Abstract
To evaluate the impact of open surgical care (OSC) compared to minimally invasive surgery (MIS) on the occurrence of wound infection (WI) and overall postoperative aggregate complications (POACs) in female cervical cancer (CC) patients, we conducted this meta-analysis study. A thorough examination of the literature up to March 2024 was conducted, and 1849 related studies were examined. The 44 studies that were selected included 11,631 females who had CC. The odds ratio (ORs) and the estimation using 95% confidence intervals (CIs) were used to calculate the impact of open surgical management and MIS on WI and POACs in females with CC, using dichotomous methodologies and a random or fixed model. When comparing MIS to open surgical care, there was a substantial decrease in WI (OR, 0.19; 95% CI, 0.13–0.29, p < 0.001) and POACs (OR, 0.49; 95% CI, 0.38–0.62, p < 0.001) in females with CC. On the other hand, among female patients with CC, MIS did not differ significantly from open surgical care in pelvic infection and abscess (PI&A) incidence (OR, 0.59; 95% CI, 0.31–1.16, p = 0.13). When compared to OSC, women with CC who underwent MIS experienced considerably fewer WI and POACs; however, there was no discernible difference in PI&A rates. However, given several of the designated examinations for the meta-analysis had relatively small sample sizes, caution must be used while handling its values.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12893-024-02713-8.
Keywords: Wound infection, Laparotomy, Minimally invasive surgery, Postoperative issues, Cervical cancer
Introduction
Cervical cancer (CC), including its extension, ranks as the fourth most prevalent form of cancer in females [1]. The chief choice for treating such situations was a radical hysterectomy (RH) through open surgical care (OSC) [2]. Robotic surgery, also known as laparoscopy, has gained global recognition as the most effective treatment for CC, even in its early stages, thanks to the development of the laparoscopic approach paired with minimally invasive surgery (MIS) [3]. The clinical study’s findings on laparoscopic methodology for the cervix were surprising in that they indicated poorer overall and disease-free rates of survival for MIS in 2018 compared to OSC [4]. The four National Comprehensive Cancer Network recommendations and subsequent literature referred to OSC as a typical and suggested strategy for RH for CC [5]. To fully understand the correct intent of OSC and MIS for CC, it is crucial to talk about surgical outcomes. A briefer hospital stay, a lesser amount of blood loss, and a quicker recovery time are just a few advantages that MIS has over OSC for the treatment of gynecological problems [5–8]. Like the mainstream of previous examinations, MIS offers the advantage of a 3D viewpoint and a more specific surgical setting over open surgical management [9–12]. MIS was also associated with operative difficulty, a lengthy learning curve, and a higher cost compared to OSC. There isn’t much proof to back up the superiority of any one surgical technique, and it is uncertain whether MIS is harmless and effective due to the poor quality of previous studies, the small sample sizes, and the dearth of randomized controlled trials (RCTs). There was no difference in postoperative problems between OSC and MIS, according to several previous studies on complications [13]. As instruments and methods have improved, many studies have discovered that MIS has lower POAC rates than OSC [14]. Incorrectly, it is still unknown whether female complication rates from OSC are lower than those from MIS. A chief component in the assessment of CC is one that emphasizes the gravity of the issues. In order to provide trustworthy information to compare the advantages of different surgical techniques for treating CC, our meta-analysis set out to assess the effects of open surgical interventions and MIS on wound infection (WI), POACs, pelvic infection, and abscess.
Methods
Eligibility criteria
The studies showing how MIS and OSC affect WI and POACs in female CC patients chosen so that an overview could be made [15]. The protocol was registered in PROSPERO (ID number: CRD2111617323).
Information sources
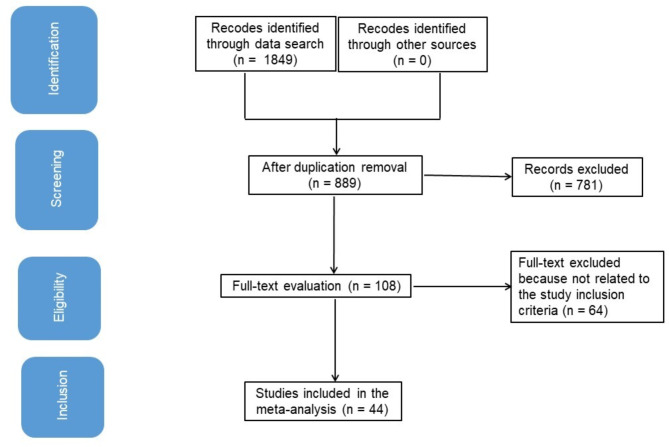
Figure 1 is an overall study representation. When the next criteria of inclusion were satisfied, literature was incorporated into the study: [16, 17]
Fig. 1.
Shows the study procedure flowchart
The study was a RCT, observational, retrospective, prospective one.
Selected female subjects for the investigation were those who had CC.
Open surgical management was added into the operation.
The study made a distinction between the impact of open surgical management and MIS on WI and POACs in CC treatment.
Laparoscopy studies, with or without the use of robotics, were included in the analysis.
Explorations on WIs and POACs in females without MIS and open surgical management, as well as explorations that did not examine features of consequence of MIS and open surgical management on WI and POACs in females with CC, were excluded from consideration [18, 19].
Search strategy
We defined the search protocol operations in accordance with the PICOS opinion. WIs, POACs, pelvic infection, and abscess (PI&A) were the “outcomes”; finally, the “study design” was unrestricted. The “population” (P) consisted of female patients with CC; the “intervention” or “exposure” was open surgical management, and the “comparison” was between MIS and open surgical management [19]. In 2002, the first robotic-hysterectomy was carried out by Diaz-Arrasti [20], followed by several published trials. Hence, we searched for studies published between 2002 and until March 2024 using the following databases: the Cochrane Library, Embase, Google Scholar, PubMed, and OVID. We accomplished this by organizing keywords and connected expressions, as shown in Table 1. We eliminated paper repetitions, compiled them into an EndNote file, and reassessed their titles and abstracts to omit studies that could not demonstrate a connection between the outcomes of open surgical management and MIS, WI, and POACs in female CC patients [19–21].
Table 1.
Search Strategy for Each Database
| Database | Search strategy |
|---|---|
| Google Scholar |
#1 “cervical cancer” OR “minimally invasive surgery” #2 “laparotomy” OR “wound infection” OR " “postoperative complication” OR “pelvic infection and abscess” #3 #1 AND #2 |
| Embase |
#1 ‘cervical cancer’ /exp OR ‘minimally invasive surgery’ /exp OR ‘postoperative complication’ #2 ‘laparotomy’/exp OR ‘wound infection’/exp OR ‘pelvic infection and abscess’ #3 #1 AND #2 |
| Cochrane library |
#1 (cervical cancer): ti, ab, kw (minimally invasive surgery): ti, ab, kw (postoperative complication): ti, ab, kw (Word variations have been searched) #2 (laparotomy): ti, ab, kw OR (wound infection): ti, ab, kw OR(pelvic infection and abscess): ti, ab, kw (Word variations have been searched) #3 #1 AND #2 |
| Pubmed |
#1 “cervical cancer“[MeSH] OR “minimally invasive surgery“[MeSH] OR “postoperative complication” [All Fields] #2 “laparotomy“[MeSH Terms] OR “wound infection“[MeSH] OR “pelvic infection and abscess “[All Fields] #3 #1 AND #2 |
| OVID |
#1 “cervical cancer“[All Fields] OR “minimally invasive surgery” [All Fields] OR “postoperative complication” [All Fields] #2 “laparotomy“[ All fields] OR “wound infection“[All Fields] or “pelvic infection and abscess“[All Fields] #3 #1 AND #2 |
Selection process
A process was established after the epidemiological statement, and it was subsequently organized and scrutinized using a meta-analysis technique.
Data collection process
The criteria used to collect data included the name of the first author, study date, study year, nation or location, population type, medical and therapeutic characteristics, categories, quantitative and qualitative estimation methods, data source, outcome estimation, and statistical analysis [22].
Data items
When a study’s values were variable, we independently collected data depended on a valuation of how OSC and MIS affected WI and POACs in female CC patients [16, 17, 23].
Study risk of bias assessment
To determine was there a possibility that any of the studies was biased, two authors individualistically reviewed the methodology of chosen examinations. “Risk of bias; RoB” instrument from the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 was utilized to estimate the bureaucratic quality. Following classification according to the judgement criteria, each research work was allocated to a specific bias risk category: low: If all quality criteria were adequately met, the research was categorized as having a low RoB. However, if any requirements were not met or not addressed, the study was classed as having a medium bias risk. The analysis identified a significant risk of bias if any of the quality requirements were not fully or partially completed.
Effect measures
Only studies that evaluated and recorded the impact of open surgical management and MIS on WI and POACs in female CC patients were exposed to sensitivity analysis. Analysis of subclass was performed to assess sensitivity of females with CC and to compare OSC to MIS.
Statistical analysis
The odds ratio (OR) with a 95% confidence interval (CI) were estimated utilizing dichotomous random- or fixed-effect models [24]. Calculated I [2] index has range of 0 to 100 and is expressed as a percentage [25]. Higher I [2] values signify increased heterogeneity, whilst lower I [2] values signify decreased heterogeneity. If I [2] was 50% or above, random effect was selected; otherwise, fixed effect was chosen [26]. First study’s consequences were classified as component of analysis of subcategory. Bias was measured by Begg’s and Egger’s tests for quantitative assessment, and it was considered to be existing if the estimated P-value was above 0.05 [27, 28]. P-values were calculated by the two-tailed method. With Jamovi 2.3, graphs and statistical analyses were created [29].
Results
The study selected 44 papers published between 2002 and 2024 from all the related research that satisfied the inclusion criteria. Other studies were excluded for various reasons including studies that involved advanced stages of cervical cancer, patients who received chemotherapy prior to surgery, lacked unique data presentation, and articles that did not describe the outcome of interest. Table 2 displays the findings of these studies (30–72). Among the 11,631 female patients with CC who were at the beginning of the selected studies, 5072 were undergoing MIS, and 6559 were undergoing OSC. There were between 26 and 3333 females in the sample.
Table 2.
Characteristics of the selected investigations for the meta-analysis
| Study | Country | Study design | Total | Minimally invasive surgery | Open-surgical management | Outcomes measured | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | BMI (SD) | Age (years) | No. | BMI (SD) | Age (years) | |||||
| Lee, 2002 [30] | Taiwan | Prospective | 60 | 30 | 54.4 (12.6) | 46.2/ | 30 | 56.3 (10.4) | 48 | POACs |
| Steed, 2004 [31] | Canada | Retrospective | 276 | 71 | NA | 43/ | 205 | NA | 44 | POACs |
| Sharma, 2006 [32] | UK | Retrospective | 67 | 35 | NA | 43.4 | 32 | NA | 42.8 | POACs |
| Frumovitz, 2007 [33] | USA | Retrospective | 89 | 35 | 28.1 | 40.8 | 54 | 28.2 | 42.5 |
POACs, PI & A |
| Li, 2007 [34] | China | Retrospective | 125 | 90 | NA | 42 | 35 | NA | 44 | POACs |
| Uccella, 2007 [35] | Italy | Retrospective | 98 | 50 | 23 | 47 | 48 | 25 | 53 | POACs |
| Morgan, 2007 [36] | Ireland | Retrospective | 60 | 30 | 25 | 35 | 30 | 24 | 38 | WI, POACs |
| Zakashansky, 2007 [37] | USA | Prospective | 60 | 30 | NA | 48.3 | 30 | NA | 46.6 | WI, POACs |
| Boggess, 2008 [38] | USA | Retrospective | 100 | 51/ robotic laparoscopy | 28.6 (7.2) | 47.4 | 49 | 26.1 | 41.9 | WI, POACs,, PI & A |
| Ko, 2008 [39] | USA | Retrospective | 48 | 16/ robotic laparoscopy | 27.6(6.4) | 42.3 | 32 | 26.6 (5.9) | 41.7 | WI, POACs,, PI & A |
| Papacharalabous, 2009 [40] | UK | Retrospective | 26 | 14 | NA | 38.6 | 12 | NA | 43.5 | WI, POACs |
| Estape, 2009 [41] | USA | Retrospective | 63 | 49/ robotic laparoscopy | 29.7 (3.2) | 55 | 14 | 28.1 (4.8) | 52.8 | POACs,, PI & A |
| Maggioni, 2009 [42] | USA | Retrospective | 80 | 40/ robotic laparoscopy | 24.1 (5.5) | 44.1 | 40 | 23.6 (5.0) | 49.8 | WI, POACs |
| Malzoni, 2009 [43] | Italy | Retrospective | 147 | 65 | 26 | 40.5 | 82 | 29 | 42.7 | POACs |
| Sobiczewski, 2009 [44] | Poland | Retrospective | 80 | 22 | NA | 45.4 | 58 | NA | 51.1 | WI, POACs |
| Pahisa, 2010 [45] | Spain | Retrospective | 90 | 67 | 25.4 (1.1) | 51 | 23 | 27.2 2.5) | 48 | WI, POACs |
| Lee, 2011 [46] | Korea | Retrospective | 72 | 24 | 23.4 (3.55) | 48.4 | 48 | 23.9 (4.7) | 50.2 | POACs |
| Sert, 2011 [47] | Norway | Retrospective | 68 | 42/ robotic laparoscopy | 25.4 (4.36) | 44.1 | 26 | 22.5 (1.84) | 45 | POACs |
| Taylor, 2011 [48] | USA | Retrospective | 27 | 9 | 26.3 | 41.4 | 18 | 26.9 | 41.1 | WI, POACs |
| Gortchev, 2012 [49] | Bulgaria | Retrospective | 294 | 119 | NA | 46 | 175 | NA | 42.5 | POACs |
| Park, 2012 [50] | Korea | Retrospective | 166 | 54 | 31.8 (1.4) | 49.4 | 112 | 31.7 (1.5) | 52.1 | WI, POACs |
| Nam, 2012 [51] | Korea | Retrospective | 526 | 263 | NA | NA | 263 | NA | NA | WI,, PI & A |
| Park, 2013 [52] | Korea | Retrospective | 303 | 115 | 23.1 | 48.5 | 188 | 23.7 | 48.1 | POACs,, PI & A |
| Lim, 2013 [53] | Singapore | Prospective | 48 | 18 | 22.9 | 48 | 30 | 22.4 | 47 | WI, POACs |
| Campos, 2013 [54] | Brazil | Randomized-controlled trial | 30 | 16 | NA | 36.2 | 14 | NA | 39.6 | POACs |
| Bogani, 2014 [55] | Italy | Retrospective | 130 | 65 | 25.1 (5.2) | 48.9 | 65 | 25.9 (6.1) | 50.9 | WI, POACs |
| Chen, 2014 [56] | Taiwan | Retrospective | 100 | 56/ robotic laparoscopy | 24.4 (4.9) | 53.7 | 44 | 23.2 (3.4) | 51.2 | WI, POACs |
| Yin, 2014 [57] | China | Retrospective | 45 | 22 | NA | 44 | 23 | NA | 46 | WI, POACs |
| Asciutto, 2015 [58] | Sweden | Retrospective | 250 | 65/ robotic laparoscopy | 27.0 (6.0) | 45.4 | 185 | 25.7 (4.7) | 45.7 | POACs |
| Xiao, 2015 [59] | China | Retrospective | 154 | 106 | 23.8 (3.9) | 43.7 | 48 | 24.7 (3.8) | 45.7 | WI, POACs |
| Ditto, 2015 [60] | Italy | Retrospective | 120 | 60 | 24.4 (2.9) | 46 | 60 | 24.0 (4.3) | 45.5 | POACs |
| Park, 2016 [61] | Korea | Retrospective | 293 | 186 | 23.7 | 45.3 | 107 | 23.6 | 47.3 | POACs |
| Shah, 2017 [62] | USA | Retrospective | 311 | 109/ robotic laparoscopy | 27.9 | 45.2 | 202 | 29.1 | 45.4 | WI, POACs,, PI & A |
| Corrado, 2018 [63] | Italy | Retrospective | 341 | 240/ robotic laparoscopy | 23.3 | 46 | 101 | 23.5 | 45 | WI, POACs |
| Guo, 2018 [64] | China | Retrospective | 551 | 412 | 22.8 | 46 | 139 | 23.2 | 45 | WI |
| Bogani, 2019 [65] | Italy | Retrospective | 70 | 35 | 22.9 (4.0) | 41.1 | 35 | 20.1 (9.3) | 44.1 | POACs |
| Matanes, 2019 [66] | Canada | Retrospective | 98 | 74/ robotic laparoscopy | 26.4 | 48 | 24 | 26.2 | 47 | WI, POACs |
| Piedimonte, 2019 [67] | Canada | Retrospective | 3333 | 749/ robotic laparoscopy | NA | NA | 2584 | NA | NA | WI, POACs |
| Yuan, 2019 [68] | China | Retrospective, single center | 198 | 99 | 44.6 (7.6) | 43.6 | 99 | 24.6 (1.5) | 44.6 | WI, POACs |
| Li, 2021 [69] | China | Retrospective | 1207 | 661 | NA | 46.9 | 546 | NA | 47.03 | POACs |
| Zaccarini, 2021 [70] | France | Retrospective | 93 | 61 | 26.4 (4.7) | 48.3 | 32 | 27.2 (6.0) | 51 | POACs,, PI & A |
| Jing, 2023 [71] | China | Retrospective | 61 | 45 | NA | 49.06 | 16 | NA | 49.37 | WI, POACs |
| Vázquez-Vicente a, 2024 [72] | Spain | Retrospective | 117 | 39 | 25.4 (4.9) | 47.1 | 78 | 25.6 (6.1) | 46.8 | WI, POACs |
| Vázquez-Vicente b, 2024 [72] | Spain | Retrospective | 1156 | 633 | 25.1 (5.3) | 46 | 523 | 25.7 (4.6) | 48 | WI, POACs |
| Total | 11,631 | 5072 | 6559 | |||||||
NA: not available, PI & A: Pelvic infection & Abscess, POACs: postoperative aggregate complications, WI: wound infection
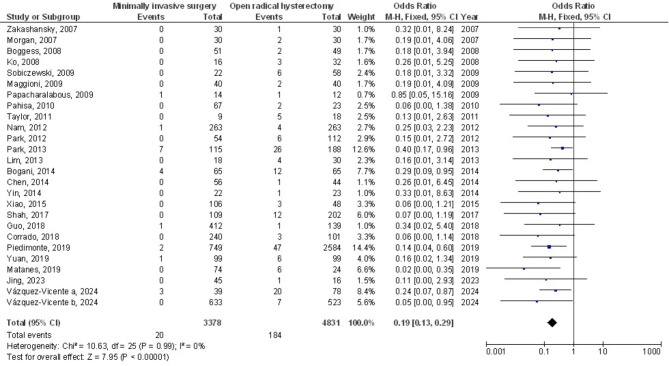
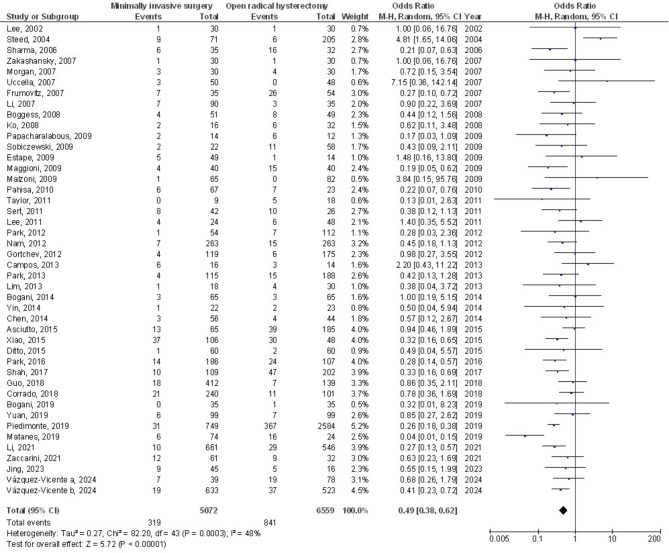
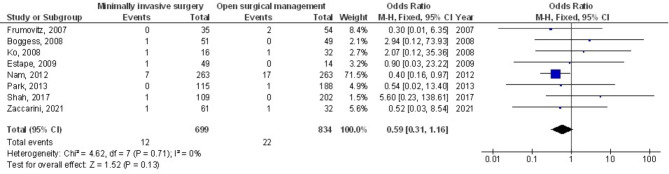
Figures 2 and 3 show that, compared to open surgery, MIS had a much lower risk of WI (OR, 0.19; 95% CI, 0.13–0.29, p < 0.001) with no heterogeneity (I2 = 0%) and POACs (OR, 0.49; 95% CI, 0.38–0.62, p < 0.001) with moderate heterogeneity (I2 = 48%) in women with CC. Figure 4 shows that there wasn’t a considerable difference in PI&A between MIS and OSC for CC patients (OR, 0.59; 95% CI, 0.31–1.16, p = 0.13), and there was also no overlap (I2 = 0%).
Fig. 2.
The forest plot analysis shows how wound infection in cervical cancer patients is affected by minimally invasive surgery as opposed to OSC
Fig. 3.
The forest plot analysis shows how POACs in cervical cancer patients were affected by minimally invasive surgery as opposed to OSC
Fig. 4.
The forest plot of the minimally invasive surgery’s impact on PI&A in cervical cancer patients in comparison to OSC
Subgroup analysis of studies based on the use of MIS techniques with or without robotics showed a consistently significant effect size for wound infection outcome [(OR, 0.23; 95% CI, 0.15–0.37, p < 0.001), and (OR, 0.11; 95% CI, 0.05–0.28, p < 0.001), respectively] with no heterogeneity between studies (I2 = 0%). Similarly, POACs subgroup analysis based on robotics use in laparoscopic procedure revealed consistent significance [(OR, 0.54; 95% CI, 0.41–0.71, p < 0.001, I2 = 34%) without robotics, and (OR, 0.38; 95% CI, 0.23–0.62, p < 0.001, I2 = 66%).) with robotics use], respectively. The effect size for both outcomes was smaller for robotic-based laparoscopy procedures versus without robotics.
Subgroup analysis based on robotic-based laparoscopy for the PI &A outcome showed a significant effect with the conventional laparoscopy (OR, 0.40; 95% CI, 0.18–0.89, p = 0.02, I2 = 0%), while robotic based procedures resulted in non-significant pooled estimate (OR, 2.42; 95% CI, 0.51–11.41, p = 0.26, I2 = 0%).
The visual interpretation of the forest plot effect and the quantitative Egger regression test revealed no indication of investigation bias (p = 0.890). The findings revealed that the majority of relevant examinations lacked practical quality and exhibited bias in their selective reporting.
Discussion
Of the studies that were considered for the meta-analysis, 5072 of the 11,631 females with CC who were at baseline of the selected investigations were using MIS, and 6559 were using open surgical management [30–72]. When compared to OSC, women with CC who underwent MIS had significantly fewer WI and POACs, but no discernible difference in PI&A. These findings are in accordance with the results of meta-analyses conducted by Kampers et al., [73], Zhang et al. [74], and Zhao et al. [75] which compared MIS versus OSC confirming the significant efficacy and safety of MIS with similar non-significance in the postoperative infection and abscess formation compared to OSC in the general and subgroup analysis. It is noteworthy that our results only included eight relevant studies in the PI&A outcome. Our analysis may be biased due to a limited number of researches comparing PI & A outcome between the two surgical techniques.
The small sample size of some of the chosen examinations (23 out of 44 ≤ 100 females) for meta-analysis requires caution when interpreting their results. Limited patient samples may introduce bias contingent upon the surgeon’s proficiency in novel surgical procedures, particularly robotic hysterectomy. This would impact how significant the evaluated assessments were [76–86]. Therefore, randomized studies with larger sample size are needed to better validate the current evidence.
The use of Veress needles throughout the process might account for the dissimilarities in abdominal damage between MIS and open surgical management [87]. The Veress needle methodology is a contained method that entails the placement and retracting of a sharp-tipped 2-mm external needle, succeeded by a hollow blunt-tipped needle that advances to provide gas. Insufflation at different pressure, duration, or volume parameters happens before to the placement of the main trocar. This technique is the predominant entry method employed by gynecologists globally, and is associated with heightened chances of mild problems, such as preperitoneal injuries, as well as entry failure [88]. The 2012 Cochrane database determined that the open entry technique significantly reduces the rate of failed entry as opposed to the closed entry technique, without variation in the occurrence of visceral or vascular harm. The minimal incidence of reported complications linked to laparoscopic entry and the limited participant pool in the trials may explain the absence of a substantial disparity in major visceral and vascular injuries between the entry procedures [89]. Previous studies have shown that a Veress needle or trocar entrenched throughout laparoscopy causes most intestinal damage and WIs. A number of risk factors could produce subcutaneous emphysema in MIS [90, 91]. The skill of the surgeon could affect the frequency of complications when considered holistically. Regretfully, this study was unable to compare different surgeons. When comparing various surgical methods, the learning curve may also significantly impact issues. MIS gained preferred over open surgical care because to the problems of the surgical approach [92, 93]. Utilization of surgical tools has been connected to injuries, which could be the consequence of thermal injury because the elevated temperature of the tools harms the deeper or sub-mucosal tissues of the gut, bladder, and bowels. Previous studies have assessed the heat damage to the intestines caused by laparoscopic surgery [90]. Recall that the open surgical treatment approach raised the risk of heat injury, so surgeons needed to be attentive to this. All of these variables were associated with the development of WIs and POACs. This aligns with the results of the former meta-analyses. Refinement of laparoscopic-assisted vaginal RH is crucial because of the intricate nature of the female pelvic floor. Urinary tract injuries are a severe problem with MIS. The uterine ligament is identified and removed in the vaginal approach, which next involves pushing on the uterus to find the bladder and ureters [94]. The gradual drop in PI&A can be ascribed to the emergence of laparoscopy as a result of advances in surgical methods, equipment, and learning curves. Compared to OSC, complete laparoscopes and robotic RHs were linked with a lower risk of POACs [92]. An earlier study by Park et al., which investigated the unfavorable impacts of the three treatments, supported these results by indicating that MIS was superior than OSC in terms of minimizing overall difficulties for females with CC [10]. The results on POACs for the group that underwent open surgical management-aided vaginal RH might be biased because of the significant heterogeneity degree and small sample size. In the future, further high-quality cohort investigations will be required to compare and estimate the risk of POACs in MIS and OSC.
Eleven studies included robotic-based laparoscopy versus OSC. We compared both robotic and non-robotic laparoscopy to OSC. The results showed that robotic-based procedures had a significant but smaller effect size on the WI and POACs outcomes. These findings are consistent with a recently published meta-analysis, which reported that conventional laparoscopic procedures have a much lower operating time and overall complication rate. Robotic laparoscopy did not improve treatment outcomes, but its application did reduce blood loss [95].
This meta-analysis validated the effects of OSC and MIS on WI and CC control. Based on the current meta-analysis findings, MIS procedures can be a preferred alternative for open surgical procedures with better outcomes in terms of wound infection and the overall postoperative morbidities. Moreover, the surgeon’s skills and proficiency may influence the incidence of complications following the procedure. Further investigation is still needed to elucidate these plausible influences. This was also highlighted in earlier studies that generated equal impact levels through the use of a correlated meta-analysis technique [96–103]. Well-led RCTs are crucial to take into account these aspects and the diversity of dissimilar ages and demographics of female participants, even if meta-analysis was unable to establish whether modifications in these features are associated to the values being studied. In conclusion, among female patients with CC, OSC resulted in dramatically decreased WIs as compared to MIS.
Limitations
Possible selection bias may have been present due to the exclusion of some studies in the meta-analysis. However, the publications that were excluded did not match the requirements to be included in the meta-analysis. However, we required the data in order to assess if demographic and age disparities had an impact on the outcomes. The objective of the exploration was to assess the influence of open surgical management and minimally invasive surgery (MIS) on wound infection (WI) and complication rate (CC) in the treatment. The presence of inaccurate or missing data in previous studies may have contributed to an increased bias. Aside from their age and race, the nutritional well-being of the girls was a possible factor contributing to discrimination. Inadequate data and unpublished investigations can lead to unwanted distortion of the value being examined.
Conclusions
When compared to OSC, MIS resulted in much lower WI and POACs; however, there was no discernible difference in PI&A rates among female patients with CC. However, the small sample size of several specified investigations (23 out of 44 ≤ 100 female patients) necessitates caution when interpreting the data in the meta-analysis, nevertheless. That would have an impact on how significant the evaluated assessments were.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
R.S., and M.M: Concept and methodology, N.Y. ,and J.L.: software, C.C., and H.W.: data curation, C.C. and H.W.: validation, N.Y., and M.M.: visualization, and J.L, R.S., H.W: writing. I confirm that the work is original; the work has not been, and will not be published, in whole, or in part, in any other journal; and all the authors have agreed to the contents of the manuscript in its submitted form.
Funding
No funding was received for this study.
Data availability
On request, the corresponding author is required to provide access to the meta-analysis database.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Ries E. THE OPERATIVE TREATMENT OF CANCER OF THE CERVIX UTERI. JAMA. 1906;XLVII(23):1869–72. [Google Scholar]
- 3.Wright JD, Herzog TJ, Neugut AI, Burke WM, Lu Y-S, Lewin SN, Hershman DL. Comparative effectiveness of minimally invasive and abdominal radical hysterectomy for cervical cancer. Gynecol Oncol. 2012;127(1):11–7. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, Buda A, Yan X, Shuzhong Y, Chetty N. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379(20):1895–904. [DOI] [PubMed] [Google Scholar]
- 5.Wood DE. National Comprehensive Cancer Network (NCCN) clinical practice guidelines for lung cancer screening. Torac Surg Clin. 2015;25(2):185–97. [DOI] [PubMed] [Google Scholar]
- 6.Galaal K, Donkers H, Bryant A, Lopes AD. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Reviews, 2018(10). [DOI] [PMC free article] [PubMed]
- 7.Diver E, Hinchcliff E, Gockley A, Melamed A, Contrino L, Feldman S, Growdon W. Minimally invasive radical hysterectomy for cervical cancer is associated with reduced morbidity and similar survival outcomes compared with laparotomy. J Minim Invasive Gynecol. 2017;24(3):402–6. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y-z, Deng L, Xu H-c, Zhang Y, Liang Z-q. Laparoscopy versus laparotomy for the management of early stage cervical cancer. BMC Cancer. 2015;15(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gala RB, Margulies R, Steinberg A, Murphy M, Lukban J, Jeppson P, Aschkenazi S, Olivera C, South M, Lowenstein L. Systematic review of robotic surgery in gynecology: robotic techniques compared with laparoscopy and laparotomy. J Minim Invasive Gynecol. 2014;21(3):353–61. [DOI] [PubMed] [Google Scholar]
- 10.Park D, Yun J, Kim S, Lee S. Surgical and clinical safety and effectiveness of robot-assisted laparoscopic hysterectomy compared to conventional laparoscopy and laparotomy for cervical cancer: a systematic review and meta-analysis. Eur J Surg Oncol (EJSO). 2017;43(6):994–1002. [DOI] [PubMed] [Google Scholar]
- 11.Madhuri TK, Butler-Manuel S. Robotic-assisted vs traditional laparoscopic surgery for endometrial cancer: A randomized controlled trial. Am J Obstet Gynecol. 2017;216(6):619. [DOI] [PubMed] [Google Scholar]
- 12.Mäenpää MM, Nieminen K, Tomás EI, Laurila M, Luukkaala TH, Mäenpää JU. Robotic-assisted vs traditional laparoscopic surgery for endometrial cancer: a randomized controlled trial. Am J Obstet Gynecol. 2016;215(5):588. e1-588. e7. [DOI] [PubMed] [Google Scholar]
- 13.Brandt B, Sioulas V, Basaran D, Kuhn T, LaVigne K, Gardner GJ, Sonoda Y, Chi DS, Roche KCL, Mueller JJ. Minimally invasive surgery versus laparotomy for radical hysterectomy in the management of early-stage cervical cancer: survival outcomes. Gynecol Oncol. 2020;156(3):591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obermair A, Asher R, Pareja R, Frumovitz M, Lopez A, Moretti-Marques R, Rendon G, Ribeiro R, Tsunoda A, Behan V. Incidence of adverse events in minimally invasive vs open radical hysterectomy in early cervical cancer: results of a randomized controlled trial. American journal of obstetrics and gynecology, 2020. 222(3): p. 249. e1-249. e10. [DOI] [PMC free article] [PubMed]
- 15.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 16.Emad M, Osama H, Rabea H, Saeed H. Dual compared with triple antithrombotics treatment effect on ischemia and bleeding in atrial fibrillation following percutaneous coronary intervention: A meta-analysis. Int J Clin Med Res. 2023;1(2):77–87. [Google Scholar]
- 17.Osama H, Saeed H, Nicola M, Emad M. Neuraxial anesthesia compared to general anesthesia in subjects with hip fracture surgery: A meta-analysis. Int J Clin Med Res. 2023;1(2):66–76. [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. [DOI] [PubMed] [Google Scholar]
- 19.Zangeneh MM, Zangeneh A. Prevalence of wound infection following right anterolateral thoracotomy and median sternotomy for resection of benign atrial masses that induce heart failure, arrhythmia, or thromboembolic events: A meta-analysis. Int J Clin Med Res. 2023;2(1):27–33. [Google Scholar]
- 20.Xie S, Wood TC, Dasgupta P, Aydin A. Robot Assisted Laparoscopic Surgery in Gynaecology: An Evolving Assistive Technology. Surg Innov. 2024;31(3):324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaaban MEA, Mohamed AIM. Determining the efficacy of N-acetyl cysteine in treatment of pneumonia in COVID-19 hospitalized patients: A meta-analysis. Int J Clin Med Res. 2023;1(2):36–42. [Google Scholar]
- 22.Gupta S, Rout G, Patel AH, Mahanta M, Kalra N, Sahu P, Sethia R, Agarwal A, Ranjan G, Kedia S. Efficacy of generic oral directly acting agents in patients with hepatitis C virus infection. J Viral Hepatitis. 2018;25(7):771–8. [DOI] [PubMed] [Google Scholar]
- 23.Guo Y. Effect of resident participation in ophthalmic surgery on wound dehiscence: A meta-analysis. Int J Clin Med Res, 2024. 2(2).
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta A, Das A, Majumder K, Arora N, Mayo HG, Singh PP, Beg MS, Singh S. Obesity is Independently Associated With Increased Risk of Hepatocellular Cancer–related Mortality. Am J Clin Oncol. 2018;41(9):874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheikhbahaei S, Trahan TJ, Xiao J, Taghipour M, Mena E, Connolly RM, Subramaniam RM. FDG-PET/CT and MRI for evaluation of pathologic response to neoadjuvant chemotherapy in patients with breast cancer: a meta-analysis of diagnostic accuracy studies. Oncologist. 2016;21(8):931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen RA, Evans RS, Burke JP, Pestotnik SL, Gardner RM, Classen DC. Improved perioperative antibiotic use and reduced surgical wound infections through use of computer decision analysis. Infect Control Hosp Epidemiol. 1989;10(7):316–20. [DOI] [PubMed] [Google Scholar]
- 28.Maki D, Schuna A. A study of antimicrobial misuse in a university hospital. Am J Med Sci. 1978;275(3):271–82. [DOI] [PubMed] [Google Scholar]
- 29.Singh RK. A meta-analysis of the impact on gastrectomy versus endoscopic submucosal dissection for early stomach cancer. Int J Clin Med Res. 2023;1(3):88–99. [Google Scholar]
- 30.Lee C-L, Huang K-G, Jain S, Lee P-S, Soong Y-K. Comparison of laparoscopic and conventional surgery in the treatment of early cervical cancer. J Am Assoc Gynecol Laparosc. 2002;9(4):481–7. [DOI] [PubMed] [Google Scholar]
- 31.Steed H, Rosen B, Murphy J, Laframboise S, De Petrillo D, Covens A. A comparison of laparascopic-assisted radical vaginal hysterectomy and radical abdominal hysterectomy in the treatment of cervical cancer. Gynecol Oncol. 2004;93(3):588–93. [DOI] [PubMed] [Google Scholar]
- 32.Sharma R, Bailey J, Anderson R, Murdoch J. Laparoscopically assisted radical vaginal hysterectomy (Coelio-Schauta): a comparison with open Wertheim/Meigs hysterectomy. Int J Gynecologic Cancer, 2006. 16(5). [DOI] [PubMed]
- 33.Frumovitz M, dos Reis R, Sun CC, Milam MR, Bevers MW, Brown J, Slomovitz BM, Ramirez PT. Comparison of Total Laparoscopic and Abdominal Radical Hysterectomy for Patients With Early-Stage Cervical Cancer. Obstet Gynecol. 2007;110(1):96–102. [DOI] [PubMed] [Google Scholar]
- 34.Li G, Yan X, Shang H, Wang G, Chen L, Han Y. A comparison of laparoscopic radical hysterectomy and pelvic lymphadenectomy and laparotomy in the treatment of Ib-IIa cervical cancer. Gynecol Oncol. 2007;105(1):176–80. [DOI] [PubMed] [Google Scholar]
- 35.Uccella S, Laterza R, Ciravolo G, Volpi E, Franchi M, Zefiro F, Donadello N, Ghezzi F. A comparison of urinary complications following total laparoscopic radical hysterectomy and laparoscopic pelvic lymphadenectomy to open abdominal surgery. Gynecol Oncol. 2007;107(1):S147–9. [DOI] [PubMed] [Google Scholar]
- 36.Morgan D, Hunter D, McCracken G, McClelland H, Price J, Dobbs S. Is laparoscopically assisted radical vaginal hysterectomy for cervical carcinoma safe? A case control study with follow up. BJOG: Int J Obstet Gynecol. 2007;114(5):537–42. [DOI] [PubMed] [Google Scholar]
- 37.Zakashansky K, Chuang L, Gretz H, Nagarsheth N, Rahaman J, Nezhat F. A case-controlled study of total laparoscopic radical hysterectomy with pelvic lymphadenectomy versus radical abdominal hysterectomy in a fellowship training program. Int J Gynecologic Cancer, 2007. 17(5). [DOI] [PubMed]
- 38.Boggess JF, Gehrig PA, Cantrell L, Shafer A, Ridgway M, Skinner EN, Fowler WC. A case-control study of robot-assisted type III radical hysterectomy with pelvic lymph node dissection compared with open radical hysterectomy. Am J Obstet Gynecol. 2008;199(4):357. e1-357. e7. [DOI] [PubMed] [Google Scholar]
- 39.Ko EM, Muto MG, Berkowitz RS, Feltmate CM. Robotic versus open radical hysterectomy: a comparative study at a single institution. Gynecol Oncol. 2008;111(3):425–30. [DOI] [PubMed] [Google Scholar]
- 40.Papacharalabous E, Tailor A, Madhuri T, Giannopoulos T, Butler-Manuel S. Early experience of laparoscopically assisted radical vaginal hysterectomy (Coelio-Schauta) versus abdominal radical hysterectomy for early stage cervical cancer. Gynecol Surg. 2009;6(2):113–7. [Google Scholar]
- 41.Estape R, Lambrou N, Diaz R, Estape E, Dunkin N, Rivera A. A case matched analysis of robotic radical hysterectomy with lymphadenectomy compared with laparoscopy and laparotomy. Gynecol Oncol. 2009;113(3):357–61. [DOI] [PubMed] [Google Scholar]
- 42.Maggioni A, Minig L, Zanagnolo V, Peiretti M, Sanguineti F, Bocciolone L, Colombo N, Landoni F, Roviglione G, Vélez JI. Robotic approach for cervical cancer: comparison with laparotomy: a case control study. Gynecol Oncol. 2009;115(1):60–4. [DOI] [PubMed] [Google Scholar]
- 43.Malzoni M, Tinelli R, Cosentino F, Fusco A, Malzoni C. Total laparoscopic radical hysterectomy versus abdominal radical hysterectomy with lymphadenectomy in patients with early cervical cancer: our experience. Ann Surg Oncol. 2009;16(5):1316–23. [DOI] [PubMed] [Google Scholar]
- 44.Sobiczewski P, Bidzinski M, Derlatka P, Panek G, Danska-Bidzinska A, Gmyrek L, Michalski W. Early cervical cancer managed by laparoscopy and conventional surgery: comparison of treatment results. Int J Gynecologic Cancer, 2009. 19(8). [DOI] [PubMed]
- 45.Pahisa J, MartÍNez-RomÁN S, TornÉ A, FustÉ P, Alonso I, LejÁRcegui JA, Balasch J. Comparative study of laparoscopically assisted radical vaginal hysterectomy and open Wertheim-Meigs in patients with early-stage cervical cancer: eleven years of experience. International Journal of Gynecologic Cancer, 2010. 20(1). [DOI] [PubMed]
- 46.Lee E-J, Kang H, Kim D-H. A comparative study of laparoscopic radical hysterectomy with radical abdominal hysterectomy for early-stage cervical cancer: a long-term follow-up study. Eur J Obstet Gynecol Reproductive Biology. 2011;156(1):83–6. [DOI] [PubMed] [Google Scholar]
- 47.Sert MB, Abeler V. Robot-assisted laparoscopic radical hysterectomy: comparison with total laparoscopic hysterectomy and abdominal radical hysterectomy; one surgeon’s experience at the Norwegian Radium Hospital. Gynecol Oncol. 2011;121(3):600–4. [DOI] [PubMed] [Google Scholar]
- 48.Taylor SE, McBee WC Jr, Richard SD, Edwards RP. Radical hysterectomy for early stage cervical cancer: laparoscopy versus laparotomy. JSLS: J Soc Laparoendoscopic Surg. 2011;15(2):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gortchev G, Tomov S, Tantchev L, Velkova A, Radionova Z. Robot-assisted radical hysterectomy—perioperative and survival outcomes in patients with cervical cancer compared to laparoscopic and open radical surgery. Gynecol Surg. 2012;9(1):81–8. [Google Scholar]
- 50.Park J-Y, Kim D-Y, Kim J-H, Kim Y-M, Kim Y-T, Nam J-H. Laparoscopic compared with open radical hysterectomy in obese women with early-stage cervical cancer. Obstet Gynecol. 2012;119(6):1201–9. [DOI] [PubMed] [Google Scholar]
- 51.Nam J-H, Park J-Y, Kim D-Y, Kim J-H, Kim Y-M, Kim Y-T. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival outcomes in a matched cohort study. Ann Oncol. 2012;23(4):903–11. [DOI] [PubMed] [Google Scholar]
- 52.Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Laparoscopic versus open radical hysterectomy in patients with stage IB2 and IIA2 cervical cancer. J Surg Oncol. 2013;108(1):63–9. [DOI] [PubMed] [Google Scholar]
- 53.Lim YK, Chia YN, Yam KL. Total laparoscopic Wertheim’s radical hysterectomy versus Wertheim’s radical abdominal hysterectomy in the management of stage I cervical cancer in Singapore: a pilot study. Singap Med J. 2013;54(12):683–8. [DOI] [PubMed] [Google Scholar]
- 54.Campos LS, Francisco Limberger L, Tetelbom Stein A, Nocchi A, Kalil. Postoperative pain and perioperative outcomes after laparoscopic radical hysterectomy and abdominal radical hysterectomy in patients with early cervical cancer: a randomised controlled trial. Trials. 2013;14(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bogani G, Cromi A, Uccella S, Serati M, Casarin J, Pinelli C, Ghezzi F. Laparoscopic versus open abdominal management of cervical cancer: long-term results from a propensity-matched analysis. J Minim Invasive Gynecol. 2014;21(5):857–62. [DOI] [PubMed] [Google Scholar]
- 56.Chen C-H, Chiu L-H, Chang C-W, Yen Y-K, Huang Y-H, Liu W-M. Comparing robotic surgery with conventional laparoscopy and laparotomy for cervical cancer management. Int J Gynecologic Cancer, 2014. 24(6). [DOI] [PubMed]
- 57.Yin X-H, Wang Z-Q, Yang S-Z, Jia H-Y, Shi M. Clinical observation of laparoscopic radical hysterectomy for cervical cancer. Int J Clin Exp Med. 2014;7(5):1373. [PMC free article] [PubMed] [Google Scholar]
- 58.Asciutto KC, Kalapotharakos G, Löfgren M, Högberg T, Borgfeldt C. Robot-assisted surgery in cervical cancer patients reduces the time to normal activities of daily living. Acta Obstet Gynecol Scand. 2015;94(3):260–5. [DOI] [PubMed] [Google Scholar]
- 59.Xiao M, Zhang Z. Total laparoscopic versus laparotomic radical hysterectomy and lymphadenectomy in cervical cancer: an observational study of 13-year experience. Medicine, 2015. 94(30). [DOI] [PMC free article] [PubMed]
- 60.Ditto A, Martinelli F, Bogani G, Gasparri ML, Di Donato V, Zanaboni F, Lorusso D, Raspagliesi F. Implementation of laparoscopic approach for type B radical hysterectomy: a comparison with open surgical operations. Eur J Surg Oncol (EJSO). 2015;41(1):34–9. [DOI] [PubMed] [Google Scholar]
- 61.Park J-Y, Kim D, Suh D-S, Kim J-H, Kim Y-M, Kim Y-T, Nam J-H. The role of laparoscopic radical hysterectomy in early-stage adenocarcinoma of the uterine cervix. Ann Surg Oncol. 2016;23(5):825–33. [DOI] [PubMed] [Google Scholar]
- 62.Shah CA, Beck T, Liao JB, Giannakopoulos NV, Veljovich D, Paley P. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J gynecologic Oncol, 2017. 28(6). [DOI] [PMC free article] [PubMed]
- 63.Corrado G, Vizza E, Legge F, Anchora LP, Sperduti I, Fagotti A, Mancini E, Gallotta V, Zampa A, Chiofalo B. Comparison of different surgical approaches for stage IB1 cervical cancer patients: a multi-institution study and a review of the literature. Int J Gynecologic Cancer, 2018. 28(5). [DOI] [PubMed]
- 64.Guo J, Yang L, Cai J, Xu L, Min J, Shen Y, Xiong Z, Dong W, Bunyamanop V, Wang Z. Laparoscopic procedure compared with open radical hysterectomy with pelvic lymphadenectomy in early cervical cancer: a retrospective study. OncoTargets therapy. 2018;11:5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bogani G, Rossetti D, Ditto A, Martinelli F, Chiappa V, Leone C, Maggiore ULR, Lorusso D, Raspagliesi F. Minimally invasive surgery improves short-term outcomes of nerve-sparing radical hysterectomy in patients with cervical cancer: a propensity-matched analysis with open abdominal surgery. J gynecologic Oncol, 2019. 30(2). [DOI] [PMC free article] [PubMed]
- 66.Matanes E, Abitbol J, Kessous R, Kogan L, Octeau D, Lau S, Salvador S, Gotlieb WH. Oncologic and surgical outcomes of robotic versus open radical hysterectomy for cervical cancer. J Obstet Gynecol Can. 2019;41(4):450–8. [DOI] [PubMed] [Google Scholar]
- 67.Piedimonte S, Czuzoj-Shulman N, Gotlieb W, Abenhaim HA. Robotic radical hysterectomy for cervical cancer: a population-based study of adoption and immediate postoperative outcomes in the United States. J Minim Invasive Gynecol. 2019;26(3):551–7. [DOI] [PubMed] [Google Scholar]
- 68.Yuan Z, Cao D, Yang J, Yu M, Shen K, Yang J, Zhang Y, Zhou H. Laparoscopic vs. open abdominal radical hysterectomy for cervical cancer: a single-institution, propensity score matching study in China. Front Oncol. 2019;9:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z, Chen C, Liu P, Duan H, Liu M, Xu Y, Li P, Zhang W, Jiang H, Bin X. Comparison of oncological outcomes and major complications between laparoscopic radical hysterectomy and abdominal radical hysterectomy for stage IB1 cervical cancer with a tumour size less than 2 cm. Eur J Surg Oncol. 2021;47(8):2125–33. [DOI] [PubMed] [Google Scholar]
- 70.Zaccarini F, Santy A, Dabi Y, Lavoue V, Carcopino X, Bendifallah S, Benbara A, Collinet P, Canlorbe G, Raimond E. Comparison of survival outcomes between laparoscopic and abdominal radical hysterectomy for early-stage cervical cancer: A French multicentric study. J Gynecol Obstet Hum Reprod. 2021;50(2):102046. [DOI] [PubMed] [Google Scholar]
- 71.Jing Z, Qiao L, Dan J, Tianmin C, Shengjun M, Chuqiang S. Comparative study of tumor-free laparoscopic and open surgery in the treatment of early-stage cervical cancer. J Cent South Univ Med Sci. 2023;48(11):1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vázquez-Vicente D, Boria F, Castellanos T, Gutierrez M, Chacon E, Manzour N, Minguez JA, Martin-Calvo N, Alcazar JL, Chiva L. SUCCOR morbidity: complications in minimally invasive versus open radical hysterectomy in early cervical cancer. Int J Gynecologic Cancer, 2024. 34(2). [DOI] [PubMed]
- 73.Kampers J, Gerhardt E, Sibbertsen P, et al. Perioperative morbidity of different operative approaches in early cervical carcinoma: a systematic review and meta-analysis comparing minimally invasive versus open radical hysterectomy. Arch Gynecol Obstet. 2022;306:295–314. 10.1007/s00404-021-06248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Sha-shaMD, Ding, Tian MD, Cui, Zheng-hui MD, Lv YMD, Jiang. Ruo-an MD∗. Efficacy of robotic radical hysterectomy for cervical cancer compared with that of open and laparoscopic surgery: A separate meta-analysis of high-quality studies. Medicine 98(4):p e14171, January 2019. 10.1097/MD.0000000000014171 [DOI] [PMC free article] [PubMed]
- 75.Zhao Y, Hang B, Xiong GW, Zhang XW. Laparoscopic radical hysterectomy in early stage cervical cancer: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech. 2017;27(11):1132–44. [DOI] [PubMed] [Google Scholar]
- 76.Sayed AM, Khalaf AM, Abdelrahim MEA, Elgendy MO. Repurposing of some anti-infective drugs for COVID-19 treatment: A surveillance study supported by an in silico investigation. Int J Clin Pract. 2020;75(4):e13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saeed H, Salem HF, Rabea H, Abdelrahim ME. Effect of human error, inhalation flow, and inhalation volume on dose delivery from Ellipta® dry-powder inhaler. J Pharm Innov. 2019;14(3):239–44. [Google Scholar]
- 78.Nicola M, Elberry A, Sayed O, Hussein R, Saeed H, Abdelrahim M. The impact of adding a training device to familiar counselling on inhalation technique and pulmonary function of asthmatics. Adv therapy. 2018;35(7):1049–58. [DOI] [PubMed] [Google Scholar]
- 79.Elgendy MO, Abdelrahim ME, Eldin RS. Potential benefit of repeated MDI inhalation technique counselling for patients with asthma. Eur J Hosp Pharm. 2015;22(6):318–22. [Google Scholar]
- 80.Saeed H, Mohsen M, Eldin AS, Elberry AA, Hussein RR, Rabea H, Abdelrahim ME. Effects of fill volume and humidification on aerosol delivery during single-limb noninvasive ventilation. Respir Care. 2018;63(11):1370–8. [DOI] [PubMed] [Google Scholar]
- 81.Hassan A, Rabea H, Hussein RR, Eldin RS, Abdelrahman MM, Said AS, Salem HF, Abdelrahim ME. -Vitro Characterization of the Aerosolized Dose During Non-Invasive Automatic Continuous Positive Airway Pressure Ventilation. Pulmonary Therapy. 2016;2:115–26. [Google Scholar]
- 82.Elgendy MO, Hassan AH, Saeed H, Abdelrahim ME, Eldin RS. Asthmatic children and MDI verbal inhalation technique counseling. Pulmonary Pharmacology & Therapeutics; 2020. p. 101900. [DOI] [PubMed]
- 83.Harb HS, Elberry AA, Rabea H, Fathy M, Abdelrahim ME. Performance of large spacer versus nebulizer T-piece in single-limb noninvasive ventilation. Respir Care. 2018;63(11):1360–9. [DOI] [PubMed] [Google Scholar]
- 84.Madney YM, Fathy M, Elberry AA, Rabea H, Abdelrahim ME. Nebulizers and spacers for aerosol delivery through adult nasal cannula at low oxygen flow rate: An in-vitro study. J Drug Deliv Sci Technol. 2017;39:260–5. [Google Scholar]
- 85.Vecellio L, Abdelrahim ME, Montharu J, Galle J, Diot P, Dubus J-C. Disposable versus reusable jet nebulizers for cystic fibrosis treatment with tobramycin. J Cyst Fibros. 2011;10(2):86–92. [DOI] [PubMed] [Google Scholar]
- 86.Zawbaa HM, Osama H, El-Gendy A, Saeed H, Harb HS, Madney YM, Abdelrahman M, Mohsen M, Ali AMA, Nicola M, Elgendy MO, Ibrahim IA, Abdelrahim MEA. Effect of mutation and vaccination on spread, severity, and mortality of COVID-19 disease. J Med Virol. 2022;94(1):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ott DE. Subcutaneous emphysema—beyond the pneumoperitoneum. J Soc Laparoendoscopic Surg. 2014;JSLS(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cuss A, BM BM. Coming to TermsWith the Fact That the Evidence for Laparoscopic Entry Is as Good as It Gets. Volume 10. JMIG; 2014. pp. 23–30. [DOI] [PubMed]
- 89.Thepsuwan J, Huang KG, Wilamarta M, Adlan AS, Manvelyan V, Lee CL. Principles of safe abdominal entry in laparoscopic gynecologic surgery. Gynecol Minim Invasive Therapy. 2013;2(4):105–9. [Google Scholar]
- 90.Van der Voort M, Heijnsdijk E, Gouma D. Bowel injury as a complication of laparoscopy. J Br Surg. 2004;91(10):1253–8. [DOI] [PubMed] [Google Scholar]
- 91.Llarena NC, Shah AB, Milad MP. Bowel injury in gynecologic laparoscopy: a systematic review. Obstet Gynecol. 2015;125(6):1407–17. [DOI] [PubMed] [Google Scholar]
- 92.Yim GW, Kim SW, Nam EJ, Kim S, Kim YT. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J gynecologic Oncol. 2013;24(4):303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chong GO, Park NY, Hong DG, Cho YL, Park IS, Lee YS. Learning curve of laparoscopic radical hysterectomy with pelvic and/or para-aortic lymphadenectomy in the early and locally advanced cervical cancer: comparison of the first 50 and second 50 cases. Int J Gynecologic Cancer, 2009. 19(8). [DOI] [PubMed]
- 94.Hwang JH. Urologic complication in laparoscopic radical hysterectomy: meta-analysis of 20 studies. Eur J Cancer. 2012;48(17):3177–85. [DOI] [PubMed] [Google Scholar]
- 95.Roh HF, Nam SH, Kim JM. (2018). Robot-assisted laparoscopic surgery versus conventional laparoscopic surgery in randomized controlled trials: a systematic review and meta-analysis. PLoS ONE, 13(1), e0191628. [DOI] [PMC free article] [PubMed]
- 96.Guo X, Tian S, Wang H, Zhang J, Cheng Y, Yao Y. Outcomes associated with different surgical approaches to radical hysterectomy: A systematic review and network meta-analysis. International Journal of Gynecology & Obstetrics; 2022. [DOI] [PubMed]
- 97.Zeng Z, Liu J, Lv T, Feng Z, Zhang L, Liao Q. Evaluation of the efficacy of laparoscopic-assisted radical vaginal hysterectomy and abdominal radical hysterectomy for treating cervical cancer: a meta-analysis. Videosurgery Other Miniinvasive Techniques. 2022;17(1):69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang S, Wang S, Lv A, Huang S. Laparoscopically assisted radical vaginal hysterectomy for early-stage cervical cancer: a systemic review and meta-analysis. Int J Gynecologic Cancer, 2016. 26(8). [DOI] [PubMed]
- 99.Zhang S, Ma L, Meng QW, Zhou D, Moyiding T. Comparison of laparoscopic-assisted radical vaginal hysterectomy and abdominal radical hysterectomy in patients with early stage cervical cancer: a retrospective study. Medicine, 2017. 96(36). [DOI] [PMC free article] [PubMed]
- 100.Pergialiotis V, Rodolakis A, Christakis D, Thomakos N, Vlachos G, Antsaklis A. Laparoscopically assisted vaginal radical hysterectomy: systematic review of the literature. J Minim Invasive Gynecol. 2013;20(6):745–53. [DOI] [PubMed] [Google Scholar]
- 101.Smith AJB, Jones TN, Miao D, Fader AN. Minimally invasive radical hysterectomy for cervical cancer: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2021;28(3):544–55. e7. [DOI] [PubMed] [Google Scholar]
- 102.Li Y, Kong Q, Wei H, Wang Y. Comparison of the complications between minimally invasive surgery and open surgical treatments for early-stage cervical cancer: A systematic review and meta-analysis. PLoS ONE. 2021;16(7):e0253143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ronsini C, Köhler C, De Franciscis P, La Verde M, Mosca L, Solazzo MC, Colacurci N. Laparo-assisted vaginal radical hysterectomy as a safe option for Minimal Invasive Surgery in early stage cervical cancer: A systematic review and meta-analysis. Gynecologic Oncology; 2022. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
On request, the corresponding author is required to provide access to the meta-analysis database.