Abstract
Background
The three-amino-acid-loop-extension (TALE) superfamily genes are broadly present in plants and play important roles in plant growth, development, and abiotic stress responses. So far, the TALE family in B.napus have not been systematically studied, especially their potential roles in response to abiotic stress.
Results
In this study, we identified 74 TALE family genes distributed on 19 chromosomes in the B. napus genome using bioinformatics methods. Phylogenetic analysis divided the BnTALE superfamily into two subfamilies, the BEL1-like (BLH/BELL homeodomain) and the KNOX (KNOTTED-like homeodomain) subfamilies. Moreover, the KNOX subfamily could be further categorized into three clades (KNOX Class I, KNOX Class II, and KNOX Class III). BnTALE members in the same subclass or branch of the phylogenetic tree generally showed similar gene structures and conserved domain compositions, which may indicate that they have similar biological functions. The BnTALE promoter regions contained many hormone-related elements and stress response elements. Duplication events identification analysis showed that WGD/segmental duplications were the main drivers of amplification during the evolution of TALE genes, and most of the duplicated BnTALE genes underwent purifying selection pressures during evolution. Potential protein interaction network analysis showed that a total of 12,615 proteins might interact with TALE proteins in B. napus. RNA-seq and qRT-PCR analyses showed that the expression of BnTALE was tissue-differentiated and can be induced by abiotic stresses such as dehydration, cold, and NaCl stress. In addition, weighted gene co-expression network analysis (WGCNA) identified four co-expression modules containing the most BnTALE genes, which would be notably related to dehydration and cold stresses.
Conclusions
Our study paves the way for future gene functional research of BnTALE and facilitate their applications in the genetic improvement of B. napus in response to abiotic stresses.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05953-1.
Keywords: Brassica napus, TALE gene family, Tissue expression, WGCNA, Abiotic stress
Background
The homeobox genes encoding the transcriptional regulatory factors with highly conserved homeodomain play an important role in the growth and development of plants [1, 2]. In PlantTFDB, the homeobox genes were divided into five classes: homeodomain-leucine zipper (HD-ZIP), three-amino-acid-loop-extension (TALE), wuschel homeobox (WOX), homeobox-plant homeodomain (HB-PHD), and HB-other [3].
The TALE superclass, which consists of 63 amino acids forming two helices and three additional amino acid residues (P-Y-P) connecting the first and second helices, is a pivotal transcription factor that broadly exist in plants [1, 4–6]. The TALE family consists of the KNOX (KNOTTED-like homeodomain) and BELL (BEL1-Like homeodomain) subfamilies [7]. The KNOX proteins include four domains: KNOX1, KNOX2, ELK, and KN homeodomain and it can be divided into three classes according to the structure characteristics of homeodomain and expression patterns. Both class I and II KNOX proteins contain four members while Class III has only one member, KNATM, which lack the homeodomain and is only found in dicotyledons [4, 8–10]. BELL proteins contain SKY, BELL, and homeodomain [11]. The BELL and KNOX proteins have been shown to specifically recognize and bind to form the BELL-KNOX heterodimeric proteins [12], which are essential for nuclear localization, binding of target genes, and playing a regulatory role in biological processes of the two transcription factor proteins [13, 14].
The TALE gene family plays a regulatory role in plant growth, development [2], and different biological processes, such as meristem formation, organ morphogenesis, secondary cell wall development [15] and signal transduction [16]. Previous research has shown that the KNOX1 gene plays an important role in the development and maintenance of meristem. The KNOX2 gene plays a vital role in regulating the secondary growth of plant cell walls and the development of roots, stems, seed coats, and heartwood [17–20]. BELL gene plays essential regulatory roles in ovule development, frond development, and fruit development [21, 22]. The LeT6/TKn2, which belongs to KNOX class I was involved in morphological development in tomato fruit [23]. Kim et al. (2013) established that the AtBLH1 protein regulates seed germination and seedling development by cooperating with the AtKNAT3 protein [13]. GmSBH1 identified in Glycine max, could influences leaf phenotype [24]. ATH1 (Arabidopsis thaliana homeobox 1) interacts with STM (homeobox protein SHOOT MERISTEMLESS) and KNAT2 (A. thaliana KNOX 2) to participate in the development of meristems and inflorescence tissue [20, 25]. In A. thaliana KNOX Class I, AtKNAT2 showed expression in the internal vegetative shoot apical meristem (SAM) [26]. In A. thaliana, AtKNAT7 (class II KNOX protein) negatively regulates secondary cell wall deposition. It inhibits secondary cell wall lignin synthesis by forming a heterodimer with AtBLH6 [27, 28]. PoptrKNAT7, GhKNAT7-A03, and OsKNAT7 (Class II KNOX protein) were reported to be crucial for cell elongation and secondary cell wall (SCW) biosynthesis [7, 17, 29].
In addition, TALE are also involved in hormone regulatory pathways and response to a variety of abiotic stresses [30]. Ectopic expression of the maize KNOX-like gene KN1 in leaves enhances auxin signaling in maize [31]. In A. thaliana, BLH1 activates the expression of abscisic acid (ABA) response gene abscisic acid insensitive 3 (ABI3) by forming a heterodimer with KNAT3, thereby promoting the plant response to ABA at seed germination and seedling stage [13]. Recent studies have identified 11 NaCl stress response genes from the poplar TALE transcription factor family. Among them, the expression of ptTALE5, a member of this family, was upregulated after NaCl stress treatment, which may play an important role in the response of poplar to NaCl stress [32]. Wang et al. (2021) reported that the expression level of GmTALE genes changes in response to NaCl stress and dehydration stress [2]. In poplars, the type I KNOX gene PagKNAT2/6b can enhance plant dehydration resistance by inhibiting gibberellin synthesis and adjusting plant phenotype [33]. Overexpression of the Triticum aestivum KNOX-like gene TaKNOX11-A in A. thaliana can enhance the NaCl tolerance and dehydration resistance of the plant [34].
Brassica napus, formed by spontaneous hybridization between B. rapa (AA genome) and B. oleracea (CC genome) [35], is the second largest oil crop in the world and plays a crucial role in the production of edible oil. During the growth and development of rapeseed, there are many abiotic stress problems, such as high or low temperature and soil salinity, which seriously affect the yield and quality of rapeseed. Previous studies on several plant species identified and analyzed TALE gene families at the genome-wide level in Triticum aestivum [34], cotton [7], soybean [2], tomato [16], and so on. However, no systematic study of the TALE family in B. napus and their expression pattern in tissues and under various stresses has been performed.
In this study, based on the published genome [35] and transcriptome datasets [36, 37], we identified members of the TALE gene family in the B. napus genome. The gene structure, position in the genome, phylogenetic relationships, cis-acting elements in the promoter region, physicochemical properties of coding proteins, tissue expression characteristics, and possible protein interaction network analysis were analyzed. Besides, the gene expression profiling in different tissues during rapeseed plant development, NaCl, and dehydration stresses were also carried out by qRT-PCR. This study could lay a foundation to explore the role of TALE gene family in B. napus growth and development and identify some promising or key TALE genes that could be useful for genetic improvement of abiotic resistance in rapeseed.
Results
Identification of the TALE gene family members in B. napus
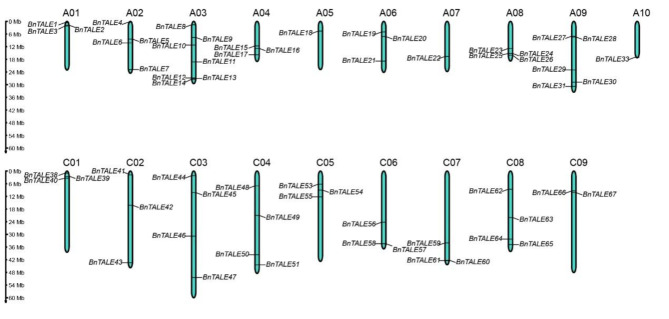
By using the Hidden Markov Model, we searched for ELK, KNOX1, KNOX2, POX, and Homeobox_KN structural domains in the rapeseed cultivar “Darmor-bzh” as a systematic screen for TALE genes at the genome-wide level. We identified a total of 74 TALE genes in the B. napus genome and named these BnTALE genes according to their location on the chromosomes. These BnTALE genes were unevenly distributed on the chromosomes (Table 1; Fig. 1). The A03 chromosome contained the largest number of TALE genes (7) while only one TALE gene was present on chromosomes A05, A07, and A10, respectively. Subcellular localization prediction showed that most of the BnTALE were located in the nucleus. According to domain differences, the TALE of B. napus can be divided into two subfamilies, BELL and KNOX. The sizes and physicochemical properties of the BnTALE vary greatly among the two sub-families. The number of exons contained in the BELL subfamily ranged from four to nine, while the number of exons for the KNOX subfamily members was between one to seven. The length of BELL subfamily proteins varied between 290 (BnTALE34 and BnTALE58) to 692 (BnTALE45) amino acids (aa), and the molecular weights varied from 32.98 to 75.64 kDa, with an average of 59.74 kDa. In the KNOX subfamily, BnTALE66 with 103 aa was the smallest TALE protein, while BnTALE59 was the largest protein with 445 AA, the molecular weights varied from 11.09 to 48.91 kDa, with an average of 32.69 kDa. Therefore, it can be concluded that the proteins of the BELL subfamily in B. napus are longer and bigger than the KNOX subfamily proteins. Isoelectric point analysis showed that the isoelectric point of BELL subfamily proteins ranged from 5.22 to 8.46, with 87.5% members (35/40) exhibiting acidic pI values. The isoelectric point of KNOX subfamily proteins ranged from 4.47 to 8.99, and with the exception of BnTALE25, all the proteins have isoelectric points less than seven.
Table 1.
The three-amino-acid-loop-extension (TALE) gene family members in B. napus
| Gene | Name | Chr | Start | End | Amino acids | Exon number | pI | MW(kDa) | Subcellular Localization | Subfamily |
|---|---|---|---|---|---|---|---|---|---|---|
| BnaA01g00980D | BnTALE1 | A01 | 535,291 | 540,515 | 670 | 4 | 6.59 | 73.51279 | Nucleus | BELL |
| BnaA01g03890D | BnTALE2 | A01 | 1,798,245 | 1,799,923 | 467 | 4 | 6.53 | 53.05888 | Nucleus | BELL |
| BnaA02g17200D | BnTALE6 | A02 | 10,314,345 | 10,316,831 | 479 | 4 | 6.56 | 54.26916 | Nucleus | BELL |
| BnaA03g16450D | BnTALE9 | A03 | 7,679,196 | 7,682,325 | 672 | 4 | 6.61 | 73.41444 | Nucleus | BELL |
| BnaA03g39060D | BnTALE11 | A03 | 19,441,616 | 19,443,428 | 450 | 4 | 5.82 | 50.26421 | Nucleus | BELL |
| BnaA03g52290D | BnTALE13 | A03 | 27,260,893 | 27,262,492 | 438 | 4 | 6.32 | 50.00129 | Nucleus | BELL |
| BnaA03g52940D | BnTALE14 | A03 | 27,658,343 | 27,665,800 | 686 | 6 | 7.31 | 64.21826 | Nucleus | BELL |
| BnaA04g13860D | BnTALE15 | A04 | 11,725,811 | 11,731,115 | 646 | 5 | 6.57 | 71.24468 | Nucleus | BELL |
| BnaA04g15670D | BnTALE16 | A04 | 12,928,777 | 12,931,158 | 449 | 4 | 7.26 | 50.4209 | Nucleus | BELL |
| BnaA04g20970D | BnTALE17 | A04 | 16,034,502 | 16,037,438 | 633 | 6 | 6.5 | 68.73016 | Nucleus | BELL |
| BnaA05g08310D | BnTALE18 | A05 | 4,587,942 | 4,591,572 | 629 | 7 | 6.33 | 69.40377 | Nucleus | BELL |
| BnaA06g13850D | BnTALE20 | A06 | 7,368,318 | 7,369,995 | 390 | 4 | 6.24 | 44.76677 | Nucleus | BELL |
| BnaA07g21690D | BnTALE22 | A07 | 16,762,939 | 16,765,267 | 474 | 4 | 6.29 | 53.73141 | Nucleus | BELL |
| BnaA08g15450D | BnTALE23 | A08 | 12,850,348 | 12,855,102 | 668 | 5 | 6.6 | 73.37995 | Nucleus | BELL |
| BnaA08g21960D | BnTALE26 | A08 | 16,112,826 | 16,115,895 | 507 | 6 | 5.65 | 57.18188 | Nucleus | BELL |
| BnaA09g41850D | BnTALE30 | A09 | 29,166,242 | 29,171,316 | 609 | 4 | 6.31 | 67.57958 | Nucleus | BELL |
| BnaA10g27410D | BnTALE33 | A10 | 17,257,903 | 17,261,512 | 579 | 5 | 7.12 | 62.49513 | Nucleus | BELL |
| BnaAnng09210D | BnTALE34 | Ann_random | 9,702,570 | 9,704,594 | 290 | 5 | 5.8 | 32.99845 | Nucleus | BELL |
| BnaAnng09220D | BnTALE35 | Ann_random | 9,706,433 | 9,709,540 | 517 | 5 | 6.33 | 58.95304 | Nucleus | BELL |
| BnaAnng29380D | BnTALE36 | Ann_random | 33,665,323 | 33,668,807 | 575 | 5 | 6.8 | 61.93265 | Nucleus | BELL |
| BnaC01g02010D | BnTALE38 | C01 | 1,008,510 | 1,013,374 | 668 | 5 | 6.65 | 73.40157 | Nucleus | BELL |
| BnaC01g05260D | BnTALE39 | C01 | 2,691,346 | 2,692,991 | 462 | 4 | 6.32 | 52.67547 | Nucleus | BELL |
| BnaC02g03640D | BnTALE41 | C02 | 1,735,436 | 1,738,924 | 576 | 5 | 6.94 | 61.96476 | Nucleus | BELL |
| BnaC03g19820D | BnTALE45 | C03 | 10,358,826 | 10,362,353 | 692 | 6 | 6.74 | 75.64013 | Nucleus | BELL |
| BnaC03g46250D | BnTALE46 | C03 | 31,174,783 | 31,176,447 | 456 | 4 | 5.64 | 50.82279 | Nucleus | BELL |
| BnaC03g61760D | BnTALE47 | C03 | 50,983,453 | 50,988,199 | 671 | 5 | 6.68 | 74.04248 | Nucleus | BELL |
| BnaC04g09330D | BnTALE48 | C04 | 7,068,991 | 7,072,342 | 589 | 9 | 6.27 | 65.53572 | Nucleus | BELL |
| BnaC04g38940D | BnTALE50 | C04 | 40,015,293 | 40,018,054 | 450 | 4 | 8.46 | 50.69834 | Nucleus | BELL |
| BnaC04g44970D | BnTALE51 | C04 | 44,808,821 | 44,812,263 | 637 | 8 | 6.53 | 69.28185 | Nucleus | BELL |
| BnaC04g56300D | BnTALE52 | C04_random | 3,995,247 | 4,001,065 | 649 | 4 | 6.56 | 71.45187 | Nucleus | BELL |
| BnaC05g15280D | BnTALE54 | C05 | 9,089,226 | 9,091,135 | 520 | 4 | 5.97 | 58.90236 | Nucleus | BELL |
| BnaC06g22380D | BnTALE56 | C06 | 24,450,176 | 24,452,898 | 504 | 5 | 6.08 | 56.71476 | Nucleus | BELL |
| BnaC06g36180D | BnTALE57 | C06 | 34,787,789 | 34,790,730 | 515 | 5 | 6.43 | 58.5538 | Nucleus | BELL |
| BnaC06g36190D | BnTALE58 | C06 | 34,794,361 | 34,796,367 | 290 | 5 | 5.94 | 32.98147 | Nucleus | BELL |
| BnaC07g44050D | BnTALE61 | C07 | 42,788,812 | 42,790,447 | 453 | 4 | 6.14 | 51.5971 | Nucleus | BELL |
| BnaC08g19170D | BnTALE63 | C08 | 22,140,847 | 22,142,559 | 419 | 5 | 5.22 | 47.23048 | Nucleus | BELL |
| BnaC08g34350D | BnTALE64 | C08 | 32,473,743 | 32,479,230 | 602 | 5 | 6.37 | 66.75979 | Nucleus | BELL |
| BnaCnng03740D | BnTALE68 | Cnn_random | 2,892,511 | 2,896,094 | 576 | 5 | 7.14 | 62.15578 | Nucleus | BELL |
| BnaCnng11290D | BnTALE69 | Cnn_random | 10,582,998 | 10,587,255 | 569 | 6 | 6.36 | 64.19103 | Nucleus | BELL |
| BnaCnng21740D | BnTALE71 | Cnn_random | 20,394,052 | 20,396,527 | 472 | 4 | 6.51 | 53.4803 | Nucleus | BELL |
| BnaA02g14950D | BnTALE5 | A02 | 8,569,079 | 8,574,265 | 328 | 5 | 4.91 | 36.93633 | Nucleus | KNOX I |
| BnaA03g23610D | BnTALE10 | A03 | 11,303,177 | 11,306,833 | 403 | 6 | 6.1 | 46.35865 | Nucleus | KNOX I |
| BnaA08g20500D | BnTALE24 | A08 | 15,447,762 | 15,449,510 | 209 | 4 | 4.59 | 22.92107 | Nucleus | KNOX I |
| BnaA09g13310D | BnTALE28 | A09 | 7,413,897 | 7,416,888 | 384 | 4 | 6.12 | 43.10947 | Nucleus | KNOX I |
| BnaA09g31100D | BnTALE29 | A09 | 23,130,703 | 23,132,290 | 236 | 5 | 4.53 | 26.11447 | Nucleus | KNOX I |
| BnaC02g19900D | BnTALE42 | C02 | 16,260,036 | 16,265,948 | 328 | 5 | 4.9 | 36.89625 | Nucleus | KNOX I |
| BnaC05g18670D | BnTALE55 | C05 | 12,356,472 | 12,357,686 | 222 | 4 | 4.47 | 24.45232 | Nucleus | KNOX I |
| BnaC08g06320D | BnTALE62 | C08 | 8,766,857 | 8,772,834 | 315 | 5 | 5.22 | 35.23275 | Nucleus | KNOX I |
| BnaC09g13580D | BnTALE67 | C09 | 10,235,596 | 10,238,634 | 384 | 4 | 6.22 | 43.19965 | Nucleus | KNOX I |
| BnaCnng59830D | BnTALE73 | Cnn_random | 59,614,805 | 59,616,584 | 350 | 5 | 6.17 | 40.19479 | Nucleus | KNOX I |
| BnaA01g04870D | BnTALE3 | A01 | 2,258,376 | 2,260,668 | 375 | 5 | 5.8 | 41.90902 | Nucleus | KNOX II |
| BnaA02g00810D | BnTALE4 | A02 | 304,150 | 306,956 | 394 | 6 | 5.91 | 44.28204 | Nucleus | KNOX II |
| BnaA02g32110D | BnTALE7 | A02 | 23,108,587 | 23,111,116 | 421 | 6 | 5.62 | 46.28523 | Nucleus | KNOX II |
| BnaA03g03190D | BnTALE8 | A03 | 1,539,765 | 1,542,572 | 387 | 6 | 5.94 | 43.14774 | Nucleus | KNOX II |
| BnaA03g51900D | BnTALE12 | A03 | 27,019,152 | 27,020,903 | 383 | 6 | 5.72 | 43.17633 | Nucleus | KNOX II |
| BnaA06g27560D | BnTALE21 | A06 | 18,939,822 | 18,941,622 | 300 | 6 | 6.08 | 33.43057 | Nucleus | KNOX II |
| BnaA08g20510D | BnTALE25 | A08 | 15,451,084 | 15,453,936 | 122 | 4 | 8.99 | 14.02783 | Nucleus | KNOX II |
| BnaA09g12980D | BnTALE27 | A09 | 7,016,927 | 7,021,165 | 294 | 5 | 5.88 | 33.01723 | Nucleus | KNOX II |
| BnaA09g52990D | BnTALE32 | A09_random | 786,104 | 789,831 | 295 | 6 | 6.14 | 33.10331 | Nucleus | KNOX II |
| BnaAnng30720D | BnTALE37 | Ann_random | 35,042,409 | 35,043,398 | 205 | 5 | 6.23 | 23.25746 | Nucleus | KNOX II |
| BnaC01g06410D | BnTALE40 | C01 | 3,350,853 | 3,353,173 | 375 | 5 | 5.98 | 41.85796 | Nucleus | KNOX II |
| BnaC02g40790D | BnTALE43 | C02 | 43,802,090 | 43,804,797 | 405 | 6 | 5.68 | 44.84264 | Nucleus | KNOX II |
| BnaC03g04580D | BnTALE44 | C03 | 2,188,077 | 2,190,704 | 303 | 6 | 6.3 | 33.77428 | Nucleus | KNOX II |
| BnaC04g20090D | BnTALE49 | C04 | 21,173,379 | 21,173,759 | 105 | 2 | 5.03 | 11.45896 | Cytoplasm | KNOX II |
| BnaC07g29530D | BnTALE59 | C07 | 34,248,589 | 34,253,723 | 445 | 7 | 5.22 | 48.90775 | Nucleus | KNOX II |
| BnaC07g43650D | BnTALE60 | C07 | 42,610,676 | 42,612,632 | 401 | 6 | 5.59 | 45.346 | Nucleus | KNOX II |
| BnaC09g12900D | BnTALE66 | C09 | 9,424,016 | 9,424,324 | 103 | 1 | 5.35 | 11.09354 | Cytoplasm | KNOX II |
| BnaCnng20070D | BnTALE70 | Cnn_random | 18,852,728 | 18,855,246 | 394 | 6 | 5.88 | 44.23395 | Nucleus | KNOX II |
| BnaCnng51440D | BnTALE72 | Cnn_random | 50,877,922 | 50,881,532 | 295 | 5 | 6.06 | 33.08529 | Nucleus | KNOX II |
| BnaCnng70390D | BnTALE74 | Cnn_random | 70,412,295 | 70,413,281 | 206 | 4 | 6.53 | 23.48277 | Nucleus | KNOX II |
| BnaA06g09570D | BnTALE19 | A06 | 5,123,287 | 5,124,136 | 142 | 3 | 4.66 | 16.25438 | Nucleus | KNOX III |
| BnaA09g45470D | BnTALE31 | A09 | 31,056,313 | 31,057,006 | 136 | 3 | 4.47 | 15.31036 | Nucleus | KNOX III |
| BnaC05g10940D | BnTALE53 | C05 | 6,337,554 | 6,338,427 | 138 | 3 | 5.07 | 15.70188 | Nucleus | KNOX III |
| BnaC08g39310D | BnTALE65 | C08 | 35,085,126 | 35,085,803 | 136 | 3 | 4.49 | 15.24631 | Nucleus | KNOX III |
Fig. 1.
Location of the B. napus TALE genes on chromosomes. The y-axes represented the chromosomes length
Phylogenetic relationship analysis of the TALE gene family
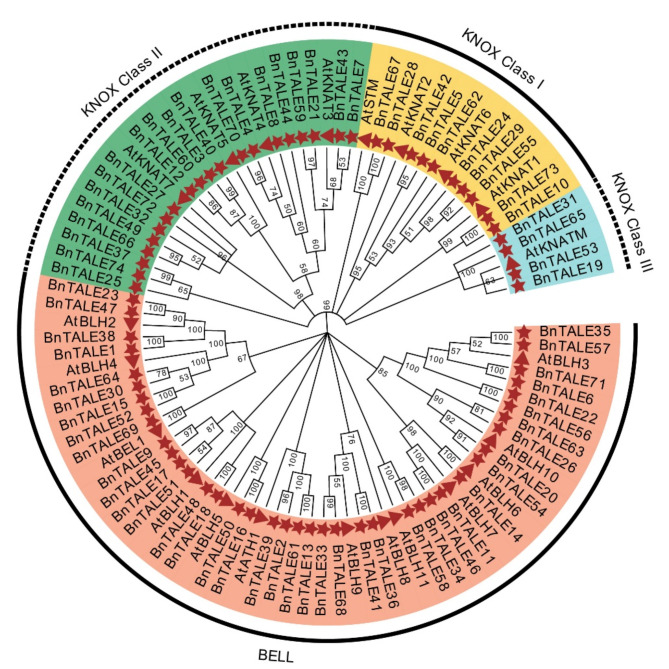
To clarify the phylogeny and taxonomic relationships among TALEs in B. napus, a phylogenetic tree was constructed with the full-length sequence of 74 BnTALE proteins and 22 AtTALE proteins. Consistent with the classification of (A) thaliana TALE gene family, the B. napus TALE proteins were clearly divided into two main clades (The BELL subfamily and the KNOX subfamily) (Fig. 2). The BELL subfamily consisted of 40 BnTALE proteins and 13 AtTALE proteins. The KNOX subfamily had a total of 34 BnTALE proteins and nine AtTALE proteins, which can be further divided into class I, II, III, we can infer that functional differentiation may be existed in this TALE gene subfamily. Among them, Class II was the largest branch, consisting of 20 BnTALE proteins and four AtTALE proteins, Class I was comprised of 10 BnTALE proteins and four AtTALE proteins, and Class III consisted of four BnTALE proteins and one AtTALE protein. The evolutionary tree showed a one-to-many relationship between the A. thaliana TALE gene family and the B. napus TALE gene family, indicating that gene family replication events occurred after genome differentiation between B. napus and A. thaliana. From the phylogenetic tree, it can be inferred that the TALEs in B. napus and A. thaliana were evolutionary conserved.
Fig. 2.
Phylogenetic tree of the B. napus and A. thaliana TALE gene families. The neighbor-joining tree was generated using the amino acid sequences of the TALE proteins through the MEGA7 program and neighbor-joining (NJ) method, with 1000 bootstrap replicates. The two major phylogenetic clades (The BELL subfamily and the KNOX subfamily) are labeled and the TALEs from B. napus and A. thaliana are marked with asterisks and triangles, respectively
Gene structure and domains analysis of the TALE gene family in B. napus
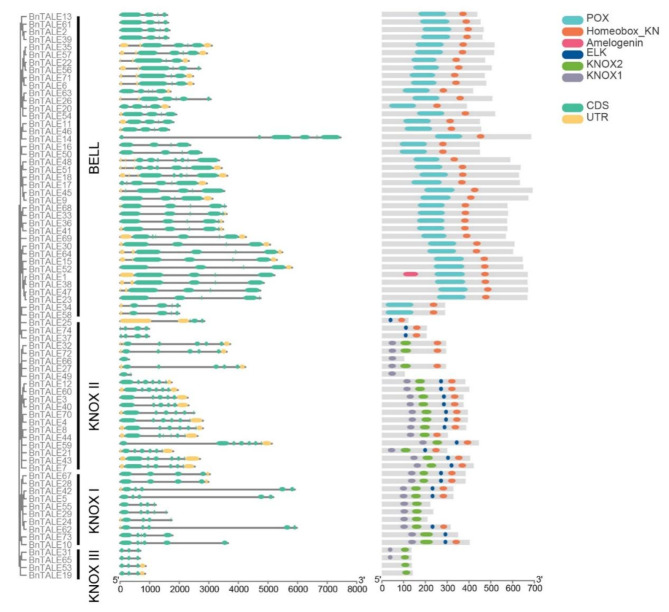
To obtain insights into structural feature of BnTALE, TBtools was used to display the gene structure. In the TALE gene family, 21 genes had 5′ and 3′ UTRs, 34 genes contained only one side of the UTR, and 19 genes did not have UTR (Fig. 3). Many genes in the same subfamily exhibit similar structures, especially in the KNOXII and KNOXIII subfamilies, indicating the high conservation. In other three subfamilies, although the length of gene sequences varied highly, the majority of the genes possessed similar exon numbers and arrangement order.
Fig. 3.
B. napus TALE family gene structures and TALE proteins domains. The yellow box, the green box and the horizontal line represent UTRs, CDSs and introns, respectively. The length of the yellow box, the green box and the horizontal line represents the relative lengths of the corresponding UTRs, CDSs and introns
Furthermore, the number of domains contained in different TALE subfamilies varied from one to four (Fig. 3). Overall, the domain composition patterns of TALE proteins in the same subfamily were very similar, suggesting that the proteins were highly conserved. Almost all TALE proteins (87.8%) contained Homeobox_KN, indicating Homeobox_KN was very conservative and these TALEs may have common functions. The BELL subfamily of BnTALE contained two to three protein-conserved domains, while the domain of BnTALE in the KNOX subfamily varied between one to four. The domain composition pattern of the BELL subfamily of BnTALE was highly similar, and all contained POX and Homeobox_KN. The POX was specific to this subfamily and may be associated with subfamily-specific functions. In the KNOX subfamily, almost all the genes possessed KNOX1 or KNOX2, indicating that these two domains were strongly conserved and related to the function of the KNOX subfamily of the BnTALE. Furthermore, different subclasses of the KNOX subfamily contained different domains and patterns, for example, KNOX III contained the least number of domains (1 to 2), KNOX I contained two to four domains, while the number of domains contained in the KNOX II subfamily proteins varied highly, ranging from 1 to 4.
Analysis of cis-acting elements within the promoters of BnTALE
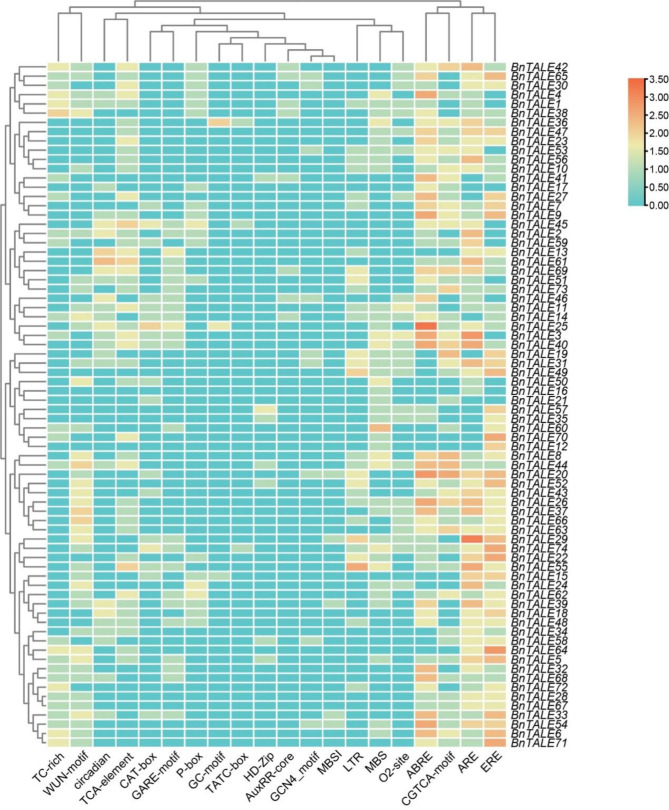
Analysis of promoter characteristics of B. napus TALE gene family by PlantCARE software showed that the number of cis-acting elements contained in the TALE gene family varied widely (Fig. 4). BnTALE25 contained the highest number of the cis-regulatory elements (25), followed by BnTALE20 (24), BnTALE3 (23), and BnTALE29 (23), while BnTALE16 contained only two elements (Table S1). As to the type of cis-acting elements, the highest number of cis-acting elements in the promoters of BnTALE genes were hormone-responsive elements (509), followed by environmental stress-responsive elements (347), and developmental-responsive elements (110). Among the hormone response elements, the most were ABRE elements (152), followed by ERE elements (148) and CGTCA-motif (87). Among the stress response elements, the most were ABRE elements (150), followed by WUN-motif elements (61). Specifically, the hormone response element ABRE, which is activated by ABA, could regulate the corresponding gene expression. BnTALE25 contained the largest number of ABREs (9), followed by BnTALE20 (6), BnTALE4, BnTALE26, BnTALE3, BnTALE9, and BnTALE40 all contained five ABREs. Previous studies showed that there is a correlation between hormone response and plant resistance to abiotic stress [38]. Such as ABRE is associated with the plant response to drought stress [39], this implies that BnTALE may assist plants with adaptation to drought stress. BnTALE64 contained the largest number of ERE components (6), BnTALE71, BnTALE22, BnTALE70, BnTALE74 also contained many ERE elements. BnTALE20 contained more CGTCA elements (5). BnTALE29 contained the largest number of ARE elements (8), followed by BnTALE3 (6). The promoter of the B. napus TALE gene family contained a small number of TCTC box (3), MBSI (5), and GC motif (6).
Fig. 4.
Heatmap of the cis-regulatory elements for 74 TALE genes in B. napus normalized to log2 transformation. The color scale represents the number of elements from low (blue color) to high (red color)
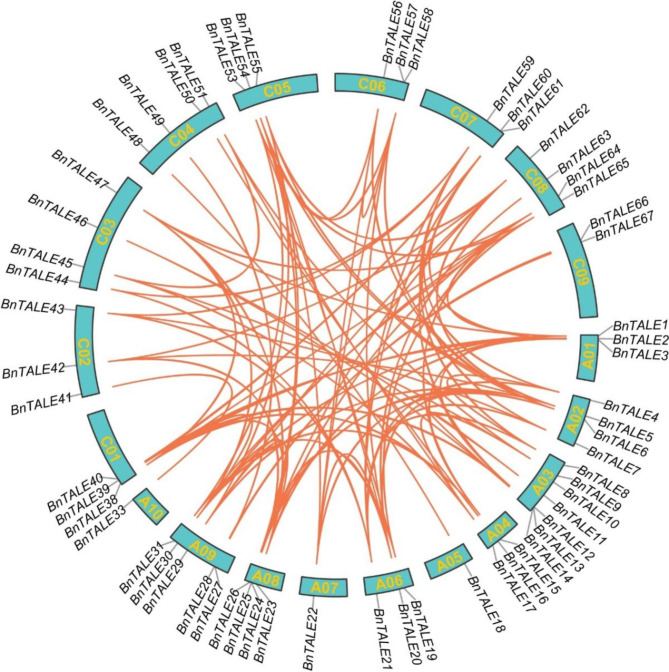
The duplication events of BnTALE genes
To explore the expansion patterns of the B. napus TALE genes, gene duplication events were identified. A total of 105 TALE gene pairs were obtained by MCScanX program. Among them, 24 pairs were found in the A subgenome, 15 pairs occurred in the C subgenome, and the other 66 pairs derived from the A and C subgenomes respectively (Fig. 5). Most of the TALE genes (65/74) were defined as whole-genome duplication (WGD) or segmental duplication events, and only nine genes were caused by dispersed duplication. Therefore, the increase of TALE genes in the B. napus genome may be mainly attributed to WGD/segmental duplication. To assess the direction and strength of natural selection pressure of the TALE family during evolution, the non-synonymous/synonymous substitution ratio (Ka/Ks) for each duplicated gene pair was counted. The Ka/Ks ratio of the duplicated TALEs in B. napus varied from 0.0384 to 1.7325 with an average of 0.2291 (Table S2). Except BnTALE27 and BnTALE66 (1.7325), the other duplicated BnTALE had experienced purifying selection pressure during their development, as shown by the fact that these Ka/Ks values of the duplicated TALEs gene pairs were less than 1.
Fig. 5.
The duplicate gene pair analysis of TALE genes in B. napus. Red lines indicate duplicated TALE gene pairs. Chromosome numbers are shown in the green box. The name and location of the TALE gene is marked on the respective chromosome
Potential protein interaction network analysis of B. napus TALE family members
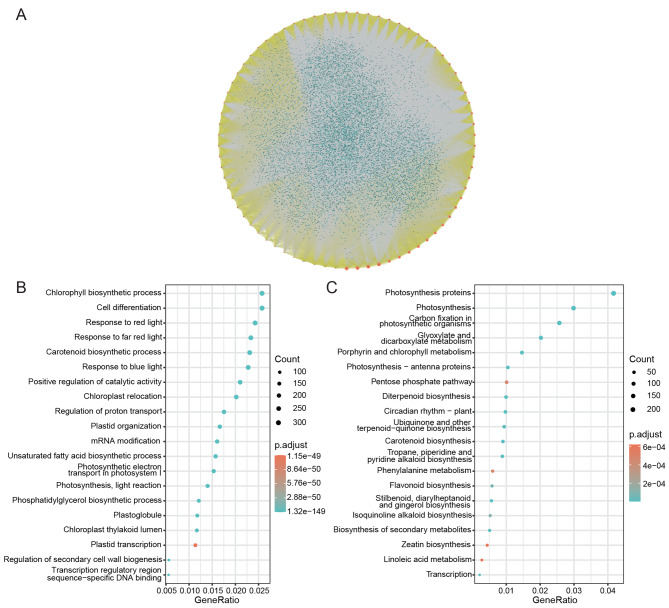
In order to uncover the potential interaction of BnTALE and the participating pathway at the molecular level, possible protein-protein interaction (PPI) network was predicted and enrichment analysis of GO and KEGG were conducted.
The PPI network analysis showed that there were 12,615 proteins possibly interacted with BnTALE proteins (Fig. 6A), and some BnTALE proteins could also interact with each other, especially between the BELL subfamilies and the KNOX subfamilies. GO enrichment of proteins interacting with BnTALE showed that the first three enriched GO terms were plastid organization, chlorophyl II biosynthetic process, response to far red light (Fig. 6B, Table S3). According to the KEGG annotations of the interacting proteins, the top enriched pathways were photosynthesis proteins, photosynthesis, carbon fixation in photosynthetic organisms (Fig. 6C, Table S4).
Fig. 6.
Potential protein interaction network of TALE family members and enrichment analysis in B. napus. A. Potential protein interaction network of TALE family members in B. napus displayed by Cytoscape. Red dots indicate TALEs in B. napus, indigo-blue dots indicate other proteins, yellow lines indicate the relationship between different TALEs and gray lines indicate the relationship between TALEs and other proteins. B. GO enrichment of proteins interacting with B. napus TALEs, the x-axes represented the gene ratio and the y-axes represented the GO categories. C. KEGG pathway enrichment of proteins interacting with B. napus TALEs, the x-axes represented the gene ratio and the y-axes represented the KEGG categories. The circle size represented the gene number, and the circle color represented the adjusted-p value
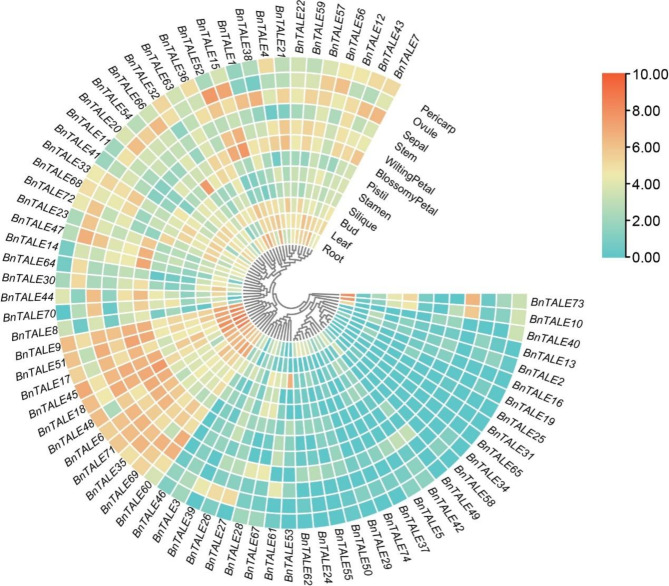
Expression of TALEs in different tissues of B. napus
The analysis of the expression characteristics of TALE gene family members based on transcriptome data showed that the expression of BnTALE was quite different in different tissues (root, leaf, bud, silique, stamen, pistil, blossomy petal, wilting petal, stem, sepal, ovule, and pericarp) of B. napus [40]. Based on the expression patterns, the BnTALE could be divided into three clusters (I–III) (Fig. 7). Cluster I was composed of 31 genes that showed very low or no expression in these 12 tissues. There are a few exceptions, like the expression of BnTALE53 in the bud was relatively high, suggesting that the encoded proteins may be required for the development of the bud, and the expressions of BnTALE73 and BnTALE10 were relatively high in the root and stem. Cluster II consisted of 11 genes which were highly expressed in multiple tissues. These genes were particularly higher expressed in the sepal, wilting petal, leaf, and root. For example, BnTALE45 and BnTALE18, with similar expression patterns, were highly expressed in various tissues, especially in the root and leaf. The expressions of BnTALE9, BnTALE51, BnTALE6, and BnTALE71 were also higher in various tissues. Cluster III was comprised of 32 moderately expressed BnTALE genes. The BnTALE15 and BnTALE52 genes were highly expressed in the ovule, wilting petal, and blossomy petal, displaying similar expression patterns, while the BnTALE66 was highly expressed in the pistil. The expression profile of BnTALE in different tissues indicated that the BnTALE may have certain tissue-specific properties and fulfill different functions in different tissues.
Fig. 7.
Heatmap representation and hierarchical clustering of TALE genes in different tissues. Expression data were processed with log2 normalization. The color scale represents relative expression levels from low (green color) to high (red color)
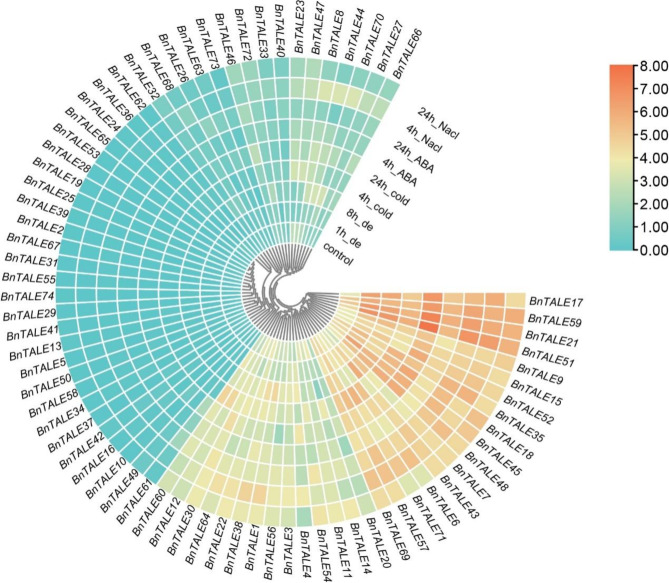
Expression profiles of the BnTALE genes under abiotic stresses
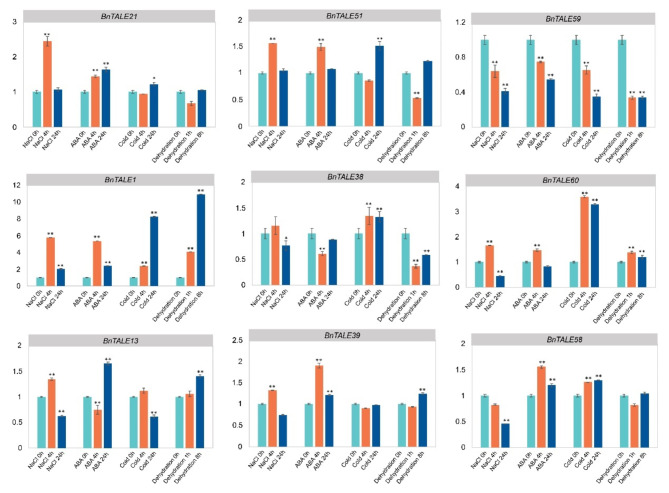
To explore the potential function of BnTALE in response to abiotic stresses, expression patterns were detected using the published RNA-seq data [41]. It displayed that the BnTALE genes were differentially expressed under dehydration, cold, ABA, and NaCl stresses (Fig. 8). Under dehydration stress, the genes with high expression were BnTALE17, BnTALE59, BnTALE21, BnTALE51, BnTALE45, and BnTALE48. The expressions of BnTALE7 and BnTALE43 were also high at 8 h of dehydration. Under 24 h cold stress, BnTALE17, BnTALE59, BnTALE21, and BnTALE51 were highly expressed. The expression levels of BnTALE18, BnTALE45, and BnTALE48 were relatively high. After ABA treatment for 24 h and NaCl treatment for 4 h, the expression levels of BnTALE17, BnTALE59, BnTALE21, and BnTALE51 were higher. According to the gene expression level under stress treatment, BnTALE genes can be divided into three clusters. Cluster I contained 17 highly expressed genes under different stresses. Cluster II consisted of 43 genes with low expression. Cluster III was comprised of 14 genes with expression levels between Cluster I and Cluster II. In Cluster I, BnTALE59, BnTALE21, and BnTALE51 had high expression levels under different stresses, especially at cold stress for 24 h, followed by BnTALE17, which also had relatively high expression levels under different stresses, indicating that they may be key genes in response to different stresses. BnTALE45 and BnTALE48 genes had relatively high expression levels under dehydration and cold stress, BnTALE7 and BnTALE43 genes were highly expressed after 8 h of dehydration and had similar expression patterns. The expression of BnTALE35 and BnTALE18 genes were higher under cold stress. Besides, the expression of BnTALE35 was higher under ABA stress for 24 h and NaCl stress for 4 h, and the expression of BnTALE18 was higher under ABA stress for 24 h and NaCl stress for 24 h. The expression patterns of BnTALE6 and BnTALE71 were similar under different stresses, and their expression levels were higher at 4 h of cold stress and 4 h of NaCl treatment. The expression patterns of BnTALE57 and BnTALE69 were also similar, and their expression were higher under 4 h of NaCl stress and 4 h of cold stress. In Cluster III, the overall expression level of the BnTALE genes were relatively low, the expressions of BnTALE1 and BnTALE38 were relatively high at 4 h of NaCl stress and the expressions of BnTALE12 and BnTALE60 were slightly higher at 4 h of cold stress. The TALE genes in Cluster II were not expressed or had very low expression levels under stresses, indicating that these genes were not involved in the stress response of B. napus. Further, qRT-PCR expression patterns of nine representative TALE genes under cold stress, dehydration stress, ABA stress, and NaCl stress in B.napus cultivar ZS11 showed that the expression profiles of the selected TALE genes were generally consistent with the results of the analysis of the previously published RNA-seq data (Fig. 9).
Fig. 8.
Heatmap of the expression of 74 TALE genes under dehydration, cold, ABA and NaCl treatment at 4 h and 24 h. Expression data were processed with log2 normalization. The color scale represents relative expression levels from low (green color) to high (red color)
Fig. 9.
qRT-PCR analysis of BnTALE genes expression under cold and osmotic stresses (dehydration, ABA and NaCl). The error bars represent standard deviations. The y-axis represents relative expression levels and x-axis represents different stresses of ZS11
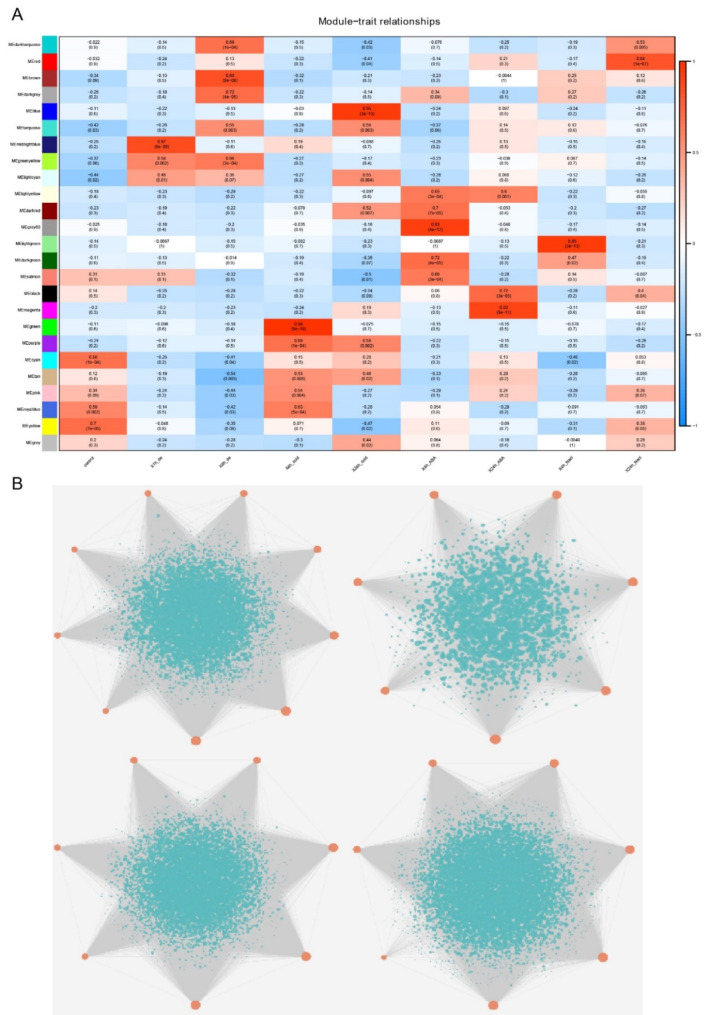
Generally, genes perform their biological functions by cooperating with a series of genes with similar expression patterns, therefore, investigating the co-expression modules associated with BnTALE could obtain a better understanding of their functions. Herein, WGCNA was performed to study the associated co-expression modules of BnTALE under abiotic stresses. A total of 25 co-expression modules were detected, and 37 BnTALE genes were clustered in these modules. Among them, 9, 7, 7, and 7 BnTALE genes were found in the brown, green, blue, and turquoise modules, respectively (Table S5). According to the relationships between modules and stresses, these four modules were significantly related to 8 h_dehydration, 4 h_cold, 24 h_cold, 8 h_dehydration, and 24 h_cold, respectively (Fig. 10). These thirty genes clustered in the four modules would be potential key genes for genetic improvement of dehydration and cold resistance in rapeseed. In the brown module, the most enriched KEGG pathways were autophagy, peroxisome, fatty acid degradation, valine, leucine, and isoleucine degradation, and biosynthesis of unsaturated fatty acids (Fig. S1A). In the green module, the most enriched KEGG pathways were circadian rhythm, flavonoid biosynthesis, stilbenoid, diarylheptanoid, gingerol biosynthesis, and vitamin B6 metabolism (Fig. S1B). In the blue module, the most enriched KEGG pathways were ribosome biogenesis in eukaryotes, citrate cycle, protein export, phagosome, and secretion system (Fig. S1C). In the turquoise module, the most enriched KEGG pathways were proteasome, GTP-binding proteins, autophagy, and SNARE interactions in vesicular transport (Fig. S1D). According to the expression heatmap (Fig. 8), cluster I was identified as highly expressed genes under different stresses, among them, BnTALE59, BnTALE21, BnTALE51, and BnTALE17 were the highest. Now with the help of WGCNA results, BnTALE59 and BnTALE21 were found in the blue module related to 24 h_cold, while BnTALE51 and BnTALE17 were in the turquoise module related to 24 h_cold and 8 h_dehydration, which would function through different pathways. These results indicated that BnTALE, as the important transcription factor, could cooperate with other genes to respond to various abiotic stresses by different pathways.
Fig. 10.
Co-expression modules under abiotic stresses identified by weight gene co-expression networks analysis (WGCNA). A. Relationship between co-expression modules and abiotic stresses. B. BnTALE genes involved in the brown, green, blue, turquoise modules. Red dots indicate TALEs and green dots indicate other co-expressed genes
Discussion
The TALE superfamily genes ubiquitously exist in plant genomes and play an important role in regulating plant growth, development, cell differentiation, and stress responses [2, 7, 32]. Till now, TALE genes have been studied in a variety of plants, however, the genome-wide identification and characterization of B. napus TALE superfamily members have not been studied.
In this study, we identified a total of 74 TALE family genes in B. napus, the number of TALE genes in the B. napus genome is approximately the same as in Triticum aestivum (70), three times that of A. thaliana (22) and rice (22) [42], and twice that of poplar [32]. We speculated that the number of gene family members correlates with the size of the genome and the degree of polyploidy, similar to the findings in soybeans [2]. Phylogenetic tree analysis showed that, the members of the B. napus TALE family can be classified into two groups, namely, the KNOX subfamily and the BELL subfamily. The KNOX subfamily can be further divided into three classes. The classification of the B. napus TALE family was consistent with the result of A. thaliana, cotton, and poplar, indicating that the amino acid sequence of the TALE family in plants is highly conserved. The TALE members in the same phylogenetic cluster of different species generally indicate their analogous biological functions, which is consistent with previous studies in soybean [2]. BnTALE39, BnTALE2, BnTALE61, and BnTALE13 clustered with AtATH1. AtATH1 has been shown to affect the growth of nutrient or reproductive organs and inhibit stem development [43]. Thus, we hypothesized that the above four TALE genes in B. napus have growth-related functions similar to AtATH1.
In the present study, BnTALE members in the same subclass or branch of the phylogenetic tree generally have similar gene structures and protein-conserved domains, which may indicate that they have similar biological functions in general and further validate our classification of the B. napus TALE family. From the physicochemical characteristics of the members of the B. napus TALE family, it can be seen that there are significant differences between the KNOX subfamily and the BELL subfamily members. The amino acid number and molecular weight of TALE members in the BELL subfamily of B. napus are much larger than those of the KNOX family, which is consistent with the characteristics of TALE members in soybean [2], cotton [7], and poplar [32].
Gene structure analysis showed that out of 74 BnTALE genes, 39 genes had 5′ UTR, and 37 genes had 3′ UTR. Given that the 5′ UTR plays a role in regulating mRNA stability and the 3′ UTR may function as a miRNA binding site, we suggest that the B. napus TALE genes exert complex regulatory properties on downstream genes [44–47]. Specific domains or motifs were reported to play important roles in DNA binding and protein interactions [48]. The Homebox KN domain is located at the C-terminal end of the protein. It is involved in DNA-binding functions and transcriptional regulation [47, 48]. The domain analysis of B. napus TALE proteins showed that 87.8% of members contained Homebox_KN domain. Specifically, all BELL subfamily members of the B. napus TALE family contain the Homebox_KN domain. With the exception of nine proteins, members of the KNOX subfamily all contain Homebox_KN domains, suggesting that B. napus TALE proteins possess DNA-binding, and potential protein-interaction functions. Previous research has shown that the ELK domain of the TALE gene family can act as a nuclear localization signal and is involved in transcriptional regulation, which is associated with transcriptional repression [49, 50]. Most members of the KNOX subfamily of the BnTALE family contain the ELK domain, whereas members of the BELL subfamily do not. TALE proteins typically function as dimers. Zhao found that in poplar, different TALE proteins can form heterodimers [32]. Yang presumed that the KNOX and BELL subfamilies of the Prunus mume TALE proteins can form heterodimers that affect early stem development [51]. In this study, most members of the KNOX subfamily of the BnTALE family contained the KNOX2 structural domain, which was considered essential for homodimerization and was critical for protein function [52]. All BELL subfamily members contain the POX domain. Studies in A. thaliana have shown that BEL1-like proteins containing the POX structural domain interact with KNAT2 and KNAT5 proteins to influence plant development [53, 54]. The potential protein interaction of the two components of the heterodimer TALE proteins can occur between TALE and non-TALE members or between members of different TALE families [55]. In this study, the PPI network analysis also indicated that some BnTALE proteins could also interact with each other, especially between the BELL subfamilies and the KNOX subfamilies.
Gene replication patterns, including tandem, fragment, and genome replication, are important factors affecting biological evolution and the amplification of different gene families in eukaryotic genomes [56]. In this study, most of the TALE genes were derived from whole-genome duplication (WGD) or segmental duplication events, suggesting that WGD/segmental duplication was the main driving force for the expansion of TALE genes in the B. napus genome. With the exception of BnTALE27 and BnTALE66 (1.7325), all BnTALE gene pairs had Ka/Ks ratios less than 1, indicating that these genes have evolved under the influence of purifying selection, which is consistent with research in sweet orange [43]. Since purification selection limited gene differentiation, it can be inferred that the duplicated TALE genes in B. napus were relatively conserved in evolution and may have similar functions [57].
Cis-element analysis of the promoter region revealed that the promoter sequence of the BnTALE genes contains several cis-elements related to hormone response, abiotic stress, and development. This was consistent with the TALE family in soybean, wheat, pomegranate [1], and cotton, indicating that the B. napus TALE genes may be associated with abiotic stress and plant development regulation. The main cis-acting elements of B. napus TALE genes are hormone response elements (509), such as ABA-responsive element (ABRE) and estrogen-responsive element (ERE), followed by environmental stress response elements. The effects of transcription factors on growth, development, and plant stress resistance are usually closely related to hormonal pathways, for example, analysis of ethylene-related gene expression models suggests that ethylene may indirectly be involved in the induction of dormancy, thereby improving cold/freeze tolerance in P. mume [58]. Previous studies have shown that the function of the TALE genes were related to the hormone pathway of plants [59]. Here, we infer that B. napus TALE genes may participate in the response to abiotic stress through the hormone pathway.
The expression profile of BnTALE genes showed that the expression of BnTALE genes were higher in roots and leaves, which may be related to the fact that the root system is an important sensory organ that responds to various abiotic stresses. Previous studies showed that sweet orange TALE genes were highly expressed in stems [60]. The majority of the PgTALE genes were expressed in pomegranate; nevertheless, distinct PgTALE genes were expressed in various tissues, indicating expression differentiation [1]. The differences in the expression positions of the TALE gene in plants may be due to species differences. The expression profiles of BnTALE genes under various abiotic stresses including NaCl, ABA, cold, and dehydration stresses revealed that BnTALE59, BnTALE21, and BnTALE51 had high expression levels under different stresses, especially at cold stress for 24 h. In this study, four co-expression modules brown, green, blue, and turquoise colors detected by WGCAN were significantly associated with dehydration and cold stress. BnTALE59 and BnTALE21 were found in the blue module, while BnTALE51 were present in the turquoise module. Thus, we infer that these genes, together with other genes in the module, are involved in certain metabolic pathways through co-expression in response to abiotic stresses. We supposed that signal-regulated pathways of plants in response to different abiotic stresses may be interrelated and the above three genes were possibly key responsive genes in different stress-specific regulatory networks. In addition, further studies on the functions of these genes should be investigated so as to provide important genetic resources for breeding for stress tolerance in B. napus.
Conclusions
In the present study, we identified 74 TALE superfamily members in the genome of B. napus. The BnTALE members were further divided into the BEL1-like subfamily and the KNOX subfamily. BnTALE members in the same subfamily or clade displayed universal similarities, indicating their analogous biological functions. Whole-genome duplication (WGD) or segmental duplications played a major role in the expansion of BnTALE superfamily. The Ka/Ks ratios indicate that the BnTALE genes have evolved under the influence of purifying selection, and it is inferred that the duplicated TALE genes in B. napus were relatively conserved in evolution and may have similar functions. Potential protein interaction analysis showed that TALE proteins were involved in response to drought, temperature, and regulation of defense response. By analyzing cis-element in gene promoter regions, combined with transcriptome data and quantitative RT-PCR investigations, several BnTALE genes such as BnTALE59, BnTALE21, and BnTALE51 have been proposed to play potential roles during B. napus development and abiotic stress responses. In addition, WGCNA analysis detected four modules what would be notably related to dehydration and cold stresses. To conclude, our work laid a foundation for the biological functions study of BnTALE genes in the future, which may provide genetic resources for the genetic improvement of B. napus and the breeding of new varieties resistant to various abiotic stresses.
Materials and methods
Genome wide identification of TALE gene family members in B. napus
The conserved TALE ELK (PF03789), KNOX1 (PF03790), KNOX2 (PF037901), POX (PF07526), Homeobox_KN (PF05920) protein domains from the Pfam website were used to build the Hidden Markov Model profiles (http://hmmer.janelia.org/) to search against the whole-genome protein database of B. napus cultivar ‘Darmor-bzh’ (the Brassicaceae Database: B. napus v4.1) [35]. The TALE gene sequences of A. thaliana were also used to search against the B. napus genome to obtain the homologous sequences in B. napus. The proteins obtained by both methods were further verified through submissions to Pfam (http://pfam.xfam.org/), NCBI-CDD (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) and Smart (http://smart.embl-heidelberg.de/) for domain prediction. After manual screening, the TALE genes for B. napus were obtained. MG2C v2 (http://mg2c.iask.in/) was used to display the location of the TALE gene on the chromosome [60]. ExPASY (https://web.expasy.org/compute_pi/) was used to analyze the isoelectric point and molecular weight of the TALE protein, and CELLO (http://cello.life.nctu.edu.tw/) was used to predict the subcellular localization of the TALE protein [61].
Construction of the B. napus TALE protein family phylogenetic tree
The TALE protein sequences of B. napus and A. thaliana were aligned together using ClustalW (http://www.clustal.org/clustal2/) [62] and a phylogenetic tree was constructed using MEGA10 (https://www.megasoftware.net/) with the neighbor-joining method and 1000 replicate iterations [63]. Evolutionary trees were decorated using Evolview (http://www.evolgenius.info/evolview/#/) [64].
TALE gene structure, domain, and promoter element analysis
BnTALE sequence information was extracted from the B. napus reference genome (the Brassicaceae Database: B. napus v4.1). Pfam (http://pfam.xfam.org/), NCBI-CDD (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) and Smart (http://smart.embl-heidelberg.de/) were used to predict the domains in the protein sequences. The locations of UTR, CDS and domains were displayed by TBtools (https://tbtools.updatestar.com/en) [65]. The 2 kb sequence upstream of TALE CDS was extracted and analyzed for cis-acting elements using PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) [66]. The pheatmap package of R was used to draw the heatmap representing the number of cis-elements.
Gene duplication events identification analysis
Duplicated genes in the TALE family of B. napus were analyzed using BLASTP and MCScanX (https://github.com/wyp1125/MCScanX? tab=readme-ov-file) [67]. The position and relationship of the duplicated genes were showed by Circos software (https://circos.ca/) [68]. The protein sequences of the duplicated gene pair were aligned by Muscle (https://link.zhihu.com/?target=https%3 A//www.drive5.com/muscle/) [69]. To evaluate the selection pressure, the Ka/Ks values were calculated with the KaKs_Calculator (https://ngdc.cncb.ac.cn/biocode/tools/BT000001) [70].
Potential protein interaction analysis
The protein interaction information for TALEs in A. thaliana was obtained by STRING (https://www.string-db.org/) and used to search the corresponding potential interaction network of B. napus TALE proteins based on the homology between A. thaliana and B. napus TALEs. The potential protein interaction relationship was displayed by Cytoscape (http://www.cytoscape.org/) [71]. KEGG and GO enrichment analysis were conducted on the genes encoding proteins that interacted with TALE proteins using the R package clusterProfiler [72].
Gene expression analysis
To investigate the expression patterns of BnTALE genes, we downloaded RNA-seq data of different tissues (root, leaf, bud, silique, stamen, pistil, blossomy petal, wilting petal, stem, sepal, ovule, and pericarp) and various abiotic stresses (dehydrate, cold, ABA, and NaCl) [36, 37]. RNA-seq reads were mapped to the B. napus genome using Hisat2 and the expression levels were calculated using Stringtie. The expression data of TALEs were extracted and displayed with the R package pheatmap.
To further uncover the critical BnTALE genes in response to abiotic stresses, weight gene co-expression network analysis (WGCNA) was performed using RNA-seq data from those four stresses. Power value was set as 8 to get the original adjacency matrix, minModuleSize and cutHeight were set as 50 and 0.25, respectively. Cytoscape (http://www.cytoscape.org/) [71] was used to visualize the interested module. Package ClusterProfile [72] in R was selected for GO and KEGG enrichment analysis.
Quantitative reverse transcription polymerase chain reaction
B. napus ZS11 seedlings were grown in a growth room at 24 °C with a 16/8 h light/dark photoperiod. The leaves, stems and roots were collected from 20-day-old seedlings, buds were collected from 70-day-old seedlings, and siliques were harvested 90 days after germination. For cold and NaCl stresses treatment research, leaf samples from three weeks old plants of B. napus ZS11 were collected at 1 h and 8 h after dehydration while 4 h and 24 h of ABA (25 µM), NaCl (200 mM), and cold (4 °C) treatment, as described in [37]. Samples were stored in liquid nitrogen immediately after collection. Total RNA was extracted using the TRIzol reagent (Invitrogen, 15596026, USA) according to the product manual. Reverse transcription was performed using the PrimeScript RT Reagent Kit with gDNA Eraser (Takara, Japan). The relative expression of BnTALE genes was quantified using quantitative real time-PCR (qRT-PCR) on the CFX96 Real-time PCR System using gene-specific primers (Table S6). The SYBR Green Real-time PCR Master Mix was used for the qRT-PCR (Bio-Rad, USA). The internal standard was the B. napus histone gene. The PCR program was as follows: 95 °C for 30 s followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. All assays were carried out for three biological repeats, each with three technical repeats. The quantification methods used for the expression of BnTALE genes in different tissues and under different stresses were 2 −△CT and 2 −△△CT [73], respectively.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Additional file 1: Figure S1 KEGG pathway enrichment of module brown (A), green (B), blue (C), and turquoise (D).
Supplementary Material 2: Additional file 2: Table S1 Numbers of cis-acting elements in the promoter of B. napus TALEs.
Supplementary Material 3: Additional file 3: Table S2 Ka/Ks analysis for paralogous gene pairs of B. napus TALEs.
Supplementary Material 4: Additional file 4: Table S3 GO enrichment of proteins interacting with B. napus TALEs.
Supplementary Material 5: Additional file 5: Table S4 KEGG pathway enrichment of proteins interacting with B. napus TALEs.
Supplementary Material 6: Additional file 6: Table S5 Gene names and IDs of four modules.
Supplementary Material 7: Additional file 7: Table S6 qRT-PCR primer sequence for the three-amino-acid-loop-extension (TALE) genes in B. napus.
Acknowledgements
Not applicable.
Author contributions
Meili Xie analyzed the data and provided manuscript preparation and editing. Xiaojuan Zhang performed the experiments and manuscript preparation. Kexin Liu performed part of the experiments. Zhixian Qiao provided data analysis assistance. Xiaohui Cheng designed the research and modified this manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (32300559, 32370693), Shaanxi Provincial Department of Science and Technology Project (2023-YBNY-070), Shaanxi University of Technology talent startup project (SLGRCQD2115), the Natural Science Foundation of Hubei Province (2023AFB433), the Natural Science Foundation of Wuhan (2024040801020314) and the Innovation Program of Chinese Academy of Agricultural Sciences (CAAS-CSIAF-202402).
Data availability
The datasets supporting the conclusions of this study are available within the article and its supplementary materials.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meili Xie and Xiaojuan Zhang contributed equally to this work.
References
- 1.Wang Y, Zhao Y, Yan M, Zhao H, Zhang X, Yuan Z. Genome-wide identification and expression analysis of TALE gene family in pomegranate (Punica granatum L). Agronomy. 2020;10:829. [Google Scholar]
- 2.Wang L, Yang X, Gao Y, Yang S. Genome-wide identification and characterization of TALE superfamily genes in soybean (Glycine max L). Int J Mol Sci. 2021;22:4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin J, Tian F, Yang DC, Meng YQ, Kong L, Luo J, Gao G. PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017;45:D1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bürglin TR. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997;25:4173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hay A, Tsiantis M. KNOX genes: versatile regulators of plant development and diversity. Development. 2010;137:3153–65. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Rosin FM, Prat S, Hannapel DJ. Interacting transcription factors from the three-amino acid loop extension superclass regulate tuber formation. Plant Physiol. 2003;132:1391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Q, Wang N, Hao P, Sun H, Wang C, Ma L, Wang H, Zhang X, Wei H, Yu S. Genome-wide identification and characterization of TALE superfamily genes in cotton reveals their functions in regulating secondary cell wall biosynthesis. BMC Plant Biol. 2019;19:432. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Gao J, Yang X, Zhao W, Lang T, Samuelsson T. Evolution, diversification, and expression of KNOX proteins in plants. Front Plant Sci. 2015;6:882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bürglin TR. The PBC domain contains a MEINOX domain: coevolution of Hox and TALE homeobox genes? Dev Genes Evol. 1998;208:113–6. [DOI] [PubMed] [Google Scholar]
- 10.Vollbrecht E, Veit B, Sinha N, Hake S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature. 1991;350:241–3. [DOI] [PubMed] [Google Scholar]
- 11.Bellaoui M, Pidkowich MS, Samach A, Kushalappa K, Kohalmi SE, Modrusan Z, Crosby WL, Haughn GW. The Arabidopsis BELL1 and KNOX TALE homeodomain proteins interact through a domain conserved between plants and animals. Plant Cell. 2001;13:2455–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatt A, Etchells J, Canales C, Lagodienko A, Dickinson H. VAAMANA–a BEL1-like homeodomain protein, interacts with KNOX proteins BP and STM and regulates inflorescence stem growth in Arabidopsis. Gene. 2004;328:103–11. [DOI] [PubMed] [Google Scholar]
- 13.Kim D, Cho YH, Ryu H, Kim Y, Kim TH, Hwang I. BLH1 and KNAT3 modulate ABA responses during germination and early seedling development in Arabidopsis. Plant J. 2013;75:755–66. [DOI] [PubMed] [Google Scholar]
- 14.Smith HM, Boschke I, Hake S. Selective interaction of plant homeodomain proteins mediates high DNA-binding affinity. Proc Natl Acad Sci U S A. 2002;99:9579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee K, Brocchieri L, Bürglin TR. A comprehensive classification and evolutionary analysis of plant homeobox genes. Mol Biol Evol. 2009;26:2775–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Zhao P, Cheng B, Zhang Y, Shen Y, Wang X, Zhang Q, Lou Q, Zhang S, Wang B, Qi S, Li Y, Islam MM, Muhammad T, Zhang F, Liang Y. Identification of TALE Transcription factor family and expression patterns related to fruit chloroplast development in tomato (Solanum lycopersicum L). Int J Mol Sci. 2022;23:4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li E, Bhargava A, Qiang W, Friedmann MC, Forneris N, Savidge RA, Johnson LA, Mansfield SD, Ellis BE, Douglas CJ. The Class II KNOX gene KNAT7 negatively regulates secondary wall formation in Arabidopsis and is functionally conserved in Populus. New Phytol. 2012;194:102–15. [DOI] [PubMed] [Google Scholar]
- 18.Zhong R, Lee C, Zhou J, McCarthy RL, Ye ZH. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell. 2008;20:2763–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhargava A, Mansfield SD, Hall HC, Douglas CJ, Ellis BE. MYB75 functions in regulation of secondary cell wall formation in the Arabidopsis inflorescence stem. Plant physiol. 2010;154:1428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li E, Wang S, Liu Y, Chen J, Douglas CJ. OVATE FAMILY PROTEIN4 (OFP4) interaction with KNAT7 regulates secondary cell wall formation in Arabidopsis thaliana. Plant J. 2011;67:328–41. [DOI] [PubMed] [Google Scholar]
- 21.Byrne ME, Groover AT, Fontana JR, Martienssen RA. Phyllotactic pattern and stem cell fate are determined by the Arabidopsis homeobox gene BELLRINGER. Development. 2003;130:3941–50. [DOI] [PubMed] [Google Scholar]
- 22.Meng L, Fan Z, Zhang Q, Wang C, Gao Y, Deng Y, Zhu B, Zhu H, Chen J, Shan W, Yin X, Zhong S, Grierson D, Jiang CZ, Luo Y, Fu DQ. BEL1-LIKE HOMEODOMAIN 11 regulates chloroplast development and chlorophyll synthesis in tomato fruit. Plant J. 2018;94:1126–40. [DOI] [PubMed] [Google Scholar]
- 23.Avivi Y, Lev-Yadun S, Morozova N, Libs L, Williams L, Zhao J, Varghese G, Grafi G. Clausa, a tomato mutant with a wide range of phenotypic perturbations, displays a cell type-dependent expression of the homeobox gene LeT6/TKn2. Plant physiol. 2000;124:541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shu Y, Tao Y, Wang S, Huang L, Yu X, Wang Z, Chen M, Gu W, Ma H. GmSBH1, a homeobox transcription factor gene, relates to growth and development and involves in response to high temperature and humidity stress in soybean. Plant Cell Rep. 2015;34:1927–37. [DOI] [PubMed] [Google Scholar]
- 25.Rutjens B, Bao D, van Eck-Stouten E, Brand M, Smeekens S, Proveniers M. Shoot apical meristem function in Arabidopsis requires the combined activities of three BEL1-like homeodomain proteins. Plant J. 2009;58:641–54. [DOI] [PubMed] [Google Scholar]
- 26.Pautot V, Dockx J, Hamant O, Kronenberger J, Grandjean O, Jublot D, Traas J. KNAT2: evidence for a link between knotted-like genes and carpel development. Plant Cell. 2001;13:1719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B, Wang L, Zhang J, Li J, Zheng H, Chen J, Lu M. WUSCHEL-related homeobox genes in Populus tomentosa: diversified expression patterns and a functional similarity in adventitious root formation. BMC Genomics. 2014;15:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petzold HE, Chanda B, Zhao C, Rigoulot SB, Beers EP, Brunner AM. DIVARICATA AND RADIALIS INTERACTING FACTOR (DRIF) also interacts with WOX and KNOX proteins associated with wood formation in Populus trichocarpa. Plant J. 2018;93:1076–87. [DOI] [PubMed] [Google Scholar]
- 29.Yu Y. OsKNAT7 bridges secondary cell wall formation and cell growth regulation. Plant Physiol. 2019;181:385–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao S, Wang Y, Yan Y, Liu Y, Wang J, Chen S. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae. 2021;7:132. [Google Scholar]
- 31.Bolduc N, Yilmaz A, Mejia-Guerra MK, Morohashi K, O’Connor D, Grotewold E, Hake S. Unraveling the KNOTTED1 regulatory network in maize meristems. Genes Dev. 2012;26:1685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao K, Zhang X, Cheng Z, Yao W, Li R, Jiang T, Zhou B. Comprehensive analysis of the three-amino-acid-loop-extension gene family and its tissue-differential expression in response to salt stress in poplar. Plant Physiol Biochem. 2019;136:1–12. [DOI] [PubMed] [Google Scholar]
- 33.Song X, Zhao Y, Wang J, Lu MZ. The transcription factor KNAT2/6b mediates changes in plant architecture in response to drought via down-regulating GA20ox1 in Populus alba × P. glandulosa. J Exp Bot. 2021;72:5625–37. [DOI] [PubMed] [Google Scholar]
- 34.Han Y, Zhang L, Yan L, Xiong X, Wang W, Zhang XH, Min DH. Genome-wide analysis of TALE superfamily in Triticum aestivum reveals TaKNOX11-A is involved in abiotic stress response. BMC Genomics. 2022;23:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chalhoub B, Denoeud F, Liu S, et al. Plant genetics. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science. 2014;345:950–3. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Dong C, Hu M, Bai Z, Tong C, Zuo R, Liu Y, Cheng X, Cheng M, Huang J, Liu S. Identification of flower-specific promoters through comparative transcriptome analysis in Brassica napus. Int J Mol Sci. 2019;20:5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Ali U, Zhang G, Yu L, Fang S, Iqbal S, Li H, Lu S, Guo L. Transcriptome analysis reveals genes commonly responding to multiple abiotic stresses in rapeseed. Mol Breeding. 2019;39:158. [Google Scholar]
- 38.Waadt R, Seller CA, Hsu PK, Takahashi Y, Munemasa S, Schroeder JI. Plant hormone regulation of abiotic stress responses. Nat Rev Mol Cell Biol. 2022;23(10):680–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hwang K, Susila H, Nasim Z, Jung JY, Ahn JH. Arabidopsis ABF3 and ABF4 transcription factors act with the NF-YC complex to regulate SOC1 expression and mediate drought-accelerated flowering. Mol Plant. 2019;12(4):489–505. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Dong C, Hu M, Bai Z, Tong C, Zuo R, Liu Y, Cheng X, Cheng M, Huang J, Liu S. Identification of flower-specific promoters through comparative transcriptome analysis in Brassica napus. Int J Mol Sci. 2019;20(23):5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang YT, Ali U, Zhang GF, Yu LQ, Fang S, Lqbal S, Li HH, Lu SP, Guo L. Transcriptome analysis reveals genes commonly responding to multiple abiotic stresses in rapeseed. Mol Breed. 2019;39(11):1–19. [Google Scholar]
- 42.Han Y, Zhang L, Yan L, Xiong X, Wang W, Zhang XH, Min DH. Genome-wide analysis of TALE superfamily in Triticum aestivum reveals TaKNOX11-A is involved in abiotic stress response. BMC Genomics. 2022;23(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng W, Yang Y, Xu J, Peng E, Dai S, Dai L, Wang Y, Yi T, Wang B, Li D, Song N. TALE transcription factors in sweet orange (Citrus sinensis): Genome-wide identification, characterization, and expression in response to biotic and abiotic stresses. Front Plant Sci. 2021;12:814252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng Y, Soper TJ, Woodson SA. RNase eootprinting of protein binding sites on an mRNA target of small RNAs. Methods Mol Biol. 2012;905:213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuster SL, Hsieh AC. The untranslated regions of mrnas in cancer. Trends Cancer. 2019;5(4):245–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orso F, Quirico L, Dettori D, Coppo R, Virga F, Ferreira LC, Paoletti C, Baruffaldi D, Penna E, Taverna D. Role of miRNAs in tumor and endothelial cell interactions during tumor progression. Semin Cancer Biol. 2020;60:214–24. [DOI] [PubMed] [Google Scholar]
- 47.Rykova E, Ershov N, Damarov I, Merkulova T. SNPs in 3’UTR miRNA target sequences associated with individual drug susceptibility. Int J Mol Sci. 2022;23(22):13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu L, White MJ, MacRae TH. Transcription factors and their genes in higher plants functional domains, evolution and regulation. Eur J Biochem. 1999;262:247–57. [DOI] [PubMed] [Google Scholar]
- 49.Kerstetter R, Vollbrecht E, Lowe B, Veit B, Yamaguchi J, Hake S. Sequence analysis and expression patterns divide the maize knotted1-like homeobox genes into two classes. Plant cell. 1994;6:1877–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scofield S, Murray JA. KNOX gene function in plant stem cell niches. Plant Mol Biol. 2006;60:929–46. [DOI] [PubMed] [Google Scholar]
- 51.Yang Q, Yuan C, Cong T, Wang J, Zhang Q. Genome-wide identification of three-amino-acid-loop-extension gene family and their expression profile under hormone and abiotic stress treatments during stem development of Prunus mume. Front Plant Sci. 2022;13:1006360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagasaki H, Sakamoto T, Sato Y, Matsuoka M. Functional analysis of the conserved domains of a rice KNOX homeodomain protein, OSH15. Plant Cell. 2001;13:2085–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamant O, Pautot V. Plant development: a TALE story. C R Biol. 2010;333:371–81. [DOI] [PubMed] [Google Scholar]
- 54.Kumar R, Kushalappa K, Godt D, Pidkowich MS, Pastorelli S, Hepworth SR, Haughn GW. The Arabidopsis BEL1-LIKE HOMEODOMAIN proteins SAW1 and SAW2 Act redundantly to regulate KNOX expression spatially in leaf margins. Plant Cell. 2007;19:2719–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hudry B, Thomas-Chollier M, Volovik Y, Duffraisse M, Dard A, Frank D, Technau U, Merabet S. Molecular insights into the origin of the Hox-TALE patterning system. Elife. 2014;3:e01939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng W, Li W, Song N, Tang Z, Liu J, Wang Y, Pan S, Dai L, Wang B. Genome-wide characterization, evolution, and expression profile analysis of GATA transcription factors in Brachypodium distachyon. Int J Mol Sci. 2021;22:2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu W, Li W, He Q, Daud MK, Chen J, Zhu S. Genome-wide survey and expression analysis of calcium-dependent protein kinase in Gossypium raimondii. PLoS ONE. 2014;9:e98189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li P, Zheng T, Zhuo X, Zhang M, Yong X, Li L, Wang J, Cheng T, Zhang Q. Photoperiod- and temperature-mediated control of the ethylene response and winter dormancy induction in Prunus mume. Hortic Plant J. 2021;7:232–42. [Google Scholar]
- 59.Zhang X, Jiang J, Yang Y, Ma Z, Meng L, Cui G, Yin X. Identification and responding to exogenous hormone of HB-KNOX family based on transcriptome data of Caucasian clover. Gene. 2022;828:146469. [DOI] [PubMed] [Google Scholar]
- 60.Chao J, Li Z, Sun Y, Aluko OO, Wu X, Wang Q, Liu G. MG2C: a user-friendly online tool for drawing genetic maps. Mol Hortic. 2021;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu CS, Chen YC, Lu CH, Hwang JK. Prediction of protein subcellular localization. Proteins Struct Funct Bioinform. 2006;64:643–51. [DOI] [PubMed] [Google Scholar]
- 62.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23:2947–8. [DOI] [PubMed] [Google Scholar]
- 63.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He Z, Zhang H, Gao S, Lercher MJ, Chen WH, Hu S. Evolview v2: an online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 2016;44:W236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13:1194–202. [DOI] [PubMed] [Google Scholar]
- 66.Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, Lee TH, Jin H, Marler B, Guo H, Kissinger JC, Paterson AH. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang D, Zhang Y, Zhang Z, Zhu J, Yu J. KaKs_Calculator 2.0: a toolkit incorporating gamma-series methods and sliding window strategies. Genomics Proteom Bioinf. 2010;8:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2–∆∆CT method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Additional file 1: Figure S1 KEGG pathway enrichment of module brown (A), green (B), blue (C), and turquoise (D).
Supplementary Material 2: Additional file 2: Table S1 Numbers of cis-acting elements in the promoter of B. napus TALEs.
Supplementary Material 3: Additional file 3: Table S2 Ka/Ks analysis for paralogous gene pairs of B. napus TALEs.
Supplementary Material 4: Additional file 4: Table S3 GO enrichment of proteins interacting with B. napus TALEs.
Supplementary Material 5: Additional file 5: Table S4 KEGG pathway enrichment of proteins interacting with B. napus TALEs.
Supplementary Material 6: Additional file 6: Table S5 Gene names and IDs of four modules.
Supplementary Material 7: Additional file 7: Table S6 qRT-PCR primer sequence for the three-amino-acid-loop-extension (TALE) genes in B. napus.
Data Availability Statement
The datasets supporting the conclusions of this study are available within the article and its supplementary materials.