, and increase crop water productivity in dry season maize in sub-tropical climate of South Asia. Agric Water Manag. 2020;238:106229. [Google Scholar]
- 14.Liu H, Li H, Ning H, Zhang X, Li S, Pang J, et al. Optimizing irrigation frequency and amount to balance yield, fruit quality and water use efficiency of greenhouse tomato. Agric Water Manag. 2019;226:105787. [Google Scholar]
- 15.Shu L, Liu R, Min W, Wang Y, Yu H, Zhu P, et al. Regulation of soil water threshold on tomato plant growth and fruit quality under alternate partial root-zone drip irrigation. Agric Water Manag. 2020;238:106200. [Google Scholar]
- 16.Alliaume F, Echeverria G, Ferrer M, González Barrios P. A study of the multivariate spatial variability of soil properties, and their association with vine vigor growing on a clayish soil. J Soil Sci Plant Nutr. 2024.
- 17.Jasse A, Berry A, Aleixandre-Tudo JL, Poblete-Echeverría C. Intra-block spatial and temporal variability of plant water status and its effect on grape and wine parameters. Agric Water Manag. 2021;246(December 2020):106696. [Google Scholar]
- 18.Lin X, Wang Z, Li J. Spatial variability of salt content caused by nonuniform distribution of irrigation and soil properties in drip irrigation subunits with different lateral layouts under arid environments. Agric Water Manag. 2022;266:107564. [Google Scholar]
- 19.Lazarovitch N, Kisekka I, Oker TE, Brunetti G, Wöhling T, Xianyue L et al. Modeling of irrigation and related processes with HYDRUS. In: Sparks DLBT-A in A, redakteur. Academic Press; 2023. bl 79–181.
- 20.Wang Z, Zhu G, Long H, Zhang R, Shen Z, Qu X, et al. Effects of temporal variation of soil moisture on the growth and water use efficiency of maize. J Agric Sci Technol. 2020;22:153–64. [Google Scholar]
- 21.Niu L, Wang Z, Zhu G, Yu K, Li G, Long H. Stable soil moisture improves the water use efficiency of maize by alleviating short-term soil water stress. Front Plant Sci. 2022;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G, Long H, Zhang R, Drohan PJ, Xu A, Niu L. Stable soil moisture alleviates water stress and improves morphogenesis of tomato seedlings. Horticulturae. 2023;9:391. [Google Scholar]
- 23.Ali S, Jan A, Manzoor, Sohail A, Khan A, Khan MI, et al. Soil amendments strategies to improve water-use efficiency and productivity of maize under different irrigation conditions. Agric Water Manag. 2018;210:88–95. [Google Scholar]
- 24.Guo Q, Huang G, Guo Y, Zhang M, Zhou Y, Duan L. Optimizing irrigation and planting density of spring maize under mulch drip irrigation system in the arid region of Northwest China. F Crop Res. 2021;266:108141. [Google Scholar]
- 25.Li G, Zhu G, Liu J, Wang Z, Long H, Zhang R et al. Effects of stable and fluctuating soil water on the agronomic and biological performance of root vegetables. Front Plant Sci. 2024;15. [DOI] [PMC free article] [PubMed]
- 26.Liu J, Wang X, Rong Z, Gao Y, Zhang G, Wang W, et al. Modified non-rectangular hyperbola equation with plant height for photosynthetic light-response curves of Potentilla anserina and Elymus nutans at various growth phases in the Heihe River Basin, Northwest China. J Arid Land. 2019;11:764–73. [Google Scholar]
- 27.Li G, Zhao B, Dong S, Zhang J, Liu P, Lu W. Controlled-release urea combining with optimal irrigation improved grain yield, nitrogen uptake, and growth of maize. Agric Water Manag. 2020;227:105834. [Google Scholar]
- 28.Ali S, Xu Y, Ma X, Henchiri M, Cai T, Ren X, et al. Cultivation modes and deficit irrigation strategies to improve 13C carbon isotope, photosynthesis, and winter wheat productivity in semi-arid regions. Environ Sci Pollut Res. 2019;26:5539–53. [DOI] [PubMed] [Google Scholar]
- 29.Xing H, Zhou W, Hao W, Li L, Wang C, Ma H, et al. Inhibition of nitrogen increasing on maize growth under water stress. Chin J Agrometeorol. 2020;41:240–52. [Google Scholar]
- 30.Xia JB, Zhang GC, Wang RR, Zhang SY. Effect of soil water availability on photosynthesis in Ziziphus jujuba var. Spinosus in a sand habitat formed from seashells: comparison of four models. Photosynthetica. 2014;52:253–61. [Google Scholar]
- 31.Moreno-Sotomayor A, Weiss A, Paparozzi ET, Arkebauer TJ. Stability of leaf anatomy and light response curves of field grown maize as a function of age and nitrogen status. J Plant Physiol. 2002;159:819–26. [Google Scholar]
- 32.Medrano H, Tomás M, Martorell S, Flexas J, Hernández E, Rosselló J, et al. From leaf to whole-plant water use efficiency (WUE) in complex canopies: limitations of leaf WUE as a selection target. Crop J. 2015;3:220–8. [Google Scholar]
- 33.Ellsworth PZ, Cousins AB. Carbon isotopes and water use efficiency in C4 plants. Curr Opin Plant Biol. 2016;31:155–61. [DOI] [PubMed] [Google Scholar]
- 34.Ubierna N, Holloway-Phillips M-M, Farquhar GD. Using stable carbon isotopes to study C3 and C4 photosynthesis: Models and calculations. In: Methods in molecular biology (Clifton, N.J.). United States; 2018. bl 155–96. [DOI] [PubMed]
- 35.Twohey RJ, Roberts LM, Studer AJ. Leaf stable carbon isotope composition reflects transpiration efficiency in Zea mays. Plant J. 2019;97:475–84. [DOI] [PubMed] [Google Scholar]
- 36.Saranga Y, Flash I, Paterson AH, Yakir D. Carbon isotope ratio in cotton varies with growth stage and plant organ. Plant Sci. 1999;142:47–56. [Google Scholar]
- 37.Dercon G, Clymans E, Diels J, Merckx R, Deckers J. Differential 13C isotopic discrimination in maize at varying water stress and at low to high nitrogen availability. Plant Soil. 2006;282:313–26. [Google Scholar]
- 38.Br ü ck H, Payne WA, Sattelmacher B. Effects of phosphorus and water supply on yield, transpirational water-use efficiency, and carbon isotope discrimination of pearl millet. Crop Sci. 2000;40:120–5. [Google Scholar]
- 39.Li S, Tan D, Wu X, Degré A, Long H, Zhang S, et al. Negative pressure irrigation increases vegetable water productivity and nitrogen use efficiency by improving soil water and NO3–-N distributions. Agric Water Manag. 2021;251:106853. [Google Scholar]
- 40.Yang P, Bian Y, Long H, Drohan PJ. Comparison of emitters of ceramic tube and polyvinyl formal under negative pressure irrigation on soil water use efficiency and nutrient uptake of crown daisy. Agric Water Manag. 2020;228:105830. [Google Scholar]
- 41.Yang P, Bai J, Yang M, Ma E, Yan M, Long H, et al. Negative pressure irrigation for greenhouse crops in China: a review. Agric Water Manag. 2022;264:107497. October 2021. [Google Scholar]
- 42.Zhang J, Ji J, Wang P, Long H, Wu X. Molecular mechanism of negative pressure irrigation inhibiting root growth and improving water use efficiency in maize. Plant Soil. 2022;472:127–43. [Google Scholar]
- 43.Challabathula D, Analin B, Mohanan A, Bakka K. Differential modulation of photosynthesis, ROS and antioxidant enzyme activities in stress-sensitive and -tolerant rice cultivars during salinity and drought upon restriction of COX and AOX pathways of mitochondrial oxidative electron transport. J Plant Physiol. 2022;268:153583. [DOI] [PubMed] [Google Scholar]
- 44.Ye Z, Yu Q. A coupled model of stomatal conductance and photosynthesis for winter wheat. Photosynthetica. 2008;46:637–40. [Google Scholar]
- 45.Sun Q, Wang Y, Chen G, Yang H, Du T. Water use efficiency was improved at leaf and yield levels of tomato plants by continuous irrigation using semipermeable membrane. Agric Water Manag. 2018;203:430–7. [Google Scholar]
- 46.Farquhar G, Richards R. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Funct Plant Biol. 1984;11:539. [Google Scholar]
- 47.Zhang G, Dai R, Ma W, Fan H, Meng W, Han J, et al. Optimizing the ridge-furrow ratio and nitrogen application rate can increase the grain yield and water use efficiency of rain-fed spring maize in the Loess Plateau region of China. Agric Water Manag. 2022;262:107430. [Google Scholar]
- 48.Guo L, Bornø ML, Niu W, Liu F. Biochar amendment improves shoot biomass of tomato seedlings and sustains water relations and leaf gas exchange rates under different irrigation and nitrogen regimes. Agric Water Manag. 2021;245:106580. [Google Scholar]
- 49.Pazzagli PT, Weiner J, Liu F. Effects of CO2 elevation and irrigation regimes on leaf gas exchange, plant water relations, and water use efficiency of two tomato cultivars. Agric Water Manag. 2016;169:26–33. [Google Scholar]
- 50.Wang Z. Effects of soil moisture temporal variation on growth, physiology and water use efficiency of maize and lettuces. Chinese Academy of Agricultural Sciences; 2020.
- 51.Guo Q, Li X, Niu L, Jameson PE, Zhou W. Transcription-associated metabolomic adjustments in maize occur during combined drought and cold stress. Plant Physiol. 2021;186:677–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu S, Huang Y, Zhang R, Niu L, Long H. Appropriate nitrogen application for alleviation of soil moisture-driven growth inhibition of okra (Abelmoschus esculentus L. (Moench)). Horticulturae. 2024;10:425. [Google Scholar]
- 53.Hao S, Cao H, Wang H, Pan X. The physiological responses of tomato to water stress and re-water in different growth periods. Sci Hortic (Amsterdam). 2019;249:143–54. [Google Scholar]
- 54.Perez P, Rabnecz G, Laufer Z, Gutierrez D, Tuba Z, Martinez-Carrasco R. Restoration of photosystem II photochemistry and carbon assimilation and related changes in chlorophyll and protein contents during the rehydration of desiccated Xerophyta scabrida leaves. J Exp Bot. 2011;62:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calcagno AM, Rivas M, Castrillo M. Structural, physiological and metabolic integrated responses of two tomato (Solanum lycopersicum L.) cultivars during leaf rehydration. Aust J Crop Sci. 2011;5.
- 56.Morabito C, Orozco J, Tonel G, Cavalletto S, Meloni GR, Schubert A, et al. Do the ends justify the means? Impact of drought progression rate on stress response and recovery in Vitis vinifera. Physiol Plant. 2022;174:e13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lamptey S, Li L, Xie J, Zhang R, Antille SY. Photosynthetic response of maize to nitrogen fertilization in the semiarid western Loess Plateau of China. Crop Sci. 2017;57:2739–52. [Google Scholar]
- 58.Fu L, Ren H, Xu S, Hu S, Yang J, Liu C. Planting models and mulching material strategies to reduce bundle sheath cell leakage and improve photosynthetic capacity and maize production in semi-arid climate. Environ Sci Pollut Res. 2021;28:2315–27. [DOI] [PubMed] [Google Scholar]
- 59.Eom K, Park S-H, Yoo S-Y. Water requirement of maize according to growth stage. Korean J Soil Sci Fertil. 2013;46:16–22. [Google Scholar]
- 60.Guo J, Fan J, Xiang Y, Zhang F, Yan S, Zhang X, et al. Maize leaf functional responses to blending urea and slow-release nitrogen fertilizer under various drip irrigation regimes. Agric Water Manag. 2022;262:107396. [Google Scholar]
- 61.Zhu K, Zuo Q, Liu F, Qin J, Wang A, Zhang J, et al. Divergences in leaf CO2 diffusion conductance and water use efficiency of soybean coping with water stress and its interaction with N addition. Environ Exp Bot. 2024;217:105572. [Google Scholar]
- 62.Wu H, Qiao M, Zhang W, Wang K, Li S, Jiang C. Systemic regulation of photosynthetic function in maize plants at graining stage under a vertically heterogeneous light environment. J Integr Agric. 2022;21:666–76. [Google Scholar]
- 63.Amitrano C, Arena C, Cirillo V, De Pascale S, De Micco V. Leaf morpho-anatomical traits in Vigna radiata L. affect plant photosynthetic acclimation to changing vapor pressure deficit. Environ Exp Bot. 2021;186:104453. [Google Scholar]
- 64.Gao H, Li N, Li J, Khan A, Ahmad I, Wang Y, et al. Improving boll capsule wall, subtending leaves anatomy and photosynthetic capacity can increase seed cotton yield under limited drip irrigation systems. Ind Crops Prod. 2021;161:113214. [Google Scholar]
- 65.Eggels S, Blankenagel S, Schön C-C, Avramova V. The carbon isotopic signature of C4 crops and its applicability in breeding for climate resilience. Theor Appl Genet. 2021;134:1663–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang YJ, Gao H, Li YH, Wang L, Kong DS, Guo YY, et al. Effect of water stress on photosynthesis, chlorophyll fluorescence parameters and water use efficiency of common reed in the Hexi Corridor. Russ J Plant Physiol. 2019;66:556–63. [Google Scholar]
- 67.Farquhar G. On the nature of carbon isotope discrimination in C4 species. Funct Plant Biol. 1983;10:205. [Google Scholar]
- 68.Galmes J, Ribas-Carbo M, Medrano H, Flexas J. Rubisco activity in Mediterranean species is regulated by the chloroplastic CO2 concentration under water stress. J Exp Bot. 2011;62:653–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu F, Jensen CR, Shahanzari A, Andersen MN, Jacobsen S-E. ABA regulated stomatal control and photosynthetic water use efficiency of potato (Solanum tuberosum L.) during progressive soil drying. Plant Sci. 2005;168:831–6. [Google Scholar]
- 70.Cao M, Wu C, Liu J, Jiang Y. Increasing leaf δ13C values of woody plants in response to water stress induced by tunnel excavation in a karst trough valley: implication for improving water-use efficiency. J Hydrol. 2020;586:124895. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within this article and within its additional files published online.