Abstract
Background
Vitamin D (VD) plays a critical role in regulating systemic inflammation, but its correlation with the systemic immune-inflammatory index (SII) remains unclear. This study aimed to explore the relationship between serum VD concentration, dietary VD intake, and SII using data from the National Health and Nutrition Examination Survey (NHANES).
Methods
Data from NHANES 2007–2018 and NHANES 2007–2020 were analyzed for serum VD levels and dietary VD intake, respectively. Restricted cubic splines (RCS) and logistic regression were used to assess associations between VD and SII. Mediation analysis was conducted to evaluate the role of SII in VD-related disease outcomes and mortality.
Results
Serum VD concentration exhibited a U-shaped correlation with SII (P-overall = 0.005; P-non-linear = 0.002). Severe VD deficiency significantly elevated SII levels compared to insufficiency or sufficiency groups. No association was observed between dietary VD intake and SII. Mediation analysis revealed that SII mediated the effects of VD on all-cause and cardiovascular disease-related mortality, but not on cancer, hypertension, or diabetes development.
Conclusion
A U-shaped relationship exists between serum VD and SII, with VD supplementation potentially reducing systemic inflammation and improving cardiovascular outcomes. Future studies should explore VD’s role in systemic inflammation and its clinical implications.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-21133-0.
Keywords: Vitamin D, Systemic immune-inflammatory index, NHANES, U-shaped correlation, Cardiovascular mortality
Background
Vitamin D (VD), a fat-soluble secosteroid, is well-recognized for its critical role in bone health, including regulating calcium and phosphate homeostasis to promote bone mineralization [1, 2].This vitamin can promote the absorption of calcium and phosphate in the intestines and renal tubules, further increasing the levels of these microelements in the blood; VD also works synergistically with parathyroid hormone and calcitonin to promote bone matrix calcification, thereby strengthening the bones [3, 4].
Beyond its classical roles, VD has garnered considerable attention for its regulatory effects on the immune system and inflammation. Acting through the vitamin D receptor, VD modulates gene expression pathways that influence immune cell function, inflammatory cytokine production, and systemic inflammation [5–7]. Deficiency in VD has been linked to numerous diseases, including cardiovascular disorders, diabetes, and cancer, where inflammation plays a pivotal role in disease progression [8, 9].
In recent years, the role of inflammation in the occurrence and development of diseases has been continuously recognized in both local and systemic inflammation. Among them, the level of systemic inflammation mainly depends on the proportion and function of various immune cells in peripheral blood. Systemic inflammation can represent the ability to generate and maintain the immune response, and is associated with the prognosis of cancer, cardiovascular diseases, and respiratory diseases [10–13]. For decades, researchers have been committed to exploring indicators to evaluate systemic inflammation, including conventional single indicators such as C-reactive protein (CRP), PCT, and IL1 and innovative combined factors such as the neutrophil/lymphocyte ratio, the monocyte/lymphocyte ratio, and the systemic inflammation response index.
The systemic immune-inflammatory index (SII), a composite marker derived from platelet, neutrophil, and lymphocyte counts, has emerged as a robust and clinically relevant indicator of systemic inflammation [14]. SII has demonstrated strong predictive power in evaluating disease prognosis, severity, and survival, particularly in cancers and cardiovascular diseases [15–17]. Despite the established roles of VD in immune regulation and SII in reflecting systemic inflammation, the precise relationship between VD levels and SII remains poorly understood.
Existing studies exploring the link between VD and inflammation often focus on single inflammatory markers, such as CRP or ILs, with inconsistent findings. For instance, while some research suggests that lower VD levels correlate with elevated inflammatory markers, others report non-linear or negligible associations [18, 19]. The limited scope of these studies, which fail to capture the complexity of systemic inflammation, highlights a significant research gap. The potential U-shaped relationship between VD and inflammation, where both deficiency and excess of VD may drive inflammatory processes, has been hypothesized but remains inadequately explored.
Investigating the association between serum VD and SII, a comprehensive indicator of systemic inflammation, can provide deeper insights into the interplay between VD status and inflammatory pathways. Moreover, understanding whether SII mediates the effects of VD on disease outcomes and mortality can shed light on VD’s broader role in chronic disease management and prevention. Using data from the National Health and Nutrition Examination Survey (NHANES), a large, representative database, this study aims to address these gaps by systematically analyzing the relationship between serum VD concentration, dietary VD intake, and SII.
By clarifying the dose-response relationship and potential mediating role of SII, this study contributes to the growing body of literature on VD and inflammation. The findings have implications for public health policies and clinical practices, particularly regarding VD supplementation strategies to mitigate systemic inflammation and improve disease outcomes.
Methods
Data selection and study design
NHANES is a nationwide public database focusing on the health and nutrition status of the US population (https://www.cdc.gov/nchs/nhanes/index.htm), involving demographic data, dietary data, laboratory data, and questionnaire data. The protocols of the NHANES study were authorized by the Research Ethics Review Board of the National Center for Health Statistics (NCHS), and participants signed informed consent forms. We selected individuals with complete serum VD (NHANES 2007–2018) or dietary VD intake (NHANES 2007–2020) data to analyze the correlation between serum VD (or VD intake) and SII. The SII was calculated as follows: platelet count × neutrophil count / lymphocyte count. Specimen collection and processing adhered to the NHANES Laboratory Procedures Manual. Complete blood count analysis was conducted using the Beckman Coulter DxH 800 in the NHANES mobile examination center. The Coulter method reliably counts and sizes cells by detecting changes in electrical resistance as each particle (e.g., a cell) in a conductive solution passes through a narrow aperture.
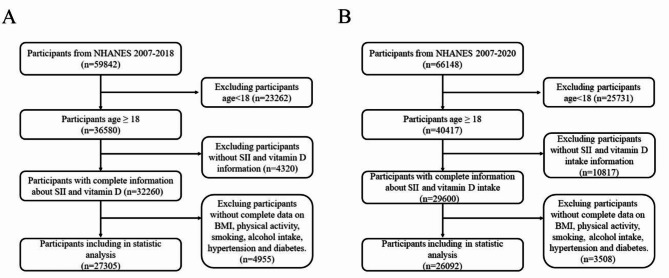
Exclusion criteria for this part included: (a) age under 18 years; (b) lack of data on serum VD or dietary VD intake; (c) lack of data for calculating SII; (d) lack of data on covariates, including BMI, physical activity, smoking history, alcohol consumption history, hypertension history, and diabetes history. The corresponding research flow chart is shown in Fig. 1.
Fig. 1.
Flow chart for study population selection. (A) Flow chart for serum vitamin D analysis; (B) Flow chart for dietary VD intake analysis
Measurement of serum vitamin D and vitamin D intake
In this study, serum VD concentrations were measured as the total VD concentration, calculated by VD2 and VD3. High-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) conducted by the National Center for Environmental Health was used to measure the total VD levels. Vitamin D levels were assessed using a single baseline serum sample collected from participants in the NHANES study. The samples were processed, stored at − 30 °C, and sent to the Nutritional Biomarkers Branch of the Division of Laboratory Sciences at the National Center for Environmental Health. Researchers quantified vitamin D concentration by measuring total serum 25-hydroxyvitamin D [25(OH)D], which includes both 25(OH)D2 and 25(OH)D3 components. More detailed information on the 25(OH)D measurement method can be found on the NHANES website. Serum VD levels were divided into four groups based on the Endocrine Society Clinical Practice guidelines: severe deficiency group, deficiency group, insufficiency group, and sufficiency group [20].
The data on dietary VD intake were obtained from two 24-hour diet recall interviews in NHANES. According to the dietary VD Recommended Nutrient Intakes (RNIs) from the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO), individuals with a vitamin D intake ≤ 5.00 µg/d were considered as a reference group, and those with a vitamin D intake > 5.00 µg/d were further divided into two groups based on the median number (9.90 µg/d) [21].
Based on serum VD levels, 27,305 individuals were divided into four groups: 1,243 in the severe deficiency group (VD ≤ 25 nmol/L), 7,482 in deficiency group (25 < VD ≤ 50 nmol/L), 10,030 in insufficiency group (50 < VD ≤ 75 nmol/L), and 8,550 in sufficiency group (VD > 75 nmol/L).
Assessment of covariates
Based on previous reports, parameters that might affect SII were included as covariates in this study, including sex, age, race, BMI, physical activity, smoking history, alcohol consumption history, hypertension history, and diabetes history. Some covariates groupings were as follows: (a) Sex (male, female); (b) Race (Non-Hispanic white, Mexican American, Other Hispanic, Non-Hispanic black, and Other races); (c) Smoking history (Never smoke, Quit smoke, Current smokers); (d) Alcohol history (No, Yes); (e) Physical activity (Sedentary, Insufficient, Moderate, and High); (f) Hypertension history (No, Yes); (g) Diabetes history (No, Yes). Body mass index (BMI) was calculated as weight (kg)/height (m2). Physical activity was defined as total metabolic equivalent (MET) minutes per week according to all physical activity questions. MET scores were calculated based on the PAQ files from the NHANES database. The PAQ files provided suggested MET scores for each type of activity and the duration per week that each activity type was performed. Leisure-time physical activity was categorized into 4 groups based on MET-minutes per week: sedentary (MET = 0), insufficient (0 < MET ≤ 500), moderate (500 < MET ≤ 1000), and high (MET > 1000).
Statistical methods
The data for this study were derived from the National Health and NHANES 2007–2018 and 2007–2020 datasets, which are publicly available and include a wide range of demographic, laboratory, and survey data. All statistical analyses were performed using R version 4.3.1 and the “mediation” package for mediation analysis. A significance level of p < 0.05 (two-sided) was considered statistically significant.
Descriptive statistics
Continuous variables, such as age and body mass index (BMI), were expressed as mean ± standard deviation (SD). Categorical variables, including sex, smoking history, and hypertension status, were presented as counts and percentages. The baseline characteristics of the study population were compared across the serum VD concentration and dietary VD intake groups using ANOVA for continuous variables and Chi-square tests for categorical variables.
Association between vitamin D and SII
The relationship between serum VD concentration and SII was assessed using restricted cubic splines (RCS) to model the non-linear association. RCS is a flexible approach that allows for the detection of both linear and non-linear relationships between continuous variables and outcomes. The statistical significance of the overall association and the non-linear component was tested using likelihood ratio tests.
For examining the association between VD and SII, logistic regression models were applied, adjusting for potential confounders. These models were run in the following steps:
Model 1 adjusted for age, gender, race, and BMI. Model 2 adjusted for age, gender, race, BMI, smoking history, alcohol consumption, hypertension history, diabetes history, and physical activity.
Regression coefficients (β) along with their 95% confidence intervals (CIs) were reported for each group based on serum VD levels and dietary VD intake categories.
Mediation analysis
To assess whether SII mediated the association between VD and disease outcomes or mortality, mediation analysis was conducted using the “mediation” package in R. This package implements a counterfactual framework for causal inference, decomposing the direct and indirect effects of VD on health outcomes through the mediator (SII). The bootstrap method was used to estimate the confidence intervals for the Average Causal Mediation Effect (ACME) and Average Direct Effect (ADE) to assess the strength and significance of mediation.
Sensitivity analyses
To assess the robustness of the findings, sensitivity analyses were conducted using different model specifications, including testing for interactions between VD and key covariates such as age, BMI, and physical activity. Stratified analyses were also performed to assess the relationship between VD and SII in specific subgroups, such as males and females, and individuals with and without hypertension or diabetes.
Results
Baseline characteristics of participants
As shown in Table 1, a total of 27,305 individuals were selected to analyze the association between serum VD and SII. The cohort included 13,512 men and 13,793 women, with an average age and BMI of 48.96 ± 18.13 years and 29.22 ± 7.00 kg/m2, respectively. Among these individuals, 4,215 (15.4%) were Non-Hispanic White, 2,886 (10.6%) were Mexican American, 11,530 (42.2%) were Other Hispanic, 5,573 (20.4%) were Non-Hispanic Black, and 3,101 (11.4%) were of Other races. Non-smokers accounted for 14,641 (56.1%), non-drinkers for 8,664 (33.2%), individuals without hypertension for 15,879 (58.2%), and those without diabetes for 23,618 (86.5%) in this cohort.
Table 1.
Baseline characteristics of participants for serum VD analysis
| Characteristic | Serum Vitamin D, nmol/L | P-value | |||||
|---|---|---|---|---|---|---|---|
| overall | Vitamin D ≤ 25 | 25 < Vitamin D ≤ 50 | 50 < Vitamin D ≤ 75 | 75 ≤ Vitamin D | |||
| Weighted, n | 189,897,106 | 5,601,117 | 39,817,868 | 70,387,178 | 74,090,943 | ||
| Age [year], mean (SD) | 47.12 (17.20) | 41.90 (16.43) | 42.77 (16.23) | 44.99 (16.49) | 51.89 (17.30) | < 0.001 | |
| Gender, n (%) | Male | 93,035,099 (49.0) | 2,431,945 (43.4) | 20,222,488 (50.8) | 38,427,096 (54.6) | 31,953,571 (43.1) | < 0.001 |
| Female | 96,862,007 (51.0) | 3,169,173 (56.6) | 19,595,381 (49.2) | 31,960,082 (45.4) | 42,137,372 (56.9) | ||
| Race, n (%) | Non-Hispanic white | 16,424,525 (8.6) | 787,421 (14.1) | 6,284,790 (15.8) | 7,111,194 (10.1) | 2,241,120 (3.0) | < 0.001 |
| Mexican American | 11,116,248 (5.9) | 318,710 (5.7) | 3,276,682 (8.2) | 5,230,178 (7.4) | 2,290,678 (3.1) | ||
| Other Hispanic | 128,467,767 (67.7) | 1,277,153 (22.8) | 16,902,315 (42.4) | 47,552,072 (67.6) | 62,736,226 (84.7) | ||
| Non-Hispanic black | 19,840,704 (10.4) | 2,670,587 (47.7) | 9,183,226 (23.1) | 5,147,745 (7.3) | 2,839,145(3.8) | ||
| Other races | 14,047,863 (7.4) | 547,246 (9.8) | 4,170,855 (10.5) | 5,345,989 (7.6) | 3,983,774 (5.4) | ||
| BMI [kg/m2], mean (SD) | 29.08 (6.88) | 31.55 (9.27) | 30.69 (7.74) | 29.26 (6.70) | 27.86 (6.07) | < 0.001 | |
| Smoke status, n (%) | Never smoke | 105,785,024 (55.7) | 3,157,358 (56.4) | 23,250,806 (58.4) | 38,334,112 (54.5) | 41,042,747 (55.4) | < 0.001 |
| Quit smoke | 47,083,831 (24.8) | 839,015 (15.0) | 7,218,942 (18.1) | 17,782,286 (25.3) | 21,243,588 (28.7) | ||
| Current smokers | 37,028,252 (19.5) | 1,604,745 (28.7) | 9,348,120 (23.5) | 14,270,780 (20.3) | 11,804,607 (15.9) | ||
| Alcohol intake, n (%) | No | 50,901,454 (26.8) | 1,865,146(33.3) | 12,102,234 (30.4) | 18,417,626 (26.2) | 18,516,447 (25.0) | < 0.001 |
| Yes | 138,995,653 (73.2) | 3,735,971 (66.7) | 27,715,634 (69.6) | 51,969,552 (73.8) | 55,574,495 (75.0) | ||
| Physical activity, n (%) | Sedentary | 69,368,430 (36.5) | 2,847,223 (50.8) | 17,057,312 (42.8) | 25,437,579 (36.1) | 24,026,317 (32.4) | < 0.001 |
| Insufficient | 30,074,727 (15.8) | 788,743 (14.1) | 6,476,954(16.3) | 10,917,873 (15.5) | 11,891,157 (16.0) | ||
| Moderate | 25,686,199 (13.5) | 586,033 (10.5) | 4,616,010 (11.6) | 9,483,252 (13.5) | 11,000,906 (14.8) | ||
| High | 64,767,750 (34.1) | 1,379,119 (24.6) | 11,667,593 (29.3) | 24,548,475 (34.9) | 27,172,564 (36.7) | ||
| Hypertension, n (%) | No | 119,123,233 (62.7) | 3,340,112 (59.6) | 25,465,387 (64.0) | 46,855,350 (66.6) | 43,462,386 (58.7) | < 0.001 |
| Yes | 70,773,873 (37.3) | 2,261,006 (40.4) | 14,352,482 (36.0) | 23,531,829 (33.4) | 30,628,557 (41.3) | ||
| Diabetes, n (%) | No | 170,817,162 (90.0) | 4,919,659 (87.8) | 35,549,482 (89.3) | 64,204,457 (91.2) | 66,143,565 (89.3) | < 0.001 |
| Yes | 19,079,945 (10.0) | 681,458 (12.2) | 4,268,387 (10.7) | 6,182,722 (8.8) | 7,947,378 (10.7) | ||
Based on serum VD levels, 27,305 individuals were divided into four groups. Differences in covariates among the groups were shown in Table 1, including age, sex, race, BMI, smoking history, alcohol consumption history, hypertension history, and diabetes history.
As shown in Table 2, a total of 26,092 individuals were selected to analyze the association between dietary VD intake and the SII. This cohort included 12,670 men and 13,422 women, with an average age of 49.25 ± 17.99 years and an average BMI of 29.43 ± 7.11 kg/m², respectively. Among these individuals, 3,747 (14.4%) were Non-Hispanic White, 2,703 (10.4%) were Mexican American, 11,310 (43.3%) were Other Hispanic, 5,641 (21.6%) were Non-Hispanic Black, and 2,691 (10.3%) were of Other races. Non-smokers accounted for 14,641 (56.1%), non-drinkers for 8,664 (33.2%), individuals without hypertension for 15,419 (59.1%), and those without diabetes for 22,557 (86.5%) in this cohort. Based on dietary VD intake levels, 26,092 individuals were divided into three groups: 12,466 (VD intake ≤ 5.00 µg/d), 6,816 (5.00 < VD intake ≤ 9.90 µg/d), and 6,810 (VD intake > 9.90 µg/d). Differences in covariates among the groups were also shown in Table 2.
Table 2.
Baseline characteristics of participants for dietary VD intake analysis
| Characteristic | Vitamin D intake, ug/d | P-value | ||||
|---|---|---|---|---|---|---|
| overall | VD Intake ≤ 5.00 | 5.00 < VD Intake ≤ 9.90 | VD Intake>9.90 | |||
| Weighted, n | 205,638,253 | 93,550,829 | 52,572,481 | 59,514,944 | ||
| Age [year], mean (SE) | 47.15 (17.23) | 43.25 (16.25) | 46.48 (17.11) | 53.86 (16.82) | < 0.001 | |
| Gender, n (%) | Male | 99,616,081 (48.4) | 45,188,934 (48.3) | 28,201,841 (53.6) | 26,225,306 (44.1) | < 0.001 |
| Female | 106,022,173 (51.6) | 48,361,895 (51.7) | 24,370,640 (46.4) | 33,289,638 (55.9) | ||
| Race, n (%) | Non-Hispanic white | 17,766,203 ( 8.6) | 9,843,621 (10.5) | 4,769,359 (9.1) | 3,153,223 (5.3) | < 0.001 |
| Mexican American | 12,310,991 ( 6.0) | 6,602,731 (7.1) | 3,174,702 (6.0) | 2,533,558 (4.3) | ||
| Other Hispanic | 138,231,221 (67.2) | 57,508,944 (61.5) | 35,431,191 (67.4) | 45,291,086 (76.1) | ||
| Non-Hispanic black | 21,935,567 (10.7) | 12,554,995 (13.4) | 5,171,314 (9.8) | 4,209,258 (7.1) | ||
| Other races | 15,394,272 (7.5) | 7,040,537 (7.5) | 4,025,916 (7.7) | 4,327,819 (7.3) | ||
| BMI [kg/m2], mean (SE) | 29.17 (6.90) | 29.43 (7.05) | 29.02 (6.83) | 28.90 (6.70) | 0.005 | |
| Smoke status, n (%) | Never smoke | 115,818,452 (56.3) | 50,974,343 (54.5) | 30,438,122 (57.9) | 34,405,987 (57.8) | < 0.001 |
| Quit smoke | 51,464,878 (25.0) | 20,375,233 (21.8) | 13,109,288 (24.9) | 17,980,356 (30.2) | ||
| Current smokers | 38,354,924 (18.7) | 22,201,252 (23.7) | 9,025,072 (17.2) | 7,128,600 (12.0) | ||
| Alcohol intake, n (%) | No | 57,527,601 (28.0) | 25,322,062 (27.1) | 13,939,945 (26.5) | 18,265,594 (30.7) | < 0.001 |
| Yes | 148,110,652 (72.0) | 68,228,766 (72.9) | 38,632,536 (73.5) | 41,249,350 (69.3) | ||
| Physical activity, n(%) | Sedentary | 72,518,579 (35.3) | 35,140,926 (37.6) | 17,921,682 (34.1) | 19,455,970 (32.7) | < 0.001 |
| Insufficient | 329,885,679 (16.0) | 14,783,481 (15.8) | 8,049,654 (15.3) | 10,155,433 (17.1) | ||
| Moderate | 277,472,089 (13.5) | 11,139,808 (11.9) | 7,595,576 (14.4) | 9,011,825 (15.1) | ||
| High | 72,383,899 (35.2) | 32,486,613 (34.7) | 19,005,569 (36.2) | 20,891,717 (35.1) | ||
| Hypertension, n(%) | No | 133,059,566 (64.7) | 64,526,144 (69.0) | 34,240,404 (65.1) | 34,293,018 (57.6) | < 0.001 |
| Yes | 72,578,688 (35.3) | 29,024,685 (31.0) | 18,332,077 (34.9) | 25,221,926 (42.4) | ||
| Diabetes, n (%) | No | 20,610,764 (10.0) | 8,229,605(8.8) | 4,849,232 (9.2) | 7,531,927 (12.7) | < 0.001 |
| Yes | 185,027,489 (90.0) | 85,321,224 (91.2) | 47,723,249 (90.8) | 51,983,016 (87.3) | ||
Relationship between serum vitamin D, dietary vitamin D intake, and SII
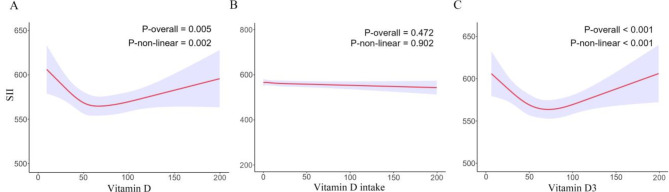
Tables 3 and 4 present the relevant results of the weighted multivariate linear regression model: the crude model did not adjust for covariates; model 1 adjusted for age, gender, ethnicity, and BMI; and model 2 adjusted for age, gender, ethnicity, BMI, smoking history, alcohol history, hypertension history, diabetes history, and physical activity. In models 1 and 2, setting the insufficiency group as the baseline, SII levels were significantly higher in the severe deficiency group (for model 1, β = 45.08, 95% CI, 17.73–72.43, P = 0.002; for model 2, β = 26.05, 95% CI, -0.65–52.75, P = 0.06) and the deficiency group (for model 1, β = 23.57, 95% CI, 10.92–36.22, P < 0.001; for model 2, β = 12.78, 95% CI, 0.68–24.89, P = 0.04), but there was no significant difference in SII levels in the sufficiency group: (for model 1, β = -5.50, 95% CI, -18.17–7.17, P = 0.39; for model 2, β = 5.43, 95% CI, -6.97–17.83, P = 0.39). Additionally, across all three models, there was no significant association between dietary VD intake and SII. Compared to the VD intake ≤ 5.00 µg/d group, SII levels did not significantly increase or decrease in the other two groups (Table 4). We then used the RCS curve to evaluate the nonlinear relationship between serum VD (or dietary VD intake) and SII. As shown in Fig. 2, a U-shaped relationship existed between serum VD and SII (P-overall = 0.005, P-non-linear = 0.002). In the contrast, no relationship existed between dietary VD intake and SII (P-overall = 0.472, P-non-linear = 0.902).
Table 3.
Association of serum vitamin D with SII in NHANES 2007–2018 participants
| crude model | model 1 | model2 | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | |
| VD ≤ 25 | -0.60 (-27.96, 26.76) | 0.97 | 45.08 (17.73, 72.43) | 0.002 | 26.05 (-0.65, 52.75) | 0.06 |
| 25 < VD ≤ 50 | 2.49 (-10.34, 15.31) | 0.70 | 23.57 (10.92, 36.22) | < 0.001 | 12.78 (0.68, 24.89) | 0.04 |
| 50 < VD ≤ 75 | 1.00 | - | 1.00 | - | 1.00 | - |
| VD>75 | 14.95 (2.03, 27.88) | 0.02 | -5.50 (-18.17, 7.17) | 0.39 | 5.43 (-6.97, 17.83) | 0.39 |
crude model: unadjust
model 1: adjust for age, gender, race and BMI;
model 2: adjust for age, sex, race, BMI, smoking history, alcohol consumption history, hypertension history, and diabetes history
Table 4.
Association of dietary VD intake with SII in NHNES 2007–2020 particanpants crude model: unadjust
| crude model | model 1 | model2 | ||||
|---|---|---|---|---|---|---|
| β(95% CI) | P value | β(95% CI) | P value | β(95% CI) | P value | |
| VD intake ≤ 5.00 | 1.00 | - | 1.00 | - | 1.00 | - |
| 5.00 < VD intake ≤ 9.90 | 6.99 (-7.64, 21.62) | 0.35 | 3.07 (-11.46, 17.59 ) | 0.68 | 6.69 (-7.67, 21.04) | 0.36 |
| 9.90 < VD intake | 6.82 (-7.36, 21.00) | 0.34 | -11.50 (-26.14, 3.14) | 0.12 | -4.99 (-19.10, 9.13) | 0.48 |
model 1: adjust for age, gender, race and BMI
model 2: adjust for age, sex, race, BMI, smoking history, alcohol consumption history, hypertension history, and diabetes history
Fig. 2.
RCS results for VD and SII. (A) RCS results for total VD and SII; (B) RCS results for dietary VD intakeand SII; (C) RCS results for serum VD3 and SII
The subgroup analysis revealed the association between serum VD and SII, including age, gender, BMI, smoking history, alcohol history, hypertension history, diabetes history, and physical activity: the effect of serum VD on SII was consistent in most subgroups, except for BMI, physical activity, and diabetes history (Supplementary Table 1, P-interaction = 0.015, 0.024, and 0.023).
Mediation analysis
Considering the key roles of the inflammatory and VD in disease development, we applied mediation analysis to further assess whether VD could influence the occurrence of cancer, hypertension, diabetes, and coronary heart disease through SII. In this analysis, the package relies on the bootstrap method to estimate the confidence intervals for both the Average Causal Mediation Effect (ACME) and Average Direct Effect (ADE). The mediation package uses a counterfactual framework for causal inference, enabling a precise decomposition of the mediation and direct effects. Based on the theoretical hypothesis, we constructed two regression models: the first model used SII as the dependent variable, VD as the independent variable, and other covariates to analyze the effect of VD on SII. The second model used disease or mortality as the dependent variable, with VD and SII as independent variables, to analyze both direct and indirect effects of VD on disease or mortality.
Based on these models, we used the mediate function to calculate ACME and ADE. To ensure robust effect estimates, the mediate function employs the bootstrap method for repeated sampling, calculating confidence intervals for both the mediation and direct effects. This bootstrap method enhances the reliability of estimates, particularly when data do not meet the normality assumption.
As shown in the Table 5, we report the estimated values of ACME and ADE along with their 95% confidence intervals. ACME reflects the indirect effect of VD on disease or mortality mediated through SII, while ADE indicates the direct effect of VD on disease or mortality. These results reveal the extent of SII’s mediating role between VD and disease. The table also includes total effects with their confidence intervals, as well as the mediation proportion, quantifying SII’s contribution as a mediator to the total effect. Together, VD did not influence the occurrence of above diseases through SII. Moreover, SII was a mediator for vitamin D affected all-cause mortality and cardiovascular disease-related mortality (Table 5).
Table 5.
Mediation analysis for serum vitamin D, SII, and disease
| ACME | ADE | Total effect | Proportion mediated | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI lower | 95% CI upper | P | Estimate | 95% CI lower | 95% CI upper | P | Estimate | 95% CI lower | 95% CI upper | P | Estimate | 95% CI lower | 95% CI upper | P | |
| Cancer | ||||||||||||||||
| -0.00000082 | -0.0000035 | 0 | 0.39 | 0.000249 | 0.000164 | 0 | <0.001 | 0.000249 | 0.000164 | 0 | <0.001 | -0.00331 | -0.0147 | 0.01 | 0.39 | |
| CHD | ||||||||||||||||
| 0.000000266 | -0.000000722 | 0 | 0.64 | -0.000191 | -0.000334 | 0 | <0.001 | -0.00019 | -0.000334 | 0 | <0.001 | -0.0014 | -0.0122 | 0 | 0.64 | |
| Hypertension | ||||||||||||||||
| -0.00000309 | -0.0000124 | 0 | 0.45 | 0.000382 | 0.000197 | 0 | <0.001 | 0.000379 | 0.000196 | 0 | <0.001 | -0.00817 | -0.0409 | 0.01 | 0.45 | |
| Diabetes | ||||||||||||||||
| -0.000000978 | -0.0000042 | 0 | 0.44 | 0.00018 | 0.0000366 | 0 | 0.02 | 0.000179 | 0.0000339 | 0 | 0.02 | -0.00545 | -0.035 | 0.02 | 0.46 | |
| All-cause mortality | -0.1371 | -0.2049 | -0.0841 | <0.001 | 5.6844 | 3.7518 | 7.9896 | <0.001 | 5.5471 | 3.6065 | 7.8438 | <0.001 | -0.0247 | -0.044 | -0.0139 | <0.001 |
| CVD mortality | -0.6912 | -1.2491 | -0.3335 | <0.001 | 39.456 | 17.6415 | 70.0899 | <0.001 | 38.7647 | 17.052 | 69.2018 | <0.001 | -0.0173 | -0.0408 | -0.0087 | <0.001 |
| Cancer mortality | -0.3667 | -0.6381 | -0.1824 | <0.001 | 6.3073 | -4.3436 | 16.6769 | 0.27 | 5.9407 | -4.8876 | 16.3519 | 0.25 | -0.0436 | -0.6791 | 0.4479 | 0.27 |
Relationship between serum vitamin D3 and SII
Given that VD3 is the main component and active form of VD, we further analyzed the relationship between serum VD3 and SII. As shown in Table 6, there was no significant difference in SII levels among the normal group (VD3 ≥ 50 nmol/L), the insufficient group (30 ≤ VD3 < 50 nmol/L), and the deficient group (D3 < 30 nmol/L) in the crude model; in models 1 and 2, compared to the normal group, SII levels were significantly higher in the insufficient group (for model 1, β = 29.54, 95% CI, 16.40-42.68, P < 0.001; for model 2, β = 15.80, 95% CI, 3.53–28.08, P = 0.01) and deficient group (for model 1, β = 44.41, 95% CI, 29.45–59.36, P < 0.001; for model 2, β = 21.50, 95% CI, 7.16–35.84, P = 0.004) RCS curve analysis showed a U-shaped relationship between serum VD3 and SII Fig. 2. Subgroup analysis indicated that the effect of serum VD3 on SII was consistent in most subgroups, except for BMI, physical activity, and diabetes history (P-interaction = 0.021, 0.02, and 0.028; Supplementary Table 2). The trends of serum VD3 results were consistent with those of total VD.
Table 6.
Association of Serum vitamin D3 with SII in NHNES 2007–2018 participants
| crude model | model 1 | model 2 | ||||
|---|---|---|---|---|---|---|
| β(95% CI) | P value | β(95% CI) | P value | β(95% CI) | P value | |
| D3 < 30 | 2.26 (-13.62, 18.14) | 0.78 | 44.41 (29.45, 59.36) | < 0.001 | 21.50 (7.16, 35.84) | 0.004 |
| 30 ≤ D3 < 50 | 4.10 (-9.83, 18.03) | 0.56 | 29.54 (16.40, 42.68) | < 0.001 | 15.80 (3.53, 28.08) | 0.01 |
| D3 ≥ 50 | 1.00 | 1.00 | 1.00 | |||
crude model: unadjusted
model 1: adjust for age, gender, race and BMI
model 2: adjust for age, sex, race, BMI, smoking history, alcohol consumption history, hypertension history, and diabetes history
Discussion
To our knowledge, this study first and systematically explored the relationship between vitamin D (VD) and systemic inflammation index (SII) from multiple perspectives. We found no significant association between dietary VD intake and SII, but there existed a U-shaped relationship between serum VD and SII. Additionally, SII was a mediator for the effect of serum VD on all-cause mortality and cardiovascular disease-related mortality, but not on the occurrence of diabetes, cancer, or cardiovascular disease.
In recent years, the relationship between VD and inflammation has garnered widespread attention, with researchers continuously employing various inflammation markers to establish connections between them. An observational study found a negative correlation between serum VD and some inflammation markers (such as WBC and fibrinogen) in adults, while a U-shaped correlation with CRP [22]. A meta-analysis indicated that the decrease in VD levels was accompanied by the increase in inflammation markers in elderly populations [23]. A cross-sectional study revealed a negative correlation between VD levels and certain inflammatory markers (CRP, A1GPA) in patients with cystic fibrosis [24]. Another cross-sectional study demonstrated weak and inconsistent correlations between VD levels and inflammation markers (CRP and AGP) in preschool children and adult women [25]. A study based on the UK Biobank showed a negative correlation between VD deficiency and systemic inflammation markers based on blood cells, but not with CRP based markers [26]. Innovatively, our analytic data revealed a U-shaped association between serum VD and SII, and the result indicated that the effect of VD on systemic inflammation is bidirectional and concentration-dependent.
Previous studies have shown that the impact of exogenous VD supplementation on systemic inflammation is also unclear. Some research suggests no correlation between VD supplementation and inflammation levels. For example, supplementing with VD did not significantly affect systemic inflammation levels in knee osteoarthritis patients [27]; a meta-analysis of RCT studies indicated no significant correlation between VD3 supplementation and some serum inflammation markers (IL6, IL10, and CRP) in cancer and precancerous patients [28]; a randomized, double-blind RCT study showed that combined magnesium and vitamin D supplementation did not influence serum inflammation marker levels [29]. Other studies suggested that VD supplementation could effectively reduce systemic inflammation levels. For example, an RCT study focused on Parkinson’s disease found a downward trend in inflammation levels in the VD supplementation group [30]; an RCT study involving type 2 diabetes showed that VD supplementation could reduce platelet-mediated inflammatory responses, and the levels of several inflammation factors (such as IL18, TNF-α, and IFN-γ) [31]; The VITAL study demonstrated that exogenous VD3 supplementation could effectively decrease high-sensitivity CRP levels in peripheral blood by 19% [32]. In this study, we further analyzed the correlation between dietary VD intake and SII. Unlike serum VD results, no correlation was found between dietary VD intake and SII levels. These results suggest that the dosage and duration of VD supplementation should be based on real-time monitoring of serum VD levels.
As mentioned in the background, VD and systemic inflammation play critical roles in the occurrence and development of various diseases. Some studies have evaluated whether VD affects disease progression through systemic inflammation. For example, some mechanistic studies indicated that VD could affect inflammation processes to promote cancer progression, involving COX-2, NF-κB, TNF-α, IL1β, and IL6 [33, 34]; Vitamin D has been reported to play important roles in cardiovascular disease by regulating immune and inflammation functions [35, 36]; VD could also prevent high glucose-induced pancreatic β cell dysfunction through the AMPK/NLRP3 inflammatory pathway [37]. In this study, we found that SII is not a mediator for the influence of VD on the incidence of these diseases, which might be due to the strong disease-promoting effect of VD. Additionally, we found that VD might affect all-cause mortality and cardiovascular disease-related mortality through SII. In general, we demonstrated that vitamin D affected disease prognosis, especially cardiovascular disease prognosis through systemic inflammation levels by using large-scale data.
One of the key strengths of this study is its use of the NHANES database, a large, nationally representative dataset that includes diverse demographic groups and allows for robust generalizability of the findings. The study employs a comprehensive approach by analyzing both serum Vitamin D levels and dietary intake, along with SII, a well-established marker of systemic inflammation. The use of RCS and logistic regression models enabled the detection of non-linear relationships between Vitamin D and SII, offering a more nuanced understanding of their correlation. Additionally, mediation analysis provided valuable insights into the role of SII in the relationship between Vitamin D and mortality outcomes, contributing to the current body of knowledge on inflammation and disease prognosis.
However, this study also has some limitations. First, the results based on database analysis only revealed the association type between VD and SII, but did not directly prove a causal relationship. This causal relationship requires supplementary evidence from earlier studies. Secondly, VD levels in this study were mostly based on single measurements rather than continuous results, which might not accurately reflect long-term vitamin D status. Thirdly, this study only selected SII as a marker for systemic inflammation, possibly leading to the omission of more representative inflammation markers. Fourthly, the study did not include the potential impact of the COVID-19 pandemic on vitamin D intake, and the use of anti-inflammatory and immunosuppressive drugs that may influence the generalizability of our findings. Fifthly, the results were derived from a single cross-sectional nature of the NHANES database, lacking external data validation and related animal model validation. Therefore, well-designed, large-sample prospective cohort studies are needed to clarify the relationship between VD, systemic inflammation, and specific disease progression. Additionally, animal studies are needed to explore the mechanisms of how VD influences disease occurrence and progression through systemic inflammation.
Conclusion
In this study, we found a U-shaped correlation between serum VD and SII based on the NHANES database for the first time. However, dietary VD intake did not affect SII levels. Furthermore, SII was a mediator for the impact of VD on all-cause mortality and cardiovascular disease mortality. This study reveals a U-shaped relationship between serum Vitamin D and systemic inflammation, highlighting the potential role of Vitamin D in regulating inflammation-related health outcomes. Future research should focus on randomized controlled trials to explore the causal relationship between Vitamin D and systemic inflammation, while clinical strategies should consider personalized Vitamin D supplementation to optimize inflammation control and improve cardiovascular health.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the individuals who participated in the construction of NHANES database.
Author contributions
Peng Yunpeng: Formal analysis, Investigation, Writing original draftDai Shangnan: Data curation, Formal analysis, Project administrationWu Di: Data curation, Formal analysisHou Chaoqun: Methodology, SoftwareGe Wanli: Data curation, SoftwareLi Qiang: Conceptualization, Supervision, Writing – review & editingGuo Feng: Conceptualization, Data curation, Project administration, Writing – review & editing.
Funding
This research received no external funding.
Data availability
Data are available at the NHANES website, https://www.cdc.gov/nchs/nhanes/index.htm.
Declarations
Ethics approval and consent to participate
Ethical review and approval were waived for this study due to prior ethical clearance from regulatory institutions. Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not Applicable.
Clinical trail number
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peng Yunpeng, Dai Shangnan and Wu Di contributed equally.
Contributor Information
Li Qiang, Email: liqiang020202@163.com.
Guo Feng, Email: Guofeng1978@sohu.com.
References
- 1.Alonso N, Zelzer S, Eibinger G, Herrmann M. Vitamin D metabolites: Analytical challenges and clinical relevance. Calcif Tissue Int. 2023;112(2):158–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delrue C, Speeckaert MM. Vitamin D and vitamin D-Binding protein in Health and Disease. Int J Mol Sci 2023, 24(5). [DOI] [PMC free article] [PubMed]
- 3.Norman AW. The history of the discovery of vitamin D and its daughter steroid hormone. Ann Nutr Metab. 2012;61(3):199–206. [DOI] [PubMed] [Google Scholar]
- 4.Liu D, Meng X, Tian Q, Cao W, Fan X, Wu L, Song M, Meng Q, Wang W, Wang Y. Vitamin D and multiple Health outcomes: an Umbrella Review of Observational studies, randomized controlled trials, and mendelian randomization studies. Adv Nutr. 2022;13(4):1044–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlberg C. Genomic signaling of vitamin D. Steroids. 2023;198:109271. [DOI] [PubMed] [Google Scholar]
- 7.Lu M, Taylor BV, Korner H. Genomic effects of the vitamin D receptor: potentially the link between vitamin D, Immune cells, and multiple sclerosis. Front Immunol. 2018;9:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charoenngam N, Holick MF. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12(7). [DOI] [PMC free article] [PubMed]
- 9.Zmijewski MA. Vitamin D and Human Health. Int J Mol Sci 2019, 20(1). [DOI] [PMC free article] [PubMed]
- 10.Wellenstein MD, Coffelt SB, Duits DEM, van Miltenburg MH, Slagter M, de Rink I, Henneman L, Kas SM, Prekovic S, Hau CS, et al. Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature. 2019;572(7770):538–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roxburgh CS, McMillan DC. Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer. 2014;110(6):1409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Z, Zhang X, Sun T, Huang X, Ma M, Yang S, Zhou Y. Prognostic value of systemic immune-inflammation index in CAD patients: systematic review and meta-analyses. Eur J Clin Invest. 2024;54(2):e14100. [DOI] [PubMed] [Google Scholar]
- 13.Tattersall MC, Jarjour NN, Busse PJ. Systemic inflammation in Asthma: what are the risks and impacts outside the Airway? J Allergy Clin Immunol Pract. 2024;12(4):849–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Islam MM, Satici MO, Eroglu SE. Unraveling the clinical significance and prognostic value of the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, systemic immune-inflammation index, systemic inflammation response index, and delta neutrophil index: an extensive literature review. Turk J Emerg Med. 2024;24(1):8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangoni AA, Zinellu A. The diagnostic role of the systemic inflammation index in patients with immunological diseases: a systematic review and meta-analysis. Clin Exp Med. 2024;24(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng L, Yang Y, Hu X, Zhang R, Li X. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: a systematic review and meta-analysis. J Transl Med. 2023;21(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu B, Wang J, Li YY, Li KP, Zhang Q. The association between systemic immune-inflammation index and rheumatoid arthritis: evidence from NHANES 1999–2018. Arthritis Res Ther. 2023;25(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bikle DD. Vitamin D regulation of Immune function. Curr Osteoporos Rep. 2022;20(3):186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ao T, Kikuta J, Ishii M. The effects of vitamin D on Immune System and Inflammatory diseases. Biomolecules 2021, 11(11). [DOI] [PMC free article] [PubMed]
- 20.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine S. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. [DOI] [PubMed] [Google Scholar]
- 21.Wang R, Wang W, Hu P, Zhang R, Dong X, Zhang D. Association of Dietary Vitamin D Intake, serum 25(OH)D(3), 25(OH)D(2) with cognitive performance in the Elderly. Nutrients 2021, 13(9). [DOI] [PMC free article] [PubMed]
- 22.Mellenthin L, Wallaschofski H, Grotevendt A, Volzke H, Nauck M, Hannemann A. Association between serum vitamin D concentrations and inflammatory markers in the general adult population. Metabolism. 2014;63(8):1056–62. [DOI] [PubMed] [Google Scholar]
- 23.Alharbi SS, Albalawi AA, Sr., Al Madshush AM, Alsaidalani WMH, Aljohani OS, Alaradi AR, Alatawi AA, Albalawi RS, Alanazi LA, Albalawi HS, et al. Association between Lower Levels of Vitamin D and inflammation in the Geriatric Population: a systematic review and Meta-analysis. Cureus. 2024;16(5):e60892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Queiroz DJM, Silva AS, Silva Junior CCD, Persuhn DC, Diniz ADS, Lima R, Paiva MP, Cartaxo CGB, Bezerra PGM, Duarte Ribeiro M, et al. Vitamin D levels and their association with oxidative stress and inflammation markers in patients with cystic fibrosis. Nutr Hosp. 2023;40(2):280–5. [DOI] [PubMed] [Google Scholar]
- 25.Young MF, Ou J, Duong C, Luo H, Beyh YS, Meng J, Gernand AD, Roth DE, Suchdev PS. Assessment of vitamin D status and association with inflammation: biomarkers reflecting inflammation and nutritional determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2023;117(1):175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sha S, Gwenzi T, Chen LJ, Brenner H, Schottker B. About the associations of vitamin D deficiency and biomarkers of systemic inflammatory response with all-cause and cause-specific mortality in a general population sample of almost 400,000 UK Biobank participants. Eur J Epidemiol. 2023;38(9):957–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saengsiwaritt W, Jittikoon J, Chaikledkaew U, Tawonsawatruk T, Honsawek S, Udomsinprasert W. Effect of vitamin D supplementation on circulating level of autophagosome protein LC3A, inflammation, and physical performance in knee osteoarthritis. Clin Transl Sci. 2023;16(12):2543–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gwenzi T, Zhu A, Schrotz-King P, Schottker B, Hoffmeister M, Brenner H. Effects of vitamin D supplementation on inflammatory response in patients with cancer and precancerous lesions: systematic review and meta-analysis of randomized trials. Clin Nutr. 2023;42(7):1142–50. [DOI] [PubMed] [Google Scholar]
- 29.Cheung MM, Dall RD, Shewokis PA, Altasan A, Volpe SL, Amori R, Singh H, Sukumar D. The effect of combined magnesium and vitamin D supplementation on vitamin D status, systemic inflammation, and blood pressure: a randomized double-blinded controlled trial. Nutrition 2022, 99–100:111674. https://www.sciencedirect.com/science/article/abs/pii/S0899900722000867?via%3Dihub [DOI] [PubMed]
- 30.Bytowska ZK, Korewo-Labelle D, Berezka P, Kowalski K, Przewlocka K, Libionka W, Kloc W, Kaczor JJ. Effect of 12-Week BMI-Based Vitamin D(3) Supplementation in Parkinson’s Disease with Deep Brain Stimulation on Physical Performance, Inflammation, and Vitamin D Metabolites. Int J Mol Sci 2023, 24(12). [DOI] [PMC free article] [PubMed]
- 31.Johny E, Jala A, Nath B, Alam MJ, Kuladhipati I, Das R, Borkar RM, Adela R. Vitamin D supplementation modulates platelet-mediated inflammation in subjects with type 2 diabetes: a Randomized, Double-Blind, placebo-controlled trial. Front Immunol. 2022;13:869591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong Y, Zhu H, Chen L, Huang Y, Christen W, Cook NR, Copeland T, Mora S, Buring JE, Lee IM et al. Effects of vitamin D(3) and Marine Omega-3 fatty acids supplementation on biomarkers of systemic inflammation: 4-Year findings from the VITAL randomized trial. Nutrients 2022, 14(24). [DOI] [PMC free article] [PubMed]
- 33.van Harten-Gerritsen AS, Balvers MG, Witkamp RF, Kampman E, van Duijnhoven FJ. Vitamin D, inflammation, and Colorectal Cancer Progression: a review of mechanistic studies and future directions for Epidemiological studies. Cancer Epidemiol Biomarkers Prev. 2015;24(12):1820–8. [DOI] [PubMed] [Google Scholar]
- 34.Lawler T, Su T, Cai Q, Steinwandel MD, Zheng W, Blot WJ, Warren Andersen S. Associations between serum vitamin D biomarkers and tumor expression of Ki67, p53, and COX-2 in colorectal cancer cases from the Southern Community Cohort Study. J Steroid Biochem Mol Biol. 2023;225:106201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pal E, Ungvari Z, Benyo Z, Varbiro S. Role of vitamin D Deficiency in the Pathogenesis of Cardiovascular and Cerebrovascular diseases. Nutrients 2023, 15(2). [DOI] [PMC free article] [PubMed]
- 36.Argano C, Mirarchi L, Amodeo S, Orlando V, Torres A, Corrao S. The role of Vitamin D and its molecular bases in insulin resistance, diabetes, metabolic syndrome, and Cardiovascular Disease: state of the art. Int J Mol Sci 2023, 24(20). [DOI] [PMC free article] [PubMed]
- 37.Wu M, Lu L, Guo K, Lu J, Chen H. Vitamin D protects against high glucose-induced pancreatic beta-cell dysfunction via AMPK-NLRP3 inflammasome pathway. Mol Cell Endocrinol. 2022;547:111596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at the NHANES website, https://www.cdc.gov/nchs/nhanes/index.htm.