Abstract
Background
Mucolipidosis (ML) II and III alpha/beta are lysosomal disorders caused by mutations in the GNPTAB gene which encodes the alpha and beta subunits of the heterohexameric enzyme, N-acetylglucosamine-1-phosphotransferase.
Method
To explore the clinical and molecular characteristics of the 20 ML II and III alpha/beta patients, clinical data was collected and GNPTAB gene was analyzed by nest PCR and direct Sanger-sequencing. The activity of several lysosomal enzymes was measured in the plasma.
Results
Among the 20 ML II and III alpha/beta patients, 6 patients were classified as ML II and 14 as ML III alpha/beta. The main clinical manifestations were joint stiffness, skeletal deformity, mental retardation and short stature. Bone X-ray examination showed radiological changes. The plasma arylsulfatase A and hexosaminidase A enzyme activities increased significantly. Urinary glycosaminoglycan values were normal. We detected mutations in GNPTAB in 35 of 40 alleles (87.5%). Mutation c.2715 + 1G > A and c.2404 C > T (p.Gln802Ter) were the most prevalent variants, accounting for 14.3% and 11.4%, respectively. Five novel mutations c.3335 + 5G > A, c.1284 + 1G > A, c.571 + 4 A > G, c.1634_1635delAA (p.Lys545Serfs*16) and c.1582T > C(p.Cys528Arg) were identified.
Conclusion
Our study expands the spectrum of GNPTAB gene in China. Mutation c.2715 + 1G > A was the most prevalent mutation in our study. The novel mutation c.1284 + 1G > A might be a severe mutation associated with ML II.
Keywords: Mucolipidosis, GNPTAB gene, Clinical and molecular characteristics
Background
Mucolipidosis (ML) is an autosomal recessive lysosomal storage disease caused by reduced enzyme activity of N-acetylglucosamine-1-phosphotransferase (GlcNAc-1-PT) (EC 2.7.8.17). The term “mucolipidosis” was first described by Wiedmann et al. in 1970, because its clinical manifestations were similar to mucopolysaccharidosis and sphingolipidosis [1, 2]. GlcNAc-1-PT catalyzes the first step in the synthesis of the mannose 6-phosphate (M6P) recognition marker required for targeting of newly synthesized lysosomal hydrolases to the lysosome. Without the M6P recognition markers, the overflowed lysosomal enzymes will leak out of the cells and elevated in the serum and body fluids [3, 4]. Among live births, the incidence is 1/123,500 in Portugal [5], 1/252,500 in Japan [6], 1/625,500 in the Netherlands [7], and 1/2,229,516 in Malaysia [8].
GlcNAc-1-PT is a 540-kDa hexameric complex composed of three subunits: 2α, 2β and 2γ. The α/β subunit is encoded by the GNPTAB gene and the γ subunit is encoded by the GNPTG gene. The alpha/beta subunit contains the catalytic domain of GlcNAc-1-PT enzyme and the gamma subunit plays an auxiliary role in the GlcNAc-1-PT enzyme. GNPTAB gene (MIM# 607840, ENSG00000111670) spans up to 85Kb of chromosome 12q23.3 and contains 21 exons encoding 1256 amino acid residues. GNPTG (MIM#252605, ENSG00000090581) gene spans 11.13 kb of chromosome 16p13.3 and contains 11 exons encoding 305 amino acid residues. Mutations in GNPTAB are known to be responsible for ML II and ML III alpha/beta. Mutations in the GNPTG gene cause ML III gamma [9–12].
The classic ML is divided into 4 types, namely ML I-IV. Currently, ML I and ML IV have been classified into other diseases. ML I, named sialidosis, is due to deficiency of neuraminidase, while ML IV is a neurodevelopmental disorder with retinal degeneration and normal lysosomal hydrolase activities [2]. Recently, mucolipidosis type V was reported in the literature. It found that TMEM251 was a regulator of M6P, which was crucial for the cleavage and activity of GlcNAc-PT enzyme. Individuals carrying pathogenic TMEM251 mutations might show severe symptoms similar to ML II, so they were classified as ML V [13]. The etiology of ML II and ML III are both caused by the deficiency of GlcNAc-1-PT enzyme activity. ML II patients are completely deficient in GlcNAc-1-PT enzyme activity and characterized by stiffness of the joints, coarse facies, serious progressive bone diseases and intellectual disability [14, 15]. Most of them developed the disease between the age of 6 to 12 months and died within 10 years of age [6]. A certain number of patients may be evident at birth or prenatally [16, 17]. ML III alpha/beta is the moderate form of ML II. Patients with ML III are partial deficient in GlcNAc-1-PT enzyme activity and have a later onset at the age of 2 to 4 years. The ML III progress slowly and could survival into adulthood [18].
At present, there is no effective treatment for ML disease, except for symptomatic treatment. It was reported that intravenous treatment with pamidronate could improve reduced bone density [19]. But there weren’t many cases, so it wasn’t widely used in clinical practice. Surgical orthopedic treatment for bones and joints, such as bilateral hip and knee replacements, spinal fusion procedures and carpal tunnel release, could help improve movement limitations. The heart valve damage can affect the function of the heart and patients should require surgical intervention [20].
In this study, we described clinical, biochemical and molecular characteristics of 20 Chinese patients with ML II and III alpha/beta to further analyze the relationship between genotype and phenotype, which helps us to predict the prognosis of patients and to offer genetic counseling.
Methods
Patients
Twenty probands from 17 unrelated families with ML II and III alpha/beta from South China (Guangdong, Hunan, Hubei, Guangxi, Sichuan, Yunnan province) were enrolled in this study. Eleven were male and nine were female. The diagnosis of ML II and III alpha/beta was based on clinical findings and confirmed by lysosomal enzyme activities in plasma measured at the Guangzhou Women and Children’s Medical Center from August 2011 to January 2023. Informed consent was obtained from parents of patients. This study was approved by the Ethics Committee of Guangzhou Women and Children’s Medical Center (2015-92).
Lysosomal enzyme activity
The activities of several lysosomal hydrolases in plasma, including arylsulfatase A (ASA) and hexosaminase A (HexA), were tested with reference to previous methods and modified slightly [21, 22]. The fluorogenic substrate 4-nitrocatechol sulfate (Sigma-Aldrich, St. Louis, MO, USA) and 4-methylumbelliferyl-6-sulfo-2-acetamido-2-deoxy-β-Dglucopyranoside (Glycosynth, Warrington, UK) were used in the detection, respectively. If the enzyme activity values were increased 10–20 times than the normal range, then the diagnosis of ML II or III alpha/beta was supported. The normal reference range of ASA enzyme activity was 50–140 nmol/mg.17 h, and the normal reference range of HexA enzyme activity was 29.8–63.8 nmol/mg.h. Enzyme activities measurements of our lab participate in the quality assurance of IEM laboratory tests (www.eqa.erndim.org).
Urinary glycosaminoglycan (GAG) determination
Urinary GAG was measured using dimethylmethylene blue/Tris by spectrophotometry and corrected for urinary creatinine (Cr) content. The ratio of GAG/Cr (mg/mmol) was compared to age-matched normal controls. Normal reference values of urine GAG/Cr were GAG/Cr normal reference range: <64.0 mg/mmol(< 28days), < 49.9 mg/mmol(28days-6months), < 37.8 mg/mmol(6months-1year), < 30.0 mg/mmol(1-3years), < 19.9 mg/mmol(3-5years), < 16.0 mg/mmol(5-7years), < 12.6 mg/mmol(7-18years), < 7.1 mg/mmol(> 18years) [23].
Molecular analysis
Genomic DNA was extracted from peripheral blood samples. GNPTAB gene was analyzed by direct Sanger-sequencing. Primers (Table 1) were designed using Primer 5 software (Biosoft International, Palo Alto, USA). The coding and splicing regions of the GNPTAB gene were amplified with Ex Taq DNA polymerase (TaKaRa Bio Inc., Otsu, Japan). The PCR products were sequenced by an ABI 3730 sequencer (Applied Biosystems, Foster City, CA, USA) and the sequencing chromatograms were analyzed by comparing with the corresponding reference sequences (NCBI: NM_024312.5) using Sequencer software DNAMAN (Lynnon Biosoft, Inc., Ouebec, Canada). Once variants were identified, the PCR and sequencing on the corresponding exons were repeated at least twice to verify reliability of the results. Pathogenic assessment of novel missense variants were analyzed by the Mutation Taster2021 [24] (https://www.mutationtaster.org/) and Polymorphism Phenotyping v2 (PolyPhen-2) (http://genetics.bwh.harvard.edu/pph2/index/shtml). To predict the pathogenicity of novel splicing mutation c.3335 + 5G > A, c.1284 + 1G > A and c.571 + 4 A > G, we performed in silico analysis using the Mutation Taster2021 (https://www.mutationtaster.org/), FATHMM (http://fathmm.biocompute.org.uk/) and NetGene2 (https://services.healthtech.dtu.dk/services/NetGene2-2.42/).
Table 1.
Primers used for PCR amplification and sequencing of GNPTAB
| Exons | Forward primers(5’-3’) | Reverse primers(5’-3’) | Product size(bp) |
|---|---|---|---|
| 1 | CGTCCGTCGCCGGAGCTGCAATG | GGCAAAACCCCGTCTCTAATAATG | 386 |
| 2 | GATCTAACACGATGTATGTGGTAGGCAG | GGCCACACTAATCTTCTCTGTATCGTTC | 458 |
| 3–4 | TACAGTTTGAAAACATGCAGTTCTGTGA | AGATAACAATGCACCAAGAGGGAACTAA | 1951 |
| 5 | GCTCTATCTTTGGAGTTGGGTTTAACAA | TGTTTTGCTTCTCTTTGTGCATTTTTAG | 552 |
| 6–7 | TCAACGTACACTGATTTACATTGTTCCC | CAGGAAACAAGCAAACCAAATAAGACTC | 863 |
| 8–10 | AGGAAAGATTAAAGAGCAGAGTGGGAAT | CGGGCCCAGATAATTATTTTACTTTTTG | 1430 |
| 11 | AAACTTAAAGCACTTGTGAGTGTGAACG | CCTCCCAAGTAGCTAGGACTACAGGTTT | 576 |
| 12 | CACCCAGTCCAGAACTGTCTTTC | GCTAAGGTAAATCTGCTTGGTCC | 522 |
| 13 | TGCCTACTTCAGCAGCACATATAC/ TCCAAGTCAGCCTTGCTGAG | CCTGAGCATGAGAAAGAATGAGG/ CTCAGCAAGGCTGACTTGGA | 1345 |
| 14–16 | TGAGTCCTGCTAAGTCCAGTTTAGATCC | ATGAATGATTCGGATTACCTGTGCTACT | 2066 |
| 17–18 | GGTGTTTTTCTTACCTCCAGAGAGCATA | CTGGGTCTCTCTGAACAGCTTGTTATTA | 812 |
| 19 | CCACATCCTTGTTTTGTTAGGTCAG | AGAAGAATCATTGTACCCAGGAGG | 450 |
| 20 | TGAACTCGTGTTGGAAATGTATATTGTG | TATTTGCTGCCTGAATATTGTGAAACAT | 428 |
| 21 | AGGCATACTGTCCCTACAAAGCT | GGCTATATTCATGCCACAAAACAG | 433 |
Results
Clinical features
Patients with ML II
As shown in Table 2, the ML II group contains 6 patients, including 4 females and 2 males. Patient 1a and 1b were fraternal twins. The onset age of the 6 patients ranged from 3 to 6 months, and the diagnosis age ranged from 6 months to 2 years and 6 months. Two patients died when they were about 3 years of age (patient no.4 and no.5). All 6 patients had joint stiffness, short stature and developmental delay or intellectual disability. Other main clinical features included coarse facies (5 cases) and skeletal deformity (4 cases). Some patients had gingival hyperplasia (2 cases), Mongolian spots (1 case) and left ventricular hypertrophy (1 case).
Table 2.
Clinical features and genotypes of 20 Chinese probands with ML II and III alpha/beta
| Patient no. | Sex | Age of onset | Age of diagnosis | Type | HS | SD | CF | DD | SS | MS | HD | GH | ASA | HexA | GAG/Cr | GNPTAB mutation | GNPTAB mutation | Mutation type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | M | 3 m | 2y6m | II | + | + | + | + | + | - | - | - | 4323 | 724.9 | 41.9 | c.1523delG (p.Gly508Aspfs*39) | c.3335 + 5G > A | NS/Spl |
| 1b | F | 3 m | 2y6m | II | + | + | + | + | + | - | - | - | 4607 | 759.7 | 34.6 | c.1523delG (p.Gly508Aspfs*39) | c.3335 + 5G > A | NS/Spl |
| 2 | F | 3 m | 6 m | II | + | - | + | + | + | - | - | - | 3230 | 420.6 | 46.6 | c.2404 C > T (p.Gln802Ter) | c.2404 C > T (p.Gln802Ter) | NS/NS |
| 3 | F | 3 m | 7 m | II | + | + | + | + | + | - | - | + | 7776 | 594.2 | ND | c.88_89delAC (p.Thr30Hisfs*24) | c.2550-2554del (p.Lys850Asnfs*10) | FSH/FSH |
| 4 | F | 5 m | 11 m | II | + | + | - | + | + | + | - | + | 4516 | 232.9 | ND | c.1090 C > T (p.Arg364Ter) | c.2404 C > T (p.Gln802Ter) | NS/NS |
| 5 | M | 6 m | 2y6m | II | + | - | + | + | + | - | + | - | 8930 | 510.1 | ND | c.1090 C > T (p.Arg364Ter) | c.1284 + 1G > A | NS/Spl |
| 6 | F | 1y | 3y | III | + | + | + | - | + | - | + | - | 8505 | 417 | ND | c.1760G > A (p.Arg587Pro) | c.1760G > A (p.Arg587Pro) | MS/MS |
| 7c | M | 7 m | 7 m | III | + | + | + | - | + | - | - | - | 5730 | 828.9 | 55.9 | c.118-1G > A | ND | Spl |
| 7d | F | 10 m | 3y10m | III | + | + | + | - | + | - | - | - | 5346 | 655 | 9.2 | c.118-1G > A | ND | Spl |
| 8 | M | 1y9m | 4y9m | III | + | + | + | - | + | - | - | - | 6581 | 695.5 | 10.4 | c.1760G > A (p.Arg587Pro) | c.2693delA (p.Lys898Serfs*13) | MS/FSH |
| 9 | M | 2y | 4y | III | + | - | + | + | + | + | - | - | 6298 | 622.2 | 17.6 | c.3094delA (p.Thr1032Hisfs*11) | c.571 + 4 A > G | FSH/Spl |
| 10 | F | 2y | 5y | III | + | + | - | - | - | + | - | - | 2309 | 650.9 | 9.7 | c.2455G > T (p.Glu819Ter) | ND | NS |
| 11 | F | 3y | 12y | III | + | + | - | + | + | - | + | - | 7107 | 279.4 | ND | c.673 C > T (p.Gln225Ter) | ND | NS |
| 12 | M | 3y | 11y | III | + | + | + | + | + | + | - | - | ND | 939.3 | 8.9 | c.2404 C > T (p.Gln802Ter) | c.2715 + 1G > A | NS/Spl |
| 13 | M | 3y | 14y | III | + | + | - | + | + | - | - | - | 5903 | 721.1 | 6.6 | c.196 C > T (p.Gln66Ter) | c.2715 + 1G > A | NS/Spl |
| 14 | M | 3y | 12y | III | + | - | - | - | + | - | - | - | 3604 | 172 | 6.6 | c.1090 C > T (p.Arg364Ter) | c.3571 C > T (p.Arg1191Cys) | NS/MS |
| 15 | M | 4y | 10y | III | + | - | - | - | + | - | - | - | 1897 | 303.6 | 6.6 | c.1582T > C (p.Cys528Arg ) | c.2715 + 1G > A | MS/Spl |
| 16 | M | 6y | 7y | III | + | - | - | - | - | + | - | - | 6085 | 597.1 | 7.7 | c.77G > A (p.Gly26 > Asp ) | ND | MS |
| 17e | M | 5y | 10y | III | + | - | - | - | - | - | + | - | 7685 | 304.2 | 4.5 | c.1634_1635delAA (p.Lys545Serfs*16) | c.2715 + 1G > A | FSH/Spl |
| 17f | F | 9y | 13y | III | + | - | - | - | - | - | - | - | 8100 | 348.7 | 5.6 | c.1634_1635delAA (p.Lys545Serfs*16) | c.2715 + 1G > A | FSH/Spl |
Novel mutations are indicated in bold letters.
a, b: fraternal twins; c,d: affected siblings; e, f: affected siblings; F: female; M: male; m: month; y: year; ASA: arylsulphatase A; HexA: β-hexosaminidase A; CF: coarse facies; Cr: urine creatinine; DD: developmental delay; GAG: urine glycosaminoglycan; GH: gingival hyperplasia; HS: hand stiffness; HD: heart disease; MS: Mongolian spots; SD: skeletal deformity; SS: short statue; ND, not detected; FSH: frameshift; MS: missense; NS: nonsense; Spl: splice site.
ASA normal reference range: (50–140)nmol/mg.17 h.
HexA normal reference range: (29.8–63.8)nmol/mg.h.
GAG/Cr normal reference range: <64.0 mg/mmol(< 28days), < 49.9 mg/mmol(28days-6months), < 37.8 mg/mmol(6months-1year), < 30.0 mg/mmol(1-3years), < 19.9 mg/mmol(3-5years), < 16.0 mg/mmol(5-7years), < 12.6 mg/mmol(7-18years), < 7.1 mg/mmol(> 18years). The detection time of GAG/Cr is the diagnosed time.
Patients with ML III alpha/beta
The ML III alpha/beta group contained 14 patients, including 5 females and 9 males. Patient 7c and 7d, 17e and 17f were affected siblings. The onset age of the 14 patients ranged from 7 months to 9 years of age, and the diagnosis age ranged from 7 months to 14 years of age. The clinical symptoms were similar to ML II and progressed with age. The 14 ML III alpha/beta patients all had joint stiffness. Eight of them had skeletal deformity, six had coarse facies, four had developmental delay and Mongolian spots and three had valvular disease or left ventricular hypertrophy. Notably, patient 7c and 7d were finally classified into ML III alpha/beta, though the onset age was within 1 year of age. Though the siblings began to develop joint stiffness and coarse facies in infancy, the disease progressed slowly. By their mid-teens, they were still alive and relied on assistive wheelchair for longer distances.
Radiographic findings
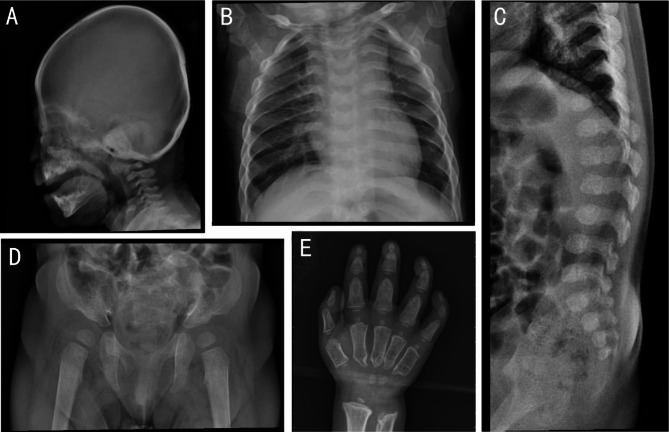
The bone X-ray examination of most patients was characterised by “dysostosis multiplex”. The combination of radiographic features included “J” shaped sella turcica, oar shaped ribs, anterior inferior beaking of lower thoracic to upper lumbar vertebral bodies, flared iliac wings, constricted iliac bodies, dysplastic femoral heads, “bullet-shaped” proximal phalanges and central pointing of proximal metacarpals (Fig. 1).
Fig. 1.
Radiographic findings of patient no.2 (A, B, C, D) and patient no.9 (E). (A) ‘J’ shaped sella turcica. (B) Oar shaped ribs. (C) Anterior inferior beaking of lower thoracic to upper lumbar vertebral bodies. (D) Flared iliac wings, constricted iliac bodies, dysplastic femoral heads. (E) ‘bullet-shaped’ proximal phalanges and central pointing of proximal metacarpals
Biochemical analysis
As shown in Table 2, plasma activities of ASA and HexA were significantly higher than the reference values. The average of ASA activities was 5712nmol/mg.17 h. The ASA activities of patients increased 13 to 63 times of reference value. The average of HexA activities was 538.9nmol/mg.h. The HexA activities of patients increased 3 to 4 times of reference value. Most GAG/Cr ratios of patients were normal. The GAG/Cr ratios of patient 1a, 1b and 7c were slightly elevated. The GAG/Cr ratios in patients with mucopolysaccharidosis (MPS) generally increase more than 2 times of the reference value. Combined with the results of enzyme activities, we could exclude MPS.
GNPTAB mutations
As shown in Table 3, out of 40 mutant alleles in 20 children with mucolipidosis we collected, only 35 mutant alleles (87.5% identification)were detected. The remaining five mutations were not detected. It may be related to large fragments are missing. This is just speculation. And we need to perform cDNA or CNVs studies to detect the variants we didn’t find in the affected cases. There were 20 different variants, including 15 reported mutations (c.2715 + 1G > A, c.3571 C > T, c.2455G > T, c.2404 C > T, c.1760G > C, c.1090 C > T, c.673 C > T, c.196 C > T, c.77G > A, c.3094delA, c.2693delA, c.2550_2554del, c.1523delG, c.88_89delAC, c.118-1G > A) and five novel mutations (c.3335 + 5G > A, c.1284 + 1G > A, c.571 + 4 A > G, c.1634_1635delAA, c.1582T > C). None of the novel mutations were found in the 1000 Genomes database (www.1000genomes.org) and HGMD database. In addition, novel missense mutation c.1582T > C (p.Cys528Arg) was predicted to be damaging by Mutation Taster and Polyphen-2 web software. The Cys528 is located in the immunoglobulin domain of GlcNAc-1-PT. This domain participates in specific recognition and binding to lysosomal hydrolases, allowing the catalytic domain to tag their glycans. Mutation p.Cys528Arg may affect the recognition and binding of lysosomal hydrolases [3]. Novel splicing mutation c.1284 + 1G > A was predicted to be deleterious by Mutation Taster, FATHMM and NetGene2. Mutation c.3335 + 5G > A and c.571 + 4 A > G were predicted to be influential by FATHMM, while to be benign by Mutation Taster. It is necessary to perform cDNA analysis of c.3335 + 5G > A and c.571 + 4 A > G mutations to be sure the effect they cause. The missense mutation was the most common form, accounting for 45.7% (16/35) in this study, followed by splicing mutation and deletion mutation, accounting for 31.4% (11/35) and 22.9% (8/35), respectively. Mutation c.2715 + 1G > A and c.2404 C > T accounted for 14.3% (5/35) and 11.4% (4/35), respectively. About 34.2% (12/35) detected mutations were localized in exon 13, suggesting that this exon was hotspot region for GNPTAB mutations.
Table 3.
GNPTAB gene mutation summary in our 20 patients
| Alteration | Location | effect | alleles | frequency | Phenotype | |
|---|---|---|---|---|---|---|
| 1 | c.2715 + 1G > A | I13 | Splicing | 5 | 14.3% | III |
| 2 | c.2404 C > T | E13 | p.Gln802Ter | 4 | 11.4% | II/III |
| 3 | c.1760G > C | E13 | p.Arg587Pro | 3 | 8.6% | III |
| 4 | c.1090 C > T | E9 | p.Arg364Ter | 3 | 8.6% | II/III |
| 5 | c.118-1G > A | I1 | Splicing | 2 | 5.7% | III |
| 6 | c.3335 + 5G > A | I17 | Splicing | 2 | 5.7% | II |
| 7 | c.1523delG | E12 | p.Gly508Aspfs*39 | 2 | 5.7% | II |
| 8 | c.1634_1635delAA | E13 | p.Lys545Serfs*16 | 2 | 5.7% | III |
| 9 | c.77G > A | E1 | p.Gly26 > Asp | 1 | 2.9% | III |
| 10 | c.196 C > T | E2 | p.Gln66Ter | 1 | 2.9% | III |
| 11 | c.673 C > T | E7 | p.Gln225Ter | 1 | 2.9% | III |
| 12 | c.1582T > C | E12 | p.Cys528Arg | 1 | 2.9% | III |
| 13 | c.2455G > T | E13 | p.Glu819Ter | 1 | 2.9% | III |
| 14 | c.3571 C > T | E19 | p.Arg1191Cys | 1 | 2.9% | III |
| 15 | c.571 + 4 A > G | I5 | Splicing | 1 | 2.9% | III |
| 16 | c.1284 + 1G > A | I10 | Splicing | 1 | 2.9% | II |
| 17 | c.88_89delAC | E1 | p.Thr30Hisfs*24 | 1 | 2.9% | II |
| 18 | c.2550_2554del | E13 | p.Lys850Asnfs*10 | 1 | 2.9% | II |
| 19 | c.2693delA | E13 | p.Lys898Serfs*13 | 1 | 2.9% | III |
| 20 | c.3094delA | E15 | p.Thr1032Hisfs*11 | 1 | 2.9% | III |
E: exon, I: intron
Discussion
In this study, joint stiffness and skeletal deformities were the earliest and the most important manifestations of patients. Plasma lysosomal hydrolase activities (ASA and HexA) increased significantly. Bone X-ray examinations showed radiological changes. The earliest age of onset of our ML II patients was 3 months of age, and no cases of neonatal onset have been found in our study. However, it was reported that severe cases of ML II could present earlier as prenatal skeletal dysplasia and neonatal severe secondary hyperparathyroidism [16, 17]. Most ML II patients presented with symptoms immediately after birth. The median age of symptom onset was 0 years [18]. In America, 14 ML II patients were born with the abnormalities and half of them died between the ages of 3 days and 8 years [2]. In Australia, 6 out of 15 ML II cases had neonatal onset and died at around 27 months [25]. In Beijing, 1 out of 8 ML II patient had neonatal onset, the remaining 7 patients had onset range from 2 months to 6 months [26]. In Eastern China, 4 out of 15 ML II patients had neonatal onset, the remaining 11 patients had onset range from 3 months to 18 months [27]. Our research observed that the proportion of Chinese patients who got sick in infancy was relatively fewer compared to those from abroad. It may be related to the difference of mutation spectrum of GNPTAB gene.
Previous research has found that a 45-year-old patient with no obvious abnormalities in the pre-birth period was diagnosed with atypical juvenile rheumatoid arthritis when he was 3 years old. The skeletal deformity progressed with age and by the age of 10 years, mucolipidosis was confirmed by enzyme examination [28]. Similarly, patient no.10 of our study had costal valgus and mild finger joints inflexibility at the age of 2 years. She was diagnosed with the sequelae stage of rickets at that time. As the stiffness of the finger joints worsened, she was diagnosed with mucolipidosis by enzyme activities around the age of 5 years. Although there are some clinical and imaging overlaps between ML and mucopolysaccharidosis diseases, urinary GAG/Cr and lysosomal hydrolase activities can be distinguished. Through the above biochemical indicators and imaging examination, mucolipidosis can be distinguished from rheumatoid arthritis and rickets as well. GNPTAB gene analysis can further confirm the diagnosis.
There were regional and ethnic differences in the variation spectrum of GNPTAB gene. According to reports, c.3503delTC variant was common in Portugal, Brazil, Italy, Turkey, Argentina and America. The c.3503delTC variant accounted for 45% and 22.7% in Portuguese and the United States, respectively. And it was speculated to be associated with ML II [2, 29]. In Japan and South Korea, p.Arg1189Ter was the most common variant, accounting for 41% and 30%, respectively [30, 31]. In eastern China, p.Arg364Ter was the most frequent mutation, accounting for 18% [32]. In northern China, c.2715 + 1G > A was the most common variant, accounting for 28%, followed by p.Arg364Ter and c.2345 C > T, accounting for 13% and 9%, respectively [26]. Similarly, c.2715 + 1G > A was the most common variant in our research, accounting for 14.3%. The GNPTAB mutations reported in ML patients of other countries were concentrated in exon 19, while it was exon 13 in Chinese.
Cathey, et al. found homozygous or compound heterozygous nonsense mutation and frameshift mutation caused more serious phenotypes. The ML II phenotype correlates directly with those GNPTAB genotypes that produce no or nearly no gene products [2]. In our study, there were one patient homozygous for p.Gln802Ter variant (patient no.2), one compound heterozygous for two different nonsense mutations (patient no.4), and one compound heterozygous for two different frameshift mutations (patient no.3). They developed the disease within one year of age and progressed rapidly. All of them were associated with ML II phenotype. And the p.Arg587Pro homozygote was detected in a patient with ML III alpha/beta. She had finger joint stiffness and scoliosis at the age of 1 year. By the age of 20 years, she could barely walk due to the worsening bone deformities. Our study similar to the previous reports that mutation p.Gln802Ter and p.Arg587Pro was associated with ML II and ML III α/β, respectively [27, 33].
Among our 5 ML III α/β patients with c.2715 + 1G > A heterozygote, they carried another mutation p.Gln802Ter, p.Gln66Ter, p.Arg364Ter and p.Lys545Serfs*16, respectively. They were characterized by a later onset form and showed mild phenotype, which speculated that the c.2715 + 1G > A mutation might be a mild mutation associated with ML III α/β. Similarly, in eastern China, c.2715 + 1G > A was identified in four patients with ML III α/β [27]. In northern China, c.2715 + 1G > A was identified in both ML II α/β (3 patients) and ML III α/β (6 patients) [26]. In Japan, c.2715 + 1G > A has been reported to be a mild form of gene mutation involved in ML III [30]. Patient no.5 was p.Arg364Ter/c.1284 + 1G > A genotype and classified as ML II. He developed the disease when he was 6 months of age, presenting with developmental delay and stiff fingers. The disease progressed rapidly and he died at about 3 years of age. The nonsense mutation p.Arg364Ter was reported to be a severe mutation associated with ML II [2]. Yang Ke, et al. also found very similar mutation p.Arg364Ter/c.1284 + 1G > T in a ML II patient [34]. We speculated that the novel mutation of c.1284 + 1G > A we found might be a severe mutation associated with ML II. Under the influence of two highly pathogenic mutations, our patient presented severe symptoms as ML II.
Conclusions
The mutation spectrum of GNPTAB gene exhibits ethnic and regional disparity in ML patients. Mutation c.2715 + 1G > A was the most prevalent mutation in our study. The novel mutation c.1284 + 1G > A might be a severe mutation associated with ML II.
Acknowledgements
We gratefully acknowledge the patients and their families for participating in this study.
Abbreviations
- ML
Mucolipidosis
- GlcNAc-1-PT
N-acetylglucosamine-1-phosphotransferase
- M6P
Mannose 6-phosphate
- ASA
Arylsulfatase A
- HexA
Hexosaminidase A
- GAG
Glycosaminoglycan
- Cr
Creatinine
Author contributions
YYF, YLH acquisition of data, analysis and interpretation of data, drafting the article. YLH, LL and WZ analysis and interpretation of data, revising the manuscript critically for important intellectual content. XYZ and XYS Performed biochemical analysis. HYS and XY performed genetic analysis. All authors reviewed and approved the manuscript.
Funding
This work was supported by Guangzhou Municipal Science and Technology Project (No.202102080028), Natural Science Foundation of Guangdong Province (No.2022A1515012524), Guangdong Medical Development Foundation (K-202108-5), Natural Science Foundation of Guangdong Province (No.2022A1515012524).
Data availability
The datasets generated and/or analysed during the current study are available in the ClinVar repository. The ClinVar accession number are SCV004041869, SCV004041870, SCV004041871, SCV004041872, SCV004041873, SCV004041874, SCV004041875, SCV004041876, SCV004041877, SCV004041878, SCV004041879, SCV004041880, SCV004041881, SCV004041882, SCV004041883, SCV004041884, SCV004041885, SCV004041886, SCV004041887, SCV004041888.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Guangzhou Women and Children’s Medical Center (2015-92). The patients’ parents provided written informed consent for all genetic studies performed in relation to this case.
Consent for publication
The written informed consents for publication of identifying images and clinical details were obtained from all parents.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gilbertbarness EF, Barness LA. The mucolipidoses. Perspect Pediatr Pathol. 1993;17:148. [PubMed] [Google Scholar]
- 2.Cathey SS, Leroy JG, Wood T, et al. Phenotype and genotype in mucolipidoses II and III alpha/beta: a study of 61 probands. J MED GENET. 2010;47:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorelik A, Illes K, Bui KH, Nagar B. Structures of the mannose-6-phosphate pathway enzyme, GlcNAc-1-phosphotransferase. Proceedings of the National Academy of Sciences - PNAS. 2022;119:e2091449177. [DOI] [PMC free article] [PubMed]
- 4.Coutinho MF, Prata MJ, Alves S. Mannose-6-phosphate pathway: a review on its role in lysosomal function and dysfunction. MOL GENET METAB. 2012;105:542–50. [DOI] [PubMed] [Google Scholar]
- 5.PINTO R, CASEIRO C, RIBEIRO I, et al. Prevalence of lysosomal storage diseases in Portugal. Eur J Hum Genetics: EJHG. 2004;12:87–92. [DOI] [PubMed] [Google Scholar]
- 6.Okada S, Owada M, Sakiyama T, Yutaka T, Ogawa M. I-cell disease: clinical studies of 21 Japanese cases. CLIN GENET. 1985;28:207–15. [DOI] [PubMed] [Google Scholar]
- 7.Pohlmann RA, Waheed A, Hasilik A, Figura KV. Synthesis of phosphorylated recognition marker in lysosomal enzymes is located in the cis part of Golgi apparatus. J Biol Chem. 1982;257:5323. [PubMed] [Google Scholar]
- 8.Affandi Omar R. Mohamed, Epidemiology and genotyping of patients with Lysosomal Storage Disease in Malaysia. 10.21203/rs.3.rs-757575/v1
- 9.Kudo M. The alpha- and beta-subunits of the human UDP-N-acetylglucosamine:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase [corrected] are encoded by a single cDNA. J biol chem. 2005;280:36141–9. [DOI] [PubMed] [Google Scholar]
- 10.Tiede S, Storch S, Lubke T, et al. Mucolipidosis II is caused by mutations in GNPTA encoding the alpha/beta GlcNAc-1-phosphotransferase. NAT MED. 2005;11:1109–12. [DOI] [PubMed] [Google Scholar]
- 11.Raas-Rothschild A, Cormier-Daire V, Bao M, et al. Molecular basis of variant pseudo-hurler polydystrophy (mucolipidosis IIIC). J Clin Investig. 2000;105:673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao M, Elmendorf BJ, Booth JL, Drake RR, Canfield WM. Bovine UDP-N-acetylglucosamine:lysosomal-enzyme N-acetylglucosamine-1-phosphotransferase. II. Enzymatic characterization and identification of the catalytic subunit. J BIOL CHEM. 1996;271:31446–51. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Yang X, Li Y, et al. GCAF(TMEM251) regulates lysosome biogenesis by activating the mannose-6-phosphate pathway. NAT COMMUN. 2022;13:5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dierks T, Schlotawa L, Frese M et al. Molecular basis of multiple sulfatase deficiency, mucolipidosis II/III and Niemann-pick C1 disease - lysosomal storage disorders caused by defects of non-lysosomal proteins. Biochimica et Biophysica Acta (BBA) -. Mol Cell Res 2009:710–25. [DOI] [PubMed]
- 15.Cathey SS, Kudo M, Tiede S, Raas-Rothschild A, Mckusick VA. Molecular order in mucolipidosis II and III nomenclature. AM J MED GENET A. 2010;146A:512–3. [DOI] [PubMed] [Google Scholar]
- 16.Heo JS, Choi KY, Sohn SH, et al. A case of mucolipidosis II presenting with prenatal skeletal dysplasia and severe secondary hyperparathyroidism at birth. Korean J Pediatr. 2012;55:438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unger Sheila PDA, Nino Michelle C, et al. Mucolipidosis II presenting as severe neonatal hyperparathyroidism. EUR J PEDIATR. 2005;164:236–43. [DOI] [PubMed] [Google Scholar]
- 18.Emma JD, Margreet AEMW, Martina W et al. Mucolipidosis type II and type III: a systematic review of 843 published cases. GENET MED 2021;23. [DOI] [PubMed]
- 19.Robinson C, Baker N, Noble J, King A, Cundy T. The osteodystrophy of mucolipidosis type III and the effects of intravenous pamidronate treatment. J INHERIT METAB DIS. 2002;25:681–93. [DOI] [PubMed] [Google Scholar]
- 20.Miura S, Yamada A, Ujihira K, Iba Y, Nakanishi K. Aortic valve replacement for two siblings with Mucolipidosis Type III. Japanese J Cardiovasc Surg. 2018;47:7–12. [Google Scholar]
- 21.Sheth J, Mistri M, Kamate M, Vaja S. FJ Sheth. Diagnostic strategy for mucolipidosis II/III. INDIAN PEDIATR 2012;49. [DOI] [PubMed]
- 22.Brown CA, Mahuran DJ. beta-hexosaminidase isozymes from cells cotransfected with alpha and beta cDNA constructs: analysis of the alpha-subunit missense mutation associated with the adult form of Tay-Sachs disease. AM J HUM GENET. 1993;53:497. [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao XY, Huang Y, Li S, Lin W, Zhou Z. Quantitative measuring urinary glycosaminoglycan by dimethylmethylene-tris spectrophotometric method. Chin J Child Health Care. 2010;18:885–8. [Google Scholar]
- 24.Robin S, Sebastian P, Markus S et al. MutationTaster2021. NUCLEIC ACIDS RES 2021;Jul 2; 49:W446-W451. [DOI] [PMC free article] [PubMed]
- 25.David-Vizcarra G, Briody J, Ault J, et al. The natural history and osteodystrophy of mucolipidosis types II and III. J PAEDIATR CHILD H. 2010;46:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S, Zhang W, Shi H, et al. Mutation analysis of 16 mucolipidosis II and III Alpha/Beta Chinese children revealed Genotype-Phenotype Correlations. PLoS ONE. 2016;11:e163204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Ye J, Qiu WJ, et al. Identification of predominant GNPTAB gene mutations in Eastern Chinese patients with mucolipidosis II/III and a prenatal diagnosis of mucolipidosis II. ACTA PHARMACOL. 2019;40:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerr DA, Memoli VA, Cathey SS, Harris BT. Mucolipidosis type III α/β:the first characterization of this rare disease by autopsy. ARCH PATHOL LAB MED. 2011;135:503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Encarnação M, Lacerda L, Costa R, et al. Molecular analysis of the GNPTAB and GNPTG genes in 13 patients with mucolipidosis type II or type III identification of eight novel mutations. CLIN GENET. 2009;76:76–84. [DOI] [PubMed] [Google Scholar]
- 30.Otomo T, Muramatsu T, Yorifuji T, et al. Mucolipidosis II and III alpha/beta: mutation analysis of 40 Japanese patients showed genotype-phenotype correlation. J HUM GENET. 2009;54:145–51. [DOI] [PubMed] [Google Scholar]
- 31.Paik KH, Song SM, Ki CS, Yu HW, Jin DK. Identification of mutations in the GNPTA (MGC4170) gene coding for GlcNAc-phosphotransferase alpha/beta subunits in Korean patients with mucolipidosis type II or type IIIA. HUM MUTAT 2010;26. [DOI] [PubMed]
- 32.Yu W, Jun Y, Wen-Juan Q et al. Identification of predominant GNPTAB gene mutations in Eastern Chinese patients with mucolipidosis II/III and a prenatal diagnosis of mucolipidosis II. ACTA PHARMACOL SIN 2018:1. [DOI] [PMC free article] [PubMed]
- 33.Lam CW, Yan MS, Li CK, Lau KC. DNA-based diagnosis of mucolipidosis type IIIA and mucopolysacchariodisis type VI in a Chinese family: a chance of 1 in 7.6 trillion. Clin Chim Acta: Int J Clin Chem Appl Mol Biology 2007;376. [DOI] [PubMed]
- 34.Yang Ke, Guiyu L. A novel compound heterozygous mutation of GNPTAB gene underlying a case with mucolipidosis type II a / β. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2022:39. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the ClinVar repository. The ClinVar accession number are SCV004041869, SCV004041870, SCV004041871, SCV004041872, SCV004041873, SCV004041874, SCV004041875, SCV004041876, SCV004041877, SCV004041878, SCV004041879, SCV004041880, SCV004041881, SCV004041882, SCV004041883, SCV004041884, SCV004041885, SCV004041886, SCV004041887, SCV004041888.